Introduction
Undifferentiated pleomorphic sarcoma (UPS) is a soft
tissue sarcoma of uncertain differentiation with prominent nuclear
pleomorphism. In the current WHO classification of soft tissue
tumors, UPS is defined as a subtype of undifferentiated soft-tissue
sarcoma (USTS) (1). UPS has been a
heterogeneous tumor category and it has usually been diagnosed by
ruling out the diagnosis of other types of soft tissue sarcoma with
specific differentiation. Immunohistochemically, the tumor cells of
UPS present no definitive expression of specific
immunohistochemical markers (1).
The prognosis of UPS remains unfavorable despite
wide resection and additional chemotherapy or radiation therapy.
Standard chemotherapy for UPS is anthracycline plus ifosfamide
neoadjuvant chemotherapy (2). The
efficacy of eribulin and the clinical outcome of pazopanib and
gemcitabine/docetaxel for UPS have also been reported (3,4).
Cancer immunotherapy has been successfully used in some cancers
such as malignant melanoma and non-small-cell lung carcinoma
(5–8). SARC028, a clinical trial of anti-PD-1
therapy for patients with metastatic or surgically unresectable
locally advanced sarcoma, demonstrated the effectiveness of this
therapeutic approach for pleomorphic sarcomas such as UPS and
dedifferentiated liposarcoma (9).
Moreover, anti-PD-1 therapy was approved for the treatment of
tumors with deficient mismatch repair (dMMR) (10). Deficiency of MLH1, MSH2, MSH6 and
PMS2 may cause a high number of genetic mutations, especially
frameshift mutations in the repetitive DNA sequences known as
microsatellites, resulting in neoantigens. Thus, tumors with
MSI-high may become a target of the patient's immune system. It is
known that MSI-high tumors are eliminated by antitumor immunity
upon treatment with immune checkpoint blockade. On the other hand,
the dMMR status of UPS remains unknown (10). As HLA class I is required for
cytotoxic immunity, the effectiveness of anti-PD-1 therapy for
tumors with the loss of HLA class I, a target of cytotoxic T
lymphocytes, is now studied and this remains controversial
(11,12). However, it is unclear whether UPS
cases exhibit a decrease or complete loss of expression of HLA
class I. Indoleamine 2,3-dioxygenase (IDO-1) is the rate-limiting
enzyme in tryptophan catabolism and has a potent immune-suppressive
effect through local inhibition of T lymphocytes and a clinical
trial of therapy combining anti-PD-1 and anti-IDO-1 was also
performed (13,14). Although the outcome was unfavorable
(15), it was recently reported
that tumors expressing IDO-1 may be a good target for anti-PD-1
therapy (12). Previous findings on
various cancers such as lung cancer and melanoma have also
described that PD-L1 and IDO-1 are induced by TILs (5,16,17).
However, only a small number of studies on the expression of PD-L1
and IDO-1 in sarcoma have been carried out.
In the present study, we examined the immune
microenvironment of UPS, such as the expression of immune
checkpoint markers, PD-L1 and IDO-1, tumor-infiltrating lymphocytes
(TILs), the status of dMMR, and the expression of HLA class I to
reveal their value as prognostic factors and therapeutic
targets.
Materials and methods
Patients and materials
This study was conducted in accordance with the
principles embodied in the Declaration of Helsinki. The study was
also approved by the Ethics Committee of Kyushu University (nos.
29-429, 29-625) and consent was obtained from the patients that
donated these tissues. A total of 52 cases of UPS, previously
diagnosed as malignant fibrous histiocytoma (MFH) or UPS, were
retrieved from among the soft tissue tumors registered in the files
of the Department of Anatomic Pathology, Graduate School of Medical
Sciences, Kyushu University, Fukuoka, Japan, from 1998 January to
2017 December.
To collect pure primary UPS, tumors were assessed
according to the flow chart presented in Fig. S1. Secondary sarcomas following the
other distinct tumors, USTS of the spindle cell type and
epithelioid USTS classified in accordance with the WHO 2013
classification were excluded (1).
We also excluded sarcomas after radiation and/or chemotherapy;
sarcomas located in the body cavity, retroperitoneum, or bone;
sarcomas in which the proportion of myxoid matrix exceeded 10%; and
sarcomas that were immunoreactive for MDM2 or positive for MDM2
gene amplification in order to rule out myxofibrosarcoma (MFS) and
dedifferentiated liposarcoma (18,19).
Formalin-fixed, paraffin-embedded samples of the 52
tumors were available. Follow-up information was available for 49
cases. Cases without wide resection were removed. Finally, 42 cases
were analyzed for overall, metastasis-free, and recurrence-free
survival. The follow-up period after surgery ranged from 7 to 140
months (38.5 months as the average).
Clinicopathological and histological
evaluation
Clinical and pathological data were obtained from
the database of the Department of Anatomic Pathology, Kyushu
University. Clinicopathological findings such as age, sex, tumor
location, tumor size, distant metastasis, and local recurrence were
evaluated. The cases were classified into younger and older groups
according to the median age. The cases were also classified into
smaller and larger groups according to the cut-off of 5 cm. We also
evaluated histopathological findings such as necrosis, mitosis, and
grade of the French Federation of Cancer Centers (FNCLCC) grading
system of the primary tumors (20).
The existence of necrosis was classified as ‘positive’ for
necrosis. Mitosis was classified as ‘high; when there were >10
mitotic figures per 10 high power fields (HPFs). The existence of a
myxoid area (1–10%) of the total area was classified as ‘positive’
for focal myxoid area.
Immunohistochemistry (IHC)
Formalin fixation was carried out with 10% formalin
at about 18°C for 48–72 h following tissue resection.
Formalin-fixed, paraffin-embedded (FFPE) samples of 52 tumors were
available for IHC staining of PD-L1 (28–8),
IDO-1, CD8, CD4, CD3, and HLA class I. Such samples of 50 tumors
were available for immunohistochemical staining of MSH2, MSH6,
MLH1, and PMS2. FFPE samples of two cases were used up and not
available for immunostaining of dMMR. FFPE tissue was sectioned at
a thickness of 3 µm. Primary antibodies for the immunohistochemical
staining of PD-L1 (28–8), IDO-1, CD8, CD4, CD3, HLA class I,
MLH1, PMS2, MSH2, and MSH6 were used as described in Table SI. The immunoperoxidase polymer
method (Envision-kit and Envision Flex-kit; Dako Japan) was
performed for all available cases. Antigen retrieval was carried
out by boiling the slides with 10 mM sodium citrate (pH 6.0) or
Target Retrieval Solution (pH 9.0; Dako, Carpinteria).
The expression of PD-L1 was assessed by determining
the ratio of membranous staining-positive tumor cells to all tumor
cells in a stained slide in which at least 100 tumor cells were
observed, in accordance with a previously reported standardized
method (21), regardless of the
staining intensity. We set the cut-off of PD-L1 expression as 1 or
50% in accordance with the trial (22,23).
The expression of IDO-1 was assessed by determining the ratio of
cytoplasmic staining-positive tumor cells to all tumor cells in a
stained slide in which at least 100 tumor cells were observed. We
also set the cut-off of IDO-1 expression as 1%, with reference to
the literature (21,24). Macrophages were also stained by the
immunohistochemical staining of PD-L1 and IDO-1, but they were
excluded from the count of positive-stained cells.
TILs were assessed by counting the number of CD8-,
CD4-, and CD3-positive lymphocytes infiltrating into the tumor
cells per five high-power fields (HPFs), which were randomly chosen
in tumors excluding the fields with lymphoid aggregates (25). For survival analysis, the number of
infiltrated lymphocytes was divided into high and low groups
according to the cut-off determined by drawing ROC curves.
The expression of MSH2, MSH6, MLH1 and PMS2 was
classified as having been lost when there was a complete absence of
nuclear staining in neoplastic cells, while the surrounding
non-neoplastic cells showed consistently preserved nuclear staining
(26). Thus, antibodies for HLA
class I-A, B and C were used. The expression of HLA class I was
classified in accordance with a previous study (27). When the cell membrane was stained as
strongly as stromal lymphocytes or endothelial cells in >75% of
the tumor cells, expression levels were defined as strong. If
heterogeneous membranous staining was found in >25% of the tumor
cells, expression was defined as weak. If <75% of the tumor
cells lacked membrane staining, it was defined as no expression.
Cases with weak and no expression were classified as those with
loss of HLA class I and cases with strong expression as having HLA
class I intact. To confirm that CD8-, CD4-, and CD3-positive
lymphocytes secreted IFN-γ, the EnVision™ G|2 Doublestain System
was used to stain IFN-γ and CD8, CD4, or CD3 at the same time in 10
samples each, in accordance with the basic method. IFN-γ was
colored red using alkaline phosphatase and CD8, CD4, and CD3 were
colored brown using peroxidase.
Cell culture and cytokine
experiments
UPS cell lines, FPS-1 and FU-MFH2, were cultured in
RPMI-1640 medium and Dulbecco's modified Eagle's medium (DMEM)/F-12
supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin (28,29).
Mycoplasma testing had been done. These cells were cultured in
six-well plates in the presence or absence of IFN-γ (200 ng/ml) for
24 h in 37°C. Then, mRNA and protein were extracted as described
below.
RNA isolation, reverse transcription,
and qPCR of cell lines
Cultured cells were suspended in QIAzol solution
(Qiagen). Total RNA of cultured cells was extracted with acidic
phenol: Chloroform and then reverse-transcribed using ReverTra Ace
qPCR RT Master Mix with gDNA Remover (Toyobo), in line with the
manufacturer's recommendations. Quantitative polymerase chain
reaction (qPCR) for PD-L1 and IDO-1 was performed using THUNDERBIRD
SYBR qPCR Master Mix (Toyobo) on an Applied Biosystems Step One
Plus Real Time PCR System (Thermo Fisher Scientific Inc.). Primers
of PD-L1 and IDO-1 are listed in Table
SII. The data were normalized to GAPDH expression levels and
are presented as the mean ± standard deviation of three independent
experiments.
Immunoblotting
Cells were washed twice with phosphate-buffered
saline (PBS) and suspended in 2X sodium dodecyl sulfate (SDS)
sample buffer of 37°C. The samples were separated by SDS-PAGE
(5–20% gel) and transferred to a polyvinylidene fluoride membrane
with the Trans-Blot Turbo Transfer System (Bio-Rad Laboratories;
2.5 A, 25 V, 7 min). Membranes were blocked for 30 min in 5%
Blocking One-P (Nacalai Tesque Inc.) and then incubated with
primary antibodies in Can Get Signal (Toyobo). The primary
antibodies used included rabbit monoclonal anti-PD-L1 (E1L3N,
1:1,000; Cell Signaling Technology), mouse anti-IDO-1 (UMAB126,
1:1,000; OriGene), and rabbit anti-HSP 90 (C45G5, 1:1,000; Cell
Signaling Technology). The membranes were then washed with TBS
containing 0.05% Tween-20 and incubated with horseradish peroxidase
(HRP)-conjugated anti-rabbit IgG (7074S, 1:1,000; Cell Signaling
Technology) or anti-mouse IgG (sc-2005, 1:1,000; Santa Cruz). The
membranes were then washed and developed to enhance the
chemiluminescence with Chemi-Lumi One Ultra (Nacalai Tesque Inc.).
The antibodies are described in Table
SI. The chemiluminescence signals were detected with an Image
Quant LAS 4000 (Fujifilm). ImageJ was used to analyze the data.
Statistical analysis
Cut-offs of PD-L1 expression of 1 and 50% were set
and statistical analyses between <1% and ≥1% as well as <50%
and ≥50% were performed (22,23).
The cut-off of IDO-1 expression of 1% was set and the two groups
were analyzed accordingly (21,24).
Clinicopathological and immunohistochemical parameters were
analyzed using the Fisher's exact test. The infiltration of
lymphocytes was analyzed by Mann-Whitney U test and linear
regression analysis. Correlation between the immune checkpoints and
TILs was assessed by applying the least squares method. Survival
curves were created using the Kaplan-Meier method. Overall,
metastasis-free, and recurrence-free survival curves were analyzed
by the log-rank test. For survival analysis and the Fisher's exact
test, the cut-offs of TILs were determined by drawing ROC curves.
The outcome of qPCR was analyzed by the Student's t-test. A P-value
of <0.05 was classified as significant for each statistical
analysis. Statistical analyses were conducted using the JMP
statistical software package (version 13; SAS Institute).
Results
Clinicopathological and histological
findings
The clinicopathological data for the 52 tumors are
summarized in Table I. The patients
included 26 males and 26 females, with ages ranging from 38 to 90
years (median: 69.5, mean: 69.1 years) at the diagnosis of the
primary lesion. Thirty-two tumors (72.7%) were >5 cm. Three
cases (5.8%) were located in the head and neck (1 case in the head
and 3 in the neck), 8 cases (15.4%) in the trunk (2 cases in the
abdominal wall and 6 in the back), 9 cases (17.3%) in an upper
extremity (3 cases in the upper arm and 6 in the forearm), and 32
cases (61.6%) in a lower extremity (25 cases in the thigh and 7 in
the lower leg). Tumor size ranged from 2.5 to 20 cm (median: 7.5
cm, mean: 8.3 cm). Tumor-related death occurred in 13 of 42 cases
(31.0%), distant metastasis in 18 of 42 cases (42.9%), and local
recurrence in 11 of 42 cases (26.2%). Metastatic sites were as
follows: Lung in 11 cases (61.1%), bone in 3 cases (16.7%), lymph
node in 2 cases (11.1%), and abdominal wall in 1 case (5.6%), as
described in Table SIII.
 | Table I.Clinicopathological and histological
features. |
Table I.
Clinicopathological and histological
features.
|
Characteristics | No. (%) |
|---|
| Age
(Average=69.5) |
|
|
(<70) | 26/52 (50.0) |
|
(≥70) | 26/52 (50.0) |
| Sex |
|
|
Female | 24/52 (46.2) |
|
Male | 28/52 (53.8) |
| Size |
|
| Smaller
group (≤5 cm) | 12/44 (27.3) |
| Larger
group (>5 cm) | 32/44 (72.7) |
| Localization |
|
| Head
and neck | 3/52 (5.8) |
|
Anterior trunk | 2/52 (3.9) |
|
Back | 6/52 (11.5) |
| Upper
arm | 3/52 (5.8) |
|
Forearm | 6/52 (11.5) |
|
Thigh | 25/52 (48.1) |
| Lower
leg | 7/52 (13.5) |
| Metastasis |
|
| − | 24/42 (57.1) |
| + | 18/42 (42.9) |
| Recurrence |
|
| − | 31/42 (73.8) |
| + | 11/42 (26.2) |
| Necrosis |
|
| − | 32/52 (61.5) |
| + | 20/52 (38.5) |
| Mitosis |
|
| Low
(<10/10HPFs) | 23/52 (44.2) |
| High
(≥10/10HPFs) | 29/52 (55.8) |
| FNCLCC |
|
| Grade
2 | 26/52 (50.0) |
| Grade
3 | 26/52 (50.0) |
| Myxoid area |
|
|
<1% | 31/52 (59.6) |
|
1–10% | 21/52 (40.4) |
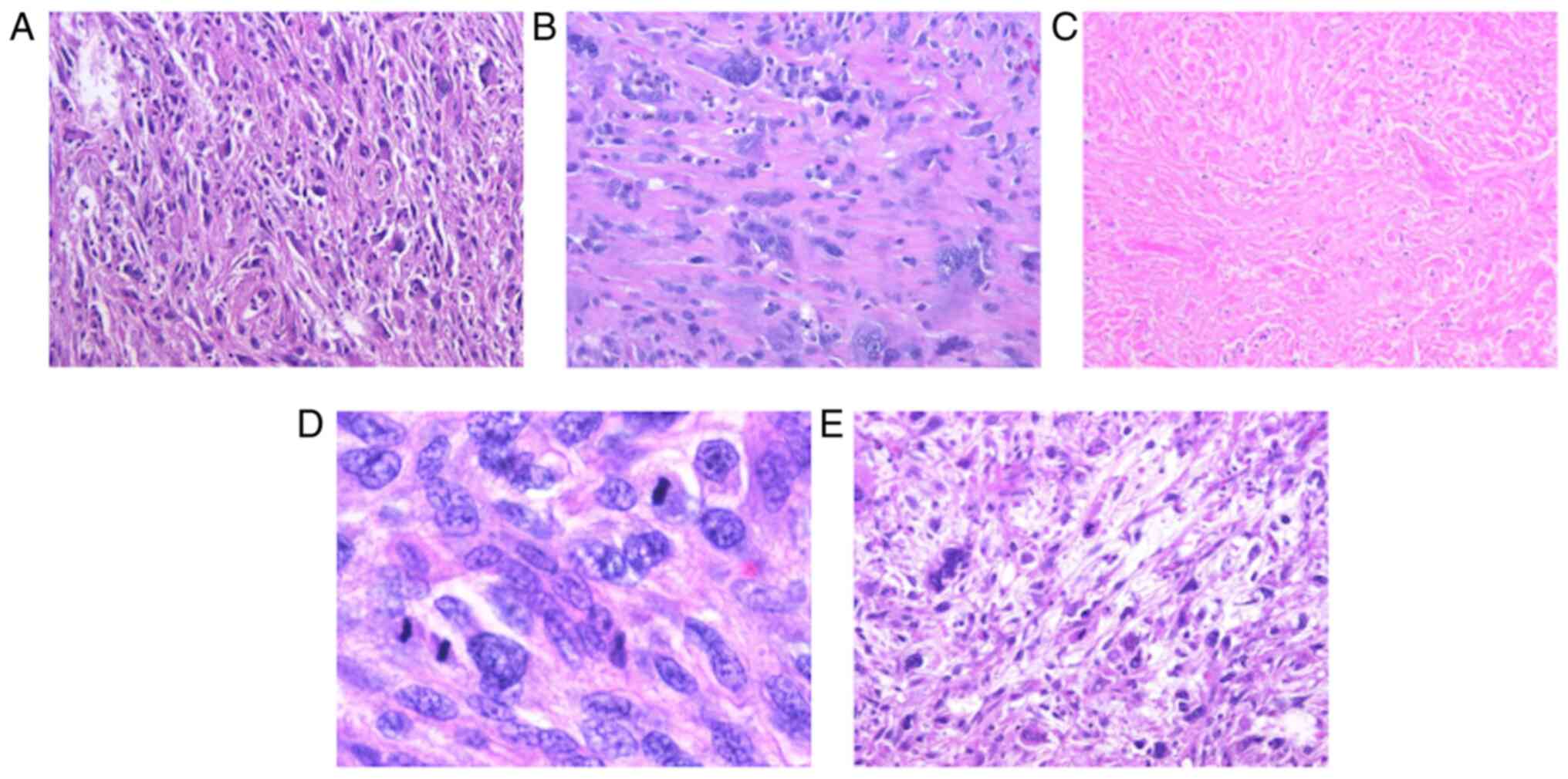
A representative case of each histological feature
is shown in Fig. 1. All 52 tumors
showed the proliferation of spindle- to polygonal-shaped tumor
cells with high-grade nuclear atypia (Fig. 1A), accompanied by no or only a focal
(≤10%) myxoid area. Atypical tumor giant cells were scattered
(Fig. 1B). Tumor necrosis (Fig. 1C) was evidenced in 32 of 52 cases
(61.5%) and mitosis (≥10/10 HPFs) (Fig.
1D) was observed in 29 of 52 (55.8%). Overall, 26 of 52 cases
were classified as FNCLCC grade 2 (50.0%) and 26 as grade 3
(50.0%). Focal myxoid area (≥1 and <10%) was observed in 21 of
52 cases (40.4%) (Fig. 1E).
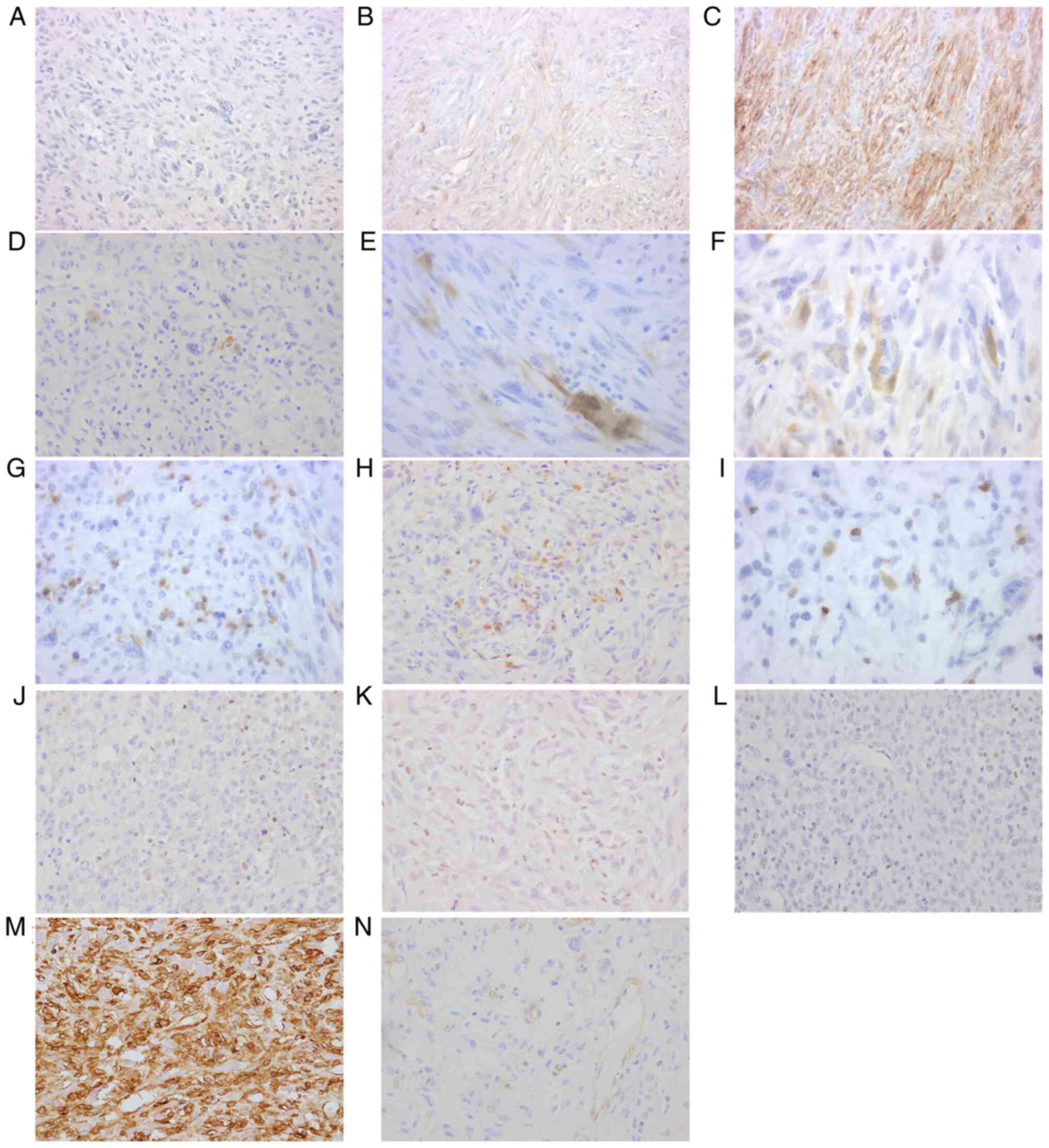
Immunohistochemistry (PD-L1, IDO-1,
TILs, HLA class I, dMMR)
The results of the immunohistochemical study, the
positive ratio of PD-L1 and IDO-1 and whether there were UPS with
dMMR of loss of HLA class I are summarized in Table II. Representative figures of
immunohistochemical staining are shown in Fig. 2. Regarding the immunohistochemical
results of PD-L1 immunostaining, 33 of 52 (63.5%) cases showed
negative staining (<1%: Fig.
2A), 14 of 52 (26.9%) showed focal staining (≥1 and <50%:
Fig. 2B), and 5 of 52 (9.6%) showed
strong staining (≥50%; Fig. 2C).
Regarding IDO-1, 27 of 52 (51.9%) cases showed negative staining
(<1%: Fig. 2D), 17 of 52 (32.7%)
showed focal staining (≥1 and <10%: Fig. 2E), 8 of 52 (15.4%) showed moderate
staining (≥10 and <25%: Fig.
2F), and none showed strong staining (≥25%). The numbers of
CD8-positive lymphocytes (Fig. 2G)
ranged from 0 to 489 per five HPFs, with a median of 62.5. The
numbers of CD4-positive lymphocytes (Fig. 2H) ranged from 1 to 221 per five
HPFs, with a median of 22.5. The numbers of CD3-positive
lymphocytes (Fig. 2I) ranged from 1
to 811 per five HPFs, with a median of 117. There was deficiency of
mismatch repair protein in 2 of 50 tumors (4.0%): One case with
loss of MSH2 and MSH6 (2.0%), and another with loss of PMS2 (2.0%)
(Fig. 2J-L). For HLA class I, two
tumors were classified to have no expression, four to have weak
expression, and the other 46 to have strong expression (Fig. 2M and N). Therefore, six tumors were
classified as having loss of HLA class I. The remaining cases were
classified as having normal HLA class I and their HLA class I
expression was completely retained. Double staining of IFN-γ and
CD8, CD4, or CD3 is shown in Fig.
S2. CD8-, CD4-, or CD3-positive TILs infiltrating into the
tumors seemed to secrete IFN-γ.
 | Table II.Immunohistochemical results. |
Table II.
Immunohistochemical results.
| Antibody | Positive ratio | No. (%) |
|---|
| PD-L1 (28–8) | <1% | 33/52(63.5) |
|
| 1%≤ and
<50% | 14/52(26.9) |
|
| ≥50% | 5/52(9.6) |
| IDO-1 | <1% | 27/52(51.9) |
|
| 1%≤ and
<10% | 17/52(32.7) |
|
| 10%≤ and
<25% | 8/52(15.4) |
|
| ≥25% | 0/52 |
| MMR | Deficient | 2/50(4.0) |
|
| Proficient | 48/50(96.0) |
| HLA Class I | Loss | 6/52(11.5) |
|
| Retain | 46/52(88.5) |
Correlations between
clinicopathological features and the expression of PD-L1 and IDO-1,
TILs, loss of HLA class I, and dMMR
The results of statistical analysis between the
clinicopathological features and the expression of PD-L1 or IDO-1
are summarized in Table III. ROC
curves of TILs are presented in Fig.
S3. Focal PD-L1 expression (≥1%) was associated with necrosis
(P=0.0402). No significant correlations between the
clinicopathological features and TILs, loss of HLA class I, and
dMMR were identified.
 | Table III.Statistical results of
clinicohistopathological and mmunohistochemical features. |
Table III.
Statistical results of
clinicohistopathological and mmunohistochemical features.
|
| PD-L1 (1%
cut-off) | PD-L1 (50%
cut-off) | IDO-1 (1%
cut-off) |
|---|
|
|
|
|
|
|---|
|
| <1% | ≥1% | <50% | ≥50% | <1% | ≥1% |
|---|
| Age |
|
<70 | 20 | 6 | 25 | 1 | 16 | 10 |
|
≥70 | 13 | 13 | 22 | 4 | 11 | 15 |
|
| P=0.0828 | P=0.350 | P=0.267 |
| Sex |
| F | 16 | 8 | 22 | 2 | 11 | 13 |
| M | 17 | 11 | 25 | 3 | 16 | 12 |
|
| P=0.775 | P=1.000 | P=0.578 |
| Size |
| Small
(≤5 cm) | 6 | 6 | 11 | 1 | 6 | 6 |
| Large
(>5 cm) | 21 | 11 | 28 | 4 | 17 | 5 |
|
| P=0.4889 | P=1.000 | P=1.000 |
| FNCLCC |
| Grade
2 | 20 | 6 | 26 | 0 | 12 | 14 |
| Grade
3 | 13 | 13 | 21 | 5 | 15 | 11 |
|
| P=0.083 | P=0.0506 | P=0.578 |
| Necrosis |
| − | 24 | 8 | 30 | 2 | 15 | 17 |
| + | 9 | 11 | 17 | 3 | 12 | 8 |
|
|
P=0.0402a | P=0.3607 | P=0.404 |
| Mitosis |
| Low
(<10/10HPFs) | 18 | 5 | 23 | 0 | 13 | 10 |
| High
(≥10/10HPFs) | 15 | 14 | 24 | 5 | 14 | 15 |
|
| P=0.0811 | P=0.0586 | P=0.588 |
| Myxoid area |
| 19 | 12 | 26 | 5 | 17 | 14 |
|
|
<1% | 14 | 7 | 21 | 0 | 10 | 11 |
| ≤1%,
<10% | P=0.774 | P=0.0732 | P=0.778 |
Correlations between the expression of
PD-L1 or IDO-1, loss of HLA class I, and dMMR
No association between the expression of PD-L1 and
IDO-1 was detected by Fisher's exact test, as shown in Table SIV. Of the six tumors with loss of
HLA class I, one tumor exhibited the loss of MSH2 and MSH6 and
expressed focal PD-L1 but did not express IDO-1, three expressed
IDO-1 but did not express PD-L1, and two expressed neither PD-L1
nor IDO-1. In the two tumors with dMMR, one tumor with the loss of
MSH2 and MSH6 expressed focal PD-L1 but did not express IDO-1. The
other one with the loss of PMS2 expressed strong PD-L1 and focal
IDO-1.
Correlations between TILs and the
expression of PD-L1 or IDO-1
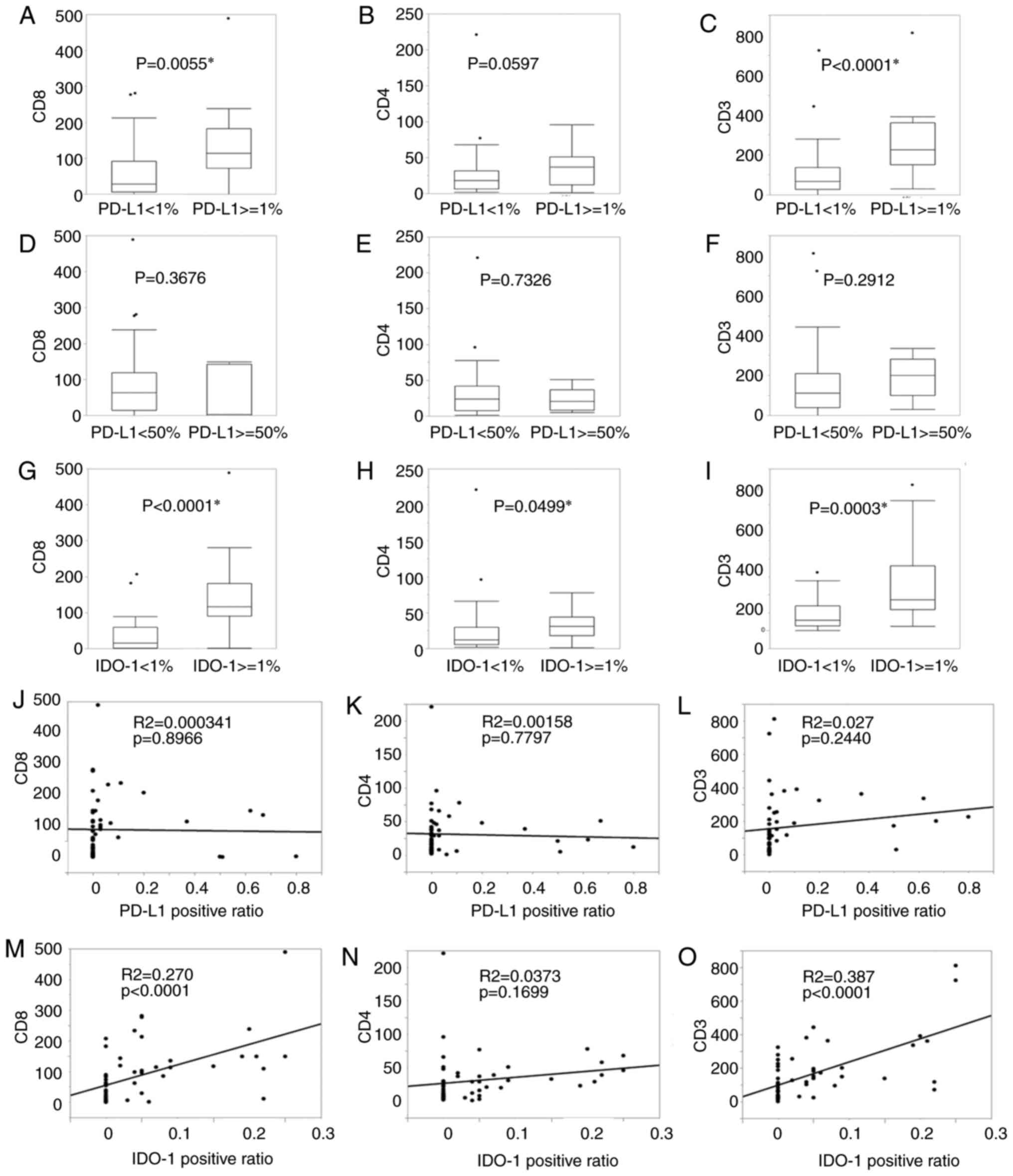
The relationships between PD-L1 or IDO-1
immunoexpression and lymphocytic infiltration are presented in
Fig. 3. In the Mann-Whitney U-test,
PD-L1 expression (≥1%) was associated with CD8-(P=0.0055, Fig. 3A) and CD3-positive lymphocytes
(P<0.0001, Fig. 3C), but not
with CD4-positive ones (Fig. 3B).
Strong PD-L1 expression (≥50%) was not associated with lymphocytic
infiltration (Fig. 3D-F). IDO-1
expression (≥1%) was related to CD8-(P<0.0001, Fig. 3G), CD4- (P<0.0499, Fig. 3H), and CD3-positive lymphocytes
(P=0.0003, Fig. 3I). From the
analysis applying the least squares method, the regressions between
PD-L1 and CD8-, CD4-, and CD3-positive TILs were not significant
(R-squared: 0.000341, 0.00158, 0.0270, P-values: 0.8966, 0.7797,
0.2440; Fig. 3J-L). Positive
correlations of IDO-1 with CD8 and CD3 were seen (R-squared: 0.270,
0.387, P-values: <0.0001, <0.0001; Fig. 3M and O). The regression between
IDO-1 and CD4 was not significant (R-squared: 0.0373, P-value:
0.1699; Fig. 3N).
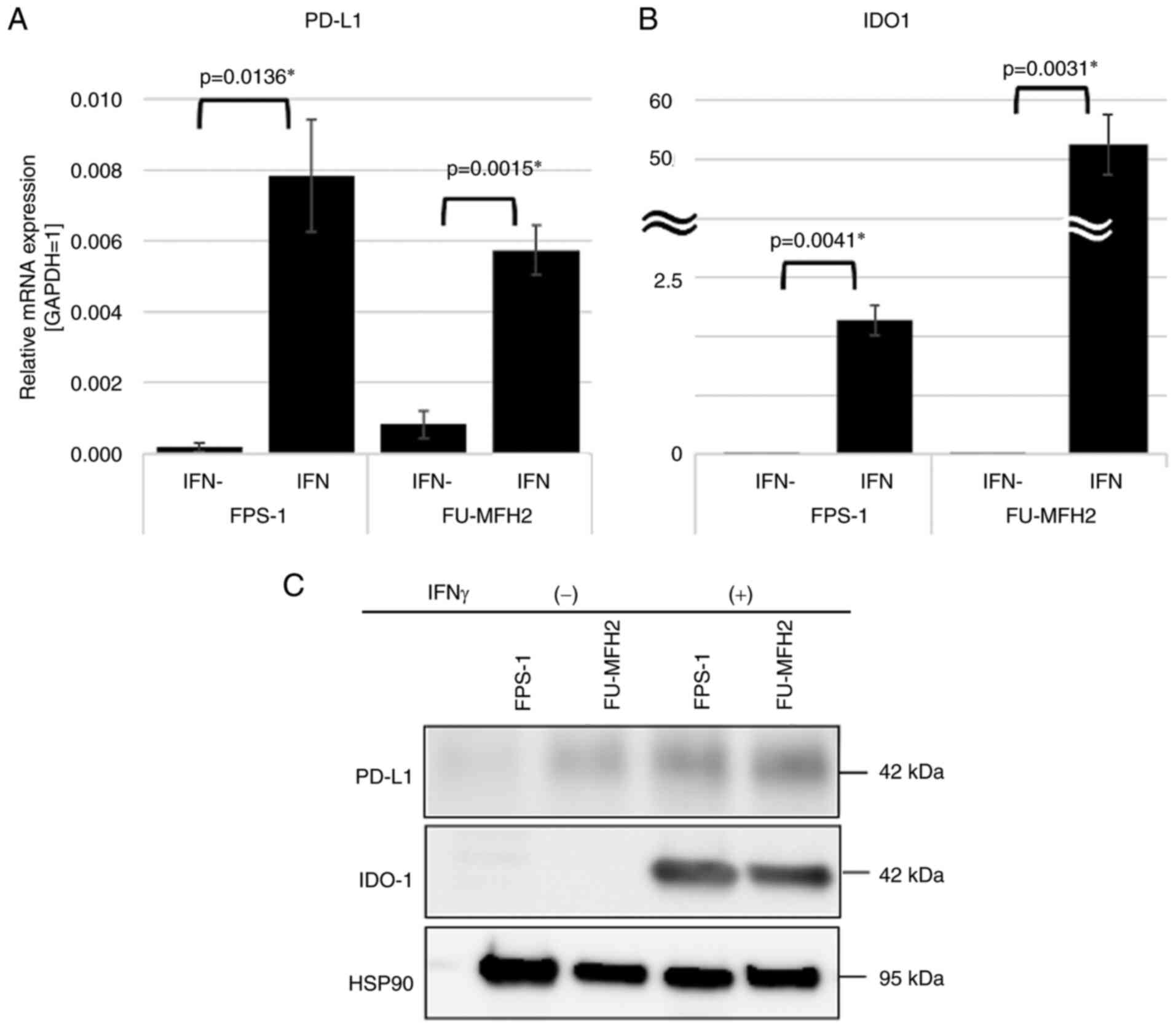
Induction of PD-L1 and IDO-1
expression by IFN-γ in vitro
The data acquired from qPCR are presented in
Fig. 4A and B. IFN-γ induced the
mRNA expression of PD-L1 (P=0.0136 and P=0.0015, Fig. 4A) and IDO-1 (P=0.0041 and P=0.0031,
Fig. 4B). The results of western
blotting showed that the expression of PD-L1 induced by IFN-γ was
4.29-fold as high as in the control in FPS-1 cells and 2.00-fold in
FU-MFH2 cells. The expression of IDO-1 induced by IFN-γ was higher
than the expressions in the control in FPS-1 cells and in FU-MFH2
cells (Fig. 4C). IDO-1 expression
of the cell lines tended to react more strongly to IFN-γ
stimulation than that of PD-L1 in both qPCR and western
blotting.
Survival analysis
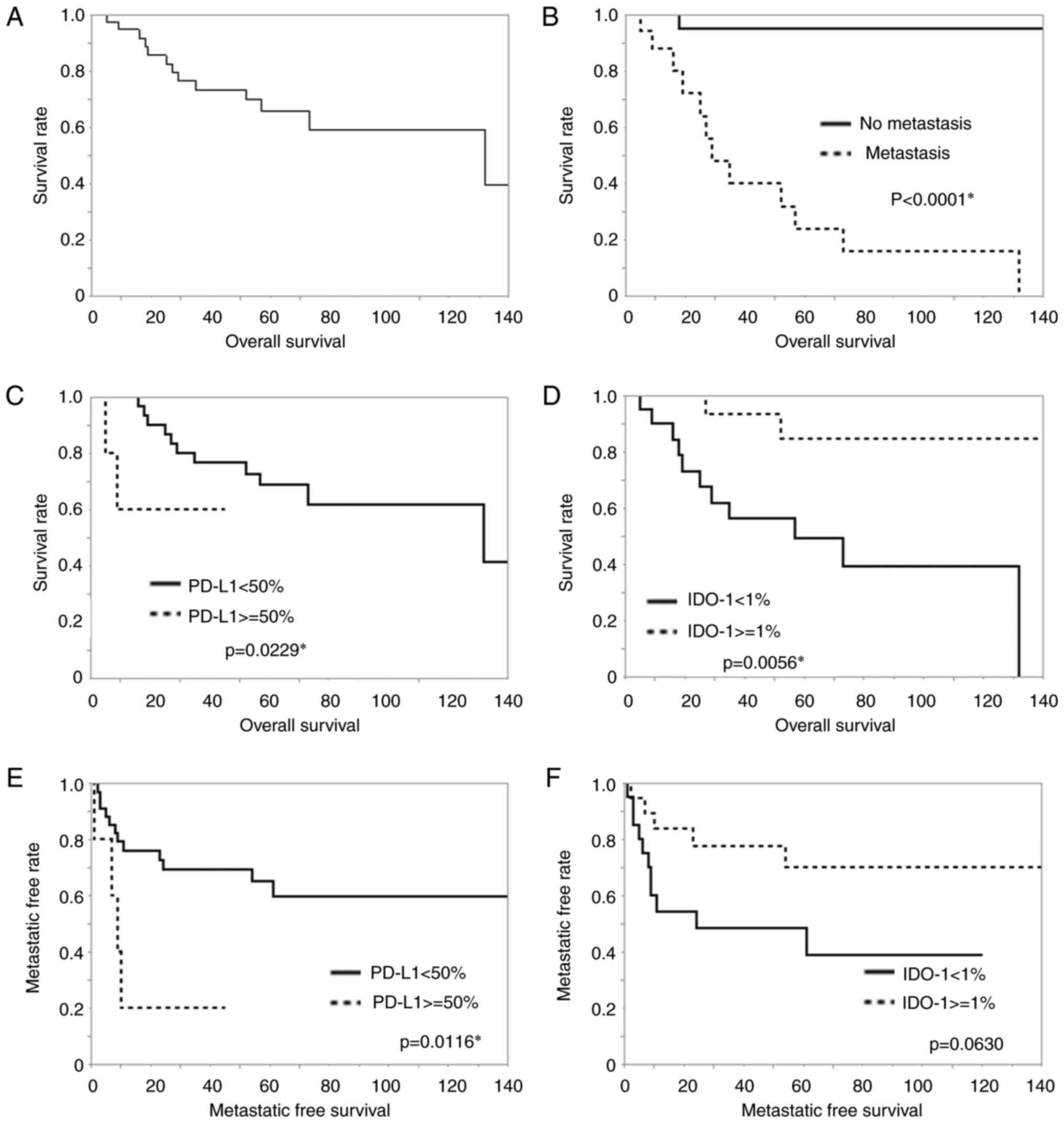
The results of survival curve analysis are presented
in Fig. 5. Overall survival rate is
shown (Fig. 5A). Metastasis was
closely related to a poorer overall survival rate (P<0.0001,
Fig. 5B), but recurrence was not in
this study. Patients with strong PD-L1 expression (≥50%) had worse
prognosis than the other patients (<50%) (P=0.0229, Fig. 5C). On the other hand, IDO-1
expression (≥1%) was associated with a better prognosis than lack
of this IDO-1 expression regarding overall survival (P=0.0056,
Fig. 5D). PD-L1 expression (≥1%),
TILs, loss of HLA class I and dMMR were not significant prognostic
factors for overall survival. In addition, patients with strong
PD-L1 expression also had worse prognosis than the other patients
(P=0.0116 Fig. 5E). On the other
hand, IDO-1 expression (≥1%) tended to be a factor associated with
a favorable prognosis compared with negative IDO-1 expression
(P=0.0630, Fig. 5F), but it was not
significant. TILs, loss of HLA class I, and dMMR were not
significant prognostic factors for metastasis-free survival. No
significant findings for recurrence-free survival were made for the
expression of PD-L1 and IDO-1, clinicopathological features, TILs,
loss of HLA class I, and dMMR.
Discussion
We confirmed the frequent expression of PD-L1 and
IDO-1 in UPS by immunohistochemical analysis. The proportion of
cases with PD-L1 expression was similar to that in a study of UPS
performed by Boxberg et al (30). In detail, over 30% of UPS cases
exhibited at least focal PD-L1 expression and nearly 10% had strong
PD-L1 expression.
It has been reported that PD-L1 and IDO-1 expression
is associated with TILs. In the current study, PD-L1 expression
(≥1%) was related to the infiltration of CD8- and CD3-positive
lymphocytes. IDO-1 expression (≥1%) was associated with the
infiltration of CD8-, CD4-, and CD3-positive lymphocytes. Of note,
the group with a strong expression of PD-L1 (≥50%) was not
significantly associated with TILs. In the regression analysis, the
correlation between PD-L1 and TILs was not significant, but there
were significant positive correlations of IDO-1 with CD8 and CD3.
It was previously reported that helper T, cytotoxic T, and NK
cells, as well as macrophages, secrete IFN-γ (17) and that IFN-γ induces the expression
of PD-L1 and IDO-1 (5,16,17).
In the current study, PD-L1 and IDO-1 expression in UPS-cell lines
were induced by IFN-γ in vitro. The results of the current
study confirmed that PD-L1 and IDO-1 were induced by TILs in
sarcoma as in other cancers (5).
The results also suggest that a strong PD-L1 expression (≥50%)
requires factors other than TILs. These other factors may be
intrinsic to cancer, such as copy number gain and CMTM6 (31). The survival rate of patients with
IDO-1 expression was better than that of patients without it. IDO-1
expression of the UPS cell lines tended to react more strongly to
IFN-γ stimulation than that of PD-L1. The findings also suggest
that IDO-1 may reflect the condition of anti-cancer immunity rather
than PD-L1, and that immune reactions against tumor cells may work
better in tumors with IDO-1 expression than in tumors without it.
It was reported that tumor-infiltrating CD8-positive cells were
predictive factor for anti-PD-1 immunotherapy (32). We considered that IDO-1 may be a
predictive factor of the efficacy of anti-PD-1 therapy. It was also
reported that tumors expressing IDO-1 may be a good target for
anti-PD-1 therapy (12). Findings
of that study may support our consideration that IDO-1 reflected
the condition of anti-cancer immunity. In a previous study, it was
reported that patients with IDO-1-positivity in stromal cells had
better prognosis (33). In that
study, the authors discussed that decreased tryptophan availability
disturbs the proliferation of tumor cells to some extent. In the
current study, macrophages and lymphocytes were stained by IDO-1 as
well as tumor cells. In addition, IFN-γ secreted by macrophages may
stimulate themselves to express IDO-1. It was difficult to score
the IDO-1 expression of stromal cells as described in a previous
study as tumor and stromal cells were not clearly separated
optically (33). In the current
study as well, it was suggested that the exhaustion of tryptophan
may interfere with tumor cell proliferation.
As in previous results from a meta-analysis
(34), high expression of PD-L1 was
related to an unfavorable prognosis in this study. This could be
explained by tumor cells evading tumor immunity due to a high
expression of PD-L1 potentially causing poor prognosis. By
contrast, IDO-1 expression was related to a favorable prognosis,
although IDO-1 may enable tumor cells to avoid the immune system.
As mentioned above, IDO-1 exhibited close correlations with CD8 and
CD3, thus the favorable effect of IDO-1 expression on prognosis may
reflect the promotion of the tumor-targeting immune system by
TILs.
As for the clinicopathological features, overall
survival and metastasis were closely related to each other as in a
previous study (35). Specifically,
the cases without metastasis tended to be less likely to result in
tumor-related death. Therefore, it was suggested that wide
resection before metastasis may be one of the most important
therapeutic options and that a definitive diagnosis of UPS in the
early therapeutic phase may be essential. In the present study, the
existence of a focal myxoid matrix was not significantly associated
with overall, metastasis-free, or recurrence-free survival.
Cases of UPS with tumor necrosis also expressed
PD-L1 more often than those of UPS without it. This may depend on
the TILs around the area of tumor necrosis. PD-L1 expression in
these tumors may not necessarily reflect that tumor immunity is
working. Therefore, anti-PD-1 therapy may not be useful for such
tumors, although they expressed PD-L1.
In the present investigation, we found a small
population of UPS cases with dMMR, which also expressed PD-L1.
Regarding HLA class I, six tumors lost their expression and one of
these expressed focal PD-L1. More studies on the effectiveness of
anti-PD-1 therapy for tumors with the loss of HLA class I are
needed.
As for the limitations of the current investigation,
UPS is extremely rare, thus, the number of cases was small. There
were only five UPS cases expressing PD-L1 (≥50%). As such, the
possibility of bias in the results cannot be ruled out. The
functional experiments on the usefulness of anti-PD1 or anti-PDL1
therapy to UPS cell lines were also missing. Our experiments were
limited to the induction of INF-γ to UPS cell lines and its effect
on PDL-1 and IDO-1 expression. Our in vitro study showed
only a preliminary result that IDO-1 expression induced by IFN-γ
stimulation in UPS cells tended to be stronger than PD-L1
expression. The results of double staining did not prove that TILs
positive for CD8-, CD4-, and CD3-secreted IFN-γ because of the data
based on only immunochemical staining of the specimens. In
addition, the outcome of anti-PD-1 therapy for patients with UPS
was not included in the analysis. Therefore, the difference of
effectiveness of anti-PD-1 therapy in UPS cases between UPS with
and without IDO-1 expression remains uncertain. A clinical study
with a larger number of UPS cases should be carried out.
In conclusion, findings of the present study showed
that UPS tumor cells frequently expressed PD-L1 and IDO-1. It was
also suggested that strong PD-L1 expression (≥50%) requires factors
other than TILs. Finally, PD-L1 expression (≥50%) may be a poor
prognostic factor and IDO-1 expression may be a better prognostic
factor of for UPS patients.
Supplementary Material
Supporting Data
Acknowledgements
We would like to thank all of the technical staff of
the Department of Pathology, Kyushu University for their
assistance. We also appreciate the technical assistance from The
Research Support Center, Kyushu University Graduate School of
Medical Sciences.
Funding
The present study was supported by the Japan Society
for the Promotion of Science KAKENHI (19H03444).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SI, YY, TI, KK HY and YO designed the study. SI, YY
and MY collected the materials. SI, TI and YT performed the
experiments. YM and YN collected the clinical information. SI wrote
the manuscript. KK and YO reviewed and checked the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Kyushu
University Committee of Bioethics (approval no. 29-429 and 29-625;
2017).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
UPS
|
undifferentiated pleomorphic
sarcoma
|
|
TIL
|
tumor-infiltrating lymphocyte
|
|
dMMR
|
deficient mismatch repair
|
References
|
1
|
The International Agency for Research on
Cancer (IARC), ; Fletcher CDM, Chibon F and Mertens F: Pathology
and genetics of tumours of soft tissue and bone (IARC WHO
Classification of Tumours). IARC Press; Lyon: pp. 235–238. 2013
|
|
2
|
Gronchi A, Palmerini E, Quagliuolo V,
Broto JM, Pousa AL, Grignani G, Brunello A, Blay JY, Tendero O,
Beveridge RD, et al: Neoadjuvant chemotherapy in high-risk soft
tissue sarcomas: Final results of a randomized trial from Italian
(ISG), Spanish (GEIS), French (FSG), and Polish (PSG) sarcoma
groups. J Clin Oncol. 1:2178–2186. 2020. View Article : Google Scholar
|
|
3
|
Kim JH, Park HS, Heo SJ, Kim SK, Han JW,
Shin KH, Kim SH, Hur H, Kim KS, Choi YD, et al: Differences in the
efficacies of pazopanib and gemcitabine/docetaxel as second-line
treatments for metastatic soft tissue sarcoma. Oncology. 96:59–69.
2019. View Article : Google Scholar
|
|
4
|
Nakamura T, Tsukushi S, Asanuma K,
Katagiri H, Ikuta K, Nagano A, Kozawa E, Yamada S, Shido Y, Yamada
K, et al: The clinical outcome of eribulin treatment in Japanese
patients with advanced soft tissue sarcoma: A tokai musculoskeletal
oncology consortium study. Clin Exp Metastasis. 36:343–350. 2019.
View Article : Google Scholar
|
|
5
|
Schalper KA, Carvajal-Hausdorf D,
McLaughlin J, Altan M, Velcheti V, Gaule P, Sanmamed MF, Chen L,
Herbst RS and Rimm DL: Differential expression and significance of
PD-L1, IDO-1, and B7-H4 in human lung cancer. Clin Cancer Res.
15:370–378. 2017. View Article : Google Scholar
|
|
6
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 22:252–264.
2012. View
Article : Google Scholar
|
|
7
|
Syn NL, Teng MW, Mok TS and Soo RA:
De-Novo and acquired resistance to immune checkpoint targeting.
Lancet Oncol. 18:e731–e741. 2012. View Article : Google Scholar
|
|
8
|
Ready N, Hellmann MD, Awad MM, Otterson
GA, Gutierrez M, Gainor JF, Borghaei H, Jolivet J, Horn L, Mates M,
et al: First-Line nivolumab plus ipilimumab in advanced
non-small-cell lung cancer (CheckMate 568): Outcomes by programmed
death ligand 1 and tumor mutational burden as biomarkers. J Clin
Oncol. 20:992–1000. 2019. View Article : Google Scholar
|
|
9
|
Tawbi HA, Burgess M, Bolejack V, Van Tine
BA, Schuetze SM, Hu J, D'Angelo S, Attia S, Riedel RF, Priebat DA,
et al: Pembrolizumab in advanced soft-tissue sarcoma and bone
sarcoma (SARC028): A multicentre, two-cohort, single-arm,
open-label, phase 2 trial. Lancet Oncol. 18:1493–1501. 2017.
View Article : Google Scholar
|
|
10
|
Marabelle A, Le DT, Ascierto PA, Di
Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M,
Penel N, Hansen AR, et al: Efficacy of pembrolizumab in patients
with noncolorectal high microsatellite instability/mismatch
repair-deficient cancer: Results from the phase II KEYNOTE-158
study. J Clin Oncol. 1:1–10. 2020. View Article : Google Scholar
|
|
11
|
Flores-Martin JF, Perea F, Exposito-Ruiz
M, Carretero FJ, Rodriguez T, Villamediana M, Ruiz-Cabello F,
Garrido F, Cózar-Olmo JM and Aptsiauri N: A combination of positive
tumor HLA-I and negative PD-L1 expression provides an immune
rejection mechanism in bladder cancer. Ann Surg Oncol.
26:2631–2639. 2019. View Article : Google Scholar
|
|
12
|
Johnson DB, Bordeaux J, Kim JY, Vaupel C,
Rimm DL, Ho TH, Joseph RW, Daud AI, Conry RM, Gaughan EM, et al:
Quantitative spatial profiling of PD-1/PD-L1 interaction and
HLA-DR/IDO-1 predicts improved outcomes of anti-PD-1 therapies in
metastatic melanoma. Clin Cancer Res. 1:5250–5260. 2018.
|
|
13
|
Munn DH and Mellor AL: Indoleamine 2,3
dioxygenase and metabolic control of immune responses. Trends
Immunol. 34:137–143. 2013. View Article : Google Scholar
|
|
14
|
Prendergast GC, Smith C, Thomas S,
Mandik-Nayak L, Laury- Kleintop L, Metz R and Muller AJ:
Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and
immune escape in cancer. Cancer Immunol Immunother. 63:721–735.
2014. View Article : Google Scholar
|
|
15
|
Long GV, Dummer R, Hamid O, Gajewski TF,
Caglevic C, Dalle S, Arance A, Carlino MS, Grob JJ, Kim TM, et al:
Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in
patients with unresectable or metastatic melanoma
(ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study.
Lancet Oncol. 20:1083–1097. 2019. View Article : Google Scholar
|
|
16
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 27:568–571. 2014. View Article : Google Scholar
|
|
17
|
Sznol M and Chen L: Antagonist antibodies
to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human
cancer. Clin Cancer Res. 19:1021–1034. 2013. View Article : Google Scholar
|
|
18
|
Yoshimoto M, Yamada Y, Ishihara S, Kohashi
K, Toda Y, Ito Y, Yamamoto H, Furue M, Nakashima Y and Oda Y:
Comparative study of myxofibrosarcoma with undifferentiated
pleomorphic sarcoma: Histopathologic and clinicopathologic review.
Am J Surg Pathol. 44:87–97. 2020. View Article : Google Scholar
|
|
19
|
Thway K, Flora R, Shah C, Olmos D and
Fisher C: Diagnostic utility of p16, CDK4, and MDM2 as an
immunohistochemical panel in distinguishing well-differentiated and
dedifferentiated liposarcomas from other adipocytic tumors. Am J
Surg Pathol. 36:462–469. 2012. View Article : Google Scholar
|
|
20
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; St. Louis, MO: 2010
|
|
21
|
Volaric A, Gentzler R, Hall R, Mehaffey
JH, Stelow EB, Bullock TN, Martin LW and Mills AM:
Indoleamine-2,3-dioxygenase in non-small cell lung cancer: A
targetable mechanism of immune resistance frequently coexpressed
with PD-L1. Am J Surg Pathol. 42:1216–1223. 2018. View Article : Google Scholar
|
|
22
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 9:1540–1550. 2016.
View Article : Google Scholar
|
|
23
|
Reck M, Rodriguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 10:1823–1833. 2016.
View Article : Google Scholar
|
|
24
|
Seeber A, Klinglmair G, Fritz J, Steinkohl
F, Zimmer KC, Aigner F, Horninger W, Gastl G, Zelger B, Brunner A
and Pichler R: High IDO-1 expression in tumor endothelial cells is
associated with response to immunotherapy in metastatic renal cell
carcinoma. Cancer Sci. 109:1583–1591. 2018. View Article : Google Scholar
|
|
25
|
Perea F, Bernal M, Sánchez-Palencia A,
Carretero J, Torres C, Bayarri C, Gómez-Morales M, Garrido F and
Ruiz-Cabello F: The absence of HLA class I expression in non-small
cell lung cancer correlates with the tumor tissue structure and the
pattern of T cell infiltration. Int J Cancer. 15:888–899. 2017.
View Article : Google Scholar
|
|
26
|
Nakano K, Yamamoto H, Fujiwara M, Koga Y,
Tsuruta S, Ihara E, Oki E, Nakamura M, Ogawa Y and Oda Y:
Clinicopathologic and molecular characteristics of synchronous
colorectal carcinoma with mismatch repair deficiency. Am J Surg
Pathol. 42:172–182. 2018. View Article : Google Scholar
|
|
27
|
Yoo SH, Keam B, Ock CY, Kim S, Han B, Kim
JW, Lee KW, Jeon YK, Jung KC, Chung EJ, et al: Prognostic value of
the association between MHC class I downregulation and PD-L1
upregulation in head and neck squamous cell carcinoma patients. Sci
Rep. 22:76802019. View Article : Google Scholar
|
|
28
|
Hakozaki M, Hojo H, Sato M, Tajino T,
Yamada H, Kikuchi S and Abe M: Establishment and characterization
of a new cell line, FPS-1, derived from human undifferentiated
pleomorphic sarcoma, overexpressing epidermal growth factor
receptor and cyclooxygenase-2. Anticancer Res. 26:3393–3401.
2006.
|
|
29
|
Nishio J, Iwasaki H, Nabeshima K, Ishiguro
M, Isayama T and Naito M: Establishment of a new human pleomorphic
malignant fibrous histiocytoma cell line, FU-MFH-2: Molecular
cytogenetic characterization by multicolor fluorescence in situ
hybridization and comparative genomic hybridization. J Exp Clin
Cancer Res. 24:1532010. View Article : Google Scholar
|
|
30
|
Boxberg M, Steiger K, Lenze U, Rechl H,
von Eisenhart-Rothe R, Wörtler K, Weichert W, Langer R and Specht
K: PD-L1 and PD-1 and characterization of tumor-infiltrating
lymphocytes in high grade sarcomas of soft tissue-prognostic
implications and rationale for immunotherapy. Oncoimmunology.
20:e13893662017.
|
|
31
|
Burr ML, Sparbier CE, Chan YC, Williamson
JC, Woods K, Beavis PA, Lam EY, Henderson MA, Bell CC, Stolzenburg
S, et al: CMTM6 maintains the expression of PD-L1 and regulates
anti-tumour immunity. Nature. 7:101–105. 2017. View Article : Google Scholar
|
|
32
|
Sun R, Limkin EJ, Vakalopoulou M, Dercle
L, Champiat S, Han SR, Verlingue L, Brandao D, Lancia A, Ammari S,
et al: A radiomics approach to assess tumour-infiltrating CD8 cells
and response to anti-PD-1 or anti-PD-L1 immunotherapy: An imaging
biomarker, retrospective multicohort study. Lancet Oncol.
19:1180–1191. 2018. View Article : Google Scholar
|
|
33
|
Patil PA, Blakely AM, Lombardo KA, Machan
JT, Miner TJ, Wang LJ, Marwaha AS and Matoso A: Expression of
PD-L1, indoleamine 2,3-dioxygenase and the immune microenvironment
in gastric adenocarcinoma. Histopathology. 73:124–136. 2018.
View Article : Google Scholar
|
|
34
|
Zheng C, You W, Wan P, Jiang X, Chen J,
Zheng Y, Li W, Tan J and Zhang S: Clinicopathological and
prognostic significance of PD-L1 expression in sarcoma: A
systematic review and meta-analysis. Medicine (Baltimore).
97:e110042018. View Article : Google Scholar
|
|
35
|
Vasileios KA, Eward WC and Brigman BE:
Surgical treatment and prognosis in patients with high-grade soft
tissue malignant fibrous histiocytoma of the extremities. Arch
Orthop Trauma Surg. 132:955–961. 2012. View Article : Google Scholar
|
|
36
|
Roland CL, May CD, Watson KL, Al Sannaa
GA, Dineen SP, Feig R, Landers S, Ingram DR, Wang WL, Guadagnolo
BA, et al: Analysis of clinical and molecular factors impacting
oncologic outcomes in undifferentiated pleomorphic sarcoma. Ann
Surg Oncol. 23:2220–2228. 2016. View Article : Google Scholar
|