Introduction
Cancer is a multifactorial disease caused by
genetic, epigenetic and environmental factors, and represents the
end point of multiple molecular changes, which results in the
dysregulation of the normal biological processes controlling cell
proliferation, cell survival, genome stability, energy metabolism,
angiogenesis and immune surveillance to promote tumorigenesis
(1). Breast cancer is the most
common type of cancer among women and the main cause of
cancer-related deaths, accounting for 2.08 million new cases and
626,679 deaths worldwide annually (2). It has been estimated that the
incidence of breast cancer will reach 3.2 million cases worldwide
by 2050 (3). Breast cancer
treatment is primarily focused on local intervention, including
surgery and radiotherapy, while systemic treatments consist of
chemotherapy, hormonal regimens, targeted-therapies and
immunotherapy (4). Patient survival
rates depend on the stage of disease, and the presence of
metastases, as well as the existence of drugs resistance.
Therefore, there is a urgent requirement for the implementation of
novel and more effective therapies, such as the use of
drugs-coupled nanoparticles (NP) that may efficiently target the
tumors, which are currently under exhaustive investigation
(5). To understand the most common
phenotypic alterations that occurred in heterogeneous tumors,
Hanahan and Weinberg (6) described
the typical features of malignancies, known as the hallmarks of
cancer, as the ability of cancer cells to: i) Exhibit sustained
proliferative signaling; ii) evade growth suppressors; iii) resist
programmed cell death; iv) enable replicative immortality; v)
induce angiogenesis; and vi) activate invasion and metastasis. In
2011, Hanahan and Weinberg (7)
proposed two additional cancer hallmarks, namely the reprogramming
of energy metabolism and the ability to evade immune responses; the
authors further suggested that both hallmarks were enabled by two
traits, namely genome instability and mutations. Cancer is not
considered as a single disease, therefore, breast tumors with
different diagnostic features differ in the hallmarks controlling
their clinical behavior and patient outcome. Personalized therapies
are directed against proteins and signaling pathways governing
cancer hallmarks, thus highlighting the importance of identifying
novel molecular targets combined with efficient delivery tools to
improve the current landscape of cancer therapies (7).
Lipid-based NPs (LBNPs) for non-coding RNA
(ncRNA) delivery in breast cancer cells
LBNPs, and nanostructured lipid carriers (NLCs) have
been recognized among a large number of non-viral vectors as
alternative, effective and safe methods for gene therapy to
potentially treat both genetic and non-genetic diseases, including
ocular and infectious diseases, lysosomal storage disorders and
cancer (8). These methods have been
developed to overcome the numerous challenges, including the low
stability of the formulations in the blood circulatory system, drug
burst release by erosion mechanisms, immune system evasion and high
toxicity, for successful gene delivery and effectiveness. LNs are
able to overcome the main biological barriers for successfully
transfecting cells, including the degradation of therapeutic ncRNAs
by nucleases, cell internalization, intracellular trafficking and
the inability to selectively target a specific cell type (8). Additionally, LNs may offer significant
advantages, including an improved bioavailability by enhancing
aqueous solubility, increasing the half-life for clearance,
increasing the specificity for its cognate receptors and targeting
drugs to specific locations in the target tissues (9).
In addition, from a safety point of view, LNs are
constructed using well-tolerated components, while from the
technological point of view, they may be easily produced on a large
scale, and subjected to sterilization and lyophilization, thus
providing the best storage stability. Liposomes are the most
studied delivery systems due to their biocompatibility and
biodegradability. These NPs are primarily composed of
phospholipids, which are organized in bilayer-forming structures
due to the amphipathic properties of the phospholipid molecules. In
the presence of water, NPs form vesicles, thus improving the
solubility and stability of the antitumor drugs once they are
loaded into the vehicle (10).
Furthermore, these lipid-based vehicles are capable of
encapsulating either hydrophobic or hydrophilic drugs (8). In addition to phospholipids, other
compounds, such as cholesterol, can be loaded to the formed
vehicles; cholesterol decreases the membrane fluidity of the NP and
increases its permeability to hydrophobic drugs, thus improving its
stability in blood (10).
Several studies have demonstrated that NPs are
useful and efficient tools for delivering ribonucleic acids into
tumor cells and tissues. Although NPs have been used to deliver
chemotherapeutic drugs, other molecules, such as ncRNAs, can be
also transported into tumor cells. Therefore, NPs encompassing
ncRNAs are considered as a promising alternative approach, as
ncRNAs mediate the specific and potent silencing of oncogenes, and
more specifically, of the tyrosine kinase receptors expressed on
the tumor cell surface, as they are well-known and important
drivers of carcinogenesis (11).
During this process, the degradation of oncogenic mRNAs by ncRNAs
is mediated by a RNA interference (RNAi) mechanism, which serves an
important role in the modulation of gene expression (12). Among the aforementioned delivery
systems, NLCs have been widely used for transporting chemotherapy
drugs and other molecules, especially in the form of liposomal
vehicles. The most frequently used nanosystems for delivering
ncRNAs include the cationic, neutral and ionizable liposomes, and
exosomal and other synthetic nanocarriers (Fig. 1 and Table I) (13–19).
These liposomes exhibit several advantages when acting as carriers
for the in vitro delivery of RNAi agents, as they can be
easily prepared from biocompatible lipids or phospholipid
components, and be modified to serve specific purposes, such as the
encapsulation of the hydrophilic drug molecules.
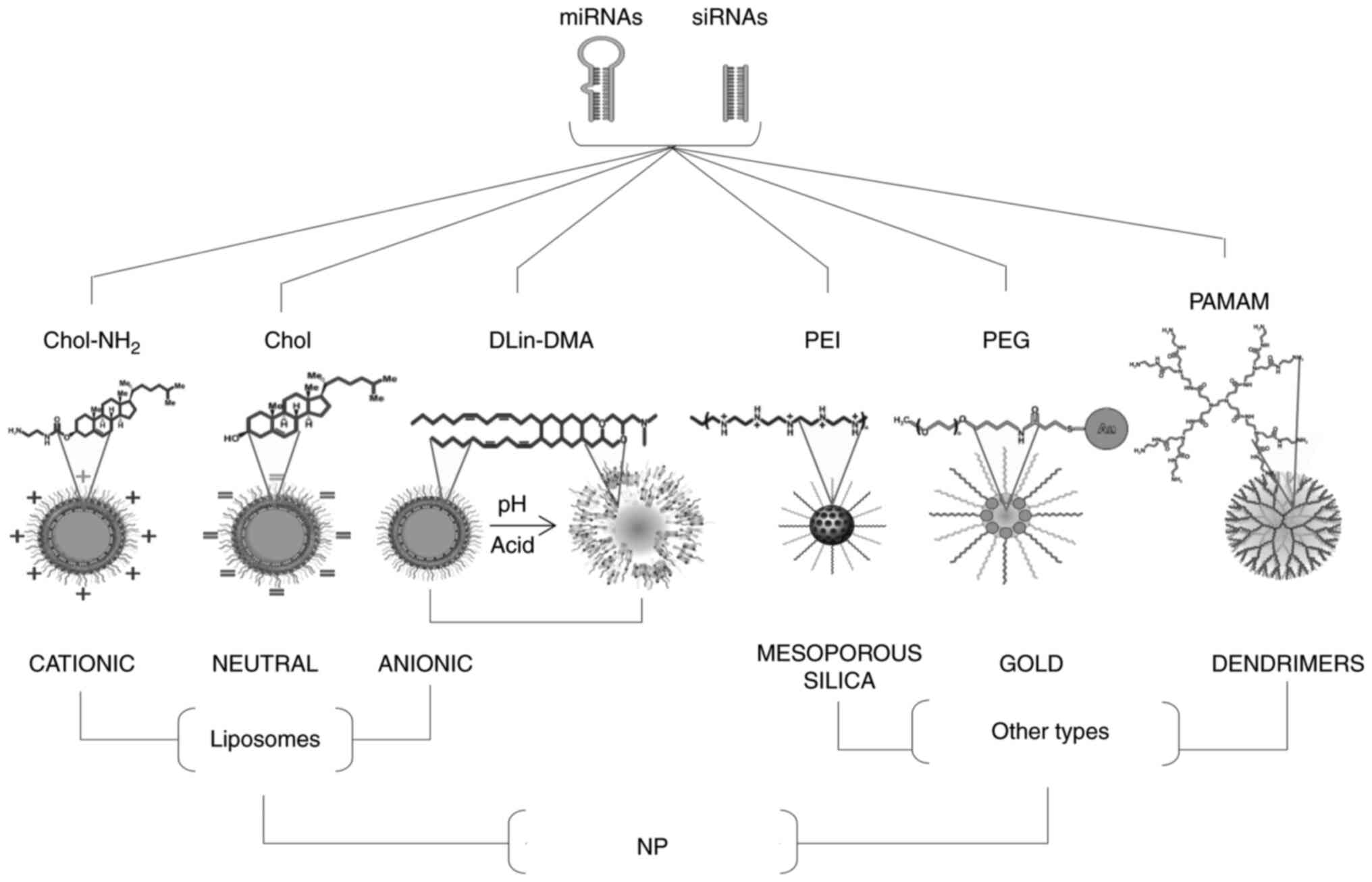 | Figure 1.Graphical representation of common
types of NPs composed of liposomes and other types (mesoporous
silica, gold and dendrimers) for the delivery of nanomiRNAs (siRNAs
or miRNAs) into tumor cells. The three kinds of liposomes known as
cationic, neutral, and anionic pH-sensitive, synthesized with
Chol-NH2, Chol and DLin-DMA, respectively, are indicated
on the left-hand side. On the right side are mesoporous silica,
gold and dendrimers-based NPs covalently or not covalently linked
to PEI, PEG and PAMAM, respectively. siRNA, small interfering RNA;
miRNA, microRNA; Chol, cholesterol; DLin-DMA, N,N-Dimetilacetamida;
PAMAM, poly-amido amine; PEG, polyethylene glycol; PEI,
polyethylenimine; NP, nanoparticles. |
 | Table I.Summary of examples of delivery
systems for therapeutic microRNAs and siRNAs in breast cancer. |
Table I.
Summary of examples of delivery
systems for therapeutic microRNAs and siRNAs in breast cancer.
| First author,
year | Therapeutic
agent | Example of delivery
system | Cancer type | (Refs.) |
|---|
| Chen et al,
2017 | siRNA |
H40-P(Asp-AED-ICA)-polyethylene
glycol | Breast cancer | (13) |
| Deng et al,
2014 | miRNA-34a
mimics | Hyaluronic
acid-chitosan | Breast cancer | (14) |
| Hogrefe et
al, 2006 | siRNA | Immunoliposome | Breast cancer
xenograft | (15) |
| Shu et al,
2015 | miRNA-21
inhibitor | RNA-NPs decorated
with EGFR-targeting aptamer | Triple-negative
breast cancer | (16) |
| Urban-Klein et
al, 2005 | siRNA | pH/redox
dual-sensitive cationic unimolecular NP | Triple-negative
breast cancer | (17) |
| Yhee et al,
2015 | siRNA | Glycol
chitosan | Drug-resistant
breast cancer | (18) |
| Guo et al,
2014 | siRNA |
1,2-Dioleoyl-3-dimethylammonium-propane,
1,2-dioleoyl-sn-glycero-3-phosphocholine,
1,2-dioleoyl-sn-glycero-3-phosphoethanolamineN-dodecanoyl | Triple-negative
breast cancer | (19) |
The innate immune system constitutes the first line
of defense and consists of a diverse set of cell types, including
monocytes, macrophages and dendritic cells. These cells express
different pattern recognition receptors on their cell surface, such
as the membrane-bound Toll-like receptors, cytoplasmic
nucleotide-binding oligomerization domain-like receptors and
scavenger receptors. The adaptive immune system provides the second
line of defense and includes several important T helper (Th) cell
subsets, including Th1, Th2, Th17, T regulatory and Th22 cells. In
a mouse model of vaccination using antigen-coupled nanobeads, it
was reported that the NP size determined the Th1 response (IFNγ
production) and Th2 response (IL-4 production); the 40 and 49 nm
beads significantly improved Th1 activation, whereas the larger
sized beads (>100 nm) stimulated the Th2 response to a great
extent (20). These blood cells and
erythrocytes interact with various types of NPs depending on their
composition, size, geometry and surface charge, and importantly,
these interactions may affect the delivery of NPs as they can forms
aggregates, mainly with the abundant erythrocytes, and be toxic for
cells of the immune system. The geometry of the NPs also defines
the efficiency of the cellular absorption and the ability to form
aggregates; for example, the rod-shaped NPs are internalized by
leukocytes with high efficacy, followed by sphere- and
cylinder-shaped NPs, whereas cube-shaped NPs are not as easily
internalized (21). Regarding the
charge, cationic NPs will interact positively with the surface of
the negatively charged cell membrane; however, cationic liposomes
can enhance immunotoxicity by stimulating neutrophils and inducing
oxidative stress. In addition, anionic NPs have been shown to have
unfavorable interactions with the cell membrane due to the
repulsive forces between the two negatively charged surfaces and
therefore, show poor cellular internalization (22). Conversely, it has been proposed that
leukocyte-NP interactions are advantageous, since these complexes
can easily cross physiological barriers and travel to tumor niches
(23).
Cationic liposomes are especially convenient and the
electrostatic interactions between the positively charged lipids
and the negatively charged RNA molecules notably improve the
efficiency of RNA encapsulation. The encapsulated ncRNAs within
cationic liposomes exhibit a preferential uptake from the target
cells through endocytosis. To further ensure that the desired cells
are targeted, ligands binding to cell surface receptors of the
target tissue are incorporated into the cationic coat of the
liposome (24). The engineering of
carriers has gained considerable attention in recent years due to
the development of effective delivery systems that transport small
interfering RNAs (siRNAs) and microRNAs (miRNAs/miRs) into the
tumor tissues and the cytoplasm of tumor cells. Furthermore, an
ideal nanocarrier system should be able to protect the therapeutic
RNAi agents from the circulatory environment, provide high
resistance to nucleases, overcome immune responses and ensure
efficient delivery to tumor cells (25).
An interesting study by Hayward et al
(26) described the delivery of
miR-125a-5p in HER2-positive metastatic breast cancer cells using a
lipid-based system; the study demonstrated that LNs coated with
hyaluronic acid used for miR-125a-5p delivery significantly
downregulated the expression levels of HER2 and suppressed cell
proliferation and migration via the PI3K/AKT and MAPK signaling
pathways. Thus, the findings of this study suggested a therapeutic
potential of miRNAs coupled to LNs for HER2-positive breast cancer
treatment. Another study, performed in cancer stem-like cell
subpopulations derived from MCF-7 breast cancer cells, demonstrated
that the delivery of miR-200c by LBNPs downregulated the expression
levels of class III β-tubulin protein, which is considered as a
candidate biomarker for predicting resistance to chemotherapy
(27). In addition, LBNPs exhibited
lower cytotoxicity compared with lipofectamine in a previous study.
These data illustrated the promising efficiency of LBNPs in
delivering miRNAs, thus suggesting that LBNPs may be considered as
a powerful therapeutic tool for the treatment of breast cancer.
Furthermore, Wang et al (28) evaluated the efficiency of LNs in
delivering specific siRNAs targeting CDK4; the results revealed
that the LN triggered exceptional gene silencing, to a greater
extent than that of commercial lipofectamine. CDK4 silencing in
HeLa cervical cancer cells and MDA-MB-468 breast cancer cells also
promoted G1 phase cell cycle arrest. Therefore, as CDK4
is involved in the cell cycle and proliferation, the development of
selective blocking agents against CDK4, such as siRNAs, is
considered a very attractive approach for cancer treatment. In
addition, Tang et al (29)
explored the efficiency of an interesting delivery system, in which
LNs were coated with calcium phosphate, in transferring a mixture
of highly potent siRNAs targeting ubiquitously expressed genes
essential for cell survival. The results reported enhanced cellular
uptake and the inhibition of MDA-MB-468 breast cancer cell growth.
Furthermore, the conjugation of either EGFR-specific single chain
fragment antibody or folic acid improved the delivery ability of
the siRNAs into the cancer cells.
The simultaneous delivery of chemotherapeutic drugs
and therapeutic ncRNAs has provided remarkable advantages; for
example, Yu et al (30)
developed a co-delivery system consisting of paclitaxel and a
specific siRNA targeting myeloid cell leukemia-1 (MCL1) coupled to
LBNPs; the results of this study demonstrated that MCL1 mRNA
expression levels were significantly downregulated in MDA-MB-231
cancer cells transfected using the aforementioned delivery system.
Additionally, in a xenograft mouse model, the intratumoral
injection of LNs encapsulating paclitaxel and siRNA-MCL1
significantly attenuated tumor growth. These results indicated that
nanomiRNAs may efficiently transport antitumor and therapeutic
drugs.
The high specificity and performance of the cellular
RNAi mechanism may explain the above effects. Since the discovery
of RNAi in mammalian cells, there has been increasing interest in
harnessing this mechanism for treating several types of disease
including ophthalmologic disease, cancer, metabolic diseases such
as hypercholesteremia, and viral infections. Briefly, RNAi is an
endogenous pathway associated with the post-transcriptional
silencing of gene expression, triggered by small double-stranded
RNAs (dsRNAs) functioning in a sequence-specific manner via
directly binding and degrading target mRNAs, thus leading to the
potent silencing of gene expression (31). miRNAs are small ncRNAs of 21–25
nucleotides in length, encoded by genes dispersed throughout the
genome. These tiny RNAs act at the post-transcriptional level as
negative regulators of gene expression through binding to the
3′-untranslated region of multiple mRNAs, thus leading in their
degradation and transcriptional repression via the activation of
the cellular RNAi mechanism (32).
Interestingly, miRNAs may function as oncogenes or tumor
suppressors, regulating the expression of a number of genes
involved in pivotal signaling pathways and cellular processes that
promote tumor development and progression, including cell
proliferation, the cell cycle, angiogenesis, the cancer stem
cell-like phenotype, epithelial-mesenchymal transition, and
invasion and metastasis (33–39).
The expression levels of miRNAs are often dysregulated in different
types of tumor and their ability to directly target multiple genes
greatly affects the cancer cell phenotype, thus resulting in
tumorigenesis (40,41). These cellular alterations on the
expression profile of miRNAs determine the clinicopathological
features of patients, resulting in a decreased overall survival and
disease-free survival rates, and an increased risk of disease
relapse, drug resistance (42,43),
disease recurrence (44) and poor
prognosis (45). Therefore, miRNAs
may serve as bona fide diagnostic and predictive biomarkers
for several types of disease, including obesity, neurological
disorders, cardiopathies and cancer (46). On the other hand, siRNAs are a class
of small dsRNAs, which are exogenously administered into cells, as
they are not encoded in the genome, to mediate gene silencing.
siRNAs inhibit gene expression and protein synthesis via
complementary binding to their target mRNAs, where they trigger
target gene silencing through the RNAi mechanism, like miRNAs
(47). A conventional siRNA
consists of 19–21 nucleotides in length with a 3′ double-nucleotide
overhang, usually TT and UU; these nucleotides are important for
sequence recognition by the RNAi machinery. As the length of the
dsRNA increases, its potency is significantly enhanced. For
instance, an in vitro study demonstrated that siRNAs of 27
nucleotides in length were up to 100 times more potent compared
with the conventional 21-nucleotide siRNAs (48). In recent years, novel strategies for
the targeted delivery of miRNAs and siRNAs into tumor cells have
been developed; however, the primary barrier to the clinical
application of these RNAi-mediated therapies remains the lack of an
effective delivery system providing sufficient protection for the
ribonucleic acids against nucleases. Among them, nanomiRNAs offer
great potential owing to their biocompatibility and low toxicity
compared with inorganic NPs and viral delivery systems (49). However, to the best of our
knowledge, the development of clinical trials using ncRNA delivery
systems through NPs has not yet been explored in patients with
breast cancer. Two registered clinical trials using different
approaches to deliver therapeutic miRNAs in different cohorts of
patients with cancer have been reported. The first report is a
phase I study of TargomiRs as 2nd or 3rd line treatment for
patients with recurrent malignant pleural mesothelioma (MPM) and
non-small cell lung cancer (clinical trial no. NCT02369198).
TargomiRs are targeted minicells (bacteria-derived NPs) containing
the tumor suppressor miR-16 mimic, which is a small RNA involved in
diverse types of cancer. The drug delivery system denominated
EnGeneIC Delivery consists of nonliving bacterial minicells forming
NPs of 100–400 nm size and the TargomiRs system is targeted to
tumors using an anti-EGFR antibody. The data illustrated that the
TargomiRs formulation displayed acceptable safety and was well
tolerated by the 26 patients with refractory MPM enrolled in the
study; however, it only promoted moderate antitumor activity
(50). The second report is a
multicenter phase I study using a miR-34a mimic-encapsulated
liposomal formulation (MRX34), which was injected into 85
participants with diverse types of solid tumor, including
hepatocellular carcinoma, melanoma, renal carcinoma and lung
cancer, among others (clinical trial no. NCT01829971). The
pharmacodynamic data indicated the efficient delivery of miR-34a
into the tumors, and a dose-dependent and longer-lasting repression
of its cognate target genes (Bcl-2, DnaJ homolog subfamily B member
1, β-catenin, forkhead box protein P1 and histone deacetylase 1) in
white blood cells (51). In
addition, a manageable toxicity profile and modest clinical
activity was observed following MRX34 treatment. Unfortunately, the
clinical trial was closed due to unexpected adverse events related
to the immune system that were not anticipated in pre-clinical
studies (52). At present, these
novel nanotechnologies are gaining increasing attention as
promising anticancer tools due to their high specificity,
efficiency and low toxicity in normal cells (53,54).
In the next section, the current applications of diverse nanomiRNAs
systems in breast cancer are summarized.
Cationic liposomes
The lipids constituting the cationic liposomes
neutralize the negative charge of the DNA, thus forming a compact
structure, which differs from the typical vesicular structure of
liposomes. These complexes, resulting from the interaction between
DNA and the cationic lipids, provide protection to the genetic
material and promote cell internalization. For gene therapy
applications, the surfactants are often positively charged to
obtain cationic LBNPs electrostatically bound to nucleic acids
(55). In addition, LBNPs usually
exhibit a ζ potential, an indirect measurement of the surface
charge (>30 mV) which decreases following the addition of
increasing concentrations of nucleic acids (10). Previous studies have taken advantage
of the features of cationic liposomes to mediate the silencing of
cancer-related genes in different types of cancer; for instance,
Meraz et al (56)
demonstrated in a murine model of breast cancer that the
intratumoral injection of cationic liposomes loaded with
monophosphoryl lipid A and IL-12 promoted cell death, inhibited
cell proliferation and increased the serum levels of IL-1β and
TNF-α. Thus, this study illustrated the efficacy of liposomal
nanocarriers to attenuate cell viability, tumor growth and drug
toxicity, and enhance immunity.
Other researchers have implemented innovative
systems for the simultaneous delivery of siRNAs and
chemotherapeutic agents, including cationic liposomes (57,58).
Such a system was applied in breast cancer, where cationic
liposomes were used to deliver paclitaxel together with a specific
siRNA targeting polo-like kinase 1 (PLK1). PLK1 is an aggressive
oncogene frequently found overexpressed in breast cancer, thus
promoting the accelerated proliferation of tumor cells. The
simultaneous delivery of paclitaxel and PLK1 siRNA synergistically
increased the number of apoptotic MCF-7 breast cancer cells and
reduced angiogenesis. This delivery method exhibited significant
advantages over monotherapies with paclitaxel and siRNAs (57).
On the other hand, the conjugation of
chemotherapeutic agents, such as doxorubicin, with liposomes is a
commonly used method in cancer treatment (58). Wang et al (58) modified the doxorubicin-liposome
system with a cationic polymer to improve the cellular absorption
and antitumor activity of NPs; the NPs were efficiently absorbed,
resulting in cell death following 5 h incubation compared with
doxorubicin monotherapy and doxorubicin-liposomes complexes. In
addition, the efficacy of the above delivery system was determined
in H22 mice following four injections of doxorubicin-liposomes
conjugated to a cationic polymer; the results revealed that the
delivery system conjugated to the cationic polymer reduced tumor
growth by up to 60% in vivo. Thus, the aforementioned
studies supported the great potential of cationic liposomes to
improve the delivery of anticancer drugs.
Neutral liposomes
Neutral liposomes are primarily constructed by
neutral lipids, including phosphatidylcholine,
phosphatidylethanolamine, cholesterol and
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) (59,60).
DOPE may be used as an adjuvant phospholipid in cationic liposomes
and it was discovered to enhance the transfection efficiency of
nanocarriers encapsulating nucleic acids (59). In addition, neutral liposomes have
exhibited good biocompatibility and excellent pharmacokinetic
characteristics; however, they are unable to interact with DNA to
efficiently adsorb and encapsulate it into the liposomes (59,60).
Yan et al (61) constructed
a novel and functional type of neutral liposomes; these neutral
liposomes, known as 1,2-distearoyl-sn-glycero-3-phosphoeth
anolamine-N-[(polyethylene glycol)-2000], efficiently silenced the
expression levels of Slug and inhibited the TGF-β1/Smad signaling
pathway in triple-negative breast cancer cells. In addition, these
liposomes demonstrated a high antitumor efficacy in vivo
when combined with vinca alkaloid (vinorelbine) chemotherapy in
triple-negative breast cancer. Another study revealed that
pegylated liposomal doxorubicin significantly extended the
disease-free survival period of patients with pathological stage
I–III breast cancer, which suggested that it could be applied at
different pathological stages of breast cancer (62).
Emerging evidence has suggested that curcumin may
overcome the drug resistance to conventional chemotherapies;
however, its poor bioavailability and decreased absorption have
limited its clinical use (63,64).
Furthermore, adriamycin, a widely known cytotoxic agent, is often
used in cancer chemotherapy (65);
however, breast tumors have exhibited high resistance to
adriamycin, leading to a poor prognosis. Zhou et al
(66) developed NPs encapsulating
the dietary compound curcumin to improve its bioavailability.
Furthermore, miRNA profiling was performed in adriamycin
(Adr)-resistant MCF-7 breast cancer cells (MCF-7/Adr) and the
results revealed that neutral liposomes containing curcumin
increased the chemosensitivity of MCF-7/Adr cells, which was
mediated by the altered expression pattern of miRNAs associated
with drug resistance. In fact, 67 differentially expressed miRNAs
were identified among the parental MCF-7, MCF-7/Adr and
curcumin-treated MCF-7/Adr cells; among them, the downregulation of
miR-29b-1-5p was associated with decreased IC50 values
of curcumin, while its upregulation attenuated the effects of
liposomal curcumin in Adr-resistance in MCF-7/Adr cells.
Another previous study described the development of
a functional neutral liposome for miR-203 delivery; these
miR-203-containing liposomes efficiently silenced Slug expression
levels and inhibited the TGF-β1/Smad signaling pathway in
triple-negative breast cancer cells. Additionally, when liposomes
were combined with vinorelbine chemotherapy, they exhibited
significant antitumor activity in triple-negative breast cancer
cells. Therefore, these findings suggested that the development of
functional miRNAs and neutral liposomes should be further
investigated in clinical trials (61).
Furthermore, it was revealed that the growth
inhibitory effects of siRNAs targeting VEGF loaded into liposomes
incorporating modified G2 polyamidoamine-cholesterol dendrimer were
comparable with those noted to commercially available transfection
systems (Metafectene) in SKBR3 breast cancer cells (67). In addition, Chen et al
(68) applied a novel polycation
liposome encapsulating a calcium phosphate (PLCP) NP to facilitate
endosomal escape via combining the protonation of polyethylenimine
and membrane destabilization of DOPE, which resulted in an
increased transfection efficiency. In addition, this siRNA delivery
system was also applied using three siRNA sequences targeting VEGF
to inhibit tumor angiogenesis. Compared with the commercial
transfection reagents, the silencing activity of the PLCP/VEGF
siRNA complex was increased two times in MCF-7 cells. In
vivo results in an MCF-7 ×enograft mouse model revealed that
the PLCP/VEGF siRNA complex significantly inhibited angiogenesis
and tumor growth. Additionally, a synergetic tumor inhibition
effect was observed in cells co-treated with doxorubicin in a mouse
model. Thus, the aforementioned data suggested that the delivery of
siRNAs targeting VEGF in combination with PLCP inhibited
angiogenesis, indicating that this delivery system may serve as a
promising approach for breast cancer treatment.
Ionizable liposomes
Ionizable liposomes are promising nanocarriers used
to deliver siRNAs due to their physical and functional properties.
Unlike the cationic and neutral liposomes that carry a single type
of charge, ionizable liposomes are protonated and deprotonated
according to the acidity of the environment (59). Early cationic lipids, such as
N-[1-dioleyloxy)propyl]-N,N,N-trimethylammonium and
dioctadecyldimethylammonium chloride, contain a positive quaternary
amine, whereas ionizable cationic lipids, such as
1,2-dioleoyl-3-dimethylammonium propane and
1,2-dioleyloxy-N,N-dimethyl-3-aminopropane, have positive and
neutral charged tertiary amines, respectively, at acidic and
physiological pH conditions (69).
Furthermore, it has been reported that the incorporation of helper
and neutral lipids, such as cholesterol and saturated
phosphatidylcholines, increase both the system stability and
transfection efficiency (70). The
effects of the ionizable liposomes in cancer therapy have been
poorly investigated; however, experimental evidence has suggested
that their application provides significant advantages compared
with other types of therapeutic methods (71). For example, an in vivo study
in a glioma murine model discovered that the delivery of conjugated
siRNAs to ionizable liposomes exhibited an improved efficiency and
resulted in enhanced cellular uptake under hypoxia and low-pH
conditions. Specifically, in this study, Liu et al (72) synthesized malate dehydrogenase
lipids with nitroimidazole groups, which provided hypoxia
sensitivity and specificity as hydrophobic tails, and tertiary
amines as hydrophilic head groups. These precursors were
self-assembled into O′1,O1-(3-(dimethylamino)propane-1,2-diyl)
16-bis(2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl)
di(hexadecanedioate) liposomes (MLP) to encapsulate PLK1 siRNA; the
results demonstrated the enhanced delivery of MLP/PLK1 siRNAs into
glioma cancer cells due to the increased positive charges induced
by hypoxia and low pH. Therefore, MLP/PLK1 siRNA was shown to
effectively inhibit glioma cell growth both in vitro and
in vivo.
In addition, it has been reported that the
implementation of ionizable liposomes increases encapsulation
(−2,200 molecules per 100 nm liposome), whereas the therapeutic
effects of encapsulated siRNAs and their useful shelf life are
greater compared with another liposomes (73,74).
Active loading remains the most commonly used method for loading
ionizable drugs in liposomes; however, the low aqueous solubility
of numerous anticancer drugs may slow liposomal uptake during
active loading (74). To overcome
this limitation, drug supersaturation is maintained during active
loading (73). Modi et al
(73) developed a method for
maintaining supersaturation of a poorly soluble camptothecin drug
analogue, namely 7-t-butyldimethylsilyl-10-hydroxycamptothecin,
using low concentrations of sulfobutylether-β-cyclodextrin to
inhibit crystallization, thus overcoming the limitations associated
with the liposomal delivery of poorly soluble ionizable drugs.
It has been reported in a breast cancer model that
the application of ionizable liposomes with vincristine at a
drug-lipid ratio of 1:1 provided an average life of 15.7 h when
combined with conventional cyclophosphamide, doxorubicin,
vincristine and prednisone chemotherapy. Therapeutically optimized
rates of drug release were suggested to be achieved by varying the
drug-to-lipid ratios in liposomal vincristine formulations
(75). Another study demonstrated
the implementation of ionizable liposomes conjugated to lipocalin 2
(Lcn2) siRNA to inhibit the C-X-C chemokine receptor type 4 and
Lcn2 pathways in metastatic MDA-MB-436 and MDA-MB-231 breast cancer
cell lines, thus indicating that the treatment with liposomes may
inhibit breast cancer progression (19).
At present, studies with liposome-miRNA complexes at
the nanometer and micrometer scale are being conducted for their
pharmaceutical application in breast cancer. Defining the critical
quality attributes of nanosystems using high level quality control
for reproducibility is pivotal for their potential application in
cancer research (76). Lujan et
al (76) studied diverse
aspects of the formation of nanoliposomal systems for the proper
storage and delivery of miRNAs in triple-negative breast cancer
cells; the study revealed that the nanometer-sized liposomes
(<350 nm) could be obtained at low concentrations, preserved
stably for up to 6 months in lyophilized form and maintained
encapsulation after extended time periods in storage. Furthermore,
the nano-formulation efficiency was determined by delivering
miR-203, a tumor suppressor, into MDA-MB-231 and Hs578t
triple-negative breast cancer cells. The results illustrated that
both micro- and nanoliposomes effectively delivered miR-203 into
breast cells via an endocytic pathway. Another previous study in a
triple-negative breast cancer mouse model revealed that the
encapsulation and administration of miR-203 in liposomes of a size
of 120 nm inhibited tumor growth compared with functional
vinorelbin liposomes. Remarkably, the treatment with both types of
liposomes resulted in complete tumor growth inhibition (61).
An in vitro study by de Antonellis et
al (77) focused on the
restoration of the Notch signaling pathway by employing a stable
2′-O-methylated-encapsulated miR-199b-5p in ionizable lipids.
miR-199b-5p was identified to regulate basic-helix-loop-helix
transcription factor 1 (Hes-1), a downstream effector of the
canonical Notch pathway, by affecting medulloblastoma cancer stem
cells (CSCs) through decreasing the number of
CD133+/CD15+ cell subpopulations. The
efficacy of the miR-199b-5p delivery using stable nucleic acid
lipid particles was confirmed by the significant attenuation of
Hes-1 and CSC marker expression levels in tumorigenic cell lines
from colon (HT-29, CaCo-2 and SW480), breast (MDA-MB231T and MCF-7)
and prostate (PC-3) cancer, glioblastoma (U-87) and medulloblastoma
(Daoy, ONS-76 and UW-228). In addition, the formulation inhibited
cell proliferation, thus indicating that this system could be
considered as a powerful tool for gene therapy. Furthermore, it was
revealed that hypoxia induced in solid tumors efficiently favored
the ionizable liposomes to transfer effector particles (72). Therefore, these studies on liposomes
may represent a potential research approach for breast cancer
(70,72).
Exosomes nanocarriers
Exosomes are extracellular small vesicles present in
the circulation, with diameters ranging from 30 to 150 nm. Exosomes
serve an important role in cell-to-cell communication and in the
regulation of several biological processes in normal cells
(78). However, in cancer cells,
this cellular communication process is exacerbated, as cancer cells
secrete 10-fold more exosomes, the so-called tumor-derived exosomes
(TDEs). TDEs carry growth factors, DNA, chemokines and ncRNAs to
promote cell communication and contribute to the tumor
microenvironment, metastasis, tumorigenesis and cancer metabolism
(79,80). Xin et al (81) demonstrated that miR-455-5p was
specifically overexpressed in tumor exosomes; likewise, the
exosomal and cellular expression of miR-1255a was also upregulated.
It was reported that miR-455-5p targeted CDK inhibitor 1B to
regulate the cell cycle, while miR-1255a regulated SMAD4 to inhibit
the TGF-β signaling pathway. Upregulated expression levels of
miR-455-5p and miR-1255a were associated with a poor overall
survival, while the upregulation of their target genes has been
associated with an excellent overall, recurrence-free and distant
metastasis-free survival. In conclusion, this study preliminarily
indicated that exosomal miR-455-5p and miR-1255a may be considered
as novel therapeutic targets for breast cancer.
Another previous study suggested that breast
cancer-derived exosomes may contribute to tumor growth,
angiogenesis, invasion and metastasis (82,83).
Therefore, Lin et al (83)
suggested that exosomes derived from human mesenchymal stem cells
may promote MCF-7 breast cancer cell migration and proliferation
via altering the gene expression profiles. In addition, exosomes
have been indicated to also affect the microenvironment to promote
tumorigenesis (82–84). A previous study also revealed
differences in the miRNA expression profile between patients with
triple-negative breast cancer and healthy controls; for example 8
exosomal miRNAs (miR-21, miR-141, miR-200a, miR-200b, miR-200c,
miR-203, miR-205 and miR-214) from malignant tumors were
significantly differentially expressed from those observed in
benign tumors, and were not detected in normal controls (85). In addition, the levels of exosomal
let-7f and/or miR-30e-3p in patients with non-small cell lung
cancer could distinguish patients with resectable tumors from those
with non-resectable tumors (86).
The expression levels of 12 exosomal miRNAs (miR-17-3p, miR-21,
miR-106a, miR-146, miR155, miR-191, miR-192, miR-203, miR-205,
miR-210, miR-212 and miR-214) were significantly different between
patients and controls (87). The
differences in miRNAs expression exhibited promising predictive
values; thus they may serve as biomarkers to distinguish patients
with recurrent breast cancer from patients with non-recurrent
breast cancer (88).
Stealth liposomes
Stealth liposomes, also called PEG-coated liposomes,
exhibit prolonged blood circulation time and improved distribution
in tissues. This type of liposome is characterized by the
incorporation of a PEG derivative, which promotes the steric
stabilization of NPs to overcome their opsonization and prolong
their time in the blood circulation (89). In addition, the presence of PEG on
the surface of liposomes was discovered to increase the
hydrophilicity, thus reducing their interaction ability with plasma
proteins (90,91). PEG anchoring can be obtained by the
polymer adsorption into vesicles and aggregation of the PEG-lipid
during development (92). These
lipids exhibit several advantages, including increased solubility,
null toxicity and low antigenicity. Furthermore, for practical
purposes, it is easier to incorporate this type of polymer with
lipids. Another interesting advantage is that PEG lipids have been
reported to potentiate therapeutic proteins without altering their
mechanism of action (93). At
present, a pegylated liposome loaded with doxorubicin,
DOXIL® has been approved by the Food and Drug
Administration since 1995. These nanodrugs exhibit prolonged
circulation time, as they can overcome their recognition by the
reticuloendothelial system due to the presence of PEG on the
surface of the lipids. Furthermore, loading of these liposomes with
doxorubicin, directed by an ammonium sulfate gradient, improved the
delivery of the drug into tumor cells (94). A study by Vakhshiteh et al
(95) demonstrated that the
encapsulation of miR-34a into a type of PEG-anchored liposomes
promoted its accumulation in MDA-MB-231 breast carcinoma cells.
Additionally, this formulation exhibited a significant inhibitory
effect on tumor cell growth, migration and invasion, whereas it
attenuated the percentage of CD44+/CD24−
cells, indicating its effectiveness in cancer hallmarks.
Triggered-release liposomes
Liposomes are NPs widely used for the delivery of
drugs, DNA fragments and siRNAs, which allow the release of these
molecules in time and space (96).
The activated liposomes represent a promising system that may
increase the release rate of their contents (97). The ability to deliver antitumor
molecules in situ remains a challenge for the development of
liposomes, thus several strategies have been applied considering
the physical and chemical characteristics of the tumor
microenvironment (98,99). For example, Elegbede et al
(100) developed an interesting
formulation with a collagen-like triple-helix conformation
encompassing a matrix metalloproteinase (MMP) substrate to induce
drug release in a MMP activity-dependent manner. Another study
revealed that thermosensitive liposomes, used for localized
delivery, triggered the release of chemotherapeutic drugs. For
example, the delivery of pegylated liposome-based drugs, like
DOXIL, was more efficient in tumors exposed to slightly higher
microenvironment temperatures (101). In addition, an in vitro
study performed in triple-negative breast cancer cells showed that
a benzoporphyrin-derived conjugate exhibited a lifespan of one
month at 37°C, suggesting that it could be successfully employed
for in vivo interventions (102).
Porphysomes
Phosphorene, also referred to as single- or
few-layer black phosphorus (FLBP), is a new member of the 2D
material family (103). FLBPs have
gained increasing attention in recent years and have been used in
optoelectronics, strength storage and biomedicine due to their
efficiency and biocompatibility (104). In 2017, Yang et al
(103) constructed FLBP nanosheets
loaded with gold (Au)-NPs and black phosphorus (BP)-NPs as
effective surface-enhanced Raman scattering (SERS) substrates; the
features of the breast tumors before and after photothermal therapy
were distinguished utilizing the SERS analysis and >85% of 4T1
cells were alive following incubation with BP-Au NSs, thus
indicating that the hybrid NP BP-Au exhibited good biocompatibility
and low cytotoxicity. Another advancement originated from the study
by Sun et al (105), who
focused on the application of photodynamic treatment (PDT) combined
with quality treatment for triple-negative breast cancer. The
objective was accomplished by utilizing cationic porphyrin lipid
microbubbles loaded with hypoxia-inducible factor-1α (HIF-1α)
siRNA, which was monitored using ultrasound imaging. HIF-1α siRNA
downregulated HIF-1α expression levels, which was mediated by the
common hypoxic tumor conditions or the reactive oxygen species
(ROS) produced by PDT, improved the PDT efficacy and partially
inhibited tumor progression.
Lipid-coated calcium phosphate (LCP)
NPs
In 2018, Tang et al (106) designed and developed biocompatible
multifunctional LCP NPs as an effective delivery system to inhibit
the development of tumors using triple-negative MDA-MB-468 cells.
Briefly, LCP NPs were conjugated with a bispecific immunizer (BsAb)
through a non-covalent bond with methoxy PEG on the surface of the
molecule; this BsAb targeted the EGFR on the surface of MDA-MB-468
cells. These LCP-BsAb NPs, loaded with cell death (CD) siRNA and
indocyanine green (ICG), were efficiently uptaken by MDA-MB-468
cells, promoting cell apoptosis and cell absorbance at 808 nm using
a near-infrared laser. These multifunctional LCP-BsAb NPs were
increasingly accumulated in the tumor tissue. Thus, the combination
of CD siRNA and photothermal (ICG) treatment using LCP-BsAb NPs
inhibited both small and large tumors in the in vivo mouse
model (106,107).
Conclusions
In conclusion, the recent advances in nanotechnology
and the development of novel LBNPs highlight the potential for its
utilization in cancer therapy. Treatments using LBNPs greatly
improve the transport of ncRNAs into cancer cells and tumor tissues
and at the same time, demonstrated significant advantages, such as
reduced therapeutic doses, low cytotoxicity to normal cells and an
ability to reverse the resistance to chemotherapy. Moreover, LBNPs
have the ability to transport therapeutic ncRNAs to modulate the
expression levels of genes involved in cell growth, proliferation,
cell death, invasion and metastasis, thereby impairing the
malignant behavior of tumors. Therefore, the development of
efficient nanomiRNAs in breast cancer will depend on the proper
synthesis of new lipid components and novel targeting ligands.
LBNPs are promising tools for the therapeutic delivery; however,
although significant advancements have been made in the field of
ncRNA delivery, there still exists a further requirement to
investigate alternative effective strategies. LBNPs also have
disadvantages; for example, due to their crystalline structure,
they have low drug loading efficiency and burst release. Also,
there is a high probability for drug expulsion from the NPs due to
the crystallization process during the storage conditions, where
polymorphic changes between the LBNPs and compounds promotes the
tendency for drug expulsion. Undoubtedly, nanotechnology-based
treatments are still far from reaching the patients; however, it is
a treatment that, together with the development and increased
research into different microRNAs, may serve a fundamental role in
the non-invasive treatment of multiple types of cancer.
Acknowledgements
We would like to thank Mr. Juan Manuel Delgado
Cervantes at the Facultad de Medicina de la Universidad Autónoma de
San Luis Potosí for the support in producing the figure.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
MBSC, MZSL, EJA, SINO, ICM and CLC wrote all the
sections of manuscript. CLC and MBSC conceived and designed the
review. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cavallo F, Giovanni C, Nanni P, Forni G
and Lollini PL: 2011: The immune hallmarks of cancer. Cancer
Immunol Immunother. 60:319–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cronin KA, Lake AJ, Scott S, Sherman RL,
Noone AM, Howlader N, Henley SJ, Anderson RN, Firth AU, Ma J, et
al: Annual report to the nation on the status of cancer, part I:
National cancer statistics. Cancer. 124:2785–2800. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Breast Cancer Treatment|Treatment Options
for Breast Cancer.
|
|
5
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yingchoncharoen P, Kalinowski DS and Des
Richardson R: Lipid-based drug delivery systems in cancer therapy:
What is available and what is yet to come. Pharmacol Rev.
68:701–787. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mudshinge SR, Deore AB, Patil S and
Bhalgat CM: Nanoparticles: Emerging carriers for drug delivery.
Saudi Pharm J. 19:129–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Del Pozo-Rodríguez A, Solinís MÁ and
Rodríguez-Gascón A: Applications of lipid nanoparticles in gene
therapy. Eur J Pharm Biopharm. 109:184–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hashemi M and Kalalinia F: Application of
encapsulation technology in stem cell therapy. Life Sci.
143:139–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aagaard L and Rossi JJ: RNAi therapeutics:
Principles, prospects and challenges. Adv Drug Deliv Rev. 59:75–86.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen G, Wang Y, Xie R and Gong S:
Tumor-targeted pH/redox dual-sensitive unimolecular nanoparticles
for efficient siRNA delivery. J Control Release. 259:105–114. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng X, Cao M, Zhang J, Hu K, Yin Z, Zhou
Z, Xiao X, Yang Y, Sheng W, Wu Y and Zeng Y: Hyaluronic
acid-chitosan nanoparticles for co-delivery of MiR-34a and
doxorubicin in therapy against triple negative breast cancer.
Biomaterials. 35:4333–4344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hogrefe RI, Lebedev AV, Zon G, Pirollo KF,
Rait A, Zhou Q, Yu W and Chang EH: Chemically modified short
interfering hybrids (siHYBRIDS): Nanoimmunoliposome delivery in
vitro and in vivo for RNAi of HER-2. Nucleosides Nucleotides
Nucleic Acids. 25:889–907. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shu D, Li H, Shu Y, Xiong G, Carson WE
III, Haque F, Xu R and Guo P: Systemic delivery of Anti-miRNA for
suppression of triple negative breast cancer utilizing RNA
nanotechnology. ACS Nano. 9:9731–9740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Urban-Klein B, Werth S, Abuharbeid S,
Czubayko F and Aigner A: RNAi-mediated gene-targeting through
systemic application of polyethylenimine (PEI)-complexed siRNA in
vivo. Gene Ther. 12:461–466. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yhee JY, Song S, Lee SJ, Park SG, Kim KS,
Kim MG, Son S, Koo H, Kwon IC, Jeong JH, et al: Cancer-targeted
MDR-1 siRNA delivery using self-cross-linked glycol chitosan
nanoparticles to overcome drug resistance. J Control Release.
198:1–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo P, You JO, Yang J, Di J, Moses MA and
Auguste DT: Inhibiting metastatic breast cancer cell migration via
the synergy of targeted, pH-triggered siRNA delivery and chemokine
axis blockade. Mol Pharm. 11:755–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reichmuth AM, Oberli MA, Jaklenec A,
Langer R and Blankschtein D: mRNA vaccine delivery using lipid
nanoparticles. Ther Deliv. 7:319–334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de la Harpe K, Kondiah P, Choonara Y,
Marimuthu T, du Toit L and Pillay V: The hemocompatibility of
nanoparticles: A review of cell-nanoparticle interactions and
hemostasis. Cells. 8:12092019. View Article : Google Scholar
|
|
22
|
Buzea C, Pacheco II and Robbie K:
Nanomaterials and nanoparticles: Sources and toxicity.
Biointerphases. 2:MR17–MR71. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Y, Gao X and Chen J:
Leukocyte-derived biomimetic nanoparticulate drug delivery systems
for cancer therapy. Acta Pharm Sin B. 8:4–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huynh A, Madu CO and Lu Y: siRNA: A
promising new tool for future breast cancer therapy. Oncomedicine.
3:74–81. 2018. View Article : Google Scholar
|
|
25
|
Singh A, Trivedi P and Jain NK: Advances
in siRNA delivery in cancer therapy. Artif Cells Nanomed
Biotechnol. 46:274–283. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hayward SL, Francis DM, Kholmatov P and
Kidambi S: Targeted delivery of MicroRNA125a-5p by engineered lipid
nanoparticles for the treatment of HER2 positive metastatic breast
cancer. J Biomed Nanotechnol. 12:554–568. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu J, Meng T, Ming Y, Wen L, Cheng B, Liu
N, Huang X, Hong Y, Yuan H and Hu F: MicroRNA-200c delivered by
solid lipid nanoparticles enhances the effect of paclitaxel on
breast cancer stem cell. Int J Nanomedicine. 11:6713–6725. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Yu B, Wu Y, Lee RJ and Lee LJ:
Efficient down-regulation of CDK4 by novel lipid
nanoparticle-mediated siRNA delivery. Anticancer Res. 31:1619–1626.
2011.PubMed/NCBI
|
|
29
|
Tang J, Howard CB, Mahler SM, Thurecht KJ,
Huang L and Xu ZP: Enhanced delivery of siRNA to triple negative
breast cancer cells in vitro and in vivo through functionalizing
lipid-coated calcium phosphate nanoparticles with dual target
ligands. Nanoscale. 10:4258–4266. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu YH, Kim E, Park DE, Shim G, Lee S, Kim
YB, Kim CW and Oh YK: Cationic solid lipid nanoparticles for
co-delivery of paclitaxel and siRNA. Eur J Pharm Biopharm.
80:268–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng ZM, Tang S and Tao M: Development of
resistance to RNAi in mammalian cells. Ann NY Acad Sci.
1058:105–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang W, Lee DY and Ben-David Y: The roles
of microRNAs in tumorigenesis and angiogenesis. Int J Physiol
Pathophysiol Pharmacol. 3:140–155. 2011.PubMed/NCBI
|
|
34
|
Kwon T, Chandimali N, Huynh DL, Zhang JJ,
Kim N, Bak Y, Yoon DY, Yu DY, Lee JC, Gera M, et al: BRM270
inhibits cancer stem cell maintenance via microRNA regulation in
chemoresistant A549 lung adenocarcinoma cells. Cell Death Dis.
9:2442018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao M, Ang L, Huang J and Wang J:
MicroRNAs regulate the epithelial-mesenchymal transition and
influence breast cancer invasion and metastasis. Tumour Biol.
39:1010428317691682. 2017. View Article : Google Scholar
|
|
36
|
Barbarotto E and Calin GA: MicroRNAs and
drug resistance. Drug Resistance Cancer Cells. 102:257–270
|
|
37
|
Kim J, Yao F, Xiao Z, Sun Y and Ma L:
MicroRNAs and metastasis: Small RNAs play big roles. Cancer
Metastasis Rev. 37:5–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McGuire A, Brown JA and Kerin MJ:
Metastatic breast cancer: The potential of miRNA for diagnosis and
treatment monitoring. Cancer Metastasis Rev. 34:145–155. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Volinia S, Galasso M, Sana ME, Wise TF,
Palatini J, Huebner K and Croce CM: Breast cancer signatures for
invasiveness and prognosis defined by deep sequencing of microRNA.
Proc Natl Acad Sci USA. 109:3024–3029. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Ann Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Klinge CM: Non-coding RNAs in breast
cancer: Intracellular and intercellular communication. Noncoding
RNA. 4:402018.
|
|
42
|
Kanasty R, Dorkin JR, Vegas A and Anderson
D: Delivery materials for siRNA therapeutics. Nat Materials.
12:967–977. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Majumder S and Jacob ST: Emerging role of
microRNAs in drug-resistant breast cancer. Gene Expr. 15:141–151.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Papadaki C, Stratigos M, Markakis G,
Spiliotaki M, Mastrostamatis G, Nikolaou C, Mavroudis D and Agelaki
S: Circulating microRNAs in the early prediction of disease
recurrence in primary breast cancer. Breast Cancer Res. 20:722018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Quan Y, Huang X and Quan X: Expression of
miRNA-206 and miRNA-145 in breast cancer and correlation with
prognosis. Oncol Lett. 16:6638–6642. 2018.PubMed/NCBI
|
|
46
|
Wang H, Peng R, Wang J, Qin Z and Xue L:
Circulating microRNAs as potential cancer biomarkers: The advantage
and disadvantage. Clinical Epigenetics. 10:592018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Abdelrahim M, Safe S, Baker C and
Abudayyeh A: RNAi and cancer: Implications and applications. J RNAi
Gene Silencing. 2:136–145. 2006.PubMed/NCBI
|
|
48
|
Ewert KK, Zidovska A, Ahmad A, Bouxsein
NF, Evans HM, McAllister CS, Samuel CE and Safinya CR: Cationic
liposome-nucleic acid complexes for gene delivery and silencing:
Pathways and mechanisms for plasmid DNA and siRNA. Top Curr Chem.
296:191–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lin Q, Chen J, Zhang Z and Zheng G:
Lipid-based nanoparticles in the systemic delivery of siRNA.
Nanomedicine. 9:105–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
van Zandwijk N, Pavlakis N, Kao SC, Linton
A, Boyer MJ, Clarke S, Huynh Y, Chrzanowska A, Fulham MJ, Bailey
DL, et al: Safety and activity of microRNA-loaded minicells in
patients with recurrent malignant pleural mesothelioma: A
first-in-man, phase 1, open-label, dose-escalation study. Lancet
Oncol. 18:1386–1396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hong DS, Kang YK, Borad M, Sachdev J,
Ejadi S, Lim HY, Brenner AJ, Park K, Lee JL, Kim TY, et al: Phase 1
study of MRX34, a liposomal miR-34a mimic, in patients with
advanced solid tumours. Br J Cancer. 122:1630–1637. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Anselmo AC and Mitragotri S: Nanoparticles
in the clinic: An update. Bioeng Transl Med. 4:e101432019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Qattan A: Gene Silencing Agents in Breast
Cancer. Modulating Gene Expression-Abridging the RNAi and
CRISPR-Cas9 Technologies. Singh A W..Khan M: Intech Open; 2019,
View Article : Google Scholar
|
|
54
|
Stratton MR, Campbell PJ and Futreal PA:
The cancer genome. Nature. 458:719–724. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pedroso de Lima MC, Simões S, Pires P,
Faneca H and Düzgüneş N: Cationic lipid-DNA complexes in gene
delivery: From biophysics to biological applications. Adv Drug
Deliv Rev. 47:277–294. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Meraz IM, Savage DJ, Segura-Ibarra V, Li
J, Rhudy J, Gu J and Serda RE: Adjuvant cationic liposomes
presenting MPL and IL-12 induce cell death, suppress tumor growth,
and alter the cellular phenotype of tumors in a murine model of
breast cancer. Mol Pharm. 11:3484–3491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yu S, Bi X, Yang L, Wu S, Yu Y, Jiang B,
Zhang A, Lan K and Duan S: Co-delivery of paclitaxel and
PLK1-targeted siRNA using aptamer-functionalized cationic liposome
for synergistic anti-breast cancer effects in vivo. J Biomed
Nanotechnol. 15:1135–1148. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang W, Shao A, Zhang N, Fang J, Ruan JJ
and Ruan BH: Cationic Polymethacrylate-modified liposomes
significantly enhanced doxorubicin delivery and antitumor activity.
Sci Rep. 7:430362017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sun Y, Zhao Y, Zhao X, Lee RJ, Teng L and
Zhou C: Enhancing the therapeutic delivery of oligonucleotides by
chemical modification and nanoparticle encapsulation. Molecules.
22:17242017. View Article : Google Scholar
|
|
60
|
Angelini G, Pisani M, Mobbili G, Marini M
and Gasbarri C: Neutral liposomes containing crown ether-lipids as
potential DNA vectors. Biochim Biophys Acta. 1828:2506–2512. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yan Y, Li XQ, Duan JL, Bao CJ, Cui YN, Su
ZB, Xu JR, Luo Q, Chen M, Xie Y and Lu WL: Nanosized functional
miRNA liposomes and application in the treatment of TNBC by
silencing Slug gene. Int J Nanomedicine. 14:3645–3667. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lu YC, Ou-Yang FU, Hsieh CM, Chang KJ,
Chen DR, Tu CW, Wang HC and Hou MF: Pegylated liposomal doxorubicin
as adjuvant therapy for stage I–III operable breast cancer. In
Vivo. 30:159–163. 2016.PubMed/NCBI
|
|
63
|
Xu D, Tian W and Shen H: Curcumin prevents
induced drug resistance: A novel function? Chin J Cancer Res.
23:218–223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tang X, Bi H, Feng J and Cao J: Effect of
curcumin on multidrug resistance in resistant human gastric
carcinoma cell line SGC7901/VCR. Acta Pharmacologica Sinica.
26:1009–1016. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Thorn CF, Oshiro C, Marsh S,
Hernandez-Boussard T, McLeod H, Klein TE and Altman RB: Doxorubicin
pathways: Pharmacodynamics and adverse effects. Pharmacogenet
Genomics. 21:440–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhou S, Li J, Xu H, Zhang S, Chen X, Chen
W, Yang S, Zhong S, Zhao J and Tang J: Liposomal curcumin alters
chemosensitivity of breast cancer cells to Adriamycin via
regulating microRNA expression. Gene. 622:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Golkar N, Samani SM and Tamaddon AM: Data
on cell growth inhibition induced by anti-VEGF siRNA delivered by
Stealth liposomes incorporating G2 PAMAM-cholesterol versus
Metafectene® as a function of exposure time and siRNA
concentration. Data Brief. 8:1018–1023. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen J, Sun X, Shao R, Xu Y, Gao J and
Liang W: VEGF siRNA delivered by polycation liposome-encapsulated
calcium phosphate nanoparticles for tumor angiogenesis inhibition
in breast cancer. Int J Nanomedicine. 12:6075–6088. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Leung AK, Tam YY and Cullis PR: Lipid
Nanoparticles for short interfering RNA delivery. Adv Genet.
88:71–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fernandez-Piñeiro I, Badiola I and Sanchez
A: Nanocarriers for microRNA delivery in cancer medicine.
Biotechnol Adv. 35:350–360. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pandey H, Rani R and Agarwal V: Liposome
and their applications in cancer therapy. Brazilian Arch Biol
Technol. 592016.http://dx.doi.org/10.1590/1678-4324-2016150477.
|
|
72
|
Liu HM, Zhang YF, Xie YD, Cai YF, Li BY,
Li W, Zeng LY, Li YL and Yu RT: Hypoxia-responsive ionizable
liposome delivery siRNA for glioma therapy. Int J Nanomedicine.
12:1065–1083. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Modi S, Xiang TX and Anderson BD: Enhanced
active liposomal loading of a poorly soluble ionizable drug using
supersaturated drug solutions. J Control Release. 162:330–339.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fenske DB, Chonn A and Cullis PR:
Liposomal nanomedicines: An emerging field. Toxicol Pathol.
36:21–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Johnston MJ, Semple SC, Klimuk SK, Edwards
K, Eisenhardt ML, Leng EC, Karlsson G, Yanko D and Cullis PR:
Therapeutically optimized rates of drug release can be achieved by
varying the drug-to-lipid ratio in liposomal vincristine
formulations. Biochim Biophys Acta. 1758:55–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lujan H, Griffin WC, Taube JH and Sayes
CM: Synthesis and characterization of nanometer-sized liposomes for
encapsulation and microRNA transfer to breast cancer cells. Int J
Nanomedicine. 14:5159–5173. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
de Antonellis P, Liguori L, Falanga A,
Carotenuto M, Ferrucci V, Andolfo I, Marinaro F, Scognamiglio I,
Virgilio A, De Rosa G, et al: MicroRNA 199b-5p delivery through
stable nucleic acid lipid particles (SNALPs) in tumorigenic cell
lines. Naunyn Schmiedebergs Arch Pharmacol. 386:287–302. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Samanta S, Rajasingh S, Drosos N, Zhou Z,
Dawn B and Rajasingh J: Exosomes: New molecular targets of
diseases. Acta pharmacologica Sinica. 39:501–513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bhome R, Del Vecchio F, Lee GH, Bullock
MD, Primrose JN, Sayan AE and Mirnezami AH: Exosomal microRNAs
(exomiRs): Small molecules with a big role in cancer. Cancer Lett.
420:228–235. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang J, Li S, Li L, Li M, Guo C, Yao J
and Mi S: Exosome and exosomal microRNA: Trafficking, sorting, and
function. Genomics Proteomics Bioinformatics. 13:17–24. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Xin Y, Wang X, Meng K, Ni C, Lv Z and Guan
D: Identification of exosomal miR-455-5p and miR-1255a as
therapeutic targets for breast cancer. Biosci Rep.
40:BSR201903032020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Harris DA, Patel SH, Gucek M, Hendrix A,
Westbroek W and Taraska JW: Exosomes released from breast cancer
carcinomas stimulate cell movement. PLoS One. 10:e01174952015.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Lin R, Wang S and Zhao RC: Exosomes from
human adipose-derived mesenchymal stem cells promote migration
through Wnt signaling pathway in a breast cancer cell model. Mol
Cell Biochem. 383:13–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wu CY, Du SL, Zhang J, Liang AL and Liu
YJ: Exosomes and breast cancer: A comprehensive review of novel
therapeutic strategies from diagnosis to treatment. Cancer Gene
Ther. 24:6–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Taylor DD and Gercel-Taylor C: MicroRNA
signatures of tumor-derived exosomes as diagnostic biomarkers of
ovarian cancer. Gynecol Oncol. 110:13–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Silva J, Garcia V, Zaballos A, Provencio
M, Lombardía L, Almonacid L, García JM, Domínguez G, Peña C, Diaz
R, et al: Vesicle-related microRNAs in plasma of nonsmall cell lung
cancer patients and correlation with survival. Eur Respir J.
37:617–623. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Rabinowits G, Gerçel-Taylor C, Day JM,
Taylor DD and Kloecker GH: Exosomal MicroRNA: A diagnostic marker
for lung cancer. Clinical Lung Cancer. 10:42–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wu H, Wang Q, Zhong H, Li L, Zhang Q,
Huang Q and Yu Z: Differentially expressed microRNAs in exosomes of
patients with breast cancer revealed by next-generation sequencing.
Oncol Rep. 43:240–250. 2019.PubMed/NCBI
|
|
89
|
Allen TM, Hansen C, Martin F, Redemann C
and Yau-Young A: Liposomes containing synthetic lipid derivatives
of poly(ethylene glycol) show prolonged circulation half-lives in
vivo. Biochim Biophys Acta. 1066:29–36. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Klibanov AL, Maruyama K, Torchilin VP and
Huang L: Amphipathic polyethyleneglycols effectively prolong the
circulation time of liposomes. FEBS Lett. 268:235–237. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Senior J, Delgado C, Fisher D, Tilcock C
and Gregoriadis G: Influence of surface hydrophilicity of liposomes
on their interaction with plasma protein and clearance from the
circulation: Studies with poly(ethylene glycol)-coated vesicles.
Biochim Biophys Acta. 1062:77–82. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Immordino ML, Dosio F and Cattel L:
Stealth liposomes: Review of the basic science, rationale, and
clinical applications, existing and potential. Int J Nanomedicine.
1:297–315. 2006.PubMed/NCBI
|
|
93
|
Caliceti P: Pharmacokinetic and
biodistribution properties of poly(ethylene glycol)-protein
conjugates. Adv Drug Delivery Rev. 55:1261–1277. 2003. View Article : Google Scholar
|
|
94
|
Barenholz Y: Doxil®−The first
FDA-approved Nano-drug: Lessons learned. J Control Release.
160:117–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Vakhshiteh F, Khabazian E, Atyabi F, Ostad
SN, Madjd Z and Dinarvand R: Peptide-conjugated liposomes for
targeted miR-34a delivery to suppress breast cancer and cancer
stem-like population. J Drug Deliv Sci Technol. 57:1016872020.
View Article : Google Scholar
|
|
96
|
Amstad E, Kohlbrecher J, Müller E,
Schweizer T, Textor M and Reimhult E: Triggered release from
liposomes through magnetic actuation of iron oxide nanoparticle
containing membranes. Nano Lett. 11:1664–1670. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Nahire R, Hossain R, Patel R, Paul S,
Meghnani V, Ambre AH, Gange KN, Katti KS, Leclerc E, Srivastava DK,
et al: pH-Triggered echogenicity and contents release from
liposomes. Mol Pharm. 11:4059–4068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Khan DR, Webb MN, Cadotte TH and Gavette
MN: Use of targeted liposome-based chemotherapeutics to treat
breast cancer. Breast Cancer (Auckl). 9 (Suppl 2):S1–S5. 2015.
|
|
99
|
Ta T and Porter TM: Thermosensitive
liposomes for localized delivery and triggered release of
chemotherapy. J Control Release. 169:112–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Elegbede AI, Banerjee J, Hanson AJ,
Tobwala S, Ganguli B, Wang R, Lu X, Srivastava DK and Mallik S:
Mechanistic studies of the triggered release of liposomal contents
by matrix metalloproteinase-9. J Am Chem Soc. 130:10633–10642.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Brown S and Khan DR: The treatment of
breast cancer using liposome technology. J Drug Deliv.
2012:2129652012. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Sneider A, Jadia R, Piel B, VanDyke D,
Tsiros C and Rai P: Engineering remotely triggered liposomes to
target triple negative breast cancer. Oncomedicine. 2:1–13. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yang G, Liu Z, Li Y, Hou Y, Fei X, Su C,
Wang S, Zhuang Z and Guo Z: Facile synthesis of black phosphorus-Au
nanocomposites for enhanced photothermal cancer therapy and
surface-enhanced Raman scattering analysis. Biomater Sci.
5:2048–2055. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Qiu M, Ren WX, Jeong T, Won M, Park GY,
Sang DK, Liu LP, Zhang H and Kim JS: Omnipotent phosphorene: A
next-generation, two-dimensional nanoplatform for multidisciplinary
biomedical applications. Chem Soc Rev. 47:5588–5601. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Sun S, Xu Y, Fu P, Chen M, Sun S, Zhao R,
Wang J, Liang X and Wang S: Ultrasound-targeted photodynamic and
gene dual therapy for effectively inhibiting triple negative breast
cancer by cationic porphyrin lipid microbubbles loaded with
HIF1α-siRNA. Nanoscale. 10:19945–19956. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Tang J, Li B, Howard CB, Mahler SM,
Thurecht KJ, Wu Y, Huang L and Xu ZP: Multifunctional lipid-coated
calcium phosphate nanoplatforms for complete inhibition of large
triple negative breast cancer via targeted combined therapy.
Biomaterials. 216:1192322019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ghasemiyeh P and Mohammadi-Samani S: Solid
lipid nanoparticles and nanostructured lipid carriers as novel drug
delivery systems: Applications, advantages and disadvantages. Res
Pharma Sci. 13:288–303. 2018. View Article : Google Scholar
|















