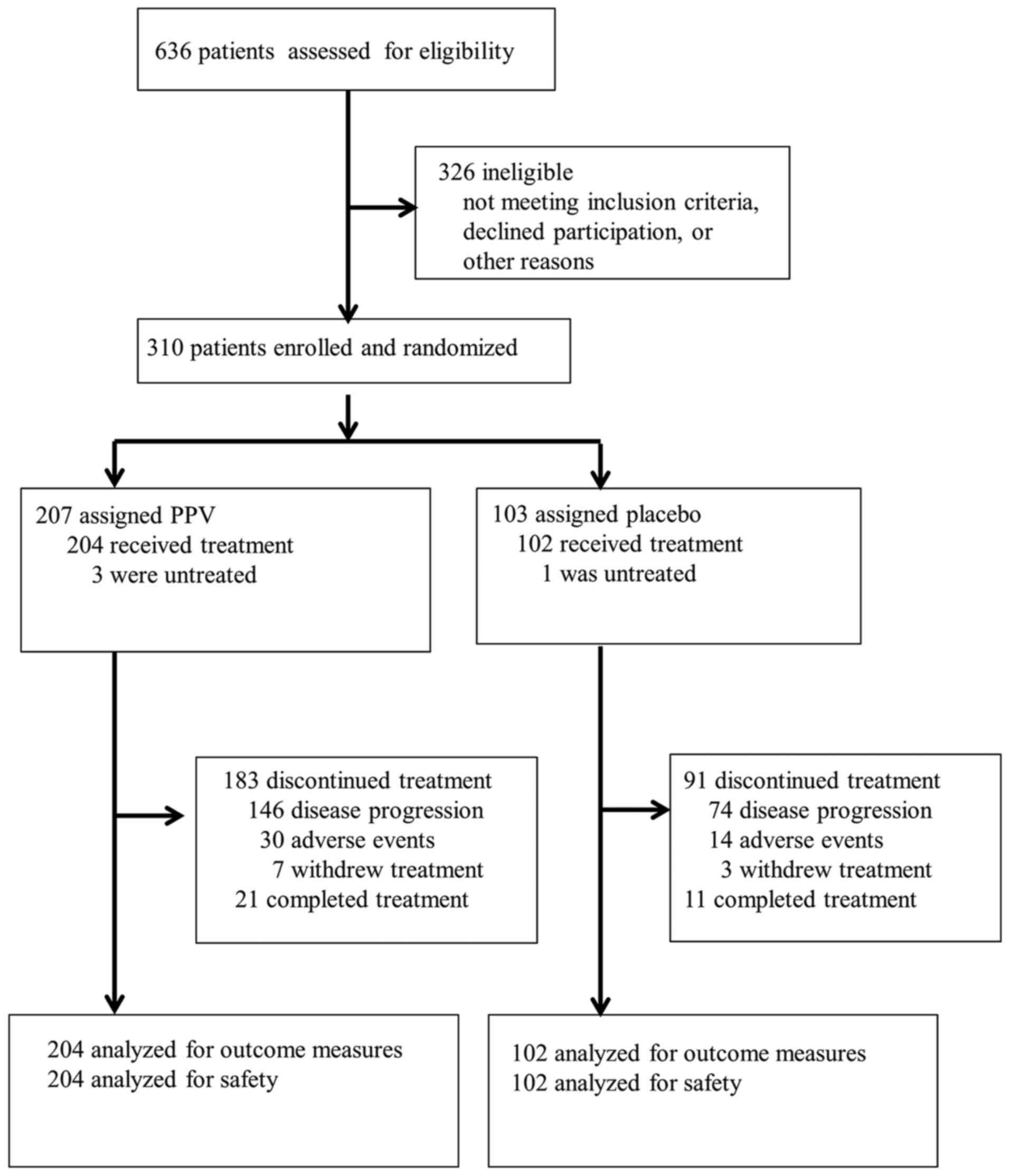
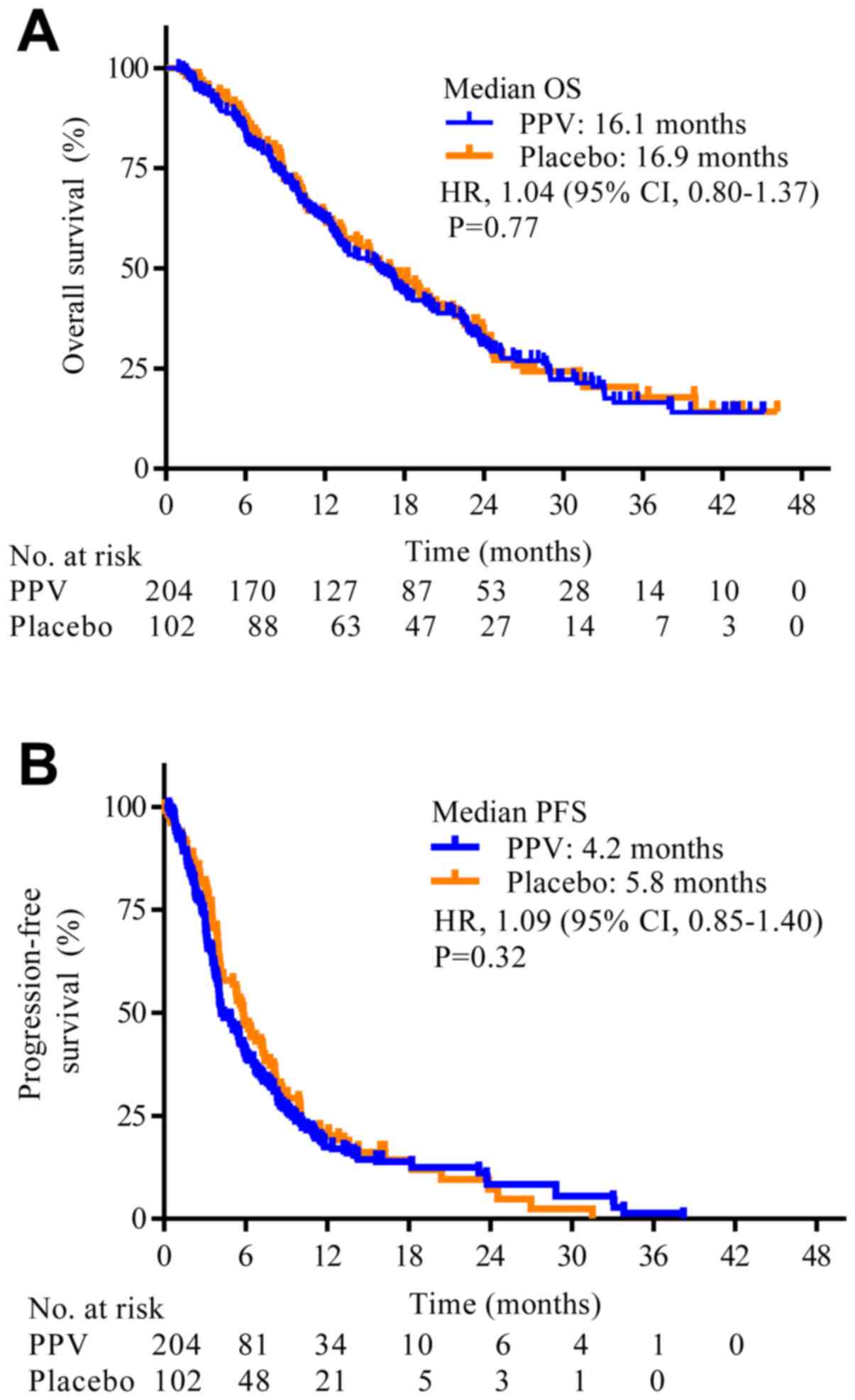
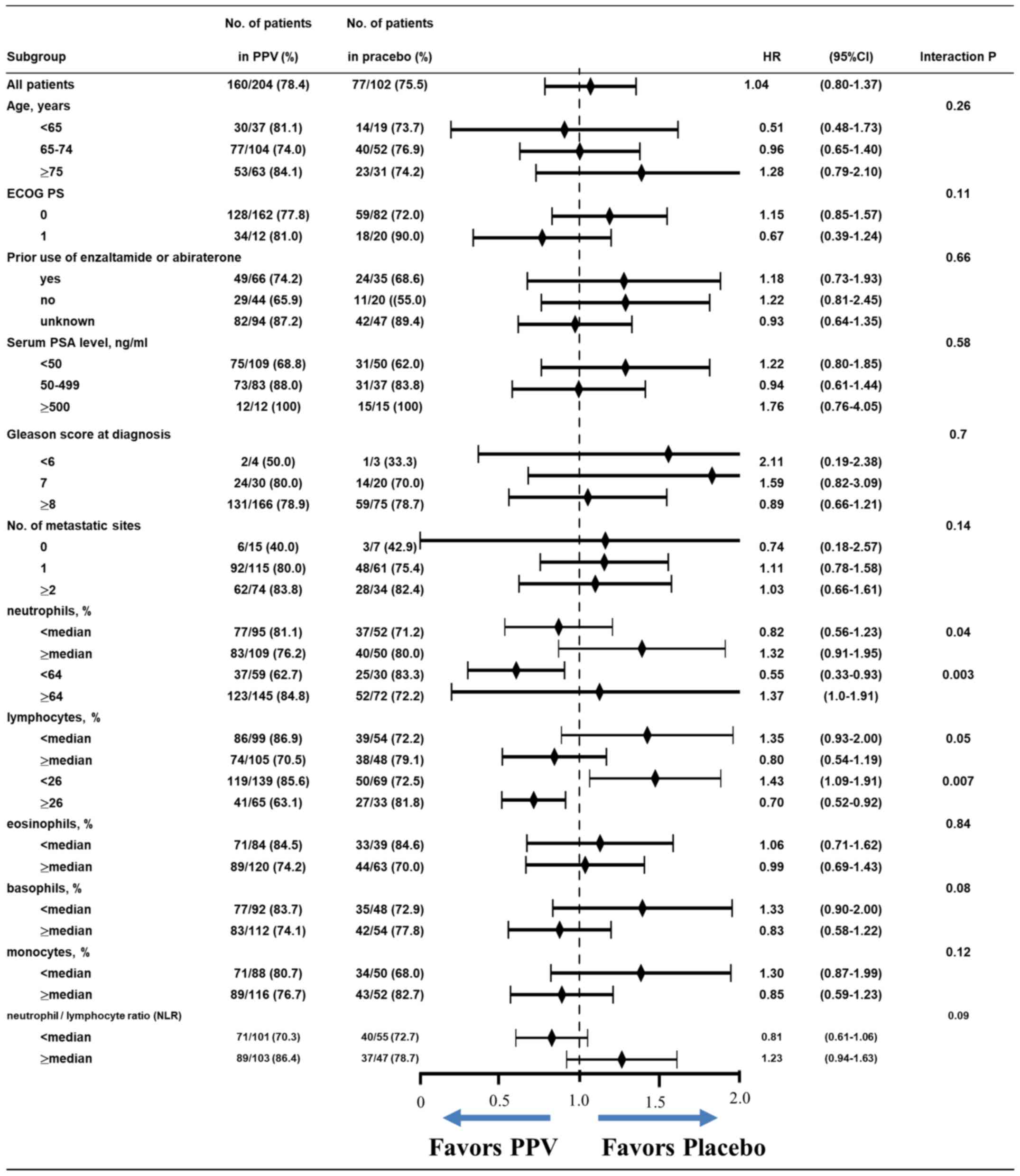
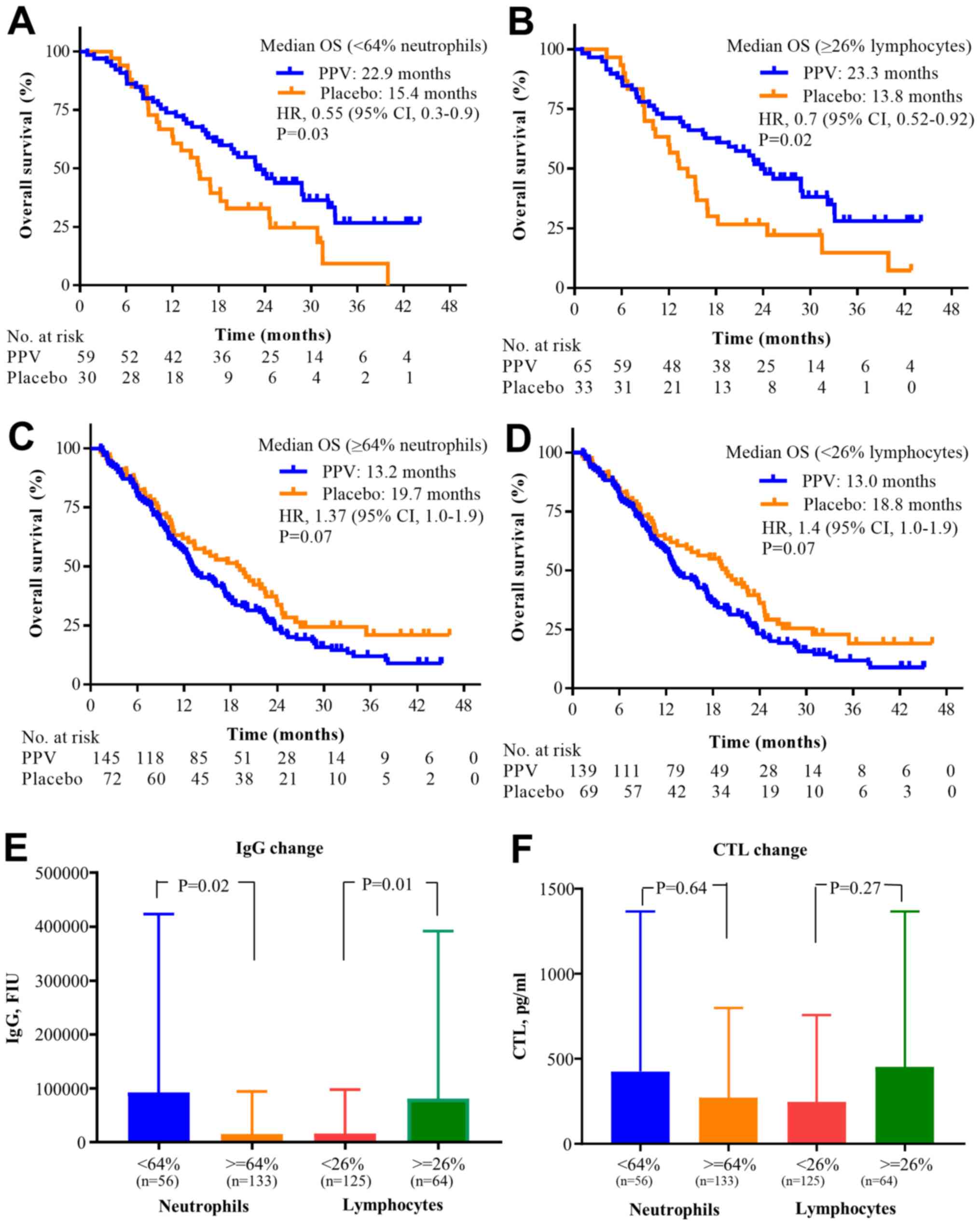
|
1
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et
al TAX 327 Investigators, : Docetaxel plus prednisone or
mitoxantrone plus prednisone for advanced prostate cancer. N Engl J
Med. 351:1502–1512. 2004. View Article : Google Scholar
|
|
2
|
Berthold DR, Pond GR, Soban F, de Wit R,
Eisenberger M and Tannock IF: Docetaxel plus prednisone or
mitoxantrone plus prednisone for advanced prostate cancer: Updated
survival in the TAX 327 study. J Clin Oncol. 26:242–245. 2008.
View Article : Google Scholar
|
|
3
|
de Bono JS, Oudard S, Ozguroglu M, Hansen
S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L,
et al TROPIC Investigators, : Prednisone plus cabazitaxel or
mitoxantrone for metastatic castration-resistant prostate cancer
progressing after docetaxel treatment: A randomised open-label
trial. Lancet. 376:1147–1154. 2010. View Article : Google Scholar
|
|
4
|
De Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: Abiraterone and increased survival in mPrCa. N Engl J Med.
354:1995–2005. 2011. View Article : Google Scholar
|
|
5
|
Scher HI, Fizazi K, Saad F, Taplin ME,
Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et
al AFFIRM Investigators, : Increased survival with enzalutamide in
prostate cancer after chemotherapy. N Engl J Med. 367:1187–1197.
2012. View Article : Google Scholar
|
|
6
|
Parker C, Nilsson S, Heinrich D, Helle SI,
O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, et
al ALSYMPCA Investigators, : Alpha emitter radium-223 and survival
in metastatic prostate cancer. N Engl J Med. 369:213–223. 2013.
View Article : Google Scholar
|
|
7
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, et al IMPACT Study Investigators, : Sipuleucel-T immunotherapy
for castration-resistant prostate cancer. N Engl J Med.
363:411–422. 2010. View Article : Google Scholar
|
|
8
|
Isaacsson Velho P and Antonarakis ES:
PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert
Rev Clin Pharmacol. 11:475–486. 2018. View Article : Google Scholar
|
|
9
|
Kwon ED, Drake CG, Scher HI, Fizazi K,
Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R,
Mahammedi H, et al CA184-043 Investigators, : Ipilimumab versus
placebo after radiotherapy in patients with metastatic
castration-resistant prostate cancer that had progressed after
docetaxel chemotherapy (CA184-043): A multicentre, randomised,
double-blind, phase 3 trial. Lancet Oncol. 15:700–712. 2014.
View Article : Google Scholar
|
|
10
|
Nuhn P, De Bono JS, Fizazi K, Freedland
SJ, Grilli M, Kantoff PW, Sonpavde G, Sternberg CN,
Yegnasubramanian S and Antonarakis ES: Update on systemic prostate
cancer therapies: Management of metastatic castration-resistant
prostate cancer in the era of precision oncology. Eur Urol.
75:88–99. 2019. View Article : Google Scholar
|
|
11
|
Noguchi M, Sasada T and Itoh K:
Personalized peptide vaccination: A new approach for advanced
cancer as therapeutic cancer vaccine. Cancer Immunol Immunother.
62:919–929. 2013. View Article : Google Scholar
|
|
12
|
Noguchi M, Uemura H, Naito S, Akaza H,
Yamada A and Itoh K: A phase I study of personalized peptide
vaccination using 14 kinds of vaccine in combination with low-dose
estramustine in HLA-A24-positive patients with castration-resistant
prostate cancer. Prostate. 71:470–479. 2011. View Article : Google Scholar
|
|
13
|
Yoshimura K, Minami T, Nozawa M, Kimura T,
Egawa S, Fujimoto H, Yamada A, Itoh K and Uemura H: A phase 2
randomized controlled trial of personalized peptide vaccine
immunotherapy with low-dose dexamethasone versus dexamethasone
alone in chemotherapy-naïve castration-resistant prostate cancer.
Eur Urol. 70:35–41. 2016. View Article : Google Scholar
|
|
14
|
Noguchi M, Moriya F, Suekane S, Matsuoka
K, Arai G, Matsueda S, Sasada T, Yamada A and Itoh K: Phase II
study of personalized peptide vaccination for castration-resistant
prostate cancer patients who failed in docetaxel-based
chemotherapy. Prostate. 72:834–845. 2012. View Article : Google Scholar
|
|
15
|
Komatsu N, Shichijo S, Nakagawa M and Itoh
K: New multiplexed flow cytometric assay to measure anti-peptide
antibody: A novel tool for monitoring immune responses to peptides
used for immunization. Scand J Clin Lab Invest. 64:535–545. 2004.
View Article : Google Scholar
|
|
16
|
Hida N, Maeda Y, Katagiri K, Takasu H,
Harada M and Itoh K: A simple culture protocol to detect
peptide-specific cytotoxic T lymphocyte precursors in the
circulation. Cancer Immunol Immunother. 51:219–228. 2002.
View Article : Google Scholar
|
|
17
|
Noguchi M, Yao A, Harada M, Nakashima O,
Komohara Y, Yamada S, Itoh K and Matsuoka K: Immunological
evaluation of neoadjuvant peptide vaccination before radical
prostatectomy for patients with localized prostate cancer.
Prostate. 67:933–942. 2007. View Article : Google Scholar
|
|
18
|
Schellhammer PF, Chodak G, Whitmore JB,
Sims R, Frohlich MW and Kantoff PW: Lower baseline
prostate-specific antigen is associated with a greater overall
survival benefit from sipuleucel-T in the Immunotherapy for
Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology.
81:1297–1302. 2013. View Article : Google Scholar
|
|
19
|
Gabrilovich DI, Bronte V, Chen SH, Colombo
MP, Ochoa A, Ostrand-Rosenberg S and Schreiber H: The terminology
issue for myeloid-derived suppressor cells. Cancer Res. 67:425–426;
author reply 426. 2007. View Article : Google Scholar
|
|
20
|
Miller AM, Lundberg K, Ozenci V, Banham
AH, Hellström M, Egevad L and Pisa P: CD4+CD25high T cells are
enriched in the tumor and peripheral blood of prostate cancer
patients. J Immunol. 177:7398–7405. 2006. View Article : Google Scholar
|
|
21
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar
|
|
22
|
Anderson MJ, Shafer-Weaver K, Greenberg NM
and Hurwitz AA: Tolerization of tumor-specific T cells despite
efficient initial priming in a primary murine model of prostate
cancer. J Immunol. 178:1268–1276. 2007. View Article : Google Scholar
|
|
23
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar
|
|
24
|
Komatsu N, Matsueda S, Tashiro K, Ioji T,
Shichijo S, Noguchi M, Yamada A, Doi A, Suekane S, Moriya F, et al:
Gene expression profiles in peripheral blood as a biomarker in
cancer patients receiving peptide vaccination. Cancer.
118:3208–3221. 2012. View Article : Google Scholar
|
|
25
|
Noguchi M, Moriya F, Koga N, Matsueda S,
Sasada T, Yamada A, Kakuma T and Itoh K: A randomized phase II
clinical trial of personalized peptide vaccination with metronomic
low-dose cyclophosphamide in patients with metastatic
castration-resistant prostate cancer. Cancer Immunol Immunother.
65:151–160. 2016. View Article : Google Scholar
|
|
26
|
Coffelt SB, Wellenstein MD and de Visser
KE: Neutrophils in cancer: Neutral no more. Nat Rev Cancer.
16:431–446. 2016. View Article : Google Scholar
|
|
27
|
Shaul ME and Fridlender ZG: Cancer-related
circulating and tumor-associated neutrophils - subtypes, sources
and function. FEBS J. 285:4316–4342. 2018. View Article : Google Scholar
|
|
28
|
Dolan RD, Laird BJA, Horgan PG and
McMillan DC: The prognostic value of the systemic inflammatory
response in randomised clinical trials in cancer: A systematic
review. Crit Rev Oncol Hematol. 132:130–137. 2018. View Article : Google Scholar
|
|
29
|
Dvorak HF: Tumors: Wounds that do not
heal-redux. Cancer Immunol Res. 3:1–11. 2015. View Article : Google Scholar
|
|
30
|
Steinman RM, Hawiger D and Nussenzweig MC:
Tolerogenic dendritic cells. Annu Rev Immunol. 21:685–711. 2003.
View Article : Google Scholar
|
|
31
|
Basile D, Garattini SK, Bonotto M, Ongaro
E, Casagrande M, Cattaneo M, Fanotto V, De Carlo E, Loupakis F,
Urbano F, et al: Immunotherapy for colorectal cancer: Where are we
heading? Expert Opin Biol Ther. 17:709–721. 2017. View Article : Google Scholar
|
|
32
|
van Doorn E, Liu H, Huckriede A and Hak E:
Safety and tolerability evaluation of the use of Montanide ISA™51
as vaccine adjuvant: A systematic review. Hum Vaccin Immunother.
12:159–169. 2016. View Article : Google Scholar
|