Introduction
Colorectal cancer (CRC) is a common digestive tract
tumor. Despite advances in surgical resection combined with
chemotherapy and radiotherapy, the median survival rate of CRC
patients remains low and it is still a serious threat to human
health (1). Although multifactorial
etiology is linked with high susceptibility to CRC development,
including genetic factors and unhealthy dietary lifestyle (2), the exact molecular mechanisms
underlying human CRC development are far from clear.
Long non-coding RNAs (lncRNAs) are ncRNAs that play
important roles including regulation of gene expression and
tumorigenesis (3). Growth arrest
specific 5 (GAS5) is a newly discovered lncRNA, which is encoded by
a poorly conserved gene mapped to chromosome 1q25.1. The gene
consists of 12 exons and 11 introns from which 29 transcripts are
produced from alternative splicing, many of which contain retained
introns (4). Several studies have
shown that GAS5 has a critical tumor-suppressive effect during
progression of prostate cancer, renal cancer, ovarian cancer,
cervical cancer as well as CRC (5–10).
Additionally, accumulating evidence has confirmed that GAS5 binds
to microRNAs (miRs), such as miR-21, miR-196A, miR-205, miR-222 and
miR-103, sponging their inhibitory effect on the target genes to
perform tumor-suppressive roles (5,11–15).
It is important to note that our previous studies have verified
that miR-34a acts as a tumor-suppressor gene in human CRC (16,17)
and our bioinformatic analyses have revealed the presence of a
common binding site for miR-34a and GAS5. These results have
broadened our exploration of the interaction between GAS5 and
miR-34a in the progression of CRC.
Recently, the roles of macroautophagy regulated by
ncRNAs in the malignant progression of human cancers have been
investigated. Our previous studies demonstrated that
miR-34a-regulated macroautophagy enhanced the sensitivity of
oxaliplatin and induced CRC cell apoptosis (16,17).
GAS5 performs key regulatory roles in macroautophagy in response to
external stimuli in various types cells (18–20).
Although increasing evidence has focused on the association between
macroautophagy regulated by miR-34a and GAS5 in malignant
progression of human cancers, as well as other pathological
processes, to our knowledge, the precise roles of GAS5/miR-34a
axis-regulated macroautophagy and its effect on CRC progression
requires further investigation.
In the present study, we demonstrated that GAS5
acted as a tumor-suppressive factor in progression of human CRC by
regulating the miR-34a/SIRT1 axis. miR-34a participated in
mediating GAS5-mediated suppression of CRC cell macroautophagy and
induced apoptosis via the mammalian target of rapamycin/sirtuin 1
(mTOR/SIRT1) pathway. Moreover, GAS5-regulated macroautophagy
maintained cells in an equilibrium state that protected against CRC
cell apoptosis. GAS5/miR-34a/SIRT1/mTOR formed a negative
regulatory feedback loop that might explain the macroautophagy
striking in the relatively activated balance in CRC cells.
Materials and methods
Patients and tissue samples
The present study protocol was approved by the
Institutional Research Ethics Committee of Harbin Medical
University (KY2018-208). All patients gave signed informed consent
according to our institutional guidelines. We obtained 75 CRC
samples and their paired adjacent normal colon tissues from
patients (median age, 64 years; range, 49–79 years) immediately
after surgical resection between March 2018 and March 2019 at the
Second Affiliated Hospital of Harbin Medical University. The
samples were stored at −80°C. The tissues were reviewed by a
pathologist and classified according to the 7th
Tumor-Node-Metastasis (TNM) classification of the International
Union against Cancer (21).
Information concerning the clinical characteristics was collected
from medical records.
Animal model of azoxymethane
(AOM)-induced CRC
Thirty-six male Wistar rats (40–60 g, aged 3 weeks)
purchased from the Animal Center of the Second Affiliated Hospital
of Harbin Medical University were housed with free access to
sterile food and water under a standard 12-h light/dark cycle and
controlled temperature (22±2°C) and humidity (55±5%). The health
and behavior of the rats were monitored every 2 days. Animal
experiments were carried out in strict accordance with the Harbin
Medical University Institutional Animal Care and Use Committee
(KY2018-208). The rats were randomly divided into three groups of
12. Once weekly, for 6 weeks, animals in the control group received
an equal volume of saline, while animals in the AOM groups received
AOM (15 mg/kg i.p.). Rats in the AOM1 group were sacrificed at 12
weeks after the first AOM treatment, while rats in the AOM2 group
were sacrificed at 24 weeks after the first AOM treatment. Animals
were sacrificed by anesthesia overdose with i.p. injection of
sodium pentobarbital (Nembutal; 200 mg/kg). The colon tissue
samples were obtained from each rat after decapitation (17).
Cell culture and treatment
The normal colon epithelial cell line FHC and human
CRC cell lines HT29, HCT116, SW480 and SW620 were purchased from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China)
and routinely cultured as previously described (17). All the cell lines used in the
present study were authenticated by short tandem repeat (STR)
profiling. The GAS5 plasmid for GAS5 overexpression and the
hsa-miR-34a mimics or hsa-miR-34a inhibitor were synthesized by
GenePharma. The CRC cell lines HT29 and SW480 were transfected with
either 200 nM pcDNA3.1-GAS5 or 100 nM miR-34a mimics (or miR-34a
inhibitors) for 48 h. The corresponding negative control was
performed in all experiments. The mTOR siRNA (50 nM, 48 h) was
purchased from Santa Cruz Biotechnology, Inc. Transient
transfection of the siRNA or miRNA was conducted using
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.). A potent mTOR
activator, MYH1485 (2 nM, 12 h; Sigma-Aldrich; Merck KGaA), was
used to enhance mTOR expression, which in turn inhibited
macroautophagy flux. Rapamycin (10 nM, 24 h; Cell Signaling
Technology, Inc.), an macroautophagy promoter, was used to further
activate CRC cell macroautophagy as previously described (17).
Binding sites prediction and
luciferase assay
The putative binding sites between GAS5 and miR-34a
were predicted by online software DIANA-LncBase v2 (http://www.microrna.gr/LncBase). Luciferase
activity was measured using a Dual-Luciferase Reporter Assay system
(Promega Corp.), and promoter activity was expressed as the ratio
of firefly luciferase activity to Renilla luciferase
activity as previously described (17).
Real-time quantitative reverse
transcription polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent and
total miRNAs were extracted using mirVana miRNA Isolation kit
(Ambion; Thermo Fisher Scientific, Inc.). cDNA was synthesized from
2 µg total miRNAs using the High Capacity cDNA Reverse
Transcription kit (Ambion; Thermo Fisher Scientific, Inc.).
Expression levels of miRNAs and mRNAs were assessed with real-time
RT-qPCR using the Power SYBR Green and a 7500 Sequence Detection
System (Thermo Fisher Scientific, Inc.) as previously described
(17). The names and the primer
sequences of the detected genes are listed in Table I. Changes in mRNAs and miRNAs were
quantified using the 2−∆∆Cq method (22) and β-actin mRNA and U6 small nuclear
RNA were used as references.
 | Table I.Primers used for RT-qPCR. |
Table I.
Primers used for RT-qPCR.
| Primer name | Primer sequence:
5′-3′ |
|---|
| Forward GAS5 |
CTTGCCTGGACCAGCTTAAT |
| Reverse GAS5 |
CAAGCCGACTCTCCATACCT |
| Forward mTOR |
TCCGAGAGATGAGTCAAGAGG |
| Reverse mTOR |
CACCTTCCACTCCTATGAGGC |
| Forward SIRT1 |
CCCAGAACATAGACACGCTGGA |
| Reverse SIRT1 |
ATCAGCTGGGCACCTAGGACA |
| Forward
β-actin |
ATGTTGAGACCTTCAACACC |
| Reverse
β-actin |
AGGTAGTCAGTCAGGTCCCGGCC |
| Forward
miR-34a |
TGGTGTCGTGGAGTCG |
| Reverse
miR-34a |
GGCATCTCTCGCTTCATCTT |
| Forward U6 |
CTCGCTTCGGCAGCACA |
| Reverse U6 |
AACGCTTCACGAATTTGCGT |
Western blotting
Total protein from each experimental group was lysed
in RIPA buffer (Beyotime Institute of Biotechnology). The BCA kit
(Beyotime Institute of Biotechnology) was used to estimate the
concentration of total protein. Total protein (20–40 µg) was
separated by 10–15% SDS-PAGE and blotted on nitrocellulose
membranes (Amersham Pharmacia Biotech). Membranes were blocked with
blocking buffer (Beyotime Institute of Biotechnology) for 2 h at
room temperature, and then the membranes were probed with
antibodies against mTOR, phospho (p)-mTOR, SIRT1, MAPLC3β, Beclin1,
Bcl-2, Bax, matrix metalloproteinase (MMP)2, MMP9 and β-actin.
Enhanced chemiluminescence detection reagents of donkey anti-mouse
IgG Alexa Fluor 680 or donkey anti-rabbit IgG Alexa Fluor 680 were
incubated with the membranes for 12 h at 4°C and visualized with an
Odyssey Infrared Imaging system (LI-COR Biosciences). Densitometry
was performed using an Alpha Imager 2200 (Applied Biosystems;
Thermo Fisher Scientific, Inc). β-actin was used as a normalization
control. The names and manufacturers of the detected genes are
listed in Table II.
 | Table II.Antibodies used for western blot
analysis. |
Table II.
Antibodies used for western blot
analysis.
| Antibody | Product/cat. no.
(manufacturer) | Species | Dilution |
|---|
| mTOR | sc-517464 (Santa
Cruz Biotechnology, Inc., USA) | Mouse | 1:200 |
| p-mTOR | sc-293089 (Santa
Cruz Biotechnology, Inc., USA) | Mouse | 1:200 |
| SIRT1 | sc-135792 (Santa
Cruz Biotechnology, Inc., USA) | Mouse | 1:500 |
| LC3 | sc-28266 (Santa
Cruz Biotechnology, Inc., USA) | Rabbit | 1:500 |
| Beclin1 | sc-11427 (Santa
Cruz Biotechnology, Inc., USA) | Rabbit | 1:500 |
| Bax | sc-526 (Santa Cruz
Biotechnology, Inc., USA) | Mouse | 1:200 |
| Bcl-2 | sc-7382 (Santa Cruz
Biotechnology, Inc., USA) | Mouse | 1:200 |
| MMP2 | sc-13594 (Santa
Cruz Biotechnology, Inc., USA) | Mouse | 1:500 |
| MMP9 | sc-393859 (Santa
Cruz Biotechnology, Inc., USA) | Mouse | 1:500 |
| β-actin | sc-69879 (Santa
Cruz Biotechnology, Inc., USA) | Mouse | 1:500 |
| Anti-rabbit IgG,
Alexa Fluor 680 | A10043 (Thermo
Fisher Scientific, Inc., USA) | Donkey | 1:5,000 |
| Anti-mouse IgG,
Alexa Fluor 680 | A32788 (Thermo
Fisher Scientific, Inc., USA) | Donkey | 1:5,000 |
Cell migration and invasion
assays
The invasive potential of cells was measured in
6.5-µm Transwell chambers with 8.0-µm Pore Polycarbonate Membrane
Insert (Corning Inc.). The filter of the top chamber was
Matrigel-coated with 50 µl diluted Matrigel and incubated at 37°C
for 2 h. The lower chambers were filled with 600 µl Dulbecco's
modified Eagle's medium (DMEM) containing 5% fetal bovine serum
(FBS) as chemoattractant for a further 24 h. Cells were
serum-free-starved overnight, harvested, and suspended in migration
medium (DMEM with 0.5% bovine serum albumin). A suspension of 5,000
CRC cells in 100 µl migration medium was added to each top chamber.
After the cells were incubated for 16 h, the non-invading cells
that remained on the upper surface were removed with a cotton swab.
The invasive cells on the lower surface of the membrane insert were
fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.2%
Triton X-100 at room temperature for 15 min, and stained with 0.1%
crystal violet for 5 min. The number of cells on the lower surface,
which had invaded through the membrane, was counted under a light
microscope in five random fields at a magnification of ×100. The
procedure for the Transwell migration assays was the same as the
Transwell invasion assay except that the filter of the top chamber
was not coated with Matrigel.
Evaluation of macroautophagy and
apoptosis by flow cytometry
CRC cell macroautophagy and apoptosis were evaluated
by flow cytometry as previously described (17). CRC cells (104) were
washed with PBS and then incubated with 0.05 mmol/l
mono-dansylcadaverine (MDC; Sigma-Aldrich; Merck KGaA) at 37°C for
45 min. The cells were washed three times with PBS and analyzed by
flow cytometry immediately (BD Biosciences). Apoptosis was detected
with flow cytometry using Annexin V-fluoroisothiocyanate
(FITC)/propidium iodide (PI) kit (Becton-Dickinson). After
treatment, the cells from each group were washed three times with
PBS and stained with Annexin V-FITC/PI in the dark. The rate of
apoptosis was quantified by flow cytometry (BD Biosciences).
Transmission electron microscopy
CRC cells were harvested and fixed with 2.5%
glutaraldehyde at 4°C for 2 h and then in 1% osmic acid PBS at pH
7.4, at 37°C for 2 h. After dehydration and embedding, the
ultrathin sections were prepared on uncoated copper grids with an
Ultrotome (Leica, Reichert Ultracuts) and stained with uranyl
acetate and lead citrate. Images were recorded under a transmission
electron microscope (JEM 1230; Geol) (17).
Statistical analysis
Statistical analysis was carried out by SPSS version
21 (IBM Corp.) or GraphPad Prism version 5.0 (GraphPad Software,
Inc.). Results are presented as the mean ± SEM. Comparisons of
quantitative data between two groups were performed using Student's
two-tailed t-tests, and among three or more groups were performed
by one-way ANOVA test followed by Tukey's test. Correlation
analysis between GAS5, miR-34a, SIRT1 and mTOR mRNA expression
levels was examined by Pearson's rank correlation analysis. The
Chi-square test analysis was used to calculate the P-values in
Table III. P<0.05 was assigned
to indicate a significant difference.
 | Table III.Association between GAS5 expression
levels and clinicopathological features in the CRC patients
(N=75). |
Table III.
Association between GAS5 expression
levels and clinicopathological features in the CRC patients
(N=75).
|
|
| GAS5 |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | n | Low (n=37) | High (n=38) | P-value |
|---|
| Age (years) |
|
|
| 0.786 |
|
<60 | 35 | 18 | 17 |
|
|
≥60 | 40 | 19 | 21 |
|
| Sex |
|
|
| 0.125 |
|
Male | 35 | 17 | 18 |
|
|
Female | 40 | 20 | 20 |
|
| Tumor size
(cm) |
|
|
| 0.062 |
|
<5 | 44 | 26 | 18 |
|
| ≥5 | 31 | 11 | 20 |
|
|
Differentiation |
|
|
| 0.170 |
|
Well/moderate | 29 | 3 | 26 |
|
|
Poor | 46 | 34 | 12 |
|
| Lymphatic node
metastasis |
|
|
| 0.016 |
|
Present | 36 | 33 | 3 |
|
|
Absent | 39 | 4 | 35 |
|
| Clinical
stages |
|
|
| 0.022 |
|
I/II | 38 | 3 | 35 |
|
|
III/IV | 37 | 34 | 3 |
|
| CA19-9 level
(U/ml) |
|
|
| 0.690 |
|
<37 | 38 | 19 | 19 |
|
|
≥37 | 37 | 18 | 19 |
|
| CEA level
(ng/ml) |
|
|
| 0.081 |
|
<5 | 23 | 3 | 20 |
|
| ≥5 | 52 | 34 | 18 |
|
Results
Expression of GAS5, miR-34a, SIRT1 and
mTOR in human CRC tissues, cell lines and rat colon tissues
We initially identified GAS5, miR-34a, SIRT1 and
mTOR expression in human CRC specimens to understand better the
underlying complex mechanisms of human CRC. Expression levels of
GAS5, miR-34a and mTOR were significantly decreased (Fig. 1A-C) while those of SIRT1 mRNA were
significantly upregulated (Fig. 1D)
in most of the CRC tissues compared to the paired adjacent
non-tumor tissues. All the CRC cells displayed significantly lower
expression levels of GAS5, miR-34a and mTOR, and SIRT1 mRNA was
significantly higher when compared to these levels in the FHC cells
(Fig. 1E-H). The AOM-induced CRC
rat model was used to determine the potential mechanism of GAS5
in vivo. All rats treated with AOM developed polyps and
preneoplastic aberrant crypt foci (ACF) (Fig. 1I). The number of ACF with ≤4 crypts
and total number of ACF in the entire colon of the rats were
significantly different. There was no significant difference in the
number of ACF with >5 crypts in the entire colon of the rats.
Consistent with the results in vitro, expression of GAS5,
miR-34a and mTOR was dramatically decreased, while expression of
SIRT1 was significantly upregulated in the colon tissues from
AOM-treated rats compared to the control group (Fig. 1J). These results showed that the
GAS5/miR-34a axis is involved in inhibition of CRC malignant
behavior through the mTOR/SIRT1 pathway in vitro and in
vivo.
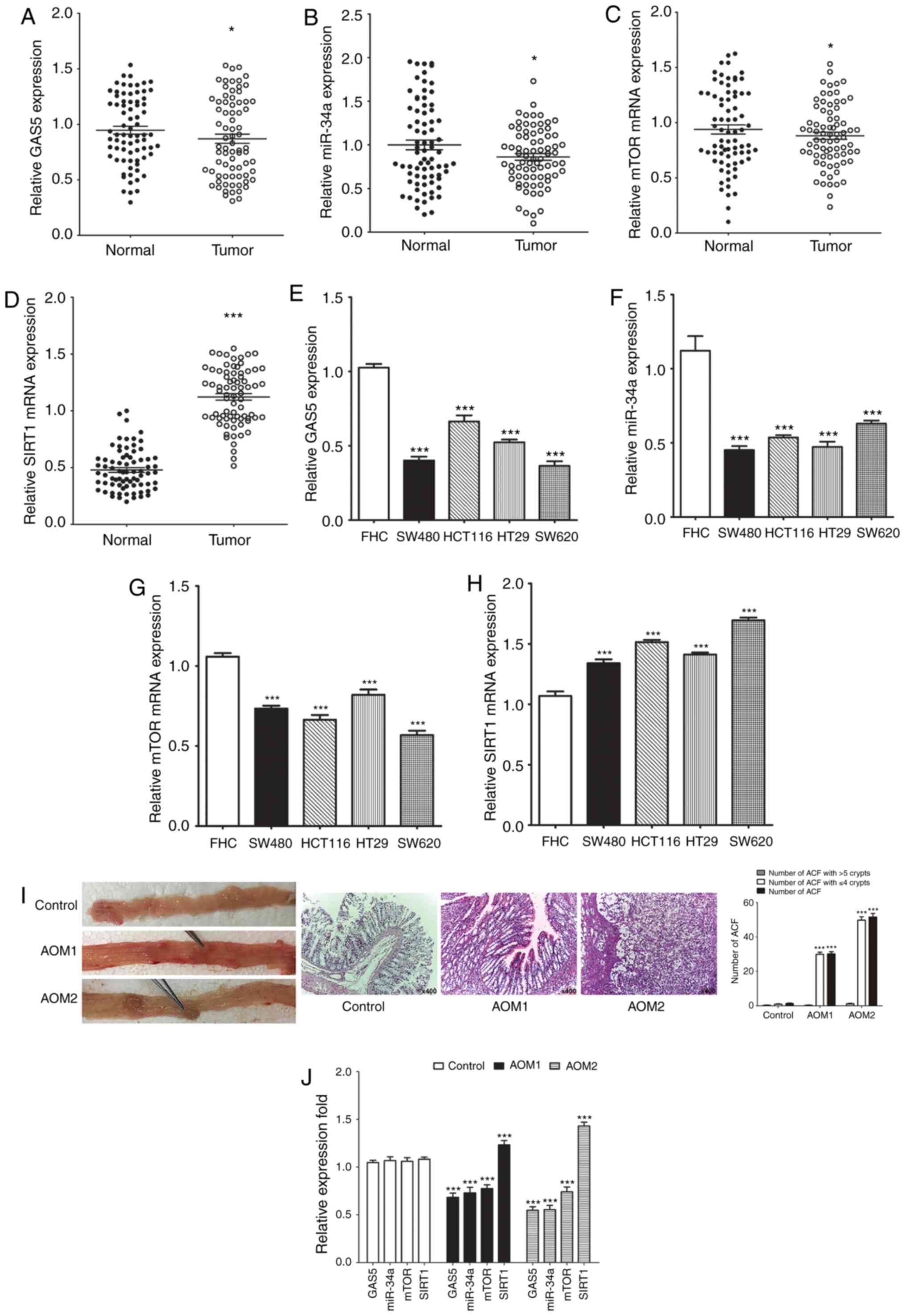 | Figure 1.Expression of GAS5, miR-34a, SIRT1
and mTOR in human CRC tissues, cell lines and rats colon tissues.
(A-D) RT-qPCR was performed and revealed downregulation of GAS5,
miR-34a and mTOR mRNA expression and upregulation of SIRT1 mRNA
expression in 75 CRC samples compared to their paired adjacent
nontumor tissues. (E-H) RT-qPCR revealed downregulation of GAS5,
miR-34a and mTOR mRNA expression and upregulation of SIRT1 mRNA
expression in HT29, HCT116, SW480 and SW620 CRC cell lines compared
to FHC cells. (I) All rats treated with AOM developed polyps and
ACF. Numbers of ACF in the entire colon of the rats were
quantified. (J) Expression of GAS5, miR-34a, SIRT1 and mTOR in the
colon samples of rats was determined by RT-qPCR. *P<0.05 and
***P<0.001 means a significant difference vs. the adjacent
nontumor tissues, FHC cells or the control group. CRC, colorectal
cancer; GAS5, growth arrest specific 5; miR/miRNA, microRNA; SIRT1,
sirtuin 1; mTOR, mammalian target of rapamycin. |
Association between expression of
GAS5, miR-34a, SIRT1 and mTOR in human CRC tissues and the
clinicopathological significance of GAS5 expression in CRC
patients
To evaluate further the clinical value and
underlying mechanism of the GAS5/miR-34a/SIRT1 axis in CRC
patients, we analyzed the correlation between expression of GAS5,
miR-34a, SIRT1 and mTOR in human CRC tissues. Although the
correlation between GAS5 and miR-34a (R=0.209, P=0.010, Fig. 2A), miR-34a and SIRT1 (R=−0.182,
P=0.026, Fig. 2B) was weakly
inverse the correlation was significant. Expression of GAS5 and
SIRT1 was positively correlated in most of the tissues (R=0.163,
P=0.046, Fig. 2C). However, our
data indicated a mild inverse correlation between GAS5 and mTOR
expression in CRC but the correlation was not significant (R=0.032,
P=0.700, Fig. 2D). The 75 CRC
patients were divided into two groups according to the median value
(0.8699) of GAS5 mRNA expression in CRC tissues to evaluate further
the association between expression of GAS5 and clinicopathological
characteristics of CRC (Table
III). The data indicated that GAS5 expression was inversely
associated with samples with lymphatic metastasis and advanced
clinical stages. However, age, sex, tumor size, poor cell
differentiation, and high expression of carcinoembryonic antigen
(CEA) and carbohydrate antigen (CA)19-9 had no association with
GAS5 mRNA expression.
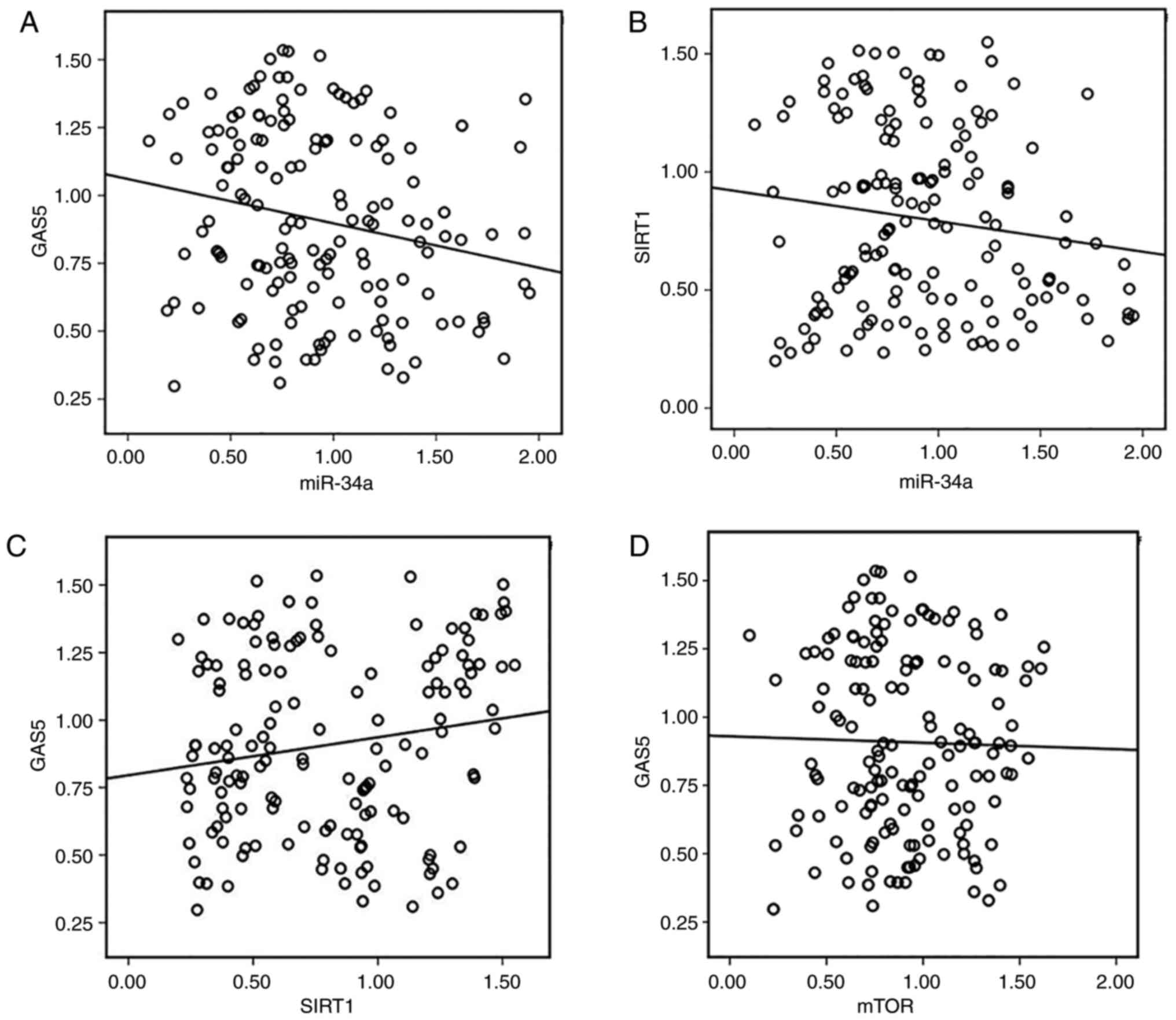 | Figure 2.Correlation between expression of
GAS5, miR-34a, SIRT1 and mTOR in human CRC tissues. (A) Inverse
correlation between GAS5 and miR-34a expression was determined by
Spearman's rank correlation analysis (R=−0.209, P=0.010). (B)
Inverse correlation between miR-34a and SIRT1 expression was
determined by Spearman's rank correlation analysis (R=−0.182,
P=0.026). (C) Expression of GAS5 and SIRT1 was positively
correlated in the experimental tissues (R=0.163, P=0.046). (D) A
mild inverse but not significant correlation between GAS5 and mTOR
expression in human colon and CRC tissues (R=−0.032, P=0.700). CRC,
colorectal cancer; GAS5, growth arrest specific 5; miR/miRNA,
microRNA; SIRT1, sirtuin 1; mTOR, mammalian target of
rapamycin. |
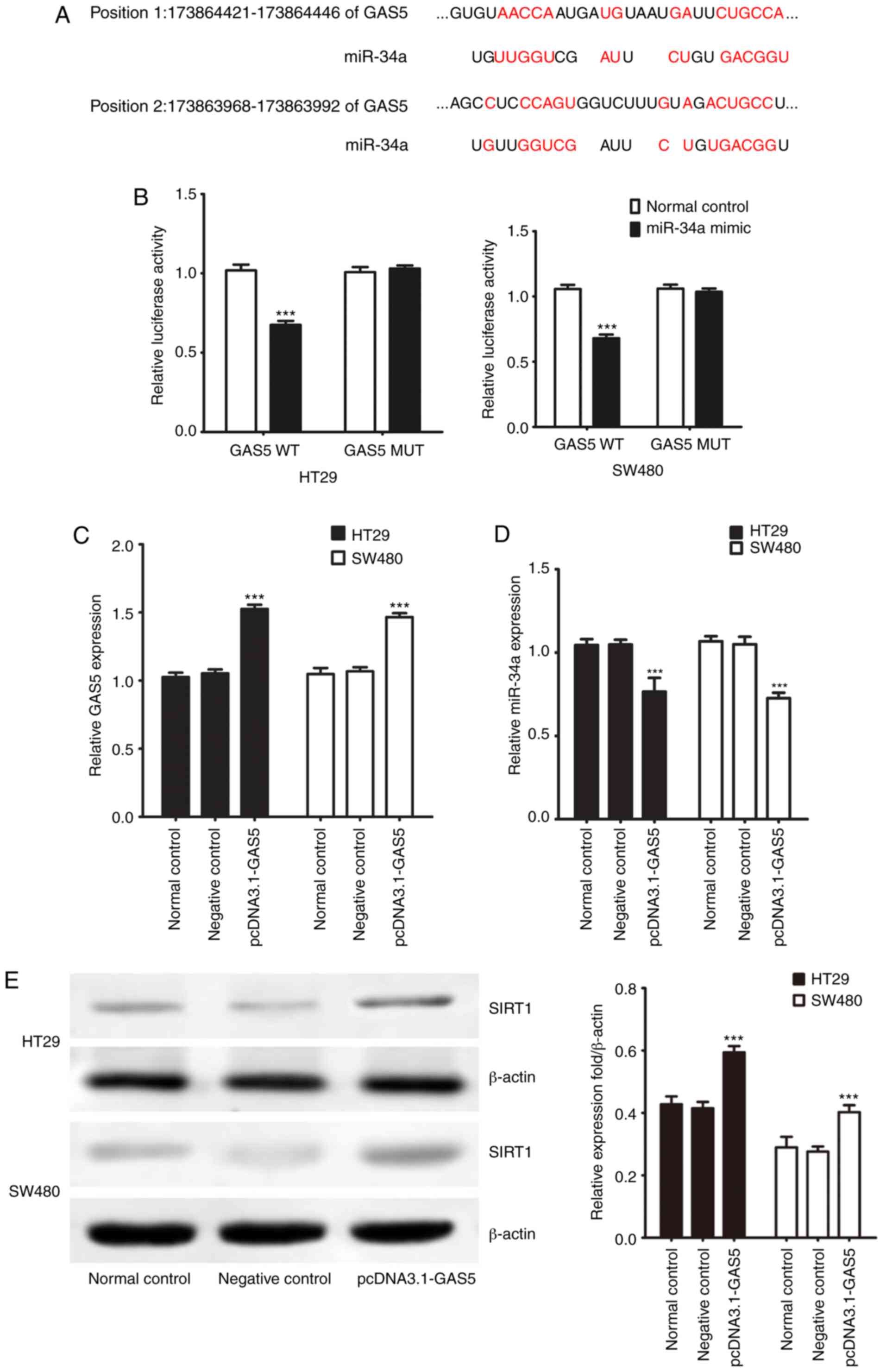
GAS5 acts as a molecular sponge of
miR-34a in CRC cells
lncRNAs act as molecular sponges of miRNAs to exert
their regulatory functions. Online bioinformatic analysis using the
DIANA Tools suggested the presence of a common binding site for
miR-34a and GAS5 (Fig. 3A). We
previously found that SIRT1 is one of the target genes of miR-34a
in CRC cells (17). Here, GAS5
WT/MUT (wild-type/mutant) was cloned into the double luciferase
reporter gene vector and cotransfected into CRC cell lines with
miR-34a mimics and mimic negative control to verify the targeting
effect of GAS5 on miR-34a. miR-34a mimic transfection decreased
luciferase activity compared to the negative control group
(Fig. 3B). Additionally, after
transfection with the mutated 3′ untranslated region (UTR) of the
GAS5 gene, the luciferase activities did not differ significantly
between the miR-34a mimics and negative control groups.
Transfection of pcDNA3.1-GAS5 increased endogenous GAS5 expression
but decreased miR-34a expression in the CRC cells (Fig. 3C and D). Western blotting showed
that transfection with pcDNA3.1-GAS5 significantly promoted SIRT1
protein expression in the CRC cells (Fig. 3E). Thus, the results indicated that
GAS5 negatively regulated expression of miR-34a at the
post-transcriptional level in CRC cells. Since our correlation
analysis of clinical specimens indicated that lncRNA GAS5 was
related to lymph node metastasis, we evaluated cell invasion and
migration activities in vitro. Protein expression of MMP2
and MMP9 was not significantly influenced by overexpression of GAS5
in CRC cells compared to the control group (Fig. S1). HT29 and SW480 cells were
transfected with pcDNA3.1-GAS5 to evaluate cell invasion and
migration activities using a cell invasion assay in Transwell
chambers. Transfection with pcDNA3.1-GAS5 had no significant effect
on cell migration and invasive capacity in the CRC cells (Fig. S2).
miR-34a participates in regulating
GAS5-mediated suppression of CRC cell macroautophagy and induces
apoptosis through the mTOR/SIRT1 pathway
Previous studies have demonstrated that both miR-34a
and GAS5 act as key regulators of macroautophagy and apoptosis
(16–20). We investigated whether miR-34a was
involved in mediating GAS5-regulated macroautophagy and apoptosis
in CRC cells. Our data indicated that the number of MDC-positive
cells (Fig. 4A) and autophagosomes
(Fig. 4B) in pcDNA3.1-GAS5
treatment and miR-34a mimics transfection groups were significantly
decreased compared to the controls. Western blotting showed lower
expression of LC3-II, Beclin I and Bcl-2, and higher expression of
SIRT1, p-mTOR and Bax (Fig. 4D) in
the pcDNA3.1-GAS5 and miR-34a mimic transfection groups. Similarly,
the apoptotic rate was significantly increased in CRC cells
following transfection with pcDNA3.1-GAS5 and miR-34a mimics
(Fig. 4C). Macroautophagy was
enhanced but apoptosis was not further induced in HT29 and SW480
cell lines by miR-34a inhibitor transfection compared to the
control group. Treatment with pcDNA3.1-GAS5 in combination with
miR-34a mimics had no significant effect on p-mTOR expression,
macroautophagy flux, or apoptosis, but impaired SIRT1 expression
compared with pcDNA3.1-GAS5 treatment alone in the CRC cells
(Fig. 4). Compared with
pcDNA3.1-GAS5 treatment alone, the induction of p-mTOR expression
and apoptosis and inhibitory effect on macroautophagy flux were
impaired by pcDNA3.1-GAS5 treatment in combination with miR-34a
inhibitor in CRC cells. This was shown by more MDC-positive cells
(Fig. 4A) and autophagosomes
(Fig. 4B), increased expression of
LC3-II, Beclin I and Bcl2, decreased expression of Bax (Fig. 4D), and apoptotic rate detected by
flow cytometry (Fig. 4C). Notably,
no significant change was found in SIRT1 expression level between
pcDNA3.1-GAS5 and pcDNA3.1-GAS5 treatment in combination with
miR-34a inhibitor. These data indicated that the miR-34a/SIRT1 axis
at least partly participated in mediating GAS5-suppressed
macroautophagy and induced apoptosis through activation of the mTOR
pathway in CRC cells.
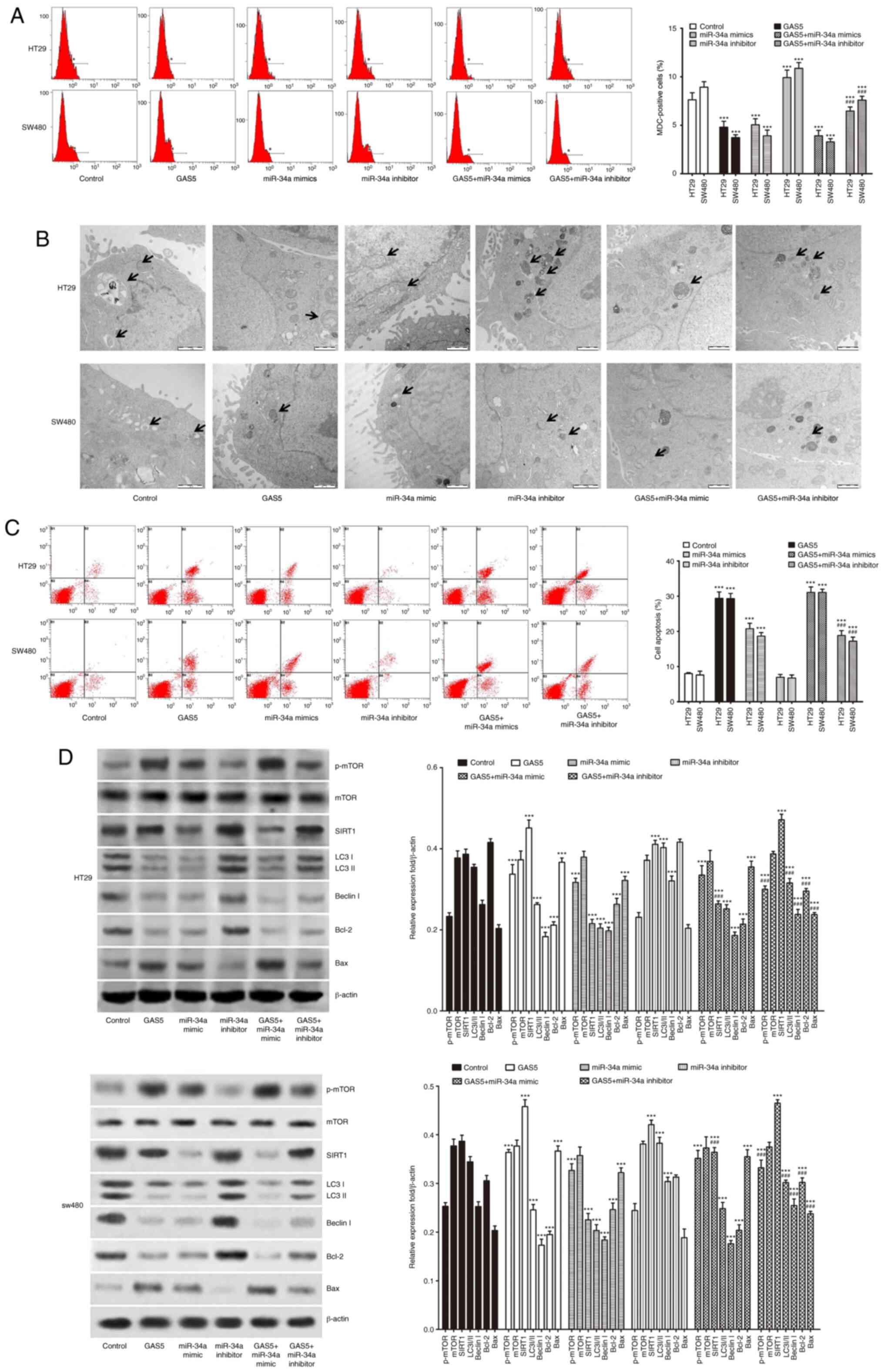 | Figure 4.miR-34a participates in regulating
GAS5-mediated suppression of CRC cell macroautophagy and induces
apoptosis through the mTOR/SIRT1 pathway. (A) CRC HT29 and SW480
cells were transfected with pcDNA3.1-GAS5 with or without miR-34a
mimics and miR-34a inhibitor. Flow cytometry detected the number of
MDC-positive cells to evaluate macroautophagy levels. (B)
Autophagic vacuole formation was shown by transmission electron
microscopy of CRC cell lines. The arrows indicate the
autophagosomes (scale bar, 1 µm). (C) Analysis of apoptosis by flow
cytometry in HT29 and SW480 cells. (D) Western blotting was
performed to detect p-mTOR, mTOR, SIRT1 and macroautophagy- and
apoptosis-related proteins in CRC cells. ***P<0.001 indicates a
significant difference vs. the control group;
###P<0.001 means a significant difference vs. the
pcDNA3.1-GAS5 treatment group. MDC, mono-dansylcadaverine; CRC,
colorectal cancer; GAS5, growth arrest specific 5; miR/miRNA,
microRNA; SIRT1, sirtuin 1; mTOR, mammalian target of rapamycin;
LC3, microtubule-associated protein light chain 3; p-,
phosphorylated. |
Activation of GAS5-suppressed
macroautophagy is involved in promoting apoptosis in CRC cells
To address whether GAS5 regulated
macroautophagy-modulated apoptosis in CRC cells, we transfected CRC
cells with pcDNA3.1-GAS5 with or without MYH1485 and rapamycin
treatment. pcDNA3.1-GAS5 transfection inhibited macroautophagy and
induced apoptosis in CRC cells, as shown by decreased MDC-positive
cells (Fig. 5A) and autophagosomes
(Fig. 5B); lower expression of
LC3-II, Beclin I and Bcl-2; and higher expression of Bax (Fig. 5D) as well as apoptosis detected by
flow cytometry (Fig. 5C). MYH1485
treatment dramatically suppressed macroautophagy and induced
apoptosis of CRC cells, while rapamycin further activated
macroautophagy and protected CRC cells against apoptosis, as shown
by more MDC-positive cells (Fig.
5A) and autophagosomes (Fig.
5B); and higher expression of macroautophagy-related protein
and Bax; and lower expression of Bcl-2 (Fig. 5D) as well as decreased apoptosis
detected by flow cytometry (Fig.
5C). Additionally, MYH1485 in combination with pcDNA3.1-GAS5
transfection enhanced the inhibitory effect on macroautophagy and
induction of apoptosis in CRC cells, while rapamycin in combination
with pcDNA3.1-GAS5 transfection attenuated the inhibitory effect on
macroautophagy and induction of apoptosis in CRC cells compared
with pcDNA3.1-GAS5 treatment alone (Fig. 5). Thus, the results strongly
suggested that GAS5-mediated macroautophagy maintained cells in an
equilibrium state that might have a protective effect on CRC cell
apoptosis.
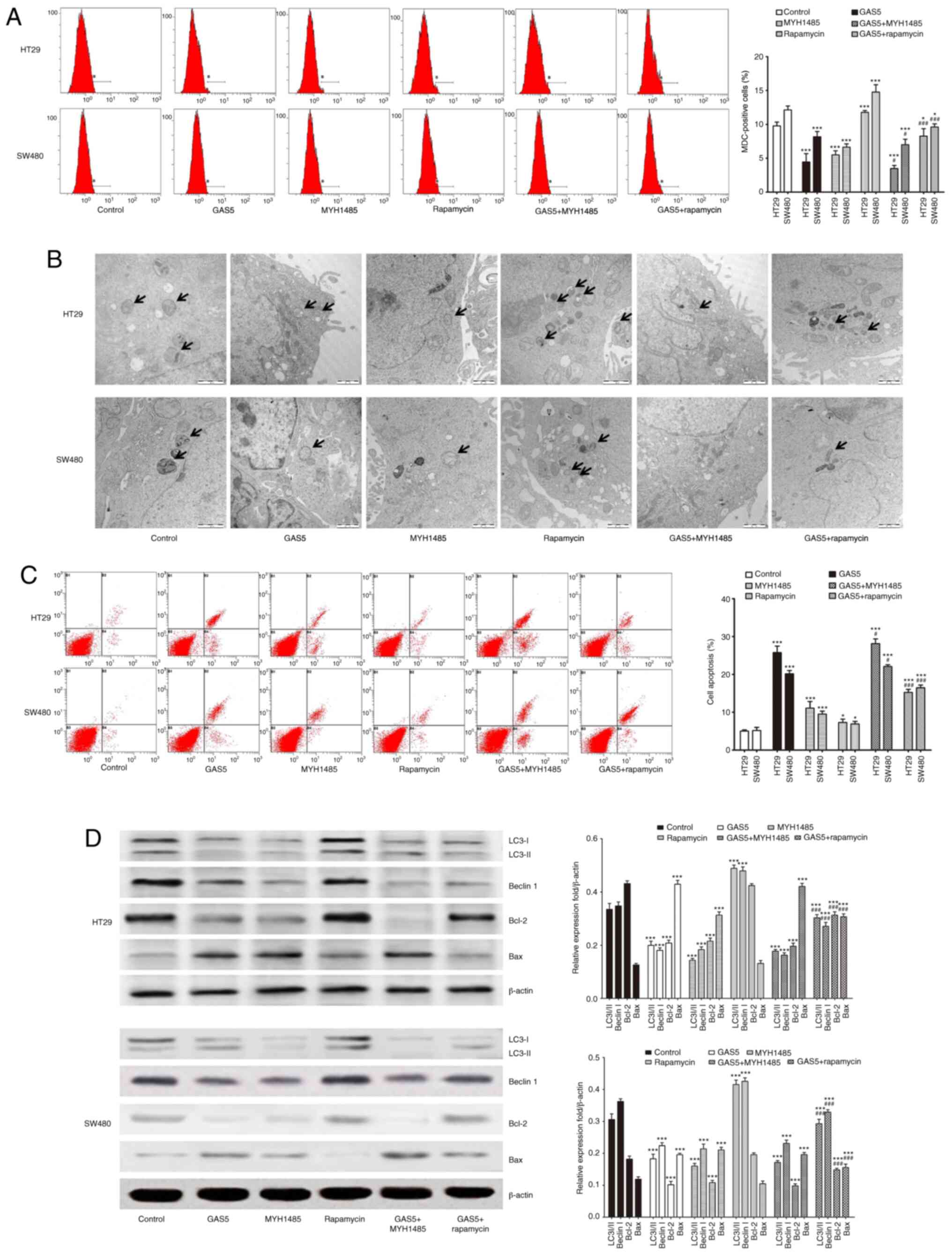 | Figure 5.Activation of GAS5-mediated
suppression of macroautophagy is involved in promoting apoptosis in
CRC HT29 and SW480 cells. (A) CRC cells were treated with
pcDNA3.1-GAS5 with or without MYH1485 and rapamycin, and then flow
cytometry was used to detect the number of MDC-positive cells to
evaluate macroautophagy levels. (B) Macroautophagy vacuole
formation is shown by transmission electron microscopy of CRC cell
lines. The arrows indicate the autophagosomes (scale bar, 1 µm).
(C) Apoptosis was analyzed by flow cytometry in CRC cells. (D)
Western blotting shows expression of LC3, Beclin1, Bcl-2 and Bax in
CRC cells. Quantification was performed. *P<0.05, ***P<0.001
means a significant difference vs. the control group;
#P<0.05, ###P<0.001 means a significant
difference vs. the pcDNA3.1-GAS5 treatment group. MDC,
mono-dansylcadaverine; GAS5, growth arrest specific 5; CRC,
colorectal cancer; LC3, microtubule-associated protein light chain
3. |
mTOR/SIRT1 pathway inhibits expression
of GAS5 and forms a negative regulation feedback loop with miR-34a
in CRC cells
The above results indicated that miR-34a
participates in regulating GAS5-mediated macroautophagy and
maintained CRC cells in an equilibrium state through the mTOR/SIRT1
pathway. More importantly, it has been reported that SIRT1 enhances
mTOR expression in different conditions (23), and under conditions in which mTOR
activity is high, GAS5 translation is promoted due to the presence
of the 5′-TOR sequence. Conversely, when mTOR activity is low, this
in turn results in accumulation of GAS5 transcripts (24). Thus, we speculated that a negative
regulation feedback loop might be involved in mediating
GAS5-regulated macroautophagy in CRC cells. To confirm our
hypothesis, we examined the effect of activation of mTOR by MYH1485
or inhibition of mTOR by transfection of mTOR siRNAs on GAS5,
miR-34a and SIRT1 expression in CRC cells. Compared with the
control group, MYH1485 significantly elevated mTOR mRNA and protein
expression in CRC cells, while transfection of mTOR siRNAs
decreased expression of mTOR mRNA and protein. Additionally, when
treated with MYH1485 and mTOR siRNAs simultaneously, mTOR
expression was upregulated compared to transfection with mTOR
siRNAs alone (Fig. 6A and E). As
shown in Fig 6B and C, after
treatment with MYH1485, GAS5 and SIRT1 mRNA expression was
significantly downregulated and miR-34a expression was upregulated
(Fig. 6D), while converse results
were obtained when HT29 and SW480 cells were transfected with mTOR
siRNAs. When treated with MYH1485 and mTOR siRNAs simultaneously,
promotion of expression of GAS5 and SIRT1 and inhibition of
expression of miR-34a were impaired compared to transfection with
mTOR siRNAs alone (Fig. 6B, C and
E). Based on these results and together with the inhibitory
effect of pcDNA3.1-GAS5 transfection on miR-34a and the promotive
effect on SIRT1 and mTOR expression in CRC cells, we suggest that a
GAS5/miR-34a/mTOR/SIRT1 negative regulatory feedback loop in CRC
cells is involved in mediating CRC progression.
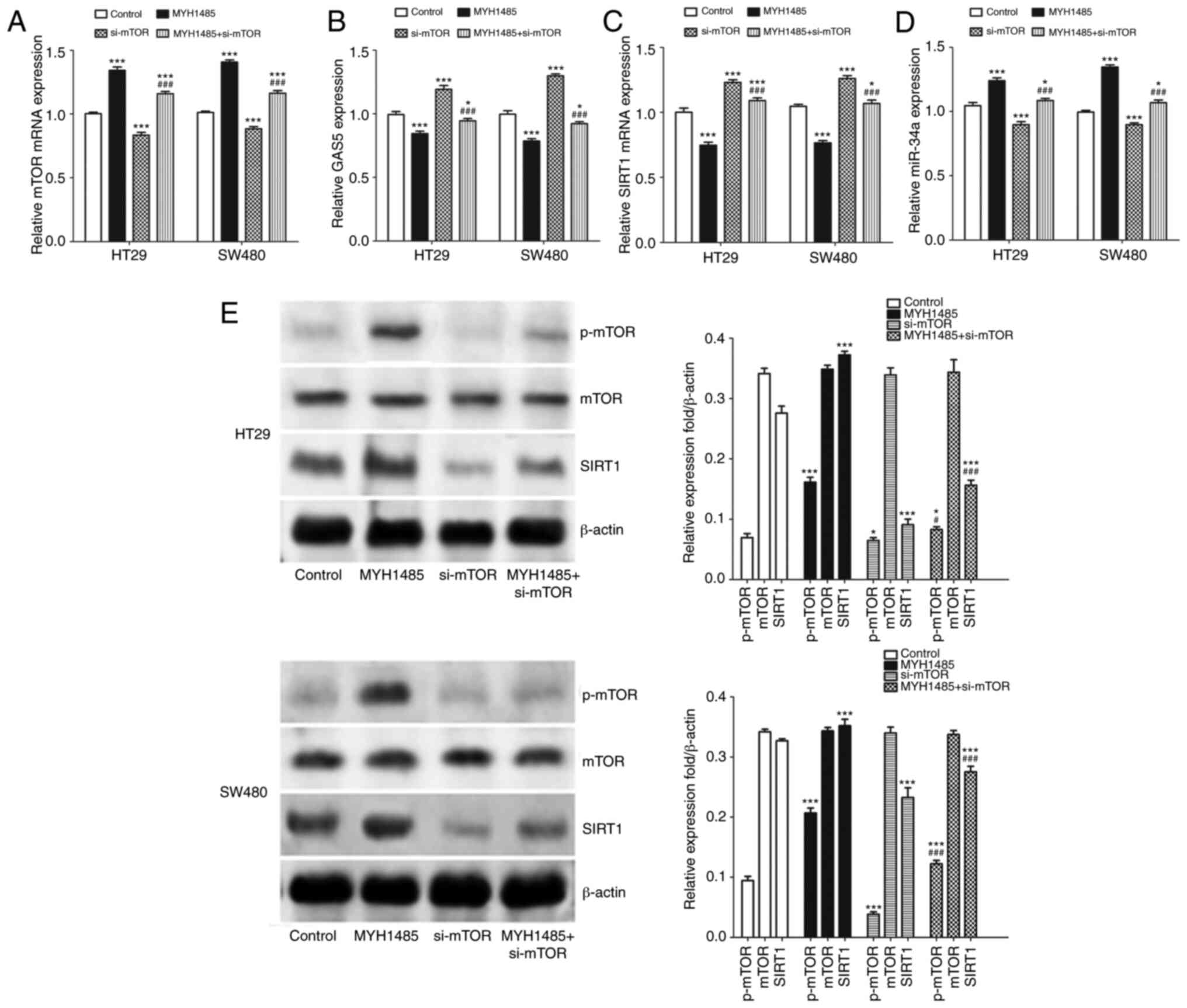 | Figure 6.mTOR/SIRT1 pathway inhibits
expression of GAS5 and forms a negative regulatory feedback loop
with miR-34a in CRC HT29 and SW480 cells. (A-D) CRC cells were
treated with MYH1485 with or without si-mTOR transfection, and then
RT-qPCR was used to analysis mTOR mRNA, GAS5, SIRT1 mRNA and
miR-34a expression. (E) Western blotting shows expression of
p-mTOR, mTOR and SIRT1 in CRC cells. Quantification of the results
was performed. *P<0.05, ***P<0.001 means a significant
difference vs. the control group; #P<0.05,
###P<0.001 means a significant difference vs. the
si-mTOR treatment group. GAS5, growth arrest specific 5; CRC,
colorectal cancer; SIRT1, sirtuin 1; mTOR, mammalian target of
rapamycin; p-, phosphorylated. |
Discussion
Most studies have demonstrated that long non-coding
RNA (lncRNA) growth arrest specific 5 (GAS5) is overexpressed in
growth-arrested cells, as its name implies. This is further
considered in the slowest dividing cells in the body as opposed to
its lowest levels in other rapidly dividing cells, the most
important of which are cancer cells (25). GAS5 performs a tumor-suppressor role
in human cancer (5–15). However, separate studies have
indicated that GAS5 promotes proliferation, migration and invasion
in esophageal cancer and hepatocellular carcinoma (26,27).
In human colorectal cancer (CRC), recent studies have verified that
GAS5 contributes to lymphatic metastasis (10). There is increasing evidence for
significant changes in miRNA expression profiles in cancer cells
when GAS5 is activated under different conditions (5,11–15)
and most of our previous studies were concerned with the
tumor-suppressive gene miR-34a during progression of human
gastrointestinal cancer (16,17).
Thus, here we selected miR-34a as the candidate gene to investigate
further the mechanism of GAS5 in the progression of CRC. We
revealed that both GAS5 and miR-34a expression levels were
aberrantly decreased in most, but not all human CRC tissues. In
line with a previous study (10),
our present results revealed that all the CRC cells had
significantly lower levels of GAS5 and miR-34a compared to FHC
cells. HT29 and SW480 cells expressed medium expression levels of
GAS5, thus, these two cell lines were selected to explore further
the role of GAS5 in the progression of CRC. The AOM-induced CRC rat
model was used to evaluate the inhibitory effect of GAS5 on
malignancy in vivo. We found that AOM intervention markedly
induced the formation of colon tumors and blocked expression of
GAS5 and miR-34a in colon tissues of rats. Our analysis of
clinicopathological characteristics of CRC patients indicated that
decreased expression of GAS5 was inversely correlated with
lymphatic metastasis and advanced clinical stage. A crucial step in
CRC invasion is the degradation of basement membrane, which is
catalyzed by proteolytic enzymes, such as matix metalloproteinases
(MMPs) (28). Thus, in the present
study, we detected protein expression of MMP2 and MMP9 in CRC
cells, and Transwell migration and invasion assays were performed
to evaluated migration and invasion of CRC cells exposed to forced
upregulation of GAS5. We verified that forced upregulation of GAS5
inhibited expression of miR-34a in CRC cells. However, no
significant difference in MMP2 and MMP9 protein expression and no
significant effect on cell migration and invasion were found by
forced upregulation of GAS5 in HT29 and SW480 cells. Although
expression of GAS5 and miR-34a was markedly decreased in most human
CRC tissues in the present study, expression of GAS5 and miR-34a
showed a significant weakly inverse correlation. The inconsistent
results observed between the in vivo and in vitro
models in our study could have many possible explanations. First,
we measured the expression of GAS5 in tumor tissues obtained
directly from CRC patients. The clinical-feature specificity of CRC
may antagonize or interfere with the effect of GAS5 on CRC
malignant behavior and miR-34a expression (29). Moreover, CRC cell lines are highly
essential for functional molecular analysis. Yet, they may be
different from primary tumors, which possibly through building up
new mutations attempt to adjust their artificial environment
(30). Such mutations could easily
alter cellular responses and regulatory mechanisms, and thereby
affect the expression of GAS5 and miR-34a. Second, miR-34a could be
a non-specific molecule which can be regulated by internal stimuli
(other regulatory molecules or polymorphisms, oxidative molecules,
other associated disease states and tumor stage) and external
stimuli (cellular response to environmental exposures, including
chemotherapy and food) (4). Third,
miR-34a acts as a target for many lncRNAs (31). We speculate that other unique
regulatory networks participate in the development of CRC and
regulate the expression of miR-34a. Every signal study focuses on
one or a few targets and infers a tumor-suppressor or an oncogenic
function based on its effect on the studied signaling pathway.
However, miR-34a is a small molecule among the larger network, and
the function of miR-34a could easily differ according to countless
variables (4). These speculations
need further investigation in the future. The final but the most
important aspect is that the GAS5/miR-34a/SIRT1/mTOR negative
regulatory feedback loop may partly explain why the expression of
GAS5 and miR-34a was markedly decreased in most human CRC tissues,
but their expression levels showed a significant weak inverse
correlation. The above results suggest that GAS5 is involved in
suppressing CRC malignant behavior at least partly through
mediating miR-34a expression.
Sirtuin 1 (SIRT1) is a class III nuclear deacetylase
that can inactivate the p53 pathway (32). Our previous study confirmed that
miR-34a suppresses progression of CRC via binding to the site
within the 3′ UTR of SIRT1 to silence SIRT1 mRNA (17). Regarding mammalian targets of
rapamycin(mTOR), it was found to be involved in regulating
macroautophagy activity independent of its enzymatic function based
on cumulative evidence. Additionally, it has been reported that
SIRT1 can activate the mTOR signaling pathway under different
conditions (23). However, some
studies have indicated that SIRT1 acts as a negative regulator of
mTOR (33). In the present study,
SIRT1 expression was significantly increased in most CRC tissues,
all CRC cell lines, and AOM-treated colon tissues of rats, whereas
mTOR expression showed opposite trends. Most importantly, we found
a significant inverse correlation between miR-34a and SIRT1, and a
significant positive correlation between GAS5 and SIRT1. Although
GAS5 and mTOR revealed an inverse correlation, the result was not
significant. In vitro, we found that forced upregulation of
miR-34a had no significant effect on p-mTOR expression but impaired
SIRT1 expression upon treatment with pcDNA3.1-GAS5 in CRC cells.
Moreover, exogenous inhibition of miR-34a reduced the induction of
p-mTOR expression but caused no significant change in SIRT1
expression after pcDNA3.1-GAS5 treatment in CRC cells. We speculate
that the expression pattern of individuals, the clinical-feature
specificity, or the different target genes involved in the
regulatory network of CRC might be involved in antagonizing or
interfering with expression of the mTOR/SIRT1 pathway, which
remains to be elucidated in the future. The luciferase reporter
results and clinical studies confirmed that activation of GAS5
inhibition of malignancy might be partly through regulating miR-34a
in combination with suppressing SIRT1 expression, while the mTOR
signaling pathway is more likely downregulated during progression
of CRC.
Mounting evidence shows the inhibitory effects on
macroautophagy and proliferation when GAS5 is activated in several
types of human malignancies (18–20).
Our own and other studies have indicated that miR-34a inhibits CRC
cell macroautophagy and induces apoptosis through different
signaling pathway in different conditions (16,17,34).
We know that the mTOR pathway acts as a classical negative
regulator of macroautophagy, and miR-34a was selected as the
candidate gene to investigate the mechanism of GAS5 in the
progression of CRC. Thus, in our present study, we investigated
whether the miR-34a/SIRT1 axis is involved in mediating
GAS5-regulated macroautophagy and apoptosis through the mTOR
signaling pathway in CRC cells. We found that overexpression of
GAS5 in combination with promotion of miR-34a expression had no
significant effect on macroautophagy flux or apoptosis in CRC
cells, but the inhibitory effect of macroautophagy and induction of
apoptosis were impaired by overexpression of GAS5 in combination
with silencing miR-34a in CRC cells compared to overexpression of
GAS5 alone. Microtubule-associated protein light chain 3 (LC3) is a
soluble protein that is distributed ubiquitously in mammalian
tissues and cultured cells. A cytosolic form of LC3 (LC3-I) is
conjugated to phosphatidylethanolamine to form
LC3-phosphatidylethanolamine conjugate (LC3-II), which is recruited
to autophagosomal membranes. At the same time, LC3-II in
autolysosomal lumen is degraded. Thus, lysosomal turnover of the
autophagosomal marker LC3-II reflects macroautophagy activity
(35). Moreover, Beclin 1 is a
central scaffold protein that assembles components for promoting or
inhibiting macroautophagy. Binding of Beclin I with Bcl-2 family
and (or) class III phosphatidylinositol 3-kinase (PI3KC3) has been
shown to play a vital role during the formation of mammalian
autophagosomes in response to external stimuli. Thus, detecting LC3
and Beclin I by immunoblotting or immunofluorescence has become a
reliable method for monitoring macroautophagy-related processes in
different conditions (36). For the
detection of apoptosis, it is established that cancer cells
reprogram the Bcl-2 family interaction network that regulates
mitochondrial apoptosis. Overexpression of antiapoptotic Bcl-2
family members such as Bcl-2 and Bcl-XL bind and neutralize the BH3
death domains of the activated proapoptotic Bcl-2 members, such as
Bax (37). Cells deficient in Bax
become less sensitive to various apoptotic stimuli and become
resistant when Bax is deleted. Selective inhibitors of
antiapoptotic Bcl-2 proteins are effective inducers of apoptosis
because they release BH3-only proteins from the antiapoptotic Bcl-2
proteins to activate Bax (38).
Thus, protein expression of Bcl-2 and Bax was measured in the
present study by western blotting. In another method to analyze
apoptosis in vitro, we used Annexin V-FITC, a protein
fluorophore with high affinity to phosphatidylserines during the
early stage of apoptosis, and PI staining, red fluorescence to
detect dead cells, which are used as a standard protocol for
measuring late apoptotic cells and dead cells (39). Caspases also have been used as an
apoptosis-specific target. In particular, caspase-3- and
caspase-7-specifc cleavable peptide substrates have been
extensively used as caspase-cleavable imaging probes for apoptosis
imaging for monitoring of caspase activity in tumor cells (40). The activity of caspases regulated by
GAS5 in CRC cells remains to be elucidated in the future. As we
noted previously, GAS5 exerts its molecular effects by targeting
multiple genes and signaling pathways. The antitumor effects and
potential mechanisms underlying GAS5-mediated macroautophagy or
miRNAs might be regulated by complicated signaling pathways
(18–20). A limitation of the present study
might be that our data only partly suggested that miR-34a
participated in mediating GAS5 suppression of macroautophagy and
induction of apoptosis in CRC cells through inhibiting SIRT1
expression and activating the mTOR signaling pathway, and more
studies are needed in the future.
It has been established that macroautophagy has a
dual role in regulating apoptosis of cancer cells (41). Although increasing evidence has
focused on the association between macroautophagy and progression
of malignant tumors, it is far from being clarified. The present
study indicated that MYH1485 suppressed CRC cell macroautophagy and
induced apoptosis, but rapamycin treatment caused the inverse
trend. More importantly, in combination with activation of GAS5,
MYH1485 enhanced the inhibitory effect on macroautophagy and
promotion of apoptosis, while rapamycin in combination with
activation of GAS5 attenuated the inhibitory effect on
macroautophagy and induction of apoptosis in CRC cells compared to
upregulation of GAS5 alone. These results showed that
GAS5-regulated macroautophagy developed a steady activation state
that exerted an antiapoptotic effect during CRC progression. Thus,
we thought that negative feedback regulation between GAS5 and its
target pathways might be involved in mediating macroautophagy
activation, but not excessive activation, which might be induced by
apoptosis of CRC cells.
A negative feedback regulatory loop might not only
amplify a response but also control a self-sustained mode that is
autonomous from the original stimuli (42). Recently, there has been a focus on
positive or negative feedback regulations between lncRNAs and their
target miRNAs (43). SIRT1 has been
reported to activate the mTOR pathway directly in different
conditions (23), and notably, GAS5
is one of the downstream targets that is negatively regulated by
the mTOR pathway (24). Thus, we
examined the effect of mTOR activation or silencing on the
GAS5/miR-34a axis and SIRT1 expression in CRC cells. Our data
indicated that silencing mTOR expression resulted in upregulation
of GAS5 and SIRT1 expression and downregulation of miR-34a
expression, while converse results were obtained when the mTOR
pathway was activated in CRC cells. Based on these results and
together with the inhibitory effect of exogenous overexpression of
GAS5 on miR-34a and promotive effect on SIRT1 and mTOR expression
in CRC cells, we proposed a GAS5/miR-34a/mTOR/SIRT1 negative
regulatory feedback loop in CRC cells that mediates CRC
progression. Our present study suggests that after deficient
expression of GAS5, the miR-34a/SIRT1 axis is activated and in turn
promotes mTOR pathway phosphorylation, which results in
accumulation of GAS5 transcripts. The GAS5/miR-34a/SIRT1/mTOR
negative regulatory feedback loop might partly explain why the CRC
cell macroautophagy was in an autonomously relative equilibrium
state, but not excessive activation state, which function as a
strong anti-apoptosis phenotype during human CRC progression.
In summary, we demonstrated that GAS5 acts as a
tumor-suppressive factor in progression of human CRC, and exogenous
overexpression of GAS5 inhibits tumorigenesis, at least in part
regulated through the miR-34a/SIRT1 axis. miR-34a participates in
regulating GAS5-suppressed CRC cell macroautophagy and induces
apoptosis through the mTOR/SIRT1 pathway. GAS5-mediated regulation
of macroautophagy maintains CRC cells in an equilibrium state that
protects against apoptosis. GAS5/miR-34a/SIRT1/mTOR forms a
negative regulatory feedback loop that provides new insight into
the mechanisms of CRC progression and the influence of
macroautophagy on the malignant behavior of CRC through external
factors. Macroautophagy may be a potential method to screen out
relevant target molecules and provide a reliable basis for the
clinical diagnosis and treatment of CRC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by The Science
Foundation of the Health Commission of Heilongjiang Province
(2018346).
Availability of data and materials
The data used in the present study are available
from the corresponding author upon reasonable request.
Authors' contributions
PFQ designed the experiments. HGZ and YW performed
the experiments. ZXZ analyzed the data. PFQ and FJW carried out the
literature review, drafted the manuscript and revised it critically
for important intellectual content. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All patients provided written informed consent
according to our institutional guidelines and the study protocol
was approved by the Institutional Review Board of Harbin Medical
University. Animal experiments were carried out in strict
accordance with the Harbin Medical University Institutional Animal
Care and Use Committee (KY2018-208).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
lncRNA
|
long non-coding RNA
|
|
GAS5
|
growth arrest specific 5
|
|
miR/miRNA
|
microRNA
|
|
MMP
|
matrix metalloproteinase
|
|
SIRT1
|
sirtuin 1
|
|
UTR
|
untranslated region
|
|
AOM
|
azoxymethane
|
|
PBS
|
phosphate-buffered saline
|
|
MDC
|
mono-dansylcadaverine
|
|
RT-qPCR
|
quantitative reverse transcription
polymerase chain reaction
|
|
NC
|
negative control
|
|
mTOR
|
mammalian target of rapamycin
|
|
CA
|
carbohydrate antigen
|
|
CEA
|
carcinoembryonic antigen
|
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar
|
|
2
|
Doubeni CA, Corley DA, Quinn VP, Jensen
CD, Zauber AG, Goodman M, Johnson JR, Mehta SJ, Becerra TA, Zhao
WK, et al: Effectiveness of screening colonoscopy in reducing the
risk of death from right and left colon cancer: A large
community-based study. Gut. 67:291–298. 2018. View Article : Google Scholar
|
|
3
|
Gibb EA, Brown CJ and Lam W L: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar
|
|
4
|
Toraih EA, Alghamdi SA, El-Wazir A, Hosny
MM, Hussein MH, Khashana MS and Fawzy MS: Dual biomarkers long
non-coding RNA GAS5 and microRNA-34a co-expression signature in
common solid tumors. PLoS One. 13:e01982312018. View Article : Google Scholar
|
|
5
|
Xue D, Zhou C, Lu H, Xu R, Xu X and He X:
lncRNA GAS5 inhibits proliferation and progression of prostate
cancer by targeting miR-103 through AKT/mTOR signaling pathway.
Tumor Biol. Oct 14–2016.(Epub ahead of print). doi:
10.1007/s13277-016-5429-8. View Article : Google Scholar
|
|
6
|
Liu L, Pang X, Shang W, Xie H, Feng Y and
Feng G: Long non-coding RNA GAS5 sensitizes renal cell carcinoma to
sorafenib via miR-21/SOX5 pathway. Cell Cycle. 18:257–263. 2018.
View Article : Google Scholar
|
|
7
|
Gao J, Liu M, Zou Y, Mao M, Shen T, Zhang
C, Song S, Sun M, Zhang S, Wang B, et al: Long non-coding RNA
growth arrest-specific transcript 5 is involved in ovarian cancer
cell apoptosis through the mitochondria-mediated apoptosis pathway.
Oncol Rep. 34:3212–3221. 2015. View Article : Google Scholar
|
|
8
|
Wen Q, Liu Y, Lyu H, Xu X, Wu Q, Liu N,
Yin Q, Li J and Sheng X: Long noncoding RNA GAS5, which acts as a
tumor suppressor via microRNA 21, regulates cisplatin resistance
expression in cervical cancer. Int J Gynecol Cancer. 27:1096–1108.
2017. View Article : Google Scholar
|
|
9
|
Ye K, Wang S, Zhang H, Han H, Ma B and Nan
W: Long noncoding RNA GAS5 suppresses cell growth and
epithelial-mesenchymal transition in osteosarcoma by regulating the
miR-221/ARHI pathway. J Cell Biochem. 118:4772–4781. 2017.
View Article : Google Scholar
|
|
10
|
Zheng Y, Song D, Xiao K, Yang C, Ding Y,
Deng W and Tong S: lncRNA GAS5 contributes to lymphatic metastasis
in colorectal cancer. Oncotarget. 7:83727–83734. 2016. View Article : Google Scholar
|
|
11
|
Zhang Z, Zhu Z, Watabe K, Zhang X, Bai C,
Xu M, Wu F and Mo YY: Negative regulation of lncRNA GAS5 by miR-21.
Cell Death Differ. 20:1558–1568. 2013. View Article : Google Scholar
|
|
12
|
Yang W, Hong L, Xu X, Wang Q, Huang J and
Jiang L: lncRNA GAS5 suppresses the tumorigenesis of cervical
cancer by downregulating miR-196a and miR-205. Tumour Biol.
39:1010428317711312016. View Article : Google Scholar
|
|
13
|
Guo C, Song WQ, Sun P, Jin L and Dai HY:
lncRNA-GAS5 induces PTEN expression through inhibiting miR-103 in
endometrial cancer cells. J Biomed Sci. 22:1002015. View Article : Google Scholar
|
|
14
|
Gao ZQ, Wang JF, Chen DH, Ma XS, Wu Y,
Tang Z and Dang XW: Long non-coding RNA GAS5 suppresses pancreatic
cancer metastasis through modulating miR-32-5p/PTEN axis. Cell
Biosci. 7:662017. View Article : Google Scholar
|
|
15
|
Li Y, Gu J and Lu H: The GAS5/miR-222 Axis
regulates proliferation of gastric cancer cells through the
PTEN/Akt/mTOR Pathway. Dig Dis Sci. 62:3426–3437. 2017. View Article : Google Scholar
|
|
16
|
Sun C, Wang FJ, Zhang HG, Xu XZ, Jia RC,
Yao L and Qiao PF: miR-34a mediates oxaliplatin resistance of
colorectal cancer cells by inhibiting macroautophagy via
transforming growth factor-β/Smad4 pathway. World J Gastroenterol.
23:1816–1827. 2017. View Article : Google Scholar
|
|
17
|
Qiao PF, Yao L and Zeng ZL:
Catalpol-mediated microRNA-34a suppresses autophagy and malignancy
by regulating SIRT1 in colorectal cancer. Oncol Rep. 43:1053–1066.
2020.
|
|
18
|
Gu J, Wang Y, Wang X, Zhou D, Wang X, Zhou
M and He Z: Effect of the lncRNA GAS5-MiR-23a-ATG3 axis in
regulating autophagy in patients with breast cancer. Cell Physiol
Biochem. 48:194–207. 2018. View Article : Google Scholar
|
|
19
|
Li L, Huang C, He Y, Sang Z, Liu G and Dai
H: Knockdown of Long non-coding RNA GAS5 increases miR-23a by
targeting ATG3 involved in autophagy and cell viability. Cell
Physiol Biochem. 48:1723–1734. 2018. View Article : Google Scholar
|
|
20
|
Zhang N, Yang GQ, Shao XM and Wei L: GAS5
modulated autophagy is a mechanism modulating cisplatin sensitivity
in NSCLC cells. Eur Rev Med Pharmacol. 20:2271–2277. 2016.
|
|
21
|
Sobin L, Gospodarowicz M and Wittekind C:
TNM Classification of Malignant Tumors. 7th edition. UICC
International Union Against Cancer. 2009, simplehttps://media.wiley.com/product_data/coverImage300/60/14443589/1444358960.jpg
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Zhang T, Du X, Zhao L, He M, Lin L, Guo C,
Zhang X, Han J, Yan H, Huang K, et al: SIRT1 facilitates primordial
follicle recruitment independent of deacetylase activity through
directly modulating Akt1 and mTOR transcription.
FASEB J. 33:14703–14716. 2019. View Article : Google Scholar
|
|
24
|
Pickard MR and Williams GT: Molecular and
cellular mechanisms of action of tumour suppressor GAS5 lncRNA.
Genes (Basel). 6:484–499. 2015. View Article : Google Scholar
|
|
25
|
Schneider C, King RM and Philipson L:
Genes specifically expressed at growth arrest of mammalian cells.
Cell. 54:787–793. 1988. View Article : Google Scholar
|
|
26
|
Li W, Zhao W, Lu Z, Zhang W and Yang X:
Long noncoding RNA GAS5 promotes proliferation, migration, and
invasion by regulation of miR-301a in esophageal cancer. Oncol Res.
26:1285–1294. 2018. View Article : Google Scholar
|
|
27
|
Tao R, Hu S, Wang S, Zhou X, Zhang Q, Wang
C, Zhao X, Zhou W, Zhang S, Li C, et al: Association between indel
polymorphism in the promoter region of lncRNA GAS5 and the risk of
hepatocellular carcinoma. Carcinogenesis. 36:1136–1143. 2015.
View Article : Google Scholar
|
|
28
|
Jia YL, Shi L, Zhou JN, Fu CJ, Chen L,
Yuan HF, Wang YF, Yan XL, Xu YC, Zeng Q, et al: Epimorphin promotes
human hepatocellular carcinoma invasion and metastasis through
activation of focal adhesion kinase/extracellular signal-regulated
kinase/matrix metalloproteinase-9 axis. Hepatology. 54:1808–1818.
2011. View Article : Google Scholar
|
|
29
|
Christodoulou F, Raible F, Tomer R,
Simakov O, Trachana K, Klaus S, Snyman H, Hannon GJ, Bork P and
Arendt D: Ancient animal microRNAs and the evolution of tissue
identity. Nature. 463:1084–1088. 2010. View Article : Google Scholar
|
|
30
|
Borrell B: How accurate are cancer cell
lines? Nature. 463:8582010. View Article : Google Scholar
|
|
31
|
Li C, Liu T, Zhang Y, Li Q and Jin LK:
lncRNA-ZDHHC8P1 promotes the progression and metastasis of
colorectal cancer by targeting miR-34a. Eur Rev Med Pharmacol Sci.
23:1476–1486. 2019.
|
|
32
|
Kim EJ, Kho JH, Kang MR and Um SJ: Active
regulator of SIRT1 cooperates with SIRT1 and facilitates
suppression of p53 activity. Mol Cell. 28:277–290. 2007. View Article : Google Scholar
|
|
33
|
Romeo-Guitart D, Leiva-Rodriguez T, Forés
J and Casas C: Improved motor nerve regeneration by
SIRT1/Hif1a-mediated autophagy. Cells. 8:13542019. View Article : Google Scholar
|
|
34
|
Liu K, Huang J, Xie M, Yu Y, Zhu S, Kang
R, Cao L, Tang D and Duan X: miR-34a regulates autophagy and
apoptosis by targeting HMGB1 in the retinoblastoma cell. Autophagy.
10:442–452. 2014. View Article : Google Scholar
|
|
35
|
Tanida I, Ueno T and Kominami E: LC3 and
autophagy. Methods Mol Biol. 445:77–88. 2008. View Article : Google Scholar
|
|
36
|
Fujiwara N, Usui T, Ohama T and Sato K:
Regulation of beclin 1 protein phosphorylation and autophagy by
protein phosphatase 2A (PP2A) and death-associated protein kinase
3. J Biol Chem. 13:10858–10866. 2016. View Article : Google Scholar
|
|
37
|
Letai AG: Diagnosing and exploiting
cancer's addiction to blocks in apoptosis. Nat Rev Cancer.
8:121–132. 2008. View Article : Google Scholar
|
|
38
|
Reyna DE, Garner TP, Lopez A, Kopp F,
Choudhary GS, Sridharan A, Narayanagari SR, Mitchell K, Dong B,
Bartholdy BA, et al: Direct activation of BAX by BTSA1 overcomes
apoptosis resistance in acute myeloid leukemia. Cancer Cell.
32:490–505.e10. 2017. View Article : Google Scholar
|
|
39
|
Shim MK, Yoon HY, Lee S, Jo MK, Park J,
Kim JH, Jeong SY, Kwon IC and Kim K: Caspase-3/-7-specific
metabolic precursor for bioorthogonal tracking of tumor apoptosis.
Sci Rep. 7:166352017. View Article : Google Scholar
|
|
40
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar
|
|
41
|
Maiuri M, Zalckvar E, Kimchi A and Kroemer
G: Self-eating and self-killing: Crosstalk between autophagy and
apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007. View Article : Google Scholar
|
|
42
|
Misso G, Zarone MR, Lombardi A, Grimaldi
A, Cossu AM, Ferri C, Russo M, Vuoso DC, Luce A, Kawasaki H, et al:
miR-125b Upregulates miR-34a and sequentially activates stress
adaption and cell death mechanisms in multiple myeloma. Mol Ther
Nucleic Acids. 16:391–406. 2019. View Article : Google Scholar
|
|
43
|
Tian F, Wang J, Zhan Z and Yang J: lncRNA
SNHG7/miR-34a-5p/SYVN1 axis plays a vital role in proliferation,
apoptosis and autophagy in osteoarthritis. Biol Res. 53:92020.
View Article : Google Scholar
|