Introduction
Anaplastic thyroid carcinoma (ATC) is one of the
most aggressive human malignancies and has a poor prognosis
(1–3). The average survival time of patients
with ATC is approximately 3–4.5 months (1–3), with
a 1-year survival rate of only 16–20% (1–3). Once
diagnosed, a large number of ATC tumors cannot be radically
resected because the disease is too advanced. There are currently
no effective chemotherapy regimens for ATC, and the majority of
patients diagnosed with ATC will succumb to the disease (1–3).
Doxorubicin has been used to treat ATC, but has only
achieved modest effects (4,5). A few effective chemotherapy regimens
and drugs have recently been approved as treatments for ATC. A
phase 2 trial on paclitaxel for ATC patients reported an overall
response rate (ORR) of 56% (6).
Another study demonstrated that the weekly administration of
paclitaxel for ATC was effective and safe; the ORR was 21% and the
clinical benefit rate was 73% (7).
Lenvatinib is a multikinase inhibitor, and its targets are vascular
endothelial growth factor receptors 1–3, fibroblast growth factor
receptors 1–4, plated-derived growth factor receptor-α, and the RET
and KIT proto-oncogenes (8). In
Japan, lenvatinib was approved as a treatment for radically
unresectable thyroid cancer, including ATC, in 2015 (3). A phase 2, single-arm, open-label study
on lenvatinib conducted in Japan reported that it resulted in a
median progression-free survival period of 7.4 months, a median
overall survival period of 10.6 months, and an ORR of 24% (3,8).
ATC is rare; i.e., it only accounts for 1–2% of all
thyroid carcinomas (9,10). The aggressive characteristics and
rarity of ATC make it difficult to accumulate a sufficient number
of cases for clinical studies aimed at developing new ATC
treatments. The phase 2 trials on paclitaxel (6) and Lenvatinib (8) for ATC patients aforementioned included
limited numbers of patients (n=17-19). Therefore, the development
and establishment of suitable preclinical tumor models that may be
used to examine the effects of anticancer drugs against ATC are
required.
Several animal models of thyroid carcinoma have been
created, for example, models involving the subcutaneous or
orthotopic implantation of cancer cells into immunodeficient mice
(11–13). In 1889, Paget proposed the ‘seed and
soil’ theory, in which an organ-specific site provides tumor cells
with an appropriate environment for local growth and metastasis
(14). Orthotopic tumor models may
provide a more relevant biological setting than subcutaneous
xenograft models in terms of the tumor microenvironment (12–18).
However, the main disadvantage of orthotopic tumor
models is that difficulties are associated with assessing changes
in tumor size, except at necropsy. Therefore, it is impossible to
repeatedly and continuously monitor the same model. It was
theorized that the use of small-animal positron-emission
tomography/computed tomography (PET/CT) may overcome these
disadvantages. Our group previously performed the non-invasive
monitoring of the anticancer effects of cisplatin on lung cancer in
an orthotopic SCID mouse model using 18F-fludeoxyglucose
(FDG) PET/CT (19). We also
monitored the anticancer effects of cisplatin and erlotinib in an
orthotopic SCID mouse model of lung cancer using both CT and PET/CT
(20).
ATC typically exhibits high glucose metabolism and
high FDG uptake in both primary and metastatic lesions (21,22).
The American Thyroid Association recommends the use of PET/CT to
evaluate distant metastatic disease (21). FDG PET/CT may be used for initial
staging, early evaluations of treatment responses, and follow-ups
(22). In patients with ATC, PET/CT
is useful for directing clinical management and evaluating the
efficacy of therapy (21,22).
In the present study, orthotopic tumor xenograft
models were established using ATC cell lines and SCID mice. Tumor
growth was also evaluated using PET/CT to repeatedly and
non-invasively monitor these models. Furthermore, the antitumor
effects of paclitaxel and lenvatinib against ATC were investigated
with PET/CT.
Materials and methods
Cell lines
Three ATC cell lines were used: ACT-1, 8305c, and
8505c. The ACT-1 cell line was established at our institute
(Tokushima University, Tokushima, Japan). The 8305c and 8505c cell
lines were provided by the BioResource Center of RIKEN. The ACT-1
and 8305c cell lines were cultured in Roswell Park Memorial
Institute-1640 medium (Nissui Pharmaceutical Co., Ltd.), and the
8505c cell line in Minimum Essential Medium (Sigma-Aldrich; Merck
KGaA) supplemented with 10% fetal bovine serum (Bio-West), 100
IU/ml of penicillin, and 100 µg/ml of streptomycin (Gibco;
Life Technologies; Thermo Fisher Scientific, Inc.). Cells were
maintained at 37°C in a humidified incubator equilibrated with 5%
CO2 and 95% air. Cells were grown in tissue culture
flasks, and passaged at 5- to 7-day intervals at 80% confluence.
Cell morphology and viability were monitored by microscopic
observations. All cell lines were used after being propagated
between 10 and 20 times. Information on all cell lines was
confirmed and checked using the International Cell Line
Authentication Committee (https://iclac.org/) and ExPASy Cellosaurus databases
(https://web.expasy.org/cellosaurus/).
Drugs
Paclitaxel was purchased from Fujifilm Wako Pure
Chemical Corporation and lenvatinib from LC Laboratories.
Cell viability after drug
exposure
Cells (ACT-1 cells: 4×103; 8305c and
8505c cells: 2×103) were seeded on 96-well plastic
culture plates (100 µl/well). ACT-1 cells were maintained for 48 h,
and 8305c and 8505c cells for 24 h at 37°C in a humidified
incubator with 5% CO2 and 95% air. They were treated
with the intended doses of each agent for 48 h. After the
incubation period,
3-(4,5-dimrthylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT;
Nacalai Tesque, Inc.) was added at a final concentration of 0.5
mg/ml, and cells were incubated for 4 h under the same conditions
as aforementioned. Dimethyl sulfoxide (Nacalai Tesque, Inc.) was
added, and spectrophotometric absorbance was measured at 535 nm
with a microplate reader (Spectra Max i3; Molecular Devices, LLC)
and calculated using the supplied software (SoftMax Pro, version
7). The control sample was untreated and incubated in an equal
volume of medium (100 µl/well). Each experiment was performed three
times independently, and the mean values for the three independent
experiments were calculated. The concentrations of the agents at
which cell growth was inhibited by 50% (IC50 values)
were assessed using growth-inhibition curves.
Animals
As described in our previous studies involving an
orthotopic mouse model of lung cancer (19,20),
female nine SCID mice (CB-17/Icr-scidJcl; CLEA Japan, Inc.) (age,
6–8 weeks; mean body weight, 20 g) were purchased and maintained at
our institution's laboratory for animal experiments (Tokushima
University, Tokushima, Japan). Animals were housed in
Micro-Isolator cages on a layer of wood shavings at 22°C and 55%
humidity under a fixed 12-h light/dark regime. A basic diet (MF;
Oriental Yeast Co.) and water were available ad libitum. All
studies were performed in accordance with the guidelines
established by the Tokushima University Committee on Animal Care
and Use. At the end of each in vivo experiment, mice were
anesthetized and then euthanized humanely by vertebral dislocation
(20) or, mice were euthanized when
they had lost >20% of their baseline body weight or had signs of
tracheal compressive symptoms. Death was confirmed by observation
of respiratory and cardiac arrest. All experimental protocols were
reviewed and approved by the Animal Research Committee of the
University of Tokushima, Japan. All sections of this study adhered
to the ARRIVE Guidelines for reporting animal research (23).
Orthotopic mouse model of anaplastic
thyroid cancer
On the day of tumor implantation, cells were
trypsinized, centrifuged (140 × g, 5 min), and resuspended in
serum-free media to produce ACT-1, 8305c, and 8505c cell
suspensions with a cell density of 5×105/µl cells, and
0.4 mg/µl Matrigel (Collaborative Biochemical Products) was then
added to each cell suspension. Cell suspensions were maintained on
ice until the implantation procedure. As described in our previous
studies involving an orthotopic mouse model of lung cancer
(19,20), 9 mice were fully anesthetized via
the inhalation of 1.5% isoflurane, before being injected with tumor
cells. A 1-cm transverse incision was produced along the middle of
the throat. The salivary glands were reflected laterally, the
trachea was exposed, and the overlaying strap muscles were
dissected away from the right thyroid lobe. Once the right thyroid
lobe was exposed, the cell suspension (5×105 cells in 2
µl of medium (Minimum Essential Medium; Sigma-Aldrich; Merck KGaA)
was injected into the right thyroid gland using a Hamilton syringe
(Thermo Fisher Scientific, Inc.) attached to a 30-gauge needle.
After the injection of the cell suspension, the salivary glands
were repositioned and the skin incision was closed with 3-0 silk
sutures. After the implantation, all mice were weighed and
monitored for signs of distress regularly every week.
FDG PET/CT-based analysis of tumor
volumes and maximum standardized uptake value
(SUVmax)
As described in our previous studies (19,20),
FDG PET/CT scans were performed with a Siemens Inveon small-animal
PET scanner (Siemens Healthcare). Mice that had been orthotopically
implanted with 8505c cells were fasted for 12 h, but were allowed
free access to water. Their body weights were measured, and mice
were then anesthetized via the inhalation of 1.5–2.0% isoflurane,
before 10 MBq/0.1–0.2 ml FDG was injected via a tail-vein catheter.
The whole body of each mouse was scanned using CT [field of view:
32.0×32.0×48.1 mm3]. PET data were acquired for 20 min
after a delay of 40 min to allow for FDG uptake. PET/CT images were
analyzed with PMOD version 3.7 (PMOD Technologies LLC). In each
PET/CT scan, the metabolic tumor volume (MTV) was calculated based
on the volume of interest (VOI), which was drawn manually around
the primary tumor on PET/CT images after referring to CT images.
Since the boundary between the thyroid gland and surrounding
structures was unclear on CT alone, MTV was used as the tumor
volume in the present study. On fused PET images, SUVmax
was calculated from the maximum voxel value (Bq/ml) in the VOI.
Field-of-view of coronal-view in the PET-CT images was 10×10 cm,
and the scale was calculated based on it.
Evaluation of tumor growth in mice
that had been orthotopically implanted with 8505c cells
The period of the tumor growth evaluation study was
set from the day of tumor implantation to 5 weeks after
implantation. In weeks 3 to 5 after the implantation of 8505c
cells, PET/CT measurements on the no treatment group [n=3] were
performed every 7 days.
Evaluation of the inhibition of tumor
growth in mice that had been orthotopically implanted with 8505c
cells
The period of the tumor growth inhibition study was
set from the day of tumor implantation to 4 weeks after
implantation, since the body weight loss of the non-treated mice
was 18 and 28% at 4 and 5 weeks after the implantation procedure,
respectively.
Two weeks after the implantation of 8505c cells, 6
mice were subjected to PET/CT measurements. To select drug doses,
we referred to a study by Jing et al (24). The interval of paclitaxel
administration was modified according to our preliminary
examination. Jing et al administered paclitaxel twice per
week. However, a sufficient antitumor effect was confirmed even
once a week and marked weight loss was observed when paclitaxel was
administered twice per week in the present study. Therefore, we
adopted a weekly administration regimen for the paclitaxel group
[n=3, 5 mg/kg, intraperitoneal administration, every week], and
daily administration regimen for the lenvatinib group [n=3, 5
mg/kg, oral administration, every day]. PET/CT measurements were
performed every 7 days from 2 to 4 weeks after the implantation
procedure.
Histological examinations
We sacrificed the mice and en bloc resected
the bilateral thyroid glands, bilateral salivary glands, trachea,
bilateral lungs, and mediastinal region. The tumors implanted in
the thyroid and surrounding organs were cut into 1–2-mm-thick
pieces in the horizontal direction. These pieces were fixed with
10% formalin at 22°C for one week and embedded in paraffin blocks.
Paraffin sections that had been stained with hematoxylin and eosin
(H&E) were examined under an Olympus BX51 polarizing microscope
(Olympus Corporation; magnifications, ×20 and ×200). It was not
possible to perform a pathological examination on the mice
subjected to PET/CT because their removal from the PET/CT facility
was prohibited in order to prevent radiation exposure.
Statistical analysis
Due to animal protection concerns, experiments were
conducted with the minimum sample size. Statistical methods based
on the standard maximum likelihood were not applicable. As an
alternative, the significance of differences in tumor volume,
SUVmax, and body weight were assessed using the mean
data for three mice.
Results
Cell viability after drug
exposure
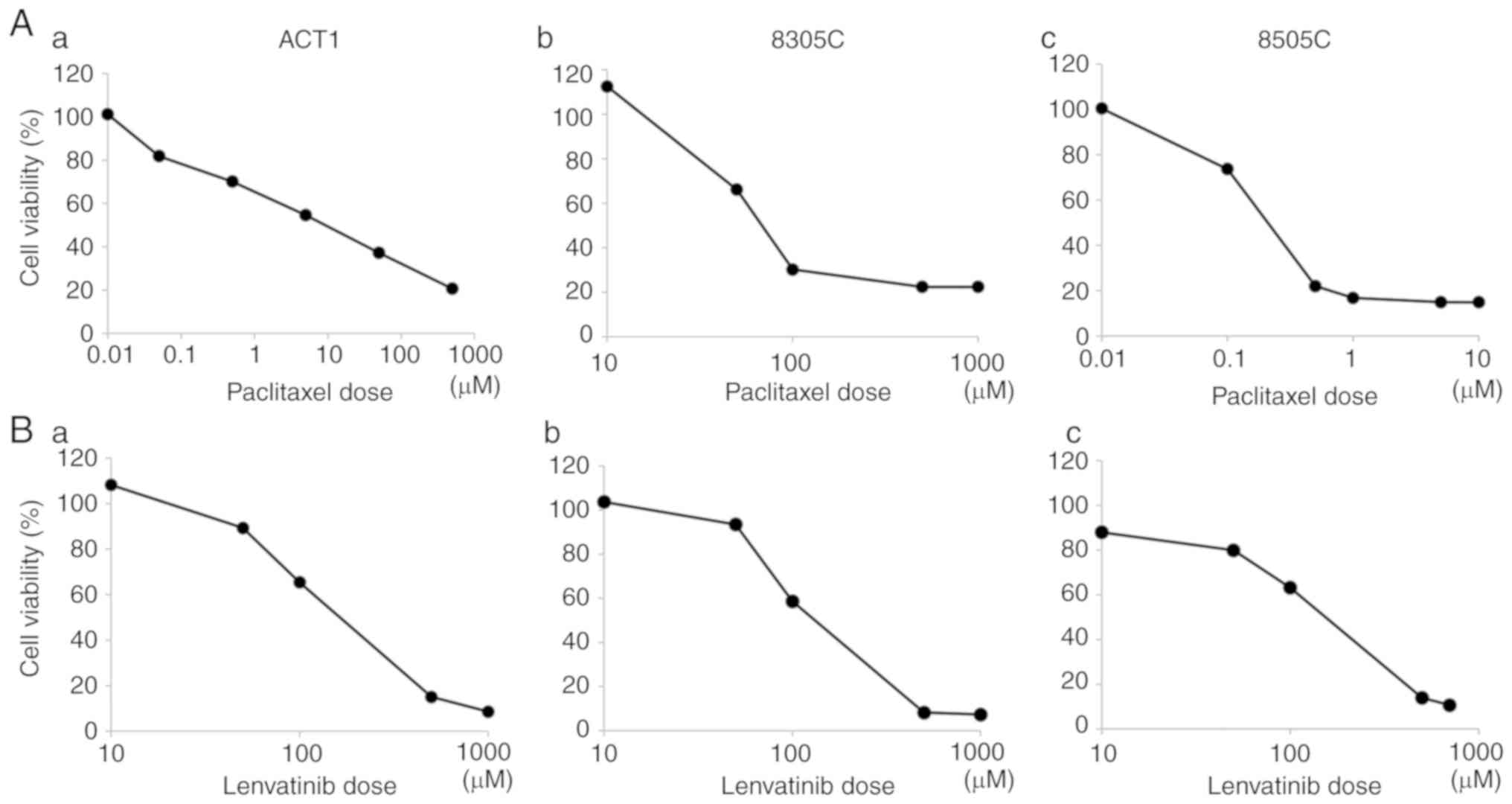
The results of the cell viability assay are
presented in Fig. 1. The paclitaxel
and lenvatinib treatments both dose-dependently inhibited cell
viability in all cell lines. Among the three cell lines assessed,
8505c cells exhibited the highest sensitivity and lowest
IC50 (250 nM/l) to paclitaxel (Fig. 1A). All cell lines displayed similar
levels of sensitivity to lenvatinib (Fig. 1B).
Tumor growth and SUVmax in the 8505c
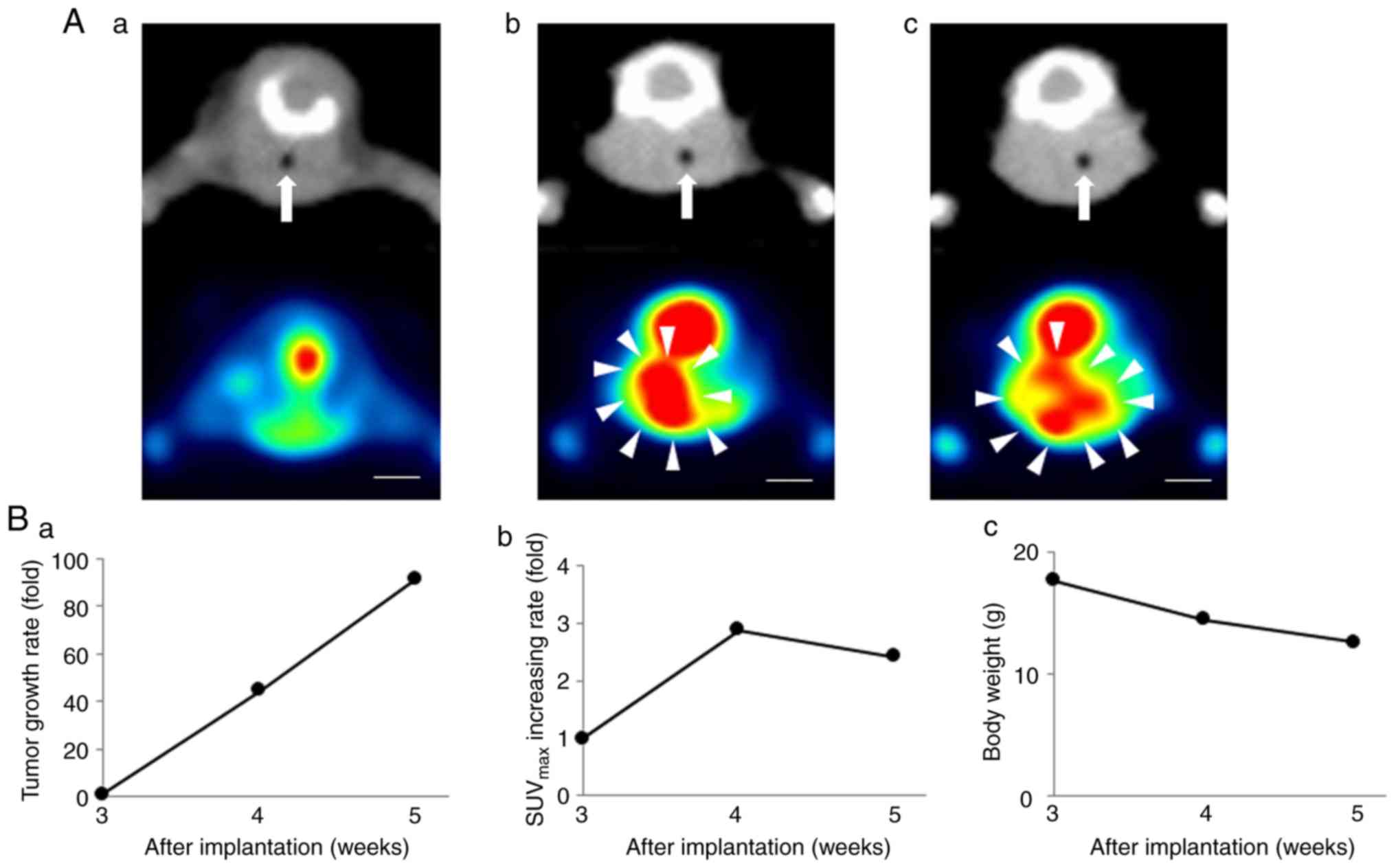
cell-based orthotopic mouse model. In the orthotopic 8505c
cell-based model mice, there was no FDG uptake in thyroid tissue 3
weeks after the implantation procedure on PET/CT (Fig. 2A-a). FDG uptake was observed in the
right thyroid lobe 4 weeks after the implantation procedure on
PET/CT, which also revealed lateral deflection of the trachea due
to increases in the size of the tumors 4 weeks after the
implantation procedure (Fig. 2A-b).
Mean tumor volumes were 44.6- and 91.5-fold higher at 4 and 5 weeks
after the implantation procedure, respectively, than those at 3
weeks after the implantation procedure (Fig. 2B-a). The maximum tumor volume and
diameter at 5 weeks after the implantation were 1042.9±58.3
mm3 and 13.3±0.6 mm, respectively (Fig. 2A-c). FDG uptake increased by
2.8-fold from 3 to 4 weeks after the implantation procedure;
however, decreased FDG uptake was observed after 5 weeks (Fig. 2B-b). On coronal PET/CT images, FDG
uptake inside the tumors had decreased at 5 weeks (Fig. 2A-c). The body weights of the mice
decreased to a maximum 28% (Fig.
2B-c). Gross examinations of the tumors revealed that they were
approximately 1.5 cm in diameter (Fig.
3A), and necrotic changes were pathologically observed in the
central parts of the tumors (Fig.
3B-a) 5 weeks after the implantation procedure. The mice in the
tumor growth evaluation experiment were anesthetized and then
euthanized humanely by vertebral dislocation at the end of the
experiment 5 weeks after the implantation procedure, and there was
no accidental death during the experiment. Orthotopic implantation
was also performed with ACT-1 and 8305c cells as well as 8505c
cells. Although tumors were pathologically diagnosed in these mouse
models, no clear macroscopic tumor formation was observed 6 weeks
after the implantation of ACT-1 or 8305c cells (data not shown).
Since tumors derived from ACT-1 or 8305c cells grew markedly slower
than those derived from 8505c cells, the mouse models involving the
implantation of ACT-1 or 8305c cells were not considered to be
appropriate for assessing the antitumor effects of chemotherapy.
Thus, PET/CT-based examinations on tumor growth and the antitumor
effects of chemotherapy were only performed using the 8505c
cell-based orthotopic mouse model.
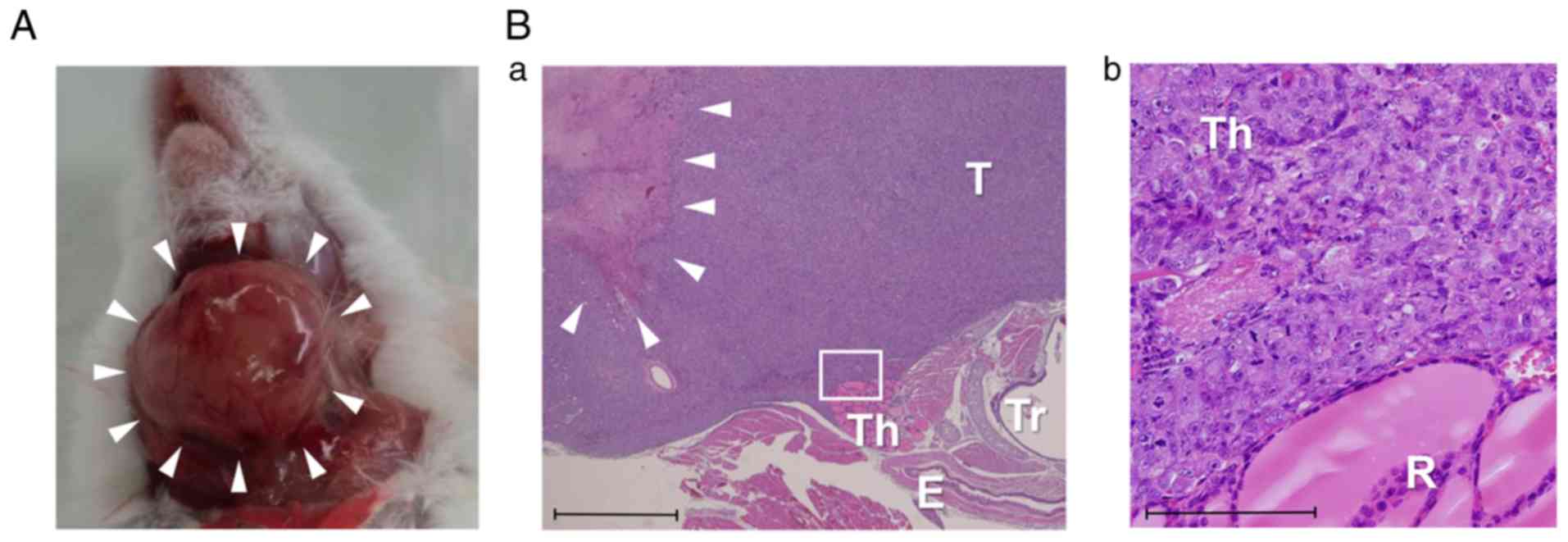 | Figure 3.Histological findings. (A) Gross
examination of a tumor derived from 8505c cells performed 5 weeks
after implantation. (B-a) Histological sections of tumors derived
from 8505c cells. H&E staining (magnification, ×20). Th,
thyroid lobe; T, tumor; E, esophagus; Tr, trachea; arrows, necrotic
changes in the central part of the tumor. scale bar, 1,000 µm.
(B-b) Magnified image of the white square presented in B-a. H&E
staining (magnification, ×200). Th, thyroid lobe; T, tumor. Scale
bar, 100 µm. H&E, hematoxylin and eosin. |
Antitumor effects of paclitaxel and lenvatinib in
the 8505c cell-based orthotopic mouse model according to FDG PET/CT
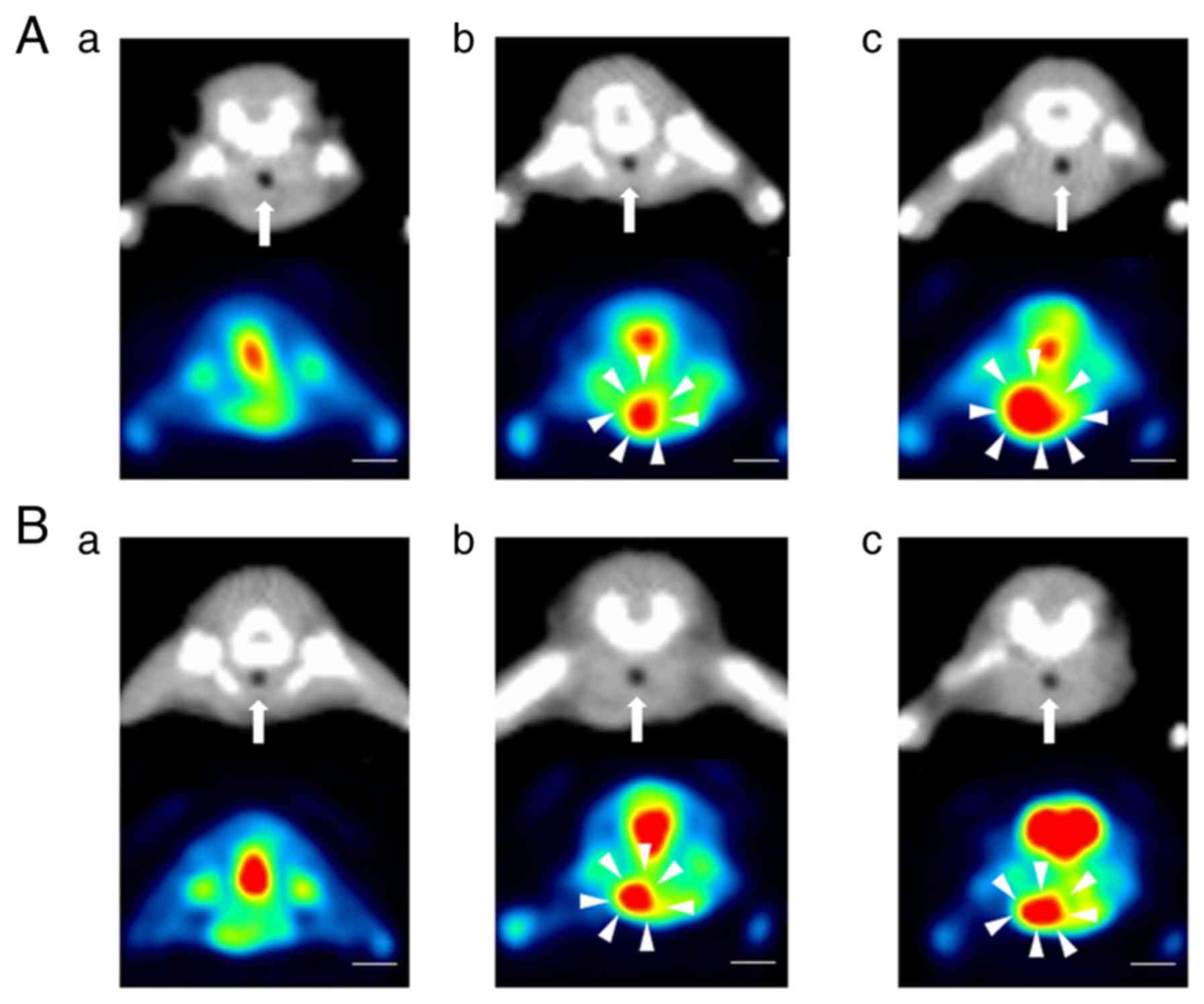
imaging. We examined the antitumor effects of paclitaxel and
lenvatinib in mice that had been implanted with 8505c cells using
PET/CT. No tumors were detected on PET/CT 2 weeks after the
implantation procedure in the paclitaxel or lenvatinib group
(Fig. 4A-a and B-a). FDG uptake was
observed at the putative tumor sites in both treated groups 3 weeks
after the implantation procedure (Fig.
4A-b and B-b). The size of the tumors on coronal PET/CT images
increased from 3 to 4 weeks after implantation in both treated
groups, whereas tumor growth rates were markedly slower than that
in the no treatment group (Fig. 4A-c
and B-c). Throughout the treatment period, the increases
observed in tumor volume and FDG uptake in the control group were
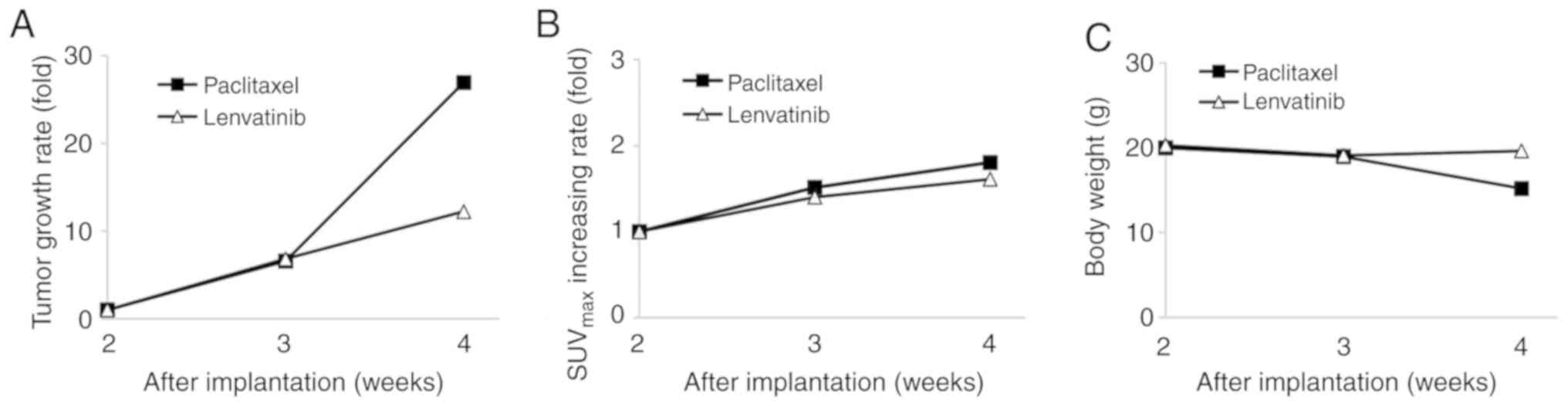
suppressed in both treated groups. In the paclitaxel group, the
mean tumor volumes were 6.6- and 26.9-fold higher at 3 and 4 weeks
after the implantation procedure, respectively, than that at 2
weeks after the implantation procedure (Fig. 5A). In the paclitaxel group, the
maximum tumor volume and diameter at 4 weeks after the implantation
were 595.4±190.0 mm3 and 8.7±1.5 mm, respectively
(Fig. 4A-c). In the lenvatinib
group, the mean tumor volumes were 6.8- and 12.2-fold higher at 3
and 4 weeks after the implantation procedure, respectively, than
that at 2 weeks after the implantation procedure (Fig. 5A). In the lenvatinib group, the
maximum tumor volume and diameter at 4 weeks after the implantation
were 216.2±83.9 mm3 and 6.7±0.6 mm, respectively
(Fig. 4B-c). In the paclitaxel
group, FDG uptake increased by 1.5- and 1.8-fold from 2 to 3 weeks
and from 2 to 4 weeks after the implantation procedure,
respectively. In the lenvatinib group, FDG uptake increased by 1.4-
and 1.6-fold from 2 to 3 weeks and from 2 to 4 weeks after the
implantation procedure, respectively (Fig. 5B). A marked antitumor effect was
observed from 3 to 4 weeks after the implantation procedure in the
lenvatinib group (Fig. 5A and B).
The maximum body weight loss of the mice in the lenvatinib group
was 3%, whereas the maximum weight loss in the paclitaxel group was
24% at 4 weeks after the implantation procedure (Fig. 5C). The mice in the tumor growth
inhibition experiment were euthanized at 4 weeks after the
implantation procedure.
Discussion
In the present study, the growth of tumors in an
orthotopic mouse model that had been implanted with 8505c cells was
monitored using FDG PET/CT. Th mean tumor volumes were 44.6- and
91.5-fold higher 4 and 5 weeks after the implantation procedure,
respectively, than that 3 weeks after the implantation procedure.
The rapid growth of these tumors reflects the aggressive
characteristics of ATC. Furthermore, the trachea, esophagus, and
other surrounding tissues were displaced as the tumors grew, which
is consistent with the clinical course of ATC (25). Conversely, although FDG uptake
increased from 3 to 4 weeks after the implantation procedure, it
decreased from 4 to 5 weeks. FDG uptake was also only observed at
the margins of the tumors, and was not detected in the center of
the tumors on PET/CT images obtained 5 weeks after the implantation
procedure. Histological examinations on tumors performed 5 weeks
after the implantation procedure revealed necrotic changes in the
central parts, which were surrounded by viable tumor cells. Central
tumor necrosis is one of the typical histological changes
associated with ATC (25,26). The decrease observed in tumor FDG
uptake from 4 to 5 weeks after the implantation procedure was
considered to have been caused by necrotic changes. Therefore, the
tumor volume, rather than FDG uptake, needs to be measured as an
index of tumor growth in orthotopic mouse models involving the
implantation of 8505c cells.
We previously reported that FDG PET/CT is useful for
monitoring antitumor effects in an orthotopic lung cancer model
(19,20). In these studies, the tumor volume
was easy to calculate based on CT images since the CT values of the
tumors and lung fields were different (19,20).
Conversely, in the present study, the boundaries between the tumors
and the surrounding structures of the thyroid gland were unclear on
CT images because their CT values were similar. Therefore, MTV,
which was calculated based on the area of FDG uptake, was adopted
as the tumor volume in the present study. MTV has been reported as
a marker that increases the accuracy of prognostication and
clinical staging in human tumors (27–29).
Manohar et al reported that MTV reflected a higher tumor
burden for patients receiving multikinase inhibitor therapy for
metastatic radioiodine-refractory differentiated thyroid cancer
(29). PET and CT were both
considered to be important for evaluating tumors in orthotopic
xenograft models involving the implantation of ATC cell lines into
SCID mice.
The antitumor effects of paclitaxel and lenvatinib
in an orthotopic mouse model that had been implanted with 8505c
cells were examined using PET/CT. In the chemotherapy groups, FDG
uptake and tumor volume were both lower than those in the no
chemotherapy group. Therefore, the present study demonstrated that
FDG uptake and MTV may be used to assess the effects of anticancer
drugs in orthotopic mouse models involving the implantation of
8505c cells. A previous study reported that lenvatinib exerted
antitumor and antiangiogenic effects in numerous thyroid cancer
xenograft models, including ATC models (30). Furthermore, Jing et al
demonstrated the effects of lenvatinib and paclitaxel in
subcutaneous xenograft models of ATC (24). The effects of lenvatinib and
paclitaxel in an orthotopic tumor xenograft model of ATC were
confirmed in the present study. In addition, the effects of the two
drugs were objectively demonstrated using PET/CT. This is the first
study, to the best of our knowledge, to demonstrate the antitumor
effects of paclitaxel and lenvatinib in an orthotopic tumor
xenograft model of ATC with PET/CT.
Orthotopic tumor models provide a suitable
preclinical environment for evaluating the effects of anticancer
drugs (15). The main disadvantage
of using orthotopic tumor models for this purpose is the difficulty
associated with monitoring tumor size. Small-animal PET/CT was
employed to overcome this issue. The use of PET/CT had the
following advantages. The main advantage was that any changes in
tumor size may be continuously evaluated in each mouse using
PET/CT. By monitoring the changes in tumor size that occurred in
each individual mouse, we were able to clearly assess tumor growth
and the therapeutic effects of the anticancer drugs. Conversely,
previous studies (16,17) that evaluated the therapeutic effects
of anticancer drugs using orthotopic thyroid cancer animal models
required the sacrifice of more than 6 mice at each time-point to
ensure the quality of the data obtained; however, the resultant
data had no real continuity. Nehas et al revealed that
anti-BRAFV600E therapy inhibited tumor growth in an orthotopic
mouse model that had been implanted with 8505c cells (16). Forty-eight mice were orthotopically
implanted with ATC cells and divided into 8 groups of 6 mice for
the purpose of establishing the time course of tumor progression
and the response to late therapeutic interventions with PLX4720,
which selectively inhibits BRAFV600E. Each week, 6 mice were
sacrificed, and tumor growth was evaluated to assess the
effectiveness of PLX4720. Cha et al (17) investigated the effects of a
perioperative treatment with echinomycin, which is a
hypoxia-inducible factor-1α inhibitor, using an orthotopic surgical
mouse model of thyroid cancer. In the latter study, 60 mice were
implanted with thyroid cancer cell lines. Mice were divided into
four groups of 15 mice: A control group, surgical group,
echinomycin group, and surgical and echinomycin group, and were
sacrificed to evaluate the effects of each treatment. In the
present study, only 9 mice were required to evaluate tumor growth
and the effects of the two drugs. Therefore, our PET/CT-based
method appears to be an ideal preclinical experimental system from
the point of view of animal welfare.
We were also able to detect tumor formation earlier
than previous studies involving mouse models in which 8505c cells
were orthotopically implanted. Nucera et al demonstrated
that tumors became palpable 35 days after the implantation of 8505c
cells (12). However, by using
PET/CT, FDG uptake on the right side of the trachea was observable
21 days after the implantation procedure in the treatment group.
Thus, whether each orthotopic mouse model was suitable was
confirmed at an early stage.
Moreover, continuous detailed examinations of tumor
growth and its effects on the surrounding organs were possible in
the same model using PET/CT. Previous studies demonstrated that
orthotopic models involving the implantation of 8505c cells
produced large tumors, which resulted in cachexia and death by 35
days after the implantation procedure (12,16).
Similar findings to those described in these studies were
reproduced in our models, and we were able to observe gradual
increases in tumor size that displaced the trachea and surrounding
tissues using PET/CT. However, when the neck tumor exceeds a
certain size, esophageal compression by the tumor causes
insufficient oral intake and rapid weight loss. Therefore,
conducting experiments within the range of weight loss allowed by
the experimental protocols requires careful experimental period
setting and mouse monitoring.
Paclitaxel and lenvatinib both improved the
prognosis of ATC patients. However, the effects of these therapies
are limited. The combination of chemotherapy and molecular targeted
agents was recently evaluated in various cancer fields (31). Combination therapy with paclitaxel
and lenvatinib may improve the treatment outcomes of ATC, and our
model has the potential to become an effective tool for the
development of this new therapy.
In conclusion, in the present study, the utility of
FDG PET-CT for repeated and non-invasive evaluations of tumor
progression and the effects of antitumor drugs (paclitaxel and
lenvatinib) in orthotopic ATC models were demonstrated. The methods
presented in this study may facilitate the development of new ATC
treatments.
Acknowledgments
The authors would like to thank Mr. Toshio Yamaguchi
and Ms Kana Tominaga for their technical assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HT had full access to all data in the study and
takes responsibility for the integrity of the data and accuracy of
the data analysis. MA, TO, and SI performed the experiments, and NK
analyzed the data. MT, KK, and AT designed the study. YB and HU
contributed to histological examinations. MA contributed to data
analysis and interpretation and writing of the manuscript.
Ethics approval and consent to
participate
All studies were performed in accordance with the
guidelines established by the Tokushima University Committee on
Animal Care and Use. The present study was conducted according to
the ARRIVE guidelines and AVMA euthanasia guidelines 2013. All
experimental protocols were reviewed and approved by the Animal
Research Committee of the University of Tokushima.
Patient consent for publication
Not applicable.
Author information
Mariko Aoyama, E-mail: aoyama.mariko@tokushima-u.ac.jp
Competing interests
The authors declare that there are no competing
interests that may be perceived as prejudicing the impartiality of
the reported research.
References
|
1
|
Kebebew E, Dreenspan FS, Clark OH, Woeber
KA and McMillan A: Anaplastic thyroid carcinoma. Treatment outcome
and prognostic factors. Cancer. 103:1330–1335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tennvall J, Lundell G, Wahlberg P,
Bergenfelz A, Grimelius L, Akerman M, Hjelm Skog AL and Wallin G:
Anaplastic thyroid carcinoma: Three protocols combining
doxorubicin, hyperfractionated radiotherapy and surgery. Br J
Cancer. 12:1848–1853. 2002. View Article : Google Scholar
|
|
3
|
Sugitani I, Onoda N, Ito K and Sugitabi S:
Management of anaplastic thyroid carcinoma: Fruits from ATC
Research Consortium of Japan. J Nippon Med Sch. 85:18–27. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimaoka K, Schoenfeld DA, DeWys WD,
Creech RH and DeConti R: A randomized trial of doxorubicin versus
doxorubicin plus cisplatin in patients with advancer thyroid
carcinoma. Cancer. 56:2155–2160. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahuja S and Emst H: Chemotherapy of
thyroid carcinoma. J Endocrinol Invest. 3:303–310. 1987. View Article : Google Scholar
|
|
6
|
Ain KB, Egorin MJ and DeSimone PA:
Treatment of anaplastic thyroid carcinoma with paclitaxel: Phase 2
trial using ninety-six-hour infusion. Collaborative Thyroid Cancer
Health Intervention Trials (CATCHIT) Group. Thyroid. 10:587–594.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Onoda N, Sugino K, Higashiyama T, Kammor
i, Toda K, Ito K, Yoshida A, Suganuma N, Nakashima N, Suzuki S, et
al: The safety and efficacy of weekly paclitaxel administration for
anaplastic thyroid cancer patients: A nationwide prospective study.
Thyroid. 26:1293–1299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tahara M, Kiyota N, Yamazaki T, Chayara N,
Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Y, et al:
Lenvatinib for anaplastic thyroid cancer. Front Oncol. 7:252017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hundahl SA, Fleming ID, Fremgen AM and
Menck HR: A national cancer data base report on 53,856 cases of
thyroid carcinoma treated in the U.S., 1985–1995. Cancer.
83:2638–2648. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kitamura Y, Shimizu K, Hagahama M, Sugino
K, Osaki O, Mimura T, Ito K, Ito K and Tanaka S: Immediate causes
of death in thyroid carcinoma: Clinicopathological analysis of 161
fetal cases. J Clin Endocrinol Metab. 84:4034–4039. 1999.
View Article : Google Scholar
|
|
11
|
Kim S, Park YW, Schiff BA, Doan DD, Yazici
Y, Jasser SA, Younes M, Mandal M, Bekele BN and Myers JN: An
orthotopic model of anaplastic thyroid carcinoma in athymic nude
mice. Clin Cancer Res. 11:1713–1721. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nucera C, Nehs MA, Mekel M, Zhang X, Hodin
R, Lawler J, Nose V and Parangi S: A novel orthotopic mouse model
of human anaplastic thyroid carcinoma. Thyroid. 19:1077–1084. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sewell W, Reeb A and Lin RY: An orthotopic
mouse model of anaplastic thyroid carcinoma. J Vis Exp.
74:500972013.
|
|
14
|
Paget S: The distribution of secondary
growths in cancer of the breast. Lancet. 133:571–573. 1889.
View Article : Google Scholar
|
|
15
|
Bibby MC: Orthotopic models of cancer for
preclinical drug evaluation: Advantages and disadvantages. Eur J
Cancer. 40:852–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nehas MA, Nucera C, Nagarkatti SS, Sado w,
Morales-Garcia D, Hodin RA and Parangi S: Late intervention with
anti-BRAFV600E therapy induces tumor regression in an orthotopic
mouse model of anaplastic thyroid cancer. Endocrinology.
153:985–994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cha W, Kim DW, Kim SD, Jeon EH, Jeong WJ
and Ahn SH: Effect of perioperative treatment with a
hypoxia-inducible factor-1-alpha inhibitor in an orthotopic
surgical mouse models of thyroid cancer. Anticancer Res.
35:2049–2054. 2015.PubMed/NCBI
|
|
18
|
Nehs MA, Nagarkatti S, Nucera C, Hodin RA
and Parangi S: Thyroidectomy with neoadjuvant PLX4720 extends
survival and decreases tumor burden in an orthotopic mouse models
of anaplastic thyroid cancer. Surgery. 148:1154–1162. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mokhtar M, Kondo K, Takizawa H, Ohtani T,
Otsuka H, Kubo H, Kajiura K, Nakagawa Y, Kawanaka Y, Yoshida M, et
al: Non-invasive monitoring of anticancer effects of cisplatin on
lung cancer in an orthotopic SICD mouse model using [18F] FDG
PET-CT. Oncol Rep. 31:2007–2014. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Otani T, Kondo K, Takizawa H, Kajiura K,
Fujino H, Otsuka H and Miyoshi H: Non-invasive monitoring of
cisplatin and erlotinib efficacy against lung cancer in orthotopic
SCID mouse models by small animal FDG-PET/CT and CT. Oncol Rep.
41:447–454. 2019.PubMed/NCBI
|
|
21
|
Marcus C, Whitworth PW, Surasi DS, Pai SI
and Subramaniam RM: PET/CT in the Management of thyroid cancers.
AJR Am J Roentgenol. 202:1316–1329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poisson T, Deandreis D, Leboulleux S,
Bidault F, Bonniaud G, Baillot S, Auperin A, AI Ghuzlan A, Travagli
JP and Lumbroso J: 18F-fluorodeoxyglucose positron emission
tomography and computed tomography in anaplastic thyroid cancer.
Eur J Nucl Med Mol Imaging. 37:2277–2285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Killkenny C, Browne WJ, Cuthill IC,
Emerson M and Altman DG: Improving bioscience research reporting:
The ARRIVE guidelines for reporting animal research. Osteoarthritis
Cartilage. 20:256–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jing C, Gao Z, Wang R, Yang Z, Shi B and
Hou P: Lenvatinib enhances the antitumor effects of paclitaxel in
anaplastic thyroid cancer. Am J Cancer Res. 4:903–912. 2017.
|
|
25
|
Ahmed S, Ghazarian MP, Cabanillas ME,
Zafereo ME, Williams MD, Vu T, Schomer DF and Debnam JM: Imaging of
anaplastic thyroid carcinoma. AJNR AM J Neuroradiol. 3:547–551.
2018. View Article : Google Scholar
|
|
26
|
Deeken-Draisey A, Yang GY, Gao J and
Alexiev BA: Anaplastic thyroid carcinoma: An epidemiologic,
histologic, immunohistochemical, and molecular single-institution
study. Hum Pathol. 82:140–148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kostakoglu L and Chauvie S: Metabolic
tumor volume metrics in lymphoma. Semin Nucl Med. 48:50–66. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dibble EH, Alvarez AC, Truong MT, Mercier
G, Cook EF and Subramaniam RM: 18F-FDG metabolic tumor volume and
total glycolytic activity of oral cavity and oropharyngeal squamous
cell cancer: Adding value to clinical staging. J Nucl Med.
53:709–715. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Manohar PM, Beesley LJ, Bellile EL, Worden
FP and Avram AM: Prognostic value of FDG-PET/CT metabolic
parameters in metastatic radioiodine-refractory differentiated
thyroid cancer. Clin Nucl Med. 43:641–647. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res. 2014:132014. View Article : Google Scholar
|
|
31
|
Miller K, Wang M, Gralow J, Dickler M,
Cobleigh M, Perez EA, Shenkiier T, Cella D and Davidson NA:
Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic
breast cancer. N Engl J Med. 26:2666–2676. 2007. View Article : Google Scholar
|