Introduction
Ovarian cancer is one of the most common malignant
tumors and poses a major threat to the health of women worldwide
(1). Patients with ovarian cancer
do not usually exhibit early symptoms and there is a lack of
effective screening and diagnostic methods (1,2).
Therefore, ~70% of patients with ovarian cancer are diagnosed at an
advanced stage (III–IV), after invasion and metastasis have
occurred. Furthermore, ovarian cancer is associated with
treatment-related complications and poor prognosis (2).
MicroRNAs (miRNA or miRs) are important regulatory
factors that regulate gene expression, cell proliferation and
differentiation, tumorigenesis and tumor progression (3). miRNAs exert their affects via base
complementary pairing with target gene mRNA, degrading
corresponding mRNA or suppressing mRNA translation (4). Recent research has indicated that
certain miRNAs are abnormally expressed in ovarian cancer and are
involved in tumor pathogenesis, development, metastasis and
resistance to treatment (4). The
association between miRNAs and ovarian cancer has been extensively
investigated, and miRNAs have become a focus of interest in the
diagnosis and treatment of ovarian cancer (3,4).
Epithelial-to-mesenchymal transition (EMT) is an
important event during invasion and metastasis of ovarian cancer
cells and involves multiple signaling pathways and complex
molecular mechanisms (5).
EMT-related proteins include interleukin-2-inducible T-cell kinase
and T-cell-specific kinase (6).
Members of the zinc finger E-box binding homeobox
(ZEB) family are important transcription factors that induce EMT
(6). ZEB proteins directly or
indirectly suppress the expression of adhesion proteins, such as
epithelial (E)-cadherin, at the transcriptional level (6) to promote EMT (7). Research into the role of the ZEB
family in the EMT of epithelial ovarian cancer cells may provide
novel therapeutic targets for slowing ovarian cancer invasion and
metastasis (7).
Ovarian cancer is one of the most common tumors of
the female reproductive system (8)
and is associated with poor prognosis. EMT is a biological process
during which epithelial cells acquire a mesenchymal phenotype
through specific pathways. Multiple tumor microenvironment
cytokines, including epidermal growth factor, endothelin 1 and bone
morphogenetic protein, are involved in EMT during the pathogenesis
and progression of ovarian cancer (8) and regulate related signaling pathways
to promote cancer development and metastasis (9). Additionally, EMT is associated with
chemoresistance in ovarian cancer (9).
One of the hallmarks of EMT is the reduced
expression of cell adhesion molecules, including E-cadherin
(10). E-cadherin belongs to the
cadherin family and its lowered expression is closely associated
with tumor invasion and dedifferentiation. It was previously
indicated that abnormal E-cadherin expression is closely associated
with the pathogenesis and development of ovarian cancer (10), and research into the regulation of
E-cadherin expression in ovarian cancer has become a focus of
interest.
Zheng et al (11) reported that miR-199b-5p inhibits
triple-negative breast cancer cell proliferation, migration and
invasion. Koshizuka et al (12) demonstrated that the miR-199 family
inhibits cancer cell migration and invasion in head and neck cancer
(12). In the present study, the
role of miRNA-199b-3p in ovarian cancer progression and its
possible mechanism of action was investigated.
Materials and methods
Patients and samples
Patients with ovarian cancer (aged 57-64 years) and
healthy volunteers (controls; aged 55-60 years) were recruited from
Weihai Municipal Hospital between July and December 2014. Serum
samples were centrifuged at 1,000 × g for 10 min at 4°C and stored
at −80°C. The current study protocol was approved by the Medical
Ethics Committee of Weihai Municipal Hospital. Written informed
consent was obtained from all participants.
The inclusion criteria were as follows: Ovarian
cancer was confirmed by pathological examination, all cases of
ovarian cancer were non-metastatic and none of the patients had
received chemoradiotherapy or radiotherapy. The exclusion criteria
were as follows: Patients with metastatic tumours and/or those who
had received prior chemoradiotherapy or radiotherapy. Overall
survival (OS) and disease-free survival (DFS) were determined by
follow-up via telephone communication.
Reverse transcription quantitative PCR
(RT-qPCR) analysis
Microarray experiments were performed at Genminix
Informatics, Co. Ltd. Gene expression profiles were analyzed with
the Human Exon 1.0 ST GeneChip (Affymetrix; Thermo Fisher
Scientific, Inc.).
Total RNA was isolated from tissue samples using
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. cDNA was synthesized
using Moloney murine leukemia virus reverse transcriptase (Promega
Corporation). qPCR was then performed using SYBR® Premix
Ex Taq™, according to the manufacturer's protocol (Takara
Biotechnology Co., Ltd.). The primers were as follows:
miRNA-199b-3p: sense, 5′-CCAGAGGACACCTCCACTCC-3-3′ and antisense,
5′-GGGCTGGGTTAGACCCTCGG3′; and U6: sense,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and antisense,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The thermocycling conditions were as
follows: 95°C for 15 min, followed by 40 cycles at 95°C for 2 sec,
60°C for 20 sec and 72°C for 20 sec. Gene expression was quantified
using the 2−ΔΔCq method (13).
Microarray experiments
High miRNA-199b-3p expression in patients with
ovarian cancer was considered as 0.5-1-fold higher compared with
its expression in controls. Low miRNA-199b-3p expression in
patients with ovarian cancer was considered as <0.5 of its
expression in controls. High ZEB1 expression in patients with
ovarian cancer was ≥2-fold higher compared with its expression in
controls, and low ZEB1 expression was considered as 1-2-fold lower
compared with its expression in controls. The correlation between
ZEB1 and miRNA-199b-3p expression was analyzed using Pearson's
correlation test.
Cell culture and transfection
OVCAR-3 cells were purchased from Shanghai Cell
Bank, Chinese Academy of Sciences, and were cultured in RPMI-1640
medium (Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Thermo Fisher Scientific, Inc.) and incubated with 5%
CO2 at 37°C.
Plasmids for ZEB1 (50 ng, 5′-TTCTCCTTCACTTATGAAGC-3′
and 5′-AGTCAAGCAAGCAGCTTAGGACAAAAAGTA−3′), E-cadherin (50 ng,
5′-GAGTATGTCCACCGTGTCCAGCGAA-3′ and
5′-TTTGTTGTTTGTTGTGTAAATGCAA-3′), miRNA-199b-3p (50 ng,
5′-GCCACCAGTGTTCAGACTACC−3′ and 5′-TAGTCTGCACATTGGTTAGGC−3′), and
negative mimics (50 ng, 5′-TTCTCCGAACGTGTCACGT-3′ and
5′-TTCTCTAGAACGTGTCAT−3′) were purchased from Sangon Biotech Co.,
Ltd. and were transfected into OVCAR-3 cells using Lipofectamine™
2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
Dual-luciferase reporter assay
Human ZEB1 3′-UTR genes were cloned into pGL3
luciferase reporter plasmids to construct pGL3-ZEB1 vectors. The
aforementioned plasmids were co-transfected with pGL3-ZEB1 vectors
using Lipofectamine™ 2000 reagent (Thermo Fisher Scientific, Inc.).
Dual-luciferase reporter assays (Promega Corporation) were
performed 48 h after transfection using Dual-luciferase reporter
kit (Promega Corporation). Renilla luciferase activity was
used to normalize data.
Cell proliferation assay
After 48 h of transfection, OVCAR-3 cells
(1×103 cells/well in 96-well plates) were incubated with
20 µl MTT reagent (5 g/l) for 4 h at 37°C. DMSO (150 µl/well) was
then added to OVCAR-3 cells for 20 min at 37°C. Optical density
(OD) was measured at 490 nm using a microplate reader (AD-340;
Beckman Coulter, Inc.).
Transwell assay
The Transwell assay was performed using 24-well
Transwell chambers (Corning Inc.). After 48 h of transfection,
OVCAR-3 cells (1×105 cells/well) were seeded into the
upper chamber and 500 µl DMEM with 10% FBS were added to the lower
chamber to act as a chemoattractant. Cells migrating to the lower
surface of the filter were fixed with 4% paraformaldehyde at room
temperature for 15 min. Following incubation for 48 h, the cells
were stained with 0.1% crystal violet solution at room temperature
for 5 min and observed using an Olympus BX51 microscope (Olympus
Corporation) at a magnification of ×40.
Wound healing assay
After 48 h of transfection, OVCAR-3 cells
(1×105 cells/well) were cultured in 6-well plates
without FBS at 37°C and a linear scratch was created in the center
of the well using a 100-µl micropipette tip. Images were captured
at 0 and 24 h of incubation with an Olympus BX51 microscope
(Olympus Corporation) at a magnification of ×40.
Flow cytometric analysis of apoptosis
using FITC
After 48 h of transfection, OVCAR-3 cells
(1×106 cells/well) were washed with PBS for 10 min and
fixed with 4% paraformaldehyde for 15 min at room temperature.
Cells were stained using an Annexin-V/propidium iodide assay (BD
Biosciences) according to the manufacturer's protocol for 15 min at
room temperature in the dark. Apoptosis rate was determined using a
BD Accuri™ C6 flow cytometer (BD Biosciences) and analyzed by
FlowJo software (version 7.6.1; FlowJo LLC).
Western blotting
Total protein was extracted using RIPA lysis buffer
(Beyotime Institute of Biotechnology) and protein concentration was
quantified using a BCA Protein Assay kit, according to the
manufacturer's protocol (Thermo Fisher Scientific, Inc.). Equal
amounts of protein (50 µg/lane) were separated by SDS-PAGE on 10%
gels and then transferred onto PVDF membranes (Bio-Rad
Laboratories, Inc.). The membranes were blocked with 5% non-fat
milk with Tris-buffered saline with 0.1% Tween-20 for 1 h at 37°C
and probed with antibodies against ZEB1 (cat. no. sc-81428; 1:500
dilution; Santa Cruz Biotechnology, Inc.), checkpoint kinase 1
(CHK1; cat. no. sc-377231; 1:500 dilution; Santa Cruz
Biotechnology, Inc.), E-cadherin (cat. no. sc-71007; 1:500
dilution; Santa Cruz Biotechnology, Inc.), EMT (sc-23902; 1:500
dilution; Santa Cruz Biotechnology, Inc.) and GAPDH (cat. no.
sc-69778; 1:5,000 dilution; Santa Cruz Biotechnology, Inc.) at 4°C
overnight. The membranes were then incubated with goat anti-rabbit
horseradish peroxidase-conjugated IgG (cat. no. sc-2004; 1:5,000
dilution; Santa Cruz Biotechnology, Inc.) at 37°C for 1 h and
visualized with an enhanced chemiluminescence detection kit
(Sigma-Aldrich; Merck KGaA). Protein results were analyzed using
Image_Lab_3.0 (Bio-Rad Laboratories, Inc.).
Lactate dehydrogenase (LDH) activity
and caspase-3/9 activity levels
LDH activity levels at 48 h post-transfection were
measured using an LDH activity kit (cat. no. C0017; Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol, and OD was measured at 450 nm using a microplate reader
(AD-340; Beckman Coulter, Inc.).
Caspase-3/9 activity levels at 48 h
post-transfection were assessed using caspase-3/9 activity kits
(cat. nos. C1116 and C1158; Beyotime Institute of Biotechnology)
according to the manufacturer's protocol, and OD was measured at
450 nm using a microplate reader (AD-340; Beckman Coulter,
Inc.).
In vivo model
A total of 12 nude male mice (aged 5-6 weeks and
weighing 17-19 g) were collected from the Animal Laboratory of
Shandong University. All mice were housed at 23-25°C, 55-60%
humidity, 12-h light/dark cycle, and were given free access to food
and water. The mice were inoculated with 200 µl OVCAR-3 cells
(1×107 cell/ml) by subcutaneous injection in the axilla.
Every 3 days, the tumor size was measured using a vernier caliper.
The maximum tumor size was ≤1 cm3. The mice were
euthanized by decapitation after being anesthetized with 50 mg/kg
pentobarbital sodium [Sangon Biotech (Shanghai) Co., Ltd.] by
intraperitoneal administration. All animal experiments were
approved by the Ethics Committee of Weihai Municipal Hospital.
Immunofluorescence
After 48 h of transfection, OVCAR-3 cells were fixed
with 4% paraformaldehyde at room temperature for 15 min. OVCAR-3
cells were blocked with 5% BSA and 0.25% Triton X-100 in PBS for 1
h at room temperature, and observed using an Olympus BX51
microscope (Olympus Corporation) at a magnification of ×40.
Statistical analysis
Data are presented as the mean ± standard deviation
and experiments were performed in triplicate using SPSS 22.0 (IBM
Corp.). Two-tailed Student's t-test or one-way ANOVA with
Bonferroni post hoc test were used to analyze data depending on the
result. P<0.05 was considered to indicate a statistically
significant difference.
Results
miRNA-199b-3p and ZEB1 expression in
patients with ovarian cancer
To investigate the mechanism of ovarian cancer
progression, changes in miRNA expression between normal and ovarian
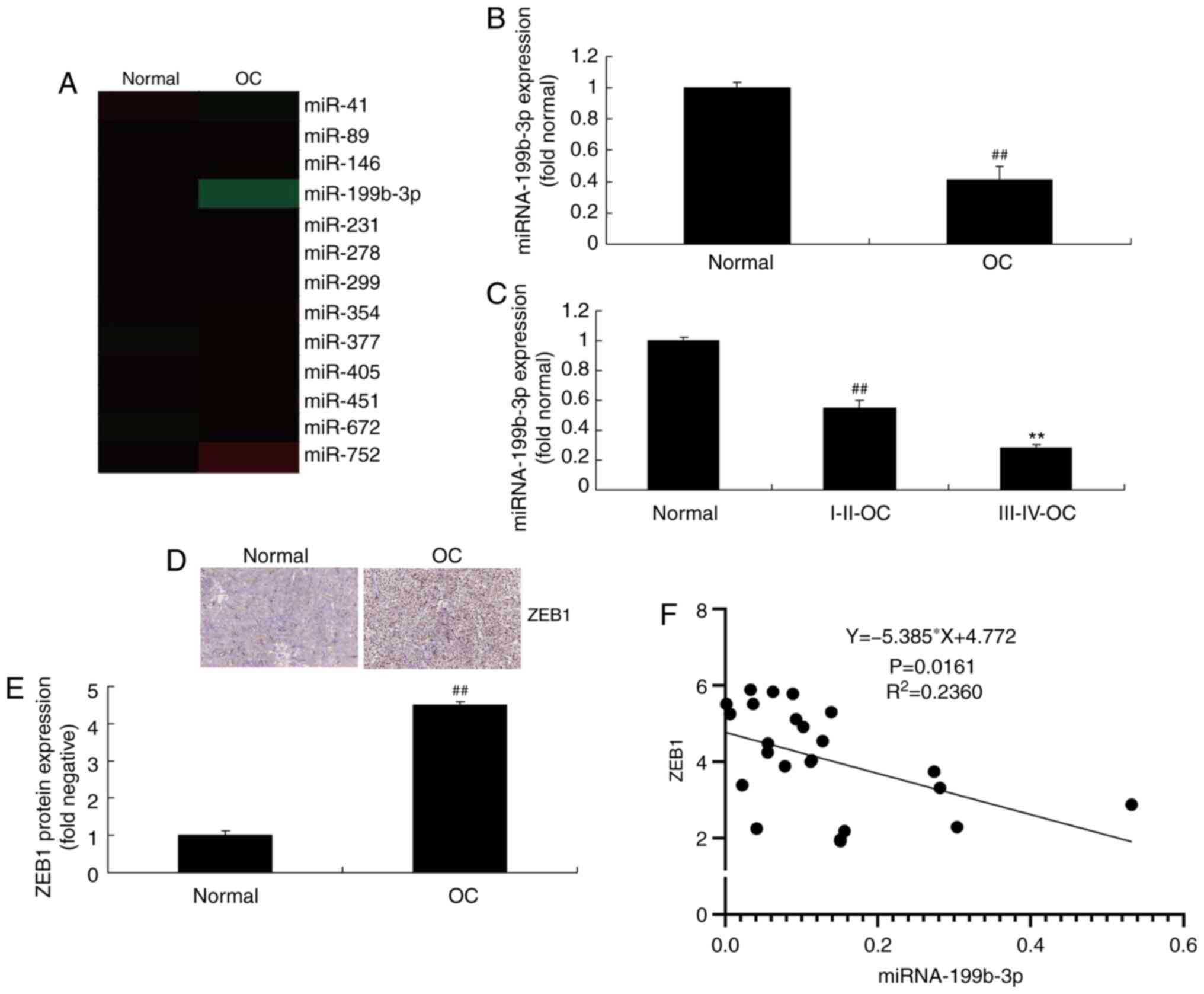
cancer tissues were analyzed. The results demonstrated that
miRNA-199b-3p expression was reduced in patients with ovarian
cancer compared with that in controls (Fig. 1A and B). Furthermore, the expression
of miRNA-199b-3p in patients with stage III–IV ovarian cancer was
decreased compared with that in patients with stage I–II disease
(Fig. 1C). In addition, ZEB1
expression was increased in patients with ovarian cancer compared
with that in controls (Fig. 1D and
E). There was a negative correlation between ZEB1 and
miRNA-199b-3p expression (Fig. 1F).
These results demonstrated that miRNA-199b-3p may be involved in
the progression of ovarian cancer.
miRNA-199b-3p and ZEB1 expression
regulates OS and DFS in patients with ovarian cancer
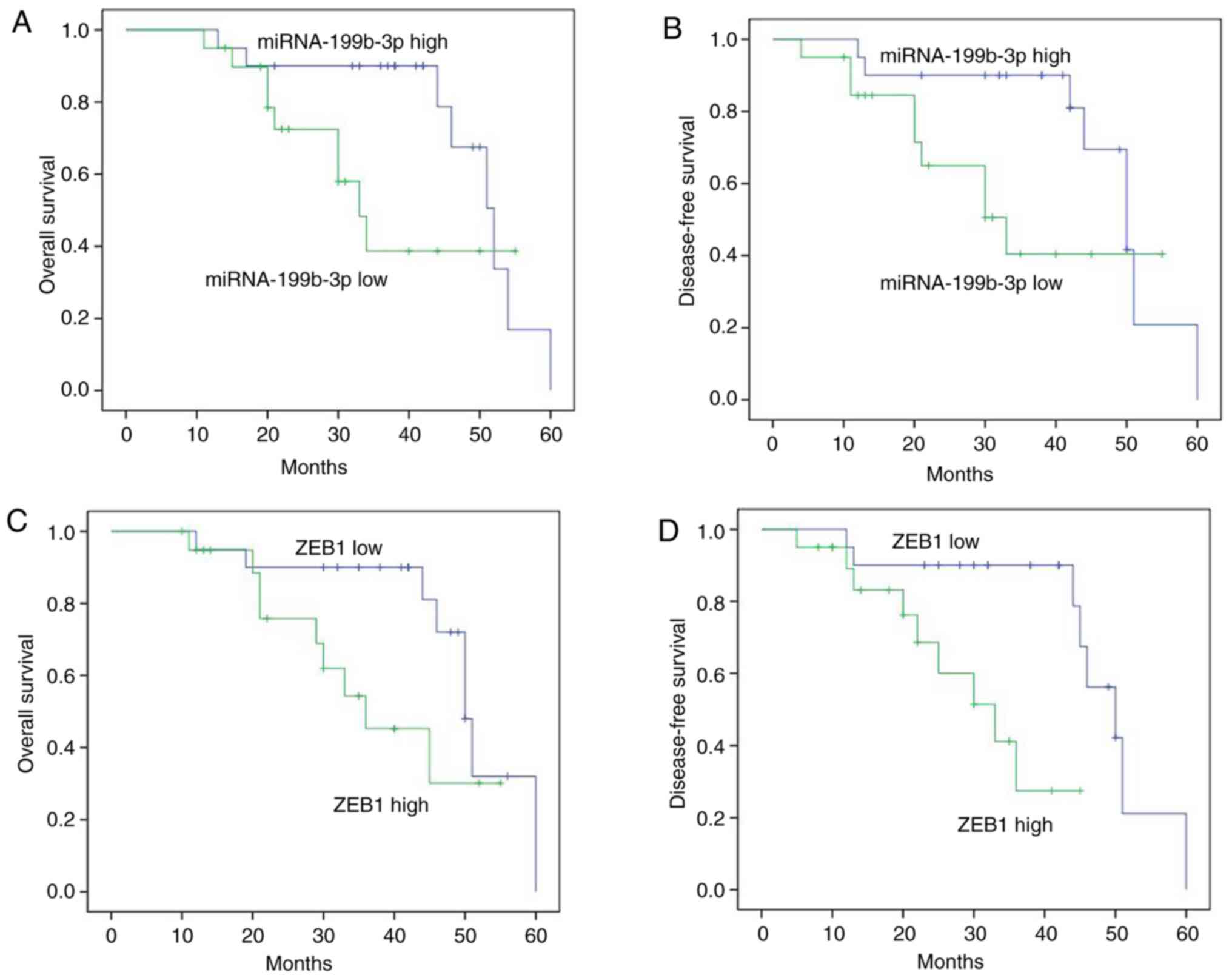
The results demonstrated that miRNA-199b-3p and ZEB1
expression regulated OS and DFS in patients with ovarian cancer.
The OS and DFS in patients with ovarian cancer exhibiting high
miRNA-199b-3p expression were longer compared with those exhibiting
low expression (Fig. 2A and B).
Furthermore, the OS and DFS in patients with ovarian cancer and low
ZEB1 expression were longer compared with those exhibiting high
expression (Fig. 2C and D).
miRNA-199b-3p expression regulates
ovarian cancer cell proliferation and apoptosis in an in vitro
model of ovarian cancer
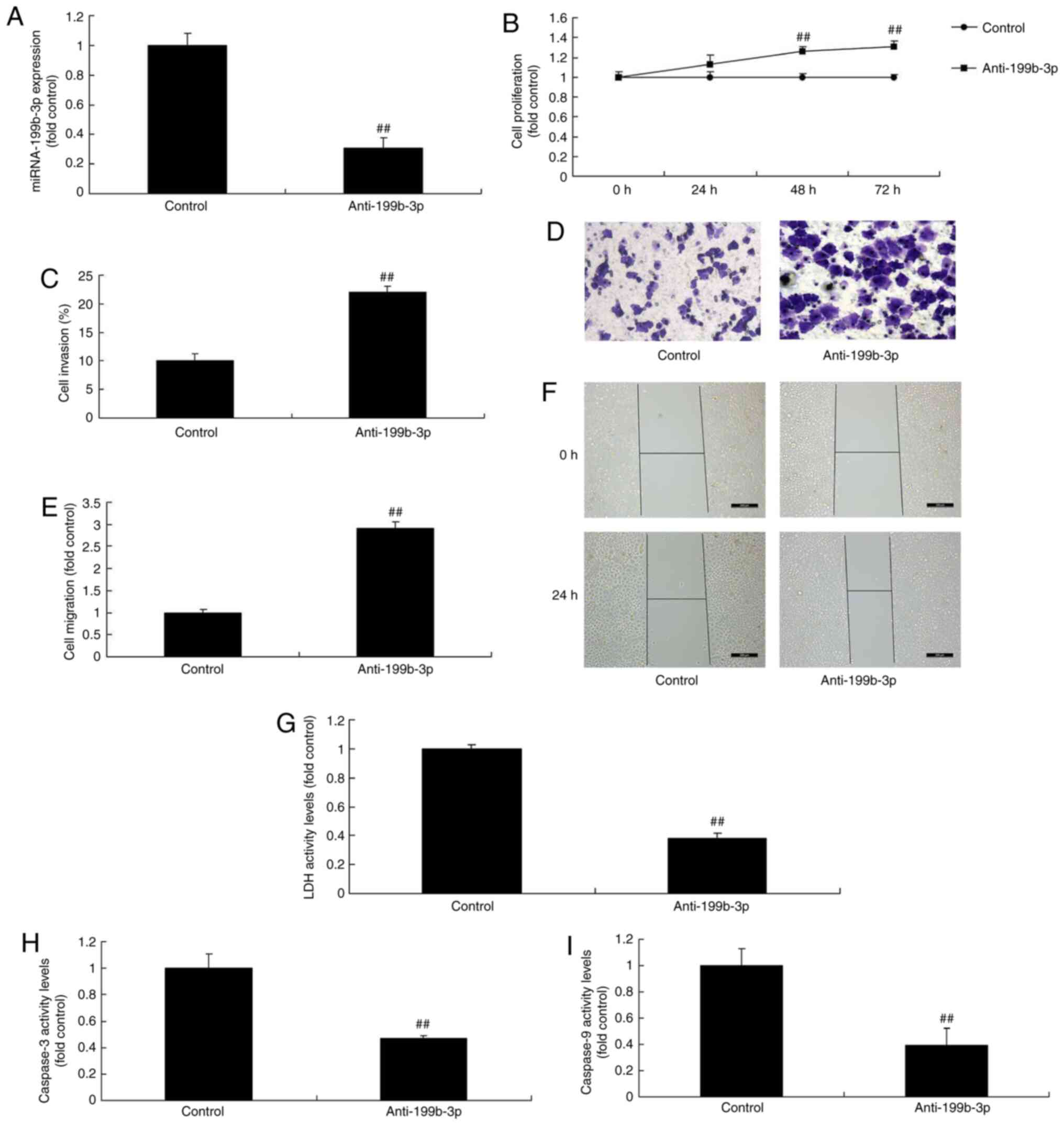
Anti-miRNA-199b-3p mimics were used to decrease
miRNA-199b-3p expression in an in vitro model of ovarian
cancer, and were compared with negative mimics (Fig. 3A) in order to investigate the
effects of miRNA-199b-3p on ovarian cancer progression. The results
demonstrated that miRNA-199b-3p inhibition increased cell
proliferation, migration and invasion, and reduced LDH and
caspase-3/9 activity levels compared with negative mimics (Fig. 3B-I). Furthermore, miRNA-199b-3p
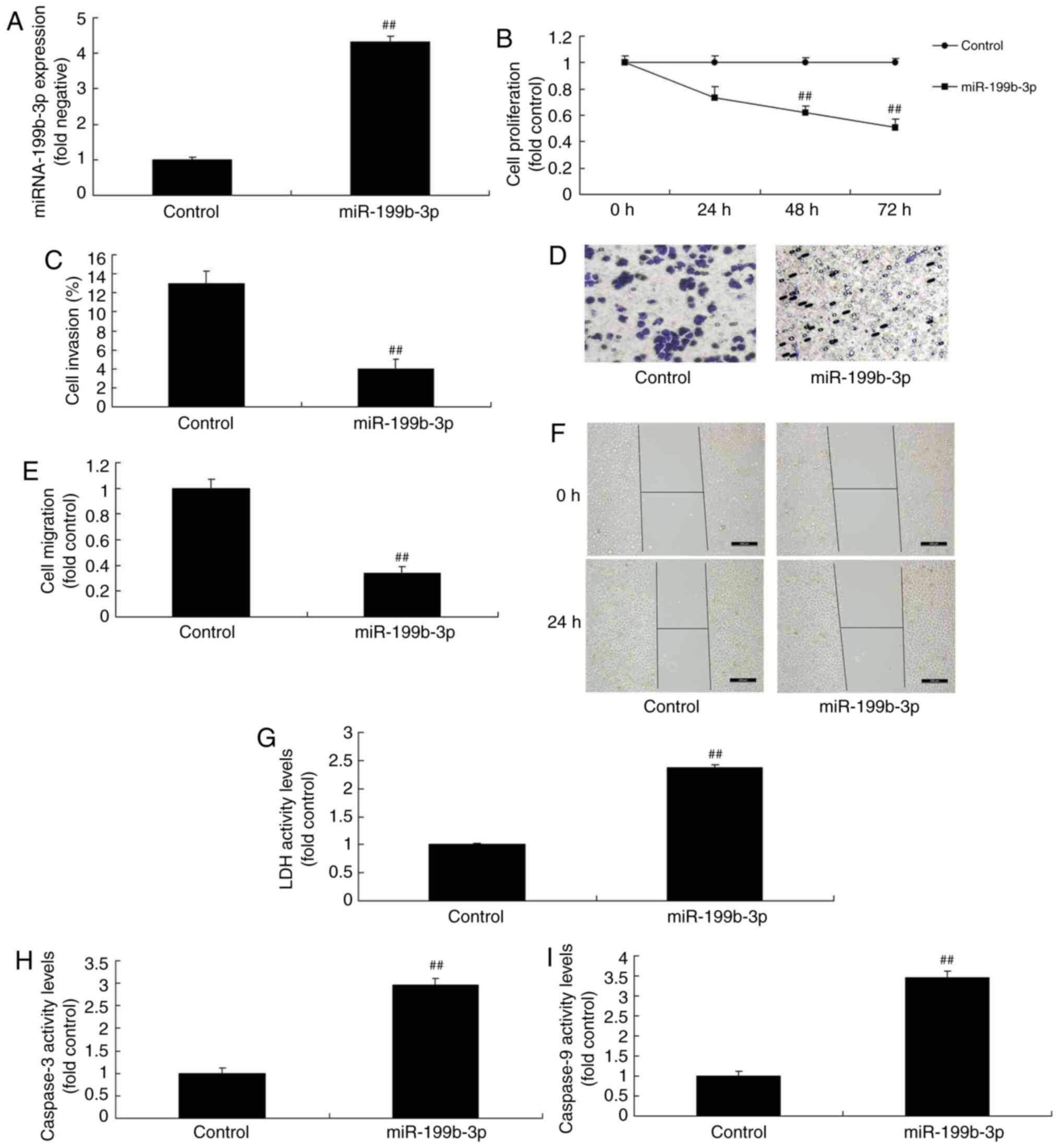
mimics were used to increase miRNA-199b-3p expression in an in
vitro model of ovarian cancer (Fig.
4A). The results revealed that miRNA-199b-3p overexpression
reduced cell proliferation, migration and invasion, and increased
LDH and caspase-3/9 activity levels in an in vitro model of
ovarian cancer compared with negative mimics (Fig. 4B-I). The results demonstrated that
miRNA-199b-3p expression regulated ovarian cancer cell
proliferation in vitro.
miRNA-199b-3p expression regulates
ZEB1 in an in vitro model of ovarian cancer
To investigate the mechanism of miRNA-199b-3p in
ovarian cancer progression, a gene chip was employed to analyze the
signaling pathway involved in an in vitro model of ovarian
cancer following miRNA-199b-3p overexpression. The results
demonstrated that miRNA-199b-3p overexpression suppressed ZEB1 and
CHK1 expression, and induced E-cadherin and EMT expression in an
in vitro model of ovarian cancer (Fig. 5A). Furthermore, the 3′-UTR of ZEB1
was a direct target site for miRNA-199b-3p (Fig. 5B). Luciferase activity levels, ZEB1
and CHK1 expression were reduced in response to miRNA-199b-3p
overexpression compared with negative controls (Fig. 5C-F and I). Additionally, E-cadherin
and EMT expression were increased compared with negative controls
(Fig. 5G-I). In comparison,
miRNA-199b-3p downregulation induced ZEB1 and CHK1 expression and
suppressed E-cadherin and EMT expression compared with negative
controls (Fig. 6). These results
suggested that miRNA-199b-3p expression regulated ZEB1 signaling in
an in vitro model of ovarian cancer.
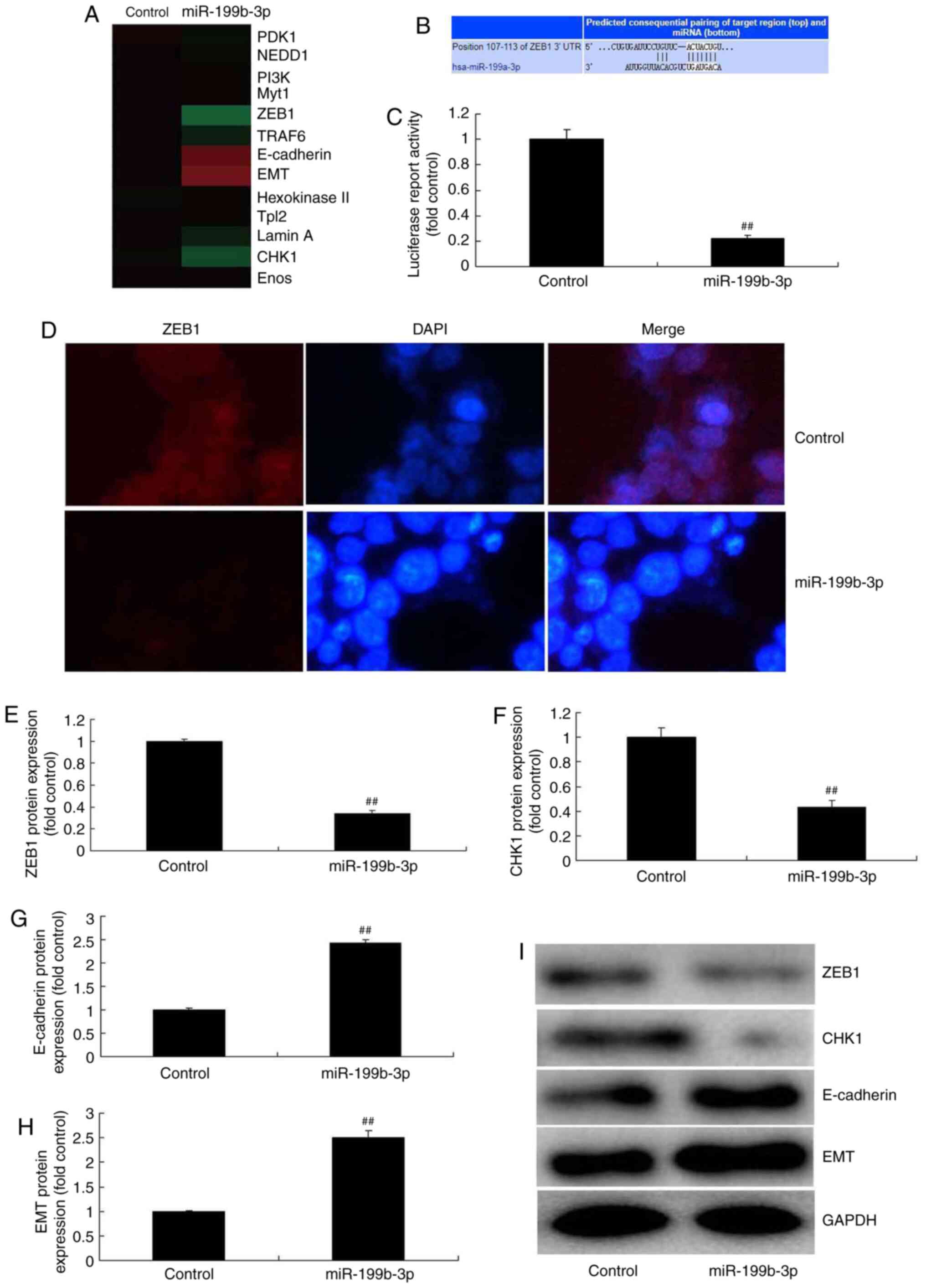 | Figure 5.miRNA-199b-3p overexpression
regulates ZEB1 in an in vitro model of ovarian cancer. (A)
Heat map for ZEB1 signalling. (B) The 3′-UTR of ZEB1 had a direct
target site for miRNA-199b-3p. (C) Luciferase activity levels. (D)
Immunofluorescence for ZEB1 (magnification, ×40). The protein
expression of (E) ZEB1, (F) CHK1, (G) E-cadherin and (H) EMT was
examined by (I) western blotting and statistical analysis.
##P<0.01 vs. negative controls. miRNA or miR,
microRNA; ZEB1, zinc finger E-box binding homeobox 1; UTR,
untranslated region; CHKI, checkpoint kinase 1; E-cadherin,
epithelial cadherin; EMT, epithelial-to-mesenchymal transition;
control, negative controls; miRNA-199b-3p, overexpressed
miRNA-199b-3p. |
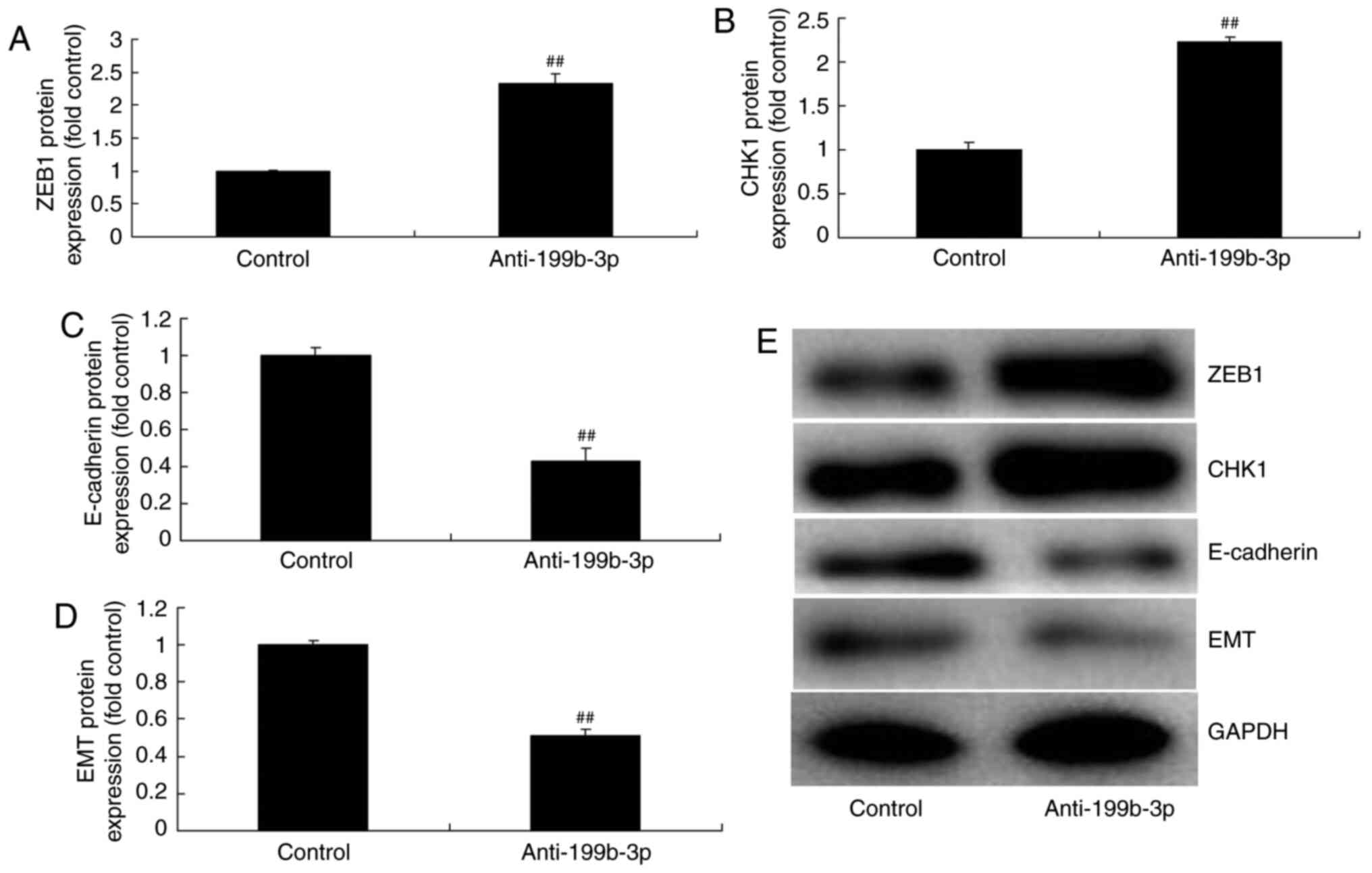 | Figure 6.miRNA-199b-3p downregulation
regulated ZEB1 signalling in an in vitro model of ovarian
cancer. The protein expression of (A) ZEB1, (B) CHK1, (C)
E-cadherin and (D) EMT was examined by (E) western blotting and
statistical analysis. ##P<0.01 vs. negative controls.
miRNA, microRNA; ZEB1, zinc finger E-box binding homeobox 1;
p-ZEB1, phosphorylated ZEB1; CHKI, checkpoint kinase 1; E-cadherin,
epithelial cadherin; EMT, epithelial-to-mesenchymal transition;
control, negative controls; anti-199b-3p, downregulated
miRNA-199b-3p. |
Anti-miRNA-199b-3p regulates ovarian
cancer progression in vivo and in vitro
miRNA-199b-3p downregulation increased tumor volume
in an in vivo model of ovarian cancer compared with negative
controls (Fig. 7A and B).
Furthermore, miRNA-199b-3p downregulation increased caspase-3/9
activity levels, induced ZEB1 and CHK1 expression and suppressed
E-cadherin and EMT expression in vitro compared with
negative controls (Fig. 7C-J).
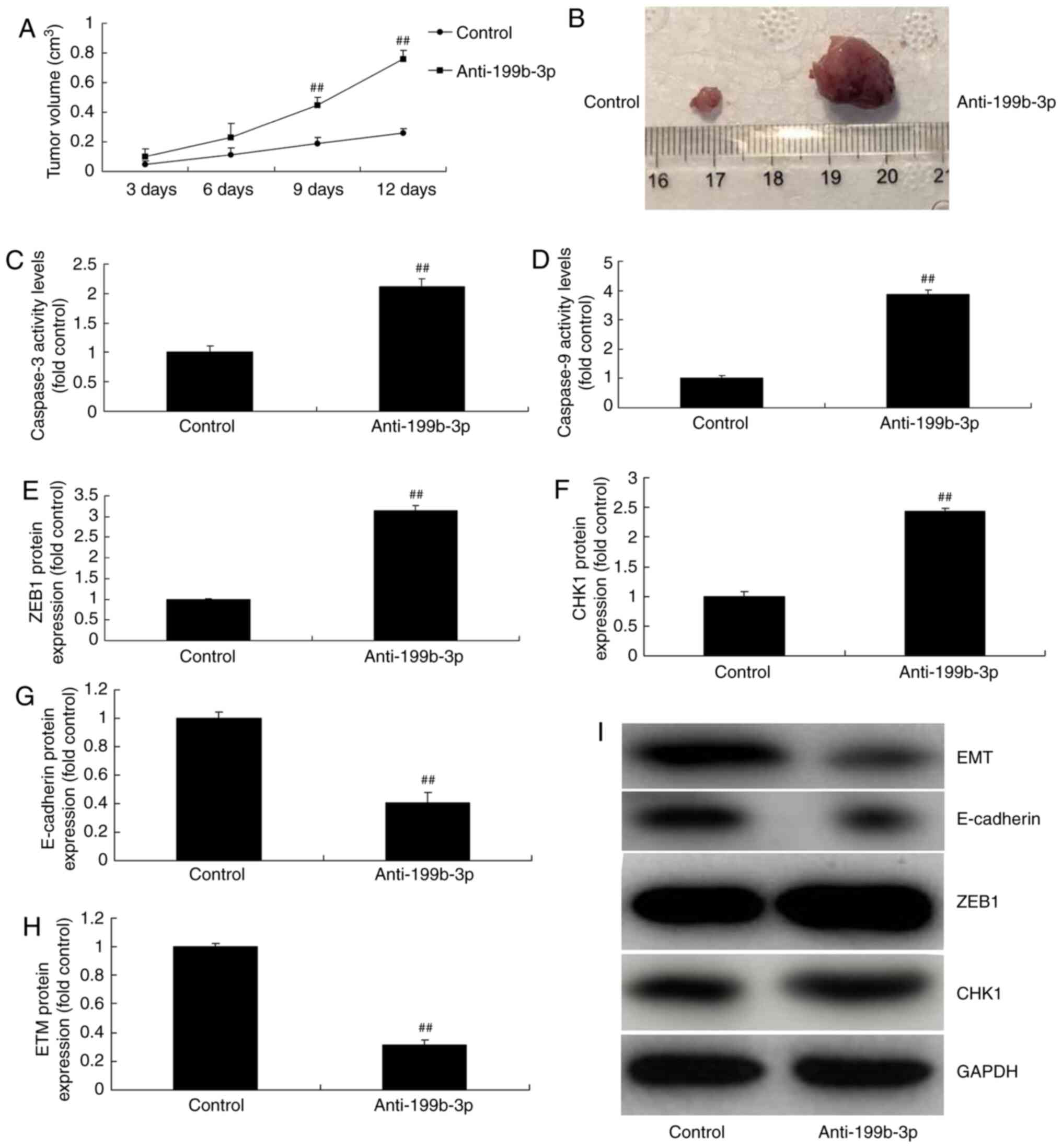 | Figure 7.Anti-199b-3p regulates ovarian cancer
progression in in vivo and in vitro models of ovarian
cancer. Tumor (A) volume and (B) macroscopic size. (C) Caspase-3
and (D) caspase-9 activity levels. The protein expression of (E)
ZEB1, (F) CHK1, (G) E-cadherin and (H) EMT were analyzed by (I)
western blotting and statistical analysis. ##P<0.01
vs. negative controls. Anti-199b-3p, negative controls; ZEB1, zinc
finger E-box binding homeobox 1; p-ZEB1, phosphorylated ZEB1; CHKI,
checkpoint kinase 1; E-cadherin, epithelial cadherin; EMT,
epithelial-to-mesenchymal transition; control, negative controls;
anti-199b-3p, downregulated miRNA-199b-3p. |
ZEB1 activation attenuates the effects
of miRNA-199b-3p-induced apoptosis in an in vitro model of ovarian
cancer
pGL3-ZEB1 plasmids were used to analyze the role of
ZEB1 in miRNA-199b-3p-induced apoptosis in an in vitro model
of ovarian cancer. ZEB1 plasmids induced ZEB1 expression compared
with negative controls (Fig. 8A).
Furthermore, miRNA-199b-3p + ZEB1 transfection induced ZEB1 and
CHK1 expression and suppressed E-cadherin and EMT expression in
ovarian cancer cells following compared with the miRNA-199b-3p
overexpression group (Fig. 8B-F).
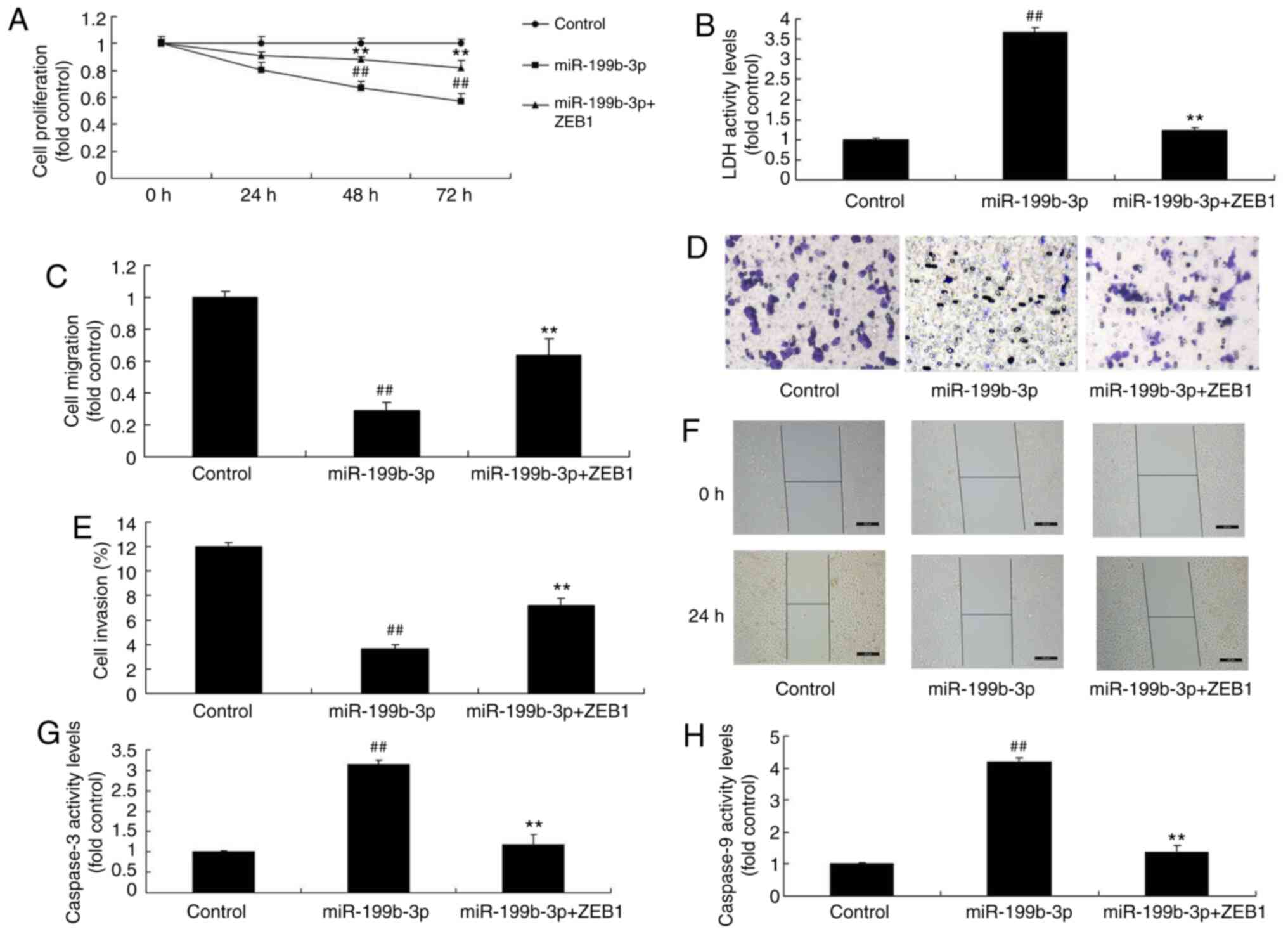
Additionally, miRNA-199b-3p + ZEB1 transfection reduced cell
proliferation, migration and cell invasion, and inhibited the LDH
and caspase-3/9 activity levels, compared with the miRNA-199b-3p
overexpression group in an in vitro model of ovarian cancer
(Fig. 9A-H).
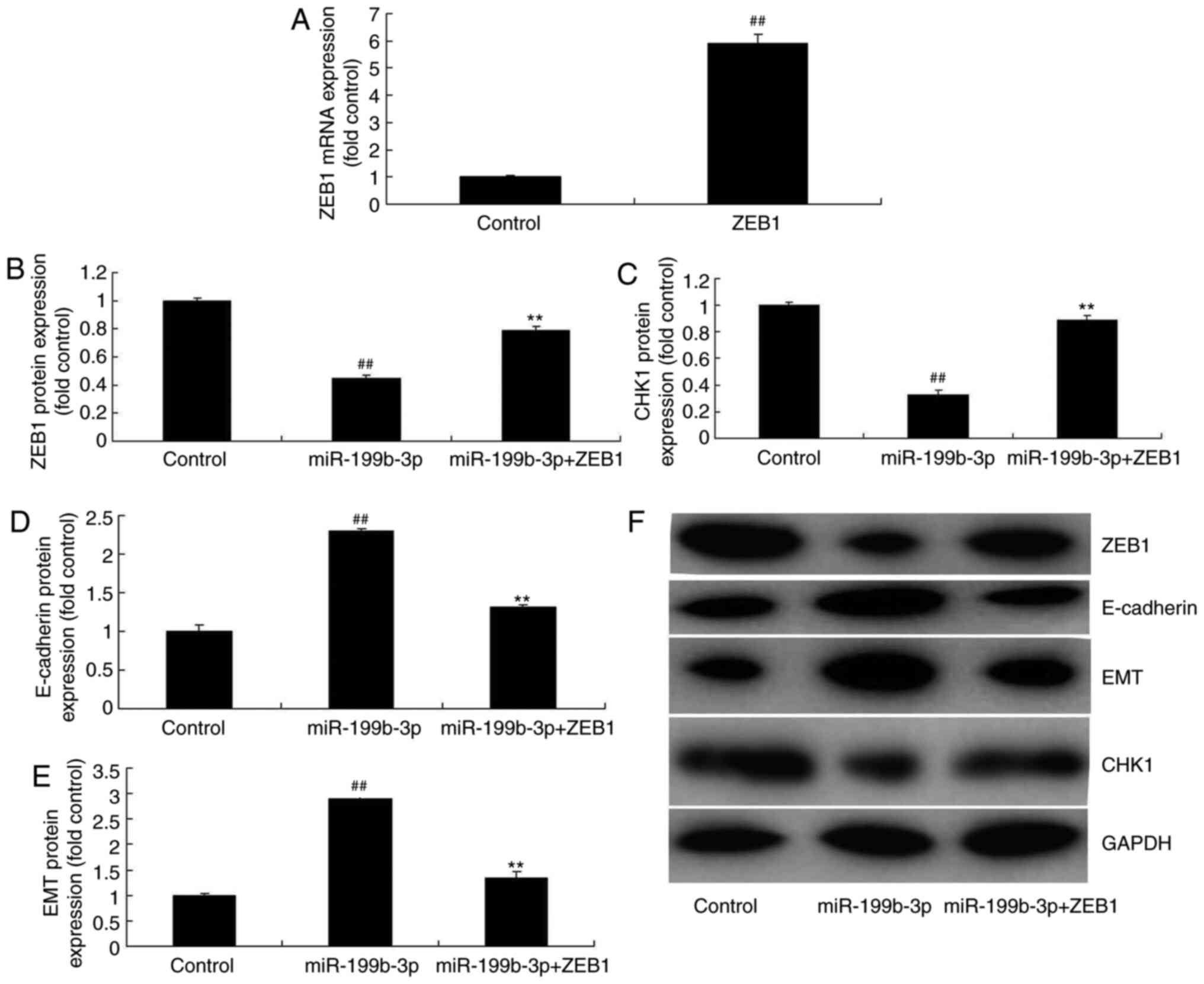 | Figure 8.ZEB1 activation reduced the effects
of miRNA-199b-3p on cell apoptosis in an in vitro model of
ovarian cancer. (A) mRNA expression of ZEB1. The protein expression
of (B) ZEB1, (C) CHK1, (D) E-cadherin and (E) EMT was examined by
(F) western blotting and statistical analysis.
##P<0.01 vs. negative control group. **P<0.01 vs.
overexpressed miRNA-199b-3p. ZEB1, zinc finger E-box binding
homeobox 1; miRNA/miR, microRNA; p-ZEB1, phosphorylated ZEB1; CHKI,
checkpoint kinase 1; E-cadherin, epithelial cadherin; EMT,
epithelial-to-mesenchymal transition; control, negative controls;
miR-199b-3p, overexpressed miR-199b-3p; miR-199b-3p + ZEB1;
miR-199b-3p/ZEB1 transfection. |
E-cadherin induction attenuates the
effects of anti-miRNA-199b-3p-induced metastasis in an in vitro
model of ovarian cancer
The function of E-cadherin on the effects of
anti-miRNA-199b-3p-induced metastasis in an in vitro model
of ovarian cancer were analyzed. E-cadherin plasmids were used to
induce E-cadherin mRNA expression, which was increased compared
with the negative controls (Fig.
10A). miRNA-199b-3p + E-cadherin transfection induced
E-cadherin and EMT expression in ovarian cancer cells compared with
downregulated miRNA-199b-3p (Fig.
10B-D). Furthermore, anti-miRNA-199b-3p + E-cadherin
transfection also attenuated the effects of anti-miRNA-199b-3p on
cell growth, migration and apoptosis in ovarian cancer compared
with the anti-miRNA-199b-3p alone group (Fig. 10E-J). These results demonstrated
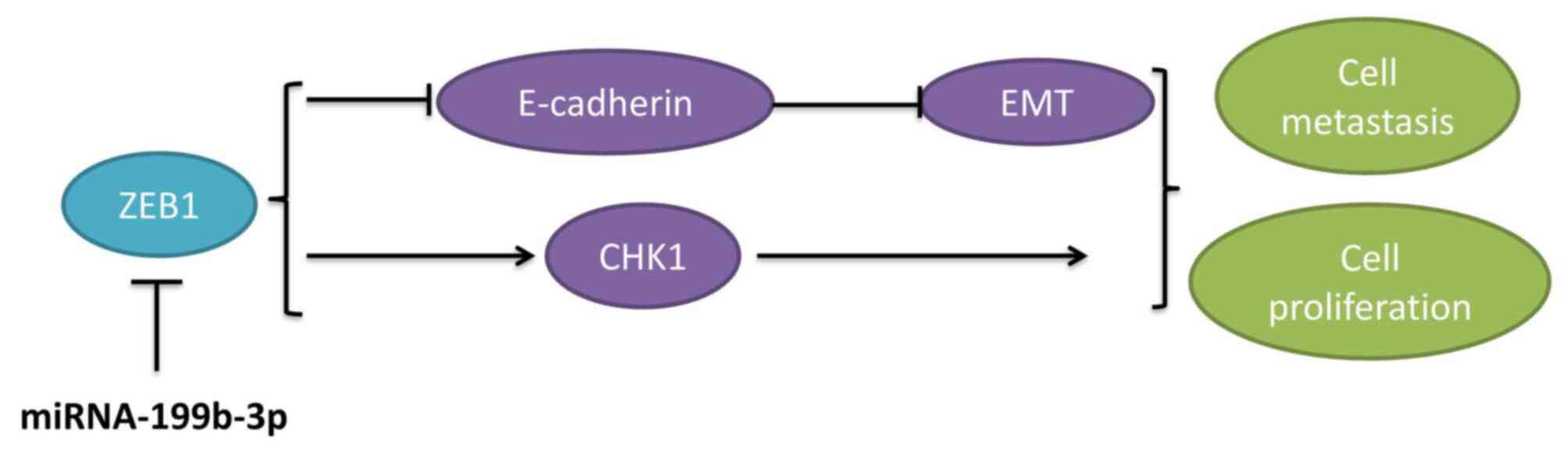
that miRNA-199b-3p suppresses ovarian cancer progression via the
CHK1/E-cadherin/EMT pathway by targeting the ZEB1 gene (Fig. 11), which may represent a potential
target for ovarian cancer treatment.
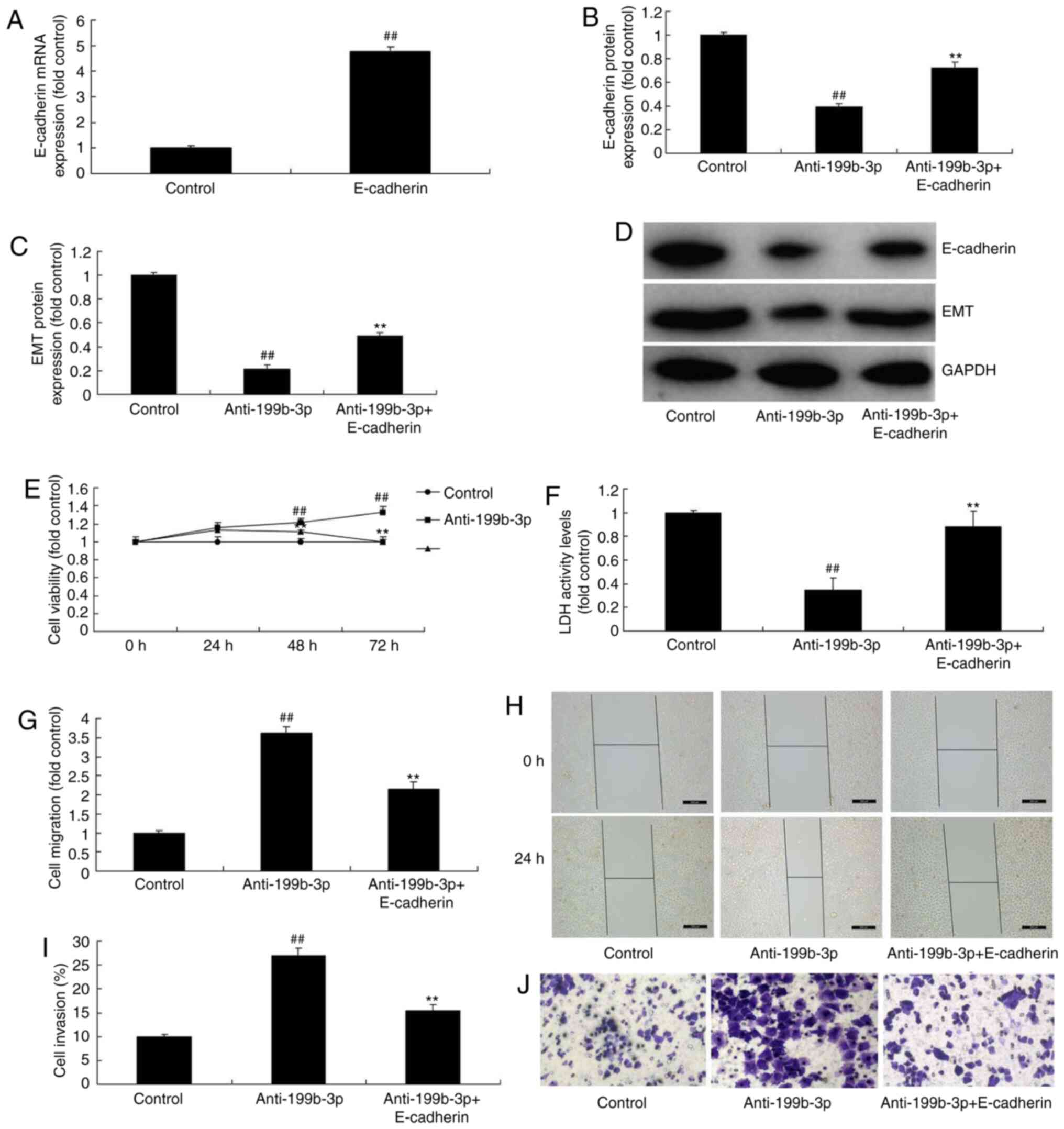 | Figure 10.E-cadherin induction reduced the
effects of anti-miRNA-199b-3p-induced metastasis in an in
vitro model of ovarian cancer. (A) mRNA expression of
E-cadherin. The protein expression of (B) E-cadherin and (C) EMT
was examined by (D) western blotting and statistical analysis. (E)
Cell viability. (F) LDH activity levels. Cell migration (G)
quantification and (H) staining (magnification, ×20). Cell invasion
(I) quantification and (J) wound healing assay (magnification,
×100). ##P<0.01 vs. negative controls; **P<0.01
vs. downregulated miRNA-199b-3p. E-cadherin, epithelial cadherin;
EMT, endothelial-to-mesenchymal transition; LDH, lactate
dehydrogenase; miRNA, microRNA; control, negative controls;
anti-199b-3p, downregulated miRNA-199b-3p; anti-199b-3p,
downregulated miRNA-199b-3p; anti-199b-3p + E-cadherin,
E-cadherin/anti-199b-3p transfection. |
Discussion
Ovarian cancer is among the major malignant tumors
affecting the female reproductive system (2). The 5-year survival rate of patients
with ovarian cancer is <30% (2).
Ovarian cancer has become a leading tumor that severely threatens
the health and life of women globally (14). Furthermore, its onset is insidious
and is difficult to detect (14).
Therefore, diagnosis is often made at a clinically advanced stage
after metastasis has already developed (15), making complete surgical resection
challenging and postoperative recurrence frequent. As a result,
ovarian cancer is considered as one of the most malignant
gynecological tumors with a poor prognosis (15). Zheng et al (11) reported that miR-199b-5p inhibits
triple-negative breast cancer cell proliferation, migration and
invasion. In the present study, miRNA-199b-3p expression was found
to be reduced in patients with ovarian cancer.
miRNAs are endogenous non-coding small RNAs that
degrade target mRNAs to suppress target gene expression at the
post-transcriptional level, and participate in the regulation of
cell growth, proliferation and apoptosis (16). Certain miRNAs regulate the
expression of various oncogenes and tumor suppressor genes, and
participate in the pathogenesis, invasion and metastasis of ovarian
cancer (16). Furthermore, the
differential expression of certain miRNAs is closely associated
with ovarian cancer resistance and prognosis (16,17).
Research into the target genes of ovarian cancer-related miRNAs and
their signaling pathways may help design novel strategies for
improving the clinical diagnosis and treatment of ovarian
cancer.
Epithelial ovarian cancer cells are often subjected
to hypoxia during their rapid growth process (18). Hypoxia increases the expression of
the EMT-related transcription factor ZEB1 in the SKOV3 and ES-2
ovarian cancer cell lines (18).
Additionally, ZEB1 reduces semaphorin 3F and activates the
hypoxia-inducing factor-1α in the oxygen reaction pathway (19). Furthermore, ZEB1 induces the
formation of classical blood vessels as well as vasculogenic
mimicry. Xu et al (20)
reported that miR-199b-5p acts as a tumor promoter in cervical
cancer. In the present study, patients with ovarian cancer and high
miRNA-199b-3p expression exhibited longer OS and DFS compared with
patients with low expression, indicating the cancer-suppressive
role of miRNA-199b-3p.
EMT plays a key role in embryogenesis, chronic
inflammation, tissue reconstruction, cancer metastasis and fibrosis
in multiple organs (9).
Furthermore, EMT is a key biological process through which
epithelium-derived malignant tumor cells can acquire the capacity
to migrate and invade (21).
Additionally, E-cadherin downregulation is an important event in
the initial stages of EMT (22).
Transcription factors that suppress E-cadherin expression are
defined as EMT-inducing factors (22). In the present study, miRNA-199b-3p
overexpression suppressed ZEB1 and CHK1 expression and induced
E-cadherin expression and EMT in an in vitro model of
ovarian cancer. Furthermore, ZEB1 activation reduced the effects of
miRNA-199b-3p-mediated apoptosis in an in vitro model of
ovarian cancer.
E-cadherin is a member of the cadherin family, which
comprises CA2+-dependent transmembrane proteins
(23). E-cadherin is the main
member of the cadherin family that is universally conserved in
epithelial cells and is a well-known epithelial cell biomarker
(23). The abnormal expression of
E-cadherin is the foundation of dissociation of cell-cell adhesion,
which is closely associated with tumor invasion and metastasis
(24). E-cadherin is rarely
expressed in normal ovarian epithelial cells (24). However, E-cadherin expression is
markedly upregulated in 85% of primary ovarian tumors. These data
indicate that E-cadherin induction also reduces the effects of
anti-miRNA-199b-3p-mediated metastasis in an in vitro model
of ovarian cancer. Portune et al (25) demonstrated that miR-199b attenuated
TGF-β1-induced EMT/E-cadherin in diabetic nephropathy. The results
of the present study revealed that the miRNA-199b-3p/E-cadherin/EMT
pathway regulates ovarian cancer cell growth and apoptosis.
In conclusion, it was herein demonstrated that
miRNA-199b-3p suppresses ovarian cancer progression via the
CHK1/E-cadherin/EMT pathway by targeting the ZEB1 gene, which may
represent a potential target for ovarian cancer treatment, and that
miRNA-199b-3p may be of value as a target for the treatment of
ovarian cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Project of
Medical and Health Technology Development program in Shandong
province (grant no. 2017WSA10016).
Availability of data and materials
The data sets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
SZ designed the experiments. LW and YH performed the
experiments. SZ, YH, SB, XL and JZ analyzed the data and SZ wrote
the manuscript.
Ethics approval and consent to
participate
The current study protocol was approved by the
Medical Ethics Committee of Weihai Municipal Hospital. Informed
written consent was obtained from all participants. All animal
experiments were approved by the Ethics Committee of Weihai
Municipal Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gray HJ, Bell-McGuinn K, Fleming GF,
Cristea M, Xiong H, Sullivan D, Luo Y, McKee MD, Munasinghe W and
Martin LP: Phase I combination study of the PARP inhibitor
veliparib plus carboplatin and gemcitabine in patients with
advanced ovarian cancer and other solid malignancies. Gynecol
Oncol. 148:507–514. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fu X, Zhang Y, Wang X, Chen M, Wang Y, Nie
J, Meng Y and Han W: Low dose decitabine combined with taxol and
platinum chemotherapy to treat refractory/recurrent ovarian cancer:
An open-label, single-arm, phase I/II study. Curr Protein Pept Sci.
16:329–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu GL, Yang HJ, Liu B and Liu T: Effects
of microRNA-19b on the proliferation, apoptosis, and migration of
Wilms' tumor cells via the PTEN/PI3K/AKT signaling pathway. J Cell
Biochem. 118:3424–3434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao J, Wu N, Liu X, Xia Y, Chen Y, Li S
and Deng Z: MicroRNA-142-3p inhibits cell proliferation and
chemoresistance in ovarian cancer via targeting sirtuin 1. Exp Ther
Med. 15:5205–5214. 2018.PubMed/NCBI
|
|
5
|
Liang H, Zhao X, Wang C, Sun J, Chen Y,
Wang G, Fang L, Yang R, Yu M, Gu Y and Shan H: Systematic analyses
reveal long non-coding RNA (PTAF)-mediated promotion of EMT and
invasion-metastasis in serous ovarian cancer. Mol Cancer.
17:962018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu DI, Liu L, Ren C, Kong D, Zhang P, Jin
X, Wang T and Zhang G: Epithelial-mesenchymal interconversions and
the regulatory function of the ZEB family during the development
and progression of ovarian cancer. Oncol Lett. 11:1463–1468. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sadlecki P, Jóźwicki J, Antosik P and
Grabiec M: Expression of selected epithelial-mesenchymal transition
transcription factors in serous borderline ovarian tumors and type
I ovarian cancers. Tumour Biol. 40:10104283187848072018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitra T, Prasad P, Mukherjee P, Chaudhuri
SR and Chatterji U: Stemness and chemoresistance are imparted to
the OC cells through TGFβ1 driven EMT. J Cell Biochem.
119:5775–5787. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leng R, Liao G, Wang H, Kuang J and Tang
L: Rac1 expression in epithelial ovarian cancer: Effect on cell EMT
and clinical outcome. Med Oncol. 32:3292015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weingarten C, Jenudi Y, Tshuva RY,
Moskovich D, Alfandari A, Hercbergs A, Davis PJ, Ellis M and
Ashur-Fabian O: The interplay between epithelial-mesenchymal
transition (EMT) and the thyroid hormones-αvβ3 axis in ovarian
cancer. Horm Cancer. 9:22–32. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng X, Huang F, Zhao A, Lei S, Zhang Y,
Xie G, Chen T, Qu C, Rajani C, Dong B, et al: Bile acid is a
significant host factor shaping the gut microbiome of diet-induced
obese mice. BMC Biol. 15:1202017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koshizuka K, Hanazawa T, Kikkawa N, Arai
T, Okato A, Kurozumi A, Kato M, Katada K, Okamoto Y and Seki N:
Regulation of ITGA3 by the anti-tumor miR-199 family inhibits
cancer cell migration and invasion in head and neck cancer. Cancer
Sci. 108:1681–1692. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blaha RZ, Arnett AB, Kirkwood MW, Taylor
HG, Stancin T, Brown TM and Wade SL: Factors influencing attrition
in a multisite, randomized, clinical trial following traumatic
brain injury in adolescence. J Head Trauma Rehabil. 30:E33–E40.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rochet N, Lindel K, Katayama S, Schubert
K, Herfarth K, Schneeweiss A, Sohn C, Harms W and Debus J:
Intensity-modulated whole abdomen irradiation following adjuvant
carboplatin/taxane chemotherapy for FIGO stage III ovarian cancer:
Four-year outcomes. Strahlenther Onkol. 191:582–589. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim Y, Guntupalli SR, Lee SJ, Behbakht K,
Theodorescu D, Lee JK and Diamond JR: Retrospective analysis of
survival improvement by molecular biomarker-based personalized
chemotherapy for recurrent ovarian cancer. PLoS One. 9:e865322014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Záveský L, Jandáková E, Weinberger V,
Minář L, Hanzíková V, Dušková D, Drábková LZ, Svobodová I and
Hořínek A: Ascites-derived extracellular microRNAs as potential
biomarkers for ovarian cancer. Reprod Sci. 26:510–522. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Loginov VI, Pronina IV, Burdennyy AM,
Filippova EA, Kazubskaya TP, Kushlinsky DN, Utkin DO, Khodyrev DS,
Kushlinskii NE, Dmitriev AA and Braga EA: Novel miRNA genes
deregulated by aberrant methylation in ovarian carcinoma are
involved in metastasis. Gene. 662:28–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bendoraite A, Knouf EC, Garg KS, Parkin
RK, Kroh EM, O'Briant KC, Ventura AP, Godwin AK, Karlan BY,
Drescher CW, et al: Regulation of miR-200 family microRNAs and ZEB
transcription factors in ovarian cancer: Evidence supporting a
mesothelial-to-epithelial transition. Gynecol Oncol. 116:117–125.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu X, Wang Y, Qiu H, Song H, Feng D, Jiang
Y, Deng S, Meng H and Geng J: AEG-1 contributes to metastasis in
hypoxia-related ovarian cancer by modulating the
HIF-1alpha/NF-kappaB/VEGF pathway. Biomed Res Int.
2018:31456892018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu LJ, Duan Y, Wang P and Yin HQ:
MiR-199b-5p promotes tumor growth and metastasis in cervical cancer
by down-regulating KLK10. Biochem Biophys Res Commun. 503:556–563.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mao M, Zheng X, Jin B, Zhang F, Zhu L and
Cui L: Effects of CD44 and E-cadherin overexpression on the
proliferation, adhesion and invasion of ovarian cancer cells. Exp
Ther Med. 14:5557–5563. 2017.PubMed/NCBI
|
|
22
|
Wang YP, Wang QY, Li CH and Li XW: COX-2
inhibition by celecoxib in epithelial ovarian cancer attenuates
E-cadherin suppression through reduced Snail nuclear translocation.
Chem Biol Interact. 292:24–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang HN, Huang WC, Lin CH, Chiang YC,
Huang HY and Kuo KT: Chromosome 20q13.2 ZNF217 locus amplification
correlates with decreased E-cadherin expression in ovarian clear
cell carcinoma with PI3K-Akt pathway alterations. Hum Pathol.
45:2318–2325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Ma J, Shen H, Wang C, Sun Y,
Howell SB and Lin X: Reactive oxygen species promote ovarian cancer
progression via the HIF-1α/LOX/E-cadherin pathway. Oncol Rep.
32:2150–2158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Portune KJ, Benítez-Páez A, Del Pulgar EM,
Cerrudo V and Sanz Y: Gut microbiota, diet, and obesity-related
disorders-The good, the bad, and the future challenges. Mol Nutr
Food Res. 61:2017. View Article : Google Scholar : PubMed/NCBI
|