Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common malignant disease and the third most common cause of
cancer-related death worldwide (1,2). A
steady increase in viral hepatitis, metabolic syndrome, and alcohol
consumption over the past decades has contributed to the increasing
incidence of HCC (3). The main
cause of treatment failure and death in hepatic cancer patients is
the highly metastatic nature and recurrence rate of the disease
(4,5). Despite specific guidance for
monitoring the disease presented in a variety of guidelines, more
than two-thirds of HCC patients are diagnosed at an advanced stage
of the disease with limited curative treatment options (6,7). Thus,
HCC continues to be a global healthcare problem. Although
significant efforts have been made to develop and evaluate chemical
compounds for the treatment of HCC, only a limited number of drugs,
such as first-line drugs sorafenib and lenvatinib, and second-line
drugs regorafenib and cabozantinib have shown success in phase III
clinical trials (8–10).
Pin1 is a peptidyl-prolyl cis/trans
isomerase, member of the parvulin family of PPIase enzymes, which
plays important roles in cell signaling, including conformational
changes in protein kinase and cellular substrates. Several studies
have suggested that Pin1 plays a significant role in various
cellular activities, including apoptosis, cell cycle progression,
differentiation, proliferation, and transformation (11–13).
Pin1 is frequently overexpressed in many types of cancer including
HCC and there is a strong relationship between the expression of
Pin1 and β-catenin/cyclin D1 (14,15).
The isomerization of Pin1 is important for the stabilization of
β-catenin and cyclin D1 (15,16).
Cancer metastasis is a complex step-by-step mechanism involving the
detachment of cells from the primary tumor, destruction of the
extracellular matrix (ECM), infiltration into the circulatory and
lymphatic systems, invasion across the basement membranes into new
tissue, and growth (17–19). Past studies have shown that
epithelial mesenchymal transition (EMT) plays a significant role in
the spread of malignant tumors by rendering tumor cells more
susceptible and facilitating the invasion and metastasis of cancer
cells (20). Matrix
metalloproteinases (MMP-2 and MMP-9) are key enzymes responsible
for tumor cell migration, invasion, and metastasis (21,22).
Therefore, the suppression of tumor cell EMT and the activation of
apoptosis have the potential to treat tumors.
Mitochondria are crucial in controlling cell
apoptosis by releasing caspase activators and caspase-independent
death effectors, which results in the loss of important
mitochondrial function (23,24).
Mitochondrial dysfunction contributes to the degradation of
mitochondrial membrane potential and, the instability of electron
transport reactions, resulting in the overproduction of reactive
oxygen species (ROS), caspase activation, and apoptosis pathway
initiation (25). Numerous studies
have identified bioactive compounds and phytochemical sources with
anticancer effects that inhibit cancer cell proliferation and
modulate metabolism (26). Our
findings suggest that PF decreased the mitochondrial membrane
potential and increased the production of mitochondrial ROS and
apoptotic cell death in hepatocellular cancer cells.
Several studies have evaluated the potential
pharmacological activity, including the antimicrobial, antifungal,
anti-inflammatory, antioxidant, and anticancer effects, of
plant-derived compounds (27–30).
The current study used Poncirus fructus (PF), a
phytochemical extract obtained from the dry immature fruits of
Poncirus trifoliata. Poncirus trifoliata grows
naturally on Gaduk and Jeju islands in South Korea. It is
cultivated by farmers in the southern villages of South Korea. PF
is believed to possess anti-inflammatory, anti-allergic, and
lipid-lowering properties. PF has traditionally been used to treat
gastrointestinal disorders, womb contraction, dyspepsia, and blood
circulation-related disorders in East Asia, including South Korea
(31–33). The biological activities of P.
trifoliata, such as oncogenic-attenuating activity, anaphylaxis
inhibition, and Helicobacter pylori suppression have been
investigated (34). Furthermore, it
has been reported that P. trifoliata extract is cytotoxic to
promyelocytic leukemia cells (35).
However, neither the cytotoxic effects of PF on HCC nor the
molecular mechanism underlying its oncogenic activity has been
elucidated. Therefore, the present study was conducted to
investigate the molecular mechanism of PF in proliferation,
apoptosis, and tumor metastasis. The findings of this study suggest
the potential therapeutic effects of PF to efficiently managing
HCC.
Materials and methods
PF extract preparation
The PF extract used was from the same batch as that
in a previous study (36). Briefly,
the dry immature fruits of P. trifoliata were purchased from
an Oriental medical store in Geuman, Korea. The fruits were
processed with methanol at room temperature for 24 h. The PF
methanol extract was concentrated under reduced pressure using a
rotary evaporator. The residues were resuspended in distilled water
and further extracted with hexane and ethyl acetate. The ethyl
acetate-soluble fraction was subjected to silica gel chromatography
using the phase solvent method and a chloroform-methanol solvent.
The active fractions were combined, concentrated, and subjected to
chromatography on a Sephadex LH-20 column eluted with methanol.
Again, the active fraction was subjected to C18 reverse-phase
column chromatography eluted with 80% aqueous methanol followed by
high-performance liquid chromatography (HPLC) eluted with 85%
aqueous methanol to obtain the desired compound.
Cell culture and drug treatments
Hep3B and Huh7 cells were obtained from the American
Type Culture Collection (ATCC). Cell line authentication was
systematically conducted using a panel of ATCC short tandem repeats
(STR) and routinely monitored for mycoplasma contamination and
tested negative for mycoplasma contamination. The cell lines were
cultured in Dulbecco's Modified Eagle's Medium (DMEM;
HyClone®, Thermo Fisher Scientific, Inc.) supplemented
with 10% fetal bovine serum (FBS; HyClone®, Thermo
Fisher Scientific, Inc.) and 1% antibiotics (HyClone®,
Thermo Fisher Scientific, Inc.) at 37°C in humidified conditions
with 5% CO2. The cells were treated for 24 h with PF
dissolved in phosphate-buffered saline (PBS) (0, 20, 30, or 40
µM).
Cell proliferation assay
Hep3B and Huh7 cells were seeded into 96-well plates
at a cell density of 5×103 per well and cultured for 24
h. The cells were treated with the indicated drug concentrations
(0, 20, 30, 40 µM). The medium was removed, and the cells were
incubated for 2 h in solution with 50 µl of 5 mg/ml 2,5-diphenyl
tetrazolium bromide (MTT; Sigma-Aldrich; Merck KGaA). The
transformed purple formazan crystals were solubilized in dimethyl
sulfoxide (DMSO) and the optical density was measured at a
wavelength of 575 nm.
Colony forming assay
Hep3B and Huh7 cells were seeded in 6-well plates
(1,000 cells per well) for 24 h. The medium was replaced with fresh
medium containing PF (20, 30, or 40 µM) for 24 h. After 24 h, the
medium was removed, and fresh medium was added to the cells for
10–14 days. Afterward, the cells were fixed for 15 min with
ice-cold methanol, washed with PBS, stained with 1% crystal violet
solution (bioWORLD) for 1 h at room temperature, and the colonies
with >10 cells were counted by using densitometric software
Clono-Counter as described previously (37).
Annexin assay
Apoptosis of the Hep3B and Huh7 cells was detected
by flow cytometry. Briefly, Hep3B and Huh7 cells were seeded at a
cell density of 5×104 in a 60-mm culture dish overnight
and treated with medium containing PF (0, 20, 30, and 40 µM) for 24
h. The cells were trypsinized and the supernatants containing the
cells were collected. The cells were centrifuged at 1,500 rpm for 3
min at 15°C and frequently washed with PBS. Then, an Annexin V-FITC
apoptosis kit was used to determine apoptosis by flow cytometry
according to the manufacturer's instructions.
Mitochondrial membrane potential (ΔΨm)
analysis
The mitochondrial membrane potential was determined
using a tetramethylrhodamine ethyl ester perchlorate (TMRE)
mitochondrial membrane potential assay kit (Abcam) according to the
manufacturer's instructions. Hep3B and Huh7 cells were seeded at a
density of 10,000/well in 96-well plates. The cells were cultured
with PF (0, 20, 30, and 40 µM) for 24 h. Then, the cells were
stained with TMRE (400 nmol/l) for 20 min and washed twice with
PBS. The TMRE dye intensity was measured at excitation and emission
wavelengths of 549/575 nm, respectively, using a fluorescence plate
reader.
Reactive oxygen species (ROS)
analysis
The levels of intracellular ROS in the Hep3B and
Huh7 cells were measured using a fluorescent dihydroethidium (DHE)
probe. Intracellular DHE is oxidized to ethidium, which binds to
DNA and stains the nuclei a bright red fluorescent color. Hep3B and
Huh7 cells were cultured in glass-bottom confocal dishes and
pretreated with PF as previously described. The cells were washed
twice with PBS and then fixed at room temperature with ice-cold
methanol for 10–15 min. Then, DHE was diluted in PBS to a final
concentration of 5 µM and applied to the cells for 35 min at 37°C
in the dark. The cells were labeled with
4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) for 5 min
after washing twice with PBS, and examined under a fluorescence
microscope using a ×63 oil immersion objective lens (Axioskop 2
Plus, Carls Zeiss, Gottingen, Germany).
In vitro cell migration and invasion
assays
The migration and invasion potential of the Hep3B
and Huh7 cells was evaluated using a Transwell chamber with 8.0-µm
pore polyester membrane inserts and 8.0-µm pore polyester membrane
inserts with Matrigel-coated chambers (Corning, Inc.). For the
migration assay, Hep3B and Huh7 cells treated with PF at the
indicated concentrations were seeded in the upper chamber at a
density of 5×104 cells per well in 200 µl of serum-free
medium, and 500 µl of 10% FBS-containing medium was added to the
lower chamber. The suspension was discarded after 24 h and the
cells on the top layer of the membrane were removed with cotton
swabs. The cells that migrated to the lower membrane surface were
stained with the Diff-Quick kit solution. The migrated cells were
counted under light microscopy in three randomly chosen fields. The
Matrigel-coated chamber was rehydrated with serum-free medium in a
humidified tissue culture incubator to perform the invasion assay,
followed by the migration assay. After 24 h, the suspension medium
was discarded and the cells on the upper layer of the membrane were
removed using cotton swabs. The cells that invaded the lower
surface of the membrane were fixed with ice-cold methanol and
stained with the Diff-Quick kit solution. The invasive cells were
detected, photographed, and counted in three randomly chosen areas
under light microscopy.
Immunoblot analysis
The cells were washed with PBS and lysed with lysis
buffer (iNtRON Biotechnology) containing a cocktail of phosphatase
inhibitor-1. Western blotting was performed as previously described
(38). The membranes were incubated
overnight with primary antibodies specific for anti-rabbit cleaved
caspase-3 (cat. no. 9661) and −9, anti-mouse caspase-9 (cat. no.
9508), cleaved PARP (cat. no. 5625), E-cadherin (cat. no. 3195),
vimentin (cat. no. 5741), Snail (cat. no. 3879), MMP-9 (cat. no.
2270) (dilution 1:1,000; Cell Signaling Technology, Inc.),
anti-mouse actin, α-SMA (cat. no. A2547, dilution 1:1,000;
Sigma-Aldrich; Merck KGaA), anti-rabbit Bcl-2 (cat. no. BS1511),
Bax (cat. no. BS6420), MMP-2 (cat. no. BS1236) (dilution 1:1,000;
Bioworld Tech. Inc.), anti-rabbit caspase-3 (cat. no. sc-7148),
anti-mouse Pin1 (cat. no. sc-46660), anti-rabbit cyclin D1 (cat.
no. sc-717) (dilution 1:1,000, Santa Cruz Biotechnology, Inc.),
anti-mouse β-catenin (cat. no. 610154), and N-cadherin (cat. no.
610920) (dilution 1:1,000, BD Biosciences). The membranes were
washed and incubated with horseradish peroxidase (HRP)-conjugated
anti-mouse (cat. no. ADI-SAB-100-J) or anti-rabbit secondary
antibodies (ADI-SAB-300-J) (dilution 1:3,000, Enzo Life Sciences).
Protein expression was detected using an enhanced chemiluminescence
detection kit (Millipore Corp.).
Gelatin zymography
Gelatin zymography was performed to determine the
levels of MMPs, such as MMP-2 and MMP-9. The HCC cell supernatants
were collected by centrifugation at 4,000 × g at 4°C and the
protein content was determined using a BCA protein assay kit. Equal
amounts of protein mixed with non-reducing 6X loading buffer were
separated by SDS-PAGE containing 0.1% gelatin. After
electrophoresis, the gels were rinsed thrice with 0.25% Triton
X-100 for 15 min at room temperature and then incubated at 37°C in
developing buffer (50 mM Tris-HCL pH 7.4, 5mM CaCl2, 200
mM NaCl) for 42 h. After incubation, the MMP-2 and MMP-9 activities
were measured by Coomassie blue staining and the gel was imaged
using an LAS 3000 (Fuji) (39).
Statistical analysis
All the experimental results were obtained by
repeating each experiment at least three times and data are
expressed as means ± standard deviation. Statistical significance
was analyzed by one-way analysis of variance (ANOVA) using the
Dunnett's test or Turkey's post hoc test in Prism 7 software
(GraphPad Software, Inc.). A P-value less than 0.05 (P<0.05) was
considered statistically significant.
Results
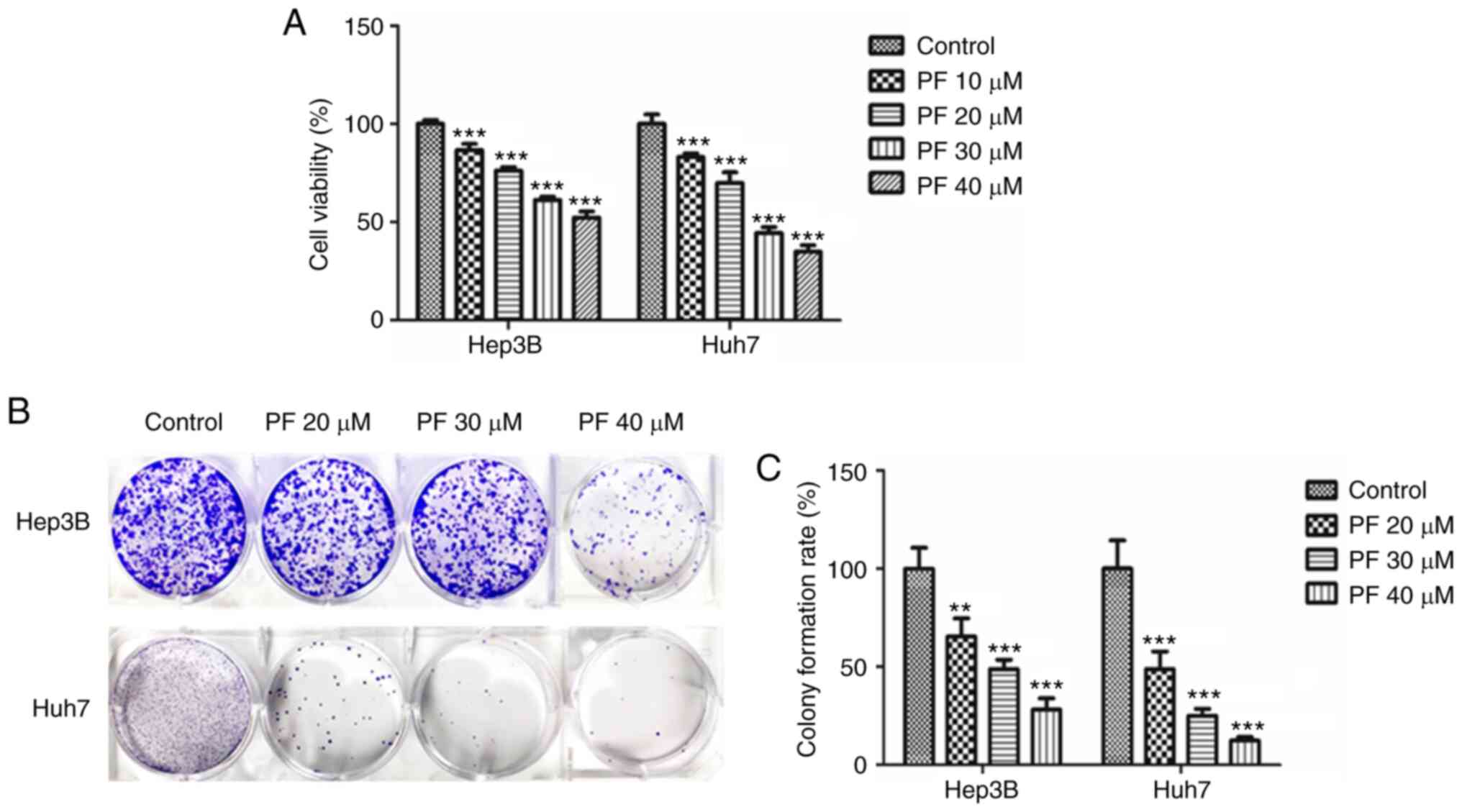
Anti-proliferative effects of PF in
the cancer cells
MTT and colony-forming assays were performed to
evaluate the anti-proliferative effects of PF in Hep3B and Huh7
cells. As shown in Fig. 1A, PF
significantly reduced the cell proliferation of Hep3B and Huh7
cells in a dose-dependent manner. The inhibitory effect of PF on
cell proliferation was also measured using a clonogenic assay. As
shown in Fig. 1B and C, PF
significantly inhibited the colony formation of Hep3B and Huh7
cells in a dose-dependent manner. These results indicate that PF
had a significant inhibitory impact on the viability and
proliferation of HCC cell lines.
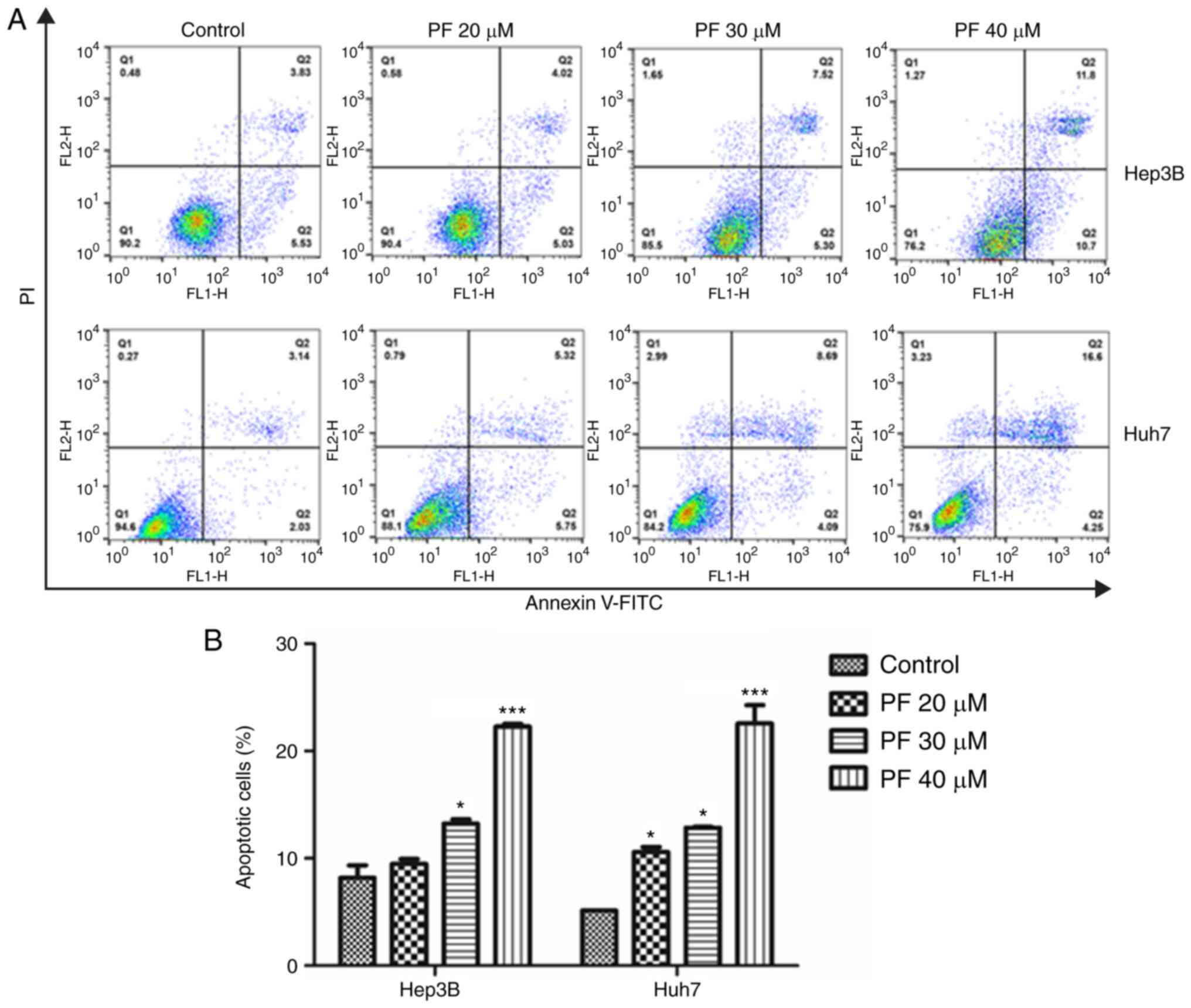
PF promotes the apoptosis of the HCC
cells
To explore whether PF induced cancer cell apoptosis,
flow cytometry was conducted. As shown in Fig. 2A and B, treatment with PF
significantly induced apoptosis in the Hep3B and Huh7 cells in a
dose-dependent manner compared to the control.
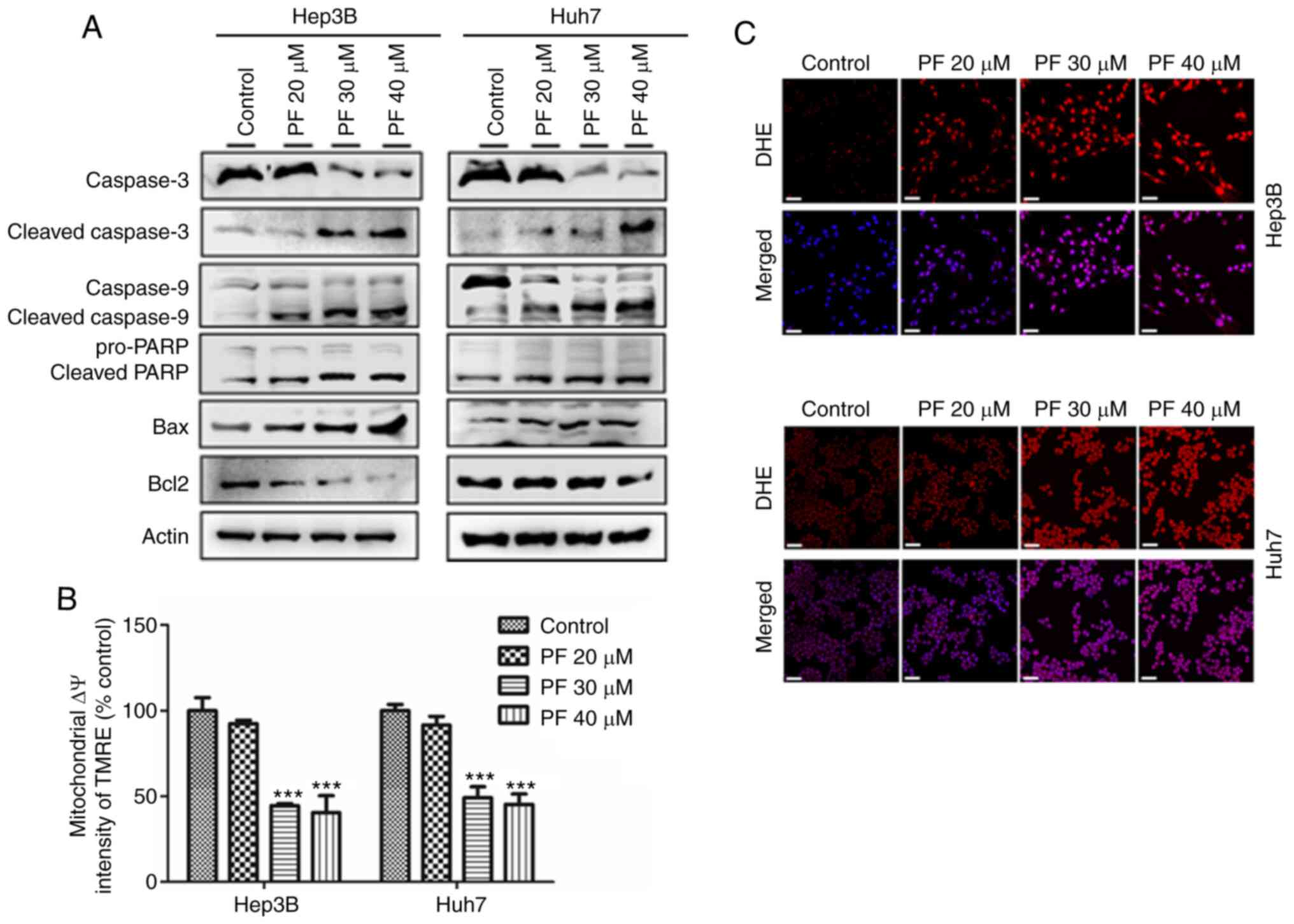
PF promotes apoptosis of HCC cells by
reducing mitochondrial membrane potential (ΔΨm) and increasing
ROS
The present study demonstrated the mechanism of
PF-induced apoptosis on HCC cells (Hep3B and Huh7) by analyzing the
apoptosis-related proteins, and quantifying the mitochondrial
membrane potential (ΔΨm) and intracellular ROS levels. As shown in
Fig. 3A, western blot analysis
revealed that PF significantly decreased the expression of
procaspase-3, procaspase-9, PARP and anti-apoptotic protein Bcl2,
whereas it increased the expression of apoptotic proteins Bax,
cleaved caspase-3, cleaved caspase-9 and cleaved PARP in a
dose-dependent manner. The loss of mitochondrial membrane potential
(ΔΨm) results in mitochondrial membrane permeabilization, which is
regarded as an important hallmark of early apoptosis and plays a
key role in the intrinsic apoptotic pathway (40–42).
Therefore, we further analyzed the disruption of the mitochondrial
membrane by PF. As shown in Fig.
3B, PF induced a significant, concentration-dependent depletion
of mitochondrial membrane potential. Mitochondria are considered
the main source of ROS, which is considered a trigger of apoptosis
(43). Therefore, we determined the
intracellular ROS generation in Hep3B and Huh7 cells by assessing
the DHE fluorescence intensity after PF treatment. As shown in
Fig. 3C, PF treatment induced
stronger fluorescence intensity in Hep3B and Huh7 cells compared to
the control, which indicated increased levels of ROS. These results
suggest that PF induced apoptosis of the Hep3B and Huh7 cells by
effects on the mitochondrial apoptosis pathway.
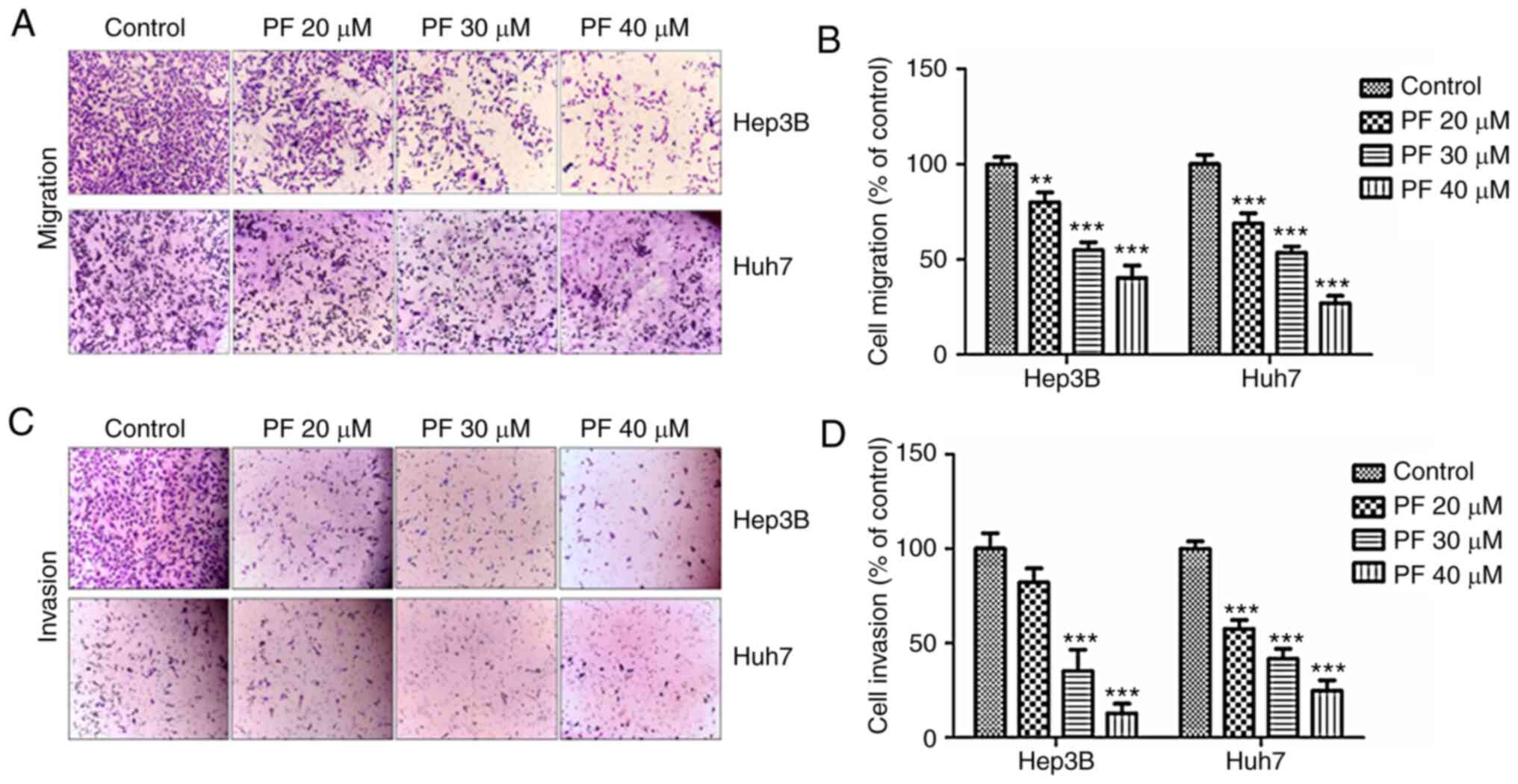
PF inhibits the migration and invasion
of HCC cells
The migratory and invasive abilities of tumor cells
are considered as significant hallmarks of HCC and play a key roles
in the metastasis of various tumors (44). To investigate whether PF exhibits
anti-migratory and anti-invasion effects, Transwell assays were
performed. As shown in Fig. 4A and
B, the migratory ability of Hep3B and Huh7 cells was inhibited
by PF treatment in a dose-dependent manner. Consistently, the
Transwell invasion assay showed that PF treatment inhibited the
number of invasive cells (Fig. 4C and
D).
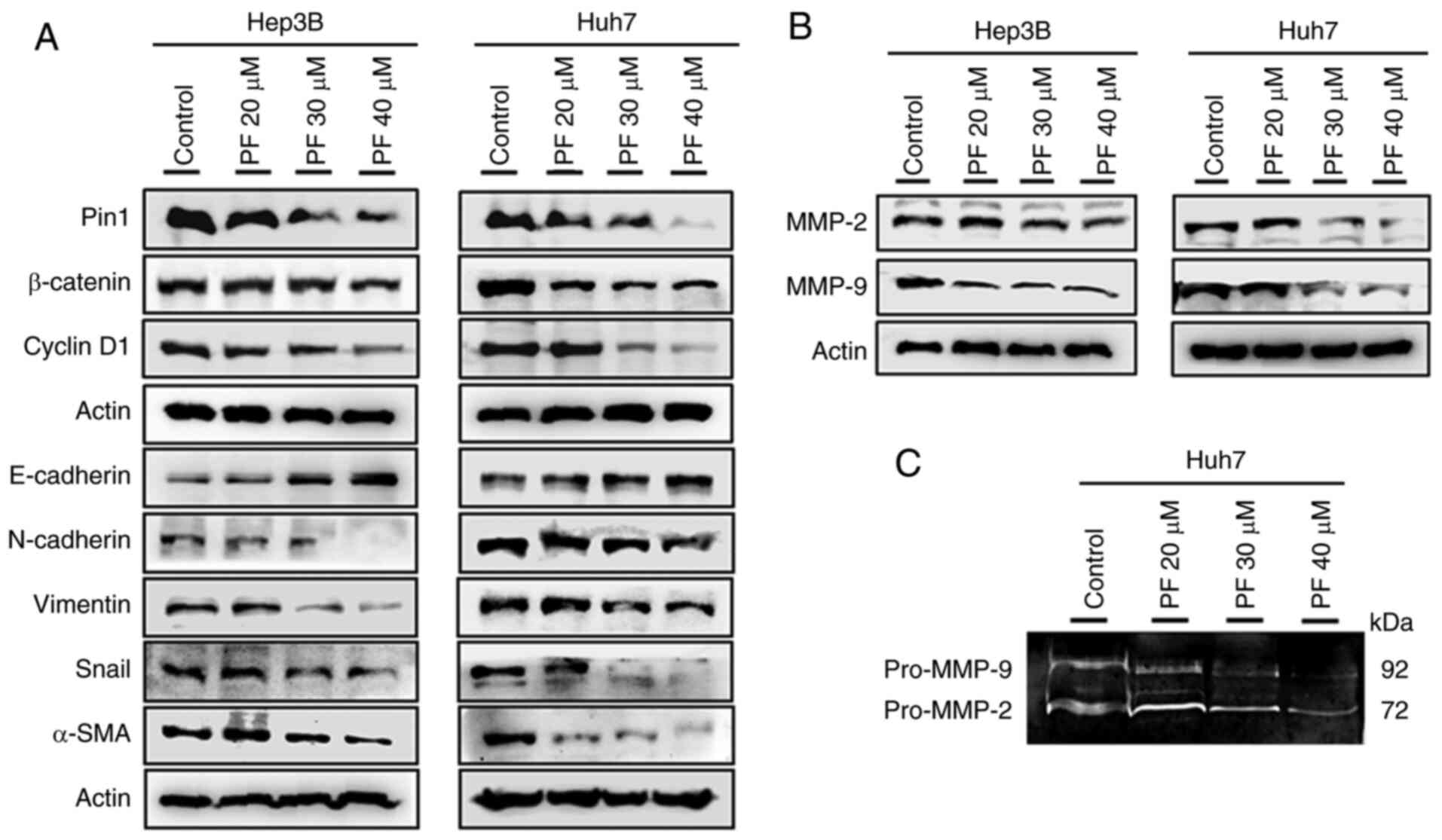
Effects of PF on the expression of Pin
1, cyclin D1, β-catenin l, and EMT marker
EMT is vital in tumor cell growth, causing the
invasion and metastasis of malignant tumors (45). Therefore, the protein levels of Pin
1, cyclin D1, and EMT markers such as β-catenin, E-cadherin,
vimentin, N-cadherin, Snail, and α-SMA hepatic cancer cells were
evaluated. As shown in Fig. 5A, the
treatment of Hep3B and Huh7 cells with PF markedly increased the
expression of E-cadherin, whereas it decreased the levels of Pin1,
cyclin D1, β-catenin, N-cadherin, vimentin, Snail, and α-SMA in a
dose-dependent manner. These results suggested that PF inhibited
cancer cell progression by decreasing the EMT process. A previous
study demonstrated that matrix metalloproteinases (MMPs) such as
MMP-2 and MMP-9 play crucial roles in basement membrane degradation
during tumor cell invasion (46).
Therefore, we determined the expression of MMP-2 and MMP-9 in Hep3B
and Huh7 cells. As shown in Fig.
5B, PF treatment markedly downregulated the protein levels of
MMP-2 and MMP-9. To confirm whether the activities of extracellular
MMPs were inhibited by PF, a gelatin zymography assay was
performed. As shown in Fig. 5C, PF
decreased the activities of MMP-2 and MMP-9 in the Huh7 culture
medium. These results suggest that PF inhibits cancer cell
migration and invasion by attenuating the activities of the
metalloproteinases.
Discussion
The advancements, discoveries, and successful trials
of plant-derived drugs have directed attention to treating various
cancers with agents with low toxicity to normal cells. Poncirus
fructus (PF) is a phytochemical compound extracted from the
dry, immature fruits of Poncirus trifoliate. PF is
traditionally used for the treatment of gastrointestinal and
vascular disorders in South Asia. Previous studies have reported
the use of PF for the clinical treatment of various cancers
(47). However, no strong evidence
has been provided on the molecular mechanism of the anticancer role
of PF (35). Several previous
studies have shown that the pharmacological efficacy of these
fruits depends on their maturity. The previous studies on PF in
cancer and non-cancer diseases are documented in Table SI. Hepatocellular carcinoma (HCC)
is the most common malignancy in many countries, with a low
survival rate and a high rate of intrahepatic and extra-hepatic
metastasis (48,49). Hepatic cancer patients have high
recurrence and mortality rates due to progression by tumor cell
migration and invasion (5). The
present study showed the antiproliferative and anti-invasive
effects of PF by inducing apoptosis in HCC Hep3B and Huh7 HCC cell
lines. These results suggest the potential of PF as a clinical
agent for cancer treatment.
We demonstrated that the high expression of Pin1,
peptidyl-prolyl cis/trans isomerase, in HCC was reduced by
treatment with PF. The dysregulation of PIN1 contributes to
pathological conditions including cancer (50). A number of cancer markers such as
cyclin D1, β-catenin, and NF-κB/P65 are also correlated with the
overexpression of PIN1 (15,51–54).
Several studies have indicated that Pin 1, β-catenin, and cyclin D1
were involved in promoting the growth of HCC by regulating cell
proliferation and reducing the activity of E-cadherin-mediated cell
adhesion and thus, were correlated with poorly differentiated
disease in HCC patients (55–59).
In the present study, we found that PF inhibited epithelial
mesenchymal transition (EMT) by reducing the expression of Pin 1,
β-catenin, and cyclin D1. EMT plays a significant role in the
malignancy of tumors by reducing the ability of cells to bind to
the basement membranes, inducing a loss of epithelial cell
polarity, and acquiring elongated mesenchymal morphology and
migratory and invasive properties to become mesenchymal stem cells
(60–62). Transwell migration and invasion
assays are commonly used to evaluate EMT. During EMT, epithelial
cells acquire motile and invasive characteristics by increasing
mesenchymal properties, including the increased expression of
mesenchymal markers α-SMA, N-cadherin, and vimentin, and EMT
transcriptional factors Slug and Snail, which result in the loss of
epithelial markers E-cadherin and ZO-1 (63). This study, demonstrated that PF
inhibited the migration and invasion of hepatic cancer cells. PF
treatment also increased the protein levels of E-cadherin and
decreased the protein levels of α-SMA, N-cadherin, and vimentin.
The extracellular matrix prevents the metastasis of tumor cells by
acting as a physical barrier, and its degradation plays a crucial
role in promoting tumor cell migration and invasion (64). MMPs are important proteinases
involved in extracellular matrix degradation, and increased MMP
expression was shown to promote the invasion of tumor cells
(65,66). MMPs such as MMP-2 and MMP-9 are
strongly expressed in metastatic tumors and are responsible for the
degradation of extracellular matrix basic skeleton IV collagen
(67,68). The present study, showed that the
expression of MMP-2 and MMP-2 in HCC cells was adequately inhibited
by PF treatment. Together, these findings suggest that PF reduced
HCC cell migration and invasion by reducing the expression of Pin1,
β-catenin, and cyclin D1 thereby inhibiting EMT and downregulating
MMP-2 and MMP-9 activity.
Apoptosis plays a key role in the elimination of
dysfunctional and damaged cells to maintain homeostasis. Numerous
evidence has shown that defects in apoptotic pathways stimulate
carcinogenesis and cancer cell survival (69). Therefore, inducing apoptosis in
tumor cells may be considered a potential therapeutic approach to
treat cancers. There are two main pathways for the initiation of
apoptosis, the intrinsic (mitochondria-mediated), and the extrinsic
apoptotic pathways (70). Previous
studies indicate that proteins of the Bcl-2 member family and
caspase cascades perform important regulatory roles in
mitochondrial apoptosis (71). The
cytosolic localization of Bax pro-apoptotic proteins combines with
membrane-bound Bcl2 to create a heterodimer that stabilizes the
cytoplasmic localization of pro-apoptotic proteins and inhibits
apoptosis (72). However, the
dissociation of Bax from the Bcl-2/Bax heterodimer by the number of
the apoptosis-inducing signals allows it to translocate to the
mitochondrial membrane to induce apoptosis (73). In this study, we evaluated the
effect of PF on apoptosis and apoptosis-related proteins in HCC
cell lines. Our data indicate that PF significantly increased the
levels of pro-apoptotic proteins, such as Bax, cleaved caspase-3,
cleaved caspase-9 and cleaved PARP, whereas PF treatment decreased
the levels of caspase-3, caspase-9, PARP and anti-apoptotic Bcl-2
protein in a concentration-dependent manner.
Mitochondria are associated with the regulation of
cellular apoptosis, tumorigenesis, and drug tolerance. Several
studies have reported that the mitochondrial signal transduction
pathway has particular importance in the metabolism of cellular
energy and the initiation of apoptosis (74). The depletion of mitochondrial
membrane potential (ΔΨm) and increased membrane permeability are
characteristics of mitochondrial injury. This process releases
apoptogenic factors from the mitochondria into the cytosol and
reduces oxidative phosphorylation, resulting in
mitochondrial-mediated apoptosis (75). Recent research has found that the
excessive production of ROS from mitochondria leads to
mitochondrial membrane depolarization, which induces apoptosis
(76). In the present study, PF
treatment reduced mitochondrial membrane potential, which resulted
in excessive ROS production in Hep3B and Huh7 cells and induced
apoptosis. However, the antitumor effects of PF need to confirmed
by future studies using an animal model.
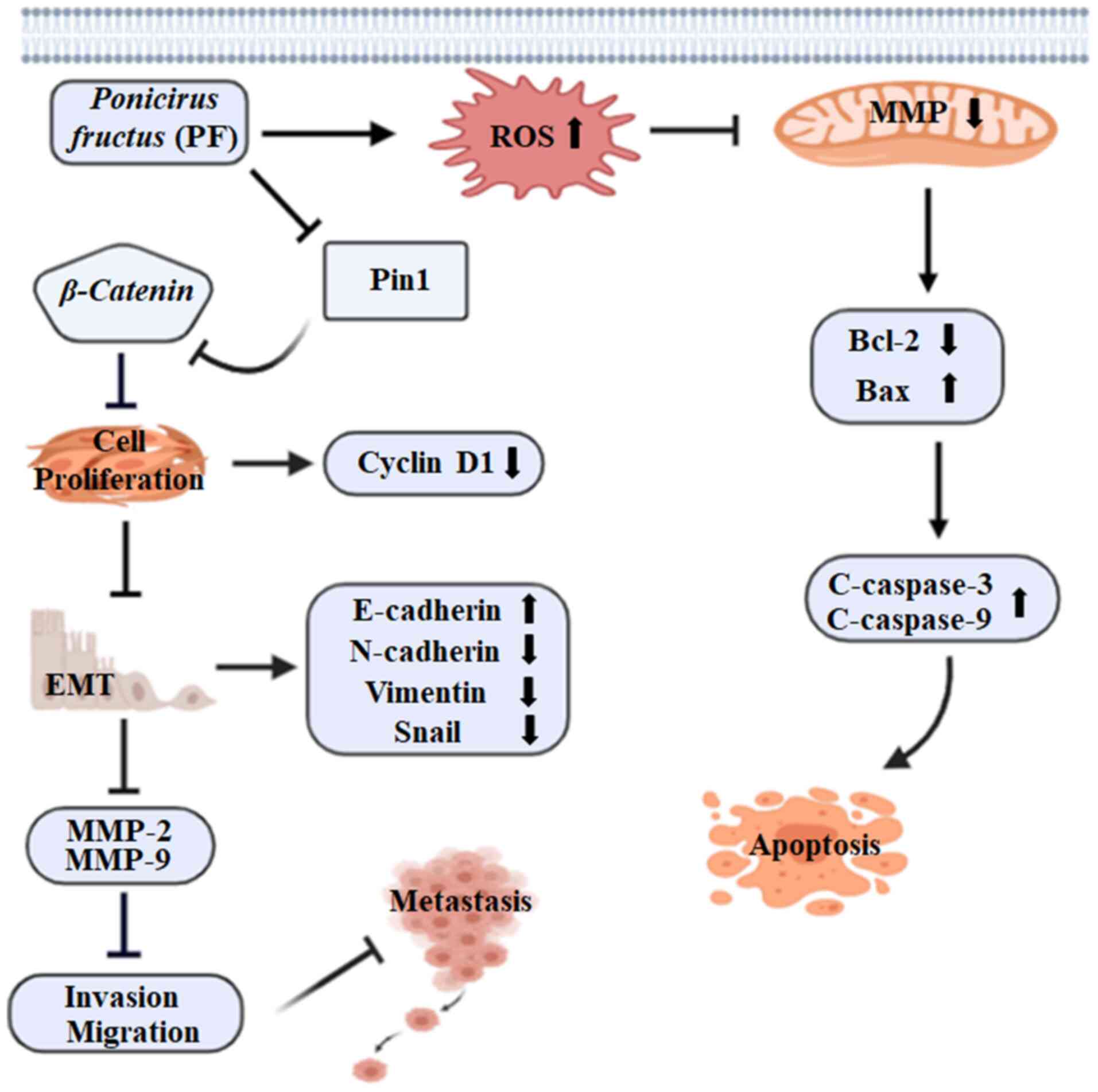
The results of this study revealed that PF
effectively inhibited the proliferation and mediated the apoptosis
of HCC cells through the mitochondrial apoptotic pathway. In
addition, PF treatment exerted anti-migration and anti-invasive
effects on HCC cells by inhibiting EMT and reducing the levels of
MMP-2 and MMP-9. The findings that PF induced the apoptosis of
Hep3B and Huh7 HCC cells by increasing ROS and disrupting the
mitochondrial membrane potential suggest the potential of PF as a
promising HCC drug candidate. In addition, PF reduced tumor growth
and metastasis by inhibiting cell proliferation and suppressing the
migration and invasion ability of Hep3B and Huh7 cells (Fig. 6). These results indicate that
Poncirus fructus could be a prospective candidate for
treating hepatic cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was funded by research grants from the
Research Institute of Clinical Medicine of Chonbuk National
University and Biomedical Research Institute of Chonbuk National
University Hospital.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SM, LC and YJJ conceived and designed the
experiments. UKH performed the zymography experiment. YJJ acted as
the chief investigator and provided strategic advice for all
aspects of the projects. SM conducted the experiments and
interpreted the results with assistance from HBS. B-SY and HRP
prepared the materials and agents for the experimental research. SM
composed the manuscript. All authors read and accepted the final
manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Naghavi M, Wang H, Lozano R, Davis A,
Liang X, Zhou M, Vollset SE, Ozgoren AA, Abdalla S, Abd-Allah F, et
al: Global, regional, and national age-sex specific all-cause and
cause-specific mortality for 240 causes of death, 1990–2013: A
systematic analysis for the global burden of disease study 2013.
Lancet. 385:117–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mederacke I: Liver fibrosis-mouse models
and relevance in human liver diseases. Z Gastroenterol. 51:55–62.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morise Z, Kawabe N, Tomishige H, Nagata H,
Kawase J, Arakawa S, Yoshida R and Isetani M: Recent advances in
liver resection for hepatocellular carcinoma. Front Surg. 1:212014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu Y, Shen H, Yu H, Zhong F, Zhang Y,
Zhang C, Zhao J, Li H, Chen J, Liu Y and Yang P: Systematic
proteomic analysis of human hepotacellular carcinoma cells reveals
molecular pathways and networks involved in metastasis. Mol
Biosyst. 7:1908–1916. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
European Association For The Study Of The
Liver; European Organisation For Research And Treatment Of Cancer,
. EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosen HR, Ghany MG, Chung RT and Lok ASF:
NAM 2017 report: A national plan to eliminate hepatitis B and C in
the United States by 2030 and the AASLD's response. Hepatology.
66:1020–1022. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nault JC, Galle PR and Marquardt JU: The
role of molecular enrichment on future therapies in hepatocellular
carcinoma. J Hepatol. 69:237–247. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu KP, Hanes SD and Hunter T: A human
peptidyl-prolyl isomerase essential for regulation of mitosis.
Nature. 380:544–547. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng H, You H, Zhou XZ, Murray SA, Uchida
T, Wulf G, Gu L, Tang X, Lu KP and Xiao ZX: The prolyl isomerase
Pin1 is a regulator of p53 in genotoxic response. Nature.
419:849–853. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bernis C, Vigneron S, Burgess A, Labbé JC,
Fesquet D, Castro A and Lorca T: Pin1 stabilizes Emi1 during G2
phase by preventing its association with SCF(betatrcp). EMBO Rep.
8:91–98. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liou YC, Ryo A, Huang HK, Lu PJ, Bronson
R, Fujimori F, Uchida T, Hunter T and Lu KP: Loss of Pin1 function
in the mouse causes phenotypes resembling cyclin D1-null
phenotypes. Proc Natl Acad Sci USA. 99:1335–1340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ryo A, Nakamura M, Wulf G, Liou YC and Lu
KP: Pin1 regulates turnover and subcellular localization of
beta-catenin by inhibiting its interaction with APC. Nat Cell Biol.
3:793–801. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ryo A, Nakamura M, Wulf G, Liou YC and Lu
KP: Pin1 regulates turnover and subcellular localization of
beta-catenin by inhibiting its interaction with APC. Nat Cell Biol.
3:793–801. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen K, Zhang S, Ji Y, Li J, An P, Ren H,
Liang R, Yang J and Li Z: Baicalein inhibits the invasion and
metastatic capabilities of hepatocellular carcinoma cells via
down-regulation of the ERK pathway. PLoS One. 8:e729272013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meyer T and Hart IR: Mechanisms of tumour
metastasis. Eur J Cancer. 34:214–221. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Radisky ES and Radisky DC: Matrix
metalloproteinase-induced epithelial-mesenchymal transition in
breast cancer. J Mammary Gland Biol Neoplasia. 15:201–212. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song J, Zhang X, Ge Q, Yuan C, Chu L,
Liang HF, Liao Z, Liu Q, Zhang Z and Zhang B: CRISPR/Cas9-mediated
knockout of HBsAg inhibits proliferation and tumorigenicity of
HBV-positive hepatocellular carcinoma cells. J Cell Biochem.
119:8419–8431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Itoh Y and Nagase H: Matrix
metalloproteinases in cancer. Essays Biochem. 38:21–36. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan H, Mutomba M, Prinz I and Gottlieb
RA: Differential processing of cytosolic and mitochondrial
caspases. Mitochondrion. 1:61–69. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zaidieh T, Smith JR, Ball KE and An Q: ROS
as a novel indicator to predict anticancer drug efficacy. BMC
Cancer. 19:12242019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs from 1981 to 2014. J Nat Prod. 79:629–661.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vallejo MJ, Salazar L and Grijalva M:
Oxidative stress modulation and ROS-mediated toxicity in cancer: A
review on in vitro models for plant-derived compounds. Oxid Med
Cell Longev. 2017:45860682017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Greenwell M and Rahman PK: Medicinal
Plants: Their use in anticancer treatment. Int J Pharm Sci Res.
6:4103–4112. 2015.PubMed/NCBI
|
|
29
|
Zheng X, Zhao MG, Jiang CH, Sheng XP, Yang
HM, Liu Y, Yao XM, Zhang J and Yin ZQ: Triterpenic acids-enriched
fraction from Cyclocarya paliurus attenuates insulin resistance and
hepatic steatosis via PI3K/Akt/GSK3β pathway. Phytomedicine.
66:1531302020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sumorek-Wiadro J, Zając A, Maciejczyk A
and Jakubowicz-Gil J: Furanocoumarins in anticancer therapy-for and
against. Fitoterapia. 142:1044922020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu DJ, Jun JH, Kim TJ, Suh DK, Youn DH and
Kim TW: The relaxing effect of Poncirus fructus and its
flavonoid content on porcine coronary artery. Lab Anim Res.
31:33–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kornblum HI, Raymon HK, Morrison RS,
Cavanaugh KP, Bradshaw RA and Leslie FM: Epidermal growth factor
and basic fibroblast growth factor: Effects on an overlapping
population of neocortical neurons in vitro. Brain Res. 535:255–263.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jang Y, Kim EK and Shim WS:
Phytotherapeutic effects of the fruits of Poncirus
trifoliata (L.) Raf. on cancer, inflammation, and digestive
dysfunction. Phytother Res. 32:616–624. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hong J, Min HY, Xu GH, Lee JG, Lee SH, Kim
YS, Kang SS and Lee SK: Growth inhibition and G1 cell cycle arrest
mediated by 25-methoxyhispidol A, a novel triterpenoid, isolated
from the fruit of Poncirus trifoliata in human
hepatocellular carcinoma cells. Planta Med. 74:151–155. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yi JM, Kim MS, Koo HN, Song BK, Yoo YH and
Kim HM: Poncirus trifoliata fruit induces apoptosis in human
promyelocytic leukemia cells. Clin Chim Acta. 340:179–185. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi AR, Lee IK, Woo EE, Kwon JW, Yun BS
and Park HR: New glabretal triterpenes from the immature fruits of
Poncirus trifoliata and their selective cytotoxicity. Chem
Pharm Bull (Tokyo). 63:1065–1069. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Niyazi M, Niyazi I and Belka C: Counting
colonies of clonogenic assays by using densitometric software.
Radiat Oncol. 2:42007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Munakarmi S, Chand L, Shin HB, Jang KY and
Jeong YJ: Indole-3-carbinol derivative DIM mitigates carbon
tetrachloride-induced acute liver injury in mice by inhibiting
inflammatory response, apoptosis and regulating oxidative stress.
Int J Mol Sci. 21:20482020. View Article : Google Scholar
|
|
39
|
Ahmed AG, Hussein UK, Ahmed AE, Kim KM,
Mahmoud HM, Hammouda O, Jang KY and Bishayee A: Mustard seed
(Brassica nigra) extract exhibits antiproliferative effect against
human lung cancer cells through differential regulation of
apoptosis, cell cycle, migration, and invasion. Molecules.
25:20692020. View Article : Google Scholar
|
|
40
|
Skulachev VP: Why are mitochondria
involved in apoptosis? Permeability transition pores and apoptosis
as selective mechanisms to eliminate superoxide-producing
mitochondria and cell. FEBS Lett. 397:7–10. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zamzami N, Susin SA, Marchetti P, Hirsch
T, Gómez-Monterrey I, Castedo M and Kroemer G: Mitochondrial
control of nuclear apoptosis. J Exp Med. 183:1533–1544. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xiong Y, Ye T, Wang M, Xia Y, Wang N, Song
X, Wang F, Liu L, Zhu Y, Yang F, et al: A novel cinnamide YLT26
induces breast cancer cells apoptosis via ROS-mitochondrial
apoptotic pathway in vitro and inhibits lung metastasis in vivo.
Cell Physiol Biochem. 34:1863–1876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Finkel T: Signal transduction by reactive
oxygen species. J Cell Biol. 194:7–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang T, Li J, Dong Y, Zhai D, Lai L, Dai
F, Deng H, Chen Y, Liu M and Yi Z: Cucurbitacin E inhibits breast
tumor metastasis by suppressing cell migration and invasion. Breast
Cancer Res Treat. 135:445–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bhuvarahamurthy V, Kristiansen GO,
Johannsen M, Loening SA, Schnorr D, Jung K and Staack A: In
situ gene expression and localization of metalloproteinases
MMP1, MMP2, MMP3, MMP9, and their inhibitors TIMP1 and TIMP2 in
human renal cell carcinoma. Oncol Rep. 15:1379–1384.
2006.PubMed/NCBI
|
|
47
|
Kim SY, Yi HK, Yun BS, Lee DY, Hwang PH,
Park HR and Kim MS: The extract of the immature fruit of
Poncirus trifoliata induces apoptosis in colorectal cancer
cells via mitochondrial autophagy. Food Sci Hum Wellness. 2020.
View Article : Google Scholar
|
|
48
|
Braillon A: Hepatocellular carcinoma.
Lancet. 380:469–471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Budhu A, Forgues M, Ye QH, Jia HL, He P,
Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY and Wang XW:
Prediction of venous metastases, recurrence, and prognosis in
hepatocellular carcinoma based on a unique immune response
signature of the liver microenvironment. Cancer Cell. 10:99–111.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Min SH, Zhou XZ and Lu KP: The role of
Pin1 in the development and treatment of cancer. Arch Pharm Res.
39:1609–1620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wulf GM, Ryo A, Wulf GG, Lee SW, Niu T,
Petkova V and Lu KP: Pin1 is overexpressed in breast cancer and
cooperates with Ras signaling in increasing the transcriptional
activity of c-Jun towards cyclin D1. EMBO J. 20:3459–3472. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu GG and Etzkorn FA: Pin1 as an
anticancer drug target. Drug News Perspect. 22:399–407. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nakayama K, Hatakeyama S, Maruyama S,
Kikuchi A, Onoé K, Good RA and Nakayama KI: Impaired degradation of
inhibitory subunit of NF-kappa B (I kappa B) and beta-catenin as a
result of targeted disruption of the beta-TrCP1 gene. Proc Natl
Acad Sci USA. 100:8752–8757. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cho YS, Park SY, Kim DJ, Lee SH, Woo KM,
Lee KA, Lee YJ, Cho YY and Shim JH: TPA-induced cell transformation
provokes a complex formation between Pin1 and 90 kDa ribosomal
protein S6 kinase 2. Mol Cell Biochem. 367:85–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nejak-Bowen KN, Thompson MD, Singh S,
Bowen WC Jr, Dar MJ, Khillan J, Dai C and Monga SP: Accelerated
liver regeneration and hepatocarcinogenesis in mice overexpressing
serine-45 mutant beta-catenin. Hepatology. 51:1603–1613. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cheng CW, Chow AK, Pang R, Fok EW, Kwong
YL and Tse E: PIN1 inhibits apoptosis in hepatocellular carcinoma
through modulation of the antiapoptotic function of survivin. Am J
Pathol. 182:765–775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Brembeck FH, Rosário M and Birchmeier W:
Balancing cell adhesion and Wnt signaling, the key role of
beta-catenin. Curr Opin Genet Dev. 16:51–59. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Inagawa S, Itabashi M, Adachi S, Kawamoto
T, Hori M, Shimazaki J, Yoshimi F and Fukao K: Expression and
prognostic roles of beta-catenin in hepatocellular carcinoma:
Correlation with tumor progression and postoperative survival. Clin
Cancer Res. 8:450–456. 2002.PubMed/NCBI
|
|
59
|
David CJ and Manley JL: Alternative
pre-mRNA splicing regulation in cancer: Pathways and programs
unhinged. Genes Dev. 24:2343–2364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ko H, Kim S, Jin CH, Lee E, Ham S, Yook JI
and Kim K: Protein kinase casein kinase 2-mediated upregulation of
N-cadherin confers anoikis resistance on esophageal carcinoma
cells. Mol Cancer Res. 10:1032–1038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shenoy AK, Jin Y, Luo H, Tang M, Pampo C,
Shao R, Siemann DW, Wu L, Heldermon CD, Law BK, et al:
Epithelial-to-mesenchymal transition confers pericyte properties on
cancer cells. J Clin Invest. 126:4174–4186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cheng Y, Chen T, Yang X, Xue J and Chen J:
Atractylon induces apoptosis and suppresses metastasis in hepatic
cancer cells and inhibits growth in vivo. Cancer Manag Res.
11:5883–5894. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kamat AA, Fletcher M, Gruman LM, Mueller
P, Lopez A, Landen CN Jr, Han L, Gershenson DM and Sood AK: The
clinical relevance of stromal matrix metalloproteinase expression
in ovarian cancer. Clin Cancer Res. 12:1707–1714. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Deryugina EI and Quigley JP: Tumor
angiogenesis: MMP-mediated induction of intravasation- and
metastasis- sustaining neovasculature. Matrix Biol. 44-46:94–112.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Iyer RP, Patterson NL, Fields GB and
Lindsey ML: The history of matrix metalloproteinases: Milestones,
myths, and misperceptions. Am J Physiol Heart Circ Physiol.
303:H919–H930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kessenbrock K, Wang CY and Werb Z: Matrix
metalloproteinases in stem cell regulation and cancer. Matrix Biol.
44-46:184–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhu M, Li W, Dong X, Chen Y, Lu Y, Lin B,
Guo J and Li M: Benzyl-isothiocyanate induces apoptosis and
inhibits migration and invasion of hepatocellular carcinoma cells
in vitro. J Cancer. 8:240–248. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
Targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang F, He L, Dai WQ, Xu YP, Wu D, Lin CL,
Wu SM, Cheng P, Zhang Y, Shen M, et al: Salinomycin inhibits
proliferation and induces apoptosis of human hepatocellular
carcinoma cells in vitro and in vivo. PLoS One. 7:e506382012.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang Z, Lapolla SM, Annis MG, Truscott M,
Roberts GJ, Miao Y, Shao Y, Tan C, Peng J, Johnson AE, et al: Bcl-2
homodimerization involves two distinct binding surfaces, a
topographic arrangement that provides an effective mechanism for
Bcl-2 to capture activated Bax. J Biol Chem. 279:43920–43928. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Dewson G and Kluck RM: Mechanisms by which
Bak and Bax permeabilise mitochondria during apoptosis. J Cell Sci.
122:2801–2808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Indran IR, Tufo G, Pervaiz S and Brenner
C: Recent advances in apoptosis, mitochondria and drug resistance
in cancer cells. Biochim Biophys Acta. 1807:735–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ye T, Zhu S, Zhu Y, Feng Q, He B, Xiong Y,
Zhao L, Zhang Y, Yu L and Yang L: Cryptotanshinone induces melanoma
cancer cells apoptosis via ROS-mitochondrial apoptotic pathway and
impairs cell migration and invasion. Biomed Pharmacother.
82:319–326. 2016. View Article : Google Scholar : PubMed/NCBI
|