Introduction
Among all malignant tumours, colorectal cancer (CRC)
ranks third in incidence and second in cancer-related mortality
(1). There are >1.8 million new
cases reported each year, accounting for 6.1% of all sites of
cancers (2). In addition, the most
common histopathological type of CRC is colon adenocarcinoma
(COAD). Although significant advancements have been made in the
early diagnosis and treatment of colon cancer, the long-term
survival and prognosis of patients remain poor due to recurrence
and metastasis (3). Drug resistance
is the primary cause of cancer treatment failure and cancer-related
deaths. Therefore, the research and development of novel antitumour
drugs for metastasis has been a long-term focus of antitumour
therapy. Plant-derived compounds have numerous advantages,
including cost, stability, safety and multitargeting abilities,
making them highly valued in clinical applications (4).
Fangchinoline (FAN), a bisbenzylisoquinoline
alkaloid, is extracted from Stephania tetrandra, which
exists widely in nature. FAN possesses a variety of biological and
pharmacological activities, including anti-hypertensive and
anti-atherosclerotic activity, enhanced immunity, platelet
aggregation inhibition and histamine release (5). In previous years, researchers have
demonstrated that FAN exhibits antitumor activity against a variety
of malignant tumours, such as bladder cancer, prostate cancer,
breast cancer, gastric cancer, hepatocellular carcinoma and
melanoma (6), but its effects on
CRC are unclear. Aggressive metastatic dissemination accounts for
~90% of CRC-related deaths (7). The
loss of cell polarity and a shift from an epithelial phenotype to a
mesenchymal fibroblastic phenotype, a process known as
epithelial-mesenchymal transition (EMT), promotes malignant cell
metastasis through the extracellular matrix (ECM) to other tissues
(8). Exogenous and endogenous
cytokines work together to stimulate receptors on the cell surface
and then activate signalling pathways to regulate EMT factors,
eventually inducing EMT. For example, growth factors (including
EGF, VEGF, TGF-β, Wnt, stromal cell-derived factor 1 and
prostaglandin E2), cytokines (such as FAM3 metabolism
regulating signalling molecule C and interleukins) and receptor
tyrosine kinases [including epidermal growth factor receptor
(EGFR), hepatocyte growth factor receptor and insulin-like growth
factor 1 receptor] have been implicated in the regulation of EMT
(9). Tyrosine kinase inhibitors and
monoclonal antibodies against the extracellular domain of EGFR are
now widely used for metastatic CRC treatment after failure of
first-line chemotherapy regimens (10,11).
Therefore, targeting EGFR is important for treating CRC.
The aim of the present study was to evaluate the
anticancer activities of FAN in COAD and the potential molecular
mechanisms involved in vitro and in vivo. The present
study confirmed that FAN, as an alkaloid chemotherapeutic drug, had
antitumour activity in COAD cells.
Materials and methods
Cells and reagents
The COAD cell lines DLD-1 and LoVo and the human
intestinal epithelial cell line NCM460 were purchased from FuHeng
BioLogy Cell Centre. DLD-1 and NCM460 cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific,
Inc.), while LoVo cells were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.). Both media were supplemented with
10% foetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.) and 1% antibiotic (100 U/ml penicillin and 100 mg/ml
streptomycin; Sigma-Aldrich; Merck KGaA). All cells were cultured
in a standard humidified incubator at 37°C under an atmosphere with
5% CO2. FAN was purchased from Aladdin Industrial
Corporation (cat. no. F110200), which was dissolved in dimethyl
sulfoxide (DMSO) as a 65.78 mmol/l stock solution.
MTT assay
The MTT assay was performed to measure the
cytotoxicity of FAN on DLD-1, LoVo and NCM460 cells. Cells were
seeded into 96-well plates (5×103 per/well), incubated
overnight, and treated with varying concentrations of FAN (0–9 µM)
at 37°C for 48 h. Subsequently, MTT solution (5 mg/ml, 20 µl/well;
Sigma-Aldrich; Merck KGaA) was added to each well, followed by DMSO
(150 µl/well) to dissolve the formazan crystals. Then, the
absorbance at 490 nm in each well was read on a microplate reader
(ELx808, BioTek Instruments, Inc.). Three biologically independent
experiments were performed.
Plate colony formation assay
COAD cells (1×103) were seeded in a 6-cm
culture dish, incubated overnight, and treated with varying
concentrations of FAN (0–7 µM) for 5 h. Then, the supernatant was
replaced with regular medium containing 10% FBS. After 2 weeks, the
cells were fixed with methanol (≥99.5%) at room temperature for 15
min and stained with a 0.5% crystal violet solution at room
temperature for 10 min. Three biologically independent experiments
were conducted.
Cell cycle assay
After DLD-1 and LoVo cells were treated with FAN (5
µM) for 48 h, they were harvested, fixed in 75% ethanol at 4°C
overnight, treated with RNase and stained with propidium iodide
(Beijing 4A Biotech Co., Ltd.). Analysis of the cell cycle
distribution was performed using flow cytometry (FACSCalibur; BD
Biosciences). ModFit LT™ version 5.0 software (Verity Software
House, Inc.) was used for analysis. Three biologically independent
experiments were conducted.
Apoptosis assay
DLD-1 and LoVo cells were seeded in 6-well plates
(2.5×105 per/well) and allowed to adhere overnight.
Then, FAN (0–7 µM) was added, and the cells were incubated for 48 h
before being harvested and stained with an Annexin V-FITC/PI
Apoptosis Detection kit (cat. no. FXP018; Beijing 4A Biotech Co.,
Ltd.). FACSCalibur and FACSDiva™ 6.1.3 software (BD Biosciences)
were used to analyse apoptosis (early and late apoptosis). Three
biologically independent experiments were conducted.
Wound healing assay
Monolayers (90% confluency) of DLD-1 and LoVo cells
in serum-free medium were scratched with a sterile 200-µl pipette
tip and then treated with different doses of FAN (0–2 µM) for 24 h.
The scratch was imaged under a light microscope (magnification,
×100), and wound closure was assessed at different time points (0,
24 and 48 h). The widths of the scratches were analysed with ImageJ
v1.8.0 (National Institutes of Health), and three biologically
independent experiments were conducted.
Transwell assay
Migration and invasion experiments were performed
with Transwell chambers. DLD-1 and LoVo cells were pretreated with
different doses of FAN (0–2 µM) for 24 h before they were
resuspended in FBS-free medium and seeded into the upper chambers
of the inserts (1×104 cells/200 µl medium per well).
Then, 500 µl medium containing 10% FBS was added to the lower
chamber to serve as a chemoattractant. For invasion analysis, 50 µl
Matrigel (BD Biosciences) was added to the upper chamber to coat
the polycarbonate membrane at room temperature for 6 h before the
cells were seeded. Following incubation for 48 h, the cells on the
upper surface of the membrane were wiped away, and the migrated or
invaded cells were fixed with methanol at room temperature for 15
min and stained with a 0.5% crystal violet solution at room
temperature for 20 min. Under a light microscope (magnification,
×100), 5 randomly selected fields were quantitatively analysed by
recording the mean number of cells. Three biological experiments
were performed.
Western blot analysis
COAD cells were treated with different
concentrations of FAN (0–7 µM) for 48 h, after which they were
lysed with RIPA buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology) containing protease and phosphatase inhibitors.
After the lysates were centrifuged at 4°C for 10 min at 12,000 × g,
the supernatants were transferred to new test tubes. A BCA kit
(cat. no. P0012S; Beyotime Institute of Biotechnology) was used to
measure protein concentration. Proteins (25 µg/lane) were separated
by SDS-PAGE on 10% gels (Beyotime Institute of Biotechnology), and
then electrotransferred onto polyvinylidene difluoride membranes
(EMD Millipore). After the membranes were blocked with 5% non-fat
milk in TBS with 0.1% Tween-20 for 1 h at room temperature, they
were incubated overnight at 4°C with primary antibodies targeting
the following proteins (as appropriate): Phosphorylated (p)-AKT
(Ser473; polyclonal, rabbit anti-human IgG; 1:1,000; cat. no.
4060S; Cell Signaling Technology, Inc.), EGFR (polyclonal, rabbit
anti-human IgG; 1:1,000; cat. no. 18986-1-AP; ProteinTech Group,
Inc.), E-cadherin (polyclonal, rabbit anti-human IgG; 1:1,000; cat.
no. 20874-1-AP; ProteinTech Group, Inc.), N-cadherin (polyclonal,
rabbit anti-human IgG; 1:1,000; cat. no. 22018-1-AP; ProteinTech
Group, Inc.), vimentin (polyclonal, rabbit anti-human IgG; 1:1,000;
cat. no. 10366-1-AP; ProteinTech Group, Inc.), MMP2 (polyclonal,
rabbit anti-human IgG; 1:1,000; cat. no. 10373-2-AP; ProteinTech
Group, Inc.), MMP9 (polyclonal, rabbit anti-human IgG; 1:1,000;
cat. no. 10375-2-AP; ProteinTech Group, Inc.), CD133 (polyclonal,
rabbit anti-human IgG; 1:1,000; cat. no. 66666-1-Ig; ProteinTech
Group, Inc.), cyclin D1 (polyclonal, rabbit anti-human IgG;
1:1,000; cat. no. 60186-1-Ig; ProteinTech Group, Inc.), Bax
(polyclonal, rabbit anti-human IgG; 1:1,000; cat. no. 50599-2-Ig;
ProteinTech Group, Inc.), Bcl-2 (polyclonal, rabbit anti-human IgG;
1:1,000; cat. no. 12789-1-AP; ProteinTech Group, Inc.), zinc finger
E-box-binding homeobox 1 (ZEB1; polyclonal, rabbit anti-human IgG;
1:1,000; cat. no. 21544-1-AP; ProteinTech Group, Inc.), ZEB2
(polyclonal, rabbit anti-human IgG; 1:1,000; cat. no. 14026-1-AP;
ProteinTech Group, Inc.), zinc finger protein SNAI1 (SNAIL;
polyclonal, rabbit anti-human IgG; 1:1,000; cat. no. 13099-1-AP;
ProteinTech Group, Inc.), zinc finger protein SNAI2 (SLUG;
polyclonal, rabbit anti-human IgG; 1:1,000; cat. no. 12129-1-AP;
ProteinTech Group, Inc.), AKT (polyclonal, rabbit anti-human IgG;
1:1,000; cat. no. 10176-2-AP; ProteinTech Group, Inc.) and β-actin
(polyclonal, mouse anti-human IgG; 1:1,000; cat. no. TA-09; OriGene
Technologies, Inc.). Then, the membranes were incubated with
anti-rabbit (HRP-conjugated goat anti-rabbit IgG; 1:10,000; cat.
no. ZB-2301; OriGene Technologies, Inc.) or anti-mouse
(HRP-conjugated goat anti-mouse IgG; 1:10,000; cat. no. ZB-2305;
OriGene Technologies, Inc.) secondary antibodies for 1 h at room
temperature. Protein signals were visualized using an enhanced
chemiluminescence substrate (Thermo Fisher Scientific, Inc.) on a
Bio-Rad gel imaging system (Bio-Rad Laboratories, Inc.). Image Lab
version 2.0.1 (Bio-Rad Laboratories, Inc.) was used for
semi-quantification. Three biologically independent experiments
were performed.
Bioinformatics prediction
GEPIA2 (http://gepia2.cancer-pku.cn/#index) analysis included
differential expression in tumours vs. normal tissues, patient
survival and multiple gene comparisons (12). The Human Protein Atlas (https://www.proteinatlas.org/) was used to determine
the EGFR protein levels in colon cancer and normal colon tissues
with an EGFR antibody (CAB000035). The Venny online tool
(http://bioinfogp.cnb.csic.es/tools/venny/) was used to
obtain the intersection between two sets of data groups.
Chemical database collection of the
properties of FAN
The PubChem website (https://pubchem.ncbi.nlm.nih.gov/) was used to search
for FAN and download its three-dimensional (3D) structure as an SDF
file, which was imported into the PharmMapper website (http://www.lilab-ecust.cn/pharmmapper/)
for compound-target gene prediction, and compound-target gene
information annotation was obtained and transformed into standard
gene symbols.
Prediction of target genes of
compounds in colonic neoplasms
The Comparative Toxicogenomics Database (CTD;
http://ctdbase.org/) was searched using the key word
‘Colonic Neoplasms’ to obtain the pathogenic genes related to this
disease. The overlapping genes for the compounds and diseases were
obtained through the Venny online tool (http://bioinfogp.cnb.csic.es/tools/venny/).
Functional enrichment analysis
The genes were entered into The Database for
Annotation, Visualization and Integrated Discovery (DAVID;
http://david.ncifcrf.gov/) for Kyoto
Encyclopaedia of Genes and Genomes (KEGG) pathway (http://www.genome.jp/kegg/pathway.html)
and Gene Ontology (GO; http://www.geneontology.org) enrichment analyses.
‘Homo sapiens’ and false discovery rate (FDR) <0.05 were
selected as the filter criteria. The ggplot2.R package (version
0.0.1; http://cran.r-project.org/web/packages/ggplot2/)
was used for image visualization.
Protein-protein interaction (PPI)
network construction
The PPI network of the target genes was constructed
using the Search Tool for the Retrieval of Interacting Genes
(STRING) online tool (https://string-db.org/). R version 3.6.0 software
(https://www.r-project.org/) was used to
connect the adjacent nodes in the PPI network.
Animal experiments
A total of 10 female BALB/C nude mice (5–6 weeks
old; weight, 18 g) were purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd. and reared in a
pathogen-free environment under a 12/12 h light/dark cycle at room
temperature. The mice had free access to food and water. The
protocol relating to all animal experiments was discussed and
approved by the Institutional Animal Care and Use Committee of
Harbin Medical University (Harbin, China). The health and behaviour
of the mice were monitored every day (12). Xenograft tumours were established by
subcutaneously injecting 0.1 ml DLD-1 cells (1×107
cells/ml) in culture medium into the flanks of the mice. When the
xenograft tumours were established, the mice were randomly divided
into the treatment group (n=5) or control group (n=5) and
intraperitoneally injected with FAN (0.1 ml, 0.5 mg/ml) or PBS,
respectively, three times per week for 4 weeks. After the
intraperitoneal injection of 1% pentobarbital sodium into mice (50
mg/kg), the tumours were monitored by ultrasound imaging (USI).
Analysis of tumour growth, angiogenesis, microangiogenesis and
tumour hardness was performed with Philips IU Elite ultrasound
system (Philips Healthcare), which includes four USI modes, namely,
B-ultrasound (B-mode), colour Doppler flow imaging (CDFI), colour
power angiography (CPA) and ultrasonic elastosonography (USE). The
mice were anesthetized with sodium pentobarbital (100 mg/kg),
followed by cervical dislocation at the end of the experiment. The
tumours, hearts, livers, spleens and lungs were collected for
pathological examination, and the tumour volumes were calculated
using the following formula: Volume = length ×
width2/2.
Pathological examination via
immunohistochemistry (IHC) haematoxylin and eosin (H&E) and
TUNEL apoptosis detection
For IHC, formaldehyde-fixed (at room temperature for
48 h) and paraffin-embedded tumour tissues were sliced into 5-µm
thick sections. The sections were deparaffinized using xylene,
rehydrated in a graded series of ethanol, incubated with 3%
H2O2 for 30 min, and blocked with 10% normal
goat serum (Beijing Solarbio Science & Technology Co., Ltd.) at
room temperature for 1 h. Sections were then immunostained with
antibodies against Ki67 (1:200; cat. no. WL01384a; Wanleibio Co.,
Ltd.), EGFR (1:200; cat. no. WL02312; Wanleibio Co., Ltd.), p-Bcl-2
(1:100; cat. no. WL01556; Wanleibio Co., Ltd.), N-cadherin (1:100;
cat. no. WL01047; Wanleibio Co., Ltd.) and cyclin D1 (1:200; cat.
no. WL01435a; Wanleibio Co., Ltd.) at 4°C overnight. Tissues were
then incubated with HRP-labeled goat anti-rabbit IgG (1:500; cat.
no. 31460; Thermo Fisher Scientific, Inc.) at 37°C for 60 min, then
immersed in PBS for 5 min three times. The colour was developed
with a 3,3′-Diaminobenzidine Substrate Kit (cat. no. DA1010;
Beijing Solarbio Science & Technology Co., Ltd.) at 4°C
overnight, following which the sections were counterstained with
haematoxylin at room temperature for 3 min. Five visual fields on
each slide were randomly selected and photographed under a light
microscope (magnification, ×400).
For H&E staining, tissues were fixed and
embedded in paraffin, sliced into 5-µm thick sections, stained with
haematoxylin at room temperature for 5 min, treated with 1% acid
ethanol for 3 sec, washed with distilled water, stained with eosin
solution at room temperature for 3 min, dehydrated with graded
alcohol and washed with xylene. After drying, the mounted slides
were examined and five visual fields were randomly selected on each
slide and imaged using a light microscope (magnification,
×200).
Apoptosis in pathological tissues was detected using
a TUNEL Apoptosis Detection Kit (Wanleibio Co., Ltd.), and stained
with hematoxylin for 2 min at room temperature. A total of five
visual fields were randomly selected on each slide and imaged using
a light microscope (magnification, ×400). Three biologically
independent experiments were performed.
Statistical analysis
Statistical analysis and probit regression analysis
were conducted using SPSS 22.0 software (IBM Corp.). Data are
presented as the mean ± standard deviation. Differences between the
means of two groups were assessed using an unpaired Student's
t-test (independent). Differences among the means of multiple
groups were assessed using one-way ANOVA followed by a Bonferroni
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
FAN suppresses viability and
proliferation of COAD cells
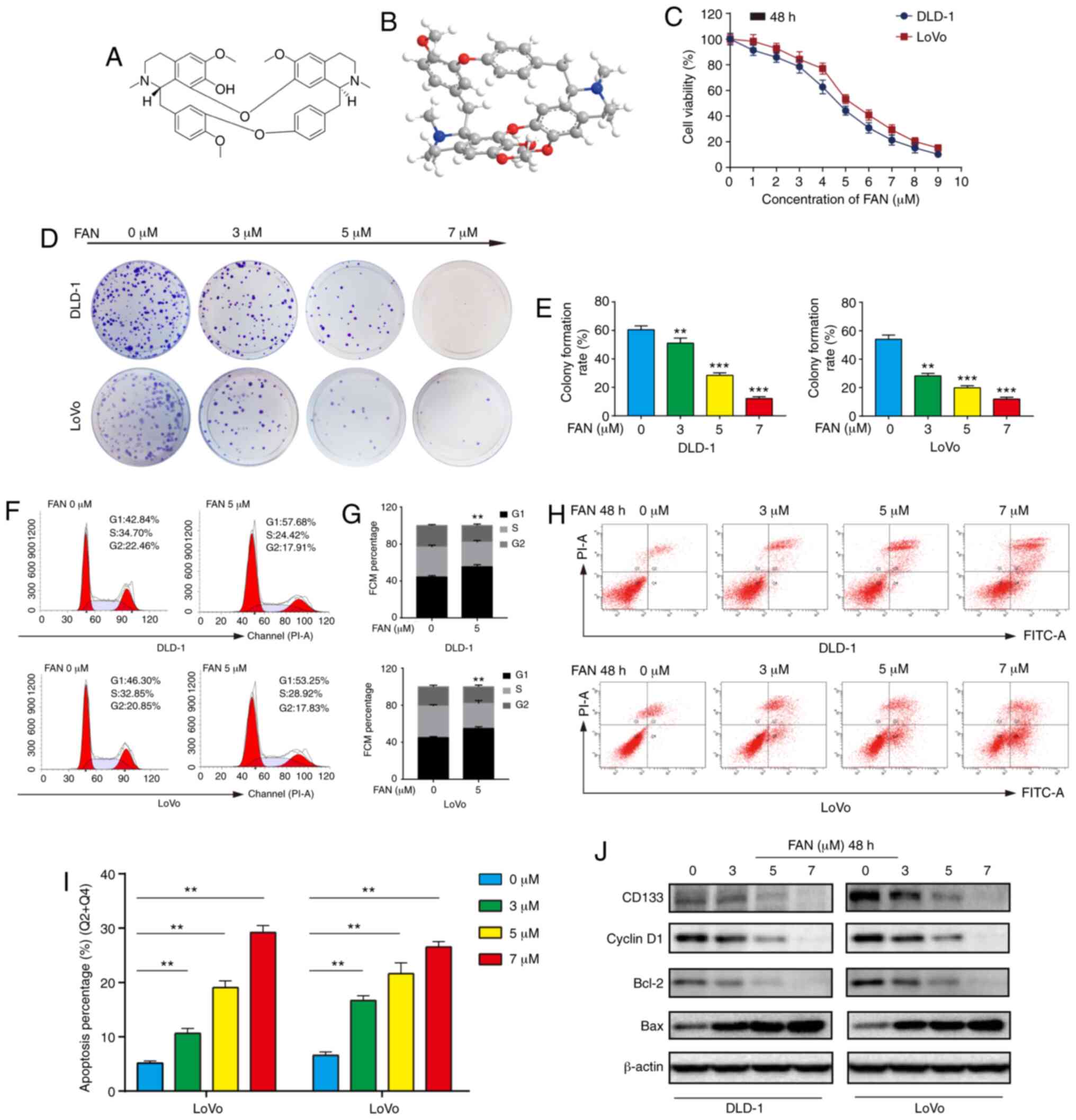
The chemical structural formula and 3D structure of
FAN are shown in Fig. 1A and B,
respectively. To evaluate cytotoxicity, COAD cells were incubated
with multiple concentrations of FAN (0–9 µM) for 48 h. The MTT
assay results showed that FAN significantly inhibited COAD cell
viability in a dose-dependent manner (Fig. 1C). The 50% inhibitory concentration
(IC50) values for DLD-1 and LoVo cells at 48 h,
according to probit regression analysis, were 4.53 and 5.17 µM,
respectively. Notably, FAN showed minimal cytotoxicity towards
NCM460 cells (Fig. S1). The colony
formation assay results indicated that the number and size of
colonies decreased in a dose-dependent manner (Fig. 1D and E).
Disordered cell cycle regulation is the basic
mechanism of infinite and rapid cancer cell proliferation (13). As shown in Fig. 1F and G, FAN induced cell cycle
arrest in DLD-1 and LoVo cells in the G1 phase, with the percentage
of cells in the G1 phase cells increasing from 42.84 to 57.68% and
from 46.30 to 53.25%, respectively. The western blot assay results
showed that the expression of CD133 and cyclin D1 in cells treated
with FAN decreased in a dose-dependent manner (Figs. 1J and S2).
FAN induces apoptosis in COAD
cells
Dysregulation of the apoptotic process is closely
associated with tumour progression and resistance to chemotherapies
(14). Therefore, exploring
drug-induced apoptosis is crucial for elucidating anticancer
mechanisms. The results showed that FAN significantly induced
apoptosis in a dose-dependent manner (Fig. 1H and I), and western blot analysis
revealed decreased Bcl-2 expression and increased Bax expression in
FAN-treated COAD cells (Figs. 1J
and S2).
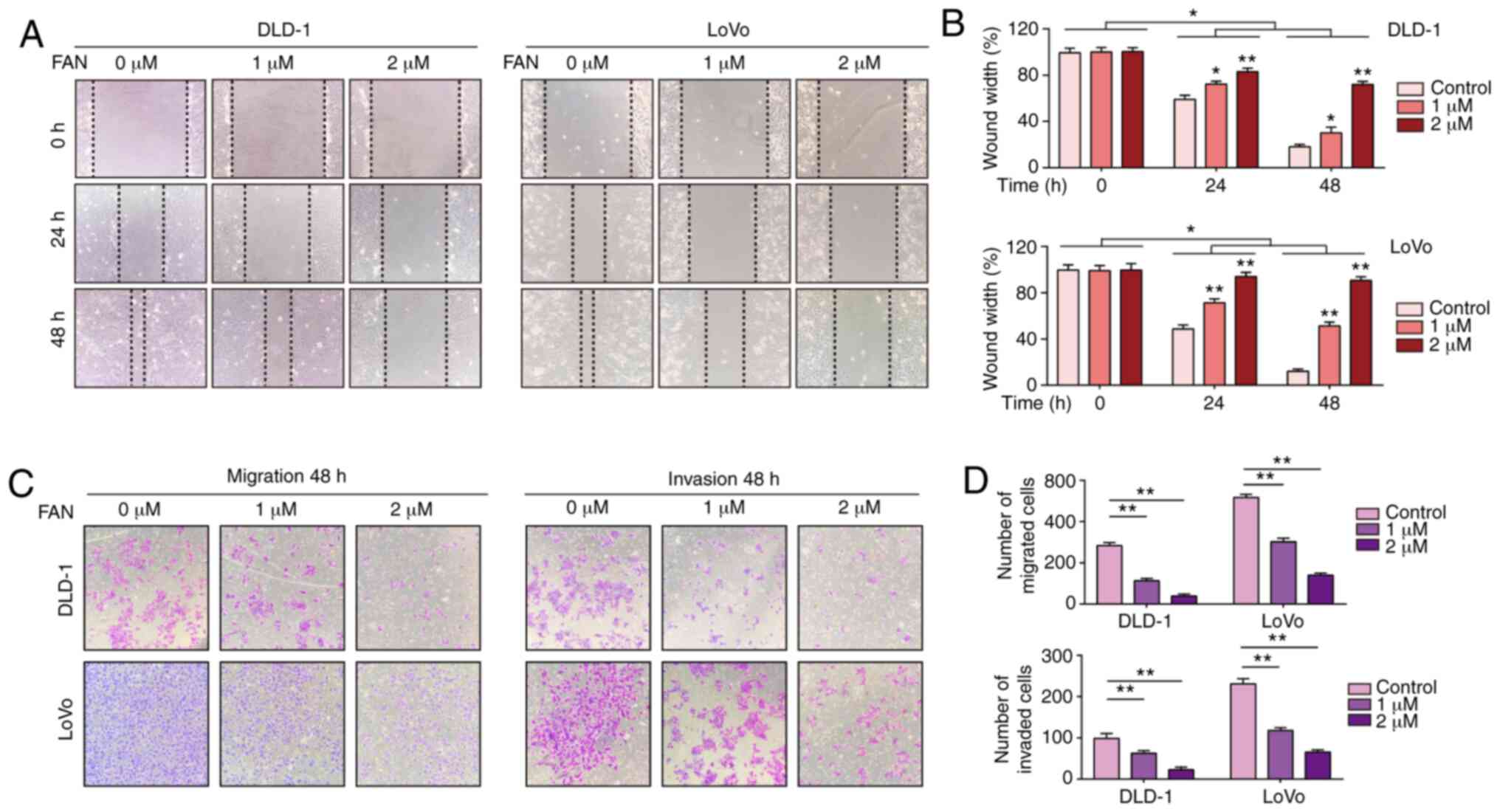
FAN impedes COAD cell mobility
Generally, chemotherapy can not only induce
apoptosis, but also weaken mobility in tumour cells (15). Tumour cell mobility is essential for
metastasis and is typically assessed by wound healing and Transwell
assays. Lower concentrations of FAN were administered (0–2 µM) to
avoid effects related to decreased viability. As shown in Fig. 2A and B, FAN significantly reduced
the wound healing rate of COAD cells in a dose-dependent manner
compared with the untreated control group. Consistent with these
observations, Transwell assay results demonstrated that the
migration and invasion of FAN-treated COAD cells decreased in a
dose-dependent manner (Fig. 2C and
D).
FAN inhibits EMT in COAD cells
Western blot analysis results showed that E-cadherin
expression increased with increasing FAN concentration, whereas
vimentin and N-cadherin expression decreased compared with the
control group. Furthermore, MMP2 and MMP9 expression levels were
decreased by FAN compared with the control cells (Fig. 3A and B). With increasing FAN
concentrations, SNAIL and ZEB2 expression decreased in a
dose-dependent manner compared with the control group, and the
expression of SLUG in DLD-1 and LoVo cell lines was not inhibited
following treatment with FAN. Inhibition of ZEB1 expression in
DLD-1 cells by FAN required a concentration >7 µM (Fig. 3C and D). The public GEPIA database
showed that SNAIL expression was higher in COAD tissues compared
with the adjacent tissues, and the overall survival (OS) of
patients with COAD was decreased in the SNAIL-overexpression group
from the GEPIA2 database (Fig.
S3).
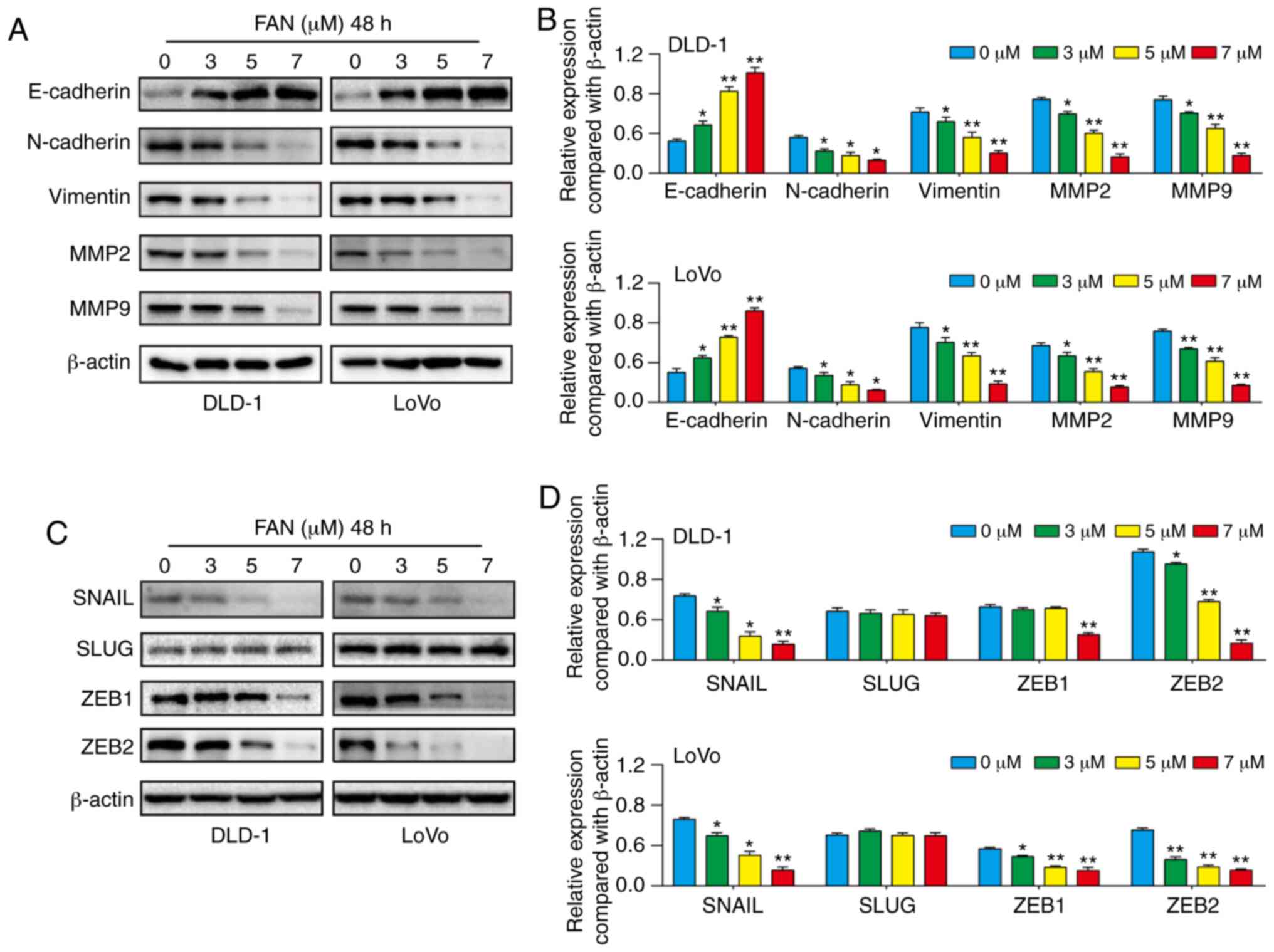 | Figure 3.FAN represses EMT and EMT regulatory
factors in colon adenocarcinoma cells. (A and B) E-Cadherin,
N-cadherin, vimentin, MMP2 and MMP9 expression following treatment
with FAN (0–7 µM, 48 h) was determined by western blotting. (C and
D) The expression of related transcription factors after FAN
treatment (0–7 µM, 48 h) was measured by western blotting.
*P<0.05 and **P<0.01 vs. 0 µM group. FAN, fangchinoline; EMT,
epithelial-mesenchymal transition; SNAIL, zinc finger protein
SNAI1; SLUG, zinc finger protein SNAI2; ZEB1, zinc finger
E-box-binding homeobox 1. |
FAN blocks the PI3K-AKT signalling
pathway in COAD cells by inhibiting EGFR targets
The obtained SDF file for FAN, which was downloaded
from the PubChem website, was imported into the PharmMapper website
to predict target genes, and 258 compound-target genes were
obtained. The CTD was searched with ‘Colonic Neoplasms’ as the key
word and 25,581 pathogenic-target genes were obtained.
Subsequently, 256 overlapping genes were identified as potential
target genes through the online tool Venny (Fig. 4A).
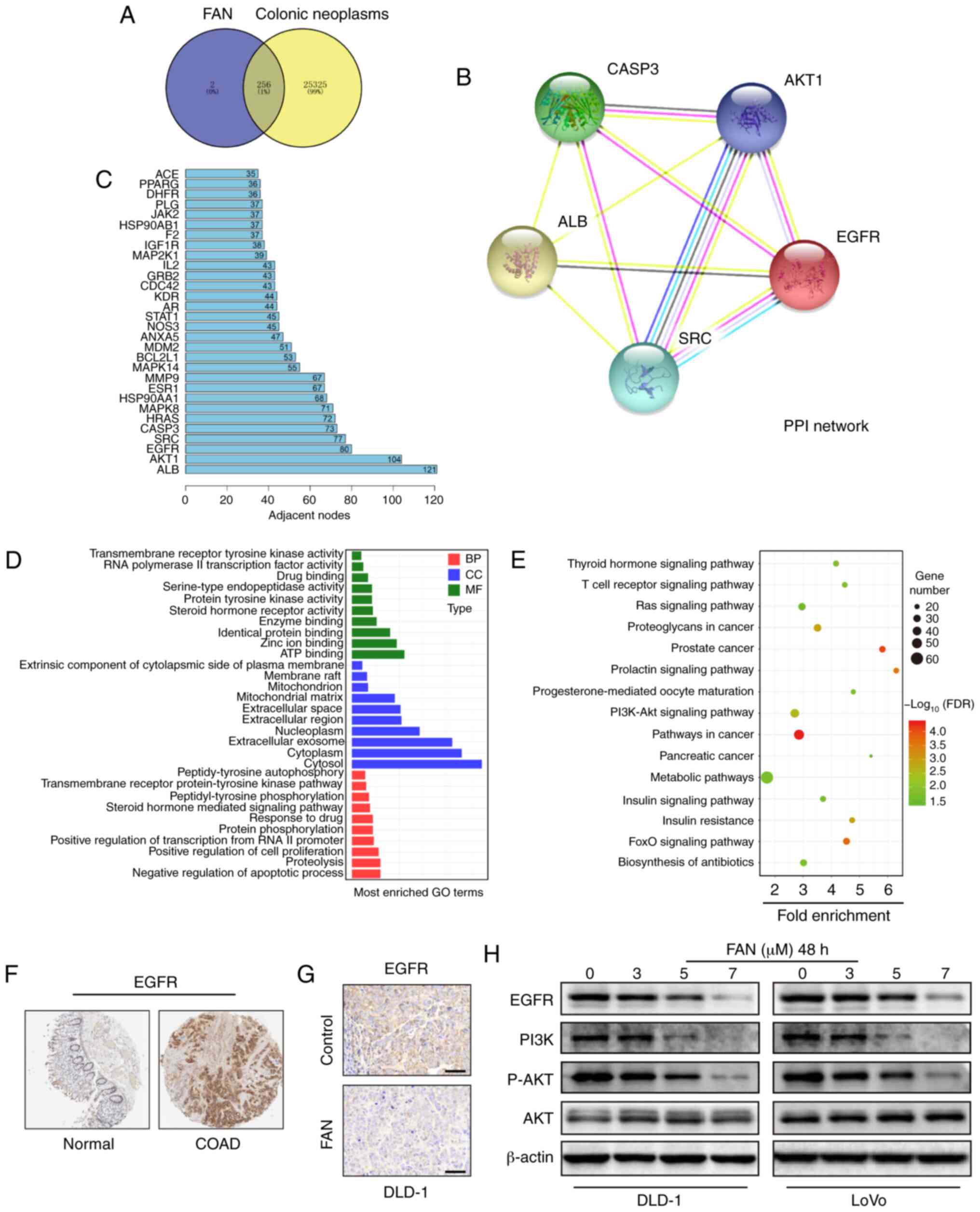 | Figure 4.EGFR is a therapeutic target of colon
cancer. (A) Venn diagram of the target genes for 258
compound-target genes and 25,581 pathogenic-target genes. (B) PPI
network of the 5 core target genes among 256 potential target
genes. (C) The column shows the adjacent nodes of the proteins. (D)
GO analysis of potential target genes. (E) Kyoto Encyclopaedia of
Genes and Genomes pathway analysis of potential target genes. (F)
EGFR protein levels in normal and colon cancer tissues from The
Human Protein Atlas database. (G) Immunohistochemistry analysis of
EGFR expression in DLD-1 ×enograft tumours following FAN treatment
(magnification, ×400; scale bar, 100 µm). (H) EGFR, PI3K, p-AKT and
AKT expression levels in COAD cells treated with FAN (0–7 µM, 48 h)
were verified by western blotting. PPI, protein-protein
interaction; EGFR, epidermal growth factor receptor; GO, Gene
Ontology; FAN, fangchinoline; PI3K, phosphoinositide 3-kinase; p-,
phosphorylated; ALB, albumin; SRC, proto-oncogene tyrosine-protein
kinase Src; CASP3, caspase-3; BP, ‘Biological Process’; MF,
‘Molecular Function’; CC, ‘Cellular Component’; COAD, colon
adenocarcinoma. |
A PPI network of five core potential target genes
out of 256 potential target genes was constructed using the STRING
online database (Fig. 4B). The
circle in Fig. 4B represents the
protein, and there are interactions between the linker proteins. R
software was used to draw the number of adjacent nodes in the
interactive network, where the abscissa is the number of adjacent
nodes of the gene, and the ordinate is the name of the gene. Genes
with more adjacent nodes are at the centre of the network. From the
column (Fig. 4C), a number of
potential target genes were obtained, among which albumin (ALB),
AKT1, EGFR, proto-oncogene tyrosine-protein kinase Src (SRC) and
caspase-3 (CASP3) were found to be at the core of the PPI
network.
The 256 potential target genes were input into DAVID
for GO enrichment analysis and KEGG signalling pathway enrichment
analysis to elucidate the multiple biological functions of
potential targets of FAN from a systematic level (Fig. 4D and E). From the GO enrichment
analysis for ‘Biological Process’, the potential targets of FAN
were enriched in ‘negative regulation of apoptotic process’
(GO:0043066), ‘proteolysis’ (GO:0006508), ‘positive regulation of
cell proliferation’ (GO:0008284), ‘protein phosphorylation’
(GO:0006468), ‘response to drug’ (GO:0042493) and others. For
‘Cellular Component’, the enriched items belonged to ‘cytosol’
(GO:0005829), ‘cytoplasm’ (GO:0005737), ‘extracellular exosome’
(GO:0070062), ‘nucleoplasm’ (GO:0005654), ‘extracellular region’
(GO:0005576) and others. For ‘Molecular Function’, ‘ATP binding’
(GO:0005524), ‘zinc ion binding’ (GO:0008270), ‘identical protein
binding’ (GO:0042802), ‘enzyme binding’ (GO:0019899) were primarily
enriched (Fig. 4D). KEGG signalling
pathway enrichment analysis results were enriched in ‘Metabolic
pathways’ (hsa01100), ‘Pathways in cancer’ (hsa05200), ‘PI3K-Akt
signalling pathway’ (hsa04151) and others (Fig. 4E).
A differential expression analysis of tumour vs.
normal tissues showed that EGFR expression was higher in COAD
tissues than in normal tissues (Fig.
4F). Furthermore, IHC results showed that EGFR expression was
decreased in tumour tissues in the FAN treatment group (Fig. 4G). Moreover, the protein expression
levels of EGFR, PI3K and p-AKT were decreased, but total AKT
expression showed no significant changes (Figs. 4H and S4).
FAN suppresses tumour growth in
vivo
USI (including B-mode, CDFI, CPA and USE) is a
non-invasive imaging technique that can enable comprehensive
analysis of xenograft tumours, and not only accurately measures the
tumour size, but also displays the blood supply around the
transplanted tumour and the hardness of the transplanted tumour. A
DLD-1 ×enograft model was established in BALB/c nude mice to
evaluate the antitumour activity of FAN in vivo. After 4
weeks, the weights and sizes of the xenografts in the FAN treatment
group were smaller than those observed in the control group, as
shown in Fig. 5A-C. According to
the different USI patterns in the FAN treatment group, the tumour
size, as measured by the B-mode pattern, decreased, the xenograft
tumour angiogenesis, as reflected by the CDFI and CPA patterns,
decreased, and the xenograft tumour hardness, according to the USE
pattern, decreased (Fig. 5F). Next,
IHC was performed to measure the protein expression of
representative tumour progression markers. Compared with that
observed in the control group, the expression of Ki67, cyclin D1,
Bcl-2 and N-cadherin in the FAN-treated group decreased (Fig. 5D). Haematoxylin and eosin (H&E)
staining of the FAN-treated group confirmed a reduction in tumour
angiogenesis (Fig. S5B) without
obvious necrosis in internal organs (Fig. S5A). The apoptotic rate of xenograft
tumours, as determined by the TUNEL assay, was 17.23 and 3.05% in
the FAN-treated and control groups, respectively, indicating the
obvious apoptosis-inducing effect of FAN (Fig. 5E).
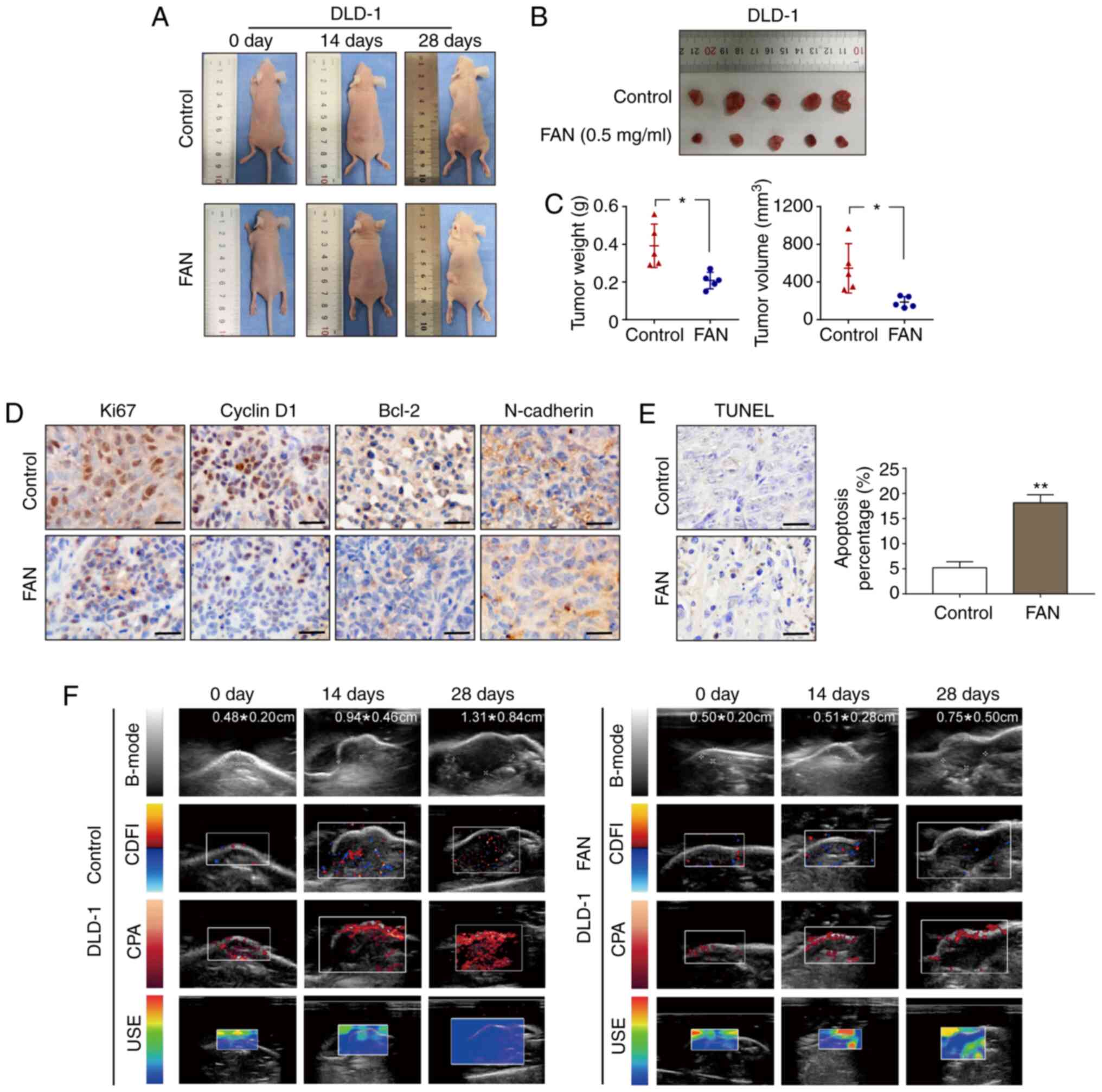 | Figure 5.FAN suppresses tumour growth in
vivo. (A-C) DLD-1 cells were subcutaneously injected into the
left flanks of mice to establish xenograft models. Following FAN
treatment (0.1 ml, 0.5 mg/ml three times per week for a total of 4
weeks), all mice were observed, and the tumour weight and volume
were measured and compared. (D) Ki67, cyclin D1, Bcl-2 and
N-cadherin expression levels in xenograft tumours were analysed by
immunohistochemistry (magnification, ×400; scale bar, 100 µm). (E)
TUNEL assays were performed to detect apoptosis in pathological
tissues. (F) Ultrasound evaluation that included B-mode, CDFI, CPA
and USE. *P<0.05 and **P<0.01 vs. control group. FAN,
fangchinoline; B-mode, B-ultrasound; CDFI, colour Doppler flow
imaging; CPA, colour power angiography; USE, ultrasonic
elastosonography. |
Discussion
Natural plant compounds (NPCs) have long been used
as a source of therapeutic substances and a structural basis for
drug development. Natural products provide unique structures that
can be used to design novel therapeutic agents (16). Boldbaatar et al (17) systematically reviewed the
anticancer, anti-inflammatory and antifibrosis effects of NPCs via
EMT inhibition and concluded that NPCs inhibit pathological EMT
through different signal transduction pathways in cells. In
addition, accumulating evidence has suggested potential
plant-derived compounds as inhibitors of several stages of
tumorigenesis and of inflammatory and fibrotic processes (4). Several clinical trials have
demonstrated that NPCs elicit anti-aging, anticancer and other
health-enhancing effects. For example, berberine shows extensive
pharmacological effects in gastroenteritis, abdominal pain and
diarrhoea, and also antibacterial, anti-diabetic and
anti-inflammatory properties (18).
Furthermore, baicalin has been found to decrease blood lipids and
inflammation in patients with coronary artery disease and
rheumatoid arthritis, supporting its further clinical application
(19). A low amount of gallic acid
can prevent oxidative DNA injury and decrease inflammatory-,
cancer- and cardiovascular disease-related markers (20). Key targets of the effects of NPCs
may include the suppression of oxidative stress and the induction
of 5′AMP-activated kinase (AMPK), or inhibition of the
WNT/β-catenin, PI3K/AKT/mTOR and RAS/MEK/ERK signalling pathways,
which result in cell death and the prevention of conditions such as
aging, diabetes, cardiovascular disease and cancer (21). During EMT, epithelial cells lose the
expression of epithelial markers (including E-cadherin, occludin
and cytokeratins) and begin to express mesenchymal markers (such as
vimentin and fibronectin). In the present study, the expression of
the epithelial marker E-cadherin increased significantly after FAN
treatment, whereas that of the mesenchymal markers N-cadherin and
vimentin decreased significantly. Although it is unclear whether
FAN can reverse EMT, it can be speculated that FAN may inhibit EMT.
Cancer stem cells (CSCs) have the potential for self-renewal and
multipotent differentiation, which leads to drug resistance
(22). As a CSC surface marker,
overexpression of CD133 is hypothesized to be involved in tumour
metastasis and CRC relapse (23).
Furthermore, degradation of the ECM due to increased MMP expression
is crucial in the process of EMT-related metastasis (24,25).
MMP2 and MMP9 are the most commonly used indicators of tumour
invasion and metastasis. Western blot analysis in the present study
showed that the expression levels of CD133, MMP2 and MMP9 were
decreased significantly in the FAN-treated group. In addition, the
expression of Bcl-2, an antiapoptotic marker, and cyclin D1, a cell
cycle G1 marker, decreased with increasing FAN concentrations.
A group of transcription factors, namely, the EMT
regulatory factors SNAIL, SLUG, ZEB1 and ZEB2, are upstream of the
aforementioned markers and can regulate EMT by directly inhibiting
E-cadherin promoter activity and promoting the mesenchymal
phenotype (26–29). In the present study, the expression
of SNAIL and ZEB2 decreased significantly and consistently in a
dose-dependent manner in both COAD cell lines. A previous study
demonstrated that the overexpression of SNAIL, a zinc finger
transcriptional repressor, is closely associated with lymph node
metastasis, indicating a poor clinical prognosis in patients with
COAD, whereas blocking SNAIL protein expression can reduce the
invasive activity of malignant tumours (30). ZEB2 expression has been demonstrated
to be significantly higher in COAD tissues than in normal adjacent
tissues (31), and Li et al
(32) confirmed that ZEB2 promotes
tumour metastasis and is associated with poor prognosis in patients
with COAD. Therefore, we speculate that SNAIL and ZEB2 are
primarily involved in regulating EMT as transcription factors in
FAN-treated cells.
The concept of network pharmacology stems from the
introduction of systems biology and the application of
bioinformatics, as well as the ‘genome’ theory, which involves the
integration of modern genomics, proteomics and metabolism (33). Based on the
‘disease-gene-target-drug’ interaction network, the intervention
and influence of drugs on the disease network were analysed. The
combination of a disease targeting database and molecular
verification can provide a basis for identifying target genes and
signalling pathways, as well as aiding the discovery of complex
underlying mechanisms (34).
In the present study, through network
pharmacological analysis, a PPI network of 256 potential target
genes was constructed, and the molecular mechanisms were further
predicted according to the enrichment of the potential target genes
in the GO analysis and KEGG signalling pathways. The PPI network
indicated that ALB, AKT1, EGFR, SRC and CASP3 were core components
of tumour progression.
ALB, the most abundant protein in human blood, helps
regulate plasma colloid osmotic pressure and acts as a carrier
protein for a broad range of endogenous molecules (35). The loss of serum ALB reflects the
degree of malnutrition and inflammatory response (36,37).
In a recent study, the combination of serum ALB and cholinesterase
levels was revealed to be a useful clinical index, as it provided
accurate prognostic information for patients with CRC (38). AKT1 is a member of the AKT family
that plays a central role in the AKT pathway. A variety of
biological processes, such as proliferation, cell cycle
progression, invasion and metastasis, are regulated by AKT kinase
(39). Caspase-3, a primary inducer
of apoptosis, is activated directly by caspase-8, caspase-9 and
caspase-10, and exerts its function via convergence of the
intrinsic and extrinsic apoptotic pathways (40,41).
SRC (a non-receptor tyrosine kinase) is a well-studied
proto-oncogene that participates in the tumorigenesis of numerous
types of cancer, such as colorectal, pancreatic cancer (42,43).
Studies have demonstrated that SRC cannot significantly increase
the proliferation, invasiveness and tumorigenicity of tumour cells
unless bound with EGFR and p-EGFR (42). As a component of EGFR signalling
transduction, cellular SRC (C-SRC) mediates EGFR-driven
tumorigenesis in vivo, and the interaction between EGFR and
C-SRC leads to a more aggressive tumour phenotype (44,45).
Previously, a study on genetic variations in the EGFR pathway
demonstrated that the interaction between SRC and single nucleotide
polymorphisms (SNPs) of EGFR is significantly related to the risk
of COAD, which further supports the joint regulatory model of EGFR
and SRC in early-stage colorectal cancer (46). Furthermore, SRC plays a central role
in regulating multiple downstream pathways, such as the PI3K/AKT,
signal transducer and activator of transcription 3 and
mitogen-activated protein kinase pathways (47). SRC activation is associated with
chemotherapy and EGFR antibody resistance, which has led to the
strategy of developing SRC family-specific inhibitors to prevent
immune inhibition (46). Research
on NPCs targeting EGFR has remained important (48). The ligands of EGFR (EGF and TGF-α)
bind to its extracellular domain and induce phosphorylation of its
intracellular domain, in turn activating an intricate downstream
signalling pathway (49,50) Curcumin treatment has been found to
significantly reduce the proliferation and migration of NSCLC
cells, possibly through toll-like receptor 4/myeloid
differentiation primary response protein MyD88-EGFR-mediated
inhibition of AP-1 protein and suppression of the EMT process
(51). A study by Li et al
(52) revealed that evodiamine can
downregulate the expression of total EGFR in human LoVo cells,
which may be the underlying mechanism of its inhibitory effects on
invasion and metastasis of CRC via the PI3K, AKT and mTOR
signalling pathways. The present study confirmed by western
blotting that the expression of EGFR decreased in a dose-dependent
manner. After a comprehensive analysis of the aforementioned
potential targets, it was concluded that EGFR is the most likely
target of FAN in the treatment of CRC.
To elucidate the molecular mechanism by which FAN
affects COAD, GO analysis and KEGG pathway enrichment analysis of
the 256 potential targets were performed. The KEGG pathway analysis
results primarily identified the PI3K/AKT signalling pathway, which
was consistent with previous reports on hepatocellular carcinoma
and gastric cancer (6,53). Abnormal activation of the PI3K/AKT
signalling pathway leads to an imbalance between cell proliferation
and apoptosis, resulting in tumorigenesis (54). In addition, terms in the ‘Biological
Process’ category ranked highest among the top 10 GO terms,
suggesting that FAN may exert its anticancer activity by regulating
different biological processes.
In a previous study, Wang et al (53) observed that FAN induced autophagy in
HepG2 and PLC/PRF/5 cells via AMPK signalling. Feng et al
(6) confirmed that FAN targets PI3K
and suppresses the PI3K/AKT signalling pathway in SGC7901 cells.
Shi et al (55) observed
that FAN inhibits the growth and metastasis of melanoma cells by
suppressing the focal adhesion kinase 1 pathway. These data
indicate that FAN is a non-specific drug. Compared with
single-target inhibitors, non-specific drugs have innate
advantages, such as greater anticancer effects and a significantly
reduced risk of multidrug resistance and serious side effects
(56). Moreover, with the
application of nanotherapy-based drug delivery systems, targeted
and sustained drug release can be achieved, the solubility and
bioavailability of drugs can be improved, and the side effects of
drugs can be reduced (57). When
combined with EGFR-targeted nanotherapy, plant-derived evodiamine
showed a stronger ability to inhibit the invasion and metastasis of
CRC than other monotherapy groups (51). Therefore, it is proposed that FAN
could potentially have useful clinical applications in the
future.
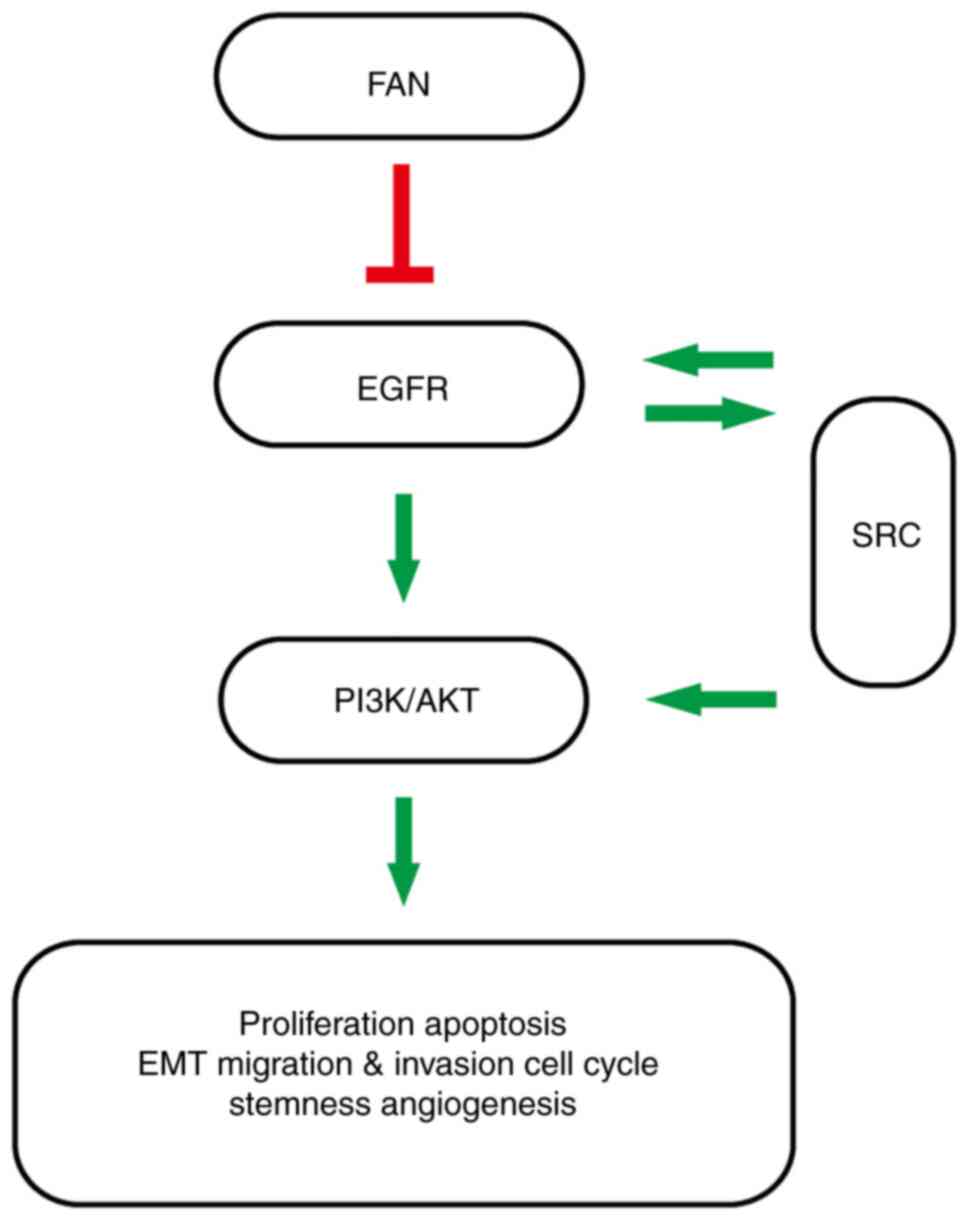
The present study confirmed that FAN has a strong
inhibitory effect on tumorigenesis in vitro and in
vivo. FAN can inhibit the proliferation and invasion of tumour
cells and induce apoptosis and cell cycle arrest through the
EGFR-PI3K/AKT signalling pathway (Fig.
6). In addition, SRC may be involved in the EGFR-SRC-PI3K/AKT
signalling pathway according to the network pharmacology assessment
and relevant literature reports; however, this finding needs
further experimental confirmation. Therefore, these results
suggested that FAN may be a potential anticancer strategy for
COAD.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present work was supported by the National
Natural Science Foundation of China (grant no. 81902468) and the
Innovation Project of Harbin Medical University (grant no.
YJSKYCX2018-37HYD).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FJ, YZ and DP conceived and designed the present
study. FJ and SR performed the experiments. FJ prepared and wrote
the manuscript. ZL and YC conducted the ultrasonic imaging
experiments. ZZ and YZ performed the pathology experiments. AZ was
involved in the analysis and interpretation of data. YZ revised the
manuscript for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal studies were approved by the Ethics
Committee of The First Affiliated Hospital of Harbin Medical
University (Harbin, China) and conducted in accordance with the
national regulations of China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
FAN
|
fangchinoline
|
|
EGFR
|
epidermal growth factor receptor
|
|
CRC
|
colorectal cancer
|
|
COAD
|
colon adenocarcinoma
|
|
NPCs
|
natural plant compounds
|
|
EMT
|
epithelial-mesenchymal transition
|
|
CDFI
|
colour Doppler flow imaging
|
|
CPA
|
colour power angiography
|
|
USE
|
ultrasonic elastosonography
|
|
SRC
|
proto-oncogene tyrosine-protein kinase
Src
|
|
ALB
|
albumin
|
References
|
1
|
Banerjee A, Pathak S, Subramanium VD, G D,
Murugesan R and Verma RS: Strategies for targeted drug delivery in
treatment of colon cancer: Current trends and future perspectives.
Drug Discov Today. 22:1224–1232. 2017. View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar
|
|
3
|
Iqbal A and George TJ: Randomized clinical
trials in colon and rectal cancer. Surg Oncol Clin N Am.
26:689–704. 2017. View Article : Google Scholar
|
|
4
|
Avila-Carrasco L, Majano P, Sánchez-Toméro
JA, Selgas R, López-Cabrera M, Aguilera A and González Mateo G:
Natural plants compounds as modulators of epithelial-to-mesenchymal
transition. Front Pharmacol. 10:7152019. View Article : Google Scholar
|
|
5
|
Wang Y, Chen J, Wang L, Huang Y, Leng Y
and Wang G: Fangchinoline induces G0/G1 arrest by modulating the
expression of CDKN1A and CCND2 in K562 human chronic myelogenous
leukemia cells. Exp Ther Med. 5:1105–1112. 2013. View Article : Google Scholar
|
|
6
|
Feng T, Ding D and Li DD: Fangchinoline
targets PI3K and suppresses PI3K/AKT signaling pathway in SGC7901
cells. Int J Oncol. 46:2355–2363. 2015. View Article : Google Scholar
|
|
7
|
Wu WK, Wang XJ, Cheng AS, Luo MX, Ng SS,
To KF, Chan FK, Cho CH, Sung JJ and Yu J: Dysregulation and
crosstalk of cellular signaling pathways in colon carcinogenesis.
Crit Rev Oncol Hematol. 86:251–277. 2013. View Article : Google Scholar
|
|
8
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar
|
|
9
|
Barr S, Thomson S, Buck E, Russo S, Petti
F, Sujka-Kwok I, Eyzaguirre A, Rosenfeld-Franklin M, Gibson NW,
Miglarese M, et al: Bypassing cellular EGF receptor dependence
through epithelial-to-mesenchymal-like transitions. Clin Exp
Metastasis. 25:685–693. 2008. View Article : Google Scholar
|
|
10
|
Kim N, Cho D, Kim H, Kim S, Cha YJ,
Greulich H, Bass A, Cho HS and Cho J: Colorectal
adenocarcinoma-derived EGFR mutants are oncogenic and sensitive to
EGFR-targeted monoclonal antibodies, cetuximab and panitumumab. Int
J Cancer. 146:2194–2200. 2020. View Article : Google Scholar
|
|
11
|
Padfield E, Ellis HP and Kurian KM:
Current therapeutic advances targeting EGFR and EGFRvIII in
glioblastoma. Front Oncol. 5:52015. View Article : Google Scholar
|
|
12
|
Li Z, Chen Y, An T, Liu P, Zhu J, Yang H,
Zhang W, Dong T, Jiang J, Zhang Y, et al: Nuciferine inhibits the
progression of glioblastoma by suppressing the SOX2-AKT/STAT3-Slug
signaling pathway. J Exp Clin Cancer Res. 38:1392019. View Article : Google Scholar
|
|
13
|
Vermeulen K, Berneman ZN and Van
Bockstaele DR: Cell cycle and apoptosis. Cell Prolif. 36:165–175.
2003. View Article : Google Scholar
|
|
14
|
Russo A, Terrasi M, Agnese V, Santini D
and Bazan V: Apoptosis: A relevant tool for anticancer therapy. Ann
Oncol. 17 (Suppl 7):vii115–vii123. 2006. View Article : Google Scholar
|
|
15
|
Qian L, Liu Y, Xu Y, Ji W, Wu Q, Liu Y,
Gao Q and Su C: Matrine derivative WM130 inhibits hepatocellular
carcinoma by suppressing EGFR/ERK/MMP-2 and PTEN/AKT signaling
pathways. Cancer Lett. 368:126–134. 2015. View Article : Google Scholar
|
|
16
|
Sak K: Cytotoxicity of dietary flavonoids
on different human cancer types. Pharmacogn Rev. 8:122–146. 2014.
View Article : Google Scholar
|
|
17
|
Boldbaatar A, Lee S, Han S, Jeong AL, Ka
HI, Buyanravjikh S, Lee JH, Lim JS, Lee MS and Yang Y: Eupatolide
inhibits the TGF-β1-induced migration of breast cancer cells via
downregulation of SMAD3 phosphorylation and transcriptional
repression of ALK5. Oncol Lett. 14:6031–6039. 2017.
|
|
18
|
Vuddanda PR, Chakraborty S and Singh S:
Berberine: A potential phytochemical with multispectrum therapeutic
activities. Expert Opin Invest Drugs. 9:1297–1307. 2010. View Article : Google Scholar
|
|
19
|
Hang Y, Qin X, Ren T and Cao J: Baicalin
reduces blood lipids and inflammation in patients with coronary
artery disease and rheumatoid arthritis: A randomized,
double-blind, placebo-controlled trial. Lipids Health Dis.
17:1462018. View Article : Google Scholar
|
|
20
|
Ferk F, Kundi M, Brath H, Szekeres T,
Al-Serori H, Mišík M, Saiko P, Marculescu R, Wagner KH and
Knasmueller S: Gallic acid improves health-associated biochemical
parameters and prevents oxidative damage of DNA in type 2 diabetes
patients: Results of a placebo-controlled pilot study. Mol Nutr
Food Res. 62:Jan 18–2018.(Epub ahead of print). doi:
10.1002/mnfr.201700482. View Article : Google Scholar
|
|
21
|
McCubrey JA, Lertpiriyapong K, Steelman
LS, Abrams SL, Yang LV, Murata RM, Rosalen PL, Scalisi A, Neri LM,
Cocco L, et al: Effects of resveratrol, curcumin, berberine and
other nutraceuticals on aging, cancer development, cancer stem
cells and microRNAs. Aging (Albany NY). 9:1477–1536. 2017.
View Article : Google Scholar
|
|
22
|
Kang M, Kim S and Ko J: Roles of CD133 in
microvesicle formation and oncoprotein trafficking in colon cancer.
FASEB J. 33:4248–4260. 2019. View Article : Google Scholar
|
|
23
|
Zhang SS, Han ZP, Jing YY, Tao SF, Li TJ,
Wang H, Wang Y, Li R, Yang Y, Zhao X, et al: CD133(+)CXCR4(+) colon
cancer cells exhibit metastatic potential and predict poor
prognosis of patients. BMC Med. 10:852012. View Article : Google Scholar
|
|
24
|
Lu P, Weaver VM and Werb Z: The
extracellular matrix: A dynamic niche in cancer progression. J Cell
Biol. 196:395–406. 2012. View Article : Google Scholar
|
|
25
|
Zucker S and Vacirca J: Role of matrix
metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis
Rev. 23:101–117. 2004. View Article : Google Scholar
|
|
26
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View Article : Google Scholar
|
|
27
|
Han YT, Chen XH, Gao H, Ye JL and Wang CB:
Physcion inhibits the metastatic potential of human colorectal
cancer SW620 cells in vitro by suppressing the transcription factor
SOX2. Acta Pharmacol Sin. 37:264–275. 2016. View Article : Google Scholar
|
|
28
|
Beyes S, Andrieux G, Schrempp M, Aicher D,
Wenzel J, Antón-García P, Boerries M and Hecht A: Genome-wide
mapping of DNA-binding sites identifies stemness-related genes as
directly repressed targets of SNAIL1 in colorectal cancer cells.
Oncogene. 38:6647–6661. 2019. View Article : Google Scholar
|
|
29
|
Chen T, You Y, Jiang H and Wang ZZ:
Epithelial-mesenchymal transition (EMT): A biological process in
the development, stem cell differentiation, and tumorigenesis. J
Cell Physiol. 232:3261–3272. 2017. View Article : Google Scholar
|
|
30
|
Zhao GX, Xu YY, Weng SQ, Zhang S, Chen Y,
Shen XZ, Dong L and Chen S: CAPS1 promotes colorectal cancer
metastasis via Snail mediated epithelial mesenchymal
transformation. Oncogene. 38:4574–4589. 2019. View Article : Google Scholar
|
|
31
|
Kahlert C, Lahes S, Radhakrishnan P, Dutta
S, Mogler C, Herpel E, Brand K, Steinert G, Schneider M,
Mollenhauer M, et al: Overexpression of ZEB2 at the invasion front
of colorectal cancer is an independent prognostic marker and
regulates tumor invasion in vitro. Clin Cancer Res. 17:7654–7663.
2011. View Article : Google Scholar
|
|
32
|
Li MZ, Wang JJ, Yang SB, Li WF, Xiao LB,
He YL and Song XM: ZEB2 promotes tumor metastasis and correlates
with poor prognosis of human colorectal cancer. Am J Transl Res.
9:2838–2851. 2017.
|
|
33
|
Wu K, Wei P, Liu M, Liang X and Su M: To
reveal pharmacological targets and molecular mechanisms of curcumol
against interstitial cystitis. J Adv Res. 20:43–50. 2019.
View Article : Google Scholar
|
|
34
|
Cheng F, Kovacs IA and Barabasi AL:
Network-based prediction of drug combinations. Nat Commun.
10:11972019. View Article : Google Scholar
|
|
35
|
Wang Y, Wang S and Huang M: Structure and
enzymatic activities of human serum albumin. Curr Pharm Des.
21:1831–1836. 2015. View Article : Google Scholar
|
|
36
|
Bauer J and Capra S: Comparison of a
malnutrition screening tool with subjective global assessment in
hospitalised patients with cancer-sensitivity and specificity. Asia
Pac J Clin Nutr. 12:257–260. 2003.
|
|
37
|
McMillan DC, Watson WS, O'Gorman P,
Preston T, Scott HR and McArdle CS: Albumin concentrations are
primarily determined by the body cell mass and the systemic
inflammatory response in cancer patients with weight loss. Nutr
Cancer. 39:210–213. 2001. View Article : Google Scholar
|
|
38
|
Yamamoto M, Saito H, Uejima C, Tanio A,
Tada Y, Matsunaga T, Sakamoto T, Honjo S, Ashida K and Fujiwara Y:
Combination of serum albumin and cholinesterase levels as
prognostic indicator in patients Ith colorectal cancer. Anticancer
Res. 39:1085–1090. 2019. View Article : Google Scholar
|
|
39
|
Pal I and Mandal M: PI3K and Akt as
molecular targets for cancer therapy: Current clinical outcomes.
Acta Pharmacol Sin. 33:1441–1458. 2012. View Article : Google Scholar
|
|
40
|
Lossi L, Castagna C and Merighi A:
Caspase-3 mediated cell death in the normal development of the
mammalian cerebellum. Int J Mol Sci. 19:39992018. View Article : Google Scholar
|
|
41
|
Chen H, Yang X, Feng Z, Tang R, Ren F, Wei
K and Chen G: Prognostic value of caspase-3 expression in cancers
of digestive tract: A meta-analysis and systematic review. Int J
Clin Exp Med. 8:10225–10234. 2015.
|
|
42
|
Lieu C and Kopetz S: The SRC family of
protein tyrosine kinases: A new and promising target for colorectal
cancer therapy. Clin Colorectal Cancer. 9:89–94. 2010. View Article : Google Scholar
|
|
43
|
Parkin A, Man J, Timpson P and Pajic M:
Targeting the complexity of Src signalling in the tumour
microenvironment of pancreatic cancer: From mechanism to therapy.
FEBS J. 286:3510–3539. 2019. View Article : Google Scholar
|
|
44
|
Kopetz S: Targeting SRC and epidermal
growth factor receptor in colorectal cancer: Rationale and progress
into the clinic. Gastrointest Cancer Res. 1 (Suppl 2):S37–S41.
2007.
|
|
45
|
Maa MC, Leu TH, McCarley DJ, Schatzman RC
and Parsons SJ: Potentiation of epidermal growth factor
receptor-mediated oncogenesis by c-Src: Implications for the
etiology of multiple human cancers. Proc Natl Acad Sci USA.
92:6981–6985. 1995. View Article : Google Scholar
|
|
46
|
Poole EM, Curtin K, Hsu L, Kulmacz RJ,
Duggan DJ, Makar KW, Xiao L, Carlson CS, Slattery ML, Caan BJ, et
al: Genetic variability in EGFR, Src and HER2 and risk of
colorectal adenoma and cancer. Int J Mol Epidemiol Genet.
2:300–315. 2011.
|
|
47
|
Chen J, Elfiky A, Han M, Chen C and Saif
MW: The role of Src in colon cancer and its therapeutic
implications. Clin Colorectal Cancer. 13:5–13. 2014. View Article : Google Scholar
|
|
48
|
Lin L, Cheng K, He Z, Lin Q, Huang Y, Chen
C, Xie Z, Chen L and Liang Z: A polysaccharide from hedyotis
diffusa interrupts metastatic potential of lung adenocarcinoma A549
cells by inhibiting EMT via EGFR/Akt/ERK signaling pathways. Int J
Biol Macromol. 129:706–714. 2019. View Article : Google Scholar
|
|
49
|
Geethadevi A, Parashar D, Bishop E,
Pradeep S and Chaluvally-Raghavan P: ERBB signaling in CTCs of
ovarian cancer and glioblastoma. Genes Cancer. 8:746–751. 2017.
View Article : Google Scholar
|
|
50
|
Sigismund S, Avanzato D and Lanzetti L:
Emerging functions of the EGFR in cancer. Mol Oncol. 12:3–20. 2018.
View Article : Google Scholar
|
|
51
|
Zhang L, Tao X, Fu Q, Ge C, Li R, Li Z,
Zhu Y, Tian H, Li Q, Liu M, et al: Curcumin inhibits cell
proliferation and migration in NSCLC through a synergistic effect
on the TLR4/MyD88 and EGFR pathways. Oncol Rep. 42:1843–1855.
2019.
|
|
52
|
Li C, Cai G, Song D, Gao R, Teng P, Zhou
L, Ji Q, Sui H, Cai J, Li Q and Wang Y: Development of
EGFR-targeted evodiamine nanoparticles for the treatment of
colorectal cancer. Biomater Sci. 7:3627–3639. 2019. View Article : Google Scholar
|
|
53
|
Wang N, Pan W, Zhu M, Zhang M, Hao X,
Liang G and Feng Y: Fangchinoline induces autophagic cell death via
p53/sestrin2/AMPK signalling in human hepatocellular carcinoma
cells. Br J Pharmacol. 164:731–742. 2011. View Article : Google Scholar
|
|
54
|
Suman S, Kurisetty V, Das TP, Vadodkar A,
Ramos G, Lakshmanaswamy R and Damodaran C: Activation of AKT
signaling promotes epithelial-mesenchymal transition and tumor
growth in colorectal cancer cells. Mol Carcinog. 53 (Suppl
1):E151–E160. 2014. View Article : Google Scholar
|
|
55
|
Shi J, Guo B, Hui Q, Chang P and Tao K:
Fangchinoline suppresses growth and metastasis of melanoma cells by
inhibiting the phosphorylation of FAK. Oncol Rep. 38:63–70. 2017.
View Article : Google Scholar
|
|
56
|
Xia J, Chen J, Zhang Z, Song P, Tang W and
Kokudo N: A map describing the association between effective
components of traditional Chinese medicine and signaling pathways
in cancer cells in vitro and in vivo. Drug Discov Ther. 8:139–153.
2014. View Article : Google Scholar
|
|
57
|
Bahrami B, Hojjat-Farsang M, Mohammadi H,
Anvari E, Ghalamfarsa G, Yousefi M and Jadidi-Niaragh F:
Nanoparticles and targeted drug delivery in cancer therapy. Immunol
Lett. 190:64–83. 2017. View Article : Google Scholar
|