Introduction
Gliomas are the most frequent primary tumours of the
central nervous system and a major cause of death among patients
with intracranial tumours (1).
Aggressive interventions, including surgery, chemotherapy and
radiotherapy, are not always curative, particularly for
glioblastoma (GBM) (2). In recent
years, increasing attention has been focused on the molecular
genetics of GBM in order to improve the prognosis and treatment of
patients with GBM (3). However, no
definite conclusions have been reached regarding the mechanisms
underlying GBM tumourigenesis. Therefore, it is urgent to identify
new molecular biomarkers that regulate the malignant biological
behaviour of GBM.
MicroRNAs (miRNAs) constitute a class of
single-stranded non-coding RNAs that play an important role in the
translation of tumour genes and the regulation of downstream
proteins. Accumulating evidence indicates that miR-138 may act as a
tumour suppressor via its interaction with critical signalling
pathways in tumourigenesis, such as the hypoxia-inducible
factor-1α, extracellular-signal regulated protein kinase and
nuclear factor-κB pathways (4,5).
Specifically, aberrantly low expression levels of miR-138 have been
reported in several malignant tumours, such as gallbladder
carcinoma, anaplastic thyroid carcinoma (ATC), non-small cell lung
cancer (NSCLC) and oral squamous cell carcinoma (OSCC) (6–9).
Recent studies have demonstrated that the upregulation of miR-138
in tumour cells may cause reversion of the malignant phenotype
(10,11). Moreover, Stojcheva et al
observed that miR-138 promoted acquired alkylator resistance in GBM
by targeting BIM, highlighting the importance of this miRNA in GBM
(12). However, the molecular
mechanisms underlying the function of miR-138 in glioma remain
unclear.
The cAMP response element-binding protein 1 (CREB1)
is a multifunctional molecule that mediates transcriptional
responses to various growth factors and stress signals involved in
tumour progression (13).
Typically, CREB1 is overexpressed in a series of human neoplasms,
and high expression of CREB1 in several cancers, such as
astrocytoma, hepatocellular carcinoma and NSCLC, has been
associated with an unfavourable overall survival (OS) outcome
(14–16). Furthermore, Rodon et al
revealed that CREB1 may promote a malignant transforming growth
factor-β2 autocrine loop in GBM (17). However, current research cannot
fully explain the molecular mechanism of action of CREB1 in GBM,
and the presence of an interactive network mainly involving CREB1
has not been established. Therefore, the role of CREB1 in GBM cell
proliferation, apoptosis and invasion and the underlying regulatory
mechanism require further investigation.
The aim of the present study was to investigate
miR-138 expression in glioma specimens and evaluate miR-138 as a
candidate biomarker. In addition, the expression of miR-138 was
upregulated in U87 and U251 cells to observe the effect of miR-138
on cell proliferation, invasion and apoptosis. Furthermore, it was
investigated whether CREB1 is a direct target miR-138 in order to
determine whether the miR-138/CREB1/mTOR pathway may play a
critical role in the tumourigenic behaviour of glioma cells.
Materials and methods
Cell culture
The 293 cell line and the malignant glioma cell
lines U87 (glioblastoma of unknown origin, ATCC HTB-14™) and U251
were obtained from the Chinese Academy of Sciences (Shanghai,
China). All glioma cell lines were grown in DMEM supplemented with
10% FBS in a humidified atmosphere containing 5% CO2 at
37°C.
Tissue samples
The study protocol complied with the National
Regulations on the Use of Clinical Samples in China. The Specialty
Committee on Ethics of Biomedicine Research, Navy Medical
University, approved the use of human specimens in this study
(PJ2011-012-03). Written informed consent was obtained from all
participants prior to the present study. The glioma cancer
specimens were obtained from patients who underwent surgery at
Changzheng Hospital and Shanghai Tongji Hospital between July 2012
and July 2018.
Cell viability assay
In the present study, MTT was used to monitor cell
proliferation. An MTT assay was conducted as previously described
to measure cell viability (18).
Cell viability was analysed with a Cell Counting Kit (cat. no.
11465007001, Roche Diagnostics GmbH). The optical density at 570 nm
was measured 24, 48, 72, 96 and 120 h after transfection.
Cell transfections
The miR-138 mimics and miRNA-negative control (NC)
mimics were obtained from Qiagen GmbH. U87 and U251 cells were
transfected with miR-138 mimics or miRNA-NC by Lipofectamine™ 2000
(Thermo Fisher Scientific, Inc.). The target sequences of miR-138
mimics were as previously described (19). The scrambled sequence
(5′-UUCUCCGAACGUGUCACGUTT-3′) was used to create non-targeting
miR-NC and GFP-siRNA. CREB1 siRNA (sc-35111) and siRNA Reagent
System (sc-45064) were purchased from Santa Cruz Biotechnology,
Inc. and all transfections were conducted according to the
manufacturer's instructions. CREB1 overexpression vectors were
purchased from GenePharma and successfully transfected into the
corresponding cells according to the manufacturer's instructions in
the presence of Lipofectamine™ 2000 (Thermo Fisher Scientific,
Inc.). A full-length human CREB1 complementary DNA containing the
entire coding sequence tagged with GFP or GFP alone (Lenti-GFP) was
cloned into the lentiviral vector pLenti6/V5-DEST (Invitrogen;
Thermo Fisher Scientific, Inc.) to create the complete functional
overexpression vector Lenti-CREB1. The efficacy of the transfection
was tested by using western blotting.
Invasion assay
The invasion assay was conducted as previously
described (20). Equal numbers
(1×105) of cells stably transfected with miR-NC, miR-138
mimics, GFP-siRNA or CREB1-siRNA were plated in separate 24-well
Matrigel-coated cell culture inserts with an 8-µm pore size. The
invasion rate was measured in three independent experiments.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from glioma tissues and
cells lines by using TRIzol® reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. cDNA
was synthesized using miRNA RT assay (TaqMan; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol, and
miRNA expression levels were analysed using the 7900 fast RT-PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) using
miR-138-specific primers. The 25-µl reaction mixture contained 14
µl 2X SYBR Green Master Mix, 1 µl forward primer (10 µM), 1 µl
reverse primer (10 µM), 3 µl cDNA template and 6 µl
ddH2O was set up. The thermocycling parameters were as
follows: 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec,
60°C for 1 min, and a detection step at 72°C for 30 sec. The
sequences for the primers of miR-138 and U6 were as previously
described (21) and were as
follows: miR-138, forward, 5′-CCCAGGGTCTGGTGCGGAGA-3′ and reverse,
5′-CAGGGGCTGAGCGGTGAGGG-3′; and U6, forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. U6
probe was used as an endogenous control. Relative fold expression
of the target genes was calculated according to the
2−∆∆Cq method (22).
Apoptosis assay
Apoptosis was assessed by dual staining using
Annexin V-FITC and propidium iodide (Invitrogen; Thermo Fisher
Scientific, Inc.). All cells were analysed by a FACSCalibur System
(BD Biosciences). The apoptosis assays were performed using U87 and
U251 cell lines with or without miR-138 overexpression. The
analyses were performed using FlowJo software (v10.6.2; FlowJo
LLC).
Dual luciferase reporter assay
A total of 100 ng CREB1 wild-type (WT)
3′-untranslated region (UTR) or CREB1 mutant (MT) 3′-UTR luciferase
reporter plasmids were co-transfected into 293 cells. At 48 h after
transfection, the luciferase activity in the cells was measured
with a Dual Luciferase Reporter Assay System (Promega Corporation).
The transfections were performed three times.
Statistical analysis
All data are expressed as the mean ± SD values of
data from triplicate experiments and paired groups were compared by
Student's t-test, whereas three or more groups were compared by
ANOVA followed by Tukey's test for multiple pairwise comparisons.
The Kruskal-Wallis with Dunn multiple comparisons test was used to
compare the immunolabeling results between the glioma tissue
grades. The correlation between miR-138 and CREB1 expression was
examined by Spearman's correlation coefficient. OS was investigated
by conducting a Kaplan-Meier analysis, and the predictors of
survival were analysed by a Cox proportional hazards regression
analysis. Statistical analysis was performed with SPSS 13.0
software (SPSS Inc.) and P<0.05 was considered to indicate
statistically significant differences.
Results
miR-138 is frequently downregulated in
clinical glioma tissue samples
RT-qPCR was first used to measure miR-138 expression
in 48 human glioma specimens and 12 normal brain tissue specimens.
miR-138 expression in the glioma specimens was significantly lower
compared with that in the normal brain specimens (Fig. 1A). In addition, high-grade glioma
specimens (WHO grades III–IV) exhibited a lower expression of
miR-138 compared with the low-grade glioma specimens (WHO grades
I–II; Fig. 1B). Subsequently, the
association between miR-138 expression and clinicopathological
characteristics was investigated. According to the miR-138
expression level in the specimens, the 48 patients with glioma were
divided into the low expression (<4.12, n=22) and high
expression (≥4.12, n=26) groups, and a statistical analysis was
conducted (Table I). The expression
of miR-138 was found to be significantly associated with
histological grade (P=0.02). Furthermore, it was demonstrated that
patients with high expression levels of miR-138 had significantly
longer OS compared with those with low miR-138 expression levels by
a Kaplan-Meier survival analysis (Fig.
1C). Collectively, these results indicate that miR-138
expression may be a key prognostic index of glioma patient
survival.
 | Table I.Association between miR-138
expression and clinicopathological variables of glioma
patients. |
Table I.
Association between miR-138
expression and clinicopathological variables of glioma
patients.
|
|
| miR-138
expression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Value | High | Low | P-value |
|---|
| No. of
patients | 48 | 25 | 23 |
|
| Age, years |
|
|
| 0.28 |
|
<50 | 26 | 15 | 11 |
|
|
≥50 | 22 | 10 | 12 |
|
| Sex |
|
|
| 0.35 |
|
Male | 28 | 13 | 15 |
|
|
Female | 20 | 12 | 8 |
|
| Mean tumour
diameter, cm |
|
|
| 0.29 |
|
<3 | 29 | 14 | 15 |
|
| ≥3 | 19 | 11 | 8 |
|
| Predominant
location |
|
|
| 0.17 |
| Frontal
lobe | 12 | 8 | 4 |
|
|
Temporal lobe | 16 | 9 | 7 |
|
|
Parietal lobe | 11 | 5 | 6 |
|
|
Cerebellum | 9 | 3 | 6 |
|
| Stage |
|
|
| 0.013 |
|
I/II | 19 | 14 | 5 |
|
|
III/IV | 29 | 11 | 18 |
|
miR-138 regulates cell viability,
invasion and apoptosis in gliomas
Subsequently, RT-qPCR was used to measure the
miR-138 expression level in four glioma cell lines (U87, U251,
A172, SW1088 and U133) (Fig. 2A).
The U87 and U251 cells exhibited significantly higher miR-138
expression compared with the A172 and U133 cells (Fig. 2A). Therefore, the U87 and U251 cells
were selected for the subsequent in vitro experiments. The
U87 and U251 cells transfected with miR-138 mimics exhibited a
significant increase in miR-138 expression (Fig. 2B). Then, an MTT assay was used to
assess the effect of miR-138 expression on U87 and U251 cell
viability. Compared with the miR-NC cells, the transfection of the
U87 and U251 cells with the miR-138 mimics resulted in a
significant reduction in cell viability (Fig. 2C). The upregulation of miR-138 in
the U87 and U251 cells was correlated with enhanced apoptosis
(Fig. 2D). Furthermore, compared
with the miR-NC cells, the glioma cells transfected with the
miR-138 mimics exhibited significantly decreased invasion ability
(Fig. 2E). These data suggest that
miR-138 can increase the proliferation, promote invasion and
inhibit apoptosis of glioma cells.
The antitumour effect of miR-138 is
associated with the dephosphorylation of AKT/mTOR
To explore the underlying mechanism through which
miR-138 acts as a tumour suppressor, the expression levels of
signalling molecules involved in the AKT/mTOR pathway were
measured. A marked decrease in the levels of p-AKT and p-mTOR was
observed in the U87 and U251 cells transfected with the miR-138
mimics, while the levels of total AKT and total mTOR did not differ
between the mimic-transfected and miR-NC-transfected groups
(Fig. 2F). In addition, using
RT-qPCR analysis, no change was observed in the expression levels
of miR-138 following treatment with the AKT inhibitor SH-5 inU87
and U251 cells (Fig. S1),
suggesting that miR-138 is an upstream regulator of the AKT/mTOR
pathway. Moreover, the levels of the anti-apoptotic protein Bcl-2
and the invasion-associated protein MMP-2 were decreased in the
miR-138-transfected U87 and U251 cells (Fig. 2F). These data suggest that the
dephosphorylation of AKT/mTOR may mediate the function of miR-138
in glioma cells. Activating miR-138 in glioma cells may promote the
dephosphorylation of AKT and mTOR, thereby enhancing apoptosis and
inhibiting invasion in these cells by decreasing the levels of
anti-apoptotic proteins and invasion-promoting factors.
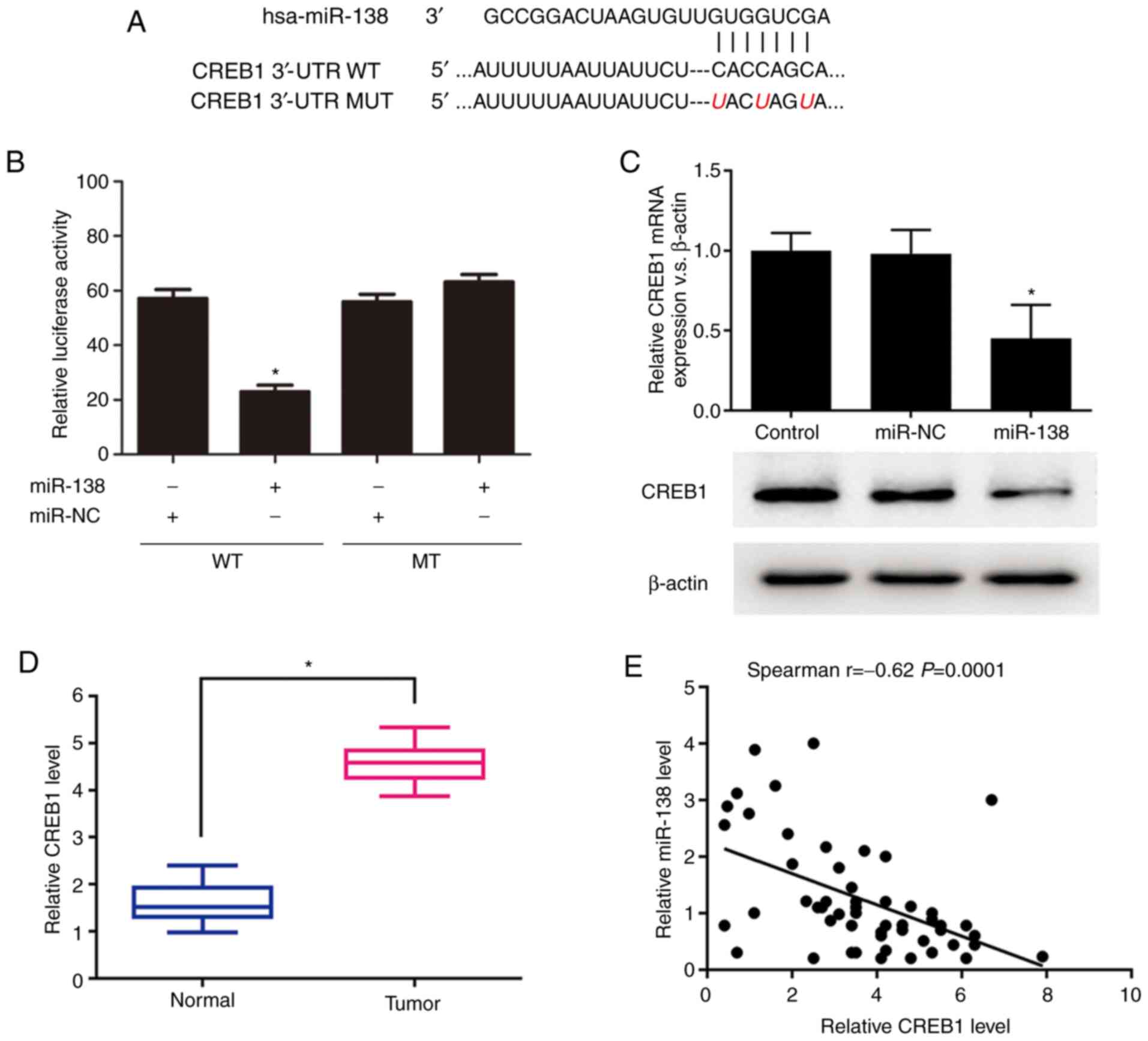
CREB1 is a novel target of
miR-138
As CREB1 was predicted by TargetScan (http://www.targetscan.org/vert_72/) to contain a
putative miR-138 target site in its 3′-UTR, it was hypothesized
that CREB1 is a potential target of miR-138. The WT or MUT target
site was inserted into identical luciferase reporter vectors
(Fig. 3A). The transfection of the
reporter vectors containing the putative target sequence resulted
in a significant decrease in the relative luciferase activity
compared to that in the 293 cells cotransfected with the MUT
sequence and miR-138 (Fig. 3B). In
addition, the miR-138 transfection significantly decreased both the
mRNA and protein expression levels of CREB1 (Fig. 3C). The expression levels of CREB1
and miR-138 were further explored in 48 glioma tissues to
investigate their clinical relevance in vivo. The RT-qPCR
results revealed that the mRNA expression level of CREB1 was
significantly increased in the glioma samples compared to that in
the normal brain samples (Fig. 3D).
Furthermore, the Spearman's correlation analysis of the mRNA
expression levels demonstrated that CREB1 expression was inversely
associated with miR-138 expression (r=−0.62, P=0.0001; Fig. 3E). Taken together, these results
suggest that CREB1 is a novel target of miR-138.
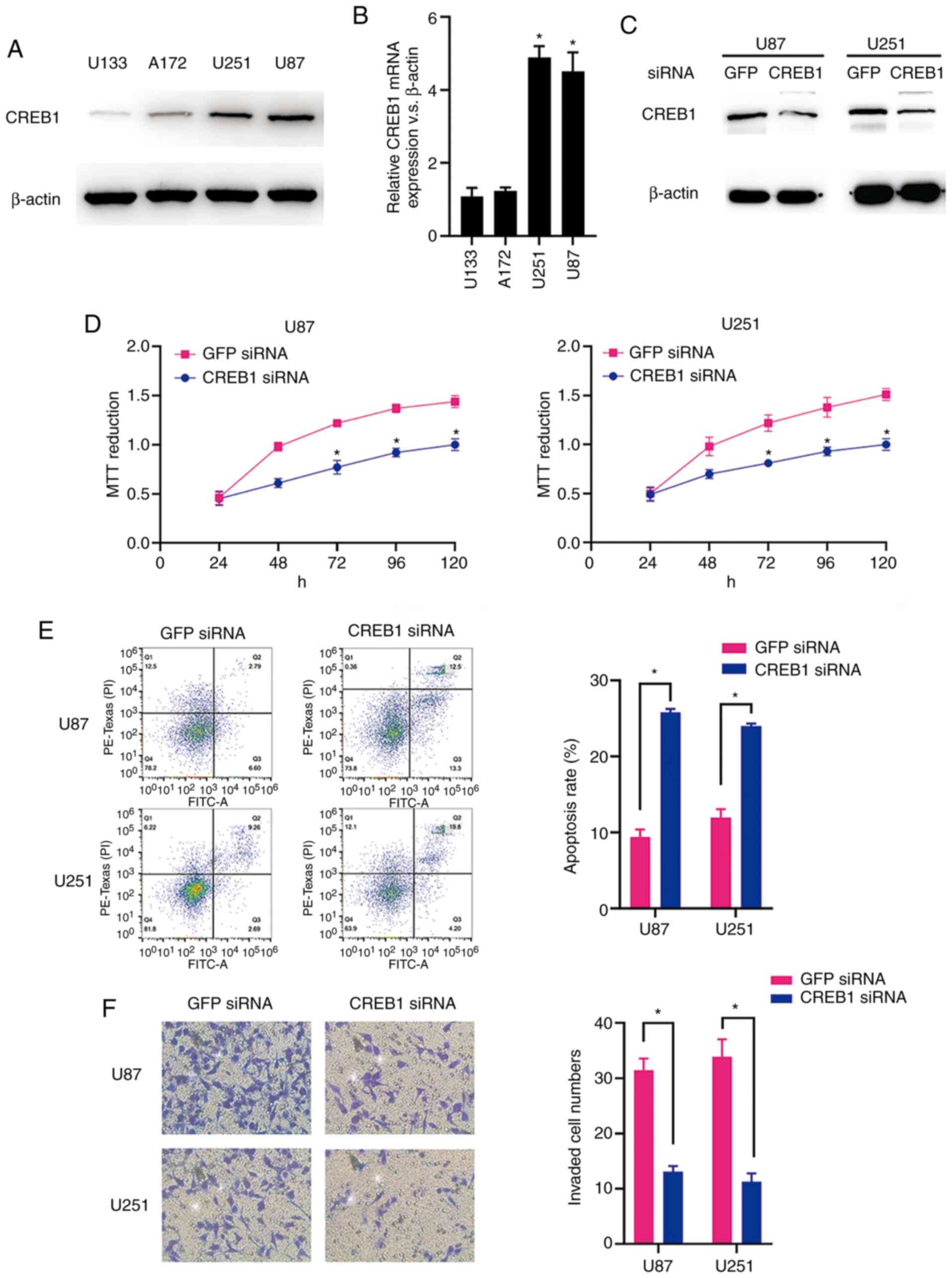
CREB1 functions as an oncogene in
glioma
The CREB1 mRNA and protein expression levels in the
glioma cell lines (U87, U251, A172 and U133) were measured by
RT-qPCR analysis and western blotting. As predicted, the U87 and
U251 cells exhibited significantly higher expression levels of
CREB1 mRNA and protein compared with the A172 and U133 cells
(Fig. 4A and B). The western blot
analysis results revealed that CREB1 protein expression was
significantly suppressed in both cell types following transfection
of siRNA targeting CREB1 (Fig. 4C).
The MTT assay results demonstrated that the downregulation of CREB1
significantly suppressed the viability of U87 and U251 cells
(Fig. 4D). In addition, the
downregulation of CREB1 promoted apoptosis of U87 and U251 cells,
as demonstrated by the flow cytometric analysis (Fig. 4E). Furthermore, compared to the
cells transfected with control siRNA, the knockdown of CREB1
significantly inhibited the invasion of U87 and U251 cells
transfected with CREB1 siRNA, as indicated by the Transwell assay
results (Fig. 4F). These data
suggest that CREB1 acts as a critical oncogene in glioma cells
in vitro.
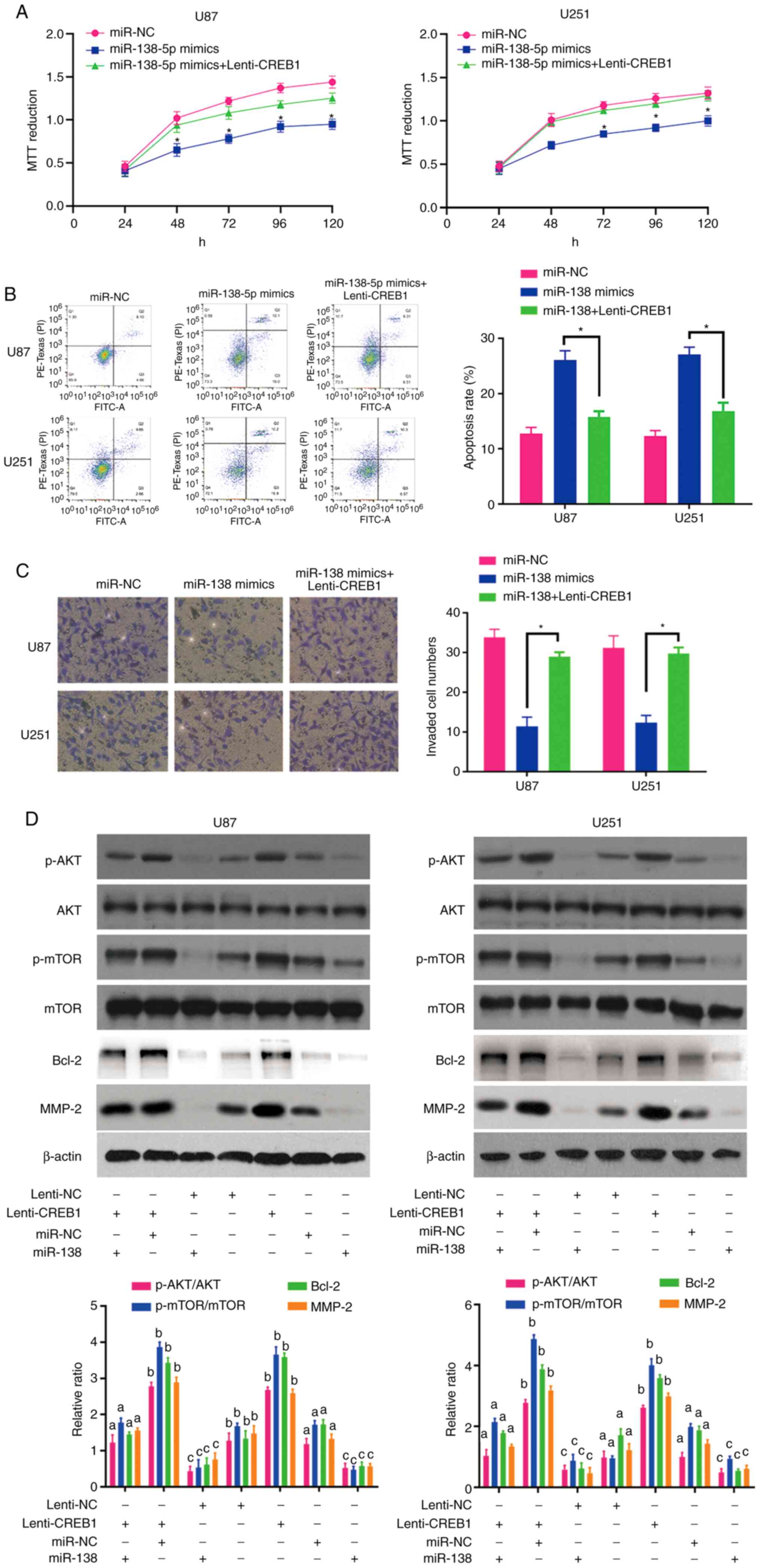
Rescue of CREB1 expression overcomes
the antitumour effects of miR-138 through the AKT/mTOR signalling
pathway
To confirm that CREB1 functions as a critical
downstream target of miR-138, rescue experiments were performed by
culturing miR-138 mimic-transfected U87 and U251 cells in the
presence of purified CREB1. The results of the MTT, apoptosis and
invasion assays revealed that the upregulation of CREB1 restored
the viability (Fig. 5A), apoptosis
(Fig. 5B) and invasion (Fig. 5C) of the miR-138 mimic-transfected
glioma cells, suggesting that CREB1 plays an important role in
mediating the antitumour effects of miR-138 in glioma cells.
The association between miR-138 and CREB1 in glioma
cells prompted us to explore the activation of the AKT/mTOR
signalling pathway and the expression of Bcl-2 and MMP-2 in
response to changes in CREB1 expression. After increasing the
expression of CREB1 in U87 and U251 cells via Lenti-CREB1, the
expression levels of MMP-2 were increased in these cells (Fig. 5D). In addition, CREB1 overexpression
activated the AKT/mTOR signalling pathway and enhanced Bcl-2
expression by increasing the levels of p-AKT and p-mTOR, without
altering the levels of total AKT and mTOR (Fig. 5D). In particular, the upregulation
of CREB1 drastically abrogated the decreases in AKT/mTOR activity
and Bcl-2 expression in cells overexpressing miR-138 (Fig. 5D). Furthermore, CREB1 transfection
rescued the inhibitory effect of miR-138 on MMP-2 expression in U87
and U251 cells (Fig. 5D). These
data indicate that miR-138 likely mediates apoptosis and invasion
of glioma cells by targeting CREB1, thus suppressing MMP-2
expression and AKT/mTOR signalling pathway activation.
Discussion
miRNAs have been reported to participate in several
malignant biological behaviours, including cell proliferation,
metabolism and differentiation, at the post-transcriptional level
(23,24). Mutations affecting miRNAs or their
functional interactions with oncogenes and tumour suppressors may
mediate tumourigenesis (25).
Recent studies have indicated that miR-138 may act as a tumour
suppressor in various cancers, such as ATC, NSCLC and OSCC
(7,9,10).
Notably, certain studies demonstrated that miR-138 inhibits GBM
cell proliferation by suppressing the EZH2-CDK4/6-pRb-E2F1
signalling loop and suppresses low-grade glioma development and
metastasis by regulating IGF2BP2 (19,26).
However, Chan et al demonstrated that miR-138 acts as an
oncogene in glioma stem cells (GSCs) (27). These contradictory results suggest
that the molecular function of miR-138 in glioma remains elusive. A
preliminary miRNA microarray analysis of 5 high-grade glioma
specimens and 5 normal brain tissue specimens was recently
conducted and revealed a markedly lower level of miR-138 expression
in high-grade glioma samples (data not shown). These results led to
the hypothesis that miR-138 may be used as a prognostic marker of
GBM. The present study demonstrated that miR-138 expression was
lower in the GBM samples and was inversely associated with advanced
pathological stage. These results are consistent with those
reported by previous studies, indicating that miR-138 may play an
important role in inhibiting the proliferation and malignant
behaviour of tumour cells. In addition, the statistical analysis
revealed that glioma patients with low expression levels of miR-138
had a markedly longer OS, indicating that miR-138 expression may
induce less aggressive tumour behaviour. Thus, the results of the
present study indicate that miR-138 expression is inversely
associated with pathological grade and that high miR-138 expression
is a prognostic factor for a more favourable outcome.
The cellular origin of gliomas remains a
controversial topic in cancer research. Recent studies involving
animal models indicate that the two different lineages of
tumour-initiating cells may contribute to the different biological
and genomic phenotypes of GBM (28). Notably, certain miRNAs, such as
miR-92a-3p, miR-221, miR-222 and miR-21, exert opposite effects on
glioma cells and GSCs (29,30). This obvious paradox may be
attributed to the fact that miRNAs may target several important
downstream signal molecules, some of which may have opposite
functions, which may partially explain why miR-138 could act as
either an onco-miR in GSCs (27) or
a tumour suppressor in glioma cells (19,26).
The present study confirmed that miR-138 exerts an antitumour
effect in glioma cells through the dephosphorylation of AKT/mTOR by
targeting CREB1, which should be further investigated in future
studies.
Recent studies have reported that miR-138 functions
as a tumour suppressor by targeting various downstream genes
related to cell growth, autophagy, invasion and apoptosis (5,6,10).
Moreover, other recent studies have suggested that miR-138 may play
an even broader role. Wang et al revealed that miR-138
participates in the process of DNA damage, which affects cell
sensitivity to DNA-damaging agents (21). In addition, miR-138 has been
confirmed to participate in crosstalk with other pathways (31,32).
Hrdlickova et al observed that miR-138 can inhibit TCF7,
MSI1 and PAX5, which are crucial target genes of the Wnt signalling
pathway involved in the regulation of cell proliferation (33). As miR-138 expression has been
demonstrated to be inversely correlated with aggressive behaviour
in other tumours, a similar correlation may be expected in GBM.
Consistent with this hypothesis, cultured U87 and U251 cells were
used to demonstrate the tumour-suppressive role of miR-138, which
attenuated the aggressive nature of GBM. Treatment with miR-138
resulted in significant decreases in the levels of p-AKT2 and
p-mTOR, without affecting the levels of total AKT2 and mTOR, which
is consistent with the results of a previous study suggesting that
miR-138 can decrease cell proliferation ability by attenuating the
phosphorylation of its target gene. In addition, the AKT inhibitor
SH-5 did not affect the expression levels of miR-138 in U87 and
U251 glioma cells. Given the importance of miR-138 in AKT/mTOR
signalling, it is reasonable to hypothesise that agents
specifically targeting miR-138 may be beneficial in GBM patients,
particularly those with GBMs exhibiting a low expression of
miR-138.
CREB1 produces oncogenic effects by enhancing
several signalling pathways in human cancer cells (13). Notably, a recent study revealed that
the knockdown of CREB1 decreased the phosphorylation of IRK1/2 and
AKT in U251 glioma cells (34). AKT
serves as a critical downstream effector of the PI3K pathway.
Approximately 88% of gliomas have somatic alterations in RTK/PI3K
pathway genes, highlighting the importance of AKT in glioma
(35). The findings of the present
study indicated that CREB1 acts as an oncogene in glioma cells and
that the overexpression of CREB1 can reverse the antitumour effects
of miR-138 treatment on glioma cells. To address the molecular
mechanisms underlying miR-138-mediated cell proliferation, CREB1,
AKT and mTOR expression was further explored and it was observed
that miR-138 can directly bind the 3′-UTR of CREB1 and inhibit the
phosphorylation of AKT and mTOR without affecting the total
expression levels of AKT and mTOR, forming a miR-138/CREB1/AKT/mTOR
interactive network that plays an important role in promoting
proliferation and suppressing apoptosis of GBM cells. The
aforementioned results are also consistent with those of a previous
study demonstrating that the C/EBPβ responsive element is essential
for miR-138 to participate in the development of gliomas (36). The present study strongly suggests
that the significant suppression of glioma cell proliferation
induced by miR-138 treatment may be attributed to an enhancement of
AKT/mTOR-induced apoptosis.
A hallmark of malignant glioma cells is their highly
invasive nature (37). The
disruption of the extracellular matrix (ECM) has been identified as
an initial step in the penetration of glioma cells into adjacent
normal brain tissue (38). Emerging
evidence indicates that MMP-2, which is an important member of the
MMP family, is required for the degradation of the physical ECM
barrier at tumour invasion fronts during this process (39,40).
Notably, the expression level of MMP-2 increases as the
pathological stage advances and is the highest in malignant gliomas
(39). In particular, MMP-2 is a
well-documented candidate predictive biomarker of glioma invasion
(41). These results indicate that
MMP-2 may be strongly associated with tumour invasion. Therefore,
the expression level of MMP-2 was measured after miR-138 treatment
to elucidate the mechanisms underlying GBM cell invasion. It was
demonstrated that the upregulation of miR-138 expression was
correlated with a decrease in MMP-2 expression, suggesting a
functional interaction between miR-138 and MMP-2.
In summary, the results of the present study
indicate that miR-138 mimic transfection into U87 and U251 cells
induced apoptosis via the AKT/mTOR signalling pathway and decreased
the invasiveness of GBM cells by decreasing MMP-2 expression.
However, the mechanisms through which miR-138 regulates GBM cell
apoptosis and invasion require further investigation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Science
Foundation of China (grant no. 81802489), the Shanghai Natural
Science Foundation (grant nos. 18ZR1434500, 19ZR1448900 and
18411962500), the Scientific Research Initial Funding of Shanghai
Tongji Hospital (grant no. RCQD1704), the Featured Clinical
Discipline Project of Shanghai Pudong (grant no. PWYst2018-01) and
the Key Discipline Group Construction Project of Shanghai Pudong
(grant no. PWZxq2017-02).
Availability of data and materials
The datasets generated and analysed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
CZ and QW were responsible for designing the study
and writing the manuscript. XZ, LZ and HC were responsible for the
data analysis. JQ and QB performed the experiments and revised the
manuscript. CL and GH contributed to the design and critically
revised the manuscript for important intellectual content YY and JG
were involved in acquisition and interpretation of RT-qPCR data.
All the authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Fearon C, Loftus T, Byrne AL, Heffernan J,
Cooney M, Heeney C, Walsh A, Lorigan J, Beausang A, Cryan J, et al:
Impact of the 2016 world health organization classification of
tumours of the central nervous system: An Irish experience. Ir J
Med Sci. 189:799–803. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegelin MD, Schneider E, Westhoff MA,
Wirtz CR and arpel-Massler G: Current state and future perspective
of drug repurposing in malignant glioma. Semin Cancer Biol. Nov
14–2019.(Epub ahead of print). doi:
10.1016/j.semcancer.2019.10.018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tamura R, Miyoshi H, Yoshida K, Okano H
and Toda M: Recent progress in the research of suicide gene therapy
for malignant glioma. Neurosurg Rev. Nov 28–2019.(Epub ahead of
print). doi: 10.1007/s10143-019-01203-3. View Article : Google Scholar
|
|
4
|
Song T, Zhang X, Wang C, Wu Y, Cai W, Gao
J and Hong B: MiR-138 suppresses expression of hypoxia-inducible
factor 1α (HIF-1α) in clear cell renal cell carcinoma 786-O cells.
Asian Pac J Cancer Prev. 12:1307–1311. 2011.PubMed/NCBI
|
|
5
|
Islam M, Datta J, Lang JC and Teknos TN:
Down regulation of RhoC by microRNA-138 results in de-activation of
FAK, Src and Erk1/2 signaling pathway in head and neck squamous
cell carcinoma. Oral Oncol. 50:448–456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu Z, Tang J, Wang J, Duan G, Zhou L and
Zhou X: MiR-138 acts as a tumor suppressor by targeting EZH2 and
enhances cisplatin-induced apoptosis in osteosarcoma cells. PLoS
One. 11:e01500262016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao L, Zhou H, Li XP, Chen J, Fang C, Mao
CX, Cui JJ, Zhang W, Zhou HH, Yin JY and Liu ZQ: MicroRNA-138 acts
as a tumor suppressor in non small cell lung cancer via targeting
YAP1. Oncotarget. 7:40038–40046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma F, Zhang M, Gong W, Weng M and Quan Z:
MiR-138 suppresses cell proliferation by targeting Bag-1 in
gallbladder carcinoma. PLoS One. 10:e01264992015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang B, Mu W, Wang J, Lu J, Jiang S, Li
L, Xu H and Tian H: MicroRNA-138 functions as a tumor suppressor in
osteosarcoma by targeting differentiated embryonic chondrocyte gene
2. J Exp Clin Cancer Res. 35:692016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu Y, Pan ZG, Shu L and Li QJ:
Podocalyxin-like, targeted by miR-138, promotes colorectal cancer
cell proliferation, migration, invasion and EMT. Eur Rev Med
Pharmacol Sci. 22:8664–8674. 2018.PubMed/NCBI
|
|
11
|
Nama S, Muhuri M, Di Pascale F, Quah S,
Aswad L, Fullwood M and Sampath P: MicroRNA-138 is a prognostic
biomarker for triple-negative breast cancer and promotes
tumorigenesis via TUSC2 repression. Sci Rep. 9:127182019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stojcheva N, Schechtmann G, Sass S, Roth
P, Florea AM, Stefanski A, Stühler K, Wolter M, Müller NS, Theis
FJ, et al: MicroRNA-138 promotes acquired alkylator resistance in
glioblastoma by targeting the Bcl-2-interacting mediator BIM.
Oncotarget. 7:12937–12950. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sakamoto KM and Frank DA: CREB in the
pathophysiology of cancer: Implications for targeting transcription
factors for cancer therapy. Clin Cancer Res. 15:2583–2587. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang JQ, Yao QH, Kuang YQ, Ma Y, Yang LB,
Huang HD, Cheng JM, Yang T, Liu EY, Liang L, et al: Prognostic
value of coexistence of abnormal expression of micro-RNA-200b and
cyclic adenosine monophosphate-responsive element-binding protein 1
in human astrocytoma. Hum Pathol. 45:2154–2161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu L, Guo X, Zhang P, Qi R, Li Z and Zhang
S: Cyclic adenosine monophosphate-responsive element-binding
protein activation predicts an unfavorable prognosis in patients
with hepatocellular carcinoma. Onco Targets Ther. 7:873–879.
2014.PubMed/NCBI
|
|
16
|
Seo HS, Liu DD, Bekele BN, Kim MK, Pisters
K, Lippman SM, Wistuba II and Koo JS: Cyclic AMP response
element-binding protein overexpression: A feature associated with
negative prognosis in never smokers with non-small cell lung
cancer. Cancer Res. 68:6065–6073. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rodon L, Gonzàlez-Juncà A, Inda Mdel M,
Sala-Hojman A, Martínez-Sáez E and Seoane J: Active CREB1 promotes
a malignant TGFβ2 autocrine loop in glioblastoma. Cancer Discov.
4:1230–1241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Q, Zhang L, Cui Y, Zhang C, Chen H,
Gu J, Qian J and Luo C: Increased RLIP76 expression in IDH1
wildtype glioblastoma multiforme is associated with worse
prognosis. Oncol Rep. 43:188–200. 2020.PubMed/NCBI
|
|
19
|
Qiu S, Huang D, Yin D, Li F, Li X, Kung HF
and Peng Y: Suppression of tumorigenicity by microRNA-138 through
inhibition of EZH2-CDK4/6-pRb-E2F1 signal loop in glioblastoma
multiforme. Biochim Biophys Acta. 1832:1697–1707. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Q, Qian J, Wang J, Luo C, Chen J, Hu
G and Lu Y: Knockdown of RLIP76 expression by RNA interference
inhibits invasion, induces cell cycle arrest, and increases
chemosensitivity to the anticancer drug temozolomide in glioma
cells. J Neurooncol. 112:73–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Huang JW, Li M, Cavenee WK,
Mitchell PS, Zhou X, Tewari M, Furnari FB and Taniguchi T:
MicroRNA-138 modulates DNA damage response by repressing histone
H2AX expression. Mol Cancer Res. 9:1100–1111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Syed SN and Brune B: MicroRNAs as emerging
regulators of signaling in the tumor microenvironment. Cancers
(Basel). 12:9112020. View Article : Google Scholar
|
|
24
|
SiamiGorji S, Jorjani I, Tahamtan A and
Moradi A: Effects of microRNAs polymorphism in cancer progression.
Med J Islam Repub Iran. 34:32020.PubMed/NCBI
|
|
25
|
Sandiford OA, Moore CA, Du J, Boulad M,
Gergues M, Eltouky H and Rameshwar P: Human aging and cancer: Role
of miRNA in tumor microenvironment. Adv Exp Med Biol. 1056:137–152.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Y, Liu X, Cheng L, Li L, Wei Z, Wang
Z, Han G, Wan X, Wang Z, Zhang J and Chen C: Tumor suppressor
microRNA-138 suppresses low-grade glioma development and metastasis
via regulating IGF2BP2. Onco Targets Ther. 13:2247–2260. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chan XH, Nama S, Gopal F, Rizk P, Ramasamy
S, Sundaram G, Ow GS, Ivshina AV, Tanavde V, Haybaeck J, et al:
Targeting glioma stem cells by functional inhibition of a
prosurvival oncomiR-138 in malignant gliomas. Cell Rep. 2:591–602.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alcantara Llaguno SR, Xie X and Parada LF:
Cell of origin and cancer stem cells in tumor suppressor mouse
models of glioblastoma. Cold Spring Harb Symp Quant Biol. 81:31–36.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aldaz B, Sagardoy A, Nogueira L, Guruceaga
E, Grande L, Huse JT, Aznar MA, Díez-Valle R, Tejada-Solís S,
Alonso MM, et al: Involvement of miRNAs in the differentiation of
human glioblastoma multiforme stem-like cells. PLoS One.
8:e770982013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song H, Zhang Y, Liu N, Zhao S, Kong Y and
Yuan L: MiR-92a-3p exerts various effects in glioma and glioma
stem-like cells specifically targeting CDH1/β-catenin and
Notch-1/Akt signaling pathways. Int J Mol Sci. 17:17992016.
View Article : Google Scholar
|
|
31
|
Sha HH, Wang DD, Chen D, Liu SW, Wang Z,
Yan DL, Dong SC and Feng JF: MiR-138: A promising therapeutic
target for cancer. Tumour Biol. 39:10104283176975752017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang H, Xiong Y, Wu Z, He Y, Gao X, Zhou
Z and Wang T: MIR-138-5P inhibits the progression of prostate
cancer by targeting FOXC1. Mol Genet Genomic Med. 8:e11932020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hrdlickova R, Nehyba J, Bargmann W and
Bose HR Jr: Multiple tumor suppressor microRNAs regulate telomerase
and TCF7, an important transcriptional regulator of the Wnt
pathway. PLoS One. 9:e869902014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng KB, Xie J, Li YT, Yuan Y, Wang Y, Li
C and Shi YF: Knockdown of CERB expression inhibits proliferation
and migration of glioma cells line U251. Bratisl Lek Listy.
120:309–315. 2019.PubMed/NCBI
|
|
35
|
Robison NJ and Kieran MW: Identification
of novel biologic targets in the treatment of newly diagnosed
diffuse intrinsic pontine glioma. Am Soc Clin Oncol Educ Book.
2012:625–628. 2012. View Article : Google Scholar
|
|
36
|
Di Pascale F, Nama S, Muhuri M, Quah S,
Ismail HM, Chan XH, Sundaram GM, Ramalingam R, Burke B and Sampath
P: C/EBPβ mediates RNA polymerase III-driven transcription of
oncomiR-138 in malignant gliomas. Nucleic Acids Res. 46:336–349.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vollmann-Zwerenz A, Leidgens V, Feliciello
G, Klein CA and Hau P: Tumor cell invasion in glioblastoma. Int J
Mol Sci. 21:19322020. View Article : Google Scholar
|
|
38
|
Ferrer VP, Moura Neto V and Mentlein R:
Glioma infiltration and extracellular matrix: Key players and
modulators. Glia. 66:1542–1565. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou W, Yu X, Sun S, Zhang X, Yang W,
Zhang J, Zhang X and Jiang Z: Increased expression of MMP-2 and
MMP-9 indicates poor prognosis in glioma recurrence. Biomed
Pharmacother. 118:1093692019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kast RE and Halatsch ME: Matrix
metalloproteinase-2 and −9 in glioblastoma: A trio of old
drugs-captopril, disulfiram and nelfinavir-are inhibitors with
potential as adjunctive treatments in glioblastoma. Arch Med Res.
43:243–247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chintala SK, Tonn JC and Rao JS: Matrix
metalloproteinases and their biological function in human gliomas.
Int J Dev Neurosci. 17:495–502. 1999. View Article : Google Scholar : PubMed/NCBI
|