Introduction
Ovarian cancer (OC) is one of the leading causes of
cancer-associated deaths. There was an estimated 52,100
newly-diagnosed cases and 22,500 fatalities from OC in China in
2015 and 21,750 newly-diagnosed cases and 13,940 fatalities in the
USA in 2020 (1,2). High-grade serous ovarian carcinomas
(HGSOC) account for 70–80% of all OC cases and the mortality rate
is markedly high (3). Despite the
important advances in diagnosis and research, the role and precise
mechanisms of HGSOC have not been fully elucidated.
The fibulin protein family widely exists in the
extracellular matrix (ECM), and plays a crucial role in the
formation and stabilization of the basement membrane, and loose
connective tissue and elastic fibers (4,5). In
addition to the role in tissue framework, fibulin proteins have
also been revealed to be involved in tumorigenesis and progression
of cancer (6). Depending on the
cell type and cellular context, different fibulins possess both
antitumor and pro-tumor properties (7). Fibulin-5 (FBLN5), a 66-kDa secreted
glycoprotein, plays significant roles in cell adhesion and
motility, and cell-to-cell and cell-to-matrix communication
(8,9). In addition, FBLN5 has been revealed to
regulate cell growth, cell migration, tissue repair and
tumorigenesis (10). Numerous
studies have indicated the prognostic potential of FBLN5, as a
tumor suppressor in a diverse range of cancers, such as breast
cancer, lung cancer and hepatocellular carcinoma (11–14).
However, the molecular mechanisms and prognostic significance of
FBLN5 in HGSOC have not been characterized.
Micro(mi)RNAs are endogenous, short (21 to 25
nucleotides) non-coding RNAs that repress gene expression at the
post-transcriptional level (15).
miRNAs target the mRNAs by complementary binding to the homology
sequence in the 3′-untranslated region (3′-UTR) (16), and have an overarching regulatory
role during carcinogenesis and tumor development (17). Specifically, micro (mi)RNA-27a-3p
(miR-27a-3p) was revealed to be a significant positive regulator of
tumorigenesis and progression in different types of cancer,
including hepatocellular (18),
nasopharyngeal (19), and oral
squamous carcinoma (20).
In the present study, the expression level of FBLN5
and the molecular mechanisms in patients with HGSOC were
elucidated. FBLN5 was identified and proved to negatively regulate
the malignant behavior of ovarian cancer both in vitro and
in vivo, implying that FBLN5 and its upstream regulator
miR-27a-3p has the potential to be a novel therapeutic target of
ovarian cancer.
Materials and methods
Tissue samples
In the present study, a total of 216 samples [57
normal fallopian tubes (FT) and 159 HGSOC tissues] were collected
at Qilu Hospital of Shandong University (Shandong, China) from May
2006 to July 2013. The ages of all included patients ranges from 35
to 78 years. The normal FT samples were obtained from patients who
had a benign gynecological tumor, while the HGSOC samples were
collected from patients undergoing surgical resection without
previous chemotherapy. Written informed consent was provided by
each patient prior to surgery. In addition, the guidelines
developed by the Ethics Committee of Shandong University Qilu
Hospital (KYLL-2018-229) were also adhered to.
Cells culture
The SKOV3 and 293T cell lines were purchased from
the American Type Culture Collection and the Chinese Academy of
Sciences, respectively. The HEY and A2780 cell lines were a kind
gift from the laboratory of Dr. Wei (Department of Gynecology and
Obstetrics, Northwestern University, Feinberg School of Medicine).
The cells were cultured in the appropriate medium (SKOV3 and A2780
cells, RPMI-1640 medium and HEY and 293T cells, DMEM) (all from
Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine
serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.), and all the
cells were maintained in an incubator under standard growth
conditions.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA from cells and samples was extracted using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
The mRNA and miRNA were reverse-transcribed using the Prime Script
RT Reagent kit and One Step Prime Script miRNA cDNA Synthesis kit,
respectively, (both from Takara Bio, Inc.) following the
manufacturer's guidelines and recommended thermocycling conditions.
The cDNA was amplified using the SYBR Green (Takara Bio, Inc.) and
Real-Time PCR (qPCR) System (QuantStudio3; Thermo Fisher
Scientific, Inc.). The thermocycling conditions were as follows:
95°C for 30 sec, 40 cycles at 95°C for 5 sec and 60°C for 30 sec,
95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec. U6 and ACTB
were used as the internal controls for normalization and
comparison. The 2−ΔΔCq method (21) was used to calculate the expression
level of the specific genes. The primers are presented in Table SIA.
Western blot analysis
The total protein from the cells and tissues was
isolated in ice-cold lysis solution containing RIPA buffer,
phenylmethylsulfonyl fluoride and sodium fluoride (100:1:1) (all
from Beyotime Bio, Inc.), and then centrifuged for 15 min at 12,000
× g at 4°C. The supernatants were used to calculate the protein
concentration using a bicinchoninic acid Assay (Merck KGaA). The
proteins (50 µg) from each sample were separated using
electrophoresis (separation gel, 10–12%; stacking gel, 5%).
Subsequently, the proteins were transferred onto a PVDF membrane
(0.22 µm), blocked (5% skim milk for 1 h at room temperature), and
incubated with the primary antibodies overnight at 4°C. Then the
membrane was incubated with the horseradish peroxidase-conjugated
secondary antibodies for 1–2 h. Finally, the protein signals were
visualized using an enhanced chemiluminescence detection kit
(PerkinElmer, Inc.), and the densitometry of the protein bands were
analyzed using the Image J v1.8.0 software (National Institutes of
Health). GAPDH was used as the endogenous control. The primary
antibodies in this study included: Anti-FBLN5 (dilution 1:1,000,
cat. no. ab66339; Abcam), anti-GAPDH (dilution 1:1,000, cat. no.
2118; Cell Signaling Technology), anti-N-cadherin (dilution
1:1,000, cat. no. 13116; Cell Signaling Technology), anti-Snail
(dilution 1:1,000, cat. no. 3879; Cell Signaling Technology),
anti-E-cadherin (dilution 1:1,000, cat. no. 3195; Cell Signaling
Technology), anti-p21 (dilution 1:1,000, cat. no. 2947; Cell
Signaling Technology), anti-p27 (dilution 1:1,000, cat. no. 3686;
Cell Signaling Technology). The secondary antibodies were purchased
from KPL: Anti-mouse IgG (cat. no. 5220-0341, dilution 1:6,000) and
anti-rabbit IgG (cat. no. 5220-0336, dilution 1:4,000).
Immunohistochemical (IHC)
staining
A tissue microarray (TMA) of HGSOC tissues (4-µm
thick, made by our laboratory) were incubated at 60°C for 35 min.
The TMAs were immediately deparaffinized, rehydrated with xylene
and a graded ethanol series. Subsequently, antigen retrieval was
performed using citric acid buffer and microwave irradiation. The
non-specific antigens were blocked using a normal goat serum and
the slides were then probed with rabbit anti-FBLN5 antibody (1:300;
cat. no. ab202977; Abcam) overnight at 4°C. The signal was detected
using 3,3′-diaminobenzidine chromogenic reagent kit (ZSGB Bio,
Inc.) and hematoxylin-counterstained (Solarbio Bio, Inc.) according
to the manufacturer's instructions. The final score of FBLN5
staining was determined on the basis of extent and intensity.
Plasmid construction and
transfection
The coding DNA sequences of FBLN5 were obtained from
Shanghai GeneChem Co., Ltd., and then ligated into a pLenti-C-Myc-
DDK-IRES-Puro (PCMV) plasmid (OriGene Technologies, Inc.).
Lentivirus expressing FBLN5 were obtained using the 293T cell line
packaged with the pMD2.G (Addgene, Inc.) and psPAX2 (Addgene, Inc.)
vectors. The FBLN5-expressing stable cells were produced using the
infected lentivirus for 24 h and selected for a week in medium
containing antibiotics. Small interfering (si)RNA targeting FBLN5
was synthesized from Shanghai Biosune Biotechnology Co., Ltd., and
were similar to those stated in previous studies (4,22). The
sequences of the siRNA and miRNA are presented in Table SIB and C.
Cell proliferation assays
The MTT (Sigma-Aldrich; Merck KGaA) assay was used
to measure cell growth. A total of (0.8–1)x103
cells/well were plated in 96-well plates in sextuplicate, for 1–5
days. At the same time every day, 20 µl (5 mg/ml) MTT reagent was
added to each well, and continued to culture for 4 h at 37°C.
Subsequently, the supernatant was removed and DMSO (Sigma-Aldrich;
Merck KGaA) was then added to each well. The optical density was
detected at 490 nm using a microplate reader (Thermo Fisher
Scientific, Inc.). All experiments were performed in
triplicate.
Clonogenic assays
To evaluate the colony formation ability, single
cells were seeded in a 6-well plate (1,000 cells/well) and cultured
for two weeks, under standard culture conditions. Thereafter, 100%
methanol was used to fix the colonies for 15 min, and stained with
0.1% crystal violet for 15 min at room temperature. Colonies of
more than 50 cells were counted. All experiments were performed in
triplicate.
Invasion and migration assays
For both the assays, the cells were seeded in a
Transwell chamber (8-µm), but only the membrane for the invasion
assays was coated with Matrigel (both from BD Biosciences). A total
of 200 µl suspension (1×105 cells) was added to the
upper chamber, and 700 µl medium supplemented with 20% FBS was
added in the lower chamber. Following routine incubation at 37°C
for the appropriate time-points (6–24h), the cells in the upper
chamber were removed using a cotton swab. Subsequently, the cells
that reached the lower surface were fixed and stained with 100%
methanol and 0.1% crystal violet for 15 min at room temperature,
respectively.
Tumor formation assays in nude
mice
Female nude mice (BALB/c; 4–5 weeks old; average
weight, 15 g) were obtained from the NBRI of Nanjing University
(Jiangsu, China), and maintained in specific-pathogen-free (SPF)
conditions with 25°C temperature, 50% humidity, 12-h light/dark
cycle, and had free access to water and food. Nude mice health and
behavior were monitored every day. Then, a 200-µl cell suspension
(5×106 cells) was injected subcutaneously into either
side of the armpit, in each nude mouse. After 2–3 weeks, all the
mice were euthanized by intraperitoneal injection of pentobarbital
sodium (200 mg/kg), which was confirmed by respiratory and cardiac
arrest, and then the tumors were excised and weighed. The diameter
of the longest of these tumors was <2 cm, and all animal
experiments adhered to the guidelines and policy of the Shandong
University Animal Care and Use Committee.
Bioinformatics analyses
The GEPIA database (http://gepia.cancer-pku.cn/) was used to evaluate the
mRNA expression levels of FBLN5 in serous ovarian cancer and normal
tissues (23). The TargetScan
(http://www.targetscan.org/) and miRanda
(http://www.microrna.org) were employed to
identify predicted miRNA sequences that may regulate FBLN5
(24,25).
Statistical analysis
The data analysis was performed using SPSS v18.0
software (SPSS, Inc.). The unpaired Student's t-test and
χ2 test were used to evaluate the association and the
significant difference between groups. The curve of overall
survival (OS) was assessed using the Kaplan-Meier method and the
log-rank test. The experimental data are presented as the mean ±
standard error of the mean and P<0.05 was considered to indicate
a statistically significant difference.
Results
FBLN5 expression is significantly
downregulated in the HGSOC tissue
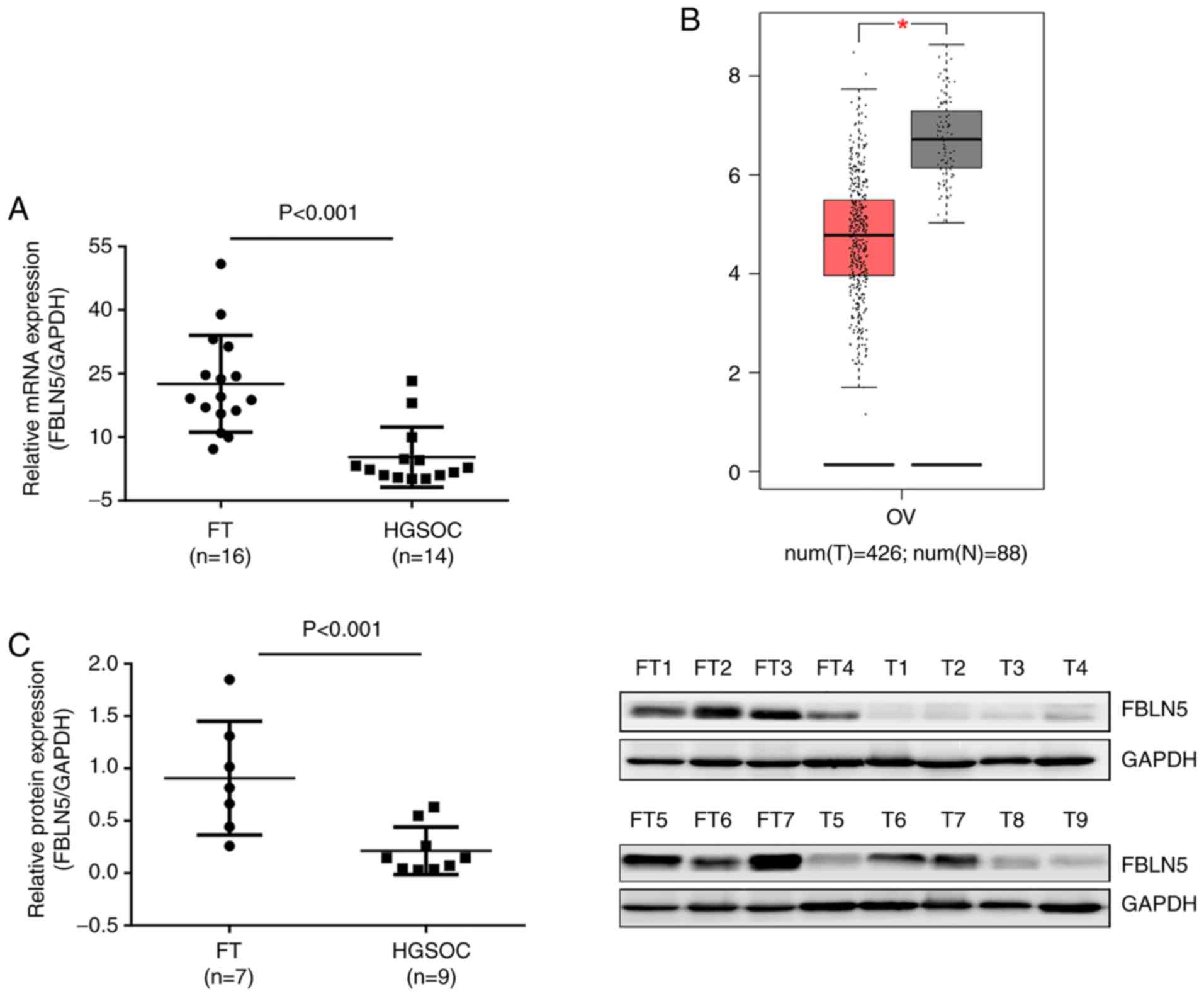
The mRNA expression level of FBLN5 in HGSOC and FT
tissues (HGSOC, n=14; FT, n=16), was initially investigated and the
expression levels of FBLN5 were upregulated in FT tissues compared
with that in HGSOC tissues (P<0.001; Fig. 1A). The results from the GEPIA
database also revealed that FBLN5 was overexpressed in normal
control tissues compared with that in OC samples (Fig. 1B). Then, the protein levels of FBLN5
in FT (n=7) and HGSOC (n=9) tissue samples were investigated using
western blot analysis and were revealed to be significantly
different (P<0.001; Fig.
1C).
Low expression level of FBLN5 is
associated with unfavorable prognosis
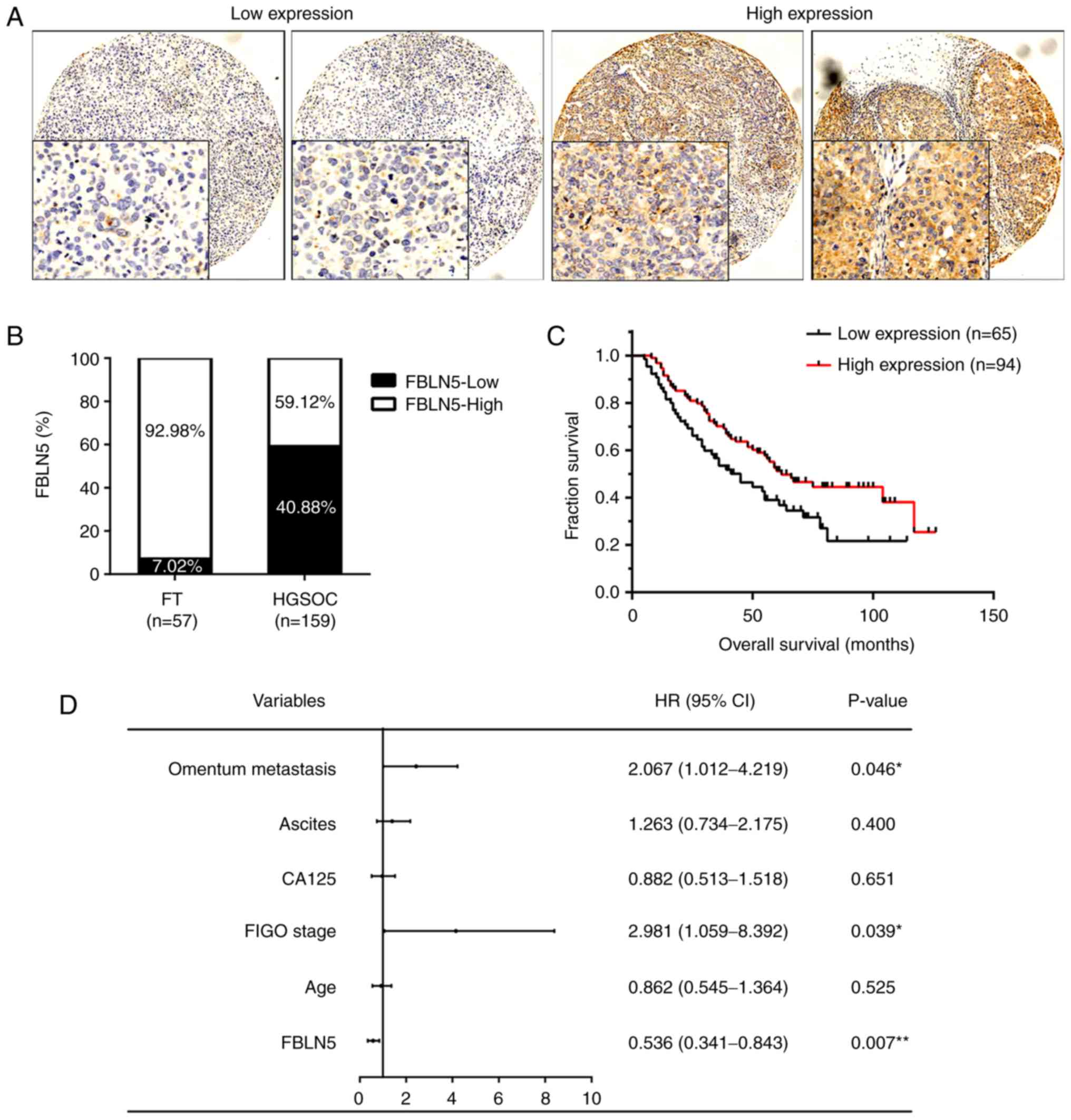
To further investigate the role of FBLN5 in HGSOC,
IHC was performed to analyze the protein expression level in a TMA
(FT, n=57; HGSOC, n=159). With respect to the staining degree, the
HGSOC tissues were divided into either high (n=94) and low FBLN5
expression groups (n=65) (Fig. 2A).
There was markedly higher expression levels of FBLN5 in the FT
tissues (92.98%; 53/57) compared with that in the HGSOC tissues
(59.12%; 94/159; Fig. 2B). In
addition, the OS of the patients in the FBLN5-high expression group
was significantly longer compared with that in patients in the
FBLN5-low expression group (P<0.05; Fig. 2C). Using multivariate analysis of
the clinicopathological parameters, the data presented in Fig. 2D revealed that OS was significantly
associated with FBLN5 expression (HR, 0.536; P=0.007), FIGO stage
(HR, 2.981; P=0.039) and omentum metastasis (HR, 2.067; P=0.046).
In addition, analysis of the clinicopathological features indicated
that FBLN5 expression was associated with age (P=0.024), omentum
metastasis (P=0.040), recurrence (P=0.008), and OS (P=0.016;
Table I).
 | Table I.Association between FBLN5 expression
and clinicopathological features. |
Table I.
Association between FBLN5 expression
and clinicopathological features.
|
|
| FBLN5
expression |
|
|---|
|
|
|
|
|
|---|
| Groups | No. | Low level | High level | P-value |
|---|
| Age (years) |
|
|
| 0.024 |
|
<55 | 74 | 23 | 51 |
|
|
≥55 | 85 | 42 | 43 |
|
| FIGO stage |
|
|
| 0.244 |
|
I+II | 35 | 11 | 24 |
|
|
III+IV | 120 | 52 | 68 |
|
| CA125 (U/ml) |
|
|
| 0.868 |
|
<600 | 64 | 27 | 37 |
|
|
≥600 | 87 | 35 | 52 |
|
| Ascites (ml) |
|
|
| 0.502 |
|
<1,000 | 66 | 24 | 42 |
|
|
≥1,000 | 84 | 36 | 48 |
|
| Omentum
metastasis |
|
|
| 0.040 |
|
Negative | 54 | 16 | 38 |
|
|
Positive | 101 | 48 | 53 |
|
| Lymph node
metastasis |
|
|
| 0.566 |
|
Negative | 41 | 11 | 30 |
|
|
Positive | 22 | 8 | 14 |
|
| Recurrence |
|
|
| 0.008 |
| No | 28 | 5 | 23 |
|
|
Yes | 97 | 45 | 52 |
|
| OS (years) |
|
|
| 0.016 |
|
<48 | 79 | 40 | 39 |
|
|
≥48 | 80 | 25 | 55 |
|
FBLN5 inhibits OS cell
proliferation
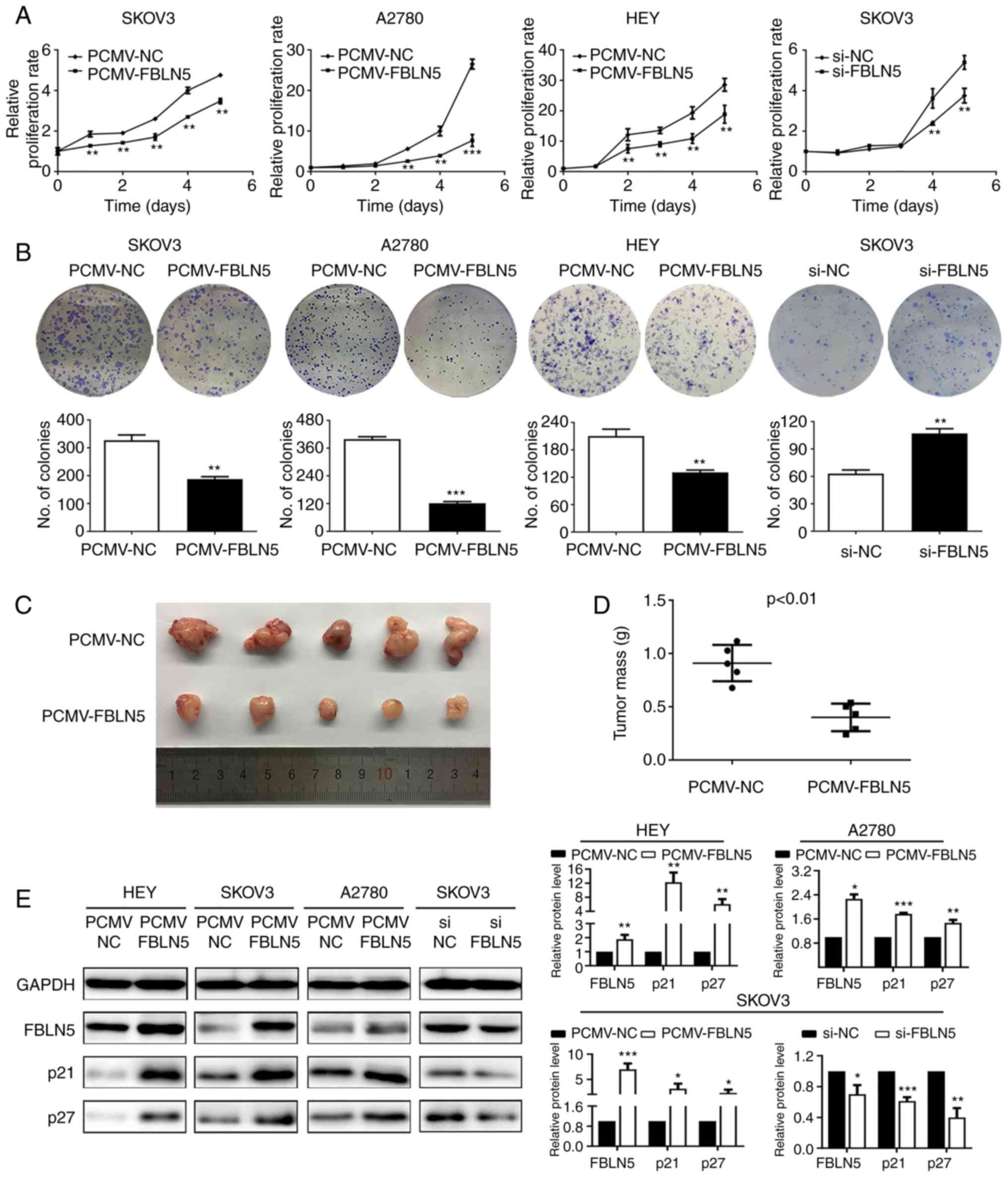
To evaluate the functional role of FBLN5, three cell
lines, including SKOV3, A2780 and HEY were transfected stably with
FBLN5 overexpression vector, while the SKOV3 cell line was
transfected transiently with FBLN5 siRNA. The growth curve analysis
demonstrated that upregulation of FBLN5 notably inhibited cell
growth compared with that in the control group (Fig. 3A). Furthermore, the clonogenic
assays revealed that overexpression of FBLN5 significantly
inhibited the clonogenicity efficiency compared with that in the
empty vector control group (Fig.
3B). In addition, the HEY cells transfected with PCMV-FBLN5 and
PCMV-NC, were subcutaneously injected into the nude mice. As
revealed in Fig. 3C and D, FBLN5
overexpression could significantly suppress the growth of OC cell
lines in the nude mice. IHC staining of the xenograft tissue was
utilized to detect the protein expression level of FBLN5. As
demonstrated in Fig. S1, the
expression level of FBLN5 in the nucleus was higher in the
PCMV-FBLN5 group compared with that in the PCMV-NC group. Lastly,
FBLN5 overexpression significantly increased p21 and p27 protein
expression levels (Fig. 3E). These
data indicated that FBLN5 could suppress the proliferation of OC
cells.
FBLN5 inhibits OC cell invasion and
migration by suppressing epithelial-mesenchymal transition
(EMT)
To determine the impact of altered FBLN5 expression
on the metastasis of the OC cell lines, the invasion and migration
abilities were detected using Transwell and Matrigel assays. As
indicated in Fig. 4A and B, FBLN5
downregulation significantly promoted cell invasion and migration
abilities. In agreement, overexpression of FBLN5 significantly
attenuated the abilities of migration and invasion of the SKOV3,
HEY and A2780 cell lines. Then, the molecular mechanism was
investigated by analyzing EMT markers. The results revealed that
FBLN5 overexpression significantly upregulated an epithelial marker
(E-cadherin) and reduced the levels of 2 mesenchymal markers
(N-cadherin and Snail) compared with that in the empty vector
group, transfected in the HEY, A2780 and SKOV3 cell lines. In
addition, FBLN5 knockdown downregulated the epithelial marker
(E-cadherin) and increased the expression of 2 mesenchymal markers
(N-cadherin, Snail) compared with that in the control group in the
SKOV3-transfected cells (Fig. 4C).
These data demonstrated that FBLN5 suppressed metastasis of OC
cells by inhibiting EMT in vitro.
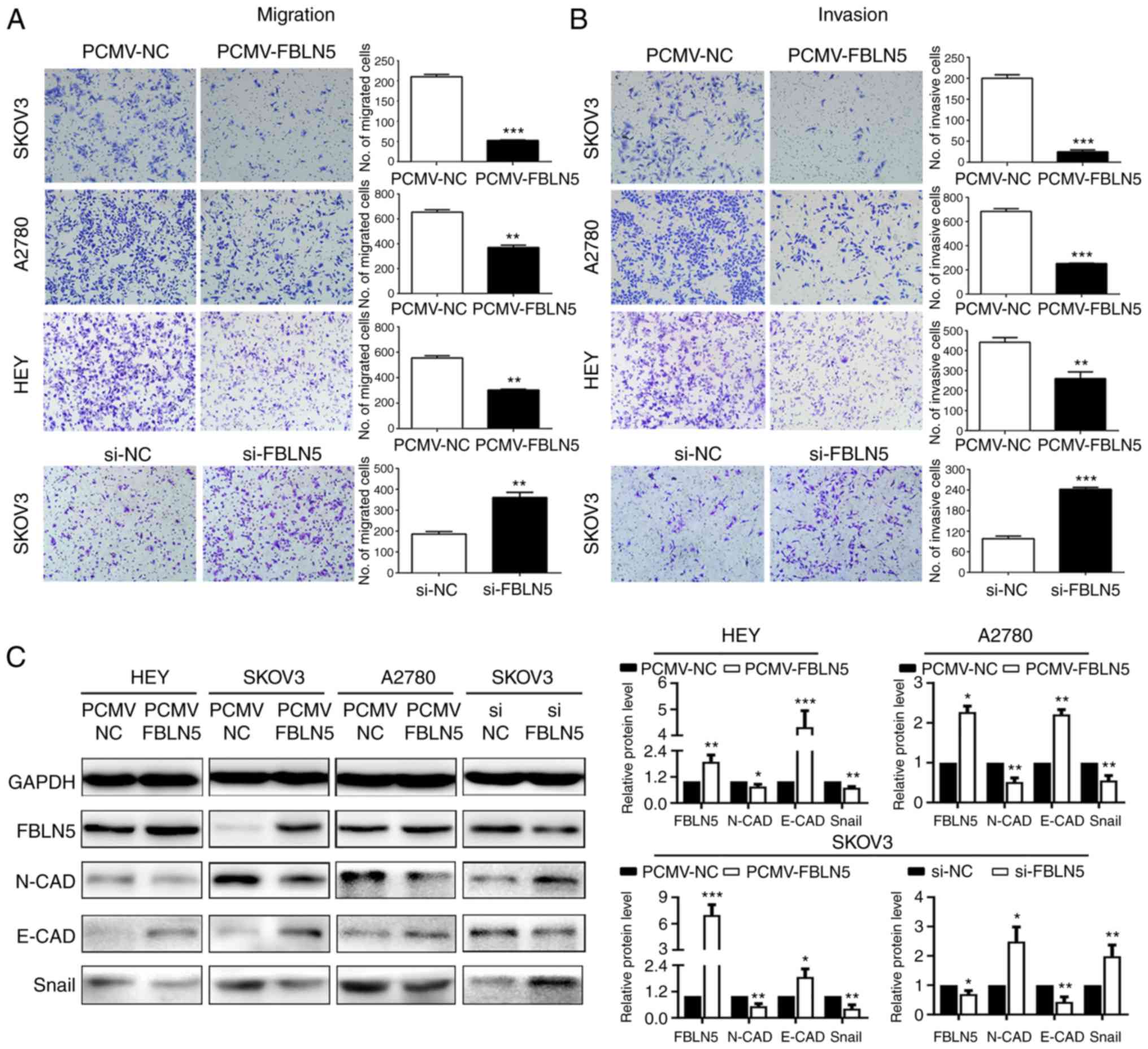 | Figure 4.FBLN5 inhibits the migration and
invasion of ovarian cancer cells in vitro. Transwell assays
were used to assess the effect of FBLN5 overexpression or knockdown
on the (A) migration and (B) invasion of ovarian cancer cells. (C)
Western blot analysis of the EMT markers, N-cadherin, E-cadherin
and Snail. Data are presented as the mean ± standard error of the
mean. n=3. *P<0.05, **P<0.01, ***P<0.001. FBLN5,
fibulin-5; EMT, epithelial-mesenchymal transition; si, small
interfering; NC, negative control; N-CAD, N-cadherin; E-CAD,
E-cadherin. |
miR-27a-3p is a direct regulator of
FBLN5
Finally, the molecular mechanisms underlying the
downregulation of FBLN5 expression in OC was investigated. The
potential miRNA that targets the mRNA of FBLN5 was predicted using
the miRanda and TargetScan online databases, and miR-27a-3p was
selected for further examination. In addition, prior research
revealed that miR-27a-3p was upregulated in OC (26,27).
As demonstrated in Fig. 5A, the
expression level of miR-27a-3p was upregulated in HGSOC compared
with that in FT tissues. Further analysis revealed that the
expression level of FBLN5 was negatively correlated with miR-27a-3p
expression level in HGSOC (Fig.
5B). To further verify the association of direct interaction
between miR-27a-3p and FBLN5, the dual-luciferase reporter assay
was subsequently performed. The data indicated that upregulation of
miR-27a-3p markedly inhibited the luciferase activities in cells
co-transfected with wild-type FBLN5 sequences and miR-27a-3p mimics
(Fig. 5C). The SKOV3 cell line was
transfected with miR-27a-3p inhibitors or mimics. Western blot and
RT-qPCR assays demonstrated that miR-27a-3p mimics significantly
reduced the protein and mRNA expression levels of FBLN5,
respectively, which was reversed by miR-27a-3p inhibitors (Fig. 5D).
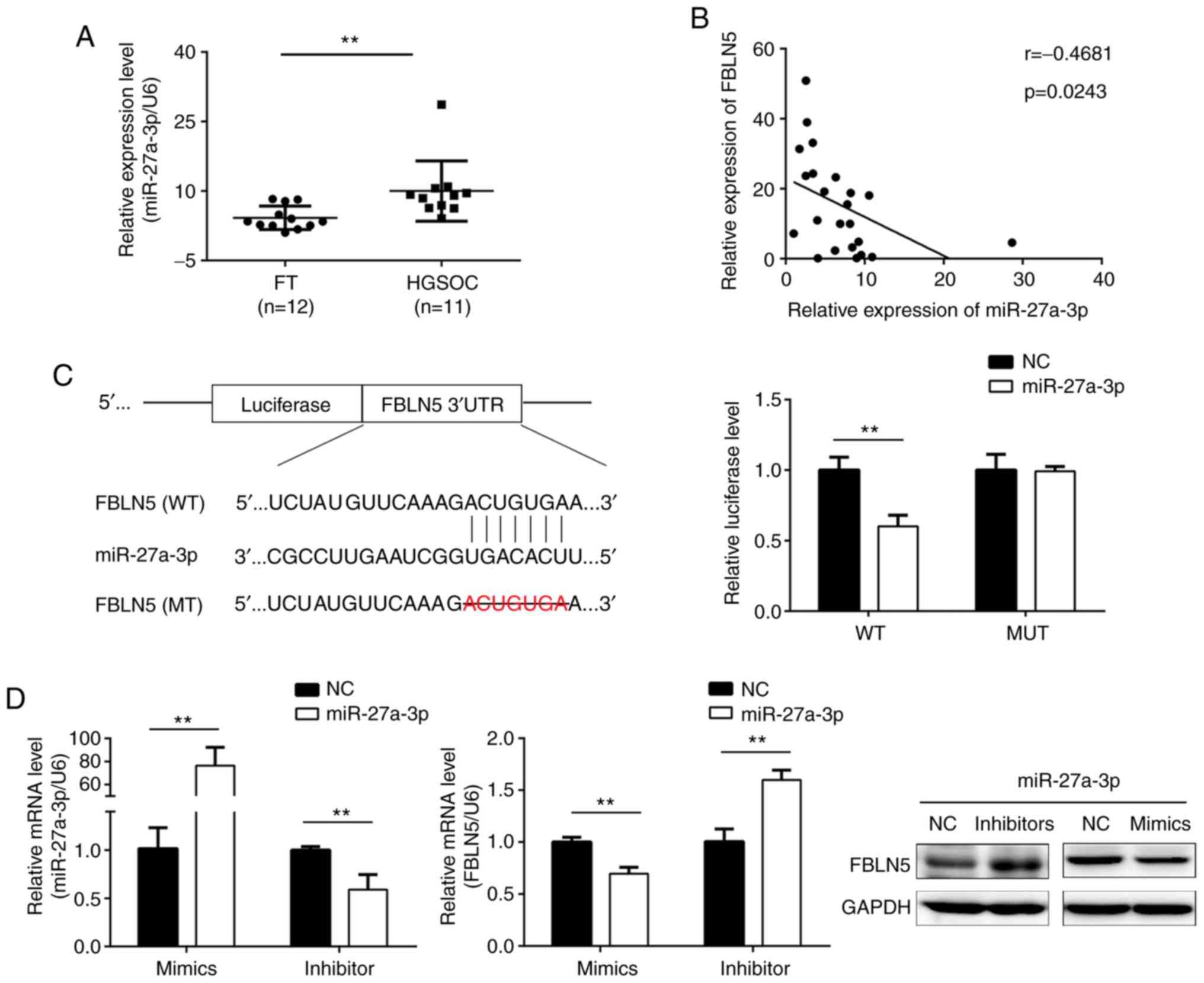 | Figure 5.FBLN5 is the target gene of
miR-27a-3p. (A) The mRNA level of miR-27a-3p was higher in HGSOC
tissues compared with that in FT tissues, using RT-qPCR. (B)
Correlation analysis of FBLN5 and miR-27a-3p expression levels. (C)
Prediction of the binding sites between miR-27a-3p and 3′-UTR of
FBLN5. The binding sequence of miR-27a-3p with the FBLN5 3′UTR
sequence was deleted in the mutated type. Dual-luciferase reporter
assays were used to analyzed the luciferase activity of the WT or
MT FBLN5 3′UTR. (D) The mRNA expression level of miR-27a-3p was
detected using RT-qPCR following SKOV3 cell transfection with
miR-27a-3p mimics or inhibitor. RT-qPCR and western blot analysis
revealed an inverse association between FBLN5 and miR-27a-3p
expression. Upregulation of miR-27a-3p inhibited FBLN5 expression
level and downregulated miR-27a-3p expression level could promote
the expression level of FBLN5. Data are presented as the mean ±
standard error of the mean. **P<0.01. FBLN5, fibulin-5;
miR-27-3p, microRNA-27-3-p; HGSOC, high-grade serous ovarian
cancer; FT, fallopian tube; RT-qPCR, reverse
transcription-quantitative PCR; UTR, untranslated region; WT,
wild-type; MT, mutant; NC, negative control. |
Discussion
Ovarian carcinoma is the fifth leading cause of
cancer-related mortality in females (28,29),
and HGSOC is the most malignant type of OC. Most patients with OC
are diagnosed at an advanced stage, which leads to poor prognosis
(30,31). FBLN5 has been verified to be an
anti-oncogene in different types of malignancy (11–14).
In the present study, it was revealed that a low expression level
of FBLN5 was associated with unfavorable prognosis of HGSOC,
providing a potential biomarker for the prediction of diagnosis and
prognosis. However, the detailed mechanism of FBLN5 in HGSOC
progression remains unknown.
FBLN5 has an anti-proliferative effect in several
types of human malignancy (11–13),
and FBLN5 overexpression was revealed to suppress DNA synthesis and
cyclin A expression in mink lung epithelial cells (32). p21 and p27 are two important cell
cycle-related genes, which regulate cell cycle progression during
G1/S and G0/S phases, respectively (33). In the present study, cell
proliferation, colony formation and tumor formation assays were
performed. Notably, the results revealed that FBLN5 overexpression
inhibited OC cell growth in vitro and in vivo, and
markedly increased the protein expression level of p21 and p27.
Based on these data, we hypothesized that FBLN5 inhibited the
proliferation of OC cells by regulating the cell cycle-related
proteins. Limitedly, we did not explore the potential relations
between FBLN5 and the other essential cell cycle regulators in the
present study, such as AURKA, AURKB and FOXM1, which would be
carried out relevantly in our future research.
Migration and invasion are important causes of death
in patients with cancer. As a vital regulator of metastasis, EMT
has been associated with tumor progression, and promotes mobility
and resistance to apoptosis in various types of cancer (34). Emerging evidence has revealed that
tumor-associated matrix metallopeptidases (MMPs) stimulate EMT
(4). Tu et al (13) and Lee et al (35), reported that FBLN5 was involved in
EMT and affected the invasion and migration by tumor-associated
MMPs in breast cancer and hepatocellular carcinoma cells. EMT is a
cellular process and is often defined as the loss of epithelial
characteristics and the increase in mesenchymal features (36,37).
Consistent with this hypothesis, the results in the present study
revealed that FBLN5 overexpression increased the epithelial marker,
E-cadherin and decreased the mesenchymal markers, N-cadherin and
Snail. Therefore, we hypothesized that FBLN5 inhibited the
migration and invasion of OC cells by inhibiting the EMT
pathway.
Currently, numerous studies have demonstrated that
miRNAs are important regulators in a diverse range of human
malignancies (18–20). Furthermore, it has been reported
that the function and aberrant expression of miRNAs play a crucial
role in several biological processes (38,39).
For example, miR-27a-3p promoted cell proliferation in glioma cells
by the cooperative regulation of MXI1 (40). Li et al (26) and Wang et al (27), reported that the miR-27a-3p
expression level was increased in OC, and primarily affected the
growth and metastasis of cancer cells. Consistent with these
previous studies, the results from the present study indicated that
miR-27a-3p was upregulated in the tissue from patients with HGSOC.
In addition, it was verified that FBLN5 was the target of
miR-27a-3p, and the miR-27a-3p expression level was negatively
correlated with FBLN5 expression level. Therefore, the increased
miR-27a-3p expression level could explain why FBLN5 was
downregulated in HGSOC.
In summary, the present study indicated that FBLN5
was targeted by miR-27a-3p and suppressed cell proliferation,
invasion and metastasis in OC cells. Furthermore, FBLN5
downregulation was associated with unfavorable prognosis. These
findings revealed that increasing FBLN5 expression could be a
potential new strategy for the treatment of HGSOC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81572554 and
81874107), and the Science Foundation of Qilu Hospital of Shandong
University (grant no. 2019QLQN03).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
RL and HW designed and conceived the experiments,
and wrote the original draft of the manuscript. HJ, QW and ZD
collected and analyzed the data. HM, CY and SY provided technical
support and revised the manuscript. NY assisted with acquisition
and analysis of clinical information. BK directed the project and
interpreted the results. All authors have read and approved the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Shandong University
Qilu Hospital Ethics Committee and Shandong University Animal Care
and Use Committee (Shandong, China).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bowtell DD, Böhm S, Ahmed AA, Aspuria PJ,
Bast RC Jr, Beral V, Berek JS, Birrer MJ, Blagden S, Bookman MA, et
al: Rethinking ovarian cancer II: Reducing mortality from
high-grade serous ovarian cancer. Nat Rev Cancer. 15:668–679. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heo JH, Song JY, Jeong JY, Kim G, Kim TH,
Kang H, Kwon AY and An HJ: Fibulin-5 is a tumour suppressor
inhibiting cell migration and invasion in ovarian cancer. J Clin
Pathol. 69:109–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Timpl R, Sasaki T, Kostka G and Chu ML:
Fibulins: A versatile family of extracellular matrix proteins. Nat
Rev Mol Cell Biol. 4:479–489. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gallagher WM, Currid CA and Whelan LC:
Fibulins and cancer: Friend or foe? Trends Mol Med. 11:336–340.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Obaya AJ, Rua S, Moncada-Pazos A and Cal
S: The dual role of fibulins in tumorigenesis. Cancer Lett.
325:132–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yanagisawa H, Schluterman MK and Brekken
RA: Fibulin-5, an integrin-binding matricellular protein: Its
function in development and disease. J Cell Commun Signal.
3:337–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Vega S, Iwamoto T and Yamada Y:
Fibulins: Multiple roles in matrix structures and tissue functions.
Cell Mol Life Sci. 66:1890–1902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Albig AR and Schiemann WP: Fibulin-5
function during tumorigenesis. Future Oncol. 1:23–35. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Song X, Yue W, Chen D, Yu J, Yao Z
and Zhang L: Fibulin-5 inhibits Wnt/β-catenin signaling in lung
cancer. Oncotarget. 6:150222015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mohamedi Y, Fontanil T, Solares L,
Garcia-Suárez O, García- Piqueras J, Vega JA, Cal S and Obaya AJ:
Fibulin-5 downregulates Ki-67 and inhibits proliferation and
invasion of breast cancer cells. Int J Oncol. 48:1447–1456. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tu K, Dou C, Zheng X, Li C, Yang W, Yao Y
and Liu Q: Fibulin-5 inhibits hepatocellular carcinoma cell
migration and invasion by down-regulating matrix
metalloproteinase-7 expression. BMC Cancer. 14:9382014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yue W, Sun Q, Landreneau R, Wu C,
Siegfried JM, Yu J and Zhang L: Fibulin-5 suppresses lung cancer
invasion by inhibiting matrix metalloproteinase-7 expression.
Cancer Res. 69:6339–6346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki HI, Yamagata K, Sugimoto K, Iwamoto
T, Kato S and Miyazono K: Modulation of microRNA processing by p53.
Nature. 460:529–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ranganathan K and Sivasankar V:
MicroRNAs-biology and clinical applications. J Oral Maxillofac
Pathol. 18:229–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Svoronos AA, Engelman DM and Slack FJ:
OncomiR or tumor suppressor? The duplicity of microRNAs in cancer.
Cancer Res. 76:3666–3670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo D, Li Y, Chen Y, Zhang D, Wang X, Lu
G, Ren M, Lu X and He S: DANCR promotes HCC progression and
regulates EMT by sponging miR-27a-3p via ROCK1/LIMK1/COFILIN1
pathway. Cell Prolif. 52:e126282019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li L and Luo Z: Dysregulated miR-27a-3p
promotes nasopharyngeal carcinoma cell proliferation and migration
by targeting Mapk10. Oncol Rep. 37:2679–2687. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng G, Xun W, Wei K, Yang Y and Shen H:
MicroRNA-27a-3p regulates epithelial to mesenchymal transition via
targeting YAP1 in oral squamous cell carcinoma cells. Oncol Rep.
36:1475–1482. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamauchi Y, Tsuruga E, Nakashima K, Sawa Y
and Ishikawa H: Fibulin-4 and-5, but not fibulin-2, are associated
with tropoelastin deposition in elastin-producing cell culture.
Acta Histochem Cytochem. 43:131–138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45((W1)):
W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar
|
|
25
|
Betel D, Wilson M, Gabow A, Marks DS and
Sander C: The microRNA.org resource: Targets and expression.
Nucleic Acids Res. 36((Database Issue)): D149–D153. 2008.PubMed/NCBI
|
|
26
|
Li E, Han K and Zhou X: microRNA-27a-3p
down-regulation inhibits malignant biological behaviors of ovarian
cancer by targeting BTG1. Open Med (Wars). 14:577–585. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Z, Ji G, Wu Q, Feng S, Zhao Y, Cao Z
and Tao C: Integrated microarray meta-analysis identifies miRNA-27a
as an oncogene in ovarian cancer by inhibiting FOXO1. Life Sci.
210:263–270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Armstrong DK, Bundy B, Wenzel L, Huang HQ,
Baergen R, Lele S, Copeland LJ, Walker JL and Burger RA;
Gynecologic Oncology Group, : Intraperitoneal cisplatin and
paclitaxel in ovarian cancer. N Engl J Med. 354:34–43. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chan JK, Cheung MK, Husain A, Teng NN,
West D, Whittemore AS, Berek JS and Osann K: Patterns and progress
in ovarian cancer over 14 years. Obstet Gynecol. 108:521–528. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kurman RJ: Origin and molecular
pathogenesis of ovarian high- grade serous carcinoma. Ann Oncol. 24
(Suppl 10):x16–x21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mezzanzanica D: Ovarian cancer: A
molecularly insidious disease. Chin J Cancer. 34:1–3. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schiemann WP, Blobe GC, Kalume DE, Pandey
A and Lodish HF: Context-specific effects of fibulin-5 (DANCE/EVEC)
on cell proliferation, motility, and invasion. Fibulin-5 is induced
by transforming growth factor-beta and affects protein kinase
cascades. J Biol Chem. 277:27367–27377. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li H, Guan H, Guo Y, Liang W, Liu L, He X,
Ke W, Cao X, Xiao H and Li Y: CITED1 promotes proliferation of
papillary thyroid cancer cells via the regulation of p21 and p27.
Cell Biosci. 8:572018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dongre A and Weinberg RA: New insights
into the mechanisms of epithelial-mesenchymal transition and
implications for cancer. Nat Rev Mol Cell Biol. 20:69–84. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee YH, Albig AR, Regner M, Schiemann BJ
and Schiemann WP: Fibulin-5 initiates epithelial-mesenchymal
transition (EMT) and enhances EMT induced by TGF-beta in mammary
epithelial cells via a MMP-dependent mechanism. Carcinogenesis.
29:2243–2251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pastushenko I and Blanpain C: EMT
transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang W, Chen H, Gao W, Wang S, Wu K, Lu C,
Luo X, Li L and Yu C: Girdin interaction with vimentin induces EMT
and promotes the growth and metastasis of pancreatic ductal
adenocarcinoma. Oncol Rep. 44:637–649. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chong GO, Jeon H-S, Han HS, Son JW, Lee
YH, Hong DG, Lee YS and Cho YL: Differential microRNA expression
profiles in primary and recurrent epithelial ovarian cancer.
Anticancer Res. 35:2611–2617. 2015.PubMed/NCBI
|
|
39
|
Llauradó M, Majem B, Altadill T, Lanau L,
Castellví J, Sánchez-Iglesias JL, Cabrera S, De la Torre J,
Díaz-Feijoo B, Pérez-Benavente A, et al: MicroRNAs as prognostic
markers in ovarian cancer. Mol Cell Endocrinol. 390:73–84. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu W, Liu M, Peng X, Zhou P, Zhou J, Xu K,
Xu H and Jiang S: miR-24-3p and miR-27a-3p promote cell
proliferation in glioma cells via cooperative regulation of MXI1.
Int J Oncol. 42:757–766. 2013. View Article : Google Scholar : PubMed/NCBI
|