Breast cancer has been identified as one of the
leading causes of cancer-related deaths worldwide. Abnormalities in
proteins result in malignant transformation of cells that results
in 25–40% of recurrence and metastasis of cancer (1). Breast cancer is a common malignant
tumor and its rate of incidence was the highest among tumors
detected in women in 2019 (2).
Approximately 627,000 people are expected to succumb
to breast cancer in 2019. Due to the lack of specific therapeutic
targets available for triple-negative breast cancer (TNBC), the
prognosis of TNBC remains unsatisfactory. The emergence of
anti-PD-L1/PD-1 therapeutics has shown promise for the treatment of
TNBC (3). In the present study, the
advancements in the efficacy of PD-L1 against TNBC are described.
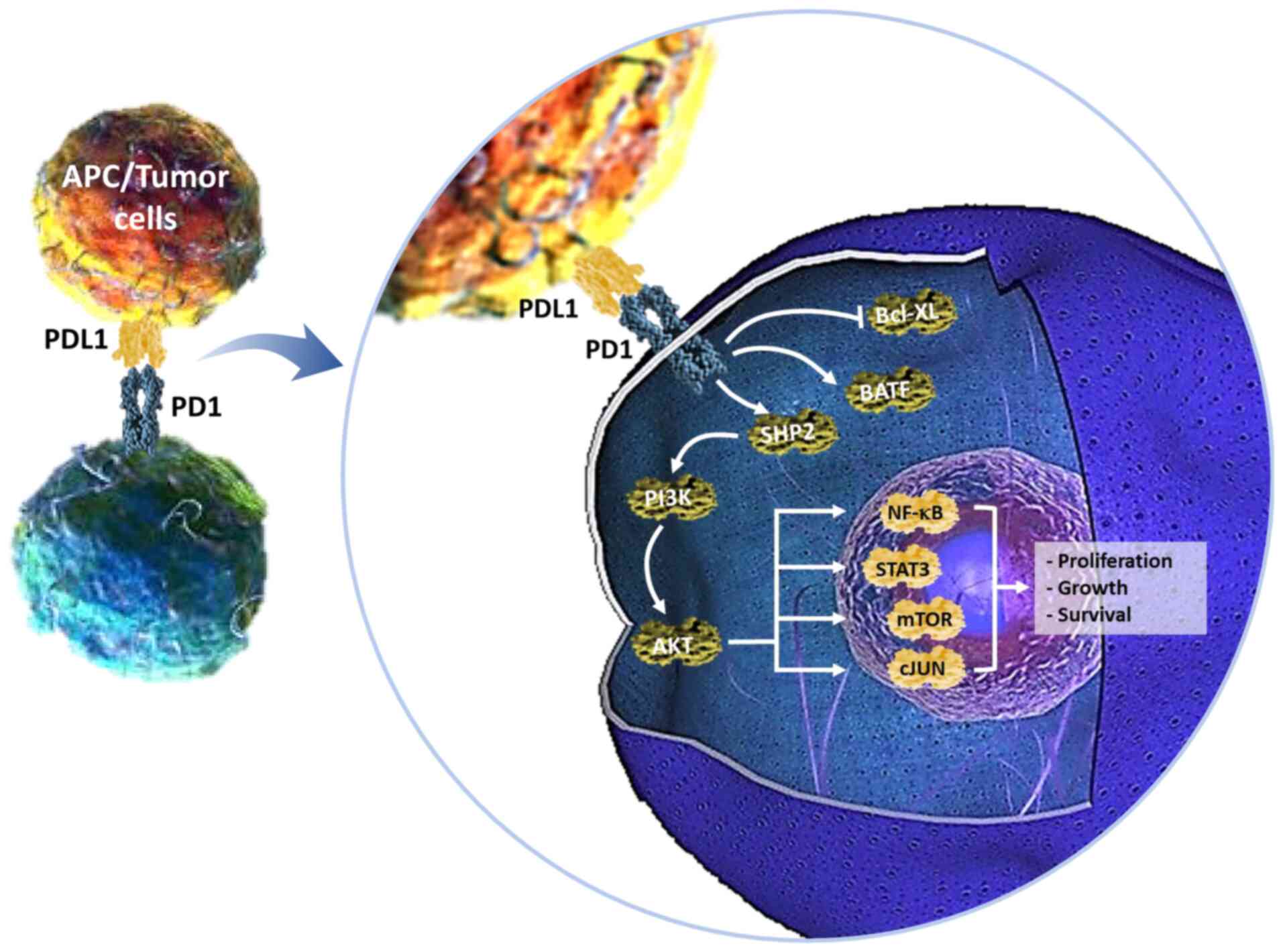
Programmed death ligand 1 (PD-L1) is a ligand of programmed cell
death-1 (PD-1) and is expressed on various tumors and immune cells.
Once PD-L1 binds to PD-1, it inhibits T-cell migration and
proliferation and the secretion of cytotoxic mediators, thereby
limiting its killing effect on tumor cells (3). PD-L1 has been revealed to be highly
expressed in a variety of tumors and enhance antitumor immunity by
inhibiting PD-L1 (4). The
development and clinical application of targeted drugs against
signaling pathways associated with the occurrence and development
of breast cancer have become a hot spot in research on breast
cancer treatment (5). Targeted
therapy has better clinical efficacy and safety than cytotoxic
drugs (6). The present study
reviewed the current molecular targeted therapeutics in treating
breast cancer.
Tumor immunotherapy is a method of treatment that
has been developed in recent years. It stimulates and regulates
immune function, enhances antitumor ability, and controls and kills
tumor cells (7). Immunotherapy has
attracted attention owing to the importance of antitumor immunity,
diverse mechanisms in escape of tumors from immune surveillance,
continuous discovery of novel therapeutic targets and
immunotherapies (8,9). Tumor immunotherapy can be divided into
active and passive immunotherapy. Active immunotherapy employs
tumor vaccines to mimic tumor antigens to activate immune effects
on tumors, thereby directly or indirectly promoting specific
antitumor immune responses in humans (10). Passive immunotherapy includes the
use of monoclonal antibodies, adoptive immune cells, and cytokines.
Monoclonal antibody therapy involves the administration of specific
antibodies to stimulate host immune response against tumor antigens
(11,12). Adoptive immune cell therapy
separates and expands immune cells to induce the production of
cytokines before introducing the cells into patients to increase
the abundance of immune cells and enhance their anticancer function
(13). Cytokine therapy involves
the direct injection of cytokines into the human body, which
enhances antitumor function in immune cells (14,15).
Compared to traditional methods of treatment, tumor immunotherapy
has specific advantages: Tumor immune-related monoclonal antibodies
and tumor vaccines exhibit fewer adverse reactions, strong
specificity, and promising clinical application (13).
An increase in research on immune checkpoints has
been observed in recent years. Immune checkpoints are inhibitory
signaling pathways present in the immune system that regulate the
persistence and intensity of immune response, maintain autoimmune
tolerance, and avoid tissue damage (16,17).
Dysfunction of key negative regulatory molecules during T-cell
activation is important for the tolerance and escape of tumor
immunity. Inhibition of the immune checkpoint can reverse the
immunosuppressive state of the tumor microenvironment and enhance
the clearance of tumor cells (18).
Immunological checkpoints include cytotoxic T lymphocyte-associated
antigen-4 (CTLA-4), PD-1, B and T lymphocyte attenuator (BTLA), and
lymphocyte activation gene 3 (LAG3). The FDA-approved immunological
checkpoint inhibitor, ipilimumab, that targets CTLA-4 is used to
treat melanoma (19,20). Researchers are further identifying
novel therapeutic targets to regulate immune checkpoints. The most
important monoclonal antibody currently in use targets the immune
checkpoint PD-1 and its ligand PD-L1 (21). PD-L1/PD-1-related immunotherapy has
become a hotspot for research on tumor immunotherapy.
PD-1 is a type I transmembrane protein expressed on
the surface of activated T cells, B cells, monocytes and dendritic
cells and is composed of extracellular, hydrophobic transmembrane,
and cytoplasmic regions (22). Its
extracellular domain consists of a single IgV-like domain with an
immunoreceptor tyrosine-based inhibitory motif (ITIM) and
immunoreceptor tyrosine-based motif (23). ITIM can be commonly found in
numerous immunosuppressive receptors (24). The immunosuppressive function of
PD-1 is primarily exerted via ITIM. PD-L1 and PD-L2 are the ligands
of PD-1 (25). PD-L1 is the primary
ligand that is upregulated in various solid tumors. This reduces
the infiltration of CD4+ and CD8+ T cells
into tumors and concomitant cytokine production. PD-L1 is a member
of the B7 superfamily and a type I transmembrane glycoprotein
(26,27). PD-L1 is expressed in various tumors,
such as urothelial, ovarian, breast, cervical, colorectal,
pancreatic, gastric, melanoma, malignant glioma, and non-small cell
lung cancers among others. This suggests the involvement of PD-1
signaling in tumors for immune evasion (7,28–30).
PD-1/PD-Ls exerts a negative immunomodulatory effect. The surface
of tumor cells in the tumor microenvironment exhibit increased
expression of PD-L1 that binds to PD-1 on activated T cells, which
leads to apoptosis or immunological inactivation of tumor
antigen-specific T cells, thereby inhibiting immune response and
promoting the evasion of tumor cells (31).
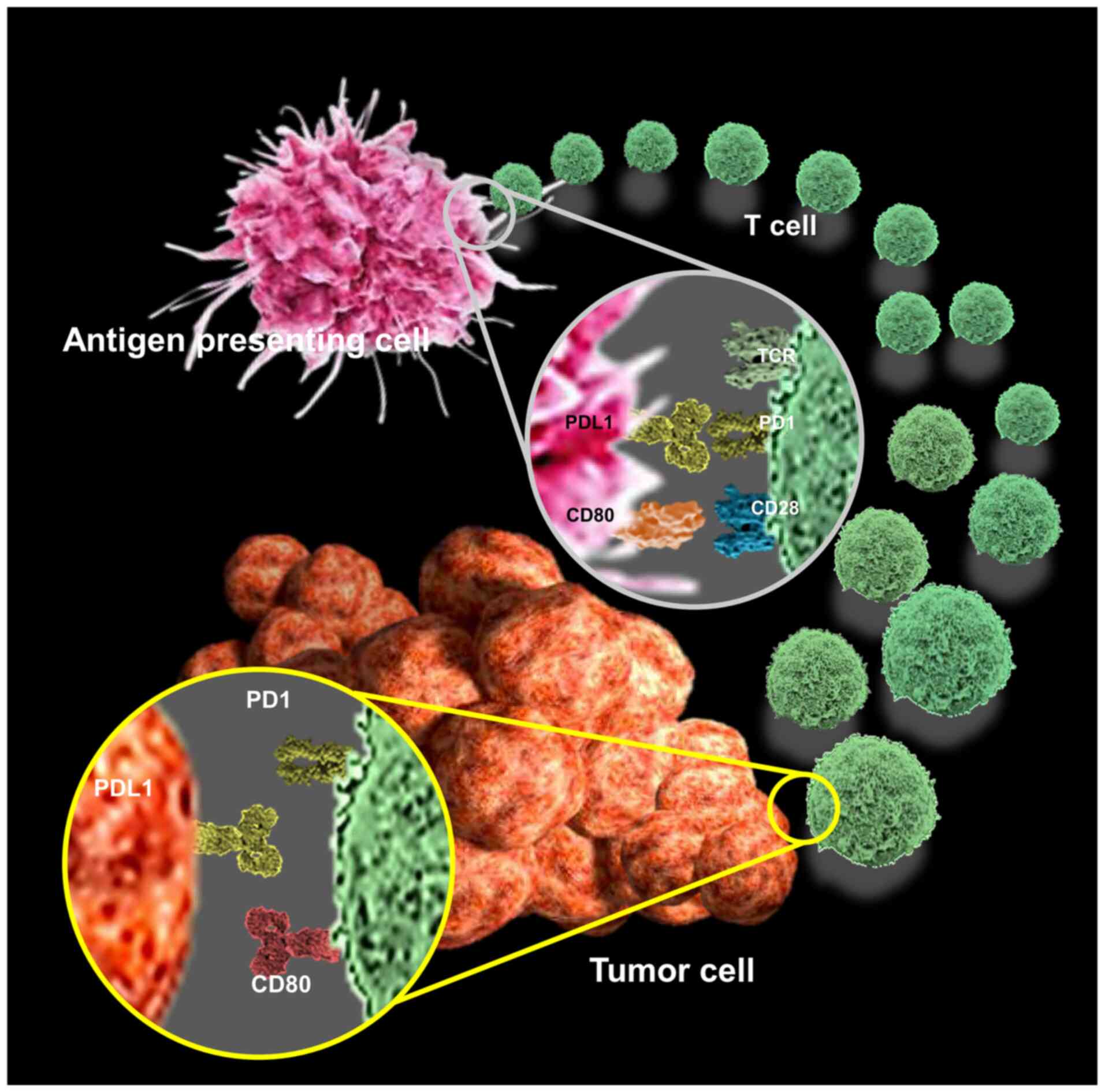
PD-L1 is expressed on the surface of various tumor
and immune cells, such as T, B, and dendritic cells (13). Tumor cells bind to PD-1 on the
surface of tumor infiltrating lymphocytes (TILs) via PD-L1.
Activation of TILs enables immunosuppressive signaling, inhibits
T-cell migration and proliferation, secretion of cytotoxic
mediators, induces T-cell depletion, and limits its antitumor
effects, thereby resulting in immune evasion (32,33).
Decreased binding of PD-L1 to PD-1 reverses immune escape, enhances
antitumor immunity, and inhibits tumor progression (34). However, PD-L1/PD-1-targeted
immunotherapy has been revealed to be effective in clinical trials
of various tumors, suggesting that the PD-L1/PD-1 pathway plays an
important role in tumor progression (13). This targeted immunotherapy has the
potential to improve the prognosis of cancer patients by inhibiting
the PD-L1/PD-1 pathway (35–37).
Thus, it is imperative to further identify and develop
anti-PD-L1/PD-1 therapeutics for TNBC.
Detection of PD-L1 levels in tumors predicts patient
response to anti-PD-L1/PD-1 monoclonal antibody therapy. This
allows the screening of selective patients to undergo immunotherapy
to reduce unnecessary waste of resources and over-treatment
(38). Using the PD-1 monoclonal
antibody, pembrolizumab, in patients with non-small cell lung
cancer has revealed a >50% correlation between the expression of
PD-L1 in tumor cells and increased efficacy (39). Immunohistochemistry (IHC) is the
most commonly used method for detecting the expression of PD-L1 in
tumors. IHC is a simple method that is associated with reduced time
for sample processing, low cost, and enhanced visualization.
However, in recent years the reliability and reproducibility of
this technique have come into question. A disadvantage is with
tissue organization (40). Studies
have revealed that surgically resected preoperative specimens for
tissue biopsy differentially express PD-L1, and biopsy specimens
underestimate the expression of PD-L1 as compared to the expression
in intraoperative resected specimens (41). PD-L1 levels are affected by focal
expression, genetic heterogeneity, and a variety of complex factors
within the tumor (42). A routine
diagnostic biopsy for measuring PD-L1 expression is highly likely
to be a false negative result that biases the sensitivity of PD-L1
targeted therapy (43). Moreover,
there is no standardized antibody currently available for the
detection of PD-L1. Different investigators use different detection
antibodies whose combined antigenic epitopes do not match. Thus,
the same sample may show opposing results. Owing to these
differences, an antibody from an effective clinical trial may not
be reliable for other patient populations (44). Finally, setting a threshold for a
positive readout is tricky and there is no standard for determining
the expression of PD-L1 (45).
Researchers have compared the efficacy of
determining PD-L1 expression using IHC and quantitative
immunofluorescence (QIF). QIF has been revealed to be more
consistent and reproducible than IHC, and the measurement of PD-L1
levels by QIF has been demonstrated to be more objective (44). Detecting the mRNA levels of PD-L1 in
tumors is also important. A novel RNA detection technology,
RNAScope, has been used to determine the mRNA levels of PD-L1 in
636 patients with stage I–III breast cancer. Surface expression of
PD-L1 on circulating tumor cells was successfully detected in
patients with hormone receptor-positive, HER-2-negative metastatic
breast cancer (46,47). Further developing this technology
may prove useful as a non-invasive technique for measuring PD-L1
expression in a liquid biopsy format to screen and treat patients
with PD-L1/PD-1 immunotherapy by means of clinical trials in the
future.
The expression of PD-L1 on the surface of immune
cells is relatively constant, whereas PD-L1 expression on the
surface of tumor cells is dynamic (48). In addition to the effects of
interferon-γ, PD-L1 expression is also affected by signaling
pathways, chemotherapy, radiation therapy, as well as other factors
(49). In anaplastic lymphoma
kinase (ALK)-positive T-cell lymphoma, the oncogene NPM/ALK was
revealed to activate transcription transducer and activator of
transcription 3 (STAT3) (50). It
has been revealed to bind to the promoter of PD-L1 to upregulate
PD-L1 and promote immunosuppression (50,51).
In breast cancer, the inactivation of PTEN or
mutation of PI3K leads to PI3K activation, which in turn activates
the downstream pathways Akt and mTOR and promotes PD-L1
transcription and protein expression in a ribosomal protein S6
Kinase 1 (S6K1)-dependent manner (52,53).
Tumor cells escape immune surveillance and produce immune
resistance (54). In melanoma,
tumor cells that are tolerant to BRAF gene inhibitors were revealed
to promote PD-L1 expression by activating MAPK signaling via the
c-Jun and STAT3 pathways (55).
Moreover, tumor intervention also affects PD-L1 expression on tumor
cells. Using chemotherapeutics, such as doxorubicin, has been
revealed to downregulate PD-L1 on the surface of breast cancer
cells. The expression of PD-L1 in the nucleus was increased; this
may lead to chemoresistance and inhibition of apoptosis of tumor
cells. The inhibition of PD-L1, doxorubicin-induced apoptosis was
revealed to be increased (55,56).
However, chemotherapeutics, such as paclitaxel, were reveealed to
upregulate PD-L1 on the surface of ovarian cancer cells via NF-κB
signaling and inhibition of T-cell function, thereby leading to
immune evasion. Combining paclitaxel and PD-L1/PD-1 inhibitors was
revealed to prolong patient survival (Fig. 1) (57). In addition, radiation therapy also
affects the expression of PD-L1. There is increased surface
expression of PD-L1 on metastatic cancer cells that are resistant
to radiation therapy, resulting in the depletion of TILs and
therapeutic resistance (58).
Inhibiting PD-L1/PD-1 signaling during radiation therapy reverses
this phenotype of T-cell depletion and promotes T-cell
proliferation (59,60). This indicates that it is necessary
to concurrently inhibit PD-L1/PD-1 signaling during chemotherapy
and radiation therapy to reduce the immune resistance of tumor
cells, enhance the therapeutic effect, and improve the prognosis of
patients.
PD-L1 is overexpressed in most breast cancers,
especially TNBC tissues, as compared to its expression in normal
breast tissue (59,61–64).
Previous research revealed that PD-L1 was expressed in 45% of
breast cancers and 59% of TNBCs among 116 breast cancer tissues.
Overexpression of PD-L1 has been revealed to be associated with
poor prognosis (63), larger
tumors, higher tumor grade, estrogen receptor (ER)-negative,
progesterone receptor-negative, and HER-2-positive status, cell
proliferation, and an increased abundance of TILs in patients with
breast cancer (59,64,65).
Using high-throughput analysis of 650 breast cancer tissue
microarrays, it was revealed that patients with breast cancer and
overexpression of PD-L1 had significantly shorter overall survival
(OS) (66). Previous research has
demonstrated that PD-L1 expression is associated with prolonged
recurrence-free survival (62). In
patients with TNBC, overexpression of PD-L1 was also associated
with metastasis-free survival and specific OS. The higher the
expression of PD-L1, the higher the reactivity to chemotherapy
(65). The potential of PD-L1 as an
independent prognostic factor is unclear. This could be attributed
to a variety of factors, such as detection methods and sample
heterogeneity; however, it is widely recognized that PD-L1 is
associated with factors related to the poor prognosis of breast
cancer (42). Tumors overexpressing
PD-L1 are often accompanied by the infiltration of PD-1-positive
TILs that are associated with shortened OS, indicating a poor
prognosis of breast cancer (67,68).
This further highlights the need for utilizing PD-L1/PD-1 signaling
in developing efficacious therapeutics for the treatment of
TNBC.
Drugs targeted to block PD-1 signaling have
exhibited sustained clinical activity in numerous advanced solid
tumors (Fig. 2) (71). The use of the monoclonal antibody
BMS-936559 to block PD-L1 in 160 patients with advanced solid
tumors during phase I clinical trials revealed an objective
response rate of 6–17% (72). The
objective response rates of using the PD-1-targeting antibody,
nivolumab, in the treatment of advanced non-small cell lung cancer,
bladder cancer, chondrosarcoma, melanoma, and renal cell carcinoma
were revealed to be 18, 28 and 27%, respectively (35,73–76).
The KEYNOTE-028 trial evaluated the safety and efficacy of
pembrolizumab in metastatic ER+ breast cancer. PD-L1 was
expressed in 19% of the samples and the efficacy of treatment in 25
evaluable patients was analyzed. The total effective rate was 12%
(77). Nanda et al evaluated
the safety and efficacy of pembrolizumab in 32 patients with
advanced TNBC who were positive for PD-L1. Most patients received
1–3 cycles of chemotherapy before being administered pembrolizumab.
A minority of patients (21.9%) were administered five or more
cycles of chemotherapy. Efficacy analysis of 27 evaluable patients
revealed a total effective rate of 18.5%, including one patient
with complete response and two patients exhibiting partial
remission. Most of the adverse reactions were grade 1–2, including
joint pain, fatigue, muscle pain, and nausea (78). The ORR for 21 PD-L1+ patients with
metastatic triple-negative breast cancer evaluable for efficacy was
19%, including 2 CRs and 2 PRs; 3 of 4 of these responses were
ongoing at the time of data cutoff. The JAVELIN study tested the
efficacy of the anti-PD-L1 antibody, avelumab, on patients with a
variety of all breast cancer types, regardless of the extent of
PD-L1 expression. ER+/HER2− patients in the
TNBC group (58 cases) had a response rate of 8.6%. Among the 72
patients and 26 HER2+ patients, the response rates were
2.8 and 3.8%, respectively. Preliminary results have revealed that
PD-L1-positive tumors exhibit a higher response rate (77).
TILs are an independent prognostic factor for
improved OS, reduced distant recurrence, and increased
metastasis-free survival in newly diagnosed patients with TNBC
(79). Retrospective analysis of
several large clinical trials and randomized neoadjuvant studies
have demonstrated that high abundance of TILs in tumors have
predictive effects on neoadjuvant chemotherapy for pCR or increased
disease-free survival and OS (80).
In addition, a recent retrospective analysis confirmed that the
presence of TILs in patients with residual lesions after
neoadjuvant chemotherapy can predict patient outcomes (81). Previous research has confirmed a
significant association between activated Ras-MAPK signaling and
low abundance of TILs in TNBC patients. Activated MEK inhibits
IFN-γ-induced antigen presentation. This is because the Ras/MAPK
pathway initiates immune evasion, and inhibition of MEK signaling
upregulates PD-L1 expression on TNBC cells (82). Combining MEK and PD-L1/PD-1
inhibitors was revealed to enhance the antitumor immune response in
a mouse model of breast cancer (82). Sagiv-Barfi et al used
ibrutinib in combination with an anti-PD-L1 antibody, ibrutinib, to
treat mice with TNBC that were not intrinsically sensitive to
ibrutinib. The combination was determined to significantly delay
tumor growth and improve survival relative to drug administration
alone (83).
For patients with metastatic breast cancer,
monotherapy, including vaccination or monoclonal antibodies against
immune checkpoints, such as anti-PD-1 or PD-L1, may not be
sufficient to eradicate the lesion (84). Vaccines stimulate antigen-specific T
cells after combination therapy. Monoclonal antibodies targeting
the immune checkpoint allow antigen-specific T cells to
proliferate. This approach may enhance antitumor immune responses,
thereby increasing tumor cell death and improving patient outcomes
(85). IHC of biopsy specimens from
42 patients evaluated the expression of PD-L1 on the surface of
tumor cells. Notably, none of the 17 PD-L1-negative patients had an
objective response to PD-1 therapy. Despite the small patient
cohort, this data suggests that PD-L1 expression on the surface of
tumor cells may be a biomarker for the efficacy of anti-PD-1
therapy (35). Melanoma patients
and PD-L1-positive tumor patients treated with nivolumab
demonstrated higher objective response rates, longer
progression-free survival, and improved OS. Ongoing and future
studies on the efficacy of nivolumab and other anti-PD-L1/PD-1
drugs will help confirm whether PD-L1 is a potent biomarker for
predicting responsiveness to anti-PD-L1/PD-1 therapy (85). Chatterjee et al have revealed
that the non-invasive detection of PD-L1 expression in the tumor
microenvironment will enable the appropriate administration of
monoclonal antibodies targeting PD-L1/PD-1 (86).
Overexpression of PD-L1 and PD-1 and abundance of
TILs suggest that PD-L1/PD-1 are reliable candidates for PD-L1/PD-1
immunotherapy (35,38,72,87,88).
The current treatment methods for breast cancer primarily include
surgery, radiation therapy, chemotherapy, endocrine therapy, and
targeted therapy. There are no specific and effective treatment
options for TNBC patients owing to the lack specific targets,
resulting in unsatisfactory disease prognosis. To that extent, the
emergence of PD-L1 as a novel target is an exciting avenue in TNBC
treatment (58,70).
A phase 1 clinical trial comprising patients with
metastatic TNBC was reported in 2015; the monoclonal antibody,
atezolizumab, targeting PD-L1 was demonstrated to be safe,
tolerable, and consistently exhibited antitumor effects (89). In addition to the PD-L1/PD-1
antibody, clinical trials have used other immunological targets to
enhance its antitumor effects, such as the CTLA-4 monoclonal
antibody ipilimumab (90). CTLA-4
monoclonal antibodies block negative co-stimulatory signaling,
promote the activation and proliferation of tumor-specific T cells,
and prevent T-cell disability. PD-1 and PD-L1-targeting monoclonal
antibodies reverse the immunosuppressive state of the tumor
microenvironment by inhibiting the PD-L1/PD-1 axis. Compared with
the CTL-4 monoclonal antibody, the PD-1 and PD-L1 monoclonal
antibodies are associated with reduced adverse reactions, improved
tolerance, and safety. Thus, PD-L1 antibodies are also used in
combination with chemotherapeutics, such as atezolizumab in
combination with white protein and abraxane or MEDI4736
(durvalumab) in combination with ibrutinib, in patients with TNBC
(91). Several clinical trials are
underway to evaluate the efficacy of PD-L1/PD-1 monoclonal
antibodies in treating TNBC (Table
I).
The past few decades have seen significant progress
in the treatment of breast cancer. This has been associated with
decreased rates of mortality rate in patients with breast cancer
and improved quality of life. However, the prognosis for most
patients with TNBC remains poor (92). Exploring TNBC-specific therapeutic
targets is key to prolonging patient survival and further improving
quality of life. PD-L1/PD-1 signaling is currently a research
hotspot for tumor immunotherapy (84). Although PD-L1/PD-1 immunotargeting
drugs have not been approved by the FDA for the treatment of TNBC,
clinical trials have revealed encouraging results. Thus, PD-L1/PD-1
immunotargeting drugs are expected to be used in the treatment of
patients with TNBC in the near future (92).
Despite this, several issues remain worthy of
further discussion: i) PD-L1 detection methods, and outcome
judgment criteria need to be further improved to accurately
determine the expression of PD-L1 and select patients to avoid
over- or ineffective treatment. ii) Recently, new members of the B7
family other than PD-L1/PD-1 have been discovered and may form
novel targets for tumor immunotherapy. For example, PD-L2 is also
expressed in TNBC; thus, anti-PD-1 antibody may be ineffective for
PD-L1-negative patients. The reason why the expressed patient is
effective; B7-H3 and B7-H4 have immunosuppressive roles and are
expressed in tumor cells. Thus, they are excellent novel candidates
to be used in tumor immunotherapy; however, their physiological
functions remain to be studied (93). iii) The accuracy and adverse
reactions of PD-L1/PD-1 immunotargeting therapy need to monitored.
It is important to target PD-L1/PD-1-specific drugs to tumor cells
during treatment and reduce the impact on autoimmune function. The
growing research on these candidates will soon establish
immunotherapy as an indispensable method for the comprehensive
treatment of cancers.
Not applicable.
This work was supported by grants from the Ministry
of Science and Technology (grant nos. MOST 106-2314-B-442-001-MY3,
MOST 109-2314-B-442-001 and MOST 109-2314-B-075B-002), the National
Health Research Institutes (grant no. NHRI-109BCCO-MF-202015-01)
and Show Chwan Memorial Hospital, Taiwan (grant nos. SRD-109023 and
RD107063).
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
CJL, LTL, MFH and PYC conceived the study. CJL and
LTL wrote the study. CJL and PYC reviewed and edited the study. PYC
supervised the study. All authors reviewed the final version of the
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Seyfried TN and Huysentruyt LC: On the
origin of cancer metastasis. Crit Rev Oncog. 18:43–73. 2013.
View Article : Google Scholar
|
|
2
|
Harbeck N, Penault-Llorca F, Cortes J,
Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J and Cardoso F:
Breast cancer. Nat Rev Dis Primers. 5:662019. View Article : Google Scholar
|
|
3
|
Nowicki TS, Hu-Lieskovan S and Ribas A:
Mechanisms of resistance to PD-1 and PD-L1 blockade. Cancer J.
24:47–53. 2018. View Article : Google Scholar
|
|
4
|
Ju X, Zhang H, Zhou Z and Wang Q:
Regulation of PD-L1 expression in cancer and clinical implications
in immunotherapy. Am J Cancer Res. 10:1–11. 2020.
|
|
5
|
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z,
Zhang L, Ji X, Liu W, Huang B, Luo W, et al: Breast cancer
development and progression: Risk factors, cancer stem cells,
signaling pathways, genomics, and molecular pathogenesis. Genes
Dis. 5:77–106. 2018. View Article : Google Scholar
|
|
6
|
Zhang Z, Zhou L, Xie N, Nice EC, Zhang T,
Cui Y and Huang C: Overcoming cancer therapeutic bottleneck by drug
repurposing. Signal Transduct Target Ther. 5:1132020. View Article : Google Scholar
|
|
7
|
Li CJ, Liao WT, Wu MY and Chu PY: New
insights into the role of autophagy in tumor immune
microenvironment. Int J Mol Sci. 18:15662017. View Article : Google Scholar
|
|
8
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunitys roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011. View Article : Google Scholar
|
|
9
|
Couzin-Frankel J: Breakthrough of the year
2013. Cancer immunotherapy. Science. 342:1432–1433. 2013.
View Article : Google Scholar
|
|
10
|
Dong Y, Sun Q and Zhang X: PD-1 and its
ligands are important immune checkpoints in cancer. Oncotarget.
8:2171–2186. 2017. View Article : Google Scholar
|
|
11
|
Matsushita M and Kawaguchi M:
Immunomodulatory effects of drugs for effective cancer
immunotherapy. J Oncol. 2018:86534892018. View Article : Google Scholar
|
|
12
|
Lin SC, Chu PY, Liao WT, Wu MY, Tsui KH,
Lin LT, Huang CH, Chen LL and Li CJ: Glycyrrhizic acid induces
human MDA-MB-231 breast cancer cell death and autophagy via the
ROS-mitochondrial pathway. Oncol Rep. 39:703–710. 2018.
|
|
13
|
Song Y, He L, Wang Y, Wu Q and Huang W:
Molecularly targeted therapy and immunotherapy for hormone
receptor-positive/human epidermal growth factor receptor 2-negative
advanced breast cancer (review). Oncol Rep. 44:3–13. 2020.
|
|
14
|
Chew V, Toh HC and Abastado JP: Immune
microenvironment in tumor progression: Characteristics and
challenges for therapy. J Oncol. 2012:6084062012. View Article : Google Scholar
|
|
15
|
Chen SN, Chang R, Lin LT, Chern CU, Tsai
HW, Wen ZH, Li YH, Li CJ and Tsui KH: MicroRNA in ovarian cancer:
Biology, pathogenesis, and therapeutic opportunities. Int J Environ
Res Public Health. 16:15102019. View Article : Google Scholar
|
|
16
|
Li YT, Lee WL and Tsui KH: Endometrial
thickness still presents a best reference to predict endometrial
cancer. Taiwan J Obstet Gynecol. 55:148–149. 2016. View Article : Google Scholar
|
|
17
|
Wu MY, Yiang GT, Cheng PW, Chu PY and Li
CJ: Molecular targets in hepatocarcinogenesis and implications for
therapy. J Clin Med. 7:2132018. View Article : Google Scholar
|
|
18
|
Xia A, Zhang Y, Xu J, Yin T and Lu XJ: T
cell dysfunction in cancer immunity and immunotherapy. Front
Immunol. 10:17192019. View Article : Google Scholar
|
|
19
|
Mahoney KM, Rennert PD and Freeman GJ:
Combination cancer immunotherapy and new immunomodulatory targets.
Nat Rev Drug Discov. 14:561–584. 2015. View
Article : Google Scholar
|
|
20
|
Roncati L: Microsatellite instability
predicts response to anti-PD1 immunotherapy in metastatic melanoma.
Acta Dermatovenerol Croat. 26:341–343. 2018.
|
|
21
|
Ribas A: Tumor immunotherapy directed at
PD-1. N Engl J Med. 366:2517–2519. 2012. View Article : Google Scholar
|
|
22
|
Okazaki T and Honjo T: The PD-1-PD-L
pathway in immunological tolerance. Trends Immunol. 27:195–201.
2006. View Article : Google Scholar
|
|
23
|
Sun H, Sun C and Xiao W: Expression
regulation of co-inhibitory molecules on human natural killer cells
in response to cytokine stimulations. Cytokine. 65:33–41. 2014.
View Article : Google Scholar
|
|
24
|
Hirsch I, Janovec V, Stranska R and
Bendriss-Vermare N: Cross talk between inhibitory immunoreceptor
tyrosine-based activation motif-signaling and toll-like receptor
pathways in macrophages and dendritic cells. Front Immunol.
8:3942017. View Article : Google Scholar
|
|
25
|
Pedoeem A, Azoulay-Alfaguter I, Strazza M,
Silverman GJ and Mor A: Programmed death-1 pathway in cancer and
autoimmunity. Clin Immunol. 153:145–152. 2014. View Article : Google Scholar
|
|
26
|
Okazaki T and Honjo T: PD-1 and PD-1
ligands: From discovery to clinical application. Int Immunol.
19:813–824. 2007. View Article : Google Scholar
|
|
27
|
Nawaf MG, Ulvmar MH, Withers DR, McConnell
FM, Gaspal FM, Webb GJ, Jones ND, Yagita H, Allison JP and Lane PJ:
Concurrent OX40 and CD30 ligand blockade abrogates the CD4-driven
autoimmunity associated with CTLA4 and PD1 blockade while
preserving excellent anti-CD8 tumor immunity. J Immunol.
199:974–981. 2017. View Article : Google Scholar
|
|
28
|
Homet Moreno B and Ribas A:
Anti-programmed cell death protein-1/ligand-1 therapy in different
cancers. Br J Cancer. 112:1421–1427. 2015. View Article : Google Scholar
|
|
29
|
Chiu HC, Li CJ, Yiang GT, Tsai AP and Wu
MY: Epithelial to mesenchymal transition and cell biology of
molecular regulation in endometrial carcinogenesis. J Clin Med.
8:4392019. View Article : Google Scholar
|
|
30
|
Tsui KH, Chiang AJ and Yu KJ: Urgent
surgical intervention for ruptured ovarian endometrioma. Taiwan J
Obstet Gynecol. 51:3282012. View Article : Google Scholar
|
|
31
|
Dai S, Jia R, Zhang X, Fang Q and Huang L:
The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol.
290:72–79. 2014. View Article : Google Scholar
|
|
32
|
Joshi S and Durden DL: Combinatorial
approach to improve cancer immunotherapy: Rational drug design
strategy to simultaneously hit multiple targets to kill tumor cells
and to activate the immune system. J Oncol. 2019:52450342019.
View Article : Google Scholar
|
|
33
|
Zhang M, Yang J, Zhou J, Gao W, Zhang Y,
Lin Y, Wang H, Ruan Z and Ni B: Prognostic Values of
CD38+CD101+PD1+CD8+ T
cells in pancreatic cancer. Immunol Invest. 48:466–479. 2019.
View Article : Google Scholar
|
|
34
|
Nguyen LT and Ohashi PS: Clinical blockade
of PD1 and LAG3-potential mechanisms of action. Nat Rev Immunol.
15:45–56. 2015. View Article : Google Scholar
|
|
35
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar
|
|
36
|
Reiss KA, Forde PM and Brahmer JR:
Harnessing the power of the immune system via blockade of PD-1 and
PD-L1: A promising new anticancer strategy. Immunotherapy.
6:459–475. 2014. View Article : Google Scholar
|
|
37
|
Powles T, Eder JP, Fine GD, Braiteh FS,
Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et
al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in
metastatic bladder cancer. Nature. 515:558–562. 2014. View Article : Google Scholar
|
|
38
|
Taube JM, Klein A, Brahmer JR, Xu H, Pan
X, Kim JH, Chen L, Pardoll DM, Topalian SL and Anders R:
Association of PD-1, PD-1 ligands, and other features of the tumor
immune microenvironment with response to anti-PD-1 therapy. Clin
Cancer Res. 20:5064–5074. 2014. View Article : Google Scholar
|
|
39
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar
|
|
40
|
Koppel C, Schwellenbach H, Zielinski D,
Eckstein S, Martin-Ortega M, D'Arrigo C, Schildhaus HU, Rüschoff J
and Jasani B: Optimization and validation of PD-L1
immunohistochemistry staining protocols using the antibody clone
28-8 on different staining platforms. Mod Pathol. 31:1630–1644.
2018. View Article : Google Scholar
|
|
41
|
Ilie M, Long-Mira E, Bence C, Butori C,
Lassalle S, Bouhlel L, Fazzalari L, Zahaf K, Lalvée S, Washetine K,
et al: Comparative study of the PD-L1 status between surgically
resected specimens and matched biopsies of NSCLC patients reveal
major discordances: A potential issue for anti-PD-L1 therapeutic
strategies. Ann Oncol. 27:147–153. 2016. View Article : Google Scholar
|
|
42
|
Wu Z, Zhang L, Peng J, Xu S, Zhou L, Lin
Y, Wang Y and Lu J, Yin W and Lu J: Predictive and prognostic value
of PDL1 protein expression in breast cancer patients in neoadjuvant
setting. Cancer Biol Ther. 20:941–947. 2019. View Article : Google Scholar
|
|
43
|
Gerlinger M, Rowan AJ, Horswell S, Math M,
Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N,
Stewart A, et al: Intratumor heterogeneity and branched evolution
revealed by multiregion sequencing. N Engl J Med. 366:883–892.
2012. View Article : Google Scholar
|
|
44
|
McLaughlin J, Han G, Schalper KA,
Carvajal-Hausdorf D, Pelekanou V, Rehman J, Velcheti V, Herbst R,
LoRusso P and Rimm DL: Quantitative assessment of the heterogeneity
of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol.
2:46–54. 2016. View Article : Google Scholar
|
|
45
|
Ilie M, Hofman V, Dietel M, Soria JC and
Hofman P: Assessment of the PD-L1 status by immunohistochemistry:
Challenges and perspectives for therapeutic strategies in lung
cancer patients. Virchows Arch. 468:511–525. 2016. View Article : Google Scholar
|
|
46
|
Schalper KA, Velcheti V, Carvajal D,
Wimberly H, Brown J, Pusztai L and Rimm DL: In situ tumor PD-L1
mRNA expression is associated with increased TILs and better
outcome in breast carcinomas. Clin Cancer Res. 20:2773–2782. 2014.
View Article : Google Scholar
|
|
47
|
Mazel M, Jacot W, Pantel K, Bartkowiak K,
Topart D, Cayrefourcq L, Rossille D, Maudelonde T, Fest T and
Alix-Panabières C: Frequent expression of PD-L1 on circulating
breast cancer cells. Mol Oncol. 9:1773–1782. 2015. View Article : Google Scholar
|
|
48
|
Yue C, Jiang Y, Li P, Wang Y, Xue J, Li N,
Li D, Wang R, Dang Y, Hu Z, et al: Dynamic change of PD-L1
expression on circulating tumor cells in advanced solid tumor
patients undergoing PD-1 blockade therapy. Oncoimmunology.
7:e14381112018. View Article : Google Scholar
|
|
49
|
Acheampong E, Spencer I, Lin W, Ziman M,
Millward M and Gray E: Is the blood an alternative for programmed
cell death ligand 1 assessment in non-small cell lung cancer?
Cancers (Basel). 11:9202019. View Article : Google Scholar
|
|
50
|
Marzec M, Zhang Q, Goradia A, Raghunath
PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA and
Wasik MA: Oncogenic kinase NPM/ALK induces through STAT3 expression
of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad
Sci USA. 105:20852–20857. 2008. View Article : Google Scholar
|
|
51
|
Tang J, Yu JX, Hubbard-Lucey VM,
Neftelinov ST, Hodge JP and Lin Y: Trial watch: The clinical trial
landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug
Discov. 17:854–855. 2018. View Article : Google Scholar
|
|
52
|
Holz MK: The role of S6K1 in ER-positive
breast cancer. Cell Cycle. 11:3159–3165. 2012. View Article : Google Scholar
|
|
53
|
Sridharan S and Basu A: Distinct roles of
mTOR targets S6K1 and S6K2 in breast cancer. Int J Mol Sci.
21:11992020. View Article : Google Scholar
|
|
54
|
Crane CA, Panner A, Murray JC, Wilson SP,
Xu H, Chen L, Simko JP, Waldman FM, Pieper RO and Parsa AT: PI(3)
kinase is associated with a mechanism of immunoresistance in breast
and prostate cancer. Oncogene. 28:306–312. 2009. View Article : Google Scholar
|
|
55
|
Jiang X, Zhou J, Giobbie-Hurder A, Wargo J
and Hodi FS: The activation of MAPK in melanoma cells resistant to
BRAF inhibition promotes PD-L1 expression that is reversible by MEK
and PI3K inhibition. Clin Cancer Res. 19:598–609. 2013. View Article : Google Scholar
|
|
56
|
Ghebeh H, Lehe C, Barhoush E, Al-Romaih K,
Tulbah A, Al-Alwan M, Hendrayani SF, Manogaran P, Alaiya A,
Al-Tweigeri T, et al: Doxorubicin downregulates cell surface B7-H1
expression and upregulates its nuclear expression in breast cancer
cells: Role of B7-H1 as an anti-apoptotic molecule. Breast Cancer
Res. 12:R482010. View Article : Google Scholar
|
|
57
|
Peng J, Hamanishi J, Matsumura N, Abiko K,
Murat K, Baba T, Yamaguchi K, Horikawa N, Hosoe Y, Murphy SK, et
al: Chemotherapy induces programmed cell death-ligand 1
overexpression via the nuclear factor-κB to foster an
immunosuppressive tumor microenvironment in ovarian cancer. Cancer
Res. 75:5034–5045. 2015. View Article : Google Scholar
|
|
58
|
Uhercik M, Sanders AJ, Owen S, Davies EL,
Sharma AK, Jiang WG and Mokbel K: Clinical significance of PD1 and
PDL1 in human breast cancer. Anticancer Res. 37:4249–4254.
2017.
|
|
59
|
Twyman-Saint Victor C, Rech AJ, Maity A,
Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi
PM, et al: Radiation and dual checkpoint blockade activate
non-redundant immune mechanisms in cancer. Nature. 520:373–377.
2015. View Article : Google Scholar
|
|
60
|
Lv S, Wang S, Qiao G, Wang X, Zhou X, Yan
F, Li Y, Wang S, Morse MA, Hobeika A, et al: Functional
CD3+CD8+PD1− T cell accumulation
and PD-L1 expression increases during tumor invasion in DCIS of the
breast. Clin Breast Cancer. 19:e617–e623. 2019. View Article : Google Scholar
|
|
61
|
Beckers RK, Selinger CI, Vilain R, Madore
J, Wilmott JS, Harvey K, Holliday A, Cooper CL, Robbins E, Gillett
D, et al: Programmed death ligand 1 expression in triple-negative
breast cancer is associated with tumour-infiltrating lymphocytes
and improved outcome. Histopathology. 69:25–34. 2016. View Article : Google Scholar
|
|
62
|
Mittendorf EA, Philips AV, Meric-Bernstam
F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM,
Akcakanat A, et al: PD-L1 expression in triple-negative breast
cancer. Cancer Immunol Res. 2:361–370. 2014. View Article : Google Scholar
|
|
63
|
Gatalica Z, Snyder C, Maney T, Ghazalpour
A, Holterman DA, Xiao N, Overberg P, Rose I, Basu GD, Vranic S, et
al: Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common
cancers and their correlation with molecular cancer type. Cancer
Epidemiol Biomarkers Prev. 23:2965–2970. 2014. View Article : Google Scholar
|
|
64
|
Bertucci F, Finetti P, Colpaert C,
Mamessier E, Parizel M, Dirix L, Viens P, Birnbaum D and van Laere
S: PDL1 expression in inflammatory breast cancer is frequent and
predicts for the pathological response to chemotherapy. Oncotarget.
6:13506–13519. 2015. View Article : Google Scholar
|
|
65
|
Sabatier R, Finetti P, Mamessier E,
Adelaide J, Chaffanet M, Ali HR, Viens P, Caldas C, Birnbaum D and
Bertucci F: Prognostic and predictive value of PDL1 expression in
breast cancer. Oncotarget. 6:5449–5464. 2015. View Article : Google Scholar
|
|
66
|
Muenst S, Schaerli AR, Gao F, Däster S,
Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE,
et al: Expression of programmed death ligand 1 (PD-L1) is
associated with poor prognosis in human breast cancer. Breast
Cancer Res Treat. 146:15–24. 2014. View Article : Google Scholar
|
|
67
|
Muenst S, Soysal SD, Gao F, Obermann EC,
Oertli D and Gillanders WE: The presence of programmed death 1
(PD-1)-positive tumor-infiltrating lymphocytes is associated with
poor prognosis in human breast cancer. Breast Cancer Res Treat.
139:667–676. 2013. View Article : Google Scholar
|
|
68
|
Sun S, Fei X, Mao Y, Wang X, Garfield DH,
Huang O, Wang J, Yuan F, Sun L, Yu Q, et al: PD-1(+) immune cell
infiltration inversely correlates with survival of operable breast
cancer patients. Cancer Immunol Immunother. 63:395–406. 2014.
View Article : Google Scholar
|
|
69
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar
|
|
70
|
Hasan A, Ghebeh H, Lehe C, Ahmad R and
Dermime S: Therapeutic targeting of B7-H1 in breast cancer. Expert
Opin Ther Targets. 15:1211–1225. 2011. View Article : Google Scholar
|
|
71
|
Lipson EJ, Forde PM, Hammers HJ, Emens LA,
Taube JM and Topalian SL: Antagonists of PD-1 and PD-L1 in cancer
treatment. Semin Oncol. 42:587–600. 2015. View Article : Google Scholar
|
|
72
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar
|
|
73
|
Wang Y, Zhang X, Yang L, Xue J and Hu G:
Blockade of CCL2 enhances immunotherapeutic effect of anti-PD1 in
lung cancer. J Bone Oncol. 11:27–32. 2018. View Article : Google Scholar
|
|
74
|
Stenehjem DD, Tran D, Nkrumah MA and Gupta
S: PD1/PDL1 inhibitors for the treatment of advanced urothelial
bladder cancer. Onco Targets Ther. 11:5973–5989. 2018. View Article : Google Scholar
|
|
75
|
Wagner MJ, Ricciotti RW, Mantilla J,
Loggers ET, Pollack SM and Cranmer LD: Response to PD1 inhibition
in conventional chondrosarcoma. J Immunother Cancer. 6:942018.
View Article : Google Scholar
|
|
76
|
Neubert NJ, Schmittnaegel M, Bordry N,
Nassiri S, Wald N, Martignier C, Tillé L, Homicsko K, Damsky W,
Maby-El Hajjami H, et al: T cell-induced CSF1 promotes melanoma
resistance to PD1 blockade. Sci Transl Med. 10:eaan33112018.
View Article : Google Scholar
|
|
77
|
Pusztai L, Karn T, Safonov A, Abu-Khalaf
MM and Bianchini G: New strategies in breast cancer: Immunotherapy.
Clin Cancer Res. 22:2105–2110. 2016. View Article : Google Scholar
|
|
78
|
Nanda R, Chow LQM, Dees EC, Berger R,
Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, et al:
Pembrolizumab in patients with advanced triple-negative breast
cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 34:2460–2467.
2016. View Article : Google Scholar
|
|
79
|
Emens LA: Breast cancer immunobiology
driving immunotherapy: Vaccines and immune checkpoint blockade.
Expert Rev Anticancer Ther. 12:1597–1611. 2012. View Article : Google Scholar
|
|
80
|
Badr NM, Berditchevski F and Shaaban AM:
The immune microenvironment in breast carcinoma: Predictive and
prognostic role in the neoadjuvant setting. Pathobiology. 87:61–74.
2020. View Article : Google Scholar
|
|
81
|
Tan W, Yang M, Yang H, Zhou F and Shen W:
Predicting the response to neoadjuvant therapy for early-stage
breast cancer: Tumor-, blood-, and imaging-related biomarkers.
Cancer Manag Res. 10:4333–4347. 2018. View Article : Google Scholar
|
|
82
|
Loi S, Dushyanthen S, Beavis PA, Salgado
R, Denkert C, Savas P, Combs S, Rimm DL, Giltnane JM, Estrada MV,
et al: RAS/MAPK activation is associated with reduced
tumor-infiltrating lymphocytes in triple-negative breast cancer:
Therapeutic cooperation between MEK and PD-1/PD-L1 immune
checkpoint inhibitors. Clin Cancer Res. 22:1499–1509. 2016.
View Article : Google Scholar
|
|
83
|
Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng
PP, Chang BY and Levy R: Therapeutic antitumor immunity by
checkpoint blockade is enhanced by ibrutinib, an inhibitor of both
BTK and ITK. Proc Natl Acad Sci USA. 112:E966–E972. 2015.
View Article : Google Scholar
|
|
84
|
Garcia-Aranda M and Redondo M:
Immunotherapy: A challenge of breast cancer treatment. Cancers
(Basel). 11:18222019. View Article : Google Scholar
|
|
85
|
Chawla A, Philips AV, Alatrash G and
Mittendorf E: Immune checkpoints: A therapeutic target in triple
negative breast cancer. Oncoimmunology. 3:e283252014. View Article : Google Scholar
|
|
86
|
Chatterjee S, Lesniak WG, Gabrielson M,
Lisok A, Wharram B, Sysa-Shah P, Azad BB, Pomper MG and Nimmagadda
S: A humanized antibody for imaging immune checkpoint ligand PD-L1
expression in tumors. Oncotarget. 7:10215–10227. 2016. View Article : Google Scholar
|
|
87
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar
|
|
88
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar
|
|
89
|
Gibson J: Anti-PD-L1 for metastatic
triple-negative breast cancer. Lancet Oncol. 16:e2642015.
View Article : Google Scholar
|
|
90
|
Ott PA, Hodi FS and Robert C: CTLA-4 and
PD-1/PD-L1 blockade: New immunotherapeutic modalities with durable
clinical benefit in melanoma patients. Clin Cancer Res.
19:5300–5309. 2013. View Article : Google Scholar
|
|
91
|
Kerr KM and Hirsch FR: Programmed death
ligand-1 immunohistochemistry: Friend or foe? Arch Pathol Lab Med.
140:326–331. 2016. View Article : Google Scholar
|
|
92
|
Voutsadakis IA: Immune blockade inhibition
in breast cancer. Anticancer Res. 36:5607–5622. 2016. View Article : Google Scholar
|
|
93
|
Yi KH and Chen L: Fine tuning the immune
response through B7-H3 and B7-H4. Immunol Rev. 229:145–151. 2009.
View Article : Google Scholar
|