Introduction
Cervical cancer is the fourth most frequently
diagnosed cancer and the fourth leading cause of cancer-related
death in women with an estimated 570,000 cases and 311,000 deaths
in 2018 worldwide (1,2). At present, the molecular mechanisms and
precise targeted therapy of cervical cancer have attracted
widespread research attention and provide new perspectives.
Transforming growth factor (TGF)-β1 is a multifunctional regulatory
peptide belonging to a newly discovered TGF-β superfamily that
regulates cell growth, differentiation, proliferation and movement,
widely involved in cell signal transduction in human cancers
(3,4).
Exosomes are small disc-shaped membrane vesicles
(30–150 nm) that contain complex RNA and proteins (5,6). Previous
studies regard exosomes as specific secretory membrane vesicles
involved in cell-to-cell communication (7,8). As the
natural intercellular information carrier, exosomes are currently
considered to have great potential in the field of drug carriers
due to their small molecular structure, natural molecular transport
characteristics and good biocompatibility (9,10). In
addition, exosomes are increasingly regarded as exclusive media in
the tumor microenvironment (TME) and molecular entities involved in
its construction (11).
Cancer-derived exosomes contain a variety of carcinogenic
information that regulates the TME to promote the occurrence and
progression of tumors.
Previous studies have confirmed that microRNAs
(miRNAs/miRs) and other molecules are loaded to exosomes and
secreted into the extracellular environment by many different cell
types. MicroRNAs act as endogenous non-coding RNAs of approximately
20–24 nucleotides and key post-transcriptional regulators that
affect gene expression. Through partial sequence complementation,
they can bind to the 3′-untranslated regions (3′-UTR) of target
mRNAs to cause mRNA degradation or translation inhibition (12). It was reported that miRNAs are widely
involved in the regulation of cell differentiation, biological
development and disease occurrence, as oncogenes or
tumor-suppressor genes to affect the proliferation, migration and
invasion of tumor cells (13–16). Epithelial-mesenchymal transition (EMT)
plays a key role in cervical cancer and its extensive role in tumor
progression has attracted more and more research attention
(17). EMT refers to the biological
process in which epithelial cells are transformed into mesenchymal
phenotype cells, through which malignant tumor cells of epithelial
origin gain the ability to migrate and invade (18–20).
Our present study demonstrated that TGF-β1 promotes
the upregulation of exosomal miR-663b in cervical cancer cell lines
by using deep RNA-seq. Further studies have shown that exosomes
enriched in miR-663b could be endocytosed by recipient cells,
causing the decreased expression of mannoside
acetylglucosaminyltransferase 3 (MGAT3) and activation of the EMT
signaling pathway, thereby promoting the metastasis of cervical
cancer cells. Overall, our data provide new insight into the role
of the TGF-β1/miR-663b/MGAT3 axis in cervical cancer.
Materials and methods
Cell lines and maintenance
293T cells and human cervical cell lines HeLa and
CaSki were obtained from the Cancer Center Laboratory of Shandong
University. 293T and HeLa cells were cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) and CaSki cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS,; Biological
Industries). All cell lines were cultured in a humidified incubator
under standard culture conditions (5% CO2, 37°C).
Exosome isolation
Cells were seeded in 10-cm diameter plates and
cultured in medium supplemented with 10% exosome-depleted FBS
(System Biosciences). Culture medium was collected and exosomes
were obtained using serial centrifugation. The medium was
centrifuged at 300 × g for 10 min and 2,000 × g for 10 min to
remove cells or other debris and 10,000 × g for 20 min to remove
other larger vesicles. Then the medium was centrifuged at 110,000 ×
g for 70 min, washed with 5 ml phosphate-buffered solution (PBS)
and centrifuged at 110,000 × g for another 70 min (all steps were
performed at 4°C). Finally, the exosomes were obtained from the
pellet and resuspended in PBS.
RNA sequencing (RNA-seq)
HeLa cells were seeded in a 10-cm diameter dish and
cultured overnight, and exosome-free serum medium with or without
10 ng/ml TGF-β1 was used to continue the culture for another 24 h.
The concentration of 10 ng/ml was much higher than the level of
TGF-β1 secreted by tumor cells, thus, the impact produced by TGF-β1
secreted by tumor cells was negligible (21,22). The
supernatant was collected to isolate exosomes and extract a total
of 1 µg RNA as input material for RNA sample preparation. Total RNA
was purified by electrophoretic separation on a 15% urea denaturing
polyacrylamide gel electrophoresis (PAGE) gel and small RNA regions
corresponding to the 18–30 nt bands in the marker lane (14–30 ssRNA
ladder marker; Takara, Japan) were excised and recovered. Then the
18–30 nt small RNAs were ligated to a 5′-adaptor and a 3′-adaptor.
The adapter-ligated small RNAs were subsequently transcribed into
cDNA by SuperScript II Reverse Transcriptase (Invitrogen; Thermo
Fisher Scientific, Inc.) and then several rounds of PCR
amplification with PCR Primer Cocktail and PCR Mix were performed
to enrich the cDNA fragments. The PCR products were selected by
agarose gel electrophoresis with target fragments 100–120 bp, and
then purified using the QIA Quick Gel Extraction Kit (Qiagen). The
library was evaluated for quality and quantitated using two
methods: Checking the distribution of the fragment size using the
Agilent 2100 bioanalyzer (Agilent Technologies, Inc.), and quantify
the library using real-time quantitative PCR (qPCR) (TaqMan Probe).
The final ligation PCR products were sequenced using the BGISEQ-500
platform. The RNA-sequence procedure and data analysis were
completed by BGI (The Beijing Genomics Institute, BGI, ShenZhen,
China).
Nanoparticle tracking analysis
Isolated exosomes diluted in PBS were gently mixed,
and the mixture was inserted into the ZetaView PMX120 instrument
(Particle Metrix GmbH) at 23°C through a 1-ml syringe. ZetaView
8.02.28 software (Particle Metrix GmbH) was utilized to analyze the
number and the size distribution of the exosomes. Each sample was
measured three times and the average value was calculated.
Transmission electron microscopy
(TEM)
Isolated exosomes fixed with 2% paraformaldehyde for
5 min were dropped onto the Formvar copper carbonate grid with glow
discharge for 1 min, and then it was negatively stained with 2%
uranyl acetate for another 1 min. After sample drying, a HT7800
electron microscope (Hitachi) was used to photograph the grid with
an acceleration voltage of 80 kV.
PKH67 staining for exosomes
PKH67 Green Fluorescent Cell Linker Kits
(Sigma-Aldrich; Merck KGaA) were purchased to label the lipid
bilayers of exosomes. PKH67 (4 µl) was added and exosomes were
isolated into 1 ml diluent C separately and incubation was carried
out for 5 min at room temperature; exosomes without PKH67 staining
were used for negative control. Bovine serum albumin (1 ml/5%)
(Solarbio) was added to halt the staining. Then the PKH67-labeled
exosome mixture was centrifuged at 110,000 × g for 2 h at 4°C. The
mixture was washed with PBS and centrifuged at 110,000 × g for
another 2 h to obtain pure PKH67-labeled exosomes. After
resuspending the mixture in complete medium and incubating with
HeLa cells at 37°C for 0, 4, 8 and 12 h, a laser confocal
microscope (LSM880; Zeiss) was used to visualize the incorporation
of exosomes into HeLa cells.
Western blot analysis
Protein extracted from the cells and exosomes was
lysed with RIPA buffer and quantified by BCA Protein Assay Kit
(Beyotime Institute of Biotechnology). Total proteins were
separated on a 10% polyacrylamide gel and transferred to a
polyvinylidene fluoride membrane and blocked with 5% defatted milk
for 1.5 h at room temperature. The membranes were probed with the
following primary antibodies at 4°C: Anti-CD63 (dilution 1:1,000,
Abcam, ab59479), anti-TSG101 (dilution 1:1,000; Abcam, ab125011),
anti-HSP70 (dilution 1:2,000; ProteinTech Group, Inc., 66183-1-Ig),
anti-GAPDH [dilution 1:1,000; Cell Signaling Technology, Inc.
(CST), #5174], anti-MGAT3 (dilution 1:1,000; ABclone, A8134),
anti-E-cadherin (dilution 1:1,000; CST, #3195), anti-N-cadherin
(dilution 1:1,000; CST, #13116), and anti-β-catenin (dilution
1:1,000; CST, #8480). Subsequently, the membranes were washed with
triethanolamine buffered saline solution (TBS) and interacted with
HRP-conjugated antibody (dilution 1:1,000; CST) for 2 h at room
temperature. The membranes were detected by ECL substrate kit
(Thermo Fisher Scientific Inc.) and quantified by ImageJ software
(v1.8.0; National Institutes of Health, USA).
RNA isolation and quantitative
real-time transcription-polymerase chain reaction (qPCR)
Total RNA extracted from cells or exosomes was
isolated with TRIzol reagent (Thermo Fisher Scientific Inc.)
according to the manufacturer's instructions and the concentration
was detected by a spectrophotometer (Thermo Fisher Scientific
Inc.). Then the RNA was transcribed to cDNA by using Prime Script
RT Master Mix for RT-PCR (Takara). qPCR was conducted using
SYBR-Green (Takara) and performed on a StepOne™ PCR amplifier
(Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) or U6 were used as internal controls. The primer sequences
for miRNA and mRNA were purchased from GenePharma (Shanghai, China)
and are as follows: miR-663b, forward (F), 5′-GGTGGCCCGGCCGTGC-3′
and reverse (R), 5′-TATCCTTGTTGACGACTCCTTGAC-3′; U6, F,
5′-CAGCACATATACTAAAATTGGAACG-3′ and R, 5′-ACGAATTTGCGTGTCATCC-3′;
MG AT3, F, 5′-TCAACCACGAGTTCGACCTG-3′ and R,
5′-CACCTTGTGGCGGATGTACT-3′; GAPDH, F, 5′-GCACCGTCAAGGCTGAGAAC-3′
and R, 5′-TGGTGAAGACGCCAGTGGA-3′.
Wound healing assay
Cells were seeded in 6-well plates until the growth
density reached 90%. Then a sterile 100-µl pipette tip was used to
quickly stroke the cells to form a straight wound. After washing
twice with PBS, 2 ml medium without serum was added to each well.
The wound healing was observed and photographed by JEM-1200 EX II
electron microscope (JEOL) at 0 and 24 h, and the wound closure was
evaluated by ImageJ software (v.8.0; National Institutes of
Health).
Transwell assays
Cells (HeLa: 6×104 cells/200 µl for
invasion and 4×104 cells/200 µl for migration, CaSki:
8×104 cells/200 µl for invasion and 6×104
cells/200 µl for migration) in 200 µl serum-free medium were seeded
in upper Transwell chambers (8-µm pore size; Corning Costar) with
or without Matrigel (60 µl, 1:9 dilution in serum-free medium, BD
Biosciences). In addition, 600 µl media containing 10% FBS were
added to the lower compartment. After 24 h of incubation in an
incubator, the chambers were washed twice by PBS, and methanol was
used to fix cells for 15 min. The cells that did not pass through
the upper layer were wiped off with a cotton swab and then stained
with crystal violet (product no. AC0121; Beyotime) for 30 min. A
total of 3–5 random fields of view were selected to photograph
under the JEM-1200 EX II electron microscope (JEOL).
miRNA target prediction and dual
luciferase reporter assay
PicTar (https://pictar.mdc-berlin.de/), TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org/microrna/home.do) were used to
predict miR-663b targets. The expression of the miR-663b-target
gene was verified in 293T cells by dual luciferase reporter gene
assay. First, we insert the 3′-UTR of wild-type (WT) and mutant
(MUT) MGAT3 into the psiCHECKTM-2 plasmid (Promega Corp.). Then,
psiCHECKTM-2-MGAT3-3′-UTR-WT or psiCHECKTM-2-MGAT3-3′-UTR-MUT were
co-transfected into 293T cells with miR-663b mimics or its NC.
After 48 h of transfection in a 96-well plate, a Dual Fluorescence
Luciferase reporter gene detection system (Promega Corp.) was used
to detect the fluorescence.
Cell transfection
Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific Inc.) and Opti-MEM (Gibco; Thermo Fisher Scientific
Inc.) were used for cell transfection. miR-663b mimics and
inhibitor were used for upregulation and downregulation of
miR-663b. miR-663b mimics NC and inhibitor NC were non-targeting
and used as the respective controls; the control (CON) group was
without any special treatment. The overexpression of MGAT3 was
produced by subcloning PCR-amplified full-length human MGAT3 cDNA
into the pCDNA3.1 plasmid (GenePharma). The empty vector was used
as a blank control (pCDNA3.1 NC). After transfection in Opti-MEM
for 6 h, completed culture medium was replaced and the cells were
cultured for the following experiments. Total RNA and total protein
were isolated from the cells 24 or 48 h after transfection for qPCR
analysis and western blot analysis. In order to obtain stably
silenced cell lines for subsequent experiments, we used the
lentiviral vector miR663b-sponge-pLVX-AcGFP-N1-Puro to transfect
HeLa and CaSki cells and cultured the cells with puromycin
dihydrochloride (2 µg/ml; Amresco) for 7–10 days. The intensity and
ratio of green fluorescence produced by cells under the
fluorescence microscope were used to prove the efficiency of
lentiviral transfection.
Statistical analysis
Statistical analysis was conducted using GraphPad
Prism 8 software (GraphPad Software, Inc.). The data are expressed
as the median values ± standard deviation (SD). Comparisons between
the groups were analyzed by Student's t-test (unpaired, two-tailed)
or one-way analysis of variance (ANOVA) followed by Tukey's post
hoc test. The results are presented as the average of three
experiments. Statistical significance was set at P<0.05.
Results
Elevated exosomal miRNAs upon TGF-β1
exposure of cervical cancer cell lines are identified by deep
RNA-sequencing (RNA-seq)
First, deep RNA-seq was performed to explore the
expression level of miRNAs in HeLa cell exosomes after treatment
with or without 10 ng/ml TGF-β1 for 24 h. The typical goblet
morphology and the size range of 30–150 nm were detected by TEM
(Fig. 1A). Next, positive signs of
exosomes including CD63, tumor susceptibility gene 101 (TSG101),
heat shock protein 70 (HSP70) and GAPDH were confirmed by western
blot analysis (Fig. 1B). NTA proved
that the concentration and size of the isolated exosomes were
consistent with previous reports (Fig.
1C) (23). The green fluorescent
signal in the HeLa cytoplasm indicated that the purified exosomes
labeled with the fluorescent membrane tracer PKH67 (green) entered
the HeLa cells after incubation (Fig.
1D). Deep RNA-seq results showed that 48 miRNAs were enriched
in exosomes of HeLa cells after treatment with TGF-β1 compared with
the non-treated cells (negative control) (Fig. 1E), proving that TGF-β1 affects the
HeLa cell exosome miRNA profile. We selected top 10 miRNAs for PCR
verification in HeLa and CaSki cells. After treatment of TGF-β1 for
24 h, miR-663b was selectively enriched 4 and 3 times in the
exosomes of HeLa and CaSki cells compared with the negative control
group (Fig. 1F and G). These results
verified that miR-663b could be selectively enriched in the
exosomes of cervical cancer cells treated with TGF-β1, thus we
selected miR-663b for the subsequent study.
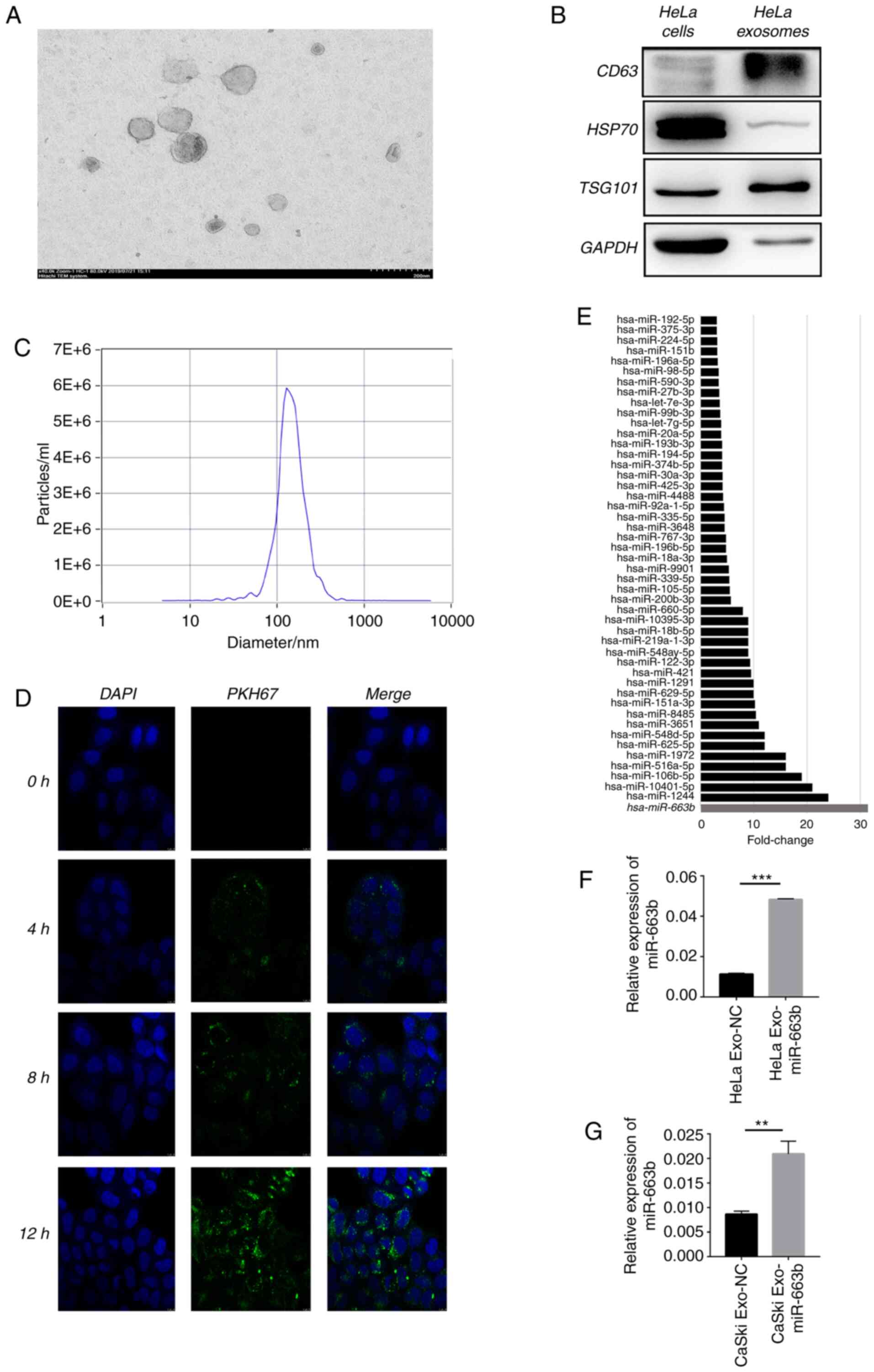 | Figure 1.Elevated exosomal miRNAs upon TGF-β1
exposure of cervical cancer cell lines are identified by deep
RNA-sequencing (RNA-seq). (A) TEM images of exosomes isolated from
HeLa cells. (B) HeLa cell-secreted exosome-positive markers CD63,
TSG101, HSP70 and GAPDH were detected by western blot analysis. (C)
Nanoparticle size analysis of HeLa cell-secreted exosomes. (D) HeLa
cells pretreated with PKH67-labeled exosomes for 0, 4, 8 and 12 h
were stained by DAPI (blue) for confocal microscopy analysis
(magnification, ×400). (E) miR-663b expression level in
HeLa-secreted exosomes with or without TGF-β1 treatment were
identified by deep RNA-seq. (F and G) miR-663b expression level in
HeLa and CaSki-secreted exosomes with or without TGF-β1 treatment
were analyzed by qPCR. **P<0.01, ***P<0.001. Exo-miR-663b,
exosomes from the TGF-β1 treatment group; Exo-NC, exosomes from the
negative control group. TGF-β1, transforming growth factor-β1; TEM,
transmission electron microscopy; TSG101, tumor susceptibility gene
101; HSP70, heat shock protein 70. |
Exosomal miR-663b promotes the
metastasis of cervical cancer cells
Previous research has confirmed that miR-663b plays
tumor-promoting roles in endometrial cancer, nasopharyngeal
carcinoma and osteosarcoma (24–26). We
collected exosomes from the TGF-β1 treatment group (Exo-miR-663b)
and the negative control group (Exo-NC) in HeLa and CaSki cells to
verify whether exosomal miR-663b affects migration, invasion or
proliferation of cervical cancer cells. In our study, HeLa and
CaSki cells were treated with exosomes from their own cells. We
found exosomes from the TGF-β1 treatment group significantly
enhanced HeLa and CaSki cell motility by wound healing assay
(Fig. 2A and B). In addition,
exosomes upon TGF-β1 exposure enhanced HeLa and CaSki cell
migration and invasion as measured by Transwell assays (Fig. 2C and D).
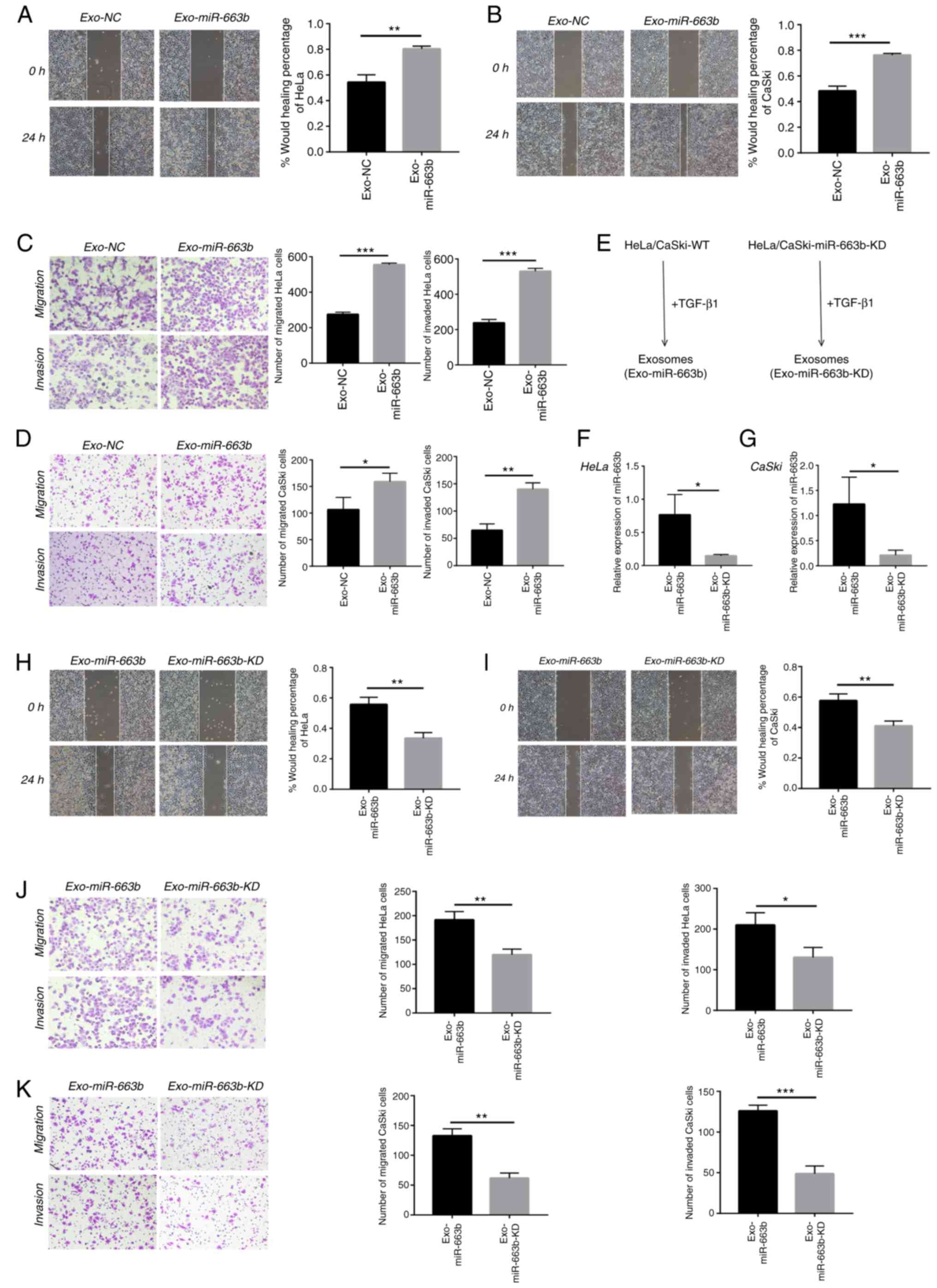 | Figure 2.Exosomal miR-663b promotes the
metastasis of cervical cancer cells. (A) HeLa cell migration with
normal or TGF-β1-treated exosomes was assessed by wound healing
assay. (B) CaSki cell migration with normal or TGF-β1 treated
exosomes was assessed by wound healing assay. (C) Migration and
invasion abilities of HeLa cells with normal or TGF-β1-treated
exosomes were detected by Transwell assays. (D) Migration and
invasion abilities of CaSki cells with normal or TGF-β1-treated
exosomes were detected by Transwell assays. (E) Schematic diagram
of the exosomes-tumor cell experiments. (F and G) miR-663b
expression levels in HeLa and CaSki cell-secreted exosomes from the
Exo-miR-663b and Exo-miR-663b-KD group were analyzed by qPCR. (H)
Wound healing assays were used to detect HeLa cell migration after
treatment with exosome from miR-663b-KD or WT cells upon TGF-β1
exposure. (I) Wound healing assays were used to detect CaSki cell
migration after treament with exosome that from miR-663b-KD or WT
cells upon TGF-β1 exposure. (J) Transwell assay was performed to
assess the effect of miR-663b-KD or WT exosomes following exposure
of TGF-β1 on migration and invasion of HeLa cells. (K) Transwell
assay was performed to measure the effect of miR-663b-KD or WT
exosomes following exposure of TGF-β1 on migration and invasion of
CaSki cells. Magnification, ×100, *P<0.05, **P<0.01,
***P<0.001. Exo-miR-663b, exosomes from the TGF-β1 treatment
group; Exo-NC, exosomes from the negative control group;
Exo-miR-663b-KD, exosomes from the miR-663b-knockdown group.
TGF-β1, transforming growth factor-β1; MGAT3, mannoside
acetylglucosaminyltransferase 3; WT, wild-type. |
To further explore that exosomal miR-663b is indeed
involved in cervical cancer cell metastasis, we used a lentiviral
vector to knock down miR-663b (Exo-miR-663b-KD). The intensity and
ratio of green fluorescence were used to prove the efficiency of
lentiviral transfection and the images are provided in Fig. S1. After treatment with TGF-β1, we
collected exosomes from the medium of the Exo-miR-663b-KD group and
wild-type group (Exo-miR-663b) (Fig.
2E). qPCR verified that the amount of miR-663b in exosomes from
the Exo-miR-663b group was much higher than that in the
Exo-miR-663b-KD group both in HeLa and CaSki cell lines (Fig. 2F and G). Then we observed that
compared with the Exo-miR-663b treatment group, the ability of
migration and invasion of HeLa and CaSki cells was attenuated when
the Exo-miR-663b-KD was incubated (Fig.
2H-K). Together, our results indicated that exosomes enriched
in miR-663b after TGF-β1 treatment could be transferred into new
target cells to promote the metastasis of cervical cancer.
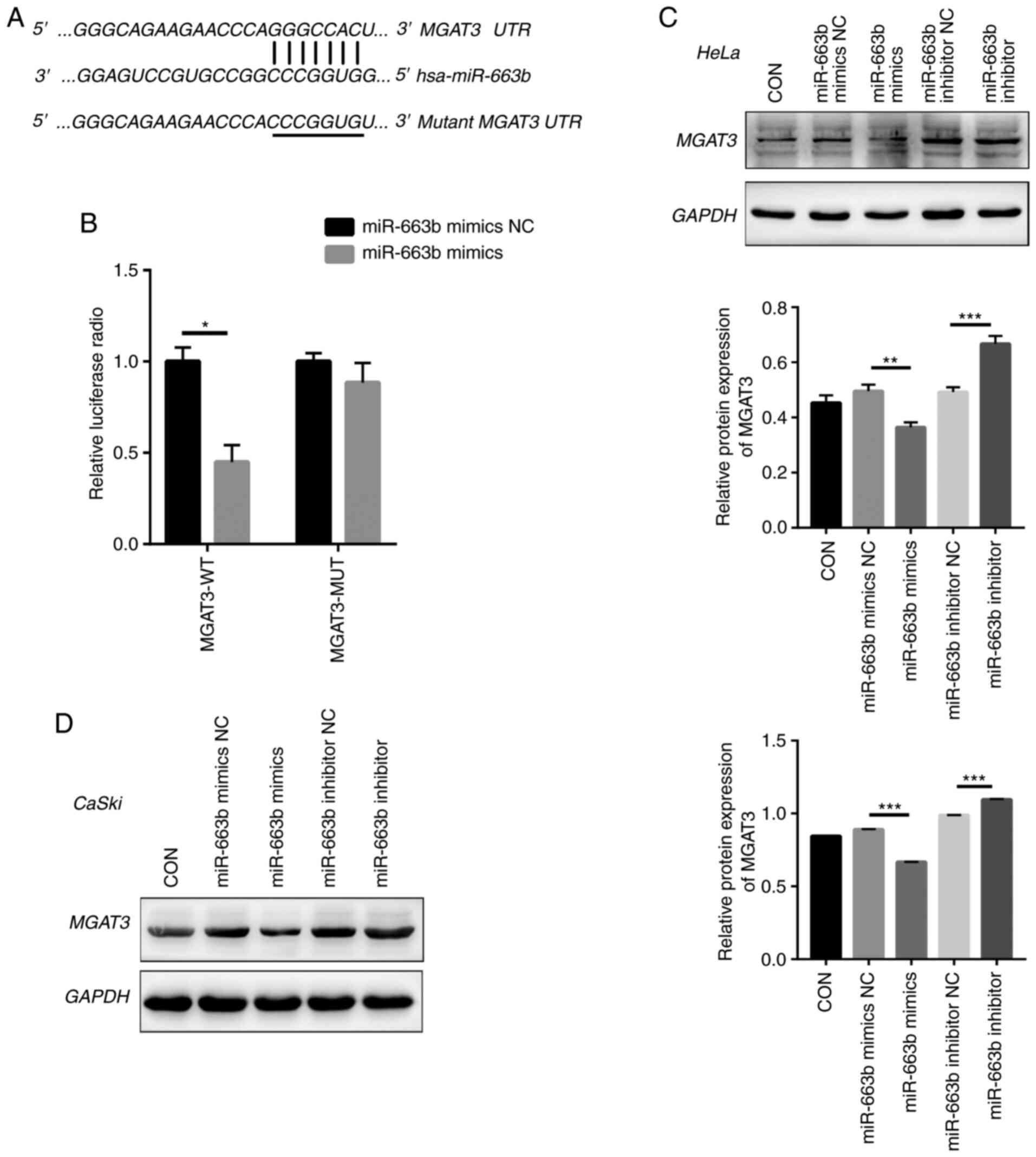
Exosomal miR-663b directly targets
MGAT3
Next, we used bioinformatic tools (TargetScan,
PicTar, and miRanda) to explore the potential miR-663b target
genes. We identified a 7-bp binding site between MGAT3 3′-UTR and
miR-663b (Fig. 3A). MGAT3, as a key
glycosyltransferase in the N-glycan biosynthetic pathway (27), is considered to be a metastasis
suppressor gene (28), which
regulates the glycosylation step and the key tumor-suppressor gene
E-cadherin to inhibit tumor invasion and metastasis (29–31). To
confirm that miR-663b could regulate the putative target of MGAT3,
the predicted miR-663b binding site (wild-type) or mutant sequence
(mutant type) in the 3′-UTR of MGAT3 was cloned into a luciferase
reporter plasmid and we detected the response to miR-663b in 293T
cells. Our results showed that the co-transfection of miR-663b
mimics significantly reduced the luciferase activity of the
wild-type 3′-UTR of MGAT3, while the luciferase activity of the
mutant 3′-UTR MGAT3 had no change (Fig.
3B).
Moreover, we directly transfected HeLa and CaSki
cells with miR-663b mimics, miR-663b inhibitor, miR-663b mimics NC
and miR-663b inhibitor NC, respectively. Consistent with the
results of dual luciferase reporter gene assay, compared with the
NC group, miR-663b mimics resulted in decreased MGAT3 protein
expression in HeLa and CaSki cells (Fig.
3C and D), while treatment with miR-663b inhibitor resulted in
increased MGAT3 protein expression. Above all, our results
demonstrated that exosomal miR-663b could inhibit MGAT3
expression.
MGAT3 is involved in cervical cancer
metastasis promoted by exosomal miR-663b
To further confirm the role of MGAT3 in cervical
cancer cells, we explored the function of exosomal miR-663b on cell
migration under the vector-mediated MGAT3 gene overexpression
conditions. Wound healing and Transwell assays were performed to
show that miR-663b could enhance the migration and invasion ability
of HeLa and CaSki cells, while co-transfection with pcDNA3.1-MGAT3
partially alleviated this effect caused by miR-663b (Fig. 4A-D). qPCR was used to verify the
overexpression efficiency of MGAT3 plasmid transfected into HeLa
and CaSki cells (Fig. 4E and F).
These results indicated that exosomal miR-663b promoted the
metastasis ability of cervical cancer cells by inhibiting the
expression of MGAT3.
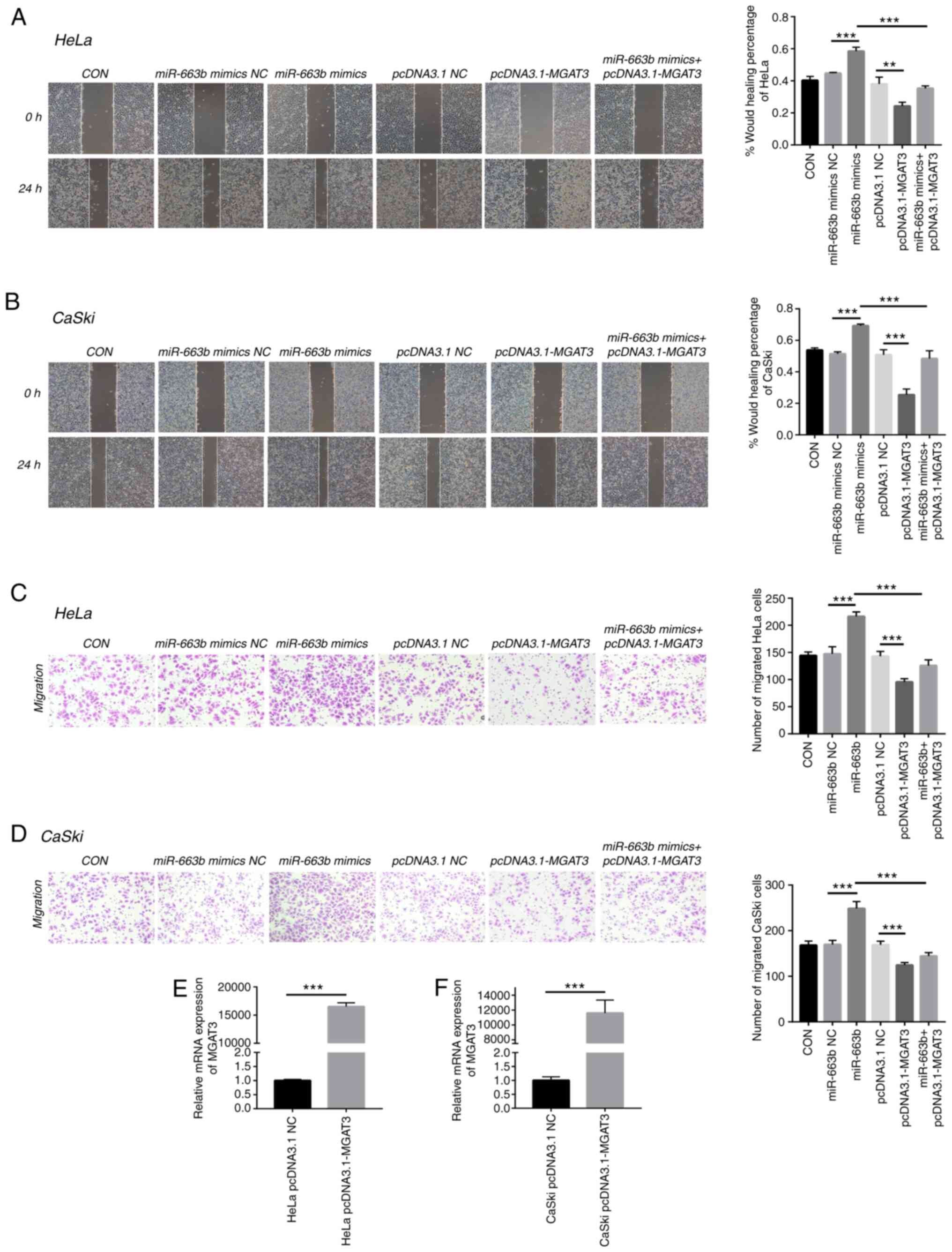 | Figure 4.MGAT3 is involved in cervical cancer
metastasis promoted by exosomal miR-663b. (A) Wound healing assays
were performed to detect the migratory ability of HeLa cells
transfected with miR-663b mimics NC, miR-663b mimics, pcDNA3.1 NC,
pcDNA3.1-MGAT3 and miR-663b mimics + pcDNA3.1-MGAT3 (magnification,
×100). (B) Wound healing assays were performed to detect the
migratory ability of CaSki cells transfected with miR-663b mimics
NC, miR-663b mimics, pcDNA3.1 NC, pcDNA3.1-MGAT3 and miR-663b
mimics + pcDNA3.1-MGAT3 (magnification, ×100). (C) Transwell assay
was performed to detect the effect of miR-663b mimics NC, miR-663b
mimics, pcDNA3.1 NC, pcDNA3.1-MGAT3 and miR-663b mimics +
pcDNA3.1-MGAT3 on the migration of HeLa cells (magnification,
×100). (D) Transwell assay was performed to detect the effect of
miR-663b mimics NC, miR-663b mimics, pcDNA3.1 NC, pcDNA3.1-MGAT3
and miR-663b mimics + pcDNA3.1-MGAT3 on the migration of CaSki
(magnification, ×100). (E) The mRNA level of MGAT3 in HeLa cells
after transfection with pCDNA3.1-MGAT3 and pCDNA3.1 NC plasmid were
analyzed by qPCR. (F) The mRNA level of MGAT3 in CaSki cells after
transfection with pCDNA3.1-MGAT3 and pCDNA3.1 NC plasmid were
analyzed by qPCR. **P<0.01 and ***P<0.001. CON, control,
MGAT3, mannoside acetylglucosaminyltransferase 3; NC, negative
control. |
MGAT3 inhibits metastasis ability of
cervical cancer by affecting the EMT pathway
As the key role in cancer recurrence and
exosome-mediated tumor progression, EMT is a biological process in
which epithelial cells transform into cells with a mesenchymal
phenotype and cause increased invasiveness and subsequent
metastasis (18,20). To understand the potential molecular
mechanism of MGAT3 in inhibiting cervical cancer invasion and
metastasis, we detected the level of epithelial differentiation
marker E-cadherin and mesenchymal marker N-cadherin and β-catenin
in cervical cancer cells. Western blot analysis verified that after
transfected with pcDNA3.1-MGAT3, the expression of E-cadherin was
significantly increased in HeLa cells, while the expression of
N-cadherin and β-catenin were significantly decreased. In contrast,
after transfection with the miR-663b mimics, the expression of
N-cadherin and β-catenin were significantly increased, while the
expression of E-cadherin was significantly decreased. Moreover, all
of these effects caused by miR-663b could be offset by the
overexpression of MGAT3 in vitro (Fig. 5A). We also observed the same results
in CaSki cells (Fig. 5B).
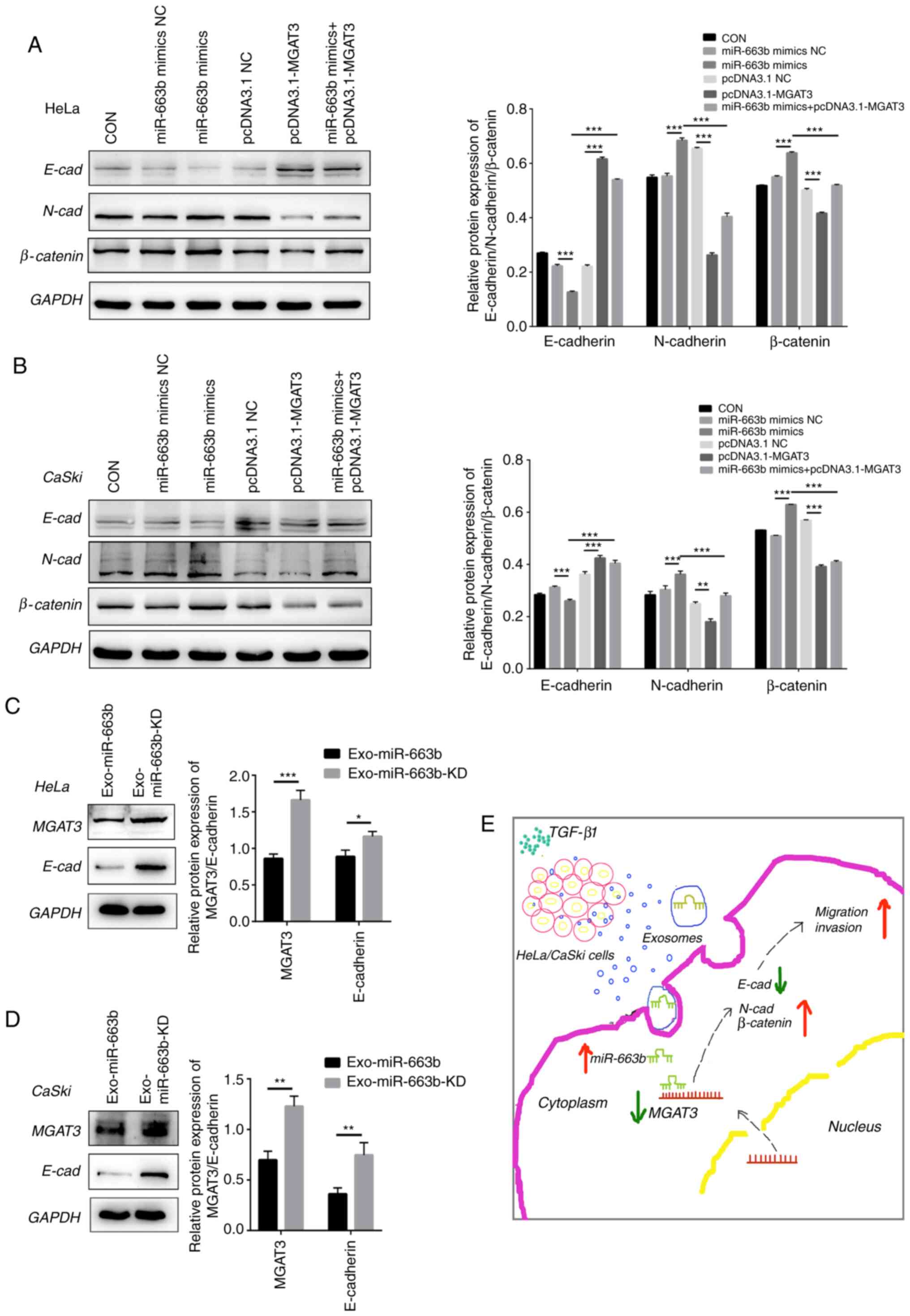 | Figure 5.MGAT3 inhibits the metastatic ability
of cervical cancer cells by affecting the EMT pathway. (A) miR-663b
mimics NC, miR-663b mimics, pcDNA3.1 NC, pcDNA3.1-MGAT3 and
miR-663b mimics + pcDNA3.1-MGAT3 were transfected into HeLa cells,
respectively. Western blot analysis was performed to analyze
E-cadherin (E-cad), N-cadherin (N-cad) and β-catenin expression.
(B) MiR-663b mimics NC, miR-663b mimics, pcDNA3.1 NC,
pcDNA3.1-MGAT3 and miR-663b mimics + pcDNA3.1-MGAT3 were
transfected into CaSki cells, respectively. Western blot analysis
was performed to analyze E-cad, N-cad and β-catenin expression. (C)
Western blot analysis was performed to analyze MGAT3, E-cad, N-cad
and β-catenin expression in HeLa cells in the Exo-miR-663b group
and Exo-miR-663b-KD group. (D) Western blot analysis was performed
to analyze MGAT3, E-cad, N-cad and β-catenin expression in CaSki
cells in the Exo-miR-663b group and Exo-miR-663b-KD group. (E)
Schematic representation of the TGF-β1/miR-663b/MGAT3 axis in
cervical cancer. *P<0.05, **P<0.01 and ***P<0.001.
Exo-miR-663b, exosomes from the TGF-β1 treatment group;
Exo-miR-663b-KD, exosomes from the miR-663b-knockdown group. EMT,
epithelial-mesenchymal transition; CON, control, MGAT3, mannoside
acetylglucosaminyltransferase 3; TGF-β1, transforming growth
factor-β1; NC, negative control. |
In order to further confirm that exosomal miR-663b
promotes metastasis by inhibiting the expression of MGAT3 and
affecting the EMT pathway, we evaluated the expression of MGAT3 and
E-cadherin after the treatment of the cell lines with Exo-miR-663b
and Exo-miR-663b-KD. Compared with the Exo-miR-663b-KD group, the
expression of MGAT3 in the Exo-miR-663b group was significantly
decreased, accompanied by the decreased expression of E-cadherin
(Fig. 5C and D). The underlying
mechanism of the TGF-β1/miR-663b/MGAT3 axis in cervical cancer is
shown in Fig. 5E.
Discussion
Accumulated research has shown that exosomes can
mediate cell-to-cell communication (32,33),
package and transport microRNAs to new cells and participate in the
regulation of gene expression. MicroRNAs in exosomes are being
suggested as novel biomarker for cervical cancer prediction and
diagnosis (34). In the present
study, RNA-seq analyzed miRNA profiles of HeLa and CaSki exosomes
and verified that miR-663b was preferentially enriched in exosomes
following treatment with TGF-β1. qPCR was performed to confirm the
expression of miRNAs in HeLa and CaSki exosomes upon TGF-β1
exposure. TGF-β1 exerts growth inhibition and displays an
anti-inflammatory function in homeostasis and early stages of
cancer (35,36), while abnormal TGF-β activation in the
advanced stage of tumors promotes aggressive growth characteristics
and metastatic spread (37). Previous
studies have reported that the TGF-β1 pathway regulates miRNA
expression, but its role in cervical cancer is controversial
(38). The concentration of 10 ng/ml
that was used in this study was much higher than the level of
TGF-β1 secreted by tumor cells and can make the effect of TGF-β1
secreted by itself negligible (21,22).
miR-663b was reported to be elevated in endometrial cancer,
nasopharyngeal carcinoma, and osteosarcoma, and its expression is
associated with cell invasion, apoptosis, and chemotherapy
resistance (24–26). miR-663b may be epigenetically
repressed by pterostilbene in human endometrial cancer cells and
repressed by long non-coding RNA HOTAIR and exerts its
tumor-suppressive function via targeting insulin-like growth factor
2 in pancreatic cancer (39,40). However, the expression and regulatory
mechanism of secreted miR-663b in cervical cancer remain unclear,
which may be the future direction of exosomal research. To explore
the role of exosomal miR-663b in the progression of cervical
cancer, exosomes collected from TGF-β1-treated and non-treated
cells were incubated with HeLa and CaSki cells, and wound healing
and Transwell assays were preformed to measure cell migration and
invasion ability. Our results revealed that miR-663b could be
enriched in exosomes upon TGF-β1 exposure and transported into new
target cells to promote cervical cancer cell metastasis. However,
flow cytometry, CCK-8 and EdU assays showed that there was no
significant difference in apoptosis and proliferation ability among
these two groups (data not shown). Our data seem to provide a
hypothesis that exosomal miRNAs can amplify metastatic elements
into cells that are adjacent or distant to effectively develop
tumors.
miRNAs cleave target mRNAs or regulate target gene
protein expression through perfect or nearly perfect complementary
binding to the 3′-UTR or open reading frame (ORF) region of the
target genes (41). In the present
study, dual luciferase activity assay showed that exosomal miR-663b
directly targets the 3′-UTR of mannoside
acetylglucosaminyltransferase 3 (MGAT3), and western blot analysis
confirmed that the protein level of MGAT3 was decreased in
recipient cells. Previous studies have reported that
glycosyltransferase MGAT3 is closely related to cell proliferation,
migration and metastasis in a variety of cancers (42–48). MGAT3
can catalyze the transfer of β-N-acetylglucosamine (GlcNAc) in the
β-1,4 bond to mannose on the N-glycan, thereby inhibiting the
β1-6GlcNAc formation catalyzed by another glycosyltransferase
MGAT5. β1-6GlcNAc is frequently detected to be overexpressed in a
variety of metastatic tumors (49,50).
Metastatic ovarian cancer and lung cancer have been reported to
exhibit significant reductions in mRNA and protein levels of MGAT3
(51). In our study, it was found
that the overexpression of MGAT3 effectively inhibited HeLa and
CaSki cell ability for migration and invasion. Taken together, our
data indicate that miR-663b promotes the metastasis of cervical
cancer cells by inhibiting MGAT3 activity in the N-glycan pathway
of cervical cancer cells.
In previous studies, MGAT3 was shown to affect the
activation of signal transduction pathways by regulating
extracellular signal-regulated kinase (ERK)1/2 or protein kinase B
(AKT) signals (52), and was involved
in EMT progression and its reverse processes (42,53).
Therefore, we examined the possible consequences of abnormal MGAT3
expression on related signaling pathways in cervical cancer. In the
miR-663b mimics group, it was observed that E-cadherin expression
was decreased and N-cadherin and β-catenin were increased. In the
pcDNA3.1-MGAT3 group, MGAT3 overexpression had a significant
blocking effect on the downregulation of E-cadherin and the
upregulation of N-cadherin and β-catenin. These findings together
demonstrated that the overexpression of MGAT3 inhibited the EMT
process in cervical cancer cell lines.
Multiple factors such as pterostilbene or long
non-coding RNAs that could affect the expression of miR-663b were
not investigated in our present study. This was the limitation of
our current research, but it also provides us with possible
directions and effective goals for future research. We will further
explore more factors affecting miR-663b expression and clarify
whether exosomal miR-663b could be used to predict the recurrence
and metastasis in cervical cancer patients, and evaluate the
effectiveness of miR-663b as a new type of targeted therapy.
In our current study, we provide evidence that
TGF-β1 can selectively promote the expression of miR-663b in
cervical cancer exosomes. After being transported to new target
cells via exosomes, miR-663b significantly inhibits the expression
of MGAT3 and enhances the metastatic ability of cervical cancer
cells. We will conduct more precise research to support our
results.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was carried out at Qilu Hospital of
Shandong University and was supported by the Shandong Provincial
Key Research Project (2017CXGC1210, 2019GSF108126), the National
Natural Science Foundation of China (NSFC, 81572559, 81902644), the
Natural Science Doctoral Program Foundation of Shandong Province
(ZR2019BC059) and the Health Commission of Weifang
(wfwsjs-2018-053).
Availability of data and materials
All data used in this study can be obtained from the
corresponding author upon reasonable request. The RNA sequencing
data has been uploaded to the GEO database (https://www.ncbi.nlm.nih.gov/geo/) with data number
GSE163507.
Authors' contributions
YZ was mainly responsible for project design and
revision of important knowledge content. XY was responsible for the
implementation of the experiment, data analysis, and wrote the
initial version of the manuscript. YW conducted the statistical
analysis, reviewed and edited the final manuscript. JM performed
the experiments and analyzed data. SH conducted the statistical
analysis, reviewed and edited the final manuscript. LL analyzed the
data and revised the content. YS maintained the cells, analyzed the
data and reviewed the manuscript. JZ, SS, XL, WS and YD analyzed
and interpreted the data. All authors participated in this research
and agreed to be responsible for all aspects of the research; they
read and approved the final manuscript to ensure the accuracy and
integrity of the work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colak S and Ten Dijke P: Targeting TGF-β
signaling in cancer. Trends Cancer. 3:56–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Batlle E and Massagué J: Transforming
growth factor-β signaling in immunity and cancer. Immunity.
50:924–940. 2018. View Article : Google Scholar
|
|
5
|
Melo SA, Luecke LB, Kahlert C, Fernandez
AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari
N, et al: Glypican-1 identifies cancer exosomes and detects early
pancreatic cancer. Nature. 23:77–82. 2015.
|
|
6
|
Sun D, Zhuang X, Zhang S, Deng ZB, Grizzle
W, Miller D and Zhang HG: Exosomes are endogenous nanoparticles
that can deliver biological information between cells. Adv Drug
Deliv Rev. 65:342–347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
OBrien K, Breyne K, Ughetto S, Laurent LC
and Breakefield XO: RNA delivery by extracellular vesicles in
mammalian cells and its applications. Nat Rev Mol Cell Biol.
21:585–606. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurywchak P and Kalluri R: An evolving
function of DNA-containing exosomes in chemotherapy-induced immune
response. Cell Res. 27:722–723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barile L and Vassalli G: Exosomes: Therapy
delivery tools and biomarkers of diseases. Pharmacol Ther.
174:63–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Veerman RE, Güçlüler Akpinar G, Eldh M and
Gabrielsson S: Immune cell-derived extracellular vesicles-functions
and therapeutic applications. Trends Mol Med. 25:382–394. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang X, Yuan X, Shi H, Wu L, Qian H and
Xu W: Exosomes in cancer: Small particle, big player. J Hematol
Oncol. 8:832015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
13
|
Shenoy A and Blelloch RH: Regulation of
microRNA function in somatic stem cell proliferation and
differentiation. Nat Rev Mol Cell Biol. 15:565–576. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wong CM, Tsang FH and Ng IO: Non-coding
RNAs in hepatocellular carcinoma: Molecular functions and
pathological implications. Nat Rev Gastroenterol Hepatol.
15:137–151. 2017. View Article : Google Scholar
|
|
15
|
Lorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
7:848–856. 2009.
|
|
16
|
Tugay K, Guay C, Marques AC, Allagnat F,
Locke JM, Harries LW, Rutter GA and Regazzi R: Role of microRNAs in
the age-associated decline of pancreatic beta cell function in rat
islets. Diabetologia. 59:161–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qureshi R, Arora H and Rizvi MA: EMT in
cervical cancer: Its role in tumour progression and response to
therapy. Cancer Lett. 356:321–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pastushenko I and Blanpain C: EMT
translation states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J, Antin P, Berx G, Blanpain C,
Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori
G, et al: Guidelines and definitions for research on
epithelial-mesenchymal transition. Nat Rev Mol Cell Biol.
21:341–352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei SC and Yang J: Forcing through tumor
metastasis: The interplay between tissue rigidity and
epithelial-mesenchymal transition. Trends Cell Biol. 26:111–120.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu F, Zhang J, Hu G, Liu L and Liang W:
Hypoxia and TGF-β1 induced PLOD2 expression improve the migration
and invasion of cervical cancer cells by promoting
epithelial-to-mesenchymal transition (EMT) and focal adhesion
formation. Cancer Cell International. 17:542017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Xing T, Chen Y and Xiao J:
Exosome-mediated miR-200b promotes colorectal cancer proliferation
upon TGF-β1 exposure. Biomed Pharmacother. 106:1135–1143. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang M, Jia M and Yuan K: MicroRNA-663b
promotes cell proliferation and epithelial mesenchymal transition
by directly targeting SMAD7 in nasopharyngeal carcinoma. Exp Ther
Med. 16:3129–3134. 2018.PubMed/NCBI
|
|
25
|
Wang YL, Shen Y, Xu JP, Han K, Zhou Y,
Yang S, Yin JY, Min DL and Hu HY: Pterostilbene suppresses human
endometrial cancer cells in vitro by down-regulating miR-663b. Acta
Pharmacol Sin. 38:1394–1400. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shu Y, Ye W, Gu YL and Sun P: Blockade of
miR-663b inhibits cell proliferation and induces apoptosis in
osteosarcoma via regulating TP73 expression. Bratisl Lek Listy.
119:41–46. 2018.PubMed/NCBI
|
|
27
|
Stanley P: Biological consequences of
overexpressing or eliminating N-acetylglucosaminyltransferase-TIII
in the mouse. Biochim Biophys Acta. 1573:363–368. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang H, Liu Y, Yu P, Qu J, Guo Y, Li W,
Wang S and Zhang J: MiR-23a transcriptional activated by Runx2
increases metastatic potential of mouse hepatoma cell via directly
targeting Mgat3. Sci Rep. 8:73662018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pinho SS, Reis CA, Paredes J, Magalhães
AM, Ferreira AC, Figueiredo J, Xiaogang W, Carneiro F, Gärtner F
and Seruca R: The role of N-acetylglucosaminyltransferase III and V
in the post-transcriptional modifications of E-cadherin. Hum Mol
Genet. 18:2599–2608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kohler RS, Anugraham M, López MN, Xiao C,
Schoetzau A, Hettich T, Schlotterbeck G, Fedier A, Jacob F and
Heinzelmann-Schwarz V: Epigenetic activation of MGAT3 and
corresponding bisecting GlcNAc shortens the survival of cancer
patients. Oncotarget. 7:51674–51686. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoshimura M, Ihara Y, Matsuzawa Y and
Taniguchi N: Aberrant glycosylation of E-cadherin enhances
cell-cell binding to suppress metastasis. J Biol Chem.
271:13811–13815. 2016. View Article : Google Scholar
|
|
32
|
Ha D, Yang N and Nadithe V: Exosomes as
therapeutic drug carriers and delivery vehicles across biological
membranes: Current perspectives and future challenges. Acta Pharm
Sin B. 6:287–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ichim TE, Zhong Z, Kaushal S, Zheng X, Ren
X, Hao X, Joyce JA, Hanley HH, Riordan NH, Koropatnick J, et al:
Exosomes as a tumor immune escape mechanism: Possible therapeutic
implications. J Transl Med. 6:372008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Devhare PB, Sasaki R, Shrivastava S, Di
Bisceglie AM, Ray R and Ray RB: Exosome-mediated intercellular
communication between hepatitis C virus-infected hepatocytes and
hepatic stellate cells. Virol. 91:e02225–16. 2017. View Article : Google Scholar
|
|
35
|
Neuzillet C, De Gramont A,
Tijeras-Raballand A, de Mestier L, Cros J, Faivre S and Raymond E:
Perspectives of TGF-β inhibition in pancreatic and hepatocellular
carcinomas. Oncotarget. 5:78–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huynh LK, Hipolito CJ and Ten Dijke P: A
perspective on the development of TGF-β inhibitors for cancer
treatment. Biomolecculars. 9:7432019. View Article : Google Scholar
|
|
37
|
Park SJ, Choi YS, Lee S, Lee YJ, Hong S,
Han S and Kim BC: BIX02189 inhibits TGF-β1-induced lung cancer cell
metastasis by directly targeting TGF-β type I receptor. Cancer
Lett. 381:314–322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim S, Lee J, You D, Jeong Y, Jeon M, Yu
J, Kim SW, Nam SJ and Lee JE: Berberine suppresses cell motility
through downregulation of TGF-β1 in triple negative breast cancer
cells. Cell Physiol Biochem. 45:795–807. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu F, Zhang X, Sun C, Xu W and Xia J:
Downregulation of miRNA-663b protects against hypoxia-induced
injury in cardiomyocytes by targeting BCL2L1. Exp Ther Med.
19:3581–3588. 2020.PubMed/NCBI
|
|
40
|
Cai H, An Y, Chen X, Sun D, Chen T, Peng
Y, Zhu F, Jiang Y and He X: Epigenetic inhibition of miR-663b by
long non-coding RNA HOTAIR promotes pancreatic cancer cell
proliferation via up-regulation of insulin-like growth factor 2.
Oncotarget. 7:86857–86870. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim VN, Han J and Siomi MC: Biogenesis of
small RNAs in animals. Nat Rev Mol Cell Biol. 10:126–139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tan Z, Wang C, Li X and Guan F: Bisecting
N-acetylglucosamine structures inhibit hypoxia-induced
epithelial-mesenchymal transition in breast cancer cells. Front
Physiol. 9:2102018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang X, Wang Y, Qian Y, Wu X, Zhang Z,
Liu X, Zhao R, Zhou L, Ruan Y, Xu J, et al: Discovery of specific
metastasis-related N-glycan alterations in epithelial ovarian
cancer based on quantitative glycomics. PLoS One. 9:e879782014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miwa HE, Koba WR, Fine EJ, Giricz O, Kenny
PA and Stanley P: Bisected, complex N-glycans and galectins in
mouse mammary tumor progression and human breast cancer.
Glycobiology. 23:1477–1490. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Akama R, Sato Y, Kariya Y, Isaji T, Fukuda
T, Lu L, Taniguchi N, Ozawa M and Gu J:
N-acetylglucosaminyltransferase III expression is regulated by
cell-cell adhesion via the E-cadherin-catenin-actin complex.
Proteomics. 8:3221–3228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Allam H, Johnson BP, Zhang M, Lu Z, Cannon
MJ and Abbott KL: The glycosyltransferase GnT-III activates Notch
signaling and drives stem cell expansion to promote the growth and
invasion of ovarian cancer. J Biol Chem. 292:16351–16359. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yoshimura M, Ihara Y, Ohnishi A, Ijuhin N,
Nishiura T, Kanakura Y, Matsuzawa Y and Taniguchi N: Bisecting
N-acetylglucosamine on K562 cells suppresses natural killer
cytotoxicity and promotes spleen colonization. Cancer Res.
56:412–418. 1996.PubMed/NCBI
|
|
48
|
Yoshimura M, Ihara Y, Nishiura T, Okajima
Y, Ogawa M, Yoshida H, Suzuki M, Yamamura K, Kanakura Y, Matsuzawa
Y and Taniguchi N: Bisecting GlcNAc structure is implicated in
suppression of stroma-dependent haemopoiesis in transgenic mice
expressing N-acetylglucosaminyltransferase III. Biochem J.
331:733–742. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nagae M, Kizuka Y, Mihara E, Kitago Y,
Hanashima S, Ito Y, Takagi J, Taniguchi N and Yamaguchi Y:
Structure and mechanism of cancer-associated
N-acetylglucosaminyltransferase-V. Nat Commun. 9:33802018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Taniguchi N and Kizuka Y: Glycans and
cancer: Role of N-glycans in cancer biomarker, progression and
metastasis, and therapeutics. Adv Cancer Res. 126:11–51. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li J, Xu J, Li L, Ianni A, Kumari P, Liu
S, Sun P, Braun T, Tan X, Xiang R and Yue S: MGAT3-mediated
glycosylation of tetraspanin CD82 at asparagine 157 suppresses
ovarian cancer metastasis by inhibiting the integrin signaling
pathway. Theranostics. 10:6467–6482. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mo C, Liu T, Zhang S, Guo K, Li M, Qin X
and Liu Y: Reduced N-acetylglucosaminyltransferase III expression
via Smad3 and Erk signaling in TGF-β1-induced HCC EMT model. Discov
Med. 23:7–17. 2017.PubMed/NCBI
|
|
53
|
Xu Q, Isaji T, Lu Y, Gu W, Kondo M, Fukuda
T, Du Y and Gu J: Roles of N-acetylglucosaminyltransferase III in
epithelial-to-mesenchymal transition induced by transforming growth
factor β1 (TGF-β1) in epithelial cell lines. J Biol Chem.
287:16563–16574. 2012. View Article : Google Scholar : PubMed/NCBI
|