Introduction
Recurrent head and neck squamous cell carcinoma
(HNSCC) in previously irradiated areas and not accessible to local
treatment usually have a poor prognosis, with a median overall
survival of less than one year in the first-line setting (1–4).
Few therapeutic options are available, mostly as
patients that have previously received irradiation are unsuitable
candidates for salvage surgery, and often have unstable overall
conditions (5). Today, salvage
surgery remains the standard of care for selected operable and fit
patients, but leads to a high rate of both local and regional
failures (1,4).
The recent watershed of immunotherapy, particularly
immune checkpoint inhibitors (ICIs), has provided renewed hope for
recurrent and metastatic HNSCC; nivolumab treatment has resulted in
a 30% reduction in risk of death (CheckMate 141 trial) (3,6) and
pembrolizumab treatment in a 20% reduction (KEYNOTE-040 trial)
(6,7). ICI use in patients with locoregional
recurrence seems to be less satisfying than for metastases
(3,7). Inhibition of the programmed
death-1/programmed death-ligand 1 (PD-1/PD-L1) pathway by these
antibodies and the presence of CD8+ tumor-infiltrating
lymphocytes (TILs) are of the greatest importance in terms of
mounting an antitumor immune response (8).
Yet, the immune landscape of pretreated areas
remains unclear, mainly because none of the randomized trials
assessing the efficacy of ICIs stratified patients according to
whether they had previously received radiation or not. Some
investigators have already described the complexity of
radio-induced alterations in growth factors and proinflammatory,
profibrotic, and proangiogenic cytokines (9,10). It
is well known that radiotherapy increases the mutational load and
improves response to ICIs (11–13).
Any changes in the frequency of CD3+ and CD8+
TILs or changes in the expression of PD-L1 by tumor and immune
cells remain to be determined and if numbers or expression appear
to be reduced, this could support the strategy for introducing an
ICI earlier in future therapeutic approaches, as is being assessed
in first-line recurrent or metastatic HNSCC and current studies
KEYNOTE-048 (14), KEYNOTE-689
(Clinical Trial NCT03765918), PembroRad (Clinical Trial
NCT02707588), KEYNOTE-412 (Clinical Trial NCT03040999), and REACH
trial (Clinical Trial NCT02999087).
We focused on HNSCC locoregional recurrences as a
recent study found a higher rate of hyperprogression following
initiation of an ICI in patients with locoregional recurrence (with
or without distant metastases) than in patients with only distant
metastases (15), although this may
be lower and more variable among solid tumors (16). We aimed to assess whether there is a
difference in the expression of antitumor immune response
biomarkers in the tumor microenvironment of de novo tumors
and tumors in irradiated areas.
Materials and methods
Study design and patients
A total of 100 HNSCC tumor tissue specimens from
patients who had undergone surgery from January 2010 to November
2017 were analyzed and divided into two cohorts: 50 de novo
tumors and 50 tumors recurring within previously irradiated areas.
The samples in the irradiated area came either from local
recurrences at the initial tumor site, or from neck metastases or
from second head and neck squamous cell carcinoma. Samples were
included if they were paraffin-embedded surgical samples of
invasive squamous cell carcinoma, from four locations (oral cavity,
oropharynx, larynx, hypopharynx). Samples were excluded if they
displayed other histology, carcinoma in situ, cancer at
stage T1N0M0, tumors from the nasal or paranasal cavity or the
nasopharynx, or if they were frozen tissue samples. All cases were
recorded at the Lorraine Institute of Oncology and tissue specimens
were obtained from the tumor bank of the Institute (tumor bank
certification FR17/81842500; norm NF S96-900). According to French
regulations, patients were informed of the research performed with
tumor tissue specimens and did not object. We compared two parallel
cohorts, de novo vs. pretreated (irradiated) area.
Histological assessment
Formalin-fixed and paraffin-embedded tumor tissue
samples stained by hematoxylin, eosin, and saffron were reviewed by
an experienced pathologist to check the quality and the
representativity of the specimens selected for supplementary
analysis with immunohistochemistry (IHC). IHC preparations were
analyzed under microscopic examination. After hematoxylin
counterstaining, tumor cells were differentiated from lymphocytes
using morphologic criteria.
Immunohistochemical analysis
We used four monoclonal antibodies: Anti-PD-L1 ones,
based on previous assessments of PD-L1 expression (17) (SP263, Ventana Medical Systems),
anti-CD3 (SP7, Thermo Fisher Scientific, Inc.), anti-CD8 (C8/144B,
Agilent Dako) and anti-p16 [anti-p16INK4a (E6H4), Ventana Medical
Systems Inc.].
P16 status was assessed as a surrogate marker of
human papillomavirus (HPV) association. Threshold positivity was at
least 70% of tumor cells stained with at least moderate to strong
and nuclear and cytoplasmic staining (18). The density of CD3+ and
CD8+ TILs were measured quantitatively by counting each
CD3+ or CD8+ cell in intratumoral and stromal
compartments separately on photographs at magnification, ×20.
Values were dichotomized (low/high) according to the median cell
number calculated from all samples and considered high if >40
CD3+ cells and >30 CD8+ cells were
observed in intratumoral regions and if >160 CD3+
cells and >90 CD8+ cells were observed in stromal
regions. PD-L1 IHC expression, defined as membranous and
cytoplasmic staining, was assessed on both tumor cells and immune
cells. For tumor cells, threshold positivity was a tumor proportion
score (TPS) ≥1%, as previously described (19,20).
For immune cells, semi-quantitative assessment was carried out in
both tumoral (categorized into three classes scored from 0 to 2)
and stromal regions (categorized into six classes scored from 0 to
5). The expression of PD-L1 by immune cells was considered high
with a score of 1–2 in intratumoral components and 2–5 in stromal
components.
We also characterized the immune phenotype of
samples of each cohort into four types as described by Teng et
al based on TIL counts and expression of PD-L1 by tumor cells:
Type I (adaptive immune resistance: PD-L1+/TILs high),
type II (immunological ignorance: PD-L1-/TILs low), type III
(intrinsic induction: PD-L1+/TILs low) and type IV
(immune tolerance: PD-L1−/TILs high) (21). We considered TPS to be positive at
≥1% and CD8+ TIL numbers to be high when they were
greater than or equal to the median cell number. Fig. 1 presents representative
microphotographs of immunohistochemical staining.
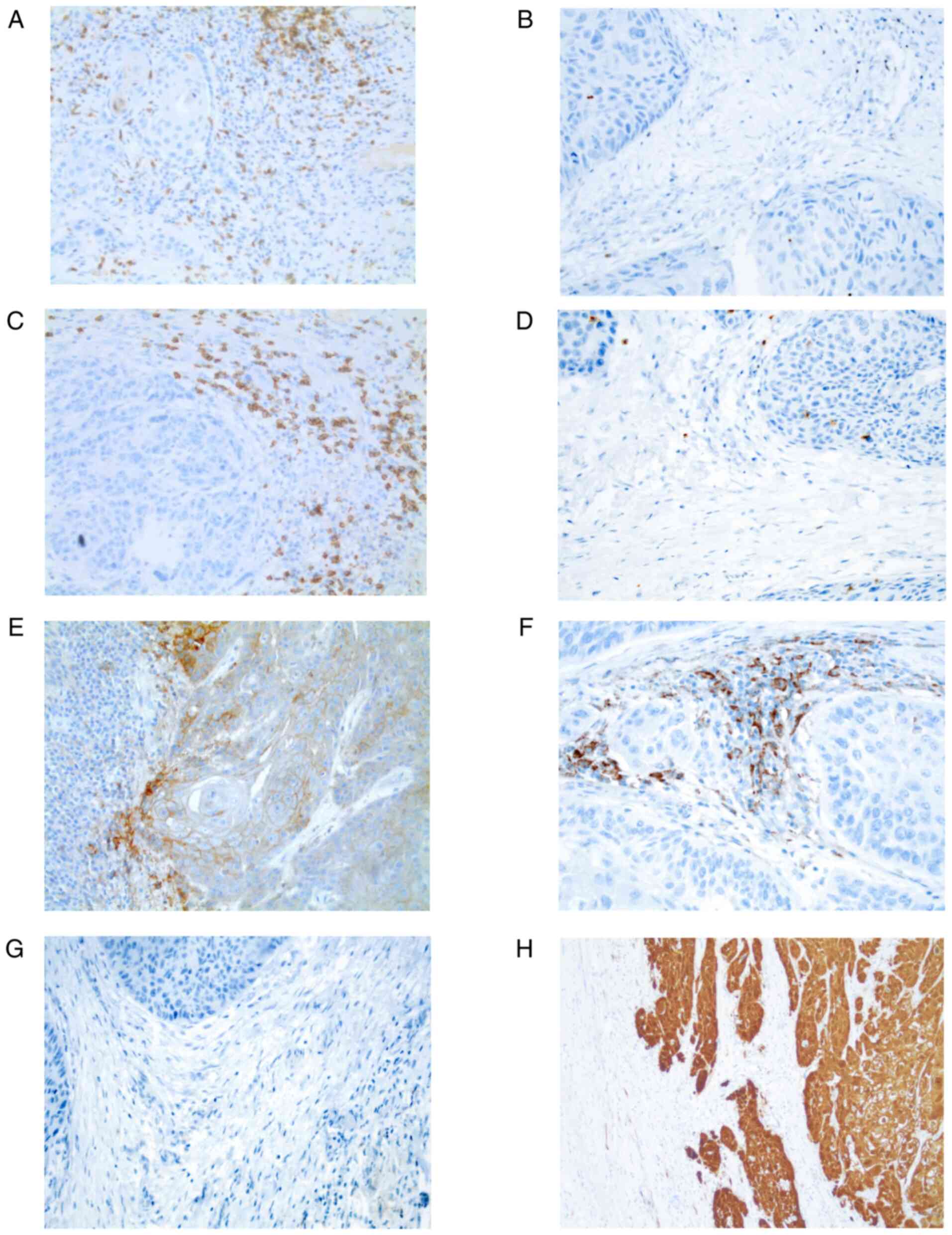 | Figure 1.Representative microphotographs of
immunohistochemical staining of CD3, CD8, PD-L1 on tumor cells and
immune cells and p16 in the irradiated area cohort (magnification,
×20). (A) High CD3+ TIL count, (B) low CD3+
TIL count, (C) high CD8+ TIL count, (D) low
CD8+ TIL count, (E) PD-L1+ tumor cells and
PD-L1− immune cells (F) PD-L1− tumor cells
and PD-L1+ immune cells, (G) PD-L1− tumor
cells and PD-L1− IC, and (H) positive P16 status. TIL,
tumor-infiltrating lymphocyte; PD-L1, programmed death-ligand
1. |
Statistical analysis
Quantitative parameters were described as median and
interquartile range or as mean and standard deviation according to
the normality of the distribution assessed by the Shapiro-Wilk
test; qualitative parameters as frequency and percentage. The two
groups were compared by the Chi-square test or Fisher's exact test
for qualitative parameters; for quantitative parameters, the
Student's t-test was performed in case of normal distribution and
the Mann-Whitney U test in the other cases. Each quantitative value
of immune response biomarkers was dichotomized according to its
median value.
In order to adjust the comparisons of immune
response biomarkers on confounding factors, the propensity score
was computed with the two groups as dependent parameters, and with
patient and tumor characteristics with a P-value <0.2 for the
comparisons of the two groups. The inverse probability of treatment
weighting (IPTW) was computed (22)
and immune response biomarkers were compared by weighting analyses.
Progression-free survival (PFS) was defined as the time between
surgery and relapse, progression, or death (whichever occurred
first).
In each group, the prognostic factors of PFS were
investigated according to the following process. First, bivariate
analyses were performed with the Cox proportional hazard model.
Parameters with a P-value <0.1 were introduced in a multivariate
Cox proportional hazard model with backward selection. Results are
presented as hazard ratio (HR) and its 95% confidence interval
(CI). The statistical analyses were performed with SAS, version 9.4
(SAS Institute Inc.) and the significance level was set at
0.05.
Results
Patient and tumor characteristics
Table I presents the
patient and tumor characteristics. Mean age was 64.2±8.3 years in
the de novo cohort and 65.8±10.7 years in the irradiated
area cohort (P=0.457). The two groups were well-balanced according
to the following criteria: Sex (P=0.806), tumor location (P=0.743),
and disease stage (P=0.873). Oropharyngeal tumors exhibited
significantly greater p16 expression in the de novo cohort
(n=7, 43.7%) than in the irradiated area cohort (n=2, 10.5%)
(P=0.025). Tumors that had developed in irradiated areas were
histologically less differentiated than the de novo tumors
[n=32 (64.0%) vs. n=37 (74.0%) were well differentiated; P=0.051],
and were more frequently stage pNx/N0/N1 [n=38 (76.0%) vs. n=26
(52.0%); P=0.012]. Resection margins were significantly more
positive [n=23 (46.0%) vs. n=11 (22.0%); P=0.011]. Lymphovascular
[n=21 (58.3%) vs. n=13 (30.2%); P=0.012] or perineural invasion
[n=28 (73.7%) vs. n=23 (53.5%); P=0.060] were more frequently
observed in the tumors that had developed in irradiated areas.
 | Table I.Patient and tumor
characteristics. |
Table I.
Patient and tumor
characteristics.
|
Characteristics | De novo
(n=50) | Irradiated area
(n=50) | P-value |
|---|
| Age (years) | 62.1; 64.2±8.3 | 64.9;
65.8±10.7 | 0.457c |
| Sex, male | 40 (80.0) | 39 (78.0) | 0.806d |
| Smoking |
|
|
|
| Never
smoker | 7
(14.2) | 4
(8.3) | 0.289d |
| Current
smoker | 21 (42.9) | 16 (33.2) |
|
| Former
smoker | 21 (42.9) | 28 (58.4) |
|
| Smoking
history (pack*year) | 40.0;
41.1±15.3 | 40.0;
42.2±17.8 | 0.964c |
| Alcohol |
|
|
|
|
Yes | 27 (55.1) | 31 (68.9) | 0.170d |
| No | 22 (44.9) | 14 (31.1) |
|
| Former
drinker | 9
(33.3) | 11 (36.7) | 0.792d |
| WHO performance
status |
|
|
|
| 0 | 28 (56.0) | 22 (44.0) | 0.453e |
| 1 | 21 (42.0) | 26 (52.0) |
|
| 2 | 1
(2.0) | 2
(4.0) |
|
| Tumor
locationa |
|
|
|
| Oral
cavity | 20 (40.0) | 15 (31.9) | 0.743d |
|
Oropharynx | 16 (32.0) | 19 (40.4) |
|
|
Larynx | 6
(12.0) | 7
(14.9) |
|
|
Hypopharynx | 8
(16.0) | 6
(12.8) |
|
| Status P16+
(>70%)b | 7
(43.7) | 2
(10.5) | 0.025e |
|
Differentiation |
|
|
|
| Well
differentiated | 37 (74.0) | 32 (64.0) | 0.051d |
|
Moderately/poorly | 5
(10.0) | 14 (28.0) |
|
|
Undifferentiated | 8
(16.0) | 4
(8.0) |
|
| pT stage |
|
|
|
|
Tx-T1-T2 | 35 (70.0) | 28 (56.0) | 0.147d |
|
T3-T4 | 15 (30.0) | 22 (44.0) |
|
| pN stage |
|
|
|
|
Nx-N0-N1 | 26 (52.0) | 38 (76.0) | 0.012d |
| N2-
N3 | 24 (48.0) | 12 (24.0) |
|
| AJCC disease stage
(8th edition) |
|
|
|
| I/
II | 20 (40.0) | 18 (36.0) | 0.873d |
|
III | 11 (22.0) | 13 (26.0) |
|
| IV | 19 (38.0) | 19 (38.0) |
|
| Resection
margins |
|
|
|
|
Positive (R1 or R2) | 11 (22.0) | 23 (46.0) | 0.011d |
|
Negative (R0 or limit) | 39 (78.0) | 27 (54.0) |
|
| Lymphovascular
invasion | 13 (30.2) | 21 (58.3) | 0.012d |
| Perineural
invasion | 23 (53.5) | 28 (73.7) | 0.060d |
| Number of
lymphadenopathies | 2.0; 4.2±9.8 | 2.0; 3.2±2.9 | 0.992f |
| Number of nodes
removed | 38.0;
41.5±17.7 | 13.0;
16.3±16.5 |
<0.001f |
| Extranodal
spread | 22 (66.7) | 11 (73.3) | 0.644e |
Treatment characteristics
Table II details
the treatment characteristics. In the irradiated cohort, 22 (44.0%)
patients had been treated with surgery and postoperative
radiotherapy, 6 (12.0%) by surgery and postoperative
chemoradiation, 10 (20.0%) by definitive chemoradiation, 5 (10.0%)
by radiotherapy only, and 7 (14.0%) by induction chemotherapy and
chemoradiation. Nineteen (44.2%) patients in the irradiated area
cohort had received a mean irradiation dose at the site of tumor
recurrence of ≥66 Gy (tumor and/or node). Table SI describes the treatments of the
initial tumors of the irradiated area cohort.
 | Table II.Characteristics of the treatments
received by patients in the irradiated area cohort. |
Table II.
Characteristics of the treatments
received by patients in the irradiated area cohort.
| Characteristics of
the irradiated cohort (n=50) | Data |
|---|
| Type of tumor |
|
| Locoregional
recurrence beyond initial treatmenta | 29 (58.0) |
| >6
months | 20 (69.0) |
| ≤6
months | 9
(31.0) |
| New location | 21 (42.0) |
| Time of new
location (years) | 10.1
(3.4–13.1) |
| Treatment of the
initial tumor |
|
| Type of
treatment |
|
| Surgery
T/N + RT | 22 (44.0) |
| Surgery
T/N + RTCT | 6
(12.0) |
|
Definitive RTCTb | 10 (20.0) |
| RT
only | 5
(10.0) |
|
Induction chemotherapy +
RTCT | 7
(14.0) |
| Surgery T/N of the
initial tumor | 28 (56.0) |
| Type of
RTc |
|
| 3D | 22 (51.1) |
|
IMRT | 21 (48.8) |
| Dose on tumor site
recurrence | 21 (48.8) |
| (T and/or N) ≥66
Gyc |
|
| Mean dose on tumor
site recurrence | 19 (44.2) |
| (T and/or N) ≥66
Gyc |
|
| Overall treatment
time (days) >6 weeksc | 20 (46.5) |
| Treatment of the
tumor in irradiated area |
|
| Salvage
surgery | 50 (100) |
| Tumor
surgery | 47 (97.9) |
| Neck
dissection | 41 (82.0) |
|
Ipsilateral | 13 (31.7) |
|
Bilateral | 28 (68.3) |
| Re-irradiation
only | 1
(2.0) |
| Re-irradiation with
CT (postoperative VOKES protocol) | 4
(8.0) |
Immune microenvironment
Table III details
the immune microenvironment (CD3+ and CD8+
TILs, PD-L1) expression on tumor and immune cells) in the
intratumoral and stromal regions.
 | Table III.Description of the expression of
immune response biomarkers in irradiated area compared to de
novo tumors. |
Table III.
Description of the expression of
immune response biomarkers in irradiated area compared to de
novo tumors.
| Biomarkers | De novo
(n=50) | Irradiated area
(n=50) | P-value | Adjusted
P-valuea |
|---|
| CD3+
TILs |
|
|
|
|
|
Intratumoral |
|
|
|
|
|
Number | 58.0
(27.0–101.0) | 20.5
[9.0–70.0] | 0.003b | 0.088 |
|
High | 33 (66) | 17 (34) | 0.001c | <0.001 |
|
Stromal |
|
|
|
|
|
Number | 185.5
(107.7–361.0) | 139.0
(63.0–215.0) | 0.020b | 0.046 |
|
High | 30 (60) | 18 (36) | 0.016c | 0.008 |
|
Intratumoral low and stromal
low CD8+ TILs | 7
(14) | 18 (36) | 0.011c | 0.001 |
|
Intratumoral |
|
|
|
|
|
Number | 30.0
(13.0–87.0) | 21.5
(3.0–64.0) | 0.273b | 0.261 |
|
High | 25 (50) | 21 (42) | 0.422c | 0.005 |
|
Stromal |
|
|
|
|
|
Number | 97.5
(48.0–158.0) | 71.0
(24.0–131.0) | 0.129b | 0.121 |
|
High | 28 (56) | 24 (48) | 0.423 | 0.577 |
|
Intratumoral low and stromal
low PD-L1 | 15 (30) | 16 (32) | 0.829 | 0.098 |
|
TPS ≥1% | 43 (86) | 28 (56) |
<0.001c | <0.001 |
| Immune
cells (ICs) |
|
|
|
|
|
Intratumoral=High | 36 (72) | 24 (48) | 0.014c | <0.001 |
|
Stromal=High | 39 (78) | 31 (62) | 0.081c | 0.058 |
| TPS <1% and
PD-L1 IC intratumoral=0 | 5
(10) | 17 (34) | 0.004c | <0.001 |
CD3+ TIL numbers
The median number of CD3+ TILs in the
intratumoral regions was significantly lower in the irradiated area
cohort [median, 20.5; interquartile range (9.0–70.0) vs. 58.0
(27.0–101.0); P=0.003].
A total of 34% of tumors in the irradiated area
(n=17) showed a high CD3+ TIL count compared to 66%
(n=33) in the de novo group (P=0.001). Similar results were
found for CD3+ TIL count in the stromal regions
(P=0.016). 36% (n=18) of irradiated tumors had a low number of
CD3+ TILs in intratumoral and stromal regions compared
to 14% (n=7) in the de novo group (P=0.001). Results were
similar with the IPTW method.
CD8+ TIL numbers
CD8+ TIL counts did not differ between
the two cohorts in either intratumoral or stromal regions. The
median number was 21.5 [3.0–64.0] in the irradiated area cohort vs.
30.0 [13.0–87.0] in the de novo cohort in intratumoral
compartments (P=0.273) and 71.0 {24.0–131.0} vs. 97.5 [48.0–158.0]
in the stromal compartments (P=0.129).
Expression of PD-L1 by tumor and
immune cells
The percentage of tumors with PD-L1+
tumor cells (TPS ≥1%) was significantly lower in the irradiated
area cohort than the de novo cohort (56.0% vs. 86.0%,
P<0.001) (Table III). The
percentage of tumors with PD-L1+ immune cells was
significantly lower in the intratumoral regions (48.0% vs. 72.0%,
P=0.014) and also lower in the stromal regions (62.0% vs. 78.0%,
P=0.081) in the irradiated area cohort compared to the de
novo cohort. One-third of irradiated tumors had a negative TPS
and a low expression of PD-L1 by immune cells (ICs) (Table III) compared to 10% in the de
novo cohort (P=0.004). Results were similar using the IPTW
method.
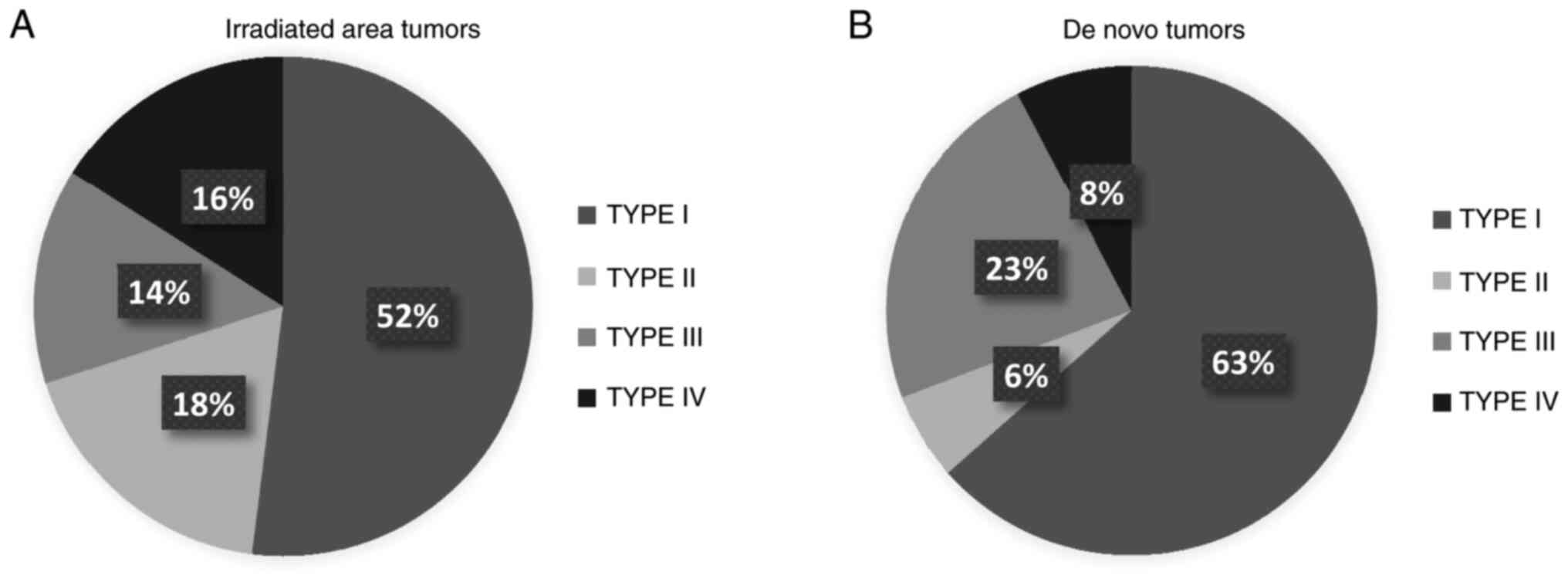
Immune phenotype
Fig. 2 presents the
proportion of microenvironment phenotypes I to IV in each cohort.
We observed type I phenotype, adaptive immune resistance, in 63% of
tumors in the de novo cohort vs. 52% in the irradiated area
cohort, whereas types II and IV were found more frequently in the
irradiated area cohort (P=0.032 in bivariate analyses and
P<0.001 after the IPTW method).
Median follow-up time was 18 months (interquartile
range 10–27). The tumor microenvironment (CD3+ and
CD8+ TILs, PD-L1+ tumor cells, and
PD-L1+ immune cells) was not significantly associated
with PFS in the de novo cohort (Table IV) or the irradiated area cohort
(Table SII).
 | Table IV.Prognostic factors of PFS for the
de novo cohort in univariate and multivariate analyses. |
Table IV.
Prognostic factors of PFS for the
de novo cohort in univariate and multivariate analyses.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years) |
| 0.139 |
|
|
|
≤60 | 1 |
|
|
|
|
>60 | 2.29
(0.76–6.87) |
|
|
|
| Sex |
| 0.334 |
|
|
|
Male | 0.60
(0.21–1.69) |
|
|
|
|
Female | 1 |
|
|
|
| Smoking |
| 0.603 |
|
|
| Current
smoker | 1 |
|
|
|
| Former
smoker | 1.59
(0.60–4.19) |
|
|
|
| Never
smoker | 0.99
(0.20–4.77) |
|
|
|
| Alcohol |
| 0.458 |
|
|
|
Yes | 1.42
(0.56–3.62) |
|
|
|
| No | 1 |
|
|
|
| Tumor location |
| 0.003 |
|
|
|
Other | 1 |
|
|
|
|
Hypopharynx | 4.08
(1.59–10.45) |
|
|
|
| Oropharynx location
and p16 |
| 0.167 |
|
|
|
Oropharynx
p16+ | 1 |
|
|
|
|
Oropharynx
p16− | 4.57
(0.53–39.26) |
|
|
|
|
Differentiation |
| 0.331 |
|
|
|
Moderately/poorly/undifferentiated | 1 |
|
|
|
|
Well-differentiated | 1.73
(0.57–5.22) |
|
|
|
| pT stage |
| 0.921 |
|
|
|
Tx-T1-T2 | 1 |
|
|
|
|
T3-T4 | 0.95
(0.37–2.48) |
|
|
|
| pN stage |
| 0.014 |
|
|
|
Nx-N1-N2 | 1 |
|
|
|
|
N3-N4 | 3.34
(1.28–8.72) |
|
|
|
| AJCC disease stage
(8th edition) |
| 0.009 |
|
|
|
I/II | 1 |
|
|
|
|
III | 0.62
(0.12–3.21) |
|
|
|
| IV | 3.62
(1.29–10.18) |
|
|
|
| Smoking history
(pack*year) |
| 0.422 |
|
|
|
<40 | 1 |
|
|
|
|
>40 | 1.44
(0.59–3.49) |
|
|
|
| Former drinker |
| 0.996 |
|
|
|
Yes | 1.00
(0.30–3.32) |
|
|
|
| No | 1 |
|
|
|
| Resection
margins |
| 0.044 |
| 0.015 |
|
Positive (R1 or R2) | 1 |
| 1 |
|
|
Negative (RO or limit) | 0.38
(0.15–0.97) |
| 4.10
(1.31–12.79) |
|
| Lymphovascular
invasion |
| 0.007 |
|
|
|
Yes | 4.36
(1.49–12.76) |
|
|
|
| No | 1 |
|
|
|
| Perineural
invasion |
| 0.019 |
| 0.021 |
|
Yes | 4.61
(1.29–16.45) |
| 5.16
(1.28–20.80) |
|
| No | 1 |
| 1 |
|
| Number of
lymphadenopathies |
| 0.001 |
| 0.037 |
| 1 | 1 |
| 1 |
|
|
>1 | 5.29
(1.92–14.62) |
| 3.90
(1.09–14.02) |
|
| Number of nodes
removed |
| 0.068 |
|
|
|
<30 | 1 |
|
|
|
|
≤30 | 2.36
(0.94–5.91) |
|
|
|
| Extranodal
spread |
| 0.503 |
|
|
|
Yes | 1 |
|
|
|
| No | 1.40
(0.52–3.76) |
|
|
|
| P16 |
| 0.116 |
|
|
|
Positive (≥70%) | 1 |
|
|
|
|
Negative (<70%) | 5.08
(0.67–38.49) |
|
|
|
| TPS |
| 0.092 |
|
|
|
<1 | 2.42
(0.86–6.80) |
|
|
|
| ≥1 | 1 |
|
|
|
| PD-L1 IC
intratumoral expression |
| 0.159 |
|
|
|
Low | 1.91
(0.78–4.71) |
|
|
|
|
High | 1 |
|
|
|
| Combined score |
| 0.075 |
|
|
| TPS
<1% AND | 2.74
(0.90–8.31) |
|
|
|
| PD-L1
IC intratumoral=0 |
|
|
|
|
| TPS ≥
1% OR | 1 |
|
|
|
| PD-L1
IC intratumoral ≥1 |
|
|
|
|
| PD-L1 IC stromal
expression |
| 0.052 |
|
|
|
Low | 2.50
(0.99–6.31) |
|
|
|
|
High | 1 |
|
|
|
| CD3 intratumoral
counts |
| 0.935 |
|
|
|
Low | 0.96
(0.38–2.41) |
|
|
|
|
High | 1 |
|
|
|
| CD3 stromal
counts |
| 0.208 |
|
|
|
Low | 1.76
(0.73–4.25) |
|
|
|
|
High | 1 |
|
|
|
| CD3 intratumoral +
stromal counts |
| 0.953 |
|
|
| CD3
intratumoral low AND | 0.96
(0.28–3.29) |
|
|
|
| CD3
stromal low |
|
|
|
|
| CD3
intratumoral high OR CD3 stromal high | 1 |
|
|
|
| CD8 intratumoral
counts |
| 0.864 |
|
|
|
Low | 1.08
(0.45–2.61) |
|
|
|
|
High | 1 |
|
|
|
| CD8 stromal
counts |
| 0.185 |
|
|
|
Low | 1.83
(0.75–4.46) |
|
|
|
|
High | 1 |
|
|
|
| CD8 intratumoral +
stromal counts |
| 0.194 |
|
|
| CD8
intratumoral low AND CD8 stromal low | 1.81
(0.74–4.44) |
|
|
|
| CD8
intratumoral high OR CD8 stromal high | 1 |
|
|
|
Discussion
The present study aimed to assess antitumor immune
response biomarkers in head and neck squamous cell carcinoma
(HNSCC) occurring in irradiated areas: Few data are currently
available. We observed a significantly lower infiltration of
CD3+ tumor-infiltrating lymphocytes (TILs) in tumors in
irradiated areas compared to de novo tumors, in both
intratumoral and stromal regions, a significantly lower expression
of programmed death-ligand 1 (PD-L1) on tumor cells and immune
cells, and no difference in CD8+ TIL infiltration,
except for the high number of intratumoral CD8+ TILs,
which was considered to be a statistical artifact.
As there are no randomized clinical trials
stratified according to whether patients had previously received
radiation or not, we had no comparative data; lower PD-L1
expression by tumor and immune cells or lower numbers of TILs,
whether in intratumoral or stromal compartments, might have been
expected, as immune checkpoint inhibitors (ICIs) seem to be less
effective for locoregional relapses than for metastatic lesions
(3). Our results confirm the
hypothesis that antitumor treatments modify the microenvironment of
recurrent tumors, especially for radiotherapy. This is why trials
are being conducted with concomitant strategies such as PembroRad
(Clinical Trial NCT02707588), KEYNOTE-412 (Clinical Trial
NCT03040999) and REACH trial (Clinical Trial NCT02999087).
In the irradiated area cohort, we observed a lower
CD3+ TIL count, which may suggest that pretreated tumors
belong to immunologically unresponsive group of tumors, as Yuan
et al described (23).
However, there was a persistent infiltration of CD8+
TILs in tumors from irradiated areas. CD8+ TILs are
cytotoxic T lymphocytes and therefore play a major role in
antigen-specific antitumor immune responses (24). Studies of several malignant tumors
have revealed that the frequency of CD8+ and
CD3+ TILs has a prognostic value (25–28).
Although we observed slightly lower densities of CD8+
TILs in the irradiated area cohort than in the de novo
cohort, previous radiation therapy or chemoradiation did not
significantly affect their presence.
This therefore highlights that irradiated tissues
retain the ability for antitumor action, as the main driver leading
to immune response is the preexistence of antitumor cytotoxic T
cells that were specifically hampered by immune checkpoints
(8). It may corroborate previous
observations that radiotherapy has the capacity to recruit
CD8+ TILs that are specific to tumor antigens and to
increase the expression of PD-L1 (29).
Moreover, it constitutes a strong rationale for the
use of ICI treatment for tumors occurring in previously treated
areas, in order to activate cytotoxic antitumor immune responses.
This is being assessed in the ADJORL1 trial (Clinical Trial
NCT03406247), which is investigating the use of immunotherapy after
salvage surgery for previously treated tumors.
CD3+ TILs are important, as CD3 is a T
cell co-receptor that aids activation of both cytotoxic cells and T
helper cells. The persistent rate of cytotoxic CD8+ TILs
and the reduction in CD3+ TILs could reflect a decrease
in other classes of T cells, such as helper T cells or regulatory T
cells.
Our results do not explain why locoregional
recurrences can be the center of hyperprogression when using ICIs
and may suggest that other mechanisms are likely to be involved. To
date, we do not yet understand why the rate of hyperprogression,
which can range from 9 to 29%, is greater in locoregional
recurrences compared to distant metastases; prospective trials
should investigate this further (15,16,30).
Significantly fewer tumors in the irradiated area
cohort expressed PD-L1 on tumor cells than in the de novo
cohort, but more than half (56%) were positive according to our
cut-off of ≥1%. This reduction compared to de novo tumors
may provide first indicators of why these tumors are less
responsive to immunotherapy and lend weight to the argument of
introducing ICIs earlier in therapeutic strategies for HNSCC,
associated or not with cytotoxic agents or radiation. This was the
rationale behind the KEYNOTE-048 phase III randomized trial, which
showed a significantly longer overall survival in the frontline
setting with anti-PD-1 pembrolizumab monotherapy vs. the EXTREME
regimen [12.3 months vs. 10.3 months for the PD-L1 combined
positive score (CPS) ≥1 population and 14.9 months vs. 10.7 months
for the PD-L1 CPS ≥20 population] (14). The pembrolizumab with chemotherapy
arm also showed a significantly longer overall survival in the
frontline setting vs. the EXTREME regimen (13.6 months vs. 10.4
months for the PD-L1 CPS ≥1 population and 14.7 months vs. 11.0
months for the PD-L1 CPS ≥20 population) (14).
The PembroRad phase II randomized trial (Clinical
Trial NCT02707588) is evaluating pembrolizumab combined with
radiotherapy vs. cetuximab combined with radiotherapy in locally
advanced HNSCC (LA HNSCC), and preliminary data seem to indicate a
good tolerance (31). Other trials
are assessing combination strategies with ICIs, such as the REACH
trial, assessing the anti-PD-L1 antibody avelumab combined with
cetuximab and radiotherapy (Clinical Trial NCT02999087), the
KEYNOTE-412 trial, assessing pembrolizumab combined with
chemoradiation (Clinical Trial NCT03040999) in LA HNSCC, or the
JAVELIN trial, assessing avelumab in combination with
chemoradiation in LA HNSCC (Clinical Trial NCT02952586).
Interestingly, our study indicates that patients
with tumors in previously irradiated areas could be the best
candidates to receive the addition of anti-CTLA4 to anti-PD-1/PD-L1
agents, as we found a significantly lower expression of PD-L1 on
tumor cells in these tumors. Indeed, anti-CTLA4 agents seem to
recruit CD8+ TILs and their combination with
anti-PD-1/anti-PD-L1 agents has a strong rationale for the
induction of an antitumor immune response, especially when
expression of PD-L1 is low. Trials assessing the combination of
anti-CTLA4 and anti-PD-1/PD-L1 agents are ongoing, including the
KESTREL phase III randomized trial (Clinical Trial NCT02551159) and
the CheckMate 651 phase III randomized trial (Clinical Trial
NCT02741570). However, CheckMate 714 phase II trial results were
negative, suggesting that a combination of chemotherapy and
immunotherapy could be of more interest than combining
immunotherapies (Clinical Trial NCT02823574).
Various tumor proportion score (TPS) thresholds are
used in different studies of HNSCC, with the cut-off ranging from 1
to 50%, illustrating that debate exists around choosing a threshold
(3,7,28,32).
We analyzed data from both intratumoral and stromal regions; there
is currently no clear consensus on whether PD-L1 expression on all
cells or specific cell populations should be analyzed, and which
emergent scores should be used (6).
CPS is one of these emergent scores, which takes into account the
ratio of PD-L1 expressing cells to the total number of tumor cells
and was able to predict response to pembrolizumab (7,14). An
elevated pretreatment neutrophil-to-lymphocyte ratio can also
predict poor prognosis with local invasion and distant metastases
(33).
Immune phenotype
The predominant tumor microenvironment type in the
irradiated area cohort was adaptive immune resistance (type I).
Yang et al noted that HNSCC had typically diverse profiles
of TILs, dividing tumors into two main categories: Inflamed or
non-inflamed (24) Lei et al
suggested that an immunoscore based on TIL phenotype may be helpful
in better classifying patients (34). This study shows that tumors in
irradiated areas can be still inflamed and links to ideas from Teng
et al, who suggested that anti-PD-1/PD-L1 agents may be most
effective in tumors with type I/inflamed microenvironments, because
of preexisting intratumoral T cells (21). The persistent infiltration of
CD8+ TILs shown in this study is encouraging for this
entity of tumors occurring in patients who have previously received
radiation, since CD8+ TILs have recently been presented
as a promising favorable prognostic biomarker in HNSCC after
adjuvant chemoradiation (28).
However, given the known poor prognosis of patients with these
tumors, CD8+ TILs may be dysfunctional T cells, as
Psyrri et al suggested when studying the mechanisms of
resistance to nivolumab (35).
Prognostic factors in the de novo cohort were
advanced stage and site of disease; our results are consistent with
previous findings that tumors in the hypopharynx have a poor
prognosis (36,37). Expression of PD-L1 on immune cells
tended to be a PFS prognostic factor. In the irradiated area
cohort, no relevant prognostic factor was highlighted, which could
be explained by the initial poor prognosis. Indeed, we found the
same tumor characteristics that have previously been shown to
confer a poor prognosis for patients with locoregional relapses:
Poor differentiation, high pN stage, positive resection margins,
and lymphovascular or perineural invasion (Table I).
Yang et al performed a meta-analysis of
studies that assessed the prognostic role of PD-L1 expression in
HNSCC (24). They concluded that
PD-L1 could not be recommended as a prognostic factor in HNSCC, nor
could it be used to stratify the risk in HPV-related HNSCC.
While PD-L1 has mainly been described as a
prognostic factor when expressed on tumor cells (PD-L1+
tumor cells), the importance of immune cells (PD-L1+
immune cells) is beginning to be revealed. Indeed, Kim et al
depicted PD-L1+ immune cells as a favorable prognostic
factor in HNSCC with a positivity cut-off of 5% (38). The two-year follow-up data from
CheckMate 141 described an OS benefit with nivolumab irrespective
of PD-L1 expression (39),
suggesting that PD-L1-negative patients may benefit from ICI
treatment because of PD-L1 expression by stromal cells. Kim et
al also found that PD-L1 expressed by immune cells was a
favorable prognostic factor in HNSCC (38) and some trials have started to use
PD-L1 expression as a stratification factor, such as the
IMpassion130 trial in triple-negative breast cancer (40).
The limitations of this study include the bias
typical of retrospective studies and the fact that patients had not
received ICI treatment, as clinical trials using immunotherapy were
ongoing.
In conclusion, our results show the persistence of
cytotoxic cells but lower expression of PD-L1 and fewer
CD3+ TILs in tumors in irradiated areas. This study
provides first potential explanations of the fact that these
lesions are less responsive to immunotherapy, although they may
still retain antitumor capacities. The assessment of immune
response biomarkers in patients treated with immunotherapy in
randomized trials is required to decipher the molecular mechanisms
involved in acquired resistance to treatments.
Supplementary Material
Supporting Data
Acknowledgements
We would like to thank the english language editing
service.
Funding
Funding for this research project was provided by
the Lorraine Institute of Oncology.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
All authors contributed equally to this research
study. CP, JS, XSG and LG conceived and designed the experiments.
CP, JT, JCF and GD made substantial contributions to acquisition of
the data. XSG performed the histological examination. CP, JS, XSG
and LG analyzed and interpreted the patient data. CB was involved
in revising the manuscript critically. All authors were involved in
the writing of the manuscript and approved the final
manuscript.
Ethics approval and consent to
participate
All patients were managed in the Lorraine Institute
of Oncology according to the standards of good clinical practice.
This retrospective study was approved by the local institutional
review board and has been declared to Commission for information
technology and civil liberties (‘CNIL’), registered as a standard
declaration by CNIL correspondent of the Institute. All patients
were informed of the research performed with tumor tissue specimens
and gave their consent. Ethical approval was not necessary for this
retrospective study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
HPV
|
human papillomavirus
|
|
ICIs
|
immune checkpoint inhibitors
|
|
PD-L1
|
programmed death-ligand 1
|
|
TILs
|
tumor-infiltrating lymphocytes
|
|
TPS
|
tumor proportion score
|
References
|
1
|
Janot F, de Raucourt D, Benhamou E, Ferron
C, Dolivet G, Bensadoun RJ, Hamoir M, Géry B, Julieron M, Castaing
M, et al: Randomized trial of postoperative reirradiation combined
with chemotherapy after salvage surgery compared with salvage
surgery alone in head and neck carcinoma. J Clin Oncol.
26:5518–5523. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tao Y, Faivre L, Laprie A, Boisselier P,
Ferron C, Jung GM, Racadot S, Gery B, Even C, Breuskin I, et al:
Randomized trial comparing two methods of re-irradiation after
salvage surgery in head and neck squamous cell carcinoma: Once
daily split-course radiotherapy with concomitant chemotherapy or
twice daily radiotherapy with cetuximab. Radiother Oncol.
128:467–471. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ho AS, Kraus DH, Ganly I, Lee NY, Shah JP
and Morris LG: Decision making in the management of recurrent head
and neck cancer. Head Neck. 36:144–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oliva M, Spreafico A, Taberna M, Alemany
L, Coburn B, Mesia R and Siu LL: Immune biomarkers of response to
immune-checkpoint inhibitors in head and neck squamous cell
carcinoma. Ann Oncol. 30:57–67. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cohen EEW, Soulières D, Le Tourneau C,
Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R,
et al: Pembrolizumab versus methotrexate, docetaxel, or cetuximab
for recurrent or metastatic head-and-neck squamous cell carcinoma
(KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet.
393:156–167. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ribas A and Wolchok JD: Cancer
immunotherapy using checkpoint blockade. Science. 359:1350–1355.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Phulpin B, Dolivet G, Marie PY, Poussier
S, Gallet P, Leroux A, Graff P, Groubach F, Bravetti P, Merlin JL
and Tran N: Re-assessment of chronic radio-induced tissue damage in
a rat hindlimb model. Exp Ther Med. 1:553–560. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gallet P, Phulpin B, Merlin JL, Leroux A,
Bravetti P, Mecellem H, Tran N and Dolivet G: Long-term alterations
of cytokines and growth factors expression in irradiated tissues
and relation with histological severity scoring. PLoS One.
6:e293992011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeng J, See AP, Phallen J, Jackson CM,
Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E,
et al: Anti-PD-1 blockade and stereotactic radiation produce
long-term survival in mice with intracranial gliomas. Int J Radiat
Oncol Biol Phys. 86:343–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng L, Liang H, Burnette B, Beckett M,
Darga T, Weichselbaum RR and Fu YX: Irradiation and anti-PD-L1
treatment synergistically promote antitumor immunity in mice. J
Clin Invest. 124:687–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Formenti SC, Rudqvist NP, Golden E, Cooper
B, Wennerberg E, Lhuillier C, Vanpouille-Box C, Friedman K, Ferrari
de Andrade L, Wucherpfennig KW, et al: Radiotherapy induces
responses of lung cancer to CTLA-4 blockade. Nat Med. 24:1845–1851.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burtness B, Harrington KJ, Greil R,
Soulières D, Tahara M, de Castro G Jr, Psyrri A, Basté N, Neupane
P, Bratland Å, et al: Pembrolizumab alone or with chemotherapy
versus cetuximab with chemotherapy for recurrent or metastatic
squamous cell carcinoma of the head and neck (KEYNOTE-048): A
randomised, open-label, phase 3 study. Lancet. 394:1915–1928. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saâda-Bouzid E, Defaucheux C, Karabajakian
A, Coloma VP, Servois V, Paoletti X, Even C, Fayette J, Guigay J,
Loirat D, et al: Hyperprogression during anti-PD-1/PD-L1 therapy in
patients with recurrent and/or metastatic head and neck squamous
cell carcinoma. Ann Oncol. 28:1605–1611. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Champiat S, Ferrara R, Massard C, Besse B,
Marabelle A, Soria JC and Ferté C: Hyperprogressive disease:
Recognizing a novel pattern to improve patient management. Nat Rev
Clin Oncol. 15:748–762. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smith J, Robida MD, Acosta K, Vennapusa B,
Mistry A, Martin G, Yates A and Hnatyszyn HJ: Quantitative and
qualitative characterization of Two PD-L1 clones: SP263 and E1L3N.
Diagn Pathol. 11:442016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fakhry C, Lacchetti C, Rooper LM, Jordan
RC, Rischin D, Sturgis EM, Bell D, Lingen MW, Harichand-Herdt S,
Thibo J, et al: Human papillomavirus testing in head and neck
carcinomas: ASCO clinical practice guideline endorsement of the
college of American pathologists guideline. J Clin Oncol.
36:3152–3161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ono T, Azuma K, Kawahara A, Sasada T,
Hattori S, Sato F, Shin B, Chitose SI, Akiba J and Hirohito U:
Association between PD-L1 expression combined with
tumor-infiltrating lymphocytes and the prognosis of patients with
advanced hypopharyngeal squamous cell carcinoma. Oncotarget.
8:92699–92714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Solomon B, Young RJ, Bressel M, Urban D,
Hendry S, Thai A, Angel C, Haddad A, Kowanetz M, Fua T, et al:
Prognostic significance of PD-L1+ and CD8+
Immune cells in HPV+ oropharyngeal squamous cell
carcinoma. Cancer Immunol Res. 6:295–304. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teng MW, Ngiow SF, Ribas A and Smyth MJ:
Classifying cancers based on T-cell infiltration and PD-L1. Cancer
Res. 75:2139–2145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rosenbaum PR: Optimal matching for
observational studies. J Am Stat Assoc. 84:1024–1032. 1989.
View Article : Google Scholar
|
|
23
|
Yuan J, Hegde PS, Clynes R, Foukas PG,
Harari A, Kleen TO, Kvistborg P, Maccalli C, Maecker HT, Page DB,
et al: Novel technologies and emerging biomarkers for personalized
cancer immunotherapy. J Immunother Cancer. 4:32016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang WF, Wong MCM, Thomson PJ, Li KY and
Su YX: The prognostic role of PD-L1 expression for survival in head
and neck squamous cell carcinoma: A systematic review and
meta-analysis. Oral Oncol. 86:81–90. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gooden MJM, de Bock GH, Leffers N, Daemen
T and Nijman HW: The prognostic influence of tumour-infiltrating
lymphocytes in cancer: A systematic review with meta-analysis. Br J
Cancer. 105:93–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thomas NE, Busam KJ, From L, Kricker A,
Armstrong BK, Anton-Culver H, Gruber SB, Gallagher RP, Zanetti R,
Rosso S, et al: Tumor-infiltrating lymphocyte grade in primary
melanomas is independently associated with melanoma-specific
survival in the population-based genes, environment and melanoma
study. J Clin Oncol. 31:4252–4259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Adams S, Gray RJ, Demaria S, Goldstein L,
Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et
al: Prognostic value of tumor-infiltrating lymphocytes in
triple-negative breast cancers from two phase III randomized
adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin
Oncol. 32:2959–2966. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Balermpas P, Rödel F, Krause M, Linge A,
Lohaus F, Baumann M, Tinhofer I, Budach V, Sak A, Stuschke M, et
al: The PD-1/PD-L1 axis and human papilloma virus in patients with
head and neck cancer after adjuvant chemoradiotherapy: A
multicentre study of the German cancer consortium radiation
oncology group (DKTK-ROG). Int J Cancer. 141:594–603. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dovedi SJ, Adlard AL, Lipowska-Bhalla G,
McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M,
Stewart R, et al: Acquired resistance to fractionated radiotherapy
can be overcome by concurrent PD-L1 blockade. Cancer Res.
74:5458–5468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Champiat S, Dercle L, Ammari S, Massard C,
Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A,
Soria JC and Ferté C: Hyperprogressive disease is a new pattern of
progression in cancer patients treated by anti-PD-1/PD-L1. Clin
Cancer Res. 23:1920–1928. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun XS, Sire C, Tao Y, Martin L, Alfonsi
M, Prevost JB, Rives M, Lafond C, Tourani JM, Biau, et al: A phase
II randomized trial of pembrolizumab versus cetuximab, concomitant
with radiotherapy (RT) in locally advanced (LA) squamous cell
carcinoma of the head and neck (SCCHN): First results of the GORTEC
2015-01 ‘PembroRad’ trial. J Clin Oncol. 36 (15 Suppl):S60182018.
View Article : Google Scholar
|
|
32
|
Zandberg DP, Algazi AP, Jimeno A, Good JS,
Fayette J, Bouganim N, Ready NE, Clement PM, Even C, Jang RW, et
al: Durvalumab for recurrent or metastatic head and neck squamous
cell carcinoma: Results from a single-arm, phase II study in
patients with ≥25% tumour cell PD-L1 expression who have progressed
on platinum-based chemotherapy. Eur J Cancer. 107:142–152. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu Y, Wang H, Yan A, Wang H, Li X, Liu J
and Li W: Pretreatment neutrophil to lymphocyte ratio in
determining the prognosis of head and neck cancer: A meta-analysis.
BMC Cancer. 18:3832018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lei Y, Xie Y, Tan YS, Prince ME, Moyer JS,
Nör J and Wolf GT: Telltale tumor infiltrating lymphocytes (TIL) in
oral, head & neck cancer. Oral Oncol. 61:159–165. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Psyrri A, Gavrielatou N, Spathis A,
Anastasiou M, Fortis E, Gkotzamanidou M, Kladi-Skandali A, Kousidou
E, Economopoulou P, Kotsantis I, et al: Predictive biomarkers for
response to nivolumab in head and neck squamous cell carcinoma
(HNSCC) (NCT#03652142). J Clin Oncol. 37 (Suppl 15):S60602019.
View Article : Google Scholar
|
|
36
|
Mehanna H, West CM, Nutting C and Paleri
V: Head and neck cancer-Part 2: Treatment and prognostic factors.
BMJ. 341:c46902010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cadoni G, Giraldi L, Petrelli L,
Pandolfini M, Giuliani M, Paludetti G, Pastorino R, Leoncini E,
Arzani D, Almadori G and Boccia S: Prognostic factors in head and
neck cancer: A 10-year retrospective analysis in a
single-institution in Italy. Acta Otorhinolaryngol Ital.
37:458–466. 2017.PubMed/NCBI
|
|
38
|
Kim HR, Ha SJ, Hong MH, Heo SJ, Koh YW,
Choi EC, Kim EK, Pyo KH, Jung I, Seo D, et al: PD-L1 expression on
immune cells, but not on tumor cells, is a favorable prognostic
factor for head and neck cancer patients. Sci Rep. 6:369562016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington KJ, Kasper S, Vokes EE,
Even C, et al: Nivolumab vs. investigator's choice in recurrent or
metastatic squamous cell carcinoma of the head and neck: 2-year
long-term survival update of CheckMate 141 with analyses by tumor
PD-L1 expression. Oral Oncol. 81:45–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, et al:
Atezolizumab and nab-paclitaxel in advanced triple-negative breast
cancer. N Engl J Med. 379:2108–2121. 2018. View Article : Google Scholar : PubMed/NCBI
|