Introduction
Prostate cancer (PCa) is one of the most common
malignant tumors of the male genitourinary system. According to
pathological characteristics, the World Health Organization
classifies PCa into five categories: i) adenocarcinoma (acinar
adenocarcinoma); ii) ductal adenocarcinoma; iii) urothelial
carcinoma; iv) squamous cell carcinoma; and v) and adenosquamous
carcinoma, of which adenocarcinomas account for >95% of cases.
Current data indicate that PCa ranks second in incidence and fifth
in mortality rate among male malignant tumors. Heterogeneity is one
of the characteristics of PCa, and it can be inert or highly
invasive (1). In almost 90% of
cases, PCa is confined to organs or locally advanced (2,3).
However, PCa is aggressive and metastasizes through the blood and
lymphatic systems, making it highly prone to recurrence in 10–15%
of cases (4). Radiotherapy or
radical prostatectomy are the preferred treatment methods for
early-stage PCa, and are administered according to clinical stage
and prostate-specific antigen levels (5). In highly metastatic PCa, antiandrogen
therapy or androgen deprivation therapy (ADT) can reduce the levels
of circulating testosterone by surgery or chemical castration
(3,6). However, these effects are short-lived,
with the majority of patients becoming resistant to ADT after 18–36
months, and gradually developing castration-resistant PCa (CRPC)
(2,7). Clinically, CRPC often exhibits a high
degree of invasiveness and is associated with a poor response to
treatment (8), and misdiagnosis
leads to the unnecessary suffering of patients due to further
treatments. Therefore, the identification of novel biomarkers that
can be used to predict disease outcomes and promote the effective
treatment of PCa, is urgently required.
Zinc finger protein 403 (ZFP403), located in
the 17q12-q21.1 region in humans, is highly conserved between
Drosophila and humans. Mice and humans share 87% homology in
their ZFP403 nucleotide sequences, and 96% homology in their
amino acid sequences (9). In
humans, ZFP403 has two known transcript types: full-length
transcripts encoding a protein composed of 696 amino acids, known
as gametogenetin binding protein 2 (GGNBP2), and short transcripts
encoding a protein consisting of 288 amino acids, termed laryngeal
cancer-related gene 1 (10–12).
ZFP403 is closely associated with the
occurrence and development of several types of cancer (13). Studies have demonstrated that
full-length ZFP403 may serve as a potential tumor
suppressor, and that ZFP403 is downregulated in various
malignant tumors, such as breast (9), ovarian cancer (14) and glioma (15). Furthermore, the absence of
ZFP403 can promote cellular proliferation (16) and tumorigenesis. However, the role
of ZFP403 in PCa has not been fully investigated.
The aim of the present study was to determine the
effect of ZFP403 on the carcinogenesis and progression of PCa, and
to support the role of ZFP403 as a potential target for PCa by
investigating its underlying mechanisms and effects on cell
proliferation, migration and invasion therein.
Materials and methods
Differential expression analysis using
the Gene Expression Profiling Interactive Analysis (GEPIA)
database
Data from the GEPIA database (http://gepia.cancer-pku.cn/) were used to determine
the association between ZFP403 and PCa. A gene symbol (ZFP403) was
entered into the ‘Enter gene name’ field. The ‘GoPIA!’ button was
clicked to generate the expression profile of the input gene across
all tumor and normal tissues, in the form of dot plots or body
maps. The ‘Boxplot’ tab in the ‘Expression DIY’ was clicked, and
the ‘PRAD’ option was selected under ‘Cancer name’; the results
were then presented in box plots.
Tissue samples
PCa and adjacent tissues were collected from 19 male
patients (aged 55 to 75 years old) who underwent treatment at the
Department of Urology, Cancer Hospital of the University of Chinese
Academy of Sciences (Hangzhou, China) between November 2013 and
July 2015. The study was approved by the Hospital's Committee for
the Protection of Human Subjects. All patients signed written
informed consent forms in accordance with the Declaration of
Helsinki.
Immunohistochemistry (IHC)
IHC was used to determine the level of ZFP403
expression in clinical PCa tissues. The experiment was performed as
previously described (14). The
data were obtained by semi-quantitative analysis and finally
presented as cut-off values. The cut-off values were determined by
the multiplication of the staining intensity score and positive
area score. Staining intensity scores were defined as follows: 0,
negative; 1, weak; 2, moderate; and 3, strong. Positive area scores
were defined as follows: 0, <25%; 1, 26–50%; 2, 51–75%; and 3,
>75%.
Cells and cell culture
The LNCaP, PC3, DU145, 22RV1 and RWPE-1 cell lines
were purchased from the Chinese Academy of Sciences. Between
December 5th and 12th, 2019, these cell lines were authenticated by
the analysis of 21 autosomal short tandem repeat loci (Biowing
Applied Biotechnology Co. Ltd.). RWPE-1 cells were cultured in
K-SFM medium (Gibco; Thermo Fisher Scientific, Inc.), and the other
cell lines were maintained in DMEM (Gibco; Thermo Fisher
Scientific, Inc.). DMEM contained 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), and the cells were
maintained at 37°C in a humidified atmosphere (5%
CO2).
Lentivirus infection and establishment
of ZFP40-knockdown cells
Lentiviruses were purchased from Shanghai GeneChem.
Short hairpin (sh)RNA sequences specifically targeting
ZFP403 (shRNA1, 5′-GAGCAUACAAUAUCCUUAU-3′; shRNA2,
5′-GGGUAUUAGCAGAUUGGAA-3′) were cloned into the
hU6-MCS-CMV-Puromycin vector. An empty vector was used as the
negative control. The cells were seeded (1×105) into a
24-well plate (Wuxi NEST Biotechnology Co., Ltd.) and cultured at
37°C overnight. Lentivirus (1×108 TU/ml, 10 µl) was
mixed with Opti-MEM (500 µl; Gibco; Thermo Fisher Scientific, Inc.)
and polybrene (5 µg/ml; Shanghai GeneChem), and then used to infect
the cells. The medium was replaced after 12 h, and complete medium
containing 1 µg/ml puromycin (Sigma-Aldrich; Merck KGaA) was used
to establish cells in which ZFP403 had been stably silenced.
After a week, the transfection efficiency was assessed by reverse
transcription-quantitative PCR (RT-qPCR) and western blot.
Reverse transcription-quantitative
(RT-q) PCR
The mRNA levels of ZFP403 in PCa cells were
analyzed by RT-qPCR. According to the manufacturer's instructions,
total RNA was extracted using TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc.) and then reverse transcribed into
cDNA using the PrimeScript™ RT reagent Kit (Takara Bio, Inc.). The
concentration of nucleic acid was quantified using a cell Imaging
Multi-mode Reader (BioTek Instruments, Inc.). Subsequently, qPCR
was performed with SYBR-Green (Bio-Rad Laboratories, Inc.) using
the CFX96 Real-Time system (Bio-Rad Laboratories, Inc.). The
thermocycling parameters were as follows: 95°C for 2 min; 40 cycles
at 95°C for 20 sec, 58°C for 20 sec, and 72°C for 15 sec; from 65°C
to 95°C, to rise 0.5°C every 5 sec. The relative expression level
of ZFP403 was quantified using the 2−∆∆Cq method
(17). The specific primer
sequences were as previously described: ZFP403 forward,
5′-ACAGGGTATTAGCAGATTGGAAC-3′ and reverse,
5′-TCATTGGTAACAATTACTTCTACAC-3′; and GAPDH forward,
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse, 5′-AGGGGCCATCCACAGTCTTC-3′
(14).
Western blot analysis
Total protein was extracted from PCa cells using
RIPA lysis buffer (Beyotime Institute of Biotechnology) and the
concentration of protein was determined using a BCA Protein Assay
kit (Beyotime Institute of Biotechnology). The samples (50 µg per
lane) were separated by 8 or 10% SDS-PAGE and then transferred onto
PVDF membranes (EMD Millipore). After blocking with TBST containing
5% skim milk at room temperature for 1 h, the membranes were
incubated overnight at 4°C with the following primary antibodies as
appropriate: GGNBP2 (1:250; cat. no. ab203104, Sigma-Aldrich; Merck
KGaA); cdc2 (cat. no. 9116), cyclin B1 (cat. no. 12231), β-catenin
(cat. no. 9562), MMP-2 (cat. no. 4022) and β-actin (cat. no. 4970)
(all 1:1,000; all from Cell Signaling Technology, Inc.); cdc25kC
(1:500; cat. no. sc-13138; Santa Cruz Biotechnology, Inc.);
E-cadherin (cat. no. 1702–1) and vimentin (cat. no. 2862-1)
(1:1,000; both from Epitomics, Inc.); and heparanase (1;500; cat.
no. ab42817; Abcam). Goat anti-rabbit IgG (H + L)-HRP and goat
anti-mouse IgG (H + L)-HRP (1:3,000; Bio-Rad Laboratories, Inc.)
were used as secondary antibodies and incubated with the membrane
at room temperature for 2 h. Protein bands were visualized using
Clarity™ Western ECL Substrate (Bio-Rad Laboratories, Inc.)
(18).
Colony formation assay
In vitro colony formation ability and
tumorigenicity were investigated by plate and soft-agar colony
formation assays. For plate colony formation assays, 500 cells per
well were seeded into 24-well plates and cultured at 37°C. After 14
days, the cells were fixed with 4% paraformaldehyde for 15 min, and
then stained with freshly prepared crystal violet for 20 min (both
at room temperature). For the soft-agar colony formation assay,
cells (1×103 per well) were plated in 0.35% low melting
point agarose above a solidified 0.7%-agarose layer in a 24-well
plate. The cells were cultured at 37°C and the medium was changed
twice a week. After 28 days, 1 mg/ml iodonitrotetrazolium chloride
(Sigma-Aldrich; Merck KGaA) was added to each well and the plate
was incubated for a further 24 h. The colonies were counted under a
Leica DM500 microscope (magnification, ×40; Leica Microsystems,
Inc.) (14).
Cell cycle analysis
The cells were stained using the PI/RNase staining
kit (BD Pharmingen; BD Biosciences), and the distribution of the
cell cycle was analyzed by flow cytometry using the Guava easyCyte
6HT-2L (Merck KGaA) (19). The
results were analyzed using ModFit LT™ software (Windows version
4.0; Verity Software House).
Migration and invasion assays
The migratory and invasive abilities of PCa cells
were investigated using a Transwell chamber (Corning, Inc.). For
invasion assays, Matrigel (BD Biosciences) was thawed at 4°C
overnight and diluted in serum-free medium. Then, 50 µl diluted
Matrigel was added to the upper chamber and incubated at 37°C for
20 min. Cells (1×105) were seeded into the upper chamber
with serum-free medium containing 0.5% BSA, while the lower chamber
was filled with 600 µl medium containing 10% FBS. After incubation
for 24 h at 37°C, the cells were fixed with 4% polyoxymethylene for
15 min at room temperature, and then stained using the Hematoxylin
and Eosin Staining Kit (Beyotime Institute of Biotechnology). The
cells that had not passed through the polycarbonate membrane were
removed with a cotton swab, and the invasive cells were visualized
using a Leica DM500 microscope and counted in five randomly
selected fields (magnification, ×100). For migration assays, all
conditions were consistent, except for the use of Matrigel
(20).
Xenograft tumor study
Male BALB/c nude mice (n=12) were obtained from the
Shanghai Laboratory Animal Center (CAS), and were used at 5 weeks
of age. The mice were housed and maintained in specific
pathogen-free conditions under a 12 h light-dark cycle at 25°C,
with free access to food and water. The tumor xenograft experiments
were approved and conducted according to the guidelines provided by
the Experimental Animal Center of Zhejiang Chinese Medical
University (no. SYXK-2018-0012). PC3 cells with or without
ZFP403-knockdown were harvested, resuspended in serum-free DMEM
medium (2×106) and subcutaneously injected into the
dorsa of the mice (21). Animal
health and behavior were monitored daily. When the tumor burden had
reached ~50 mm3, the sizes were measured every 3 days
and the tumor volume was calculated using the following formula:
V=1/2 (length × width2). After 40 days, the mice were
sacrificed by cervical dislocation. Death was verified by the
absence of a heartbeat and the onset of rigor mortis.
Statistical analysis
The data are presented as the mean ± SD. Comparison
between multiple groups was performed using one-way ANOVA followed
by Tukey's post hoc test, while a paired Student's t-test was used
for comparisons between 2 groups. All data analyses were performed
using GraphPad Prism version 5 (GraphPad Software, Inc.), and
P<0.05 was considered to indicate a statistically significant
difference.
Results
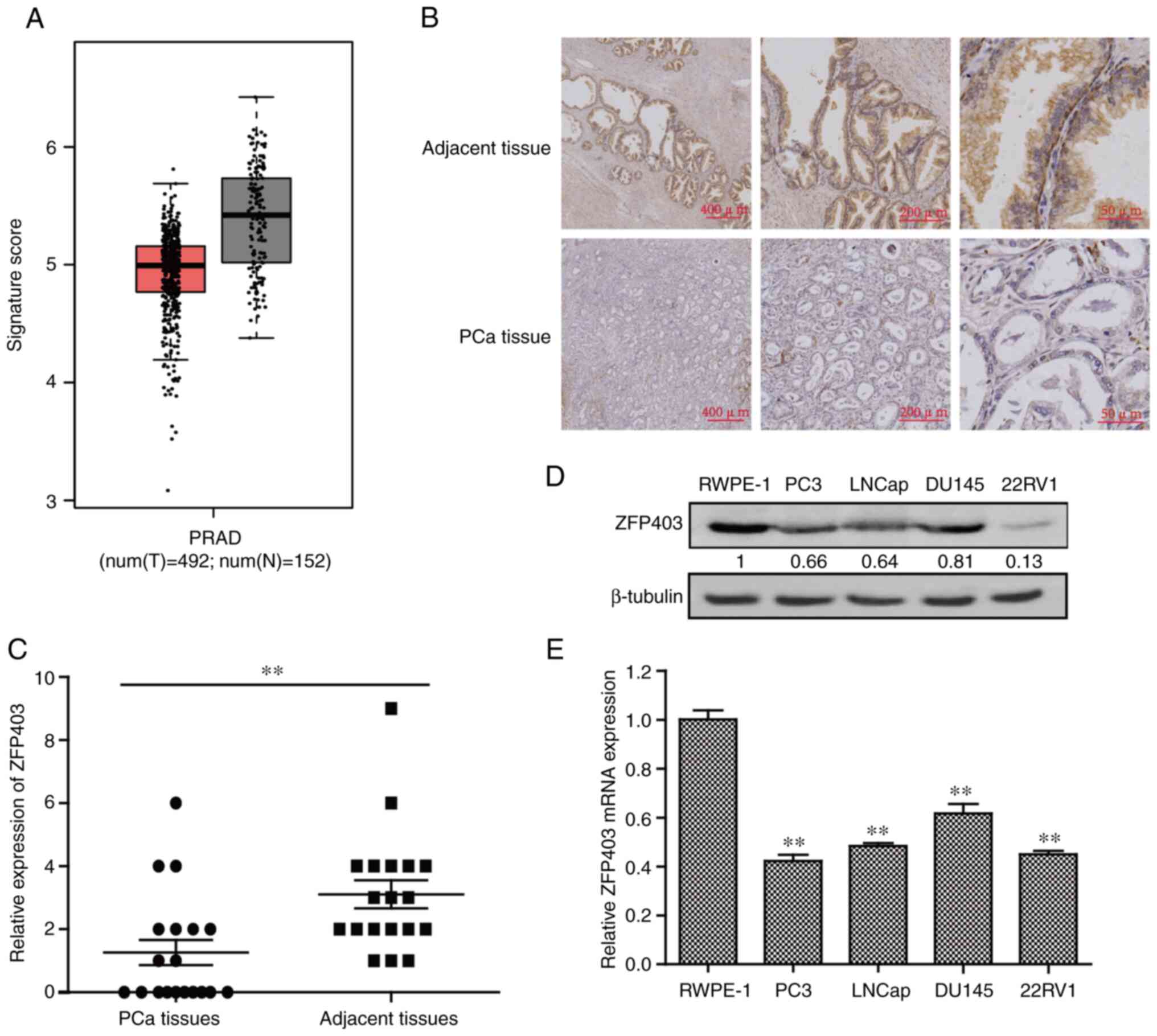
ZFP403 is downregulated in human
PCa
Data from the GEPIA2) database were used to
determine the association between ZFP403 and PCa.
Differential gene expression analysis revealed that ZFP403
expression was lower in PCa tissues than in normal tissues
(Fig. 1A). To further examine the
expression level of ZFP403 in PCa, 19 groups of PCa and
corresponding adjacent tissues were analyzed using IHC. The results
revealed that the expression of ZFP403 in PCa tissues was
significantly lower than that in adjacent normal tissues
(P<0.01; Fig. 1B and C). RT-qPCR
and western blot analysis were then used to detect the
ZFP403 level in normal prostatic epithelial cells (RWPE-1)
and PCa cell lines (LNCaP, PC3, DU145 and 22RV1). As shown in
Fig. 1D and E, the expression
levels of ZFP403 were lower in cancer cells than in RWPE-1
cells (P<0.01). These data indicate that ZFP403 may
function as a tumor suppressor in PCa.
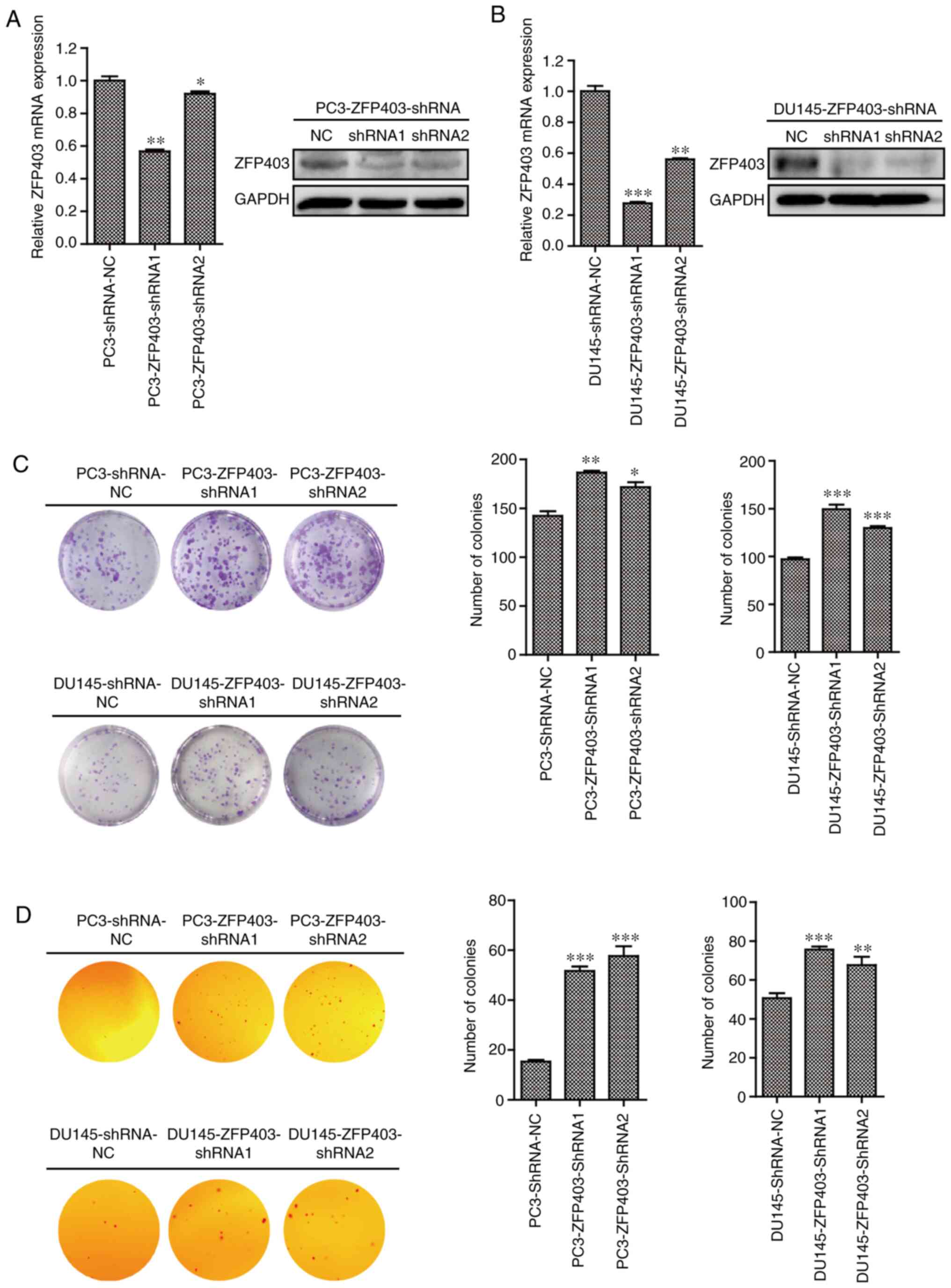
ZFP403-knockdown promotes the
proliferation of PCa cells
To evaluate the role of ZFP403 in the
proliferation PCa cells, human PCa cell lines (PC3 and DU145),
which are insensitive to androgens, were selected for transfection
with ZFP403-shRNA lentivirus. The results revealed that the
expression of ZFP403 was markedly decreased in the
ZFP403-knockdown cells compared with that in the negative
control (NC) cells, though to greater degree in cells transfected
with ZFP403-shRNA-1 (Fig. 2A and
B). Plate colony formation assays were performed to determine
the effects of ZFP403 on colony formation. The results
demonstrated that knocking down ZFP403 significantly
increased PC3 and DU145 cell colony numbers (Fig. 2C). Furthermore, in the soft-agar
colony formation assay, ZFP403-knockdown resulted in a
marked increase in the number of colonies (Fig. 2D), indicating that ZFP403
inhibits tumorigenicity in vitro.
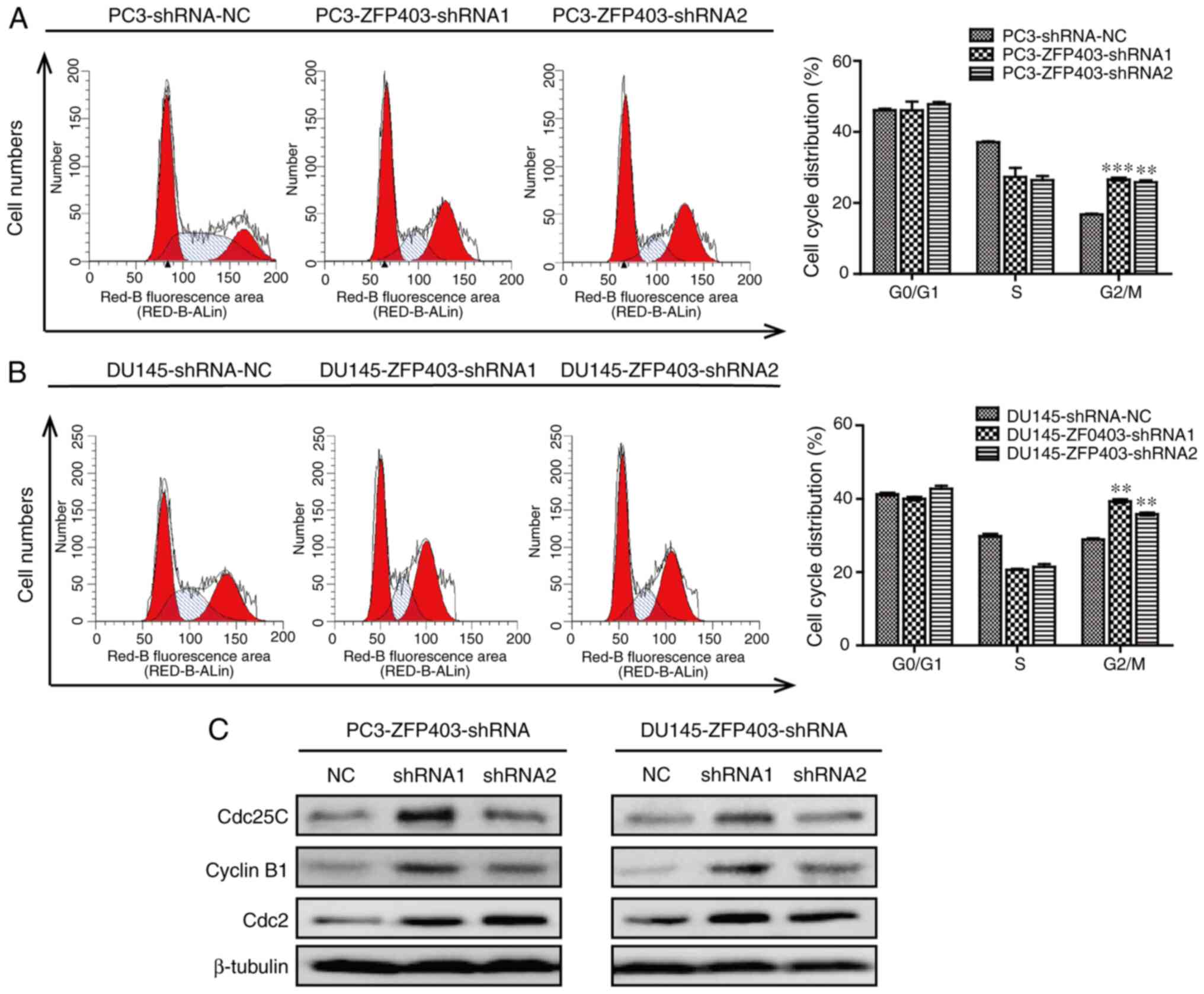
In order to further examine the effect of
ZFP403 on the proliferation of PCa cells, the cell cycle
distribution was detected by flow cytometry. The results revealed
that the number of cells transitioning from the S phase to the
G2/M phase was significantly increased in PCa cells in
which ZFP403 was knocked down (Fig. 3A and B). On this basis, western blot
analysis was performed to detect the expression of G2/M
phase-related proteins. The results demonstrated that knocking down
ZFP403 upregulated the expression of cyclin B1, cdc2 and
cdc25C (Fig. 3C). These data
indicate that silencing ZFP403 retains the viability and
promotes the proliferation of PCa cells.
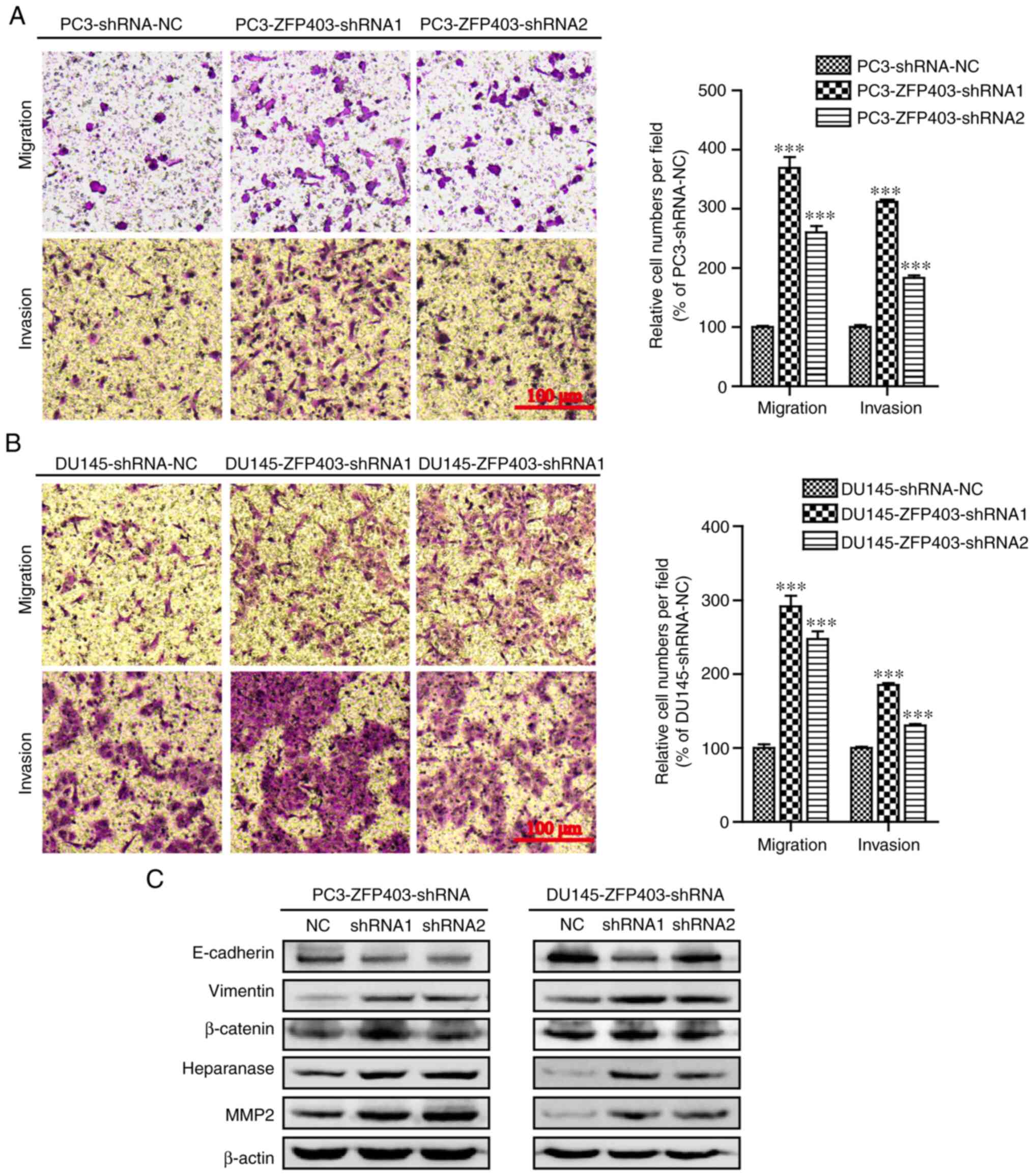
ZFP403-knockdown promotes the
migration and invasion abilities of PCa cells
To determine the potential role of ZFP403 in
the metastasis of PCa, the effects of ZPF403 on migration
and invasion were evaluated using Transwell chamber migration and
invasion assays. The results revealed that the cell migratory and
invasive abilities were significantly promoted following
ZFP403-knockdown (Fig. 4A and
B).
Further experiments demonstrated that
ZFP403-knockdown in PC3 and DU145 cells resulted in
downregulation of the epithelial cell marker E-cadherin, and the
upregulation of the interstitial cell marker vimentin. In addition,
β-catenin, heparanase and matrix metalloproteinase 2 (MMP2) were
also upregulated, as shown in Fig.
4C. Taken together, these results suggest that silencing
ZFP403 promotes PCa metastasis by enhancing cell migration
and invasiveness.
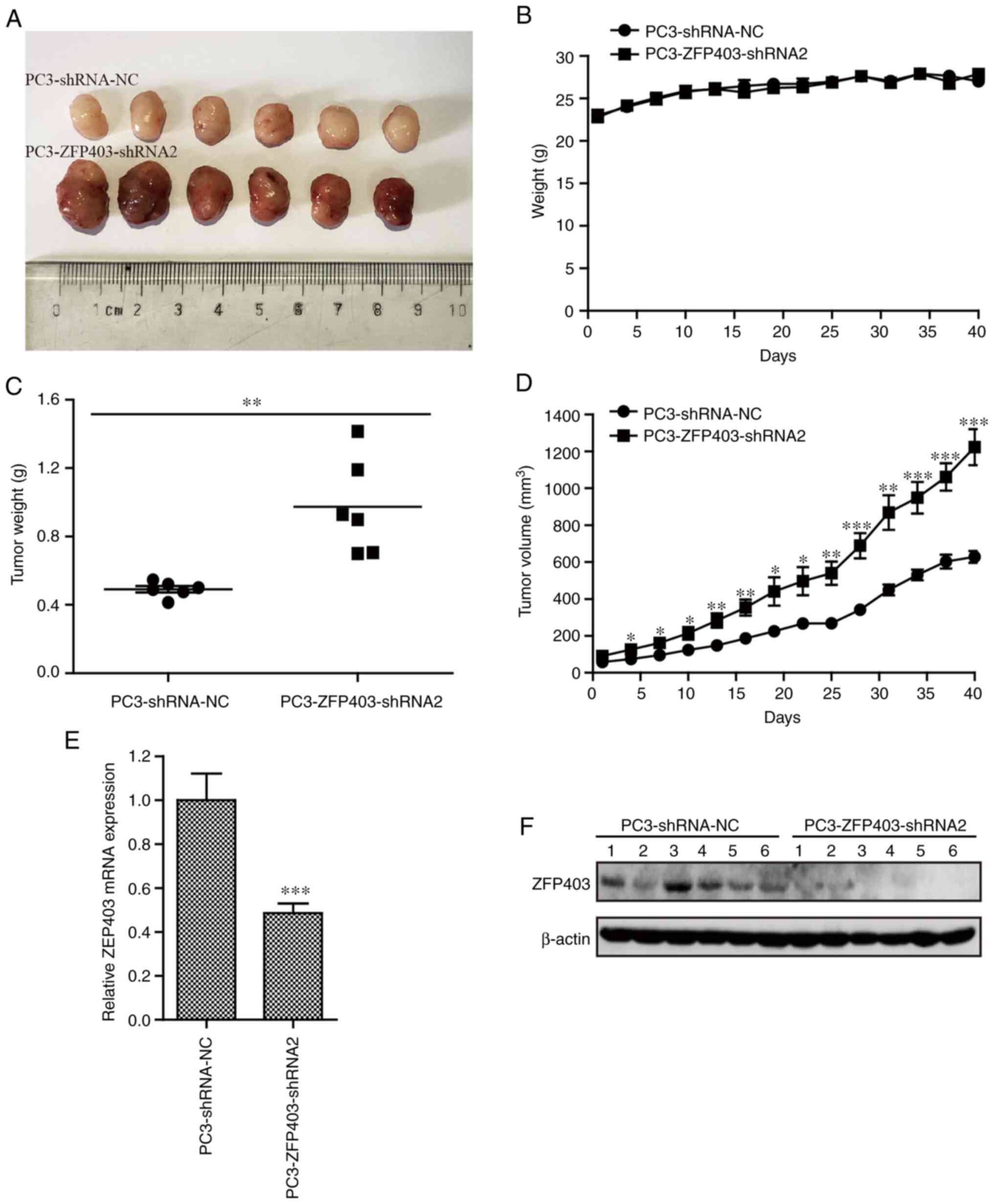
Antitumor effect of ZFP403 on tumor
xenografts in vivo
In order to examine the effect of ZFP403 on
tumor development and progression in vivo, a tumor xenograft
model of PCa cells was established by subcutaneous inoculation of
BALB/c nude mice. Body weight and tumor volume were measured every
3 days for 40 days. The growth rate of subcutaneous tumors in the
ZFP403-knockdown group was significantly higher than that in
the NC group. However, no significant difference in body weight was
observed between the groups (Fig.
5A-D). In addition, compared with the NC group, the mRNA and
protein levels of ZFP403 were decreased in the
ZFP403-knockdown group, as shown by RT-qPCR and western blot
analysis, respectively (Fig. 5E-F).
These data suggest that decreased ZFP403 expression is
closely associated with the tumorigenicity and development of PCa
in vivo.
Discussion
Due to the aging of the population and changes in
lifestyle, PCa has gradually become one of the most common cancer
types among men worldwide (22,23).
Currently, radical prostatectomy, radiotherapy and hormone therapy
are effective options for the treatment of early-stage PCa;
however, there are still challenges for the clinical treatment of
advanced PCa (24). Anti-androgen
therapy is usually effective in 80–90% of patients with advanced or
aggressive PCa, and has become the standard treatment of choice
(25). However, ADT is only
effective against androgen-dependent PCa, and does not eliminate
androgen-sensitive or androgen-independent cancer. Therefore, the
majority of patients enter a hormone-insensitive state after a few
years of hormone therapy, and eventually fail to respond to hormone
therapy (26,27). In addition, radiotherapy and
chemotherapy are largely ineffective in patients with advanced PCa,
and may even result in severe toxicity and side-effects (28,29).
With the development of molecular biological
technologies, and further understanding of the mechanisms of
tumorigenesis at the cellular and molecular levels, the targeted
molecular therapy of tumors has entered a new era. Worldwide,
>60% of ongoing clinical trials of molecular targeted therapy
are for cancer, including brain, lung, breast, pancreatic, liver,
colorectal, bladder, head and neck, skin, ovarian and kidney
cancer, as well as PCa (30).
Targeted anticancer drugs commonly used in clinical practice
include Herceptin, Glivec, Iressa and Tagrisso (31). However, few of these drugs are
applicable for the treatment of PCa, thus the identification of
novel potential targets for PCa is critical.
In the present study, in order to further determine
the progression of patients with advanced and castration-resistant
PCa, androgen-independent PCa cells (PC3 and DU145) (32) were used to investigate the functions
of ZFP403. The expression of ZFP403 in PCa tissues
was significantly lower than that in adjacent tissues. Furthermore,
the expression of ZFP403 at both the protein and mRNA level
in different PCa cells was lower than that in normal epithelial
prostate cells, which was consistent with the aforementioned IHC
results. The decreased expression of ZFP403 maintained the
growth of PCa, indicating that ZFP403 may be involved in the
progression of PCa as a tumor suppressor gene. shRNA interference
and lentivirus packaging technology were then used to silence
ZFP403 for further analysis.
To further investigate the biological functions of
ZFP403, the effects of ZFP403-knockdown on the
proliferation and metastasis of PCa cells were evaluated. The
results revealed that in vitro cell colony formation ability
and tumorigenicity were enhanced following the knockdown of
ZFP403. The cell cycle is one of the key modes of regulating
cell growth, thus the effect of ZFP403 on cell cycle
distribution was examined by flow cytometry. The results
demonstrated that the number of cells transitioning from the S to
the G2/M phase was increased in ZFP403-knockdown
cells compared with NC cells.
Cell cycle progression is regulated by
cyclin-dependent kinase, whose activity is strictly regulated by
cyclin. The ectopic expression of cyclin is associated with the
progression of a number of malignant tumors (33). Studies have indicated that the
expression of cyclin B1 plays a key role in the
G2/M-phase transition of human PCa cells (34,35).
Studies using the transgenic PCa mouse model have also demonstrated
that the expression of cyclin B1 is increased in
poorly-differentiated androgen-independent PCa (36). Thus, in the present study,
cyclin-related proteins were assessed in the G2/M phase
before and after ZFP403-knockdown. The results revealed that
the expression levels of cdc2 and cyclin B1 were upregulated, while
the expression of cdc25C was downregulated, suggesting that
ZFP403-knockdown accelerates the transition to the
G2/M phase and promotes cancer cell proliferation.
However, the presence of phosphorylated cdc25C could not be
detected (data not shown). This result is consistent with the
upregulation of cdc25C expression in PCa tissues, primarily in the
form of dephosphorylation (33).
ZFP403 is a classical Cys2His2 (C2H2)-type
zinc finger protein, which is encoded by 2% of human genes
(37), constituting the largest
sequence-specific DNA binding protein family (38). Numerous studies have demonstrated
that C2H2-type zinc finger proteins can regulate the transcription
of downstream genes involved in cellular proliferation and
metastasis. At the same time, C2H2-type zinc finger proteins act as
recruiters of chromatin modifiers or structural proteins,
regulating the migration and invasion abilities of cancer cells
(39,40). Therefore, it was hypothesized that
ZFP403 may be involved in the migration and invasiveness of
PCa. Indeed, the results of Transwell migration and invasion assays
confirmed this hypothesis, where ZFP403-knockdown
significantly enhanced the migration and invasion capacities of PCa
cells. Further experiments also demonstrated that
ZFP403-knockdown promoted metastasis by regulating the
expression of epithelial markers, mesenchymal markers, MMP2,
heparanase and β-catenin. β-catenin and E-cadherin are usually
present as an E-cadherin/β-catenin complex located in cell-cell
adherent junctions in the cell membrane. However, the loss of
E-cadherin leads to epithelial-mesenchymal transition (EMT),
accompanied by the deregulation of the Wnt signaling pathway. In
addition, as a key component of the Wnt pathway, β-catenin plays an
important role in the negative regulation of E-cadherin and EMT
(41,42). Research has indicated that
disassociation of the E-cadherin/β-catenin complex leads to the
suppression of E-cadherin and the nuclear translocation of
β-catenin, which enhances the invasive and migratory potential of
tumors (42).
In order to improve our understanding of the
function of ZFP403 in tumor development and progression
in vivo, a xenograft model was used in the present study.
ZFP403-knockdown induced the formation and development of
transplanted tumors in nude mice, suggesting that ZFP403 may
be a potential tumor suppressor in PCa.
In conclusion, the effect of ZFP403 in PCa
was preliminarily examined in the present study. The results
demonstrate that ZFP403-knockdown promotes the progression
of PCa by enhancing proliferation, migration and invasiveness.
Furthermore, ZFP403 was confirmed to function as a tumor
suppressor in PCa, and this finding is consistent with the results
of the overexpression of ZFP403 in PC3 (43). Future studies will aim to further
investigate the functions of ZFP403 in PCa, in order to
establish a novel therapeutic target for patients with advanced
metastatic and hormone-independent PCa.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of China (grant no. 81774003), the Zhejiang
Provincial Natural Science Foundation of China (grant no.
LY16H160034) and the Zhejiang Basic Public Welfare Research
Projects (grant no. LQ19H160006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX and ZZ performed the experiments and contributed
to the study design, as well as the acquisition, analysis and
interpretation of the data. YX and YJ collected and analyzed the
clinical samples. ST contributed to the acquisition, analysis and
interpretation of the data. HZ contributed to study conception and
revised the manuscript critically. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Zhejiang Cancer Hospital (Hangzhou, China), and all
enrolled patients provided written informed consent. The animal
experiments were conducted with the approval of the Experimental
Animal Ethical Committee of Zhejiang Chinese Medical University
(SYXK20180012).
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vlaeminck-Guillem V, Gillet G and Rimokh
R: SRC: Marker or actor in prostate cancer aggressiveness. Front
Oncol. 4:2222014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yap TA, Smith AD, Ferraldeschi R,
Al-Lazikani B, Workman P and de Bono JS: Drug discovery in advanced
prostate cancer: Translating biology into therapy. Nat Rev Drug
Discov. 15:699–718. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nevedomskaya E, Baumgart SJ and Haendler
B: Recent advances in prostate cancer treatment and drug discovery.
Int J Mol Sci. 19:13592018. View Article : Google Scholar
|
|
4
|
Haymart MR, Miller DC and Hawley ST:
Active surveillance for low-risk cancers-a viable solution to
overtreatment? N Engl J Med. 377:203–206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crawford ED, Heidenreich A, Lawrentschuk
N, Tombal B, Pompeo ACL, Mendoza-Valdes A, Miller K, Debruyne FMJ
and Klotz L: Androgen-targeted therapy in men with prostate cancer:
Evolving practice and future considerations. Prostate Cancer
Prostatic Dis. 22:24–38. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pan TF, Liang CZ, Chen XG and Fan S:
Mammalian target of rapamycin regulates androgen receptor and Akt
phosphorylation in prostate cancer 22RV1 cells. Zhonghua Nan Ke
Xue. 19:1068–1071. 2013.(In Chinese). PubMed/NCBI
|
|
7
|
Sumanasuriya S and De Bono J: Treatment of
advanced prostate cancer-a review of current therapies and future
promise. Cold Spring Harb Perspect Med. 8:a0306352018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu CT, Chen WC and Chen MF: The response
of prostate cancer to androgen deprivation and irradiation due to
immune modulation. Cancers (Basel). 11:202018. View Article : Google Scholar
|
|
9
|
Lan ZJ, Hu YH, Zhang S, Li X, Zhou H, Ding
J, Klinge CM, Radde BN, Cooney AJ, Zhang J and Lei Z: GGNBP2 acts
as a tumor suppressor by inhibiting estrogen receptor α activity in
breast cancer cells. Breast Cancer Res Treat. 158:263–276. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guan R, Wen XY, Wu J, Duan R, Cao H, Lam
S, Hou D, Wang Y, Hu J and Chen Z: Knockdown of ZNF403 inhibits
cell proliferation and induces G2/M arrest by modulating cell-cycle
mediators. Mol Cell Biochem. 365:211–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Wang Y, Zhou Y, Cao Z, Huang P
and Lu B: Yeast two-hybrid screens imply that GGNBP1, GGNBP2 and
OAZ3 are potential interaction partners of testicular germ
cell-specific protein GGN1. FEBS Lett. 579:559–566. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y and Chen Z: Molecular cloning and
characterization of LCRG1 a novel gene localized to the tumor
suppressor locus D17S800-D17S930. Cancer Lett. 209:75–85. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Plummer SJ, Paris MJ, Myles J, Tubbs R,
Crowe J and Casey G: Four regions of allelic imbalance on
17q12-qter associated with high-grade breast tumors. Genes
Chromosomes Cancer. 20:354–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu Z, Lou C, Zheng Z, Zhu R, Tian S, Xie
C and Zhao H: ZFP403, a novel tumor suppressor, inhibits the
proliferation and metastasis in ovarian cancer. Gynecol Oncol.
147:418–425. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhan A, Lei B, Wu H, Wen YT, Zheng L, Wang
S, Wan X and Wei Z: GGNBP2 suppresses the proliferation, invasion,
and migration of human glioma cells. Oncol Res. 25:831–842. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo K, He Y, Liu L, Liang Z, Li X, Cai L,
Lan ZJ, Zhou J, Wang H and Lei Z: Ablation of Ggnbp2 impairs
meiotic DNA double-strand break repair during spermatogenesis in
mice. J Cell Mol Med. 22:4863–4874. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang B, Zhao Y, Lou C and Zhao H:
Eupalinolide O, a novel sesquiterpene lactone from eupatorium
lindleyanum DC., induces cell cycle arrest and apoptosis in
human MDA-MB-468 breast cancer cells. Oncol Rep. 36:2807–2813.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tian SS, Chen Y, Yang B, Lou C, Zhu R,
Zhao Y and Zhao H: F1012-2 inhibits the growth of triple negative
breast cancer through induction of cell cycle arrest, apoptosis,
and autophagy. Phytother Res. 32:908–922. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li LZ, Zhang CZ, Liu LL, Yi C, Lu SX, Zhou
X, Zhang ZJ, Peng YH, Yang YZ and Yun JP: miR-720 inhibits tumor
invasion and migration in breast cancer by targeting TWIST1.
Carcinogenesis. 35:469–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang B, Zhu R, Tian S, Wang Y, Lou S and
Zhao H: Jatamanvaltrate P induces cell cycle arrest, apoptosis and
autophagy in human breast cancer cells in vitro and in vivo. Biomed
Pharmacother. 89:1027–1036. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baade PD, Youlden DR and Krnjacki LJ:
International epidemiology of prostate cancer: Geographical
distribution and secular trends. Mol Nutr Food Res. 53:171–184.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang AJ, Autio KA, Roach M III and Scher
HI: High-risk prostate cancer-classification and therapy. Nat Rev
Clin Oncol. 11:308–323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyamoto H, Messing EM and Chang C:
Androgen deprivation therapy for prostate cancer: Current status
and future prospects. Prostate. 61:332–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Uemura H, Ishiguro H, Nakaigawa N,
Nagashima Y, Miyoshi Y, Fujinami K, Sakaguchi A and Kubota Y:
Angiotensin II receptor blocker shows antiproliferative activity in
prostate cancer cells: A possibility of tyrosine kinase inhibitor
of growth factor. Mol Cancer Ther. 2:1139–1147. 2003.PubMed/NCBI
|
|
27
|
Li J, Luo J, Gu D, Jie F, Pei N, Li A,
Chen X, Zhang Y, Du H, Chen B, et al: Adenovirus-mediated
angiotensin II type 2 receptor overexpression inhibits tumor growth
of prostate cancer in vivo. J Cancer. 7:184–191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Walsh PC, DeWeese TL and Eisenberger MA:
Clinical practice. Localized prostate cancer. N Engl J Med.
357:2696–2705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bommareddy A, Rule B, VanWert AL, Santha S
and Dwivedi C: α-Santalol, a derivative of sandalwood oil, induces
apoptosis in human prostate cancer cells by causing caspase-3
activation. Phytomedicine. 19:804–811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wirth T and Ylä-Herttuala S: Gene therapy
used in cancer treatment. Biomedicines. 2:149–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Workman P: New drug targets for genomic
cancer therapy: Successes, limitations, opportunities and future
challenges. Curr Cancer Drug Targets. 1:33–47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saraon P, Musrap N, Cretu D, Karagiannis
GS, Batruch I, Smith C, Drabovich AP, Trudel D, van der Kwast T,
Morrissey C, et al: Proteomic profiling of androgen-independent
prostate cancer cell lines reveals a role for protein S during the
development of high grade and castration-resistant prostate cancer.
J Biol Chem. 287:34019–34031. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ozen M and Ittmann M: Increased expression
and activity of CDC25C phosphatase and an alternatively spliced
variant in prostate cancer. Clin Cancer Res. 11:4701–4706. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mashal RD, Lester S, Corless C, Richie JP,
Chandra R, Propert KJ and Dutta A: Expression of cell
cycle-regulated proteins in prostate cancer. Cancer Res.
56:4159–4163. 1996.PubMed/NCBI
|
|
35
|
Kallakury BV, Sheehan CE, Ambros RA,
Fisher HA, Kaufman RP Jr, Muraca PJ and Ross JS: Correlation of
p34cdc2 cyclin-dependent kinase overexpression, CD44s
downregulation, and HER-2/neu oncogene amplification with
recurrence in prostatic adenocarcinomas. J Clin Oncol.
16:1302–1309. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maddison LA, Huss WJ, Barrios RM and
Greenberg NM: Differential expression of cell cycle regulatory
molecules and evidence for a ‘cyclin switch’ during progression of
prostate cancer. Prostate. 58:335–344. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lander ES, Linton LM, Birren B, Nusbaum C,
Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al:
Initial sequencing and analysis of the human genome. Nature.
409:860–921. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tupler R, Perini G and Green MR:
Expressing the human genome. Nature. 409:832–833. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jen JY and Wang YC: Zinc finger proteins
in cancer progression. J Biomed Sci. 23:532016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cassandri M, Smirnov A, Novelli F, Pitolli
C, Agostini M, Malewicz M, Melino G and Raschellà G: Zinc-finger
proteins in health and disease. Cell Death Discov. 3:170172017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Clarke NW, Hart CA and Brown MD: Molecular
mechanisms of metastasis in prostate cancer. Asian J Androl.
11:57–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen HN, Yuan K, Xie N, Wang K, Huang Z,
Chen Y, Dou Q, Wu M, Nice EC, Zhou ZG and Huang C: PDLIM1
stabilizes the E-Cadherin/β-catenin complex to prevent
epithelial-mesenchymal transition and metastatic potential of
colorectal cancer cells. Cancer Res. 76:1122–1134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang Z, Wang Y and Ma L: Effects of
gametogenetin-binding protein 2 on proliferation, invasion and
migration of prostate cancer PC-3 cells. Andrologia. 52:e134882020.
View Article : Google Scholar : PubMed/NCBI
|