Introduction
Lung cancer ranks first among all emerging cancer
cases and cancer-related deaths worldwide (1). Surgery is an important therapeutic
method for early lung cancer. Surgery in combination with cisplatin
(DDP)-based chemotherapy contributes to the long-term survival of
patients. Chemotherapy is the main treatment for advanced and
metastatic lung cancer. However, some patients still progress to
relapse and metastasis. The development of multidrug resistance
(MDR) is the major obstacle of successful cancer chemotherapy
(2,3).
Cancer cells can achieve MDR bytransporting a broad range of
cytotoxic drugs out of cells mediated through the overexpression of
ATP-binding cassette (ABC) transporter proteins, thus reducing
intracellular drug concentrations. Inhibition of the expression of
ABC transporter proteins may reverse the MDR of cancer cells. Among
the 48 known ABC transporter proteins, P-glycoprotein (P-gp)
encoded by the MDR1 gene, multidrug resistance-associated
protein 1 (MRP1), breast cancer-resistant protein
(BCRP) and lung resistance-related protein (LRP) are
the four main efflux transporters that function in the MDR of
cancers (4–6). Leucine-rich PPR motif-containing protein
(LRPPRC), a member of the PPR family, is a multifunctional protein
involved in mitochondrial gene transcription and tumorigenesis.
LRPPRC mutation causes Leigh syndrome in French-Canadians. Many
tumors highly express LRPPRC, which is associated with poor
prognosis. Downregulation of LRPPRC inhibits the growth and induces
the apoptosis of tumor cells (7–10). More
importantly, increasing evidence indicates that LRPPRC binds to the
region of the MDR1 gene promoter and regulates the
transcription of MDR1. This regulation is influenced by the
methylation status of the GC −100 box in the MDR1 promoter
(11,12). LRPPRC has been demonstrated to play
functional roles in the tumorigenesis of lung cancer (9,13). In this
study, it was demonstrated that downregulation of LRPPRC
successfully reverses DDP resistance in lung cancer cells by
regulating MDR1 transcription.
Materials and methods
Cell culture
Human non-small cell lung cancer (NSCLC) cell lines
A549 and H1299 were purchased from the Institute of Life Sciences
Cell Resource Centre of the Chinese Academy of Sciences (Shanghai,
China). DDP-resistant cell lines (A549/DDP and H1299/DDP) were
generated by exposing these cell lines to increasing concentrations
of DDP for several months. Cells were cultured in RPMI-1640 medium
supplemented with 10% foetal bovine serum (FBS, Gibco; Thermo
Fisher Scientific, Inc.), penicillin (100 U/ml) and streptomycin
(100 µg/ml) in a humidified atmosphere of 5% CO2 at
37°C. The medium for DDP-resistant cells was further supplemented
with DDP (1 µM).
Quantitative real-time polymerase
chain reaction (qPCR)
Total RNA was extracted from cells using Trizol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. One microgram of total RNA was
converted to cDNA using TaKaRa PrimeScript RT reagent kit (Takara
Bio, Inc.). The mRNA levels of LRPPRC, MDR1, MRP1, BCRP and
LRP were analyzed through qPCR using an ABI 7500HT Fast
Real-time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with 50 cycles of 20 sec at 95°C, 30 sec at 60°C and 30 sec
at 72°C. GAPDH was used as the internal control. The
2−ΔΔCq method was used to analyze the data (14). Each sample was run in triplicate. The
primers used are presented in Table
SI.
Western blot analysis
Cellular total protein was extracted with
radioimmunoprecipitation assay buffer containing protease
inhibitors. Protein concentrations were investigated by
bicinchoninic acid protein assay (Sigma-Aldrich; Merck KGaA). Equal
amounts of protein lysates were separated by 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis and then transferred
onto polyvinylidene fluoride membranes (EMD Millipore). The
membranes were incubated with primary antibodies, including
anti-LRPPRC (Abcam, ab97505, diluted 1:1,000), anti-P-gp (Abcam,
ab262880, diluted 1:1,200) and anti-β-actin (Abcam, ab8227, diluted
1:1,000), overnight at 4°C and then with horseradish
peroxidase-conjugated secondary antibodies (Boster, BA1054, diluted
1:5,000) for 1 h at room temperature. Visualization was performed
using an enhanced chemiluminescence detection system (EMD
Millipore). The expression of the target proteins relative to that
of β-actin was determined via densitometric analysis using ImageJ
software (National Institutes of Health, version 1.52n).
Experiments were repeated in triplicate.
Cell transfection
A549/DDP and H1299/DDP cells were transfected with
lentiviral vectors expressing the LRPPRC-specific short hairpin
(shRNA) for LRPPRC silencing (Shanghai Genechem Co., Ltd.; forward,
5′-CCGGCCAUCUCGCUGCAGUCUAUTTCTCGAGAUAGACUGCAGCGAGAUGGTTTTTTTG-3′
and reverse,
5′-AATTCAAAAACCAUCUCGCUGCAGUCUAUTTCTCGAGAUAGACUGCAGCGAGAUGGTT-3′).
The shRNA lentiviral control vectors were used. Cells were
transfected using X-tremeGENE HP DNA Transfection Reagent (Roche
Diagnostics GmbH) as recommended by the manufacturer. After
transfection, A549/DDP cells with stable endogenous LRPPRC
silencing were obtained after treatment with 5 µg/ml of puromycin
(Sigma-Aldrich; Merck KGaA).
Cell proliferation assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT)
assay was used to evaluate the proliferation capacity of the cells.
Cells were incubated with 0.5 mg/ml MTT (Sigma-Aldrich; Merck KGaA)
for 4 h at 37°C and mixed with 150 µl of dimethylsulphoxide
(Sigma-Aldrich; Merck KGaA) to dissolve the generated formazan.
Cell absorbance at 570 nm was measured using a microplate reader
(Bio-Rad Laboratories, Inc.). Proliferation inhibition rate (%) =
(1-Experimental optical density [OD]/Control OD) ×100%. The
half-maximal inhibitory concentration (IC50) values of
DDP for the inhibition of cell proliferation were calculated.
Experiments were repeated in triplicate.
Flow cytometry
The effects of LRPPRC inhibition on apoptosis were
determined through flow cytometry. A549/DDP and H1299/DDP cells
were transfected with shLRPPRC or shControl for 24 h and then
cultured with medium containing DDP (IC20 concentration)
for 48 h. Cells were stained with a combination of Annexin V-FITC
and 7-AAD/PI for 15 min at 37°C in the dark to detect apoptosis,
and the percentages of apoptotic cells were analyzed through flow
cytometry using FACSCalibur (BD Biosciences; Becton, Dickinson and
Company). Experiments were repeated in triplicate.
Chromatin
immunoprecipitation-qPCR
Chromatin immunoprecipitation (ChIP) assay was
evaluated through a Simple ChIP enzymatic ChIP kit (Cell Signalling
Technology, Inc.) according to the manufacturer's protocol.
Anti-LRPPRC antibody (Santa Cruz Biotechnology, Inc., sc-166178)
was used to perform immunoprecipitation. Nonspecific mouse IgG was
used as the control. DNA was extracted from bound fractions
according to Abcam protocol. Then, the immunoprecipitated DNA was
amplified using the sequence primers of the MDR1 promoter
(MDR1p (F) 5′-GCTGATGCGCGTTTCTCTACT-3′ and MDR1p (R)
5′-CCGGGCCGGGAGCAGTCATC-3′). DNA amplification was quantified by
qPCR analysis, and the percentage of DNA brought down by ChIP
(percent input) was calculated. Experiments were repeated in
triplicate.
Animal studies
Eighteen male BALB/c nude mice weighing 19–22 g, 6
weeks of age, were obtained from Silaike Experimental Animal
Company, and housed in an air-conditioned room with a temperature
of 23±1°C, relative humidity of 55±5% and a 12-h light/dark cycle.
The bedding, food and water were autoclaved. All the animals were
acclimatized for 7 days. The mice were frequently assessed by
animal care staff using daily inspections and health records. The
Ethics Committee of the First Affiliated Hospital of Xi'an Medical
University approved the protocol (no. 20180606). The study was
carried out in accordance with the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The mice
were randomly divided into three groups (n=6 mice/group), including
the A549/DDP, shControl-A549/DDP and shLRPPRC-A549/DDP groups. In
each group, 5×106 cells were injected subcutaneously
into the right back flank of each mouse. When xenografts had grown
to a size of 100–200 mm3, the mice were treated with DDP
(10 mg/kg) every week by intraperitoneal injection for 4 weeks. The
volumes of the implanted tumors were measured twice a week. After 4
weeks of treatment with DDP, all of the mice were euthanized by
intravenous injection of sodium pentobarbital (150 mg/kg), and
tumor weights were measured.
Immunohistochemical examination
Immunohistochemistry (IHC) was performed to detect
the expression of the indicated proteins in the xenograft tumors.
Sections (4-µm thick) were deparaffinized and dehydrated. The
slides were incubated in 3% hydrogen peroxide solution for 10 min,
washed with PBS buffer and then incubated for 15 min with normal
goat serum. The sections were incubated with the primary
antibodies, including anti-LRPPRC (Abcam, ab97505, diluted 1:500)
and anti-P-gp antibody (Abcam, ab262880, diluted 1:500), overnight
at 4°C. Thereafter, the sections were incubated for 40 min with an
anti-rabbit secondary antibody (SA1050, Boster) and stained with
diaminobenzidine (DAB; Boster). The negative control was processed
identically but without the primary antibody.
Statistical analysis
Data are expressed as mean ± standard deviation.
Differences among the groups were analyzed by Student's t-test or
one-way analysis of variance (ANOVA) followed by Tukey's
multiple-comparison test using SPSS version 19.0 (SPSS, Inc.), and
P<0.05 was used to indicate a statistically significant
difference.
Results
Overexpression of LRPPRC and MDR1 in
A549/DDP and H1299/DDP cells
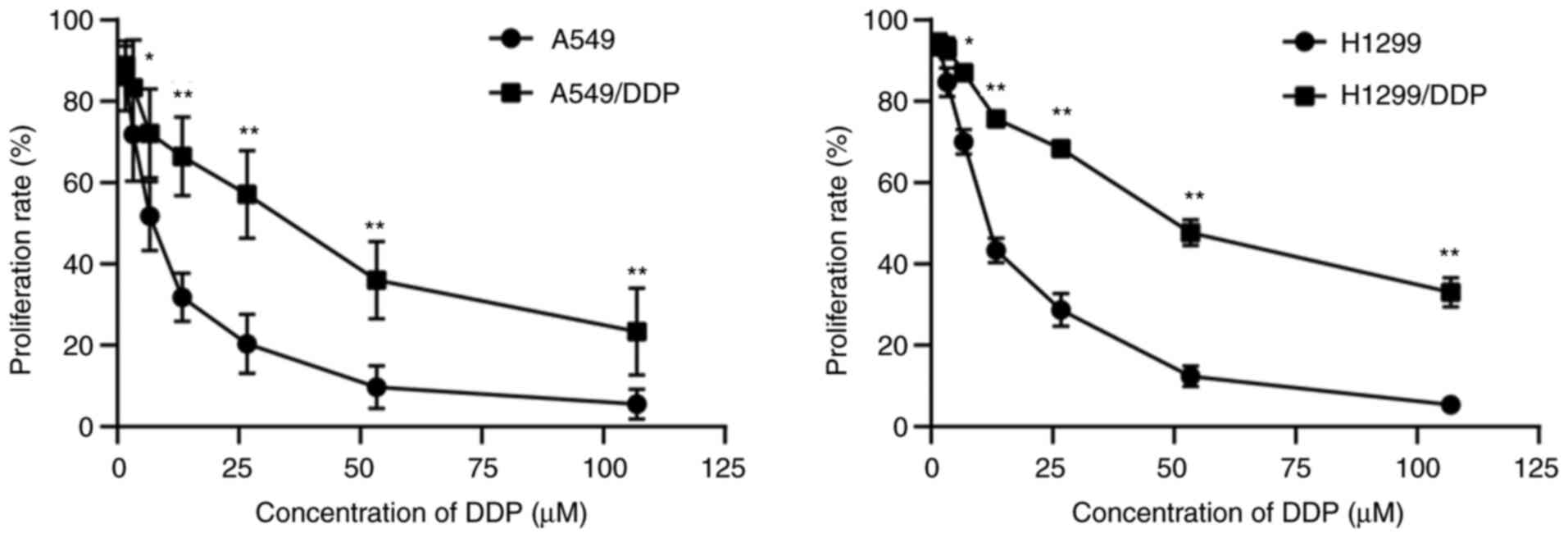
The DDP-resistant cell lines A549/DDP and H1299/DDP
were established by long-term continuous exposure of the original
DDP-sensitive cells to DDP. The proliferation inhibition of cells
was investigated using MTT assay. Cells were cultured with medium
containing different concentrations of DDP (1.7, 3.4, 6.8, 13.6,
27.2, 54.4, and 108.8 µM) for 48 h. Then, MTT assay was performed,
and IC50 values were calculated. The cytotoxicity of DDP
significantly differed between the DDP-resistant cells and their
parental cells (Fig. 1). The
IC50 of DDP in the resistant cells was significantly
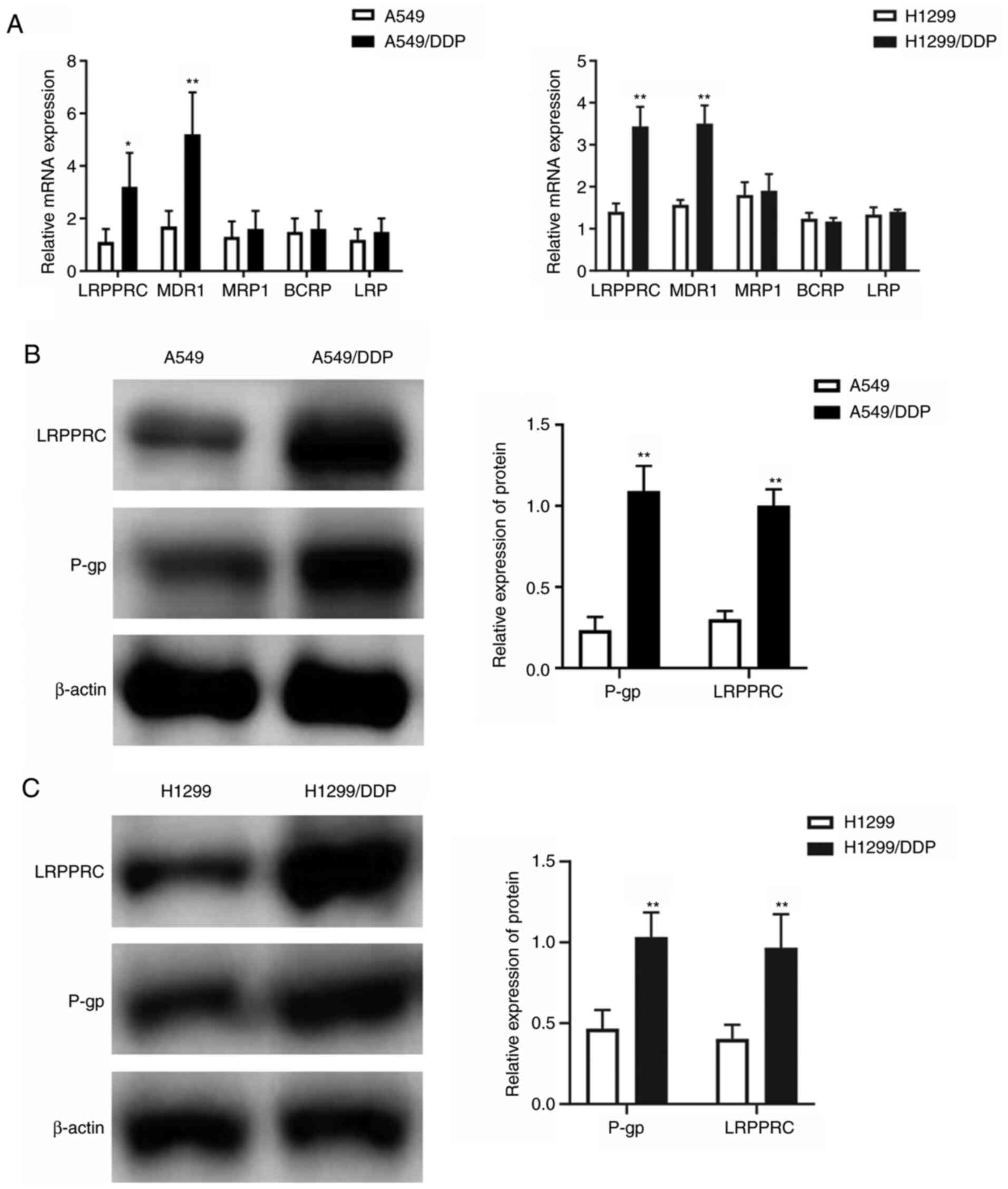
higher when compared with that in the parental cells (Table I). The mRNA expression of
LRPPRC and MDR-related efflux ABC transporter proteins,
including MDR1, BCRP, MRP1 and LRP, in DDP-resistant
and parental cells was investigated using qPCR. The mRNA expression
of LRPPRC (~3.2-fold change in A549/DDP and 2.4-fold change
in H1299/DDP) and MDR1 (~2.8-fold change in A549/DDP and
2.3-fold change in H1299/DDP) was significantly increased in the
resistant cells relative to that in the parental cells (Fig. 2A). The expression changes in LRPPRC
and P-gp encoded by the MDR1 gene were confirmed through
western blot analysis, and the results indicated significant
increases in LRPPRC and P-gp expression in the A549/DDP and
H1299/DDP cells compared with those in their parental cells
(Fig. 2B and C).
 | Table I.The IC50 values of the
lung cancer cells. |
Table I.
The IC50 values of the
lung cancer cells.
| IC50
(mM) |
|---|
|
|---|
| Cell line | Parental cells | Resistant
cells | P-value |
|---|
| A549 | 7.0±1.0 | 39.3±5.7 | <0.01 |
| H1299 | 11.7±2.7 | 54.0±11.0 | <0.01 |
LRPPRC silencing increases DDP
sensitivity in vitro and in vivo
The effect of LRPPRC silencing on DDP sensitivity
was investigated using MTT assay. The proliferation inhibition of
tumor cells treated with different concentrations of DDP treatment
for 48 h in combination with shLRPPRC was investigated, and
IC50 values were calculated. LRPPRC silencing
significantly increased the DDP sensitivity of A549/DDP and
H1299/DDP cells. The IC50 values of DDP in the
LRPPRC-silenced resistant cells were significantly decreased
compared with those in LRPPRC-normal expression cells (9.3±1.7 vs.
41.1±3.9 µM in A549/DDP cells; 17.0±3.2 vs. 54.5±4.0 µM in
H1299/DDP cells, P<0.01, Fig. 3A).
The percentage of apoptosis induced by DDP in LRPPRC-silenced
A549/DDP and H1299/DDP cells was analyzed using flow cytometry.
LRPPRC silencing synergized with DDP in increasing the percentage
of apoptotic cells (Fig. 3B). We
verified our results in vitro by examining the effect of
LRPPRC silencing on DDP sensitivity in A549/DDP xenograft tumors.
After DDP treatment for 4 weeks, the tumor volumes and weights of
LRPPRC-silenced cells were significantly decreased compared with
those of the shControl and mock groups (Fig. 3C). These findings indicate that
downregulation of LRPPRC reverses DDP resistance in lung cancer
cells.
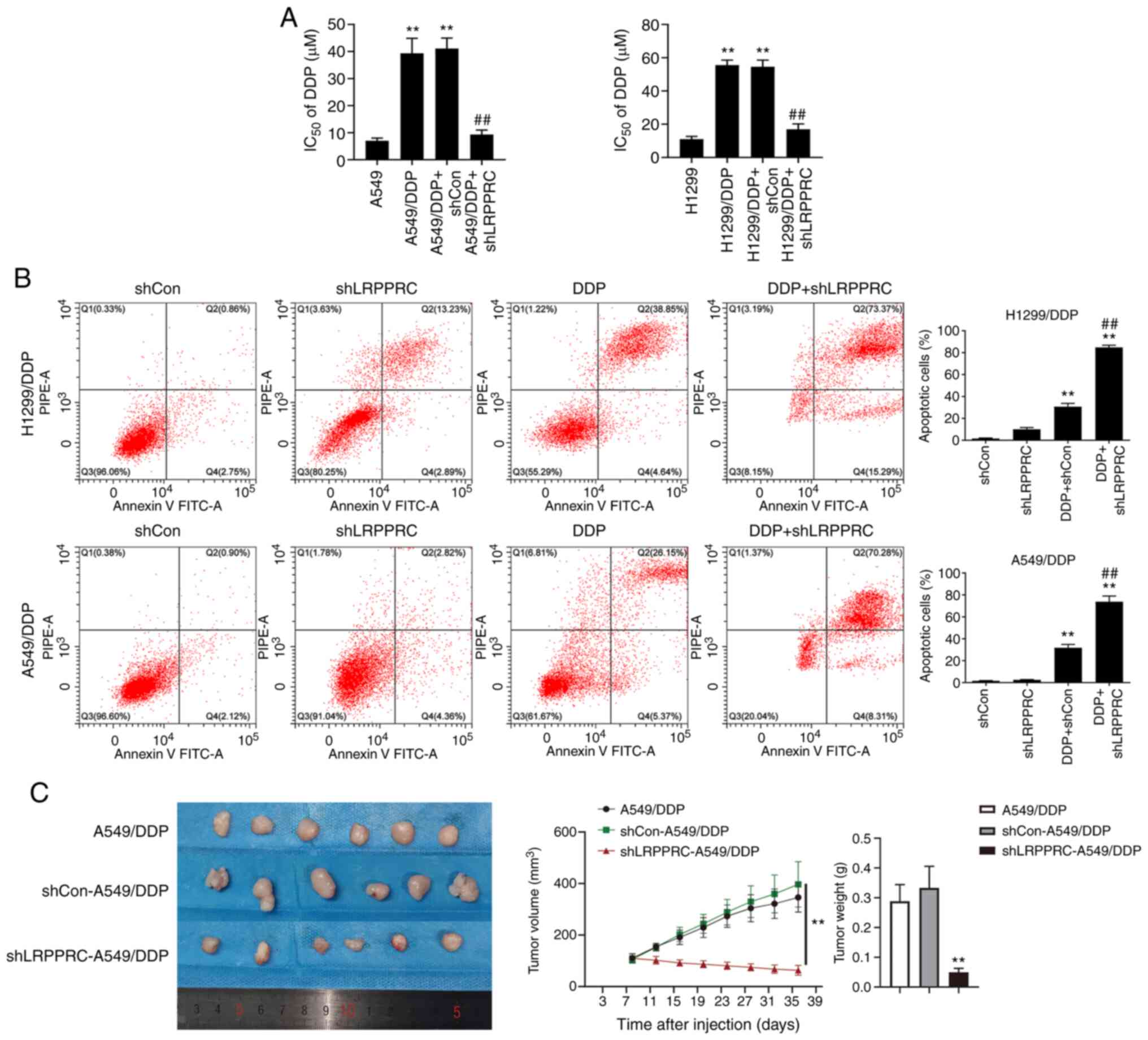 | Figure 3.LRPPRC silencing increases the DDP
sensitivity of DDP-resistant lung cancer cells in vitro and
in vivo. (A) Effects of LRPPRC silencing on the
IC50 value of DDP using MTT assay. Data are shown as
mean ± SD. **P<0.01 vs. the parental cells;
##P<0.01 vs. the resistant cells + shCon group. (B)
Effects of LRPPRC silencing on apoptosis induced by DDP using flow
cytometry. Left: Representative dot plots show the apoptotic status
of cells with different treatments. Right: Percentages of apoptotic
cells. Cells were treated with shLRPPRC, DDP or their combination.
Data are shown as mean ± SD. **P<0.01 vs. the shCon or shLRPPRC
group; ##P<0.01 vs. the DDP+shCon group. (C) Effects
of LRPPRC silencing on DDP sensitivity in A549/DDP cell-derived
tumors. Left panel: Images of the implanted tumors. Middle graph:
Tumor growth curves. Right: Histogram representing mean tumor
weights. BALB/c nude mice were divided into the A549/DDP,
shCon-A549/DDP and shLRPPRC-A549/DDP groups and treated with DDP.
Data are shown as mean ± SD (n=6/group). **P<0.01,
shLRPPRC-A549/DDP vs. shCon-A549/DDP or A549/DDP group. LRPPRC,
leucine-rich PPR-motif-containing protein; DDP, cisplatin;
IC50, half-maximal inhibitory concentration; DDP,
cisplatin; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl
tetrazolium bromide. |
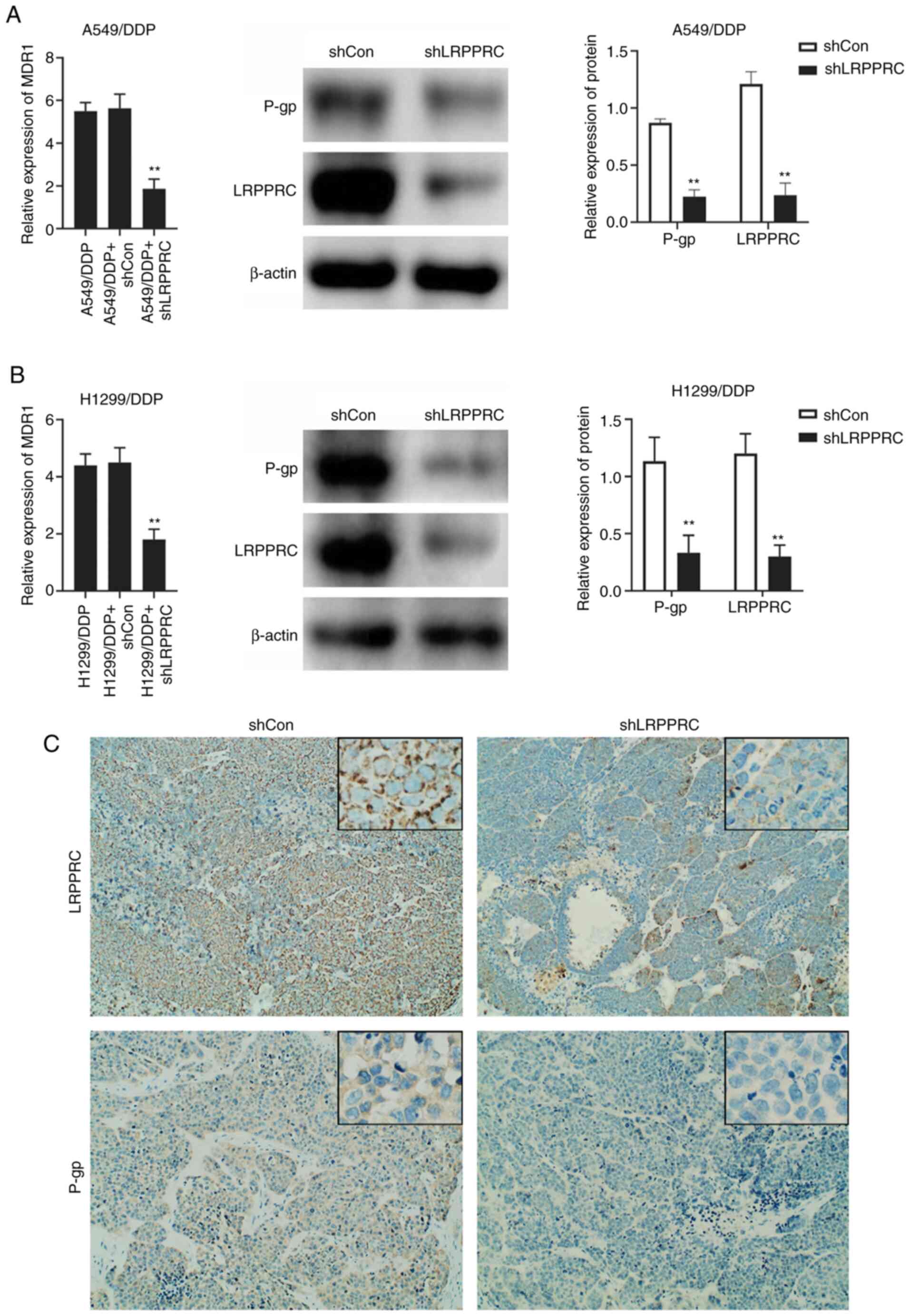
LRPPRC silencing inhibits MDR1
expression
The mRNA expression of MDR1 was investigated
in LRPPRC-silenced A549/DDP and H1299/DDP cells using qPCR.
MDR1 mRNA levels were significantly decreased in
LRPPRC-silenced resistant cells compared with that in LRPPRC-normal
expression cells. Changes in P-gp expression were confirmed through
western blot analysis (Fig. 4A and
B). The in vitro results were verified through IHC in
A549/DDP-derived xenograft tumors. P-gp expression was decreased in
the tumors derived from LRPPRC-silenced cells compared with that in
the shControl group (Fig. 4C).
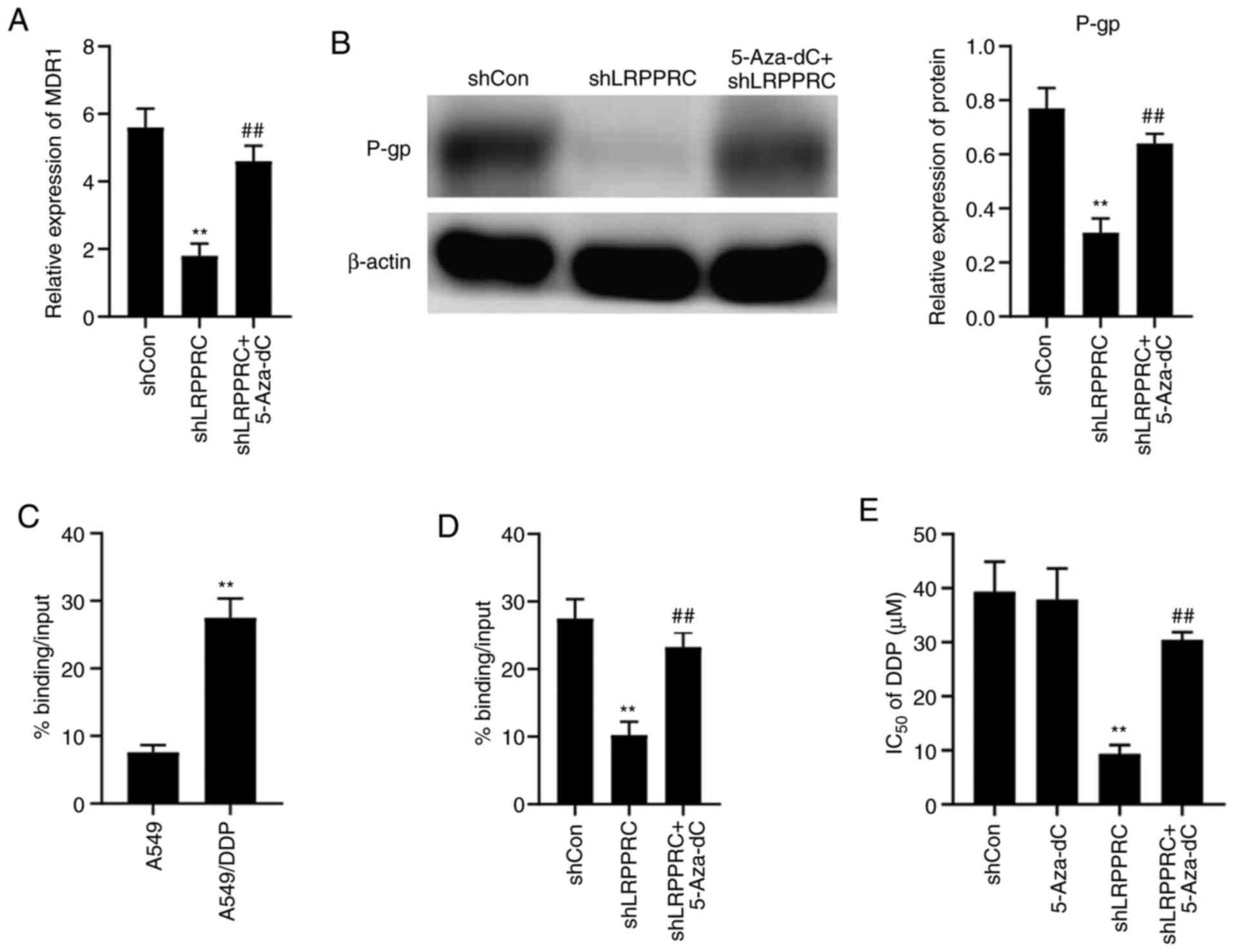
Demethylation treatment counteracts
the effect of LRPPRC silencing
We investigated whether demethylation treatment
could counteract the effect of LRPPRC silencing in lung cancer
cells using the demethylation agent 5-Aza-dC (Sigma-Aldrich; Merck
KGaA; cat. no. A3656). The viability of A549/DDP cells was
decreased by approximately 10% after 1 µM 5-Aza-dC treatment (data
not shown). Therefore, we used 0.5 µM 5-Aza-dC in the subsequent
experiments. A549/DDP cells were treated with 5-Aza-dC combined
with shLRPPRC for 48 h, and the resulting MDR1 expression
was investigated using qPCR. 5-Aza-dC treatment rescued the
decrease in MDR1 mRNA level mediated by LRPPRC silencing
(Fig. 5A). P-gp expression changes
were confirmed by western blot analysis (Fig. 5B). The interaction between LRPPRC and
the MDR1 promoter was evaluated using ChIP-qPCR. The level
of LRPPRC binding with theMDR1 promoter showed a 4.0-fold
increase in A549/DDP cells compared with that in the parental cells
(Fig. 5C). 5-Aza-dC treatment rescued
the decrease in level of LRPPRC binding with the MDR1
promoter in A549/DDP cells brought about by LRPPRC silencing
(Fig. 5D).
A549/DDP cells were pre-treated with 0.5 µM 5-Aza-dC
for 3 weeks and then treated with different concentrations of DDP
in combination with shLRPPRC for 48 h to investigate whether
demethylation treatment could affect DDP sensitivity regulated by
LRPPRC. The inhibition of cell proliferation was evaluated using
MTT assay, and IC50 values were calculated. The
IC50 values of DDP of the LRPPRC-silenced cells treated
with 5-Aza-dC increased compared with the LRPPRC-silenced cells
treated with PBS (Fig. 5E). These
results indicate that demethylation treatment decreases LRPPRC
silencing-induced increase in DDP sensitivity.
Discussion
Chemotherapy is an important therapeutic strategy
for lung cancer. Although chemotherapy can improve patient
survival, the development of resistance is generally inevitable,
ultimately causing relapse and metastasis. Therefore, a better
understanding of the relevant resistance mechanisms and
identification of agents that reverse resistance are emergent
issues concerning lung cancer.
Lung cancer highly expresses the leucine-rich
PPR-motif-containing protein (LRPPRC). Downregulation of LRPPRC was
found to decrease the anti-apoptotic, invasive and colony-forming
abilities of lung adenocarcinoma cells (9). Overexpression of P-glycoprotein (P-gp)
encoded by the multidrug resistance-associated protein 1
(MDR1) gene is one of the major mechanisms of cancer
resistance. LRPPRC promotes MDR1 transcription by binding
with the MDR1 promoter (11,12). Thus,
we investigated whether LRPPRC plays a role in the chemo-resistance
of lung cancer cells. We successfully established two DDP-resistant
lung cancer cell lines, including A549/DDP and H1299/DDP, via
long-term DDP exposure of the cells. The obtained cells were more
resistant to DDP than their parental cells. We compared the
expression of LRPPRC and MDR-related proteins between DDP-resistant
and parental cells using qPCR. LRPPRC and MDR1 mRNA
levels were significantly upregulated in A549/DDP and H1299/DDP
cells. These results were confirmed by western blot analysis, thus
revealing that long-term exposure of DDP increased MDR1
expression and the LRPPRC overexpression may contribute to DDP
resistance. We further determined whether downregulation of LRPPRC
could increase DDP sensitivity. Downregulation of LRPPRC remarkably
promoted the inhibition of proliferation and increased apoptosis of
resistant cells induced by DDP. The results were verified by in
vivo experiments, which showed that LRPPRC silencing improves
DDP sensitivity in implanted A549/DDP tumors. Taken together, our
findings indicate that LRPPRC contributes to DDP resistance in lung
cancer cells.
LRPPRC has been demonstrated to be a transcription
factor involved in the regulation of MDR1 expression through
invMED1 binding sites in the promoter. This regulation is affected
by the methylation status of MDR1 promoter GC −100 box
(11). We investigated changes in the
mRNA levels of MDR-related genes, including MDR1, BCRP, MRP1
and LRP, in LRPPRC-silenced A549/DDP and H1299/DDP cells.
LRPPRC suppression significantly decreased the mRNA levels of
MDR1 but had no effect on BCRP, MRP1 and LRP
expression. These results were confirmed by western blot analysis.
We further investigated the effect of demethylation treatment on
MDR1 expression. Demethylation treatment using 5-Aza-dC
rescued the decreased MDR1 expression caused by LRPPRC
silencing. Next, we compared the level of LRPPRC binding with the
MDR1 promoter between A549/DDP and parental cells and found
that the level of LRPPRC binding with the MDR1 promoter was
significantly upregulated in resistant cells. Demethylation
treatment rescued the decreased level of LRPPRC binding with the
MDR1 promoter in resistant cells due to LRPPRC silencing. We
investigated whether demethylation treatment could affect DDP
sensitivity regulated by LRPPRC and found that this treatment
decreased the LRPPRC-silenced-mediated increase in DDP sensitivity.
These results suggest that LRPPRC regulates MDR1
transcription, which contributes to the chemo-resistance of lung
cancer cells.
The effect of LRPPRC on multidrug resistance (MDR)
has been studied in other tumor types. Overexpression of LRPPRC has
been observed in chronic myeloid leukaemia MDR/IM cross-resistant
cells (15,16). Li et al found that MDR gastric
cancer cells highly express LRPPRC. Downregulation of LRPPRC was
found to considerably increase cytotoxic drug sensitivity, reduce
MDR1 expression and the ability of P-gp protein to efflux
adriamycin in gastric cancer cells (17,18). Our
findings are consistent with previous reports but contrast those of
Michaud et al, who found that the decreased expression of
LRPPRC did not affect P-gp expression and the capacity of
haepatocarcinoma cells to extrude cytotoxic drugs (19). Different cancer cells express
different MDR-related genes for resistance (20,21), and
the cytotoxic drug resistance of hepatocellular carcinoma cells may
not depend on MDR1 gene expression. This difference may
contribute to the contradictory results obtained.
In conclusion, LRPPRC contributes to DDP resistance
in lung cancer cells by regulating MDR1 transcription.
LRPPRC may serve as a potential molecular target for
chemo-resistance reversal, which may benefit lung cancer
patients.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Scientific Research
Program Funded by Shaanxi Provincial Education Department (grant
no. 18JK0864).
Availability of data and materials
The data that support the findings of this study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YH was concerned with the conceptualization, data
curation, formal analysis, funding acquisition, software,
investigation and writing of the original draft. JC was responsible
for the data curation, formal analysis and writing of the original
draft. LJ was responsible for the software, formal analysis,
validation, visualization of the findings and writing of the
revision and editing. YS was responsible for the data curation,
validation and visualization of the findings. XZ was concerning
with the supervision of the research project, project
administration, validation, methodology, writing, review and
editing of the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The Ethics Committee of the First Affiliated
Hospital of Xi'an Medical University approved the protocol (no.
20180606) for the animal study. The study was carried out in
accordance with the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health.
Patient consent for publication
Not applicable.
Competing interests
The authors report no competing interests in this
work.
Glossary
Abbreviations
Abbreviations:
|
LRPPRC
|
leucine-rich PPR motif-containing
protein
|
|
DDP
|
cisplatin
|
|
NSCLC
|
non-small cell lung cancer
|
|
MDR
|
multidrug resistance
|
|
ABC
|
ATP-binding cassette
|
|
P-gp
|
P-glycoprotein
|
|
MRP1
|
multidrug resistance-associated
protein 1
|
|
BCRP
|
breast cancer-resistant protein
|
|
LRP
|
lung resistance-related protein
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide
|
|
qPCR
|
quantitative real-time polymerase
chain reaction
|
|
ChIP
|
chromatin immunoprecipitation
|
|
IC50
|
half-maximal inhibitory
concentration
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tian R, Zhang C, Xiong F and Chen H:
PCAT1/miR-129/ABCB1 axis confers chemoresistance in non-small cell
lung cancer. Front Biosci (Landmark Ed). 25:948–960. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, He LR, Gao Y, Zhou NN, Liu Y, Zhou
XK, Liu JF, Guan XY, Ma NF and Xie D: CHD1L contributes to
cisplatin resistance by upregulating the ABCB1-NF-κB axis in human
non-small-cell lung cancer. Cell Death Dis. 10:992019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vasiliou V, Vasiliou K and Nebert DW:
Human ATP-binding cassette (ABC) transporter family. Hum Genomics.
3:281–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pérez-Tomás R: Multidrug resistance:
Retrospect and prospects in anti-cancer drug treatment. Curr Med
Chem. 13:1859–1876. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Z, Shi T, Zhang L, Zhu P, Deng M,
Huang C, Hu T, Jiang L and Li J: Mammalian drug efflux transporters
of the ATP binding cassette (ABC) family in multidrug resistance: A
review of the past decade. Cancer Lett. 370:153–164. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Manna S: An overview of pentatricopeptide
repeat proteins and their applications. Biochimie. 113:93–99. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cui J, Wang L, Ren X, Zhang Y and Zhang H:
LRPPRC: A multifunctional protein involved in energy metabolism and
human disease. Front Physiol. 10:5952019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tian T, Ikeda J, Wang Y, Mamat S, Luo W,
Aozasa K and Morii E: Role of leucine-rich pentatricopeptide repeat
motif-containing protein (lrpprc) for anti-apoptosis and
tumourigenesis in cancers. Eur J Cancer. 48:2462–2473. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang HY, Ma YD, Zhang Y, Cui J and Wang
ZM: Elevated Levels of autophagy-related marker ULK1 and
mitochondrion-associated autophagy inhibitor LRPPRC are associated
with biochemical progression and overall survival after androgen
deprivation therapy in patients with metastatic prostate cancer. J
Clin Pathol. 70:383–389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Corrêa S, Binato R, Du Rocher B, Ferreira
G, Cappelletti P, Soares-Lima S, Pinto LF, Mencalha A and Abdelhay
E: ABCB1 regulation through LRPPRC is influenced by the methylation
status of the GC −100 box in its promoter. Epigenetics.
9:1172–1183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Labialle S, Dayan G, Gayet L, Rigal D,
Gambrelle J and Baggetto LG: New invMED1 element cis-activates
human multidrug-related ABCB1 and MVP genes, involving the LRP130
protein. Nucleic Acids Res. 32:3864–3876. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fahrmann JF, Grapov D, Phinney BS, Stroble
C, DeFelice BC, Rom W, Gandara DR, Zhang Y, Fiehn O, Pass H and
Miyamoto S: Proteomic profiling of lung adenocarcinoma indicates
heightened DNA repair, antioxidant mechanisms and identifies LASP1
as a potential negative predictor of survival. Clin Proteomics.
13:312016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Corrêa S, Pizzatti L, Du Rocher B,
Mencalha A, Pinto D and Abdelhay E: A comparative proteomic study
identified LRPPRC and MCM7 as putative actors in imatinib mesylate
cross-resistance in lucena cell line. Proteome Sci. 10:232012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Corrêa S, Pizzatti L, Du Rocher B and
Abdelhay E: Comparative proteomic study of multidrug resistance in
chronic myeloid leukemia. Eur J Cancer Supplements. 8:2042010.
View Article : Google Scholar
|
|
17
|
Li X, Lv L, Zheng J, Zhou J, Liu B, Chen
H, Liang C, Wang R, Su L, Li X and Fan D: The significance of
LRPPRC overexpression in gastric cancer. Med Oncol. 31:8182014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Zhou J and Li X: Reversal of
multidrug resistance in gastric cancer cells by LRPPRC
downregulation. J Digest Dis. 15:842014.
|
|
19
|
Michaud M, Barakat S, Magnard S, Rigal D
and Baggetto LG: Leucine-rich protein 130 contributes to apoptosis
resistance of human hepatocarcinoma cells. Int J Oncol. 38:169–178.
2011.PubMed/NCBI
|
|
20
|
Choi HS, Kim YK and Yun PY: Upregulation
of MDR- and EMT-related molecules in cisplatin-resistant human oral
squamous cell carcinoma cell lines. Int J Mol Sci. 20:E30342019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Januchowski R, Sterzyńska K, Zaorska K,
Sosińska P, Klejewski A, Brązert M, Nowicki M and Zabel M: Analysis
of MDR genes expression and cross-resistance in eight drug
resistant ovarian cancer cell lines. J Ovarian Res. 9:652016.
View Article : Google Scholar : PubMed/NCBI
|