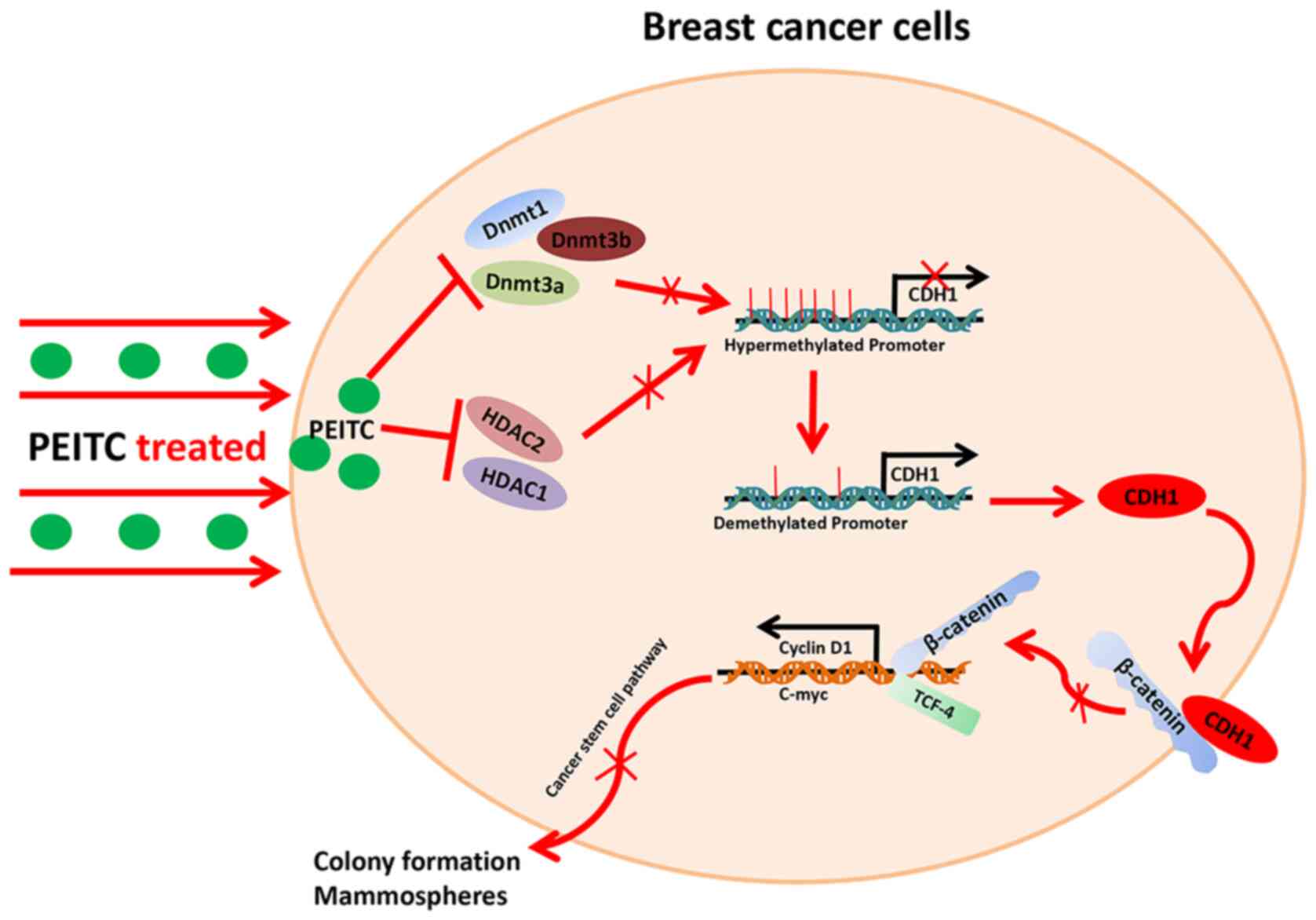
Introduction
Breast cancer is the most frequently diagnosed
malignancy and leading cause of cancer-related deaths among women
on a global scale. Data from the International Agency for Research
on Cancer (IARC), a World Health Organization agency, indicated
that in 2018 there were more than 2,088,849 new cases of breast
cancer and approximately 626,679 breast cancer-related deaths in
women (1). Therefore, breast cancer
is a global health issue of women that must be addressed. There are
five primary treatment options, most of which include a combination
of: surgery, radiation, chemotherapy, hormone therapy and targeted
therapies (2). Despite recent
advances in breast cancer treatments, a subgroup of breast cancer
cells, termed breast cancer stem cells (BCSCs), often cause tumor
recurrence and metastasis in 30–40% of early-stage breast cancer
patients following treatment (3).
The BCSC characteristics of heterogeneity, self-renewal, and
pluripotency lead to malignant progression, treatment resistance
and a poor clinical prognosis (4).
Recent studies have revealed that several signaling pathways for
maintaining self-renewal and differentiation such as Wnt, Notch and
Hedgehog are excessively activated in BCSCs (5). Additionally, multidrug resistance and
DNA repair genes including multidrug resistance-1 (MDR1) and Rad 51
are overexpressed in BCSCs, conferring resistance to conventional
chemotherapeutic drugs and radiotherapies (6,7).
Moreover, BCSCs can promote angiogenesis and grow in an
anchorage-independent manner that contributes to cancer
dissemination and secondary tumors (8). These key activities of BCSCs in breast
carcinogenesis suggest that devising novel methods to eradicate
BCSCs may improve the poor prognosis of recurrent or metastatic
breast cancer patients.
Phenethyl isothiocyanate (PEITC), one of the major
bioactive compounds derived from cruciferous vegetables such as
broccoli, watercress and Brussels sprouts, possesses marked
anticancer activities by inhibiting cell cycle progression,
inducing apoptosis and reversing drug resistance (9). Recent studies have revealed that PEITC
is selectively lethal for malignant cells with reduced cytotoxicity
for normal cells (10–12), suggesting that it has potential as a
‘high-efficiency and lower toxicity’ chemotherapy drug. At present,
clinical trials are evaluating PEITC including ‘The Safety and
Efficacy Test of Nutri-PEITC Jelly in Head and Neck Cancer
Patients’ (NCT03034603) and ‘Phenethyl isothiocyanate in Preventing
Lung Cancer in Smokers’ (NCT00691132). Moreover, a completed phase
I study (NCICN-55120) has revealed PEITC antitumor activity at
micromolar concentrations, further indicating its promise for
clinical use (13). In recent
years, epigenetic silencing of tumor suppressor genes during the
initiation and progression of cancers, including breast cancer, has
been established (14, 15). Emerging evidence further indicates that
epigenetic regulation, including DNA hypermethylation, plays a
pivotal role in promoting cancer stem cell characteristics
(16,17). As such, DNA hypermethylation and
histone deacetylation have been hypothesized to be effective
targets for cancer treatments, including the eradication of cancer
stem cells (CSCs) (18). PEITC is a
novel epigenetic regulator that inhibits both DNA
methyltransferases (DNMTs) and histone deacetylases (HDACs), both
of which mediate the silencing of tumor suppressor genes during
tumor progression (19,20). However, no study to date has
explored the mechanism by which PEITC epigenetically reactivates
tumor suppressor genes to eradicate BCSCs.
The cadherin 1 (CDH1) gene is located at chromosome
16q22.1 and encodes a transmembrane glycoprotein called E-cadherin,
which functions as a tumor suppressor by maintaining cell adhesion
and adherent junctions in normal tissues (21). The expression of CDH1 is frequently
observed to be silenced in solid malignant tumors, including breast
cancer, due to DNA hypermethylation of the promoter region during
tumor initiation and progression (16, 22). Previous studies have
revealed that a loss of CDH1 expression in breast cancer can
initiate the epithelial-mesenchymal transition (EMT) and is
associated with metastasis and poor prognosis (23,24).
Recent studies have also reported that demethylation of the CDH1
promoter region to restore CDH1 expression inactivates the
Wnt/β-catenin pathway and suppresses carcinoma cell stemness
(16), indicating another potential
way to eradicate BCSCs.
In summary, PEITC functions as an epigenetic
regulator and CDH1 is silenced by DNA hypermethylation.
Additionally, following histone deacetylation in breast cancer, the
Wnt/β-catenin pathway is activated to maintain cancer stem
cell-like properties. The purpose of the present study was to
examine the effects of PEITC on the CSC-like properties of breast
cancer cells and elucidate the mechanisms.
Materials and methods
Cell culture and drugs
Human breast cancer cell lines MCF-7 and MDA-MB-231
were obtained from the American Type Culture Collection. These cell
lines were cultured at 37°C under a 5% CO2 atmosphere in
Dulbeccos modified Eagles medium (DMEM) supplemented with 10% fetal
bovine serum and propagated for <8 generations after
resuscitation. Reagents, including PEITC, Aza-deoxycytidine
(5-Aza), and Trichostatin A (TSA) were purchased from Sigma-Aldrich
(Merck KGaA).
MTT assay
Three thousand viable breast cancer cells (MCF-7 and
MDA-MB-231) were plated onto a 96-well plate (Corning, Inc.) in
triplicate for 24 h, and then treated with various concentrations
of PEITC (0, 1, 2, 5, 10, 25, 50 and 100 µM) dissolved in complete
medium and cultured at 37°C under a 5% CO2 atmosphere
for 72 h. Cells were then incubated with 100 µl of
3-(4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) (MTT)
solution (0.5 mg/ml) for 4 h at 37°C in the dark. The MTT solution
was then discarded and the formazan was solubilized in 100 µl DMSO
for 1 h on a shaker. The optical density was measured at 570 nm
using SpectraMax M Series Multi-Mode Microplate Reader (Molecular
Devices, LLC).
Colony formation assay
Three hundred viable breast cancer cells (MCF-7 and
MDA-MB-231) were inoculated into a 6-well plate (Corning, Inc.) in
triplicate for 24 h, then incubated with 0.1% DMSO (control) or
PEITC (5 or 10 µM) and dissolved in complete medium cultured at
37°C under a 5% CO2 atmosphere for 10 days. The colonies
were fixed with a fixation solution (acetic acid:methanol, 1:7) for
5 min and stained with 0.5% crystal violet for 20 min at room
temperature. The stained colonies were washed 3 times with PBS and
counted with a countermark pen. Colonies with a minimum cell number
>50 observed under an inverted phase-contrast microscope (Nikon
Corporation) were included in the final statistical analysis. The
images of the colonies presented were visualized using a Canon
scanner (CanoScan 5600F).
Sphere formation assay
Breast cancer Cells (MCF-7 and MDA-MB-231) were
seeded in 6-well ultra-low cluster plates (Corning, Inc.) at a
density of 5×103 cells and cultured at 37°C under a 5%
CO2 atmosphere in CSC enrichment medium including
DMEM/F12 serum-free medium, 2% B27, 20 ng/ml epidermal growth
factor (EGF), and 20 ng/ml recombinant human fibroblast basic
growth factor (RH-bFGF) (all from Gibco; Thermo Fisher Scientific,
Inc.). Cells were treated with either PEITC at 5 or 10 µM or 0.1%
DMSO as the vehicle control. After 7 days, the number of tumor
spheres (≥50 µm) was imaged and counted under an inverted
phase-contrast microscope (Nikon Corporation) at a magnification of
×100.
Methylation-specific PCR (MSP)
Breast cancer cells (MCF-7 and MDA-MB-231) were
seeded in 6-well plates (Corning, Inc.) at a density of
2×105 cells in triplicate for 24 h, then incubated with
0.1% DMSO (control) or PEITC (5 or 10 µM) or positive control
epigenetic-regulating drugs (2.5 µM 5-Aza and 0.5 µM TSA) for 5
days at 37°C under a 5% CO2 atmosphere. Genomic DNA was
isolated from treated cells using the QIAamp DNA Mini Kit (Qiagen,
Inc.). Next, 1 µg of genomic DNA was denatured by bisulfite
conversion with the EZ DNA Methylation Gold Kit (Zymo Research
Corp.) following the manufacturer›s instructions and then used as a
template for PCR amplification. The primer pairs used for
methylated CDH1 were: forward, 5′-TAACTACAACCAAATAAACCCCG-3′ and
reverse, 5′-TCGAATTTAGTGGAATTAGAATCGT-3′. The primer pairs used for
unmethylated E-cadherin were: forward,
5′-TAACTACAACCAAATAAACCCCAAA-3′ and reverse,
5′-TTGAATTTAGTGGAATTAGAATTGT-3′. The PCR cycling conditions
consisted of an initial denaturation at 95°C for 2 min, followed by
30 cycles of denaturation at 95°C for 1 min, annealing at 50°C for
1 min, and extension at 74°C for 2 min. The amplification products
were separated on a 1.5% agarose gel by electrophoresis and
visualized using ethidium bromide staining and a Gel Documentation
2000 system (Bio-Rad Laboratories, Inc.). The band densities were
quantified using ImageJ software (version 1.52; National Institutes
of Health).
Western blot analysis
Cells were lysed in ice-cold RIPA lysis buffer (50
mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol,
1% Triton-X-100 and 0.1% sodium deoxycholate sulfate) supplemented
with phosphatase inhibitor (Na3Vo4, 1 mM) and
protease inhibitor (phenylmethylsulfonyl fluoride, 1 mM). The cell
lysate was then centrifuged at 12,000 × g for 10 min at 4°C. The
Pierce™ Rapid Gold bicinchoninic acid (BCA) kit (Thermo Fisher
Scientific, Inc.) was used to measure protein concentrations.
Proteins (20–50 µg per lane) were then separated by electrophoresis
on an SDS-PAGE gel (6 or 12%) and transferred onto a Sequi-Blot™
PVDF membrane (Bio-Rad Laboratories, Inc.). Then, the membranes
were blocked by 5% milk in TBST (Tris base-0.1% Tween-20) at room
temperature for 2 h. Antibodies to c-Myc (D84C12; rabbit mAb;
product no. 5605), ALDH-1 (D9J7R; XP® rabbit mAb;
product no. 36671), Oct-4A (C30A3; rabbit mAb; product no. 2840),
Sox-2 (D6D9; XP® rabbit mAb; product no. 3579),
phospho-β-catenin (Ser675) (D2F1XP®; rabbit mAb; product
no. 4176), phospho-β-catenin (Ser33/37) (rabbit mAb; product no.
2009), cyclin D1 (E3P5S; XP® rabbit mAb; product no.
55506), and CDH1 (mouse mAb; product no. 14472) were obtained from
Cell Signaling Technology, Inc. and diluted 1:1,000 for binding
with target proteins overnight at 4°C. Antibodies against Dnmt1
(mouse mAb; cat. no. sc-514784), Dnmt3a (mouse mAb; cat. no.
sc-365769), Dnmt3b (mouse mAb; cat. no. sc-81252), HDAC1 (mouse
mAb; cat. no. sc-81598), and HDAC2 (mouse mAb; cat. no. sc-9959)
were purchased from Santa Cruz Biotechnology, Inc. and diluted
1:200 for binding with target proteins overnight at 4°C. β-actin
(mouse mAb; product no. A1978) and secondary anti-mouse (product
no. A2429) or anti-rabbit (product no. A3937) antibodies were
obtained from Sigma-Aldrich (Merck KGaA) and diluted 1:1,000 for
binding with target proteins for 2 h at room temperature. After
washing the membranes with TBST (Tris base-0.1% Tween-20) 3 times,
protein bands were imaged with enhanced chemiluminescence (Thermo
Fisher Scientific, Inc.) and an X-ray film system (Ece Scientific
Co., Inc). The band densities were quantified using ImageJ software
(version 1.52; National Institutes of Health).
Co-immunoprecipitation (Co-IP)
Cells were lysed for 30 min in ice-cold
immunoprecipitation (IP) lysis buffer for IP (40 mM HEPES, 0.3%
Chaps, 2 mM EDTA, 1 mM dithiothreitol, 10 mM pyrophosphate, 10 mM
β-glycerophosphate, and 50 mM NaF) supplemented with phosphatase
inhibitor (Na3Vo4, 1 mM) and protease
inhibitor (phenylmethylsulfonyl fluoride, 1 mM). The supernatant
was collected after centrifugation at 4°C at 13,000 × g for 30 min
and the total protein concentration was adjusted to 1 µg/μl.
Finally, 200 µg of total protein was used per IP reaction. The
dilution rate of the CDH1 antibody (mouse mAb; product no. 14472;
Cell Signaling Technology) was 1:50 per IP reaction. The protein G
agarose magnetic beads (Santa Cruz Biotechnology, Inc.) were
prepared according to the manufacturers instructions. The
bead-bound immune complexes were incubated with rotation for 4 h at
4°C and then the beads were pelleted using a magnetic separation
rack and washed with 500 µl of 1X cell lysis buffer five times
(keeping on ice between washes). The bead-bound immune complexes
were resuspended in 30 µl of 1X SDS-PAGE loading buffer and
incubated for 5 min on a heating block at 95°C to denature and
release proteins. Finally, the magnetic beads were removed using
the magnetic separator and the supernatant was transferred to a
clean 1.5 ml tube for western blotting.
RNA isolation and RT-qPCR
Total RNAs were isolated from MCF-7 and MDA-MB-231
cells using TRIzol Reagent (Thermo Fisher Scientific, Inc.) and the
concentration was measured by Nanodrop (BioSpec-nano; Shimadzu
Corporation). SuperScript™ III Reverse Transcriptase (Thermo Fisher
Scientific, Inc.) was used for reverse transcription and RT-qPCR
was performed with Platinum SYBR-Green qPCR SuperMix-UDG (Thermo
Fisher Scientific, Inc.) on an ABI PRISM® 7000. The
comparative Cq method (2−ΔΔCq) (25) was used to analyze the data and GAPDH
mRNA expression was used as the normalization control. The CDH1
primers were: forward, 5′-TGGGTTATTCCTCCCATCAG-3′ and reverse,
5′-GTCACCTTCAGCCATCCTGT-3′. The GAPDH primers were: forward,
5′-AGGTCGGAGTCAACGGATTTG-3′ and reverse,
5′-GTGATGGCATGGACTGTGGT-3′. The PCR cycling conditions consisted of
an initial denaturation at 94°C for 2 min, followed by 30 cycles of
denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and
extension at 72°C for 2 min.
Statistical analyses
GraphPad Prism 5 software (GraphPad Software, Inc.)
was used to conduct the statistical analyses. All data were
expressed as the mean ± standard deviation (SD) except where
indicated. One-way ANOVA with Dunnetts multiple comparison test or
two-tailed unpaired Students t-test was used to compare means
between groups as indicated. P<0.05 or a fold change >2 was
considered to indicate a statistically significant difference.
Results
Selection of PEITC concentrations
Typically, sublethal doses of a compound are used to
investigate epigenetic regulation properties in vitro
(26). We performed a preliminary
experiment to evaluate the effects of PEITC at 0, 1, 2, 5, 10, 25,
50 and 100 µM on the cell viability of MCF-7 and MDA-MB-231 cells
using an MTT assay. PEITC at 5 and 10 µM significantly inhibited
the growth of breast cancer cells after treatment for 72 h, but it
did not markedly induce cell death (data not shown). Thus, 5 and 10
µM PEITC were used to evaluate the epigenetic effects of PEITC on
breast cancer cells in vitro.
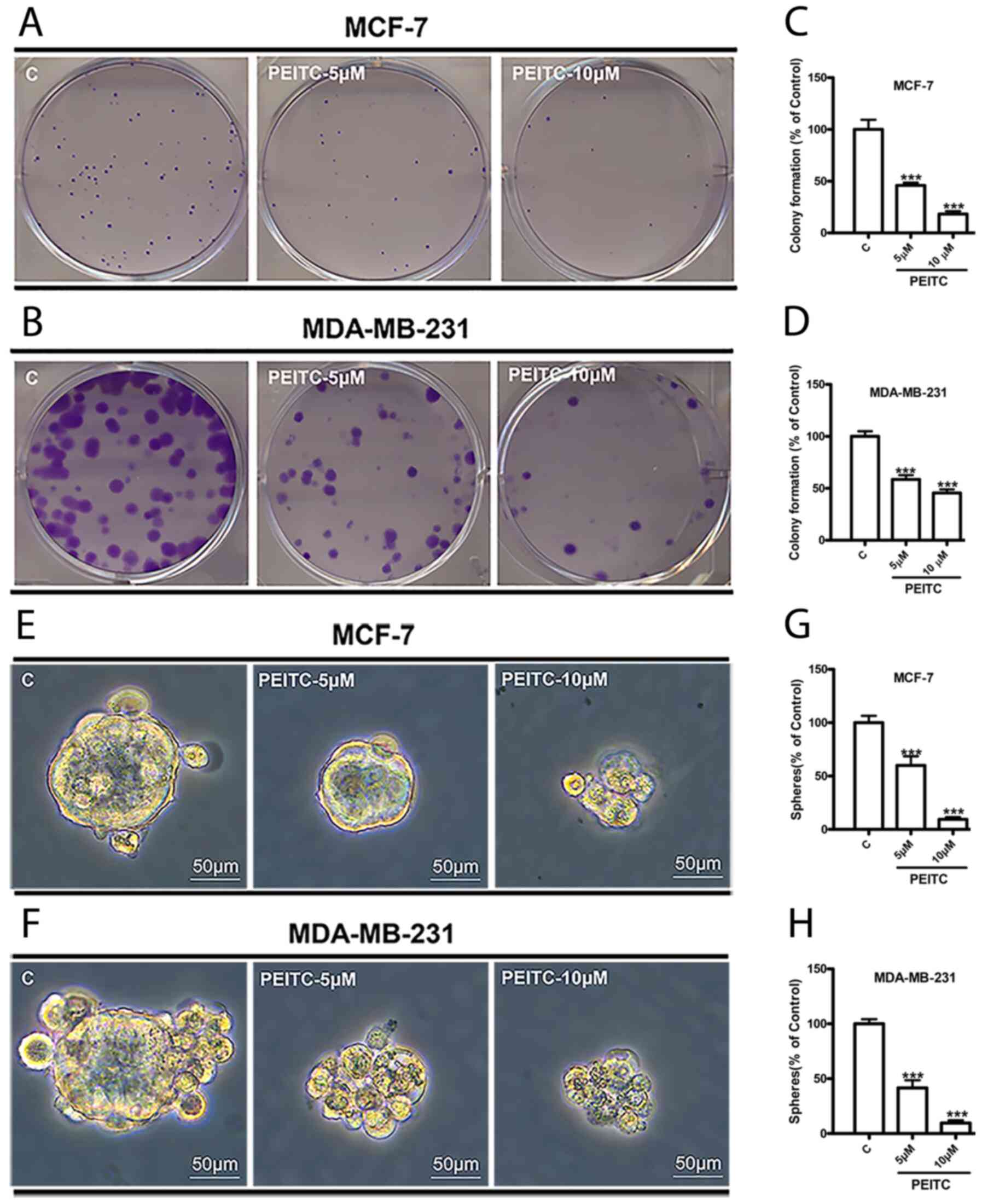
PEITC reduces colony and sphere
formation abilities
Colony and sphere formation assays are two methods
frequently used to identify cancer stem cells (CSCs) and study
their properties in vitro (27). To evaluate the effects of PEITC on
the colony formation ability of breast cancer cells, MCF-7 and
MDA-MB-231 cells were seeded at 300 cells/well in 6-well dishes.
Cells were treated with PEITC (5 and 10 µM) or 0.1% DMSO (control)
for 10 days. PEITC at 5 and 10 µM significantly reduced the number
of MCF-7 colonies to 45 and 20% (Fig.
1A and C) and MDA-MB-231 colonies to 60 and 45% (Fig. 1B and D), respectively. In the sphere
formation assay, MCF-7 and MDA-MB-231 cells were dissociated into
single cells, seeded into ultra-low cluster 6-well plates and
treated with PEITC (5 and 10 µM) or 0.1% DMSO (control) in CSC
enrichment medium. After seven days, it was observed that PEITC (5
and 10 µM) significantly decreased the size of MCF-7 and MDA-MB-231
mammospheres (Fig. 1E and F) and
reduced the number of mammospheres to 60 and 10% in MCF-7 cells
(Fig. 1G) and 40 and 10% in
MDA-MB-231 (Fig. 1H).
PEITC suppresses the expression of
BCSC-related proteins
Previous studies have identified that the protein
expression levels of c-Myc, ALDH-1, Oct-4A and Sox-2 are associated
with BCSC-like properties (28–31).
Since PEITC could reduce the colony and sphere formation abilities
of breast cancer cells, it was hypothesized that BCSC-related
proteins may also be downregulated. To test our hypothesis, western
blotting and quantitative densitometry with ImageJ were used to
observe the protein expression of c-Myc, ALDH-1, Oct-4A and Sox-2.
All targeted BCSC-related proteins were downregulated in both MCF-7
(Fig. 2A and C) and MDA-MB-231
cells (Fig. 2B and D) following
PEITC (5 and 10 µM) treatment for 3 days.
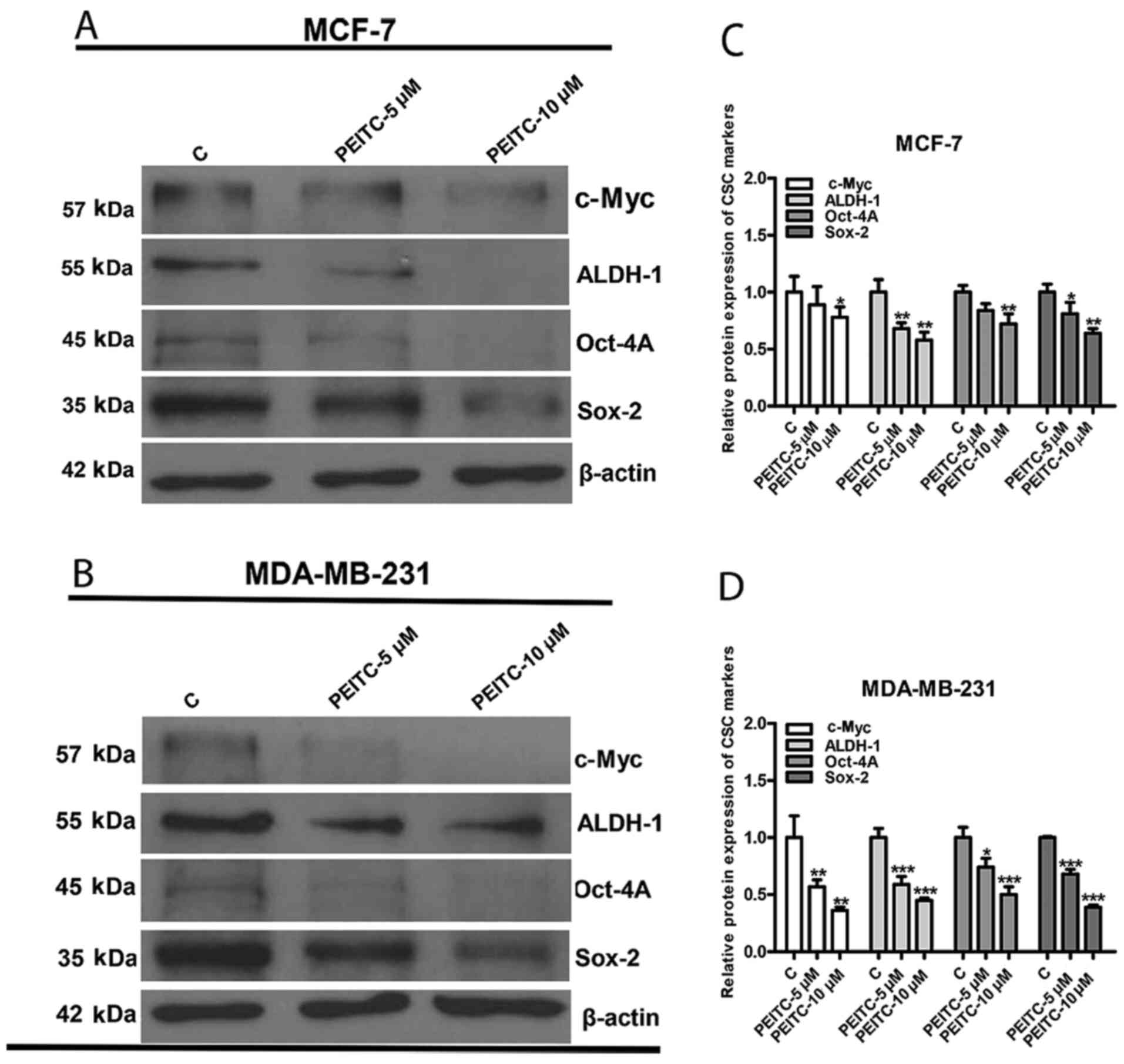 | Figure 2.Effect of PEITC on BCSC-associated
protein markers. c-Myc, ALDH-1, Oct-4A and Sox-2 are
BCSC-associated protein markers. Representative western blots
revealed the reduction in the protein expression of c-Myc, ALDH-1,
Oct-4A and Sox-2 by PEITC treatment in (A) MCF-7 and (B) MDA-MB-231
cells. Bar graphs of quantitative densitometry indicated the
downregulation of c-Myc, ALDH-1, Oct-4A, and Sox-2 in (C) MCF-7 and
(D) MDA-MB-231 cells. Data were presented as the mean ± SD, n=3.
*P<0.05, **P<0.01 and ***P<0.001 vs. the control. PEITC,
phenethyl isothiocyanate; BCSC, breast cancer stem cell; CSC,
cancer stem cell; C, control |
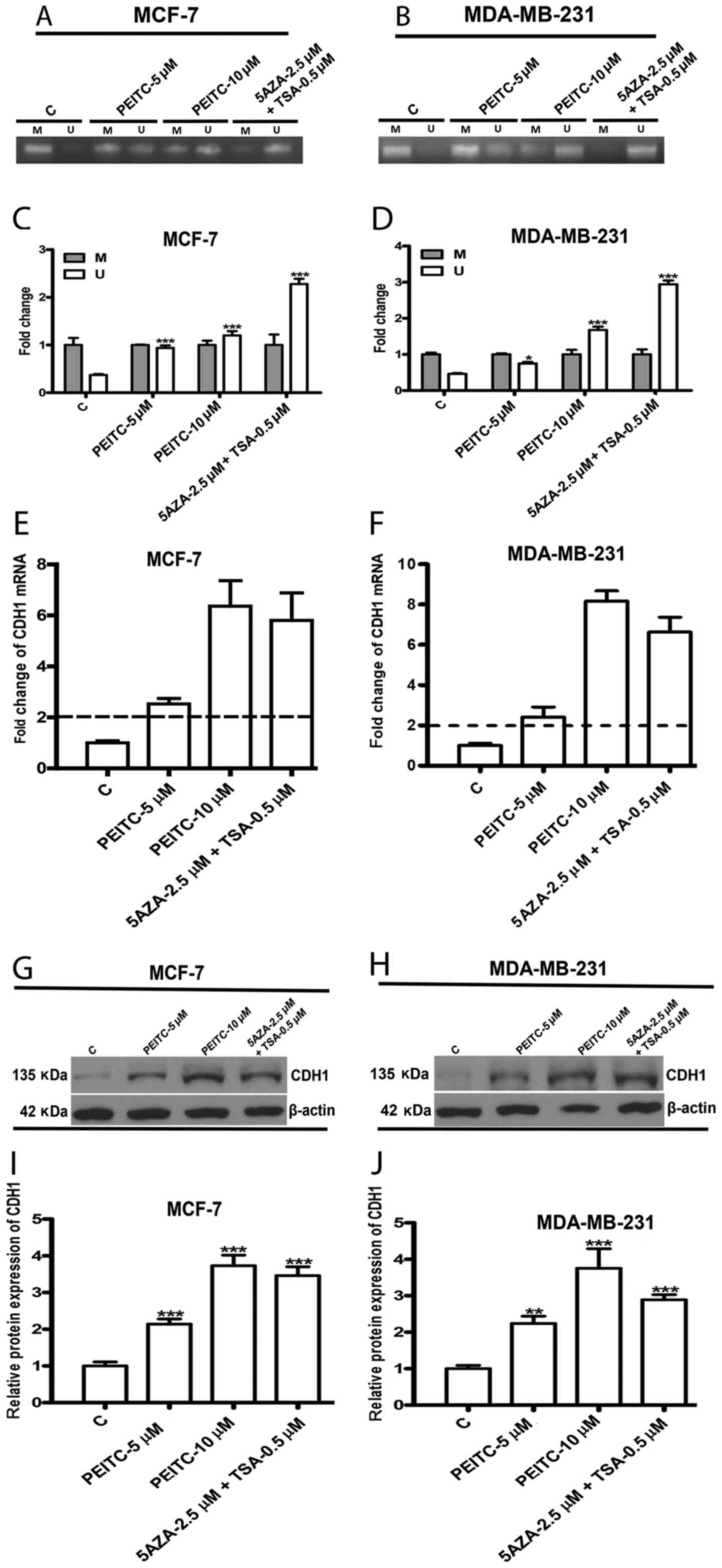
PEITC restores CDH1 expression by
demethylation of the promoter region
The hypermethylation of the CDH1 promoter region has
been identified in numerous malignant cancers including breast
cancer, leading to its encoded metastasis suppressor, E-cadherin,
being silenced along with cancer progression (32). Recent studies have revealed that
epigenetic silencing of the CDH1 gene contributes to CSC-like
properties (16). Moreover, PEITC
has been identified as a new epigenetic regulator that can
demethylate the hypermethylated promoter regions of tumor
suppressor genes to eliminate cancer cells (19). Therefore, it was hypothesized that
PEITC reduces BCSC-like properties via epigenetic reactivation of
CDH1. To test our hypothesis, we first examined the extent of
methylation of the CDH1 promoter region in the presence of either
0.1% DMSO (control) or PEITC (5 or 10 µM) or positive control
epigenetic regulating drugs (2.5 µM 5-Aza and 0.5 µM TSA) for 5
days. Methylation-specific PCR (MSP) and quantitative analysis
revealed high methylation levels of the CDH1 promoter region in
untreated breast cancer cells, suggesting hypermethylation of the
CDH1 promoter. However, a significant increase in the unmethylation
of the CDH1 promoter region was observed in PEITC (5 or 10 µM),
5-Aza (2.5 µM), and TSA (0.5 µM) MCF-7 (Fig. 3A and C) and MDA-MB-231 (Fig. 3B and D) treated-cells compared to
the control. The change in mRNA and protein levels of CDH1 in MCF-7
and MDA-MB-231 cells following treatment with either PEITC (5 or 10
µM) and 5-Aza (2.5 µM) and TSA (0.5 µM) for 5 days was further
analyzed using qPCR and western blotting, respectively. PEITC
significantly enhanced CDH1 mRNA expression in both MCF-7 (Fig. 3E) and MDA-MB-231 (Fig. 3F) cells. Similarly, CDH1 protein
levels were also significantly upregulated by PEITC in MCF-7
(Fig. 3G and I) and MDA-MB-231
(Fig. 3H and J) cells. These
results indicated that PEITC reactivated CDH1 by affecting
epigenetic regulation.
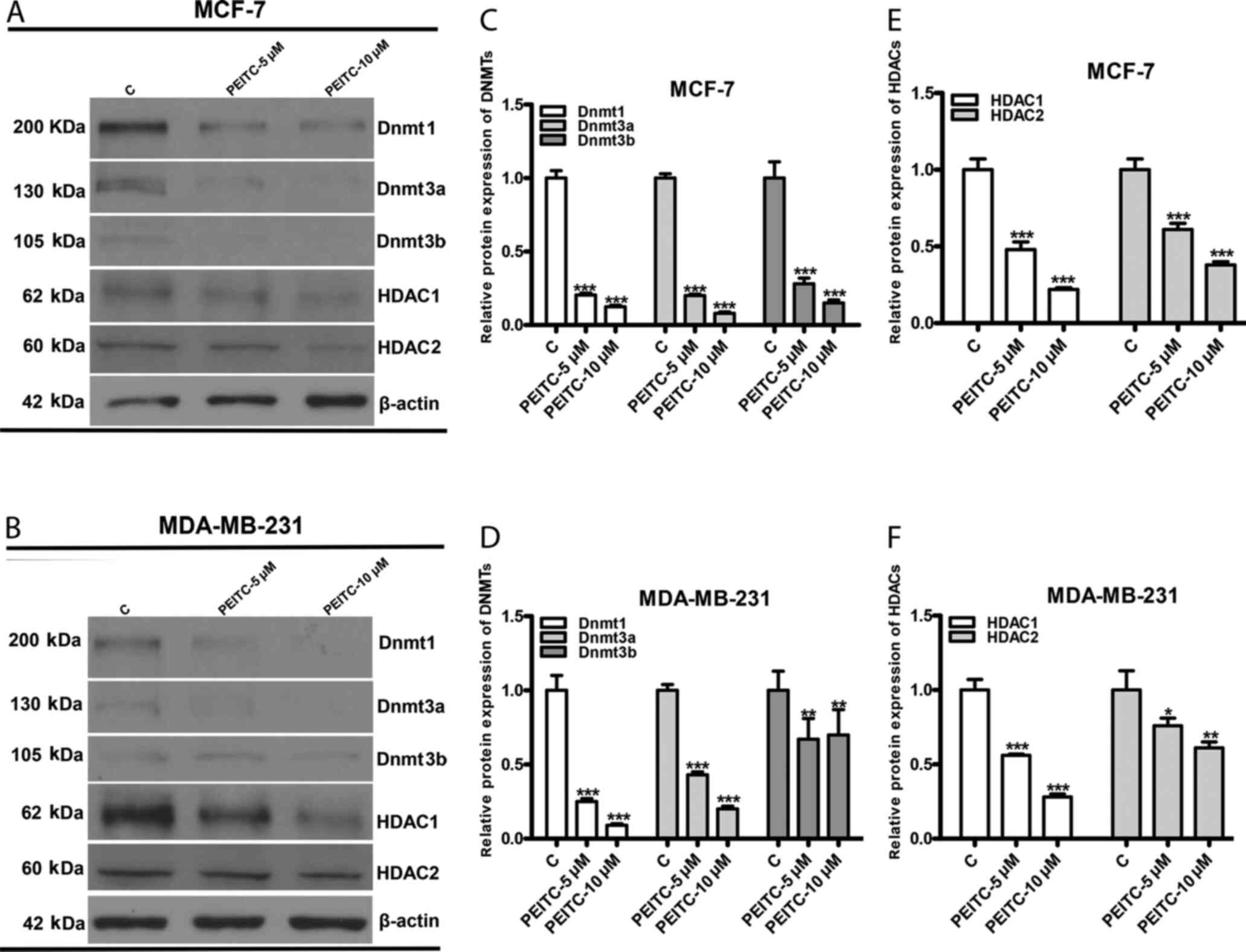
PEITC inhibits DNMTs and HDACs
DNMTs (Dnmt1, Dnmt3a, and Dnmt3b) and HDACs (HDAC1
and HDAC2) are enzymes that catalyze the addition of a methyl group
and removal of acetyl groups, respectively (19). These enzymes play crucial roles in
mediating the silencing of tumor suppressor genes with tumor
progression (33). To further
investigate whether PEITC restored CDH1 expression via epigenetic
regulation, the effects of PEITC on DNMTs and HDACs were evaluated
by western blotting and quantitative densitometry. PEITC (5 and 10
µM) significantly inhibited the protein expression of DNMTs (Dnmt1,
Dnmt3a and Dnmt3b) and HDACs (HDAC1 and HDAC2) in both MCF-7
(Fig. 4A, C and E) and MDA-MB-231
(Fig. 4B, D and F) cells.
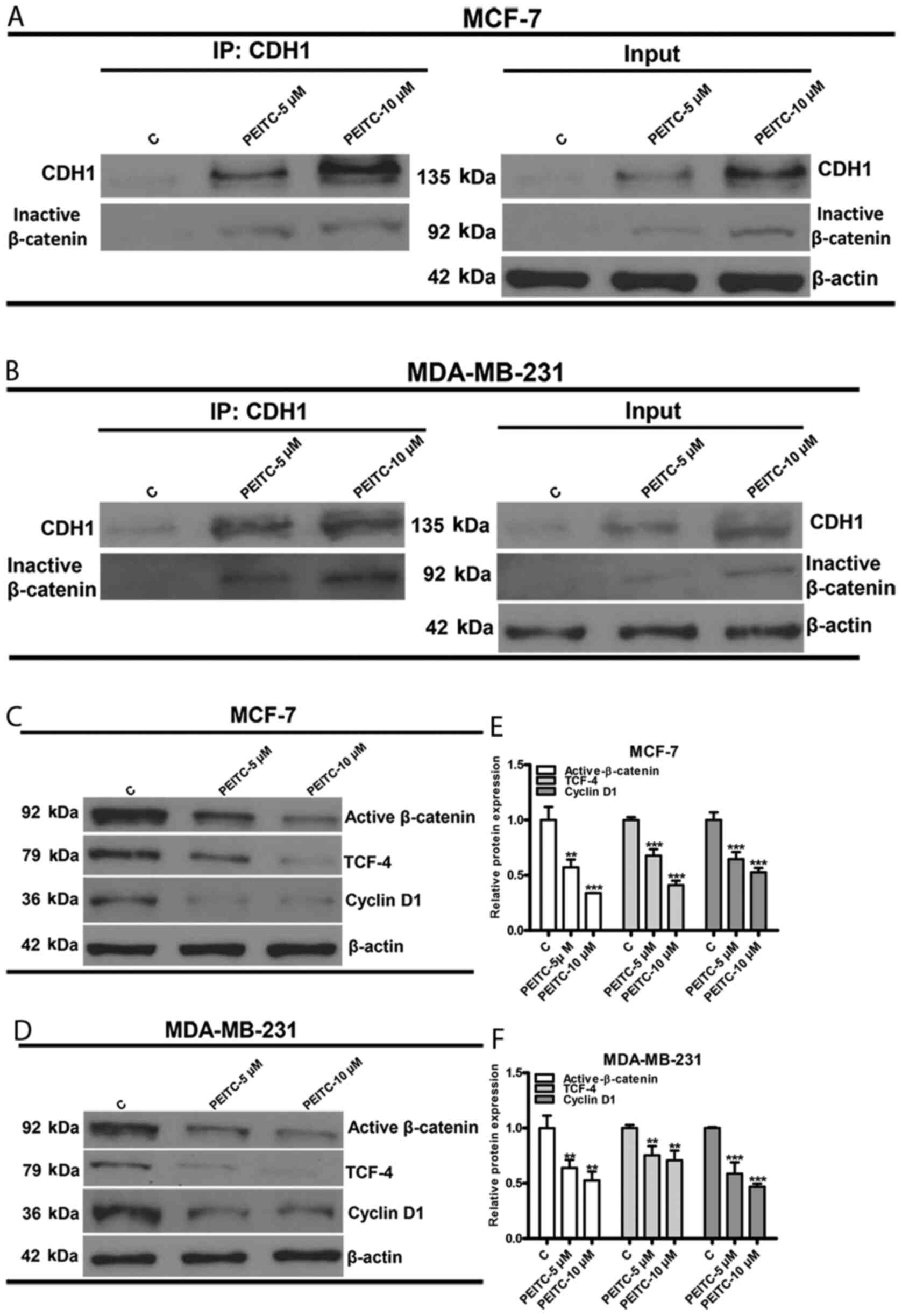
PEITC-reactivated CDH1 inhibits the
Wnt/β-catenin signaling pathway
Based on the epigenetic reactivation of CDH1 by
PEITC, the underlying mechanism of CDH1 regulation that reduces
BCSC-like properties was further explored. It is well-documented
that CDH1 affects the Wnt/β-catenin signaling pathway by binding to
β-catenin at the cytoplasmic membrane. This prevents β-catenin
translocation into the nuclei and formation of the β-catenin/TCF
transcription factor complex (16).
Previous research has also revealed that the Wnt/β-catenin
signaling pathway plays a pivotal role in maintaining BCSC-like
properties (27). Thus, it was
speculated whether PEITC-reactivated CDH1 would disrupt
intracellular Wnt/β-catenin signaling in breast cancer cells. To
address this question, MCF-7 and MDA-MB-231 cells were treated with
PEITC (0, 5 and 10 µM) for 3 days and then Co-IP was performed to
detect the ability of CDH1 and inactive β-catenin
(phospho-Ser33/37) to interact. PEITC increased the amount of
inactive β-catenin that co-precipitated with CDH1 (Fig. 5A and B). Next the effects of PEITC
on Wnt/β-catenin signaling in MCF-7 and MDA-MB-231 cells were
detected by western blotting and quantitative densitometry. Active
β-catenin (phosphor-Ser675) induced β-catenin accumulation in the
nucleus and promoted β-catenin/TCF4 transcription activity. Cyclin
D1 is a well-established downstream gene of the Wnt/β-catenin
pathway (34). Protein levels of
active β-catenin, TCF-4 and cyclin D1 were significantly decreased
in MCF-7 (Fig. 5C and E) and
MDA-MB-231 (Fig. 5D and F) cells
following PEITC (0, 5 and 10 µM) treatment for 3 days.
Collectively, the data indicated that PEITC reactivated CDH1, which
inhibited the Wnt/β-catenin signaling pathway.
Discussion
Breast cancer is the most frequently diagnosed
malignancy and leading cause of cancer-related deaths in women
worldwide. BCSCs are considered the origin of heterogeneous breast
cancer cells and are attractive prospects for therapy (27). Previous studies have identified that
PEITC, a novel epigenetic regulator, exerts antitumor effects on
malignant cells (9,19). Previous studies have also revealed
that CDH1, a tumor suppressor frequently silenced by promoter
hypermethylation in solid tumors including breast cancer, plays a
crucial role in reversing CSC properties (16,23).
It has also been demonstrated that PEITC has the potential to
eradicate CSCs in cervical, colorectal and ovarian cancer (13,35,36) as
well as breast cancer cells including the aggressive subtype such
as triple negative breast cancer cells (37,38).
However, to the best of our knowledge, no study to date, has shown
the epigenetic effects of PEITC on CDH1 in BCSCs. In the present
study, it was demonstrated for the first time that PEITC reduced
BCSC-like properties via epigenetic reactivation of CDH1, thus
inhibiting the Wnt/β-catenin pathway (Fig. 6).
Accumulating studies have demonstrated that tumors
are composed of a hodgepodge of cancer cells with diverse functions
and phenotypes. As a remarkably small proportion of cancer cells,
CSCs have the potential to generate self-renewal and heterogeneous
tumor cell lineages that contribute to cancer initiation,
metastasis, progression, therapy resistance, and tumor relapse
(39). Therefore, it is critical to
develop effective ways to eradicate or differentiate them during
antitumor therapy. Natural products have drawn considerable
attention for their marked antitumor activities (40). Recently emerging evidence indicates
that natural products are promising new candidates to eliminate
CSCs through multiple biological mechanisms such as targeting key
signaling pathways or epigenetic regulation (41). Curcumin, a compound derived from the
rhizome of turmeric, has been reported to reduce CSC-like
properties by downregulating the Wnt/β-catenin pathway in lung,
colorectal, and breast cancer cells (42–44).
Cruciferae sulforaphane (SFN), a natural compound present in
cruciferous vegetables, has been revealed to inhibit nasopharyngeal
carcinoma (NPC) stem cells through the DNMT1/Wnt inhibitory factor
1 (Wif1) axis (45). In the present
study, we examined the effects of PEITC, also a natural bioactive
compound, on breast cancer cells and revealed that it could
significantly reduce BCSC-like properties in vitro. Previous
research has demonstrated PEITC to function as a tumor killer by
inhibiting the CD44v-xCT axis in colorectal CSCs and the Sp1
transcription factor in cervical CSCs (46,47).
The mechanism of PEITC on BCSC-like properties was further
investigated in the present study.
Extensive studies have indicated that epigenetic
alteration in cancer cells including DNA methylation, histone
modifications, and chromatin remodeling maintains CSC-like
properties (48). CDH1, a tumor
suppressor gene, has been revealed to be transcriptionally silenced
due to promoter hypermethylation during breast cancer progression
(22). Previous studies have
revealed that reversing CDH1 methylation could reactivate CDH1
expression and reduce CSC-associated properties (16,23).
Recent investigations into the use of PEITC as an epigenetic
modifier have provided new insights into its anticancer effects
(19,26). Thus, it was hypothesized that PEITC
may epigenetically regulate CDH1 to reduce BCSC-associated
properties. By using MSP, it was observed that the CDH1 promoter
region was hypermethylated in untreated MCF-7 and MDA-MB-231 cells,
consistent with a previous study by Pradhan et al (49). However, after PEITC (5 or 10 µM)
treatment, both MCF-7 and MDA-MB-231 cells exhibited significant
demethylation of the CDH1 promoter. CDH1 promoter demethylation was
also observed in the positive controls, 5-Aza (2.5 µM) and TSA (0.5
µM) treated-cells. It was confirmed by qPCR and western blotting
that PEITC could restore CDH1 mRNA and protein levels in both MCF-7
and MDA-MB-231 cells. These results are similar to several other
studies that have revealed reactivation of CDH1 by epigenetic
reagents such as epigallocatechin-gallate (EGCG) and Bisphenol S
(BPS) (50,51). Notably, although the consistent
biological behavior of epigenetic regulations in breast cancer
cells between PEITC-treated groups and positive controls (2.5 µM
5-Aza and 0.5 µM TSA) were observed, it still could not be excluded
whether there are other mechanisms involved in PEITC reactivation
of CDH1 in addition to the epigenetic effects which played a major
role. Moreover, while the demethylating effects of the positive
controls (2.5 µM 5-Aza and 0.5 µM TSA) were stronger than that of
10 µM PEITC, CDH1 mRNA and protein levels were lower, suggesting
that PEITC exerts a greater effect on either histone modification
or chromatin relaxation. Previous research has characterized the
histone modification effects of PEITC to a certain degree (48), but further study is required to
elucidate this observed phenomenon. DNA methylation and histone
deacetylation, mediated by DNMTs and HDACs, respectively, are the
most common epigenetic events that occur with tumor progression and
lead to the silencing of tumor suppressor genes. Thus, the effects
of PEITC on DNMTs and HDACs were further examined. It was revealed
that PEITC (5 or 10 µM) could significantly reduce DNMT and HDAC
protein levels. However, the mechanism by which PEITC inhibits
DNMTs and HDACs remains unclear, requiring further study.
Numerous studies have shown that the Wnt/β-catenin
pathway is constitutively active in the development of breast
cancer and plays a role in regulating the self-renewal of BCSCs
(23,27). A previous study revealed that the
epigenetic reactivation of CDH1 could promote assembly of the
CDH1/β-catenin complex in the cytoplasmic membrane and prevent
β-catenin translocation into the nucleus, thereby inactivating the
Wnt/β-catenin pathway and suppressing carcinoma cell stemness
(16). In the present study, after
identifying that PEITC could epigenetically restore CDH1, the
effects of PEITC on the CDH1/β-catenin complex and Wnt/β-catenin
pathway were assessed. Co-IP and western blotting indicated that
PEITC significantly increased the amount of inactive β-catenin
(phospho-Ser33/37) that co-precipitated with CDH1. TCF-4 is a key
effector in CSC-like trait maintenance by forming the
β-catenin/TCF4 complex, which transcriptionally activates
downstream factors such as cyclin D1 in the Wnt pathway (34,52).
The present study revealed that PEITC (5 or 10 µM) significantly
decreased the protein expression levels of active β-catenin
(phospho-Ser675), TCF-4 and cyclin D1. A previous study revealed
that PEITC could inhibit the Wnt/β-catenin pathway to eliminate
colorectal CSCs (13). However, the
present study elucidated for the first time that epigenetic
regulation by PEITC reactivated CDH1, which inhibited the
Wnt/β-catenin pathway and reduced BCSC-like properties.
There are some limitations to the present study. It
is well known that the dose-dependent effects of drugs and the
optimal effective concentration revealed by in vitro
experiments are useful for further development of promising
anticancer drugs. However, we only used 2 sublethal doses of PEITC,
5 and 10 µM, to perform the experiments in the present study. Thus,
we cannot rigorously conclude that the effects and mechanism of
PEITC function to reduce BCSC-like properties in a dose-dependent
manner or identify the optimal concentration of PEITC. In addition,
due to limitations of the experimental conditions at our laboratory
and some objective reasons such as the COVID-19 pandemic, we did
not isolate special BCSC subtypes such as
CD44+/CD24− by flow cytometry to further
evaluate and characterize the function and mechanism of PEITC in
eradicating BCSCs in a nude mouse tumor xenograft model, which
needs to be conducted in the future.
In conclusion, the present study revealed a novel
epigenetic regulation-mediated mechanism by which PEITC reduced
CSC-like properties in breast cancer cells. It was revealed that
PEITC significantly inhibited colony and tumor sphere formation
abilities and reduced the expression of CSC-associated protein
markers via epigenetic reactivation of CDH1. Inhibitory effects of
PEITC on the expression of DNMTs and HDACs resulted in
demethylation of the CDH1 promoter region. CDH1 then inhibited the
Wnt/β-catenin pathway and formation of the β-catenin/TCF
transcription factor complex to suppress CSC-like properties. The
present findings suggest that PEITC is a potential natural product
that can be further developed for the eradication of breast cancer
stem cells.
Acknowledgments
Not applicable.
Funding
The present study was supported from the Natural
Science Foundation of Hunan Province (grant no. 2019JJ50542), the
Science and Technology program of Hunan Health Commission (grant
no. 20201978), and the China Scholarship Council (grant no.
201808430085).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors contributions
TZ conceived and designed the experiments. TZ and WZ
performed the experiments. TZ and MH analyzed the data. TZ wrote
the manuscript. TZ and MH revised the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar
|
|
2
|
Chu D and Lu J: Novel therapies in breast
cancer: What is new from ASCO 2008. J Hematol Oncol. 1:162008.
View Article : Google Scholar
|
|
3
|
Chinese Anti-Cancer Association, Committee
of Breast Cancer Society. Chinese expert consensus on the clinical
diagnosis and treatment of advanced breast carcinoma(2018).
Zhonghua Zhong Liu Za Zhi. 40((9)): 703–713. 2018.(In Chinese).
|
|
4
|
Luo M, Clouthier SG, Deol Y, Liu S,
Nagrath S, Azizi E and Wicha MS: Breast cancer stem cells: Current
advances and clinical implications. Methods Mol Biol. 1293:1–49.
2015. View Article : Google Scholar
|
|
5
|
Yang F, Xu J, Tang L and Guan X: Breast
cancer stem cell: The roles and therapeutic implications. Cell Mol
Life Sci. 74:951–966. 2017. View Article : Google Scholar
|
|
6
|
Bunting KD: ABC transporters as phenotypic
markers and functional regulators of stem cells. Stem Cells.
20:11–20. 2002. View Article : Google Scholar
|
|
7
|
Liu Y, Burness ML, Martin-Trevino R, Guy
J, Bai S, Harouaka R, Brooks MD, Shang L, Fox A, Luther TK, et al:
RAD51 mediates resistance of cancer stem cells to PARP inhibition
in triple-negative breast cancer. Clin Cancer Res. 23:514–522.
2017. View Article : Google Scholar
|
|
8
|
Butti R, Gunasekaran VP, Kumar TVS,
Banerjee P and Kundu GC: Breast cancer stem cells: Biology and
therapeutic implications. Int J Biochem Cell Biol. 107:38–52. 2019.
View Article : Google Scholar
|
|
9
|
Gupta P, Wright SE, Kim SH and Srivastava
SK: Phenethyl isothiocyanate: A comprehensive review of anti-cancer
mechanisms. Biochim Biophys Acta. 1846:405–424. 2014.
|
|
10
|
Trachootham D, Zhang H, Zhang W, Feng L,
Du M, Zhou Y, Chen Z, Pelicano H, Plunkett W, Wierda WG, et al:
Effective elimination of fludarabine-resistant CLL cells by PEITC
through a redox-mediated mechanism. Blood. 112:1912–1922. 2008.
View Article : Google Scholar
|
|
11
|
Trachootham D, Zhou Y, Zhang H, Demizu Y,
Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, et
al: Selective killing of oncogenically transformed cells through a
ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer
Cell. 10:241–252. 2006. View Article : Google Scholar
|
|
12
|
Zhang T, Shao Y, Chu TY, Huang HS, Liou
YL, Li Q and Zhou H: MiR-135a and MRP1 play pivotal roles in the
selective lethality of phenethyl isothiocyanate to malignant glioma
cells. Am J Cancer Res. 6:957–972. 2016.
|
|
13
|
Chen Y, Li Y, Wang XQ, Meng Y, Zhang Q,
Zhu JY, Chen JQ, Cao WS, Wang XQ, Xie CF, et al: Phenethyl
isothiocyanate inhibits colorectal cancer stem cells by suppressing
Wnt/β-catenin pathway. Phytother Res. 32:2447–2455. 2018.
View Article : Google Scholar
|
|
14
|
Liou YL, Zhang TL, Yan T, Yeh CT, Kang YN,
Cao L, Wu N, Chang CF, Wang HJ, Yen C, et al: Combined clinical and
genetic testing algorithm for cervical cancer diagnosis. Clin
Epigenetics. 8:662016. View Article : Google Scholar
|
|
15
|
Pasculli B, Barbano R and Parrella P:
Epigenetics of breast cancer: Biology and clinical implication in
the era of precision medicine. Semin Cancer Biol. 51:22–35. 2018.
View Article : Google Scholar
|
|
16
|
Liu T, Wu X, Chen T, Luo Z and Hu X:
Downregulation of DNMT3A by miR-708-5p inhibits lung cancer stem
cell-like phenotypes through repressing Wnt/β-catenin signaling.
Clin Cancer Res. 24:1748–1760. 2018. View Article : Google Scholar
|
|
17
|
Yu F, Jiao Y, Zhu Y, Wang Y, Zhu J, Cui X,
Liu Y, He Y, Park EY, Zhang H, et al: MicroRNA 34c gene
down-regulation via DNA methylation promotes self-renewal and
epithelial-mesenchymal transition in breast tumor-initiating cells.
J Biol Chem. 287:465–473. 2012. View Article : Google Scholar
|
|
18
|
Sun L, Mathews LA, Cabarcas SM, Zhang X,
Yang A, Zhang Y, Young MR, Klarmann KD, Keller JR and Farrar WL:
Epigenetic regulation of SOX9 by the NF-κB signaling pathway in
pancreatic cancer stem cells. Stem Cells. 31:1454–1466. 2013.
View Article : Google Scholar
|
|
19
|
Boyanapalli SS, Li W, Fuentes F, Guo Y,
Ramirez CN, Gonzalez XP, Pung D and Kong AN: Epigenetic
reactivation of RASSF1A by phenethyl isothiocyanate (PEITC) and
promotion of apoptosis in LNCaP cells. Pharmacol Res. 114:175–184.
2016. View Article : Google Scholar
|
|
20
|
Gupta R, Bhatt LK and Momin M: Potent
antitumor activity of Laccaic acid and Phenethyl isothiocyanate
combination in colorectal cancer via dual inhibition of DNA
methyltransferase-1 and Histone deacetylase-1. Toxicol Appl
Pharmacol. 377:1146312019. View Article : Google Scholar
|
|
21
|
Berx G and van Roy F: Involvement of
members of the cadherin superfamily in cancer. Cold Spring Harb
Perspect Biol. 1:a0031292009. View Article : Google Scholar
|
|
22
|
Shinozaki M, Hoon DS, Giuliano AE, Hansen
NM, Wang HJ, Turner R and Taback B: Distinct hypermethylation
profile of primary breast cancer is associated with sentinel lymph
node metastasis. Clin Cancer Res. 11:2156–2162. 2005. View Article : Google Scholar
|
|
23
|
Ma F, Li W, Liu C, Li W, Yu H, Lei B, Ren
Y, Li Z, Pang D and Qian C: MiR-23a promotes TGF-β1-induced EMT and
tumor metastasis in breast cancer cells by directly targeting CDH1
and activating Wnt/β-catenin signaling. Oncotarget. 8:69538–69550.
2017. View Article : Google Scholar
|
|
24
|
Huang R, Ding P and Yang F:
Clinicopathological significance and potential drug target of CDH1
in breast cancer: A meta-analysis and literature review. Drug Des
Devel Ther. 9:5277–5285. 2015.
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Park JE, Sun Y, Lim SK, Tam JP, Dekker M,
Chen H and Sze SK: Dietary phytochemical PEITC restricts tumor
development via modulation of epigenetic writers and erasers. Sci
Rep. 7:405692017. View Article : Google Scholar
|
|
27
|
Zheng A, Song X, Zhang L, Zhao L, Mao X,
Wei M and Jin F: Long non-coding RNA LUCAT1/miR-5582-3p/TCF7L2 axis
regulates breast cancer stemness via Wnt/β-catenin pathway. J Exp
Clin Cancer Res. 38:3052019. View Article : Google Scholar
|
|
28
|
Lawson DA, Bhakta NR, Kessenbrock K,
Prummel KD, Yu Y, Takai K, Zhou A, Eyob H, Balakrishnan S, Wang CY,
et al: Single-cell analysis reveals a stem-cell program in human
metastatic breast cancer cells. Nature. 526:131–135. 2015.
View Article : Google Scholar
|
|
29
|
Tsang JY, Huang YH, Luo MH, Ni YB, Chan
SK, Lui PC, Yu AM, Tan PH and Tse GM: Cancer stem cell markers are
associated with adverse biomarker profiles and molecular subtypes
of breast cancer. Breast Cancer Res Treat. 136:407–417. 2012.
View Article : Google Scholar
|
|
30
|
Almozyan S, Colak D, Mansour F, Alaiya A,
Al-Harazi O, Qattan A, Al-Mohanna F, Al-Alwan M and Ghebeh H: PD-L1
promotes OCT4 and Nanog expression in breast cancer stem cells by
sustaining PI3K/AKT pathway activation. Int J Cancer.
141:1402–1412. 2017. View Article : Google Scholar
|
|
31
|
Das S, Mukherjee P, Chatterjee R, Jamal Z
and Chatterji U: Enhancing chemosensitivity of breast cancer stem
cells by downregulating SOX2 and ABCG2 using
wedelolactone-encapsulated nanoparticles. Mol Cancer Ther.
18:680–692. 2019. View Article : Google Scholar
|
|
32
|
Matsumura T, Makino R and Mitamura K:
Frequent down-regulation of E-cadherin by genetic and epigenetic
changes in the malignant progression of hepatocellular carcinomas.
Clin Cancer Res. 7:594–599. 2001.
|
|
33
|
Gelato KA, Shaikhibrahim Z, Ocker M and
Haendler B: Targeting epigenetic regulators for cancer therapy:
Modulation of bromodomain proteins, methyltransferases,
demethylases, and microRNAs. Expert Opin Ther Targets. 20:783–799.
2016. View Article : Google Scholar
|
|
34
|
Lecarpentier Y, Schussler O, Hébert JL and
Vallée A: Multiple targets of the canonical WNT/β-catenin signaling
in cancers. Front Oncol. 9:12482019. View Article : Google Scholar
|
|
35
|
Wang D, Upadhyaya B, Liu Y, Knudsen D and
Dey M: Phenethyl isothiocyanate upregulates death receptors 4 and 5
and inhibits proliferation in human cancer stem-like cells. BMC
Cancer. 14:5912014. View Article : Google Scholar
|
|
36
|
Koschorke A, Faraci S, Giani D, Chiodoni
C, Iorio E, Canese R, Colombo MP, Lamolinara A, Iezzi M, Ladomery
M, et al: Phenethyl isothiocyanate hampers growth and progression
of HER2-positive breast and ovarian carcinoma by targeting their
stem cell compartment. Cell Oncol (Dordr). 42:815–828. 2019.
View Article : Google Scholar
|
|
37
|
Sarkar R, Mukherjee S, Biswas J and Roy M:
Phenethyl isothiocyanate, by virtue of its antioxidant activity,
inhibits invasiveness and metastatic potential of breast cancer
cells: HIF-1α as a putative target. Free Radic Res. 50:84–100.
2016. View Article : Google Scholar
|
|
38
|
Gupta P, Adkins C, Lockman P and
Srivastava SK: Metastasis of breast tumor cells to brain is
suppressed by phenethyl isothiocyanate in a novel in vivo
metastasis model. PLoS One. 8:e672782013. View Article : Google Scholar
|
|
39
|
Henkin RI: Clinical and therapeutic
implications of cancer stem cells. N Engl J Med. 381:e192019.
View Article : Google Scholar
|
|
40
|
Pratheeshkumar P, Sreekala C, Zhang Z,
Budhraja A, Ding S, Son YO, Wang X, Hitron A, Hyun-Jung K, Wang L,
et al: Cancer prevention with promising natural products:
Mechanisms of action and molecular targets. Anticancer Agents Med
Chem. 12:1159–1184. 2012. View Article : Google Scholar
|
|
41
|
Palermo R, Ghirga F, Piccioni MG, Bernardi
F, Zhdanovskaya N, Infante P and Mori M: Natural products inspired
modulators of cancer stem cells-specific signaling pathways Notch
and Hedgehog. Curr Pharm Des. 24:4251–4269. 2018. View Article : Google Scholar
|
|
42
|
Zhu JY, Yang X, Chen Y, Jiang Y, Wang SJ,
Li Y, Wang XQ, Meng Y, Zhu MM, Ma X, et al: Curcumin suppresses
lung cancer stem cells via inhibiting Wnt/β-catenin and aonic
Hedgehog pathways. Phytother Res. 31:680–688. 2017. View Article : Google Scholar
|
|
43
|
Huang YT, Lin YW, Chiu HM and Chiang BH:
Curcumin induces apoptosis of colorectal cancer stem cells by
coupling with CD44 marker. J Agric Food Chem. 64:2247–2253. 2016.
View Article : Google Scholar
|
|
44
|
Dandawate PR, Subramaniam D, Jensen RA and
Anant S: Targeting cancer stem cells and signaling pathways by
phytochemicals: Novel approach for breast cancer therapy. Semin
Cancer Biol. 40-41:192–208. 2016. View Article : Google Scholar
|
|
45
|
Chen L, Chan LS, Lung HL, Yip TTC, Ngan
RKC, Wong JWC, Lo KW, Ng WT, Lee AWM, Tsao GSW, et al: Crucifera
sulforaphane (SFN) inhibits the growth of nasopharyngeal carcinoma
through DNA methyltransferase 1 (DNMT1)/Wnt inhibitory factor 1
(WIF1) axis. Phytomedicine. 63:1530582019. View Article : Google Scholar
|
|
46
|
Ju HQ, Lu YX, Chen DL, Tian T, Mo HY, Wei
XL, Liao JW, Wang F, Zeng ZL, Pelicano H, et al: Redox regulation
of stem-like cells though the CD44v-xCT axis in colorectal cancer:
mechanisms and therapeutic implications. Theranostics. 6:1160–1175.
2016. View Article : Google Scholar
|
|
47
|
Upadhyaya B, Liu Y and Dey M: Phenethyl
isothiocyanate exposure promotes oxidative stress and suppresses
Sp1 transcription Factor in Cancer Stem Cells. Int J Mol Sci.
20:202019. View Article : Google Scholar
|
|
48
|
Feinberg AP, Koldobskiy MA and Göndör A:
Epigenetic modulators, modifiers and mediators in cancer aetiology
and progression. Nat Rev Genet. 17:284–299. 2016. View Article : Google Scholar
|
|
49
|
Pradhan N, Parbin S, Kar S, Das L, Kirtana
R, Suma Seshadri G, Sengupta D, Deb M, Kausar C and Patra SK:
Epigenetic silencing of genes enhanced by collective role of
reactive oxygen species and MAPK signaling downstream ERK/Snail
axis: Ectopic application of hydrogen peroxide repress CDH1 gene by
enhanced DNA methyltransferase activity in human breast cancer.
Biochim Biophys Acta Mol Basis Dis. 1865:1651–1665. 2019.
View Article : Google Scholar
|
|
50
|
Khan MA, Hussain A, Sundaram MK, Alalami
U, Gunasekera D, Ramesh L, Hamza A and Quraishi U:
(−)-Epigallocatechin-3-gallate reverses the expression of various
tumor-suppressor genes by inhibiting DNA methyltransferases and
histone deacetylases in human cervical cancer cells. Oncol Rep.
33:1976–1984. 2015. View Article : Google Scholar
|
|
51
|
Huang W, Zhao C, Zhong H, Zhang S, Xia Y
and Cai Z: Bisphenol S induced epigenetic and transcriptional
changes in human breast cancer cell line MCF-7. Environ Pollut.
246:697–703. 2019. View Article : Google Scholar
|
|
52
|
Lv YF, Dai H, Yan GN, Meng G, Zhang X and
Guo QN: Downregulation of tumor suppressing STF cDNA 3 promotes
epithelial-mesenchymal transition and tumor metastasis of
osteosarcoma by the Wnt/GSK-3β/β-catenin/Snail signaling pathway.
Cancer Lett. 373:164–173. 2016. View Article : Google Scholar
|