Introduction
Gliomas are the most common primary tumor in the
human brain. According to the World Health Organization (WHO)
standards, gliomas are classified into grades I–IV, in which grades
I and II are considered low-grade gliomas (LGG), while grades III
and IV are high-grade glioma (HGG) (1). Glioblastoma multiforme (GBM), which is
also known as WHO IV gliomas, accounts for approximately 57% of all
gliomas. Despite surgery, radiation therapy and chemotherapy, the
overall prognosis for patients with GBM remains poor, with a median
survival of less than 2 years (2).
In the past decade, photodynamic therapy (PDT), a minimally
invasive treatment method, has been used in glioma treatment.
Compared with traditional therapies, PDT has higher selectivity and
fewer side effects (3).
PDT is based on the principle of generating
cytotoxic reactive oxygen species (ROS) (3,4), which
induces cell apoptosis and tissue destruction photosensitizers
(PSs) by laser activation (5,6).
Recently, PDT has emerged as an effective medical tool for various
cancer treatments (7,8) considering its relatively non-invasive,
selective and repeatable characteristics (9). Despite numerous advantages, PDT has
not yet been accepted for wide clinical practice due to certain
limitations associated with PS (10–12).
Most of the PSs are highly hydrophobic, which leads to poor water
solubility, rapid degradation with clearance in blood circulation
and low bioavailability (12). In
addition, the clinically available PSs have poor tumor specificity
(13), due to their low molecular
weight and fast metabolism (14).
Chlorin e6 (Ce6), as one of the most used second
generation PS molecules in PDT, has been reported to generate ROS
under laser activation, which can effectively damage the structure
and function of cancer cells (15).
It also has been modified in multiple ways to assemble
nanostructures, such as albumin-based nanostructures [PTX
(HSA-Ce6-PTX-RGD-1)] (16) and
peptide-based nanostructures [Fmoc-L-Lys/Ce6 (FCNPs) and CDP/Ce6
(CCNPs)] (17). These nanosystems
increase PS uptake of tumor cells via the enhanced permeability and
retention (EPR) effect, resulting in the increase in photodynamic
therapy efficacy and a decrease in non-specific phototoxicity
(18,19). To formulate nanostructure PSs, low
molecular weight PSs are modified with polymers or antibodies, or
are incorporated into micelles and liposomes (20–23).
MRI is a non-invasive imaging modality and has
several advantages over other imaging modalities, as it provides
three-dimensional anatomic images with high spatial resolution,
which allows for the application of nanomaterials for early and
specific cancer detection and therapy (24,25).
Among them, gadolinium (III)-based contrast-enhanced MRI is a
preferred choice for the clinical diagnosis of gliomas and
preoperative localization. The development of the MRI technique has
increasingly relied on contrast agents (CAs) to improve the
sensitivity (26). Previous studies
have revealed that contrast-enhanced MRI-guided photodynamic
therapy using a bifunctional polymer conjugate containing an MRI
contrast agent and a photosensitizer is effective for tumor imaging
and treatment (27–29).
A variety of theranostics based on different
nanoplatforms have been reported (4,16,17,28).
However, after integrating the imaging contrast agent and therapy
function, the general characteristics of reported theranostics are
their complex designs and heavy structures, which limits their
further application (4). Instead of
simply combining imaging contrast agent and photosensitizers, ideal
theranostics should be refined in design and demonstrate high
efficiency.
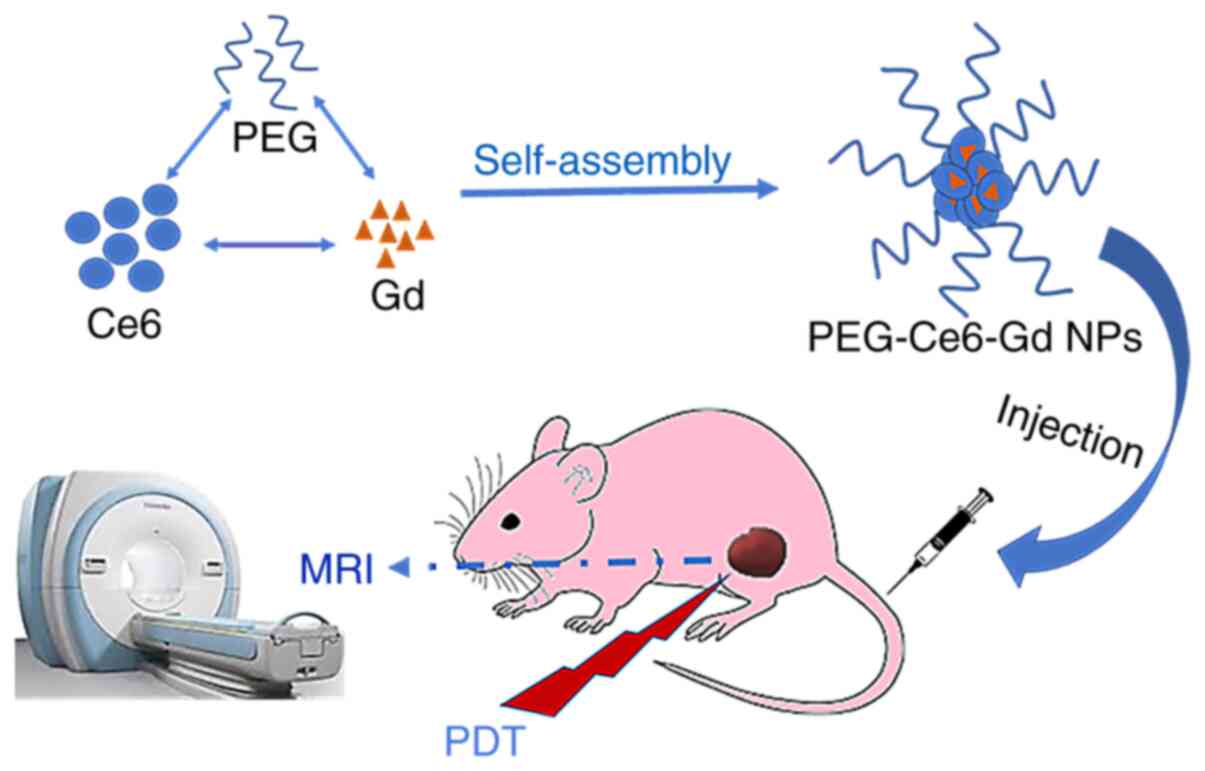
Thus, the aim of the present study was to design
theranostic agent PEG-Ce6-Gd nanoparticles (PEG-Ce6-Gd NPs) to
identify both imaging and therapy functions in gliomas or other
cancer types. PEG is a Food and Drug Administration (FDA)-approved
hydrophilic polymer without immunogenicity, antigenicity or
cytotoxicity (30). PEGylated
nanoparticles present high biocompatibility and water solubility,
as well as prolonged circulation time and enhanced accumulation in
tumor sites via the EPR effect. After covalent binding with Ce6,
the hydrophilicity of Ce6 is improved by reacting to PEG-Ce6. The
PEG-Ce6 is then bound to the Gd(III) (31) to obtain an MRI contrast agent of
PEG-Ce6-Gd. The PEG-Ce6-Gd NPs are then obtained via the
self-assembly of PEG-Ce6-Gd monolayer. These simple but powerful
PEG-Ce6-Gd NPs can facilitate MRI diagnosis and PDT treatment of
gliomas simultaneously, which has great potential in the diagnosis
and PDT treatment of gliomas and potentially other cancer
types.
Materials and methods
Materials
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene
glycol)-2000] (DSPE-PEG2000) was purchased from Shanghai Advanced
Vehicle Technology Co., Ltd. Gadolinium (III) acetate tetrahydrate
[Gd(C2H3O2)3·4H2O],
Ce6, [4,5-Dimethylthiazol-2yl]-2,5-diphenyltetrazolium bromide
(MTT), N,N′-Dicyclohexylcarbodiimide (DCC), N-Hydroxysuccinimide
(NHS), Hoechst-33342, N, N-dimethyl formamide (DMF) and anhydrous
dimethyl sulfoxide (DMSO) were purchased from Shanghai Chemical
Co., Ltd. All the chemicals were of reagent grade and used without
further purification.
The consumables for cell culture and fetal bovine
serum (FBS; Scitecher) were purchased from Beijing Dingguo
Changsheng Biotechnology Co., Ltd.
The entire experiment lasted for 1 year from
synthetic materials PEG-Ce6-Gd NPs to in vitro and in
vivo experiments.
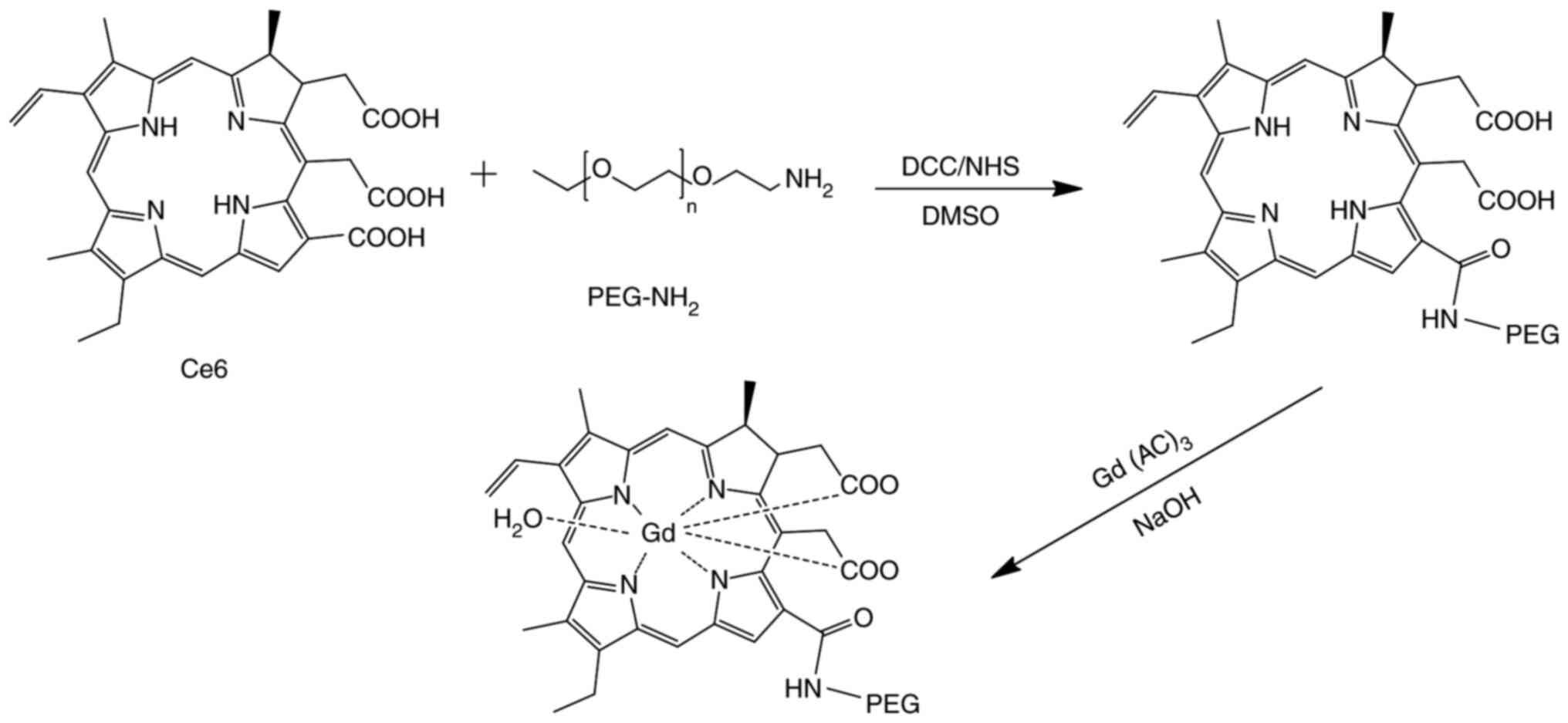
Synthesis and preparation of
PEG-Ce6-Gd NPs
For the synthesis of the NPs, 96 mg Ce6 (0.16 mM),
40 mg DCC (0.192 mM) and 22 mg NHS (0.192 mM) were separately added
to a three-flask containing anhydrous DMF (50 ml). The mixture was
stirred for 2 h in an ice bath. Then, 320 mg PEG-NH2
(0.16 mM) was added to the mixture and stirred for another 24 h at
room temperature. After filtration, NaOH, regulated at pH 8.0, was
added directly to the solution at 50°C. Subsequently, 400 mg
Gd(AC)3 was added and the resulting solution was stirred
for 48 h to obtain the final product after purification.
NPs were prepared via a self-assembly process.
PEG-Ce6-Gd (100 mg) was dissolved thoroughly in 5 ml acetonitrile.
DSPE-PEG (25 mg) in 10 ml ultrapure water was added dropwise into
the solution of acetonitrile under vigorous stirring. The mixture
was then stirred at room temperature for 24 h to remove the
acetonitrile via volatilization (32).
Characterization techniques
A Zeta-Sizer Nano ZS [dynamic light scattering
(DLS), Malvern Panalytical Ltd.] was used to measure the size of
NPs. The morphology was investigated via transmission electron
microscopy (TEM) using JEM-100CXII 100 kV (JEOL, Ltd.). UV-vis
absorption spectra were measured using a Hitachi U-3900
spectrophotometer. Fourier transform infrared spectra (FTIR) was
performed on a Nicolet IS 10 spectrometer (Thermo Fisher
Scientific, Inc.) (6,15).
Measurement of ROS generation of
PEG-Ce6-Gd NPs
A total of 0.78-100 µg/ml of PEG-Ce6-Gd NPs were
incubated with 40 µM 2′,7′-dichlorofluorescin diacetat (DCFH) and
irradiated with 630 nm (200 mW/cm2 for 30 sec).
Subsequently, the fluorescence intensity of dichlorofluorescein
(DCF) was detected using a fluorescence spectrophotometer
(RF-5301pc, Shimadzu) (7,15).
Cell culture
The rat glioma C6 cell line was purchased from
Shanghai Fuheng Cell Center, and was cultured in F12K (Shanghai
Fuheng Cell Center, Shanghai, China). All of the media were
supplemented with 10% FBS and 2% (v/v) streptomycin-penicillin.
Cells were maintained in medium in an air atmosphere with 5%
CO2 at 37°C.
Photocytotoxicity
MTT assay was performed to evaluate the
photocytotoxicity of PEG-Ce6-Gd NPs in comparison to free Ce6. In
two previous studies, cells were incubated with free Ce6 at the
same concentrations (0.5 and 1 µg/ml) for 12 and 24 h, and results
showed that the cell survival rate was identical, approximately 90
and 80% respectively (6,10). Thus, the cells were incubated with
PEG-Ce6-Gd NPs for 12 h in the present study. Briefly, cells were
cultured in 96-well microplates at a concentration of
5×105 per well for 24 h. Then, the different
concentrations of PEG-Ce6-Gd NPs (1.25 µg/ml was the highest) were
added and incubated for 12 h. The cells were washed three times
with PBS and each well underwent laser treatment for 12 min in
total, and then 100 µl of fresh culture medium was added in each
well before incubation for another 24 h. Subsequently, 20 µl of MTT
(5 mg/ml in PBS) was added in each well and incubated for 4 h.
Then, 100 µl of DMSO was added in each well to dissolve the purple
crystal of formazan. Absorbance at 570 nm was measured using a
microplate reader to evaluate cellular metabolic activity (reflects
the number of viable cells). Cell viability was calculated using
the formula: Cell viability
(%)=Asample/Acontrol ×100%, where
Asample and Acontrol represent the absorbance
values for the treated cells and the untreated control cells,
respectively. The Asample and Acontrol values
were obtained by subtracting the absorbance of DMSO. Data are
presented as the mean ± SD. The replicate number was four (6,7).
T1-weighted and
T1-mapping images of PEG-Ce6-Gd
The MRI capability of PEG-Ce6-Gd NPs was
characterized by their capacity to alter the T1
relaxation rate(r1). A clinical MR scanner (3T, Prisma;
Siemens, Healthcare Ltd.) was used to measure the relationship
between the T1 relaxation rate and PEG-Ce6-Gd NPs
concentration. For MRI measurements, each of the 96-well plates was
filled with 150 µl solution to achieve T1-weighted and
T1-mapping images. T1-weighted images were
acquired using a sequence with repetition time (TR) as 700 msec,
echo time (TE) as 12 msec, slice thickness as 2.0 mm, matrix size
as 0.3×0.3×2.0 mm, field of view (FOV) as 120 mm and number of
acquisition as 2. T1-mapping images were acquired using
a sequence with TR as 15 msec, TE as 2.7 msec, slice thickness as
2.0 mm, matrix size as 0.2×0.2×2.0 mm, FOV as 160 mm and number of
acquisition as 14 (31).
Animals and tumor model
All animals received care in compliance with the
guidelines outlined in the Guide for the Care and Use of Laboratory
Animals, and the procedures were approved by the Wuhan University
of China Animal Care and Use Committee.
All animals received care in compliance with the
guidelines outlined in the Guide for the Care and Use of Laboratory
Animals, and the procedures were approved by the Wuhan University
of China Animal Care and Use Committee. A total of 20 female BALB/c
nude mice (age, 6-8 weeks; weight, 16-18 g) were purchased from
Beijing Huafukang Bioscience Co., Ltd., and housed under specific
pathogen-free conditions (60% relative humidity; 20°C, room
temperature) with a 12-h light/dark cycle, provided and maintained
with free access to food and water. The animal health and behavior
were monitored once a day. If the tumor-bearing mice had a rapid
weight loss of >20% or could not eat or drink, they were
euthanized by cervical dislocation after anesthesia with 5%
isoflurane; the euthanasia was confirmed by checking there was no
heart rate. No mice were found dead during the experiment. The
treatment experiment lasted for 10 days. For tumor-bearing mice, C6
cells were suspended in fresh culture medium F12K after
trypsinization. Then, a density of 2×106 C6 glioma cells
was subcutaneously injected in the right flank of each mice
(31).
In vivo T1-weighted and
T1-mapping MRI
MRI was performed out on a Siemens Prima 3.0T MRI
scanner. Mice were anesthetized with 5% isoflurane at room
temperature and 2% isoflurane was maintained during subsequent
scans. Prior to administration of PEG-Ce6-Gd NPs, the pre-contrast
images of mice were obtained. Then, the mice were scanned at 15,
30, and 45 min, as well as 1 and 3 h after PEG-Ce6-Gd NPs injection
via the tail vein, respectively. T1-weighted images were
acquired using a sequence with TR as 700 msec, TE as 12 msec, slice
thickness as 2.0 mm, matrix size as 0.3×0.3×2.0 mm, FOV as 120 mm
and number of acquisition as 2. T1-mapping images were
acquired using a sequence with TR as 15 msec, TE as 2.7 msec, slice
thickness as 2.0 mm, matrix size as 0.2×0.2×2.0 mm, FOV as 160 mm
and number of acquisition as 14. The replicate number was four
(31).
Therapeutic studies in vivo
The tumor was left to inoculate for 5-7 days to
achieve an average volume of 200 mm3. The anti-tumor
effect of PEG-Ce6-Gd NPs was studied under laser irradiation.
Mice-bearing subcutaneous tumors were randomly separated into four
groups with five mice in each: i) Control group, received PBS
injection; ii) PEG-Ce6-Gd NPs group, received PEG-Ce6-Gd NPs
injection; iii) laser group, received laser irradiation; and iv)
PDT group, received PEG-Ce6-Gd NPs and laser irradiation. The
treatment was performed for 10 days. After the first therapy, tumor
size was measured every 2 days using an external digital caliper.
In addition, every 2 days 200 µl of PEG-Ce6-Gd NPs (1 mg/ml of free
Ce6 equivalent) was intravenously injected via the tail vein into
the tumor-bearing mice in groups 2 and 4. In group 3, irradiation
was performed with an intensity of 0.80 W/cm2 for 10
min. Specifically, in group 4, irradiation was executed after 1 h
of injection with an intensity of 0.80 W/cm2 for 10 min.
Tumor volumes were measured every 2 days of post-treatment and
estimated by using the formula: Tumor volume=1/2 × a ×
b2, where ‘a’ represents the largest tumor diameter and
‘b’ represents the shortest tumor diameter. This experiment didn't
provide other treatments such as ulcer treatment. The replicate
number was four (33).
Histological analysis
The mice were sacrificed when the in vivo
observation was completed. The tumors and major tissues (heart,
lung, liver, spleen and kidney) were collected, washed, fixed with
a 4% paraformaldehyde solution at 4°C overnight, and embedded in
paraffin. Then, 5-µm sections were obtained and stained using
hematoxylin and eosin (H&E) (11,14).
Statistics analysis
Statistical analysis of the results was performed
using SPSS 23.0 (IBM Corp.). Data are presented as the mean ± SD,
and the differences among the groups were analyzed with Bonferroni
comparison tests following ANOVA. P<0.05 was considered to
indicate a statistically significant difference. T1-mapping images
of various concentrations of PEG-Ce6-Gd NPs incubated without cells
and with C6 cells via MRI were the data generated for Fig. 5C and D.
Results
Preparation and characterization of
PEG-Gd-Ce6 NPs
A flow chart of the whole experiment is presented in
Fig. 1. The synthesis and chemical
structure of PEG-Ce6-Gd NPs are shown in Fig. 2. The nanoparticle was self-assembled
and chelated with Gd ion into Ce6 via a chelating agent Ce6. The
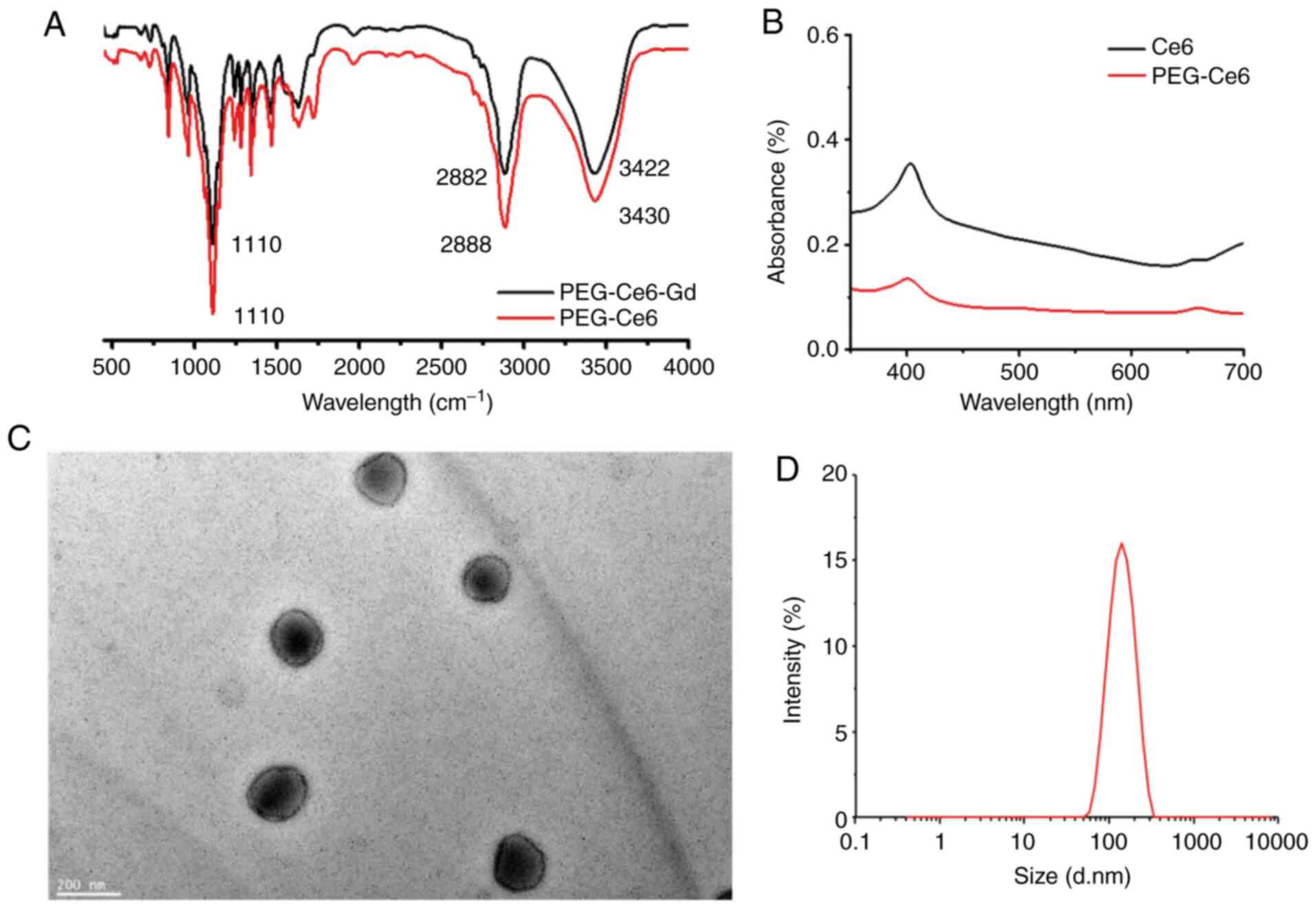
formation of the PEG-Ce6-Gd NPs and PEG-Ce6 was confirmed from the
characteristic bands of PEG-Ce6-Gd NPs (in 1110, 2882, and 3422
cm−1) and PEG-Ce6 (in 1110, 2882, and 3430
cm−1) in the FTIR spectra (Fig. 3A). The UV/V is absorption spectra of
Ce6 and PEG-Ce6 in the solution displayed a typical porphyrin ring,
appearing more intense in the range of 350-450 nm and less intense
in the range of 450-700 nm (Fig.
3B). The morphology was evaluated via TEM images. The TEM
images of PEG-Ce6-Gd NPs demonstrated a spherical shape within a
cyclohexane with a uniform size of about 120 nm in diameter
(Fig. 3C). The average hydrodynamic
diameter of PEG-Ce6-Gd NPs was measured to be 130 nm using DLS
(Fig. 3D), which was large compared
with that measured via TEM due to a difference in the measurement
mechanism.
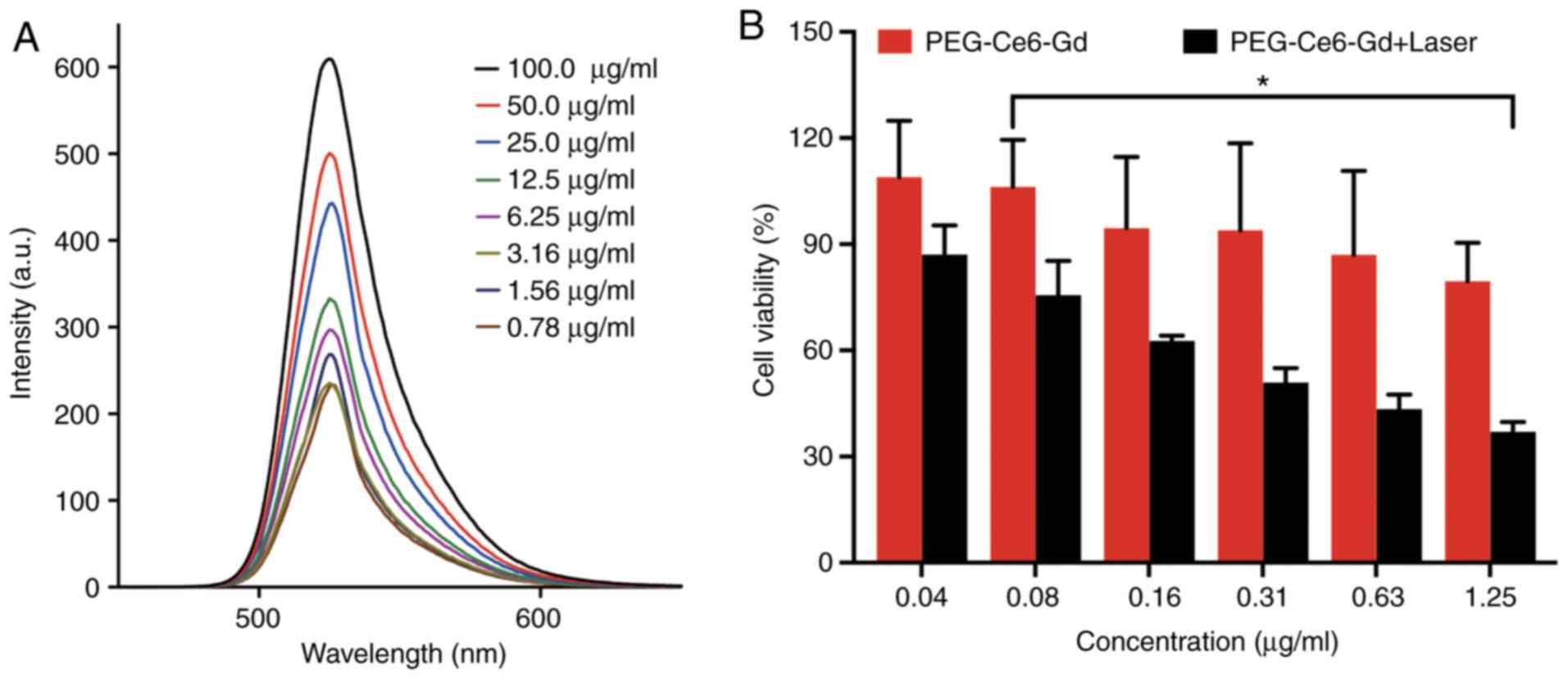
ROS generation of PEG-Ce6-Gd NPs
PDT destroys tissues via the production of ROS
generated under laser irradiation. Laser irradiation of a
sensitizer results in the production of ROS that, in the presence
of DCFH, leads to the formation of DCF, a highly fluorescent
compound that is easy to detect (34). Thus, the DCF method was used to
measure the ROS generation of PEG-Ce6-Gd NPs under 630 nm laser
irradiation. As presented in Fig.
4A, the fluorescence intensity of DCF, which was proportional
to the ROS production, increased with an increasing concentration
of PEG-Ce6-Gd NPs.
Cell viability
C6 cells were incubated with PEG-Ce6-Gd NPs at
different concentrations for 12 h in a 96-well plate. The
cytotoxicity of the PEG-Ce6-Gd NPs was evaluated separately in the
absence and presence of laser irradiation. The results indicated
that in the absence of laser irradiation, PEG-Ce6-Gd NPs has a
favorable biocompatibility and low cytotoxicity in tumor cells,
with >80% cell viability even at the highest concentration (1.25
µg/ml). However, in the presence of laser irradiation, PEG-Ce6-Gd
NPs significantly affected cell viability which was reduced to 40%
at the highest concentration (1.25 µg/ml). These results indicated
the photocytotoxicity effects of PEG-Ce6-Gd NPs on tumor cells
under laser irradiation, which suggested that PEG-Ce6-Gd NPs may be
promising for the photodynamic therapy in cancer treatment
(Fig. 4B).
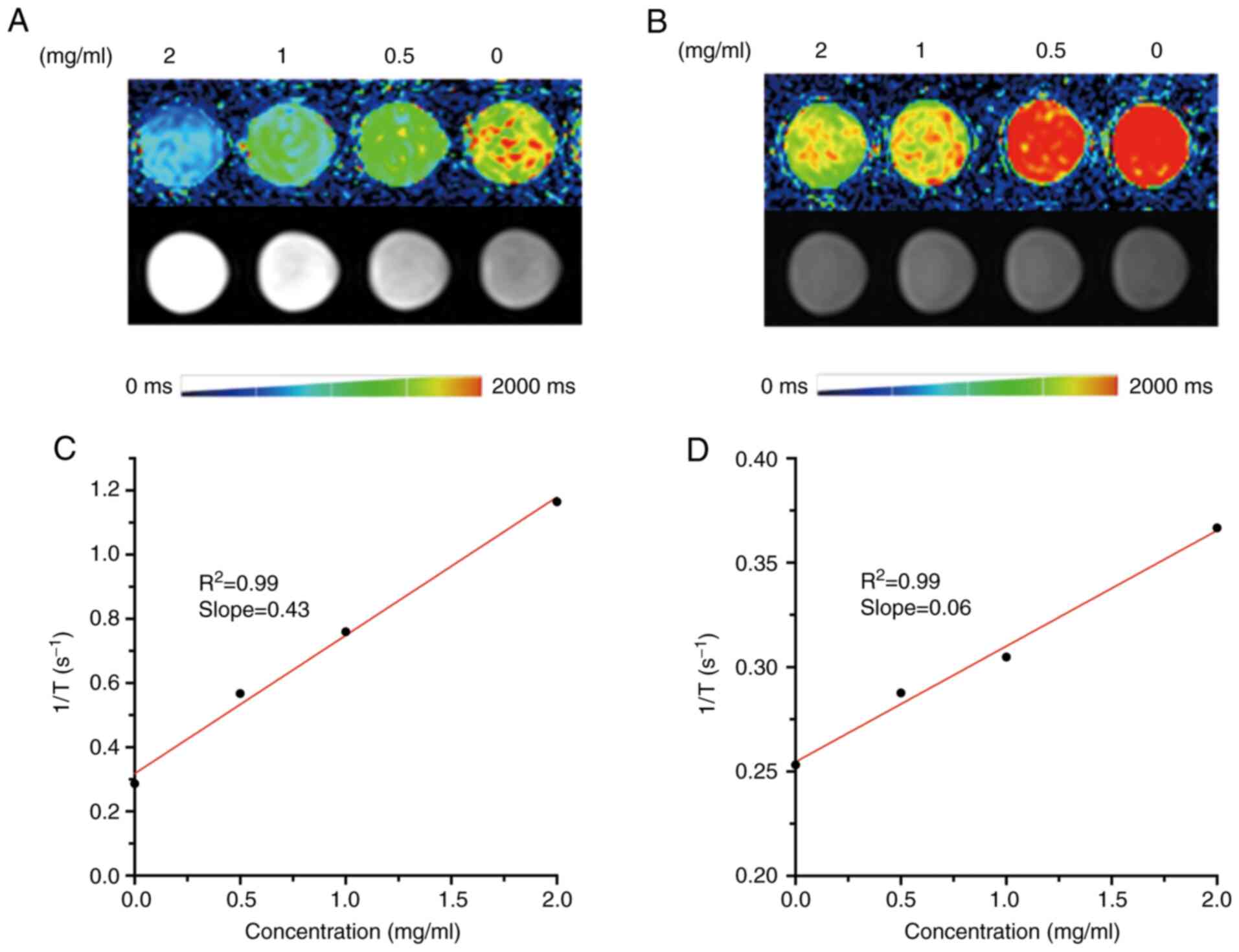
In vitro MRI of PEG-Ce6-Gd NPs
The MRI contrast efficacy of PEG-Ce6-Gd NPs was
evaluated in vitro before its application for cancer
treatment. The MRI contrast of the PEG-Ce6-Gd NPs was evaluated by
measuring the T1 relaxation rate(r1) values
as a function of Gd3+ concentration. Various
concentrations of nanomaterials PEG-Ce6-Gd NPs incubated without
cells and with C6 cells were selected as shown in Fig. 5. The data obtained via a 3.0 T
Siemens Prisma demonstrated that in T1-weighted MRI, the
MR signal intensity of PEG-Ce6-Gd NPs can be detected with
different enhancement, which increased linearly according to the
concentration influencing the T1 relaxation time. The
corresponding image-intensity color mapping also indicated that the
PEG-Ce6-Gd NPs significantly shortened the T1 relaxation
time (Fig. 5A and B). Further
analysis suggested that the T1-weighted MR signal
intensity of PEG-Ce6-Gd NPs increased linearly with Gd3+
concentration. As shown in Fig. 5C,
the r1 of PEG-Ce6-Gd NPs was 0.43 mg/ml−1
s−1 which indicated that PEG-Ce6-Gd NPs had a promising
contrast agent property. C6 cells had efficient light uptake after
PEG-Ce6-Gd NPs were added and an enhancement on MRI was observed
(Fig. 5D).
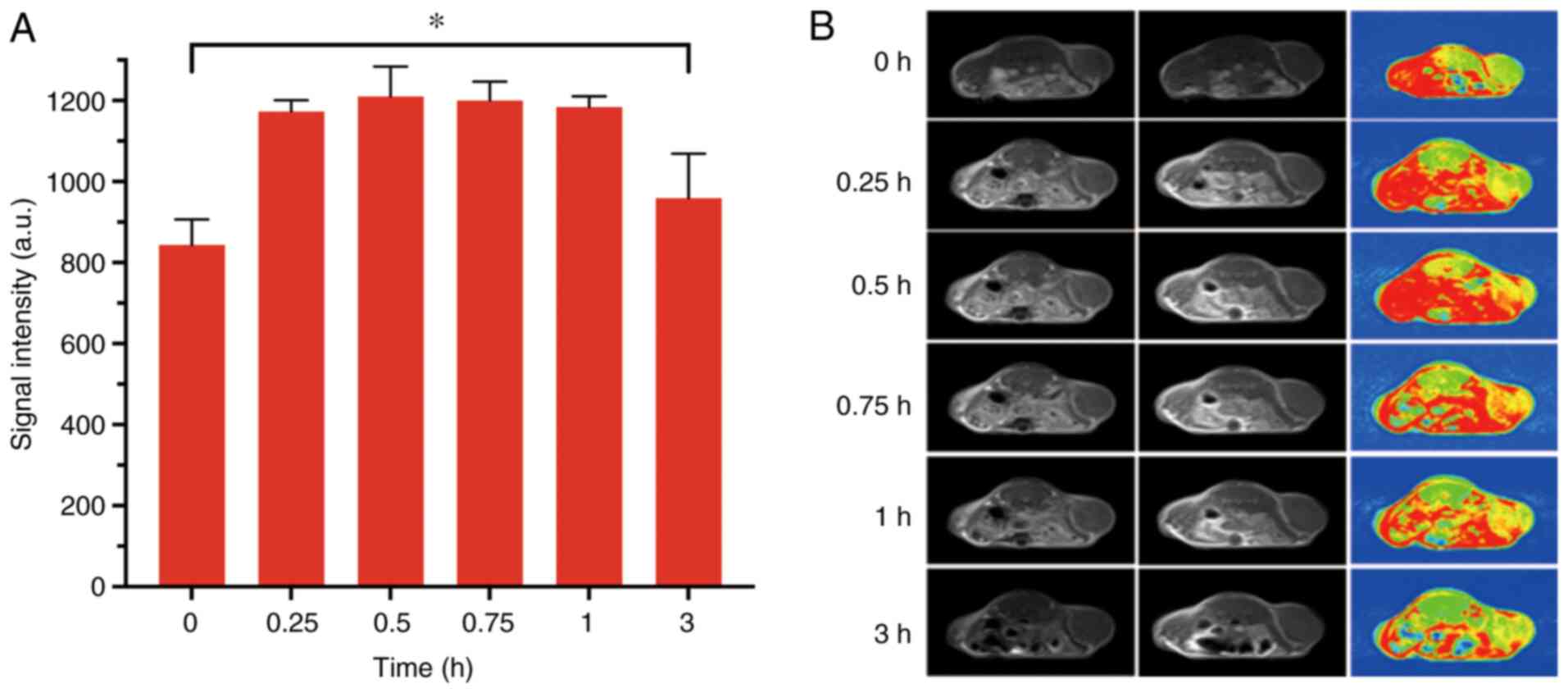
MRI in subcutaneous mouse models of
glioma
To obtain further insight into the MRI contrast
property of PEG-Ce6-Gd NPs, in vivo MRI was performed on C6
tumor xenograft-bearing nude mice after separate injection of
PEG-Ce6-Gd NPs at different time points (pre-injection, 0.25, 0.5,
0.75, 1 and 3 h) via the tail vein. As is evident in Fig. 6, significant enhancement was
identified in the T1-weighted MR images of the tumors.
The tumor site turned markedly bright after 0.25 h of injection of
PEG-Ce6-Gd NPs. After 0.5 h of injection, the contrast enhancement
in the T1-weighted MR image reached a maximum level.
After 3 h of injection, the signal intensity decreased gradually
with the prolonging time. These results indicated that PEG-Ce6-Gd
NPs has a favorable MRI contrast characteristic.
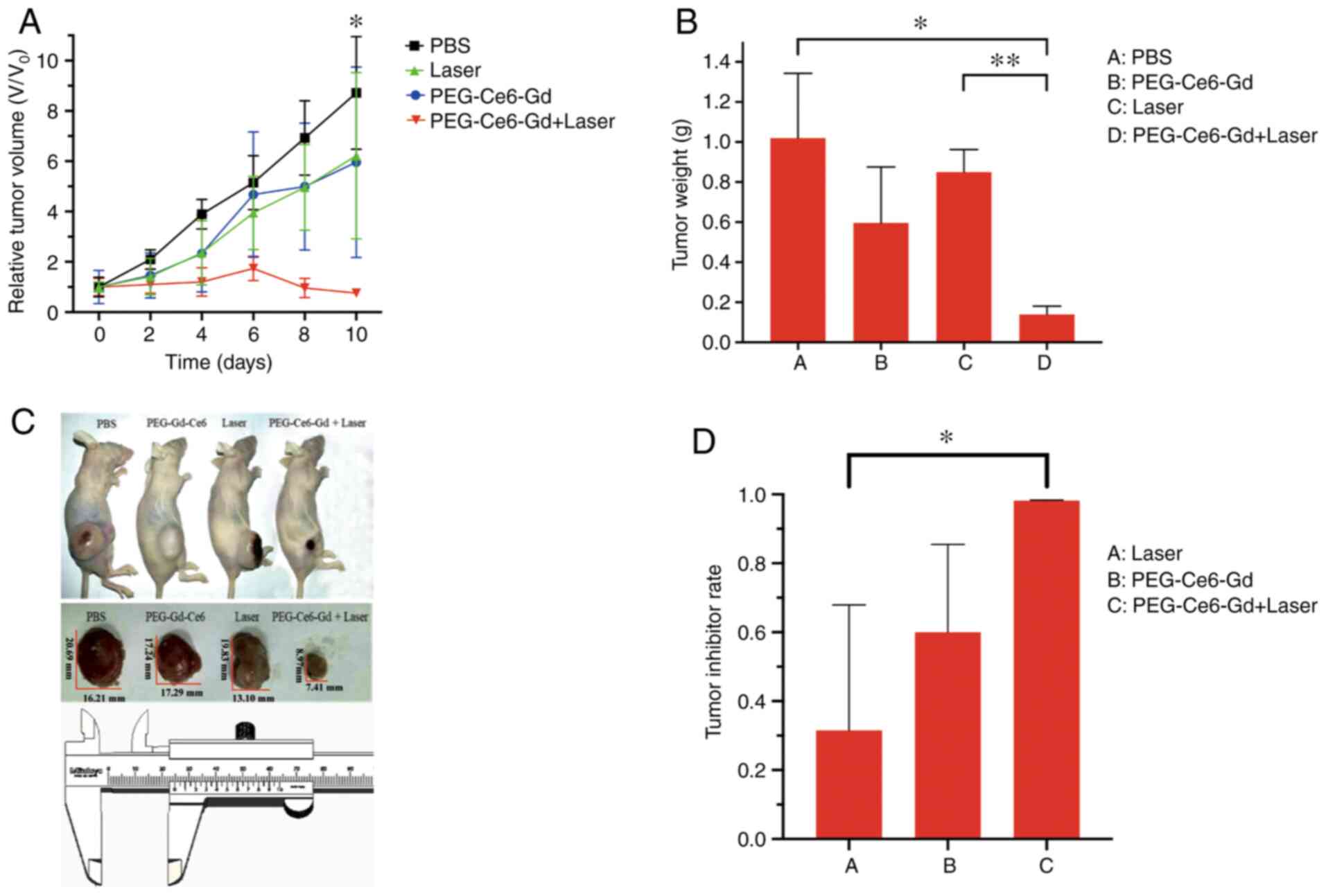
Anti-tumor effect in vivo
To quantitatively evaluate the photodynamic
therapeutic efficacy of the nanomaterial, the mice-bearing tumors
were separated into four groups: i) Control group, received PBS
injection; ii) PEG-Ce6-Gd NPs group, received PEG-Ce6-Gd NPs
injection; iii) laser group, received laser irradiation; and iv)
PDT group, received PEG-Ce6-Gd NPs injection and laser irradiation.
As shown in Fig. 7A and Table SI, the tumors in the PBS group
without treatment grew rapidly, which were about 8 times larger
compared with the initial tumor volume. Moreover, the tumors of the
two groups only irradiated with laser or injected with PEG-Ce6-Gd
NPs without laser irradiation were not suppressed, and were about 6
times larger compared with the initial tumor volume. Tumors in PDT
treatment using PEG-Ce6-Gd NPs injection under laser irradiation
group were significantly suppressed and the tumor volume showed
little increase after 10 days of treatment.
After treatment for 10 days, the tumors were removed
and weighed. The tumor destruction was obvious in PDT group and the
weight of tumor in PDT group was the lightest, indicating that
laser irradiation combined with PEG-Ce6-Gd NPs had a cytotoxic
effect on the tumor (Fig. 7B and C
and Table SII). Following further
detailed analysis, it was found that the laser only treatment group
or PEG-Ce6-Gd NPs only treatment group demonstrated a tumor
inhibition rate of 31 and 60%, respectively. However, the PDT group
had a high tumor inhibition rate of 98%. These results demonstrated
that the combined laser irradiation and PEG-Ce6-Gd NPs enhanced the
anti-tumor ability (Fig. 7D).
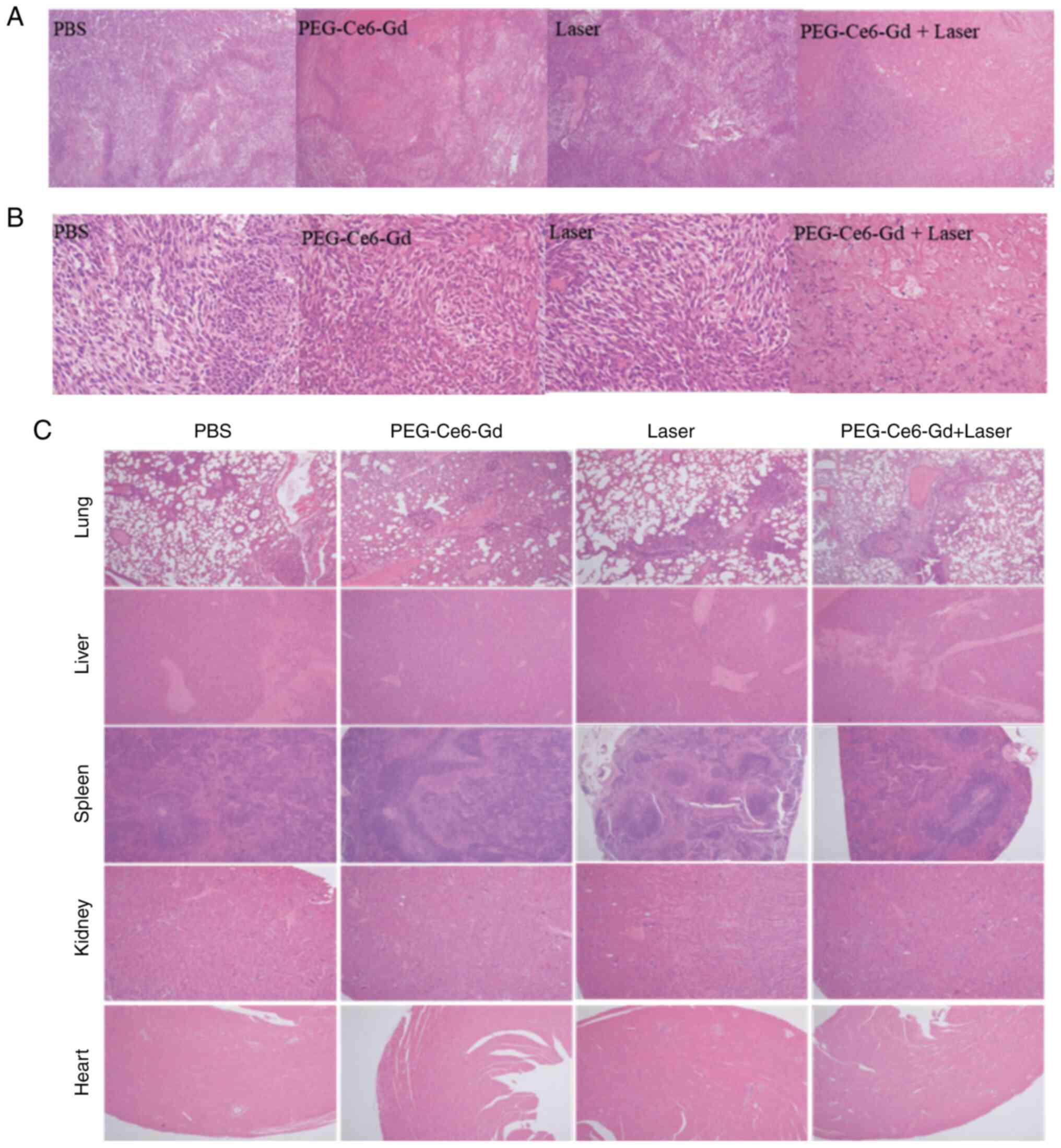
Histological analysis
H&E staining identified that the tumor in the
PDT group was significantly suppressed compared with that the in
control, PEG-Ce6-Gd NPs or laser groups (Fig. 8A and B). In addition, the mice did
not have an inflammatory response in major organs, such as the
lung, liver, spleen, kidney and heart, further indicating that
PEG-Ce6-Gd NPs at a tested dose had very low toxicity in
vivo (Fig. 8C). These results
demonstrated that PEG-Ce6-Gd NPs can effectively inhibit tumors
under laser irradiation with low side effects.
Discussion
The present study reported a type of PEGylated
Ce6-Gd NPs, which utilized Ce6 as a binding chelator of
paramagnetic metal ion (Gd3+), and simultaneously
retained the photodynamic therapy function of Ce6. The high
hydrophobic property of Ce6 enables it to easily to form aggregates
in aqueous solution, which limits its 1O2
production. Moreover, hydrophobicity could hinder its solubility in
physiological solvents and body fluids, thereby limiting its
clinical application (35). PEG is
a widely used hydrophilic polymer, which can prolong blood
circulation time and improve biosecurity. In addition, PEGylated
NPs accumulate within the tumor stroma via the EPR effect, which
improves the therapeutic effect (36). Therefore, in the present study,
introducing a hydrophilic group, such PEG, to improve the
hydrophilicity of Ce6 is a possible method for developing advanced
theranostics. In addition, the available unpaired electrons in Ce6
were employed to bind Gd3+ and served as a ligands. The
PEG-Ce6-Gd NPs exhibited acceptable longitudinal relaxation while
maintaining the excellent yields of ROS. These characteristics
ensure the imaging contrast effect and PDT therapy of
PEG-Ce6-Gd.
Enhanced MRI at different time points can
effectively and non-invasively visualize the real-time
pharmacokinetics of NPs in mice tumor models. In the present study,
the MRI results demonstrated that PEG-Ce6-Gd NPs was relatively
prolonged in the blood circulation, and the signal remained
consistent within 0.5-1 h. By contrast, the clinic contrast agent
Gd-DOTA was rapidly metabolized within 30 min (37). This difference may be due to the
hydrophilicity of PEG, as well as the reduced recognition by liver
macrophages (Kupffer cells) and splenic macrophages.
In the present study, the tumors were inhibited to a
greater extent with PEG-Ce6-Gd NPs under irradiation. Moreover,
PEG-Ce6-Gd NPs had no effect on other healthy tissues. Compared
with traditional treatment methods, PDT has its own advantages.
Chemotherapy and radiotherapy can respectively induce systemic
toxicity and destroy healthy tissues, while PDT itself has no toxic
effect on the biological system. PDT also has minimal invasiveness,
has repeatability without cumulative toxicity and can be used as an
adjunctive therapy following surgical resection, which decreases
residual tumor tissue, reduces recurrence rate and improves the
quality of life of patients (36).
However, some limitations of this study were first
that PEG-Ce6-Gd NPs were not used in the orthotopic glioma model.
The typical penetration depth of red light in living tissues used
in PDT is only 1-3 mm (36);
therefore, PDT cannot treat deep tumors. Second, 5-aminolevulinic
acid (ALA), acts as a photosensitizer, has been evaluated
clinically for glioma PDT (38). We
should compare the therapeutic effect of PEG-Ce6-Gd NPs with 5-ALA
in glioma. Thus, future studies will use PEG-Ce6-Gd NPs in an
orthotopic glioma model to observe its therapeutic effect and
compare wtih 5-ALA.
In conclusion, multifunctional NPs (PEG-Ce6-Gd NPs)
were synthesized via a self-assembly process, which was designed
for cancer diagnosis and treatment. The prepared non-toxic
PEG-Ce6-Gd NPs were identified as promising nano-agents for
photodynamic therapy and contrast-enhanced MRI diagnosis. The
synthesized NPs were able to significantly increase its
phototoxicity under laser irradiation, thus inducing death of
cancer cell death. In vitro and in vivo studies
demonstrated the beneficial therapeutic efficacy of PEG-Ce6-Gd NPs
under laser irradiation. All the observations indicated its
potential in clinical PDT cancer treatment. Thus, this novel
theranostic agent, PEG-Ce6-Gd NPs, could facilitate diagnosis and
PDT treatment of gliomas, and potentially other cancer types.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was financially supported by National
Natural Science Foundation of China (grant nos. 81701685 and
81771819) and the National Key Research and Development Program of
China (grant no. 2017YFC0108803). The authors from the corporation
only provide the technical support and have no financial
interest.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AB and DX wrote the initial draft of the paper. KD
collected and analyzed the data of material characterization, AB,
and YS Li collected and analyzed in vitro experimental data.
AB provided a nude mouse tumor model; AB, and DX provided the MRI
scanning for the contrast and the animal; Bo Wu and H-BX conceived
the idea of the study and provided the funding. All authors have
given approval to the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests, and all authors should confirm its accuracy.
References
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan AC, Ashley DM, López GY, Malinzak M,
Friedman HS and Khasraw M: Management of glioblastoma: State of the
art and future directions. CA Cancer J Clin. 70:299–312. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Celli JP, Spring BQ, Rizvi I, Evans CL,
Samkoe KS, Verma S, Pogue BW and Hasan T: Imaging and photodynamic
therapy: Mechanisms, monitoring, and optimization. Chem Rev.
110:2795–2838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhaumik J, Mittal AK, Banerjee A, Chisti Y
and Banerjee UC: Applications of phototheranostic nanoagents in
photodynamic therapy. Nano Res. 8:1373–1394. 2015. View Article : Google Scholar
|
|
5
|
Lovell JF, Liu TW, Chen J and Zheng G:
Activatable photosensitizers for imaging and therapy. Chem Rev.
110:2839–2857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hou W, Xia F, Alves CS, Qian X, Yang Y and
Cui D: MMP2-Targeting and redox-responsive PEGylated chlorin e6
nanoparticles for cancer near-infrared imaging and photodynamic
therapy. ACS Appl Mater Interfaces. 8:1447–1457. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan F, Yu Y, Zhong F, Gao M, Sun T, Liu J,
Zhang H, Qian H, Tao W and Yang X: Design of tumor
acidity-responsive sheddable nanoparticles for
fluorescence/magnetic resonance imaging-guided photodynamic
therapy. Theranostics. 7:1290–1302. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dolmans DE, Fukumura D and Jain RK:
Photodynamic therapy for cancer. Nat Rev Cancer. 3:380–387. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Castano AP, Demidova TN and Hamblin MR:
Mechanisms in photodynamic therapy: Part two-cellular signaling,
cell metabolism and modes of cell death. Photodiagnosis Photodyn
Ther. 2:1–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu K, Xing R, Zou Q, Ma G, Möhwald H and
Yan X: Simple peptide-tuned self-assembly of photosensitizers
towards anticancer photodynamic therapy. Angew Chem Int Ed Engl.
55:3036–3039. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xing R, Liu K, Jiao T, Zhang N, Ma K,
Zhang R, Zou Q, Ma G and Yan X: An injectable self-assembling
collagen-gold hybrid hydrogel for combinatorial antitumor
photothermal/photodynamic therapy. Adv Mater. 28:3669–3676. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Ma K, Jiao T, Xing R, Shen G and
Yan X: Water-insoluble photosensitizer nanocolloids stabilized by
supramolecular interfacial assembly towards photodynamic therapy.
Sci Rep. 7:429782017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allison RR, Downie GH, Cuenca R, Hu XH,
Childs CJ and Sibata CH: Photosensitizers in clinical PDT.
Photodiagnosis Photodyn Ther. 1:27–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vaidya A, Sun Y, Feng Y, Emerson L, Jeong
EK and Lu ZR: Contrast-enhanced MRI-guided photodynamic cancer
therapy with a pegylated bifunctional polymer conjugate. Pharm Res.
25:2002–2011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yue C, Zhang C, Alfranca G, Yang Y, Jiang
X, Yang Y, Pan F, de la Fuente JM and Cui D: Near-infrared light
triggered ROS-activated theranostic platform based on Ce6-CPT-UCNPs
for simultaneous fluorescence imaging and chemo-photodynamic
combined therapy. Theranostics. 6:456–469. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Q, Wang X, Wang C, Feng L, Li Y and
Liu Z: Drug-induced self-assembly of modified albumins as
nano-theranostics for tumor-targeted combination therapy. ACS Nano.
9:5223–5233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abbas M, Zou Q, Li S and Yan X:
Self-assembled peptide- and protein-based nanomaterials for
antitumor photodynamic and photothermal therapy. Adv Mater.
29:2017. View Article : Google Scholar
|
|
18
|
Maeda H, Wu J, Sawa T, Matsumura Y and
Hori K: Tumor vascular permeability and the EPR effect in
macromolecular therapeutics: A review. J Control Release.
65:271–284. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vrouenraets MB, Visser GW, Snow GB and van
Dongen GA: Basic principles, applications in oncology and improved
selectivity of photodynamic therapy. Anticancer Res. 23:505–522.
2003.PubMed/NCBI
|
|
20
|
Shiah JG, Sun Y, Peterson CM, Straight RC
and Kopecek J: Antitumor activity of N-(2-hydroxypropyl)
methacrylamide copolymer-Mesochlorine e6 and adriamycin conjugates
in combination treatments. Clin Cancer Res. 6:1008–1015.
2000.PubMed/NCBI
|
|
21
|
Jiang FN, Liu DJ, Neyndorff H, Chester M,
Jiang SY and Levy JG: Photodynamic killing of human squamous cell
carcinoma cells using a monoclonal antibody-photosensitizer
conjugate. J Natl Cancer Inst. 83:1218–1225. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Nostrum CF: Polymeric micelles to
deliver photosensitizers for photodynamic therapy. Adv Drug Deliv
Rev. 56:9–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Derycke AS and de Witte PA: Liposomes for
photodynamic therapy. Adv Drug Deliv Rev. 56:17–30. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Estelrich J, Sánchez-Martín MJ and
Busquets MA: Nanoparticles in magnetic resonance imaging: From
simple to dual contrast agents. Int J Nanomedicine. 10:1727–1741.
2015.PubMed/NCBI
|
|
25
|
Sun BO, Fang Y, Li Z, Chen Z and Xiang J:
Advances in the application of nanotechnology in the diagnosis and
treatment of gastrointestinal tumors. Mol Clin Oncol. 3:274–280.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang J, Zhong X, Wang L, Yang L and Mao
H: Improving the magnetic resonance imaging contrast and detection
methods with engineered magnetic nanoparticles. Theranostics.
2:86–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li G, Slansky A, Dobhal MP, Goswami LN,
Graham A, Chen Y, Kanter P, Alberico RA, Spernyak J, Morgan J, et
al: Chlorophyll-a analogues conjugated with aminobenzyl-DTPA as
potential bifunctional agents for magnetic resonance imaging and
photodynamic therapy. Bioconjug Chem. 16:32–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kopelman R, Lee Koo YE, Philbert MA,
Moffat B, Reddy GR, Mcconville P, Hall DE, Chenevert TL, Bhojani
MS, Buck SM, et al: Multifunctional nanoparticle platforms for in
vivo MRI enhancement and photodynamic therapy of a rat brain
cancer. J Magn Magn Mater. 293:404–410. 2005. View Article : Google Scholar
|
|
29
|
Gross S, Gilead A, Scherz A, Neeman M and
Salomon Y: Monitoring photodynamic therapy of solid tumors online
by BOLD-contrast MRI. Nat Med. 9:1327–1331. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Larson N and Ghandehari H: Polymeric
Conjugates for Drug Delivery. Chem Mater. 24:840–853. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu B, Li XQ, Huang T, Lu ST, Wan B, Liao
RF, Li YS, Baidya A, Long QY and Xu HB: MRI-guided tumor
chemo-photodynamic therapy with Gd/Pt bifunctionalized porphyrin.
Biomater Sci. 5:1746–1750. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim KS, Kim J, Kim DH, Hwang HS and Na K:
Multifunctional trastuzumab-chlorin e6 conjugate for the treatment
of HER2-positive human breast cancer. Biomater Sci. 6:1217–1226.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi H, Liu Q, Qin X, Wang P and Wang X:
Pharmacokinetic study of a novel sonosensitizer chlorin-e6 and its
sonodynamic anti-cancer activity in hepatoma-22 tumor-bearing mice.
Biopharm Drug Dispos. 32:319–332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bourré L, Thibaut S, Briffaud A, Rousset
N, Eléouet S, Lajat Y and Patrice T: Indirect detection of
photosensitizer ex vivo. J Photochem Photobiol B. 67:23–31. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lim CK, Heo J, Shin S, Jeong K, Seo YH,
Jang WD, Park CR, Park SY, Kim S and Kwon IC: Nanophotosensitizers
toward advanced photodynamic therapy of cancer. Cancer Lett.
334:176–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lucky SS, Soo KC and Zhang Y:
Nanoparticles in photodynamic therapy. Chem Rev. 115:1990–2042.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu D, Lu ST, Li YS, Baidya A, Mei H, He Y
and Wu B: Evaluation of methotrexate-conjugated gadolinium(III) for
cancer diagnosis and treatment. Drug Des Devel Ther. 12:3301–3309.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mahmoudi K, Garvey KL, Bouras A, Cramer G,
Stepp H, Jesu Raj JG, Bozec D, Busch TM and Hadjipanayis CG:
5-aminolevulinic acid photodynamic therapy for the treatment of
high-grade gliomas. J Neurooncol. 141:595–607. 2019. View Article : Google Scholar : PubMed/NCBI
|