Introduction
Since 2004, docetaxel has been the standard
first-line chemotherapy for men with metastatic
castration-resistant prostate cancer (mCRPC) based on improvements
in overall survival (OS) and progression-free survival (PFS)
compared with mitoxantrone and prednisone (1,2).
Although most patients receive docetaxel chemotherapy for mCRPC,
the cancer will eventually progress and no consensus exists for the
optimal intervention after docetaxel failure. The US Food and Drug
Administration (FDA) approved several new drugs for patients with
mCRPC for whom docetaxel chemotherapy failed, including cabazitaxel
(3), abiraterone with prednisone
(4), enzalutamide (5), and radium-223 (6). Although these advancements have been
made, the improvement in survival by these drugs is only several
months and mCRPC continues to be incurable. Therefore, treatments
that can provide stable disease control and long-term survival
benefits are needed.
In the last few decades, immunotherapy has become an
important part of treating several types of cancer. Sipuleucel-T is
currently the only approved cellular product immune therapy for the
treatment of asymptomatic or minimally symptomatic CRPC (7). Although immune checkpoint inhibitors,
including cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed
death 1 (PD1), and programmed death-ligand 1 (PD-L1) were recently
approved by the FDA to treat different types of solid tumors and
hematologic malignancies, none of these immune checkpoint
inhibitors have been approved for mCRPC (8,9). In
addition, neither peptide-based vaccine trials nor recent conducted
immunotherapy studies demonstrated clinical benefits for mCRPC in
large randomized trials (10). This
failure may be due to the large diversity of immunological features
of mCRPC patients and the lack of readily available biomarkers of
immunotherapy benefits.
To overcome these difficulties, we are developing a
new concept of personalized peptide vaccination (PPV) for patients
with advanced cancer, in which up to 4 peptides are selected from a
collection of warehouse peptides based on pre-existing immunity
(11). A phase I and follow-up
study of PPV consisting of 14 warehouse peptides for human
leukocyte antigen (HLA) -A24-positive patients with advanced CRPC
demonstrated its safety and potential clinical benefit (12). A randomized phase II trial of PPV
with low-dose dexamethasone for patients with chemotherapy-naïve
CRPC also resulted in a longer PFS of prostate-specific antigen
(PSA) and OS (13). In addition,
another phase II study suggested that the OS of docetaxel-resistant
CRPC patients treated with PPV was longer than that of historical
controls (14).
Based on these results, a randomized, double-blind,
placebo-controlled, phase III trial of PPV for HLA-A24-positive
patients with CRPC progressing after docetaxel chemotherapy with OS
as the primary endpoint was conducted in Japan.
Materials and methods
Patient population
For this phase III, randomized, double-blind,
placebo-controlled study, we enrolled HLA-A24-positive patients
with CRPC progressing within 12 months after docetaxel chemotherapy
from 68 medical centers in Japan. Eligible patients were aged 20
years or older with histologically confirmed adenocarcinoma of the
prostate. Other inclusion criteria were as follows: positive
immunoglobulin G (IgG) responses to at least 2 of 12 warehouse
peptides (Table SI) on the
screening test, an Eastern Cooperative Oncology Group (ECOG)
performance status (PS) of 0 or 1, life expectancy of ≥12 weeks,
serum testosterone level of ≤50 ng/dl, and satisfactory bone marrow
function, hepatic function, and renal function. Patients without
previous bilateral orchiectomy continued treatment with luteinizing
hormone-releasing agonists. Exclusion criteria included acute
infection, history of severe allergic reactions, pulmonary, cardiac
or other systemic diseases, or other inappropriate conditions for
enrollment as determined by the clinicians. Prior enzalutamide or
abiraterone was permitted.
The trial was conducted in accordance with the
Declaration of Helsinki and Good Clinical Practice guidelines. The
protocol was approved by institutional review boards or ethical
committees at all of the institutions, and it was registered in the
UMIN Clinical Trials Registry (no. UMIN000011308). All patients
were Japanese and provided written informed consent before
participating in this study.
Study design and treatment
Patients were randomly assigned at a 2:1 ratio to
receive PPV or placebo using the minimization technique with the
following stratification factors: age (<75 or ≥75, PS (0 or 1),
and use of enzalutamide or abiraterone (with or without) at each
participating institution. The present study was double-blinded,
and all physicians, patients, and investigators providing the
interventions, assessing outcomes, and analyzing data were blinded
to treatment assignment. Up to 4 of 12 warehouse peptides selected
based on pre-existing peptide-specific IgG levels or the
corresponding placebos were emulsified with Montanide ISA 51
incomplete Freund's adjuvant (Seppic), and each study drug (up to
4) in a 1.5-ml emulsion (3 mg/peptide or saline solution) was
subcutaneously injected in 6 doses weekly and then bi-weekly
following the maximum of 30 doses until disease progression.
Outcomes
The primary end point was OS, which was defined as
the time from random assignment to death by any cause. Secondary
end points were PFS, one-year survival rate, immune responses, and
safety. PFS was defined as the time from random assignment until
objective disease progression based on the PSA Working Group
Consensus Criteria 2 (PCWG2), the Response Evaluation Criteria in
Solid Tumors (RECIST) 1.1 criteria, or death. Immune responses were
assessed by IgG titers measured by the Luminex system (15) and cytotoxic T lymphocyte (CTL)
activity measured by the interferon (IFN)-γ release assay (16) using blood sampled at pre-treatment
and every 6 treatments. Safety was assessed based on physical
examination, vital sign measurements, clinical laboratory analyses,
and adverse events (AEs) graded using the Common Terminology
Criteria for Adverse Events (CTCAE) version 4.0.
Statistical analysis
All efficacy analyses were based on the full
analysis set (FAS), defined as patients who received at least one
dose of the treatment. The planned sample size of 300 patients (200
in PPV and 100 in placebo arms) provided 90% of power at a
two-sided significance level of 0.05 to detect a hazard ratio (HR)
of 0.6 for the primary endpoint of OS, corresponding to an increase
in the median OS from 11 to 17 months. All analyses were performed
after 23 months from the last patient enrolled or when 80% of
events for the primary endpoint of OS was reached. The follow-up
ended in October 2017 and final analyses were performed using a
database adjusted to May 2018.
Survival curves were described according to the
Kaplan-Meier method and 95% confidence intervals (CIs) were
calculated. Comparison of OS was performed by the
Harrington-Fleming test. A log-rank test using stratified
randomization was used to compare OS for PPV versus placebo. The
effects of treatments were reported as HRs, 95% CIs, and
interaction P for subgroup categories in a Forest plot. The
Student's t-test was used to compare quantitative variables. The
mean change from baseline in immune responses at each time point
was compared between the two arms in a linear regression model. All
toxicity grades and severe (grade ≥3) toxicities were compared
between the two arms (Fisher's exact test). All statistical tests
were interpreted as significant with a P-value of <5%. All
analyses were performed using JMP version 13 (SAS Institution).
Results
Patient characteristics
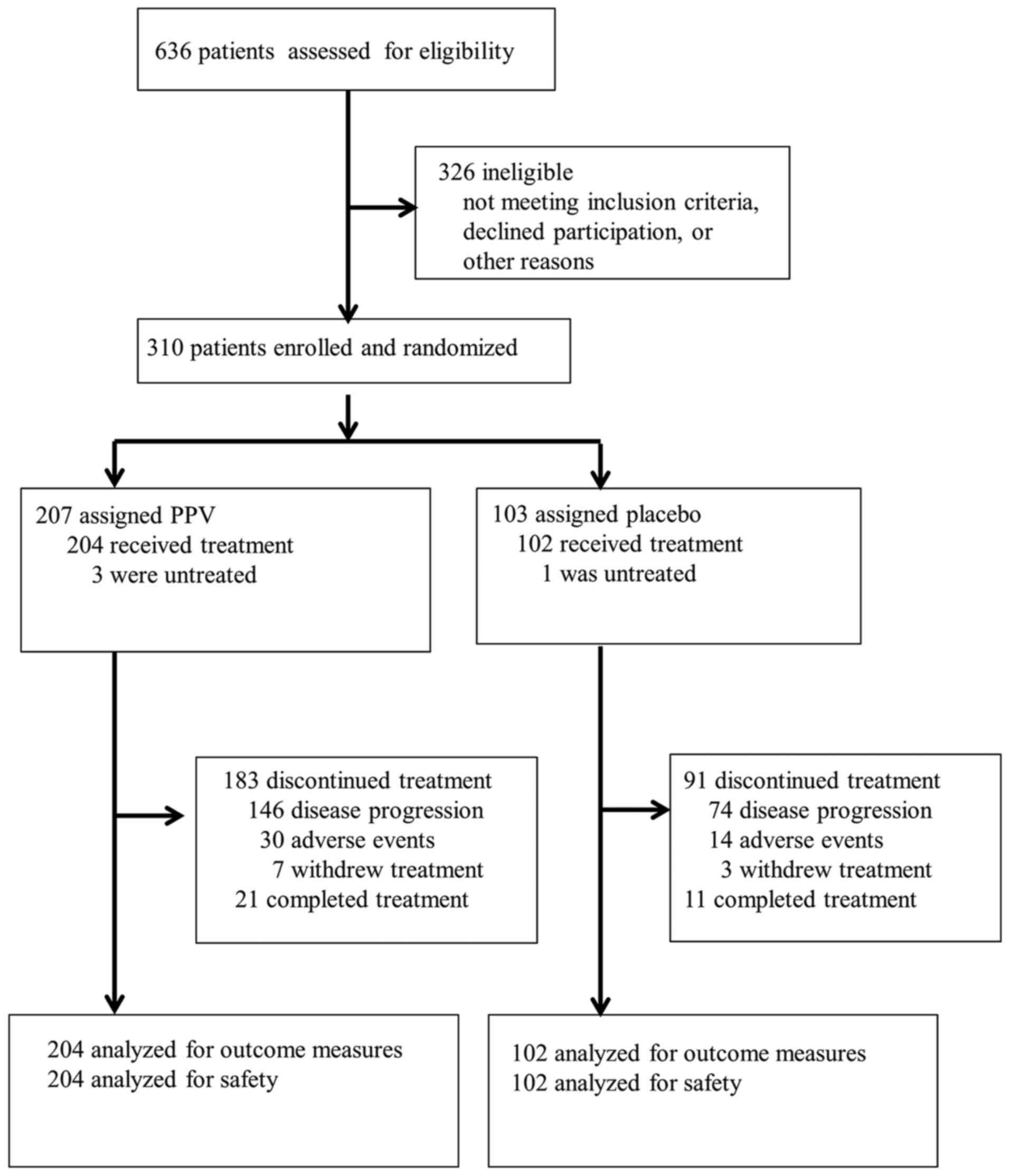
Between August 2013 and April 2016, 636 patients
were screened for eligibility in this study, and 310 eligible
patients were randomly assigned to the PPV arm (n=207) or placebo
arm (n=103). The most common reason for ineligibility was HLA
mismatch. Three patients in the PPV arm and 1 in the placebo arm
did not receive treatment, and 306 patients were analyzed by the
full analysis set (204 for PPV and 102 for placebo). A patient
flowchart is shown in Fig. 1. No
imbalances existed between randomization arms except for a lower
PSA level in the PPV arm (Table
I).
 | Table I.Baseline patient characteristics by
treatment arm. |
Table I.
Baseline patient characteristics by
treatment arm.
|
| PPV (n=204) | Placebo
(n=102) |
|---|
|
|
|
|
|---|
|
Characteristics | No. | % | No. | % |
|---|
| Median age, years
(range) | 71.0 (53–84) | 72.0 (56–82) |
| Age group
(years) |
|
<65 | 37 | 18.1 | 19 | 18.6 |
|
66-74 | 104 | 51 | 52 | 51 |
|
≥75 | 63 | 30.9 | 31 | 30.4 |
| ECOG performance
status |
| 0 | 162 | 79.4 | 82 | 80.4 |
| 1 | 42 | 20.6 | 20 | 19.6 |
| Prior use of
enzalutamide or abiraterone |
|
Yes | 66 | 32.4 | 35 | 34.3 |
| No | 44 | 21.6 | 20 | 19.6 |
|
Unknown | 94 | 46 | 47 | 46.1 |
| Serum PSA level,
ng/ml |
|
<50 | 109 | 53.4 | 50 | 49 |
|
50-499 | 83 | 40.7 | 37 | 36.3 |
|
≥500 | 12 |
5.9 | 15 | 14.7 |
| Gleason score at
diagnosis |
|
<6 | 4 |
2.4 | 3 |
2.9 |
| 7 | 30 | 14.6 | 20 | 19.6 |
| ≥8 | 166 | 80.6 | 75 | 73.5 |
|
Unknown | 4 |
2.4 | 4 | 4 |
| No. of metastatic
sites |
| 0 | 15 |
7.3 | 7 |
6.9 |
| 1 | 115 | 56.4 | 61 | 59.8 |
| ≥2 | 74 | 36.3 | 34 | 33.3 |
| Median proportion
of WBC type, % (range) |
|
Neutrophils | 70.0 (35-94.1) | 70.6 (39–91) |
|
Lymphocytes | 21.7
(3.6–50.5) | 21.4 (6-47.1) |
|
Eosinophils | 0.2 (0–2) | 0.3 (0-1.5) |
|
Basophils | 1.0 (0-13.8) | 1.0 (0-11.6) |
|
Monocytes | 6.1 (1.7–15) | 6.0 (2–17) |
|
Neutrophil to lymphocyte
ratio | 3.2 (0.7–26.1) | 3.3 (0.9–15.2) |
Efficacy
At the final analysis (cut-off date: October 1,
2017), the median length of follow-up for censored patients was
29.8 months in the PPV arm and 27.4 months in the placebo arm, and
89.5% of patients had discontinued treatment. The median total
number of doses of treatment drugs was 12 (IQR, 8 to 19) in the PPV
arm and 14 (IQR, 10 to 21) in the placebo arm. The reasons for
discontinuation (PPV vs. placebo) were disease progression (71.6
vs. 72.5%), AEs (14.7 vs. 13.7%) or withdrawal of consent for
treatment (3.4 vs. 2.9%). After the study treatment, 148 of 204
patients (72.5%) in the PPV arm and 71 of 102 patients (69.6%) in
the placebo arm used enzalutamide or abiraterone.
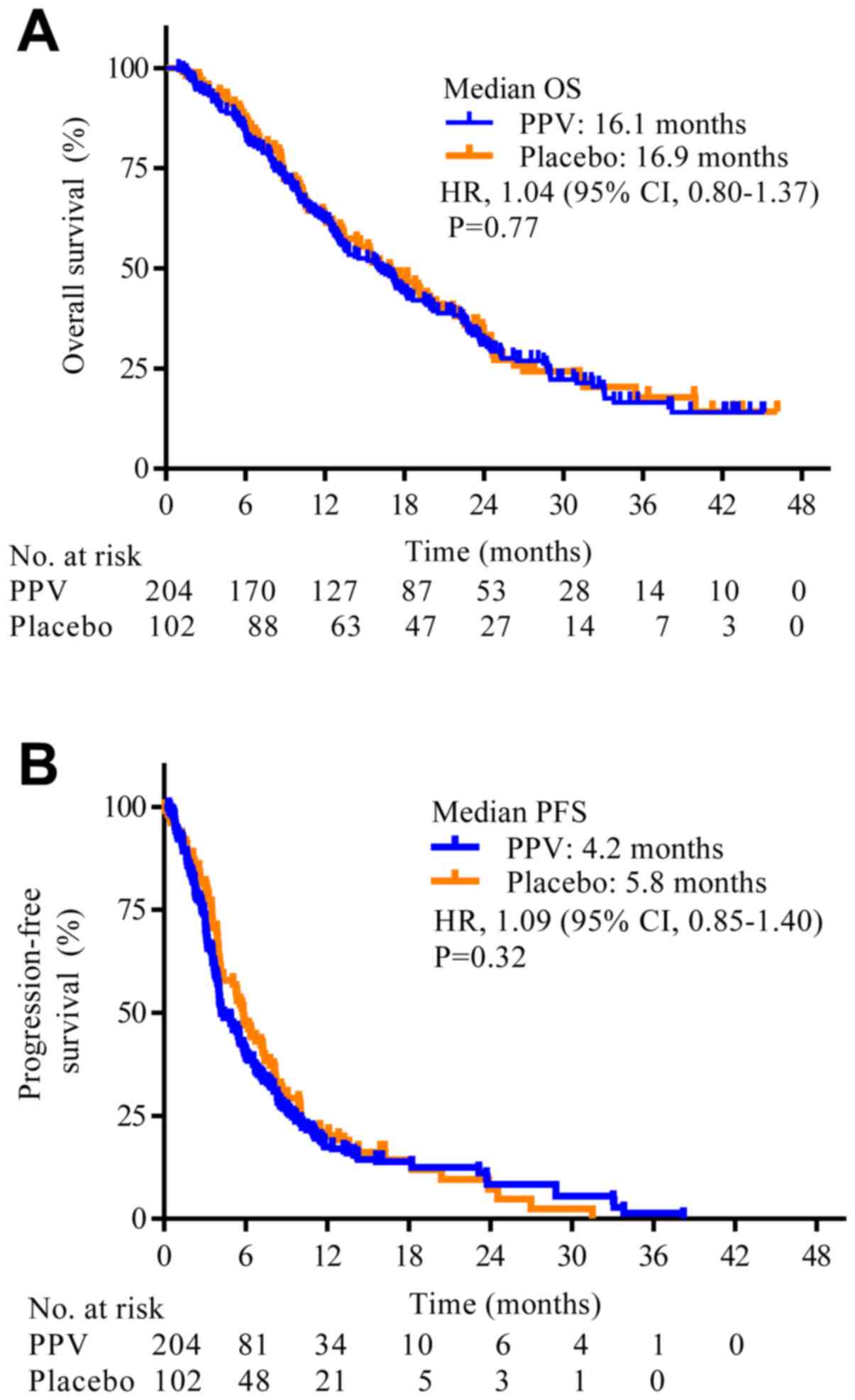
For OS, there were 160 deaths (78.4%) in the PPV arm
and 77 (75.5%) in the placebo arm. PPV did not improve OS compared
with placebo (median OS, 16.1 months (95% CI, 13–18.2 months) with
PPV and 16.9 months (95% CI, 13.1–20.4 months) with placebo; HR,
1.04; 95% CI, 0.80–1.37; P=0.77; Fig.
2A). The estimated median PFS was 4.2 months (95% CI, 4.0–5.6
months) with PPV and 5.8 months (95% CI, 4.1–7.3 months) with
placebo (HR, 1.09; 95% CI, 0.85–1.40; P=0.32; Fig. 2B). The median one-year survival rate
was also similar in the two arms (62.3 vs. 62.4%).
For immune responses, pre-treatment plasma and
peripheral blood mononuclear cell (PBMC) samples were available
from all 306 patients. The mean peptide-specific IgG and CTL levels
to the selected peptides were 154 fluorescence intensity units
(FIU) and 0.7 pg/ml in the PPV arm, and 311 FIU and 0.8 pg/ml in
the placebo arm, respectively. After the 6-dose treatment, 189
samples from the PPV arm and 96 samples from the placebo arm were
available for immune response analysis, and the mean values of IgG
and CTL were significantly higher in the PPV arm than in the
placebo arm (9716 vs. 284 FIU for IgG responses; P<0.01; 80.0
vs. 0.7 pg/ml for CTL activity; P<0.01). Significant
peptide-specific IgG and CTL increases were observed in 27.5 and
55.6% of the 189 PPV patients, respectively, but no increases were
observed in the placebo patients throughout the study period. There
was no relationship among IgG, CTL, and OS in the PPV arm following
application of the linear regression model [Fig. S1A; R2=0.00012, between
IgG change and OS after the 6-dose treatment, and
R2=0.0026, between CTL change and OS after the 6-dose
treatment (Fig. S1B)].
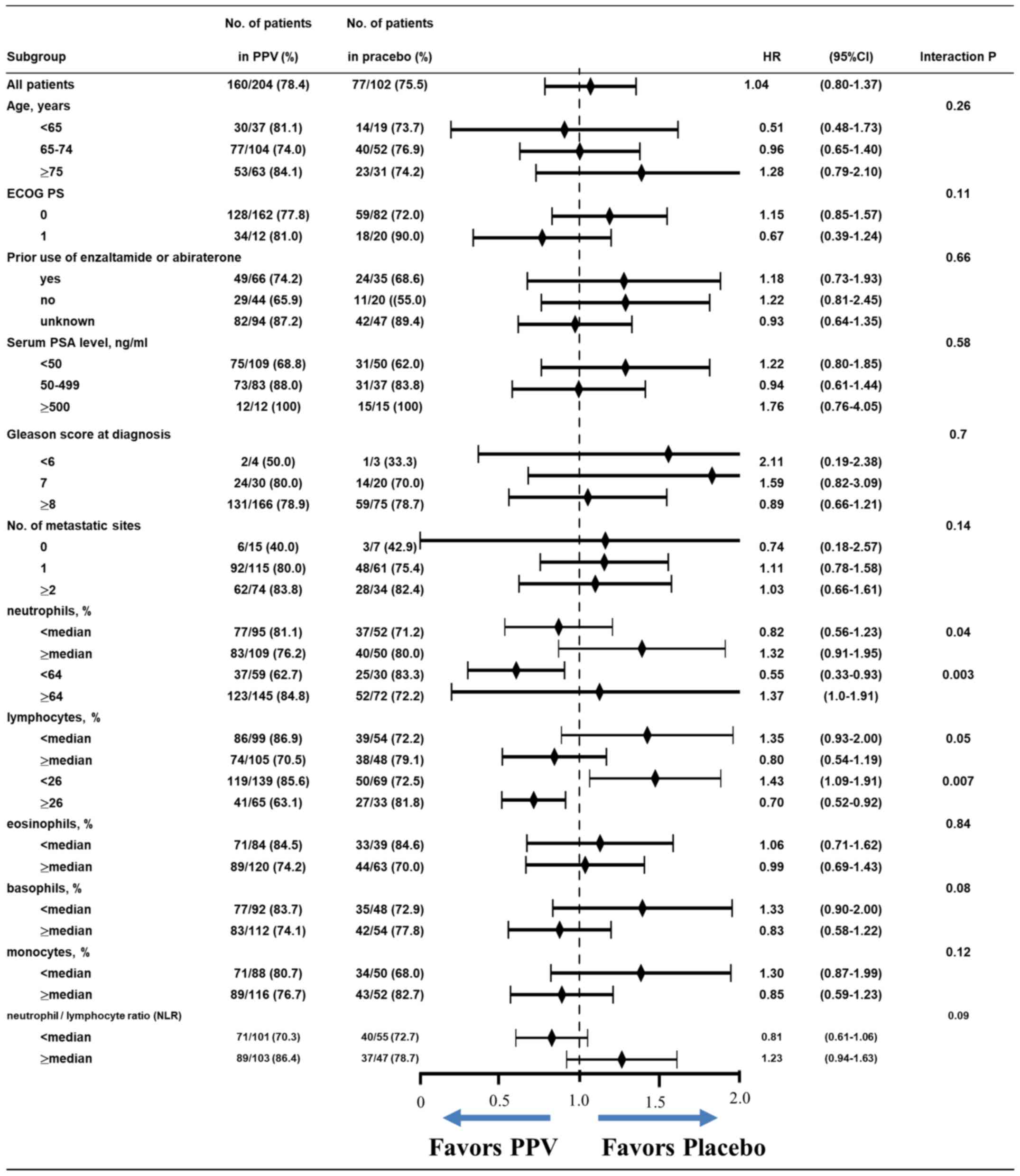
To further investigate the effects of PPV, we
evaluated treatment arm effects among patient subgroups. Initially,
we separated subgroups by the subsets listed in Table I. This analysis revealed lower HRs
for OS in favor of the PPV arm in patients with <the median%
neutrophils or ≥the median% lymphocytes among white blood cell
types at baseline (HR, 0.82; 95% CI, 0.56–1.23 for <median%
neutrophil or HR, 0.80; 95% CI, 0.54–1.19 for ≥median% lymphocyte)
by the significant interaction test (P=0.04 or P=0.05,
respectively; Fig. 3). Based on
this unexpected interaction among % neutrophils, % lymphocytes, and
the efficacy of PPV, we analyzed the most relevant % neutrophil or
% lymphocyte cut-off. We plotted interaction P-values from
neutrophil proportions of 50–80% (median value, 70%) or those from
lymphocyte proportions of 10–40% (median value, 21.6%) and the
number of target patients at each point (Fig. S1). The most relevant % neutrophil
and % lymphocyte cut-offs were 64 and 26%, respectively, with an
interaction of P<0.01 and a larger number of patients (Fig. S2). We reanalyzed treatment arm
effects using the cut-off of 64% neutrophils or 26% lymphocytes,
and found lower HRs for OS in PPV arm patients (HR, 0.55; 95% CI,
0.33–0.93 for <64% neutrophils or HR, 0.70; 95% CI, 0.52–0.92
for ≥26% lymphocytes) than in the initial analysis using the median
cut-off with an interaction P=0.003 or P=0.007, respectively
(Fig. 3). This interaction among
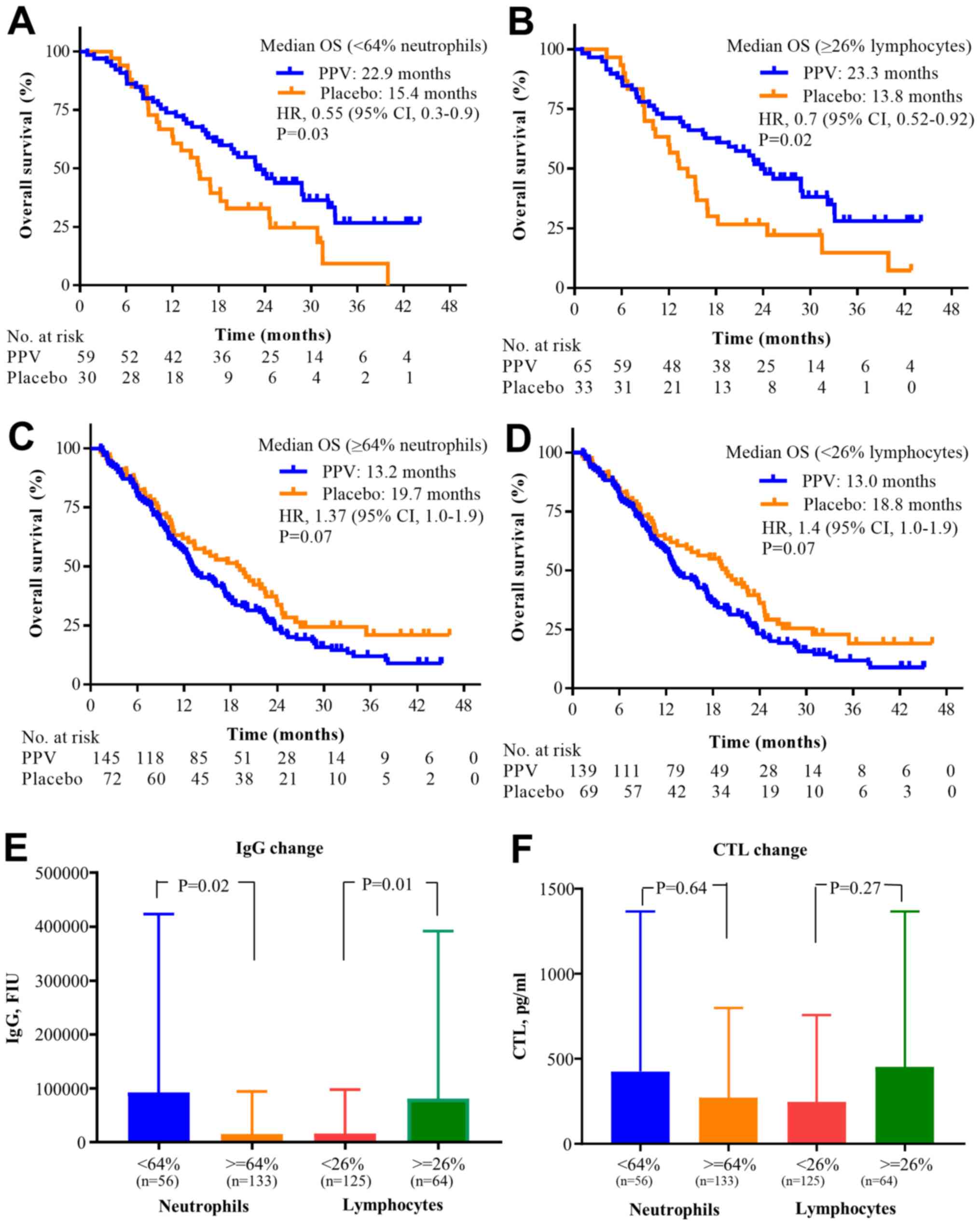
64% neutrophils, 26% lymphocytes, and the PPV treatment is shown in
Fig. 4. The median OS in the PPV
arm of patients with <64% neutrophils or ≥26% lymphocytes was
significantly longer than that in the placebo arm of patients in
the same subgroups (median OS, 22.9 vs. 15.4 months (Fig. 4A); P=0.03 or median OS, 23.3 vs.
13.8 months; P=0.02, respectively; Fig.
4B). By contrast, the median OS in the PPV arm of patients with
≥64% neutrophils or with <26% lymphocytes was not different from
that in the placebo arm of patients in the same subgroups (Fig. 4C and D). On analysis of immune
responses for these factors, IgG changes in the PPV arm of patients
with <64% neutrophils or ≥26% lymphocytes during the first 6
doses were significantly higher than those in patients in the
complementary subgroup (P=0.02 or P=0.01, respectively; t-test;
Fig. 4E), but CTL changes in these
subgroups were not significantly different (P=0.64 or P=0.27,
respectively; t-test; Fig. 4F).
All AEs related to the study treatment (peptide plus
adjuvant or placebo plus adjuvant) are shown in Table II. Injection site reactions and
nausea were more frequent with PPV (86.8 vs. 74.5% and 9.3 vs.
2.9%, respectively). The most common AEs were <grade 3 injection
site reactions in both arms. Treatment-related AEs of ≥grade 3 were
observed in 83 patients in the PPV arm (40.7%) and 42 patients in
the placebo arm (41.2%).
 | Table II.Any grade or severe AEs recorded in
patients by treatment arm (CTCAE version 4.0). |
Table II.
Any grade or severe AEs recorded in
patients by treatment arm (CTCAE version 4.0).
|
| PPV (n=204) Any
grade | Placebo (n=102) Any
grade |
| PPV (n=204) Grade
≥3 | Placebo (n=102)
Grade ≥3 |
|
|---|
|
|
|
|
|
|
|
|
|---|
| AEa | No. | % | No. | % |
P-valueb | No. | % | No. | % |
P-valueb |
|---|
| All AEs | 178 | 87.3 | 79 | 77.5 | 0.02 | 83 | 40.7 | 42 | 41.2 | 0.52 |
| Injection site
reaction | 177 | 86.8 | 76 | 74.5 | 0.008 | 5 | 0.0 | 1 | 0.0 | 0.35 |
| Cancer pain | 54 | 26.5 | 22 | 21.6 | 0.21 | 18 | 8.8 | 9 | 8.8 | 0.59 |
| Decreased
appetite | 37 | 18.1 | 13 | 12.7 | 0.15 | 19 | 9.3 | 5 | 4.9 | 0.13 |
| Edema
peripheral | 35 | 17.2 | 13 | 12.7 | 0.20 | 1 | 0.5 | 2 | 2.0 | 1.0 |
| Pyrexia | 31 | 15.2 | 12 | 11.8 | 0.26 | 2 | 1.0 | 0 | 0.0 | 0.45 |
| Anemia | 29 | 14.2 | 20 | 19.6 | 0.91 | 21 | 10.3 | 12 | 11.8 | 0.72 |
| Constipation | 29 | 14.2 | 14 | 13.7 | 0.53 | 1 | 0.5 | 1 | 1.0 | 0.89 |
|
Nasopharyngitis | 28 | 13.7 | 9 | 8.8 | 0.15 | 0 | 0.0 | 0 | 0.0 | n.a. |
| Malaise | 28 | 13.7 | 9 | 8.8 | 0.15 | 2 | 1.0 | 1 | 1.0 | 0.74 |
| Weight gain | 22 | 10.8 | 12 | 11.8 | 0.68 | 2 | 1.0 | 1 | 1.0 | 0.74 |
| Nausea | 19 | 9.3 | 3 | 2.9 | 0.03 | 3 | 1.5 | 1 | 1.0 | 0.59 |
| Subcutaneous
hemorrhage | 19 | 9.3 | 20 | 19.6 | 1.0 | 0 | 0.0 | 0 | 0.0 | n.a. |
| Fall | 17 | 8.3 | 5 | 4.9 | 0.20 | 0 | 0.0 | 1 | 1.0 | 1.0 |
| Diabetes
mellitus | 16 | 7.8 | 7 | 6.9 | 0.48 | 4 | 2.0 | 2 | 2.0 | 0.68 |
| Diarrhea | 16 | 7.8 | 8 | 7.8 | 0.60 | 1 | 0.5 | 0 | 0.0 | 0.67 |
| Prostate
cancer | 15 | 7.4 | 4 | 3.9 | 0.18 | 15 | 7.4 | 4 | 3.9 | 0.18 |
| Hypertension | 15 | 7.4 | 6 | 5.9 | 0.41 | 10 | 4.9 | 5 | 4.9 | 0.62 |
| Vomiting | 15 | 7.4 | 7 | 6.9 | 0.54 | 1 | 0.5 | 1 | 1.0 | 0.90 |
| Back pain | 15 | 7.4 | 6 | 5.9 | 0.41 | 2 | 1.0 | 1 | 1.0 | 0.74 |
| Insomnia | 14 | 6.9 | 5 | 4.9 | 0.35 | 0 | 0.0 | 0 | 0.0 | n.a. |
| Weight loss | 12 | 5.9 | 9 | 8.8 | 0.88 | 1 | 0.5 | 1 | 1.0 | 0.90 |
| Abnormal hepatic
function | 11 | 5.4 | 2 | 2.0 | 0.13 | 4 | 2.0 | 0 | 0.0 | 0.20 |
| Urinary tract
infection | 10 | 4.9 | 7 | 6.9 | 0.83 | 3 | 1.5 | 1 | 1.0 | 0.59 |
| Urinary
retention | 9 | 4.4 | 6 | 5.9 | 0.92 | 1 | 0.5 | 1 | 1.0 | 0.90 |
| Hydronephrosis | 8 | 3.9 | 7 | 6.9 | 0.92 | 4 | 2.0 | 3 | 2.9 | 0.83 |
| Platelet count
reduction | 8 | 3.9 | 9 | 8.8 | 0.80 | 5 | 2.5 | 2 | 2.0 | 0.57 |
| Hematuria | 7 | 3.4 | 6 | 5.9 | 0.90 | 3 | 1.5 | 2 | 2.0 | 0.79 |
| Dental caries | 3 | 1.5 | 6 | 5.9 | 0.99 | 1 | 0.5 | 0 | 0.0 | 0.67 |
Discussion
Peptide-based vaccines are designed to elicit CTL
against antigens selectively expressed by tumor cells. PPV, in
which a maximum of 4 HLA class IA-matched peptides are selected for
vaccination from a pool of peptides based on both HLA class IA type
and the pre-existing host immunity before vaccination, are designed
to stimulate antigen-specific memory T cells (11). In a trial of neoadjuvant peptide
vaccination before radical prostatectomy for patients with
localized prostate cancer, we previously reported that PPV quickly
induced the infiltration of CD45RO+ memory T cells, rather than
naïve T or B cells, into cancer tissues (17). In previous phase II trials, PPV was
demonstrated to improve OS in chemotherapy-naïve patients with CRPC
or in patients with docetaxel-resistant CRPC (12–14).
The only approved cancer vaccine for CRPC is sipuleucel-T
(autologous dendritic-cell vaccine). This cancer vaccine is
considered for patients with less advanced disease who may benefit
from sipuleucel-T treatment, providing rationale for immunotherapy
as an early treatment strategy in patients with CRPC (18). This phase III randomized trial
investigated PPV as a second-line treatment after docetaxel
chemotherapy in patients with progressing CRPC. To the best of our
knowledge, this is the first trial addressing this strategy in a
relatively large number of patients for whom docetaxel induction
chemotherapy failed. However, the present study demonstrated no
difference in OS or PFS between PPV and placebo.
There are several explanations for the lack of OS or
PFS improvement in this study. First, the target patients may have
had heterogeneous immune cell repertoires, a large tumor burden,
and many immune suppressive elements, such as increased
myeloid-derived suppressor cells (MDSC) or regulatory T cells in
the tumor microenvironment, and it is well known that
tumor-associated immunosuppression is significantly involved in
tumor progression and resistance to immunotherapy (19,20).
Second, efficiently primed T cells may lose their responsiveness to
tumor antigens. This may be explained by the downregulation or loss
of tumor antigens and T-cell inhibition mediated by checkpoint
molecules such as CTLA-4, PD-1 and PDL-1 (21,22).
Third, another contributory factor may be the availability of more
effective salvage therapies that prolong OS after the study
treatment, many of which were not widely available at the time of
the previous phase II study of PPV. The current availability of
such drugs (e.g., cabazitaxel, abiraterone, enzalutamide, and
radium-223) may have affected the disease course in patients
receiving PPV or placebo. Fourth, the treatment was discontinued
early before sufficient doses of PPV were administered, thereby
affecting the efficacy due to the lack of notable objective
responses or PSA responses.
Further studies of predictive biomarkers of PPV
efficacy may be necessary to determine whether subgroups may
improve the OS. The recent discovery that cancers deficient in DNA
mismatch-repair function (dMMR) or with microsatellite instability
(MSI-high) demonstrate high rates of objective tumor responses to
immune checkpoint therapies (23)
led to the FDA approval of pembrolizumab for the treatment of
advanced dMMR/MSH-high cancers of any histological type, among
which mCRPC patients are a small subset. Previous findings showed
that the abnormal granulocytes present in the PBMC fraction at
baseline may lead to the poor prognosis of advanced prostate cancer
patients receiving PPV treatment using DNA microarray analysis
(24). In addition, the increase in
granulocytic MDSC after PPV treatment was an unfavorable marker for
the OS of mCRPC patients (25).
Those results suggested that the proportion of neutrophils, the
majority of granulocytes, affects the efficacy of PPV treatment.
The post hoc analysis in this trial revealed that patients with a
low neutrophil proportion (<64%) or a high lymphocyte proportion
(≥26%) at baseline in the PPV arm had a significantly longer OS
than their counterparts in the placebo arm; however, the
proportions of eosinophils, basophils, and monocytes did not affect
the efficacy of PPV treatment even though the neutrophil to
lymphocyte ratio (NLR) was used. Although NLR was reported as a
risk factor for OS of patients with mCRPC, similar to many other
advanced cancers, when it was higher than 2 (26–28),
we found it to be less sensitive than the proportion of neutrophils
or lymphocytes as a biomarker to predict the efficacy of PPV with
an interaction P=0.09. This discrepancy may have occurred, in part,
because the NLR value as a risk factor was based on a comparison of
the lower and higher levels among patients in the same treatment
arm in the previous studies rather than between patients in
different treatment arms (i.e., PPV and placebo). The levels of
PPV-induced IgG were significantly higher in patients with <64%
neutrophils or ≥26% lymphocytes treated by PPV than in their
counterparts, and the median OS of these groups was significantly
longer than that of the counter groups. This suggested that
patients with <64% neutrophils or ≥26% lymphocytes can receive
survival benefits from PPV treatment. Although the reason for the
baseline neutrophil and lymphocyte proportions most strongly
affecting the clinical benefits is presently unknown, the following
hypothesis was considered: Inflammatory responses associated with
tumor are considered to be one of the major events to escape from
immune attack and promote the production of inflammatory cytokines.
These responses result in the circulation of a few lymphocytes
along with many neutrophils. Therefore, the baseline neutrophil and
lymphocyte proportions may be one of the keys for successful immune
induction (29–31).
The tolerability of PPV treatment was good overall
and the most common treatment-related AE in both arms was injection
site reaction, which was mainly caused by incomplete Freund's
adjuvant (32). Dose interruptions
or reductions were infrequent, and the overall safety profile was
consistent with that observed in previous phase II trials.
Treatment-related deaths were not increased by PPV, suggesting a
lack of toxicity as the main contributing factor.
In conclusion, PPV did not prolong OS or PFS in
HLA-A24-positive patients with CRPC progressing after docetaxel
chemotherapy. Subgroup analyses demonstrated that patients with a
low neutrophil proportion or a high lymphocyte proportion at
baseline in the PPV arm had a significantly longer OS than their
counterparts in the placebo arm in this setting. Additional
confirmation of this finding is required to better define subgroups
of patients who can receive PPV treatment for progressive CRPC
after docetaxel chemotherapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Japan
Agency for Medical Research and Development (no. 18im0110802h0008)
and Fujifilm Company. The funders of the study had no role in the
study design, data collection, data analysis, data interpretation,
or writing of the report.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contribution
MN, HU, SN, SE, HF and KI contributed to the
conception and design of the study. KF, GA, HU, KH, HM, SF, YK, HN,
AT, MF, SN, SE and HF provided study materials or patients. MN and
SH completed the statistical analyses. MN reviewed the clinical
data. All authors interpreted data and reviewed and approved the
final article.
Ethics approval and consent to
participate
This study was approved by the Ethics Committees of
Kurume University (approval no. 213019) and each institution, and
it was registered in the UMIN Clinical Trials Registry (no.
UMIN000011308) and the Pharmaceuticals and Medical Devices Agency
in Japan (no. 25-0917). The study was carried out in accordance
with the Declaration of Helsinki and the International Conference
on Harmonization of Good Clinical Practice guidelines. Written
informed consent to participate in the clinical trial and to use
their data for research and publication purpose was received from
all individual participants before participating in the study.
Patient consent for publication
Not applicable.
Competing interests
MN served as an advisory board consultant for
BrightPath Biotherapeutics Co., Ltd. KI received research funding
from Taiho Pharmaceutical Company. SN served as a consultant to
BrightPath Biotherapeutics Co., Ltd and received honorarium from
Sanofi. All other authors declare no competing interests.
Glossary
Abbreviations
Abbreviations:
|
AEs
|
adverse events
|
|
CRPC
|
castration-resistant prostate
cancer
|
|
CTL
|
cytotoxic T lymphocytes
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
HLA
|
human leukocyte antigen
|
|
IgG
|
immunoglobulin G
|
|
MDSC
|
myeloid-derived suppressor cells
|
|
NLR
|
neutrophil to lymphocyte ratio
|
|
OS
|
overall survival
|
|
PD
|
progressive disease
|
|
PFS
|
progression-free survival
|
|
PPV
|
personalized peptide vaccination
|
|
PSA
|
prostate-specific antigen
|
References
|
1
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et
al TAX 327 Investigators, : Docetaxel plus prednisone or
mitoxantrone plus prednisone for advanced prostate cancer. N Engl J
Med. 351:1502–1512. 2004. View Article : Google Scholar
|
|
2
|
Berthold DR, Pond GR, Soban F, de Wit R,
Eisenberger M and Tannock IF: Docetaxel plus prednisone or
mitoxantrone plus prednisone for advanced prostate cancer: Updated
survival in the TAX 327 study. J Clin Oncol. 26:242–245. 2008.
View Article : Google Scholar
|
|
3
|
de Bono JS, Oudard S, Ozguroglu M, Hansen
S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L,
et al TROPIC Investigators, : Prednisone plus cabazitaxel or
mitoxantrone for metastatic castration-resistant prostate cancer
progressing after docetaxel treatment: A randomised open-label
trial. Lancet. 376:1147–1154. 2010. View Article : Google Scholar
|
|
4
|
De Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: Abiraterone and increased survival in mPrCa. N Engl J Med.
354:1995–2005. 2011. View Article : Google Scholar
|
|
5
|
Scher HI, Fizazi K, Saad F, Taplin ME,
Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et
al AFFIRM Investigators, : Increased survival with enzalutamide in
prostate cancer after chemotherapy. N Engl J Med. 367:1187–1197.
2012. View Article : Google Scholar
|
|
6
|
Parker C, Nilsson S, Heinrich D, Helle SI,
O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, et
al ALSYMPCA Investigators, : Alpha emitter radium-223 and survival
in metastatic prostate cancer. N Engl J Med. 369:213–223. 2013.
View Article : Google Scholar
|
|
7
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, et al IMPACT Study Investigators, : Sipuleucel-T immunotherapy
for castration-resistant prostate cancer. N Engl J Med.
363:411–422. 2010. View Article : Google Scholar
|
|
8
|
Isaacsson Velho P and Antonarakis ES:
PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert
Rev Clin Pharmacol. 11:475–486. 2018. View Article : Google Scholar
|
|
9
|
Kwon ED, Drake CG, Scher HI, Fizazi K,
Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R,
Mahammedi H, et al CA184-043 Investigators, : Ipilimumab versus
placebo after radiotherapy in patients with metastatic
castration-resistant prostate cancer that had progressed after
docetaxel chemotherapy (CA184-043): A multicentre, randomised,
double-blind, phase 3 trial. Lancet Oncol. 15:700–712. 2014.
View Article : Google Scholar
|
|
10
|
Nuhn P, De Bono JS, Fizazi K, Freedland
SJ, Grilli M, Kantoff PW, Sonpavde G, Sternberg CN,
Yegnasubramanian S and Antonarakis ES: Update on systemic prostate
cancer therapies: Management of metastatic castration-resistant
prostate cancer in the era of precision oncology. Eur Urol.
75:88–99. 2019. View Article : Google Scholar
|
|
11
|
Noguchi M, Sasada T and Itoh K:
Personalized peptide vaccination: A new approach for advanced
cancer as therapeutic cancer vaccine. Cancer Immunol Immunother.
62:919–929. 2013. View Article : Google Scholar
|
|
12
|
Noguchi M, Uemura H, Naito S, Akaza H,
Yamada A and Itoh K: A phase I study of personalized peptide
vaccination using 14 kinds of vaccine in combination with low-dose
estramustine in HLA-A24-positive patients with castration-resistant
prostate cancer. Prostate. 71:470–479. 2011. View Article : Google Scholar
|
|
13
|
Yoshimura K, Minami T, Nozawa M, Kimura T,
Egawa S, Fujimoto H, Yamada A, Itoh K and Uemura H: A phase 2
randomized controlled trial of personalized peptide vaccine
immunotherapy with low-dose dexamethasone versus dexamethasone
alone in chemotherapy-naïve castration-resistant prostate cancer.
Eur Urol. 70:35–41. 2016. View Article : Google Scholar
|
|
14
|
Noguchi M, Moriya F, Suekane S, Matsuoka
K, Arai G, Matsueda S, Sasada T, Yamada A and Itoh K: Phase II
study of personalized peptide vaccination for castration-resistant
prostate cancer patients who failed in docetaxel-based
chemotherapy. Prostate. 72:834–845. 2012. View Article : Google Scholar
|
|
15
|
Komatsu N, Shichijo S, Nakagawa M and Itoh
K: New multiplexed flow cytometric assay to measure anti-peptide
antibody: A novel tool for monitoring immune responses to peptides
used for immunization. Scand J Clin Lab Invest. 64:535–545. 2004.
View Article : Google Scholar
|
|
16
|
Hida N, Maeda Y, Katagiri K, Takasu H,
Harada M and Itoh K: A simple culture protocol to detect
peptide-specific cytotoxic T lymphocyte precursors in the
circulation. Cancer Immunol Immunother. 51:219–228. 2002.
View Article : Google Scholar
|
|
17
|
Noguchi M, Yao A, Harada M, Nakashima O,
Komohara Y, Yamada S, Itoh K and Matsuoka K: Immunological
evaluation of neoadjuvant peptide vaccination before radical
prostatectomy for patients with localized prostate cancer.
Prostate. 67:933–942. 2007. View Article : Google Scholar
|
|
18
|
Schellhammer PF, Chodak G, Whitmore JB,
Sims R, Frohlich MW and Kantoff PW: Lower baseline
prostate-specific antigen is associated with a greater overall
survival benefit from sipuleucel-T in the Immunotherapy for
Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology.
81:1297–1302. 2013. View Article : Google Scholar
|
|
19
|
Gabrilovich DI, Bronte V, Chen SH, Colombo
MP, Ochoa A, Ostrand-Rosenberg S and Schreiber H: The terminology
issue for myeloid-derived suppressor cells. Cancer Res. 67:425–426;
author reply 426. 2007. View Article : Google Scholar
|
|
20
|
Miller AM, Lundberg K, Ozenci V, Banham
AH, Hellström M, Egevad L and Pisa P: CD4+CD25high T cells are
enriched in the tumor and peripheral blood of prostate cancer
patients. J Immunol. 177:7398–7405. 2006. View Article : Google Scholar
|
|
21
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar
|
|
22
|
Anderson MJ, Shafer-Weaver K, Greenberg NM
and Hurwitz AA: Tolerization of tumor-specific T cells despite
efficient initial priming in a primary murine model of prostate
cancer. J Immunol. 178:1268–1276. 2007. View Article : Google Scholar
|
|
23
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar
|
|
24
|
Komatsu N, Matsueda S, Tashiro K, Ioji T,
Shichijo S, Noguchi M, Yamada A, Doi A, Suekane S, Moriya F, et al:
Gene expression profiles in peripheral blood as a biomarker in
cancer patients receiving peptide vaccination. Cancer.
118:3208–3221. 2012. View Article : Google Scholar
|
|
25
|
Noguchi M, Moriya F, Koga N, Matsueda S,
Sasada T, Yamada A, Kakuma T and Itoh K: A randomized phase II
clinical trial of personalized peptide vaccination with metronomic
low-dose cyclophosphamide in patients with metastatic
castration-resistant prostate cancer. Cancer Immunol Immunother.
65:151–160. 2016. View Article : Google Scholar
|
|
26
|
Coffelt SB, Wellenstein MD and de Visser
KE: Neutrophils in cancer: Neutral no more. Nat Rev Cancer.
16:431–446. 2016. View Article : Google Scholar
|
|
27
|
Shaul ME and Fridlender ZG: Cancer-related
circulating and tumor-associated neutrophils - subtypes, sources
and function. FEBS J. 285:4316–4342. 2018. View Article : Google Scholar
|
|
28
|
Dolan RD, Laird BJA, Horgan PG and
McMillan DC: The prognostic value of the systemic inflammatory
response in randomised clinical trials in cancer: A systematic
review. Crit Rev Oncol Hematol. 132:130–137. 2018. View Article : Google Scholar
|
|
29
|
Dvorak HF: Tumors: Wounds that do not
heal-redux. Cancer Immunol Res. 3:1–11. 2015. View Article : Google Scholar
|
|
30
|
Steinman RM, Hawiger D and Nussenzweig MC:
Tolerogenic dendritic cells. Annu Rev Immunol. 21:685–711. 2003.
View Article : Google Scholar
|
|
31
|
Basile D, Garattini SK, Bonotto M, Ongaro
E, Casagrande M, Cattaneo M, Fanotto V, De Carlo E, Loupakis F,
Urbano F, et al: Immunotherapy for colorectal cancer: Where are we
heading? Expert Opin Biol Ther. 17:709–721. 2017. View Article : Google Scholar
|
|
32
|
van Doorn E, Liu H, Huckriede A and Hak E:
Safety and tolerability evaluation of the use of Montanide ISA™51
as vaccine adjuvant: A systematic review. Hum Vaccin Immunother.
12:159–169. 2016. View Article : Google Scholar
|