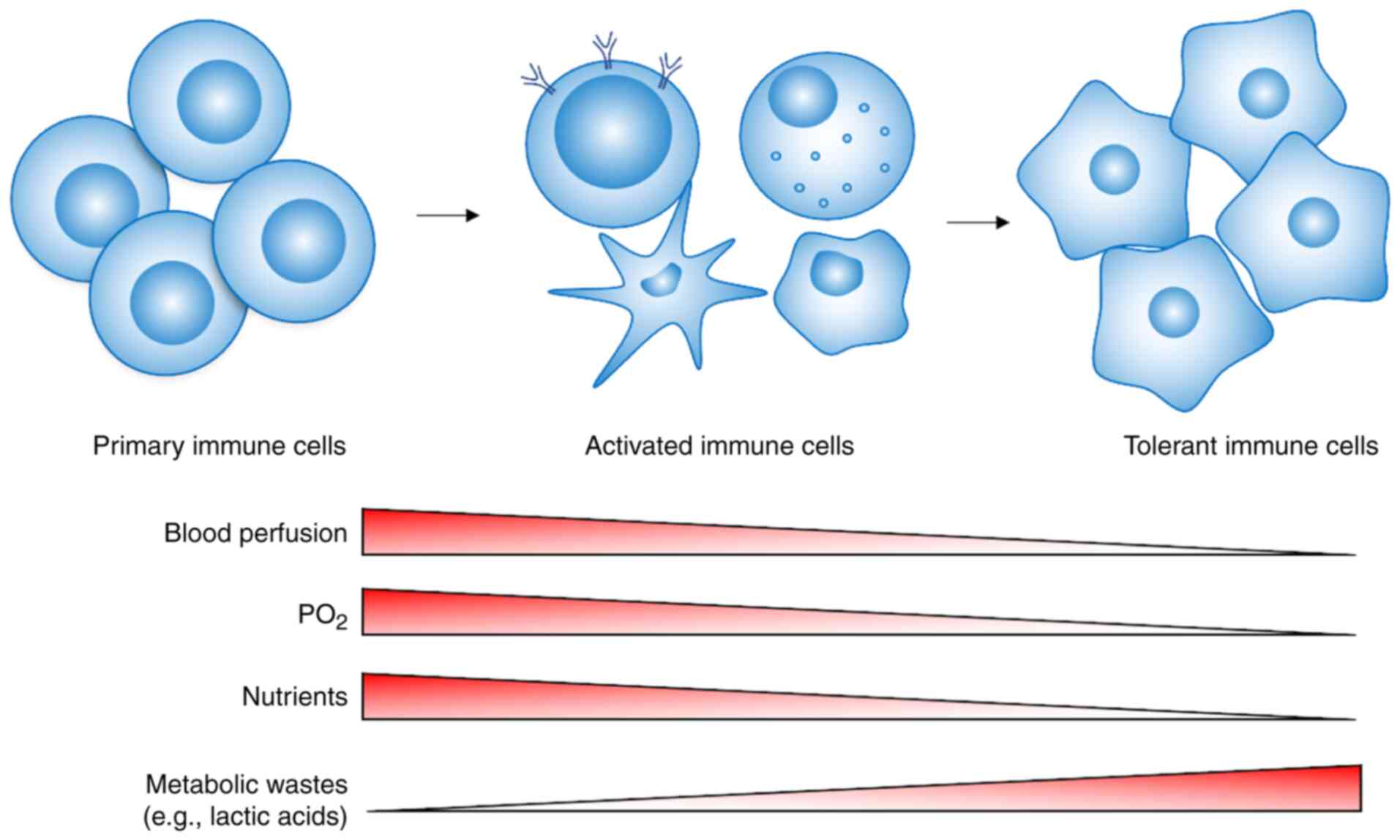
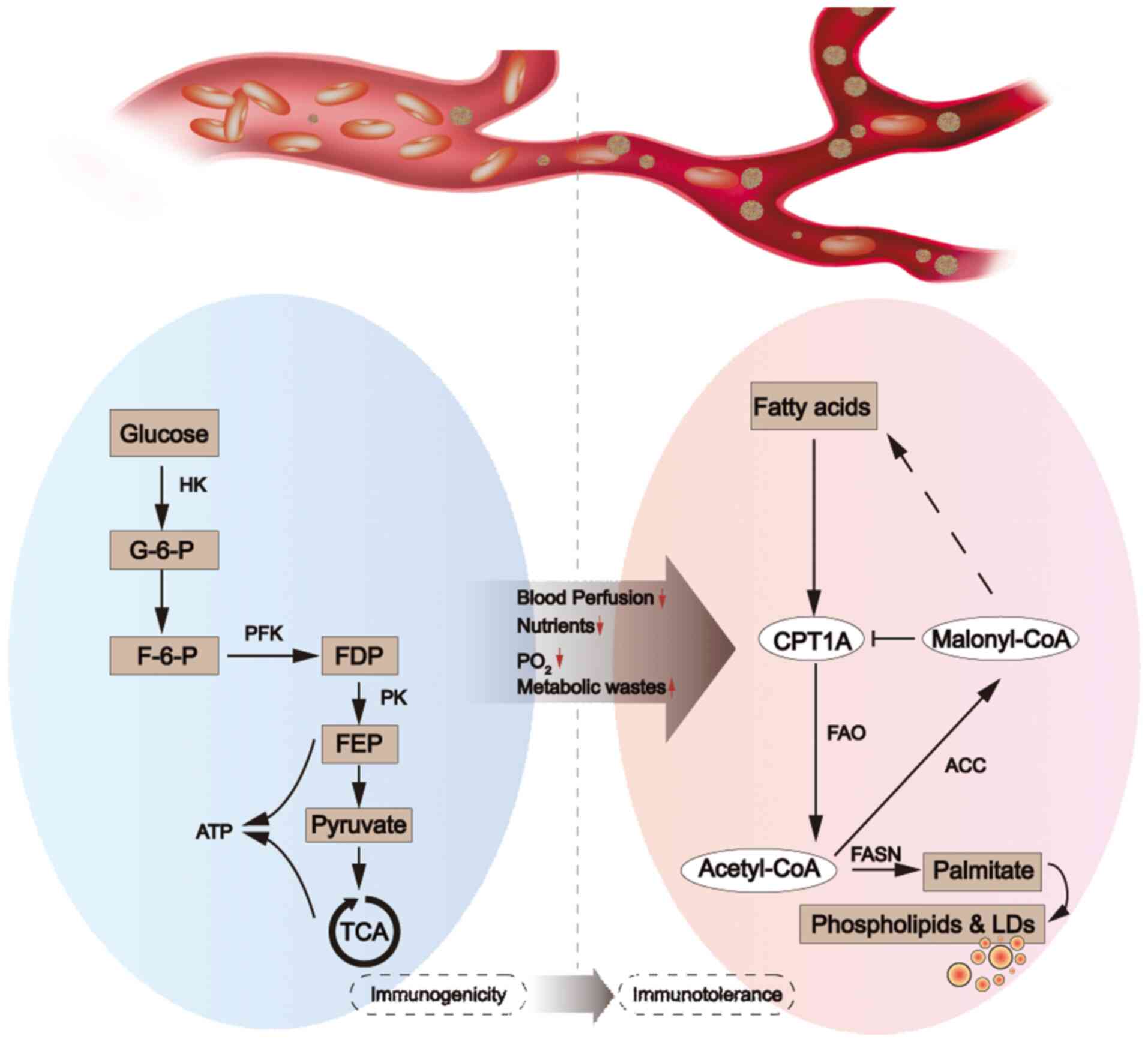
Since Otto Warburg proposed in 1924 that tumor cells
tend to produce energy rapidly through glycolysis (9), the important role of metabolic
reprogramming in tumor development has gradually been recognized
(10,11). However, the importance of lipid
metabolism was generally ignored over the years, but lipid
metabolism has been widely studied in the past few years. Based on
previous research, we speculate that changes in lipid metabolism,
especially fatty acid metabolism, are crucial in determining the
immune activity or tolerance of immune cells. The metabolic pattern
of infiltrating immune cells in breast cancer changes significantly
across different environments. However, at present, research on
this topic is not cohesive. Therefore, this review focuses on the
metabolic changes in several immune cells with relatively high
infiltration in different microenvironments in breast cancer and
addresses how cells are changed into a ‘bystander’ or an
‘accomplice’ by regulating lipid metabolism. It is helpful to
further understand the metabolic heterogeneity of infiltrating
immune cells in breast cancer in the contexts of different
backgrounds and provide novel ideas for immunotherapy.
Abbreviations used in the present review are included in Table I.
To understand the metabolic heterogeneity of immune
cells in breast cancer, the relationship among tumor cells, immune
cells and metabolism was analyzed in this review. Every aspect of
tumor tissue that differs from that of normal tissue may be the
cause of tumor metabolic heterogeneity. In recent years, an
increasing number of studies have shown that the metabolic patterns
of different groups in tumors are coupled with each other through
metabolism, and metabolism plays a very important role in the
functional maintenance and directional differentiation of immune
cells; that is, immune cells in the TME will withstand metabolic
reprogramming, undergoing either activation to play an antitumor
role or immune tolerance to promote tumor progression (12–15).
Metabolic heterogeneity is one of the markers of
breast cancer, and the genetic and phenotypic diversity of breast
cancer is the main obstacle when treating tumors (16). Due to random genetic changes,
intratumoral heterogeneity is generally considered to be chaotic.
By contrast, changes in tumor cell metabolism produces predictable
extracellular metabolite gradients, which combined with the
distance of the tumor cells from the blood supply (17), forms a unique TME and thus
coordinates the diverse phenotypes of various cells (18,19).
Previous studies have shown that breast cancer cells have a higher
extracellular environment acidification capacity than normal breast
cells (20,21). On the other hand, the rapid growth
of cells is faster than the rate of capillary formation in cancer,
leading to gradual hypoxia in breast cancer tissue (22,23),
and creating a nutrient-deficient environment (24). The series of gradient changes
described above leads to immune cells making metabolic adjustments
that correspond to these different microenvironments. The present
review described the changes in the metabolic patterns of
tumor-infiltrating immune cells in different microenvironments and
the induction of a tolerance phenotype by lipid metabolism.
Tumor-infiltrating lymphocytes (TILs) have a strong
prognostic value in various types of cancer (25), and they are also the most common
type of infiltrating immune cell in breast cancer (26). T cells comprise heterogeneous cell
groups with a wide range of effector mechanisms, ranging from
immunosuppression to cytotoxicity.
T lymphocytes are activated to become mature T
lymphocytes that show associated functions via stimulation of T
cell receptor (TCR) signaling. Activated T cells significantly
upregulate glycolysis and lactate production (27). ADP-dependent glucokinase (ADPGK),
which is a protein typically found in Archaea whose function in
eukaryotes was unknown, is activated in this process, which is
accompanied by rapid glucose uptake and decreased mitochondrial
oxygen consumption, thus resulting in the increase of glycolysis
flux (28). However, it cannot be
ignored that mitochondria-dependent metabolism still plays an
important role in the T cell response (29). In the absence of TCR stimulation,
immature T cells remain in a dormant state, reducing the expression
of nutrient transporters, but still maintaining certain catabolic
processes, including autophagy, oxidative phosphorylation (OXPHOS)
and fatty acid oxidation (FAO) (30). According to the literature,
different lymphocyte subtypes exhibit different metabolic patterns
during the activation process, in which the effector T lymphocytes
show high glycolysis and lipogenesis (31), while regulatory T cells (Tregs) show
higher lipolysis and lipid oxidation (32).
Due to factors such as hypoxia, lactate and
adenosine accumulation, nutrient deficiency, and immunosuppression,
the activation and survival of T cells face great challenges. Both
normal and tumor cells can adapt to hypoxia or a hypoxic
microenvironment by regulating hypoxia inducible factor (HIF). A
lack of oxygen supply, or hypoxia, will increase the expression and
stability of HIF in T lymphocytes, thus activating certain
molecular programs, including glycolysis (33). HIF mainly reduces oxygen consumption
by increasing the expression of pyruvate dehydrogenase kinase 1
(PDK1), thus promoting T cell adaptation to hypoxia (34). At this time, T lymphocytes also play
a corresponding role by regulating their own metabolic mode.
However, in the central tumor area with extreme hypoxia, T cell
survival is threatened. To survive, T cells reduce their dependence
on glycolysis, developing an immunosuppressive phenotype (35). Studies have demonstrated that breast
cancer tumors secreting a large amount of lactate have relatively
great metastatic potential, and the prognosis of patients with
these tumors is worse than that of patients with tumors secreting
less lactate (36,37). One of the possible reasons is that
the acidic environment formed by the accumulation of a large amount
of lactate inhibits the proliferation and function of T cells
(38,39). Activated T cells also depend on
glycolysis. Due to the high demand for energy in the processes of
proliferation and cytokine production, to ensure continuous
glycolysis, cells pump out lactate molecules. The large
accumulation of lactate in the breast cancer environment leads to
an inappropriate lactate gradient between the extracellular
environment and the cytoplasm, thus reducing energy metabolism and
ultimately infiltrated T cells gradually polarize into the
immunosuppressive Treg phenotype (40).
In addition, glucose deprivation increases the
ability of T helper (Th) cells to secrete transforming growth
factor β, thus confirming the shift of the microenvironment from
immunostimulatory to immunosuppressive (41). It has been reported that T cells can
transition into a metabolic mode to perform lactate uptake in a
glucose-deficient TME (42).
Besides, the reversal of the lactic dehydrogenase reaction to
generate pyruvate depletes nicotinamide adenine dinucleotide and
effectively inhibits GAPDH activity and glycolytic flux (43), which is particularly harmful to
cytotoxicity and effector T cells. Of note, immunosuppressive Tregs
show resistance to lactate inhibition via downregulation of c-Myc
expression by forkhead box P3 (Foxp3), which reduces glycolysis
dependence (44). At the same time,
T cells stimulated by TCR signaling in glutamine- and
glucose-deficient conditions preferentially differentiate into
Tregs, which may be because their oxidative phenotypes are
metabolically suited for survival in this environment (36). When the survival of cells is
threatened, their metabolic mode changes, and glucose is no longer
the first-choice energy source; instead, cells are more inclined to
use lipids for energy supply and maintenance (45). This is because the outer edge of
tumor tissue with abundant blood vessels in breast cancer mostly
contains effector T cells, and in the area lacking a sufficient
blood supply, Tregs have more advantages; these differences reflect
the metabolic heterogeneity of TILs (46).
Various studies have found that OXPHOS and
glycolysis, especially glycolytic flux, are significantly increased
in effector T cells (47–49). By contrast, Tregs show a unique
metabolic program that mainly utilizes mitochondrial oxidation of
lipids and pyruvate (50,51). Other evidence has suggested that
inhibition of glycolysis obstructs the development of Th1 and Th17
cells, but promotes the production of Tregs (52). Adenosine 5′-monophosphate
(AMP)-activated protein kinase (AMPK) is a major sensor and
regulator of energy metabolism in mammalian cells. AMPK interferes
with T cell differentiation and effector functions by suppressing
mechanistic target of rapamycin kinase (mTOR) and subsequently
inhibiting glycolysis and enhancing lipid oxidation (52).
Foxp3 is highly expressed in Tregs. Studies have
reported that Foxp3 plays an important role in the regulation of
metabolism (53,54). In a previous study, CD4+
T cells were stimulated and transduced to express Foxp3 under
neutral conditions, and the results showed that the expression of
lipid metabolism-related genes was significantly increased in the
Foxp3-expressing cells, while the expression of genes related to
glucose and nucleic acid metabolism was downregulated (55). It is speculated that Foxp3 may
directly regulate the PI3K-AKT-mTORC1 pathway or indirectly
regulate the expression of metabolic genes and establish a
phenotype of glycolysis inhibition (55). Immune cells respond to activation
and toll-like receptor (TLR) signaling by increasing solute carrier
family 2 member 1 (GLUT1) expression and glycolysis (55). By contrast, Foxp3 reduces GLUT1
expression, glycolysis and anabolism, indicating an activated
mitochondrial oxidation pathway (56). In vitro studies have also
demonstrated that Foxp3+ Tregs mainly rely on lipid
oxidation to promote mitochondrial OXPHOS, and it has been
speculated that Foxp3 expression is the basis of this metabolic
preference (55).
Tumor-associated macrophages (TAMs), another ‘main
force’ in the TME, have been observed in the invasive front of
breast cancer tumors in patients (57). Previous reports demonstrated that
compared with malignant cells that have not undergone
epithelial-mesenchymal transition (EMT), breast cancer cells with
EMT changes have the ability to polarize macrophages into the M2
phenotype, suggesting that macrophages in the breast cancer
microenvironment play an important role in tumor invasion (58,59).
As commonly known, the main subtypes of macrophages are
proinflammatory M1 macrophages and anti-inflammatory M2
macrophages. M1 macrophages mainly secrete cytokines such as
interferon-γ (IFN-γ), interleukin (IL)-8 and TNF-α, which play
pro-inflammatory and antitumor roles. On the other hand, M2
macrophages mainly secrete factors such as IL-13, C-C motif
chemokine (CCL)17 and CCL18 to promote tumor development (60,61).
Due to a combination of numerous factors and the complexity of the
TME, the phenotype of TAMs may be between M1 and M2 types, or
different from M1 or M2 types that can't be regarded as either type
specifically. Thus, TAMs can no longer be simply considered
either/or populations (62).
To clarify the metabolic characteristics of
macrophage subtypes, cells can still be divided into M1 and M2 type
macrophages. M1 macrophages show enhanced aerobic glycolysis,
increased pentose phosphate pathway activity and fatty acid
synthesis flux. However, at the level of succinate dehydrogenase
and isocitrate dehydrogenase, M1 macrophages also exhibit
incomplete OXPHOS, and mitochondrial adenosine triphosphate (ATP)
synthesis is blocked (63). M2
macrophages break down arginine into urea and urethane via arginase
1 (ARG1). ARG1 is a representative marker of M2 macrophages, and
nitric oxide (NO) production in M2 macrophages is blocked,
resulting in inhibition of nitroso-mediated OXPHOS, which is
conducive to maintaining the M2 phenotype (64). M2 macrophages show relatively low
levels of glycolysis and enhanced FAO to fuel OXPHOS (65). Highly glycolytic tumor cells may
prevent polarization into the M1 phenotype by inducing glucose
deprivation, while the abundance of fatty acids may affect the
differentiation of cells into the M2 phenotype (66,67).
Similar to TILs, tumor-infiltrating macrophages with
different spatial distributions face different challenges and
respond accordingly. Carmona-Fontaine et al (19) found that TAMs expressing ARG1 were
almost completely located in the ischemic tumor area, while TAMs
expressing mannose receptor C-type 1 (MRC1) were found in the
perivascular and other well-nourished tumor areas, and the research
also showed that the subgroup of TAMs expressing MRC1 in the
perivascular region of patients with breast cancer was important
for tumor recurrence after chemotherapy (19). Some studies have reported that
lactate produced by breast cancer cells, a key metabolite in the
TME, can promote M2-like polarization of macrophages by inducing
high expression of VEGF and ARG1 in macrophages, and this series of
changes may be mediated by HIF-1α (68,69).
Almost all studies have provided extensive evidence of the
synergistic effect of hypoxia and lactate (70,71).
When macrophages in normoxic or hypoxic environments are treated
with various lactate doses, the ARG1 protein level in macrophages
increases in hypoxic conditions, but not in normoxic conditions
(19). Additionally, macrophages
activated by lactate and/or hypoxia can induce aerobic glycolysis
and epithelial stromal transformation in tumor cells by regulating
the CCL5/C-C chemokine receptor type 5 (CCR5) axis, forming a
regulatory feedback loop to promote the progression of breast
cancer (72). The metabolic pattern
of M1 macrophages is similar to that of tumor cells, showing highly
activated glycolysis, which indicates that M1 macrophages and tumor
cells compete with and suppress each other (73). By contrast, M2 macrophages
preferentially use FAO, which is more conducive to their survival
in the TME, and became a favorable promoter of tumor progression
(74).
TAMs promote tumor growth and metastasis by
inhibiting tumor immune surveillance. There is evidence that the
immunosuppressive phenotype of TAMs is regulated by long-chain
fatty acid metabolism, especially unsaturated fatty acid metabolism
(75). In vitro, the
addition of unsaturated fatty acids was found to polarize myeloid
cells derived from the bone marrow into M2 macrophages with a
strong inhibitory ability. Lipid droplets play a vital role by
regulating the catabolism of free fatty acids during mitochondrial
respiration (76). IL-4-induced M2
macrophages increase their expression of CD36, thus enhancing the
uptake of very low-density lipoprotein (VLDL) and LDL, activate
FAO, and rely on FAO to support proliferation (77). Inhibition of mTOR eliminates the
mitochondrial respiration induced by lipid droplets, thus
eliminating the immunosuppressive effect of TAMs (75). A previous study reported that
simvastatin repolarizes TAMs and promotes M2 to M1 phenotypic
conversion through cholesterol-related liver X receptor/ATP binding
cassette transporter A1 regulation (78). These results suggested that lipid
metabolism plays an important role in the differentiation and
functional maintenance of M2 macrophages and that further study of
lipid metabolism has the potential to identify potential targets
and generate novel antitumor treatments.
NK cells are key components in innate immunity. They
are mainly generated from hematopoietic stem cells that develop in
the bone marrow and distribute to multiple peripheral tissues after
maturation (79). They have the
potential to kill tumor cells in different ways without prior
sensitization, so they have become an important tool in cancer
immunotherapy (80).
In general, resting NK cells use OXPHOS to meet
their own steady-state needs because this pathway can effectively
generate energy without requiring an excessive investment in
synthesis (81). When NK cells are
activated, they change their metabolic mode to be able to create
the large number of biosynthetic precursors required for the
synthesis of effector molecules. NK cells have been proven to be
able to strongly upregulate glycolysis and the OXPHOS pathway
(82,83). Activated NK cells transform
glycolysis-derived NADH into mitochondrial NADH via the
citrate-malate shuttle mechanism, which promotes OXPHOS and the
synthesis of ATP (84). NK cells
are widely characterized as CD56dim and
CD56bright (85). These
subsets also differ in terms of metabolism. CD56bright
cells are more sensitive to metabolic changes, and their
upregulation of the expression of centralized metabolic markers is
stronger than that of CD56dim cells (82). Schafer et al (86) recently reported that NK cells with
low reactivity used mitochondrial respiration to activate cytotoxic
function, while functional NK cells showed increased glycolysis
accompanied by OXPHOS. One of the main limitations of NK cell
activity is the immunosuppressive TME. Tumors and other immune
cells create conditions that favor tumor proliferation, while also
blocking the activation of NK cells.
The activity of NK cells is lower near the ischemic
area in the tumor center, and it is difficult for even NK cells to
infiltrate into the central area. The lack of nutrients and oxygen,
and high concentrations of tumor-derived metabolites (such as
lactate and adenosine) disrupt the metabolism of NK cells in the
TME (80). Short-term exposure to
hypoxia enhances the function of NK cells (87), but after long-term hypoxia, NK cells
upregulate HIF-1α expression, resulting in a change in the
transcriptional profile and obvious downregulation of the
expression of cytotoxic receptors, such as natural killer cell
p30-related protein (NKp30), NKp44, NKp46 and natural killer group
2 member D (88). At the same time,
HIF-1α also affects the following: i) Glycolytic enzymes M2 isoform
of pyruvate kinase and phosphoglycerate kinase 1; ii) the
metabolite transporters, including GLUT1, solute carrier family 2
member 3, solute carrier family 1 member 5 and solute carrier
family 16 member 4; and iii) enzymes involved in biosynthesis, such
as fatty acid synthetase (FASN) and glucose
6-phosphatedehydrogenase (89). The
expression of IFN-γ in mature NK cells is decreased, the production
of IFN-γ is decreased, and OXPHOS is reduced (90).
A recent study showed that adenosine attenuated the
metabolic activity of IL-12/15-stimulated human NK cells by
inhibiting OXPHOS and glycolysis (91). Furthermore, it has also been
demonstrated that the uptake of lactic acid by NK cells in the TME
leads to intracellular acidification and energy metabolism
disruption (92). On the one hand,
the increasing demand of tumors for amino acids and the lack of a
fuel supply in the microenvironment reduces the functions of NK
cells. We also speculated that the consumption of amino acids by
tumor cells and tumor-related cells will lead to the accumulation
of immunosuppressive metabolites in the TME, which indirectly
affects the function of NK cells. For example, myeloid-derived
suppressor cells (MDSCs) upregulate the expression of arginase and
inducible NO synthetase, which use arginine as a substrate, and the
latter metabolizes arginine into NO. It has been found that NO
weakens the cytotoxicity of antibody-dependent NK cells (93). NK cells in breast cancer gradually
experience an increasingly harsh environment. The metabolism of NK
cells is negatively affected, with the cells transitioning from an
antitumor function to being unable to undergo activation or even
becoming unable to survive in the central tumor area without blood
perfusion (94).
NK cells are significantly inhibited in the severe
TME and are even found in the resting state (95). It was found that genes related to
glycolysis and OXPHOS were downregulated in NK cells undergoing
severe TME. By contrast, the activity of lipid metabolism is
increased (96,97). Studies have also confirmed that when
NK cells survival is threatened, lipid metabolism becomes the
preferred mode of metabolism (98,99).
In a mouse model of breast cancer, NK cells appeared to accumulate
lipids in vivo, which was mediated by CD36 and CD68, after
surgery. These NK cells showed an inhibitory effector function with
downregulation of the expression of perforin- and granzyme-related
genes (100). Peroxisome
proliferator-activated receptor drives lipid accumulation in NK
cells, leading to complete ‘paralysis’ of cell metabolism and
transportation (101). Preventing
lipids from entering the mitochondria reverses NK cell metabolic
paralysis and restores cytotoxicity (101). Compared with immune cells with an
immunotolerant phenotype, NK cells have shown a smaller effect on
cell function mediated by lipid reprogramming, and the mechanism of
action is not sufficiently understood (101). However, it is of great
significance and value to identify the potential targets of lipid
metabolism in NK cells and thereby improve immunotherapy.
Tumor-associated DCs have major defects in function
and activity, and promote tumor immunosuppression. Studies have
reported that abnormal lipid accumulation in DCs is one of the main
mechanisms leading to DC dysfunction (102,103). DCs are important regulators of
activation or tolerance in the adaptive immune response (104), and are also one of the most
important types of antigen-presenting cells in the breast cancer
microenvironment (105). DCs can
not only regulate immunogenicity, but also induce tolerance. One of
the key factors determining this functional fate is the metabolic
process of DCs (13).
In the process of DC activation, glycolysis and
glucose, as the preferred carbon source, can promote an immunogenic
or inflammatory state, while OXPHOS and FAO are conducive to the
transformation of tolerant DCs (106,107). Glycolysis is important for the
maturation and function of both conventional DCs (cDCs) and bone
marrow-derived DCs (BMDCs). Treatment with 2-deoxyglucose (2-DG, an
inhibitor of glycolysis) impairs the expression of costimulatory
markers, the production of IL-12, and functioning by BMDCs and cDCs
(108). Glucose can promote the
migration of BMDCs and cDCs along a C-C motif chemokine ligand 21
gradient, which can be inhibited by 2-DG treatment (108). In addition, glycolysis is also
needed to maintain the slender cell shape of BMDCs and promote CCR7
oligomerization so that DCs can move and migrate to the draining
lymph nodes (109).
The duality of DC immunoregulatory functions mainly
depend on the differentiation and activation state of DCs (104). DCs undergo metabolic
transformation in the process of maturation, from FAO and OXPHOS to
glycolysis. Unlike the transformations of tumor cells and effector
T cells, this transformation of DCs does not promote cell division,
but is crucial in the activation and survival of DCs after TLR
stimulation (110). The
concentration of lactate after glycolysis, rather than the
availability of oxygen tends to shift the differentiation of DCs in
the direction of tolerance (111).
The high concentration of extracellular lactate in the TME may
prevent lactate output from glycolysis-dependent DCs. Tumor-derived
lactate is an important factor regulating the DC phenotype in the
TME, which may play a key role in the tumor escape mechanism
(112). Additionally, the effect
of hypoxia on DCs is very important in regulating the quality and
intensity of the immune response. DCs derived from human monocytes
exposed to hypoxia express high levels of HIF-1α. Short-term
hypoxia can indeed enhance the migration of DCs through a
HIF-1α-mediated glycolytic pathway, thus showing obvious
immunogenicity. However, long-term hypoxia can cause cell death
(113).
Of note, recent evidence has shown that the role of
glucose in DCs depends on the state of adjacent cells (108). For example, glucose can promote
the immune function of DCs, but this function is severely inhibited
in areas of high ischemia and hypoxia in tumors (114). The reason is that the high rate of
glycolysis of tumor cells leads to local glucose deprivation, and
thus the substrate of glycolysis may not be used by DCs. Therefore,
the TME may not be suitable for activation of DCs dependent on
glycolysis, and thus DCs may be required to transition to fatty
acid-dependent oxidation (8).
DCs isolated from various tumor models and patients
with tumors have shown that the accumulation of lipids in DCs
limits the cross presentation of antigens (102). Consistent with these findings, the
accumulation of lipid droplets prevents DCs from inducing antitumor
T cell responses (119). DCs with
an immunotolerant phenotype show strong activation of endoplasmic
reticulum stress and X-box binding protein 1 spliced by endoplasmic
reticulum stress response factor, which induces the biosynthesis of
triglycerides and leads to abnormal lipid accumulation (103). The aforementioned studies suggest
that lipid metabolism plays an important role in the development of
tolerance in DCs.
There are numerous types of infiltrated immune cells
in the TME. In addition to the main immune cells mentioned above,
there are also some immune cells that are less common, but still
play important roles in antitumor immunity, such as mast cells,
monocytes, eosinophils and basophils (120,121). At present, the metabolic
reprogramming of these cells in the TME is relatively unstudied.
Some of these cell types have been studied briefly, while there are
a few that have not yet been reported on, but they are worthy of
further exploration. Mast cells are unique tissue-resident immune
cells that can secrete a variety of bioactive compounds, which can
stimulate, regulate or inhibit the immune response. Increasing
evidence has reported that mast cells infiltrate breast cancer, but
whether they are a driving force or have an opposing role in breast
cancer progression is controversial (122–124).
In breast cancer, myeloid cells can transform into
MDSCs and play a key role in tumor immunosuppression. Decades ago,
studies demonstrated that cancer cells rather than stromal cells
prefer to export fatty acids to form fatty acid-rich niches
(125,126). MDSCs absorb fatty acids from the
TME and use these molecules in a variety of ways to maximize their
effects. An increase in fatty acid uptake by MDSCs leads to the
accumulation of intracellular lipids, and lipid-overloaded MDSCs
have a stronger immunosuppressive effect on CD8+ T cells
(127). Cao et al (128) revealed that the expression of
fatty acid transporter 4 (FATP4) was significantly upregulated in
MDSCs. Other studies have also reported that the upregulation of
FATP2 expression in polymorphonuclear myeloid-derived suppressor
cells (PMN-MDSCs) is the key regulator of PMN-MDSC
immunosuppressive function. FATP2 promotes the accumulation of
arachidonic acid, resulting in the synthesis of prostaglandin E2 in
MDSCs, which enhances MDSC immunosuppressive activity (129). A series of changes lead to MDSCs
surviving in the harsh TME. The role of lipid metabolism in
determining whether immune cells differentiate into an
immunotolerant phenotype has been ignored, as has whether
immunosuppressive cell infiltration of tumor tissue is induced by a
harsh environment, which provide novel ideas and insights for tumor
immune research.
The complex and dynamic changes in the TME cause
infiltrating immune cells to face different challenges. Compared
with the randomness and chaos of genetic changes, predictable
extracellular metabolite gradients allow speculation on the
metabolic changes experienced by immune cells. By studying and
summarizing the changes in the metabolic patterns of several
typical tumor-infiltrating immune cell types in different TMEs
(Table II), the present review
concluded that mild hypoxia and a low lactic acid concentration are
beneficial for immune cell activation and a metabolic mode favoring
glycolysis. In such a scenario, the immune cells have certain
immunogenicity and play an antitumor role. However, facing severe
survival challenges, such as hypoxia, an extreme lack of nutrients
and high acidity in the surrounding environment, the immune cells
will gradually shift their metabolism mode into lipid metabolism,
in some cases, even depending on FAO, and the immune cells will
show a tolerant or inhibited phenotype (Fig. 2). The function of immune cells also
changes when the metabolism mode changes, which provides us with a
number of potential immunotherapy targets and novel treatment
ideas. If the key genes and enzymes of lipid metabolism can be
targeted, it may be possible to reverse the tolerant phenotype of
immune cells and enhance the antitumor effect. In the future, it is
necessary to study the metabolic heterogeneity of immune cells and
the causes of this heterogeneity, in order to provide more
effective methods for tumor immunotherapy.
Not applicable.
This work was supported by the Natural Science
Foundation Project of Chongqing, CSTC (grant nos.
cstc2020jcyj-msxmX0485 and cstc2020jcyj-msxmX0668).
Not applicable.
HC, YS, ZY, SY, YL, MT, JZ and FZ contributed to the
conceptualization, literature review, original draft preparation,
editing and review of this manuscript. All authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vranic S, Cyprian FS, Gatalica Z and
Palazzo J: PD-L1 status in breast cancer: Current view and
perspectives. Semin Cancer Biol. Dec 26–2019.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Engelhard VH, Rodriguez AB, Mauldin IS,
Woods AN, Peske JD and Slingluff CL Jr: Immune Cell Infiltration
and tertiary lymphoid structures as determinants of antitumor
immunity. J Immunol. 200:432–442. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Savas P, Virassamy B, Ye C, Salim A,
Mintoff CP, Caramia F, Salgado R, Byrne DJ, Teo ZL, Dushyanthen S,
et al: Publisher correction: Single-cell profiling of breast cancer
T cells reveals a tissue-resident memory subset associated with
improved prognosis. Nat Med. 24:19412018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang SC, Hu ZQ, Long JH, Zhu GM, Wang Y,
Jia Y, Zhou J, Ouyang Y and Zeng Z: Clinical implications of
tumor-infiltrating immune cells in breast cancer. J Cancer.
10:6175–6184. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu D: Innate and adaptive immune cell
metabolism in tumor microenvironment. Adv Exp Med Biol.
1011:211–223. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kishton RJ, Sukumar M and Restifo NP:
Metabolic regulation of T cell longevity and function in tumor
immunotherapy. Cell Metab. 26:94–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hobson-Gutierrez SA and Carmona-Fontaine
C: The metabolic axis of macrophage and immune cell polarization.
Dis Model Mech. 11:dmm0344622018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Warburg O: Über den Stoffwechsel der
Carcinomzelle. Naturwissenschaften. 12:1131–1137. 1924. View Article : Google Scholar
|
|
10
|
Lane AN, Higashi RM and Fan TW: Metabolic
reprogramming in tumors: Contributions of the tumor
microenvironment. Genes Dis. 7:185–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim J and DeBerardinis RJ: Mechanisms and
implications of metabolic heterogeneity in cancer. Cell Metab.
30:434–446. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mehla K and Singh PK: Metabolic regulation
of macrophage polarization in cancer. Trends Cancer. 5:822–834.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Basit F, Mathan T, Sancho D and de Vries
IJM: Human dendritic cell subsets undergo distinct metabolic
reprogramming for immune response. Front Immunol. 9:24892018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L and Romero P: Metabolic control of
CD8(+) T cell fate decisions and antitumor immunity. Trends Mol
Med. 24:30–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Poznanski SM, Barra NG, Ashkar AA and
Schertzer JD: Immunometabolism of T cells and NK cells: Metabolic
control of effector and regulatory function. Inflamm Res.
67:813–828. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Norton KA, Jin K and Popel AS: Modeling
triple-negative breast cancer heterogeneity: Effects of stromal
macrophages, fibroblasts and tumor vasculature. J Theor Biol.
452:56–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Skala MC, Fontanella A, Lan L, Izatt JA
and Dewhirst MW: Longitudinal optical imaging of tumor metabolism
and hemodynamics. J Biomed Opt. 15:0111122010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roulot A, Héquet D, Guinebretière JM,
Vincent-Salomon A, Lerebours F, Dubot C and Rouzier R: Tumoral
heterogeneity of breast cancer. Ann Biol Clin (Paris). 74:653–660.
2016.PubMed/NCBI
|
|
19
|
Carmona-Fontaine C, Deforet M, Akkari L,
Thompson CB, Joyce JA and Xavier JB: Metabolic origins of spatial
organization in the tumor microenvironment. Proc Natl Acad Sci USA.
114:2934–2939. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Montcourrier P, Silver I, Farnoud R, Bird
I and Rochefort H: Breast cancer cells have a high capacity to
acidify extracellular milieu by a dual mechanism. Clin Exp
Metastasis. 15:382–392. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Logozzi M, Spugnini E, Mizzoni D, Di Raimo
R and Fais S: Extracellular acidity and increased exosome release
as key phenotypes of malignant tumors. Cancer Metastasis Rev.
38:93–101. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao T, Li JZ, Lu Y, Zhang CY, Li Q, Mao J
and Li LH: The mechanism between epithelial mesenchymal transition
in breast cancer and hypoxia microenvironment. Biomed Pharmacother.
80:393–405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu ZJ, Semenza GL and Zhang HF:
Hypoxia-inducible factor 1 and breast cancer metastasis. J Zhejiang
Univ Sci B. 16:32–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Daşu A, Toma-Daşu I and Karlsson M:
Theoretical simulation of tumour oxygenation and results from acute
and chronic hypoxia. Phys Med Biol. 48:2829–2842. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Byrne A, Savas P, Sant S, Li R, Virassamy
B, Luen SJ, Beavis PA, Mackay LK, Neeson PJ and Loi S:
Tissue-resident memory T cells in breast cancer control and
immunotherapy responses. Nat Rev Clin Oncol. 17:341–348. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Frauwirth KA, Riley JL, Harris MH, Parry
RV, Rathmell JC, Plas DR, Elstrom RL, June CH and Thompson CB: The
CD28 signaling pathway regulates glucose metabolism. Immunity.
16:769–777. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kamiński MM, Sauer SW, Kamiński M, Opp S,
Ruppert T, Grigaravičius P, Grudnik P, Gröne HJ, Krammer PH and
Gülow K: T cell activation is driven by an ADP-dependent
glucokinase linking enhanced glycolysis with mitochondrial reactive
oxygen species generation. Cell Rep. 2:1300–1315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gentric G, Mieulet V and Mechta-Grigoriou
F: Heterogeneity in cancer metabolism: New concepts in an old
field. Antioxid Redox Signal. 26:462–485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
MacPherson S, Kilgour M and Lum JJ:
Understanding lymphocyte metabolism for use in cancer
immunotherapy. FEBS J. 285:2567–2578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Michalek RD, Gerriets VA, Jacobs SR,
Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG and
Rathmell JC: Cutting edge: Distinct glycolytic and lipid oxidative
metabolic programs are essential for effector and regulatory CD4+ T
cell subsets. J Immunol. 186:3299–3303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Berod L, Friedrich C, Nandan A, Freitag J,
Hagemann S, Harmrolfs K, Sandouk A, Hesse C, Castro CN, Bähre H, et
al: De novo fatty acid synthesis controls the fate between
regulatory T and T helper 17 cells. Nat Med. 20:1327–1333. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Phan AT and Goldrath AW: Hypoxia-inducible
factors regulate T cell metabolism and function. Mol Immunol.
68:527–535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim JW, Tchernyshyov I, Semenza GL and
Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase
kinase: A metabolic switch required for cellular adaptation to
hypoxia. Cell Metab. 3:177–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Westendorf AM, Skibbe K, Adamczyk A, Buer
J, Geffers R, Hansen W, Pastille E and Jendrossek V: Hypoxia
enhances immunosuppression by inhibiting CD4+ effector T cell
function and promoting treg activity. Cell Physiol Biochem.
41:1271–1284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Molon B, Calì B and Viola A: T cells and
cancer: How metabolism shapes immunity. Front Immunol. 7:202016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mack N, Mazzio EA, Bauer D, Flores-Rozas H
and Soliman KF: Stable shRNA silencing of lactate dehydrogenase A
(LDHA) in human MDA-MB-231 breast cancer cells fails to alter
lactic acid production, glycolytic activity, ATP or survival.
Anticancer Res. 37:1205–1212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Siska PJ and Rathmell JC: T cell metabolic
fitness in antitumor immunity. Trends Immunol. 36:257–264. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Peppicelli S, Toti A, Giannoni E,
Bianchini F, Margheri F, Del Rosso M and Calorini L: Metformin is
also effective on lactic acidosis-exposed melanoma cells switched
to oxidative phosphorylation. Cell Cycle. 15:1908–1918. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fischer K, Hoffmann P, Voelkl S,
Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G,
Hoves S, et al: Inhibitory effect of tumor cell-derived lactic acid
on human T cells. Blood. 109:3812–3819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ho PC, Bihuniak JD, Macintyre AN, Staron
M, Liu X, Amezquita R, Tsui YC, Cui G, Micevic G, Perales JC, et
al: Phosphoenolpyruvate Is a metabolic checkpoint of anti-tumor t
cell responses. Cell. 162:1217–1228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Beckermann KE, Dudzinski SO and Rathmell
JC: Dysfunctional T cell metabolism in the tumor microenvironment.
Cytokine Growth Factor Rev. 35:7–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Neugent ML, Goodwin J, Sankaranarayanan I,
Yetkin CE, Hsieh MH and Kim JW: A new perspective on the
heterogeneity of cancer glycolysis. Biomol Ther (Seoul). 26:10–18.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Angelin A, Gil-de-Gómez L, Dahiya S, Jiao
J, Guo L, Levine MH, Wang Z, Quinn WJ III, Kopinski PK, Wang L, et
al: Foxp3 reprograms T cell metabolism to function in low-glucose,
high-lactate environments. Cell Metab. 25:1282–1293.e7. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hao Y, Li D, Xu Y, Ouyang J, Wang Y, Zhang
Y, Li B, Xie L and Qin G: Investigation of lipid metabolism
dysregulation and the effects on immune microenvironments in
pan-cancer using multiple omics data. BMC Bioinformatics. 20 (Suppl
7):S1952019. View Article : Google Scholar
|
|
46
|
Saleh R and Elkord E: FoxP3+ T
regulatory cells in cancer: Prognostic biomarkers and therapeutic
targets. Cancer Lett. 490:174–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Iranparast S, Tayebi S, Ahmadpour F and
Yousefi B: Tumor-Induced metabolism and T cells located in tumor
environment. Curr Cancer Drug Targets. 20:741–756. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shang W, Xu R, Xu T, Wu M, Xu J and Wang
F: Ovarian cancer cells promote glycolysis metabolism and
TLR8-mediated metabolic control of human CD4+ T cells.
Front Oncol. 10:5708992020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vardhana SA, Hwee MA, Berisa M, Wells DK,
Yost KE, King B, Smith M, Herrera PS, Chang HY, Satpathy AT, et al:
Impaired mitochondrial oxidative phosphorylation limits the
self-renewal of T cells exposed to persistent antigen. Nat Immunol.
21:1022–1033. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Beier UH, Angelin A, Akimova T, Wang L,
Liu Y, Xiao H, Koike MA, Hancock SA, Bhatti TR, Han R, et al:
Essential role of mitochondrial energy metabolism in Foxp3+
T-regulatory cell function and allograft survival. FASEB J.
29:2315–2326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gerriets VA, Kishton RJ, Nichols AG,
Macintyre AN, Inoue M, Ilkayeva O, Winter PS, Liu X, Priyadharshini
B, Slawinska ME, et al: Metabolic programming and PDHK1 control
CD4+ T cell subsets and inflammation. J Clin Invest.
125:194–207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Duan W, Ding Y, Yu X, Ma D, Yang B, Li Y,
Huang L, Chen Z, Zheng J and Yang C: Metformin mitigates autoimmune
insulitis by inhibiting Th1 and Th17 responses while promoting Treg
production. Am J Transl Res. 11:2393–2402. 2019.PubMed/NCBI
|
|
53
|
Lu L, Barbi J and Pan F: The regulation of
immune tolerance by FOXP3. Nat Rev Immunol. 17:703–717. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Georgiev P, Charbonnier LM and Chatila TA:
Regulatory T cells: The many faces of Foxp3. J Clin Immunol.
39:623–640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gerriets VA, Kishton RJ, Johnson MO, Cohen
S, Siska PJ, Nichols AG, Warmoes MO, de Cubas AA, MacIver NJ,
Locasale JW, et al: Foxp3 and Toll-like receptor signaling balance
Treg cell anabolic metabolism for suppression. Nat
Immunol. 17:1459–1466. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen X, Feng L, Li S, Long D, Shan J and
Li Y: TGF-β1 maintains Foxp3 expression and inhibits glycolysis in
natural regulatory T cells via PP2A-mediated suppression of mTOR
signaling. Immunol Lett. 226:31–37. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Choi J, Gyamfi J, Jang H and Koo JS: The
role of tumor-associated macrophage in breast cancer biology.
Histol Histopathol. 33:133–145. 2018.PubMed/NCBI
|
|
58
|
Su S, Liu Q, Chen J, Chen J, Chen F, He C,
Huang D, Wu W, Lin L, Huang W, et al: A positive feedback loop
between mesenchymal-like cancer cells and macrophages is essential
to breast cancer metastasis. Cancer Cell. 25:605–620. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Klingen TA, Chen Y, Aas H, Wik E and
Akslen LA: Tumor-associated macrophages are strongly related to
vascular invasion, non-luminal subtypes, and interval breast
cancer. Hum Pathol. 69:72–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tarique AA, Logan J, Thomas E, Holt PG,
Sly PD and Fantino E: Phenotypic, functional, and plasticity
features of classical and alternatively activated human
macrophages. Am J Respir Cell Mol Biol. 53:676–688. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Geeraerts X, Bolli E, Fendt SM and Van
Ginderachter JA: Macrophage metabolism as therapeutic target for
cancer, atherosclerosis, and obesity. Front Immunol. 8:2892017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mantovani A, Marchesi F, Malesci A, Laghi
L and Allavena P: Tumour-associated macrophages as treatment
targets in oncology. Nat Rev Clin Oncol. 14:399–416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
De Santa F, Vitiello L, Torcinaro A and
Ferraro E: The role of metabolic remodeling in macrophage
polarization and its effect on skeletal muscle regeneration.
Antioxid Redox Signal. 30:1553–1598. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Van den Bossche J, Baardman J, Otto NA,
van der Velden S, Neele AE, van den Berg SM, Luque-Martin R, Chen
HJ, Boshuizen MC, Ahmed M, et al: Mitochondrial dysfunction
prevents repolarization of inflammatory macrophages. Cell Rep.
17:684–696. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kim J: Regulation of immune cell functions
by metabolic reprogramming. J Immuno Res. 2018:86054712018.
|
|
66
|
Rodríguez-Prados JC, Través PG, Cuenca J,
Rico D, Aragonés J, Martín-Sanz P, Cascante M and Boscá L:
Substrate fate in activated macrophages: A comparison between
innate, classic, and alternative activation. J Immunol.
185:605–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Vats D, Mukundan L, Odegaard JI, Zhang L,
Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ and Chawla A:
Oxidative metabolism and PGC-1beta attenuate macrophage-mediated
inflammation. Cell Metab. 4:13–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Colegio OR, Chu NQ, Szabo AL, Chu T,
Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC,
Phillips GM, et al: Functional polarization of tumour-associated
macrophages by tumour-derived lactic acid. Nature. 513:559–563.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Feng R, Morine Y, Ikemoto T, Imura S,
Iwahashi S, Saito Y and Shimada M: Nrf2 activation drive
macrophages polarization and cancer cell epithelial-mesenchymal
transition during interaction. Cell Commun Signal. 16:542018.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Maftouh M, Avan A, Sciarrillo R, Granchi
C, Leon LG, Rani R, Funel N, Smid K, Honeywell R, Boggi U, et al:
Synergistic interaction of novel lactate dehydrogenase inhibitors
with gemcitabine against pancreatic cancer cells in hypoxia. Br J
Cancer. 110:172–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mediani L, Gibellini F, Bertacchini J,
Frasson C, Bosco R, Accordi B, Basso G, Bonora M, Calabrò ML,
Mattiolo A, et al: Reversal of the glycolytic phenotype of primary
effusion lymphoma cells by combined targeting of cellular
metabolism and PI3K/Akt/mTOR signaling. Oncotarget. 7:5521–5537.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lin S, Sun L, Lyu X, Ai X, Du D, Su N, Li
H, Zhang L, Yu J and Yuan S: Lactate-activated macrophages induced
aerobic glycolysis and epithelial-mesenchymal transition in breast
cancer by regulation of CCL5-CCR5 axis: A positive metabolic
feedback loop. Oncotarget. 8:110426–110443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Viola A, Munari F, Sánchez-Rodríguez R,
Scolaro T and Castegna A: The metabolic signature of macrophage
responses. Front Immunol. 10:14622019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang Q, Wang H, Mao C, Sun M, Dominah G,
Chen L and Zhuang Z: Fatty acid oxidation contributes to IL-1β
secretion in M2 macrophages and promotes macrophage-mediated tumor
cell migration. Mol Immunol. 94:27–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wu H, Han Y, Rodriguez Sillke Y, Deng H,
Siddiqui S, Treese C, Schmidt F, Friedrich M, Keye J, Wan J, et al:
Lipid droplet-dependent fatty acid metabolism controls the immune
suppressive phenotype of tumor-associated macrophages. EMBO Mol
Med. 11:e106982019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Rombaldova M, Janovska P, Kopecky J and
Kuda O: Omega-3 fatty acids promote fatty acid utilization and
production of pro-resolving lipid mediators in alternatively
activated adipose tissue macrophages. Biochem Biophys Res Commun.
490:1080–1085. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Huang SC, Everts B, Ivanova Y, O'Sullivan
D, Nascimento M, Smith AM, Beatty W, Love-Gregory L, Lam WY,
O'Neill CM, et al: Cell-intrinsic lysosomal lipolysis is essential
for alternative activation of macrophages. Nat Immunol. 15:846–855.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jin H, He Y, Zhao P, Hu Y, Tao J, Chen J
and Huang Y: Targeting lipid metabolism to overcome EMT-associated
drug resistance via integrin β3/FAK pathway and tumor-associated
macrophage repolarization using legumain-activatable delivery.
Theranostics. 9:265–278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chiossone L, Dumas PY, Vienne M and Vivier
E: Natural killer cells and other innate lymphoid cells in cancer.
Nat Rev Immunol. 18:671–688. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Terrén I, Orrantia A, Vitallé J,
Zenarruzabeitia O and Borrego F: NK cell metabolism and tumor
microenvironment. Front Immunol. 10:22782019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gardiner CM: NK cell metabolism. J Leukoc
Biol. 105:1235–1242. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Keating SE, Zaiatz-Bittencourt V, Loftus
RM, Keane C, Brennan K, Finlay DK and Gardiner CM: Metabolic
reprogramming supports IFN-γ production by CD56bright NK cells. J
Immunol. 196:2552–2560. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Keppel MP, Saucier N, Mah AY, Vogel TP and
Cooper MA: Activation-specific metabolic requirements for NK Cell
IFN-γ production. J Immunol. 194:1954–1962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Assmann N, O'Brien KL, Donnelly RP, Dyck
L, Zaiatz-Bittencourt V, Loftus RM, Heinrich P, Oefner PJ, Lynch L,
Gardiner CM, et al: Srebp-controlled glucose metabolism is
essential for NK cell functional responses. Nat Immunol.
18:1197–1206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cooper MA, Fehniger TA and Caligiuri MA:
The biology of human natural killer-cell subsets. Trends Immunol.
22:633–640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Schafer JR, Salzillo TC, Chakravarti N,
Kararoudi MN, Trikha P, Foltz JA, Wang R, Li S and Lee DA:
Education-dependent activation of glycolysis promotes the cytolytic
potency of licensed human natural killer cells. J Allergy Clin
Immunol. 143:346–358.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Parodi M, Raggi F, Cangelosi D, Manzini C,
Balsamo M, Blengio F, Eva A, Varesio L, Pietra G, Moretta L, et al:
Hypoxia modifies the transcriptome of human NK cells, modulates
their immunoregulatory profile, and influences NK cell subset
migration. Front Immunol. 9:23582018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Balsamo M, Manzini C, Pietra G, Raggi F,
Blengio F, Mingari MC, Varesio L, Moretta L, Bosco MC and Vitale M:
Hypoxia downregulates the expression of activating receptors
involved in NK-cell-mediated target cell killing without affecting
ADCC. Eur J Immunol. 43:2756–2764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Dengler VL, Galbraith M and Espinosa JM:
Transcriptional regulation by hypoxia inducible factors. Crit Rev
Biochem Mol Biol. 49:1–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yang C, Tsaih SW, Lemke A, Flister MJ,
Thakar MS and Malarkannan S: mTORC1 and mTORC2 differentially
promote natural killer cell development. Elife. 7:e356192018.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chambers AM, Wang J, Lupo KB, Yu H,
Atallah Lanman NM and Matosevic S: Adenosinergic signaling alters
natural killer cell functional responses. Front Immunol.
9:25332018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Brand A, Singer K, Koehl GE, Kolitzus M,
Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et
al: LDHA-associated lactic acid production blunts tumor
immunosurveillance by T and NK cells. Cell Metab. 24:657–671. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Stiff A, Trikha P, Mundy-Bosse B,
McMichael E, Mace TA, Benner B, Kendra K, Campbell A, Gautam S,
Abood D, et al: Nitric oxide production by myeloid-derived
suppressor cells plays a role in impairing Fc receptor-mediated
natural killer cell function. Clin Cancer Res. 24:1891–1904. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Piñeiro Fernández J, Luddy KA, Harmon C
and O'Farrelly C: Hepatic tumor microenvironments and effects on NK
cell phenotype and function. Int J Mol Sci. 20:41312019. View Article : Google Scholar
|
|
95
|
Vitale M, Cantoni C, Pietra G, Mingari MC
and Moretta L: Effect of tumor cells and tumor microenvironment on
NK-cell function. Eur J Immunol. 44:1582–1592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang Z, Guan D, Wang S, Chai LYA, Xu S and
Lam KP: Glycolysis and oxidative phosphorylation play critical
roles in natural killer cell receptor-mediated natural killer cell
functions. Front Immunol. 11:2022020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Terrén I, Orrantia A, Vitallé J,
Astarloa-Pando G, Zenarruzabeitia O and Borrego F: Modulating NK
cell metabolism for cancer immunotherapy. Semin Hematol.
57:213–224. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kobayashi T, Lam PY, Jiang H, Bednarska K,
Gloury RE, Murigneux V, Tay J, Jacquelot N, Li R, Tuong ZK, et al:
Increased lipid metabolism impairs NK cell function and mediates
adaptation to the lymphoma environment. Blood. Aug 20–2020.(Epub
ahead of print). View Article : Google Scholar
|
|
99
|
Inoue H, Miyaji M, Kosugi A, Nagafuku M,
Okazaki T, Mimori T, Amakawa R, Fukuhara S, Domae N, Bloom ET and
Umehara H: Lipid rafts as the signaling scaffold for NK cell
activation: Tyrosine phosphorylation and association of LAT with
phosphatidylinositol 3-kinase and phospholipase C-gamma following
CD2 stimulation. Eur J Immunol. 32:2188–2198. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Niavarani SR, Lawson C, Bakos O, Boudaud
M, Batenchuk C, Rouleau S and Tai LH: Lipid accumulation impairs
natural killer cell cytotoxicity and tumor control in the
postoperative period. BMC Cancer. 19:8232019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Michelet X, Dyck L, Hogan A, Loftus RM,
Duquette D, Wei K, Beyaz S, Tavakkoli A, Foley C, Donnelly R, et
al: Metabolic reprogramming of natural killer cells in obesity
limits antitumor responses. Nat Immunol. 19:1330–1340. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Herber DL, Cao W, Nefedova Y, Novitskiy
SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, et
al: Lipid accumulation and dendritic cell dysfunction in cancer.
Nat Med. 16:880–886. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gao F, Liu C, Guo J, Sun W, Xian L, Bai D,
Liu H, Cheng Y, Li B, Cui J, et al: Radiation-driven lipid
accumulation and dendritic cell dysfunction in cancer. Sci Rep.
5:96132015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Dong H and Bullock TN: Metabolic
influences that regulate dendritic cell function in tumors. Front
Immunol. 5:242014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Brown TP, Bhattacharjee P, Ramachandran S,
Sivaprakasam S, Ristic B, Sikder MOF and Ganapathy V: The lactate
receptor GPR81 promotes breast cancer growth via a paracrine
mechanism involving antigen-presenting cells in the tumor
microenvironment. Oncogene. 39:3292–3304. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ibrahim J, Nguyen AH, Rehman A, Ochi A,
Jamal M, Graffeo CS, Henning JR, Zambirinis CP, Fallon NC, Barilla
R, et al: Dendritic cell populations with different concentrations
of lipid regulate tolerance and immunity in mouse and human liver.
Gastroenterology. 143:1061–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Mellor AL and Munn DH: Creating immune
privilege: Active local suppression that benefits friends, but
protects foes. Nat Rev Immunol. 8:74–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Everts B, Amiel E, Huang SC, Smith AM,
Chang CH, Lam WY, Redmann V, Freitas TC, Blagih J, van der Windt
GJ, et al: TLR-driven early glycolytic reprogramming via the
kinases TBK1-IKKε supports the anabolic demands of dendritic cell
activation. Nat Immunol. 15:323–332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Guak H, Al Habyan S, Ma EH, Aldossary H,
Al-Masri M, Won SY, Ying T, Fixman ED, Jones RG, McCaffrey LM and
Krawczyk CM: Glycolytic metabolism is essential for CCR7
oligomerization and dendritic cell migration. Nat Commun.
9:24632018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Krawczyk CM, Holowka T, Sun J, Blagih J,
Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG
and Pearce EJ: Toll-like receptor-induced changes in glycolytic
metabolism regulate dendritic cell activation. Blood.
115:4742–4749. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Nasi A, Fekete T, Krishnamurthy A, Snowden
S, Rajnavölgyi E, Catrina AI, Wheelock CE, Vivar N and Rethi B:
Dendritic cell reprogramming by endogenously produced lactic acid.
J Immunol. 191:3090–3099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Gottfried E, Kunz-Schughart LA, Ebner S,
Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A and Kreutz M:
Tumor-derived lactic acid modulates dendritic cell activation and
antigen expression. Blood. 107:2013–2021. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Naldini A, Morena E, Pucci A, Miglietta D,
Riboldi E, Sozzani S and Carraro F: Hypoxia affects dendritic cell
survival: Role of the hypoxia-inducible factor-1α and
lipopolysaccharide. J Cell Physiol. 227:587–595. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lawless SJ, Kedia-Mehta N, Walls JF,
McGarrigle R, Convery O, Sinclair LV, Navarro MN, Murray J and
Finlay DK: Glucose represses dendritic cell-induced T cell
responses. Nat Commun. 8:156202017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ramakrishnan R, Tyurin VA, Veglia F,
Condamine T, Amoscato A, Mohammadyani D, Johnson JJ, Zhang LM,
Klein-Seetharaman J, Celis E, et al: Oxidized lipids block antigen
cross-presentation by dendritic cells in cancer. J Immunol.
192:2920–2931. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Menendez JA and Lupu R: Fatty acid
synthase (FASN) as a therapeutic target in breast cancer. Expert
Opin Thera Targets. 21:1001–1016. 2017. View Article : Google Scholar
|
|
117
|
Ventura R, Mordec K, Waszczuk J, Wang Z,
Lai J, Fridlib M, Buckley D, Kemble G and Heuer TS: Inhibition of
de novo palmitate synthesis by fatty acid synthase induces
apoptosis in tumor cells by remodeling cell membranes, inhibiting
signaling pathways, and reprogramming gene expression.
EBioMedicine. 2:808–824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Jiang L, Fang X, Wang H, Li D and Wang X:
Ovarian cancer-intrinsic fatty acid synthase prevents anti-tumor
immunity by disrupting tumor-infiltrating dendritic cells. Front
Immunol. 9:29272018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Cubillos-Ruiz JR, Silberman PC, Rutkowski
MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE,
Gupta D, Holcomb K, et al: ER Stress Sensor XBP1 controls
Anti-tumor immunity by disrupting dendritic cell homeostasis. Cell.
161:1527–1538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Xiong Y, Liu L, Xia Y, Qi Y, Chen Y, Chen
L, Zhang P, Kong Y, Qu Y, Wang Z, et al: Tumor infiltrating mast
cells determine oncogenic HIF-2α-conferred immune evasion in clear
cell renal cell carcinoma. Cancer Immunol Immunother. 68:731–741.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Schwartz M, Zhang Y and Rosenblatt JD: B
cell regulation of the anti-tumor response and role in
carcinogenesis. J Immunother Cancer. 4:402016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Aponte-López A, Fuentes-Pananá EM,
Cortes-Muñoz D and Muñoz-Cruz S: Mast cell, the neglected member of
the tumor microenvironment: Role in breast cancer. J Immunol Res.
2018:25842432018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Okano M, Oshi M, Butash AL, Katsuta E,
Tachibana K, Saito K, Okayama H, Peng X, Yan L, Kono K, Ohtake T
and Takabe K: Triple-negative breast cancer with high levels of
Annexin A1 expression is associated with mast cell infiltration,
inflammation, and angiogenesis. Int J Mol Sci. 20:41972019.
View Article : Google Scholar
|
|
124
|
Glajcar A, Szpor J, Pacek A, Tyrak KE,
Chan F, Streb J, Hodorowicz-Zaniewska D and Okoń K: The
relationship between breast cancer molecular subtypes and mast cell
populations in tumor microenvironment. Virchows Arch. 470:505–515.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Spector AA: The importance of free fatty
acid in tumor nutrition. Cancer Res. 27:1580–1586. 1967.PubMed/NCBI
|
|
126
|
Li Z and Zhang H: Reprogramming of
glucose, fatty acid and amino acid metabolism for cancer
progression. Cell Mol Life Sci. 73:377–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Al-Khami AA, Zheng L, Del Valle L, Hossain
F, Wyczechowska D, Zabaleta J, Sanchez MD, Dean MJ, Rodriguez PC
and Ochoa AC: Exogenous lipid uptake induces metabolic and
functional reprogramming of tumor-associated myeloid-derived
suppressor cells. Oncoimmunology. 6:e13448042017. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Cao W and Gabrilovich D: Abstract 3649:
Contribution of fatty acid accumulation to myeloid-derived
suppressor cell function in cancer. Cancer Res. 71:3649.
2011.PubMed/NCBI
|
|
129
|
Veglia F, Tyurin VA, Blasi M, De Leo A,
Kossenkov AV, Donthireddy L, To TKJ, Schug Z, Basu S, Wang F, et
al: Fatty acid transport protein 2 reprograms neutrophils in
cancer. Nature. 569:73–78. 2019. View Article : Google Scholar : PubMed/NCBI
|