Introduction
Liver cancer is the sixth most common type of cancer
and the third leading cause of cancer mortality worldwide (1). Although surgical resection is the
primary and most effective treatment for patients with liver
cancer, prognosis following surgical resection remains poor, due to
the high 5-year recurrence rate (~70%) (2,3).
However, most patients with liver cancer are diagnosed with
advanced disease, consequently missing the window of opportunity
for surgery, with the overall 5-year survival rate of patients with
liver cancer at only 17% (1,4).
Sorafenib, an orally active multi-targeted tyrosine kinase
inhibitor, is the first FDA-approved molecular targeted therapy
available for advanced liver cancer, and has been used as
first-line therapy (5).
Nevertheless, the therapeutic effect of sorafenib is limited, and
the median survival time of patients is increased by ~3 months
(6,7). Moreover, several side effects of
sorafenib have been reported. Patients who discontinue sorafenib
due to chemoresistance or severe side effects may suffer tumor
recurrence, with rapid tumor progression after they stop taking the
drug (8). Sorafenib can also
promote liver cancer cell invasion and migration, as demonstrated
by western blot analysis and in an in vivo tumor model
(9). Therefore, the molecular
mechanism of sorafenib should be fully understood, and novel
therapeutic strategies for liver cancer should be explored.
Interleukin-6 (IL-6) is a pleiotropic cytokine
present in the tumor microenvironment and involved in various
biological responses, including tumor progression, metastasis and
chemoresistance (10). IL-6 plays a
crucial role in linking chronic inflammation to liver cancer
progression (11–13), and the expression of the IL-6 gene
is associated with tumor stage in liver cancer (14). IL-6 levels in cancer tissues and
serum were increased in patients with liver cancer, as compared
with healthy controls; IL-6 levels were also correlated with tumor
metastasis and reduced patient survival (15,16).
Epithelial-mesenchymal transition (EMT) is a
biological process involved in various physiological and
pathological processes and tumorigenesis (17,18).
During EMT, tumor cells lose their epithelial traits, such as cell
polarity and cell-cell adhesion, and gain mesenchymal
characteristics, such as migration, invasion and anti-apoptosis
(19). EMT is involved in invasive,
metastatic and therapeutic resistance in liver cancer (20–23).
IL-6 is likely a potent triggering factor in the mediation of EMT
in various types of cancer, such as breast, head and neck, and
colon cancer (24–26).
The aim of the present study was to further explore
the molecular mechanism of the sorafenib-mediated pro-metastatic
effect and resistance in liver cancer, and the role of IL-6 in
sorafenib treatment.
Materials and methods
Cell culture and drugs
Hepatocellular carcinoma (HCC)LM3 and HepG2 cells
(both obtained from the Liver Cancer Institute, Fudan University,
Shanghai, China), and authenticated by STR profiling, were cultured
in Dulbecco's modified Eagle's medium (DMEM; GE Healthcare Life
Sciences; Cytiva) containing 10% fetal bovine serum (Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 50 mg/ml streptomycin in
a humidified atmosphere of 5% CO2 at 37°C. Sorafenib
(Bayer AG) was prepared as previously described (9). Recombinant human IL-6 (cat. no.
206-IL-010) was purchased from R&D Systems (R&D Systems,
Inc.). The JAK inhibitor AG490 was procured from Selleck
Chemicals.
Stable cell line construction by
transcription activator-like effector nucleases (TALEN)
Stable cell lines were constructed in accordance
with previously described procedures (27). First, the TALEN design was in
accordance with the sequence of IL-6. The TALEN arms were designed
as 2×3 (2 left and 3 right arms) combination targets on the IL-6
(NCBI gene ID, 3569). The plasmids for the left and right arms of
the TALEN were constructed using the FAST TALEN Kit according to
the manufacturer's instructions (SIDANSAI Biotechnology). Following
sequencing, 5 plasmids were transfected into the 293T cell line
(obtained from the Chinese Academy of Sciences) for 24 h at 37°C
using FuGene HD transfection reagent (Roche Diagnostics) in a 2×3
cross combination. A pair of TALEN plasmids was selected as the
most effective knockout group after 3 days of puromycin screening
and subsequent genomic PCR sequencing. The HCCLM3 cell line was
routinely culturedand was plated for 16 h before transfection. The
HCCLM3 cell line was transfected with the indicated plasmids for 24
h at 37°C using Fugene HD (Roche Diagnostics), according to the
manufacturer's instructions. The selected pair of TALEN plasmids,
which had the highest cleavage efficiency, was co-loaded into the
HCCLM3 cell line. The amount of plasmids per well for the 6-well
plates included in each transfection were 2 µg pTALEN-Left, 2 µg
pTALEN-Right, and 0.5 µg of pEGFP as a transfection marker. The
cells were exposed to 2 µg/ml puromycin for 3 days. The medium
containing puromycin was then replaced with growth media. After a
week of monoclonal culturing, stably transfected clones
[HCCLM3-IL-6(−)] were validated through western blot analysis and
reverse transcription quantitative polymerase chain reaction
(RT-qPCR), as compared with HCCLM3-wild-type (wt), which was not
transfected with TALEN plasmids. The following primers of IL-6 were
used: 5′-GAACTCCTTCTCCACAAGCG-3′ forward and
5′-TTTTCTGCCAGTGCCTCTTT-3′ reverse.
Cell proliferation assay and flow
cytometry
The cell proliferation assay was performed using a
Cell Counting Kit-8 (CCK-8) assay kit according to the
manufacturer's instructions. In this procedure, 4×103
cells were seeded in each well of a 96-well plate and cultured for
different time-points (0, 24 and 48 h). The cells were then
incubated with 100 µl DMEM containing 10% CCK-8 reagent (Dojindo
Molecular Technologies, Inc.) in each well at 37°C for 2 h, and
absorbance was detected at a wavelength of 450 nm using a
microplate reader (Nexcelom, Inc.).
The reagents of apoptosis and the cell cycle were
used in accordance with the manufacturer's protocol before flow
cytometry was performed. For the apoptosis assay, single-cell
suspensions were prepared, and then 1×105 cells were
washed with phosphate-buffered saline (PBS) twice and stained with
Annexin V and propidium iodide (BD Biosciences). For the cell cycle
assay, 1×105 cells were incubated in 75% ethanol at
−20°C overnight and stained with PI/RNase staining buffer (BD
Biosciences) at room temperature for 15 min. Fluorescence was
measured using FACSCalibur (BD Biosciences) and analyzed using
FlowJo v7.6.1 software (Tree Star, Inc.).
Western blot analysis
Western blot analysis was performed in accordance
with previously described procedures (28). The brief steps were as follows: 30
µg Protein extracted from the cells was subjected to 10% SDS-PAGE
and transferred to a PVDF membrane (EMD Millipore). The extract was
then blocked with 5% defatted milk for 1 h at room temperature. The
membrane was incubated with a primary antibody at 4°C overnight. On
the following day, the membrane was incubated with HRP-conjugated
anti-mouse/rabbit secondary antibody at a dilution of 1:5,000 with
5% defatted milk for 1 h at room temperature. The bands were
detected using a ChemiDoc MP system (Bio-Rad Laboratories,
Inc.).
The following primary antibodies were used: IL-6
(1:2,000; cat. no. NB600-1131; Novus Biologicals), E-cadherin
(1:1,000; product no. 3195T), N-cadherin (1:1,000; product no.
13116T), Vimentin (1:1,000; product no. 5741T), Snail (1:1,000;
product no. 3879T), Janus kinase 2 (JAK2) (1:1,000; product no.
3230T), phospho (p)-JAK2 (1:1,000; product no. 3771S), STAT3
(1:1,000; product no. 9139T), p-STAT3 (1:1,000; product no. 9145T),
cyclin dependent kinase 2 (CDK2) (1:1,000; product no. 18048T),
cyclin D1 (1:1,000; product no. 55506T), cleaved caspase-3
(1:1,000; product no. 9661T), cleaved poly (ADP-ribose) polymerase
(PARP) (1:1,000; product no. 5625T), B-cell lymphoma-2 (Bcl-2)
(1:1,000; product no. 15071T) and β-actin (1:1,000; product no.
4970T; all from Cell Signaling Technology, Inc.). A
peroxidase-conjugated goat anti-rabbit/mouse secondary antibody was
purchased from YEASEN Biotech (1:5,000; cat. nos.
33101ES60/33201ES60; YEASEN Biotech, Inc.).
RT-qPCR
RNA isolation and RT-qPCR procedures were performed
as previously described (27). TB
Green Premix Ex Taq II (cat. no. RR820A) and PrimeScript™ RT
Reagent Kit with gDNA Eraser (Perfect Real Time) (cat. no. RR047A)
were purchased from Takara Bio, Inc., and primers were synthesized
by Sangon Biotech. The primers for IL-6 were used as described
above and the primers for GAPDH were used as follows: Forward,
5′-GGAGCGAGATCCCTCCAAAAT−3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′.
ELISA
The cell supernatant protein levels of IL-6 were
analyzed by ELISA using IL-6 Quantikine® ELISA kit
according to the manufacturer's instructions (cat. no. D6050;
R&D Systems, Inc.). Equal numbers (1×106) of
HCCLME3-wt cells and HepG2-wt cells were plated and cultured for 24
h. The cells were then incubated with or without sorafenib (5 or 10
µmol/l) at 37°C for 24 h. ELISA was performed using the cell
supernatant collected. All analyses were performed in
triplicate.
Cell migration assay
Cell migration was assessed by Transwell assay in
Boyden chambers with an 8-µm pore (Corning Inc.). The cells were
incubated with or without sorafenib (5 or 10 µmol/l) or IL-6 (50
ng/ml) at 37°C for 24 h. Next, 5×104 cells in 200 µl
serum-free DMEM were seeded onto the upper chamber, and 650 µl DMEM
containing 10% BSA was perfused to each well in the lower chamber.
After the non-migrating cells were removed, the remaining cells
were fixed with 100% methanol for 15 min at room temperature,
stained with 0.1% crystal violet dye for 5 min at room temperature,
and finally counted under a light microscope at a magnification of
×100. Three independent experiments were performed in
triplicate.
Xenograft model of human liver cancer
in nude mice
To form subcutaneous tumors, HCCLM3-wt and
HCCLM3-IL-6(−) cells (1×107 cells) were mixed with PBS
and injected into the right flank of four, 4-week-old male BALB/c
nude mice (2 mice per group) (Beijing Vital River Laboratory Animal
Technology) weighing approximately 20 g which were housed in an
appropriate environment (28°C; ~40–60% humidity; 10-h light/14-h
dark cycle; plenty of sterilized food and water, laminar flow
cabinet under specific pathogen-free conditions). After 4 weeks,
the mice were sacrificed by cervical vertebra dislocation, and the
tumor tissues were cut into 1-cm3 pieces and implanted
into the livers of the nude mice (a total 24 of mice were used; 12
mice per group) anesthetized using intraperitoneal anesthesia with
pentobarbital sodium (2.5 mg/kg) as previously described (29). The treatment started 1 week after
the tumor was orthotopically implanted. Each group of mice was
divided into two subsets containing 6 mice and treated with 30
mg/kg/day sorafenib or vehicle for 5 weeks. Following mouse
sacrifice by cervical vertebra dislocation, the lung tissues were
extracted and analyzed after hematoxylin and eosin (H&E)
staining at room temperature for 5 min. Lung metastases were
examined as previously described (29). Ten slices from each lung were
observed. The animal experiments were approved by the Animal Care
Committee of Zhongshan Hospital (Shanghai, China).
Immunohistochemistry
Immunohistochemical staining was performed as
previously described (29).
Paraffin-embedded orthotopically implanted tumors were cut into
5-µm sections and then deparaffinized and rehydrated.
Immunohistochemical staining was performed using the Ultra Vision
Quanto Detection HRP DAB System (cat. no. TL-015-QHD; Thermo Fisher
Scientific, Inc.), following the manufacturer's instructions.
Briefly, the slices from orthotopically implanted tumors were
treated with a diluted primary antibody against IL-6 (1:100; cat.
no. NB600-1131; Novus Biologicals) at 4°C overnight and
anti-rabbit/mouse secondary antibodies (included in the Ultra
Vision Quanto Detection HRP DAB System) at room temperature for 60
min. Signals were detected by DAB at room temperature for 5 min.
Immunohistochemical images were recorded using a computerized image
system composed of a Leica CCD camera DFC420 connected to a Leica
DM IRE2 microscope (Leica Microsystems, Inc.). The total positive
staining area of IL-6 was calculated by Image-Pro Plus v6.2
software (Media Cybernetics, Inc.).
Statistical analysis
Data were analyzed with SPSS 18.0 (SPSS Inc.).
Quantitative variables were analyzed by unpaired two-tailed
Student's t-test or one-way ANOVA with Tukey's post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
IL-6 knockout attenuates the
pro-invasive effect induced by sorafenib treatment in vitro and in
vivo
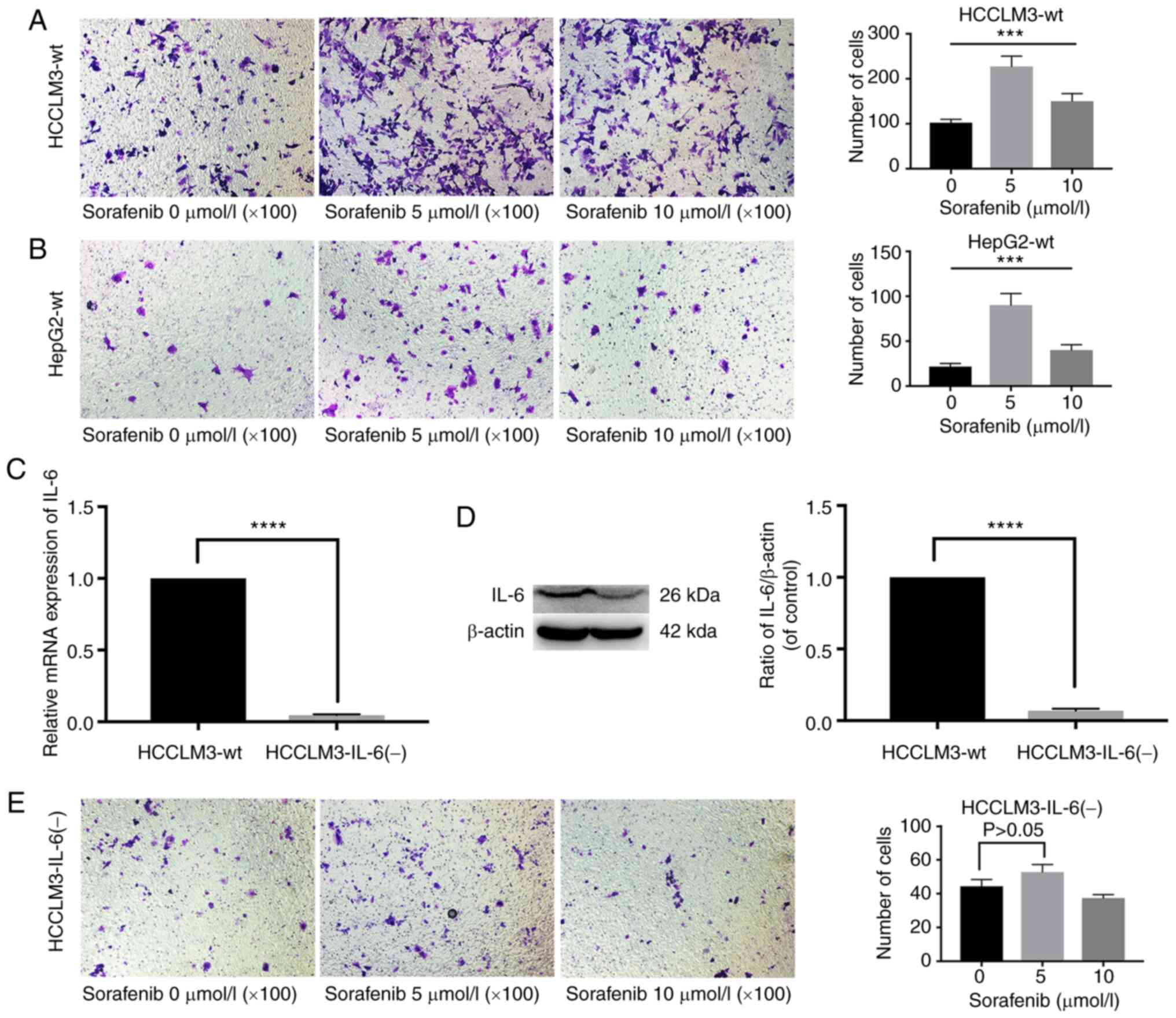
In Fig. 1A and B,
sorafenib significantly increased the number of metastatic cells in
HCCLM3-wt and HepG2-wt cells after treatment for 24 h. After IL-6
expression was disrupted by TALEN, which is a highly efficient and
specific gene editing tool with low genotoxicity in targeted genome
manipulation (30,31), in a human liver cancer HCCLM3-wt
cell line, the stably transfected clones were validated by RT-qPCR
and western blot analysis (Fig. 1C and
D). Notably, sorafenib did not increase the number of
metastatic cells in HCCLM3-IL-6(−) (Fig. 1E).
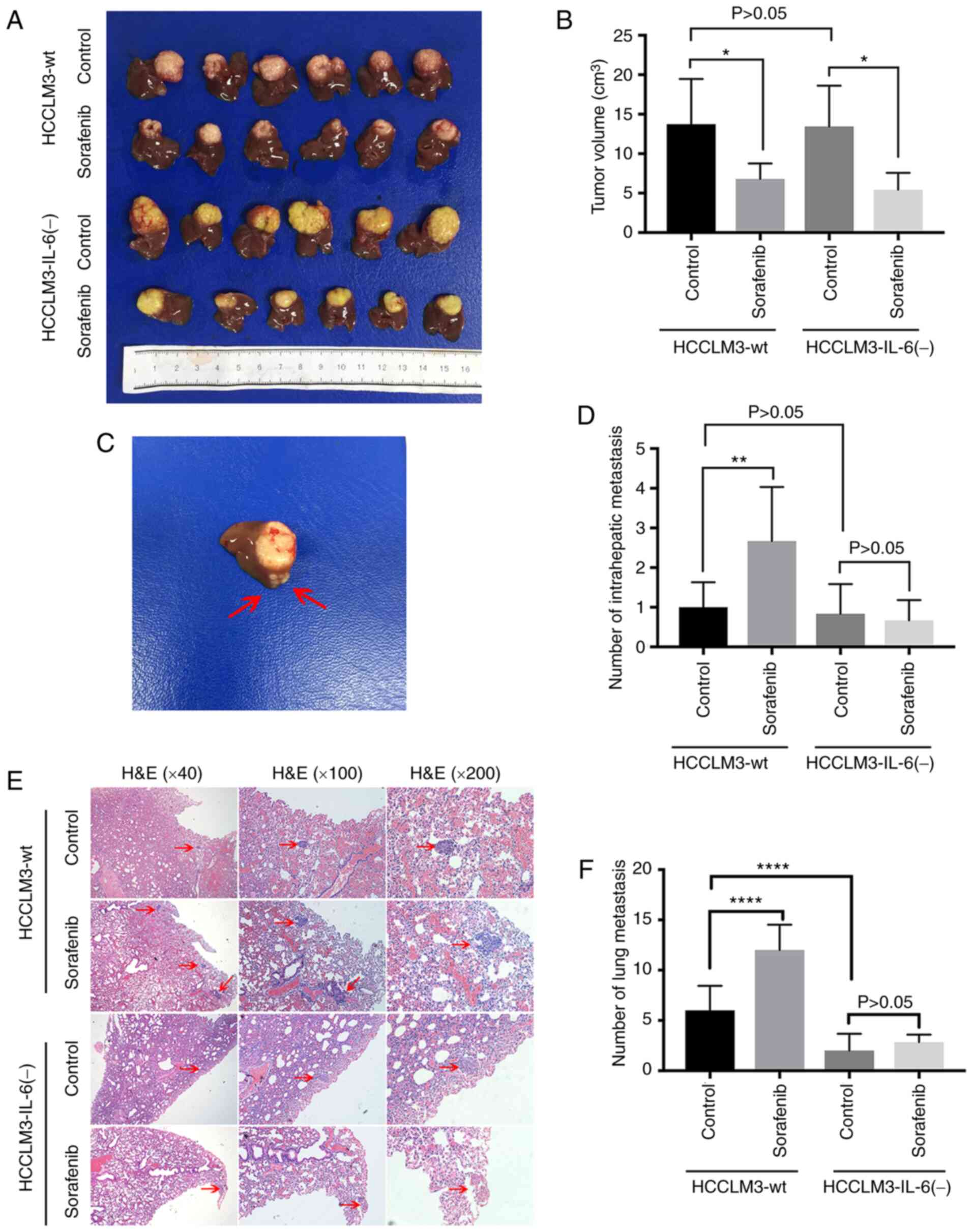
Subsequently, the orthotopic growth of liver cancer
tumors was modeled in nude mice to further investigate whether IL-6
influenced the pro-migratory effect of sorafenib in vivo. It
was revealed that sorafenib significantly reduced the volume of
tumors in the HCCLM3-wt and HCCLM3-IL-6(−) groups, respectively
(Fig. 2A and B). However,
intrahepatic metastasis (IHM) was increased in the HCCLM3-wt group
but not in the HCCLM3-IL-6(−) group following the administration of
sorafenib (Fig. 2C and D). The
number of IHMs in the HCCLM3-wt control was not higher than that in
the HCCLM3-IL-6(−) control (Fig. 2C and
D). Moreover, sorafenib treatment significantly increased the
number of lung metastatic nodules in HCCLM3-wt cells (Fig. 2E and F). Conversely, no significant
difference was observed in the two groups of mouse HCCLM3-IL-6(−)
cells (Fig. 2E and F). The number
of lung metastases in the HCCLM3-wt group was higher than that in
the HCCLM3-IL-6(−) group (Fig. 2E and
F). These results indicated that IL-6 knockout attenuated the
pro-migratory effect induced by sorafenib treatment, as detected by
western blot analysis and in vivo.
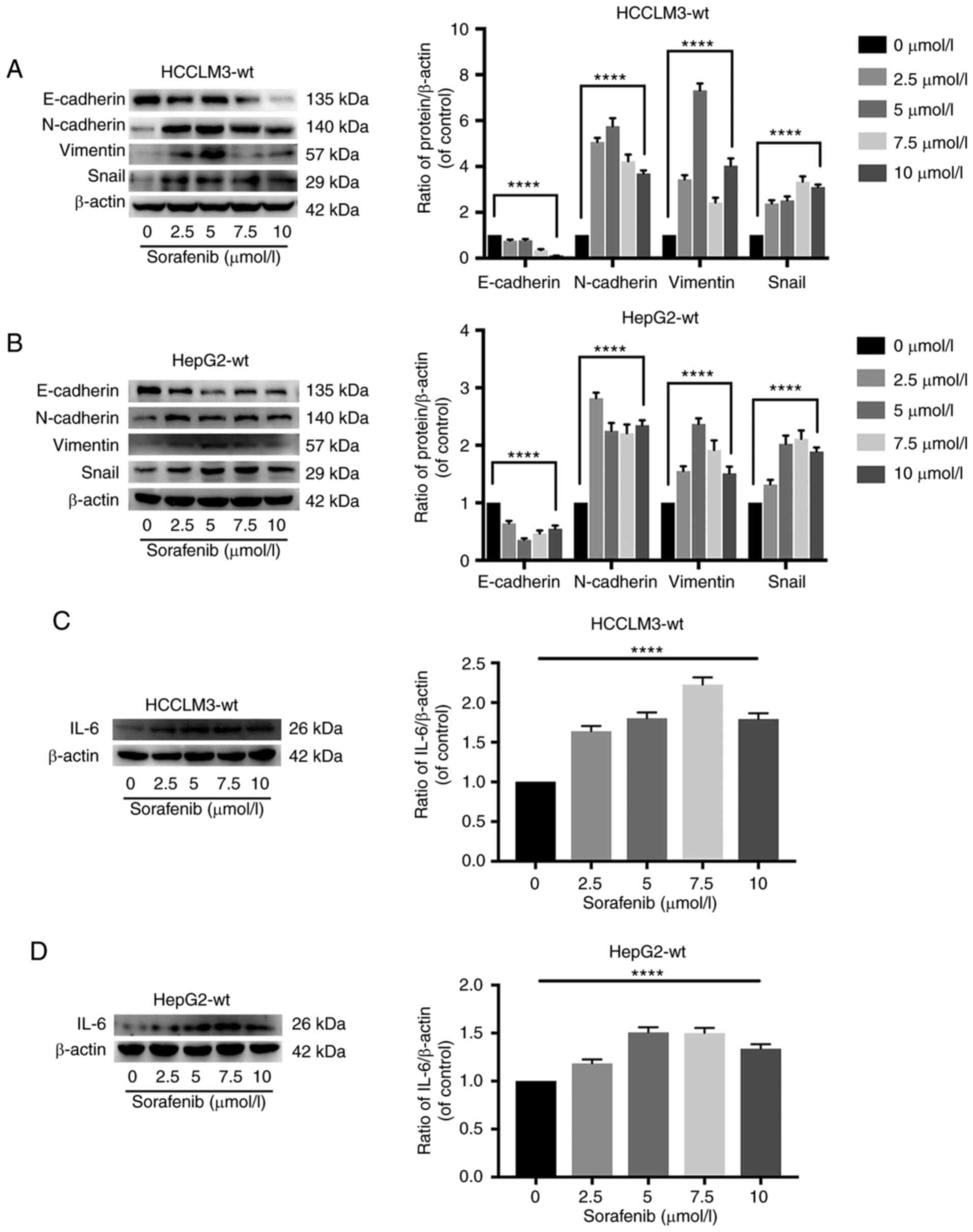
Sorafenib may promote liver cancer
cell metastasis and EMT through the upregulation of IL-6, as
detected by western blot analysis, ELISA analysis and
immunohistochemistry
To explore the role of IL-6 in sorafenib-mediated
pro-metastasis and EMT, HCCLM3-wt and HepG2-wt cells were treated
with sorafenib. As revealed in Fig. 1A
and B, sorafenib significantly promoted metastasis in HCCLM3-wt
and HepG2-wt cells at 24 h. Furthermore, western blot analysis
indicated that 0–10 µmol/l sorafenib induced EMT in HCCLM3-wt and
HepG2-wt cells at 24 h (Fig. 3A and
B). It was also determined that IL-6 was upregulated after 24 h
of treatment with sorafenib in HCCLM3-wt and HepG2-wt cells
(Fig. 3C and D). In addition, ELISA
and immunohistochemistry were performed to detect the change of
IL-6 in the cell supernatant and inside the tumor following
sorafenib administration. Consistent with the western blot analysis
results, it was revealed that IL-6 was upregulated in the cell
supernatant and inside the tumor in vivo (Fig. S1). These results indicated that the
pro-metastatic effects of sorafenib may be exerted through the
upregulation of IL-6 expression in liver cancer cells.
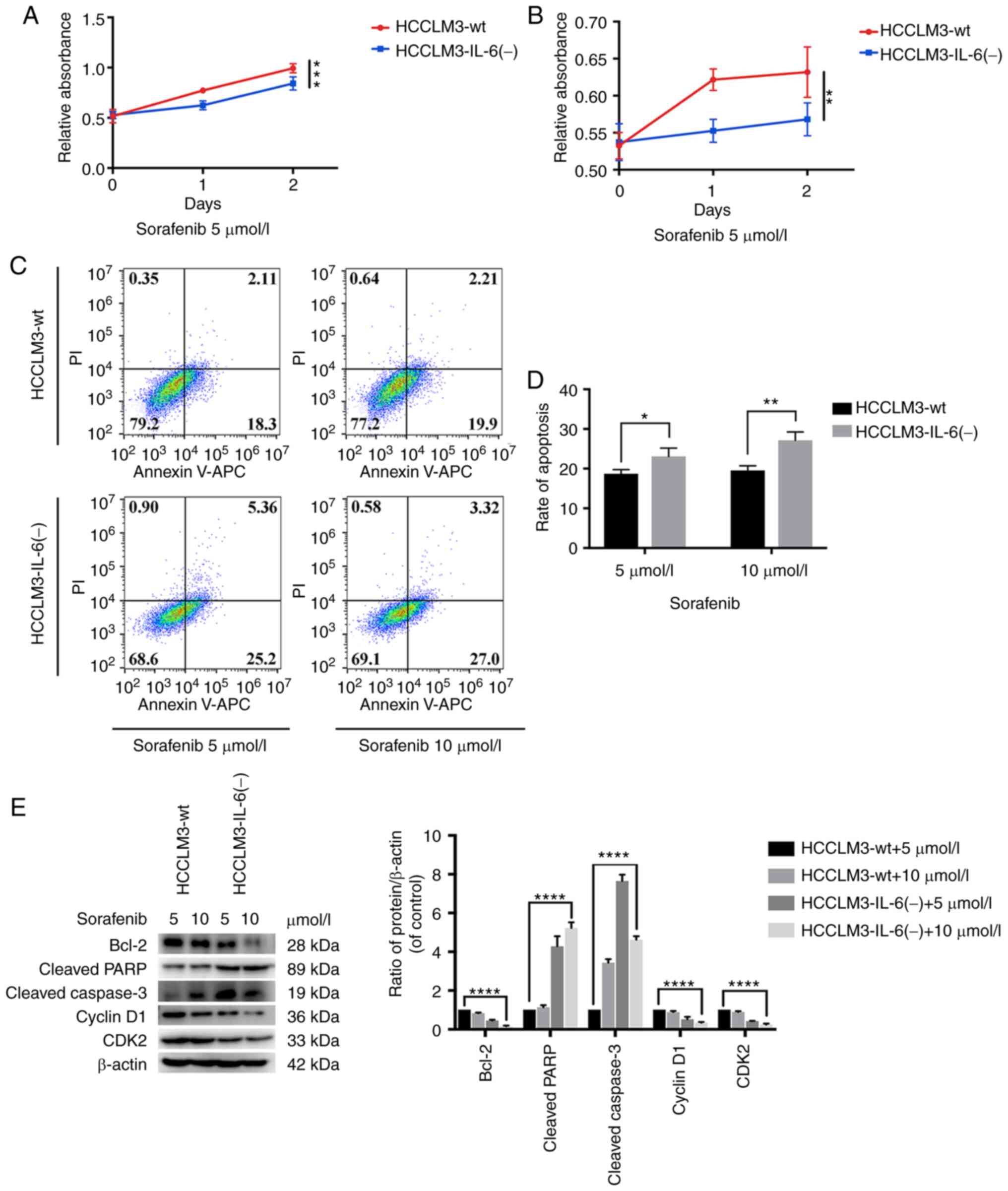
IL-6 knockout inhibits the
proliferation of HCCLM3 cells and promotes apoptosis in HCCLM3
cells, as detected by CCK-8 assay, flow cytometric analysis and
western blot analysis
The influence of the disruption of the IL-6
expression was examined by TALEN in HCCLM3 cells. Western blot
analysis and RT-qPCR were performed to confirm the stable knockout
of IL-6 expression in HCCLM3 cells (Fig. 1C and D). A CCK-8 assay indicated
that IL-6 promoted tumor cell proliferation (Fig. 4A). To further explore the effects of
IL-6 expression on liver cancer growth, flow cytometry was
conducted to detect the cell cycle and apoptosis of HCCLM3 cells.
In Fig. 4B and C, HCCLM3-IL-6(−)
increased the G1 phase and apoptosis rates, as compared with those
of HCCLM3-wt cells. In addition, the level of cell cycle (CDK2 and
cyclin D1) and apoptotic (cleaved caspase-3, cleaved PARP and
Bcl-2) markers was investigated by western blot analysis. The
results revealed that Bcl-2, cyclin D1 and CDK2 levels were higher
in HCCLM3-wt cells than in HCCLM3-IL-6(−) cells, whereas those of
cleaved caspase-3 and cleaved PARP were upregulated in
HCCLM3-IL-6(−) cells (Fig. 4D).
These results revealed that IL-6 knockout inhibited the
proliferation and promoted the apoptosis of HCCLM3 cells.
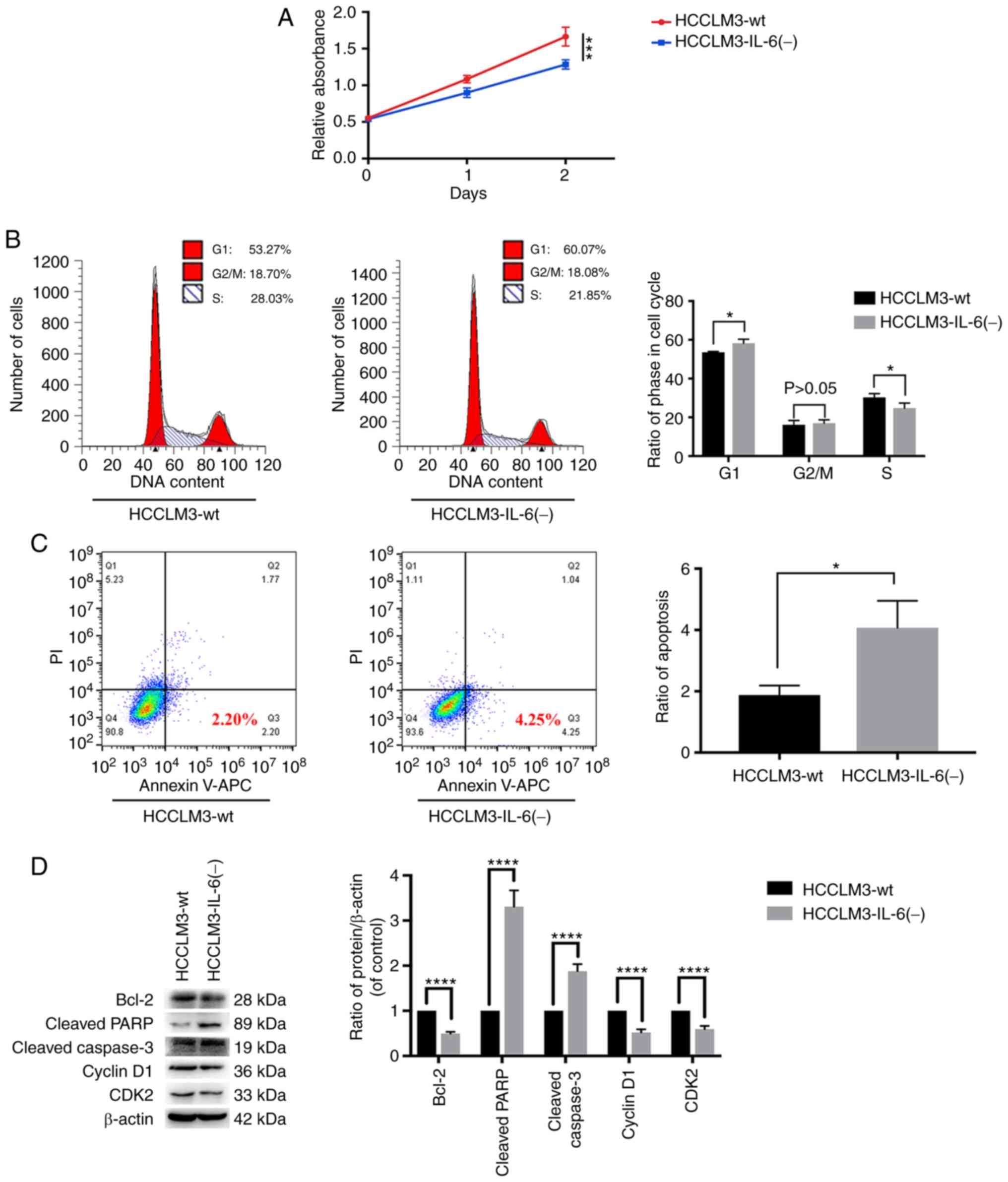 | Figure 4.IL-6 knockout inhibits tumor cell
growth, as revealed by CCK-8 assay, flow cytometric analysis and
western blot analysis. (A) CCK-8 assay for cell proliferation of
HCCLM3-wt and HCCLM3-IL-6(−) cells. IL-6 knockout inhibited liver
cancer cell proliferation, as revealed by CCK-8 assay
(***P<0.001). (B) Flow cytometric cycle assay of HCCLM3-wt and
HCCLM3-IL-6(−) cells revealed that the knockout of IL-6 increased
the proportion of cells at the G1 phase and decreased that of cells
in the S phase (both *P<0.05). (C) Flow cytometric apoptosis
assay of HCCLM3-wt and HCCLM3-IL-6(−) cells revealed that the
knockout of IL-6 increased the cell apoptosis ratio as indicated by
western blot analysis (*P<0.05). (D) Western blot analysis
revealed that anti-apoptotic marker (Bcl-2) and cell cycle markers
(cyclin D1 and CDK2) were downregulated in HCCLM3-IL-6(−) cells, as
compared with HCCLM3-wt cells, whereas pro-apoptotic markers
cleaved caspase-3 and cleaved PARP were upregulated in
HCCLM3-IL-6(−) cells (all ****P<0.0001). CCK-8, Cell Counting
Kit-8; wt, wild-type; IL-6, interleukin-6; HCC, hepatocellular
carcinoma; Bcl-2, B-cell lymphoma-2. |
IL-6 knockout decreases the metastatic
ability of HCCLM3 cells, and exogenous IL-6 increases that of HepG2
and HCCLM3 cells, as detected by Transwell assay and western blot
analysis
The influence of IL-6 disruption on the metastatic
ability of HCCLM3 cells was explored. A Transwell assay was
performed to evaluate the metastatic ability of HCCLM3 cells. When
IL-6 was knocked out, the migration of HCCLM3-IL-6(−) cells
significantly decreased, as compared with that of HCCLM3-wt cells
(Fig. 5A). Consistent with the
Transwell assay results, western blot analysis revealed that IL-6
induced EMT in HCCLM3 cells (Fig.
5B). As revealed in Fig. 5B,
E-cadherin levels were higher in HCCLM3-IL-6(−) than in HCCLM3-wt
cells, whereas the mesenchymal associated proteins vimentin and
N-cadherin were downregulated in HCCLM3-IL-6(−) cells. These
results indicated that the knockout of endogenous IL-6 could
decrease the metastatic ability of HCCLM3 cells.
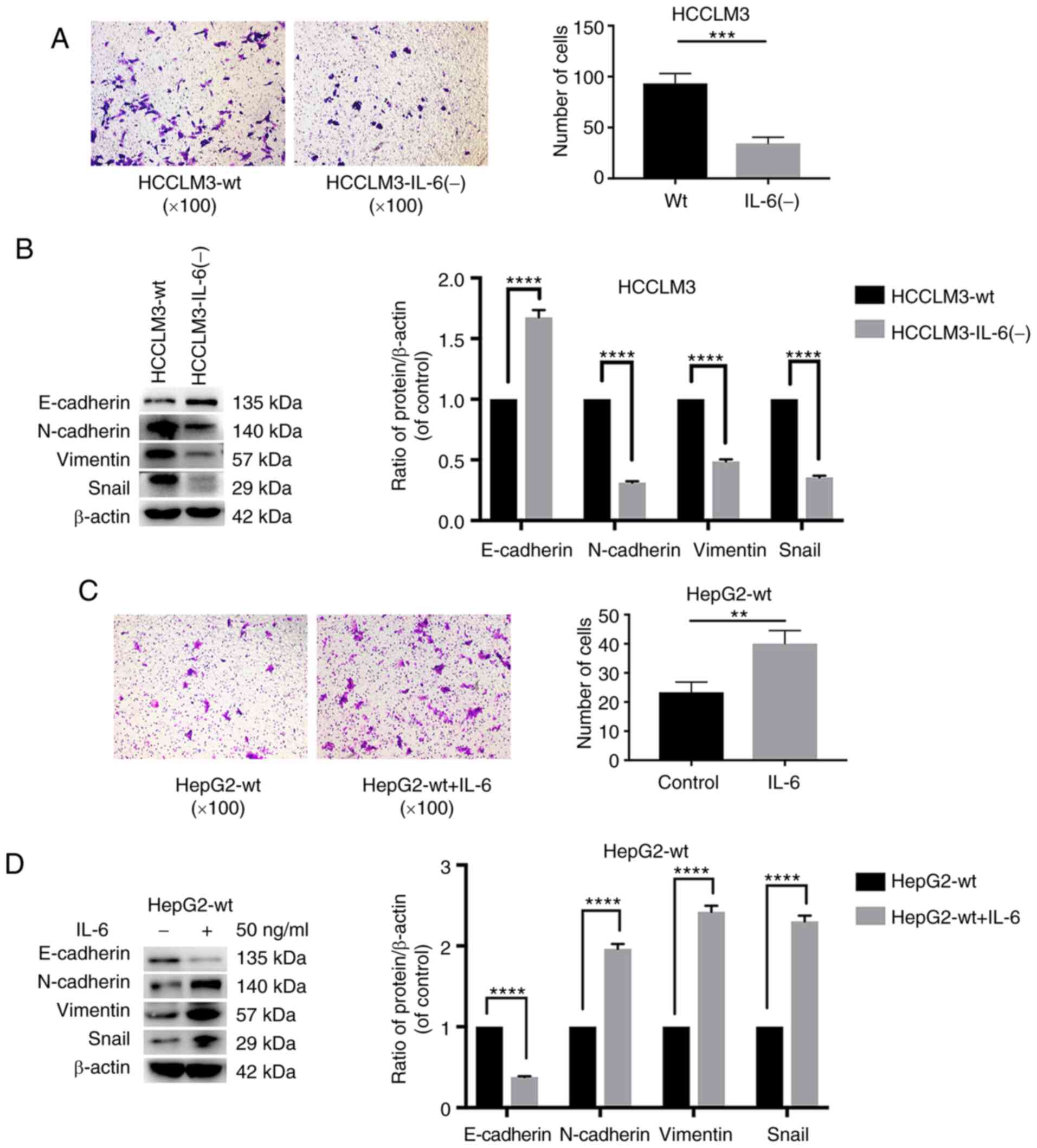 | Figure 5.The knockout of IL-6 decreases the
metastatic ability of HCCLM3 cells, and exogenous IL-6 increases
the metastasis ability of HepG2 cells, as revealed by Transwell
assay and western blot analysis. (A) The knockout of IL-6 decreased
the metastatic potential of HCCLM3-wt cells, as revealed by
Transwell assay (***P<0.001). (B) IL-6 knockout upregulated
E-cadherin, and downregulated N-cadherin, vimentin and Snail in
HCCLM3-IL-6(−) cells, as compared with HCCLM3-wt cells (all
****P<0.0001). (C) Exogenous IL-6 increased the metastatic
ability of HepG2-wt cells as revealed by Transwell assay
(**P<0.01). (D) Exogenous IL-6 downregulated E-cadherin, and
upregulated N-cadherin, vimentin and Snail in HepG2-wt cells (all
****P<0.0001). IL-6, interleukin-6; HCC, hepatocellular
carcinoma; wt, wild-type. |
The IL-6 expression level was revealed to be low in
isolated HepG2-wt supernatants (14). Therefore, to further examine the
pro-metastatic and -EMT role of IL-6 in HepG2-wt cells, HepG2-wt
cells were cultured in the presence of IL-6 to simulate the
overexpression of the IL-6 gene in HepG2-wt cells. After 24 h, a
Transwell assay was performed to evaluate the metastatic ability of
HepG2-wt cells. In Fig. 5C, the
metastatic ability of HepG2-wt cells cultured with exogenous IL-6
was greater than that of the control cells (Fig. 5C). Western blot analysis was
performed to evaluate the markers associated with EMT: E-cadherin,
N-cadherin, vimentin and Snail. Exogenous IL-6 induced EMT in
HepG2-wt cells. As such, the levels of the epithelial marker
E-cadherin were decreased, whereas those of vimentin and N-cadherin
were significantly increased (Fig.
5D). The expression of Snail, a key regulator of EMT, was also
increased following IL-6 treatment (Fig. 5D). HCCLM3-wt cells were also
cultured with IL-6. The results of the Transwell assay and western
blot analysis were consistent with HepG2-wt (Fig. S2). These results indicated that
exogenous IL-6 promoted liver cancer metastasis and EMT.
IL-6 knockout increases the
susceptivity of HCCLM3 cells to sorafenib, as detected by CCK-8
assay, flow cytometric analysis and western blot analysis
Furthermore, the proliferation inhibition and
apoptosis induced by sorafenib in HCCLM3-wt and HCCLM3-IL-6(−)
cells was detected. When IL-6 was knocked out, the tumor cells were
prone to an inhibited proliferation (Fig. 6A and B). In addition, flow cytometry
was conducted to detect the apoptosis of HCCLM3 cells treated with
sorafenib, and it was revealed that HCCLM3-IL-6(−) was prone to
sorafenib-induced apoptosis (Fig. 6C
and D). In addition, western blot analysis was performed to
investigate the level of cell cycle (CDK2 and cyclin D1) and
apoptotic (cleaved caspase-3, cleaved PARP and Bcl-2) markers.
Following the administration of sorafenib, the level of cleaved
caspase-3 and cleaved PARP in HCCLM3-IL-6(−) cells was higher than
that in HCCLM3-wt cells (Fig. 6E).
In addition, the level of Bcl-2 in HCCLM3-IL-6(−) cells was lower
than that in HCCLM3-wt cells (Fig.
6E). In addition, the level of cell cycle markers, such as CDK2
and cyclin D1, in HCCLM3-IL-6(−) cells was lower than that in
HCCLM3-wt cells (Fig. 6E). These
results indicated that the knockout of endogenous IL-6 could
increase their susceptibility to sorafenib.
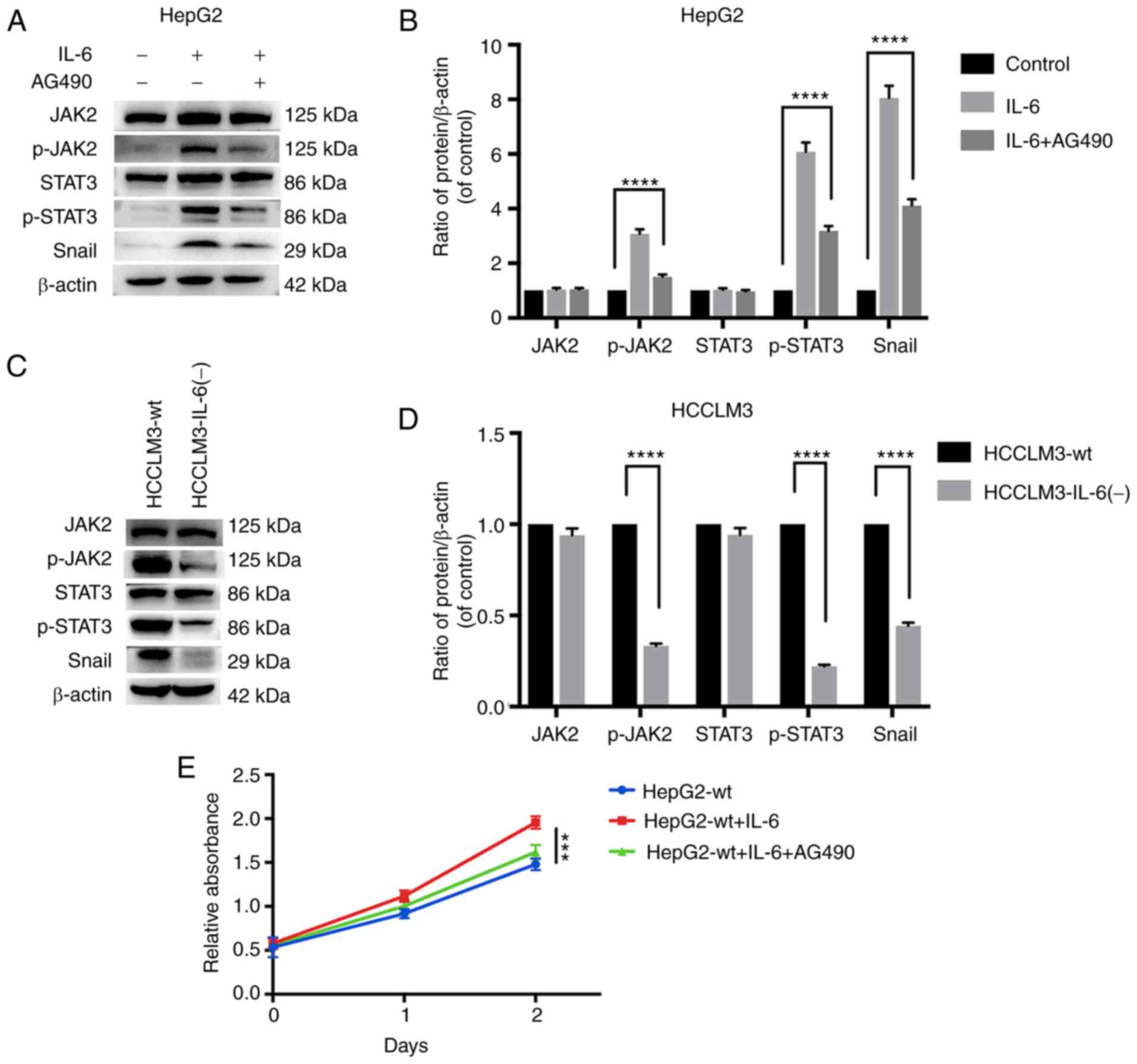
IL-6 induces EMT in liver cancer cells
and promotes proliferation through JAK/STAT3/Snail pathway
hyperactivation
The hyperactivation of JAK/STAT3/Snail signaling has
been revealed to be responsible for IL-6-induced EMT and
proliferation (25). The aim of the
present study was to test this hypothesis by inhibiting JAK/STAT3
signaling through AG490 (10 µmol/l), which is an inhibitor of JAK2
protein tyrosine kinase. Exogenous IL-6 was significantly increased
in p-JAK2, p-STAT3 and Snail in HepG2 cells (Fig. 7A and B). The combination of IL-6 and
AG490 exhibited the distinctly blocked JAK2 and STAT3
phosphorylation and inhibited the upregulation of Snail (Fig. 7A and B). IL-6 knockout significantly
decreased p-JAK2, p-STAT3 and Snail in HCCLM3 cells (Fig. 7C and D). In addition, a CCK-8 assay
revealed that exogenous IL-6 could promote HepG2-wt cell
proliferation, while this effect was significantly blocked by AG490
(Fig. 7E). These results indicated
that IL-6 may induce EMT and promote proliferation by activating
JAK/STAT3/Snail signaling.
Discussion
Sorafenib is multi-targeted tyrosine kinase
inhibitor (TKI) that affects numerous signal pathways (5). Sorafenib leads to the blocking of key
signaling pathways, namely, Ras/Raf/MAPK and PI3K/Akt/mTOR, which
have been implicated in the pathogenesis of liver cancer (32,33).
However, there remain numerous mechanisms that have not been
revealed. As a first-line treatment drug, sorafenib has been
revealed to inhibit tumor growth and prolong patient survival
(6,7). However, the drug has been revealed to
elicit several side effects and promote the invasive and metastatic
potential of cells, as demonstrated by western blot analysis and
in vivo assessment (9,34).
Sorafenib has also been revealed to induce EMT in patients with
liver cancer (35). Some patients
with liver cancer quickly develop resistance to sorafenib with
discontinued treatment (5). Some
patients with renal cancer experience tumor recurrence and succumb
after discontinuing sorafenib treatment (8). Therefore, understanding how sorafenib
interacts with other treatments may be necessary to improve its
efficacy and attenuate its side effects. The present study, to the
best of our knowledge, is the first to reveal that sorafenib may
affect tumors through the upregulation of the IL-6/STAT3 signaling
pathway. The effect of IL-6 as a single factor on liver cancer was
not only studied, but also the effect of IL-6 combined with
sorafenib on liver cancer, to further demonstrate that sorafenib
may induce tumor metastasis and chemotherapy resistance through the
IL-6/STAT3 signaling pathway. Sorafenib could upregulate the
expression of IL-6, and IL-6-induced EMT and tolerance to sorafenib
may be a ‘side effect’ of sorafenib. Therefore, it is proposed that
combined anti-IL-6/STAT3 may improve the efficacy of sorafenib.
EMT plays a critical role in tumor progression,
especially in tumor invasion, metastasis and drug resistance.
During tumor progression, epithelial cells gradually lose their
features, such as the downregulation of E-cadherin, and obtain
mesenchymal characteristics, such as the upregulation of N-cadherin
and vimentin (23,36,37).
Snail has been identified as a key regulator of EMT during
embryonic development and cancer progression, and it is effective
in inhibiting E-cadherin expression and enhancing tumor invasion
and metastasis (18,38). The present results revealed that
sorafenib promoted liver cancer cell metastasis and induced EMT.
Sorafenib treatment increased IL-6 expression in liver cancer
cells.
IL-6 is a pleiotropic cytokine present in the tumor
microenvironment; it is associated with poor prognosis, recurrence
and metastasis in various types of cancer (10,39).
Plasma levels of IL-6 and its soluble receptor were associated with
cancer progression and bone metastasis in prostate cancer (40). Sullivan et al (24) revealed that exogenous IL-6 exposure
increased breast cancer cell metastasis and induced EMT in MCF-7
cells that do not express IL-6. To verify the function of IL-6 in
liver cancer, we not only exposed liver cancer cells to exogenous
IL-6, but also knocked out the endogenous IL-6 of liver cancer
cells. The present results revealed that either endogenous or
exogenous IL-6 affected the metastatic ability and EMT progression
of liver cancer cells. The disrupted IL-6 expression in liver
cancer cells markedly attenuated the pro-invasive effect of
sorafenib treatment, as detected by western blot analysis and in
vivo assessment. Intrahepatic invasion and lung metastasis are
poor prognostic indicators for patients with liver cancer (41,42).
In the present study, it was revealed that IL-6 knockout in HCCLM3
cells could decrease the number of lung metastasis but not
intrahepatic invasion in vivo, probably due to the trait of
the HCCLM3 cells, which originate from nude mouse lung metastasis
with a highly distant metastatic potential (43).
The chemical resistance of cancer cells to
conventional chemotherapy and targeted drugs is the main
disadvantage of current chemotherapeutic strategies for various
types of tumors, including liver cancer (44). Zhang et al (45) revealed that EMT is responsible for
sorafenib resistance. The present results also indicated that
sorafenib resistance may be associated with IL-6-mediated EMT. IL-6
plays a vital role in trastuzumab resistance in HER2/neu positive
breast cancer, which mediates the expansion of cancer stem cells by
downregulating PTEN expression and triggering Akt and STAT3,
thereby leading to nuclear factor-κB activation (46). It was revealed herein by western
blotting that IL-6 knockout increased the apoptosis induced by
sorafenib in liver cancer cells. These findings indicated that an
IL-6 signaling network may be a potential therapeutic target for
liver cancer and a biomarker for predicting the response to
sorafenib treatment.
Some contradicting findings have yet to be
elucidated. It was revealed by western blot analysis that IL-6
affected tumor cell proliferation and apoptosis. The disruption of
IL-6 expression in liver cancer cells increased the susceptibility
to sorafenib in vitro. IL-6 knockout did not influence the
volume of tumors in a xenograft model of nude mice. In addition,
IL-6 knockout decreased the number of lung metastatic nodules but
did not affect the number of IHM. Possible reasons include the
following: First, the tumor microenvironment is a complex
environment, IL-6 is secreted by various cells in the tumor
environment, including cancer cells, tumor-associated fibroblasts,
macrophages and cancer stem cells (10). Although the IL-6 gene is knocked out
in tumor cells, other cells in the microenvironment can still
secrete and affect tumor cell growth. However, in distant
metastasis locations, such as the lung, no inflammatory environment
is formed at the beginning. The source of IL-6 is mainly from the
tumor itself. Therefore, knocking out the IL-6 gene in tumor cells
may have a greater affect in lung metastasis than intrahepatic
metastasis. Moreover, tumor-associated macrophages (TAMs) play a
crucial role in liver cancer progression, growth and invasiveness
(47,48). IL-6 was revealed to be undetectable
in isolated HepG2-wt supernatants and had a low expression in TAM
supernatants, whereas co-cultured HepG2-wt cells and TAMs increased
IL-6 expression 10 times more than cultured HepG2-wt alone
(14). Aside from tumor cells,
tumor microenvironments should also be considered. Therefore, CNTO
328, a humanized monoclonal antibody targeting IL-6, which can
systemically neutralize IL-6 bioactivity, should be further
investigated with sorafenib in liver cancer.
The present study has certain limitations. First,
the molecular mechanism between IL-6 and sorafenib resistance
should be further explored. Studies should verify whether IL-6
overexpression or knockdown in various liver cancer cells is
associated with sorafenib resistance. Secondly, IL-6 is a poor
prognostic factor of patients with liver cancer (14–16).
However, the association between IL-6 and sorafenib treatment in
patients remains unclear. Thirdly, aside from the JAK/STAT pathway,
the ERK1/2/MAPK and PI3K/Akt pathways can be activated by IL-6, and
may potentially account for IL-6-mediated EMT and resistance
(49).
In conclusion, the present findings demonstrated
that sorafenib promoted the tumor metastasis potential via
IL-6-mediated EMT by activating JAK2/STAT3 signaling. CNTO 328 a
promising antibody-drug conjugate targeting cytokine IL-6, has been
tested in clinical trials of several cancer models, including renal
cell cancer, ovarian cancer and multiple myeloma and a direction of
our future research will be to assess it in liver cancer (50–52).
The present results provided novel insights into the role of IL-6
in liver cancer and emphasized that the efficiency of a
sorafenib-based strategy may be improved by combining it with
anti-IL-6 therapies for liver cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Science Foundation for Young Scientists of China (grant no.
81101851).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JS and PYZ designed the study and conducted a
critical revision of the manuscript. KWZ and DW conducted the cell
viability, flow cytometry, western blotting, RT-PCR and migration
assays. KWZ, HC and MQC conducted the animal experiments. DW and
YYZ were responsible for the collection and assembly of data. KWZ
and DW prepared the figures and wrote the manuscript. All authors
read and approved the final manuscript and agree to be accountable
for all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Care Committee of Zhongshan Hospital (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases, : Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Faber W, Seehofer D, Neuhaus P, Stockmann
M, Denecke T, Kalmuk S, Warnick P and Bahra M: Repeated liver
resection for recurrent hepatocellular carcinoma. J Gastroenterol
Hepatol. 26:1189–1194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gauthier A and Ho M: Role of sorafenib in
the treatment of advanced hepatocellular carcinoma: An update.
Hepatol Res. 43:147–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Desar IM, Mulder SF, Stillebroer AB, van
Spronsen DJ, van der Graaf WT, Mulders PF and van Herpen CM: The
reverse side of the victory: Flare up of symptoms after
discontinuation of sunitinib or sorafenib in renal cell cancer
patients. A report of three cases. Acta Oncol. 48:927–931. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang W, Sun HC, Wang WQ, Zhang QB, Zhuang
PY, Xiong YQ, Zhu XD, Xu HX, Kong LQ, Wu WZ, et al: Sorafenib
down-regulates expression of HTATIP2 to promote invasiveness and
metastasis of orthotopic hepatocellular carcinoma tumors in mice.
Gastroenterology. 143:1641–1649.e5. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bharti R, Dey G and Mandal M: Cancer
development, chemoresistance, epithelial to mesenchymal transition
and stem cells: A snapshot of IL-6 mediated involvement. Cancer
Lett. 375:51–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lanton T, Shriki A, Nechemia-Arbely Y,
Abramovitch R, Levkovitch O, Adar R, Rosenberg N, Paldor M,
Goldenberg D, Sonnenblick A, et al: Interleukin 6-dependent genomic
instability heralds accelerated carcinogenesis following liver
regeneration on a background of chronic hepatitis. Hepatology.
65:1600–1611. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang TS, Wu YC, Chi CC, Su WC, Chang PJ,
Lee KF, Tung TH, Wang J, Liu JJ, Tung SY, et al: Activation of
IL6/IGFIR confers poor prognosis of HBV-related hepatocellular
carcinoma through induction of OCT4/NANOG expression. Clin Cancer
Res. 21:201–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mitra A, Yan J, Xia X, Zhou S, Chen J,
Mishra L and Li S: IL6-mediated inflammatory loop reprograms normal
to epithelial-mesenchymal transition+ metastatic cancer
stem cells in preneoplastic liver of transforming growth factor
beta-deficient β2-spectrin +/− mice. Hepatology.
65:1222–1236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wan S, Zhao E, Kryczek I, Vatan L,
Sadovskaya A, Ludema G, Simeone DM, Zou W and Welling TH:
Tumor-associated macrophages produce interleukin 6 and signal via
STAT3 to promote expansion of human hepatocellular carcinoma stem
cells. Gastroenterology. 147:1393–1404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sheng T, Wang B, Wang SY, Deng B, Qu L, Qi
XS, Wang XL, Deng GL and Sun X: The relationship between serum
interleukin-6 and the recurrence of hepatitis B virus related
hepatocellular carcinoma after curative resection. Medicine
(Baltimore). 94:e9412015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kao JT, Feng CL, Yu CJ, Tsai SM, Hsu PN,
Chen YL and Wu YY: IL-6, through p-STAT3 rather than p-STAT1,
activates hepatocarcinogenesis and affects survival of
hepatocellular carcinoma patients: A cohort study. BMC
Gastroenterol. 15:502015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Prieto-García E, Díaz-García CV,
García-Ruiz I and Agulló-Ortuño MT: Epithelial-to-mesenchymal
transition in tumor progression. Med Oncol. 34:1222017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma JL, Zeng S, Zhang Y, Deng GL and Shen
H: Epithelial-mesenchymal transition plays a critical role in drug
resistance of hepatocellular carcinoma cells to oxaliplatin. Tumour
Biol. 37:6177–6184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kodama T, Newberg JY, Kodama M, Rangel R,
Yoshihara K, Tien JC, Parsons PH, Wu H, Finegold MJ, Copeland NG
and Jenkins NA: Transposon mutagenesis identifies genes and
cellular processes driving epithelial-mesenchymal transition in
hepatocellular carcinoma. Proc Natl Acad Sci USA. 113:E3384–E3393.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu QD, Chen W, Yan TL, Ma T, Chen CL,
Liang C, Zhang Q, Xia XF, Liu H, Zhi X, et al: NSC 74859 enhances
doxorubicin cytotoxicity via inhibition of epithelial-mesenchymal
transition in hepatocellular carcinoma cells. Cancer Lett.
325:207–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giannelli G, Koudelkova P, Dituri F and
Mikulits W: Role of epithelial to mesenchymal transition in
hepatocellular carcinoma. J Hepatol. 65:798–808. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sullivan NJ, Sasser AK, Axel AE, Vesuna F,
Raman V, Ramirez N, Oberyszyn TM and Hall BM: Interleukin-6 induces
an epithelial-mesenchymal transition phenotype in human breast
cancer cells. Oncogene. 28:2940–2947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yadav A, Kumar B, Datta J, Teknos TN and
Kumar P: IL-6 promotes head and neck tumor metastasis by inducing
epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling
pathway. Mol Cancer Res. 9:1658–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rokavec M, Öner MG, Li H, Jackstadt R,
Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et
al: IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated
colorectal cancer invasion and metastasis. J Clin Invest.
124:1853–1867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhuang PY, Zhang KW, Wang JD, Zhou XP, Liu
YB, Quan ZW and Shen J: Effect of TALEN-mediated IL-6 knockout on
cell proliferation, apoptosis, invasion and anti-cancer therapy in
hepatocellular carcinoma (HCC-LM3) cells. Oncotarget.
8:77915–77927. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai H, Zhu XD, Ao JY, Ye BG, Zhang YY,
Chai ZT, Wang CH, Shi WK, Cao MQ, Li XL and Sun HC:
Colony-stimulating factor-1-induced AIF1 expression in
tumor-associated macrophages enhances the progression of
hepatocellular carcinoma. Oncoimmunology. 6:e13332132017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang YY, Kong LQ, Zhu XD, Cai H, Wang CH,
Shi WK, Cao MQ, Li XL, Li KS, Zhang SZ, et al: CD31 regulates
metastasis by inducing epithelial-mesenchymal transition in
hepatocellular carcinoma via the ITGB1-FAK-Akt signaling pathway.
Cancer Lett. 429:29–40. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang D, Zhu W, Wang Y, Sun C, Zhang KQ
and Yang J: Molecular tools for functional genomics in filamentous
fungi: Recent advances and new strategies. Biotechnol Adv.
31:1562–1574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Katsuyama T, Akmammedov A, Seimiya M, Hess
SC, Sievers C and Paro R: An efficient strategy for TALEN-mediated
genome engineering in Drosophila. Nucleic Acids Res. 41:e1632013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wilhelm S, Carter C, Lynch M, Lowinger T,
Dumas J, Smith RA, Schwartz B, Simantov R and Kelley S: Discovery
and development of sorafenib: A multikinase inhibitor for treating
cancer. Nat Rev Drug Discov. 5:835–844. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gedaly R, Angulo P, Hundley J, Daily MF,
Chen C, Koch A and Evers BM: PI-103 and sorafenib inhibit
hepatocellular carcinoma cell proliferation by blocking
Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Anticancer Res.
30:4951–4958. 2010.PubMed/NCBI
|
|
34
|
Huang XY, Ke AW, Shi GM, Zhang X, Zhang C,
Shi YH, Wang XY, Ding ZB, Xiao YS, Yan J, et al: αB-crystallin
complexes with 14-3-3ζ to induce epithelial-mesenchymal transition
and resistance to sorafenib in hepatocellular carcinoma.
Hepatology. 57:2235–2247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dazert E, Colombi M, Boldanova T, Moes S,
Adametz D, Quagliata L, Roth V, Terracciano L, Heim MH, Jenoe P and
Hall MN: Quantitative proteomics and phosphoproteomics on serial
tumor biopsies from a sorafenib-treated HCC patient. Proc Natl Acad
Sci USA. 113:1381–1386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lourenco AR and Coffer PJ: SOX4: Joining
the master regulators of epithelial-to-mesenchymal transition?
Trends Cancer. 3:571–582. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Barrallo-Gimeno A and Nieto MA: The Snail
genes as inducers of cell movement and survival: Implications in
development and cancer. Development. 132:3151–3161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo Y, Xu F, Lu T, Duan Z and Zhang Z:
Interleukin-6 signaling pathway in targeted therapy for cancer.
Cancer Treat Rev. 38:904–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shariat SF, Andrews B, Kattan MW, Kim J,
Wheeler TM and Slawin KM: Plasma levels of interleukin-6 and its
soluble receptor are associated with prostate cancer progression
and metastasis. Urology. 58:1008–1015. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Llovet JM, Brú C and Bruix J: Prognosis of
hepatocellular carcinoma: The BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yau T, Tang VY, Yao TJ, Fan ST, Lo CM and
Poon RT: Development of Hong Kong liver cancer staging system with
treatment stratification for patients with hepatocellular
carcinoma. Gastroenterology. 146:1691–1700.e3. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang BW, Liang Y, Xia JL, Sun HC, Wang L,
Zhang JB, Tang ZY, Liu KD, Chen J, Xue Q, et al: Biological
characteristics of fluorescent protein-expressing human
hepatocellular carcinoma xenograft model in nude mice. Eur J
Gastroenterol Hepatol. 20:1077–1084. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mir N, Jayachandran A, Dhungel B, Shrestha
R and Steel JC: Epithelial-to-mesenchymal transition: A mediator of
sorafenib resistance in advanced hepatocellular carcinoma. Curr
Cancer Drug Targets. 17:698–706. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang PF, Li KS, Shen YH, Gao PT, Dong ZR,
Cai JB, Zhang C, Huang XY, Tian MX, Hu ZQ, et al: Galectin-1
induces hepatocellular carcinoma EMT and sorafenib resistance by
activating FAK/PI3K/AKT signaling. Cell Death Dis. 7:e22012016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Korkaya H, Kim GI, Davis A, Malik F, Henry
NL, Ithimakin S, Quraishi AA, Tawakkol N, D'Angelo R, Paulson AK,
et al: Activation of an IL6 inflammatory loop mediates trastuzumab
resistance in HER2+ breast cancer by expanding the cancer stem cell
population. Mol Cell. 47:570–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang
W, Xiong YQ, Wu WZ, Wang L, Tang ZY and Sun HC: High expression of
macrophage colony-stimulating factor in peritumoral liver tissue is
associated with poor survival after curative resection of
hepatocellular carcinoma. J Clin Oncol. 26:2707–2716. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yeung OW, Lo CM, Ling CC, Qi X, Geng W, Li
CX, Ng KT, Forbes SJ, Guan XY, Poon RT, et al: Alternatively
activated (M2) macrophages promote tumour growth and invasiveness
in hepatocellular carcinoma. J Hepatol. 62:607–616. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nguyen DP, Li J and Tewari AK:
Inflammation and prostate cancer: The role of interleukin 6 (IL-6).
BJU Int. 113:986–992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rossi JF, Négrier S, James ND, Kocak I,
Hawkins R, Davis H, Prabhakar U, Qin X, Mulders P and Berns B: A
phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6
monoclonal antibody, in metastatic renal cell cancer. Br J Cancer.
103:1154–1162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Coward J, Kulbe H, Chakravarty P, Leader
D, Vassileva V, Leinster DA, Thompson R, Schioppa T, Nemeth J,
Vermeulen J, et al: Interleukin-6 as a therapeutic target in human
ovarian cancer. Clin Cancer Res. 17:6083–6096. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Moreau P, Harousseau JL, Wijdenes J,
Morineau N, Milpied N and Bataille R: A combination of
anti-interleukin 6 murine monoclonal antibody with dexamethasone
and high-dose melphalan induces high complete response rates in
advanced multiple myeloma. Br J Haematol. 109:661–664. 2000.
View Article : Google Scholar : PubMed/NCBI
|