Introduction
For many years, chemotherapy has been one of the
most frequently chosen types of anticancer treatment. However, its
cure rate still remains unsatisfactory and additionally severe side
effects are observed (1,2). Therefore, the development of effective
anticancer therapy is still a challenge, mainly due to the complex
nature of tumors (3). During cancer
growth, a specific niche-the tumor microenvironment (TME)-is
created, consisting mainly of proliferating tumor cells,
extracellular matrix, stromal cells and infiltrating immune cells
(3–6). Among the latter, high influx of
regulatory T cells (Tregs), tumor-associated macrophages (TAMs) and
myeloid derived suppressor cells (MDSCs), which promote tumor
progression and suppress the antitumor immune response, are
observed (5–7). On the other hand, immune cells, such
as effector T cells, natural killer (NK) cells, M1-type macrophages
and dendritic cells (DCs) infiltrating the tumor, can be activated
in situ in order to inhibit tumor growth and prevent immune
evasion and expansion of the disease (5,7).
Increasing evidence indicates that the fate of tumor progression is
highly correlated with the specific TME, whose composition is a
predominant factor in prognosis and efficacy of chemotherapy
(3).
DCs as professional antigen-presenting cells are
potent inducers of a T cell response, and are considered an
essential component of antitumor immunity (8). However, despite high potential in
promoting the antitumor response, the proper function of DCs
present in the TME may be impaired, mainly due to the abundance of
immunosuppressive factors and aforementioned cells with suppressor
activity. DCs under the influence of a hostile tumor milieu become
ineffective in their differentiation and activation, and in turn
are weak stimulators of immune responses (8,9). For
this reason, great efforts are made to design therapeutic
strategies able to overcome the negative impact of TME on
endogenous DCs. One of the strategies is ex vivo generation
and maturation of DCs for their administration as cellular-based
antitumor vaccines (10–12). Other strategies involve, not only
developing new chemotherapeutics or innovative solutions for
targeted drug delivery, but also combining different types of
anticancer therapy-for example chemotherapy and immunotherapy with
DC-based cellular vaccines. This latter strategy has several
immune-potentiating effects, such as increasing the susceptibility
of tumor cells to the activity of cytotoxic T lymphocytes (CTLs).
Furthermore, by depleting certain population of immune cells, e.g.
MDSCs or Tregs, chemotherapy creates a cytokine milieu for optimal
expansion of effector cells and facilitates the generation of a
specific antitumor immune response by DCs (13). However, major problems in anticancer
chemotherapy with low-molecular weight compounds are their fast
metabolism and excretion from an organism, as well as unfavorable
biodistribution and low specificity (14). To overcome these disadvantages, many
different drug delivery systems, including micelles (15,16),
dendrimers (17,18), nanocapsules (19) and nanoconjugates with a
macromolecular carrier (14,20)
have been developed. The nanoconjugates were designed to enhance
delivery and to improve the selectivity and pharmacological
properties of both conventional and innovative drugs (14). One of these innovative formulations
is a nanoconjugate of hydroxyethyl starch (HES) and methotrexate
(MTX). The HES-MTX nanoconjugate was obtained by covalent coupling
of well-known therapeutic compounds-methotrexate as an anticancer
agent and hydroxyethyl starch as a high-molecular carrier (20,21).
MTX is one of the oldest antifolate drugs widely used in the
treatment of autoimmune disorders as well as in anticancer
therapy-in solid tumors and hematologic malignancies (22). However, therapy with MTX is often
associated with severe systemic toxicity, bone marrow suppression,
and drug resistance (15,23,24).
HES is an amylopectin-based modified polymer used as colloidal
plasma volume expanders (20). The
structural similarity of amylopectin to glycogen ensures lack of
immunogenicity (25). Moreover,
unfavorable accumulation in the liver or spleen was not observed
(26). The mean hydrodynamic
diameter of the HES-MTX nanoconjugate is 15.2±6.2 nm, thus HES-MTX
meets the criterion for inclusion in nanoparticles (20). The main advantage of the HES-MTX
nanoconjugate over MTX in free form is the prolonged half-time in
plasma and specific biodistribution. Methotrexate enters cells via
folate receptors (FRs) overexpressed on cancer cells or through the
ubiquitously expressed reduced folate carriers (RFCs), to which MTX
has a low and high affinity, respectively (27). However, when multiple MTX molecules
are covalently conjugated to a macromolecular carrier (e.g. HES),
transport through RFCs does not occur. This is possible due to
acquisition of the polyvalence feature as a consequence of the
conjugation process (17,18,28,29).
We postulate that the internalization of HES-MTX nanoconjugate in
tumors is achieved mainly by its interaction with FRα, or through
an enhanced vascular permeability and retention effect (EPR). EPR
is related to the capacity of macromolecules larger than 40 kDa
(hydrodynamic diameter above 10 nm) for selective leakage from
tumor vessels and accumulation in tumor tissue (2,29–32).
Complete physicochemical characteristics of the novel form of
HES-MTX nanoconjugate as well as its antitumor activity in murine
P388 leukemia and human MV-4-11 leukemia models have been described
(20).
Recently considerable attention has been focused on
the immunomodulatory properties of certain chemotherapeutic agents,
including MTX (1,19), which can act not only as modulators
of immune cell phenotype (33–35),
but also through stimulation of effector immune cells and
elimination of Tregs from the TME (36–40).
Utilization of the HES-MTX nanoconjugate in a murine MC38 colon
carcinoma model and supplementing such anticancer therapy with
DC-based immunotherapy, as well determination of its
immunomodulatory effect on generation of an antitumor immune
response, has not been investigated to date. For this reason, the
main objective of our study was to determine the modulation of the
immune response after HES-MTX administration to tumor-bearing mice
and how those changes affect the activity of DC-based vaccines
injected after chemotherapy. The gathered data indicate that
chemotherapy with HES-MTX applied in treatment of mice with a
subcutaneously growing MC38 tumor affected, more strongly than MTX,
the TME by increasing the influx of CTLs and NK cells and
eliminating certain cells with suppressor activity. In addition,
the enlargement of T-helper (Th), CTL and natural killer T (NKT)
cell percentages among splenic leukocytes accompanied by a decrease
in Tregs was found. Moreover, therapy with HES-MTX resulted in
increased cytotoxic activity of splenic lymphocytes. All these
factors led to the creation of a favorable niche necessary to
promote the development of an efficient antitumor immune response
by DCs used in immunotherapy. The combined therapy with HES-MTX and
DC-based cellular vaccines contributed to enhanced influx of
effector lymphocytes into tumor tissue and reduced infiltration of
immune cells with suppressor activity. The above changes together
with activation of systemic specific antitumor response resulted in
a significant delay in tumor growth.
Materials and methods
Mice
Female C57BL/6 mice (total number of animals, 55
mice; initial weight, 20–22 g) were obtained from the Center of
Experimental Medicine of the Medical University of Białystok
(Białystok, Poland). Mice were kept in a room with a standard
light/dark cycle, with a constant temperature (22±2°C), air
humidity (55±10%) and access to food and water ad libitum.
All experiments were performed in accordance with EU Directive
2010/63/EU for animal experiments and were approved by the 1st
Local Ethics Committee for Experiments with the Use of Laboratory
Animals, Wrocław, Poland (authorization no. 31/2016). After the
experiments, mice were sacrificed by cervical dislocation.
Cell culture
The in vivo growing MC38 murine colon
carcinoma from the Tumor Bank of the TNA Radiobiology Institute
(Rijswijk, The Netherlands) was adapted to in vitro
conditions as described by Pajtasz-Piasecka et al (41). The culture of MC38/0 (named here
MC38) cells was maintained in RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 100 U/ml penicillin, 100 mg/ml
streptomycin, 0.5% sodium pyruvate, 0.05 mM 2-mercaptoethanol and
5% fetal bovine serum (FBS; all reagents from Sigma-Aldrich; Merck
KGaA). Tumor antigen (TAg) was prepared by repeated freezing and
thawing of an MC38 cell suspension (5×106 MC38
cells/ml), which was followed by sonication. DCs for in vivo
experiments were generated from bone marrow isolated from femurs
and tibias of healthy C57BL/6 mice according to the protocol
described in our previous publication (42). The cells (named here DCs) were
cultured in RPMI supplemented with 10% FBS in the presence of
recombinant murine (rm)GM-CSF (ImmunoTools, 40 ng/ml) and rmIL-4
(ImmunoTools, 10 ng/ml). After 6 days the loosely attached immature
DCs were stimulated with tumor antigens (10% v/v) and applied to
mice as antitumor vaccines.
Nanoconjugate preparation
FITC-HES was synthesized using a modification of
methods previously described (43).
Briefly, FITC-HES was prepared by the addition of 30 mg of
fluorescein isothiocyanate (FITC, isomer I, Sigma-Aldrich; Merck
KGaA) dissolved in 5 ml of DMSO to a solution that contained
hydroxyethyl starch (1.2 g in 20 ml of solution containing 150 mM
NaCl and 50 mM Na2CO3). This mixture was
stirred for 48 h at room temperature (RT). Next, the mixture was
cooled down to 4°C and precipitated with cold acetone (100 ml). The
crude product was solubilized in water and dialyzed against
ultrapure water for 5 h at a flow rate of 30 ml/min (Pellicon XL
with Ultracel-10 PLCGC membrane, Millipore). Finally, the conjugate
of FITC and HES containing 3.0×10−3 covalently bound
FITC residues per anhydroglucose unit was obtained.
HES-MTX nanoconjugate and FITC-HES-MTX were
synthesized using HES 130/0.4 (Voluven, Fresenius Kabi) or FITC-HES
and activated MTX (EBEWE Pharma) according to previously described
methods (20,44). The following absorption coefficients
were used: 8,571 M−1 cm−1 (372 nm), 70,000
M−1 cm−1 (494 nm) for MTX and FITC,
respectively. Eventually, the following conjugates were obtained:
HES-MTX containing 52×10−3 covalently bound MTX residues
per anhydroglucose unit and FITC-HES-MTX containing
53×10−3 MTX and 2.9×10−3 FITC residues per
anhydroglucose unit. In this study, the presented MTX concentration
referring to the HES-MTX conjugate was based on the total contents
of the covalently bound MTX in conjugate. The analysis and
characterization of conjugates were performed using a combination
of spectrophotometric, chromatographic and light scattering methods
based on previously published procedures (20,45).
Hydrodynamic parameters of HES and HES-MTX were characterized by
dynamic light scattering (DLS). The sample solution was illuminated
by a 633-nm laser, and the light intensity scattered at an angle of
173° was measured. At least six consecutive measurements of each
sample were carried out. All samples were measured at 25°C using a
Zetasizer Nano ZS (Malvern Instruments, UK) in a 12-µl quartz
cuvette (size measurement) and folded capillary cells (zeta
potential). HES and HES-MTX conjugate concentration was 5.5 mM
(AGU). DLS data were analyzed using the dts 6.10 software (Malvern
Instruments, UK). The intensity particle size distributions were
obtained using the General Purpose algorithm included in the DTS
software.
MTT assay
To calculate the half maximal inhibitory
concentration (IC50) value, MTT assays were performed.
The MC38 cells were placed in 96-well plates (0.005×106
cells/well) and after 24 h MTX or HES-MTX in various concentrations
was added (in the range from 0.001 to 1,000 ng/ml) and incubated
for 72 h. After this time, MTT dye
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; 5
mg/ml) was added for 4 h. Next, cells were lysed overnight in lysis
buffer (N,N-dimethylmethanamide, sodium dodecyl sulfate and water).
Absorbance at 570 nm was determined using a Thermo Labsystems
Multiskan RC microplate reader (Thermo Fisher Scientific, Inc.)
with Genesis Lite 3.05 Software (Thermo Life Sciences) and the
IC50 value was calculated.
Interaction of nanoconjugate with MC38
cells and DCs
The interaction of FITC-conjugated compounds
(FITC-HES-MTX and FITC-HES) with MC38 cells and DCs was evaluated
by flow cytometry. The MC38 cells were placed in 24-well plates
(0.2×106 cells/well), immature DCs were placed in
12-well plates (0.5×106 cells/well) and after 24 h
FITC-HES-MTX or FITC-HES (10 µg/ml) was added and incubated for the
next 24 h. After this time, cells were harvested and washed, and
dead cells were stained with DAPI dye. The analysis was performed
using FACS Fortessa with Diva software (Becton Dickinson).
Determination of FRα expression
The expression of FRα was measured by real-time PCR.
Total RNA was isolated using a NucleoSpin RNA kit (Macherey-Nagel)
and reverse-transcribed with a First Strand cDNA Synthesis Kit
(Thermo Fisher Scientific, Inc.). Real-time PCR was performed using
TaqMan Universal PCR Master Mix and TaqMan Gene Expression Assay
primers for FRα in reference to the HPRT gene. The analyses were
performed using the ViiA7 Real-Time PCR System (Applied
Biosystem).
Surface plasmon resonance
spectroscopy
Surface plasmon resonance (SPR) experiments were
conducted in a Biacore T200 instrument (GE Healthcare Life
Sciences). During measurements, the flow buffer HBS-N was used (10
mM HEPES, pH 7.4 with 150 mM NaCl). Immobilization of bovine folate
binding protein (FBP, Sigma-Aldrich) was carried out at 25°C by an
EDC-based amide coupling method using standard Biacore reagents (GE
Healthcare Life Sciences). FBP-presenting chips (CM5) were prepared
with protein density at 9.03 FBP ng/mm2. SPR experiments
were performed by injection of a ligand solution, HES or HES-MTX
nanoconjugate, each prepared in HBS-N buffer (concentrations were
presented as MTX-equiv), at a flow rate of 40 µl/min. The
conjugates were injected over a reference channel and over a
channel with immobilized FBP for 200 sec. Each analysis cycle
consisted of a 60 sec initial period, in which the stability of the
baseline was monitored. The injection of buffer was performed
between each analysis cycle for a double reference. At the end of
each dissociation phase, the chip surface was treated with 10 µl of
10 mM glycine-HCl (pH 2.5) for surface regeneration. Sensorgrams
for the reference channel were subtracted from sensorgrams for the
channel with FBP. Subsequently, sensorgrams of buffer were
subtracted from sensorgrams of the HES-MTX conjugates.
Annexin V binding assay
To evaluate apoptosis in MC38 cells after 72 h
incubation with MTX or HES-MTX the Annexin V binding assay was
performed. Briefly, the MC38 cells were placed in 24-well plates
(0.1×106 cells/well) and after 24 h MTX or HES-MTX was
added (500 ng/ml). Next, harvested cells were suspended in binding
buffer and stained with Annexin V protein conjugated with APC
fluorochrome (Thermo Fisher Scientific, Inc.) (15 min, RT). To
determine the percentage of dead cells, propidium iodide (PI) was
applied (10 µg/ml, Thermo Fisher Scientific, Inc.) and the
percentage of Annexin V+ MC38 cells was analyzed using
FACSCalibur with CellQuest 3.3 Software (Becton Dickinson).
Modulation of maturation and phenotype
of DCs generated in the presence of metabolites released by MC38
cells after MTX or HES-MTX treatment
The conditioned medium (CM) necessary to assess
modulation of the DC phenotype generated in the presence of
metabolites of tumor cells treated with MTX or HES-MTX was freshly
prepared before each test. For this purpose, the MC38 cells were
placed in 6-well plates (1.15×106 cells/well) and 24 h
later MTX or HES-MTX was added (500 ng/ml). Additionally, culture
medium containing MTX or HES-MTX without any cells was also
prepared. After 72 h of incubation, CM from the treated MC38 cells
was collected, cellular debris was removed by centrifugation (15
min, 2,000 × g) and the obtained CM was used in differentiating
culture of DCs from bone marrow. Medium containing MTX or HES-MTX
maintained without cells was prepared according to the same
procedure. Bone marrow cells (0.5×106 cells/well,
12-well plates) were suspended in a mixture of culture medium
(including cytokines necessary for DC generation) and conditioned
medium (or medium with MTX or HES-MTX) in a 50:50 ratio. After the
first 48 h of DC generation, the mixture of culture medium and CM
or medium containing MTX or HES-MTX without cells was replaced with
RPMI supplemented with 10% FBS, 40 ng/ml rmGM-CSF and 10 ng/ml
rmIL-4. Further DC culture was conducted according to the protocol
in our previous publication (42).
After 6 days, the loosely attached immature DCs were collected and
then stimulated for 24 h with tumor antigens as described above.
The phenotype of mature DCs was analyzed. For this purpose, DCs
were stained with anti-CD11c BV650, anti-MHC II FITC, anti-CD40 PE,
anti-CD80 APC (all from BioLegend) and anti-CD11c BV650 (BioLegend)
with anti-CD86 PE (BD Biosciences). The analysis was performed
using FACS Fortessa with Diva software (Becton Dickinson).
Therapeutic treatment schedule
Eight-to 10-week old female C57BL/6 mice (45 mice)
were subcutaneously (s.c.) inoculated in the right flank with MC38
cells (1.1×106 cells/0.2 ml NaCl 0.9%/mouse). The mice
were treated according to the schemes presented in Figs. 3A and 5A. In the course of the chemotherapeutic
treatment scheme (results presented in Figs. 3 and 4), on the 14th day of the experiment, mice
received intravenously (in tail vein, intravenously; i.v.) MTX or
HES-MTX (20 mg/kg body weight) and three days later (17th day of
experiment) 5 mice from each group were sacrificed and tumor
nodules and spleens were dissected, homogenized and stored in
liquid nitrogen for further analyses. In the chemoimmunotherapeutic
treatment scheme (results presented in Figs. 5 and 6) mice received chemotherapy on the 14th
day of the experiment and on the 17, 24th and 31st day of the
experiment tumor antigen-stimulated DC-based vaccines were applied
peritumorally (p.t.) (DC/TAg, 2×106 cells/0.2 ml NaCl
0.9%/mouse/p.t. injection). In the group of mice from non-treated
and chemotherapy-receiving groups (MC38 control, MTX and HES-MTX)
tumor nodules and spleens were dissected on the 31st day of the
experiment, and from DC/TAg-receiving groups (DC/TAg, MTX+DC/TAg,
HES-MTX+DC/TAg) tumor nodules and spleens were dissected on the
35th day of the experiment (3–5 mice per group). Then tumors and
spleens were homogenized and stored in liquid nitrogen for further
analyses. The health of the mice was monitored during the
experiments (weight loss, bristling hair, lethargy) and tumors were
measured by using a calliper two times a week. Mice were sacrificed
when the tumor volume was >2 cm3. The procedure of
tumor growth monitoring was presented by Rossowska et al
(42). The therapeutic effect of
the treatment was evaluated using tumor growth inhibition (TGI),
calculated according to the formula: TGI
[%]=100-(TVt⁄TVnt ×100), where
TVt is the median tumor volume in the treated
group of mice and TVnt is the median tumor volume
in the non-treated group of mice.
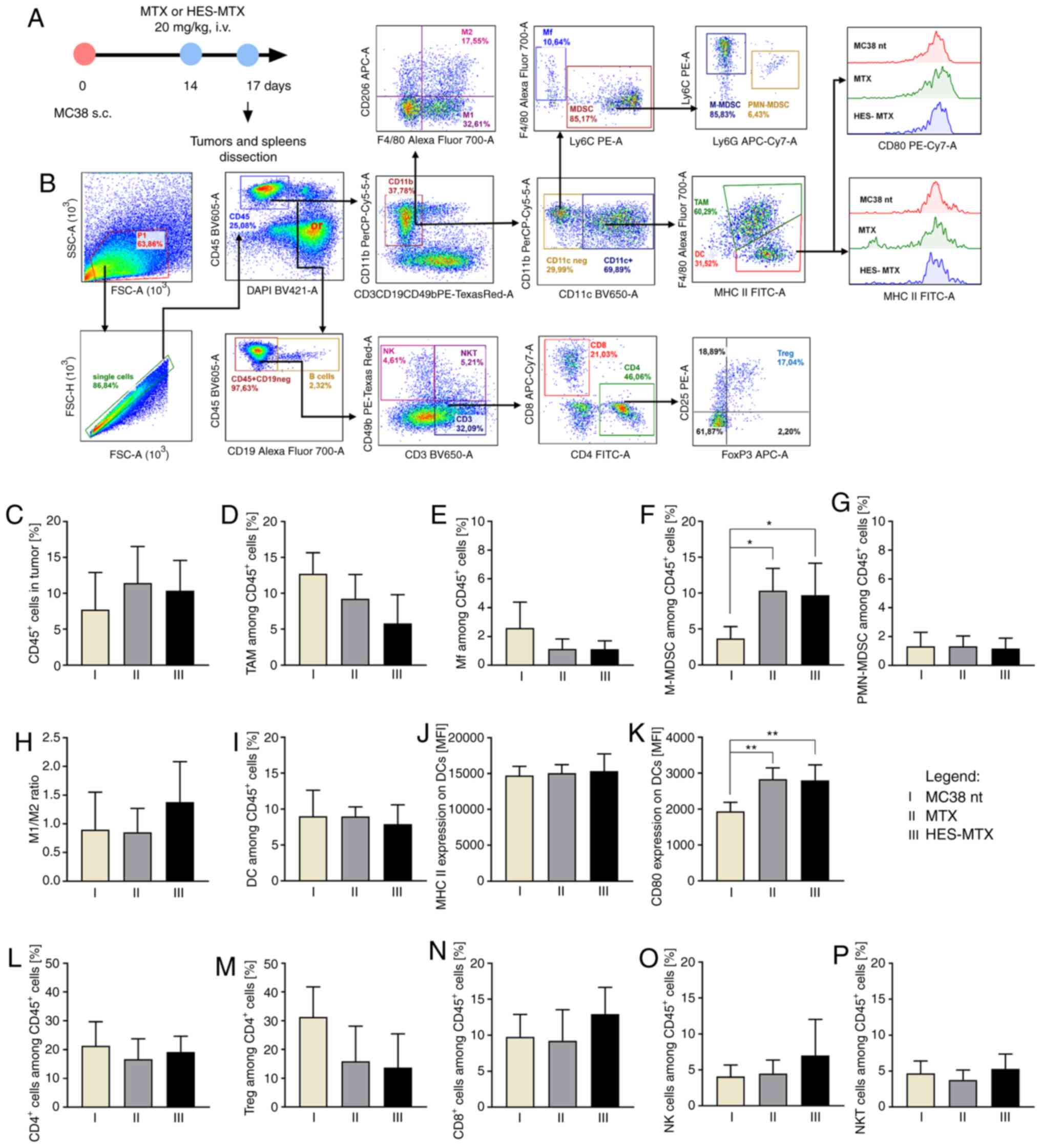 | Figure 3.Impact of chemotherapy on
infiltration of MC38 tumor nodules with immune cells. (A) Scheme of
treatment. (B) Schemes of multiparameter flow cytometry analyses
showing the method of distinguishing myeloid or lymphoid cell
subpopulation in tumors dissected from MC38 tumor-bearing mice
treated according to the scheme presented in A. (C) Percentage of
CD45+ cells in tumor nodules. (D-G) Percentage of
myeloid cell subpopulations among CD45+ cells in tumors.
(H) M1/M2 ratio showing changes in polarization of
tumor-infiltrating macrophages after therapy. (I-K) Percentage of
DCs infiltrating into tumor tissue and expression of MHC II and
CD80 molecules on the surface of DCs. (L-P) Percentage of lymphoid
cell subpopulations among CD45+ cells in tumors. Results
are expressed as mean ± SD (5 mice per group were analyzed from one
experiment). In all presented data the differences between groups
were calculated using the one-way ANOVA followed by Tukey's
multiple comparison post-hoc test (*P<0.05, **P<0.01). HES,
hydroxyethyl starch; MTX, methotrexate; DCs, dendritic cells; TAMs,
tumor-associated macrophages; Mf, macrophages; M-MDSC, monocytic
myeloid derived suppressor cells; PMN-MDSC, polymorphonuclear
myeloid derived suppressor cells; Tregs, regulatory T cells; NK,
natural killer; i.v., intravenously s.c., subcutaneously. |
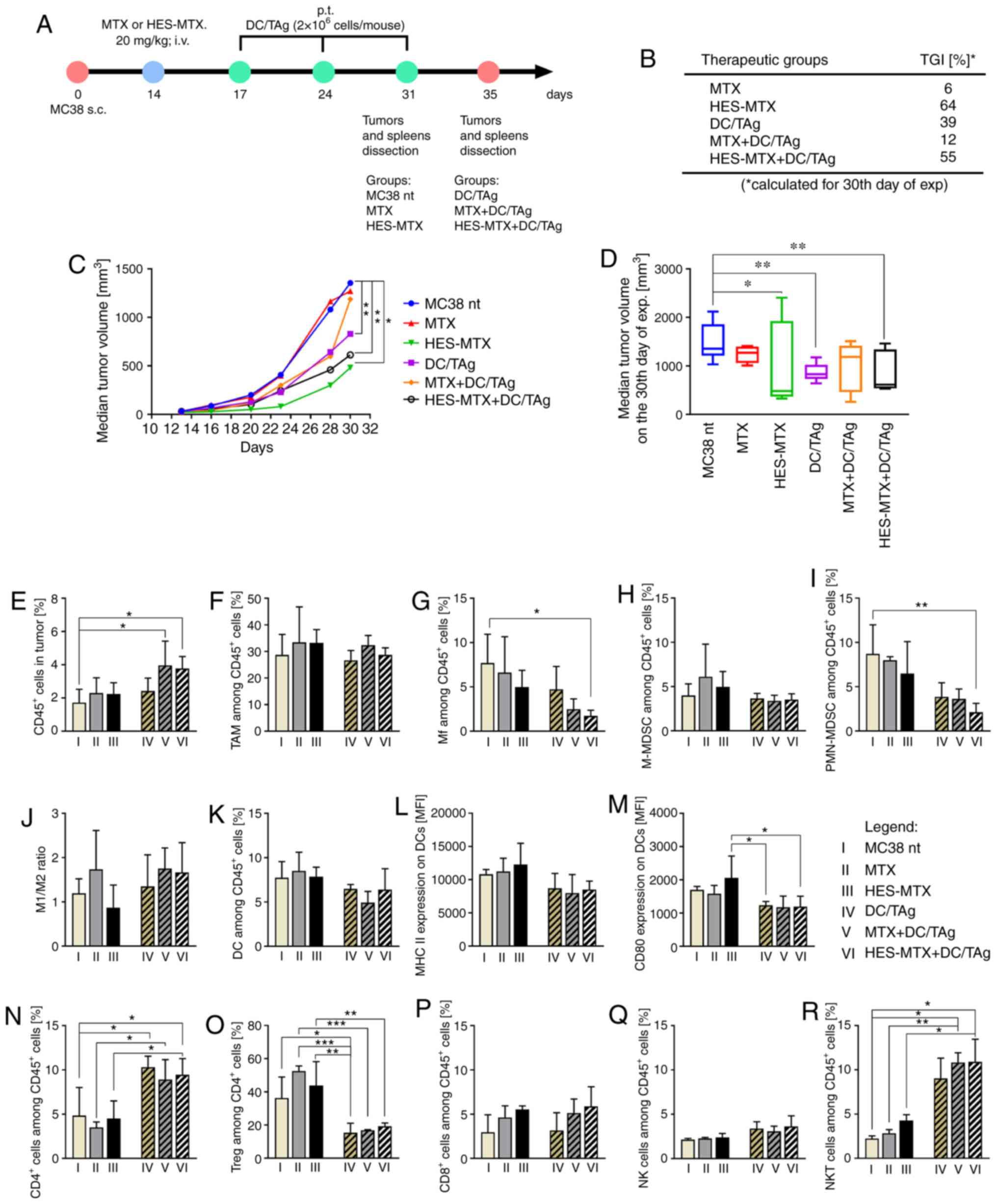 | Figure 5.Impact of combined therapy on tumor
growth and infiltration of MC38 tumor nodules with immune cells.
(A) Scheme of treatment. (B) Table presenting MC38 tumor growth
inhibition (TGI) calculated on 30th day of experiment in relation
to the MC38 nt group; (C) Graph presenting median tumor volume
after chemoimmunotherapy. (D) Box graph presenting median tumor
volume, calculated on the 30th day of the experiment. (E)
Percentage of CD45+ cells in tumor nodules. (F-I)
Percentage of myeloid cell subpopulations among CD45+
cells in tumors. (J) M1/M2 ratio showing changes in polarization of
tumor-infiltrating macrophages after therapy. (K-M) Percentage of
DCs infiltrating into tumor tissue and expression of MHC II and
CD80 molecules on their surface. (N-R) Percentage of lymphoid cell
subpopulations among CD45+ cells in tumors. Scheme of
multiparameter flow cytometry analyses showing the method of
distinguishing myeloid or lymphoid cell subpopulation in tumors is
presented in Fig. S3A. Results are
expressed as mean ± SD (3–5 mice per group were analyzed from one
experiment). The differences between groups were calculated by (C
and D) two-way ANOVA followed by Bonferroni's multiple comparisons
test, (E-Q) one-way ANOVA followed by Tukey's multiple comparison
post-hoc test or (R) Brown-Forsythe and Welch ANOVA test followed
by Dunnett's T3 multiple comparisons post-hoc test (*P<0.05,
**P<0.01, ***P<0.001). HES, hydroxyethyl starch; MTX,
methotrexate; DCs, dendritic cells; TAg, tumor antigen; TAMs,
tumor-associated macrophages; Mf, macrophages; M-MDSC, monocytic
myeloid derived suppressor cells; PMN-MDSC, polymorphonuclear
myeloid derived suppressor cells; Tregs, regulatory T cells; NK,
natural killer; i.v., intravenously s.c., subcutaneously. |
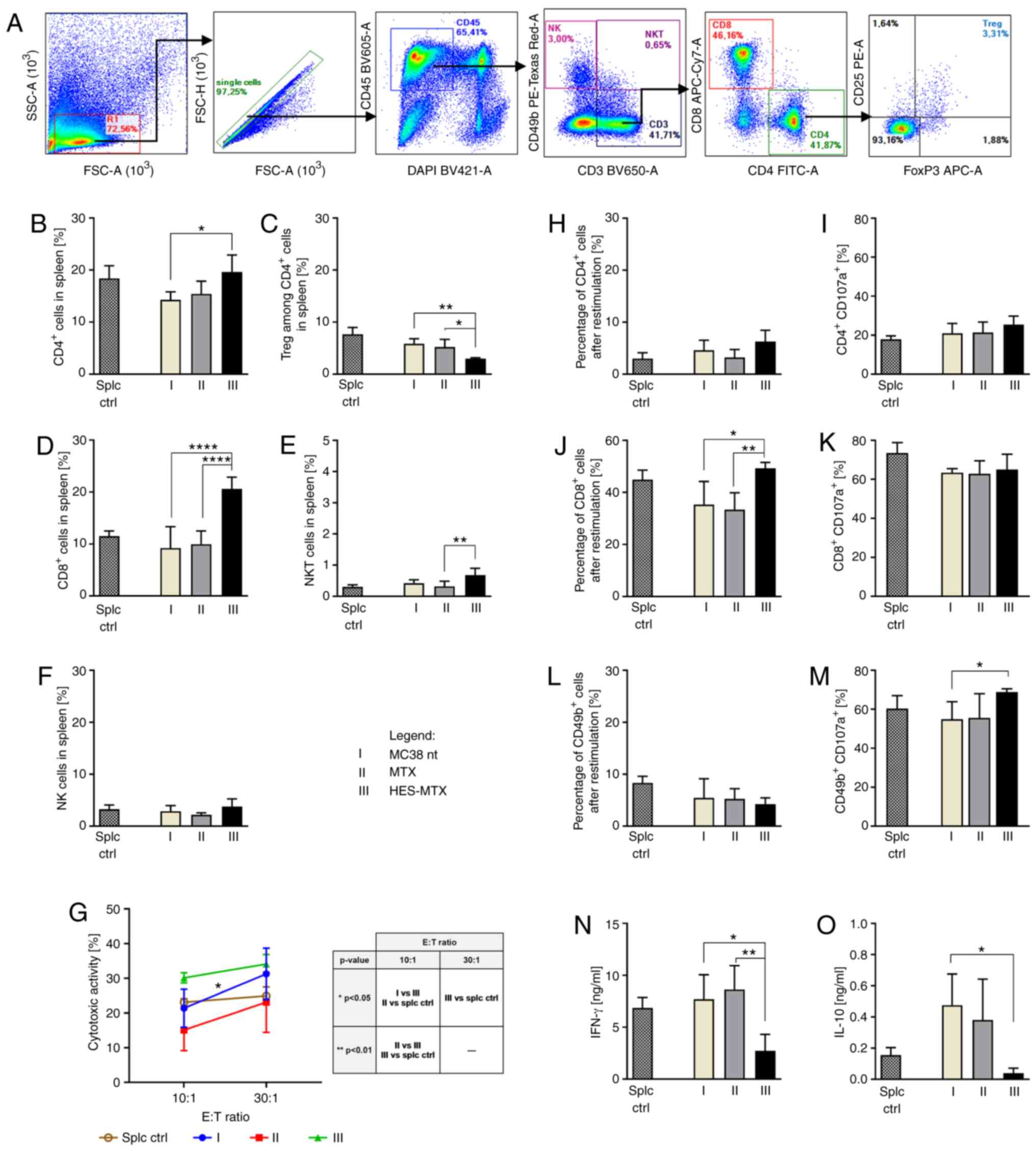 | Figure 4.Effect of applied chemotherapy on
induction of systemic antitumor response. (A) Scheme of
multiparameter flow cytometry analyses showing the method of
distinguishing lymphoid cell subpopulation in spleens dissected
from MC38 tumor-bearing mice treated according to the scheme
presented in Fig. 3A. (B-F)
Percentage of effector and suppressor lymphoid cell subpopulations
in the spleens. (G) Cytotoxic activity of splenocytes (effector
cells) against DiO+ MC38 cells (target cells). Asterisk
above the line indicates statistical significance between different
E:T ratios within a given group, while statistical significance
between groups within a given E:T ratio is presented in the table.
(H-M) Percentage of Th, CTL and NK cells (CD49b+) among
splenocytes after restimulation of spleen cells with MC38 cells and
the percentage of CD107a+ among CD4+,
CD8+ and CD49b+ cells measured by CD107a
degranulation assay. (N and O) IFN-γ and IL-10 concentration in
supernatants after restimulation. Results are expressed as mean ±
SD (5 mice per group were analyzed from one experiment). Splc ctrl,
splenocytes isolated from spleen derived from healthy mice (i.e.
without MC38-tumor). Differences between groups were calculated
using: (B-F and H-M) one-way ANOVA followed by Tukey's multiple
comparison post-hoc test (N) nonparametric Kruskal-Wallis test
followed by Dunn's multiple comparison test; (O) Brown-Forsythe and
Welch ANOVA test followed by Dunnett's T3 multiple comparisons
post-hoc test; or (G) two-way ANOVA followed by Tukey's multiple
comparison post-hoc test (*P<0.05, **P<0.01,
****P<0.0001). HES, hydroxyethyl starch; MTX, methotrexate; NK,
natural killer; Tregs, regulatory T cells; IFN, interferon; IL,
interleukin. |
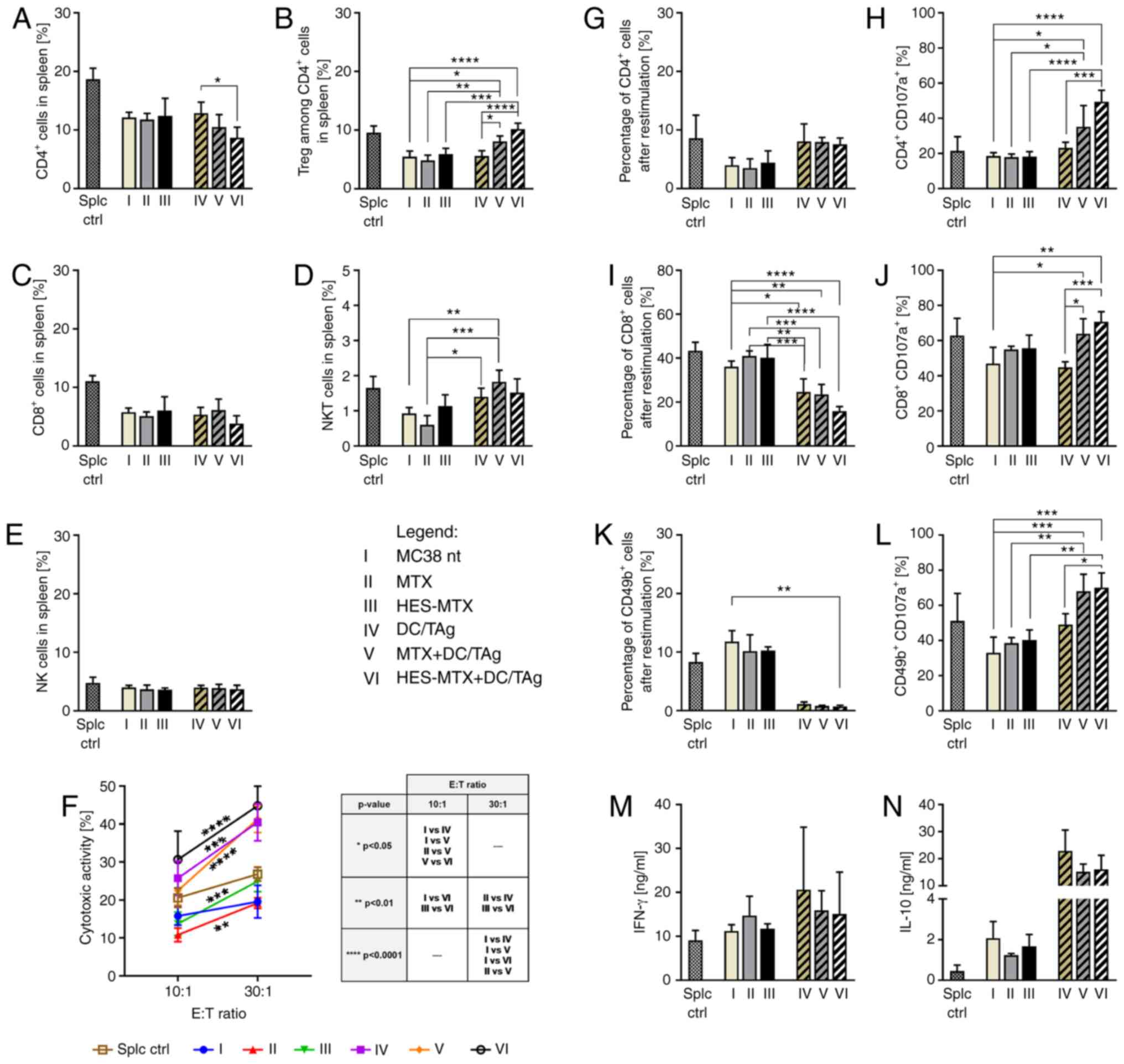 | Figure 6.Effect of applied chemoimmunotherapy
on induction of systemic antitumor response. (A-E) Percentage of
effector and suppressor lymphoid cell subpopulations in spleens of
MC38 tumor-bearing mice treated according to the scheme presented
in Fig. 5A. (F) Cytotoxic activity
of splenocytes (effector cells) against DiO+ MC38 cells
(target cells). Asterisks above or under the lines indicate
statistical significance between different E:T ratios within a
given group, while statistical significance between groups within a
given E:T ratio is presented in the table. (G-L) Percentage of Th,
CTL and B NK cells (CD49b+) among splenocytes after
restimulation of spleen cells with MC38 cells and the percentage of
CD107a+ among CD4+, and cytotoxic
CD8+ and CD49b+ cells measured by CD107a
degranulation assay. (M and N) IFN-γ and IL-10 concentration in
supernatants after restimulation. Scheme of multiparameter flow
cytometry analyses showing the method of distinguishing lymphoid
cell subpopulation in spleens is presented in Fig. S3B. Results are expressed as mean ±
SD (3–5 mice per group were analyzed from one experiment). Splc
ctrl, splenocytes isolated from spleen derived from healthy mice
(i.e. without MC38-tumor). Differences between groups were
calculated using: (A-E, G-J and L) one-way ANOVA followed by
Tukey's multiple comparison post-hoc test (K, M and N) the
nonparametric Kruskal-Wallis test followed by Dunn's multiple
comparison test; or (F) two-way ANOVA followed by Tukey's multiple
comparison post-hoc test (*P<0.05, **P<0.01, ***P<0.001,
****P<0.0001). HES, hydroxyethyl starch; MTX, methotrexate; DCs,
dendritic cells; TAg, tumor antigen; Tregs, regulatory T cells; Th,
T helper; CTL, cytotoxic T lymphocyte; NK, natural killer; IFN,
interferon; IL, interleukin. |
Analysis of myeloid cells and
lymphocytes in tumors and spleens of mice after the therapy
Tumor cells and spleen cells isolated from mice were
thawed and stained for identification of myeloid or lymphoid cell
subpopulations according to the procedure described previously
(46). Briefly, tumor single-cell
suspensions were stained with the LIVE/DEAD Fixable Violet Dead
Staining Kit (Thermo Fisher Scientific, Inc.) and then labelled
with cocktails of fluorochrome-conjugated monoclonal antibodies:
anti-CD3 PE-CF594, anti-CD19 PE-CF594, anti-CD49b PE-CF594 (all
from BD Biosciences), anti-CD45 BV605, anti-CD11b PerCP-Cy5.5,
anti-CD11c BV650, anti-F4/80 Alexa Fluor 700, anti-Ly6C PE,
anti-Ly6G APC-Cy7, anti-MHC II FITC, anti-CD80 PE-Cy7 (all from
BioLegend) for myeloid cell identification, and anti-CD45 BV605,
anti-CD3 BV650, anti-CD4 FITC, anti-CD8 APC/Fire 750, anti-CD25 PE
(all from BioLegend) for lymphocyte identification. Then, the cells
were fixed using the Foxp3/Transcription Factor Staining Buffer Set
(eBioscience). Cells stained with myeloid or lymphocyte cocktail
were additionally incubated with anti-CD206 APC (BioLegend) or
anti-FoxP3 APC (eBioscience) antibodies, respectively. In spleen
single cell suspension only the lymphocyte identification was
performed according to the procedure described above. The analysis
was performed using a FACS Fortessa flow cytometer with Diva
software (Becton Dickinson).
Analysis of antitumor response of
effector spleen cells
Spleen cells obtained from non-treated or treated
tumor-bearing mice were cocultured with mitomycin C-treated MC38
cells (50 mg mitomycin C/3×106 cells, 30 min., 37°C) in
the presence of recombinant human IL-2 (200 U/ml). After 5 days of
restimulation, supernatants were collected and stored at 4°C until
ELISA was performed. Cytotoxic activity of cells stained with DiO
lipophilic dye (Molecular Probes) was analyzed according to a
previously described procedure (47). Two E:T (effector to target) ratios
were investigated: 10:1 and 30:1. The percentage of dead double
positive (DiO+PI+) MC38 cells was determined
after analysis using a FACSCalibur with CellQuest 3.3 software
(Becton Dickinson). In order to determine the percentage of
CD107a+ cells, restimulated spleen cells were incubated
for 2 h with MC38 cells in the presence of monoclonal anti-CD107a
antibody conjugated with APC (BioLegend). Afterwards, cells were
stained with anti-CD45 V500, anti-CD4 FITC, anti-CD8 PE-Cy7 and
anti-CD49b PE and analyzed using a FACS Fortessa with Diva software
(Becton Dickinson).
Determination of cytokine
production
Production of cytokines by restimulated spleen cells
was evaluated using commercially available ELISA kits (IL-10, IL-4;
BD Biosciences and IFN-γ; eBioscience) according to the
manufacturer's instructions.
Statistics
All the data were analyzed using GraphPad Prism 8
software (GraphPad Software, Inc.). The normality of residuals was
confirmed by the D'Agostino-Pearson omnibus test. When data were
consistent with a Gaussian distribution and had equal SD values,
the statistical significance was calculated using the parametric
one-way ANOVA followed by Tukey's multiple comparison post-hoc
test. When data were consistent with a Gaussian distribution but SD
values were not equal, the Brown-Forsythe and Welch ANOVA test
followed by Dunnett's T3 multiple comparisons post-hoc test was
performed. Data inconsistent with a Gaussian distribution were
analyzed using the nonparametric Kruskal-Wallis test for multiple
independent groups followed by Dunn's multiple comparison post-hoc
test. In analyses where only two groups were compared, the
statistical differences were calculated using the Mann-Whitney test
or unpaired t-test. The statistical significance in kinetics of
tumor growth was calculated using the two-way ANOVA followed by
Bonferroni's multiple comparisons post-hoc test. The type of
statistical analysis used is described in the captions under the
figures. All statistically significant differences are presented in
the graphs; otherwise the differences were not significant.
Results
Antiproliferative activity of
nanoconjugate against MC38 cells in vitro
The first step of our research was to determine
in vitro activity of the HES-MTX nanoconjugate against MC38
cells. Therefore, its antiproliferative activity was evaluated
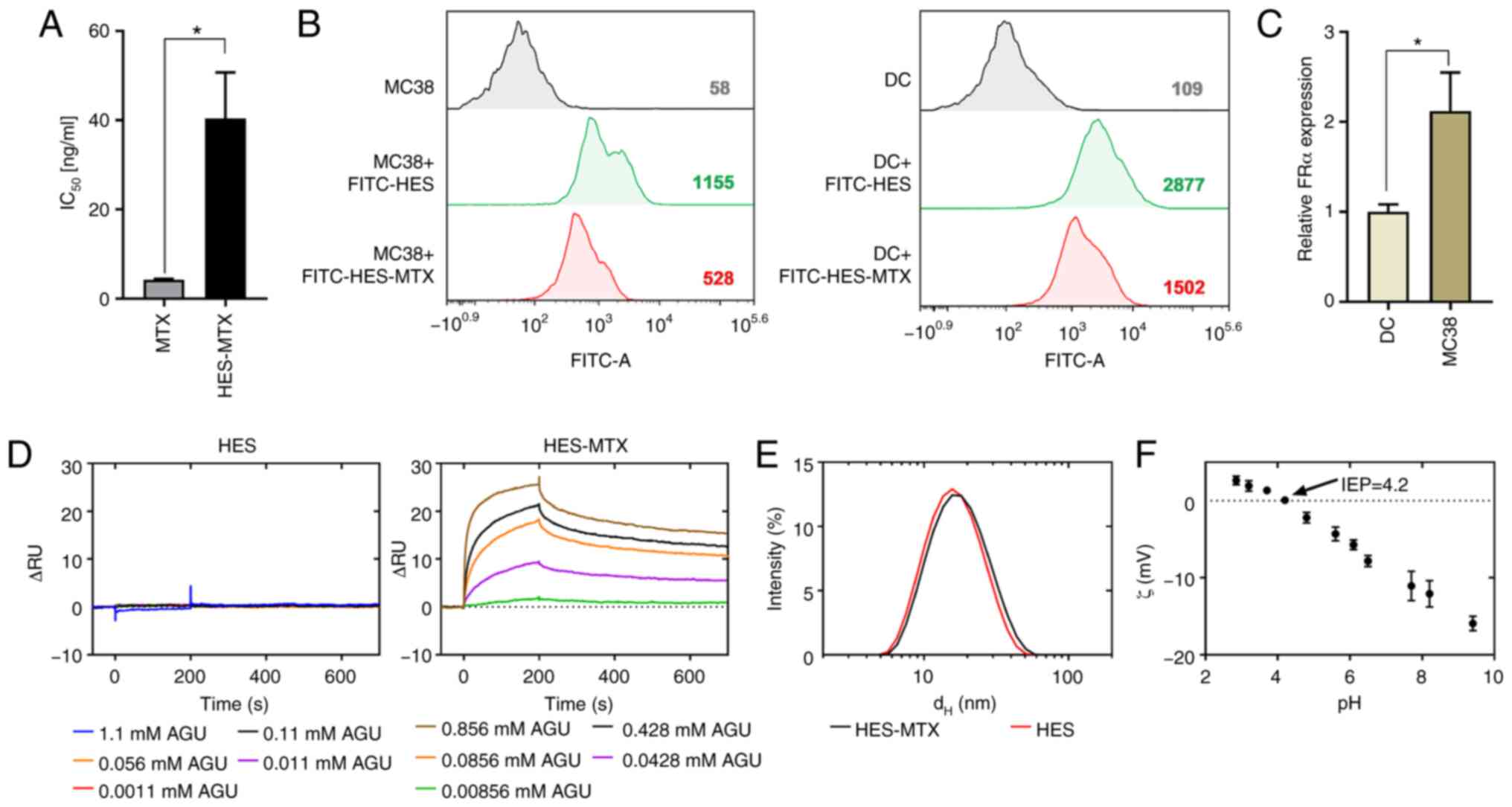
using the MTT assay (Fig. 1A).
Based on the calculated IC50 value, the HES-MTX
nanoconjugate demonstrated a 10-fold weaker antiproliferative
effect against MC38 cells than MTX. In order to confirm the
interaction of HES-MTX with MC38 cells, FITC dye was attached to
the HES molecule and analysis by flow cytometry revealed that
despite its larger diameter, HES-MTX appeared to be able to enter
tumor cells (Fig. 1B). To evaluate
the interaction of HES-MTX with DCs, the same analysis was
performed. In comparison to the MC38 cells, DCs interacted more
strongly with HES and HES-MTX, which was reflected in higher mean
fluorescence intensity (MFI) values. However, this phenomenon may
be related to the increased uptake capacity of these cells when
compared to tumor cells. Taking into account the polyvalence of
HES-MTX, the nanoconjugate could interact with cells through FRα,
which is abundant on cancer cells. Determination of the expression
of FRα in MC38 cells revealed that in comparison to DCs, MC38 cells
showed significantly higher expression of FRα (Fig. 1C). The affinity of the HES and the
HES-MTX nanoconjugate to folate binding protein (FBP) was
investigated using SPR. HES (a negative control without MTX
molecules attached) did not show any significant response over the
studied concentration range (1.1–0.0011 mM AGU), indicating a lack
of affinity to the FBP surface (Fig.
1D). In contrast, the HES-MTX showed an increasing
concentration-dependent response. The shape of the sensorgrams
indicated a fast and strong association of the HES-MTX on the FBP
presenting surface. Dissociation curves for HES-MTX indicated that
the conjugate dissociates with complex kinetics, initially at a
fast and subsequently at slower rates. At the end of analysis, the
dissociation phase was still incomplete. This suggests a high
affinity of the nanoconjugate to FBP. Characterization of HES and
HES-MTX by light scattering technique revealed that HES-MTX
represents a typical batch of HES polymers with a mean hydrodynamic
diameter of about 15 nm when compared to the initial (unmodified)
polymer (~14 nm) (Fig. 1E). The
surface of HES-MTX nanoconjugate has a negative zeta ζ potential
[about-10 mV at pH=7.4, isoelectric point (IEP)=4.2] (Fig. 1F). The gathered data demonstrated
that the HES-MTX nanoconjugate has weaker antiproliferative
activity against tumor cells than the free form of MTX. Moreover,
HES after conjugation with MTX gains a high affinity to FBP and is
a good candidate as a folate targeting macromolecule (an
FR-targeted chemotherapeutic). We assume that, due to the acquired
properties of HES-MTX, it should act more specifically towards
tumor cells and have greater potential as a chemotherapeutic
agent.
Modulation of maturation and phenotype
of DCs generated in the presence of metabolites released by MC38
cells after MTX or HES-MTX treatment
It was found that certain chemotherapeutics,
including MTX, used in appropriate concentrations, can act as
immunomodulators and contribute to an increase in DC maturation
(33,34). To investigate the impact of the
nanoconjugate on DC generation and phenotypic changes as well as to
assess whether the nanoconjugate could reverse the inhibitory
effect of MC38 cells on maturation of DCs, in vitro studies
were conducted. For this purpose, the percentage of Annexin
V+ MC38 cells previously treated with MTX or HES-MTX was
determined (Fig. 2A). Similar to
the observation made in the MTT assay and calculated
IC50 value, the Annexin V binding assay revealed that
the HES-MTX nanoconjugate was less effective in induction of
apoptosis than MTX. To reflect the changes in the TME occurring
after nanoconjugate treatment and the potential effect on DC
maturation, the MC38 cells were treated with MTX or HES-MTX for 72
h. Subsequently, the conditioned medium (CM) harvested from treated
MC38 cells was used in ex vivo generation of DCs (named
hereafter as DC/MC38/TAg, DC/MC38/MTX/TAg and DC/MC38/HES-MTX/TAg).
Due to the immunomodulatory effect of the nanoconjugate and MTX on
DC phenotype, the culture medium containing MTX or HES-MTX without
any cells was incubated for 72 h and then used in in vitro
studies (named hereafter as DC/MTX/TAg and DC/HES-MTX/TAg). Next,
in order to obtain the mature DCs, immature DCs were stimulated
with tumor antigens (TAg) and phenotype alterations of mature DCs
were evaluated by flow cytometry.
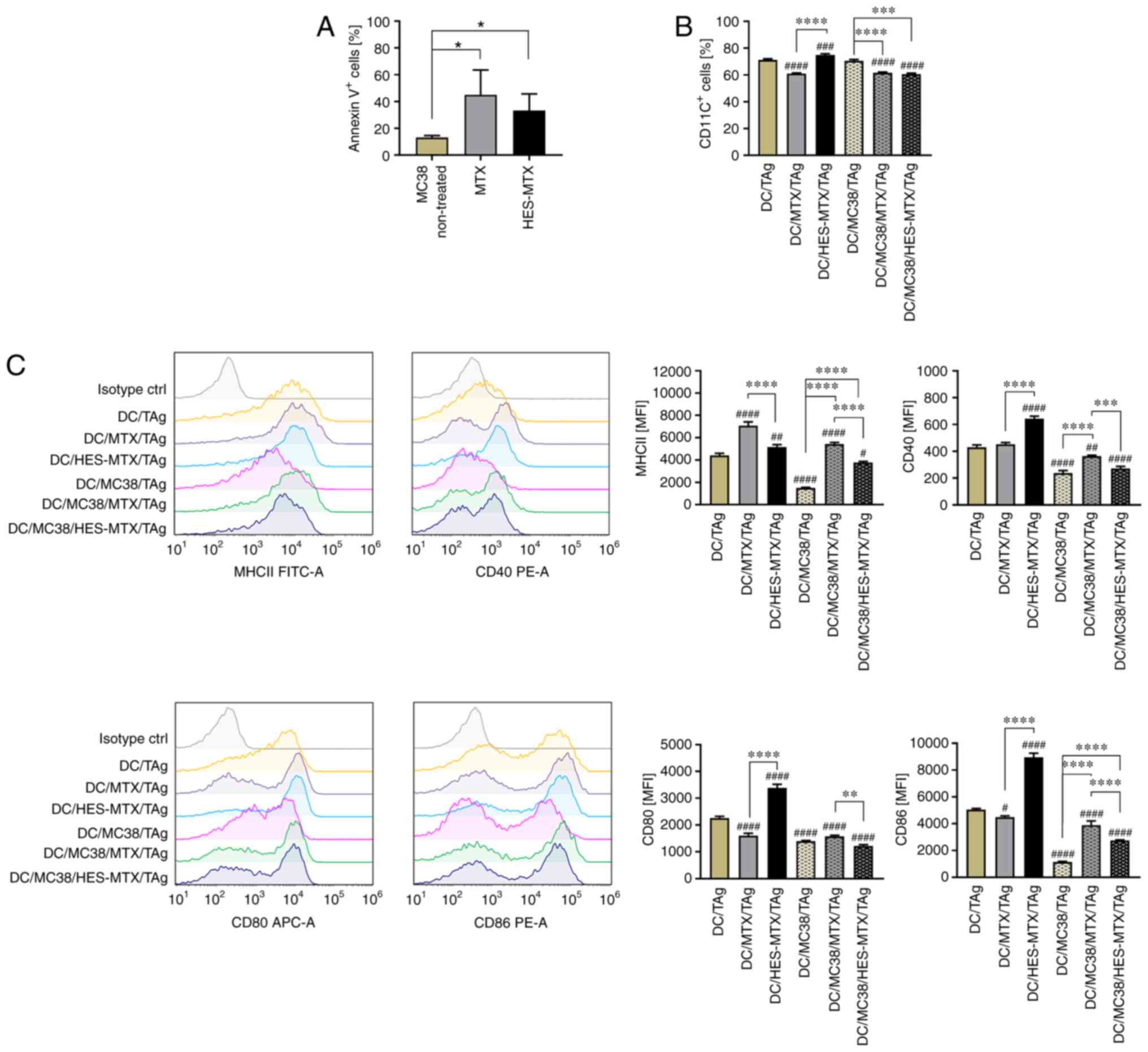
The phenotype analysis of mature DCs showed that in
comparison to the non-treated DCs (DC/TAg group), the presence of
tumor metabolites (DC/MC38/TAg) did not influence the percentage of
CD11c+ DCs, but considerably affected the maturation of
these cells (Fig. 2B and C). DCs
generated in CM harvested from MC38 cells (DC/MC38/TAg) were
characterized by statistically significantly lower expression of
MHCII and CD40, CD80 and CD86 co-stimulatory molecules compared to
the non-treated DC/TAg group. However, this effect was partially
restored when MTX or HES-MTX was used. When CM from above MC38
cells treated with MTX or HES-MTX was used in generation of DCs
(DC/MC38/MTX/TAg and DC/MC38/HES-MTX/TAg), those cells were
characterized by a statistically significantly lower percentage of
CD11c+ cells compared to DC/TAg and DC/MC38/TAg
(Fig. 2B). When we applied medium
from the above MC38 cells treated with MTX (DC/MC38/MTX/TAg),
expression of the analyzed antigens (Fig. 2C) was significantly higher than that
observed after using HES-MTX (DC/MC38/HES-MTX/TAg). We postulate
that this effect was related to the lower antiproliferative
activity of HES-MTX than MTX against tumor cells. In comparison to
MTX (DC/MTX/TAg), the nanoconjugate itself has an immunomodulatory
effect on the phenotype of DCs (DC/HES-MTX/TAg), which was observed
especially with a statistically significantly greater percentage of
CD11c+ cells and higher expression of co-stimulatory
molecules.
Summarizing these results, in comparison to the
nanoconjugate, the treatment of MC38 cells with MTX
(DC/MC38/MTX/TAg) resulted in more efficient abolition of the
negative effect of tumor cells on DC generation and maturation.
However, when considering the influence of MTX or HES-MTX on the DC
phenotype and their response to stimulation with TAg, the
nanoconjugate (DC/HES-MTX/TAg) was a more efficient immunomodulator
of DC maturation.
Influence of nanoconjugate
administration on activation of local and systemic antitumor
response in the MC38-bearing mice
To answer the question whether therapy with the
novel MTX conjugate modulates the local and systemic antitumor
response and how it would affect the efficacy of DC-based vaccines
administered after chemotherapy, in vivo experiments were
conducted. In our previous chemoimmunotherapy schedules in the
MC38-tumor model, DC-based vaccines were applied three days after
cyclophosphamide administration (39,40,46).
Therefore, in the present experiment, we aimed to determine what
changes in the tumor and spleen would occur as a result of the
application of MTX or the nanoconjugate HES-MTX. For this purpose,
mice with subcutaneously growing MC38 tumor received MTX or HES-MTX
i.v. and three days later the tumor nodules and spleens were
dissected for further analyses (Fig.
3A). Flow cytometric analyses allowing the simultaneous
identification of multiple immune cell subpopulations in tumor and
spleen tissues were performed (Fig.
3B for tumors and Fig. 4A for
spleens).
In the tumor tissue, we examined the percentage of
CD45+ cells (leukocytes) and among them we identified
myeloid cells, TAMs
(CD11b+CD11c+F4/80+), macrophages
(Mfs) (CD11b+CD11c−F4/80+),
subpopulations of MDSCs [monocytic (M-)MDSCs
(CD11b+CD11c−F4/80−Ly6C+Ly6G−),
polymorphonuclear (PMN-)MDSCs
(CD11b+CD11c−F4/80−Ly6CintLy6G+)],
DCs
(CD11b+CD11c+F4/80intMHCII+),
and expression of MHC II and CD80 molecules on the surface of DCs.
Moreover, polarization of macrophages toward type M1
(CD11b+F4/80+CD206¯) and M2
(CD11b+F4/80+CD206+) was
identified by evaluation of CD206 intracellular antigen expression.
Then the M1/M2 ratio was calculated. In addition, among
CD45+ cells in tumor tissues we identified lymphoid
cells, CD4+ (CD3+CD4+), Tregs
(CD3+CD4+CD25+FoxP3+),
CD8+ (CD3+CD8+), NK
(CD49b+) and NKT (CD3+CD49b+)
cells.
After application of MTX or HES-MTX, a high influx
of CD45+ cells in tumor tissue was observed (Fig. 3C). In both MTX and HES-MTX groups, a
lower percentage of TAMs and Mfs was found (Fig. 3D and E); however, none of these
changes was statistically significant. Although this effect was
accompanied by high infiltration of M-MDSCs, the percentage of
PMN-MDSCs remained at the same, low level (Fig. 3F and G). It should be highlighted,
that after HES-MTX application we noted the lowest influx into
tumor tissue of TAMs, which presumably belong to M1 type
macrophages. The M1/M2 ratio was not significantly elevated only
after application of HES-MTX, which would suggest a potent impact
of the nanoconjugate on the level of macrophage polarization toward
M1 type (Fig. 3H). It should be
highlighted, that neither MTX nor HES-MTX elevated the percentage
of tumor-infiltrating DCs. Despite the lack of alterations in MHC
II expression on DCs, the expression of CD80 on these cells was
significantly upregulated after MTX or HES-MTX application
(Fig. 3I-K). Although, after
treatment with MTX or HES-MTX no differences in the percentage of
CD4+ T cells compared to the non-treated group (MC38 nt)
were found, a strong, but not statistically significant, decrease
in the percentage of Tregs in the MTX and HES-MTX groups (Fig. 3L and M) was observed. Moreover,
administration of HES-MTX contributed to the increase in the
percentage of CD8+ T cells, NK and NKT cells (Fig. 3N-P); however, these changes were not
statistically significant. The activation status of CD4+
and CD8+ T cells based on the expression of CD44 and
CD62L antigens was evaluated additionally. The percentage of T
cells with effector phenotype (CD44+CD62Lneg)
and memory phenotype (CD44+CD62L+) was
determined (Fig. S1). The vast
majority of CD4+ (approximately 90%) and CD8+
(approximately 80%) T cells infiltrating into tumor tissue showed
effector phenotype (CD44+CD62Lneg), however
compared to the MC38 nt group, the applied therapy did not cause
statistically significant changes in the percentage of effector or
memory T cells (Fig. S1A-C). The
obtained data suggest that although MTX and HES-MTX can reduce the
percentage of myeloid (TAM, Mf, PMN-MDSC, M2-type macrophages) and
lymphoid (Treg) cells with suppressor activity, only after
application of HES-MTX was an increase in the percentage of CTL and
NK cells noted.
With the use of the multiparametric flow cytometry
analysis protocol presented in Fig.
4A, the percentages of CTL, Th and Treg cells among spleen
cells were estimated. In contrast to the effects caused by MTX
application, the use of HES-MTX induced significant changes in the
percentage of lymphocytes among spleen cells (Fig. 4B-F). The application of HES-MTX
contributed to a significantly higher percentage of CD4+
and CD8+ T cells compared to the non-treated group and,
in the latter subpopulation, to the MTX-treated group. It should be
noted that after HES-MTX application a statistically significant
reduction in the Treg percentage among spleen CD4+ T
cells was observed. Also, among splenocytes from the
HES-MTX-treated group the highest NK and NKT cell percentage was
observed. The use of nanoconjugate resulted in the restoration of
the size of these lymphocyte populations to the level typical for
healthy mice (splc control) or even higher. Analysis of the
activation status of splenic CD4+ and CD8+ T
cells revealed that after therapy with the nanoconjugate the
percentage of memory CD4+ T cells was significantly
higher, than after therapy with MTX (Fig. S1D-F). In order to estimate the
ability of activated splenic lymphocytes to induce a systemic
antitumor response after treatment with HES-MTX, the spleen cells
were restimulated ex vivo with MC38 cells. After five days
of co-culture of spleen cells with mitomycin C-treated MC38 cells,
their cytotoxic activity towards MC38 cells as well as their
phenotype, CD107a degranulation and cytokine production were
evaluated. Determination of cytotoxic activity of splenocytes
against MC38 cells through direct contact revealed statistically
significantly higher cytotoxicity in the HES-MTX-treated group
compared to the other groups, especially in the 10:1 E:T ratio
(Fig. 4G). Similar to observations
made during splenocyte phenotype analysis, the elevated percentages
of CD4+ and CD8+ cells in the HES-MTX group
were maintained after restimulation, and in the latter cell
population this difference was statistically significant. It was
accompanied by a slight, but not statistically significant,
decrease in the percentage of CD49b+ cells (Fig. 4H, J and L). However, the evaluation
of immune cell antitumor activity by CD107a degranulation assay did
not show a statistically significant increase in the percentage of
CD4+CD107a+ cells in the HES-MTX group, while
the percentage of CD8+CD107a+ cells remained
unchanged in all groups. It should be highlighted that therapy with
the nanoconjugate caused a significant increase in the percentage
of CD49b+CD107a+ cells (Fig. 4I, K and M). Moreover, in the HES-MTX
group a significant decrease in IFN-γ and IL-10 production after
restimulation was observed (Fig. 4N and
O).
The results presented above suggest that three days
after chemotherapy application, especially when HES-MTX was used,
the modulation of antitumor response occurred. It was reflected in
a reduction in the size of the population of immune cell with
suppressor activity (Tregs, TAMs, Mfs and M2-type macrophages) in
tumors, as well as in an expansion in the population of immune
cells with cytotoxic activity (CD8+ T cells, NK, NKT
cells) in spleen and tumor tissues. Moreover, generation of
cytotoxic activity of spleen lymphocytes against tumor cells
indicated that HES-MTX contributed to activation of the systemic
antitumor immune response.
Antitumor activity of therapy composed
of HES-MTX and DC-based vaccines and its influence on local and
systemic antitumor immune response
Taking into consideration the immunomodulatory
activity of HES-MTX, in the next step of the research, the
nanoconjugate was applied in combination with DC-based vaccines
according to the scheme presented in Fig. 5A. The tumors and spleens were
dissected at two time points: From non-treated (MC38 nt), MTX and
HES-MTX groups on the 31st day, and from immunotherapy receiving
groups (DC/TAg, MTX+DC/TAg, HES-MTX+DC/TAg) on the 35th day of the
experiment. The time discrepancies were caused by different tumor
growth rates, especially in the non-treated group and MTX group.
Thus, in the course of the experiment, we decided to separate the
dissections-the major reason for this decision was prolonged
observation of tumor growth after the last injection of DC-based
vaccines and maintaining the interval between organ dissections as
short as possible. The tumor growth inhibition (TGI) calculated on
the 30th day of the experiment for all groups of mice indicated the
strongest influence of HES-MTX on tumor growth (the TGI in HES-MTX
group was 64% in relation to the MC38 nt group), while MTX
exhibited only 6% inhibition of tumor growth (Fig. 5B). A moderate effect was observed
after application of the DC-based vaccines as sole therapy (DC/TAg;
TGI 39%). However, when compared to the HES-MTX, group combining
the chemotherapy with DC/TAg-based vaccines did not contribute to
the enhancement of tumor growth inhibition as we expected. In the
HES-MTX+DC/TAg group the TGI was 55%, whereas in the MTX+DC/TAg
group the inhibition of tumor growth was negligible (TGI 12%). The
kinetics of MC38 tumor growth and median tumor volume on the 30th
day of the experiment are shown in Fig.
5C and D.
To compare the effect of multiple injections of
DC-based vaccines, the TGI for the 35th day of the experiment was
determined in relation to the DC/TAg group (Fig. S2A). TGI for the MTX+DC/TAg group
was negative and was −14%; hence application of MTX prior to
DC-based vaccines probably reduced the effectiveness of the
immunotherapy. On the other hand, in the HES-MTX+DC/TAg group, TGI
for the 35th day of the experiment was 22%, which indicates that
HES-MTX application prior to the start of immunotherapy enhanced
tumor growth delay compared to the DC/TAg group, although these
differences were not statistically significant. This was also
reflected in the median tumor volume on the 35th day (Fig. S2B).
The multiparameter flow cytometry analyses of tumor
tissue (according to the scheme presented in Fig. S3A) showed the overall increase in
the percentage of leukocytes in all treated groups of mice
(Fig. 5E). Although after
monotherapy (MTX, HES-MTX, DC/TAg groups) the percentage of
CD45+ cells was slightly higher than in the non-treated
group, application of combined therapy, both MTX+DC/TAg and
HES-MTX+DC/TAg, caused the most intensive influx of the cells and
observed changes were statistically significant in comparison to
the MC38 nt group. One of the most numerous cell populations among
myeloid cells found in the tumor tissue was tumor-associated
macrophages (TAMs), which accounted for approximately 30% of all
leukocytes in tumors from the non-treated group of mice (Fig. 5F). The applied therapy, both chemo-
and chemoimmunotherapy, did not induce changes in the TAM influx.
However, the applied therapies demonstrated a significant impact on
decrease in the percentage of resident Mfs in the tumor nodules,
especially in the HES-MTX+DC/TAg group, in which the Mf percentage
was about four times lower than that noted in the MC38 non-treated
group and three times lower than in the DC/TAg group (Fig. 5G). Although there were no
statistically significant changes in size of the M-MDSC
population-an increase in the M-MDSC percentage occurred only when
MTX or HES-MTX was applied as monotherapy; significant changes in
the PMN-MDSC percentage in tumor tissue were observed (Fig. 5H and I). After application of each
type of therapy, a reduced population of PMN-MDSCs was found.
Although application of chemotherapy alone caused a moderate
decrease of PMN-MDSC percentage (especially after HES-MTX), the use
of immunotherapy induced a significant reduction in the PMN-MDSC
percentage. The lowest percentage of these cells was noted after
combined therapy with HES-MTX and DC-based vaccines. Considering
the influence of applied therapies on the stage of macrophage
polarization, the M1/M2 ratio was not significantly increased after
application of MTX or combined therapy (MTX+DC/TAg, HES-MTX+DC/TAg
group), while it decreased after HES-MTX treatment (Fig. 5J). In comparison to non-treated and
chemotherapy groups, DC-based vaccines caused a slight, but not
statistically significant reduction in the percentage of DCs
infiltrating tumor tissue, which was accompanied by decreased
expression of MHC II and CD80 molecules on their surface (Fig. 5K-M). Despite the fact that the
lowest percentage of DCs was found in the MTX+DC/TAg group, the
expression of MHC II and CD80 antigens did not change and remained
at the same level as in the DC/TAg-receiving group. It should be
noted that in the HES-MTX group, tumor-infiltrating DCs were
characterized by the highest expression of MHC II and CD80
molecules, which is consistent with our observations about the
modulatory potential of the nanoconjugate for the DC phenotype, and
observed changes in the expression of CD80 antigen were
statistically significant.
The changes occurring in the myeloid populations
were accompanied by modifications in the percentage of lymphoid
cell infiltrating tumor nodules. When compared to the MC38 nt
group, the use of cytostatics alone did not cause statistically
significant changes in the percentage of CD4+ cells
infiltrating tumor tissue, unlike in other lymphoid cell
subpopulations. Application of DC-based vaccines resulted in
statistically significant enlargement of CD4+ T cells,
NKT cell percentage and a decrease in the percentage of Tregs,
while the increase of NK cells percentage was not statistically
significant (Fig. 5N, O, Q and R).
Trends in changes in the percentage of Tregs suggest that
application of chemotherapeutics alone was not sufficient to
maintain the size of the tumor-infiltrating Treg population at a
low level, like those observed on the third day after chemotherapy
in the previous experiment. In comparison to the control group, the
percentage of Tregs cells was much higher, especially in the MTX
group (Fig. 5O). Multiple
application of DC-based vaccines was found to cause a statistically
significant strong reduction in the Treg percentage, but in the
HES-MTX+DC/TAg group the size of the Treg population was slightly
greater than that noted in the other DC/TAg-receiving groups. It is
noteworthy that an increase in CD8+ T cells was noted
when chemotherapy was applied alone (MTX, HES-MTX groups) or in
combination with DC-based vaccines (MTX+DC/TAg, HES-MTX+DC/TAg)
(Fig. 5P), but these changes were
not statistically significant. The highest percentage of
CD8+ T cells was noted in the HES-MTX-receiving groups
(HES-MTX and HES-MTX+DC/TAg), which is consistent with our previous
observation that HES-MTX affects the enhanced influx of
CD8+ T cells into tumor tissue. Furthermore, in
comparison to the control or DC/TAg group, a significant increase
in the percentage of NKT cells was observed when MTX or HES-MTX was
used (as mono- and combined therapy) (Fig. 5R). Analysis of the activation status
of CD4+ T cells infiltrating into tumor tissue showed
that compared to the non-treated and chemotherapy-receiving groups
(MC38 control, MTX and HES-MTX), the use of DC/TAg-based vaccines
resulted in a significantly higher percentage of effector
CD4+ T cells, while percentage of memory CD4+
T cells was decreased. Moreover, therapy consisting of HES-MTX and
DC/TAg caused a significant increase in the percentage of effector
CD8+ T cells infiltrating into the tumor tissue
(Fig. S4B and C).
The obtained results indicated that supplementing
the chemotherapy with DC-based vaccines contributed to an enhanced
influx of leukocytes into the tumor tissue, especially CTL and NKT
cells, and reduced the population of immune cells with suppressor
activity, such as Mfs, PMN-MDSCs and Tregs.
The estimation of the percentage of CTLs, Th and
Tregs among spleen cells (according to the scheme presented in
Fig. S3B) revealed that
significant changes in the lymphoid cell population occurred only
when DC-based vaccines were used (Fig.
6A-E). In comparison to the non-treated group, the application
of MTX or HES-MTX as monotherapy did not contribute to significant
alterations among CTLs, Th or Tregs, like those observed on the
third day after chemotherapy. When DC-based vaccines were applied
alone, we did not observe significant changes in the population
size of the mentioned lymphocytes in the spleens. However, therapy
consisting of HES-MTX and DC/TAg was found to cause a decrease in
the percentage of CD4+ and CD8+ T cells
compared to the non-treated and DC/TAg groups. This effect was
accompanied by a significant increase in the Treg percentage in
this group. Similar tendencies were observed in the MTX+DC/TAg
group. Among the DC/TAg-receiving groups, the highest percentage of
splenic effector CD4+ T cells was observed in the
HES-MTX+DC/TAg group and this change was statistically significant
compared to the DC/TAg and MTX+DC/TAg group. Moreover, in the case
of memory CD8+ T cells found in the spleen, after use of
the DC/TAg-based vaccines the percentage of these was significantly
lower than observed in the non-treated and HES-MTX groups (Fig. S4E and F).
It should be noted that assessment of cytotoxic
activity of restimulated spleen cells towards MC38 tumor cells
revealed that immunotherapy generated more efficient cytotoxic
activity than chemotherapy applied alone (Fig. 6F). Moreover, the highest cytotoxic
activity was noted in the HES-MTX+DC/TAg group in both E:T ratios.
The restimulation of splenocytes by MC38 cells confirmed the impact
of DC-based vaccines on the percentage of CD4+,
CD8+ and CD49b+ cells, while chemotherapy
applied alone did not cause any significant changes in these
subpopulations (Fig. 6G, I and K).
Despite the lack of alterations in the CD4+ T cell
percentage after restimulation between groups receiving DC-based
vaccines, a strong reduction in the population size of
CD8+ T cells and CD49b+ cells was found,
especially in the HES-MTX+DC/TAg group. Nevertheless, the CD107a
degranulation assay demonstrated that application of combined
therapy caused a robust increase in the percentage of
CD107a+ cells among the CD4+, CD8+
and CD49b+ cells (Fig. 6H, J
and L). Application of HES-MTX together with multiple
injections of DCs induced the highest percentage of
CD4+CD107a+,
CD8+CD107a+ and
CD49b+CD107a+ cells and these changes were
statistically significant. However, in the latter subpopulation, a
similar effect was observed in the MTX+DC/TAg group. This effect
was accompanied by increased production of IFN-γ and IL-10 in all
immunotherapy-receiving groups (Fig. 6M
and N).
The gathered data indicate that although
monotherapy with the DC-based vaccine also generated alterations in
certain crucial immune cell subpopulations in tumor nodules and
spleens, the augmentation of these effects was observed when
chemotherapy was applied prior to immunotherapy. Combined therapy
with HES-MTX and DC-based vaccines resulted in a reduction in
percentages of myeloid cells with suppressor activity in
infiltrating tumor and enhanced influx of CD8, NK and NKT cells.
Moreover, therapy consisting of HES-MTX and DC-based vaccines
contributed to generation of a specific antitumor response. It was
confirmed by increased ability of spleen cells to secrete cytolytic
granules and enhanced cytotoxic activity as a result of secondary
contact with the tumor antigen. All of these factors caused the
statistically significant delay of tumor growth after therapy with
HES-MTX and DC-based vaccines.
Discussion
In the present work, we demonstrated for the first
time that an innovative drug delivery system-in the form of a
nanoconjugate of well-known therapeutic compounds, i.e.
methotrexate (MTX) as an anticancer agent and hydroxyethyl starch
(HES) as a high-molecular carrier-was able to modulate the
antitumor immune response. Moreover, we were the first to apply
chemotherapy with the HES-MTX nanoconjugate together with DC-based
cellular vaccines in a murine MC38 colon carcinoma model.
According to Goszczyński et al the mean
hydrodynamic diameter of HES-MTX is 15.2±6.2 nm (20); therefore undoubtedly this type of
conjugate can be defined as a nanoconjugate. It is noteworthy that
conjugation of MTX with HES is achieved by esterification of HES's
hydroxyl groups, but the linker between the carrier and MTX is
glutamic acid-an integral part of the MTX molecule- and hence no
other additional linking substances are needed (20). MTX release from the nanoconjugate
occurs as a result of chemical and enzymatic hydrolysis by
esterases or amylases. Enzymatic degradation of HES leads to the
release of glucose derivatives only, allowing for easy elimination
of these derivatives from the body. At the beginning of the
research on the anticancer potential of HES-MTX, the key issue was
to determine what the main advantage of HES-MTX over the free form
of MTX is. In fact, chemotherapy in its conventional form,
including methotrexate application, is related to overall toxicity
to healthy cells, rapid elimination of chemotherapeutics from the
body and low specificity towards target cancer cells (23,48).
Thus, it was challenging to design a chemotherapeutic-carrier
system overcoming these difficulties. It is well known that MTX
enters the cell mainly through the ubiquitously expressed reduced
folate carrier (RFC) to which MTX has high affinity. MTX can also
enter cells via folate receptors (FRs) overexpressed on cancer
cells, although with low affinity (27). As a result of conjugation of one
molecule of hydroxyethyl starch with 50 molecules of MTX, the
nanoconjugate becomes polyvalent (17,28).
This polyvalence allows interactions of the nanoconjugate with
folate receptor alpha (FRα) with a much higher binding constant
than free MTX, and therefore we postulate that HES-MTX interacts
more strongly with the tumor cells overexpressing FRα than normal
cells. Another important advantage of HES-MTX is its
biodistribution, which is attained not only, although mainly, by
interaction of HES-MTX with FRs on target cells but also by an
enhanced vascular permeability and retention effect (EPR). This
phenomenon is considered as an ability of macromolecules larger
than 40 kDa (hydrodynamic diameter above 10 nm) to selectively leak
from tumor vessels and accumulate in tumor tissue (2,30–32).
EPR is often observed in solid tumors due to extensive
angiogenesis, malfunctional vascular architecture and increased
expression of proteins associated with vascular permeability
(49,50). Moreover, the use of a carrier
reduces the toxicity of therapy, since the EPR effect for drug
delivery does not occur in normal tissue.
The main objective of the present study was to
determine whether the HES-MTX nanoconjugate, applied as
chemotherapy, modulates the systemic antitumor immune response, and
affects changes in the landscape of immune cells infiltrating tumor
tissue. This, in turn, should support the generation of a proper
immune response against a growing tumor by DC-based vaccines
injected peritumorally after chemotherapy administration.
Furthermore, there are reports confirming that certain cytostatics,
including methotrexate, used at appropriate doses, can act as
modulators of the DC phenotype and function (33,34),
thus DCs reinforced in this way should generate an efficient
antitumor immune response (51).
Taking all the above into account, we designed
in vitro studies in which we found that the
antiproliferative activity of the HES-MTX nanoconjugate against
MC38 colon carcinoma cells was considerably lower than that of the
free form of MTX. Previous results reported by Goszczyński et
al revealed that HES-MTX possessed approximately 10-fold weaker
antiproliferative activity towards human (MV4-11) and murine (P388)
leukemia cell lines than MTX alone (20), which is consistent with our
observations. Nevertheless, weak in vitro efficiency of the
conjugates does not necessarily predict diminished in vivo
activity, as it has been shown for the fibrinogen-MTX conjugate or
dextran-MTX conjugate used in a P388 mouse leukemia model by
Nevozhay et al (44) or
Goszczyński et al (14),
respectively. Furthermore, we confirmed the interaction of HES-MTX
labelled with FITC dye with MC38 cells and dendritic cells (DCs).
In comparison to MC38 cells, DCs interacted more strongly with HES
and HES-MTX. It was reflected in greater MFI values for DCs, but it
may be associated with increased antigen uptake capacity, which is
typical for this type of cell. We also confirmed a high affinity of
nanoconjugate to folate binding protein and we verified the
overexpression of FRα in MC38 cells in comparison to DCs ex
vivo generated from murine bone marrow precursors.
In the next step in the in vitro research,
we estimated the influence of metabolites released by MC38 cells
treated with nanoconjugate on the generation and maturation of DCs.
It is well known that in the presence of the tumor
microenvironment, DC functions are hindered and thereby creation of
an efficient antitumor immune response by DCs is impaired (8,9,52). It
has also been confirmed by us that in comparison to untreated DCs,
murine bone marrow DC precursors cultured in the presence of CM
harvested from above MC38 cells responded more weakly to
stimulation with TAg (DC/MC38/TAg), which was demonstrated in
statistically significantly lower expression of surface molecules
necessary for efficient antigen presentation. Using the Annexin V
binding assay, we confirmed that HES-MTX induced weaker apoptosis
of MC38 cells than MTX. DC precursors cultured in CM harvested from
above MC38 cells treated with HES-MTX and stimulated with TAg
(DC/MC38/HES-MTX/TAg) exhibited modest changes in expression of DC
antigens in comparison to DC/MC38/MTX/TAg. It was associated with
lower toxicity of HES-MTX, than MTX, towards tumor cells.
Therefore, in relation to DC/MC38/MTX/TAg, only partial abolition
of the negative effect of tumor cell metabolites on DC generation
and maturation was observed. It should be highlighted that in
contrast to MTX, DCs cultured in the presence of HES-MTX and
stimulated with TAg (DC/HES-MTX/TAg) were characterized by
statistically significant elevated expression of costimulatory
molecules, which indicates that tumor antigens would be more
efficiently presented to naïve lymphocytes by these cells. Similar
results were also described by other researchers, who showed that
certain chemotherapeutic agents such as methotrexate, paclitaxel or
doxorubicin used in low, noncytotoxic concentrations during DC
generation can upregulate maturation, antigen processing, and
antigen presentation by DCs, and this phenomenon was called
chemomodulation (33). For
instance, Shurin et al reported that MTX present during
murine DC differentiation not only contributed to an increase in
expression of antigen-processing machinery proteins and
costimulatory molecules on these cells, but also resulted in
upregulated expression of IL-12p70 in DCs. In turn, this cytokine
enhanced the ability of murine DCs to present antigens to T cells
in vitro (33). Moreover,
Kaneno and co-workers found that human DCs cultured in the presence
of methotrexate showed increased expression of costimulatory
molecules. Furthermore, the ability of these DCs to stimulate
proliferation of allogeneic T lymphocytes was also increased
(35). In addition, Zhong et
al demonstrated that paclitaxel used in appropriate low doses
supported murine DC maturation and function, and
-importantly-pretreatment of 3LL cells with paclitaxel abrogated
the suppressive effect of the tumor milieu on DC generation
(53).
Altogether, the main advantage of using the
nanoconjugate rather than MTX in free form is the fact that the
physicochemical properties of HES-MTX make it possible to target
tumor cells and prolong accumulation of the nanoconjugate in tumor
tissue. Moreover, the nanoconjugate itself modulates the phenotype
of DCs and improves the maturation of these cells, and thus it may
contribute to generation of an efficient antitumor immune response
by DCs present in the body as well as by DCs administered in the
form of cellular vaccines. This knowledge allowed us to put forward
a hypothesis that more efficient accumulation of HES-MTX in tumor
tissue and greater tumor cell specificity (despite the lower
antiproliferative activity towards MC38 cells in vitro) will
affect the efficacy of HES-MTX in vivo. It should be
reflected not only by enhanced inhibition of the growing tumor in
comparison to MTX, but also by modulation of the ability of DCs to
elicit an effective antitumor immune response by the host's immune
system.
Taking into consideration the above results, our
primary purpose in the subsequent experiments was to combine
anticancer therapy with the nanoconjugate and multiple peritumoral
injection of DC-based vaccines in a murine colon carcinoma model.
In our previous chemoimmunotherapy schedules in this tumor model,
the DC-based vaccines were applied three days after
cyclophosphamide administration (39,40,46).
Moreover, certain chemotherapeutics, such as MTX (1,19),
paclitaxel (36) or
cyclophosphamide (37,38), may act as immunomodulators through
stimulation of effector immune cells and elimination of regulatory
T cells (Tregs) (39,40,54).
Thus, it was necessary to find out whether HES-MTX administration
would change the local and systemic antitumor response and how it
would affect the activity of DC-based vaccines injected three days
after chemotherapy.
In the tumor nodules dissected three days after MTX
or HES-MTX application, an increase in leukocyte influx was
observed. Nevertheless, only the HES-MTX treatment contributed to
polarization of tumor-infiltrating macrophages towards M1-type
cells, and greater influx of cytotoxic T lymphocytes (CTLs) and
natural killer (NK) cells into tumor tissue occurred. Regardless of
that, after MTX and HES-MTX treatment, the percentage of monocytic
myeloid derived suppressor cells (M-MDSCs) in tumors was
significantly elevated, the size of the other cell populations with
potent suppressor activity i.e. macrophages (Mfs), tumor-associated
macrophages (TAMs) and Tregs, was reduced and importantly, in the
HES-MTX group the percentages of TAMs and Tregs were the lowest;
however, all the above-mentioned differences were not statistically
significant.
Considering the influence of HES-MTX on the
tumor-directed systemic immune response, we observed a significant
increase of T helper (Th), CTL and NKT cell percentages among
splenic leukocytes, and this effect was accompanied by a decrease
in the percentage of Tregs among splenic CD4+ T cells.
Despite the substantial decrease in the production of interferon
(IFN)-γ and interleukin (IL)-10 by restimulated splenocytes
obtained from mice treated with HES-MTX, we observed higher
cytotoxic activity towards tumor cells in this group. Thus, we
postulate that this resulted from the higher percentage of
CD8+ T cells and an increase in the percentage of
CD49b+CD107a+ after restimulation. These
observations confirm that the use of the methotrexate nanoconjugate
not only can affect the systemic immune response by activation of a
specific antitumor response, but also can change the landscape of
tumor-infiltrating immune cells from an unfavorable environment.
This in turn should contribute to creation of more appropriate
conditions for generation of a specific antitumor immune response
by DCs inoculated peritumorally, the use of which was planned in
further experiments.
These findings led us to the next stage of in
vivo studies in which we estimated the antitumor activity of
combined chemoimmunotherapy, not only by defining the alterations
occurring in the antitumor immune response, but also by determining
the effect of the therapy by tumor growth inhibition (TGI)
calculation. Despite the different tumor growth rate in the MC38
non-treated (control) group and MTX group and, as consequence of
this, dissections of organs at two time points, we were able to
define TGI for the 30th day of the experiment as a common
denominator for all tested groups. As a result of applied
monotherapy with HES-MTX the TGI value was 64%, but extending the
treatment scheme by multiple peritumoral injection of DC/TAg did
not improve the therapeutic effect of HES-MTX (TGI was 55%). Based
on our previous studies on the use of combined therapy composed of
cyclophosphamide (CY) and DC-based vaccines in this tumor model
(46), we postulate that the lower
TGI value observed in the HES-MTX+DC/TAg group (compared to the
HES-MTX group) may be related to other suppressive factors, e.g.
cytokines, which are present in the tumor microenvironment (TME).
We made such an observation in a previous study, Rossowska et
al (46) where CY
administration generated slightly higher TGI than combined
treatment with CY+DC/TAg. Thus, we hypothesize that weaker
effectiveness of therapy consisting of nanoconjugate and
DC/TAg-based vaccines might be associated with TME-derived
immunosuppressive factors, e.g. IL-10, which affect the function of
DCs administered as cellular vaccines.
The enhancement of the CD45+ cell influx
into tumor nodules only after combined therapies (MTX+DC/TAg and
HES-MTX+DC/TAg groups) again indicates that the application of
chemotherapy prior to DC injection generates a favorable immune
microenvironment in tumors. This was also reflected in the
insignificantly increased M1/M2 ratio value. The lowest percentage
of Mfs and polymorphonuclear (PMN)-MDSCs was found in the
HES-MTX+DC/TAg group. The decrease in the percentage of DCs
infiltrating into tumor tissue in the DC/TAg-receiving group of
mice suggests the intensified migration of in situ activated
DCs to draining lymph nodes. Moreover, the highest expression of
MHC II and CD80 molecules on DCs was observed in the HES-MTX group,
which confirms our previous observations concerning the modulatory
potential of the nanoconjugate towards the DC phenotype. According
to our assumptions, when immunotherapy was used, the high influx of
Th, CTL and NKT cells into tumor nodules was accompanied by reduced
infiltration of Tregs. It should be highlighted that the use of
sole chemotherapy (i.e. MTX and HES-MTX groups of mice in the
chemoimmunotherapeutic treatment scheme) was not sufficient to
maintain the size of the tumor-infiltrating Treg population at a
low level for a long time, as it was observed on the third day
after administration of the chemotherapeutic treatment scheme.
Regardless of a minor reduction in the CD4+ T cell
percentage in the MTX+DC/TAg and HES-MTX+DC/TAg groups compared to
the DC/TAg group, we observed higher percentage of the NKT and
CD8+ T cells in those groups of mice; however, these
differences were statistically significant only in the NKT cell
population. Importantly, the highest percentage of CD8+
T cells was found in the HES-MTX-treated groups of mice, as in the
previous experiment. At this point it is worth mentioning about the
immunohistochemistry (IHC) techniques, which undoubtedly could
provide the additional information about the localization of
crucial immune cells in tumor nodules. However, due to the small
size of the tumor tissue in some groups of mice and due to the
planned extensive multi-parameter flow cytometric analyses, we
decided to focus on the quantitative-flow cytometric assessment of
immune cell infiltrating tumor tissue rather than qualitative
determination of lymphocyte infiltration into tumor nodules (e.g.
IHC). For this reason, we used the entire tumor tissue for flow
cytometric analyses, which provided information about the
percentage of immune cells present in the tumor in the context of
other immune cell subpopulations, e.g. the percentage of
CD8+ T cells among CD45+ cells or percentage
of Tregs among CD4+ T cells. Changes in the landscape of
immune cells which occurred in tumor nodules were also reflected in
the splenic lymphocyte populations. In the HES-MTX+DC/TAg group the
percentages of splenic CD4+ and CD8+ cells
were the lowest, while the percentage of Tregs were the highest.
These changes may result from migration of activated splenic
effector cells to the tumor growth site. Despite the fact that in
the HES-MTX+DC/TAg group after restimulation the percentages of
CD8+ and CD49b+ cells were strongly reduced,
the degranulation assay revealed the highest percentages of
CD4+CD107a+,
CD8+CD107a+ and
CD49b+CD107a+. Despite the low percentage of
CD49b+ cells after restimulation, these cells possessed
high cytotoxic potential, which was reflected in significant
enhancement of cytotoxic activity of splenocytes in the
HES-MTX+DC/TAg group. Similar observations were made by Zhong et
al (53), who found that a
combination of low-dose paclitaxel prior to intratumoral DC-based
vaccine injection in a murine 3LL lung cancer model was more
effective in inhibition of tumor growth. Moreover, combined therapy
with paclitaxel and DC-based vaccines contributed to greater influx
of CD4+ and CD8+ T cells into tumor nodules
and activation of a tumor-specific immune response in regional
lymph nodes than either DC-vaccine or paclitaxel applied alone. The
above results indicate that HES-MTX administration prior to
DC-based immunotherapy affected the TME by eliminating certain
cells with suppressor activity and increasing the influx of CTLs,
which was beneficial for proper functioning of exogenous DCs.
Moreover, this combined treatment had a positive impact on
cytotoxic activity of splenic CTLs. Therefore, we postulate that
activation of both the local and the systemic immune response
finally contributed to inhibition of tumor growth.
In conclusion, our results show for the first time
that the methotrexate nanoconjugate can modulate the systemic
antitumor immune response and cause changes in the landscape of the
TME through increasing influx of effector cells and eliminating
certain cells with suppressor activity. Application of HES-MTX to
MC38-tumor bearing mice resulted in induction of a systemic
specific antitumor response. After therapy consisting of HES-MTX
and DC-based vaccines CTL- and NK-mediated cytotoxicity was
activated additionally. Moreover, in contrast to MTX application,
HES-MTX used alone as well as together with DC-based cellular
vaccines contributed to significant delay in tumor growth. However,
only after treatment with HES-MTX and DC/TAg were there significant
alterations noted in the proportions of crucial host immune cell
populations necessary for an efficient antitumor response.
Nevertheless, further research is required to determine which other
factors present in the MC38 tumor microenvironment may have an
adverse influence on functions of DCs administered as cellular
vaccines and how we can overcome these difficulties.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was supported by the National Science
Centre, Poland (grant no. 2015/19/N/NZ6/02908 and
2017/27/B/NZ6/02702), and Wroclaw Centre of Biotechnology, The
Leading National Research Centre (KNOW) program for the years
2014–2018.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
AS, JR and EPP conceptualized the research; JR, EPP
and TMG developed the research methodology. AS and JR performed the
formal analysis; AS, NAG, KWC, JM, TMG, MŚ, JR and EPP performed
the experiments. AS and EPP wrote the manuscript. All the authors
read and approved the manuscript and provided critical feedback and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All experiments were performed according to EU
Directive 2010/63/EU for animal experiments and were approved by
the 1st Local Ethics Committee for Experiments with the Use of
Laboratory Animals, Wroclaw, Poland (number 31/2016).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nars MS and Kaneno R: Immunomodulatory
effects of low dose chemotherapy and perspectives of its
combination with immunotherapy. Int J Cancer. 132:2471–2478. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Golombek SK, May JN, Theek B, Appold L,
Drude N, Kiessling F and Lammers T: Tumor targeting via EPR:
Strategies to enhance patient responses. Adv Drug Deliv Rev.
130:17–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roma-Rodrigues C, Mendes R, Baptista PV
and Fernandes AR: Targeting tumor microenvironment for cancer
therapy. Int J Mol Sci. 20:8402019. View Article : Google Scholar
|
|
4
|
Hanahan D and Coussens LM: Accessories to
the crime: Functions of cells recruited to the tumor
microenvironment. Cancer Cell. 21:309–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jahchan NS, Mujal AM, Pollack JL,
Binnewies M, Sriram V, Reyno L and Krummel MF: Tuning the tumor
myeloid microenvironment to fight cancer. Front Immunol.
10:16112019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Netea-Maier RT, Smit JWA and Netea MG:
Metabolic changes in tumor cells and tumor-associated macrophages:
A mutual relationship. Cancer Lett. 413:102–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Veglia F and Gabrilovich DI: Dendritic
cells in cancer: The role revisited. Curr Opin Immunol. 45:43–51.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szczygieł A and Pajtasz-Piasecka E:
Between biology and medicine: Perspectives on the use of dendritic
cells in anticancer therapy. Postepy Hig Med Dosw (Online).
71:921–941. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Constantino J, Gomes C, Falcão A, Cruz MT
and Neves BM: Antitumor dendritic cell-based vaccines: Lessons from
20 years of clinical trials and future perspectives. Transl Res.
168:74–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sabado RL, Balan S and Bhardwaj N:
Dendritic cell-based immunotherapy. Cell Res. 27:74–95. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Palucka K and Banchereau J:
Dendritic-cell-based therapeutic cancer vaccines. Immunity.
39:38–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bol KF, Schreibelt G, Gerritsen WR, de
Vries IJM and Figdor CG: Dendritic cell-based immunotherapy: State
of the art and beyond. Clin Cancer Res. 22:1897–1906. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goszczyński T, Nevozhay D, Wietrzyk J,
Omar MS and Boratyński J: The antileukemic activity of modified
fibrinogen-methotrexate conjugate. Biochim Biophys Acta.
1830:2526–2530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hao F, Lee RJ, Yang C, Zhong L, Sun Y,
Dong S, Cheng Z, Teng L, Meng Q, Lu J, et al: Targeted Co-delivery
of siRNA and methotrexate for tumor therapy via mixed micelles.
Pharmaceutics. 11:922019. View Article : Google Scholar
|
|
16
|
Chen Y, Zhang W, Huang Y, Gao F, Sha X,
Lou K and Fang X: The therapeutic effect of methotrexate-conjugated
Pluronic-based polymeric micelles on the folate receptor-rich
tumors treatment. Int J Nanomedicine. 10:4043–4057. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomas TP, Huang B, Choi SK, Silpe JE,
Kotlyar A, Desai AM, Zong H, Gam J, Joice M and Baker JR Jr:
Polyvalent dendrimer-methotrexate as a folate receptor-targeted
cancer therapeutic. Mol Pharm. 9:2669–2676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li MH, Choi SK, Thomas TP, Desai A, Lee
KH, Kotlyar A, Banaszak Holl MM and Baker JR Jr: Dendrimer-based
multivalent methotrexates as dual acting nanoconjugates for cancer
cell targeting. Eur J Med Chem. 47:560–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Figueiró F, de Oliveira CP, Bergamin LS,
Rockenbach L, Mendes FB, Jandrey EH, Moritz CE, Pettenuzzo LF,
Sévigny J, Guterres SS, et al: Methotrexate up-regulates
ecto-5′-nucleotidase/CD73 and reduces the frequency of T
lymphocytes in the glioblastoma microenvironment. Purinergic
Signal. 12:303–312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goszczyński TM, Filip-Psurska B, Kempińska
K, Wietrzyk J and Boratyński J: Hydroxyethyl starch as an effective
methotrexate carrier in anticancer therapy. Pharmacol Res Perspect.
2:e000472014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goszczyński T, Boratyński J, Wietrzyk J,
Filip-Psurska B and Kempińska K: Patent WO/2013/127885; A conjugate
of methotrexate and Hydroxyethyl starch for use in the treatment
cancer. 2013.
|
|
22
|
Koźmiński P, Halik PK, Chesori R and
Gniazdowska E: Overview of dual-acting drug methotrexate in
different neurological diseases, autoimmune pathologies and
cancers. Int J Mol Sci. 21:34832020. View Article : Google Scholar
|
|
23
|
Abolmaali SS, Tamaddon AM and Dinarvand R:
A review of therapeutic challenges and achievements of methotrexate
delivery systems for treatment of cancer and rheumatoid arthritis.
Cancer Chemother Pharmacol. 71:1115–1130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Visentin M, Zhao R and Goldman ID: The
antifolates. Hematol Oncol Clin North Am. 26629–648. (ix)2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brecher ME, Owen HG and Bandarenko N:
Alternatives to albumin: Starch replacement for plasma exchange. J
Clin Apher. 12:146–153. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoffmann S, Caysa H, Kuntsche J,
Kreideweiss P, Leimert A, Mueller T and Mäder K: Carbohydrate
plasma expanders for passive tumor targeting: In vitro and in vivo
studies. Carbohydr Polym. 95:404–413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nogueira E, Sárria MP, Azoia NG, Antunes
E, Loureiro A, Guimarães D, Noro J, Rollett A, Guebitz G and
Cavaco-Paulo A: Internalization of methotrexate conjugates by
folate receptor-α. Biochemistry. 57:6780–6786. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thomas TP, Goonewardena SN, Majoros IJ,
Kotlyar A, Cao Z, Leroueil PR and Baker JR: Folate-targeted
nanoparticles show efficacy in the treatment of inflammatory
arthritis. Arthritis Rheum. 63:2671–2680. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang S and Dormidontova EE: Nanoparticle
targeting using multivalent ligands: Computer modeling. Soft
Matter. 7:4435–4445. 2011. View Article : Google Scholar
|
|
30
|
Fang J, Nakamura H and Maeda H: The EPR
effect: Unique features of tumor blood vessels for drug delivery,
factors involved, and limitations and augmentation of the effect.
Adv Drug Deliv Rev. 63:136–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maeda H: Tumor-selective delivery of
macromolecular drugs via the EPR effect: Background and future
prospects. Bioconjug Chem. 21:797–802. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maeda H and Matsumura Y: EPR effect based
drug design and clinical outlook for enhanced cancer chemotherapy.
Adv Drug Deliv Rev. 63:129–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shurin GV, Tourkova IL, Kaneno R and
Shurin MR: Chemotherapeutic agents in noncytotoxic concentrations
increase antigen presentation by dendritic cells via an
IL-12-dependent mechanism. J Immunol. 183:137–144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaneno R, Shurin GV, Tourkova IL and
Shurin MR: Chemomodulation of human dendritic cell function by
antineoplastic agents in low noncytotoxic concentrations. J Transl
Med. 7:582009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaneno R, Shurin GV, Kaneno FM, Naiditch
H, Luo J and Shurin MR: Chemotherapeutic agents in low noncytotoxic
concentrations increase immunogenicity of human colon cancer cells.
Cell Oncol (Dordr). 34:97–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang L, Dermawan K, Jin M, Liu R, Zheng
H, Xu L, Zhang Y, Cai Y, Chu Y and Xiong S: Differential impairment
of regulatory T cells rather than effector T cells by
paclitaxel-based chemotherapy. Clin Immunol. 129:219–229. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sistigu A, Viaud S, Chaput N, Bracci L,
Proietti E and Zitvogel L: Immunomodulatory effects of
cyclophosphamide and implementations for vaccine design. Semin
Immunopathol. 33:369–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu JY, Wu Y, Zhang XS, Yang JL, Li HL,
Mao YQ, Wang Y, Cheng X, Li YQ, Xia JC, et al: Single
administration of low dose cyclophosphamide augments the antitumor
effect of dendritic cell vaccine. Cancer Immunol Immunother.
56:1597–1604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rossowska J, Anger N, Kicielińska J,
Pajtasz-Piasecka E, Bielawska-Pohl A, Wojas-Turek J and Duś D:
Temporary elimination of IL-10 enhanced the effectiveness of
cyclophosphamide and BMDC-based therapy by decrease of the
suppressor activity of MDSCs and activation of antitumour immune
response. Immunobiology. 220:389–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rossowska J, Pajtasz-Piasecka E, Anger N,
Wojas-Turek J, Kicielińska J, Piasecki E and Duś D:
Cyclophosphamide and IL-12-transduced DCs enhance the antitumor
activity of tumor antigen-stimulated DCs and reduce Tregs and MDSCs
number. J Immunother. 37:427–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pajtasz-Piasecka E, Szyda A, Rossowska J,
Krawczenko A, Indrová M, Grabarczyk P, Wysocki P, Mackiewicz A and
Duś D: Loss of tumorigenicity of murine colon carcinoma MC38/0 cell
line after transduction with a retroviral vector carrying murine
IL-12 genes. Folia Biol (Praha). 50:7–14. 2004.PubMed/NCBI
|
|
42
|
Rossowska J, Pajtasz-Piasecka E, Szyda A,
Krawczenko A, Zietara N and Dus D: Tumour antigen-loaded mouse
dendritic cells maturing in the presence of inflammatory cytokines
are potent activators of immune response in vitro but not
in vivo. Oncol Rep. 21:1539–1549. 2009.PubMed/NCBI
|
|
43
|
Thomas E, Jones G, de Souza P, Wardrop C
and Wusteman F: Measuring blood volume with fluorescent-labeled
hydroxyethyl starch. Crit Care Med. 28:627–631. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nevozhay D, Budzynska R, Kanska U,
Jagiello M, Omar MS, Boratynski J and Opolski A: Antitumor
properties and toxicity of dextran-methotrexate conjugates are
dependent on the molecular weight of the carrier. Anticancer Res.
26:1135–1143. 2006.PubMed/NCBI
|
|
45
|
Ciekot J, Goszczyński T and Boratyńskit J:
Methods for methotrexate determination in macromolecular conjugates
drug carrier. Acta Pol Pharm. 69:1342–1346. 2012.PubMed/NCBI
|
|
46
|
Rossowska J, Anger N, Szczygieł A,
Mierzejewska J and Pajtasz-Piasecka E: Reprogramming the murine
colon cancer microenvironment using lentivectors encoding shRNA
against IL-10 as a component of a potent DC-based
chemoimmunotherapy. J Exp Clin Cancer Res. 37:1262018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pajtasz-Piasecka E, Rossowska J, Szyda A,
Krawczenko A and Dus D: Generation of anti-tumor response by JAWS
II mouse dendritic cells transduced with murine interleukin 12
genes. Oncol Rep. 17:1249–1257. 2007.PubMed/NCBI
|
|
48
|
Ji X, Guo H, Tang Q, Ma D and Xue W: A
targeted nanocarrier based on polyspermine for the effective
delivery of methotrexate in nasopharyngeal carcinoma. Mater Sci Eng
C Mater Biol Appl. 81:48–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Goos JACM, Cho A, Carter LM, Dilling TR,
Davydova M, Mandleywala K, Puttick S, Gupta A, Price WS, Quinn JF,
et al: Delivery of polymeric nanostars for molecular imaging and
endoradiotherapy through the enhanced permeability and retention
(EPR) effect. Theranostics. 10:567–584. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kobayashi H, Watanabe R and Choyke PL:
Improving conventional enhanced permeability and retention (EPR)
effects; what is the appropriate target? Theranostics. 4:81–89.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pajtasz-Piasecka E and Indrová M:
Dendritic cell-based vaccines for the therapy of experimental
tumors. Immunotherapy. 2:257–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zong J, Keskinov AA, Shurin GV and Shurin
MR: Tumor-derived factors modulating dendritic cell function.
Cancer Immunol Immunother. 65:821–833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhong H, Han B, Tourkova IL, Lokshin A,
Rosenbloom A, Shurin MR and Shurin GV: Low-dose paclitaxel prior to
intratumoral dendritic cell vaccine modulates intratumoral cytokine
network and lung cancer growth. Clin Cancer Res. 13:5455–5462.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rossowska J, Anger N, Szczygieł A,
Mierzejewska J and Pajtasz-Piasecka E: Intratumoral
lentivector-mediated TGF-β1 gene downregulation as a potent
strategy for enhancing the antitumor effect of therapy composed of
cyclophosphamide and dendritic cells. Front Immunol. 8:7132017.
View Article : Google Scholar : PubMed/NCBI
|