Introduction
Malignant tumours are uncontrolled cell
proliferation diseases caused by oncogenes and ultimately lead to
organ and body dysfunction (1). In
recent decades, great progress has been made in the study of genes
and signalling pathways in tumorigenesis. Eps8 was identified by
Fazioli et al in NIH-3T3 murine fibroblasts via an approach
that allows direct cloning of intracellular substrates for receptor
tyrosine kinases (RTKs) that was designed to study the EGFR
signalling pathway. Eps8 is mainly distributed in epithelial cells
and fibroblasts as well as in some, but not all, haematopoietic
cells located in the cytoplasm, nuclear membrane and around the
cell membrane (2). As a tyrosine
kinase receptor, Eps8 maps to human chromosome 12p12.3 and consists
of 821 amino acids (3). In mammals,
there are at least three genes highly homologous to Eps8, namely
Eps8L1, Eps8L2, and Eps8L3, and they preserve the main structure of
Eps8, thus defining a new gene family (4,5). Eps8s
are expressed differently during development but are typically
co-expressed in adults (4). Studies
have indicated that, at the mRNA level, the expression pattern of
Eps8 overlaps with that of Eps8L2, and their expression is
relatively extensive; in contrast, Eps8L1 and Eps8L3 displays
restricted expression in adult tissues (4). Notably, Eps8L1 and Eps8L2, which have
the highest homology with the C-terminal effect region of Eps8, may
compensate for Eps8 function in the whole organism (4,5), and
among them, Eps8 is the only selectively upregulated subtype in the
brain (6). There are two subtypes
of proteins recognized by Eps8 antibodies: p97Eps8 and p68Eps8
(2). Both of these subtypes are
certified as substrates for several RTKs (7). The gene encoding p97Eps8 is an
oncogene whose PH domain is critical for ERK activation, cell
localization, and cell transformation (7). Furthermore, Eps8 binds to actin in
vivo and accumulates in PDGF-induced ruffles (6). In brief, Eps8 is an essential protein
encoding the Ras and Rac signalling pathways and is effectively
phosphorylated by a variety of tyrosine kinases (receptor and
non-receptor types), resulting in actin remodelling (2).
Eps8 is highly conserved and is widely expressed
during mouse development (8,9).
Studies mainly focused on humans have confirmed that Eps8 is
markedly expressed in diverse types of solid tumours (10–23)
and even haematological malignancies (24–27)
but minimally expressed in normal tissues (Table I). The aberrant expression of Eps8
is related to numerous signalling pathways, which affect a series
of biological processes by regulating various downstream cascades,
such as EGFR transduction, actin dynamics, cell cycle regulation
and cell proliferation, and eventually tumours tend to undergo
malignant transformation (10–27).
In addition, Eps8 is also a new pathogenic gene for autosomal
recessive profound deafness that encodes the actin of cochlear hair
cell stereocilia (28). In summary,
Eps8 potentially represents a novel biomarker for cancer diagnosis
and a promising candidate for cancer therapy.
 | Table I.Summary of Eps8 overexpression in
human malignant tumours. |
Table I.
Summary of Eps8 overexpression in
human malignant tumours.
| Type of tumour | Year | Relative
overexpression value of Eps8 (total no. of cases) | (Refs.) |
|---|
| PTC | 2001 | 8 (8) | (10) |
| Breast cancer | 2002 | 2.77-fold | (11) |
| Colon cancer | 2007 | 47 (76) | (12) |
| PDAC | 2007 | 4-fold | (13) |
| Cervical
cancer | 2008 | 45 (75) | (14) |
| OSCC | 2009 | >5-fold | (15) |
| Pituitary
tumour | 2009 | 5.9-fold | (16) |
| ESCC | 2010 | 35 (65) | (17) |
| Ovarian cancer | 2010 | 63.50% | (18) |
| OSCC | 2012 | 186 (205) | (19) |
| Breast cancer | 2015 | 60% | (20) |
| ALL | 2015 | High risk | (24) |
| CML | 2018 | 50 (91) | (25) |
| AML | 2018 | High | (26) |
| MM | 2019 | High | (27) |
| NSCLC | 2019 | High | (21) |
| GBM | 2019 | Significantly
higher | (22) |
| PDAC | 2019 | 31 (46) | (23) |
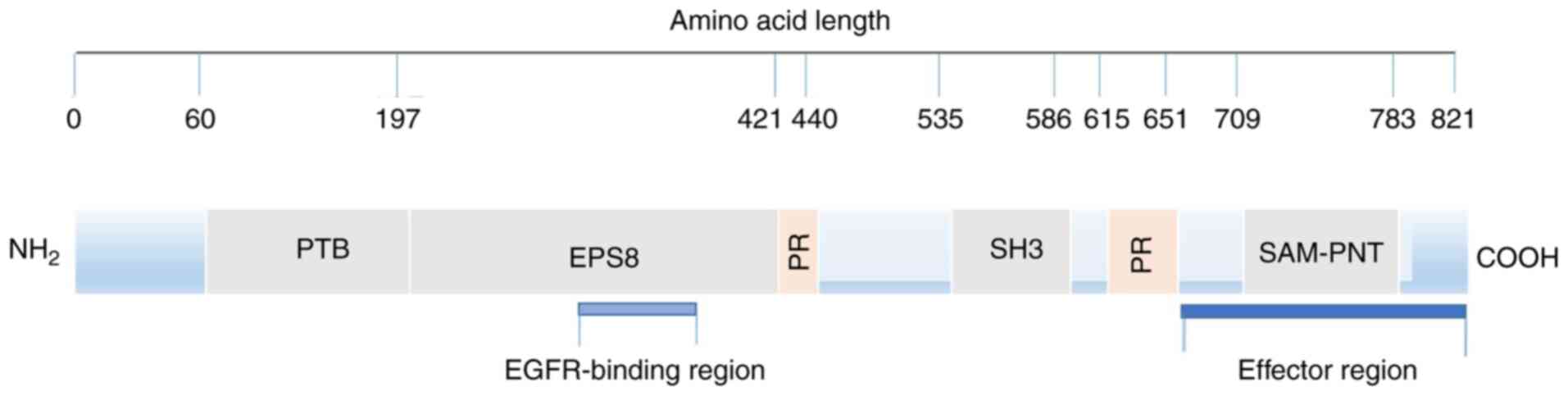
Structure and function of Eps8
Structure of EPS8
The Eps8 amino acid sequence predicted by
computer-aided analysis reveals a typical signal molecular
structure. From the N-terminus to the C-terminus, there is a
phosphate binding protein (PTB) region, proline-rich sequences and
SH3 region, stereo alpha-pointed (SAM-PNT) domain (5) (Fig.
1). It should be noted that the research on the SH3 domain is
relatively extensive, and we have a greater understanding of this
domain.
The SH3 domain is a protein component identified in
the study of Src. Proteins with this domain recognize peptides
containing XPXXP via sequence similarity and bind these peptides
(29). In particular, the SH3
domain of Eps8 exhibits a novel and unique binding preference,
mainly binding to peptides containing PXXDY rather than canonical
XPXXP, establishing specific interactions in the signal network.
Notably, the SH3 domain of Eps8 can interact with a number of
binding partners. When combined with shc (30), shb (31), RN-tre (32,33),
E3B1 (Abi-1) (32,34), Dvl-1 (35) and IRSp53 (36), Rac-mediated actin remodelling is
activated; however, when combined with RN-tre, Rab5-mediated EGFR
internalization is inhibited (33).
Proline-rich sequences are well characterised for
the adaptor protein IRSp53, which is linked to Rho family small
GTPases and exists in fibroblasts and various cancer cell lines.
The proline-rich sequence in the N-terminal region directly binds
to IRSp53 to form a complex. This binding subsequently mediates the
positive regulation of Rac activity by enhancing the formation of
the Eps8/Abi-1/Sos-1 complex and coactivating Rac. Furthermore, the
formation of the IRSp53/Eps8 complex at the leading edge of motor
cells is closely related to cell movement and invasiveness
(36,37).
The N-terminus contains a functional PTB domain
(4), which acts as a module for
protein-protein interactions, connecting the catalytic domain of
tyrosine kinase (38), and
combining different peptides in a phosphorylation-dependent or
phosphorylation-independent manner. The interaction involves
different biological processes ranging from types of receptor
signals to protein localization (39). Nevertheless, no binding partner for
the PTB domain of Eps8 is yet known.
The SAM-PNT domain belongs to the subfamily of SAM
domains and mediates the homo- and hetero-oligomerization of
proteins and the interaction of specific proteins (40). Others aspects have not been
discovered.
Physiological function of EPS8
A structure-function study concluded that Eps8
contains two functional regions. The first region discussed is the
EGFR binding region, which is rarely studied. This region acts as a
binding surface for the juxtamembrane region of EGFR and is
connected with mitosis, but its mechanism remains unclear (41). It is hypothesized that this region
may help the recruitment of Eps8 and Eps8-based complexes to EGFR,
facilitating downstream signal propagation and mitotic stimulation
(42).
The other region is defined as the C-terminus
‘effector region’. First, it regulates Rac-specific catalytic GEF
activity by binding SOS-1, which directly affects filamentous actin
remodelling (32). Moreover, the
localization of Eps8 cells is implied by mediating the interaction
between Eps8 and F-actin in vivo. Consequently,
phosphorylation may not only participate in the catalytic
activation of the Eps8-Abi1-Sos1 signalling complex but also
indicate Eps8 and Eps8-based complex localization, thereby
mediating the actin-based movement process in the cells (42,43).
Additionally, Eps8 plays a pivotal role in membrane flow, the
formation of pseudopods, the morphogenesis of microvilli, the
function and length of static cilia, cell adhesion and motility
(15).
Abnormal expression of Eps8 in malignant
tumours and tumorigenesis
Eps8 is universally expressed in human tissues,
especially in the gall bladder, fat, colon, small intestine,
kidney, endometrium, placenta, ovary and bladder (4,6).
Numerous studies have demonstrated that Eps8 is unconventionally
expressed in various tumour types, and high levels of Eps8 promote
tumour proliferation in breast cancer (11,20),
pancreatic cancer (13), colon
cancer (14), pituitary tumour
(16), oesophageal cancer (17), non-small cell lung cancer (NSCLC)
(21), and glioblastoma (22). In addition, Eps8 can also improve
the migration ability of cancer cells, including oral squamous cell
carcinoma (OSCC) (15) and colon,
breast and ovarian cancer (12,18,20).
Moreover, the aberrant expression of Eps8 alters the sensitivity of
cervical cancer cells to anticancer drugs (14) and is closely related to the
prognosis of OSCC and pancreatic adenocarcinoma (PDAC) (19,23),
thus affecting the quality of life of patients. Aside from common
malignant solid tumours, recent research has revealed that Eps8 is
also aberrantly expressed in malignant haematological tumours;
similarly, it regulates the development of a series of tumours,
including acute lymphoblastic leukaemia (ALL) (24), chronic myeloid leukaemia (CML)
(25), acute myeloid leukaemia
(AML) (26) and multiple myeloma
(MM) (27). Overall, Eps8 can
predict tumorigenesis and even tumour progression. Blocking Eps8
can inhibit tumour proliferation, metastasis, and drug resistance
and improve the overall survival rate of tumour patients.
Therefore, Eps8 is anticipated to become a detection index and a
new therapeutic target for malignant tumours.
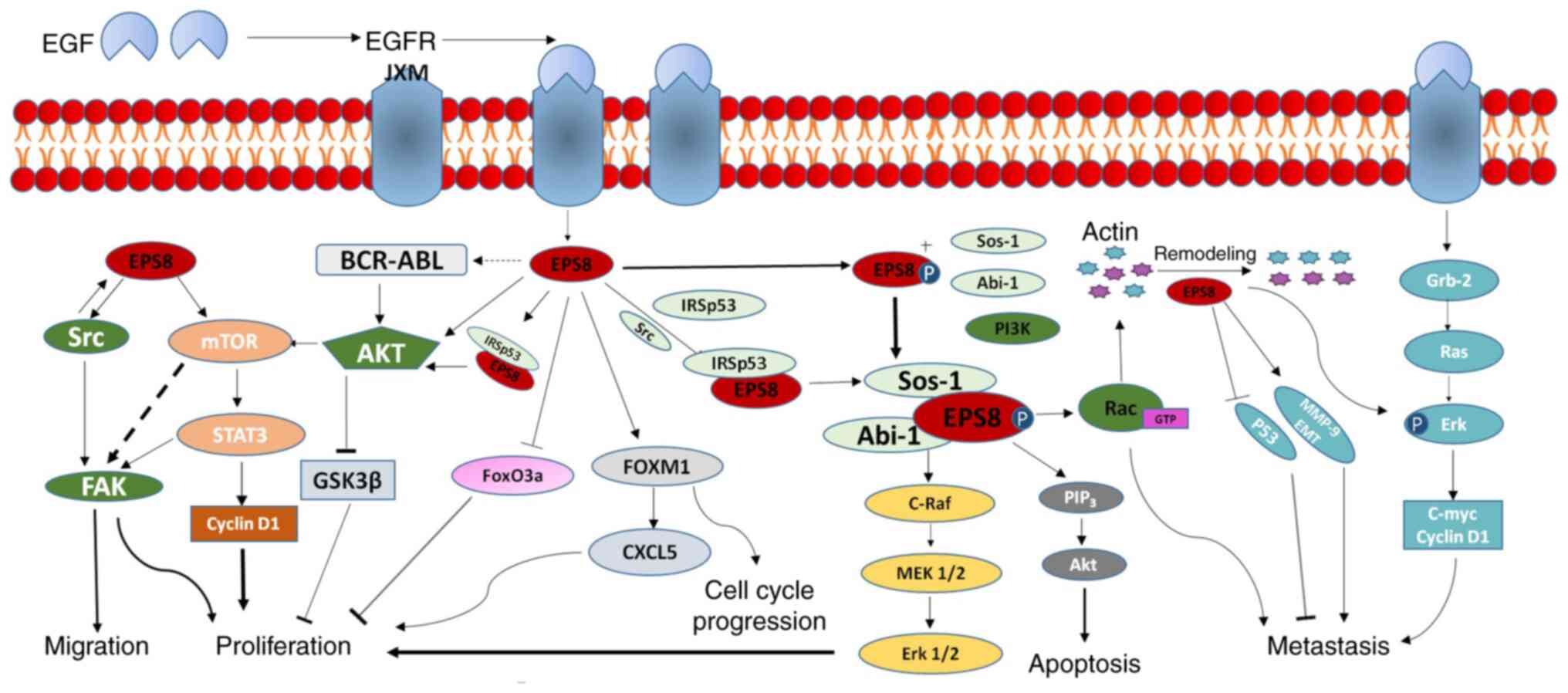
Hyperactivation of intracellular signalling pathways
is a key driver of numerous cancers. EGF, which is composed of a
single peptide, is a mitogen of fibroblasts, epithelial cells and
endothelial cells (44,45). EGF binds specific receptors on the
cell surface, controls EGFR dimerization, and activates tyrosine
kinase and receptor trans-autophosphorylation, triggering multiple
downstream cascades that promote cell proliferation (46,47).
Potentiation of the proliferative effects of EGF as a result of
receptor overexpression and/or activation of its tyrosine kinase
has long been considered to drive carcinogenesis (48). In general, Eps8 overexpression
confers EGF-dependent mitotic signals (2,30),
which bind directly to the JXM region of EGFR and are
phosphorylated. Several intracellular signalling pathways may
become activated following EGFR stimulation, including inositol
phosphoinositide 3-OH kinase (PI3K). Phospholipase C-γ (PLC γ),
activators of transcription (STATS) (49–51),
ERK (52), JNK MAP kinases
(53) and Src are also activated.
In addition, the activation of c-jun N terminal kinase in a
Rac-dependent manner is also mediated by EGFR (54). Given that Eps8 mediates important
biological processes, it is not surprising that it serves as an
attractive molecular therapeutic target and even a prognostic
marker.
Role and molecular mechanism of Eps8 in
solid tumours
The aberrant expression of Eps8 in most tumours is
involved in tumour progression; notably, its expression level may
contribute to tumour proliferation, invasion and metastasis, drug
resistance and prognosis. Importantly, the mechanisms involved are
intricate (Fig. 2). Therefore,
studying the biological significance of Eps8 in solid tumours and
its molecular mechanisms will facilitate further exploration of
cancer therapy strategies targeting Eps8.
Role and mechanisms of EPS8 in tumour
proliferation
Eps8 regulates tumour
proliferation
As early as the beginning of the 21st century,
studies emphasized that Eps8 is highly expressed in tumour cells
(10–20) and can modulate proliferation
(11,13,14,16,17,20),
which ignited the enthusiasm of researchers for the ability of Eps8
to regulate the malignant phenotype of tumours. In general, the
pathways involved in regulating proliferation include the
mTOR/STAT3/FAK pathway and PI3K/AKT pathway.
mTOR/STAT3/FAK signalling pathway and
tumour proliferation
Eps8 determines the expression of downstream factors
required for cell proliferation, and the expression level of Eps8
in colorectal cancer can reflect cell proliferation ability. The
attenuation of Eps8 reduces FAK, an intracellular tyrosine kinase
that exhibits prominent local adhesion (55,56),
and is involved in a variety of integrin-induced biological
activities, including cell migration, growth and survival (57,58).
Further research revealed that this phenomenon is integrated
through the mTOR/STAT3 pathway. Eps8 overexpression activates mTOR,
a proprotein kinase that promotes protein synthesis, which
subsequently triggers mTOR to induce FAK and cyclin D1 expression,
leading to tumorigenesis and tumour proliferation (12). Similarly, Eps8 and FAK expression
trends were detected in tumour specimens, especially in advanced
patients (12). In addition, it has
been reported that STAT3 is continuously activated in
src-transformed cells (59), and
the expression of dominant-negative STAT3 markedly abrogates
src-induced transformation (60,61).
Eps8 overexpression markedly increases src activity (62,63),
and src subsequently promotes tyrosine phosphorylation and Eps8
protein synthesis, stimulating FAK expression and activity
(64). Collectively, Eps8 and FAK
are important in the process of tumour proliferation.
Growth factor and cell
proliferation
Continuous proliferation signals in cancer cells may
be the constitutive activation of growth factor receptor mutations
or overproduction of growth factor in an autocrine manner (65). Therefore, the other mechanism of
Eps8 involvement in cell proliferation will be discussed from these
two angles.
The most typical example of growth factor receptor
activation is the increased expression or activity of various RTKs.
Growth factors, such as EGF, bind to RTKs; stimulate intrinsic
protein-tyrosine kinase activity in cells; and autophosphorylate
several tyrosine residues in the cytoplasmic domain of RTKs.
Multiple signal transduction cascades are initiated, particularly
the RAS/MAPK pathway, which induces cell proliferation (66,67).
RAS/MAPK signalling affects gene transcription by
regulating the activity of transcription factors encoded by direct
early genes and FOXM1, a forkhead box transcription factor
(68). FOXM1 is a key regulator of
the cell cycle process factor (69)
and plays a pivotal role in cell proliferation (70). Notably, FOXM1 and Eps8 are activated
by mitosis signals and upregulated in cancer. Eps8 enhances the
activity of the FOXM1 promoter. First, Eps8 is a protein associated
with FOXM1 that stimulates FOXM1 to upregulate the expression of
CXCL5, thereby increasing cell proliferation in a
PI3K/AKT-dependent manner (43,71).
It is worth mentioning that Eps8 contains a hypothetical nuclear
localization signal (NLS), which colocalizes with FOXM1 in the G2/M
phase (2). The inhibition of
CRM1/exportin1-mediated nuclear output enhanced the nuclear
translocation of Eps8. In addition, Eps8 depletion inhibited the
expression of FOXM1 and the FOXM1 target CCNB1 and slowed the G2/M
transition in cervical cancer cells (72). In conclusion, these findings support
the novel nuclear partnering role of Eps8 with FOXM1 in regulating
cell proliferation.
Eps8 is a downstream component of EGF and stimulates
growth factors (2). Eps8 protein
levels and downstream phosphorylated ERK are upregulated in human
pituitary tumours, and Eps8 cells proliferate more strongly under
conditions full of growth factors and growth restriction (16). Further studies demonstrated that
epidermal growth factor activated robust amplification of ERK and
moderate upregulation of AKT in Eps8-overexpressing cells (16). Moreover, the inhibition or silencing
of Eps8 by MAPK kinase could weaken the proliferation of cells
stimulated by growth factor; in addition, blocking the PI3K pathway
or silencing Eps8 could result in the loss of protection of Eps8
against apoptosis induced by growth factor depletion, subsequently
decreasing cell apoptosis. Consequently, the increased expression
of Eps8 results in an overreaction of the cells to the activation
of local growth factors. Accordingly, the MAPK pathway is activated
through ERK, and the PI3K pathway is activated through AKT, finally
triggering cell proliferation and antiapoptotic responses (16).
FoxO3a/PI3K/AKT signalling pathway and
tumour proliferation
It is generally considered that Eps8 facilitates the
EGFR-induced PI3K/AKT pathway and achieves tumour growth at least
in part by inhibiting FoxO3a (73,74).
FoxO3a is a well-known downstream transcription factor of the
PI3K/AKT pathway (74) and is
essential for differentiation (75). Recent studies on NSCLC have revealed
that the Eps8 expression level is greatly increased in both cells
and tissues (21). The mechanism is
that Eps8 expression is negatively correlated with FoxO3a. FoxO3a
inhibits the level of Eps8 by directly binding to the Eps8 gene
promoter and forms a negative cycle in the EGFR pathway (21). Apart from non-small cell lung
cancer, glioblastoma and breast cancer have also been revealed to
downregulate Eps8 to inhibit proliferation both in vivo and
in vitro (20,22). Notably, the silencing of Eps8 in
pancreatic ductal adenocarcinoma (PDAC) and oral squamous cells did
not affect cell proliferation but inhibited other biological
functions (15,19). Herein, we emphasize that the
aberrant expression of genes in tumours may affect some or all
biological functions.
Role and mechanisms of EPS8 in tumour
invasion and metastasis
Eps8 regulates tumour cell invasion
and metastasis
Cell migration is a complex process involving
reorganization of the actin cytoskeleton (76–78).
Rho GTPase along with other cell processes regulates the
organization of the actin cytoskeleton to promote coordinated
changes in cell behaviour, in which members of Rho GTPases,
including Rac, Cdc42 and Rho, affect different aspects of tumour
cell motility (76–78). Rac promotes the formation of
actin-rich membrane ruffle at the leading edge of migrating cells,
called Lamellipodia (77). Cdc42
modulates cell polarity and the formation of filopodia, thus
controlling the direction of cell movement, and Rho facilitates
stress fibre formation and maintains focal adhesion at the rear of
the cells (78). In addition, Ras
has also been revealed to participate in cell motility and function
downstream of Gi to mediate ovarian cancer cell
migration (79). Ras can activate
Rac through Tiam1 (80), b-PIX
(81), or the SOS1/Eps8/Abi1
tricomplex (32,82). Notably, avβ6 and a5b1
integrin-dependent activation of Rac1 is mediated by Eps8. The
downregulation of Eps8 or Rac1 inhibits integrin-dependent cell
migration, whereas the transient expression of active Rac1 restores
migration in cells with suppressed Eps8 expression (83).
Coactivation of Eps8 with F-actin has been revealed
to occur primarily in pancreatic cancer cells. Eps8 is involved in
cell morphology and protein skeleton, which determines cell
migration. Moreover, Eps8 was located at the tips of F-actin
filaments, filopodia, and the leading edge of cells. Eps8 knockdown
altered cell shape and actinomycin-based cytoskeletal structures
and impaired the formation of protuberance and intercellular
connections (13).
The earliest studies reported that Eps8
overexpression encodes cell growth in fibroblasts (7), and its potential role in the
development of human cancer has gradually been confirmed. Eps8 mRNA
expression levels are sequentially increased in primary tumours,
metastases, and malignant ascites, and Eps8 mRNA is predominantly
expressed in advanced colorectal cancer (84). Similarly, Eps8 overexpression is
highly associated with lymph node metastasis and parametrium
invasion of cervical cancer (14).
Conversely, silencing Eps8 expression blocks migration and invasion
of human glioblastoma cell lines (85). In conclusion, Eps8 serves as a
signalling intermediate for tumour invasion and metastasis.
SOS1/EPS8/Abi1 tricomplex signalling
pathway and tumour invasion and metastasis
Lysophosphatidic acid (LPA), a growth factor-like
phospholipid produced by ovarian cancer cells and secreted into the
abdominal cavity, is uniquely associated with ovarian malignancies
(86–89). High levels of LPA have been revealed
in the ascites of ovarian cancer patients (87), which can effectively drive cell
migration (90,91). The process is accomplished through a
signalling pathway consisting of the Ras-SOS1/Eps8/Abi1 tricomplex
that stimulates Rac activation and cytoskeletal recombination, and
LPA-induced Rac activation is a prerequisite for ovarian cancer
metastasis. The integrity of SOS1/Eps8/Abi1 tricomplex may
determine the possibility of ovarian cancer metastasis because
silencing any member of the SOS1/Eps8/Abi1 tricomplex is not
sufficient to reduce ovarian cancer cell migration and metastatic
colonization. The three members of the complex play their roles and
are interrelated. SOS1 serves as a Rac-specific guanine nucleotide
exchange factor (GEF) and ultimately induces Rac-regulated
cytoskeletal recombination and cell migration (92–94).
Eps8 acts as a substrate for tyrosine kinase receptors. Abi1 is a
scaffold protein that connects SOS1 and Eps8 (73,95).
Abi1 binds with SOS1 through its SH3 domain (96) and subsequently binds to the SH3
domain of Eps8.
Currently, studies have confirmed that the use of
inhibitory peptides can disrupt specific protein-protein
interactions and related biological behaviour (97–99).
As small molecules, inhibitory peptides exhibit significant
potential for clinical application (100). Moreover, short peptides have been
successfully used to interfere with signalling pathways as a new
cancer treatment (101,102). Based on this activity, researchers
selected Abi1 as the target for the design of short inhibitory
peptides and successfully developed peptides capable of inhibiting
the interaction between Eps8-Abi1 and ABI1-SOS1 to prevent the
formation of the SOS1/Eps8/Abi1 tricomplex, thereby suppressing the
invasion and metastasis of ovarian cancer (103). Biomedicine has made notable
progress in tumour treatment, offering more opportunities to
identify disease treatment targets (102,103).
EPS8/IRSp53 complexes and tumour
invasion and metastasis
IRSp53, a protein crucial in cell mobilization, not
only acts as a physical link between Rho GTPases and actin dynamics
(104) but also serves as one of
the Eps8 adapters from the human brain cDNA library. First, the
interplay between Eps8 and IRSp53 increases Rac activation and cell
migration in human fibrosarcoma cells (105). Additionally, this interaction
contributes to Src-mediated transformation. Through Src activation,
EGF increases the formation of the Eps8/IRSp53 complex in HeLa
cells; the activation of AKT, ERK and Stat3; and the enhancement of
cyclin D1 (106). Furthermore, the
Eps8-IRSp53 complex collaboratively activates Rac by enhancing the
formation of the Eps8-Abi-1-SOS-1 complex in fibroblasts and
various cancer cell lines (36).
ERK/MMP9/P53 signalling pathway and
tumour invasion and metastasis
In addition to affecting cell proliferation, Eps8
modulates the ERK signalling cascade and upregulates MMPs (such as
MMP-9) and other matrix metalloproteinases to promote tumour cell
invasion, leading to extracellular matrix remodelling (20,107).
MMP-9 principally functions in EGF- and SF/HGF-induced migration,
and attenuation of MMP-9 activity impairs receptor tyrosine
kinase-dependent SCC mobility (108). Research has revealed that Eps8
overexpression induces cell migration and invasion in vitro
and tumorigenicity in vivo, which depends on the activity of
MMP-9 (107). Notably, Eps8
knockdown had no effect on ERK but reduced the levels of
phosphorylated ERK and MMP9 while enhancing p53. Subsequently, the
expression levels of the mitotic target genes c-Myc and cyclin D1,
which are downstream of ERK signalling, were downregulated and
finally inhibited the migration of breast cancer cells.
Furthermore, attenuation of Eps8 suppressed part of the EMT-like
transformation, significantly increased E-cadherin, and diminished
N-cadherin and vimentin. Notably, the number and size of
EGF-induced Eps8-provoked filamentous pseudopods were also
decreased (20). In short, Eps8 has
been revealed to regulate breast cancer cell migration and invasion
at least in part by affecting ERK signalling, MMP9, p53 and EMT
markers.
Other factors target EPS8 to regulate
tumour invasion and metastasis
MicroRNAs (miRNAs) are a group of 14–25 bp noncoding
RNA (ncRNA) molecules (109) that
regulate gene expression by inhibiting translation or cutting mRNA
in a sequence-dependent manner (110) and are important regulators in the
process of tumour metastasis (111). Studies have provided evidence for
their roles in numerous types of tumours (112,113). Initially, miR-345 was revealed to
prevent GC cell metastasis by inhibiting the epithelial-mesenchymal
transition (EMT) (114).
Subsequently, Zhang et al revealed that Eps8 was a
downstream target of miR-345 and that miR-345 inhibited GC cell
migration, EMT and the CSC phenotype by inactivating the Rac1
signalling pathway (115). In
addition, antitumour miR-130b-5p and numerous downstream genes
mediated by Eps8 were closely involved in the aggressiveness of
PDAC (23).
In addition to the pathways aforementioned, Eps8
also mediates tumour cell metastasis through other approaches.
FOXM1 overexpression not only promotes cell proliferation but also
increases cell migration (71,116),
and targeted inhibition of CXCL5 or AKT reduces Eps8-expressing
cell migration (71). Therefore,
Eps8 and FOXM1 may mediate cell migration through a series of
common downstream components. In addition, the well-known PI3K/AKT
cascade is activated by the binding of EGF to its receptor to
increase migration and invasion in cancers (117). In particular, FoxO3a is also an
important downstream target of the PI3K/AKT pathway (118) that inhibits the expression of Eps8
to prevent the migration and invasion of non-small cell lung
cancer. The phosphorylation and translocation of FoxO3a are caused
by EGF (118). Additionally,
Eps8-induced FAK is overexpressed in numerous tumours and is
strongly correlated with tumour aggressiveness (57,58).
Maa et al demonstrated that Eps8 and FAK form a complex in
regulating cell migration; however, the underlying mechanism
remains unknown (12).
EPS8 regulates tumour resistance,
prognosis and angiogenesis
Eps8 is clearly associated with cellular responses
to cisplatin, paclitaxel and imatinib. and cancer cells were more
sensitive to drug therapy after Eps8 knockout (14,25,119).
Notably, Gorsic et al developed the Eps8 inhibitor miramycin
A as a potential drug to improve the treatment index of cisplatin,
which decreased the expression of Eps8, resulting in an
augmentation in cell sensitivity to cisplatin that was
significantly more pronounced in tumour cell lines than in
lymphoblastoid cell lines (LCLs) (119). Mechanistically, on one hand, Eps8
increases p53 and decreases Src and AKT in such a way that HeLa and
SiHa cells are sensitive to chemotherapeutic drugs (14). On the other hand, PI3K/AKT is an
important cascade reaction of tumour chemotherapy resistance
(120), and EGFR is the key to
activating the PI3K/AKT signalling pathway (121). In general, EGFR TKI resistance is
divided into ‘on target’ and ‘off-target’. ‘On-target’ signifies
that drug resistance is mainly caused by the variation of original
drug targets, and ‘off-target’ refers to the activation of parallel
signalling pathways (122).
PI3K/AKT plays a regulatory role in both on-target and off-target
resistance. PI3K/AKT is highly activated in human cancer and is
used as a therapeutic target (123–126). As a downstream molecule of
PI3K/AKT, FoxO3a (75) inhibits the
expression of the Eps8 signalling protein by directly binding to
the promoter of the Eps8 gene. The signalling pathways inhibited
and promoted by Eps8 are connected together to form a negative
regulatory loop, which bypasses EGFR and reduces the activity of
the PI3K/AKT pathway. Thus, it may affect the EGFR-TKI resistance
in both ‘on target’ and ‘off-target’. Therefore, FoxO3a may
represent a core of the EGFR TKI resistance signalling network.
EGFR and FoxO3a negatively regulate each other in the growth factor
signalling network to maintain the physiological and biochemical
functions of cells (21).
Eps8 expression in cancer cells may be a crucial
biomarker of the prognosis of patients, which is closely concerned
with the survival of patients and has clinical significance.
Generally, the higher the expression of Eps8 in the early stage,
the lower the survival rate. The overall survival (OS) of patients
with expression of Eps8 was significantly lower than that of
patients not expressing Eps8. Thus, Eps8 is considered an
independent predictor of poor OS (19).
Tumours need to absorb nutrients from blood vessels
to grow (127), therefore,
controlling tumour-related angiogenesis is an attractive strategy
to limit tumour progression. Li et al confirmed that FOXM1b
has a direct and significant relationship with the transactivation
of vascular endothelial growth factor expression and increased
angiogenesis (116). In view of
the fact that Eps8, as an adapter protein of FOXM1, mediates
tumorigenesis and development, we hypothesise that Eps8 may also
mediate tumour angiogenesis. Regrettably, the relationship between
Eps8 and tumour angiogenesis remains unknown but is worthy of
further exploration.
EPS8 regulates tumour
immunotherapy
Immunotherapy is a popular method for cancer therapy
and has achieved outstanding clinical effects. There is evidence
that the host immune response can affect the survival of patients
(128). Tumour-associated antigen
(TAA) can produce specific cytotoxic T lymphocytes (CTLs). The
recognition and identification of TAAs are important for the
development of cancer immunotherapy (129). Eps8 is involved in the regulation
of tumour progression and may be an ideal antigen because the new
HLA-A*242-restricted epitope from Eps8 can be used as a new peptide
inhibitor to inhibit the Eps8/EGFR interaction (130). In addition, studies have revealed
that Eps8 protein increases the secretion of interleukin (IL)-12
into the culture supernatant of dendritic cells (DCs) and induces a
significant CTL response, T-cell proliferation and high levels of
interferon (IFN)-γ (131). Given
the relationship between Eps8 and tumour immunity, it may provide
potential immunosuppressants for tumour treatment and create new
diagnostic and treatment methods for clinicians, thus benefiting an
increasing number of tumour patients.
Role and molecular mechanism of Eps8 in
haematological tumours
Eps8 extensively functions as an oncogene in a wide
range of solid tumours (10–23).
In recent years, growing evidence has indicated that Eps8 is
important for the proliferation, apoptosis and prognosis of
haematological tumours, demonstrating the potential of
Eps8-targeted therapy for leukaemia (24–27).
Wang et al detected the expression of Eps8 mRNA and protein
in 6 types of malignant haematological tumour cells (132). The results indicated that Eps8
mRNA and protein levels are not completely consistent. This study
demonstrated the aberrant expression of Eps8 in malignant
haematological tumours for the first time, providing a preliminary
theoretical basis for the screening of new targets for the
treatment of malignant haematological tumours.
Huang et al knocked out Eps8 in CML and MM
cells, resulting in decreased proliferation and increased
apoptosis. In addition, the absence of Eps8 inhibited cell
survival, migration and invasion, and induced drug sensitivity
(25,27). Eps8 was revealed to regulate the
proliferation, apoptosis and chemosensitivity of BCR-ABL-positive
cells by mediating the BCR-ABL/PI3K/AKT/mTOR pathway (25). Therefore, Eps8-targeted inhibitors
alone or in combination with tyrosine kinase inhibitors may
represent an exclusive strategy for refractory and drug-resistant
CML patients. Notably, mithramycin (MTM), a specific Eps8
inhibitor, exhibited anti-MM activity in xenograft tumour models
and suppressed the expression of Eps8 and related pathways
(27). Sun et al
hypothesized that trichostatin A (TSA), a panhistone deacetylase
inhibitor (HDACi), could attenuate Eps8 and its downstream
phosphorylated ERK1/2 pathway, thereby reducing the survival rate
of Burkitt's lymphoma (BL) cells and inducing apoptosis and cell
arrest at G0/G1 (133).
To further improve the prognosis of cancer
patients, researchers analysed ALL and AML and revealed that high
expression of the Eps8 gene predicted poor prognosis (132). As aforementioned Eps8 contains
nuclear localization signals, and the release of Eps8 via tyrosine
kinases creates a nuclear targeting signal responsible for the
intracellular molecular mechanism of nuclear translocation
(26). Castagnino et al
observed that part of Eps8 was indeed translocated to the nucleus,
upregulating the expression of Eps8 (41). Notably, the synthetic
cell-penetrating peptide (CP-Eps8-NLS) derived from the nuclear
localization signal of Eps8 could pass through the cell membrane
and specifically interfere with the nuclear transport of Eps8.
CP-Eps8-NLS exhibited anti-AML activity in various AML cell types
and a synergistic effect with chemotherapeutic drugs in vivo
and in vitro. CP-Eps8-NLS has been revealed to downregulate
the expression of Eps8, PI3K/AKT and MAPK/ERK-related pathway
targets; promote apoptosis and cell cycle arrest; and inhibit
proliferation and cell viability (26). In particular, the NLS of Eps8 may
represent a new target for further inhibitor design to interfere
with Eps8-dependent AML progression.
In summary, Eps8 is likely to become a target for
monitoring and treating haematological malignant tumours and
exhibits markedly broad research and application prospects.
Research on Eps8 and malignant haematological tumours has just
started, and related basic and clinical research is markedly
limited. Thus, more knowledge is required.
Conclusion and future perspective
The oncogene Eps8 is unusually expressed in solid
tumours and haematological malignant tumours and represents an
intriguing tumour biomarker linked to cellular signalling pathways.
Recent evidence has indicated the importance of Eps8 in
tumorigenesis, proliferation, migration, metastasis, drug
resistance and poor prognosis in cancer patients. However, more
tumour models and a large number of clinical trials are still
required to verify its effect and clarify the broader mechanisms,
and in the future, every effort will be made to find new directions
for cancer treatment. Collectively, Eps8 is closely related to the
occurrence and development of malignant tumours, and the potential
of Eps8 in targeted applications and cancer drug development may be
expanded with further research.
Acknowledgements
Not applicable.
Funding
The review was funded by the National Natural
Science Foundation of China (grant no. 81860463), the Science and
Technology Department of Yunnan Province and Kunming Medical
University Project Foundation [grant no. 2018FE001(−003)], the
Science and Technology Department of Yunnan Province and Kunming
Medical University Project Foundation [grant no.
2017FE468(−067)].
Availability of data and materials
Not applicable.
Authors' contributions
CQ and ML designed the study and revised the
manuscript. KL and LZ performed research and drafted the
manuscript, analyzed data and wrote the final version of the
manuscript. YL, HZ and HY participated in the conception of the
study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Correction: Mitochondrial sirtuins in
cancer: Emerging roles and therapeutic potential. Cancer Res.
76:36552016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fazioli F, Minichiello L, Matoska V,
Castagnino P, Miki T, Wong WT and Di Fiore PP: Eps8, a substrate
for the epidermal growth factor receptor kinase, enhances
EGF-dependent mitogenic signals. EMBO J. 12:3799–3808. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wong WT, Carlomagno F, Druck T, Barletta
C, Croce CM, Huebner K, Kraus MH and Di Fiore PP: Evolutionary
conservation of the EPS8 gene and its mapping to human chromosome
12q23-q24. Oncogene. 9:3057–3061. 1994.PubMed/NCBI
|
|
4
|
Tocchetti A, Confalonieri S, Scita G, Di
Fiore PP and Betsholtz C: In silico analysis of the EPS8 gene
family: Genomic organization, expression profile, and protein
structure. Genomics. 81:234–244. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Fiore PP and Scita G: Eps8 in the midst
of GTPases. Int J Biochem Cell Biol. 34:1178–1183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Offenhäuser N, Borgonovo A, Disanza A,
Romano P, Ponzanelli I, Iannolo G, Di Fiore PP and Scita G: The
eps8 family of proteins links growth factor stimulation to actin
reorganization generating functional redundancy in the Ras/Rac
pathway. Mol Biol Cell. 15:91–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maa MC, Hsieh CY and Leu TH:
Overexpression of p97Eps8 leads to cellular transformation:
Implication of pleckstrin homology domain in p97Eps8-mediated ERK
activation. Oncogene. 20:106–112. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Avantaggiato V, Torino A, Wong WT, Di
Fiore PP and Simeone A: Expression of the receptor tyrosine kinase
substrate genes eps8 and eps15 during mouse development. Oncogene.
11:1191–1198. 1995.PubMed/NCBI
|
|
9
|
Ion A, Crosby AH, Kremer H, Kenmochi N,
Van Reen M, Fenske C, Van Der Burgt I, Brunner HG, Montgomery K,
Kucherlapati RS, et al: Detailed mapping, mutation analysis, and
intragenic polymorphism identification in candidate Noonan syndrome
genes MYL2, DCN, EPS8, and RPL6. J Med Genet. 37:884–886. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang Y, Prasad M, Lemon WJ, Hampel H,
Wright FA, Kornacker K, LiVolsi V, Frankel W, Kloos RT, Eng C, et
al: Gene expression in papillary thyroid carcinoma reveals highly
consistent profiles. Proc Natl Acad Sci USA. 98:15044–15049. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang W, Wyckoff JB, Frohlich VC, Oleynikov
Y, Hüttelmaier S, Zavadil J, Cermak L, Bottinger EP, Singer RH,
White JG, et al: Single cell behavior in metastatic primary mammary
tumors correlated with gene expression patterns revealed by
molecular profiling. Cancer Res. 62:6278–6288. 2002.PubMed/NCBI
|
|
12
|
Maa MC, Lee JC, Chen YJ, Chen YJ, Lee YC,
Wang ST, Huang CC, Chow NH and Leu TH: Eps8 facilitates cellular
growth and motility of colon cancer cells by increasing the
expression and activity of focal adhesion kinase. J Biol Chem.
282:19399–19409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Welsch T, Endlich K, Giese T, Büchler MW
and Schmidt J: Eps8 is increased in pancreatic cancer and required
for dynamic actin-based cell protrusions and intercellular
cytoskeletal organization. Cancer Lett. 255:205–218. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YJ, Shen MR, Chen YJ, Maa MC and Leu
TH: Eps8 decreases chemosensitivity and affects survival of
cervical cancer patients. Mol Cancer Ther. 7:1376–1385. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yap LF, Jenei V, Robinson CM, Moutasim K,
Benn TM, Threadgold SP, Lopes V, Wei W, Thomas GJ and Paterson IC:
Upregulation of Eps8 in oral squamous cell carcinoma promotes cell
migration and invasion through integrin-dependent Rac1 activation.
Oncogene. 28:2524–2534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu M, Shorts-Cary L, Knox AJ,
Kleinsmidt-DeMasters B, Lillehei K and Wierman ME: Epidermal growth
factor receptor pathway substrate 8 is overexpressed in human
pituitary tumors: Role in proliferation and survival.
Endocrinology. 150:2064–2671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bashir M, Kirmani D, Bhat HF, Baba RA,
Hamza R, Naqash S, Wani NA, Andrabi KI, Zargar MA and Khanday FA:
P66shc and its downstream Eps8 and Rac1 proteins are upregulated in
esophageal cancers. Cell Commun Signal. 8:132010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen H, Wu X, Pan ZK and Huang S:
Integrity of SOS1/EPS8/ABI1 tri-complex determines ovarian cancer
metastasis. Cancer Res. 70:9979–9990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chu PY, Liou JH, Lin YM, Chen CJ, Chen MK,
Lin SH, Yeh CM, Wang HK, Maa MC, Leu TH, et al: Expression of Eps8
correlates with poor survival in oral squamous cell carcinoma. Asia
Pac J Clin Oncol. 8:e77–e81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen C, Liang Z, Huang W, Li X, Zhou F, Hu
X, Han M, Ding X and Xiang S: Eps8 regulates cellular proliferation
and migration of breast cancer. Int J Oncol. 46:205–214. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wen Q, Jiao X, Kuang F, Hou B, Zhu Y, Guo
W, Sun G, Ba Y, Yu D, Wang D, et al: FoxO3a inhibiting expression
of EPS8 to prevent progression of NSCLC: A new negative loop of
EGFR signaling. EBioMedicine. 40:198–209. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang G, Lu YB and Guan QL: EPS8 is
a potential oncogene in glioblastoma. Onco Targets Ther.
12:10523–10534. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukuhisa H, Seki N, Idichi T, Kurahara H,
Yamada Y, Toda H, Kita Y, Kawasaki Y, Tanoue K, Mataki Y, et al:
Gene regulation by antitumor miR-130b-5p in pancreatic ductal
adenocarcinoma: The clinical significance of oncogenic EPS8. J Hum
Genet. 64:521–534. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He YZ, Liang Z, Wu MR, Wen Q, Deng L, Song
CY, Wu BY, Tu SF, Huang R and Li YH: Overexpression of EPS8 is
associated with poor prognosis in patients with acute lymphoblastic
leukemia. Leuk Res. 39:575–581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang R, Liu H, Chen Y, He Y, Kang Q, Tu
S, He Y, Zhou X, Wang L, Yang J, et al: EPS8 regulates
proliferation, apoptosis and chemosensitivity in BCR-ABL positive
cells via the BCR-ABL/PI3K/AKT/mTOR pathway. Oncol Rep. 39:119–128.
2018.PubMed/NCBI
|
|
26
|
Chen Y, Xie X, Wu A, Wang L, Hu Y, Zhang H
and Li Y: A synthetic cell-penetrating peptide derived from nuclear
localization signal of EPS8 exerts anticancer activity against
acute myeloid leukemia. J Exp Clin Cancer Res. 37:122018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang H, Zhou L, Zhou W, Xie X, Wu M, Chen
Y, Hu Y, Du J, He Y and Li Y: EPS8-mediated regulation of multiple
myeloma cell growth and survival. Am J Cancer Res. 9:1622–1634.
2019.PubMed/NCBI
|
|
28
|
Behlouli A, Bonnet C, Abdi S, Bouaita A,
Lelli A, Hardelin JP, Schietroma C, Rous Y, Louha M, Cheknane A, et
al: EPS8, encoding an actin-binding protein of cochlear hair cell
stereocilia, is a new causal gene for autosomal recessive profound
deafness. Orphanet J Rare Dis. 9:552014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morton CJ, Pugh DJ, Brown EL, Kahmann JD,
Renzoni DA and Campbell ID: Solution structure and peptide binding
of the SH3 domain from human Fyn. Structure. 4:705–714. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matoskova B, Wong WT, Salcini AE, Pelicci
PG and Di Fiore PP: Constitutive phosphorylation of eps8 in tumor
cell lines: Relevance to malignant transformation. Mol Cell Biol.
15:3805–3812. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi X, Betzi S, Lugari A, Opi S, Restouin
A, Parrot I, Martinez J, Zimmermann P, Lecine P, Huang M, et al:
Structural recognition mechanisms between human Src homology domain
3 (SH3) and ALG-2-interacting protein X (Alix). FEBS Lett.
586:1759–1764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Scita G, Nordstrom J, Carbone R, Tenca P,
Giardina G, Gutkind S, Bjarnegård M, Betsholtz C and Di Fiore PP:
EPS8 and E3B1 transduce signals from Ras to Rac. Nature.
401:290–293. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lanzetti L, Rybin V, Malabarba MG,
Christoforidis S, Scita G, Zerial M and Di Fiore PP: The Eps8
protein coordinates EGF receptor signalling through Rac and
trafficking through Rab5. Nature. 408:374–377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kishan KV, Newcomer ME, Rhodes TH and
Guilliot SD: Effect of pH and salt bridges on structural assembly:
Molecular structures of the monomer and intertwined dimer of the
Eps8 SH3 domain. Protein Sci. 10:1046–1055. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Inobe M, Ki K, Miyagoe Y, Yi N and Takeda
S: Identification of EPS8 as a Dvl1-associated molecule. Biochem
Biophys Res Commun. 266:216–221. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Funato Y, Terabayashi T, Suenaga N, Seiki
M, Takenawa T and Miki H: IRSp53/Eps8 complex is important for
positive regulation of Rac and cancer cell motility/invasiveness.
Cancer Res. 64:5237–5244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Disanza A, Mantoani S, Hertzog M, Gerboth
S, Frittoli E, Steffen A, Berhoerster K, Kreienkamp HJ, Milanesi F,
Di Fiore PP, et al: Regulation of cell shape by Cdc42 is mediated
by the synergic actin-bundling activity of the Eps8-IRSp53 complex.
Nat Cell Biol. 8:1337–1347. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Prieto-Echagüe V, Chan PM, Craddock BP,
Manser E and Miller WT: PTB domain-directed substrate targeting in
a tyrosine kinase from the unicellular choanoflagellate Monosiga
brevicollis. PLoS One. 6:e192962011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Forman-Kay JD and Pawson T: Diversity in
protein recognition by PTB domains. Curr Opin Struct Biol.
9:690–695. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Slupsky CM, Gentile LN, Donaldson LW,
Mackereth CD, Seidel JJ, Graves BJ and McIntosh LP: Structure of
the Ets-1 pointed domain and mitogen-activated protein kinase
phosphorylation site. Proc Natl Acad Sci USA. 95:12129–12134. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Castagnino P, Biesova Z, Wong WT, Fazioli
F, Gill GN and Di Fiore PP: Direct binding of eps8 to the
juxtamembrane domain of EGFR is phosphotyrosine- and
SH2-independent. Oncogene. 10:723–729. 1995.PubMed/NCBI
|
|
42
|
Disanza A, Carlier MF, Stradal TE, Didry
D, Frittoli E, Confalonieri S, Croce A, Wehland J, Di Fiore PP and
Scita G: Eps8 controls actin-based motility by capping the barbed
ends of actin filaments. Nat Cell Biol. 6:1180–1188. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Scita G, Tenca P, Areces LB, Tocchetti A,
Frittoli E, Giardina G, Ponzanelli I, Sini P, Innocenti M and Di
Fiore PP: An effector region in Eps8 is responsible for the
activation of the Rac-specific GEF activity of Sos-1 and for the
proper localization of the Rac-based actin-polymerizing machine. J
Cell Biol. 154:1031–1044. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kirkland G, Paizis K, Wu LL, Katerelos M
and Power DA: Heparin-binding EGF-like growth factor mRNA is
upregulated in the peri-infarct region of the remnant kidney model:
In vitro evidence suggests a regulatory role in myofibroblast
transformation. J Am Soc Nephrol. 9:1464–1473. 1998.PubMed/NCBI
|
|
45
|
Miao H, Wei BR, Peehl DM, Li Q, Alexandrou
T, Schelling JR, Rhim JS, Sedor JR, Burnett E and Wang B:
Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK
pathway. Nat Cell Biol. 3:527–530. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Carpenter G and Cohen S: Epidermal growth
factor. Annu Rev Biochem. 48:193–216. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Buday L and Downward J: Epidermal growth
factor regulates p21ras through the formation of a complex of
receptor, Grb2 adapter protein, and Sos nucleotide exchange factor.
Cell. 73:611–620. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ozanne B, Richards CS, Hendler F, Burns D
and Gusterson B: Over-expression of the EGF receptor is a hallmark
of squamous cell carcinomas. J Pathol. 149:9–14. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rubin Grandis J, Zeng Q and Drenning SD:
Epidermal growth factor receptor-mediated stat3 signaling blocks
apoptosis in head and neck cancer. Laryngoscope. 110:868–874. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Song JI and Grandis JR: STAT signaling in
head and neck cancer. Oncogene. 19:2489–2895. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Grandis JR, Drenning SD, Chakraborty A,
Zhou MY, Zeng Q, Pitt AS and Tweardy DJ: Requirement of Stat3 but
not Stat1 activation for epidermal growth factor receptor- mediated
cell growth In vitro. J Clin Invest. 102:1385–1392. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Minden A, Lin A, McMahon M, Lange-Carter
C, Dérijard B, Davis RJ, Johnson GL and Karin M: Differential
activation of ERK and JNK mitogen-activated protein kinases by
Raf-1 and MEKK. Science. 266:1719–1723. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lin A, Minden A, Martinetto H, Claret FX,
Lange-Carter C, Mercurio F, Johnson GL and Karin M: Identification
of a dual specificity kinase that activates the Jun kinases and
p38-Mpk2. Science. 268:286–290. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Minden A, Lin A, Claret FX, Abo A and
Karin M: Selective activation of the JNK signaling cascade and
c-Jun transcriptional activity by the small GTPases Rac and
Cdc42Hs. Cell. 81:1147–1157. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schaller MD, Borgman CA and Parsons JT:
Autonomous expression of a noncatalytic domain of the focal
adhesion-associated protein tyrosine kinase pp125FAK. Mol Cell
Biol. 13:785–791. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hanks SK, Calalb MB, Harper MC and Patel
SK: Focal adhesion protein-tyrosine kinase phosphorylated in
response to cell attachment to fibronectin. Proc Natl Acad Sci USA.
89:8487–8491. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Parsons JT: Focal adhesion kinase: The
first ten years. J Cell Sci. 116:1409–1416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hanks SK, Ryzhova L, Shin NY and Brábek J:
Focal adhesion kinase signaling activities and their implications
in the control of cell survival and motility. Front Biosci.
8:d982–d996. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yu CL, Meyer DJ, Campbell GS, Larner AC,
Carter-Su C, Schwartz J and Jove R: Enhanced DNA-binding activity
of a Stat3-related protein in cells transformed by the Src
oncoprotein. Science. 269:81–83. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bromberg JF, Horvath CM, Besser D, Lathem
WW and Darnell JE Jr: Stat3 activation is required for cellular
transformation by v-src. Mol Cell Biol. 18:2553–2558. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Turkson J, Bowman T, Garcia R, Caldenhoven
E, De Groot RP and Jove R: Stat3 activation by Src induces specific
gene regulation and is required for cell transformation. Mol Cell
Biol. 18:2545–2552. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Leu TH, Yeh HH, Huang CC, Chuang YC, Su SL
and Maa MC: Participation of p97Eps8 in Src-mediated
transformation. J Biol Chem. 279:9875–9881. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Maa MC, Lai JR, Lin RW and Leu TH:
Enhancement of tyrosyl phosphorylation and protein expression of
eps8 by v-Src. Biochim Biophys Acta. 1450:341–351. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sachdev S, Bu Y and Gelman IH:
Paxillin-Y118 phosphorylation contributes to the control of
Src-induced anchorage-independent growth by FAK and adhesion. BMC
Cancer. 9:122009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ma RY, Tong TH, Cheung AM, Tsang AC, Leung
WY and Yao KM: Raf/MEK/MAPK signaling stimulates the nuclear
translocation and transactivating activity of FOXM1c. J Cell Sci.
118:795–806. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Koo CY, Muir KW and Lam EW: FOXM1: From
cancer initiation to progression and treatment. Biochim Biophys
Acta. 1819:28–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Laoukili J, Kooistra MR, Brás A, Kauw J,
Kerkhoven RM, Morrison A, Clevers H and Medema RH: FoxM1 is
required for execution of the mitotic programme and chromosome
stability. Nat Cell Biol. 7:126–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Costa RH: FoxM1 dances with mitosis. Nat
Cell Biol. 7:108–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kwok CT, Leung MH, Qin J, Qin Y, Wang J,
Lee YL and Yao KM: The Forkhead box transcription factor FOXM1 is
required for the maintenance of cell proliferation and protection
against oxidative stress in human embryonic stem cells. Stem Cell
Res. 16:651–661. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang H, The MT, Ji Y, Patel V,
Firouzabadian S, Patel AA, Gutkind JS and Yeudall WA: EPS8
upregulates FOXM1 expression, enhancing cell growth and motility.
Carcinogenesis. 31:1132–1141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ngan AWL, Grace Tsui M, So DHF, Leung WY,
Chan DW and Yao KM: Novel nuclear partnering role of EPS8 with
FOXM1 in regulating cell proliferation. Front Oncol. 9:1542019.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Innocenti M, Frittoli E, Ponzanelli I,
Falck JR, Brachmann SM, Di Fiore PP and Scita G: Phosphoinositide
3-kinase activates Rac by entering in a complex with Eps8, Abi1,
and Sos-1. J Cell Biol. 160:17–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chiu CF, Chang YW, Kuo KT, Shen YS, Liu
CY, Yu YH, Cheng CC, Lee KY, Chen FC, Hsu MK, et al: NF-κB-driven
suppression of FOXO3a contributes to EGFR mutation-independent
gefitinib resistance. Proc Natl Acad Sci USA. 113:E2526–E2535.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Accili D and Arden KC: FoxOs at the
crossroads of cellular metabolism, differentiation, and
transformation. Cell. 117:421–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hall A: Rho GTPases and the actin
cytoskeleton. Science. 279:509–514. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Nobes CD and Hall A: Rho, rac, and cdc42
GTPases regulate the assembly of multimolecular focal complexes
associated with actin stress fibers, lamellipodia, and filopodia.
Cell. 81:53–62. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hall A and Nobes CD: Rho GTPases:
Molecular switches that control the organization and dynamics of
the actin cytoskeleton. Philos Trans R Soc Lond B Biol Sci.
355:965–970. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bian D, Su S, Mahanivong C, Cheng RK, Han
Q, Pan ZK, Sun P and Huang S: Lysophosphatidic acid stimulates
ovarian cancer cell migration via a ras-MEK kinase 1 pathway.
Cancer Res. 64:4209–4217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lambert JM, Lambert QT, Reuther GW,
Malliri A, Siderovski DP, Sondek J, Collard JG and Der CJ: Tiam1
mediates Ras activation of Rac by a PI(3)K-independent mechanism.
Nat Cell Biol. 4:621–625. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
81
|
Shin EY, Shin KS, Lee CS, Woo KN, Quan SH,
Soung NK, Kim YG, Cha CI, Kim SR, Park D, et al: Phosphorylation of
p85 beta PIX, a Rac/Cdc42-specific guanine nucleotide exchange
factor, via the Ras/ERK/PAK2 pathway is required for basic
fibroblast growth factor-induced neurite outgrowth. J Biol Chem.
277:44417–44430. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Nimnual AS, Yatsula BA and Bar-Sagi D:
Coupling of Ras and Rac guanosine triphosphatases through the Ras
exchanger Sos. Science. 279:560–563. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tod J, Hanley CJ, Morgan MR, Rucka M,
Mellows T, Lopez MA, Kiely P, Moutasim KA, Frampton SJ, Sabnis D,
et al: Pro-migratory and TGF-β-activating functions of αvβ6
integrin in pancreatic cancer are differentially regulated via an
Eps8-dependent GTPase switch. J Pathol. 243:37–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Nasri E, Wiesen LB, Knapik JA and
Fredenburg KM: Eps8 expression is significantly lower in p16+ head
and neck squamous cell carcinomas (HNSCCs) compared with p16-
HNSCCs. Hum Pathol. 72:45–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cattaneo MG, Cappellini E and Vicentini
LM: Silencing of Eps8 blocks migration and invasion in human
glioblastoma cell lines. Exp Cell Res. 318:1901–1912. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Jesionowska A, Cecerska-Heryc E, Matoszka
N and Dolegowska B: Lysophosphatidic acid signaling in ovarian
cancer. J Recept Signal Transduct Res. 35:578–584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Pua TL, Wang FQ and Fishman DA: Roles of
LPA in ovarian cancer development and progression. Future Oncol.
5:1659–1673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Fang X, Schummer M, Mao M, Yu S, Tabassam
FH, Swaby R, Hasegawa Y, Tanyi JL, LaPushin R, Eder A, et al:
Lysophosphatidic acid is a bioactive mediator in ovarian cancer.
Biochim Biophys Acta. 1582:257–264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yu S, Murph MM, Lu Y, Liu S, Hall HS, Liu
J, Stephens C, Fang X and Mills GB: Lysophosphatidic acid receptors
determine tumorigenicity and aggressiveness of ovarian cancer
cells. J Natl Cancer Inst. 100:1630–1642. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wang P, Wu X, Chen W, Liu J and Wang X:
The lysophosphatidic acid (LPA) receptors their expression and
significance in epithelial ovarian neoplasms. Gynecol Oncol.
104:714–720. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Pierre S, Bats AS and Coumoul X:
Understanding SOS (Son of Sevenless). Biochem Pharmacol.
82:1049–1056. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Schmidt A and Hall A: Guanine nucleotide
exchange factors for Rho GTPases: turning on the switch. Genes Dev.
16:1587–1609. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Innocenti M, Tenca P, Frittoli E, Faretta
M, Tocchetti A, Di Fiore PP and Scita G: Mechanisms through which
Sos-1 coordinates the activation of Ras and Rac. J Cell Biol.
156:125–136. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kotula L: Abi1, a critical molecule
coordinating actin cytoskeleton reorganization with PI-3 kinase and
growth signaling. FEBS Lett. 586:2790–2794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Fan PD and Goff SP: Abl interactor 1 binds
to sos and inhibits epidermal growth factor- and v-Abl-induced
activation of extracellular signal-regulated kinases. Mol Cell
Biol. 20:7591–7601. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Stone TA and Deber CM: Therapeutic design
of peptide modulators of protein-protein interactions in membranes.
Biochim Biophys Acta Biomembr. 1859:577–585. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Cunningham AD, Qvit N and Mochly-Rosen D:
Peptides and peptidomimetics as regulators of protein-protein
interactions. Curr Opin Struct Biol. 44:59–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Helmer D and Schmitz K: Peptides and
peptide analogs to inhibit protein-protein interactions. Adv Exp
Med Biol. 917:147–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Fosgerau K and Hoffmann T: Peptide
therapeutics: Current status and future directions. Drug Discov
Today. 20:122–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ellert-Miklaszewska A, Poleszak K and
Kaminska B: Short peptides interfering with signaling pathways as
new therapeutic tools for cancer treatment. Future Med Chem.
9:199–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bae DG, Kim TD, Li G, Yoon WH and Chae CB:
Anti-flt1 peptide, a vascular endothelial growth factor receptor
1-specific hexapeptide, inhibits tumor growth and metastasis. Clin
Cancer Res. 11:2651–2561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yu X, Liang C, Zhang Y, Zhang W and Chen
H: Inhibitory short peptides targeting EPS8/ABI1/SOS1 tri-complex
suppress invasion and metastasis of ovarian cancer cells. BMC
Cancer. 19:8782019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Raftopoulou M and Hall A: Cell migration:
Rho GTPases lead the way. Dev Biol. 265:23–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Miki H, Yamaguchi H, Suetsugu S and
Takenawa T: IRSp53 is an essential intermediate between Rac and
WAVE in the regulation of membrane ruffling. Nature. 408:732–735.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Liu PS, Jong TH, Maa MC and Leu TH: The
interplay between Eps8 and IRSp53 contributes to Src-mediated
transformation. Oncogene. 29:3977–3989. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang H, Patel V, Miyazaki H, Gutkind JS
and Yeudall WA: Role for EPS8 in squamous carcinogenesis.
Carcinogenesis. 30:165–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
McCawley LJ, Li S, Wattenberg EV and
Hudson LG: Sustained activation of the mitogen-activated protein
kinase pathway. A mechanism underlying receptor tyrosine kinase
specificity for matrix metalloproteinase-9 induction and cell
migration. J Biol Chem. 274:4347–4353. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Liu WW, Meng J, Cui J and Luan YS:
Characterization and Function of MicroRNA*s in Plants. Front Plant
Sci. 8:22002017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wu WK, Lee CW, Cho CH, Fan D, Wu K, Yu J
and Sung JJ: MicroRNA dysregulation in gastric cancer: A new player
enters the game. Oncogene. 29:5761–5771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Yu M, Xue H, Wang Y, Shen Q, Jiang Q,
Zhang X, Li K, Jia M, Jia J, Xu J and Tian Y: miR-345 inhibits
tumor metastasis and EMT by targeting IRF1-mediated mTOR/STAT3/AKT
pathway in hepatocellular carcinoma. Int J Oncol. 50:975–983. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ying X, Zhang W, Fang M, Zhang W, Wang C
and Han L: miR-345-5p regulates proliferation, cell cycle, and
apoptosis of acute myeloid leukemia cells by targeting AKT2. J Cell
Biochem. 2018:(Epub ahead of print).
|
|
114
|
Feng A, Yuan X and Li X: MicroRNA-345
inhibits metastasis and epithelial-mesenchymal transition of
gastric cancer by targeting FOXQ1. Oncol Rep. 38:2752–2760. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhang J, Wang C, Yan S, Yang Y, Zhang X
and Guo W: miR-345 inhibits migration and stem-like cell phenotype
in gastric cancer via inactivation of Rac1 by targeting EPS8. Acta
Biochim Biophys Sin (Shanghai). 52:259–267. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Li Q, Zhang N, Jia Z, Le X, Dai B, Wei D,
Huang S, Tan D and Xie K: Critical role and regulation of
transcription factor FoxM1 in human gastric cancer angiogenesis and
progression. Cancer Res. 69:3501–3509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kedmi M, Ben-Chetrit N, Körner C, Mancini
M, Ben-Moshe NB, Lauriola M, Lavi S, Biagioni F, Carvalho S,
Cohen-Dvashi H, et al: EGF induces microRNAs that target
suppressors of cell migration: miR-15b targets MTSS1 in breast
cancer. Sci Signal. 8:ra292015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Santo EE, Stroeken P, Sluis PV, Koster J,
Versteeg R and Westerhout EM: FOXO3a is a major target of
inactivation by PI3K/AKT signaling in aggressive neuroblastoma.
Cancer Res. 73:2189–2198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Gorsic LK, Stark AL, Wheeler HE, Wong SS,
Im HK and Dolan ME: EPS8 inhibition increases cisplatin sensitivity
in lung cancer cells. PLoS One. 8:e822202013. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Smolensky D, Rathore K, Bourn J and
Cekanova M: Inhibition of the PI3K/AKT Pathway Sensitizes Oral
Squamous Cell Carcinoma Cells to Anthracycline-Based Chemotherapy
In Vitro. J Cell Biochem. 118:2615–2624. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Li F, Zhao X, Sun R, Ou J, Huang J, Yang
N, Xu T, Li J, He X, Li C, et al: EGFR-rich extracellular vesicles
derived from highly metastatic nasopharyngeal carcinoma cells
accelerate tumour metastasis through PI3K/AKT pathway-suppressed
ROS. J Extracell Vesicles. 10:e120032020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Rotow J and Bivona TG: Understanding and
targeting resistance mechanisms in NSCLC. Nat Rev Cancer.
17:637–658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Li H, Zeng J and Shen K: PI3K/AKT/mTOR
signaling pathway as a therapeutic target for ovarian cancer. Arch
Gynecol Obstet. 290:1067–1078. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Slomovitz BM and Coleman RL: The
PI3K/AKT/mTOR pathway as a therapeutic target in endometrial
cancer. Clin Cancer Res. 18:5856–5864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Mabuchi S, Kuroda H, Takahashi R and
Sasano T: The PI3K/AKT/mTOR pathway as a therapeutic target in
ovarian cancer. Gynecol Oncol. 137:173–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Narayanankutty A: PI3K/Akt/ mTOR Pathway
as a therapeutic target for colorectal cancer: A review of
preclinical and clinical evidence. Curr Drug Targets. 20:1217–1226.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Jiang X, Wang J, Deng X, Xiong F, Zhang S,
Gong Z, Li X, Cao K, Deng H, He Y, et al: The role of
microenvironment in tumor angiogenesis. J Exp Clin Cancer Res.
39:2042020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kirkwood JM, Butterfield LH, Tarhini AA,
Zarour H, Kalinski P and Ferrone S: Immunotherapy of cancer in
2012. CA Cancer J Clin. 62:309–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Novellino L, Castelli C and Parmiani G: A
listing of human tumor antigens recognized by T cells: March 2004
update. Cancer Immunol Immunother. 54:187–207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Xie X, Zhou W, Hu Y, Chen Y, Zhang H and
Li Y: A dual-function epidermal growth factor receptor pathway
substrate 8 (Eps8)-derived peptide exhibits a potent cytotoxic T
lymphocyte-activating effect and a specific inhibitory activity.
Cell Death Dis. 9:3792018. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
He YJ, Zhou J, Zhao TF, Hu LS, Gan JY,
Deng L and Li Y: Eps8 vaccine exerts prophylactic antitumor effects
in a murine model: A novel vaccine for breast carcinoma. Mol Med
Rep. 8:662–668. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Wang L, Cai SH, Xiong WY, He YJ, Deng L
and Li YH: Real-time quantitative polymerase chain reaction assay
for detecting the eps8 gene in acute myeloid leukemia. Clin Lab.
59:1261–1269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Sun P, Zhou X, He Y, Liu H, Wang Y, Chen
Y, Li M, He Y, Li G and Li Y: Effect of trichostatin A on Burkitt's
lymphoma cells: Inhibition of EPS8 activity through Phospho-Erk1/2
pathway. Biochem Biophys Res Commun. 497:990–996. 2018. View Article : Google Scholar : PubMed/NCBI
|