Introduction
Radiotherapy has been demonstrated to provide
long-term survival benefits for patients with primary or secondary
brain tumors (1–3). However, cognitive dysfunction
following cranial irradiation is the most serious complication in
these patients (4). The main
clinical manifestations of this is the progressive reduction in
hippocampal-dependent learning and memory and capacity to process
information (5,6). These delayed and long-term adverse
effects can have a serious impact on the quality of life of the
patient. Therefore, the neurocognitive state following cranial
irradiation has been recommended by the Neurological Therapeutic
Response Assessment Working Group to be one of the primary
endpoints of clinical trials investigating brain tumors (7). Unfortunately, alternative effective
treatment and predictive strategies for patients with brain tumors
remain limited. In addition, a thorough understanding of the
molecular events underlying irradiation-induced memory decline is
required to identify novel therapeutic strategies.
Numerous studies have previously indicated that
neurogenesis impairment in the hippocampus is critical for
irradiation-induced cognitive deficit (8,9). There
are two main areas in the brain where neurogenesis primarily
occurs: i) The dentate gyrus of the hippocampus; and ii) the
subventricular area of the olfactory bulb. These new neurons
migrate to the specific brain regions and incorporate into existing
neural networks involved in mediating normal brain function
(10,11).
Nerve growth factor (NGF) is a neurotrophic receptor
that is highly expressed in the hippocampus, cortex and pituitary
gland (12). NGF has been
demonstrated to bind tropomyosin receptor kinase A (TrkA) and serve
a key role in the development and functional maintenance of the
brain (13,14). Although NGF can also bind to the p75
neurotrophin receptor (p75NTR) at a lower affinity
compared with TrkA, it is improbable that p75NTR would
preferentially transmit death signals in cells expressing both
receptors (14,15). The binding of NGF to TrkA activates
the extracellular signal-regulated protein kinase (ERK)1/2
signaling pathway, which in turn activates the cyclic adenosine
monophosphate response element-binding protein (CREB). Activation
of CREB regulates various downstream signaling pathways required
for neural precursor cell proliferation, survival and memory
formation (14,16).
However, the role of NGF-TrkA signaling in
irradiation-induced memory deficit has not been fully determined.
Therefore, in the present study, a rat model was used to
investigate the influence of irradiation on NGF, TrkA and their
associated downstream signaling events, in addition to their
subsequent effects on hippocampal neurogenesis and memory
deficit.
Materials and methods
Animals and whole-brain irradiation
(WBI)
The present study was approved by the Animal Care
and Ethics Committee of The Affiliated Suzhou Hospital of Nanjing
Medical University and Shandong University (Jinan, China). A total
of 36 male Sprague-Dawley rats (weight, 50–60 g; age, 21 days) were
obtained from The School of Medicine, Shandong University. All the
rats were housed with 3–4 animals per cage with ad libitum
access to tap water and food. The temperature was set at 24°C and
the relative humidity was 50–70%. They were kept under natural
light in 12-h light/dark cycles. All rats were anesthetized with 4%
isoflurane and placed in prone position in a 23EX linear
accelerator (Varian Medical Systems, Inc.). A total of 1–2%
isoflurane with 0.6–0.8 l/min flow rate maintained anesthesia
during this process. Each rat was treated with WBI using a 4-MeV
electron beam at a single dose of 10 or 0 Gy for the control group.
The beam was directed downwards towards the head, whilst the body
was shielded using a customized block.
Study design
After WBI, each rat received a twice daily
intraperitoneal injection of bromo-deoxyuridine (BrdU; 50 mg/kg
body weight; Sigma-Aldrich; Merck KGaA) for 4 days prior to
irradiation exposure. All rats were anesthetized with 4% isoflurane
followed by 1–2% isoflurane with 0.6–0.8 l/min flow rate to
maintain anesthesia. Adeno-associated virus (serotype 8, AAV8)
encoding GFP was stereotaxically infused into the dorsal
hippocampus within 24 h after WBI. All Sprague-Dawley rats were
randomly apportioned into the following groups (n=12 per group): i)
AAV-control, consisting of control rats infused with AAV encoding
TrkA scramble sequence; ii) AAV-irradiation, consisting of
irradiated rats infused with AAV encoding TrkA scramble sequence;
and iii) AAV-overexpression (ovp)-TrkA, consisting of irradiated
rats infused within AAV encoding overexpression TrkA. Morris water
maze and open field test was performed at 1 and 3 months, following
which all rats were sacrificed with carbon dioxide. The animals
were placed into a box and CO2 was infused into the box
at a rate of 10–30% of the volume of the euthanasia box every
minute. After 5 min, the animals exhibited no movements, no
breathing, no heartbeat, and pupil dilation. Then, the infusion of
CO2 was terminated and the rats were observed for
another 2 min to confirm the death of the animal. Thus, a total of
7 min observation was used to confirm animal sacrifice. All the
euthanasia procedures on the use of experimental animals adhered to
the AVMA Guidelines for the Euthanasia of Animals (17) and the best efforts were made to
minimize animal suffering throughout the experiment. The humane
endpoints of this study included rats quickly losing 20% of their
original body weight and rats that were unable to eat and drink on
their own due to weakness. Immunofluorescence staining or western
blotting were performed to evaluate the effects of irradiation on
neurogenesis and protein expression.
BrdU labeling
Each rat received an intraperitoneal injection of
BrdU (50 mg/kg body weight), twice daily at 8-h intervals, for a
total period of 4 days before WBI.
AAV and stereotaxic injection
An AAV serotype 8 encoding GFP vector was used in
the present study. AAV8 encoding TrkA scramble sequence and
overexpressing TrkA were constructed by Jikai Biological Technology
Co., Ltd. AAV8 expressing a TrkA scramble sequence served as
control. After anesthesia, rats were bilaterally infused with AAV8
into the dorsal hippocampus as previously described (18). Briefly, the injection site was −3.7
mm anteroposterior from the bregma, ±2.2 mm mediolateral from the
bregma and 3.5 mm below the surface of the skull. The titer of AAV8
virus was 3.92×1012 v.g./ml (vector genomes/ml), where a
total volume of 2 µl was injected. The rate of infusion was 0.2
µl/min, where the cannula was left in place for 5 min to ensure the
complete diffusion of the virus into the brain.
Morris water maze test
Place navigation tests were conducted on days 1–5,
where all rats underwent 4 trials per day. A square platform, ~9 cm
in diameter, was submerged 1.5 cm beneath the water. The location
of the platform remained constant throughout the trials, but the
starting location changed for each trial. Each rat was given 60 sec
to locate the platform, where it was then permitted to rest for a
further 10 sec before being assisted back into the home cage. If
the rat failed to locate the platform in the allotted time, it was
guided to the platform and allowed to remain on it for 10 sec.
A spatial probe test took place on day 6, where the
platform was removed and the rats were allowed to swim freely for
60 sec. The test was performed within 24 h after the trials for 4
days. The time taken to reach the platform, path length, swimming
speed and the number of times the target zone was crossed were
recorded.
Open field test
Using an opaque open field (410×410×505 mm), the
middle and inner areas were termed the central region. Each rat was
placed in the central region, and was allowed to move freely around
the open field for 10 min. An automated video-tracking system
(Shanghai Jiliang Software Technology Co., Ltd.) was used to assess
voluntary locomotor activity. Total track length, recorded as the
total distance traveled and the time spent in the central region,
were measured using the automated video-tracking system.
Western blotting analysis
Immediately after the behavior experiment, the
experimental animals were sacrificed with carbon dioxide. The
hippocampus tissue homogenates were lysed in RIPA buffer (cat. no.
P0013B; Beyotime Institute of Biotechnology) and subjected to 10%
SDS-PAGE (cat. no. ST628; Beyotime Institute of Biotechnology).
Protein concentrations were determined using the bicinchoninic acid
protein assay kit (cat. no. P0009; Beyotime Institute of
Biotechnology). The polyvinylidene difluoride membranes were
blocked in 5% milk in TBST for 1 h at room temperature, incubated
with primary antibodies overnight at 4°C, and then incubated with
secondary antibodies for 1 h at room temperature. The following
antibodies were used: Anti-NGF (1:1,000; product code ab6199;
Abcam), anti-TrkA (1:2,000; product code ab76291; Abcam),
anti-phosphorylated (p)-PI3K (1:1,000; cat. no. 511120;
ZenBioscience), anti-p-AKT (1:1,000; cat. no. 310022,
ZenBioscience) and anti-p-Erk1/2 (1:1,000; cat. no. 310064;
ZenBioscience). Anti-GAPDH (1:5,000; cat. no. ABS16), anti-β-actin
(1:10,000; cat. no. AV40173) and anti-tubulin (1:10,000; cat. no.
T3526) were purchased from Sigma-Aldrich, Merck KGaA. Goat
anti-mouse HRP (1:10,000; code no. 115-035-166) and goat
anti-rabbit HRP (1:10,000; code no. 111-035-003) were purchased
from Jackson ImmunoResearch, Laboratories Inc. Immunolabeling of
membranes was detected by ECL chemiluminescence (P0018S; Beyotime
Institute of Biotechnology). The integrated densities of each band
were quantified using ImageJ Software (version 2006.02.01; National
Institutes of Health).
Immunohistochemistry
Immediately after the behavior experiment, the rats
were anesthetized and perfused with ice-cold saline followed by
ice-cold 4% paraformaldehyde. Brain tissues were then removed and
post-fixed overnight in 4% paraformaldehyde at 4°C followed by
equilibration in 30% sucrose. Sagittal sections at 30-µm-thick were
cut at −20°C by frozen slicer (Leica CM1950; Leica Microsystems,
Inc.). The sections were treated with 2 M HCl at 37°C for half an
hour and then washed in 1X Tris-buffered saline pH 8.5 before
incubation in 5% BSA (cat. no. ST023; Beyotime Institute of
Biotechnology) and 5% Triton X-100 (Beyotime Institute of
Biotechnology) in PBS for 1 h at room temperature. Tissue sections
were incubated with rabbit anti-doublecortin (DCX; 1:100; cat. no.
D9818; Sigma-Aldrich; Merck KGaA), mouse anti-neuronal nuclei
(NeuN; 1:50; cat. no. MAB377; Thermo Fisher Scientific, Inc.),
rabbit anti-glial cell marker glial fibrillary acidic protein
(GFAP; 1:100; cat. no. ab207165, Abcam) or rabbit anti-BrdU (1:500;
cat. no. B8434; EMD Millipore) primary antibodies at 4°C overnight,
followed by a fluorescent secondary antibody conjugated with Alexa
Fluor® 488 goat anti-mouse (1:500; cat. no. A11001;
Invitrogen; Thermo Fisher Scientific, Inc.) or Cy3-conjugated goat
anti-rabbit (1:500; cat. no. 111-165-144; Jackson ImmunoResearch
Laboratories, Inc.) secondary antibodies for 2 h at room
temperature. Cell nuclei were stained with 100 ng/ml DAPI (cat. no.
C1002; Beyotime Institute of Biotechnology) for 10 min at room
temperature.
A confocal laser-scanning microscope (Olympus
Corporation) with ×20 objective was used to analyze the tissue
staining. Cell counts were evaluated in 5–10 tissue sections per
rat using a multi-channel configuration with ImageJ software
(version 2006.02.01). Cell counts were limited to regions in the
granule cell layer and hilus. The volumes of the granule cell layer
and hilus were used to normalize the numbers of positive cells.
Statistical analysis
SPSS 22.0 software (IBM Corp.) was used to analyze
all statistical data, and the results are expressed as the mean ±
standard error of the mean (SEM). Data were analyzed using unpaired
t-test, one-way analysis of variance (ANOVA), or two-way ANOVA.
Student-Newman-Keuls was used as the post hoc test following ANOVA.
Mann-Whitney U-test was used to analyze non-normally distributed
behavior test data. P<0.05 was considered to indicate a
statistically significant difference.
Results
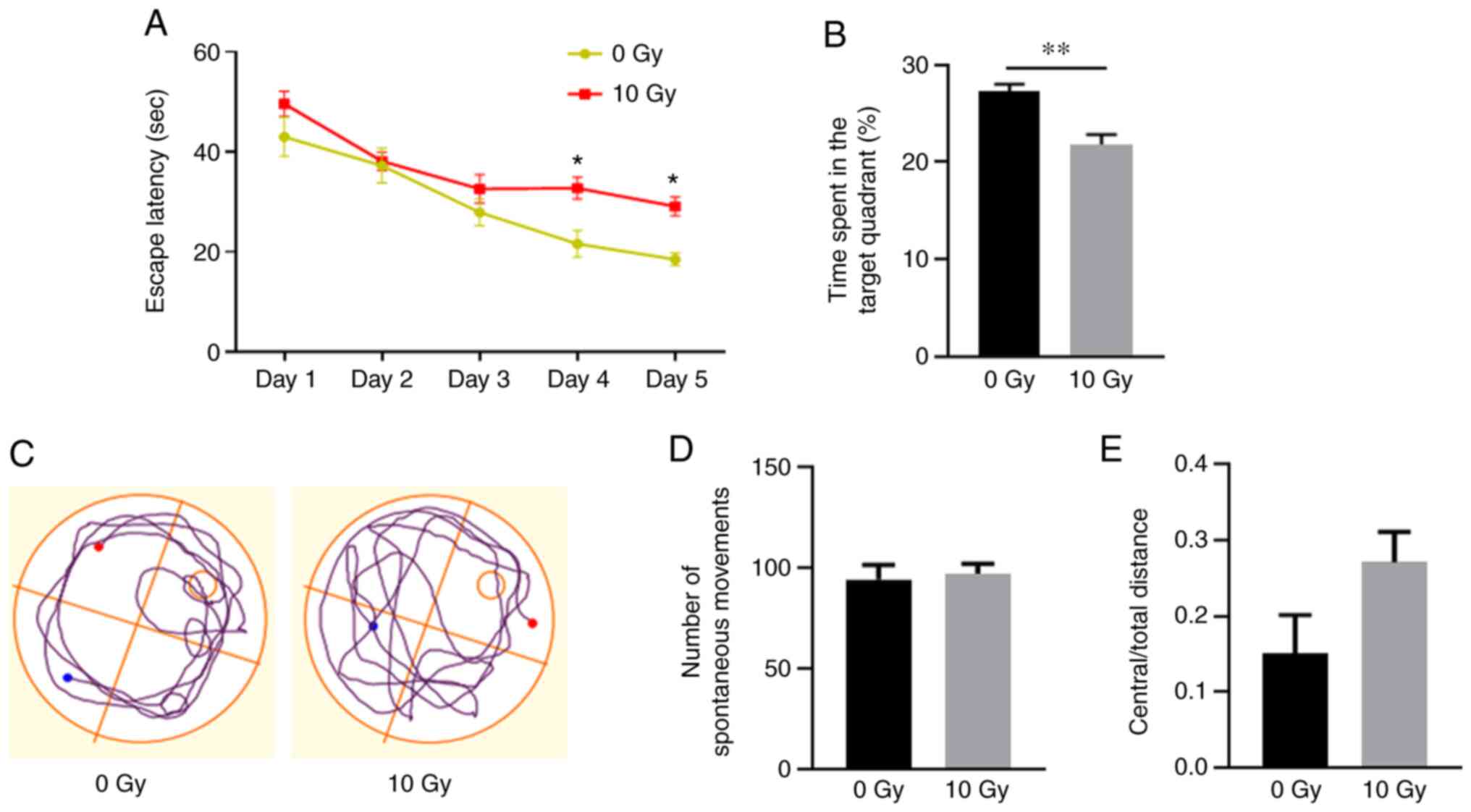
Cranial irradiation induces spatial
memory dysfunction
The effects of radiation on spatial learning and
memory were investigated using spatial navigation tests 3 months
after WBI. In the place navigation test, all rats displayed
improved performance during days 1–4. On days 4 and 5, the
irradiated rats exhibited significantly increased latency times
before locating the submerged platform, compared with those of the
non-irradiated rats (P<0.05; Fig.
1A). Furthermore, in the spatial probe test, the irradiated
rats spent significantly less time in the target quadrant compared
with that of the non-irradiated rats (P<0.01; Fig. 1B and C). These results demonstrated
that irradiation was associated with spatial memory
dysfunction.
An open field test was subsequently applied to
assess the anxiety levels and spontaneous movement. The results
revealed no significant differences in spontaneous movement or
anxiety-like behavior between the two groups (Fig. 1D and E), which eliminates the
influences of exercise and emotion on spatial memory
dysfunction.
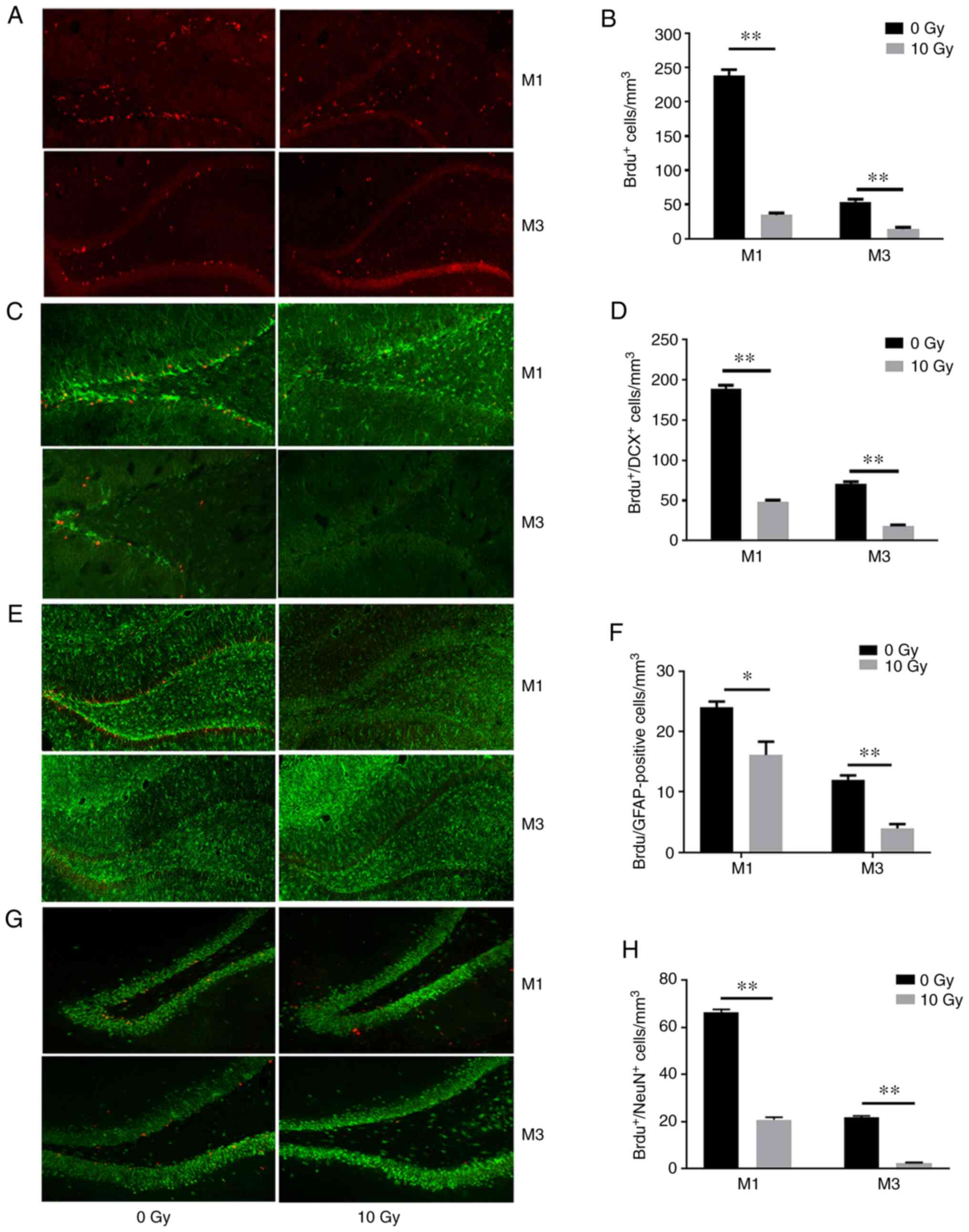
Cranial irradiation inhibits cellular
proliferation and neurogenesis in the hippocampus
The results of the BrdU-labeling assay revealed that
irradiated rats exhibited 74.2 and 73.9% fewer proliferating cells
(P<0.01; Fig. 2A and B) compared
with those in non-irradiated rats after 1 and 3 months,
respectively. Immature neurons were labeled with BrdU and DCX, a
microtubule-associated protein expressed in migrating neuroblasts
(19). The results revealed an 85.4
and 73.7% decrease in the number of BrdU- and DCX-positive cells
after cranial irradiation (P<0.01; Fig. 2C and D). In addition, double
labeling of BrdU and the glial cell marker GFAP also revealed
reduced staining (32.9 and 66.7%) in irradiated rats compared with
those in the non-irradiated rats at 1 and 3 months (P<0.01;
Fig. 2E and F). Mature neurons that
had undergone recent division were labeled with BrdU and NeuN
(BrdU+/NeuN+). The irradiated rats exhibited
68.5 and 88.6% fewer BrdU+/NeuN+ cells
compared with those in the non-irradiated rats at 1 and 3 months,
respectively (P<0.01; Fig. 2G and
H). These results indicated WBI inhibited normal neuronal
proliferation, differentiation, maturation and survival in the
hippocampus.
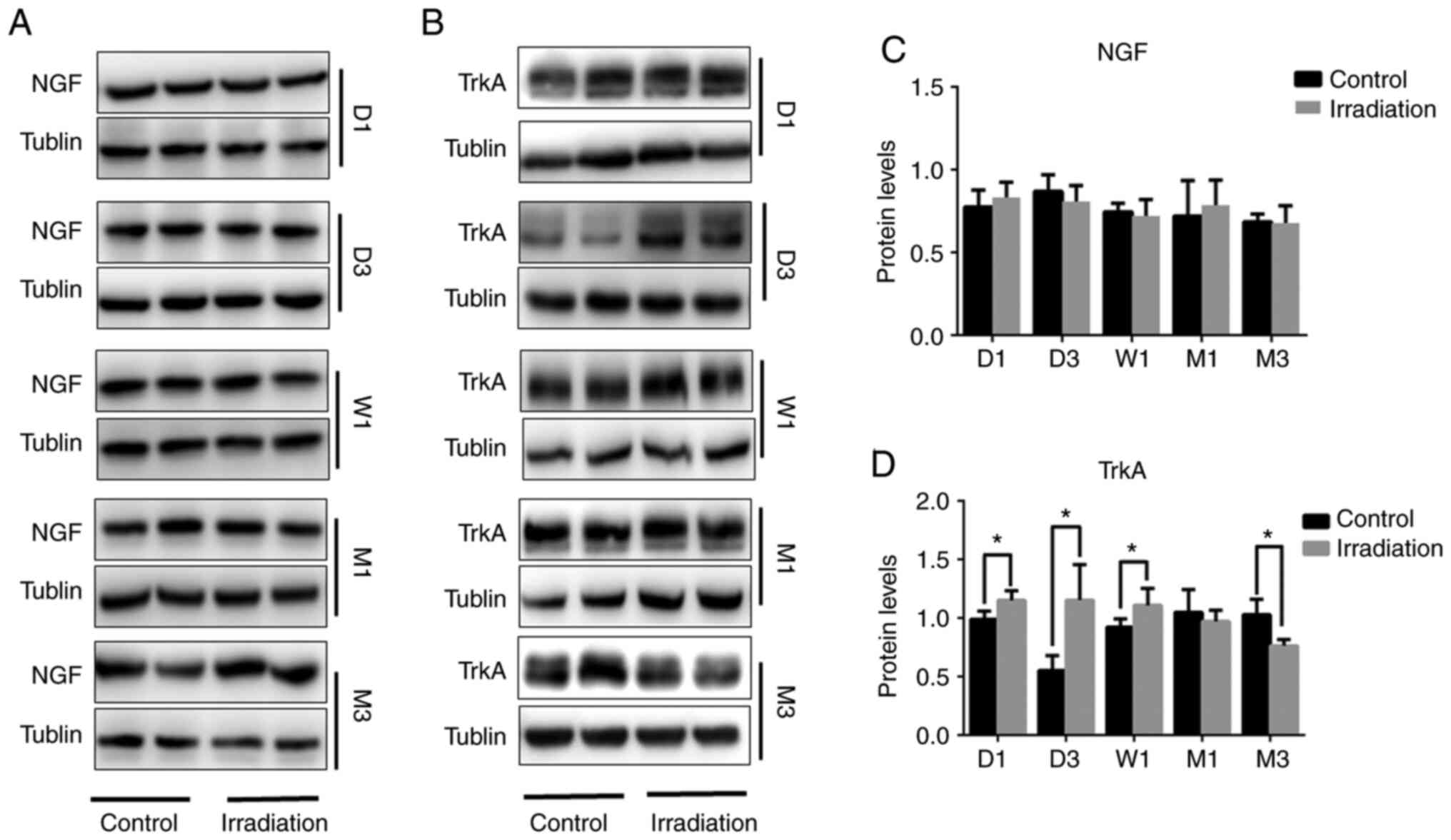
Changes in hippocampal NGF and TrkA
expression following cranial irradiation
Continuity detection was performed via western
blotting, which was used to assess changes in the expression of NGF
and TrkA in the rat hippocampus after irradiation. No differences
in NGF protein expression were detected between irradiated and
non-irradiated rats from 1 day to 3 months post-WBI (Fig. 3A and C). However, compared with that
in the non-irradiated group, TrkA protein expression was found to
be increased on days 1 and 3, in addition to after 1 week
(P<0.05), which was then decreased after the 1-month time-point
and was significantly reduced after 3 months (P<0.05; Fig. 3B and D).
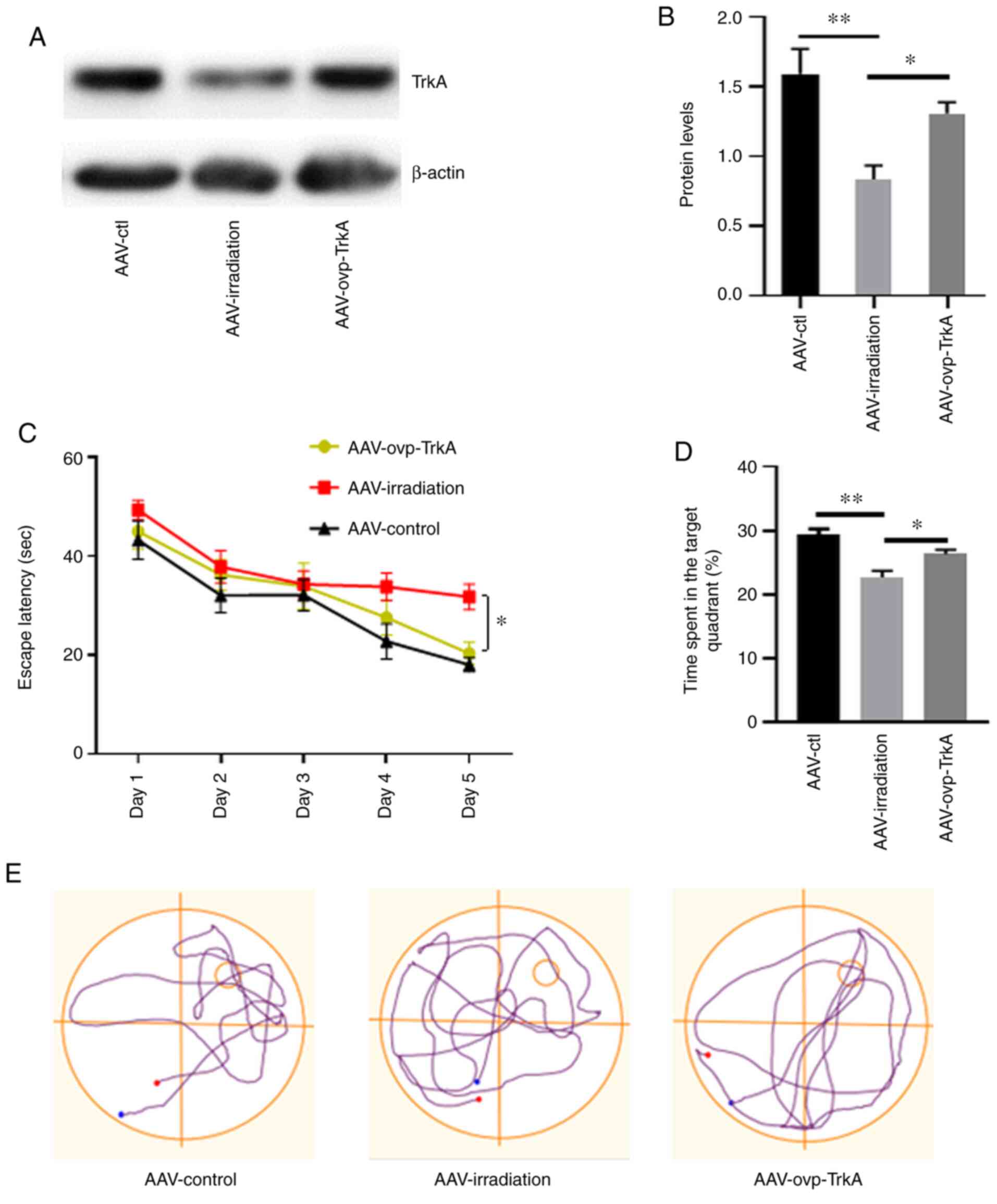
TrkA overexpression prevents memory
deficit in irradiated rats
The effects of TrkA overexpression on
irradiation-induced memory deficit were subsequently investigated.
AAV8s encoding TrkA scramble sequence and overexpressing TrkA
(AAV-ovp-TrkA) were injected into the hippocampus of irradiated
rats within 24 h after WBI (Fig.
S1). In control rats, AAV8s encoding a TrkA scramble sequence
were injected into irradiated rats (AAV-irradiation) and
non-irradiated rats (AAV-ctl), respectively. Western blotting
demonstrated the successful overexpression of TrkA in the
hippocampus (Fig. S2). The
influence of AAV8 injection on irradiated rats was also ruled out
(Fig. S3). The levels of
hippocampal TrkA expression in these three groups of rats were then
detected by western blotting (Fig. 4A
and B), which verified the effectiveness of AAV-ovp-TrkA
infection. Morris water maze test was used to evaluate spatial
memory function 3 months after WBI.
In the place navigation test, rats in the
AAV-ovp-TrkA group exhibited shorter latency times prior to arrival
at the hidden platform compared with those in the AAV-irradiation
group on days 4 and 5 (P<0.05; Fig.
4C). In the spatial probe trial, rats in the AAV-ovp-TrkA group
also spent significantly more time in the target quadrant compared
with those in the AAV-irradiation group (P<0.05; Fig. 4D). There was no statistical
difference between the AAV-ctl and AAV-ovp-TrkA groups. The swim
tracks revealed that rats in the AAV-irradiation group spent more
time searching for the submerged platform compared with those in
the other two groups (Fig. 4E).
AAV-induced TrkA overexpression in the
irradiated rat hippocampus restores neurogenesis
At 3 months after WBI, intrahippocampal infusion of
AAV-ovp-TrkA significantly prevented a reduction in neurogenesis
when compared with rats in the AAV-irradiation group (Fig. 5A-D). The rats in AAV-ovp-TrkA group
exhibited a 41.6% increase BrdU+/NeuN+ cells
compared with those in the AAV-irradiation group (P<0.05;
Fig. 5D). These results indicated
TrkA overexpression in the irradiated rat hippocampus restored
neurogenesis.
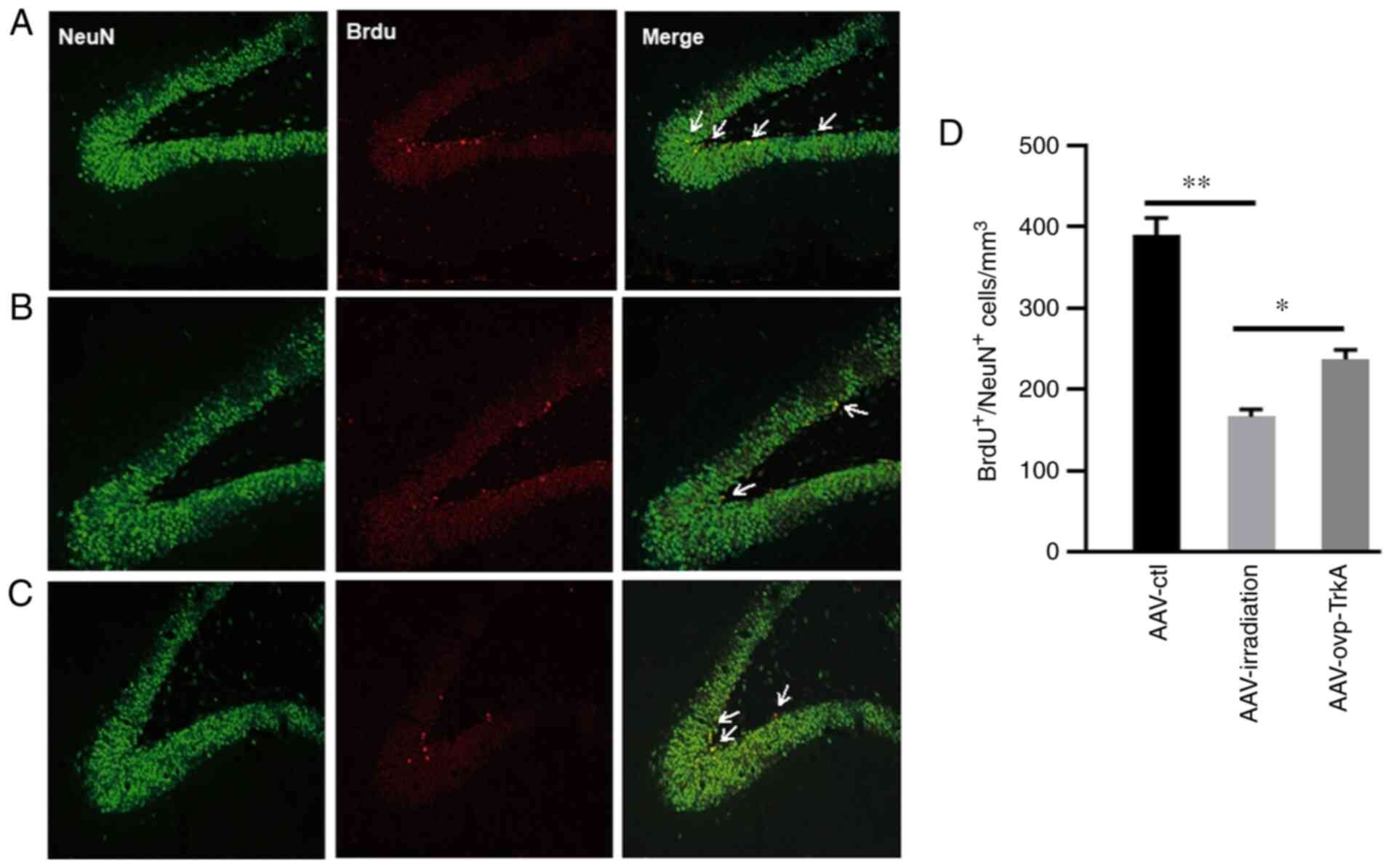 | Figure 5.AAV-TrkA expression in the irradiated
rat hippocampus prevents neurogenesis impairment. TrkA
overexpression rescued irradiation-induced neurogenesis impairment.
NeuN, BrdU and NeuN+/BrdU+ double staining in
(A) AAV-ctl, (B) AAV-irradiation and (C) AAV-ovp-TrkA groups. (D)
Number of NeuN+/BrdU+ cells in the AAV-ctl,
AAV-irradiation and AAV-ovp-TrkA groups. Data are presented as the
mean ± SEM. *P<0.05 and **P<0.01. Green, NeuN; red, BrdU;
yellow, NeuN+/BrdU+. BrdU,
5-bromodeoxyuridine; AAV, adeno-associated virus; TrkA, tropomyosin
receptor kinase A; NeuN, neuronal nuclei; ctl, control; ovp,
overexpressing. |
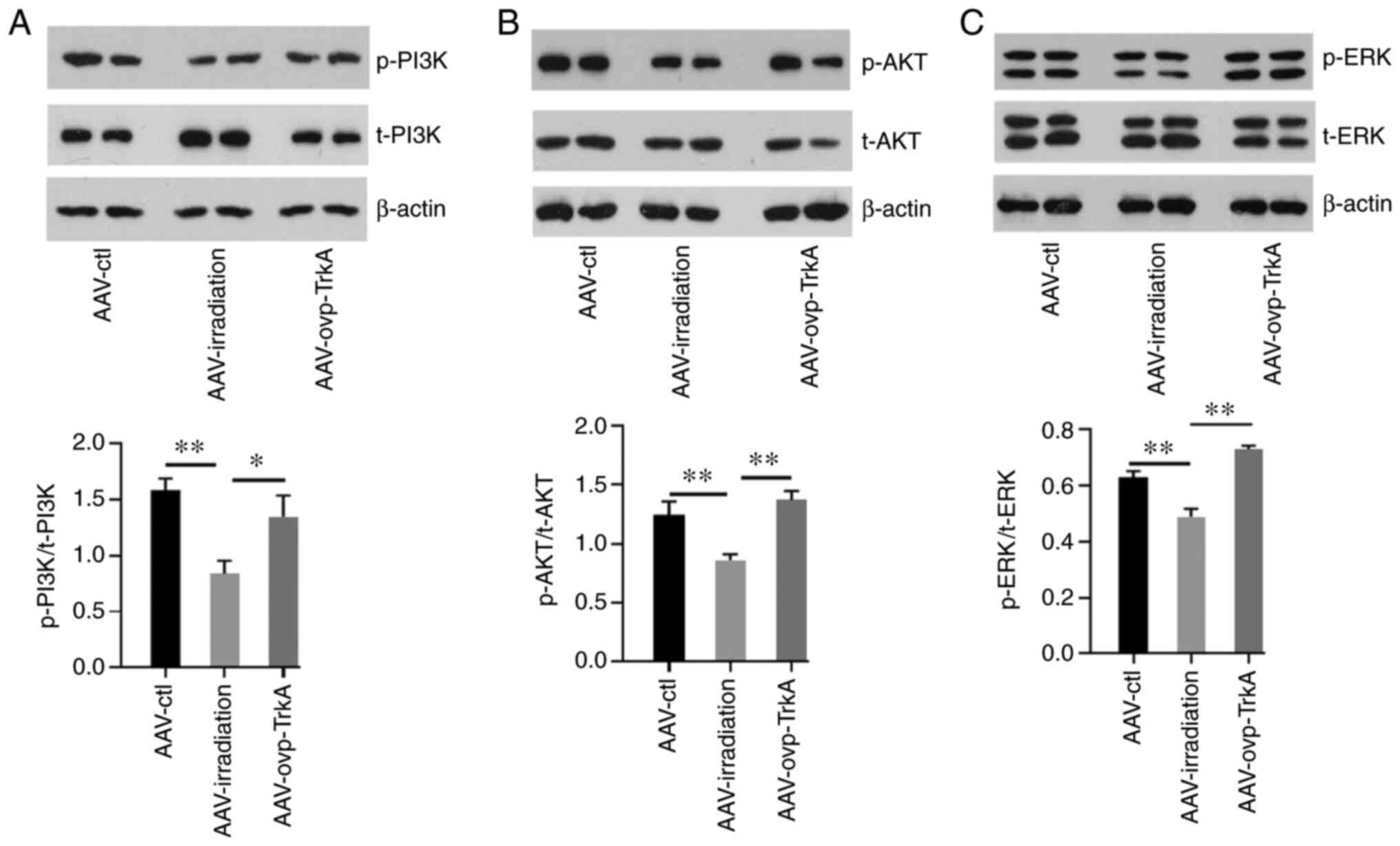
Phosphorylation of PI3K, AKT and
ERK1/2 is mediated by TrkA
The effect of cranial irradiation on the PI3K/AKT
and ERK1/2 pathways was subsequently evaluated. The results
revealed that the phosphorylation of PI3K, AKT and ERK1/2 was
decreased following WBI (all P<0.05; Fig. 6A and B). However, tissues from the
AAV-ovp-TrkA group exhibited increased PI3K, AKT and ERK1/2
phosphorylation compared with those in the AAV-irradiation group
(Fig. 6A and B).
Discussion
Radiation cognitive impairment is a common side
effect of radiotherapy for head and neck tumors, including glioma,
nasopharyngeal carcinoma and brain metastatic tumors, which limits
the efficacy of radiotherapy and damages the quality of life of
patients (20). At present, it has
attracted the attention of oncologists. The present study adds
important clinical significance to the pathogenesis of radiation
cognitive impairment.
Impairments in hippocampal neurogenesis have been
demonstrated to serve a critical role in the progression of
neurodegenerative diseases (8,10,21). A
number of studies have also indicated an association between
neurogenesis impairment and memory decline in radiotherapy-treated
animals (8,9). Rola et al (9) first reported this phenomenon, showing
a correlation between hippocampal neurogenesis and cognitive
impairment after a single dose of WBI (5–10 Gy X-ray) in young
mice. Since then, a series of studies have suggested that
hippocampal neurogenesis impairment is significantly associated
with the occurrence of cognitive defects (8,22,23).
However, the majority of these previous aforementioned studies only
focused on the acute radiation response.
Neural stem/progenitor cells are highly sensitive to
irradiation in a dose-dependent manner (8,9,24).
Brain development in rats on postnatal day 21 is comparable to that
of the preadolescent in humans (25). Because the rat brain is more
resistant to radiation than the human brain, a 10-Gy dose in the
rat approximates a clinically relevant dose in humans according to
the Biological Effective Dose (BED) assuming an α/β ratio of 2 for
late effects in normal brain tissue (26). Previous studies have found that 10
Gy WBI induced a decrease in neurogenesis in the rat hippocampus
and resulted in spatial memory retention deficits after 3 months
(9,24,25,27).
Therefore, in the present study, all rats received single WBI at 10
Gy to determine the persistence and severity of the relatively
early effects on neurogenesis 3 months after irradiation. The
results revealed that 10 Gy irradiation induced long-term and
sustained impairments in cell proliferation as revealed by BrdU
labeling, neuronal maturation as demonstrated by DCX labeling and
neurogenesis as determined by BrdU and NeuN co-labeling. The Morris
water maze test also revealed that WBI induced spatial memory
deficits. These results indicated that cranial irradiation induces
long-term impairment in hippocampal neurogenesis and spatial
memory.
NGF is an important neurotrophin involved in the
development of neural stem cells and synaptic plasticity (12,14,28).
In the adult brain, NGF also supports neural maintenance and repair
(12). NGF binds to and activates
specific receptor tyrosine kinases to facilitate neural stem cell
differentiation. TrkA has been identified as the primary NGF
receptor involved in both neuroprotection and neurodegenerative
diseases (16,29). Therefore, investigating changes in
the expression of NGF and TrkA may provide a therapeutically
beneficial target for radiotherapy-induced neurogenesis impairment
and memory decline. NGF mainly binds to TrkA to activate downstream
related signaling pathways. Conversely, p75NTR has only a low
affinity (30). Thus, in the
present study, the potential inhibitory effects of irradiation on
NGF-TrkA signaling were investigated in hippocampal tissues. NGF
expression levels were revealed to be similar between irradiated
and non-irradiated rats from 1 day to 3 months post-WBI. This
result is not consistent with those from studies of other
neurodegenerative diseases (12,14,16).
However, in clinical application, the treatment strategy developed
for neurotrophins, such as NGF and brain-derived neurotrophic
factor, has not shown the desired effect (28,31,32).
Therefore, it was hypothesized that defects in the neurotrophins
themselves may prevent their use as effective biotherapeutic
agents. The therapeutic application of natural neurotrophins is
also limited by poor oral bioavailability, poor permeability
through the blood-brain barrier, undetermined pharmacokinetics and
a short plasma half-life (28,31,33).
Notably, the expression of various specific neurotrophin receptors
was revealed to be absent in patients with neurodegenerative and
brain injury diseases, thereby limiting the biological roles of
neurotrophins in a therapeutic setting (16,34,35).
In the present study, the expression levels of TrkA
were detected in the hippocampus following irradiation. Dynamic
changes in TrkA expression were revealed, with TrkA protein
expression upregulated during week 1, before gradually decreasing 1
and 3 months post-WBI. In several previous studies of
neurodegenerative diseases, changes in TrkA receptor expression
have been revealed to be closely associated with neurological
function (33,34,35).
In addition, TrkA expression is closely related to neuronal
survival and development. In a study conducted by Haque et
al (36), calotropis
gigantean was revealed to promote the neuritogenic and
synaptogenic potential of hippocampus neurons via the activation of
NGF-TrkA-Erk1/2 signaling, which was inhibited by a TrkA-specific
inhibitor. Another previous study demonstrated that organophosphate
pesticides reduced the survival time of basal forebrain cholinergic
neurons by inhibiting TrkA (35).
Results of the present study revealed that irradiation specifically
decreased TrkA expression but that of NGF remained unchanged. This
finding indicated that TrkA serves a prominent role in
radiotherapy-induced neurogenesis impairment.
To verify the role of TrkA in radiotherapy-induced
neurogenesis impairment and memory decline further, an AAV virus
was constructed to overexpress TrkA. TrkA overexpression in the
irradiated rat hippocampus was revealed to ameliorate spatial
memory decline and prevent hippocampal neurogenesis impairment,
which further supports the crucial protective role of TrkA.
NGF-TrkA interactions may activate multiple
intracellular signaling pathways, such as the MAPK, PI3K-AKT and
phospholipase Cγ cascades (12,16,37).
Furthermore, key signaling molecules downstream of TrkA were
investigated, including those involved in cell survival and death.
The present study demonstrated that 10 Gy WBI inhibited ERK1/2 and
PI3K-Akt activation. Following the activation of TrkA, ERK1/2 was
able to upregulate the transcription factor CREB, which regulates
the transcription of various genes responsible for the maintenance
of neurogenesis, plasticity and memory consolidation (38). AKT phosphorylation activated by TrkA
is also considered to be one of the key factors in the regulation
of cell survival and apoptosis (37).
In conclusion, results from the present study
indicated a previously unreported radiation-induced memory deficit
and neurogenesis impairment in a manner that is dependent on
TrkA-dependent signaling. Therefore, future development of
TrkA-dependent signaling agonists may serve as a promising
therapeutic approach for reducing radiation-induced adverse effects
in patients with brain tumors.
Supplementary Material
Supporting Data
Acknowledgements
We would like to thank Dr Junjun Zhang and Dr Qixian
Zhang at The Second Affiliated Hospital of Soochow University
(Suzhou, China) for their technical assistance. We would like to
thank Dr Rui Sun at The Third Affiliated Hospital of Soochow
University (Changzhou, China) for manuscript revision.
Funding
The present study was supported by the Natural
Science Foundation of Jiangsu Province (grant no. BK20171224), the
China Postdoctoral Science Foundation (grant no. 2018M632683), the
‘Six One Project’ of Jiangsu Province (grant no. LGY2018009), the
Youth Talent Project of Changzhou Health Commission (grant no.
QN201804) and the Youth Medical Fund of Yancheng 1st People's
Hospital (grant no. QN2018007).
Availability of data and materials
The data generated and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SJ, JY and MY designed the present study. SJ and HW
carried out the experiments and wrote the manuscript. XD, QC and XJ
coordinated the study and analyzed the data. SJ, JY and MY revised
it critically for important intellectual content. All authors were
involved in writing the paper and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Ethics Committee of The Affiliated Suzhou Hospital of Nanjing
Medical University and Shandong University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NGF
|
nerve growth factor
|
|
TrkA
|
tropomyosin receptor kinase A
|
|
WBI
|
whole-brain irradiation
|
|
ERK
|
extracellular signal-regulated protein
kinase
|
|
CREB
|
cyclic adenosine monophosphate
response element-binding protein
|
|
AAV
|
adeno-associated virus
|
|
p75NTR
|
p75 neurotrophin receptor
|
|
GFP
|
green fluorescent protein
|
|
BrdU
|
5-bromodeoxyuridine
|
References
|
1
|
Koyfman SA, Ismaila N, Crook D, D'Cruz A,
Rodriguez CP, Sher DJ, Silbermins D, Sturgis EM, Tsue TT, Weiss J,
et al: Management of the neck in squamous cell carcinoma of the
oral cavity and oropharynx: ASCO Clinical Practice Guideline. J
Clin Oncol. 37:1753–1774. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Caudell JJ, Torres-Roca JF, Gillies RJ,
Enderling H, Kim S, Rishi A, Moros EG and Harrison LB: The future
of personalised radiotherapy for head and neck cancer. Lancet
Oncol. 18:e266–e273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gillison ML, Trotti AM, Harris J, Eisbruch
A, Harari PM, Adelstein DJ, Jordan RCK, Zhao W, Sturgis EM,
Burtness B, et al: Radiotherapy plus cetuximab or cisplatin in
human papillomavirus-positive oropharyngeal cancer (NRG Oncology
RTOG 1016): A randomised, multicentre, non-inferiority trial.
Lancet. 393:40–50. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hansen CC, Chrane K, Gunn GB, Mohamed ASR,
Rosenthal DI, Wefel JS, Phan J, Frank SJ, Garden AS, Smith B, et
al: Cognitive function and patient-reported memory problem
following radiation therapy for cancers at the skull base: A
survivorship study using the telephone interview for cognitive
status and the MDASI-HN. Int J Radiat Oncol. 94:967. 2016.
View Article : Google Scholar
|
|
5
|
Wang M, Cairncross G, Shaw E, Jenkins R,
Scheithauer B, Brachman D, Buckner J, Fink K, Souhami L, Laperriere
N, et al: Cognition and quality of life after chemotherapy plus
radiotherapy (RT) vs. RT for pure and mixed anaplastic
oligodendrogliomas: Radiation therapy oncology group trial 9402.
Int J Radiat Oncol. 77:662–669. 2010. View Article : Google Scholar
|
|
6
|
Cheung YT, Sabin ND, Reddick WE, Bhojwani
D, Liu W, Brinkman TM, Glass JO, Hwang SN, Srivastava D, Pui CH, et
al: Leukoencephalopathy and long-term neurobehavioural,
neurocognitive, and brain imaging outcomes in survivors of
childhood acute lymphoblastic leukaemia treated with chemotherapy:
A longitudinal analysis. Lancet Haematol. 3:e456–e466. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alexander BM, Brown PD, Ahluwalia MS,
Aoyama H, Baumert BG, Chang SM, Gaspar LE, Kalkanis SN, Macdonald
DR, Mehta MP, et al: Clinical trial design for local therapies for
brain metastases: A guideline by the Response Assessment in
Neuro-Oncology Brain Metastases working group. Lancet Oncol.
19:e33–e42. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji S, Tian Y, Lu Y, Sun R, Ji J, Zhang L
and Duan S: Irradiation-induced hippocampal neurogenesis impairment
is associated with epigenetic regulation of bdnf gene
transcription. Brain Res. 1577:77–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rola R, Raber J, Rizk A, Otsuka S,
VandenBerg SR, Morhardt DR and Fike JR: Radiation-induced
impairment of hippocampal neurogenesis is associated with cognitive
deficits in young mice. Exp Neurol. 188:316–330. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Snyder JS: Recalibrating the relevance of
adult neurogenesis. Trends Neurosci. 42:164–178. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Genin EC, Caron N, Vandenbosch R, Nguyen L
and Malgrange B: Concise review: Forkhead pathway in the control of
adult neurogenesis. Stem Cells. 32:1398–1407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aloe L, Rocco ML, Bianchi P and Manni L:
Nerve growth factor: From the early discoveries to the potential
clinical use. J Trans Med. 10:2392012. View Article : Google Scholar
|
|
13
|
Troy CM, Friedman JE and Friedman WJ:
Mechanisms of p75-mediated death of hippocampal neurons. Role of
caspases. J Bio Chem. 277:34295–34302. 2002. View Article : Google Scholar
|
|
14
|
Pramanik S, Sulistio YA and Heese K:
Neurotrophin signaling and stem cells-implications for
neurodegenerative diseases and stem cell therapy. Mol Neurobiol.
54:7401–7459. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Donnelly CR, Gabreski NA, Suh EB,
Chowdhury M and Pierchala BA: Non-canonical Ret signaling augments
p75-mediated cell death in developing sympathetic neurons. J Cell
Biol. 217:3237–3253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sang Q, Sun D, Chen Z and Zhao W: NGF and
PI3K/Akt signaling participate in the ventral motor neuronal
protection of curcumin in sciatic nerve injury rat models. Biomed
Pharmacother. 103:1146–1153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
AVMA Guidelines for the Euthanasia of
Animals 2020 Edition. simplehttps://www.avma.org/resources-tools/avma-policies/avma-guidelines-euthanasia-animals
|
|
18
|
Ding X, Wu HH, Ji SJ, Cai S, Dai PW, Xu
ML, Zhang JJ, Zhang QX, Tian Y and Ma QH: The p75 neurotrophin
receptor regulates cranial irradiation-induced
hippocampus-dependent cognitive dysfunction. Oncotarget.
8:40544–40557. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katharina M and Chichung LD: Evidence that
Doublecortin is dispensable for the development of adult born
neurons in mice. PLoS One. 8:e626932013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Soussain C, Ricard D, Fike JR, Mazeron JJ,
Psimaras D and Delattre JY: CNS complications of radiotherapy and
chemotherapy. Lancet. 374:1639–1651. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bao H and Song J: Treating brain disorders
by targeting adult neural stem cells. Trends Mol Med. 24:991–1006.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kitamura T, Saitoh Y, Takashima N,
Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H and
Inokuchi K: Adult neurogenesis modulates the hippocampus-dependent
period of associative fear memory. Cell. 139:814–827. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alam MJ, Kitamura T, Saitoh Y, Ohkawa N,
Kondo T and Inokuchi K: Adult neurogenesis conserves hippocampal
memory capacity. J Neurosci. 38:6854–6863. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Monje MJ, Mizumatsu S, Fike JR and Palmer
TD: Irradiation induces neural precursor-cell dysfunction. Nat Med.
8:955–962. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Achanta P, Fuss M and Martinez JL Jr:
Ionizing radiation impairs the formation of trace fear memories and
reduces hippocampal neurogenesis. Behav Neurosci. 123:1036–1045.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun R, Zhang LY, Chen LS and Tian Y:
Long-term outcome of changes in cognitive function of young rats
after various/different doses of whole brain irradiation. Neurol
Res. 38:647–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Winocur G, Wojtowicz JM, Sekeres M, Snyder
JS and Wang S: Inhibition of neurogenesis interferes with
hippocampus- dependent memory function. Hippocampus. 16:296–304.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Skup M: Neurotrophins: Evolution of
concepts on rational therapeutic approaches. Postepy Biochem.
64:231–241. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khotskaya YB, Holla VR, Farago AF, Mills
Shaw KR, Meric-Bernstam F and Hong DS: Targeting TRK family
proteins in cancer. Pharmacol Therap. 173:58–66. 2017. View Article : Google Scholar
|
|
30
|
Wehrman T, He X, Raab B, Dukipatti A, Blau
H and Garcia KC: Structural and mechanistic insights into nerve
growth factor interactions with the TrkA and p75 receptors. Neuron.
53:25–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ochs G, Penn RD, York M, Giess R, Beck M,
Tonn J, Haigh J, Malta E, Traub M, Sendtner M and Toyka KV: A phase
I/II trial of recombinant methionyl human brain derived
neurotrophic factor administered by intrathecal infusion to
patients with amyotrophic lateral sclerosis. Amyotroph Lateral
Scler Other Motor Neuron Disord. 1:201–206. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mishra BR, Maiti R, Nath S, Sahoo P, Jena
M and Mishra A: Effect of sertraline, dosulepin, and venlafaxine on
non-BDNF neurotrophins in patients with depression: A cohort study.
J Clin Psychopharmacol. 39:220–225. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Houlton J, Abumaria N, Hinkley SFR and
Clarkson AN: Therapeutic potential of neurotrophins for repair
after brain injury: A helping hand from biomaterials. Front
Neurosci. 13:7902019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shah AG, Friedman MJ, Huang S, Roberts M,
Li XJ and Li S: Transcriptional dysregulation of TrkA associates
with neurodegeneration in spinocerebellar ataxia type 17. Hum Mol
Genet. 18:4141–4152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kumar V, Gupta AK, Shukla RK, Tripathi VK,
Jahan S, Pandey A, Srivastava A, Agrawal M, Yadav S, Khanna VK and
Pant AB: Molecular mechanism of switching of TrkA/p75(NTR)
signaling in monocrotophos induced neurotoxicity. Sci Rep.
5:140382015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Haque MN, Mohibbullah M, Hong YK and Moon
IS: Calotropis gigantea promotes neuritogenesis and synaptogenesis
through activation of NGF-TrkA-Erk1/2 signaling in rat hippocampal
neurons. Am J Chin Med. 46:1861–1877. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim MS, Shutov LP, Gnanasekaran A, Lin Z,
Rysted JE, Ulrich JD and Usachev YM: Nerve growth factor (NGF)
regulates activity of nuclear factor of activated T-cells (NFAT) in
neurons via the phosphatidylinositol 3-kinase (PI3K)-Akt-glycogen
synthase kinase 3β (GSK3β) pathway. J Biol Chem. 289:31349–31360.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arthur JS, Fong AL, Dwyer JM, Davare M,
Reese E, Obrietan K and Impey S: Mitogen- and stress-activated
protein kinase 1 mediates cAMP response element-binding protein
phosphorylation and activation by neurotrophins. J Neurosci.
24:4324–4332. 2004. View Article : Google Scholar : PubMed/NCBI
|