Introduction
Lung cancer is one of the most frequently diagnosed
malignant tumors and almost 80–85% of lung cancer is non-small cell
lung cancer (NSCLC). Primary lung cancer is the leading cause of
cancer-related death worldwide (1,2). Lung
cancer is complemented with a high rate of mortality and is often
not diagnosed until reaching a late stage. With advances in many
aspects of the treatment, diagnosis, and classification, the
overall survival of NSCLC patients at early-stage NSCLC has
improved after complete resection. However, postoperative
recurrence and metastasis are also major obstacles to prolonged
survival in early-stage NSCLC (3–5). Even
though conventional chemotherapy has favorable therapeutic effects,
the side effects of the drugs that influence the life quality of
patients greatly limit the application. As drug resistance is
commonly seen with NSCLC cases, more effective treatment strategies
should be explored.
The epidermal growth factor receptor (EGFR), a
member of the transmembrane receptor tyrosine kinases of the ErbB
family, is involved in the regulation of signal pathways, including
proliferation, metastasis, epithelial-mesenchymal transformation,
and apoptosis. Gefitinib (Iressa) and erlotinib (Tarceva) are two
US FDA-approved EGFR-specific tyrosine kinase inhibitors (TKIs)
that have exhibited clinical benefit in treating NSCLC (6,7).
Erlotinib treatment prolonged the progression-free survival of
NSCLC patients, who harbor EGFR exon 19 deletion or L858R mutations
(EGFR Mut+ NSCLC) (8).
However, almost all tumors eventually develop acquired resistance
to the treatment, with 50% of the patients developing EGFR T790M
resistance mutations (9). Several
mechanisms of EGFR-TKI resistance have been reported, including
EGFR T790M mutation and MET amplification. Overactivation of
several protein kinases, such as signal transducer and activator of
transcription 3 (STAT3), has been reported to be one of the causes
of drug resistance in NSCLC (10).
HCC827 cells carry the erlotinib-sensitive mutation (E746-A750
deletion), but not the drug-resistant mutation (T790M) (11). Subsequently, HCC827 cells are
sensitive to erlotinib and other EGFR-TKIs (12). Interestingly, compared with HCC827
cells, erlotinib-resistant HCC827 cells (HCC827ER) showed higher
levels of pSTAT3 (13). Erlotinib
also induces resistance in PC-9 cells by activating STAT3 (14). In addition, HCC827ER cells showed
greater invasiveness in Matrigel-coated Boyden chambers than the
parental HCC827 cell line (15).
H1975 is a human adenocarcinoma cell line with the EGFR T790M/L858R
mutation that is regarded as an EGFR-TKI acquired drug-resistant
cell line (16).
Huanglian Jiedu Decoction (HJD) is one of the
traditional Chinese medicines used to treat diabetes in ancient
China. It is known for its liver protective effects, including
liver detoxification (17). It was
first recorded in Wai-tai-mi-yao (Arcane Essentials from the
Imperial Library) written by Wang Tao of the Tang dynasty and
widely used in clinical practice (18). The main active components of HLD are
known to have antitumor effects. For example, baicalin and
baicalein can reduce STAT3 activity, further downregulate the
expression of IFN-γ-induced PD-L1, and then restore the sensitivity
of T cells to kill tumor cells (19). Berberine hydrochloride can inhibit
the proliferation of cervical cancer cells HeLa229 and induce
apoptosis by upregulating p53 and downregulating the expression
levels of Bcl-2 and COX-2 mRNA (20). Baicalin inhibits cell growth of
SW1353 by downregulating Bcl-2 and activating caspase-3 and −9
(21). In addition, wogonin
(5,7-dihydroxy-8-methoxyflavone) (an O-methylated flavone)
inhibited tumor proliferation in Raji xenograft mice by decreasing
the expression of Ki67 (22).
Therefore, we hypothesized that the STAT3/Bcl-2 signaling pathway
and its downstream targets may be involved in the antitumor effect
of HJD.
As erlotinib and HJD act via two different pathways
key to tumor growth, using these drugs concurrently may confer
promising clinical benefits to NSCLC patients. The aim of this
study was to discuss the potential of combined inhibition in
clinically relevant xenograft models of EGFR TKI resistance and to
explore whether dual blockade may provide a significant advantage
over monotherapy.
Materials and methods
Preparation and HPLC analysis of
HJD
The herbal materials of HJD composed of Fructus
gardeniae (90 g), Cortex phellodendri (60 g), Radix scutellariae
(60 g), and Rhizoma coptidis (90 g). The herbs were all purchased
from Ma'anshan Jingquan Traditional Chinese Medicine Decoction
pieces, Co., Ltd. The authenticity of the plant species was
confirmed by Dr Huang Yawei from Ma'anshan Jingquan Traditional
Chinese Medicine Decoction pieces Co., Ltd. HJD was refluxed with
water (1:10, w/v) for 2 h. Afterwards, the HJD extract was obtained
by drying the concentrate with vacuum concentration. Powered HJD
samples were sonicated with methanol for 1 h. The sample was
finally filtrated through a 0.45 µM membrane filter prior to HPLC
analysis. The separation was performed on a Waters Acquity C18
column (250×4.6 mm, 5 µm). Separation was carried out by
acetonitrile (A) and 0.05% K2HPO4 and 0.05%
triethanolamine in water (B). The linear gradient elution was 82%
A, in 0–9 min; 82–73% A, in 9–27 min; 73–60% A, in 27–45 min;
60–82% A, in 45–60 min with a flow rate of 1.0 ml/min. The UV
detection was operated at 254 nm, and the injection volume was 20
µl. The external standard method was applied for the identification
by comparing their retention time and spectrum against known
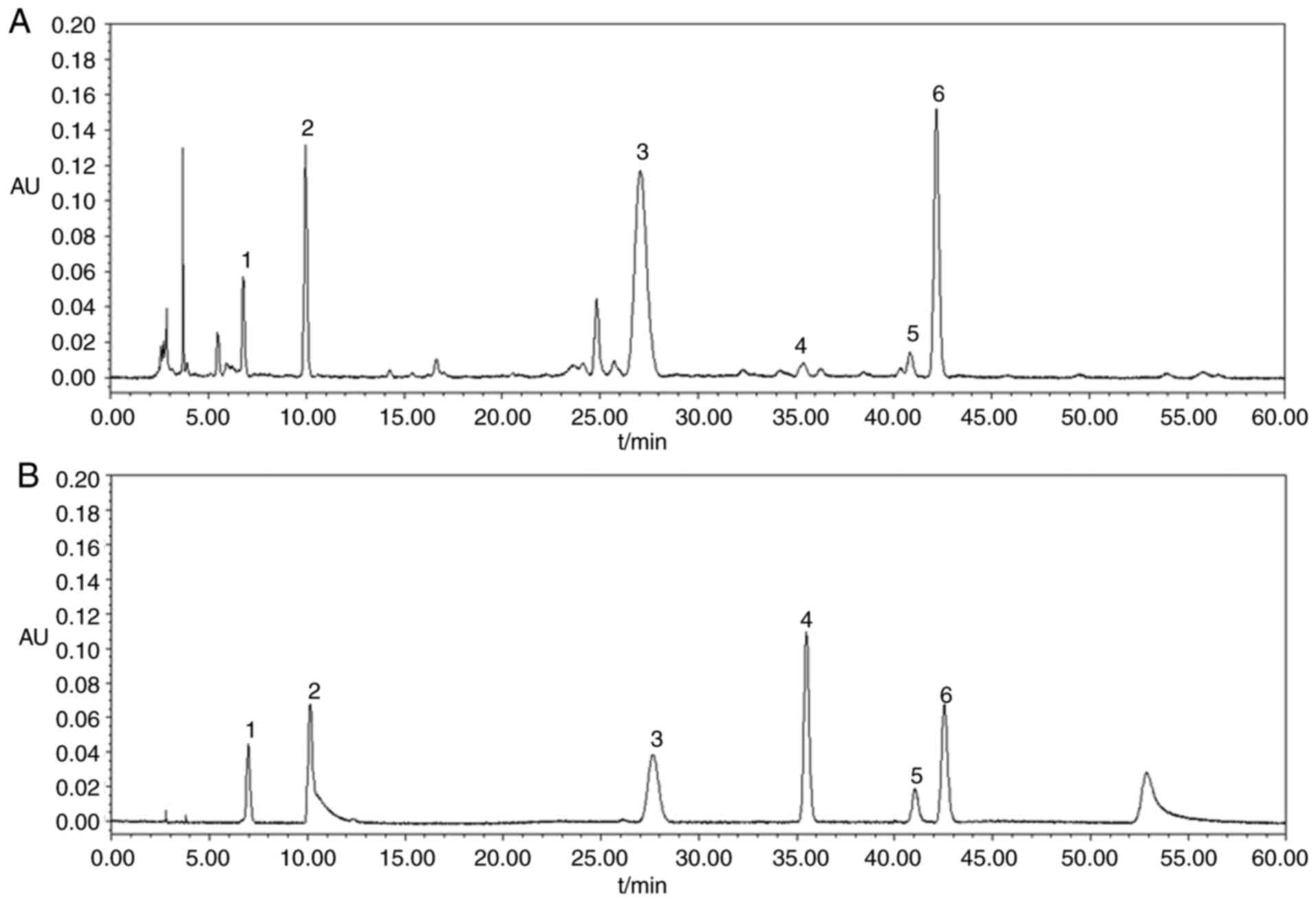
standards (Fig. 1 and Table I).
 | Table I.The structure of the standards in
HPLC chromatograms of HJD extracts. |
Cell culture and reagents
The NSCLC HCC827, A549, H460, and H1975 cell lines
were obtained from the American Type Culture Collection (Manassas).
Cell lines were maintained in DMEM medium supplemented with 10%
fetal bovine serum (FBS) at 37°C with 5% CO2. The
acquired erlotinib-resistant HCC827 cell line (HCC827/ER) was
established by exposing erlotinib-sensitive HCC827 cells to
gradually increasing concentrations of erlotinib from 0.1 to 10 µM
over a 6-month period at 37°C. Erlotinib was purchased from Selleck
(cat. no. S7786). The caspase-3 inhibitor Z-DEVD-FMK was obtained
from Gene Operation (cat. no. IAP1201). Stock solution was prepared
in dimethyl sulfoxide at 10 mM and was stored at −20°C.
Cytotoxicity assay
The Cell Counting Kit-8 (CCK-8) assay was used to
determine cell proliferation. Cells (5×104) were
prepared and mixed evenly, and then 0.1 ml cell suspension was
added to each of the 96-well plates. Cells were cultured in a 5%
CO2 incubator at 37°C for 72 h. Subsequently, 10 µl
CCK-8 solution was added to each well and incubated for 4 h (in the
dark). Cell proliferation was evaluated by using
FLUOstar® Omega microplate reader to measure the
absorbance of cells at the wavelength of 450 nm.
Detection of cell apoptosis by flow
cytometry
Apoptosis was detected using the apoptosis detection
kit (Biosea Biotechnology). The HCC827, H1975 and HCC827ER cells
were separately inoculated on a 6-well plate and cultured in
complete medium for 72 h, then treated with DMSO (control), HJD
only, erlotinib only, and HJD+E, respectively. The cells were
collected, washed twice in cold PBS, then stained with Annexin V
and propyl iodide (PI). The stained cells were collected and
analyzed by flow cytometry. FlowJo software was used to analyze the
results.
Caspase-3/7 activity assay
Caspase-3/7 activity was measured by the Apo-One
homogeneous caspase-3/7 Assay kit (Promega, no. G7790). Then,
1×104 cells per well were inoculated, incubated for 24 h
and then treated with HJD, erlotinib and HJD+E, respectively, for
72 h. The cells were then washed with PBS three times. The activity
of caspase-3/7 was evaluated according to the manufacturer's
instructions.
Cell lysates preparation and
immunoblotting
The cells were mixed with RIPA lysis buffer to
extract total protein. The protein concentration was measured by
bicinchoninic acid (BCA) method. Then protein samples (50 µg) were
subjected to 10% SDS-PAGE gel electrophoresis and transferred to
PVDF membranes. Subsequently, PVDF membranes were blocked in 5%
skimmed milk for 1 h at room temperature and then incubated with
primary antibody overnight at 4°C. Cleaved-caspase 3 (cat. no.
ab49822, 1:1,000), p-STAT3 (cat. no. ab76315, 1:3,000), t-STAT3
(cat. no. ab68153, 1:2,000), Bcl-2 (cat. no. ab182858, 1:2,000),
Bcl-XL (cat. no. ab32370, 1:1,000), GAPDH (cat. no. ab8245,
1:5,000), p-STAT1 (cat. no. ab109461, 1:3,000), t-STAT1 (cat. no.
ab180814, 1:1,000), p-STAT2 (cat. no. ab53132, 1:3,000), t-STAT2
(cat. no. ab32367, 1:2,000), p-STAT5 (cat. no. ab32364, 1:2,000),
t-STAT5 (cat. no. ab32364, 1:1,000) were purchased from Abcam. Next
day, the membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG H&L (cat. no.
ab205718; 1:6,000, Abcam) or goat anti-rat IgG H&L (cat. no.
ab97057; 1:5,000, Abcam) secondary antibodies at room temperature
for 2 h. Finally, the protein bands were detected using the
Tanon-5200 Multi chemiluminescent gel imaging system.
In vivo study protocol
Six-week-old male nude mice were purchased from the
Model Animal Reasearch Center of Nanjing University (Nanjing,
China). Twenty-four mice (22 g) were maintained in a specific
pathogen-free environment with a 12-h light/dark cycle and fed with
standard food and water ad libitum. This study and the
animal experimental protocols were approved by the Institutional
Animal Care and Use Committee of Jiangsu Provincial Academy of
Chinese Medicine.
Approximately 5×106 HCC827/ER cells or
H1975 cells were suspended in 0.2 ml DMEM medium and injected
subcutaneously into the right flank region of nude mice,
respectively. When the average tumor size reached ~75
mm3, the mice were randomized into four groups (n=6
each): i) Vehicle group, ii) HJD group (50 mg/kg), iii) erlotinib
group (20 mg/kg), iv) combination of HJD (50 mg/kg) and erlotinib
(20 mg/kg). All of the treatments were orally administrated once a
day for 27 consecutive days. The formula, 1/2(length ×
width2), was used to estimate tumor sizes. The body
weight and the tumor volume (V) were recorded every 3 days. At the
end of the experiment, the mice were sacrificed by manual cervical
dislocation, which resulted in euthanasia within approximately 10
sec. The tumors were then dissected and weighed to calculate the
inhibitory rate.
The animal experiments were approved by the ethics
committee of the Affiliated Hospital of Integrated Traditional
Chinese and Western Medicine (Nanjing, China).
Immunohistochemistry
Tissues were collected and fixed in 10% formalin for
24 h at room temperature, then embedded in paraffin, and sectioned.
The tissue sections (5 µm) were dewaxed, incubated with 3%
methanol-hydrogen peroxide and washed with PBS three times.
Sections were placed in citric acid buffer, heated and boiled, and
washed with PBS. Then, the sections were blocked with goat serum
(cat. no. 7481; Abcam) for 20 min, and incubated with STAT3 and
Bcl-2 antibodies at 4°C overnight. Next day, the sections were
incubated with goat anti-rabbit IgG H&L (horseradish
peroxidase) antibodies (cat. no. ab205718; 1:5,000, Abcam). A
3,3′-diaminobenzidine (DAB) kit was used to observe the specific
labeling (brownish yellow staining), and hemotaxylin was used for
counterstain cell nuclei (blue staining). Images were obtained from
each slide using a digital trinocular microscope (Zeiss Axio
Observer A1).
H&E staining
The collected nude mouse tissues were fixed with
formalin, paraffin-embedded, cut into 4 µm slices, and roasted in
an oven for 3 h. Sections with xylene were first washed three
times, then different gradient ethanol (75, 85, 95 and 100%) was
used to rinse the tissues twice, prior to staining the sections
with hematoxylin. Then, sections were immersed in hydrochloric
acid-ethanol solution, dyed with ammonia water, and redyed with
eosin. Finally, sections were immersed in different gradient of
ethanol (75, 85, 95 and 100%) and sealed with neutral resin.
Statistical analysis
SPSS Statistics version 13.0 was used for all
statistical analyses (SPSS Inc.). Statistical comparisons were
performed using one-way ANOVA followed by Tukey's multiple
comparison post hoc test and P<0.05 was considered statistically
different. The results were presented as the mean ± SD.
Results
HJD strengthens EGFR-TKI sensitivity
and reduces the drug resistance of NSCLC cells to erlotinib
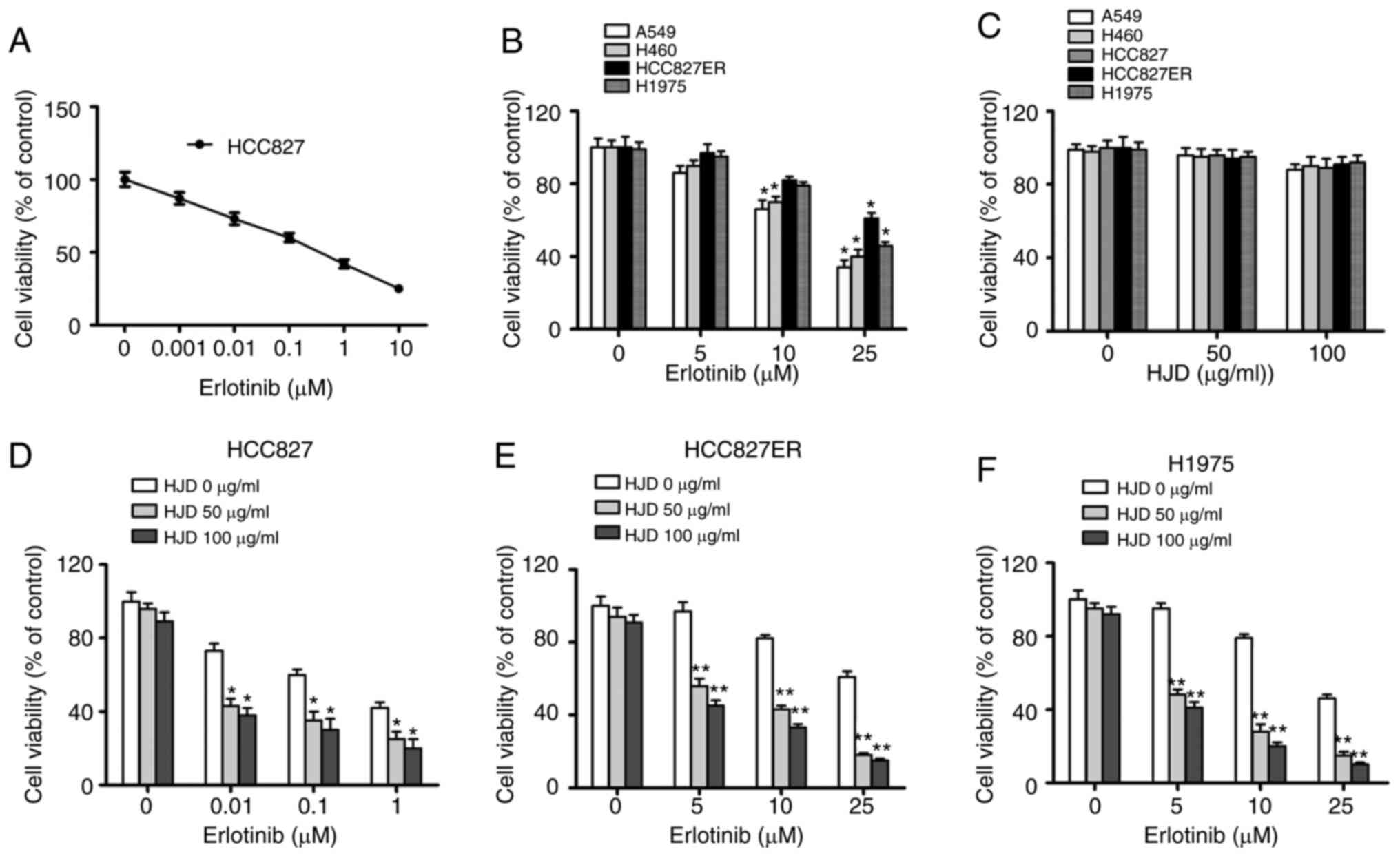
To evaluate whether HJD could overcome the
resistance of NSCLC cells to erlotinib, the inhibitory effect of
co-treatments on HCC827, HCC827ER, H1975, H460 and A549 cell growth
was assessed using a standard CCK-8 assay. The mutation profile of
NSCLC cell lines is shown in Table
II. Consistent with earlier studies, cells bearing mutated EGFR
(HCC827) were more sensitive to erlotinib than those bearing
wild-type EGFR (H460, A549). However, H1975 cells with both L858R
and T790M mutations were relatively insensitive to erlotinib
treatment. HCC827 erlotinib-resistant cells (HCC827ER) were
established by a series of stepwise increase of the erlotinib
concentration until HCC827ER was no longer responsive to 5 µM
erlotinib. HCC827ER cells were more resistant to erlotinib than
HCC827 cells (IC50=31.26 vs. 0.11 µM) (Fig. 2A and B).
 | Table II.Mutation profile of NSCLC cell
lines. |
Table II.
Mutation profile of NSCLC cell
lines.
| Cell lines | EGFR
gene | K-Ras
gene |
|---|
| A549 | WT | G12S |
| H1975 | Exon19
T790M-L858R | WT |
| HCC827 | Exon19 (E746-A750)
del | WT |
| HCC827ER | Exon19 (E746-A750)
del | WT |
| H460 | WT | G61H |
To determine whether HJD can inhibit the growth of
NSCLC cells, cell viability was determined. HJD (0–100 µg/ml) did
not produce significant toxicity in NSCLC cells, independent of the
EGFR-mutated status (Fig. 2C).
However, HJD acting on NSCLC cells showed synergistic effects with
erlotinib. The combination of HJD (50 µg/ml) with erlotinib led to
57% inhibition on the ability of HCC827ER cells (Fig. 2D), although erlotinib (10 µM) alone
exhibited a slight effect (~20% inhibition) on cell ability
(Fig. 2E). In parallel, the H1975
cell line was also sensitive to the combined administration
(Fig. 2F).
HJD amplifies erlotinib-induced
apoptosis
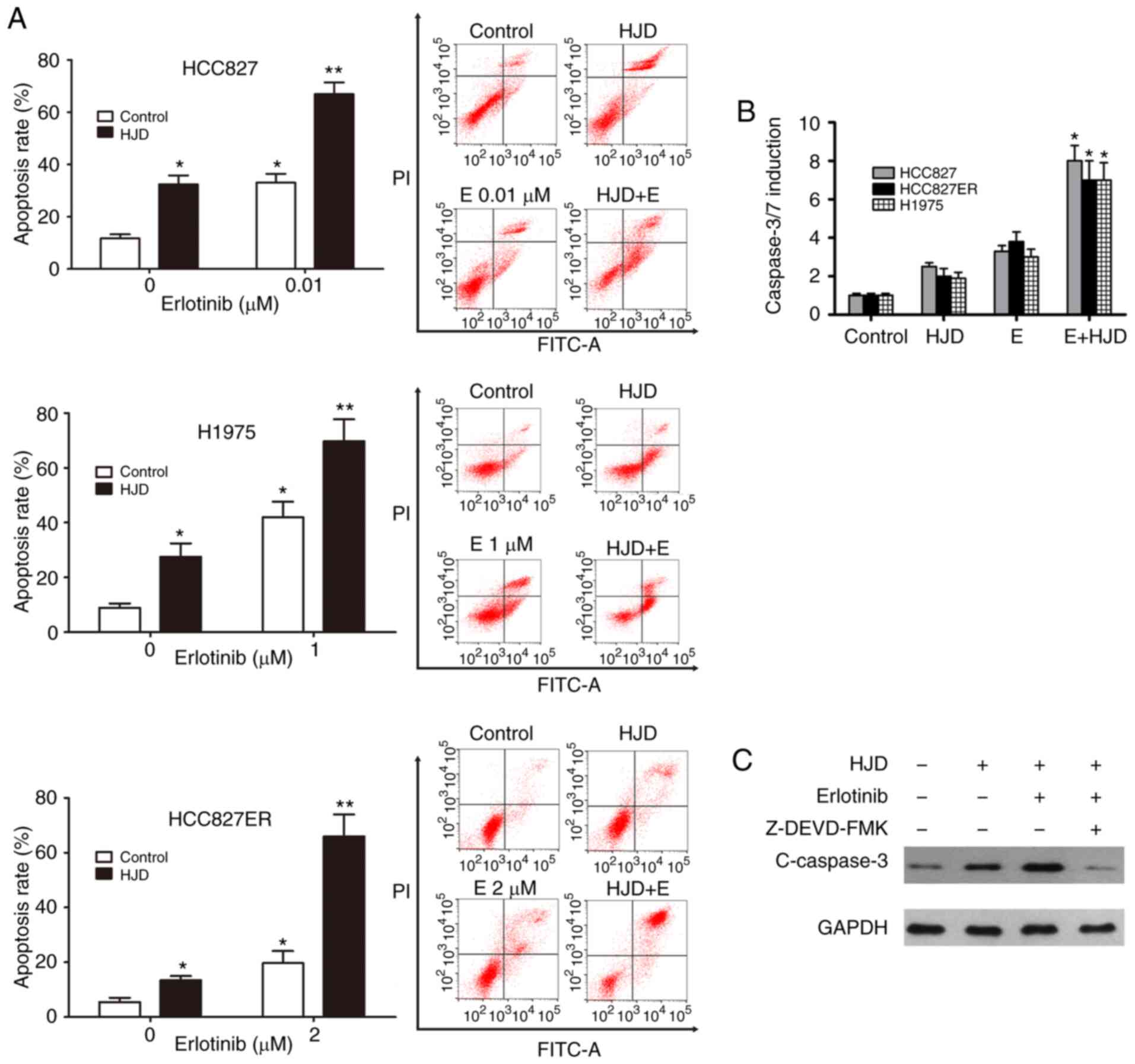
Apoptosis is involved in the anticancer effect of
erlotinib (23). To measure whether
the synergistic effect of HJD on erlotinib was associated with
apoptosis, cells were stained with Annexin V-FITC/PI and analyzed
using flow cytometry. As shown in Fig.
3A, erlotinib-stimulated apoptosis was significantly enhanced
by HJD in H1975, HCC827 and HCC827ER cells (Fig. 3A). As demonstrated in ELISA assays,
Caspase-3/7 activation was also significantly induced by
combination treatment in these cell lines (P<0.05, Fig. 3B), suggesting that
erlotinib-augmented activation of caspase was meaningfully promoted
by HJD. Moreover, the caspase-3 inhibitor Z-DEVD-FMK could almost
abrogate the activation of caspase 3 caused by HJD (Fig. 3C).
Erlotinib-induced STAT3 activation was
inhibited by HJD
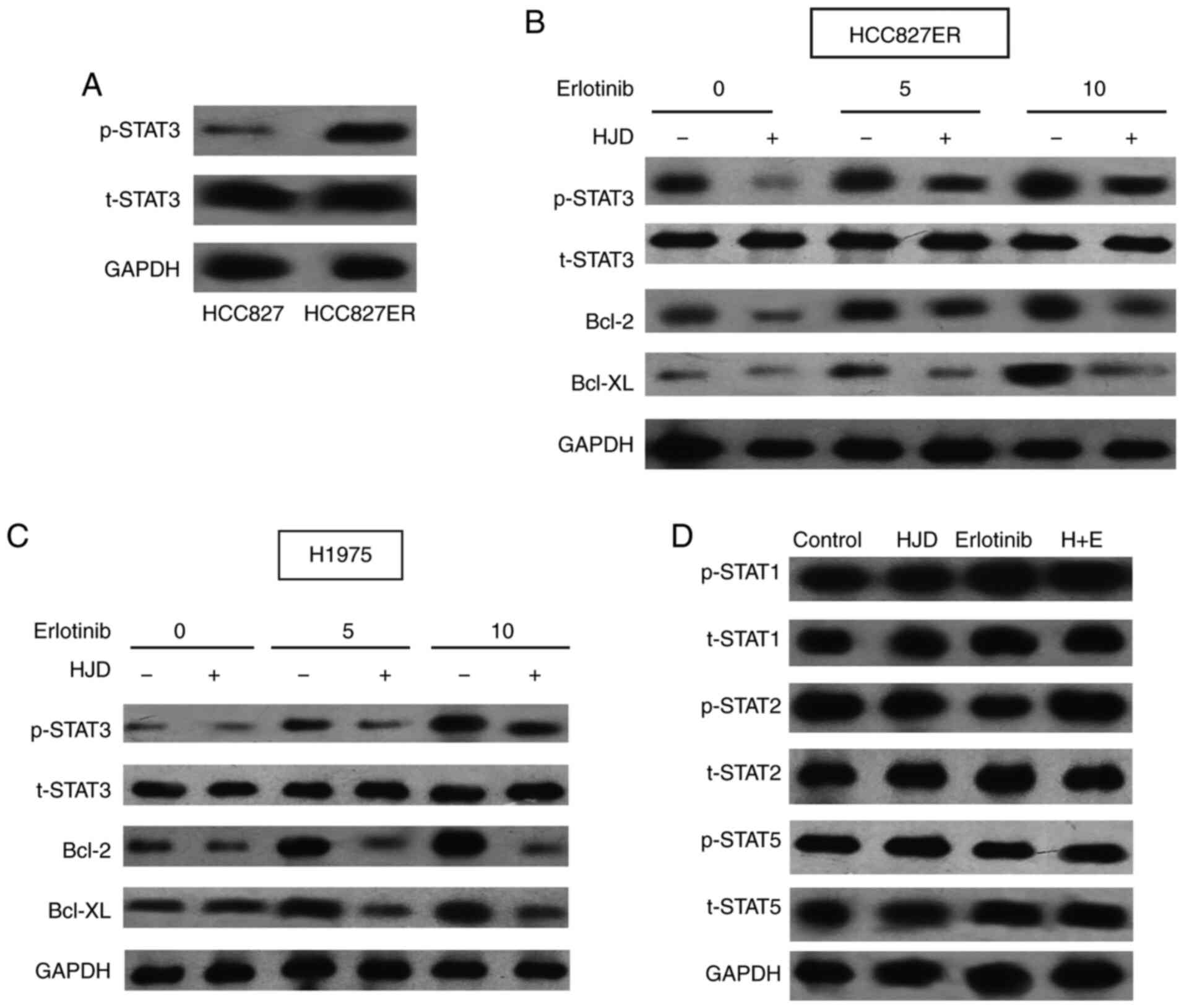
To better understand the drug resistance mechanism
of HCC827ER cells, we carefully analyzed its characteristics. We
found that the level of p-STAT3 in erlotinib-sensitive primary
HCC827 cells was much lower than that in HCC827ER cells (Fig. 4A). Abnormal activation of STAT3 in
tumor cells ensures the survival of malignant cells under stress
conditions and also leads to multi-drug resistance (24). Erlotinib did not reduce the higher
levels of STAT3 phosphorylation in HCC827ER cells (Fig. 4B). Therefore, we detected the effect
of STAT3 on HJD-mediated erlotinib sensitization. Erlotinib therapy
resulted in the feedback activation of STAT3 in H1975 cells
(Fig. 4C), consistent with previous
studies (13,25). However, HJD significantly inhibited
the levels of p-STAT3 and its downstream targets, Bcl-2 and Bcl-XL
proteins induced by erlotinib, suggesting that STAT3 may be related
to HJD-mediated erlotinib sensitizing. In addition, we evaluated
other members of the STAT family. The effect of Erlotinib and HJD
was independent of the phosphorylation of STAT1, STAT2, and STAT5
(Fig. 4D). Furthermore, HJD did not
potentiate the inhibitory effect of erlotinib on EGFR
phosphorylation of HCC827ER and H1975 cells (Fig. S1).
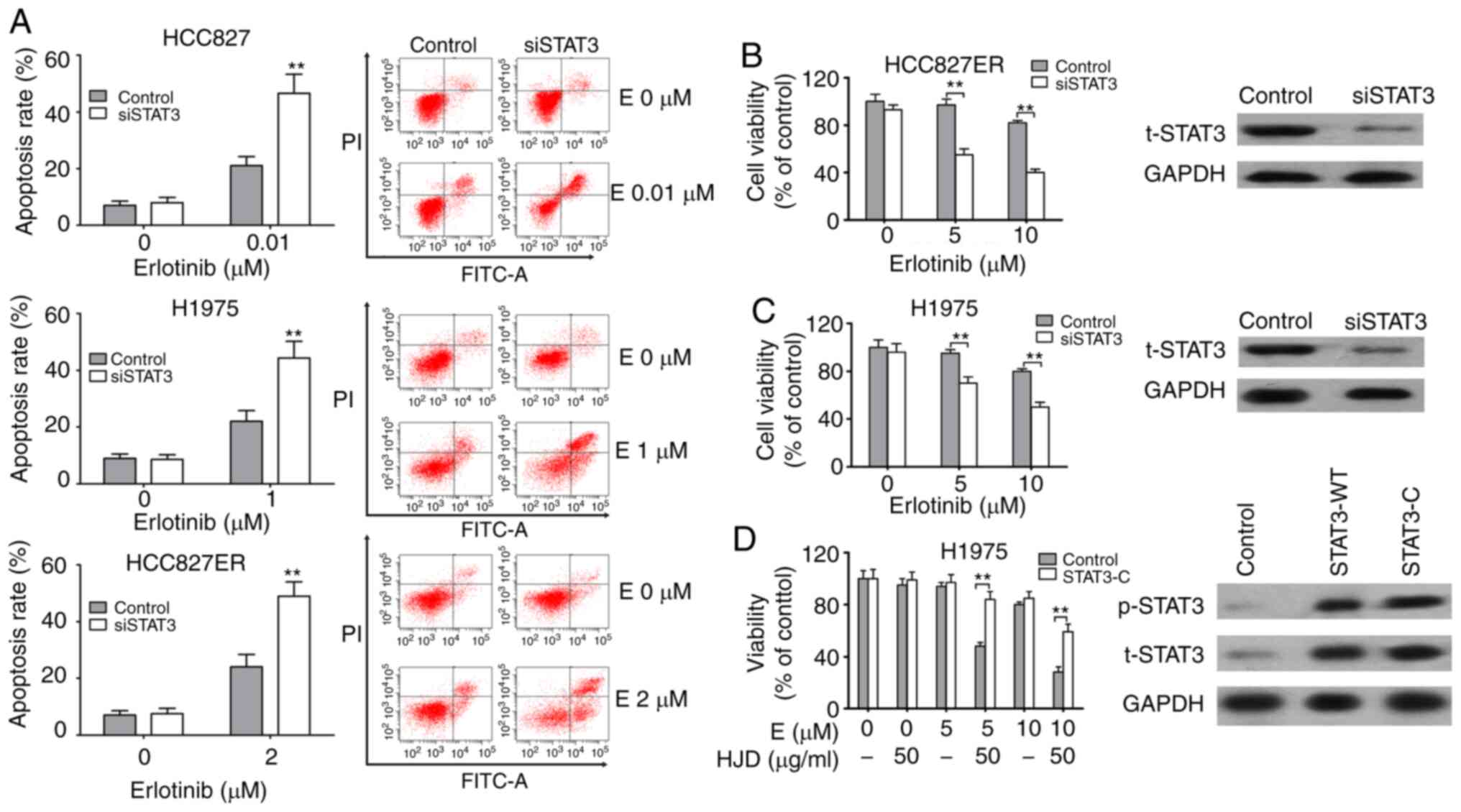
To verify the effect of STAT3 on erlotinib
resistance, we transfected HCC827, HCC827ER, and H1975 cells with
STAT3-specific siRNAs. The effect of siSTAT3 on apoptosis was
assessed. The apoptosis rate of HCC827ER cells induced by erlotinib
alone was 24.1±4.4%, and the apoptosis rate increased to 49.1±5.2%
after siSTAT3 transfection (P<0.01). A similar increment in
erlotinib-induced apoptosis was also observed in both H1975 and
HCC827 cells after siSTAT3 transfection (Fig. 5A). siSTAT3 significantly inhibited
endogenous STAT3 and enhanced the inhibition of erlotinib on H1975
and HCC827ER cell viability (Fig. 5B
and C). Furthermore, plasmids expressing constitutively
activated STAT3 (STAT3-C) were employed. Overexpression of STAT3 in
H1975 cells significantly inhibited HJD-mediated erlotinib
sensitization (Fig. 5D).
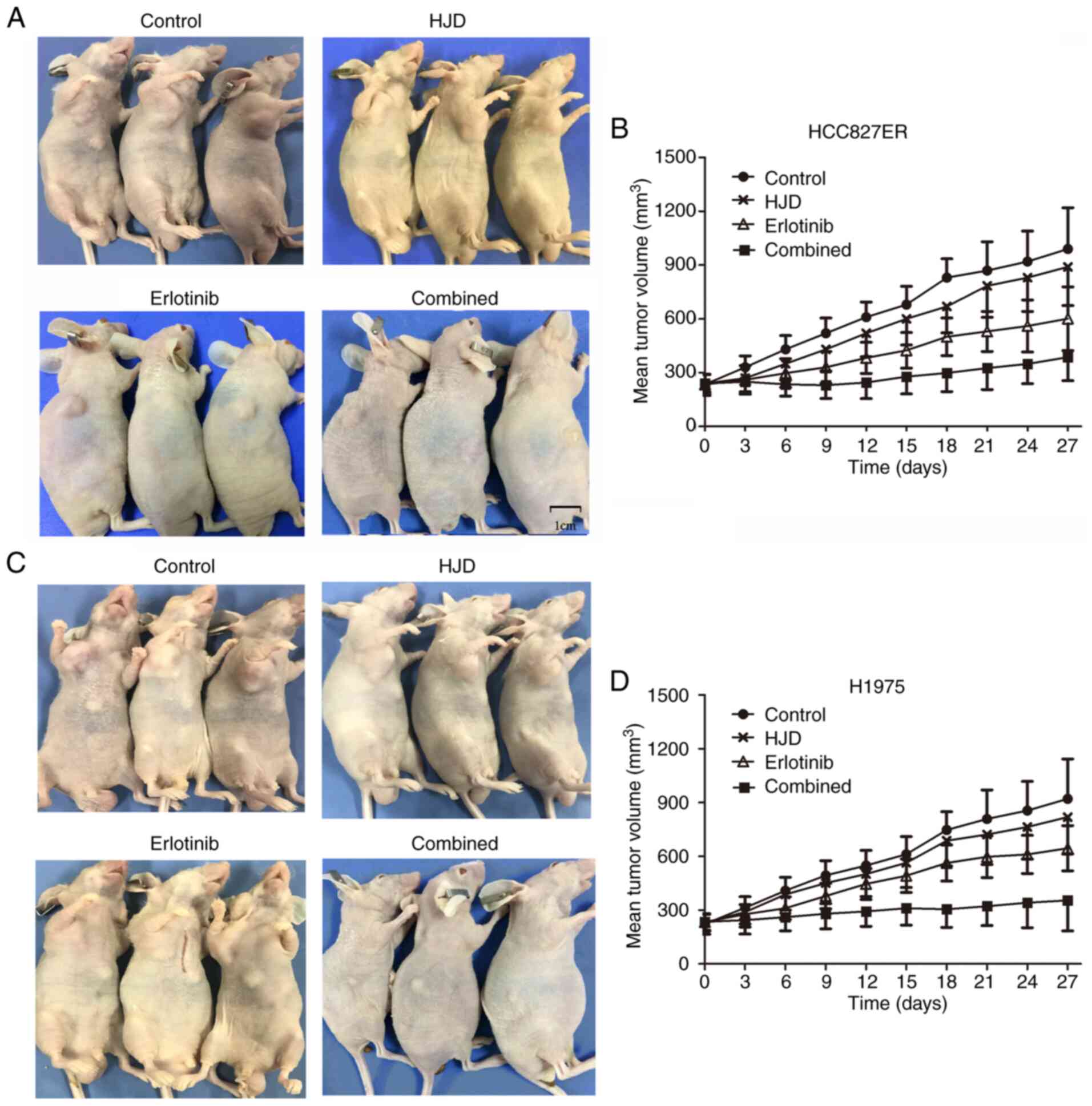
HJD potentiates the tumor-inhibiting
activity of erlotinib in vivo
Next, we evaluated whether combination therapy could
alleviate erlotinib resistance in HCC827ER and H1975 ×enograft nude
mice. The nude mice were divided into the control, HJD, erlotinib,
and HJD+erlotinib groups. After erlotinib treatment of HCC827ER
xenograft, tumor growth was retarded. Although the tumor volume
treated with HJD was smaller than that of the control group, there
were no substantial differences. The combination of HJD with
erlotinib in particular elicited an extremely strong tumor
regression (Fig. 6). At the end of
the experiment (27 days of co-treatment), tumors were barely
palpable. In summary, these results indicate that erlotinib and HJD
contributed to substantial tumor shrinkage of established
xenografts bearing EGFR T790M mutation.
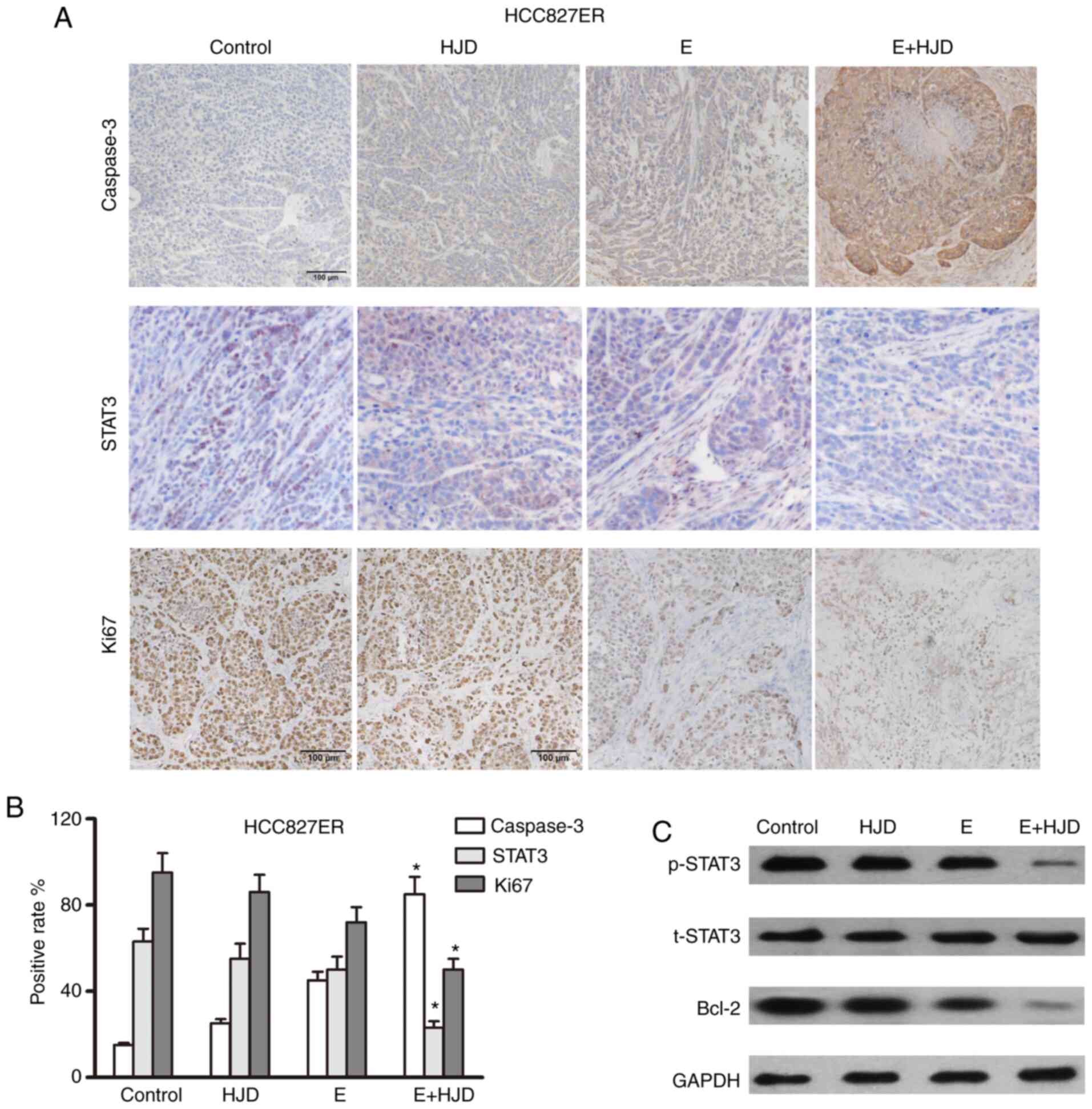
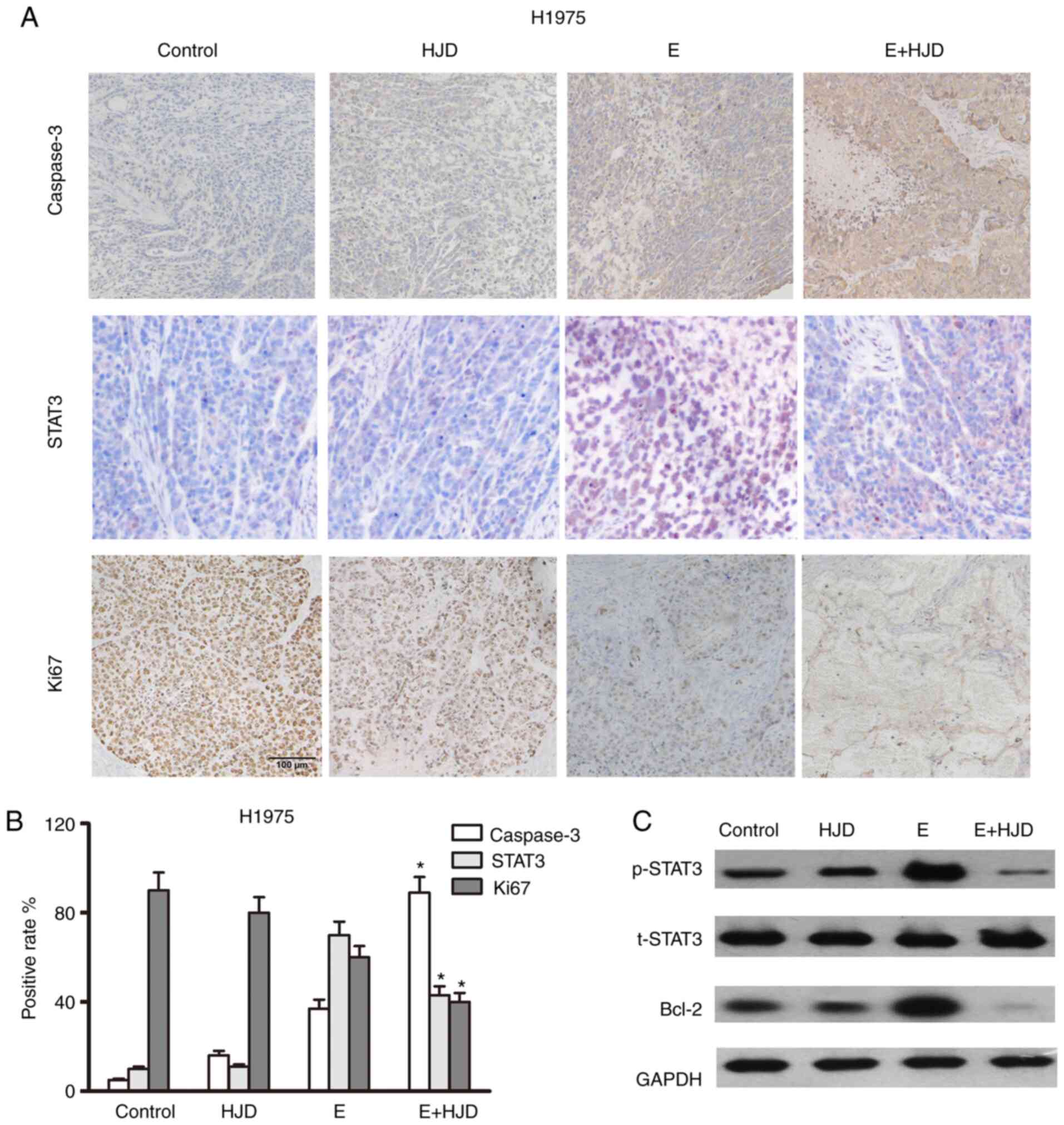
Caspase 3-positive cells were greatly increased in
the co-treatment group, whereas Ki67 expression was decreased in
the co-treatment group, as shown in immunohistochemical assays
(Figs. 7 and 8). The levels of STAT3 and its downstream
signaling target, Bcl-2, were upregulated in the erlotinib group in
mice-bearing HCC827ER tumors, compared to the saline-treated group
(Fig. 7). As expected, the
combination of HJD and erlotinib blunted the expression of STAT3
and Bcl-2 in mice-bearing H1975 ×enografts (Fig. 8). These results were also confirmed
by western blot assays. For safety evaluation of the combinatorial
treatment, the kidneys, liver, lungs and heart of nude mice were
histologically analyzed. No obvious toxicity was observed in the
main organs of mice-bearing xenografts subjected to HJD plus
erlotinib treatment, as shown in Figs.
S2 and S3. In vivo
studies suggested that HJD could overcome TKI resistance by
inhibiting the STAT3 signaling pathway. The STAT3/Bcl-2 pathway is
involved in the in vivo antitumor effect of HJD combined
with erlotinib.
Discussion
Four members of the ERBB receptor family, namely
EGFR, HER2, HER3, and HER4 are the most studied therapeutic targets
for human malignancies (26,27).
ErbB receptors induce EGFR activation by binding to ligands of
different affinities (28). There
are six major ligands for EGFR, namely epidermal growth factor,
heparin-binding EGF, transforming growth factor, heregulin,
amphiregulin, and betacellulin (29). In EGFR, when ligands bind to
receptors, the receptors undergo conformational changes that
produce homologous dimers and heterodimers (30), thereby activating downstream
signaling pathways and promoting the development of cancer
features, such as proliferation and survival, metastasis, and
therapeutic resistance (28,31).
Therefore, EGFR has become a new therapeutic target for cancer.
EGFR inhibitors can be divided into two categories according to
their sites of action: One is EGFR-TKIs, including erlotinib,
afatinib, gefitinib and osimertinib (32,33).
The other is extracellular EGRF-mab, such as cetuximab and
panitumumab (34,35).
EGFR inhibitors have shown clinical benefit for
NSCLC, but less than 10% of previously treated NSCLC patients have
an objective tumor response (36,37)
and patients develop resistance in the end. Erlotinib is an oral
low-molecular-weight quinazoline-based agent, which acts as a
reversible and selective kinase inhibitor of EGFR and has a better
anticancer effect, less toxicity, and longer progression-free
survival than chemotherapy (38,39).
It has been applied for NSCLC patients with activating EGFR
mutations. Although erlotinib is widely used in the treatment of
NSCLC, many patients also develop resistance to it, which poses a
challenge in oncology. Mutation of T790M in EGFR kinase is reported
to be the most common mechanism of resistance to erlotinib
(40). Our experiments show that
T790M mutant H1975 cells are not sensitive to erlotinib, which is
consistent with previous findings (41). The combination of HJD and erlotinib
could overcome drug resistance induced by the T790M mutation in
primary and acquired resistance cells in vitro and in
vivo, which may represent an alternative therapeutic strategy
in patients with acquired resistance to EGFR-TKIs.
The majority of components of HJD are known to
influence critical processes of angiogenesis and metastasis by
affecting numerous effector molecules. Findings of previous studies
have shown that the main component of HJD has antitumor effects
(42,43). The inhibitory effect of HJD on
cancer cells has been confirmed in human myeloma cells, and its
active compounds, baicalin and wogonin, are related to the
inhibition of cancer cell growth, cell cycle arrest, and apoptosis
(44,45). Results of this study revealed that
HJD efficaciously increased the sensitivity of NSCLC cells to
erlotinib. A synergistic effect was found in H1975 and HCC827ER
cells. The combination displayed an additive effect on A549 cells
bearing wild-type EGFR. In accordance with previous research
(46,47), both HCC827ER and H1975 cells harbor
the T790M mutation and exhibit a higher level of EGFR activity than
A549 cells expressing wild-type EGFR (data not shown). It is widely
acknowledged that T790M mutation is a critical factor for erlotinib
resistance. This discrepancy between cells harboring wild-type or
mutant EGFR possibly ascribe to variant cellular dependency for
survival to EGFR pathways (48).
The inhibitory effect of HJD+E combination surmounted the threshold
that cancer cells could bear, and induced more lethal damage to
cells with mutant EGFR.
STAT3 are important proto-carcinoma transcription
factors in a variety of cancers (49–51).
STAT3 activation has been reported in approximately 50% of NSCLC
primary tumors and lung cancer-derived cell lines, which is one of
the most important carcinogenic factors in NSCLC (52,53).
STAT3 activation induces the transcription of a variety of genes
(such as c-myc, cyclin D1, bcl-2) that play a key role in
the pathogenesis of lung cancer (54). Previous findings have shown that
EGFR mutations activate STAT3 (55). In addition, it has been shown that
abnormal activation of STAT3 can be observed in a variety of
drug-resistant cells, and inhibition of STAT3 activation can
alleviate drug resistance (14,56,57).
Erlotinib therapy resulted in the feedback activation of STAT3 in
H1975 cells, and we later transfected HCC827, HCC827ER and H1975
cells with siRNAs. siSTAT3 significantly inhibited STAT3 and
enhanced drug response. The result proved that STAT3 caused
erlotinib resistance, in accordance with previous findings
(58). In our study, HJD promoted
erlotinib to enhance caspase 3 activation and tumor cell apoptosis.
The combination of HJD and erlotinib blunted the expression of
STAT3 and Bcl-2, suggesting HJD can inhibit the STAT3 signaling
pathway to overcome TKI resistance.
In our study, HJD significantly alleviates
resistance by regulating the STAT3/Bcl-2 signaling pathway. In
addition, the results suggest that inhibition of the STAT3/Bcl-2
pathway contributed greatly to the abolishment of acquired
resistance to erlotinib. Combination therapy of HJD and erlotinib
offers a promising prospect for enhancing drug response and
extending survival in patients with lung cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding was provided by the Medical innovation team
of Jiangsu province (CXTDB2017003), Jiangsu Province's colleges and
universities (integration of Chinese and Western medicine).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
RZ and LW designed and performed experiments. ZX and
BL analyzed the data and wrote the manuscript. XZ and QN also
performed the experiments. LZ analyzed the data. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The animal experiments were approved by the ethics
committee of the Affiliated Hospital of Integrated Traditional
Chinese and Western Medicine (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
Glossary
Abbreviations
Abbreviations:
|
SDC
|
Shuangdan capsule
|
|
EGFR
|
epidermal growth factor receptor
|
|
TKI
|
tyrosine kinase inhibitors
|
|
NSCLC
|
non-small cell lung cancer
|
|
HCC827ER
|
erlotinib-resistant HCC827 cell
line
|
|
FBS
|
fetal bovine serum
|
References
|
1
|
Miao S, Qiu T, Zhao Y, Wang H, Sun X, Wang
Y, Xuan Y, Qin Y and Jiao W: Overexpression of S100A13 protein is
associated with tumor angiogenesis and poor survival in patients
with early-stage non-small cell lung cancer. Thorac Cancer.
9:1136–1144. 2018. View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar
|
|
3
|
Morgant MC, Pages PB, Orsini B, Falcoz PE,
Thomas PA, Barthes Fle P, Dahan M and Bernard A; Epithor project
(French Society of Thoracic and Cardiovascular Surgery), : Time
trends in surgery for lung cancer in France from 2005 to 2012: A
nationwide study. Eur Respir J. 46:1131–1139. 2015. View Article : Google Scholar
|
|
4
|
Wu AJ, Garay E, Foster A, Hsu M, Zhang Z,
Chaft JE, Huang J, Rosenzweig KE and Rimner A: Definitive
radiotherapy for local recurrence of NSCLC after surgery. Clin Lung
Cancer. 18:e161–e168. 2017. View Article : Google Scholar
|
|
5
|
Hattori A, Matsunaga T, Takamochi K, Oh S
and Suzuki K: Locoregional recurrence after segmentectomy for
clinical-T1aN0M0 radiologically solid non-small-cell lung
carcinoma. Eur J Cardiothorac Surg. 51:518–525. 2017.
|
|
6
|
Mghwary AE, Gedawy EM, Kamal AM and
Abuel-Maaty SM: Novel thienopyrimidine derivatives as dual EGFR and
VEGFR-2 inhibitors: Design, synthesis, anticancer activity and
effect on cell cycle profile. J Enzyme Inhib Med Chem. 34:838–852.
2019. View Article : Google Scholar
|
|
7
|
Reckamp KL, Frankel PH, Ruel N, Mack PC,
Gitlitz BJ, Li T, Koczywas M, Gadgeel SM, Cristea MC, Belani CP, et
al: Phase II trial of cabozantinib plus erlotinib in patients with
advanced epidermal growth factor receptor (EGFR)-mutant non-small
cell lung cancer with progressive disease on epidermal growth
factor receptor tyrosine kinase inhibitor therapy: A California
Cancer Consortium phase II trial (NCI 9303). Front Oncol.
9:1322019. View Article : Google Scholar
|
|
8
|
Yang KM, Shin IC, Park JW, Kim KS, Kim DK,
Park K and Kim K: Nanoparticulation improves bioavailability of
erlotinib. Drug Dev Ind Pharm. 43:1557–1565. 2017. View Article : Google Scholar
|
|
9
|
Cardona AF, Arrieta O, Zapata MI, Rojas L,
Wills B, Reguart N, Karachaliou N, Carranza H, Vargas C, Otero J,
et al: Acquired resistance to erlotinib in EGFR mutation-positive
lung adenocarcinoma among hispanics (CLICaP). Target Oncol.
12:513–523. 2017. View Article : Google Scholar
|
|
10
|
Hu H, Miao XK, Li JY, Zhang XW, Xu JJ,
Zhang JY, Zhou TX, Hu MN, Yang WL and Mou LY: YC-1 potentiates the
antitumor activity of gefitinib by inhibiting HIF-1α and promoting
the endocytic trafficking and degradation of EGFR in
gefitinib-resistant non-small-cell lung cancer cells. Eur J
Pharmacol. 874:1729612020. View Article : Google Scholar
|
|
11
|
Gandhi J, Zhang J, Xie Y, Soh J,
Shigematsu H, Zhang W, Yamamoto H, Peyton M, Girard L, Lockwood WW,
et al: Alterations in genes of the EGFR signaling pathway and their
relationship to EGFR tyrosine kinase inhibitor sensitivity in lung
cancer cell lines. PLoS One. 4:e45762009. View Article : Google Scholar
|
|
12
|
Li Y, Fan S, Koo J, Yue P, Chen ZG,
Owonikoko TK, Ramalingam SS, Khuri FR and Sun SY: Elevated
expression of eukaryotic translation initiation factor 4E is
associated with proliferation, invasion and acquired resistance to
erlotinib in lung cancer. Cancer Biol Ther. 13:272–280. 2012.
View Article : Google Scholar
|
|
13
|
Li R, Hu Z, Sun SY, Chen ZG, Owonikoko TK,
Sica GL, Ramalingam SS, Curran WJ, Khuri FR and Deng X: Niclosamide
overcomes acquired resistance to erlotinib through suppression of
STAT3 in non-small cell lung cancer. Mol Cancer Ther. 12:2200–2212.
2013. View Article : Google Scholar
|
|
14
|
Lee HJ, Zhuang G, Cao Y, Du P, Kim HJ and
Settleman J: Drug resistance via feedback activation of Stat3 in
oncogene-addicted cancer cells. Cancer Cell. 26:207–221. 2014.
View Article : Google Scholar
|
|
15
|
Lou W, Chen Y, Zhu KY, Deng H, Wu T and
Wang J: Polyphyllin I overcomes EMT-associated resistance to
erlotinib in lung cancer cells via IL-6/STAT3 pathway inhibition.
Biol Pharm Bull. 40:1306–1313. 2017. View Article : Google Scholar
|
|
16
|
Chen G, Bao Y, Weng Q, Zhao Y, Lu X, Fu L,
Chen L, Liu Z, Zhang X and Liang G: Compound 15c, a novel dual
inhibitor of EGFRL858R/T790M and FGFR1, efficiently
overcomes epidermal growth factor receptor-tyrosine kinase
inhibitor resistance of non-small-cell lung cancers. Front
Pharmacol. 10:15332020. View Article : Google Scholar
|
|
17
|
Hsu YL, Kuo PL, Tzeng TF, Sung SC, Yen MH,
Lin LT and Lin CC: Huang-lian-jie-du-tang, a traditional Chinese
medicine prescription, induces cell-cycle arrest and apoptosis in
human liver cancer cells in vitro and in vivo. J Gastroenterol
Hepatol. 23:e290–e299. 2008. View Article : Google Scholar
|
|
18
|
He MY, Deng YX, Shi QZ, Zhang XJ and Lv Y:
Comparative pharmacokinetic investigation on baicalin and
wogonoside in type 2 diabetic and normal rats after oral
administration of traditional Chinese medicine Huanglian Jiedu
decoction. J Ethnopharmacol. 155:334–342. 2014. View Article : Google Scholar
|
|
19
|
Ke M, Zhang Z, Xu B, Zhao S, Ding Y, Wu X,
Wu R, Lv Y and Dong J: Baicalein and baicalin promote antitumor
immunity by suppressing PD-L1 expression in hepatocellular
carcinoma cells. Int Immunopharmacol. 75:1058242019. View Article : Google Scholar
|
|
20
|
Wang HY, Yu HZ, Huang SM and Zheng YL:
P53, Bcl-2 and cox-2 are involved in berberine
hydrochloride-induced apoptosis of HeLa229 cells. Mol Med Rep.
14:3855–3861. 2016. View Article : Google Scholar
|
|
21
|
Zhu M, Ying J, Lin C, Wang Y, Huang K,
Zhou Y and Teng H: Baicalin induces apoptotic death of human
chondrosarcoma cells through mitochondrial dysfunction and
downregulation of the PI3K/Akt/mTOR pathway. Planta Med.
85:360–369. 2019. View Article : Google Scholar
|
|
22
|
Wu X, Liu P, Zhang H, Li Y, Salmani JM,
Wang F, Yang K, Fu R, Chen Z and Chen B: Wogonin as a targeted
therapeutic agent for EBV (+) lymphoma cells involved in
LMP1/NF-κB/miR-155/PU.1 pathway. BMC Cancer. 17:1472017. View Article : Google Scholar
|
|
23
|
Shan F, Shao Z, Jiang S and Chen Z:
Erlotinib induces the human non-small-cell lung cancer cells
apoptosis via activating ROS-dependent JNK pathways. Cancer Med.
5:3166–3175. 2016. View
Article : Google Scholar
|
|
24
|
Ji XL and He M: Sodium cantharidate
targets STAT3 and abrogates EGFR inhibitor resistance in
osteosarcoma. Aging (Albany NY). 11:5848–5863. 2019. View Article : Google Scholar
|
|
25
|
Zhang FQ, Yang WT, Duan SZ, Xia YC, Zhu RY
and Chen YB: JAK2 inhibitor TG101348 overcomes erlotinib-resistance
in non-small cell lung carcinoma cells with mutated EGF receptor.
Oncotarget. 6:14329–14343. 2015. View Article : Google Scholar
|
|
26
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar
|
|
27
|
Citri A and Yarden Y: EGF-ERBB signalling:
Towards the systems level. Nat Rev Mol Cell Biol. 7:505–516. 2006.
View Article : Google Scholar
|
|
28
|
Wang Z: ErbB receptors and cancer. Methods
Mol Biol. 1652:3–35. 2017. View Article : Google Scholar
|
|
29
|
Carcereny E, Moran T, Capdevila L, Cros S,
Vilà L, de Los Llanos Gil M, Remón J and Rosell R: The epidermal
growth factor receptor (EGRF) in lung cancer. Transl Respir Med.
3:12015. View Article : Google Scholar
|
|
30
|
Marmor MD, Skaria KB and Yarden Y: Signal
transduction and oncogenesis by ErbB/HER receptors. Int J Radiat
Oncol Biol Phys. 58:903–913. 2004. View Article : Google Scholar
|
|
31
|
Prickett TD, Agrawal NS, Wei X, Yates KE,
Lin JC, Wunderlich JR, Cronin JC, Cruz P, Rosenberg SA and Samuels
Y: Analysis of the tyrosine kinome in melanoma reveals recurrent
mutations in ERBB4. Nat Genet. 41:1127–1132. 2009. View Article : Google Scholar
|
|
32
|
Skoulidis F and Papadimitrakopoulou VA:
Targeting the gatekeeper: Osimertinib in EGFR T790M
mutation-positive non-small cell lung cancer. Clin Cancer Res.
23:618–622. 2017. View Article : Google Scholar
|
|
33
|
Wu P, Nielsen TE and Clausen MH:
FDA-approved small-molecule kinase inhibitors. Trends Pharmacol
Sci. 36:422–439. 2015. View Article : Google Scholar
|
|
34
|
Agustoni F, Suda K, Yu H, Ren S, Rivard
CJ, Ellison K, Caldwell C Jr, Rozeboom L, Brovsky K and Hirsch FR:
EGFR-directed monoclonal antibodies in combination with
chemotherapy for treatment of non-small-cell lung cancer: An
updated review of clinical trials and new perspectives in
biomarkers analysis. Cancer Treat Rev. 72:15–27. 2019. View Article : Google Scholar
|
|
35
|
Russo A, Franchina T, Ricciardi GR, Picone
A, Ferraro G, Zanghì M, Toscano G, Giordano A and Adamo V: A decade
of EGFR inhibition in EGFR-mutated non small cell lung cancer
(NSCLC): Old successes and future perspectives. Oncotarget.
6:26814–26825. 2015. View Article : Google Scholar
|
|
36
|
Thatcher N, Chang A, Parikh P, Rodrigues
Pereira J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH,
Pemberton K, Archer V and Carroll K: Gefitinib plus best supportive
care in previously treated patients with refractory advanced
non-small-cell lung cancer: Results from a randomised,
placebo-controlled, multicentre study (Iressa Survival Evaluation
in Lung Cancer). Lancet. 366:1527–1537. 2005. View Article : Google Scholar
|
|
37
|
Shepherd FA, Rodrigues Pereira J, Ciuleanu
T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S,
Smylie M, Martins R, et al: Erlotinib in previously treated
non-small-cell lung cancer. N Engl J Med. 353:123–132. 2005.
View Article : Google Scholar
|
|
38
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar
|
|
39
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar
|
|
40
|
Li YL, Pan YN, Wu WJ, Mao SY, Sun J, Zhao
YM, Dong JY, Zhang DY, Pan JP, Zhang C and Lin NM: Evodiamine
induces apoptosis and enhances apoptotic effects of erlotinib in
wild-type EGFR NSCLC cells via S6K1-mediated Mcl-1 inhibition. Med
Oncol. 33:162016. View Article : Google Scholar
|
|
41
|
Yang K, Chen Y, Zhou J, Ma L, Shan Y,
Cheng X, Wang Y, Zhang Z, Ji X, Chen L, et al: Ursolic acid
promotes apoptosis and mediates transcriptional suppression of
CT45A2 gene expression in non-small-cell lung carcinoma harbouring
EGFR T790M mutations. Br J Pharmacol. 176:4609–4624. 2019.
View Article : Google Scholar
|
|
42
|
Ma Z, Otsuyama K, Liu S, Abroun S,
Ishikawa H, Tsuyama N, Obata M, Li FJ, Zheng X, Maki Y, et al:
Baicalein, a component of Scutellaria radix from
Huang-Lian-Jie-Du-Tang (HLJDT), leads to suppression of
proliferation and induction of apoptosis in human myeloma cells.
Blood. 105:3312–3318. 2005. View Article : Google Scholar
|
|
43
|
Lee WR, Shen SC, Lin HY, Hou WC, Yang LL
and Chen YC: Wogonin and fisetin induce apoptosis in human
promyeloleukemic cells, accompanied by a decrease of reactive
oxygen species, and activation of caspase 3 and Ca(2+)-dependent
endonuclease. Biochem Pharmacol. 63:225–236. 2002. View Article : Google Scholar
|
|
44
|
Bonham M, Posakony J, Coleman I,
Montgomery B, Simon J and Nelson PS: Characterization of chemical
constituents in Scutellaria baicalensis with antiandrogenic and
growth-inhibitory activities toward prostate carcinoma. Clin Cancer
Res. 11:3905–3914. 2005. View Article : Google Scholar
|
|
45
|
Himeji M, Ohtsuki T, Fukazawa H, Tanaka M,
Yazaki S, Ui S, Nishio K, Yamamoto H, Tasaka K and Mimura A:
Difference of growth-inhibitory effect of Scutellaria
baicalensis-producing flavonoid wogonin among human cancer cells
and normal diploid cell. Cancer Lett. 245:269–274. 2007. View Article : Google Scholar
|
|
46
|
Bosse K, Haneder S, Arlt C, Ihling CH,
Seufferlein T and Sinz A: Mass spectrometry-based secretome
analysis of non-small cell lung cancer cell lines. Proteomics.
16:2801–2814. 2016. View Article : Google Scholar
|
|
47
|
Serizawa M, Murakami H, Watanabe M,
Takahashi T, Yamamoto N and Koh Y: Peroxisome
proliferator-activated receptor ү agonist efatutazone impairs
transforming growth factor β2-induced motility of epidermal growth
factor receptor tyrosine kinase inhibitor-resistant lung cancer
cells. Cancer Sci. 105:683–689. 2014. View Article : Google Scholar
|
|
48
|
Song J, Zhong R, Huang H, Zhang Z, Ding D,
Yan H, Sun E and Jia X: Combined treatment with Epimedium koreanum
Nakai extract and gefitinib overcomes drug resistance caused by
T790M mutation in non-small cell lung cancer cells. Nutr Cancer.
66:682–689. 2014. View Article : Google Scholar
|
|
49
|
Chen Y, Zeng Z and Lu Y: Is mIndy a
mediator of energy metabolism reprogramming in hepatocellular
carcinoma induced by interleukin-6/signal transducer and activator
of transcription 3 signaling? Hepatology. 67:451–452. 2018.
View Article : Google Scholar
|
|
50
|
Liu X, Wei W, Li X, Shen P, Ju D, Wang Z,
Zhang R, Yang F, Chen C, Cao K, et al: BMI1 and MEL18 promote
colitis-associated cancer in mice via REG3B and STAT3.
Gastroenterology. 153:1607–1620. 2017. View Article : Google Scholar
|
|
51
|
Atsaves V, Tsesmetzis N, Chioureas D, Kis
L, Leventaki V, Drakos E, Panaretakis T, Grander D, Medeiros LJ,
Young KH and Rassidakis GZ: PD-L1 is commonly expressed and
transcriptionally regulated by STAT3 and MYC in ALK-negative
anaplastic large-cell lymphoma. Leukemia. 31:1633–1637. 2017.
View Article : Google Scholar
|
|
52
|
Yamaguchi M, Suzuki R, Kwong YL, Kim WS,
Hasegawa Y, Izutsu K, Suzumiya J, Okamura T, Nakamura S, Kawa K and
Oshimi K: Phase I study of dexamethasone, methotrexate, ifosfamide,
L-asparaginase, and etoposide (SMILE) chemotherapy for
advanced-stage, relapsed or refractory extranodal natural killer
(NK)/T-cell lymphoma and leukemia. Cancer Sci. 99:1016–1020. 2008.
View Article : Google Scholar
|
|
53
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar
|
|
54
|
Xiao Q, Zheng F, Wu J, Tang Q, Wang W and
Hann SS: Activation of ERK and mutual regulation of Stat3 and SP1
contribute to inhibition of PDK1 expression by atractylenolide-1 in
human lung cancer cells. Cell Physiol Biochem. 43:2353–2366. 2017.
View Article : Google Scholar
|
|
55
|
Sordella R, Bell DW, Haber DA and
Settleman J: Gefitinib-sensitizing EGFR mutations in lung cancer
activate anti-apoptotic pathways. Science. 305:1163–1167. 2004.
View Article : Google Scholar
|
|
56
|
Van Schaeybroeck S, Kalimutho M, Dunne PD,
Carson R, Allen W, Jithesh PV, Redmond KL, Sasazuki T, Shirasawa S,
Blayney J, et al: ADAM17-dependent c-MET-STAT3 signaling mediates
resistance to MEK inhibitors in KRAS mutant colorectal cancer. Cell
Rep. 7:1940–1955. 2014. View Article : Google Scholar
|
|
57
|
Li G, Zhao L, Li W, Fan K, Qian W, Hou S,
Wang H, Dai J, Wei H and Guo Y: Feedback activation of STAT3
mediates trastuzumab resistance via upregulation of MUC1 and MUC4
expression. Oncotarget. 5:8317–8329. 2014. View Article : Google Scholar
|
|
58
|
Zhao C, Li H, Lin HJ, Yang S, Lin J and
Liang G: Feedback activation of STAT3 as a cancer drug-resistance
mechanism. Trends Pharmacol Sci. 37:47–61. 2016. View Article : Google Scholar
|