Introduction
Cutaneous T-cell lymphoma (CTCL) is the most common
type of lymphoma involving the skin. It accounts for 80 to 85% of
all primary cutaneous lymphomas, with approximately 1,000
individuals in the US newly diagnosed annually (1,2). There
are multiple variants of CTCL, which are classified on the basis of
histopathologic and clinical features. The World Health
Organization's classical categorization of CTCL is divided into
indolent clinical behavior and aggressive behavior, such as that
expressed by Sèzary syndrome lymphomas/leukemias (1,3–5).
Mycosis fungoides is the most common subtype of CTCL, comprising
nearly 50% of all primary cutaneous lymphomas (2,3,6). There
are 4 distinct clinical (T) stages of mycosis fungoides in the
skin, which are based on the appearance of patch, plaque, tumor and
erythroderma, respectively (7). A
complete history and physical examination, including a full skin
evaluation, must be performed to identify lesions suspicious for
CTCL, and a diagnostic skin biopsy is required to make a definitive
diagnosis. Histologic criteria that have proven useful for the
diagnosis of mycosis fungoides include epidermotropism, Pautrier
microabscesses, dense lymphoid infiltrate, perinuclear halos, and
enlarged hyperchromatic cerebriform nuclei (6). However, these histologies are more
commonly observed in advanced plaque and tumor stages of CTCL and
are subtle or even absent in early patch-stage CTCL (8). Early-stage CTCL demonstrates
borderline histologic changes with variable inflammatory reaction
patterns (9). Pautrier
microabscesses are absent in the majority of patch-stage cases
(10).
Accordingly, early-stage CTCL is challenging to
diagnose for both the dermatologist and dermatopathologist
(11). There is significant overlap
between the clinical and histopathologic features of CTCL and many
inflammatory skin diseases, especially during the early-patch
stage. Clinically, CTCL typically presents as erythematous, scaly
macules, patches, and plaques that can be nearly indistinguishable
from the presentations of eczema, psoriasis, and lichenoid
dermatoses. The histology of early-stage CTCL can also show
variable spongiotic, psoriasiform, and/or interface patterns of
dermatitis. Immunohistochemical (IHC) stains can aid diagnosis by
demonstrating predominant CD4 positivity within the T-cell
infiltrate; however, IHC analyses are limited by the significant
overlap between the early-stage features of CTCL and chronic
dermatitis. A typical IHC panel consists of pan-T-cell markers as
well as CD4 and CD8. Findings that support a diagnosis of CTCL
include an elevated CD4 to CD8 ratio (12) and/or loss of pan-T-cell markers,
especially CD5 or CD7. However, loss of T-cell markers, such as
CD2, CD3, CD4, or CD5, by the neoplastic T cells typically occurs
in advanced-stage CTCL but is less common in early-stage lesions.
Furthermore, loss of CD7 is not specific for CTCL and has been
described in inflammatory conditions (13–16).
Because of these diagnostic challenges, the
diagnosis of CTCL is often delayed; the mean time from initial
presentation to an accurate diagnosis of CTCL is approximately 4 to
6 years (17,18). Fortunately, skin-limited CTCL is
often indolent, and patients with patch/plaque-stage disease have a
life expectancy similar to controls matched for age, sex and race
(7). Nonetheless, the advent of
effective therapies for early-stage disease, as well as the desire
to alleviate patient anxiety, has led to the search for more
sensitive and specific ancillary methods for an early
diagnosis.
These putative ancillary diagnostic techniques are
often employed in the initial workup of CTCL. They include flow
cytometry of skin biopsy tissue and peripheral blood and molecular
evaluation of T-cell clonality using T-cell receptor gene
rearrangement by polymerase chain reaction (TCGR-PCR), with PCR
being the standard molecular technique for TCRGR analysis. Although
PCR is a useful tool, its ability to identify TCRGR can be limited.
Especially in early-stage lesions, the identification of a clone
using PCR is associated with a high false-negative rate (19). Flow cytometry of peripheral blood is
mostly used in the evaluation of erythrodermic patients, but its
sensitivity and specificity in early-stage disease has only rarely
been investigated. A recent study demonstrated sensitivity of 78%
and specificity of 71% in a group of 128 samples of various stages,
including early-stage mycosis fungoides (20) while an earlier study showed lower
sensitivity of only 6% in 440 cases (21). Likewise, flow cytometry performed on
skin biopsies is not in widespread use, although a small study
showed promising sensitivity of 75% (22). Therefore, there remains considerable
debate concerning the value of all of these ancillary studies,
particularly in regards to early-stage or suspected CTCL.
With the advent of next generation sequencing, the
use of high-throughput single cell T-cell receptor sequencing-high
throughput screening (HTS) to identify TCRGR has shown better
specificity in distinguishing definitive CTCL from inflammatory
processes (23). The tumor clone
frequency quantified by using HTS may be used to identify patients
with early-stage CTCL who are at a higher risk of progression
(24). Additionally, HTS has been
shown to be more sensitive and specific than flow cytometry in
identifying minimal residual disease in patients with Sèzary
syndrome (25).
However, the optimal use of T-cell receptor (TCR)
clonality and flow cytometry studies, especially for evaluation of
the patient with suspected and/or early-stage CTCL, has not been
clearly defined. A better understanding of the yield of these
ancillary tests can facilitate more cost-effective care.
Furthermore, defining the tests with optimal yield may obviate the
need for repeated clinical visits and/or additional biopsies to
establish a definitive diagnosis. In the current study, we describe
the yield of the more commonly used ancillary tests and the more
recently available TCR-HTS studies that are performed on skin
biopsy tissue and peripheral blood specimens from patients who were
evaluated for suspected CTCL at a cutaneous lymphoma referral
clinic.
Patients and methods
Patients
Following approval of the Institutional Review Board
of the University of South Florida, we retrospectively reviewed the
medical records of patients who were referred to the
multidisciplinary cutaneous lymphoma clinic at our tertiary care
cancer center between April 1, 2017 and December 6, 2017. We
analyzed sequentially all new patients who were referred for skin
disease which was suspected clinically to be a new diagnosis of
CTCL, as well as patients with a prior history of CTCL who had
responded to treatment but were being evaluated for a suspected new
clinical recurrence, and who underwent skin biopsy, flow cytometry
of skin and/or peripheral blood, and gene rearrangement analysis of
skin biopsy as part of their workup. Patients were excluded if they
were not clinically suspicious for CTCL before undergoing a
diagnostic biopsy or had no identifiable lymphoid infiltrates on
pathologic examination. Patients with a diagnosis of CTCL who had
biopsies to evaluate for progression of already established CTCL
were also excluded.
Demographic data such as age, sex, and race were
obtained. The clinical appearance of skin lesions (patch, plaque,
tumor) at the time of presentation and the diagnostic results of
flow cytometry, TCR, and HTS analyses, along with the final
consensus diagnosis of each patient, were recorded. If lesions of
>1 morphology were present, the clinical categorization was that
of the most advanced morphology.
Skin biopsy evaluation
Each patient underwent one skin biopsy, either a
3-mm punch biopsy or a shave biopsy measuring at least 4 mm, of
each skin lesion with patch, plaque, and/or tumor morphology.
Biopsies were submitted in formalin for hematoxylin and eosin
(H&E) and IHC evaluation, TCR-PCR and/or TCR-HTS analysis. Most
patients underwent an additional 3-mm punch biopsy which was
submitted fresh for flow cytometry analysis in some cases; the
shave or punch for pathologic diagnosis was bisected and one half
was submitted fresh for flow cytometry with the remainder for
H&E. H&E-stained slides were evaluated for histologic
features diagnostic of CTCL, including band-like dermal
lymphocytes, epidermotropism, Pautrier microabscesses, follicular
mucinosis, cytologic atypia of lymphocytes, and wiry papillary
dermal fibrosis.
Immunohistochemistry
IHC staining was performed on a Ventana
immunostainer (Ventana Benchmark Ultra, Roche Diagnostics).
Antibodies included CD2 (Cell Marque); CD3, CD4, CD5, CD7, CD20,
CD30 (Ventana/Roche); and CD8 (Dako/Aligent). Positive staining was
visualized with diaminobenzidine chromogen.
Flow cytometry
Fresh tissue biopsies submitted for flow cytometry
analyses were transported at room temperature in Roswell Park
Memorial Institute (RPMI)-1640 media to the flow cytometry
laboratory and manually cut into 1 to 2 mm3 pieces with
a scalpel. They were then loaded on a Medimachine sample
preparation system (BD Biosciences) and processed per
manufacturer's instructions. Briefly, this system uses disposable
polyethylene Medicon chambers with immobile stainless-steel screens
that contain microblades around each hole in the screen, which
allow for tissue disaggregation. After 45 sec, the disaggregated
tissue was collected and filtered through a 50-µm Filcon filter
membrane, then centrifuged for 5 min at 250 × g. The pellet was
resuspended in RPMI-1640 medium. Cell count and viability
assessments were performed; the concentration was between
5×105 to 1×106 cells/ml. Aliquots of the
resulting cell suspension were stained in the dark for 15 min at
room temperature with combinations of monoclonal antibodies
conjugated to fluorescein isothiocyanate, phycoerythrin-Texas Red,
peridinin chlorophyll protein CyChrome 5.5, phycoerythrin-cyanine
7, allophycocyanin, allophycocyanin-Alexa Fluor 700,
allophycocyanin-Alexa Fluor 750, Pacific Blue, and Krome Orange.
Red blood cells were lysed with BD FACS lysing solution (BD
Biosciences), and nucleated cells were resuspended in
phosphate-buffered saline containing 2% paraformaldehyde. Events
were acquired on a Gallios flow cytometer (Beckman Coulter). The
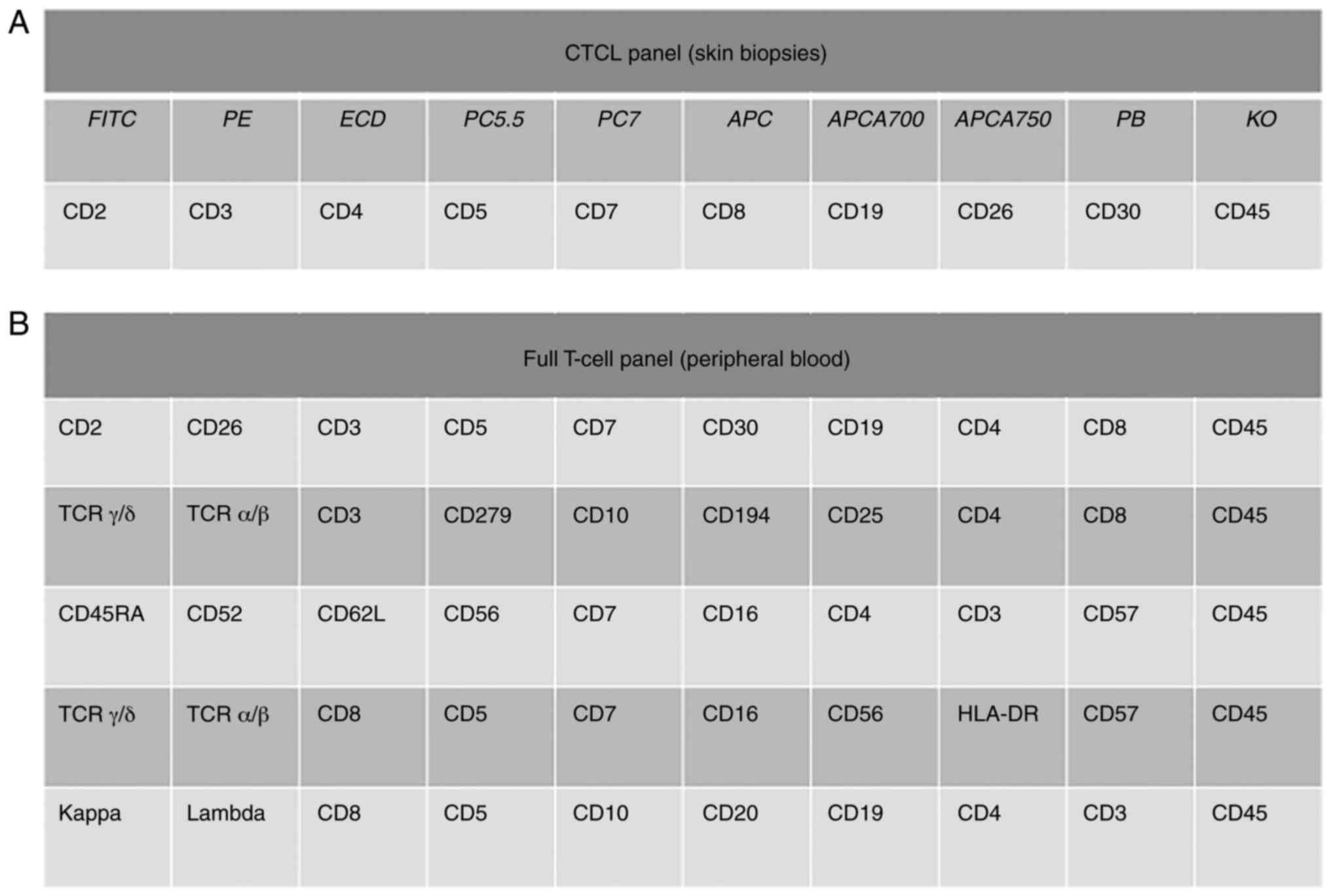
one-tube panel for skin consists of CD2, CD26, CD3, CD5, CD7, CD30,
CD19, CD4, CD8, and CD45 (Beckman Coulter). Listmode files were
analyzed using Kaluza version 1.2 (Beckman Coulter). The lymphoid
population was obtained on a dot plot of ungated forward
scatter/side scatter and CD45/side scatter gating for all
lymphocytes. A positive flow cytometry is diagnosed when a distinct
aberrant T-cell cluster is identified, such as a T cell population
with aberrant loss of CD7, CD26, or any other pan–T-cell
markers.
Flow cytometry analyses of peripheral blood were
conducted as follows. Peripheral blood cell counts were calculated
by using an XE-2100 automated hemocytometer (Sysmex, Hyogo, Japan).
Aliquots of 100 µl of whole blood in sodium heparin were incubated
in the dark for 15 min at room temperature with combinations of
monoclonal antibodies conjugated to the above-described
fluorochromes. Red blood cells were lysed with BD FACS lysing
solution (BD Biosciences), and nucleated cells were resuspended in
phosphate-buffered saline containing 2% paraformaldehyde. Events
were acquired on a Gallios flow cytometer (Beckman Coulter). The
panel consisted of 5 tubes containing the following combinations:
CD2, CD26, CD3, CD5, CD7, CD30, CD19, CD4, CD8, and CD45; TCR γ/δ,
TCR α/β, CD3, CD279, CD10, CD194, CD25, CD4, CD8, and CD45; CD45RA,
CD52, CD62L, CD56, CD7, CD16, CD4, CD3, CD57, and CD45; TCR γ/δ,
TCR α/β, CD8, CD5, CD7, CD16, CD56, HLA-DR, CD57, and CD45; and
κ-light chain, λ-light chain, CD8, CD5, CD10, CD20, CD19, CD4, CD3,
and CD45. All antibodies were obtained from Beckman Coulter.
Lymphoid populations were identified and categorized. Fig. 1 provides a summary of the antigens
evaluated via flow cytometry analyses of skin biopsies and
peripheral blood.
TCRGR by PCR
This test was performed on DNA prepared from
paraffin-embedded skin biopsy tissue and/or an aliquot of the cell
suspension in RPMI-1640 medium produced by the flow cytometry
laboratory. Total cellular DNA was extracted and PCR amplification
was performed on 5 multiplex PCR tubes with analyte specific
reagent Biomed-2 primers (Invivoscribe Technologies) targeting TCR
Vβ, Dβ, Jβ, Vγ, and Jγ regions. The products were separated and
detected by capillary gel electrophoresis on the ABI PRISM
3130xl Genetic Analyzer (Applied Biosystems). A positive PCR
result is defined as a single peak that is >2.5× larger than
adjacent polyclonal peaks.
TCRGR by HTS
DNA extracted from paraffin-embedded skin biopsy
specimens was submitted for TCR-HTS to Adaptive Biosciences for
Clonoseq testing. TCR-β complementary determining region 3 (CDR3)
was targeted and amplified by using multiplex PCR, with
bias-controlled V and J gene primers. The amplified segments were
sequenced using HTS. Cases were considered positive if there was at
least one unique sequence comprising >5% of all sequences
identified. For these cases, the number and sequence(s) of clones
containing unique CDR3 regions/1 million cells reported. Cases with
unique sequences at lower than 5% frequency were considered
polyclonal.
Data analysis
For each of the testing modalities, the number of
adequate specimens per patient tested, as well as the rate of
positivity of adequate specimens was compiled for each lesion
morphology. The sensitivity (true positives/true positives + false
negatives) and specificity (true negative/true negatives + false
positives) of TCR-PCR, TCR-HTS, and flow cytometry studies
performed were calculated for each patient using the final
integrated diagnosis (CTCL or not CTCL), which was based on the
integration of clinical, histopathologic, and molecular analyses of
all biopsy specimens from that patient. A final integrated
diagnosis of mycosis fungoides included at least 2 of the following
findings: A chronic cutaneous eruption clinically suspicious for
CTCL exhibiting an abnormal clone of CD26-expressing T cells on
flow cytometry analyses, a positive TCRGR for β and γ genes by PCR
and/or HTS, an epidermotropic lymphoid infiltrate on H&E
staining with an altered CD4 to CD8 ratio (either >5 or <2),
and/or a loss of pan-T-cell antigen(s), such as CD2, CD5, or
CD7.
Results
A total of 55 patients referred to the cutaneous
lymphoma clinic between April 1, 2017 and December 6, 2017 met the
inclusion criteria. The patients ranged from 21 to 85 years of age.
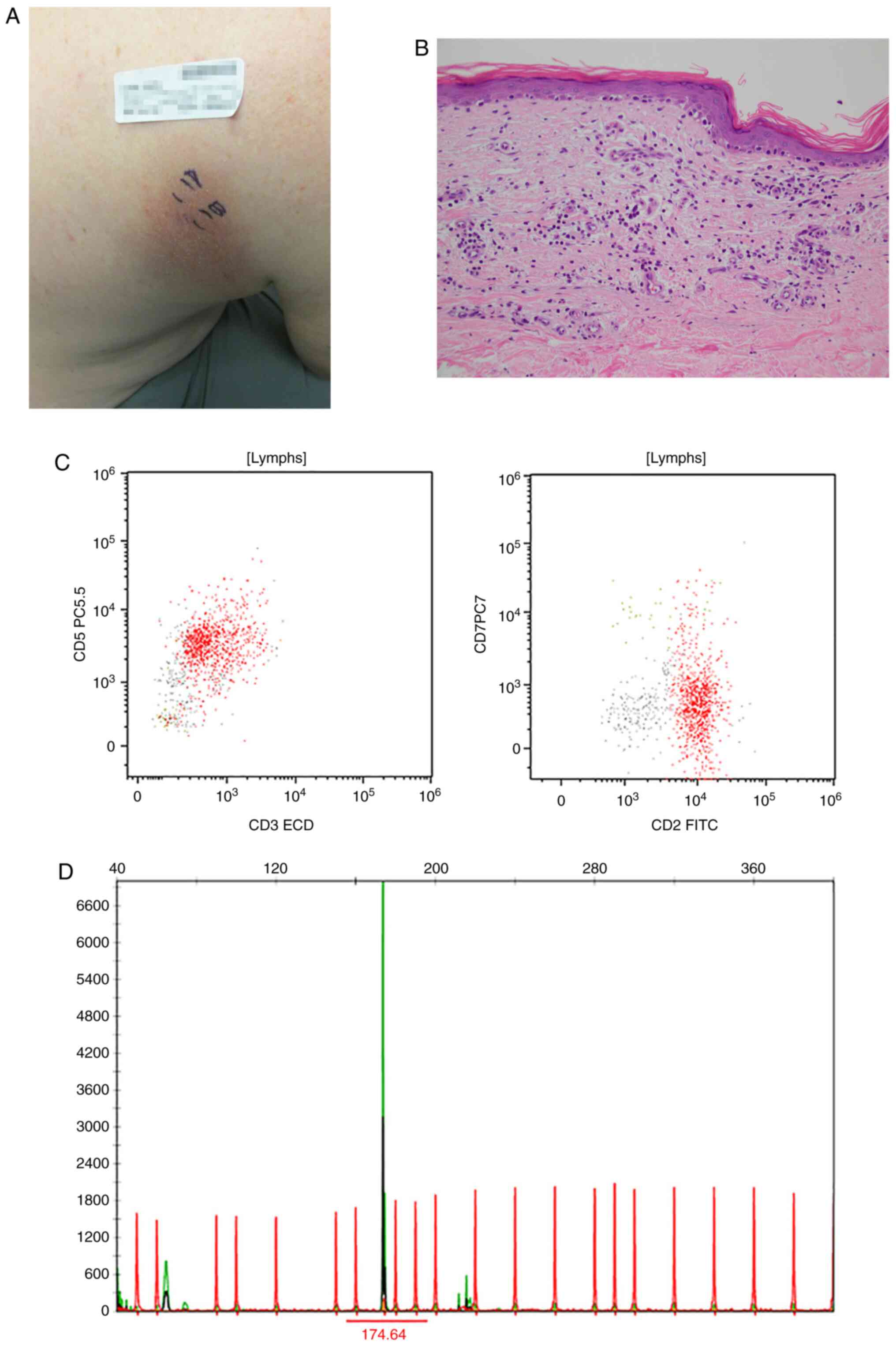
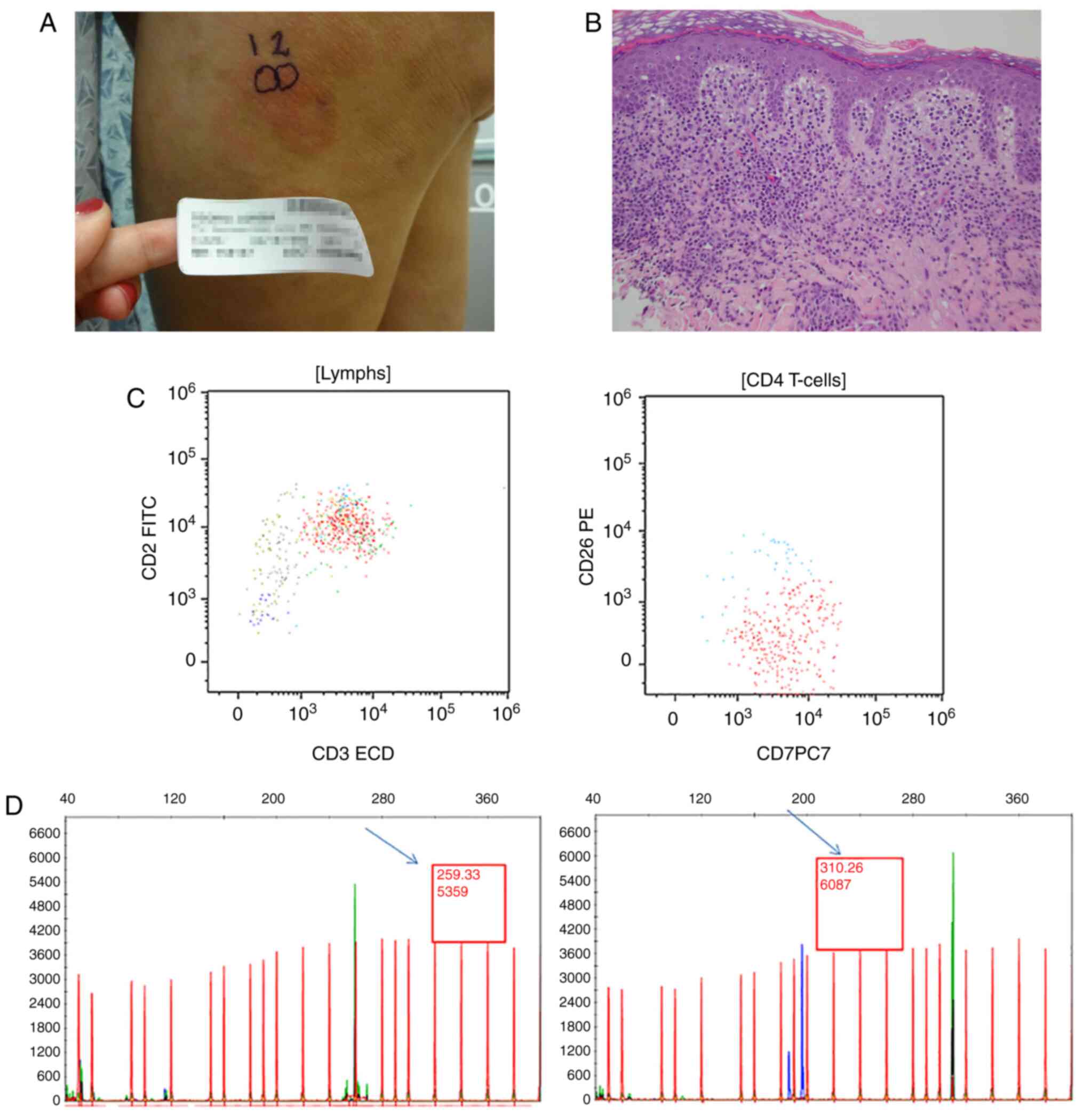
Clinicopathologic characteristics are summarized in Table I. Representative examples of results
for a patient with patch-stage disease (clinical images, histology,
flow cytometry and PCR results) are shown in Fig. 2, and for plaque disease in Fig. 3.
 | Table I.Clinicopathologic characteristics of
the cases. |
Table I.
Clinicopathologic characteristics of
the cases.
| Characteristics | Patch morphology | Plaque
morphology | Tumor morphology | Erythroderma |
|---|
| Age in years |
|
|
|
|
|
Median | 62 | 58 | 68 | 77 |
|
Range | 29-80 | 21-76 | 52-69 | 66-85 |
| Sex, n (%) |
|
|
|
|
| Male | 15 (68) | 13 (54) | 3 (100) | 3 (50) |
|
Female | 7
(32) | 11 (46) | 0 (0) | 3 (50) |
| BSA, n (%) |
|
|
|
|
|
<1% | 3
(14) | 1
(4) | 0 | 0 |
| 1–9% | 10 (45) | 12 (50) | 1 (33) | 0 |
|
10–79% | 8
(36) | 5
(21) | 0 | 0 |
|
>80% | 0 | 0 | 0 | 6 (100) |
|
Unknown | 1
(5) | 7
(29) | 2 (67) | 0 |
| Clinical
presentation, n (%) |
|
|
|
|
| New
patients | 16 (73) | 22 (88) | 2 (67) | 3 (50) |
|
Recurrent patients | 6
(27) | 3
(12) | 1 (33) | 3 (50) |
| Final diagnosis, n
(%) |
|
|
|
|
| MF | 14 (64) | 16 (67) | 1 (33) | 4 (67) |
|
Non-MF | 8
(36) | 8
(33) | 2 (67) | 2 (33) |
|
Patients (total, N=55), n | 22 | 24 | 3 | 6 |
Clinical lesions that were biopsied included patches
(n=45 biopsies from 28 patients), plaques (n=43 biopsies from 24
patients), nodules/tumors (n=5 biopsies from 3 patients), and
erythroderma (n=9 biopsies from 6 patients); 6 of these patients
had both patches and plaques submitted. One hundred and two (102)
skin biopsy specimens were submitted for H&E histologic
evaluation, with associated flow cytometry (n=59 tests from 53
patients), TCR-PCR (n=50 tests from 50 patients), and/or TCR-HTS
(n=26 tests from 23 patients) studies performed. There were 21
concurrent tissue TCR-PCR and TCR-HTS specimens. Peripheral blood
specimens were submitted for flow cytometry (51 from 51 patients),
TCR-PCR (50 from 50 patients) and TCR-HTS (16 from 16 patients),
with 15 concurrent TCR-PCR and TCR-HTS blood specimens. Thirty-five
(64) patients had a final integrated diagnosis of CTCL.
Representative clinical images, H&E slides, flow cytometry, and
TCRGR results are presented for a patient with patch-stage mycosis
fungoides (Fig. 3) and plaque stage
mycosis fungoides. A representative example of immunohistochemical
staining used to support a diagnosis of plaque-stage mycosis
fungoides is shown in Fig. S1. An
example of a high-throughput sequencing report on a patient with
plaque-stage mycosis fungoides is shown in Fig. S2. The diagnoses of the remaining 20
patients were pseudolymphoma (n=7), no evidence of/inactive disease
(n=4), atypical lymphoid infiltrate not diagnostic for CTCL (n=1),
lymphomatoid papulosis (n=1), superficial perivascular dermatitis
(n=2), phototoxic dermatitis (n=1), postinflammatory pigment
alteration (n=1), interstitial granulomatous dermatitis (n=1),
Staphylococcus folliculitis (n=1) and morphea (n=1).
Performance of flow cytometry analyses
of skin biopsy samples
The sensitivity and specificity of each diagnostic
modality were calculated for patients on the basis of lesion type
and were divided into 4 groups on the basis of clinical
presentation (Table II). The per
patient sensitivity of flow cytometry analyses of skin biopsy
specimens was 55% for clinical patches, 75% for plaques, and 0% for
nodular disease, and 100% for erythroderma, for an overall
sensitivity of 66%. The per patient specificity of flow cytometry
analyses was 100% for patches, plaques, nodular disease, and
erythroderma, with an overall specificity of 100%. Specimens with
insufficient cellularity were excluded from the final calculations;
these included 33% of patches, 9% of plaques, 0% of nodules/tumors,
and 33% of erythrodermic skin. Therefore, a total of 21% of
specimens submitted for flow cytometry analysis were
insufficient.
 | Table II.Sensitivity and specificity of flow
cytometry, TCR-PCR and TCR-HTS analyses of skin biopsy
specimens. |
Table II.
Sensitivity and specificity of flow
cytometry, TCR-PCR and TCR-HTS analyses of skin biopsy
specimens.
| Clinical
morphology | Study | No. of adequate
specimens/patients tested (%) | No. of positive
specimens/adequate specimens % | Per patient
sensitivity in adequate specimens, % | Per patient
specificity in adequate specimens, % |
|---|
| Patch | Flow cytometry | 14/21 (67) | 5/14
(36) | 55 | 100 |
|
| TCR-PCR | 15/20 (75) | 7/15
(47) | 55 | 66 |
|
| TCR-HTS | 7/7
(100) | 1/7
(14) | 25 | 100 |
| Plaque | Flow cytometry | 21/23 (91) | 9/21
(43) | 75 | 100 |
|
| TCR-PCR | 19/20 (95) | 15/19 (79) | 82 | 50 |
|
| TCR-HTS | 17/14 (100) | 9/14
(64) | 89 | 80 |
| Nodule/tumor | Flow cytometry | 3/3
(100) | 0/3
(0) | 0 | 100 |
|
| TCR-PCR | 3/3
(100) | 0/3
(0) | 0 | 100 |
|
| TCR-HTS | 1/1
(100) | 0/1
(0) | 0 | NP |
| Erythroderma | Flow cytometry | 4/6
(67) | 2/4
(50) | 100 | 100 |
|
| TCR-PCR | 4/3
(100) | 1/3
(33) | 0 | 50 |
|
| TCR-HTS | 1/1
(100) | 0/1
(0) | 0 | NP |
| All lesions | Flow cytometry | 42/53 (100) | 16/42 (38) | 66 | 100 |
|
| TCR-PCR | 41/46 (89) | 23/41 (56) | 61 | 64 |
|
| TCR-HTS | 26/23 (100) | 10/23 (43) | 60 | 87 |
Performance of molecular analyses of
skin biopsy samples
A total of 72% of skin biopsies had sufficient DNA
for performance of TCR-PCR. The sensitivity of TCR-PCR analyses of
skin biopsy samples was 55% for patients with clinical patches, 82%
for plaques, and 0% for both the 1 tumor-stage CTCL case and the 1
erythrodermic CTCL case; overall sensitivity was 61%. The
specificity of TCR-PCR analyses was 66% for patients with patches,
50% for plaques, 100% for nodular disease, and 50% for
erythroderma, for an overall specificity of 64%. TCR-HTS on skin
biopsy samples had a per patient sensitivity of 25% for clinical
patches, 89% for plaques, and 0% for the 1 erythrodermic CTCL case
tested, for an overall sensitivity of 60%. TCR-HTS analyses had a
specificity of 100% for patients with patches and 80% for plaques,
with an overall specificity of 87%. Samples submitted for TCR had a
higher rate of sufficient DNA: 89% for PCR and 100% for HTS,
although inadequate specimens were more frequent with patch-stage
disease (25%). There were 16 patients with concurrent PCR and HTS
results; these were concordant in 75% of cases. In the four
discordant cases, there were equal numbers of false-positive and
false-negative results for both types of testing.
Performance of flow cytometry analyses
of peripheral blood samples
Table III
demonstrates the yield, sensitivity and specificity of peripheral
blood analysis by flow cytometry and molecular analysis in the
lesion subgroups and entire group of patients. Flow cytometry
analyses of peripheral blood samples identified an abnormal clone
in 23% of patients diagnosed with patch-stage CTCL, 0% with
plaque-stage CTCL, 0% with tumor-stage CTCL, and 50% with
erythrodermic CTCL/Sèzary syndrome, for an overall sensitivity of
14%. The specificity of flow cytometry analyses of peripheral blood
samples was 85% for clinical patches, 83% for plaques, 100% for
nodular disease, and 75% for erythroderma, for an overall
specificity of 88%.
 | Table III.Sensitivity and specificity of flow
cytometry, TCR-PCR, and TCR-HTS analyses of peripheral blood. |
Table III.
Sensitivity and specificity of flow
cytometry, TCR-PCR, and TCR-HTS analyses of peripheral blood.
| Clinical
morphology | Study | No. of positive
specimens/total specimens (%) | Per patient
sensitivity in adequate specimens, % | Per patient
specificity in adequate specimens, % |
|---|
| Patch | Flow cytometry | 4/20
(20) | 23 | 85 |
|
| TCR-PCR | 7/20
(35) | 38 | 71 |
|
| TCR-HTS | 0/5
(0) | 0 | 100 |
| Plaque | Flow cytometry | 0/22
(0) | 0 | 83 |
|
| TCR-PCR | 8/21
(38) | 36 | 57 |
|
| TCR-HTS | 3/9
(33) | 50 | 100 |
| Nodule/tumor | Flow cytometry | 0/3
(0) | 0 | 100 |
|
| TCR-PCR | 2/3
(66) | 0 | 0 |
|
| TCR-HTS | 0/2
(0) | 0 | 0 |
| Erythroderma | Flow cytometry | 3/6
(50) | 50 | 75 |
|
| TCR-PCR | 3/6
(50) | 75 | 100 |
|
| TCR-HTS | NP | NP | NP |
| All lesions | Flow cytometry | 7/53
(13) | 14 | 88 |
|
| TCR-PCR | 20/50 (40) | 40 | 61 |
|
| TCR-HTS | 3/16
(19) | 30 | 100 |
Performance of molecular analyses of
peripheral blood samples
TCR-PCR analyses of peripheral blood samples were
positive in 38% of patients diagnosed with patch-stage CTCL, 36%
with plaque-stage disease, 0% with tumor-stage disease, and 75%
diagnosed with erythrodermic CTCL/Sèzary syndrome, for an overall
sensitivity of 40%. The specificity of TCR-PCR analyses of
peripheral blood samples was 71% for clinical patches, 57% for
plaques, 0% for nodular disease, and 100% for erythroderma, for an
overall specificity of 61%.
TCR-HTS analyses of peripheral blood samples was
positive in 0% of patients diagnosed with patch-stage CTCL and 50%
with plaque-stage CTCL; TCR-HTS analyses were not performed in
cases of tumor-stage CTCL or erythrodermic CTCL/Sèzary syndrome.
The overall sensitivity of TCR-HTS analyses was 30%. The
specificity of TCR-HTS analyses of peripheral blood samples was
100% for patches, 100% for plaques, and 100% for nodular disease.
TCR-HTS analyses were not performed on peripheral blood samples of
patients with erythrodermic skin (Table III). Forty-nine (49) patients had
concurrent peripheral blood flow cytometry and clonality analysis,
either by PCR or HTS. Thirty-four (34) (69%) of these patients had
concordant results. The most common cause of discordant results was
false-negative flow cytometry (9/15 discordant results) followed by
false-positive molecular results (4/15 discordant samples) and
false-negative molecular results (2/15 samples).
Discussion
Cutaneous T-cell lymphoma (CTCL) is a cutaneous
lymphoma that clinically mimics a variety of inflammatory
dermatoses. A skin biopsy demonstrating characteristic histologic
features, including a predominant CD4+ superficial
lymphoid infiltrate with atypical cerebriform nuclei,
epidermotropism, Pautrier microabscesses, and loss of ≥1 pan-T-cell
antigens (CD2, CD3, CD5), can be used to affirm the diagnosis
(8). Although these pathognomonic
features are seen in well-established cases of CTCL, they are
rarely observed in cases of early-stage CTCL, in which
differentiation between neoplastic and reactive T-cell infiltrates
is difficult.
The diagnostic workup for suspected CTCL includes
the use of ancillary diagnostic techniques, which have been shown
to have promising results in the diagnosis of CTCL (26,27).
The identification of T-cell monoclonality within the lymphoid
infiltrate can provide strong evidence of the diagnosis of CTCL. In
well-established CTCL cases, T-cell receptor (TCR) has been shown
to detect T-cell monoclonality in 52 to 90% of established CTCL
biopsy specimens (5,28–30).
Kirsch et al demonstrated superior results using
high-throughput sequencing (HTS), which detected expanded T-cell
clones in 100% (46 out of 46) of established CTCL cases (19).
Little is known about the usefulness of these
ancillary studies in early or pathologically borderline/equivocal
CTCL cases. Ashton-Key et al analyzed clinically suspicious,
histologically borderline CTCL biopsy specimens of lesions from 22
patients who subsequently developed mycosis fungoides and 32 newly
suspected CTCL patients. TCR detected monoclonality in 50% (11/22)
and 19% (6/32) of cases, respectively (13). The use of HTS in examining
clinically suspicious, histologically borderline lesions has not
been studied.
Once a diagnosis of CTCL is established, additional
diagnostic testing is conducted in accordance with current National
Comprehensive Cancer Network guidelines for the workup and staging
of cutaneous lymphomas (31). These
diagnostic tests include PET/CT scans and laboratory studies, such
as complete blood count, differential, comprehensive metabolic
panel, lactic dehydrogenase, serum protein
electrophoresis/quantitative immunoglobulins, molecular testing
with cytogenetics, fluorescence in situ hybridization, flow
cytometry, and viral studies (32),
as clinically indicated. A bone marrow biopsy is also performed in
select cases of CTCL (33).
The majority of patients in our cohort presented
with low-volume patch or plaque-stage disease. For tissue
evaluation, the sensitivity of each technique was similar for the
population as a whole, while flow cytometry was most specific.
Although flow cytometry was useful for analyzing skin biopsy
specimens from patients with plaques and erythroderma, it suffered
from low sensitivity for patch-stage disease and had a high rate of
insufficient tissue (33%). We no longer perform flow cytometry of
patch-stage skin lesions. TCR-PCR remains a valuable technique to
assess the tissue of patients with suspected CTCL, especially with
patch and plaque lesions, but had a lower sensitivity in
patch-stage disease and a high rate of insufficient tissue (25%).
Since our population was skewed towards patients with suspected
malignancy, we do not have adequate numbers of benign diagnoses to
make valid conclusions about the specificity of TCR-PCR. Compared
to TCR-PCR, TCR-HTS offered similar sensitivity but had greater
specificity in patients with plaque but not patch lesions. Both
TCR-PCR and TCR-HTS had greater sensitivity than flow cytometry in
assessing patients with raised lesions; flow cytometry offered
greater specificity than TCR-PCR among those with clinically raised
lesions. The PCR and HTS techniques showed a high rate of
concordance (75%) in the low number of samples concurrently tested.
For all of these tests, it must be emphasized that our reported
performance characteristics can only be generalized to patients
with early or partially treated disease, as this population was the
focus of this study. Furthermore, the low numbers of patients
tested with TCR-HTS limits drawing strong conclusions about test
performance, although the results are promising.
With the exception of erythrodermic patients, the
overall rate of positive TCR and flow cytometry studies performed
on peripheral blood specimens was low. These data suggest that flow
cytometry analyses of peripheral blood may safely be eliminated
from the workup of patients with patch and plaque disease; the low
numbers of patients with nodules/tumor disease precluded the
evaluation of this technique. In 9 cases, TCR-PCR analyses
performed on blood yielded a positive result when concurrent flow
cytometry was negative. Although TCR-PCR was more sensitive than
TCR-HTS, TCR-HTS had a significantly higher specificity when
performed on peripheral blood. For patients with positive TCR-PCR
but negative flow cytometry of peripheral blood, a comparison of a
TCR peak(s) with the tissue specimen is suggested to evaluate for
matching peaks. Indeed, all 3 positive TCR-HTS results had matching
peaks in tissue and peripheral blood; in all 3 of these flow
cytometry was negative. An accurate comparison of TCR-PCR peaks may
often be challenging, whereas TCR-HTS identifies matching gene
sequences with superior specificity and has the ability to reveal
low levels of dominant sequences found in skin biopsies as well as
within corresponding peripheral blood specimens.
Given the rising health care costs in the US, a
cost-conscious approach must be considered in diagnostic medicine.
Despite the decrease in the cost of next-generation sequencing in
the last decade, the techniques of established flow cytometry
methods and PCR-based studies are less costly. However, the higher
rates of insufficient tissue associated with flow cytometry and PCR
studies, leading to the need for additional biopsies, may negate
any cost difference.
These data suggest that molecular analyses remain a
useful tool for evaluating patients with suspected CTCL. In the 16
patients tested, TCR by HTS showed better specificity for both the
skin biopsy and peripheral blood samples than the traditional PCR
method, although sensitivities were similar. By identifying the
gene sequences present in lesional tissue, HTS may be superior for
monitoring for recurrence and minimal residual disease. Flow
cytometry of tissue can be a useful adjunct to molecular analyses
for patients with plaque, tumor, or erythrodermic lesions. In our
limited sample, flow cytometry analyses of peripheral blood samples
showed relatively low sensitivity and moderate specificity in
patch, plaque, and tumor disease, and flow cytometry analyses of
peripheral blood remains a useful tool for evaluating patients with
erythroderma. Although our findings are limited by the low number
of cases studied, they point to the continued need for more
sensitive tests for early-stage CTCL.
Supplementary Material
Supporting Data
Acknowledgements
We thank Paul Fletcher and Daley Drucker (Moffitt
Cancer Center) for editorial assistance. They were not compensated
beyond their regular salaries.
Funding
No funding was received.
Availability of data and materials
All data generated and/or analyzed during the
current study are included in this published article. The
high-throughput sequencing that was performed on a subset of
patients was obtained through testing at a commercial laboratory,
and results are included in the article.
Authors' contributions
JLM carried out the creation of the research
project, data gathering and analysis, substantial preparation and
final approval of manuscript and is the corresponding author. JDG
carried out the data gathering and analysis, preparation of
manuscript, and gave final approval of the manuscript. SM conducted
the data gathering and analysis, preparation of manuscript, and
gave final approval of the manuscript. AK carried out the data
collection and analysis, and gave final approval of the manuscript.
LS and LSV achieved conception of the study and gave final approval
of the manuscript. ES, HZ and XZ made contribution to the methods,
analysis of data, and gave final approval of the manuscript. PLC
conducted analysis of the data, and gave final approval of the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
This study was carried out with the approval of the
institutional ethics review boards: Moffitt Cancer Center
Scientific Review Committee (MCC 19315) and University of South
Florida Bulls IRB (Pro00031911).
Patient consent for publication
According to institutional guidelines, the patient
images contained herein show no personally identifying data and
therefore the requirement for patient consent was waived.
Competing interests
The authors declare that the research was conducted
in the absence of any commercial or financial relationships that
could be construed as a potential conflict of interest.
References
|
1
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H and Thiele J: World Health Organization
Classification of Tumours of Hematopoietic and Lymphoid Tissue.
IARC Press; Lyon: 2008
|
|
2
|
Cerroni L: Lymphoproliferative lesions of
the skin. J Clin Pathol. 59:813–826. 2006. View Article : Google Scholar
|
|
3
|
Willemze R and Meijer CJ: Classification
of cutaneous T-cell lymphoma: From Alibert to WHO-EORTC. J Cutan
Pathol. 33 (Suppl 1):18–26. 2006. View Article : Google Scholar
|
|
4
|
Olsen EA: Evaluation, diagnosis, and
staging of cutaneous lymphoma. Dermatol Clin. 33:643–654. 2015.
View Article : Google Scholar
|
|
5
|
Algara P, Soria C, Martinez P, Sanchez L,
Villuendas R, Garcia P, Lopez C, Orradre JL and Piris MA: Value of
PCR detection of TCR gamma gene rearrangement in the diagnosis of
cutaneous lymphocytic infiltrates. Diagn Mol Pathol. 3:275–282.
1994. View Article : Google Scholar
|
|
6
|
Galper SL, Smith BD and Wilson LD: Wilson,
Diagnosis and management of mycosis fungoides. Oncology (Williston
Park). 24:491–501. 2010.
|
|
7
|
Willemze R, Jaffe ES, Burg G, Cerroni L,
Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL,
Duncan LM, et al: WHO-EORTC classification for cutaneous lymphomas.
Blood. 105:3768–3785. 2005. View Article : Google Scholar
|
|
8
|
Cerroni L: Mycosis fungoides-clinical and
histopathologic features, differential diagnosis, and treatment.
Semin Cutan Med Surg. 37:2–10. 2018. View Article : Google Scholar
|
|
9
|
Jawed SI, Myskowski PL, Horwitz S,
Moskowitz A and Querfeld C: Primary cutaneous T-cell lymphoma
(mycosis fungoides and Sézary syndrome): Part I. Diagnosis:
Clinical and histopathologic features and new molecular and
biologic markers. J Am Acad Dermatol. 70:205.e1–16; quiz 221-222.
2014. View Article : Google Scholar
|
|
10
|
Kempf W and Mitteldorf C: Mitteldorf,
pathologic diagnosis of cutaneous lymphomas. Dermatol Clin.
33:655–681. 2015. View Article : Google Scholar
|
|
11
|
Hodak E and Amitay-Laish I: Amitay-Laish,
Mycosis fungoides: A great imitator. Clin Dermatol. 37:255–267.
2019. View Article : Google Scholar
|
|
12
|
Campbell SM, Peters SB, Zirwas MJ and Wong
HK: Immunophenotypic diagnosis of primary cutaneous lymphomas: A
review for the practicing dermatologist. J Clin Aesthet Dermatol.
3:21–25. 2010.
|
|
13
|
Ashton-Key M, Diss TC, Du MQ, Kirkham N,
Wotherspoon A and Isaacson PG: The value of the polymerase chain
reaction in the diagnosis of cutaneous T-cell infiltrates. Am J
Surg Pathol. 21:743–747. 1997. View Article : Google Scholar
|
|
14
|
Alaibac M, Pigozzi B, Belloni-Fortina A,
Michelotto A, Saponeri A and Peserico A: CD7 expression in reactive
and malignant human skin T-lymphocytes. Anticancer Res.
23:2707–2710. 2003.
|
|
15
|
Moll M, Reinhold U, Kukel S, Abken H,
Müller R, Oltermann I and Kreysel HW: CD7-negative helper T cells
accumulate in inflammatory skin lesions. J Invest Dermatol.
102:328–332. 1994. View Article : Google Scholar
|
|
16
|
Lu C, Zhang J, Nagahawatte P, Easton J,
Lee S, Liu Z, Ding L, Wyczalkowski MA, Valentine M, Navid F, et al:
The genomic landscape of childhood and adolescent melanoma. J
Invest Dermatol. 135:816–823. 2015. View Article : Google Scholar
|
|
17
|
Skov AG and Gniadecki R: Gniadecki, Delay
in the histopathologic diagnosis of mycosis fungoides. Acta Derm
Venereol. 95:472–475. 2015. View Article : Google Scholar
|
|
18
|
van Doorn R, Van Haselen CW, van Voorst
Vader PC, Geerts ML, Heule F, de Rie M, Steijlen PM, Dekker SK, van
Vloten WA and Willemze R: Mycosis fungoides: Disease evolution and
prognosis of 309 Dutch patients. Arch Dermatol. 136:504–510. 2000.
View Article : Google Scholar
|
|
19
|
Kirsch IR, Watanabe R, O'Malley JT,
Williamson DW, Scott LL, Elco CP, Teague JE, Gehad A, Lowry EL,
LeBoeuf NR, et al: TCR sequencing facilitates diagnosis and
identifies mature T cells as the cell of origin in CTCL. Sci Transl
Med. 7:308ra1582015. View Article : Google Scholar
|
|
20
|
Maitre E, Le-Page AL, Comoz F, Truquet F,
Damaj G, Cornet E, Verneuil L, Salaün V and Troussard X: Usefulness
of flow cytometry for the detection of cutaneous localization in
malignant hematologic disorders. Cytometry B Clin Cytom.
96:283–293. 2019. View Article : Google Scholar
|
|
21
|
Bawazir MA, Almohideb M, Walsh S, Shear NH
and Alhusayen R: Early-stage mycosis fungoides screening
investigations: A retrospective analysis of 440 cases. J Eur Acad
Dermatol Venereol. 32:e217–e218. 2018. View Article : Google Scholar
|
|
22
|
Horna P, Kurant D, Sokol L, Sotomayor EM,
Moscinski L and Glass LF: Flow cytometric identification of
immunophenotypically aberrant T-cell clusters on skin shave biopsy
specimens from patients with mycosis fungoides. Am J Clin Pathol.
143:785–796. 2015. View Article : Google Scholar
|
|
23
|
Rea B, Haun P, Emerson R, Vignali M,
Farooqi M, Samimi S, Elenitsas R, Kirsch I and Bagg A: Role of
high-throughput sequencing in the diagnosis of cutaneous T-cell
lymphoma. J Clin Pathol. 71:814–820. 2018. View Article : Google Scholar
|
|
24
|
de Masson A, O'Malley JT, Elco CP, Garcia
SS, Divito SJ, Lowry EL, Tawa M, Fisher DC, Devlin PM and Teague
JE: High-throughput sequencing of the T cell receptor β gene
identifies aggressive early-stage mycosis fungoides. Sci Transl
Med. 10:eaar58942018. View Article : Google Scholar
|
|
25
|
Weng WK, Armstrong R, Arai S, Desmarais C,
Hoppe R and Kim YH: Minimal residual disease monitoring with
high-throughput sequencing of T cell receptors in cutaneous T cell
lymphoma. Sci Transl Med. 5:214ra1712013. View Article : Google Scholar
|
|
26
|
Torres-Cabala CA: Diagnosis of T-cell
lymphoid proliferations of the skin: Putting all the pieces
together. Mod Pathol. 33 (Suppl 1):83–95. 2019. View Article : Google Scholar
|
|
27
|
Cocks M, Porcu P, Wick MR and Gru AA:
Recent advances in cutaneous T-cell lymphoma: Diagnostic and
prognostic considerations. Surg Pathol Clin. 12:783–803. 2019.
View Article : Google Scholar
|
|
28
|
Liebmann RD, Anderson B, McCarthy KP and
Chow JW: The polymerase chain reaction in the diagnosis of early
mycosis fungoides. J Pathol. 182:282–287. 1997. View Article : Google Scholar
|
|
29
|
Theodorou I, Delfau-Larue MH, Bigorgne C,
Lahet C, Cochet G, Bagot M, Wechsler J and Farcet JP: Cutaneous
T-cell infiltrates: Analysis of T-cell receptor gamma gene
rearrangement by polymerase chain reaction and denaturing gradient
gel electrophoresis. Blood. 86:305–310. 1995. View Article : Google Scholar
|
|
30
|
Wood GS, Tung RM, Haeffner AC, Crooks CF,
Liao S, Orozco R, Veelken H, Kadin ME, Koh H, Heald P, et al:
Detection of clonal T-cell receptor gamma gene rearrangements in
early mycosis fungoides/Sezary syndrome by polymerase chain
reaction and denaturing gradient gel electrophoresis (PCR/DGGE). J
Invest Dermatol. 103:34–41. 1994. View Article : Google Scholar
|
|
31
|
Zelenetz AD: Guidelines for NHL: Updates
to the management of diffuse large B-cell lymphoma and new
guidelines for primary cutaneous CD30+ T-cell
lymphoproliferative disorders and T-cell large granular lymphocytic
leukemia. J Natl Compr Canc Netw. 12 (Suppl 5):797–800. 2014.
View Article : Google Scholar
|
|
32
|
Liu WP, Song YQ, Zheng W, Wang XP, Ding N
and Zhu J: Aggressive behavior and elevated lactate dehydrogenase
at baseline confer inferior prognosis in patients with primary
cutaneous lymphoma. Clin Lymphoma Myeloma Leuk. 13:534–540. 2013.
View Article : Google Scholar
|
|
33
|
Quereux G, Frot AS, Brocard A, Leux C,
Renaut JJ and Dreno B: Routine bone marrow biopsy in the initial
evaluation of primary cutaneous B-cell lymphoma does not appear
justified. Eur J Dermatol. 19:216–220. 2009. View Article : Google Scholar
|