Introduction
Bladder cancer (BC) is the fifth most commonly
diagnosed tumor worldwide and the second leading cause of death in
patients with genitourinary tract malignancies (1). Annually, approximately 430,000 new BC
cases and 165,000 cancer deaths are reported globally, according to
the International Agency for Research on Cancer and the World
Health Organization (2). The vast
majority (an estimated 80%) of BC is diagnosed as nonmuscle
invasive (NMIBC), also known as superficial BC, and the remaining
20% are muscle (i.e., muscularis propria)-invasive BC (MIBC)
(3). The overall survival rate of
patients diagnosed with NMIBC is high, although 30–50% of NMIBC
will recur after transurethral resection and 10–20% will progress
to MIBC (4). MIBC is responsible
for the vast majority of BC-specific deaths, and approximately 50%
of patients with MIBC present a higher incidence of distant
metastasis than those with NMIBC at the time of diagnosis (5). Currently, transurethral resection of a
bladder tumor (TURBT) and intravesical chemotherapy are still
considered the standard of care for bladder cancer. However, not
all patients are suitable for chemotherapy. Viable treatment
alternatives are warranted in the treatment of BC.
Tumor metastasis is a complex and continuous
multistep process encompassing local adhesion, migration, and
invasion that also accompany a variety of proteases, particularly
matrix metalloproteinases (MMPs) (6). MMPs, a group of calcium
(Ca2+)- and zinc (Zn2+)-dependent
endopeptidases, are responsible for the tissue remodeling and
degradation of the extracellular matrix (ECM) leading to tumor cell
migration and invasion in the metastatic environment (7,8). In
total, 24 genes encoding MMPs in humans have been identified and
many are implicated in cancer (9).
For instance, MMP-1, MMP-3, and MMP-9 expression levels are high in
human chondrosarcoma cells, and MMP-9 is positively correlated with
clinical outcome parameters in chondrosarcoma (10). The secretion of MMP-2 and MMP-9 is
elevated in various types of human cancers, and their expression is
closely related to clinical staging, lymph node metastasis, and
poor prognosis (11–13).
MicroRNAs (miRNAs/miRs), consisting of approximately
22 nucleotides, are non-coding RNAs (14) that bind to target mRNAs and suppress
protein translation by either blocking the translation or
destabilizing mRNA levels (15).
Many miRNAs are classified as either tumor-suppressive (TS) miRNAs
or oncogenic miRNAs, also known as oncomirs (16). miR-34a is a TS miRNA, and its
expression is epigenetically silenced in several human cancers
(17,18). Expression of hsa-miR-34a stimulates
cell apoptosis, cell cycle arrest, cellular senescence,
epithelial-mesenchymal transition (EMT) repression, and impedes
cell proliferation in cancer stem cells (19). Moreover, hsa-miR-34a is
downregulated in BC tissues and has been demonstrated to inhibit
migration and invasion by silencing Notch1 (20) or CD44 (21). Overexpression of hsa-miR-34a may
also inhibit cell migration and invasion and thus suppress
metastasis in hepatocellular carcinoma (22). In breast cancer, low hsa-miR-34a
expression was found to advance cell invasion in vitro and
distant metastasis in vivo by directly silencing
proto-oncogene Fos-related antigen-1 (Fra-1) expression (23). These findings highlight hsa-miR-34a
as a potential therapeutic target and a clinical biomarker of
cancer progression and metastasis.
The present study revealed that hsa-miR-34a
expression is negatively associated with clinical disease stage,
regional lymph node metastasis, and overall survival rate in
patients with BC. In vitro evidence revealed that
hsa-miR-34a suppressed MMP-2 expression, leading to inhibition of
BC cell motility. Another critical clinical characteristic of MMP-2
was its higher expression in late stage than in early stage BC.
Moreover, high expression of MMP-2 was found to be significantly
associated with a lower survival rate for patients with BC. This
study provides insight into the mechanisms underlying
hsa-miR-34a-mediated cell migration and invasion through MMP-2
silencing in BC.
Materials and methods
Cell culture
Human BC cell lines (5637 and UMUC3) and human
bladder epithelial cell line (SV-HUC-1) were obtained from
Bioresource Collection and Research Center (Hsinchu, Taiwan) and
incubated at 37°C under 5% CO2. SV-HUC-1 cells were
cultured in F-12 medium (Gibco; Thermo Fisher Scientific, Inc.).
5637 and UMUC3 cells were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.). All culture media were
supplemented with 10% fetal bovine serum (FBS), 2 mM GlutaMAX-1,
100 U/ml penicillin, and 100 µg/ml streptomycin.
hsa-miR-34a stable clones in BC
cells
MicroRNA expression vectors were constructed by
annealing a paired oligonucleotide consisting of mature
hsa-miR-34a-5p sequences and cloning it into a small-RNA expression
vector (pSM-vector), as previously described (24). Culture cells with 70% confluence
were transfected with pSM-34a-5p in 6-well plates for 24 h prior to
the administration of G418 selection antibiotic. The transfection
was performed using polymer-based transfection reagents (Ultra293,
GeneDirex, Taipei, Taiwan) according to manufacturer's
instructions. The cell viability of each transfected cell line was
tested to investigate whether miRNA disrupted BC cell
proliferation.
Transfection of hsa-miR-34a mimic and
inhibitor
MISSION synthetic negative control (NC) mimic
(5′-GGUUCGUACGUACACUGUUCA-3′), hsa-miR-34a-5p mimic
(5′-UGGCAGUGUCUUAGCUGGUUGU-3′), NC inhibitor
(5′-GGUUCGUACGUACACUGUUCA-3′) and hsa-miR-34a-5p inhibitor
(5′-ACAACCAGCUAAGACACUGCC-3′) were purchased from
Sigma-Aldrich/Merck KGaA. Cells were seeded in 6-well dishes with 2
ml culture medium. Viromer® BLUE (Lipocalyx GmbH,
Germany), miRNA transfection reagent, was used to transfect
hsa-miR-34a mimic (100 nM) or inhibitor (100 nM) in cells for 24 h.
Cell samples were then evaluated for MMP-2 expression and the
ability of cell migration and invasion by Western blot analysis and
Transwell migration assay, respectively.
Luciferase reporter assay
Wild-type (5′…CACUGCC…3′) and mutant (5′…GAGAGGC…3′)
plasmids of human MMP-2 3′-UTR (untranslated region) containing the
miR-34a-5p binding site were constructed in pmiR-GLO (Promega
Corp.) by MDBio, Inc. (Taipei, Taiwan). BC cells were seeded in
6-well dishes and transfected with the plasmid (1 µg/µl), using
Viromer® RED (Lipocalyx GmbH), as per manufacturer's
protocol. After 24 h of transfection, cell lysates were harvested
and the activities of firefly and Renilla luciferase were
detected using Dual-Luciferase kit (Promega Corp.). Firefly
luciferase activity was normalized to Renilla luciferase
activity.
Western blot analysis
Total proteins were extracted from BC cell lines.
The samples containing 20 µg of total protein were electrophoresed
on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) gels and proteins were transferred to Immobilon
polyvinyldifluoride membranes. Bovine serum albumin (4%) was used
to block membranes for 1 h at room temperature; the membranes were
then probed with primary anti-MMP-2 (dilution 1:3,000; GeneTex;
cat. no. GTX104577), anti-b-actin (dilution 1:5,000; Merck; cat.
no. MAB1501) and anti-GAPDH (dilution 1:5,000; Abcam; cat. no.
ab8245) antibodies at 4°C overnight. The membranes were incubated
with the appropriate horseradish peroxidase (HRP)-conjugated
anti-rabbit antibody (dilution 1:3,000; Cell Signaling; cat. no.
7074S) or anti-mouse antibody (dilution 1:3,000;
Sigma-Aldrich/Merck KGaA; A9044) at 37°C for 1 h. Enhanced
chemiluminescence was then added to the blots, which were imaged
using a ChemiDoc-It Imaging System (UVP Inc.).
Quantitative real-time polymerase
chain reaction
Total RNA extracted from BC cells was reverse
transcribed and subjected to quantitative real-time polymerase
chain reaction (qPCR) to detect MMP-2 and GAPDH as previously
described (25).
Migration and invasion assays
Transwell inserts in 24-well dishes (8-µm pore size;
Costar) were used to perform cell migration and invasion. For
invasion assays, Transwell inserts were precoated with 30 µl
Matrigel basement membrane matrix (BD Biosciences) for 30 min. The
human BC cells in 200 µl serum-free medium were seeded in Transwell
inserts with a 300 µl medium containing 1% FBS and placed in the
lower chamber. Cell migration and invasion were imaged under ×200
magnification by Eclipse Ti2 microscope (Nikon) after 24 h.
Analysis of publicly available
database
Using the TCGA dataset of bladder urothelial
carcinoma, the correlations among MMP-2, hsa-mir-34a expression,
race, tumor histology, molecular subtype, clinical stage, and nodal
metastasis status were analyzed for each tumor sample through the
UALCAN web server (http://ualcan.path.uab.edu/) (26). Overall survival rate between
patients with BC with high and low hsa-miR-34a or MMP-2 expression
was analyzed by OncoLnc (http://www.oncolnc.org/).
Statistical analysis
Statistical analyses were performed using SigmaPlot
10.0 (Systat Software, Inc.) and GraphPad Prism 7 (GraphPad
Software. Inc.). The values are expressed as the mean ± standard
deviation (SD). All differences between experimental groups and
controls were assessed for significance using Student's t-test.
Between-group differences were considered to be significant if the
P-value was <0.05.
Results
Clinical importance of hsa-miR-34a in
human BC based on the TCGA Database
Numerous studies have classified hsa-mir-34a as a
tumor suppressor because it inhibits tumorigenesis, and hsa-mir-34a
is considered a potential therapeutic candidate for cancer
(27). However, the clinical
importance of hsa-mir-34a in human BC is largely unknown. We
therefore analyzed the expression pattern of hsa-mir-34a by using
different variables from The Cancer Genome Atlas (TCGA) Urothelial
Bladder Carcinoma datasets through the UALCAN web server. Results
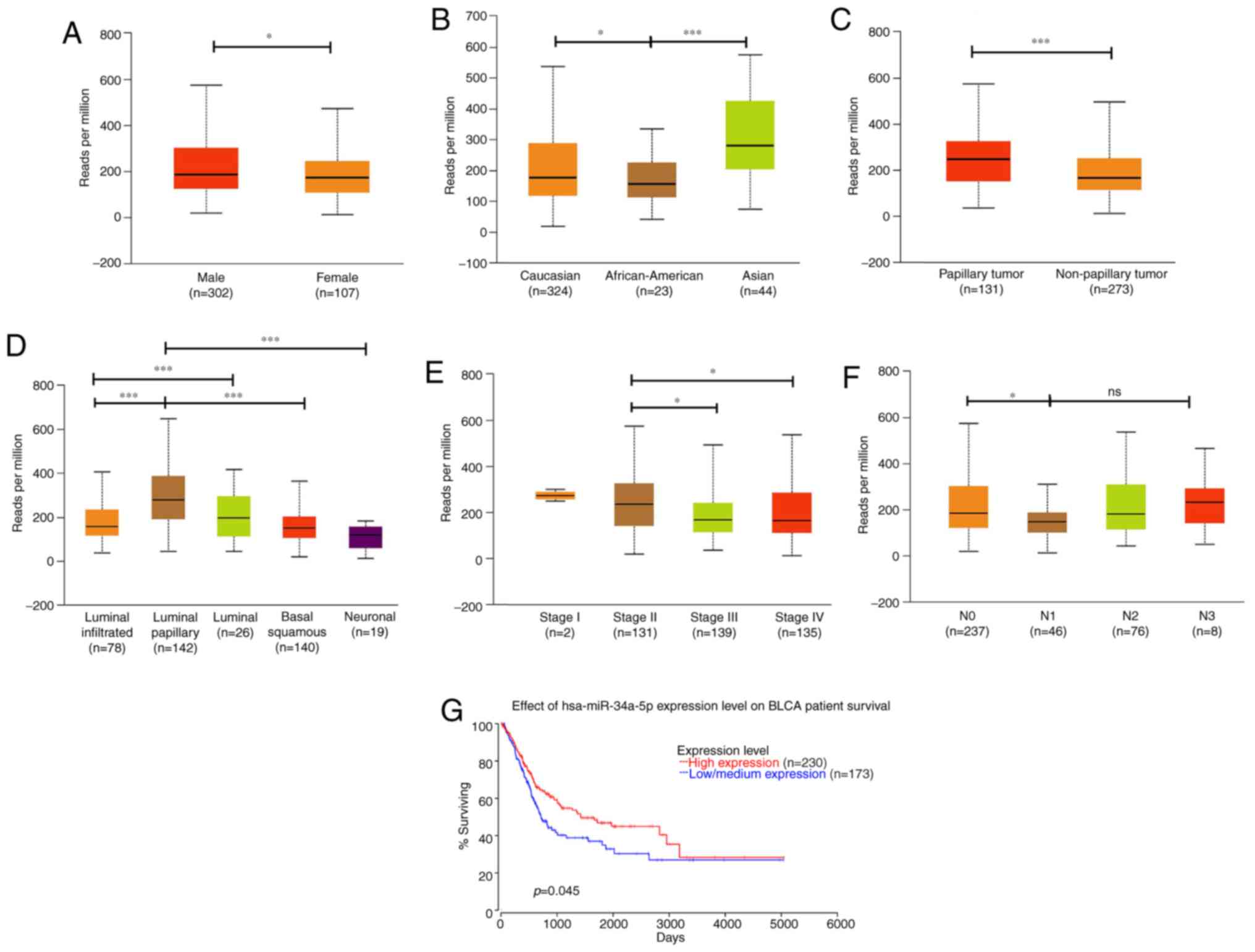
revealed that hsa-miR-34a had a lower expression in female
individuals diagnosed with BC (n=107) than in male individuals
(n=302) (P=0.04628; Fig. 1A).
Notably, hsa-miR-34a expression was more enhanced in Asians (n=44)
than in Caucasians (n=324; P=0.0195) and African-Americans (n=23;
P=0.00007; Fig. 1B). This result
indicates that hsa-miR-34a expression varies according to genetic
differences between races. We further analyzed the expression
pattern of hsa-miR-34a in tumor histology and molecular subtypes of
BC. Papillary urothelial carcinoma (n=131), which progresses more
slowly and has a more straightforward treatment and a more
favorable prognosis than other types of BC (28), exhibited higher hsa-miR-34a
expression than nonpapillary samples (n=273; P=0.00061; Fig. 1C). Moreover, MIBC has five molecular
subtypes, namely luminal-papillary, luminal-infiltrated, luminal,
basal-squamous, and neuronal (28).
We found that the luminal-papillary subtype (n=142) exhibited
higher levels of hsa-miR-34a expression than did the other four
molecular subtypes (Fig. 1D). By
contrast, the neuronal subtype (n=19), which has the poorest
clinical outcome of all the subtypes, exhibited lower levels of
hsa-miR-34a expression than did the luminal-papillary subtype
(P=0.000002; Fig. 1D). These data
indicate that hsa-miR-34a may be positively associated with
favorable clinical outcomes.
Next, we analyzed hsa-miR-34a expression in clinical
stages and nodal metastasis status of BC samples. Results indicated
that late stage (stage III and IV) had lower hsa-miR-34a expression
than did early stage (stage II) tumors (stage II vs. III:
P=0.025879; stage II vs. IV: P=0.046764; Fig. 1E). Moreover, significant
associations were observed between low hsa-miR-34a expression
levels and regional lymph node metastasis (N0 vs. N1: P=0.010535;
Fig. 1F). OncoLnc, a new TCGA data
portal, focuses on survival correlations by using mRNA or miRNA
expression data. Using OncoLnc analysis, we found that low
hsa-miR-34a expression levels were significantly correlated with a
low survival rate for patients with BC (P=0.045; Fig. 1G). These data indicate that
hsa-miR-34a, as a tumor suppressor, has significant associations
with clinical and histopathologic characteristics in patients with
BC.
hsa-miR-34a impedes cell migration and
invasion in BC cells
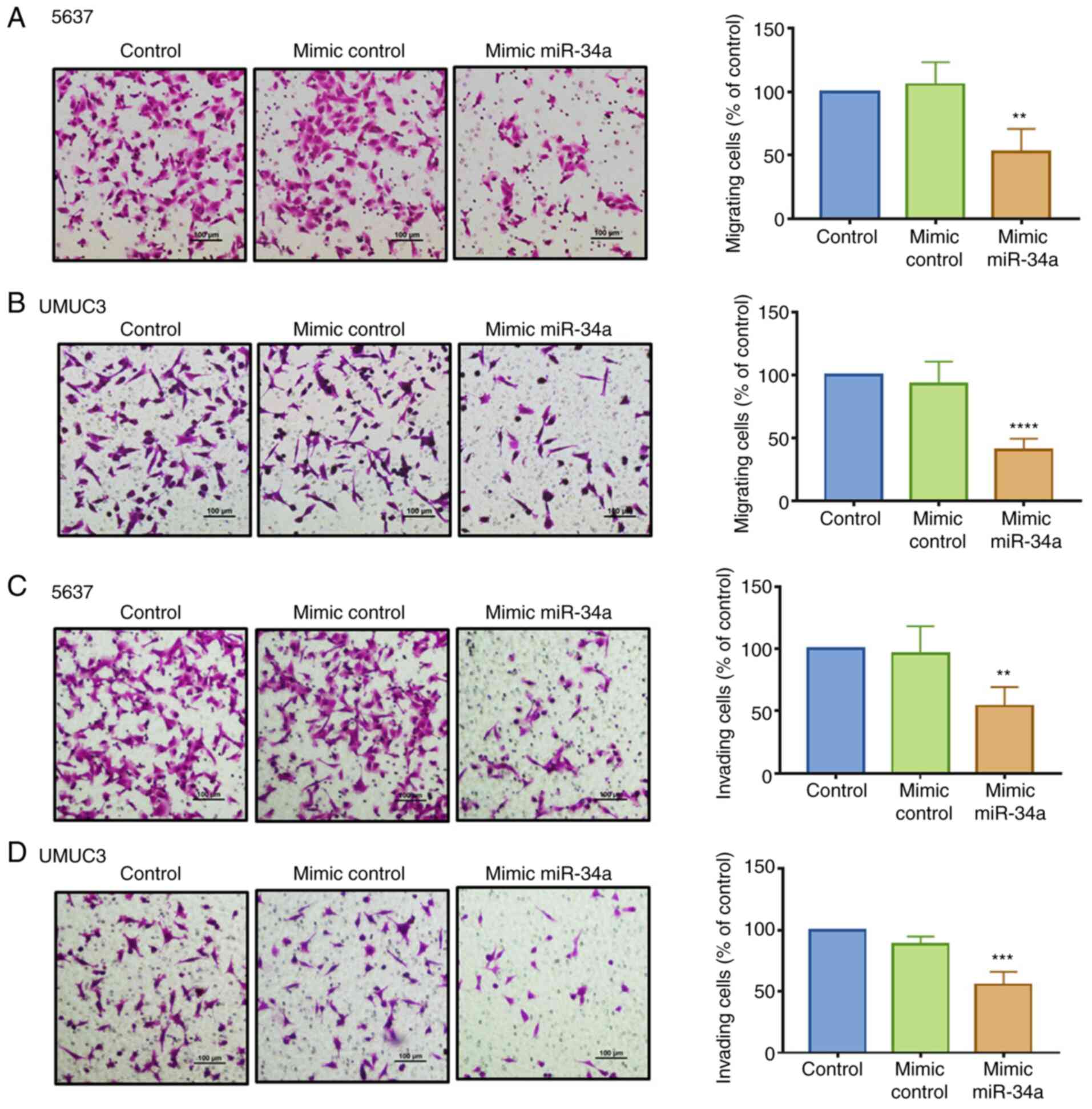
hsa-miR-34a can inhibit cancer cell proliferation
and migration (29). Therefore, we
investigated whether hsa-miR-34a exhibits antitumor effects in
inhibiting BC cell motility. An hsa-miR-34a mimic was used to
analyze its function in regulating cell migration and invasion by
employing a Transwell assay. We found that compared with the
control mimic, hsa-miR-34a mimic significantly suppressed cell
migration (Fig. 2A and B) and
invasion ability (Fig. 2C and D) in
the 5637 and UMUC3 cell lines. The groups between the control and
control mimic exhibited no such effects. The successful
transfection of hsa-miR-34a mimic was confirmed (Fig. S1A). These data demonstrated that
hsa-miR-34a reduced cell migration and invasion in BC cells.
hsa-miR-34a directly targets
MMP-2
The action of miRNAs appears to involve mRNA
silencing by binding to the 3′-UTRs (untranslated regions) at the
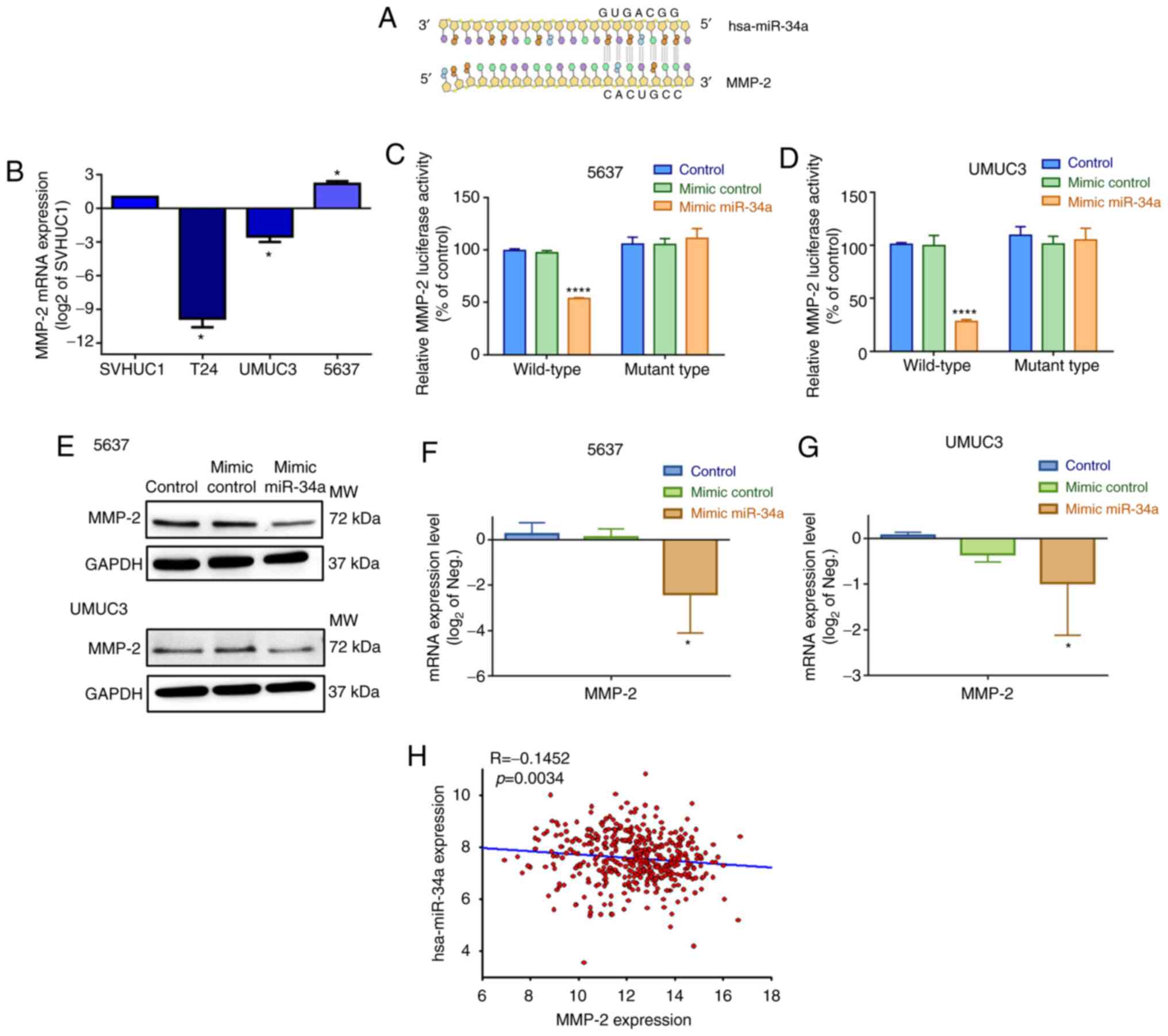
miRNA recognition elements of target mRNAs (30). Subsequently, we investigated which
target mRNA hsa-mir-34a binds to when impeding cell motility. On
the basis of an analysis using the miRNA target prediction program
(microRNA.org), we confirmed that hsa-miR-34a
directly targets the 3′-UTR of MMP-2 (Fig. 3A). Therefore, we hypothesized that
hsa-miR-34a affects BC cell migration and invasion through MMP-2
silencing. First, we assessed basal MMP-2 expression levels in
three BC cell lines. The 5637 and UMUC3 cells exhibited
significantly higher MMP-2 mRNA and protein expression than T24
cells (Figs. 3B and S1B).
To confirm that hsa-miR-34a directly binds to the
3′-UTR of MMP-2 and inhibits MMP-2 mRNA translation, we constructed
wild-type and mutant MMP-2-3′-UTR luciferase plasmids containing
hsa-miR-34a binding sites. After transfection of 5637 and UMUC3
cells with wild-type luciferase plasmids, we found that the
hsa-miR-34a mimic inhibited luciferase activity, but the mutant
type had no such affect (Fig. 3C and
D). Moreover, the hsa-miR-34a mimic diminished the levels of
endogenous MMP-2 protein and mRNA expression in 5637 and UMUC3
cells (Fig. 3E-G). hsa-miR-34a
expression was also negatively correlated with MMP-2 mRNA in human
BC samples (Fig. 3H). According to
our data, high hsa-miR-34a expression levels are associated with
improved clinical outcomes and significant suppression of MMP-2
expression by combining with the 3′-UTR region of MMP-2 mRNA.
Hsa-miR-34a regulates cell motility by
suppressing MMP-2 expression
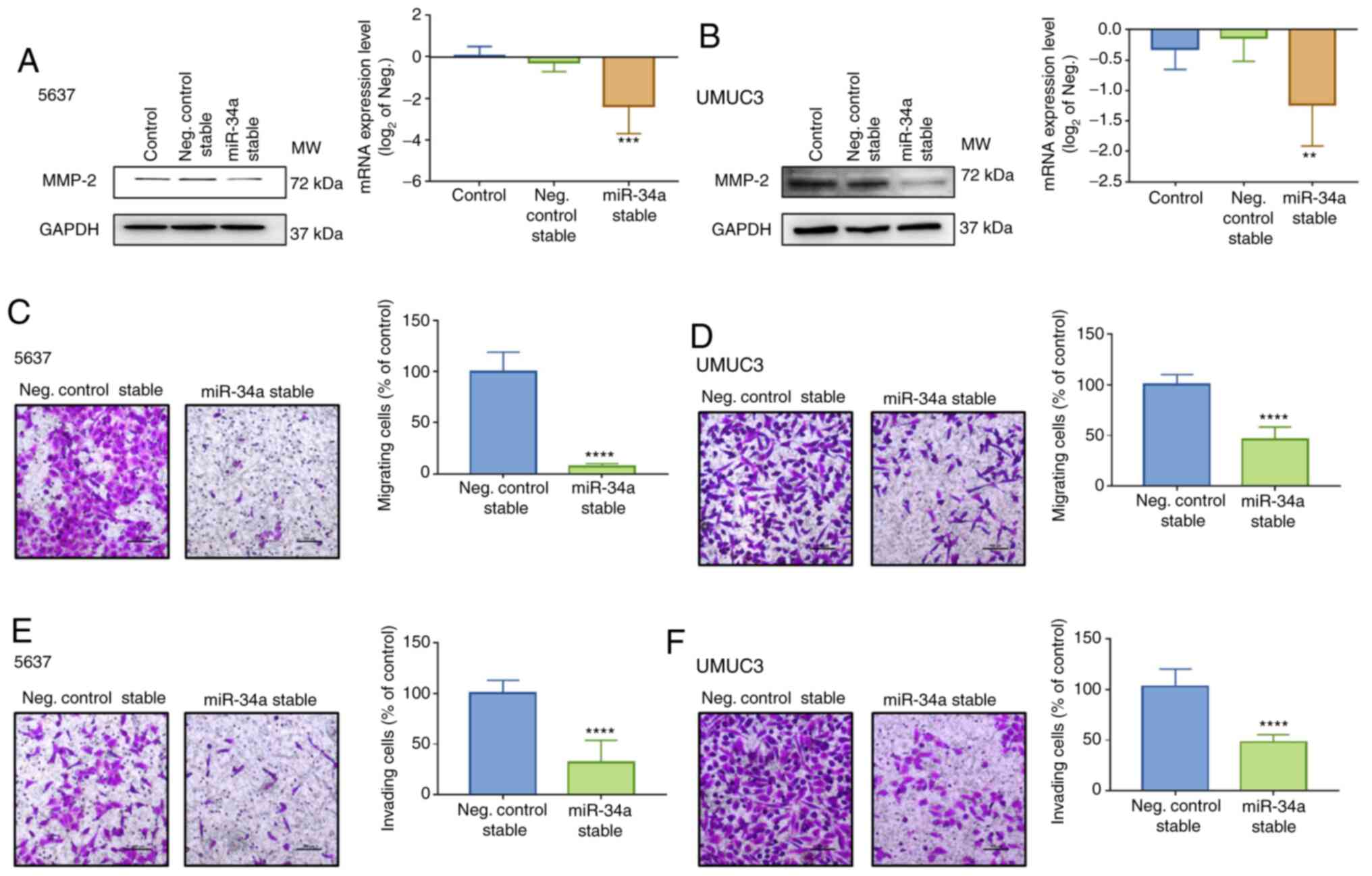
To confirm that MMP-2 is a critical mediator
involved in hsa-miR-34a-regulated cell migration and invasion, BC
cell lines 5637 and UMUC3 that stably expressed control or
hsa-miR-34a were established. Low levels of MMP-2 expression were
measured in hsa-miR-34a stable cells (Fig. 4A and B) and hsa-miR-34a inhibitor
reversed the MMP-2 downregulation (Fig. S1C). The successful transfection of
hsa-miR-34a inhibitor was verified (Fig. S1D). Moreover, hsa-mir-34a stable
cells exhibited low mobility (Fig. 4C
and D) and invasiveness (Fig. 4E
and F). Thus, hsa-miR-34a regulates cell migration and invasion
by suppressing MMP-2 expression in BC cells.
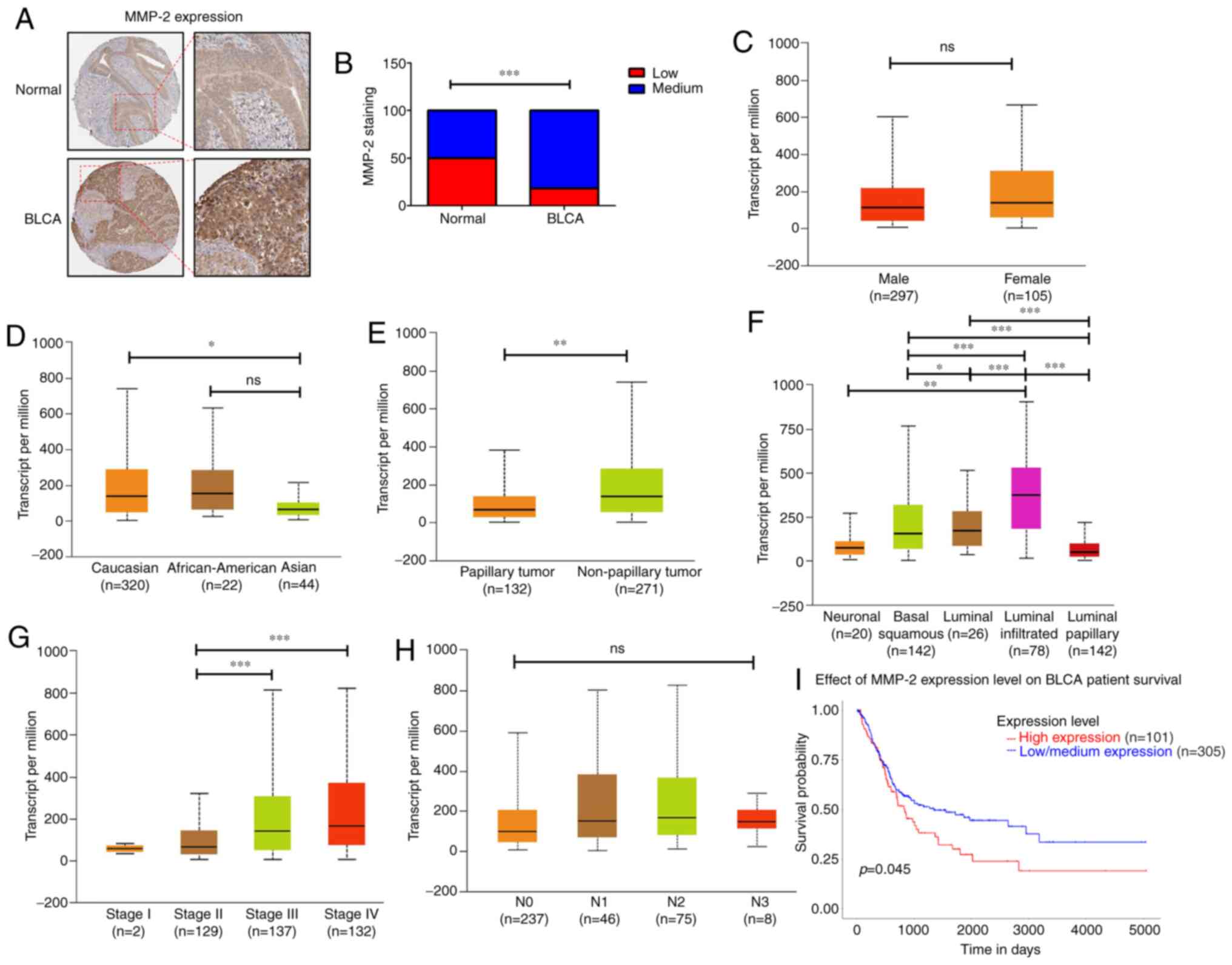
Clinicopathologic characteristics of
MMP-2 in patients with BC
To further investigate the role of MMP-2 in human
BC, we analyzed the clinical importance of MMP-2 in human BC tissue
samples derived from the Human Protein Atlas database. MMP-2
protein levels were higher in tumor specimens than in normal
tissues (Fig. 5A and B). The data
presented in Fig. 5C-H revealed
that, in BC patients, MMP-2 expression differed according to sex,
race, tumor histology, molecular subtype, tumor stages, and
regional lymph node metastasis. Overexpression of MMP-2 was higher
in Caucasians than in Asians (P=0.012; Fig. 5D). Similarly, MMP-2 expression was
significantly elevated in BC tissues of nonpapillary (P=0.0043;
Fig. 5E) or luminal-infiltrated
subtypes (luminal-infiltrated vs. luminal-papillary: P=1.76E-12;
Fig. 5F). Additionally, the mRNA
levels of MMP-2 in patients with stage III and IV BC were clearly
higher than those in patients with stage II (stage II vs. stage
III: P=0.00002; stage II vs. stage IV: P=0.00001; Fig. 5G). We also found that high levels of
MMP-2 expression were significantly correlated with low survival
rates for patients with BC (P=0.045; Fig. 5I). However, no relationship was
observed between MMP-2 expression and other clinical features such
as sex (Fig. 5C) and regional lymph
node metastasis (Fig. 5H). Taken
together, our results indicate that increased MMP-2 might predict a
poor prognosis for patients with BC, and MMP-2 is an excellent
target for developing BC treatments.
Discussion
Bladder cancer (BC) is a common urologic cancer
(31). Surgery and chemotherapy are
useful standard treatments; however, tumor recurrence rates (an
estimated 70% within 5 years) remain high, and progression to
invasive disease is extremely common (32). The poor prognosis in patients with
BC is related to high local recurrence rates and distant metastases
(33). Developing a novel and safer
BC treatment that improves patient outcomes and recurrence rates is
essential. This study investigated the therapeutic potential of
hsa-miR-34a in inhibiting BC cell migration and invasion.
The extracellular matrix (ECM) in the tumor
microenvironment plays a vital role in regulating cancer
progression (34). Notably, MMPs
are involved in every step of tumor progression, such as promoting
angiogenesis (35), tumor growth,
and distant metastasis (36).
MMP-14 silencing significantly reduced tumor cell migration and
invasion ability (37). Similarly,
MMP-9 silencing was found to diminish cell migration and invasion
in glioma cells (38). Furthermore,
in vivo analysis revealed that increased MMP-2 levels are
correlated with melanoma progression (39). MMP-9 knockdown significantly
inhibited tumor growth and metastasis in a mouse xenograft model of
triple-negative breast cancer (40). In the present study, we found that
high MMP-2 expression levels were significantly correlated with low
survival rates in patients with BC. The mRNA levels of MMP-2 in
patients with stage III and IV BC were markedly higher than those
in patients with stage II. We further demonstrated that MMP-2
mediates BC cell migration and invasion. From a clinical
perspective, MMP expression is correlated with tumor
aggressiveness, disease stage, and poor prognosis in patients with
cancers (41,42). Together, these findings indicate
that MMPs are an attractive target for cancer therapeutics. Cancer
clinical trials have been conducted with numerous MMP inhibitors,
including peptidomimetics, nonpeptidomimetics inhibitors, and
tetracycline derivatives, which target MMPs in the extracellular
space (43). However, the results
have been disappointing and associated complications, such as
musculoskeletal pain and inflammation, have been of great concern
(43). Therefore, new drugs for the
treatment of BC should be developed.
As crucial modulators in cellular pathways,
microRNAs are instrumental in tumorigenesis and metastasis by
targeting numerous oncogenes simultaneously through translation
repression or mRNA degradation (44). A thorough understanding of
associated downstream and upstream miRNAs is essential for
successful treatment. In BC, hsa-miR-34a has been reported to
attenuate metastasis and chemoresistance by reducing the levels of
TCF1 and LEF1 (45). Also,
hsa-miR-34a was found to inhibit BC cell migration and invasion by
upregulating tumor-suppressor gene PTEN (46). Our study results concur with these
findings, by showing that hsa-miR-34a inhibits MMP-2 expression and
thereby suppresses cell migration and invasion in BC cells. The
relevant results indicate that the hsa-miR-34a/MMP-2 axis is
involved in the regulation of the antitumor effect of esophageal
squamous cell carcinoma and glioma (47,48).
In addition, we also found that hsa-miR-34a was significantly
correlated with clinical and histopathologic characteristics in
human BC. However, hsa-miR-34a-mediated MMP-2 expression and tumor
invasiveness should be evaluated in vivo to confirm these
findings. In multiple myeloma, intratumor or exogenous delivery of
lipidic-formulated synthetic hsa-miR-34a mimic triggered antitumor
action in an in vivo animal model (49,50).
Related results revealed that systemic transfer of hsa-miR-34a
mimic to tumors in an in vivo lung cancer animal model
resulted in extraordinary suppression of tumor growth, suggesting
the effectiveness of hsa-miR-34a replacement therapy in lung cancer
(51,52). Notably, MRX34, a liposomal
formulation of nanoparticles packed with hsa-miR-34a mimics, is
progressing to a phase I clinical trial (NCT01829971) for primary
liver cancer, several solid tumors, and hematopoietic malignancies
(27). Therefore, hsa-miR-34a is
attractive as a potential treatment in cancers.
Although prior studies have emphasized the potential
benefit of miRNA expression profiles in the prognosis for BC
patients (53,54), their value concerning ethnicity is
unfamiliar. Caucasians are about twice as likely to develop BC as
African-Americans, and Asians have slightly lower rates of BC
(55). The reasons for these
differences are not well understood. In the present investigation,
we found that tumor-suppressor hsa-miR-34a has higher expression in
Asians than Caucasians and African-Americans. This result suggests
a racial-association for hsa-miR-34a in BC progression. hsa-miR-34a
may repress tumorigenicity, especially in the Asian population,
leading to a lower prevalence of BC. However, further investigation
is still needed to prove the specificity of interaction between
hsa-miR-34a and ethnicity.
In conclusion, hsa-miR-34a inhibits MMP-2 expression
and thus suppresses cell migration and invasion (Fig. 6). These results may provide
significant therapeutic opportunities for human BC.
Supplementary Material
Supporting Data
Acknowledgements
We gratefully acknowledge the contributions of the
TCGA Research Network: https://www.cancer.gov/tcga. We thank Jiun-Lin Lai for
his preparation of the schematic summary of Fig. 6.
Funding
This work was supported by the Ministry of Science
and Technology, Taiwan (MOST-108-2314-B-341-001-) and Shin Kong Wu
Ho-Su Memorial Hospital (SKH-8302-106-DR-09).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
Conceptualization of the study was achieved by KYC
and TISH. Data curation was conducted by ACC and PCC. Formal
analysis was carried out by TFT and YCL. Investigation of the data
and results was conducted by KYC and ACC. Methodology was overseen
by KYC. Project administration was carried out by HEC and CYH.
Resources were provided by PCC and TFT. Software analysis was
overseen by ACC and PCC. Supervision was carried out by CYH, YCL,
HEC and TISH. Writing of the original draft was carried out by KYC
and ACC. Writing of the review and editing were conducted by KYC
and TISH.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors of this article declare that they do not
have any competing interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Isharwal S and Konety B: Non-muscle
invasive bladder cancer risk stratification. Indian J Urol.
31:289–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sylvester RJ, van der Meijden AP,
Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW and
Kurth K: Predicting recurrence and progression in individual
patients with stage Ta T1 bladder cancer using EORTC risk tables: A
combined analysis of 2,596 patients from seven EORTC trials. Eur
Urol. 49:466–465, Discussion 475-467. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi T: Understanding the biology of
urothelial cancer metastasis. Asian J Urol. 3:211–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Zijl F, Krupitza G and Mikulits W:
Initial steps of metastasis: Cell invasion and endothelial
transmigration. Mutat Res. 728:23–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yadav L, Puri N, Rastogi V, Satpute P,
Ahmad R and Kaur G: Matrix metalloproteinases and cancer-roles in
threat and therapy. Asian Pac J Cancer Prev. 15:1085–1091. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nannuru KC, Futakuchi M, Varney ML,
Vincent TM, Marcusson EG and Singh RK: Matrix metalloproteinase
(MMP)-13 regulates mammary tumor-induced osteolysis by activating
MMP9 and transforming growth factor-beta signaling at the
tumor-bone interface. Cancer Res. 70:3494–3504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Verma S, Kesh K, Gupta A and Swarnakar S:
An overview of matrix metalloproteinase 9 polymorphism and gastric
cancer risk. Asian Pac J Cancer Prev. 16:7393–7400. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malcherczyk D, Heyse TJ, El-Zayat BF,
Kunzke V, Moll R, Fuchs-Winkelmann S and Paletta JRJ: Expression of
MMP-9 decreases metastatic potential of Chondrosarcoma: An
immunohistochemical study. BMC Musculoskelet Disord. 19:92018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Qiu Z, Li F and Wang C: The
relationship between MMP-2 and MMP-9 expression levels with breast
cancer incidence and prognosis. Oncol Lett. 14:5865–5870.
2017.PubMed/NCBI
|
|
12
|
Reis ST, Leite KR, Piovesan LF,
Pontes-Junior J, Viana NI, Abe DK, Crippa A, Moura CM, Adonias SP,
Srougi M and Dall'Oglio MF: Increased expression of MMP-9 and IL-8
are correlated with poor prognosis of Bladder Cancer. BMC Urol.
12:182012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou W, Yu X, Sun S, Zhang X, Yang W,
Zhang J, Zhang X and Jiang Z: Increased expression of MMP-2 and
MMP-9 indicates poor prognosis in glioma recurrence. Biomed
Pharmacother. 118:1093692019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ranganathan K and Sivasankar V:
MicroRNAs-Biology and clinical applications. J Oral Maxillofac
Pathol. 18:229–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eichhorn SW, Guo H, McGeary SE,
Rodriguez-Mias RA, Shin C, Baek D, Hsu SH, Ghoshal K, Villén J and
Bartel DP: mRNA destabilization is the dominant effect of mammalian
microRNAs by the time substantial repression ensues. Mol Cell.
56:104–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hammond SM: MicroRNAs as oncogenes. Curr
Opin Genet Dev. 16:4–9. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lodygin D, Tarasov V, Epanchintsev A,
Berking C, Knyazeva T, Körner H, Knyazev P, Diebold J and Hermeking
H: Inactivation of miR-34a by aberrant CpG methylation in multiple
types of cancer. Cell Cycle. 7:2591–2600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chim CS, Wong KY, Qi Y, Loong F, Lam WL,
Wong LG, Jin DY, Costello JF and Liang R: Epigenetic inactivation
of the miR-34a in hematological malignancies. Carcinogenesis.
31:745–750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Nat Med. 17:211–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang C, Yao Z, Zhu M, Ma X, Shi T, Li H,
Wang B, Ouyang J and Zhang X: Inhibitory effects of microRNA-34a on
cell migration and invasion of invasive urothelial bladder
carcinoma by targeting Notch1. J Huazhong Univ Sci Technolog Med
Sci. 32:375–382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu G, Yao W, Xiao W, Li H, Xu H and Lang
B: MicroRNA-34a functions as an anti-metastatic microRNA and
suppresses angiogenesis in bladder cancer by directly targeting
CD44. J Exp Clin Cancer Res. 33:7792014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou J, Zhou W, Kong F, Xiao X, Kuang H
and Zhu Y: microRNA-34a overexpression inhibits cell migration and
invasion via regulating SIRT1 in hepatocellular carcinoma. Oncol
Lett. 14:6950–6954. 2017.PubMed/NCBI
|
|
23
|
Yang S, Li Y, Gao J, Zhang T, Li S, Luo A,
Chen H, Ding F, Wang X and Liu Z: MicroRNA-34 suppresses breast
cancer invasion and metastasis by directly targeting Fra-1.
Oncogene. 32:4294–4303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin CJ, Gong HY, Tseng HC, Wang WL and Wu
JL: miR-122 targets an anti-apoptotic gene, Bcl-w, in human
hepatocellular carcinoma cell lines. Biochem Biophys Res Commun.
375:315–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin JF, Lin YC, Yang SC, Tsai TF, Chen HE,
Chou KY and Hwang TI: Autophagy inhibition enhances RAD001-induced
cytotoxicity in human bladder cancer cells. Drug Des Devel Ther.
10:1501–1513. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Liao Y and Tang L: MicroRNA-34
family: A potential tumor suppressor and therapeutic candidate in
cancer. J Exp Clin Cancer Res. 38:532019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Inamura K: Bladder cancer: New insights
into its molecular pathology. Cancers (Basel). 10:1002018.
View Article : Google Scholar
|
|
29
|
Li L, Yuan L, Luo J, Gao J, Guo J and Xie
X: MiR-34a inhibits proliferation and migration of breast cancer
through down-regulation of Bcl-2 and SIRT1. Clin Exp Med.
13:109–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yaxley JP: Urinary tract cancers: An
overview for general practice. J Family Med Prim Care. 5:533–538.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen Z, Shen T, Wientjes MG, O'Donnell MA
and Au JL: Intravesical treatments of bladder cancer: Review. Pharm
Res. 25:1500–1510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Di Pierro GB, Gulia C, Cristini C,
Fraietta G, Marini L, Grande P, Gentile V and Piergentili R:
Bladder cancer: A simple model becomes complex. Curr Genomics.
13:395–415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu J, Xiong G, Trinkle C and Xu R:
Integrated extracellular matrix signaling in mammary gland
development and breast cancer progression. Histol Histopathol.
29:1083–1092. 2014.PubMed/NCBI
|
|
35
|
Quintero-Fabián S, Arreola R,
Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V,
Lara-Riegos J, Ramírez-Camacho MA and Alvarez-Sánchez ME: Role of
matrix metalloproteinases in angiogenesis and cancer. Front Oncol.
9:13702019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ueda J, Kajita M, Suenaga N, Fujii K and
Seiki M: Sequence-specific silencing of MT1-MMP expression
suppresses tumor cell migration and invasion: Importance of MT1-MMP
as a therapeutic target for invasive tumors. Oncogene.
22:8716–8722. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lakka SS, Rajan M, Gondi C, Yanamandra N,
Chandrasekar N, Jasti SL, Adachi Y, Siddique K, Gujrati M, Olivero
W, et al: Adenovirus-mediated expression of antisense MMP-9 in
glioma cells inhibits tumor growth and invasion. Oncogene.
21:8011–8019. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hofmann UB, Westphal JR, Zendman AJ,
Becker JC, Ruiter DJ and van Muijen GN: Expression and activation
of matrix metalloproteinase-2 (MMP-2) and its co-localization with
membrane-type 1 matrix metalloproteinase (MT1-MMP) correlate with
melanoma progression. J Pathol. 191:245–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mehner C, Hockla A, Miller E, Ran S,
Radisky DC and Radisky ES: Tumor cell-produced matrix
metalloproteinase 9 (MMP-9) drives malignant progression and
metastasis of basal-like triple negative breast cancer. Oncotarget.
5:2736–2749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vihinen P and Kähäri VM: Matrix
metalloproteinases in cancer: Prognostic markers and therapeutic
targets. Int J Cancer. 99:157–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Itoh T, Tanioka M, Yoshida H, Yoshioka T,
Nishimoto H and Itohara S: Reduced angiogenesis and tumor
progression in gelatinase A-deficient mice. Cancer Res.
58:1048–1051. 1998.PubMed/NCBI
|
|
43
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Skaftnesmo KO, Prestegarden L, Micklem DR
and Lorens JB: MicroRNAs in tumorigenesis. Curr Pharm Biotechnol.
8:320–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu X, Liu X, Wu Y, Fang Z, Wu Q, Wu C,
Hao Y, Yang X, Zhao J, Li J, et al: MicroRNA-34a attenuates
metastasis and chemoresistance of bladder cancer cells by targeting
the TCF1/LEF1 axis. Cell Physiol Biochem. 48:87–98. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ding ZS, He YH, Deng YS, Peng PX, Wang JF,
Chen X, Zhao PY and Zhou XF: MicroRNA-34a inhibits bladder cancer
cell migration and invasion, and upregulates PTEN expression. Oncol
Lett. 18:5549–5554. 2019.PubMed/NCBI
|
|
47
|
Yang L, Song X, Zhu J, Li M, Ji Y, Wu F,
Chen Y, Cui X, Hu J, Wang L, et al: Tumor suppressor microRNA-34a
inhibits cell migration and invasion by targeting
MMP-2/MMP-9/FNDC3B in esophageal squamous cell carcinoma. Int J
Oncol. 51:378–388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao H, Xing F, Yuan J, Li Z and Zhang W:
Sevoflurane inhibits migration and invasion of glioma cells via
regulating miR-34a-5p/MMP-2 axis. Life Sci. 256:1178972020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Di Martino MT, Leone E, Amodio N, Foresta
U, Lionetti M, Pitari MR, Cantafio ME, Gullà A, Conforti F, Morelli
E, et al: Synthetic miR-34a mimics as a novel therapeutic agent for
multiple myeloma: In vitro and in vivo evidence. Clin Cancer Res.
18:6260–6270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yan D, Zhou X, Chen X, Hu DN, Dong XD,
Wang J, Lu F, Tu L and Qu J: MicroRNA-34a inhibits uveal melanoma
cell proliferation and migration through downregulation of c-Met.
Invest Ophthalmol Vis Sci. 50:1559–1565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wiggins JF, Ruffino L, Kelnar K, Omotola
M, Patrawala L, Brown D and Bader AG: Development of a lung cancer
therapeutic based on the tumor suppressor microRNA-34. Cancer Res.
70:5923–5930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Trang P, Wiggins JF, Daige CL, Cho C,
Omotola M, Brown D, Weidhaas JB, Bader AG and Slack FJ: Systemic
delivery of tumor suppressor microRNA mimics using a neutral lipid
emulsion inhibits lung tumors in mice. Mol Ther. 19:1116–1122.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dyrskjøt L, Ostenfeld MS, Bramsen JB,
Silahtaroglu AN, Lamy P, Ramanathan R, Fristrup N, Jensen JL,
Andersen CL, Zieger K, et al: Genomic profiling of microRNAs in
bladder cancer: miR-129 is associated with poor outcome and
promotes cell death in vitro. Cancer Res. 69:4851–4860. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xie Y, Ma X, Chen L, Li H, Gu L, Gao Y,
Zhang Y, Li X, Fan Y, Chen J and Zhang X: MicroRNAs with prognostic
significance in bladder cancer: A systematic review and
meta-analysis. Sci Rep. 7:56192017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Al-Husseini MJ, Kunbaz A, Saad AM, Santos
JV, Salahia S, Iqbal M and Alahdab F: Trends in the incidence and
mortality of transitional cell carcinoma of the bladder for the
last four decades in the USA: A SEER-based analysis. BMC Cancer.
19:462019. View Article : Google Scholar : PubMed/NCBI
|