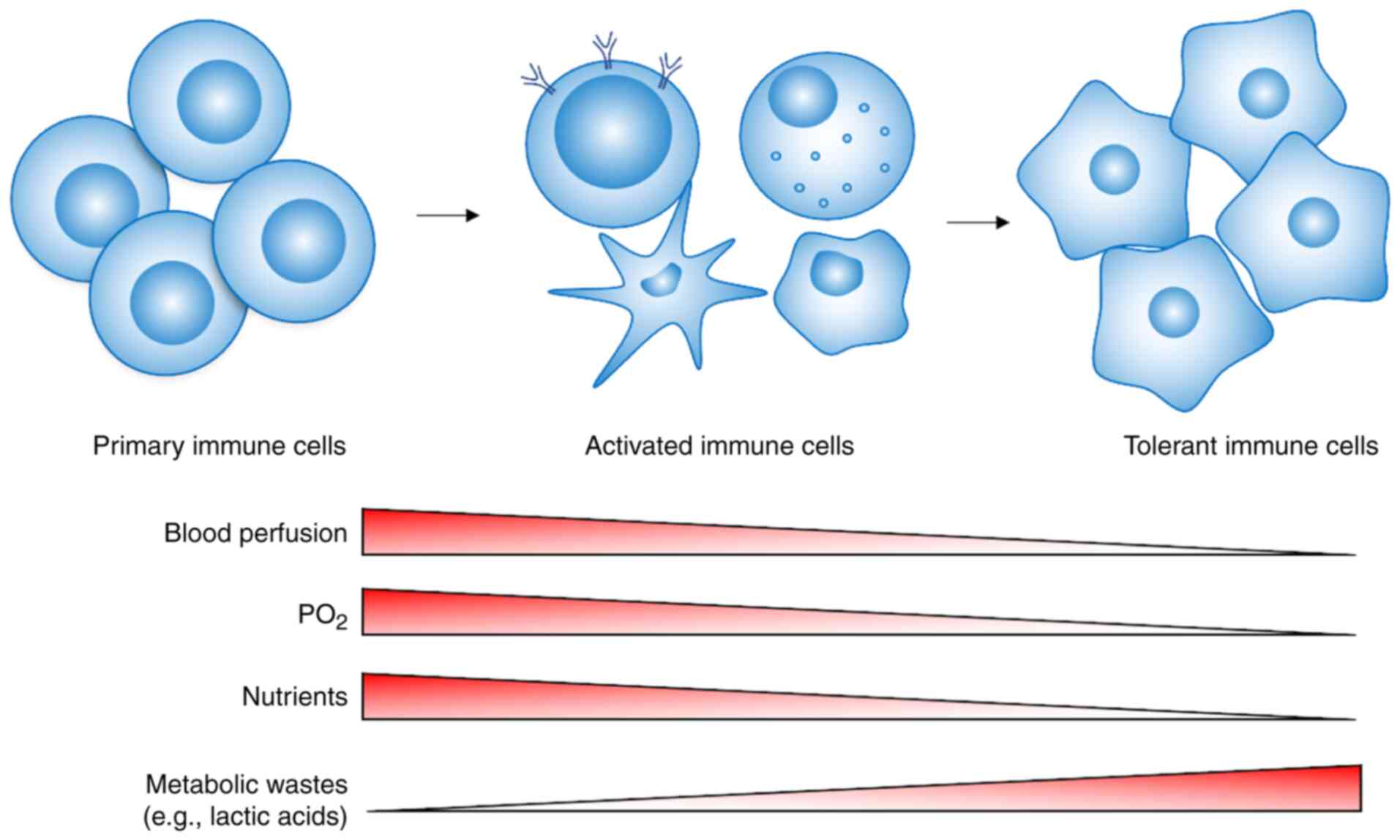
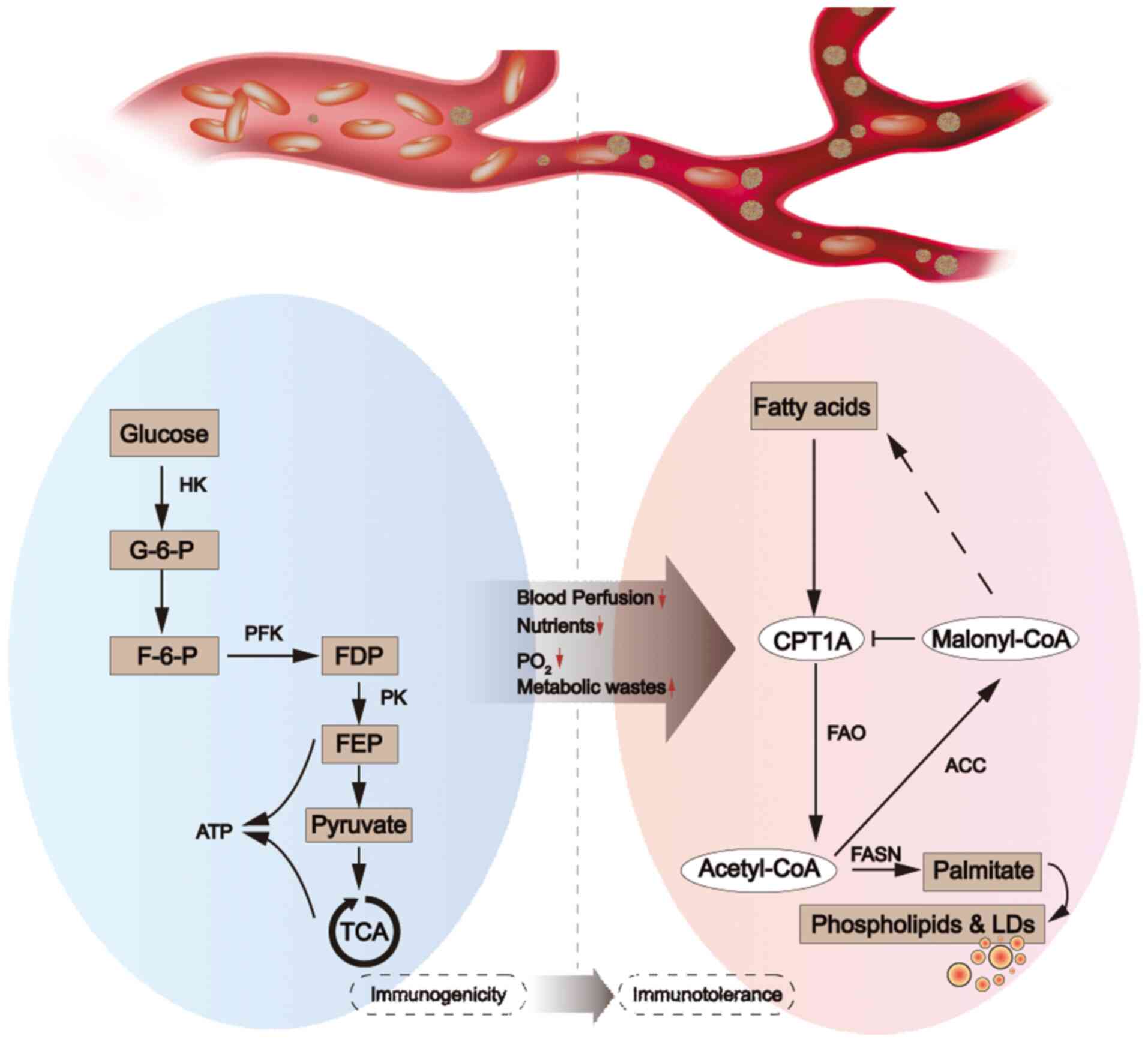
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vranic S, Cyprian FS, Gatalica Z and
Palazzo J: PD-L1 status in breast cancer: Current view and
perspectives. Semin Cancer Biol. Dec 26–2019.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Engelhard VH, Rodriguez AB, Mauldin IS,
Woods AN, Peske JD and Slingluff CL Jr: Immune Cell Infiltration
and tertiary lymphoid structures as determinants of antitumor
immunity. J Immunol. 200:432–442. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Savas P, Virassamy B, Ye C, Salim A,
Mintoff CP, Caramia F, Salgado R, Byrne DJ, Teo ZL, Dushyanthen S,
et al: Publisher correction: Single-cell profiling of breast cancer
T cells reveals a tissue-resident memory subset associated with
improved prognosis. Nat Med. 24:19412018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang SC, Hu ZQ, Long JH, Zhu GM, Wang Y,
Jia Y, Zhou J, Ouyang Y and Zeng Z: Clinical implications of
tumor-infiltrating immune cells in breast cancer. J Cancer.
10:6175–6184. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu D: Innate and adaptive immune cell
metabolism in tumor microenvironment. Adv Exp Med Biol.
1011:211–223. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kishton RJ, Sukumar M and Restifo NP:
Metabolic regulation of T cell longevity and function in tumor
immunotherapy. Cell Metab. 26:94–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hobson-Gutierrez SA and Carmona-Fontaine
C: The metabolic axis of macrophage and immune cell polarization.
Dis Model Mech. 11:dmm0344622018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Warburg O: Über den Stoffwechsel der
Carcinomzelle. Naturwissenschaften. 12:1131–1137. 1924. View Article : Google Scholar
|
|
10
|
Lane AN, Higashi RM and Fan TW: Metabolic
reprogramming in tumors: Contributions of the tumor
microenvironment. Genes Dis. 7:185–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim J and DeBerardinis RJ: Mechanisms and
implications of metabolic heterogeneity in cancer. Cell Metab.
30:434–446. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mehla K and Singh PK: Metabolic regulation
of macrophage polarization in cancer. Trends Cancer. 5:822–834.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Basit F, Mathan T, Sancho D and de Vries
IJM: Human dendritic cell subsets undergo distinct metabolic
reprogramming for immune response. Front Immunol. 9:24892018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L and Romero P: Metabolic control of
CD8(+) T cell fate decisions and antitumor immunity. Trends Mol
Med. 24:30–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Poznanski SM, Barra NG, Ashkar AA and
Schertzer JD: Immunometabolism of T cells and NK cells: Metabolic
control of effector and regulatory function. Inflamm Res.
67:813–828. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Norton KA, Jin K and Popel AS: Modeling
triple-negative breast cancer heterogeneity: Effects of stromal
macrophages, fibroblasts and tumor vasculature. J Theor Biol.
452:56–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Skala MC, Fontanella A, Lan L, Izatt JA
and Dewhirst MW: Longitudinal optical imaging of tumor metabolism
and hemodynamics. J Biomed Opt. 15:0111122010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roulot A, Héquet D, Guinebretière JM,
Vincent-Salomon A, Lerebours F, Dubot C and Rouzier R: Tumoral
heterogeneity of breast cancer. Ann Biol Clin (Paris). 74:653–660.
2016.PubMed/NCBI
|
|
19
|
Carmona-Fontaine C, Deforet M, Akkari L,
Thompson CB, Joyce JA and Xavier JB: Metabolic origins of spatial
organization in the tumor microenvironment. Proc Natl Acad Sci USA.
114:2934–2939. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Montcourrier P, Silver I, Farnoud R, Bird
I and Rochefort H: Breast cancer cells have a high capacity to
acidify extracellular milieu by a dual mechanism. Clin Exp
Metastasis. 15:382–392. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Logozzi M, Spugnini E, Mizzoni D, Di Raimo
R and Fais S: Extracellular acidity and increased exosome release
as key phenotypes of malignant tumors. Cancer Metastasis Rev.
38:93–101. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao T, Li JZ, Lu Y, Zhang CY, Li Q, Mao J
and Li LH: The mechanism between epithelial mesenchymal transition
in breast cancer and hypoxia microenvironment. Biomed Pharmacother.
80:393–405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu ZJ, Semenza GL and Zhang HF:
Hypoxia-inducible factor 1 and breast cancer metastasis. J Zhejiang
Univ Sci B. 16:32–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Daşu A, Toma-Daşu I and Karlsson M:
Theoretical simulation of tumour oxygenation and results from acute
and chronic hypoxia. Phys Med Biol. 48:2829–2842. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Byrne A, Savas P, Sant S, Li R, Virassamy
B, Luen SJ, Beavis PA, Mackay LK, Neeson PJ and Loi S:
Tissue-resident memory T cells in breast cancer control and
immunotherapy responses. Nat Rev Clin Oncol. 17:341–348. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Frauwirth KA, Riley JL, Harris MH, Parry
RV, Rathmell JC, Plas DR, Elstrom RL, June CH and Thompson CB: The
CD28 signaling pathway regulates glucose metabolism. Immunity.
16:769–777. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kamiński MM, Sauer SW, Kamiński M, Opp S,
Ruppert T, Grigaravičius P, Grudnik P, Gröne HJ, Krammer PH and
Gülow K: T cell activation is driven by an ADP-dependent
glucokinase linking enhanced glycolysis with mitochondrial reactive
oxygen species generation. Cell Rep. 2:1300–1315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gentric G, Mieulet V and Mechta-Grigoriou
F: Heterogeneity in cancer metabolism: New concepts in an old
field. Antioxid Redox Signal. 26:462–485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
MacPherson S, Kilgour M and Lum JJ:
Understanding lymphocyte metabolism for use in cancer
immunotherapy. FEBS J. 285:2567–2578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Michalek RD, Gerriets VA, Jacobs SR,
Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG and
Rathmell JC: Cutting edge: Distinct glycolytic and lipid oxidative
metabolic programs are essential for effector and regulatory CD4+ T
cell subsets. J Immunol. 186:3299–3303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Berod L, Friedrich C, Nandan A, Freitag J,
Hagemann S, Harmrolfs K, Sandouk A, Hesse C, Castro CN, Bähre H, et
al: De novo fatty acid synthesis controls the fate between
regulatory T and T helper 17 cells. Nat Med. 20:1327–1333. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Phan AT and Goldrath AW: Hypoxia-inducible
factors regulate T cell metabolism and function. Mol Immunol.
68:527–535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim JW, Tchernyshyov I, Semenza GL and
Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase
kinase: A metabolic switch required for cellular adaptation to
hypoxia. Cell Metab. 3:177–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Westendorf AM, Skibbe K, Adamczyk A, Buer
J, Geffers R, Hansen W, Pastille E and Jendrossek V: Hypoxia
enhances immunosuppression by inhibiting CD4+ effector T cell
function and promoting treg activity. Cell Physiol Biochem.
41:1271–1284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Molon B, Calì B and Viola A: T cells and
cancer: How metabolism shapes immunity. Front Immunol. 7:202016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mack N, Mazzio EA, Bauer D, Flores-Rozas H
and Soliman KF: Stable shRNA silencing of lactate dehydrogenase A
(LDHA) in human MDA-MB-231 breast cancer cells fails to alter
lactic acid production, glycolytic activity, ATP or survival.
Anticancer Res. 37:1205–1212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Siska PJ and Rathmell JC: T cell metabolic
fitness in antitumor immunity. Trends Immunol. 36:257–264. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Peppicelli S, Toti A, Giannoni E,
Bianchini F, Margheri F, Del Rosso M and Calorini L: Metformin is
also effective on lactic acidosis-exposed melanoma cells switched
to oxidative phosphorylation. Cell Cycle. 15:1908–1918. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fischer K, Hoffmann P, Voelkl S,
Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G,
Hoves S, et al: Inhibitory effect of tumor cell-derived lactic acid
on human T cells. Blood. 109:3812–3819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ho PC, Bihuniak JD, Macintyre AN, Staron
M, Liu X, Amezquita R, Tsui YC, Cui G, Micevic G, Perales JC, et
al: Phosphoenolpyruvate Is a metabolic checkpoint of anti-tumor t
cell responses. Cell. 162:1217–1228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Beckermann KE, Dudzinski SO and Rathmell
JC: Dysfunctional T cell metabolism in the tumor microenvironment.
Cytokine Growth Factor Rev. 35:7–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Neugent ML, Goodwin J, Sankaranarayanan I,
Yetkin CE, Hsieh MH and Kim JW: A new perspective on the
heterogeneity of cancer glycolysis. Biomol Ther (Seoul). 26:10–18.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Angelin A, Gil-de-Gómez L, Dahiya S, Jiao
J, Guo L, Levine MH, Wang Z, Quinn WJ III, Kopinski PK, Wang L, et
al: Foxp3 reprograms T cell metabolism to function in low-glucose,
high-lactate environments. Cell Metab. 25:1282–1293.e7. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hao Y, Li D, Xu Y, Ouyang J, Wang Y, Zhang
Y, Li B, Xie L and Qin G: Investigation of lipid metabolism
dysregulation and the effects on immune microenvironments in
pan-cancer using multiple omics data. BMC Bioinformatics. 20 (Suppl
7):S1952019. View Article : Google Scholar
|
|
46
|
Saleh R and Elkord E: FoxP3+ T
regulatory cells in cancer: Prognostic biomarkers and therapeutic
targets. Cancer Lett. 490:174–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Iranparast S, Tayebi S, Ahmadpour F and
Yousefi B: Tumor-Induced metabolism and T cells located in tumor
environment. Curr Cancer Drug Targets. 20:741–756. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shang W, Xu R, Xu T, Wu M, Xu J and Wang
F: Ovarian cancer cells promote glycolysis metabolism and
TLR8-mediated metabolic control of human CD4+ T cells.
Front Oncol. 10:5708992020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vardhana SA, Hwee MA, Berisa M, Wells DK,
Yost KE, King B, Smith M, Herrera PS, Chang HY, Satpathy AT, et al:
Impaired mitochondrial oxidative phosphorylation limits the
self-renewal of T cells exposed to persistent antigen. Nat Immunol.
21:1022–1033. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Beier UH, Angelin A, Akimova T, Wang L,
Liu Y, Xiao H, Koike MA, Hancock SA, Bhatti TR, Han R, et al:
Essential role of mitochondrial energy metabolism in Foxp3+
T-regulatory cell function and allograft survival. FASEB J.
29:2315–2326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gerriets VA, Kishton RJ, Nichols AG,
Macintyre AN, Inoue M, Ilkayeva O, Winter PS, Liu X, Priyadharshini
B, Slawinska ME, et al: Metabolic programming and PDHK1 control
CD4+ T cell subsets and inflammation. J Clin Invest.
125:194–207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Duan W, Ding Y, Yu X, Ma D, Yang B, Li Y,
Huang L, Chen Z, Zheng J and Yang C: Metformin mitigates autoimmune
insulitis by inhibiting Th1 and Th17 responses while promoting Treg
production. Am J Transl Res. 11:2393–2402. 2019.PubMed/NCBI
|
|
53
|
Lu L, Barbi J and Pan F: The regulation of
immune tolerance by FOXP3. Nat Rev Immunol. 17:703–717. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Georgiev P, Charbonnier LM and Chatila TA:
Regulatory T cells: The many faces of Foxp3. J Clin Immunol.
39:623–640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gerriets VA, Kishton RJ, Johnson MO, Cohen
S, Siska PJ, Nichols AG, Warmoes MO, de Cubas AA, MacIver NJ,
Locasale JW, et al: Foxp3 and Toll-like receptor signaling balance
Treg cell anabolic metabolism for suppression. Nat
Immunol. 17:1459–1466. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen X, Feng L, Li S, Long D, Shan J and
Li Y: TGF-β1 maintains Foxp3 expression and inhibits glycolysis in
natural regulatory T cells via PP2A-mediated suppression of mTOR
signaling. Immunol Lett. 226:31–37. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Choi J, Gyamfi J, Jang H and Koo JS: The
role of tumor-associated macrophage in breast cancer biology.
Histol Histopathol. 33:133–145. 2018.PubMed/NCBI
|
|
58
|
Su S, Liu Q, Chen J, Chen J, Chen F, He C,
Huang D, Wu W, Lin L, Huang W, et al: A positive feedback loop
between mesenchymal-like cancer cells and macrophages is essential
to breast cancer metastasis. Cancer Cell. 25:605–620. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Klingen TA, Chen Y, Aas H, Wik E and
Akslen LA: Tumor-associated macrophages are strongly related to
vascular invasion, non-luminal subtypes, and interval breast
cancer. Hum Pathol. 69:72–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tarique AA, Logan J, Thomas E, Holt PG,
Sly PD and Fantino E: Phenotypic, functional, and plasticity
features of classical and alternatively activated human
macrophages. Am J Respir Cell Mol Biol. 53:676–688. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Geeraerts X, Bolli E, Fendt SM and Van
Ginderachter JA: Macrophage metabolism as therapeutic target for
cancer, atherosclerosis, and obesity. Front Immunol. 8:2892017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mantovani A, Marchesi F, Malesci A, Laghi
L and Allavena P: Tumour-associated macrophages as treatment
targets in oncology. Nat Rev Clin Oncol. 14:399–416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
De Santa F, Vitiello L, Torcinaro A and
Ferraro E: The role of metabolic remodeling in macrophage
polarization and its effect on skeletal muscle regeneration.
Antioxid Redox Signal. 30:1553–1598. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Van den Bossche J, Baardman J, Otto NA,
van der Velden S, Neele AE, van den Berg SM, Luque-Martin R, Chen
HJ, Boshuizen MC, Ahmed M, et al: Mitochondrial dysfunction
prevents repolarization of inflammatory macrophages. Cell Rep.
17:684–696. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kim J: Regulation of immune cell functions
by metabolic reprogramming. J Immuno Res. 2018:86054712018.
|
|
66
|
Rodríguez-Prados JC, Través PG, Cuenca J,
Rico D, Aragonés J, Martín-Sanz P, Cascante M and Boscá L:
Substrate fate in activated macrophages: A comparison between
innate, classic, and alternative activation. J Immunol.
185:605–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Vats D, Mukundan L, Odegaard JI, Zhang L,
Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ and Chawla A:
Oxidative metabolism and PGC-1beta attenuate macrophage-mediated
inflammation. Cell Metab. 4:13–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Colegio OR, Chu NQ, Szabo AL, Chu T,
Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC,
Phillips GM, et al: Functional polarization of tumour-associated
macrophages by tumour-derived lactic acid. Nature. 513:559–563.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Feng R, Morine Y, Ikemoto T, Imura S,
Iwahashi S, Saito Y and Shimada M: Nrf2 activation drive
macrophages polarization and cancer cell epithelial-mesenchymal
transition during interaction. Cell Commun Signal. 16:542018.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Maftouh M, Avan A, Sciarrillo R, Granchi
C, Leon LG, Rani R, Funel N, Smid K, Honeywell R, Boggi U, et al:
Synergistic interaction of novel lactate dehydrogenase inhibitors
with gemcitabine against pancreatic cancer cells in hypoxia. Br J
Cancer. 110:172–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mediani L, Gibellini F, Bertacchini J,
Frasson C, Bosco R, Accordi B, Basso G, Bonora M, Calabrò ML,
Mattiolo A, et al: Reversal of the glycolytic phenotype of primary
effusion lymphoma cells by combined targeting of cellular
metabolism and PI3K/Akt/mTOR signaling. Oncotarget. 7:5521–5537.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lin S, Sun L, Lyu X, Ai X, Du D, Su N, Li
H, Zhang L, Yu J and Yuan S: Lactate-activated macrophages induced
aerobic glycolysis and epithelial-mesenchymal transition in breast
cancer by regulation of CCL5-CCR5 axis: A positive metabolic
feedback loop. Oncotarget. 8:110426–110443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Viola A, Munari F, Sánchez-Rodríguez R,
Scolaro T and Castegna A: The metabolic signature of macrophage
responses. Front Immunol. 10:14622019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang Q, Wang H, Mao C, Sun M, Dominah G,
Chen L and Zhuang Z: Fatty acid oxidation contributes to IL-1β
secretion in M2 macrophages and promotes macrophage-mediated tumor
cell migration. Mol Immunol. 94:27–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wu H, Han Y, Rodriguez Sillke Y, Deng H,
Siddiqui S, Treese C, Schmidt F, Friedrich M, Keye J, Wan J, et al:
Lipid droplet-dependent fatty acid metabolism controls the immune
suppressive phenotype of tumor-associated macrophages. EMBO Mol
Med. 11:e106982019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Rombaldova M, Janovska P, Kopecky J and
Kuda O: Omega-3 fatty acids promote fatty acid utilization and
production of pro-resolving lipid mediators in alternatively
activated adipose tissue macrophages. Biochem Biophys Res Commun.
490:1080–1085. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Huang SC, Everts B, Ivanova Y, O'Sullivan
D, Nascimento M, Smith AM, Beatty W, Love-Gregory L, Lam WY,
O'Neill CM, et al: Cell-intrinsic lysosomal lipolysis is essential
for alternative activation of macrophages. Nat Immunol. 15:846–855.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jin H, He Y, Zhao P, Hu Y, Tao J, Chen J
and Huang Y: Targeting lipid metabolism to overcome EMT-associated
drug resistance via integrin β3/FAK pathway and tumor-associated
macrophage repolarization using legumain-activatable delivery.
Theranostics. 9:265–278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chiossone L, Dumas PY, Vienne M and Vivier
E: Natural killer cells and other innate lymphoid cells in cancer.
Nat Rev Immunol. 18:671–688. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Terrén I, Orrantia A, Vitallé J,
Zenarruzabeitia O and Borrego F: NK cell metabolism and tumor
microenvironment. Front Immunol. 10:22782019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gardiner CM: NK cell metabolism. J Leukoc
Biol. 105:1235–1242. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Keating SE, Zaiatz-Bittencourt V, Loftus
RM, Keane C, Brennan K, Finlay DK and Gardiner CM: Metabolic
reprogramming supports IFN-γ production by CD56bright NK cells. J
Immunol. 196:2552–2560. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Keppel MP, Saucier N, Mah AY, Vogel TP and
Cooper MA: Activation-specific metabolic requirements for NK Cell
IFN-γ production. J Immunol. 194:1954–1962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Assmann N, O'Brien KL, Donnelly RP, Dyck
L, Zaiatz-Bittencourt V, Loftus RM, Heinrich P, Oefner PJ, Lynch L,
Gardiner CM, et al: Srebp-controlled glucose metabolism is
essential for NK cell functional responses. Nat Immunol.
18:1197–1206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cooper MA, Fehniger TA and Caligiuri MA:
The biology of human natural killer-cell subsets. Trends Immunol.
22:633–640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Schafer JR, Salzillo TC, Chakravarti N,
Kararoudi MN, Trikha P, Foltz JA, Wang R, Li S and Lee DA:
Education-dependent activation of glycolysis promotes the cytolytic
potency of licensed human natural killer cells. J Allergy Clin
Immunol. 143:346–358.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Parodi M, Raggi F, Cangelosi D, Manzini C,
Balsamo M, Blengio F, Eva A, Varesio L, Pietra G, Moretta L, et al:
Hypoxia modifies the transcriptome of human NK cells, modulates
their immunoregulatory profile, and influences NK cell subset
migration. Front Immunol. 9:23582018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Balsamo M, Manzini C, Pietra G, Raggi F,
Blengio F, Mingari MC, Varesio L, Moretta L, Bosco MC and Vitale M:
Hypoxia downregulates the expression of activating receptors
involved in NK-cell-mediated target cell killing without affecting
ADCC. Eur J Immunol. 43:2756–2764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Dengler VL, Galbraith M and Espinosa JM:
Transcriptional regulation by hypoxia inducible factors. Crit Rev
Biochem Mol Biol. 49:1–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yang C, Tsaih SW, Lemke A, Flister MJ,
Thakar MS and Malarkannan S: mTORC1 and mTORC2 differentially
promote natural killer cell development. Elife. 7:e356192018.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chambers AM, Wang J, Lupo KB, Yu H,
Atallah Lanman NM and Matosevic S: Adenosinergic signaling alters
natural killer cell functional responses. Front Immunol.
9:25332018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Brand A, Singer K, Koehl GE, Kolitzus M,
Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et
al: LDHA-associated lactic acid production blunts tumor
immunosurveillance by T and NK cells. Cell Metab. 24:657–671. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Stiff A, Trikha P, Mundy-Bosse B,
McMichael E, Mace TA, Benner B, Kendra K, Campbell A, Gautam S,
Abood D, et al: Nitric oxide production by myeloid-derived
suppressor cells plays a role in impairing Fc receptor-mediated
natural killer cell function. Clin Cancer Res. 24:1891–1904. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Piñeiro Fernández J, Luddy KA, Harmon C
and O'Farrelly C: Hepatic tumor microenvironments and effects on NK
cell phenotype and function. Int J Mol Sci. 20:41312019. View Article : Google Scholar
|
|
95
|
Vitale M, Cantoni C, Pietra G, Mingari MC
and Moretta L: Effect of tumor cells and tumor microenvironment on
NK-cell function. Eur J Immunol. 44:1582–1592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang Z, Guan D, Wang S, Chai LYA, Xu S and
Lam KP: Glycolysis and oxidative phosphorylation play critical
roles in natural killer cell receptor-mediated natural killer cell
functions. Front Immunol. 11:2022020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Terrén I, Orrantia A, Vitallé J,
Astarloa-Pando G, Zenarruzabeitia O and Borrego F: Modulating NK
cell metabolism for cancer immunotherapy. Semin Hematol.
57:213–224. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kobayashi T, Lam PY, Jiang H, Bednarska K,
Gloury RE, Murigneux V, Tay J, Jacquelot N, Li R, Tuong ZK, et al:
Increased lipid metabolism impairs NK cell function and mediates
adaptation to the lymphoma environment. Blood. Aug 20–2020.(Epub
ahead of print). View Article : Google Scholar
|
|
99
|
Inoue H, Miyaji M, Kosugi A, Nagafuku M,
Okazaki T, Mimori T, Amakawa R, Fukuhara S, Domae N, Bloom ET and
Umehara H: Lipid rafts as the signaling scaffold for NK cell
activation: Tyrosine phosphorylation and association of LAT with
phosphatidylinositol 3-kinase and phospholipase C-gamma following
CD2 stimulation. Eur J Immunol. 32:2188–2198. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Niavarani SR, Lawson C, Bakos O, Boudaud
M, Batenchuk C, Rouleau S and Tai LH: Lipid accumulation impairs
natural killer cell cytotoxicity and tumor control in the
postoperative period. BMC Cancer. 19:8232019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Michelet X, Dyck L, Hogan A, Loftus RM,
Duquette D, Wei K, Beyaz S, Tavakkoli A, Foley C, Donnelly R, et
al: Metabolic reprogramming of natural killer cells in obesity
limits antitumor responses. Nat Immunol. 19:1330–1340. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Herber DL, Cao W, Nefedova Y, Novitskiy
SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, et
al: Lipid accumulation and dendritic cell dysfunction in cancer.
Nat Med. 16:880–886. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gao F, Liu C, Guo J, Sun W, Xian L, Bai D,
Liu H, Cheng Y, Li B, Cui J, et al: Radiation-driven lipid
accumulation and dendritic cell dysfunction in cancer. Sci Rep.
5:96132015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Dong H and Bullock TN: Metabolic
influences that regulate dendritic cell function in tumors. Front
Immunol. 5:242014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Brown TP, Bhattacharjee P, Ramachandran S,
Sivaprakasam S, Ristic B, Sikder MOF and Ganapathy V: The lactate
receptor GPR81 promotes breast cancer growth via a paracrine
mechanism involving antigen-presenting cells in the tumor
microenvironment. Oncogene. 39:3292–3304. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ibrahim J, Nguyen AH, Rehman A, Ochi A,
Jamal M, Graffeo CS, Henning JR, Zambirinis CP, Fallon NC, Barilla
R, et al: Dendritic cell populations with different concentrations
of lipid regulate tolerance and immunity in mouse and human liver.
Gastroenterology. 143:1061–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Mellor AL and Munn DH: Creating immune
privilege: Active local suppression that benefits friends, but
protects foes. Nat Rev Immunol. 8:74–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Everts B, Amiel E, Huang SC, Smith AM,
Chang CH, Lam WY, Redmann V, Freitas TC, Blagih J, van der Windt
GJ, et al: TLR-driven early glycolytic reprogramming via the
kinases TBK1-IKKε supports the anabolic demands of dendritic cell
activation. Nat Immunol. 15:323–332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Guak H, Al Habyan S, Ma EH, Aldossary H,
Al-Masri M, Won SY, Ying T, Fixman ED, Jones RG, McCaffrey LM and
Krawczyk CM: Glycolytic metabolism is essential for CCR7
oligomerization and dendritic cell migration. Nat Commun.
9:24632018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Krawczyk CM, Holowka T, Sun J, Blagih J,
Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG
and Pearce EJ: Toll-like receptor-induced changes in glycolytic
metabolism regulate dendritic cell activation. Blood.
115:4742–4749. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Nasi A, Fekete T, Krishnamurthy A, Snowden
S, Rajnavölgyi E, Catrina AI, Wheelock CE, Vivar N and Rethi B:
Dendritic cell reprogramming by endogenously produced lactic acid.
J Immunol. 191:3090–3099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Gottfried E, Kunz-Schughart LA, Ebner S,
Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A and Kreutz M:
Tumor-derived lactic acid modulates dendritic cell activation and
antigen expression. Blood. 107:2013–2021. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Naldini A, Morena E, Pucci A, Miglietta D,
Riboldi E, Sozzani S and Carraro F: Hypoxia affects dendritic cell
survival: Role of the hypoxia-inducible factor-1α and
lipopolysaccharide. J Cell Physiol. 227:587–595. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lawless SJ, Kedia-Mehta N, Walls JF,
McGarrigle R, Convery O, Sinclair LV, Navarro MN, Murray J and
Finlay DK: Glucose represses dendritic cell-induced T cell
responses. Nat Commun. 8:156202017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ramakrishnan R, Tyurin VA, Veglia F,
Condamine T, Amoscato A, Mohammadyani D, Johnson JJ, Zhang LM,
Klein-Seetharaman J, Celis E, et al: Oxidized lipids block antigen
cross-presentation by dendritic cells in cancer. J Immunol.
192:2920–2931. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Menendez JA and Lupu R: Fatty acid
synthase (FASN) as a therapeutic target in breast cancer. Expert
Opin Thera Targets. 21:1001–1016. 2017. View Article : Google Scholar
|
|
117
|
Ventura R, Mordec K, Waszczuk J, Wang Z,
Lai J, Fridlib M, Buckley D, Kemble G and Heuer TS: Inhibition of
de novo palmitate synthesis by fatty acid synthase induces
apoptosis in tumor cells by remodeling cell membranes, inhibiting
signaling pathways, and reprogramming gene expression.
EBioMedicine. 2:808–824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Jiang L, Fang X, Wang H, Li D and Wang X:
Ovarian cancer-intrinsic fatty acid synthase prevents anti-tumor
immunity by disrupting tumor-infiltrating dendritic cells. Front
Immunol. 9:29272018. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Cubillos-Ruiz JR, Silberman PC, Rutkowski
MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE,
Gupta D, Holcomb K, et al: ER Stress Sensor XBP1 controls
Anti-tumor immunity by disrupting dendritic cell homeostasis. Cell.
161:1527–1538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Xiong Y, Liu L, Xia Y, Qi Y, Chen Y, Chen
L, Zhang P, Kong Y, Qu Y, Wang Z, et al: Tumor infiltrating mast
cells determine oncogenic HIF-2α-conferred immune evasion in clear
cell renal cell carcinoma. Cancer Immunol Immunother. 68:731–741.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Schwartz M, Zhang Y and Rosenblatt JD: B
cell regulation of the anti-tumor response and role in
carcinogenesis. J Immunother Cancer. 4:402016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Aponte-López A, Fuentes-Pananá EM,
Cortes-Muñoz D and Muñoz-Cruz S: Mast cell, the neglected member of
the tumor microenvironment: Role in breast cancer. J Immunol Res.
2018:25842432018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Okano M, Oshi M, Butash AL, Katsuta E,
Tachibana K, Saito K, Okayama H, Peng X, Yan L, Kono K, Ohtake T
and Takabe K: Triple-negative breast cancer with high levels of
Annexin A1 expression is associated with mast cell infiltration,
inflammation, and angiogenesis. Int J Mol Sci. 20:41972019.
View Article : Google Scholar
|
|
124
|
Glajcar A, Szpor J, Pacek A, Tyrak KE,
Chan F, Streb J, Hodorowicz-Zaniewska D and Okoń K: The
relationship between breast cancer molecular subtypes and mast cell
populations in tumor microenvironment. Virchows Arch. 470:505–515.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Spector AA: The importance of free fatty
acid in tumor nutrition. Cancer Res. 27:1580–1586. 1967.PubMed/NCBI
|
|
126
|
Li Z and Zhang H: Reprogramming of
glucose, fatty acid and amino acid metabolism for cancer
progression. Cell Mol Life Sci. 73:377–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Al-Khami AA, Zheng L, Del Valle L, Hossain
F, Wyczechowska D, Zabaleta J, Sanchez MD, Dean MJ, Rodriguez PC
and Ochoa AC: Exogenous lipid uptake induces metabolic and
functional reprogramming of tumor-associated myeloid-derived
suppressor cells. Oncoimmunology. 6:e13448042017. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Cao W and Gabrilovich D: Abstract 3649:
Contribution of fatty acid accumulation to myeloid-derived
suppressor cell function in cancer. Cancer Res. 71:3649.
2011.PubMed/NCBI
|
|
129
|
Veglia F, Tyurin VA, Blasi M, De Leo A,
Kossenkov AV, Donthireddy L, To TKJ, Schug Z, Basu S, Wang F, et
al: Fatty acid transport protein 2 reprograms neutrophils in
cancer. Nature. 569:73–78. 2019. View Article : Google Scholar : PubMed/NCBI
|