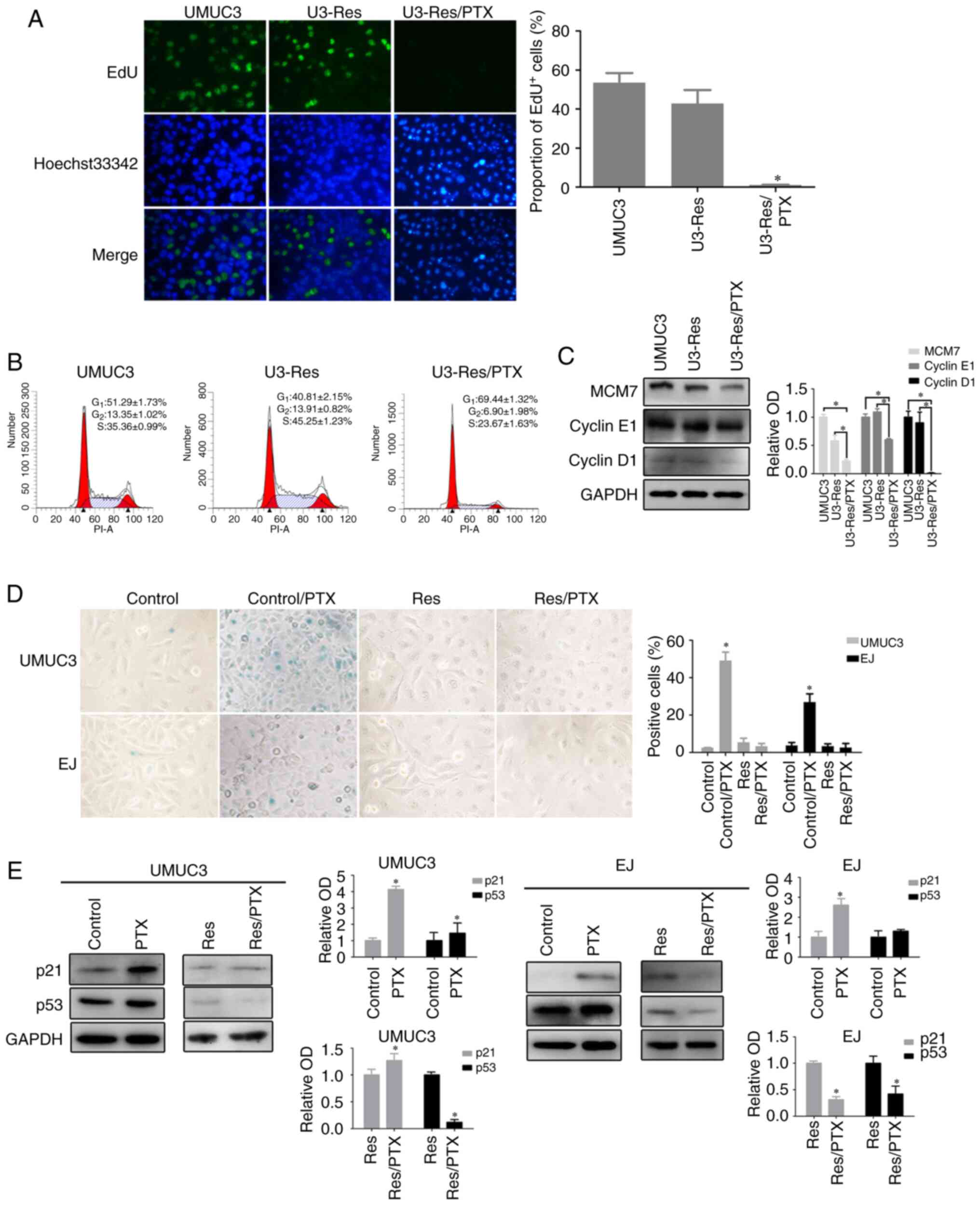
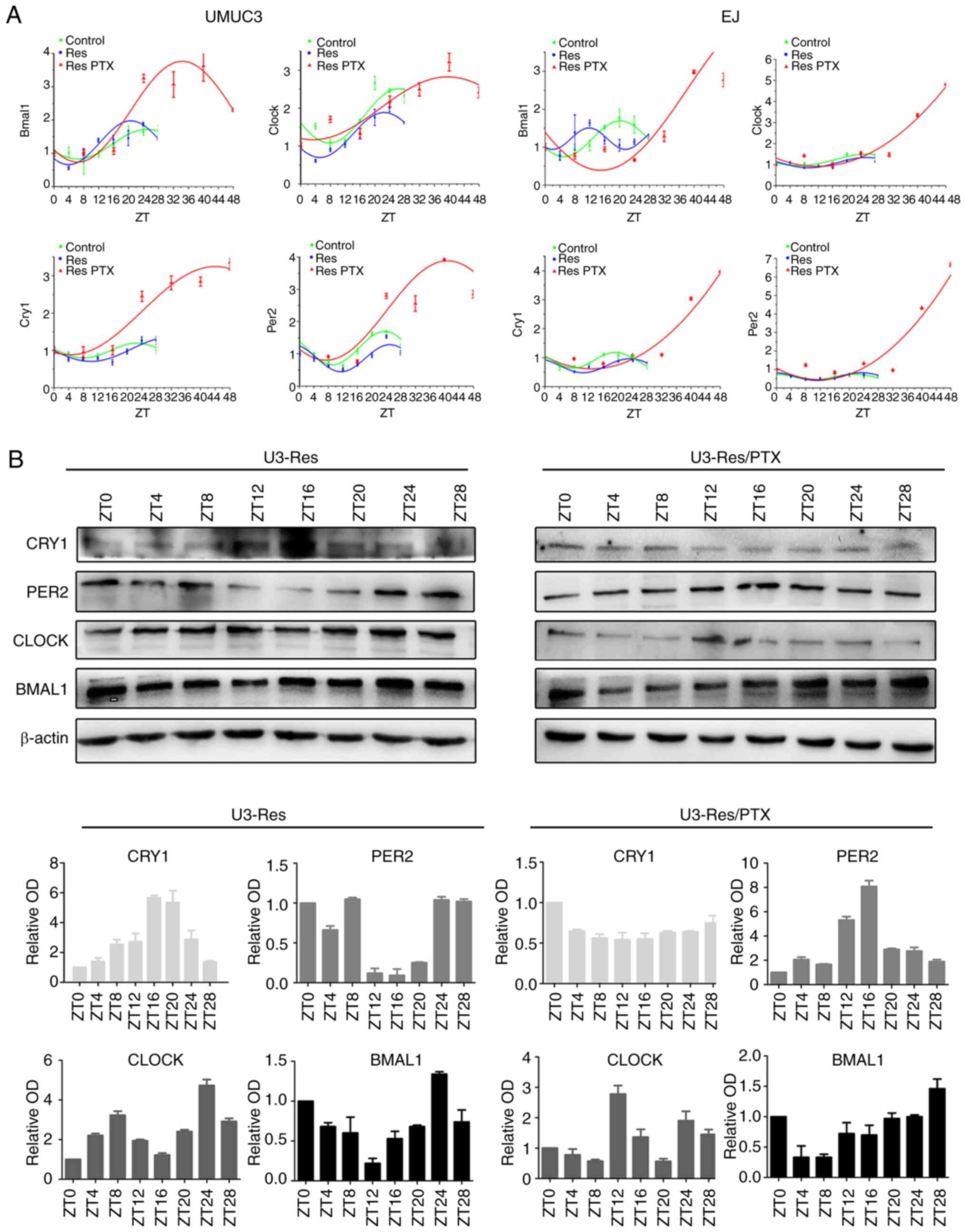
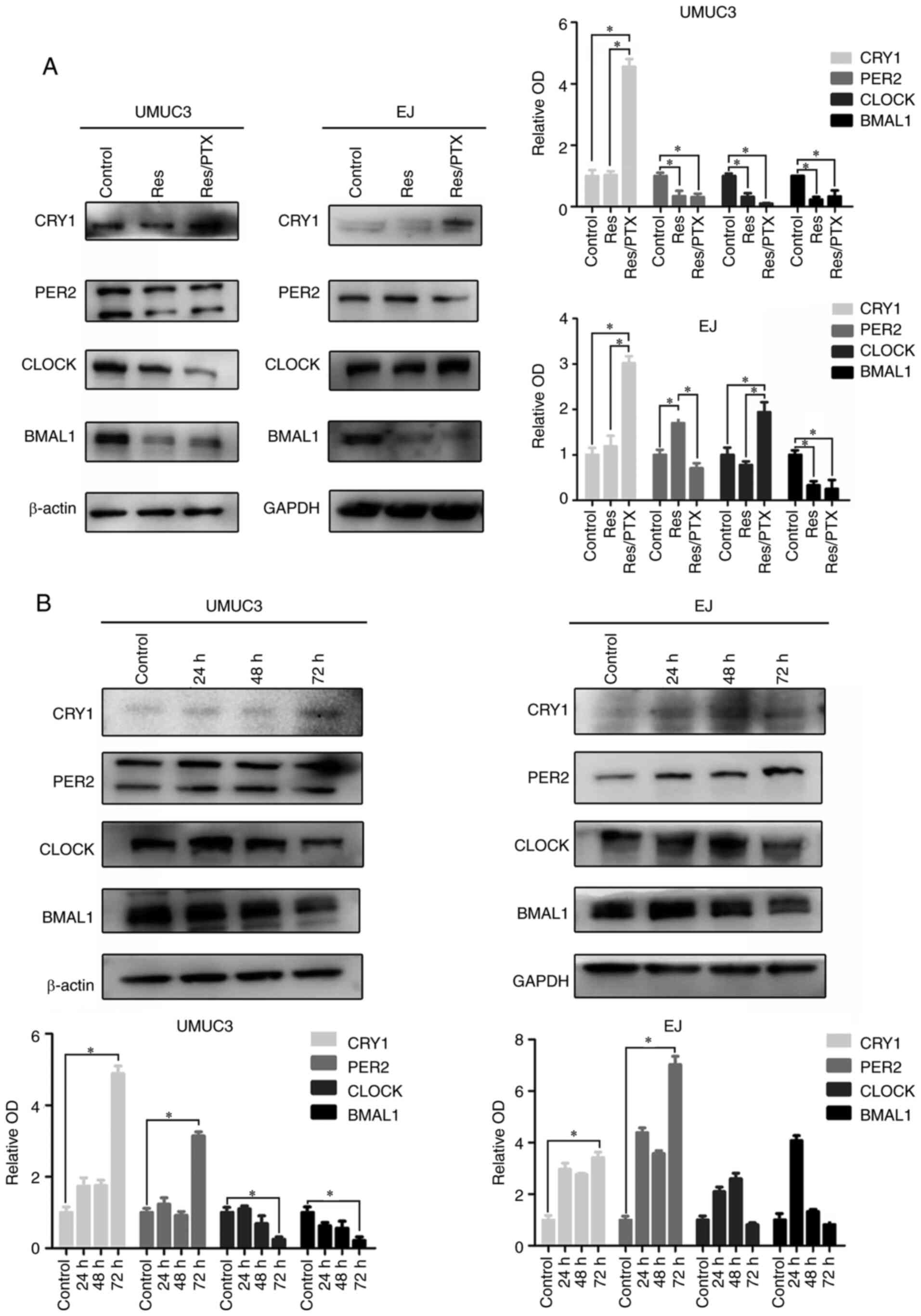
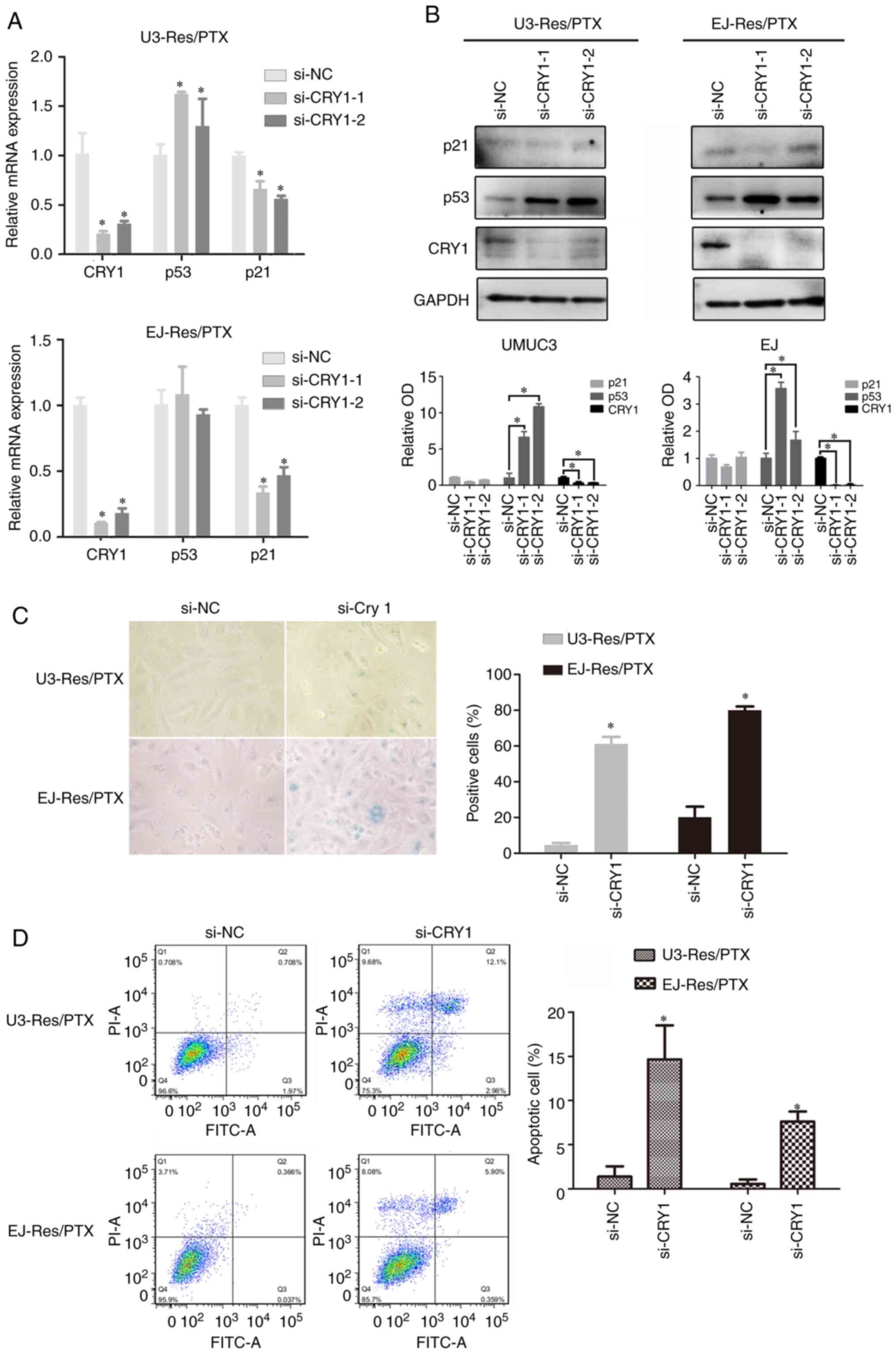
|
1
|
Casadei C, Dizman N, Schepisi G, Cursano
MC, Basso U, Santini D, Pal SK and De Giorgi U: Targeted therapies
for advanced bladder cancer: New strategies with FGFR inhibitors.
Ther Adv Med Oncol. 11:17588359198902852019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alfred Witjes J, Lebret T, Compérat EM,
Cowan NC, De Santis M, Bruins HM, Hernández V, Espinós EL, Dunn J,
Rouanne M, et al: Updated 2016 EAU guidelines on muscle-invasive
and metastatic bladder cancer. Eur Urol. 71:462–475. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ke B, Wei T, Huang Y, Gong Y, Wu G, Liu J,
Chen X and Shi L: Interleukin-7 resensitizes non-small-cell lung
cancer to cisplatin via inhibition of ABCG2. Mediators Inflamm.
2019:72414182019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Li LJ, Qiu MX and Gong BS: Effects
of paclitaxel combined with miR-448 on growth and proliferation of
bladder cancer EJ cells. Eur Rev Med Pharmacol Sci. 22:3363–3369.
2018.PubMed/NCBI
|
|
5
|
Zeng Q, Liu J, Cao P, Li J, Liu X, Fan X,
Liu L, Cheng Y, Xiong W, Li J, et al: Inhibition of REDD1
sensitizes bladder urothelial carcinoma to paclitaxel by inhibiting
autophagy. Clin Cancer Res. 24:445–459. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He Q, Li J, Yin W, Song Z, Zhang Z, Yi T,
Tang J, Wu D, Lu Y, Wang Z, et al: Low-dose paclitaxel enhances the
anti-tumor efficacy of GM-CSF surface-modified whole-tumor-cell
vaccine in mouse model of prostate cancer. Cancer Immunol
Immunother. 60:715–730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Michaelson MD, Hu C, Pham HT, Dahl DM,
Lee-Wu C, Swanson GP, Vuky J, Lee RJ, Souhami L, Chang B, et al: A
phase 1/2 trial of a combination of paclitaxel and trastuzumab with
daily irradiation or paclitaxel alone with daily irradiation after
transurethral surgery for noncystectomy candidates with
muscle-invasive bladder cancer (Trial NRG Oncology RTOG 0524). Int
J Radiat Oncol Biol Phys. 97:995–1001. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Albers P, Park SI, Niegisch G, Fechner G,
Steiner U, Lehmann J, Heimbach D, Heidenreich A, Fimmers R and
Siener R; AUO Bladder Cancer Group, : Randomized phase III trial of
2nd line gemcitabine and paclitaxel chemotherapy in patients with
advanced bladder cancer: Short-term versus prolonged treatment
[German association of urological oncology (AUO) trial AB 20/99].
Ann Oncol. 22:288–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mari A, D'Andrea D, Abufaraj M, Foerster
B, Kimura S and Shariat SF: Genetic determinants for chemo- and
radiotherapy resistance in bladder cancer. Transl Androl Urol.
6:1081–1089. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao YH, Qin XL, Yang JY, Liao YW, Wu XZ
and Zheng HP: Identification and expression analysis of ceftriaxone
resistance-related genes in Neisseria gonorrhoeae integrating
RNA-Seq data and qRT-PCR validation. J Glob Antimicrob Resist.
16:202–209. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang S, Xie J, Miao J, Li R, Liao W and
Luo R: A knockdown of Maml1 that results in melanoma cell
senescence promotes an innate and adaptive immune response. Cancer
Immunol Immunother. 62:183–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mikula-Pietrasik J, Niklas A, Uruski P,
Tykarski A and Książek K: Mechanisms and significance of
therapy-induced and spontaneous senescence of cancer cells. Cell
Mol Life Sci. 77:213–229. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Collado M and Serrano M: Senescence in
tumours: Evidence from mice and humans. Nat Rev Cancer. 10:51–57.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee S and Lee JS: Cellular senescence: A
promising strategy for cancer therapy. BMB Rep. 52:35–41. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin S, Schulte BA and Wang GY: Role of
senescence induction in cancer treatment. World J Clin Oncol.
9:180–187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khongkow P, Gomes AR, Gong C, Man EP,
Tsang JW, Zhao F, Monteiro LJ, Coombes RC, Medema RH, Khoo US and
Lam EW: Paclitaxel targets FOXM1 to regulate KIF20A in mitotic
catastrophe and breast cancer paclitaxel resistance. Oncogene.
35:990–1002. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kavanagh EL, Lindsay S, Halasz M, Gubbins
LC, Weiner-Gorzel K, Guang MHZ, McGoldrick A, Collins E, Henry M,
Blanco-Fernández A, et al: Protein and chemotherapy profiling of
extracellular vesicles harvested from therapeutic induced senescent
triple negative breast cancer cells. Oncogenesis. 6:e3882017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baba K and Tosini G: Aging alters
circadian rhythms in the mouse eye. J Biol Rhythms. 33:441–445.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rakshit K, Wambua R, Giebultowicz TM and
Giebultowicz JM: Effects of exercise on circadian rhythms and
mobility in aging Drosophila melanogaster. Exp Gerontol.
48:1260–1265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takahashi JS: Transcriptional architecture
of the mammalian circadian clock. Nat Rev Genet. 18:164–179. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Relles D, Sendecki J, Chipitsyna G, Hyslop
T, Yeo CJ and Arafat HA: Circadian gene expression and
clinicopathologic correlates in pancreatic cancer. J Gastrointest
Surg. 17:443–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Taniguchi H, Fernández AF, Setién F,
Ropero S, Ballestar E, Villanueva A, Yamamoto H, Imai K, Shinomura
Y and Esteller M: Epigenetic inactivation of the circadian clock
gene BMAL1 in hematologic malignancies. Cancer Res. 69:8447–8454.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu Y, Stevens RG, Hoffman AE, Fitzgerald
LM, Kwon EM, Ostrander EA, Davis S, Zheng T and Stanford JL:
Testing the circadian gene hypothesis in prostate cancer: A
population-based case-control study. Cancer Res. 69:9315–9322.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Terzi MY, Izmirli M and Gogebakan B: The
cell fate: Senescence or quiescence. Mol Biol Rep. 43:1213–1220.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang L, Li Y, Bhattacharya A and Zhang Y:
PEPD is a pivotal regulator of p53 tumor suppressor. Nat Commun.
8:20522017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grim J, D'Amico A, Frizelle S, Zhou J,
Kratzke RA and Curiel DT: Adenovirus-mediated delivery of p16 to
p16-deficient human bladder cancer cells confers chemoresistance to
cisplatin and paclitaxel. Clin Cancer Res. 3:2415–2423.
1997.PubMed/NCBI
|
|
28
|
Sanchez-Carbayo M, Socci ND, Charytonowicz
E, Lu M, Prystowsky M, Childs G and Cordon-Cardo C: Molecular
profiling of bladder cancer using cDNA microarrays: Defining
histogenesis and biological phenotypes. Cancer Res. 62:6973–6980.
2002.PubMed/NCBI
|
|
29
|
Gaucher J, Montellier E and Sassone-Corsi
P: Molecular cogs: Interplay between circadian clock and cell
cycle. Trends Cell Biol. 28:368–379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sulli G, Rommel A, Wang X, Kolar MJ, Puca
F, Saghatelian A, Plikus MV, Verma IM and Panda S: Pharmacological
activation of REV-ERBs is lethal in cancer and oncogene-induced
senescence. Nature. 553:351–355. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jang H, Lee GY, Selby CP, Lee G, Jeon YG,
Lee JH, Cheng KK, Titchenell P, Birnbaum MJ, Xu A, et al:
SREBP1c-CRY1 signalling represses hepatic glucose production by
promoting FOXO1 degradation during refeeding. Nat Commun.
7:121802016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Manjili MH: Tumor dormancy and relapse:
From a natural byproduct of evolution to a disease state. Cancer
Res. 77:2564–2569. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang L, Shang Z, Zhou Y, Hu X, Chen Y, Fan
Y, Wei X, Wu L, Liang Q, Zhang J and Gao Z: Autophagy mediates
glucose starvation-induced glioblastoma cell quiescence and
chemoresistance through coordinating cell metabolism, cell cycle,
and survival. Cell Death Dis. 9:2132018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Atkins RJ, Stylli SS, Kurganovs N,
Mangiola S, Nowell CJ, Ware TM, Corcoran NM, Brown DV, Kaye AH,
Morokoff A, et al: Cell quiescence correlates with enhanced
glioblastoma cell invasion and cytotoxic resistance. Exp Cell Res.
374:353–364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Touil Y, Igoudjil W, Corvaisier M, Dessein
AF, Vandomme J, Monté D, Stechly L, Skrypek N, Langlois C, Grard G,
et al: Colon cancer cells escape 5FU chemotherapy-induced cell
death by entering stemness and quiescence associated with the
c-Yes/YAP axis. Clin Cancer Res. 20:837–846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang YJ, Xu LL, Wang P, Jian H, Shi X,
Jia M, Mo L, Hu Z, Li H and Li J: Phenotypic transition of tumor
cells between epithelial- and mesenchymal-like state during
adaptation to acidosis. Cell Cycle. 18:1938–1947. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
De Angelis ML, Francescangeli F, La Torre
F and Zeuner A: Stem cell plasticity and dormancy in the
development of cancer therapy resistance. Front Oncol. 9:6262019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Toledo M, Batista-Gonzalez A, Merheb E,
Aoun ML, Tarabra E, Feng D, Sarparanta J, Merlo P, Botrè F,
Schwartz GJ, et al: Autophagy regulates the liver clock and glucose
metabolism by degrading CRY1. Cell Metab. 28:268–281.e4. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hirota T, Lee JW, St John PC, Sawa M,
Iwaisako K, Noguchi T, Pongsawakul PY, Sonntag T, Welsh DK, Brenner
DA, et al: Identification of small molecule activators of
cryptochrome. Science. 337:1094–1097. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiang S, Dauchy RT, Hoffman AE, Pointer D,
Frasch T, Blask DE and Hill SM: Epigenetic inhibition of the tumor
suppressor ARHI by light at night-induced circadian melatonin
disruption mediates STAT3-driven paclitaxel resistance in breast
cancer. J Pineal Res. 67:e125862019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiang S, Dauchy RT, Hauch A, Mao L, Yuan
L, Wren MA, Belancio VP, Mondal D, Frasch T, Blask DE and Hill SM:
Doxorubicin resistance in breast cancer is driven by light at
night-induced disruption of the circadian melatonin signal. J
Pineal Res. 59:60–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dauchy RT, Xiang S, Mao L, Brimer S, Wren
MA, Yuan L, Anbalagan M, Hauch A, Frasch T, Rowan BG, et al:
Circadian and melatonin disruption by exposure to light at night
drives intrinsic resistance to tamoxifen therapy in breast cancer.
Cancer Res. 74:4099–4110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu SL, Lin HX, Lin CY, Sun XQ, Ye LP, Qiu
F, Wen W, Hua X, Wu XQ, Li J, et al: TIMELESS confers cisplatin
resistance in nasopharyngeal carcinoma by activating the
Wnt/β-catenin signaling pathway and promoting the epithelial
mesenchymal transition. Cancer Lett. 402:117–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miyamoto N, Izumi H, Noguchi T, Nakajima
Y, Ohmiya Y, Shiota M, Kidani A, Tawara A and Kohno K: Tip60 is
regulated by circadian transcription factor clock and is involved
in cisplatin resistance. J Biol Chem. 283:18218–18226. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chun SK, Chung S, Kim HD, Lee JH, Jang J,
Kim J, Kim D, Son GH, Oh YJ, Suh YG, et al: A synthetic
cryptochrome inhibitor induces anti-proliferative effects and
increases chemosensitivity in human breast cancer cells. Biochem
Biophys Res Commun. 467:441–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chang BD, Xuan Y, Broude EV, Zhu H, Schott
B, Fang J and Roninson IB: Role of p53 and p21waf1/cip1 in
senescence-like terminal proliferation arrest induced in human
tumor cells by chemotherapeutic drugs. Oncogene. 18:4808–4818.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chang BD, Broude EV, Dokmanovic M, Zhu H,
Ruth A, Xuan Y, Kandel ES, Lausch E, Christov K and Roninson IB: A
senescence-like phenotype distinguishes tumor cells that undergo
terminal proliferation arrest after exposure to anticancer agents.
Cancer Res. 59:3761–3767. 1999.PubMed/NCBI
|
|
48
|
Katamune C, Koyanagi S, Shiromizu S,
Matsunaga N, Shimba S, Shibata S and Ohdo S: Different roles of
negative and positive components of the circadian clock in
oncogene-induced neoplastic transformation. J Biol Chem.
291:10541–10550. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wyld L, Bellantuono I, Tchkonia T, Morgan
J, Turner O, Foss F, George J, Danson S and Kirkland JL: Senescence
and cancer: A review of clinical implications of senescence and
senotherapies. Cancers (Basel). 12:21342020. View Article : Google Scholar
|
|
50
|
Janich P, Pascual G, Merlos-Suárez A,
Batlle E, Ripperger J, Albrecht U, Cheng HY, Obrietan K, Di Croce L
and Benitah SA: The circadian molecular clock creates epidermal
stem cell heterogeneity. Nature. 480:209–214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Antoch MP, Gorbacheva VY, Vykhovanets O,
Toshkov IA, Kondratov RV, Kondratova AA, Lee C and Nikitin AY:
Disruption of the circadian clock due to the Clock mutation has
discrete effects on aging and carcinogenesis. Cell Cycle.
7:1197–1204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huber AL, Papp SJ, Chan AB, Henriksson E,
Jordan SD, Kriebs A, Nguyen M, Wallace M, Li Z, Metallo CM and
Lamia KA: CRY2 and FBXL3 cooperatively degrade c-MYC. Mol Cell.
64:774–789. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Du H, Jie L, Xu W, Wu Y, Liu T and Li M: A
monoclonal antibody against a potential cancer biomarker, human
ubiquitin-conjugating enzyme E2. Hybridoma (Larchmt). 31:196–202.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lamia KA, Papp SJ, Yu RT, Barish GD,
Uhlenhaut NH, Jonker JW, Downes M and Evans RM: Cryptochromes
mediate rhythmic repression of the glucocorticoid receptor. Nature.
480:552–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chaudhari A, Gupta R, Patel S, Velingkaar
N and Kondratov R: Cryptochromes regulate IGF-1 production and
signaling through control of JAK2-dependent STAT5B phosphorylation.
Mol Biol Cell. 28:834–842. 2017. View Article : Google Scholar : PubMed/NCBI
|