Introduction
During cancer treatment, radiotherapy is used in
conjunction with surgery to reduce recurrence and the risk of
metastasis (1,2). Although radiotherapy is a suitable
treatment strategy for many cancer patients, the persistence of
radiation-resistant tumor cells often poses a significant obstacle
to effective radiation-based therapy and leads to poor prognosis
(3). Thus, understanding the
mechanisms governing radiation resistance is essential in enhancing
the utility of radiotherapy.
Collapsin response mediator protein 4 (CRMP4), one
of the five members of the cytosolic phosphoprotein family, is also
known as dihydropyrimidinase-like protein 3 (DPYSL3) and shares 58%
sequence homology with dihydropyrimidinase (DHPase) (4). DHPase catalyzes the ring-opening of
5,6-dihydrouracil to N-carbamyl-β-alanine and that of
5,6-dihydrothymine to N-carbamyl-β-amino isobutyrate; however,
whether CRMP4 demonstrates DHPase-like properties remains to be
elucidated (5). In contrast to the
structure of DHPase, CRMPs have a positively charged C-terminal
that renders them highly susceptible to proteolysis (6). The C-terminal region of CRMP4 has been
associated with neuronal cell injury and neurite damage (7). CRMP4 deletion in vivo exerts a
neuroprotective effect against spinal cord injury owing to
decreased apoptotic cell death rate and suppressed inflammatory
responses (8). CRMP4 is thus
considered an important therapeutic target for neuroregeneration.
In addition, several studies have indicated that CRMP4 is involved
in various types of cancers. For example, pancreatic and colon
cancers show elevated CRMP4 expression, which strongly correlates
with severe venous invasion, liver metastasis, and poor prognosis
(9,10). Conversely, CRMP4 is regarded as a
metastasis suppressor in prostate and breast cancer (11,12). These
results indicate that a deeper analysis of CRMP4 function may offer
new insights into potential cancer therapies.
The mitochondrial membrane potential (MMP) is the
major component of the proton-motive force, which is the central
intermediate of aerobic energy production and the driving force
behind other physiological processes in the mitochondria, such as
Ca2+ uptake and antioxidant activity (13). Cellular injury or stress stimulation
directly elicits alterations in the mitochondrial architecture,
membrane potential, and oxidative capacity, which are associated
with an irreversible loss of mitochondrial matrix contents and
integral membrane protein constituents, such as cytochrome c
oxidase (14). The release of
cytochrome c from the mitochondria leads to the activation
of caspase-3 and caspase-9, resulting in apoptosis (15). Ca2+ ions serve as an
important second messenger for multiple physiological processes.
Several studies have indicated that intracellular Ca2+
levels are regulated by ionizing radiation (16); moreover, the rise in intracellular
Ca2+ levels after radiation exposure is crucial for a
diverse array of signaling pathways that regulate critical cellular
processes, including apoptosis (17,18).
Ca2+ influx has been known to be facilitated by voltage-
and ligand-gated Ca2+ channels. Although CRMP4 has not
been reported to be associated with Ca2+ channels, CRMP2
was shown to interact with a novel N-type voltage-gated
Ca2+ channel (19,20); nevertheless, the functional role of
Ca2+ binding to CRMPs remains elusive.
In the present study, radiation-resistant colon
cancer cell lines were established, and RNA sequencing was
conducted to examine the radioresistant-associated genes.
CRMP4 was identified as one of the strongly downregulated
genes (<0.5-fold) in the radioresistant cells compared to their
parental cells. To know the function of CRMP4 under radiation
exposure, MMP and cytochrome c release were analyzed using
CRMP4-knockdown SW620-shCRMP4 and RKO-shCRMP4 cells and
radiation-resistant IR-SW620 and IR-RKO cells. In addition,
Ca2+ ionophore A23187 and Ca2+ chelator
BAPTA-AM were used to examine the relationship between CRMP4,
Ca2+-related MMP, and apoptosis.
Materials and methods
Cell culture
Colon cancer cell lines SW480 (KCLB-10228; colon
adenocarcinoma), SW620 (KCLB-10227; colorectal carcinoma), HT-29
(KCLB-30038; rectosigmoid colon adenocarcinoma), DLD1 (KCLB-10221;
colon adenocarcinoma), Caco2 (KCLB-30037.1; colon adenocarcinoma),
LoVo (KCLB-10229; colon adenocarcinoma, KM12C (KCLB-80015; colon
carcinoma), and KM12SM (KCLB-80016; colon carcinoma) cells were
purchased from the Korean Cell Line Bank (Seoul, Korea). RKO (ATCC
CRL-2577; colon carcinoma), HCT116 (ATCC CCL-247; colorectal
carcinoma), and LS174T (ATCC CL-188; colorectal adenocarcinoma)
cells were purchased from the American Type Culture Collection
(ATCC, USA). Each cell line was authenticated through STR profiling
from the cell line bank. Cells were cultured in Dulbecco's modified
Eagle's medium (DMEM) (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS) (HyClone) and 100
µg/ml antibiotics [100 U/ml penicillin and 100 µg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc.)] at 37°C under an
atmosphere of 5% CO2 in a humidified incubator.
Radiation exposure and radioresistant
cell line generation
For radiation treatment, cells were seeded in 60-mm
dishes and exposed to radiation from a 60Co source
(model 109 irradiator; JL Shepherd and Associates) at the indicated
doses (0–5 Gy). Subsequently, cells were incubated at 37°C under a
humidified, 5% CO2/air atmosphere. To generate
radiation-resistant colon cancer cell lines (21,22), cells
(SW620, RKO, SW480, and HT-29) were plated in 60-mm dishes at a
density of 5×103 cells/dish and exposed to a 5-Gy dose
of ionizing radiation, followed by a 15-day recovery period. This
process was repeated for 24 treatment cycles totaling 120 Gy;
finally, radioresistant IR-SW620, IR-RKO, IR-SW480, and IR-HT-29
cell lines were established.
RNA sequencing and data analysis
The mRNAs of the established radioresistant
IR-SW620, IR-SW480, IR-RKO, and IR-HT-29 cells were extracted using
TRIzol (Sigma-Aldrich; Merck KGaA) method. The total RNA quantity
and integrity of each RNA sample were evaluated using the
bioanalyzer 2100 (Agilent Technologies, Inc.). The RNA
concentration was quantified as 1 µg/µl, rRNA ratio (28S/18S) was
2.0, and the RIN number was 10 (HT-29, RKO, IR-RKO, SW480,
IR-SW480, SW620) and 9.9 (IR-HT-29, IR-SW620). The RNA sequencing
library was prepared using the TruSeq RNA Sample Prep Kit
(Illumina, Inc.) and sequenced using Hiseq-2000 (Illumina, Inc.) to
generate 76- or 101-bp paired-end reads. Reads were trimmed to
remove adapter sequences and base with low sequencing quality
(per-base quality <20) using Cutadapt (23). The clean reads were aligned to the
reference genome (hg38) sequencing using STAR 2.4.1 (24), and the gene expression levels (GRCh38)
were quantified with the HTSeq package (25). The edgeR package (26) was used to select differentially
expressed genes from sequencing count data.
RNA interference experiments
In this study, we used two RNA interference methods
to knock down the CRMP4 gene, one for transient siRNA and
the other for stable shRNA method. Small interfering RNA (siRNA)
duplexes of CRMP4 were purchased from Bioneer (Daejeon,
Korea). The specific target sequences of CRMP4 siRNA (#2,
Fig. S1A) were sense
5′-GUGGAAGGAUUGUAGUCAUdTdT-3′ and antisense
5′-AUGACUACAAUCCUUCCACdTdT-3′. siRNA duplexes were transfected into
cells (50 nM for RKO, 200 nM for SW620, SW480, Caco2, and KM12C)
using Lipofectamine RNAiMAX reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The short hairpin RNA (shRNA) targeting
CRMP4 was obtained from Origene (TL313373V). The shRNA
expression vector was transfected into the lentiviral packaging
Lenti-X 293T cell line (Takara Bio USA). The culture supernatant
containing virus particles was harvested 48 h post-transfection.
For the stable transduction of the lentivirus, cells at 60–70%
confluence were grown in 6-well plates. After 48 h, 1 µg/ml
puromycin (Clontech Laboratories, Inc.) was added, and finally,
CRMP4-knockdown RKO (#1 clone) and SW620 (#4 clone) cells were
selected for further study (Fig.
S1B).
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
(RIPA) lysis buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP40,
0.25% sodium deoxycholate, 1 mM PMSF, protease inhibitor mixture
(Sigma-Aldrich; Merck KGaA), and 1 mM sodium orthovanadate].
Proteins were separated using sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE), transferred to a polyvinylidene
fluoride membrane, and blocked with 5% skim milk/PBS-T buffer for 1
h. Subsequently, the membrane was incubated with the following
primary antibodies: β-actin, CRMP4, cytochrome c (Santa Cruz
Biotechnology, Inc.), and cleaved-PARP (cleaved-polyADP-ribose
polymerase) (Cell Signaling Technologies, Inc.). The bound
antibodies were visualized with a horseradish peroxidase-conjugated
secondary antibody using enhanced chemiluminescence (Clarity
Western ECL; Bio-Rad Laboratories, Inc.) and the Ez-Capture MG
system (Atto Corp.).
Flow cytometry for the measurement of
MMP, intracellular Ca2+ levels, and apoptosis assay
The fluorescent probe Fluo 3-AM was used for the
assessment of intracellular levels of Ca2+ and the
lipophilic cationic dye 3,3′-dihexyloxacarbocyanine iodide
[DiOC6(3)] was used for measuring disruption of the MMP (Δψm).
Briefly, cells were exposed to a 5-Gy radiation dose and incubated
for 72 h, then stained with 1 µM DiOC6(3) at 37°C for 15 min in the
dark, and analyzed using FACSverse flow cytometry (BD Biosciences).
For apoptosis analysis, cells were harvested and centrifuged at 800
rpm for 3 min following radiation treatment (48 h). Cells were
carefully resuspended, and hen Annexin-V (5 µl) and propidium
iodide (PI) (5 µl) (BD Biosciences) were added, and the cells were
incubated at 37°C for 15 min in the dark. The stained cells were
analyzed using FACSverse flow cytometry (BD Biosciences) and the
Flowjo software v7.6.1 (FlowJo LLC). At least 10,000 cells per
sample were analyzed, and duplicate analyses were performed at each
time point.
Clonogenic assay
Cells were seeded into 60-mm dish plates at
densities of 1, 2, 5, 8, and 10×103 cells/plate. After
24 h, cells were treated with the indicated ionizing radiation
dose. After 14 days, the colonies were subsequently fixed, stained
with 0.1% crystal violet in 20% ethanol, and counted.
Alternatively, cells were stained with crystal violet; after a wash
step, the dye was solubilized, and absorbance was measured at 590
nm using a microplate reader (Molecular Devices, LLC).
Cell cycle analysis
Irradiated cells were trypsinized, washed in
ice-cold PBS, and fixed with 70% ethanol on ice. Fixed cells were
stained with PI (BD Biosciences) containing RNase (0.1 mg/ml) for
15 min at 37°C, and cell population analysis was performed using
FACSCalibur flow cytometry.
Measurement of cytochrome c
release
The cytochrome c- releasing apoptosis assay
kit (Abcam; ab65311) was used for detecting cytochrome c
translocation from mitochondria into the cytosol. After irradiated
or A23187-treated cells were lysed in a cytosolic extraction buffer
and homogenized, the supernatant cytosolic fraction was separated
by centrifugation, and the pellet was resuspended in a
mitochondrial extraction buffer, according to the manufacturer's
instructions. Separated cytosolic and mitochondrial fractions were
immunoblotted for the measurement of cytochrome c
release.
Cell viability assay
Cell viability was assessed using the water-soluble
tetrazolium salt (WST)-1 assay (Roche Diagnostics) according to the
manufacturer's instructions. Briefly, 10 µl WST-1 reagent was added
to each well of a 96-well plate (1×104 cells/well).
After incubation for 1 h, the conversion of WST-1 reagent into
chromogenic formazan was evaluated using a microplate reader.
Statistical analysis
All experiments were performed in triplicate and
data are expressed as the means ± standard deviation. Differences
in cell apoptosis, survival, and MMP depolarization between control
and CRMP4-deficiency cells were analyzed using a paired Student's
t-test. Differences in cell survival among different cell groups
(parent, IR-resistant, CRMP4-shRNA) were analyzed using one-way
ANOVA with Tukey's post hoc test. Data were analyzed using GraphPad
Prism (GraphPad Software Inc.). P<0.05 was considered
statistically significant.
Results
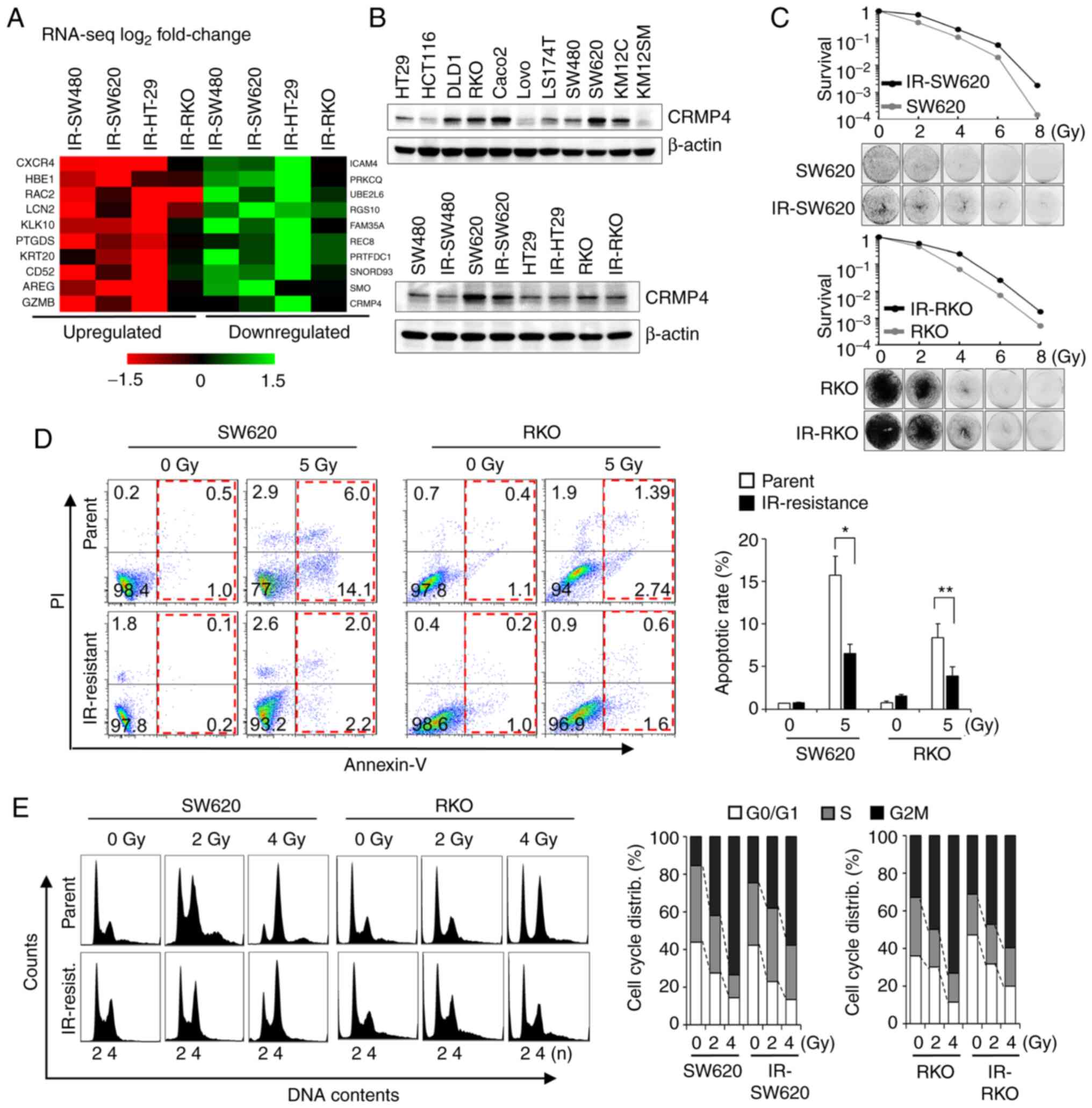
Radiation-resistant cells exhibit
CRMP4 downregulation and increased survival rate
To identify genes associated with radiation-induced
cell death, radiation-resistant IR-SW620, IR-RKO, IR-SW480, and
IR-HT-29 cell lines were established by repeated exposure to
ionizing radiation over several weeks for a total exposure of 120
Gy (21,22). From the RNA sequencing analysis, a
total of 25,207 genes were identified. Among them, 70 genes were
upregulated (>1.5-fold), while 45 were downregulated
(<0.5-fold). These 115 genes were confirmed using RT-PCR
analysis. Finally, the upregulated genes (CXCR4, RAC2, HBE1
(21), PTGDS, and LCN2)
and downregulated genes (SMO, RGS10, PRTFDC1, and
CRMP4) were identified as candidate genes associated with
radiation resistance (Fig. 1A). To
verify CRMP4 downregulation at the protein level, several colon
cancer and IR-resistant cell lines were analyzed by western
blotting (Fig. 1B). CRMP4 was
differentially expressed in those; however, radiation-resistant
IR-SW480, IR-SW620, and IR-RKO cells displayed apparent decreased
CRMP4 expression compared to that of their parental cells. When
SW620 and RKO cells showing high CRMP4 expression were compared
with IR-SW620 and IR-RKO cells in regards to cell survival,
IR-resistant cells exhibited better survival in the 5 Gy-irradiated
clonogenic assay (Fig. 1C). The
degree of apoptosis under exposure to 5 Gy of radiation, as
analyzed by Annexin-V and PI, was significantly decreased by about
half in IR-SW620 and IR-RKO cells compared to their parental cells
(Fig. 1D). To investigate the effect
of radiation on cell cycle distribution in IR-resistant cells and
parental cells, cells were exposed to 0, 2, and 4 Gy of radiation.
After 24 h, cells were analyzed by flow cytometry using PI, and it
revealed that radiation-mediated G2M accumulation was reduced in
both IR-SW620 and IR-RKO cells compared to their parent cells
(Fig. 1E).
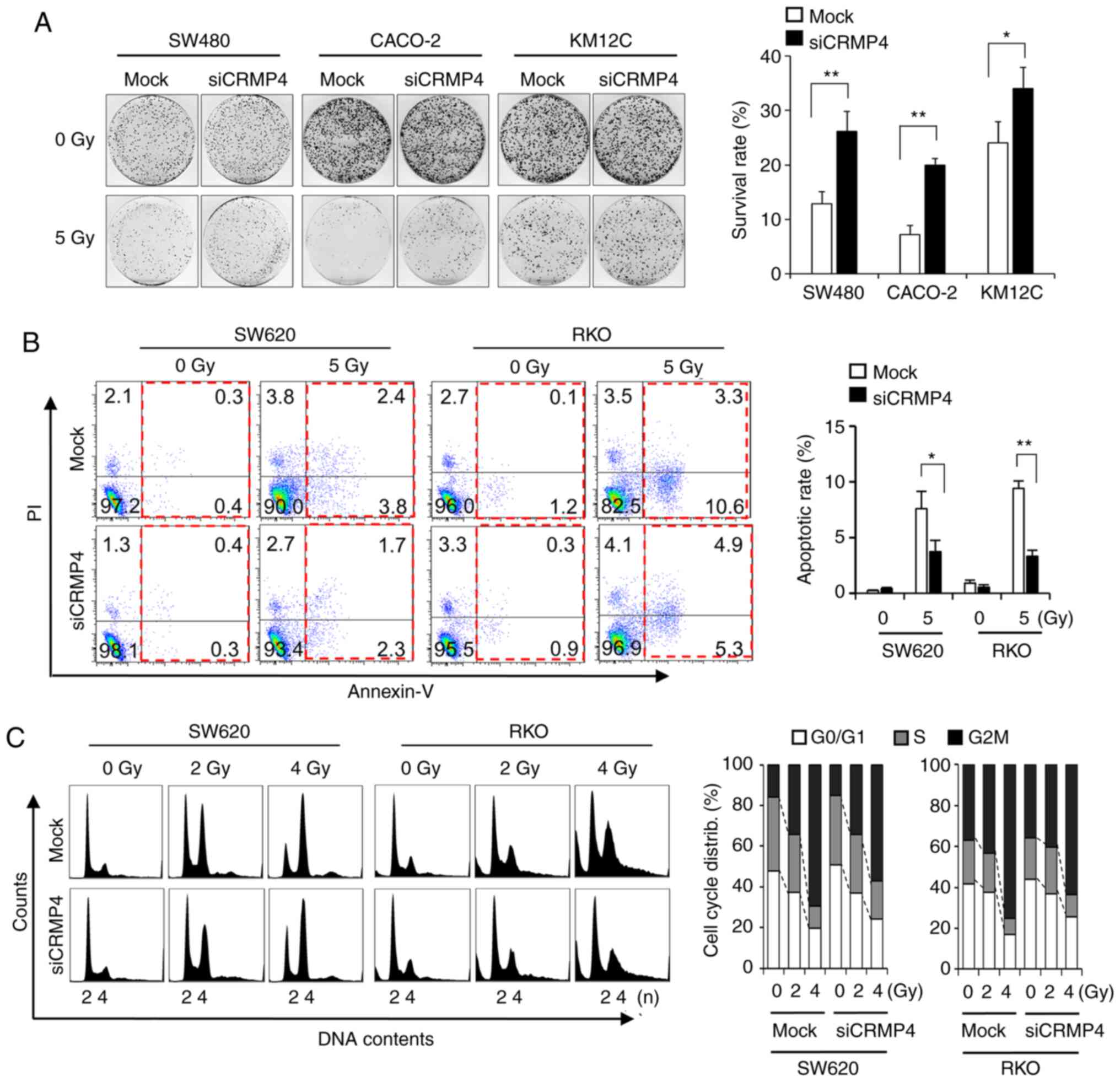
siRNA-mediated CRMP4 knockdown causes
radiation resistance
Loss-of-function experiments using siRNAs were
performed on colon cancer cells to determine whether CRMP4
reduction is involved in radiation resistance. When RKO and SW620
cells were treated with CRMP4-siRNA, there was a 5-fold
reduction in CRMP4 protein expression (Fig. S1A), although the siRNA amount was
treated differently based on transfection efficiency for each cell
line types. To verify the results from CRMP4-siRNA
transfected SW620 and RKO cells, colon cancer cell lines SW480,
Caco2 and KM12C, all of which express CRMP4, were selected and
transfected with CRMP4-siRNA. The clonogenic assays revealed
that CRMP4 reduction was associated with resistance to radiation in
these cells (Fig. 2A). To ascertain
whether increased clonogenic survival by CRMP4 knockdown was
associated with reduced apoptosis, Annexin V and PI staining was
used to examine the degree of apoptosis induced by radiation in
CRMP4-knockdown and mock cells (Fig. 2B). The number of apoptotic cells
following radiation treatment in the CRMP4-knockdown cells
was significantly decreased by half compared to that found in the
mock cells transfected with nonspecific control siRNA. In cell
cycle analysis, radiation-exposed CRMP4-knockdown cells
displayed decreased G2/M accumulation compared to the control cells
(Fig. 2C). These results suggest that
CRMP4 may be associated with the development of radiation
resistance in colon cancer cells.
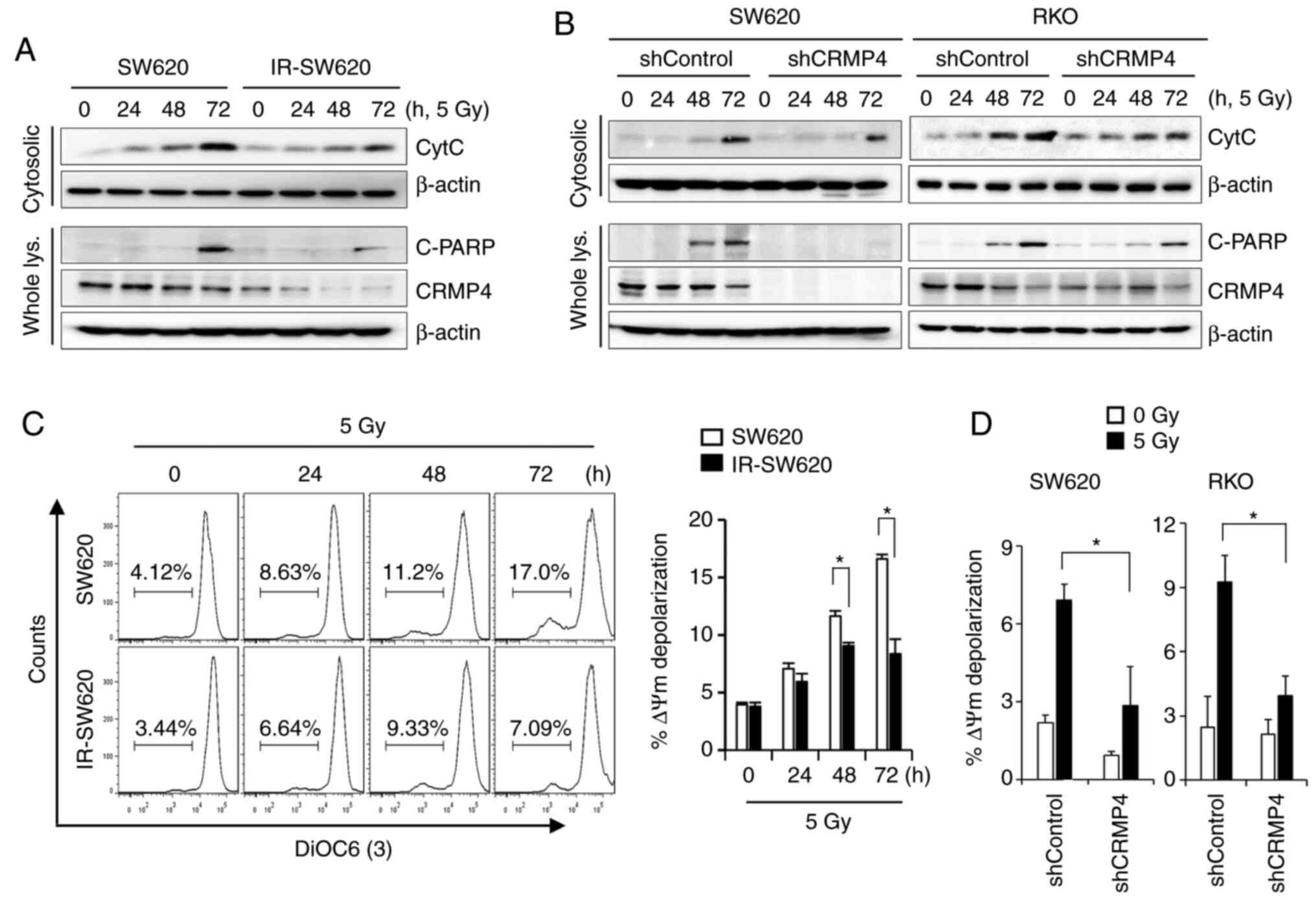
CRMP4-deficiency attenuates
radiation-induced cytochrome c release from mitochondria
Irradiation has been shown to induce various
cellular and molecular damage outcomes, including apoptosis, in
which cytochrome c release from mitochondria constitutes a
critical event (27). The amount of
cytochrome c released from the mitochondria of irradiated
cells was measured using a mitochondria/cytosol fractionation
method. As shown in Fig. 3A,
irradiated cells induced cytochrome c release in a
time-dependent manner. The maximum amount of cytochrome c
release was attained at 72 h of exposure to 5 Gy of radiation.
However, decreased cytochrome c release and PARP cleavage
were observed in the CRMP4-deficiency IR-SW620 cells compared to
the parental cells. To verify the role of CRMP4 in cancer cells as
regards radiation resistance, CRMP4 expression was stably knocked
down in SW620 and RKO cells through lentiviral-mediated shRNA
infection (Fig. S1B). After CRMP4
knockdown was verified in both cell lines, cytochrome c
release was evaluated under radiation exposure. As expected,
mitochondrial cytochrome c release and PARP cleavage were
decreased in CRMP4-knockdown SW620-shCRMP4 and RKO-shCRMP4 cells
compared to the nonspecific control shRNA cells (Fig. 3B). It has been known that the release
of cytochrome c from mitochondria during apoptosis is
associated with low MMP (ΔΨm). The lipophilic cationic dye
3DiOC6(3) was used to monitor MMP and determine whether CRMP4
reduction is associated with MMP loss. Radiation treatment
gradually caused the SW620 cells to lose MMP in a time-dependent
manner, whereas IR-SW620 cells showed a weak depolarization of MMP
(Fig. 3C). Similarly,
CRMP4-knockdown cells also exhibited insignificant
depolarization of MMP, but shControl cells showed a 2.3-fold
increase in depolarization (Fig. 3D).
These results indicate that CRMP4 deficiency may diminish
radiation-induced MMP depolarization and cytochrome c
release.
CRMP4 deficiency attenuates
Ca2+-mediated cell death pathway
Consequent to its role as an incredibly versatile
signaling ion, uncontrolled cytosolic Ca2+ influx
induces mitochondrial dysfunction and cell death pathways (3,18). To
determine whether CRPM4 could modulate intracellular
Ca2+ influx, Ca2+ concentration was measured
by flow cytometry using a cell-permeable fluorescent
Ca2+ indicator, Fluo 3-AM. As shown in Fig. 4A, radiation exposure strongly induced
intracellular Ca2+ influx regardless of CRMP4 expression
(Fig. 4A). When Ca2+
levels were analyzed in cells expressing the siRNA- or
shRNA-CRMP4 as a preliminary experiment (data not shown),
the significant difference in Ca2+ concentration
according to CRMP4 expression could not be determined. Under
radiation exposure, intracellular Ca2+ concentration was
strongly elevated in all radiation-treated cells irrespective of
CRMP4 expression (Fig. 4A),
indicating that CRMP4 is not likely involved in intracellular
Ca2+ influx regulation. Because improper Ca2+
influx into mitochondria causes MMP depolarization, we verified the
consequent radiation-induced cell death using the clonogenic assay
(Fig. 4B). When cells were treated
with cyclosporin A (CsA), known to inhibit
Ca2+-dependent mitochondrial permeability transition
(MPT) pores, cells exhibited strong radiation-resistant survival
compared to CsA-untreated control cells, indicating that
Ca2+ is critical to radiation-induced cell death. To
examine whether CRMP4 is required for Ca2+-mediated
apoptosis or MMP depolarization, cells were treated with the
Ca2+ ionophore A23187 to increase intracellular
Ca2+ levels. As shown in Fig.
4C, IR-resistant and shCRMP4 cells exhibited dose-dependent
improved survival compared to control parent cells upon A23187
treatment. Western blotting analysis further revealed that
cytochrome c release from mitochondria was obviously reduced
in the IR-resistant and shCRMP4 cells compared to the control cells
under A23187 treatment (Fig. 4D).
Consistent with this, when A23187-treated cells were analyzed by
flow cytometry using DiOC6(3), MMP depolarization was significantly
increased in shControl cells but not in shCRMP cells (Fig. 4E), indicating that CRMP4 may play a
role in Ca2+-mediated MMP depolarization followed by
mitochondrial cytochrome c release and apoptosis.
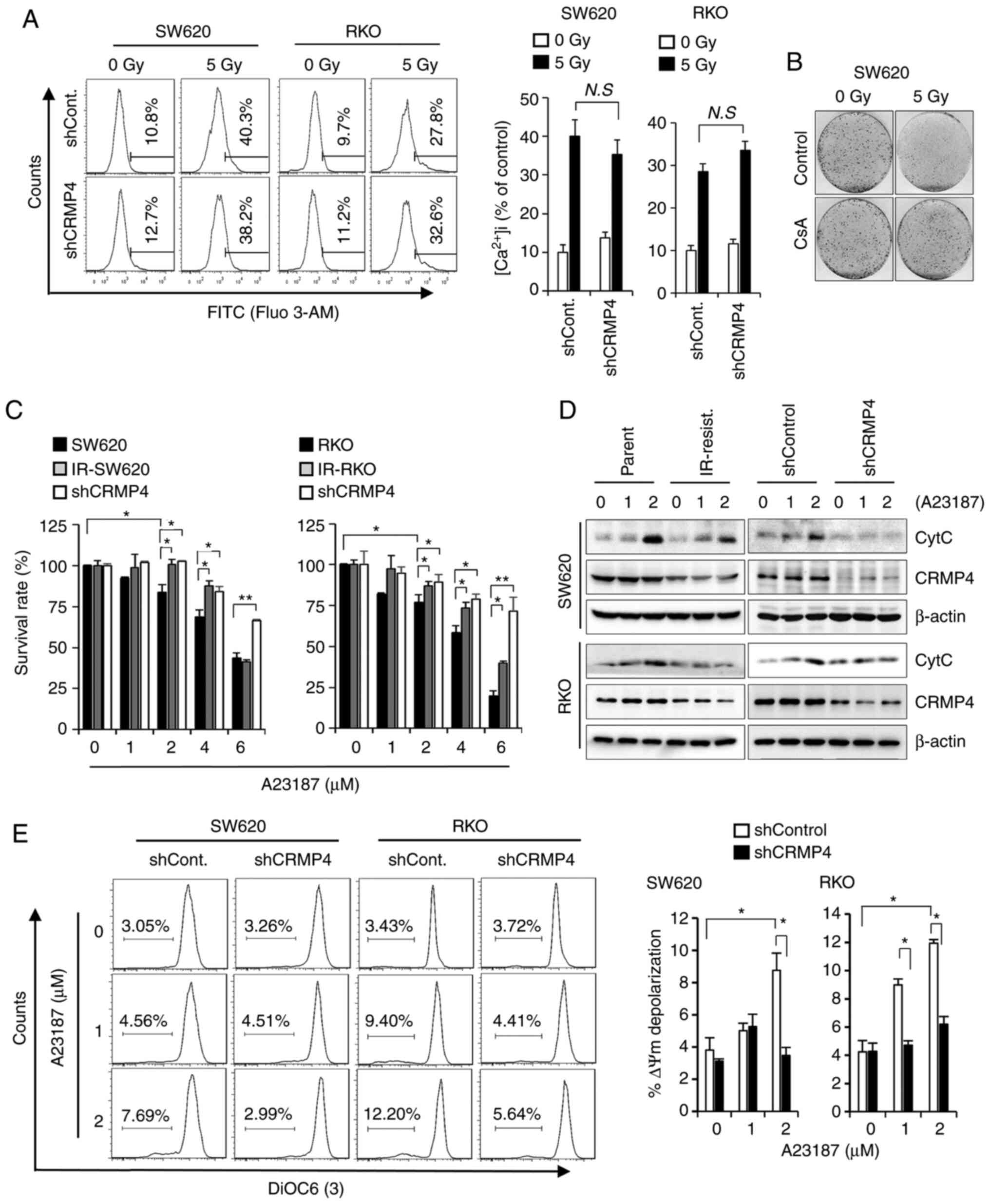 | Figure 4.CRMP4-deficiency inhibits the
Ca2+-mediated cell death pathway. (A) Intracellular
Ca2+ level was not affected by CRMP4-deficiency. Cells
were irradiated with 5 Gy of radiation for 72 h, treated with
Fluo-3 AM for Ca2+ detection, and analyzed by flow
cytometry. Histograms of intracellular Ca2+ content are
shown on the right. N.S, not significant. (B) Increased cell
survival by cyclosporine A (CsA) in radiation-treated cells. Cells
were treated with or without CsA (5 µM) for 1 h and exposed to 5 Gy
of radiation, and after 14 days, cells were stained with 0.1%
crystal violet. (C) CRMP4-deficiency inhibited A23187-mediated cell
death. Several cells were treated with the Ca2+
ionophore A23187 for 24 h, and their proliferation was measured by
a plate reader using the WST-1 reagent. The survival rate is
expressed as the % of control cells. Student's t-test was
performed. *P<0.05, **P<0.01. (D) Cytochrome c (CytC)
release inhibition in CRMP4-downregulated cells. After cells were
treated with A23187 (0–2 µM) for 12 h, the cytosolic fractions were
isolated and western blotting was conducted. CRMP4-deficient
ionizing radiation (IR)-resistant and shCRMP4 cells showed a
decreased cytochrome c release. (E) MMP depolarization was
increased in shControl cells but not in shCRMP4 cells. After cells
were treated with A23187 for 24 h, cells were stained with DIOC6(3)
and analyzed by flow cytometry. The quantitative graph is shown on
the right. Student's t-test was performed. *P<0.05. CRMP4,
collapsin response mediator protein 4; MMP, mitochondrial membrane
potential. |
Ca2+ chelator BAPTA-AM
downregulates CRMP4 and enhances radioresistance
Previously, we found that CsA-mediated MPT
inhibition caused the downregulation of CRMP4. To examine whether
CRMP4 expression is affected by intracellular Ca2+
levels, cells were treated with the cell-permeant intracellular
Ca2+ chelator, BAPTA-AM, and cell lysates were analyzed
with western blotting. Interestingly, CRMP4 was downregulated by
BAPTA-AM treatment in a dose-dependent manner (Fig. 5A). Although we could not determine
whether CRMP4 was proteolyzed or degraded in this condition, CRMP4
expression may be regulated by the Ca2+-related pathway.
The buffering of intracellular Ca2+ concentration by
BAPTA-AM (5 µM) also obviously reduced A23187-induced cytochrome
c release in SW620 cells (Fig.
5B). Western blotting results showed that cleaved-PARP was
augmented following radiation exposure, but IR-SW620 cells revealed
a strong inhibition of PARP cleavage under radiation exposure
following BAPTA-AM treatment (Fig.
5C). Consistently, when cells were analyzed with flow cytometry
using DiOC6, BAPTA-AM attenuated radiation-induced MMP
depolarization in both shCRMP4 and shControl cells (Fig. 5D). These data suggest that low
BAPTA-AM (<5 µM) could alleviate A23187- and radiation-induced
MMP depolarization and cytochrome c release.
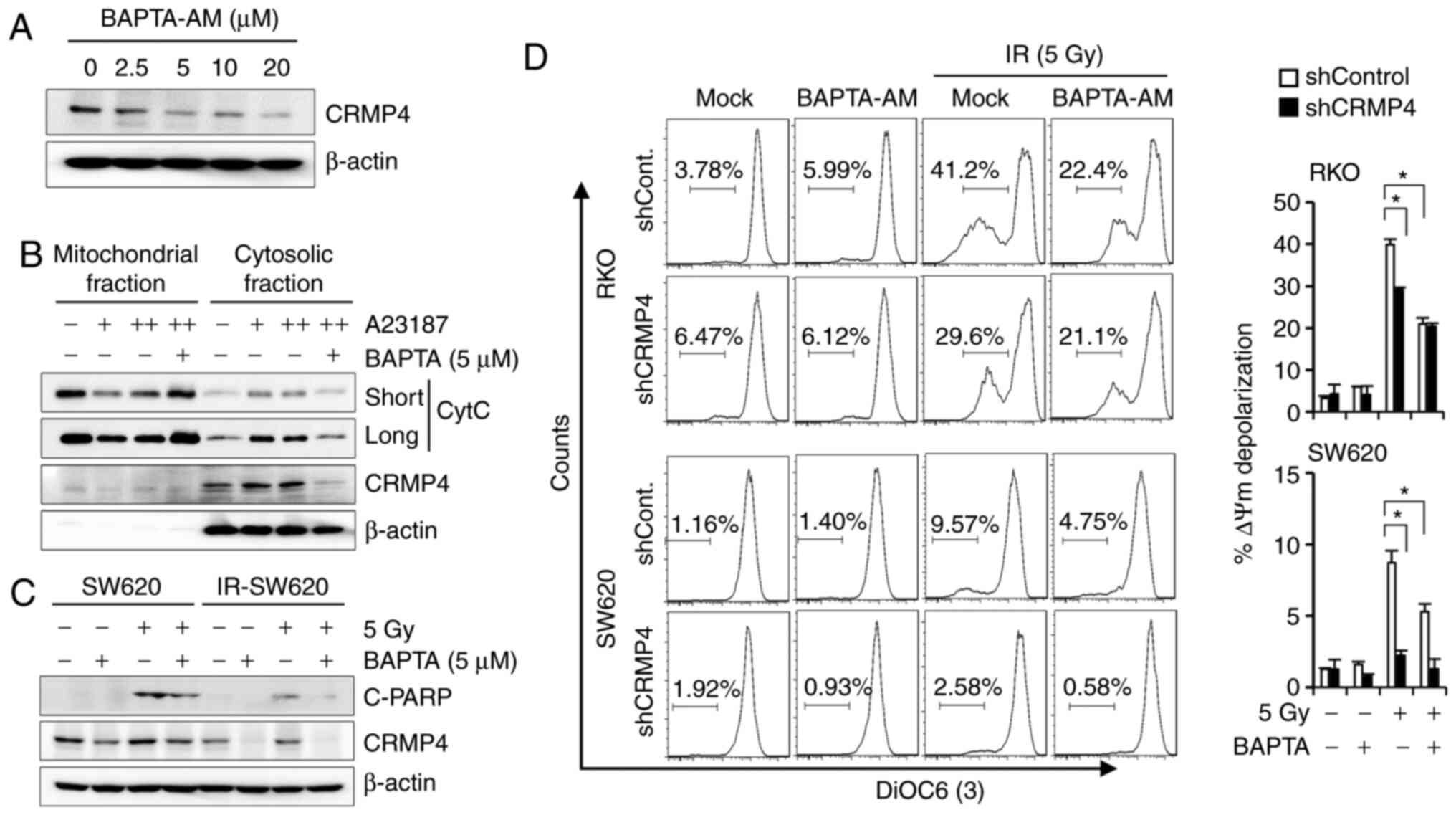 | Figure 5.The Ca2+ chelator,
BAPTA-AM, downregulates CRMP4 and inhibits MMP depolarization. (A)
CRMP4 downregulation by BAPTA-AM treatment. SW620 cells were
treated with BAPTA-AM (0–20 µM) for 24 h, and western blotting was
conducted. (B) A23187-mediated cytochrome c (CytC) release
was inhibited by BAPTA-AM. SW620 cells were treated with A23187 (+,
1 µM; ++, 2 µM) in the absence or presence of BAPTA-AM (5 µM) for
12 h, and mitochondrial and cytosolic fractions were isolated for
western blotting. (C) Radiation-mediated PARP cleavage (C-PARP) was
blocked by BAPTA-AM. Cells were treated with BAPTA-AM (5 µM) and/or
radiation (5 Gy), and after 72 h, cell lysates were analyzed by
western blotting. (D) Radiation-mediated MMP depolarization was
inhibited by BAPTA-AM. Cells were treated with BAPTA-AM (5 µM)
and/or radiation (5 Gy), and after 72 h, cells were stained with
DiOC6(3) for flow cytometry. The quantitative graph is shown on the
right. Student's t-test was performed. *P<0.05. CRMP4, collapsin
response mediator protein 4; MMP, mitochondrial membrane
potential. |
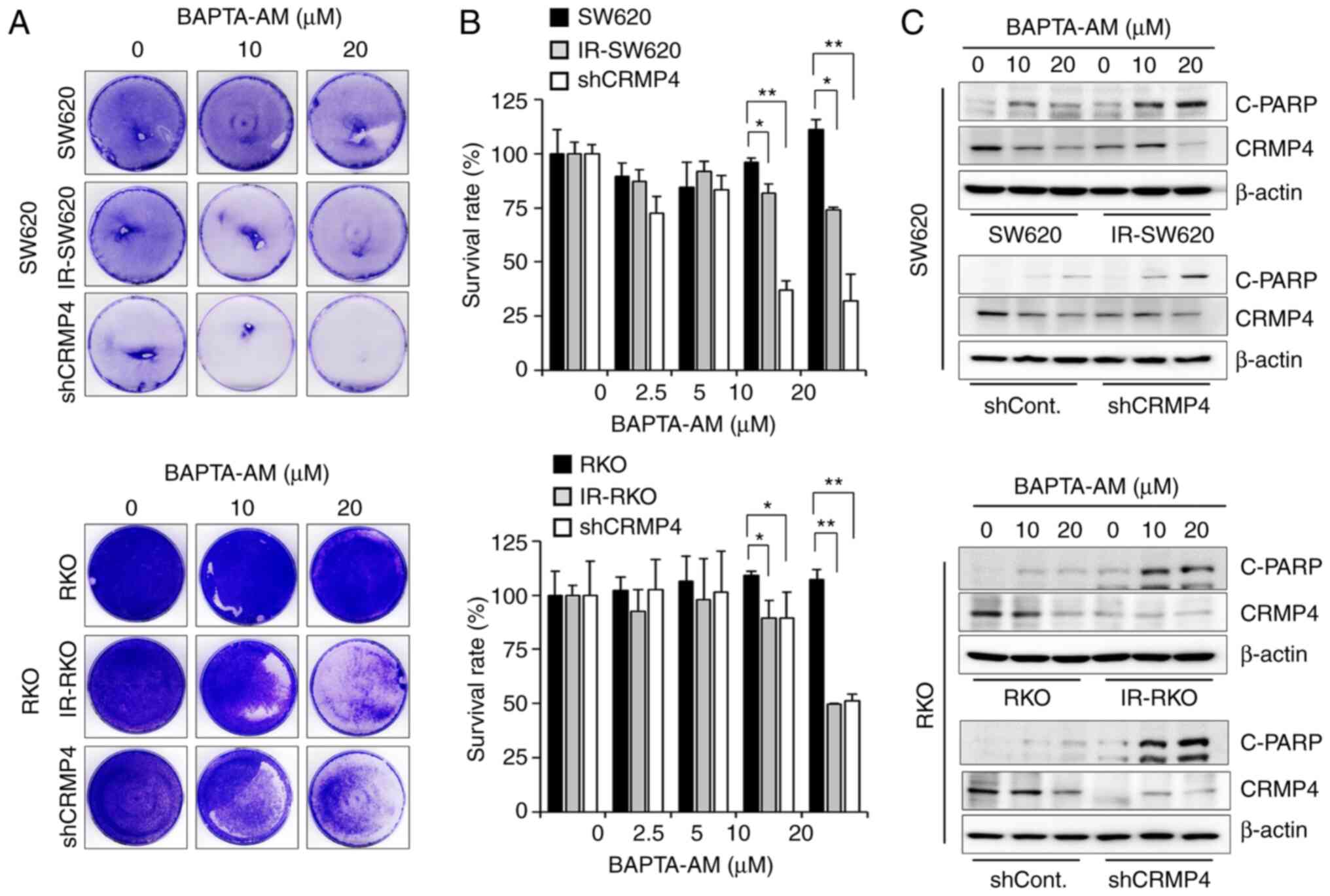
Cell death is increased in
CRMP4-deficient cells treated with high BAPTA-AM
Several studies have demonstrated the extensive use
of BAPTA-AM for buffering Ca2+; however, high
concentrations of BAPTA-AM have been found to deplete intracellular
Ca2+ stores (10,28). To ascertain whether the apoptosis
inhibition in CRMP4-deficient cells is changed under high BAPTA-AM
levels (>10 µM), several CRMP-deficient cell lines were treated
with 10–20 µM of BAPTA-AM, and surviving cells were stained with
crystal violet dye (Fig. 6A).
Furthermore, WST-1 assay was conducted for cell viability
measurement (Fig. 6B). High
BAPTA-AM-induced Ca2+ deficiency-mediated cell death in
both IR-resistant and CRMP4-knockdown cells in a
dose-dependent manner, but no significant effect was observed in
the parental cells, indicating that Ca2+
deficiency-induced apoptosis may be associated with the CRMP4
expression level. In addition, there was an obvious dose-dependent
cleaved-PARP elevation in both IR-resistant and shCRMP4 cells
treated with BAPTA-AM (Fig. 6C).
Parental and shControl cells showed a weak cleavage of PARP.
Therefore, these results suggest that CRMP4 may be important for
cell survival during cellular stress response due to
BAPTA-AM-induced Ca2+ deficiency.
Discussion
Previously, γ-irradiation-resistant colon cancer
cell lines were established, and novel candidate molecules
implicated in radioresistance were identified using RNA sequencing
analysis (21). Among the genes
significantly downregulated in radioresistant cell lines than in
their parental cells, collapsin response mediator protein 4 (CRMP4)
was further examined in the present study concerning the
association between tumor growth and radioresistance. It is known
that CRMPs influence various intracellular signal transduction
pathways, including VEGF, RhoA, GSK3β, and Sema3A (29,30);
moreover, CRMP4 is involved in neurodevelopmental disorders such as
schizophrenia, neurological disorders such as Alzheimer's disease
(31), and various types of cancers
such as breast, prostate, gastric, and hepatocellular carcinomas
(11,32–35).
Until now, the relationship between CRMP4 and
Ca2+ influx stress or Ca2+ homeostasis has
not been defined. We assayed 5-Gy radiation-mediated intracellular
Ca2+ concentration in shControl- and shCRMP4-SW620 and
-RKO cells (Fig. 4A). Ca2+
levels were increased highly in all radiation-treated cells, but no
statistical significance between the Ca2+ levels of
shControl and shCRMP4 cells was identified in both
radiation-treated and -untreated cells. Similarly, the siRNA
experiment did not show statistical significance between the
Ca2+ levels of siControl and siCRMP4 cells (data not
shown). As revealed by the data, under the radiation-untreated
condition, survival, apoptosis, and cell cycle distribution were
not significantly different between siControl and siCRMP4 cells.
Therefore, it is not likely that CRMP4 regulates intracellular
Ca2+ influx.
Ca2+ is a ubiquitous diffusible
intracellular second messenger released inside cells upon ligand
interaction with membrane receptors; it is especially associated
with diverse cellular functions related to cell growth; however, it
can induce apoptosis. A primary cause of Ca2+-induced
mitochondrial damage is the nonspecific pore opening of the
mitochondrial membrane leading to the activation of mitochondrial
permeability transition (MPT), causing loss of mitochondrial
membrane potential (MMP), rupture of the outer mitochondrial
membrane, and leakage of intermembrane proteins, such as cytochrome
c, to the cytoplasm (36–38).
Therefore, the disruption of Ca2+ homeostasis in cells
affects various signaling pathways, including those associated with
proliferation and apoptosis (15,28). It
was recently reported that patients with Hodgkin's lymphoma who
received a total dose of more than 30 Gy of radiation displayed
significantly higher Ca2+ scores than other patients,
putting them at a higher risk of coronary artery disease (39). Nonetheless, our understanding of the
correlation between radiation exposure and Ca2+
homeostasis remains insufficient. Our data showed a considerable
relationship between radiation-resistance and CRMP4 downregulation.
When CRMP4-deficient cells such radiation-resistant and
CRMP4-shRNA-infected cells were treated with radiation or
Ca2+ ionophore A23187, intracellular Ca2+
influx caused high Ca2+ concentrations regardless of
CRMP4, but MMP depolarization followed by apoptosis was
significantly inhibited according to CRMP4 deficiency (Figs. 3 and 4).
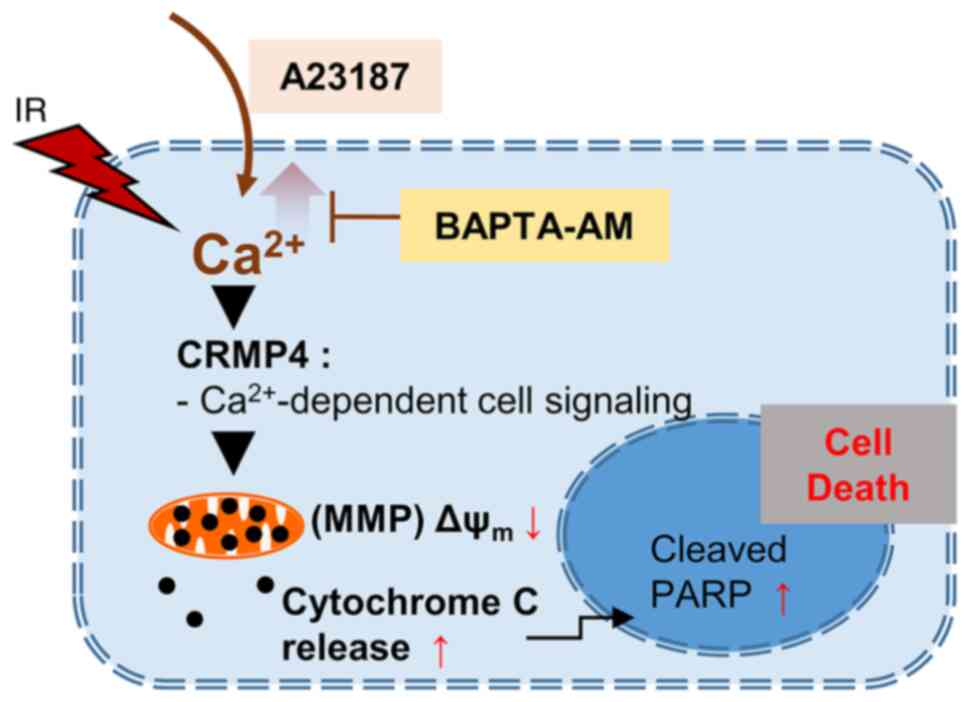
Therefore, it can be inferred that CRMP4 plays an important role in
the Ca2+-mediated cell death pathway in colon cancer
cells (Fig. 7).
In addition, the cellular effects of BAPTA-AM, a
cell-permeant intracellular Ca2+ chelator that acts as
an intracellular Ca2+ buffer, were investigated.
Interestingly, CRMP4 was obviously downregulated in
BAPTA-AM-treated cells (Fig. 5),
suggesting that CRMP4 expression may be regulated depending on the
Ca2+-related process. Although it was not determined why
CRMP4 was downregulated or whether CRMP4 protein was cleaved (or
degraded) in a BAPTA-AM-treated or radiation-treated condition, it
is clear that CRMP4 downregulation was strongly involved in the
inhibition of Ca2+-mediated MMP depolarization. In
addition, low BAPTA-AM (<5 µM) treatment diminished A23187- or
radiation-mediated apoptosis. However, under high BAPTA-AM (>10
µM) conditions (Fig. 6),
CRMP4-deficiency from IR-resistant or shCRMP4 cell lines caused
increased cell death, suggesting that proper intracellular
Ca2+ levels modulated by BAPTA-AM may be critical for
cell survival and cell death. Therefore, there may be a close
relationship between CRMP4 and Ca2+-mediated apoptosis
pathway under radiation exposure (Fig.
7), although further study is needed. According to previous
studies, several intracellular protein transport systems can be
affected by the chelation of Ca2+ with BAPTA-AM
(40), potentially including vesicle
formation. It was therefore predicted herein that CRMP4 could be
involved in Ca2+ signaling since CRMP4 activity would be
essential for cell survival during intracellular Ca2+
deficiency.
Our results also indicated that CRMP4 expression
levels were altered by several factors, including radiation
exposure, CsA or BAPTA-AM treatment, implying that CRMP4 may be an
adjustable target protein. It has been reported that CRMP4
expression levels are regulated by miRNAs. miR-130a upregulation
has been reported to target the 3′UTR region of CRMP4 in gastric
cancer cells and promote tumor progression via CRMP4 inhibition
(41). Conversely, VEGF enhances
CRMP4 expression levels in gastric cancer cells, which is inhibited
by the MAPK inhibitor, PD98059, and the PI3K inhibitor, LY294002.
In mice, CRMP4 overexpression was found to facilitate tumor growth
and metastasis (42). Taken together,
these results imply that CRMP4 can be controlled and that the
modulation of CRMP4 expression at the cellular level may affect
cancer-cell radiation sensitivity.
Although further biochemical studies are required
to characterize the physiological properties of CRMP4 as an
important player in radiation-resistant colon cancer cells, our
findings suggest that radioresistance-associated CRMP4 might be
related to the Ca2+-mediated apoptotic pathway and that
CRMP4 may be an attractive target for radiation-mediated or
-combined colon cancer therapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Research Foundation of Korea (NRF) grant funded by the Korean
government (MSIT) (no. 2020R1A2C2010321) and the KRIBB Research
Initiative Program.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
SYP, JTK, BYK, and HGL designed the study; SYP,
JTK, YSH, ESP, HRY, HJ, and KEB performed the experiments; SYP,
JTK, HJ, SRY, and HJC contributed essential reagents or tools; SYP,
JTK, HJC, BYK, SRY, and HGL analyzed the data; SYP, JTK, HJC, and
HGL wrote the manuscript. All authors critically revised the
manuscript and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BAPTA-AM
|
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
tetrakis(acetoxymethyl ester)
|
|
CRMP4
|
collapsin response mediator protein
4
|
|
DPYSL3
|
dihydropyrimidinase-like protein
3
|
|
DHPase
|
dihydropyrimidinase
|
|
MMP
|
mitochondrial membrane potential
|
|
MPT
|
mitochondrial permeability
transition
|
|
siRNA
|
small-interfering RNA
|
|
shRNA
|
short hairpin RNA
|
|
WST
|
water-soluble tetrazolium
|
|
PARP
|
polyADP-ribose polymerase
|
|
RIPA
|
radioimmunoprecipitation assay
|
|
DiOC6(3)
|
3,3′-dihexyloxacarbocyanine
iodide
|
|
CsA
|
cyclosporin A
|
References
|
1
|
Begg AC, Stewart FA and Vens C: Strategies
to improve radiotherapy with targeted drugs. Nat Rev Cancer.
11:239–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Habibullah G, Gul R, Cassum S and Elahi R:
Experiences of the breast cancer patients undergoing radiotherapy
at a Public Hospital Peshawar Pakistan. Asia Pac J Oncol Nurs.
5:184–194. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baskar R, Dai J, Wenlong N, Yeo R and Yeoh
KW: Biological response of cancer cells to radiation treatment.
Front Mol Biosci. 1:242014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ponnusamy R, Lebedev AA, Pahlow S and
Lohkamp B: Crystal structure of human CRMP-4: Correction of
intensities for lattice-translocation disorder. Acta Crystallogr D
Biol Crystallogr. 70:1680–1694. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ponnusamy R and Lohkamp B: Insights into
the oligomerization of CRMPs: Crystal structure of human collapsin
response mediator protein 5. J Neurochem. 125:855–868. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deo RC, Schmidt EF, Elhabazi A, Togashi H,
Burley SK and Strittmatter SM: Structural bases for CRMP function
in plexin-dependent semaphorin3A signaling. EMBO J. 23:9–22. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Minturn JE, Fryer HJ, Geschwind DH and
Hockfield S: TOAD-64, a gene expressed early in neuronal
differentiation in the rat, is related to unc-33, a C.
elegans gene involved in axon outgrowth. J Neurosci.
15:6757–6766. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagai J, Kitamura Y, Owada K, Yamashita N,
Takei K, Goshima Y and Ohshima T: Crmp4 deletion promotes recovery
from spinal cord injury by neuroprotection and limited scar
formation. Sci Rep. 5:82692015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hiroshima Y, Nakamura F, Miyamoto H, Mori
R, Taniguchi K, Matsuyama R, Akiyama H, Tanaka K, Ichikawa Y, Kato
S, et al: Collapsin response mediator protein 4 expression is
associated with liver metastasis and poor survival in pancreatic
cancer. Ann Surg Oncol. 20 (Suppl 3):S369–S378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan M, Ma S, Huang Q, Hu K, Song B and Li
M: GSK-3α/β-mediated phosphorylation of CRMP-2 regulates
activity-dependent dendritic growth. J Neurochem. 125:685–697.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsunuma R, Chan DW, Kim BJ, Singh P, Han
A, Saltzman AB, Cheng C, Lei JT, Wang J, Roberto da Silva L, et al:
DPYSL3 modulates mitosis, migration, and epithelial-to-mesenchymal
transition in claudin-low breast cancer. Proc Natl Acad Sci USA.
115:E11978–11987. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou W, Xie P, Pang M, Yang B, Fang Y, Shu
T, Liu C, Wang X, Zhang L, Li S and Rong L: Upregulation of CRMP4,
a new prostate cancer metastasis suppressor gene, inhibits tumor
growth in a nude mouse intratibial injection model. Int J Oncol.
46:290–298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gerencser AA, Chinopoulos C, Birket MJ,
Jastroch M, Vitelli C, Nicholls DG and Brand MD: Quantitative
measurement of mitochondrial membrane potential in cultured cells:
Calcium-induced de- and hyperpolarization of neuronal mitochondria.
J Physiol. 590:2845–2871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Molkentin JD: Calcineurin, mitochondrial
membrane potential, and cardiomyocyte apoptosis. Circ Res.
88:1220–1222. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Orrenius S, Zhivotovsky B and Nicotera P:
Regulation of cell death: The calcium-apoptosis link. Nat Rev Mol
Cell Biol. 4:552–565. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heise N, Palme D, Misovic M, Koka S,
Rudner J, Lang F, Salih HR, Huber SM and Henke G: Non-selective
cation channel-mediated Ca2+-entry and activation of
Ca2+/calmodulin-dependent kinase II contribute to G2/M
cell cycle arrest and survival of irradiated leukemia cells. Cell
Physiol Biochem. 26:597–608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meyn RE, Stephens LC, Voehringer DW, Story
MD, Mirkovic N and Milas L: Biochemical modulation of
radiation-induced apoptosis in murine lymphoma cells. Radiat Res.
136:327–334. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Porporato PE, Filigheddu N, Pedro JMB,
Kroemer G and Galluzzi L: Mitochondrial metabolism and cancer. Cell
Res. 28:265–280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Z, Majava V, Greffier A, Hayes RL,
Kursula P and Wang KK: Collapsin response mediator protein-2 is a
calmodulin-binding protein. Cell Mol Life Sci. 66:526–536. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Brittain JM, Wilson SM and Khanna
R: Emerging roles of collapsin response mediator proteins (CRMPs)
as regulators of voltage-gated calcium channels and synaptic
transmission. Commun Integr Biol. 3:172–175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park SY, Lee SJ, Cho HJ, Kim JT, Yoon HR,
Lee KH, Kim BY, Lee Y and Lee HG: Epsilon-globin HBE1 enhances
radiotherapy resistance by down-regulating BCL11A in colorectal
cancer cells. Cancers (Basel). 11:4982019. View Article : Google Scholar
|
|
22
|
Yokoi K, Yamashita K, Ishii S, Tanaka T,
Nishizawa N, Tsutsui A, Miura H, Katoh H, Yamanashi T, Naito M, et
al: Comprehensive molecular exploration identified promoter DNA
methylation of the CRBP1 gene as a determinant of radiation
sensitivity in rectal cancer. Br J Cancer. 116:1046–1056. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martin M: Cutadapt removes adapter
sequences from high-throughput sequencing reads. EMBnet J.
17:10–12. 2011. View Article : Google Scholar
|
|
24
|
Dobin A, Davis CA, Schlessinger F, Drenkow
J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR: STAR:
Ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anders S, Pyl PT and Huber W: HTSeq-a
Python framework to work with high-throughput sequencing data.
Bioinformatics. 31:166–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A Bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smaili SS, Hsu YT, Carvalho AC, Rosenstock
TR, Sharpe JC and Youle RJ: Mitochondria, calcium and pro-apoptotic
proteins as mediators in cell death signaling. Braz J Med Biol Res.
36:183–190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Romero-Garcia S and Prado-Garcia H:
Mitochondrial calcium: Transport and modulation of cellular
processes in homeostasis and cancer (Review). Int J Oncol.
54:1155–1167. 2019.PubMed/NCBI
|
|
29
|
Schmidt EF and Strittmatter SM: The CRMP
family of proteins and their role in Sema3A signaling. Adv Exp Med
Biol. 600:1–11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alabed YZ, Pool M, Ong Tone S, Sutherland
C and Fournier AE: GSK3 beta regulates myelin-dependent axon
outgrowth inhibition through CRMP4. J Neurosci. 30:3635–3643. 2010.
View Article : Google Scholar
|
|
31
|
Ohtani-Kaneko R: Crmp4-KO mice as an
animal model for investigating certain phenotypes of autism
spectrum disorders. Int J Mol Sci. 20:24852019. View Article : Google Scholar
|
|
32
|
Huang QX, Xiao CT, Chen Z, Lu MH, Pang J,
Di JM, Luo ZH and Gao X: Combined analysis of CRMP4 methylation
levels and CAPRA-S score predicts metastasis and outcomes in
prostate cancer patients. Asian J Androl. 20:56–61. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo H and Xia B: Collapsin response
mediator protein 4 isoforms (CRMP4a and CRMP4b) have opposite
effects on cell proliferation, migration, and invasion in gastric
cancer. BMC Cancer. 16:5652016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sato S, Nakamura F, Hiroshima Y, Nagashima
Y, Kato I, Yamashita N, Goshima Y and Endo I: Caerulein-induced
pancreatitis augments the expression and phosphorylation of
collapsin response mediator protein 4. J Hepatobiliary Pancreat
Sci. 23:422–431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oya H, Kanda M, Sugimoto H, Shimizu D,
Takami H, Hibino S, Hashimoto R, Okamura Y, Yamada S, Fujii T, et
al: Dihydropyrimidinase-like 3 is a putative hepatocellular
carcinoma tumor suppressor. J Gastroenterol. 50:590–600. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Crompton M: The mitochondrial permeability
transition pore and its role in cell death. Biochem J. 341:233–249.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ravagnan L, Roumier T and Kroemer G:
Mitochondria, the killer organelles and their weapons. J Cell
Physiol. 192:131–137. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Halestrap AP, Connern CP, Griffiths EJ and
Kerr PM: Cyclosporin A binding to mitochondrial cyclophilin
inhibits the permeability transition pore and protects hearts from
ischaemia/reperfusion injury. Mol Cell Biochem. 174:167–172. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rademaker J, Schöder H, Ariaratnam NS,
Strauss HW, Yahalom J, Steingart R and Oeffinger KC: Coronary
artery disease after radiation therapy for Hodgkin's lymphoma:
Coronary CT angiography findings and calcium scores in nine
asymptomatic patients. AJR Am J Roentgenol. 191:32–37. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tagliarino C, Pink JJ, Dubyak GR, Nieminen
AL and Boothman DA: Calcium is a key signaling molecule in
beta-lapachone-mediated cell death. J Biol Chem. 276:19150–19159.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou Y, Li R, Yu H, Wang R and Shen Z:
microRNA-130a is an oncomir suppressing the expression of CRMP4 in
gastric cancer. Onco Targets Ther. 10:3893–3905. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen S, Zhang X, Peng J, Zhai E, He Y, Wu
H, Chen C, Ma J, Wang Z and Cai S: VEGF promotes gastric cancer
development by upregulating CRMP4. Oncotarget. 7:17074–17086. 2016.
View Article : Google Scholar : PubMed/NCBI
|