Introduction
The incidence of thyroid cancer has continued to
increase in the USA over the past 30 years (1). A Surveillance, Epidemiology, and End
Results Program-based study reported that between 1975 and 2009
there was a three-fold increase in incidence rate, from 4.9 to 14.3
per 100,000 individuals in the USA (1). Although the majority of cases (>90%)
are those of differentiated thyroid cancer with a favorable
prognosis, ~1% of all cases are anaplastic thyroid cancer (ATC)
(2). ATC is one of the most
aggressive cancers, with a 1-year survival rate of only 5–20%
(3,4).
Surgical treatment improves the prognosis of ATC (5); however, early diagnosis remains
challenging (4). The progression and
metastasis of ATC occur early and rapidly, making it likely that
the optimal timing for surgery is missed (3–6).
Multimodal therapy consisting of surgery, systemic chemotherapy and
external beam radiation therapy is required; however, ATC is known
to be highly resistant to any form of therapy (6). Targeted kinase inhibitors based on
tumor-derived molecular alterations and emerging immunomediated
combination therapies were introduced in 2013, but the prognosis
continues to be poor (6).
For a tissue to maintain homeostasis, cell growth
and death rates must be balanced (7).
The inhibition of a cellular death pathway, apoptosis, is a crucial
mechanism underlying the development and progression of cancer,
which helps cancer cells escape the immune system (8). Furthermore, the inhibition of apoptosis
can lead to resistance to chemotherapy or radiation therapy
(9). Inhibitors of apoptosis proteins
(IAPs) are regulatory proteins that impede tumor cell apoptosis by
inhibiting caspases in the apoptosis signaling pathway (10). Several studies have revealed links
between members of the IAP family and cancer (10,11). IAPs
play an important role in resistance to chemotherapy and
radiotherapy (9,12–15); they
are characterized by a domain termed the baculoviral IAP repeat
(BIR) (9). Livin, a novel IAP family
member, contains of a single BIR domain and a COOH-terminal RING
finger domain (9,16). Human Livin manifests as two isoforms,
Livin α and β, as a result of two alternatively spliced transcripts
(9). Livin is highly expressed in
various tumor cells; however, it has minimal/no expression in most
normal adult tissues (17,18). Several studies have shown that Livin
expression is associated with aggressive disease course,
chemoresistance and poor outcome (17,19–22);
moreover, therapeutic sensitivity has been shown to be altered
following Livin downregulation (23,24).
Therefore, Livin may serve as a potential therapeutic target
(25). To the best of our knowledge,
there is no study to date on Livin expression in human ATC
specimens or cell lines. The aim of the present study was to
analyze the role of Livin in the ATC cell line and process the
clinical data of patients with ATC, thereby investigating its
potential as a therapeutic agent.
Materials and methods
Cell culture and transfection
The BHT101 human ATC cell line was provided by Dr
Won Gu Kim (Asan Medical Center, University of Ulsan College of
Medicine, Seoul, Korea). BHT101 cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 20% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin in a humidified atmosphere of 5%
CO2 at 37°C. To knock down the endogenous genetic
expression of Livin in ATC cells, small interfering RNAs (siRNAs)
were used. Seeded in 6-well plates at a density of
2.0×105 cells/well, ATC cells were then transfected with
50 µM Livin-specific siRNA (Bioneer Corporation) or 50 µM negative
control siRNA (scrambled siRNA; cat. no. 1027281; Qiagen, Inc.)
using Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher Scientific,
Inc.) for 48 h at 37°C. Subsequent experiments were performed after
48 h. Livin-specific siRNA sequences were as follows: Sense,
5′-GGAUGGCUUAACUCUACCU-3′, and antisense,
5′-AGGUACAGUUAAGCCAUCC-3′.
Protein isolation and western blot
analysis
Cells were lysed using radioimmunoprecipitation
assay buffer (Biosesang Inc.), following which the bicinchoninic
acid assay was performed to measure protein concentrations. Protein
lysates (20–30 µg/lane) after 10–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis were separated and
electrophoretically transferred onto polyvinylidene fluoride
membranes. The membranes were then incubated with 5% bovine serum
albumin (Bioshop Canada Inc.) in Tris-buffered saline (TBS)-0.5%
Tween-20 at room temperature for 1 h. Subsequently, the membrane
was washed four times for 15 min each with TBS-0.5% Tween-20.
Specific proteins were detected sequentially using primary
antibodies against GAPDH (cat. no. sc-25778; Santa Cruz
Biotechnology, Inc.), Livin (α, 36 kDa; β, 34 kDa; cat. no. 5471;
Cell Signaling Technology, Inc.), cleaved caspase-3 (cat. no. 9664;
Cell Signaling Technology, Inc.), caspase-3 (cat. no. 9662; Cell
Signaling Technology, Inc.), cleaved caspase-7 (cat. no. 9491; Cell
Signaling Technology, Inc.), caspase-7 (cat. no. 9492; Cell
Signaling Technology, Inc.), cleaved poly(ADP-ribose) polymerase
(PARP; cat. no. 5625; Cell Signaling Technology, Inc.), and PARP
(cat. no. 9542; Cell Signaling Technology, Inc.). After diluting
the primary antibodies at 1:1,000 in TBS-0.5% Tween-20, they were
incubated with the membranes for 24 h at 4°C. Anti-rabbit (cat. no.
7074; Cell Signaling Technology, Inc.) or anti-mouse (cat. no.
7076, Cell Signaling Technology, Inc.) horseradish peroxidase
(HRP)-conjugated secondary antibodies were diluted at 1:2,000. The
membranes were incubated with secondary antibodies at room
temperature for 2 h. Using an enhanced chemiluminescence detection
system for HRP (EMD Millipore), immunoreactive proteins were
visualized and analyzed with an LAS-4000 luminescence image
analyzer (FUJIFILM Wako Pure Chemical Corporation). All western
blot analysis experiments were run independently and in
triplicate.
RNA isolation, reverse transcription
(RT) semi-quantitative polymerase chain reaction (qPCR) and
RT-qPCR
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for RNA extraction from cells, according
to the manufacturer's protocol. RT was performed using 1 µg total
RNA, M-MLV reverse transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc.), 1 µl 2 mM dNTP mix (Enzynomics Co., Ltd.), 2 µl
0.1 M dithiothreitol (Invitrogen; Thermo Fisher Scientific, Inc.),
4 µl 5X first-strand buffer (Invitrogen; Thermo Fisher Scientific,
Inc,), 1 µl RNase inhibitor (Promega Corporation) and 1 µl
oligo(dT) (Bioneer Corporation). The resulting cDNA was amplified
using primers specific for Livin and GAPDH (Bioneer Corporation).
GoTaq DNA Polymerase and 5X Green GoTaq reaction buffer (Promega
Corporation) were used for PCR. qPCR was performed for 5 min at
94°C for one cycle, 30 sec at 94°C, 20 sec at 58°C and 30 sec at
72°C for 32 cycles, and 7 min at 72°C for one cycle. The primer
sequences were as follows: Livin α and β forward,
5′-CACACAGGCCATCAGGACAAG-3′ and reverse,
5′-ACGGCACAAAGACGATGGAC-3′; and GAPDH forward,
5′-ACCACAGTCCATGCCATCAC-3′ and reverse, 5′-TCCACCCTGTTGCTGTA-3′.
The polymerase chain reaction products were separated
electrophoretically on a 1% agarose gel containing ethidium
bromide. Densitometry was performed using WiseUV (WUV-L20; Daihan
Scientific Co., Ltd.) and Multigauge V3.2, (Fuji Co., Ltd.).
RT-qPCR analysis was performed using the QuantiSpeed
SYBR-Green Kit (cat. no. 105-02; PhileKorea) with the Rotor-Gene
6000 real-time rotary analyzer (Corbett Life Science) and confirmed
using melting curve analysis. RT, prior to qPCR, was performed as
described above. Amplification plots were used to evaluate the
quantification cycle (Cq). The sequences of qPCR primers used were
as follows: Livin α and β forward, 5′-CACACAGGCCATCAGGACAAG-3′ and
reverse, 5′-ACGGCACAAAGACGATGGAC-3′; and 18S rRNA forward,
5′-GTAACCCGTTGAACCCCATT-3′ and reverse, 5′-CCATCCAATCGGTAGTAGCG-3′.
qPCR was performed at 95°C for 2 min for one cycle, followed by
95°C for 10 sec and 60°C for 25 sec for 42 cycles. Each reaction
was repeated independently at least three times. The
2−ΔΔCq method was used to determine the mRNA expression
levels (26).
Cell invasion assay
The number of cells that migrated through a 8.0-µm
pore Transwell invasion apparatus (cat. no. 3422; Costar, Inc.) was
used to determine the extent of cell invasion. A day before the
experiment, the upper chamber was coated with 1% gelatin solution
for 12 h at 37°C and then dried for 12 h at room temperature. After
transfection for 48 h, the upper chamber cells transfected with 50
µM Livin siRNA or 50 µM negative control siRNA were seeded at
2×105 cells in 120 µl 0.2% bovine serum albumin (BioShop
Canada, Inc.) with FBS-free DMEM. As the chemoattractant, 400 µl
0.2% bovine serum albumin with FBS-free DMEM containing fibronectin
(cat. no. 361635; EMD Millipore) was loaded into the lower chamber.
After a 24 h incubation period, cells at the bottom of the
Transwell surface were stained with Diff-Quik solution (Sysmex
Corporation). Subsequently, the cells were counted in five random
microscopic fields of view at ×100 magnification using a light
microscope. The results are presented as mean ± standard error of
the number of cells per field after three individual
experiments.
Apoptosis assay
An Annexin V-fluorescein isothiocyanate (FITC) assay
was performed to assess apoptosis. Cells were transfected with
either 50 µM Livin siRNA or 50 µM negative control siRNA for 48 h.
After 48 h of transfection, the cells were collected following
trypsinization, washed twice in phosphate buffered saline, and
resuspended in binding buffer (BD Biosciences). After the addition
of Annexin V-FITC and 7-amino-actinomycin D (BD Biosciences), the
cells were incubated in the dark for 15 min and then resuspended in
400 ml binding buffer. A FACS Calibur flow cytometer (BD
Biosciences) and BD Cell Quest version 3.3 software
(Becton-Dickinson) were used for cell analysis. The data analysis
was executed using WinMDI version 2.9 (The Scripps Research
Institute). All apoptosis assay experiments were run in triplicate
and independently.
Cell viability assay
After 48 h of transfection, cells seeded in 24-well
plates (1×104 cells/well) were transfected the following
day with either 50 µM Livin siRNA or 50 µM negative control siRNA.
Cell viability was measured after incubation for 48 h using an
EZ-CyTox (tetrazolium salts, WST-1) enhanced cell viability assay
kit (cat. no. EZ-3000; Daeil Lab, Inc.) for 1–2 h at 37°C. The
absorbance was read at 460 nm using a microplate reader. All cell
viability assay experiments were run in triplicate and
independently.
Cell irradiation or lenvatinib
treatment
After 48 h of transfection, cells were cultured at
37°C and treated with γ-irradiation at various doses (5, 10, 20, 30
and 40 Gy) (137Cs, 2.875 Gy/min) using a Gammacell 3000
Elan (Therathronics) at room temperature. A stock solution of
lenvatinib (4 mg/ml; Eisai Co., Ltd.) was dissolved in dimethyl
sulfoxide (27,28) and diluted at various concentrations
(5, 10, 20, 40, 80 and 160 µM) for 24 h at 37°C for experimental
use.
Patients and tumor specimens
Paraffin-embedded tissue sections from 25 patients
who underwent surgery for definitive ATC at Chonnam National
University Hwasun Hospital (Jeonnam, Korea) between September 2005
and December 2015, were collected to evaluate Livin protein
expression. A total of 6 men and 17 women, with a mean age of
73.0±10.7 years (range, 37–85 years), were enrolled in the present
study. To ensure the diagnostic accuracy of ATC, two pathologists
reviewed the pathological slides of all enrolled patients
independently. Two patients were excluded because they were
diagnosed with carcinoma showing thymus-like differentiation rather
than ATC. All ATC tumor tissues were obtained before either
radiotherapy or chemotherapy. All 23 enrolled patients were treated
with definitive surgery (total thyroidectomy, 20 patients; subtotal
thyroidectomy, 2 patients; hemothyroidectomy, 1 patient), among
them, 16 were followed up with adjuvant radiotherapy or concurrent
chemoradiotherapy. Hospital records were reviewed thoroughly for
clinicopathological characteristics and complete medical history.
The date of starting treatment until the date of death or the date
of last follow-up was calculated as the survival duration
(months).
Immunohistochemistry
Tissue processing and immunohistochemical analysis
were performed according to a previously described method (29). The tissue sections were incubated with
1:100-diluted primary antibodies against Livin (cat. no. 5471; Cell
Signaling Technology, Inc.) in antibody diluent reagent solution
(cat. no. 003118; Invitrogen; Thermo Fisher Scientific, Inc.) for
24 h at 4°C. The staining results were interpreted by two
independent observers without any knowledge of the associated
clinical records. Some patients had both ATC and papillary thyroid
carcinoma (PTC) in the thyroid gland. Since the Livin staining
status may be different in ATC or PTC tissues, in these cases the
staining results were interpreted according to the Livin staining
status in the ATC tissue. Scores for staining intensity were as
follows: 0, no staining of tumor cells; 1+, weak to comparable
staining in the cytoplasm and/or nucleus relative to non-tumor cell
staining; and 2+, readily appreciable or dark brown staining
distinctly marking the tumor cell cytoplasm and/or nucleus. The
percentile of stained cells was scored as follows: 0, 0%; 1, 1–25%;
2, 26–50%; and 3, ≥51%. The products of the intensity and
percentile scores were the final intensity scores, which were
defined as low Livin expression if ≤2 and as high Livin expression
if >2.
Assessment of tumor cell proliferation
and apoptosis
Tissue processing and immunohistochemical analysis
were performed according to a previously described method (29). The tissue sections were incubated with
1:100-diluted primary antibodies against Ki-67 in antibody diluent
reagent solution (cat. no. 003118; Invitrogen; Thermo Fisher
Scientific, Inc.) for 24 h at 4°C. Tumor cell proliferation was
visualized using Ki-67 (cat. no. ab16667; Abcam) and a light
microscope (magnification, ×200). The number of Ki-67-positive
nuclei per 1,000 tumor cell nuclei was used to determine the Ki-67
labeling index.
To detect and quantify apoptosis, the DeadEnd™
Colorimetric terminal deoxynucleotidyl transferase dUTP nick end
labeling (TUNEL) system (cat. no. G7130; Promega Corporation) was
used according to the manufacturer's instructions. TUNEL-positive
apoptotic cells exhibit darkly stained nuclei or nuclear fragments
with a cytoplasmic halo, as observed with a light microscope
(magnification, ×200). The number of TUNEL-positive nuclei
containing apoptotic bodies among 1,000 tumor cell nuclei was
defined as the apoptotic index.
Statistical analysis
An unpaired Student's t-test was used to determine
the significance of experimental differences. Data are shown as the
mean ± standard error. All experimental assays were run in
triplicate and independently. The survival curves were calculated
using the Kaplan-Meier method and assessed with a log-rank test.
All analyses were performed using SPSS version 21.0 (IBM, Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Livin knockdown suppresses tumor cell
invasion and enhances tumor cell apoptosis in human ATC cells
In the present study, the role of Livin in tumor
progression was investigated using siRNA to inhibit the endogenous
expression of Livin in the BHT101 human ATC cells. In the
Livin-specific siRNA-treated BHT101 cells, both mRNA and protein
levels of Livin α and Livin β were significantly lower than those
in the negative control siRNA-treated cells (Fig. 1A). In the cell invasion assay, there
were 145.0±6.2 invading Livin-knockdown BHT101 cells compared with
258.2±12.5 invading negative control BHT101 cells (Fig. 1B), which was significantly different
(P<0.05).
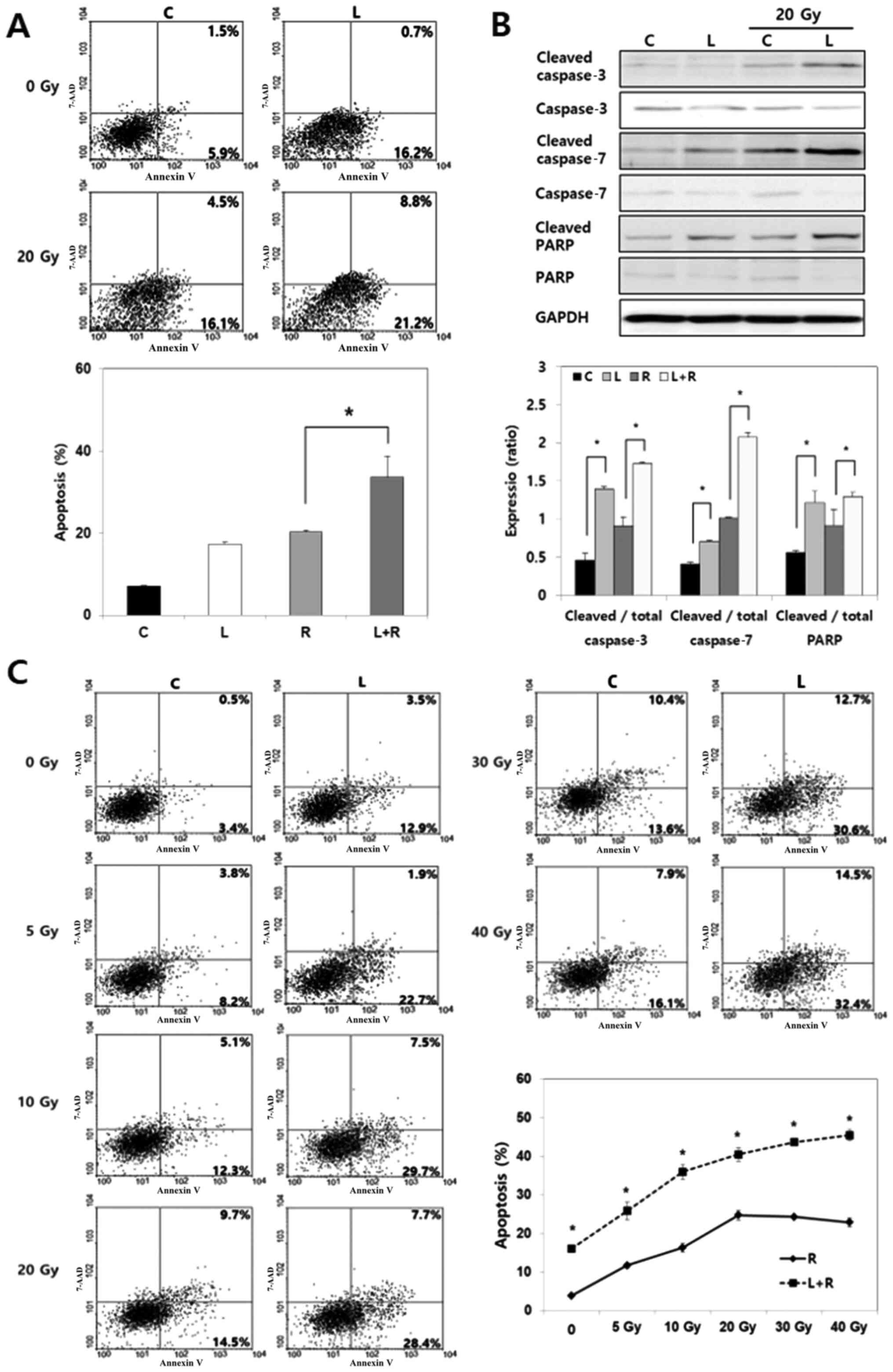
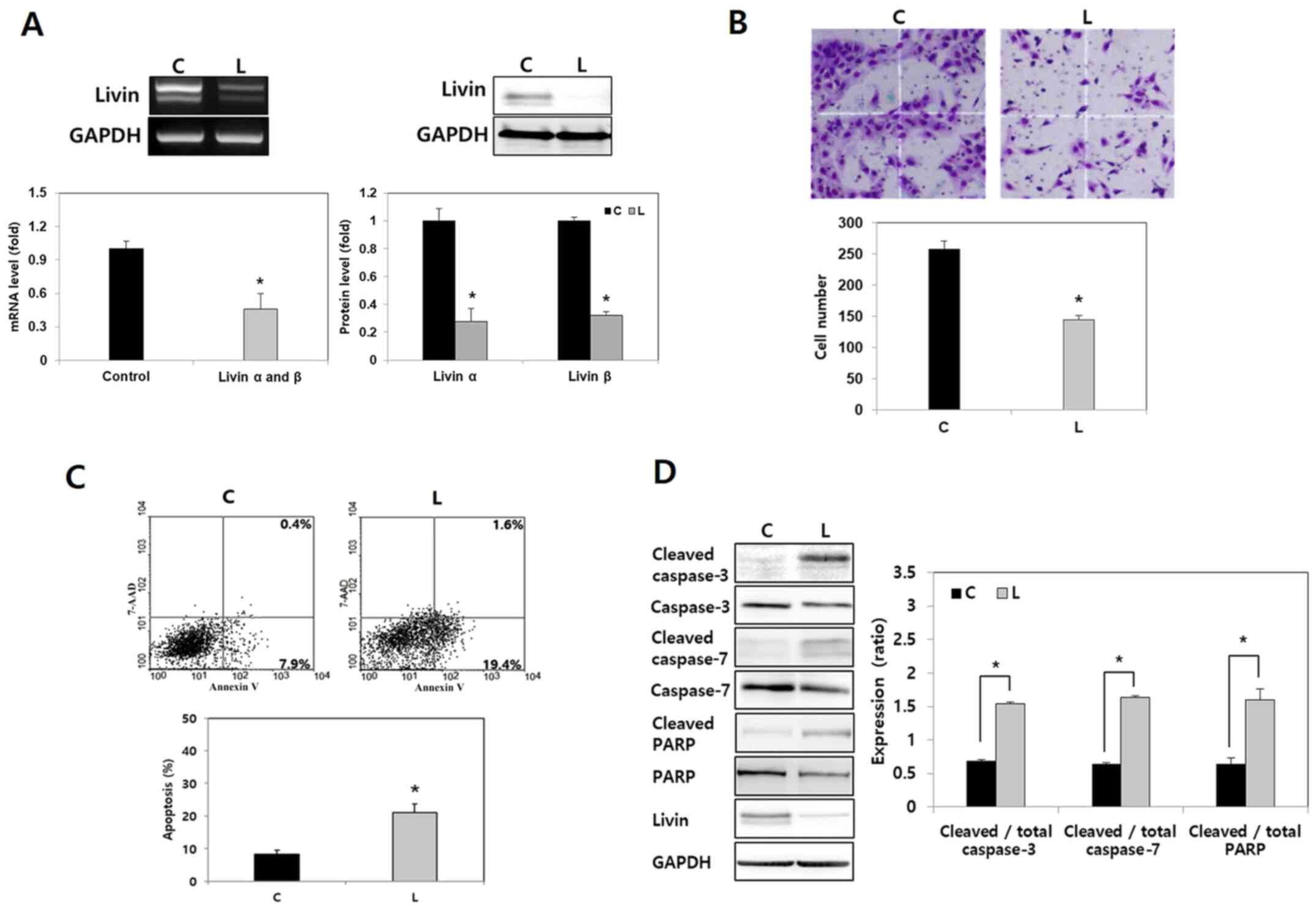 | Figure 1.Effect of Livin knockdown on cell
invasion and apoptosis in human anaplastic thyroid cancer cells.
(A) Compared with negative control siRNA, the mRNA and protein
levels of Livin α and Livin β were reduced by Livin siRNA in BHT101
cells, as shown by RT-semi-qPCR, RT-qPCR and western blotting.
*P<0.05 vs. C. (B) Compared with negative control cells,
significantly fewer Livin knockdown BHT101 cells demonstrated
invasion capacity in a cell invasion assay. Magnification, ×100.
Stained invading cells were counted and presented as the mean ±
standard error for three independent experiments. *P<0.05 vs. C.
(C) In a cell apoptosis assay, flow cytometry demonstrated that
Livin-knockdown BHT101 cells exhibited more apoptosis compared with
control cells. *P<0.05 vs. C. (D) Compared with the levels in
the control cells, levels of cleaved caspase-3, cleaved caspase-7
and cleaved PARP were higher in Livin-knockdown BHT101 cells.
Cleaved proteins were normalized to the corresponding total protein
level. *P<0.05. RT, reverse transcription; siRNA, small
interfering RNA; C, negative control siRNA-transfected cells; L,
Livin-specific siRNA-transfected cells; 7-AAD, 7-amino-actinomycin
D; PARP, poly(ADP-ribose)polymerase. |
To evaluate the effect of Livin on apoptosis, an
Annexin V apoptosis assay was conducted. The results of the flow
cytometric analysis revealed that Livin knockdown significantly
increased the proportion of apoptotic cells (P<0.05; Fig. 1C). Next, the expression levels of
apoptosis regulatory proteins following transfection with siRNA
were evaluated. Levels of cleaved caspase-3, cleaved caspase-7 and
cleaved PARP were significantly higher in Livin-knockdown BHT101
cells compared with in the negative control cells (P<0.05;
Fig. 1D). These results demonstrated
that Livin knockdown leads to tumor cell apoptosis by regulating
apoptosis regulatory proteins such as caspase-3, caspase-7 and PARP
in human ATC cells.
Livin knockdown enhances
radiosensitivity in human ATC cells
It was further examined whether Livin knockdown
enhances radiosensitivity by inducing apoptosis in BHT101 cells.
Radiation (20 Gy) was applied to cells after transfection with
Livin siRNA or negative control siRNA for 48 h. A significantly
higher extent or apoptosis was detected with the combination of
Livin siRNA and radiation compared with radiation alone (P<0.05;
Fig. 2A). Consistently, cleaved
caspase-3, cleaved caspase-7 and cleaved PARP levels following
radiation treatment were significantly higher in the
Livin-knockdown cells compared with in the control cells
(P<0.05; Fig. 2B). The effect of
Livin knockdown on apoptosis under various radiation doses was also
assessed. Cells treated with radiation alone exhibited
radioresistance without any further increase in apoptosis under the
>20 Gy radiation dose; conversely, the combination of Livin
knockdown and radiation resulted in a continuous increase in
apoptosis, which was significantly different (P<0.05; Fig. 2C). These findings indicated that a
combination of Livin knockdown and radiotherapy enhances
radiation-induced apoptosis in human ATC cells.
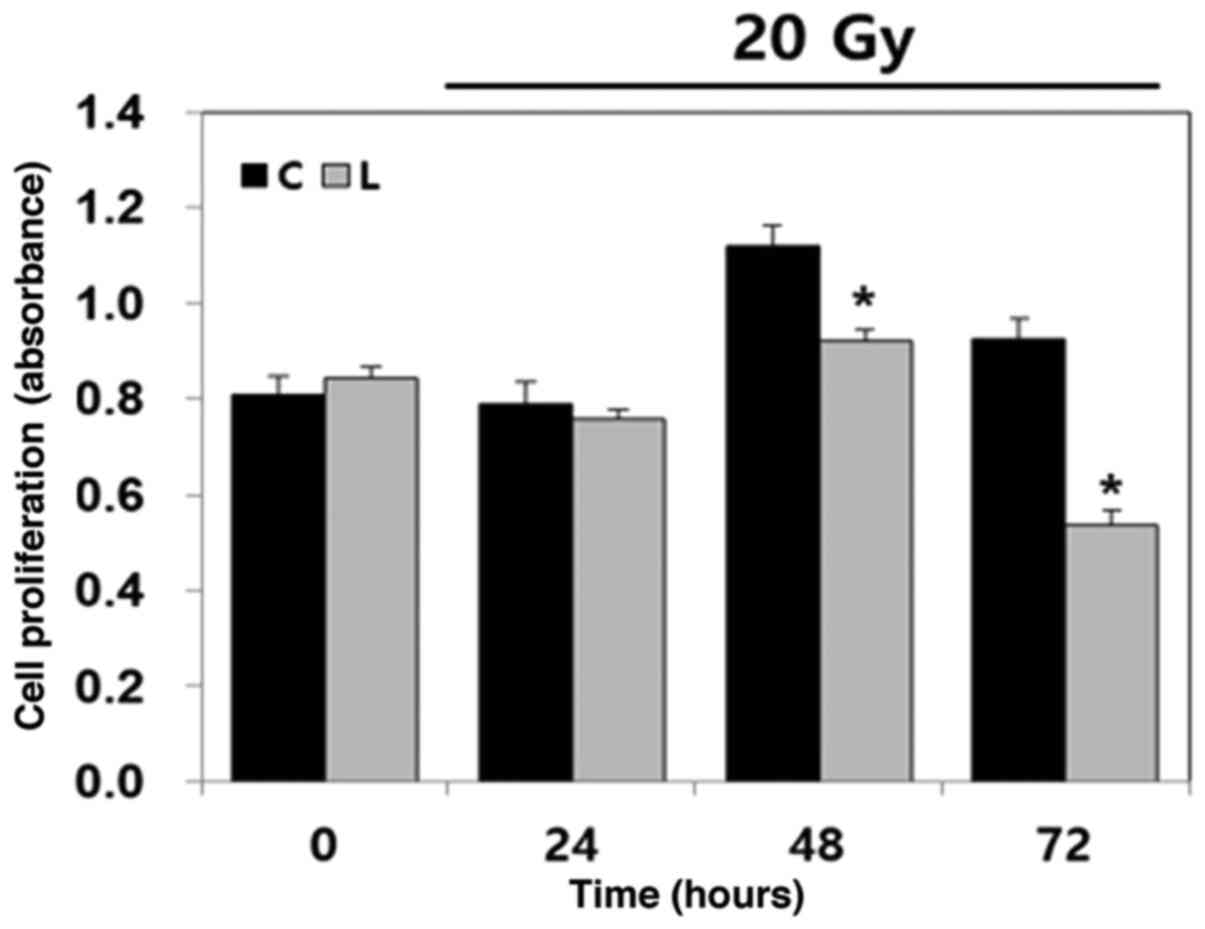
To determine the effect of Livin knockdown on the
cytotoxicity of radiation over time, a cell viability assay was
performed with BHT101 cells. Following 20 Gy radiation treatment,
the number of viable Livin-knockdown BHT101 cells, calculated by
absorbance, was significantly decreased at 48 and 72 h compared
with the negative control cells (P<0.05; Fig. 3). As the radiation time increased, the
differences in cell viability between the two groups increased.
This indicated that Livin knockdown enhances the cytotoxicity of
radiotherapy in human ATC cells.
Livin knockdown enhances
chemosensitivity of lenvatinib in human ATC cells
It was evaluated whether Livin knockdown enhances
chemosensitivity by inducing apoptosis in BHT101 cells. Cells were
transfected with Livin siRNA or negative control siRNA for 48 h,
following which the cells were treated with lenvatinib (5–80 µM)
for 24 h. The combination of Livin siRNA and lenvatinib resulted in
a significantly higher level of apoptosis compared with lenvatinib
alone at 20 µM (P<0.05; Fig. 4A).
Cells treated with lenvatinib alone exhibited chemoresistance with
no significant increase in apoptosis was observed even at an
increased dose, whereas cells treated with Livin knockdown and
lenvatinib showed a continuous increase in apoptosis with
increasing doses of lenvatinib. Similarly, the cell viability assay
showed that the number of viable Livin-knockdown BHT101 cells,
calculated by absorbance, treated with 40 or 80 µM lenvatinib was
significantly lower than that of the negative control cells
(Fig. 4B). These findings implied
that the combination of Livin knockdown and lenvatinib enhances the
chemosensitivity of lenvatinib in human ATC cells.
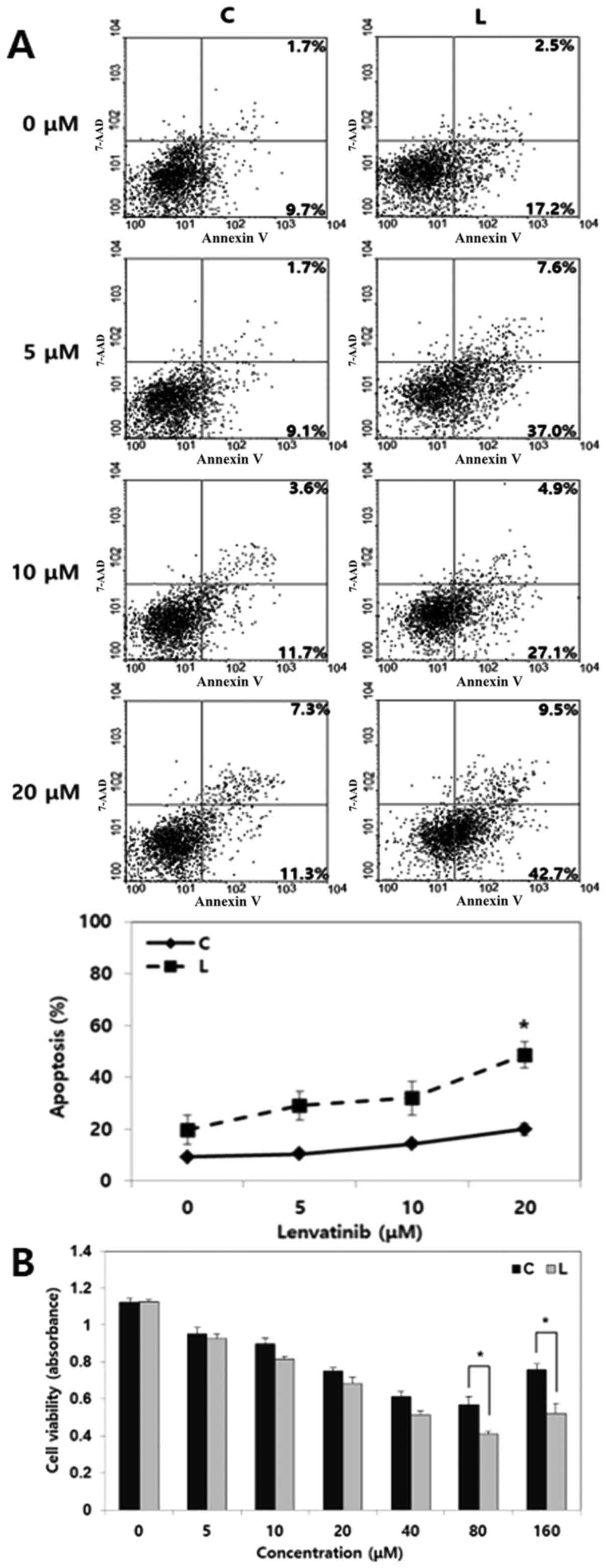 | Figure 4.Effect of Livin knockdown on the
chemosensitivity of lenvatinib in human anaplastic thyroid cancer
cells. (A) In a cell apoptosis assay, a combination treatment of
Livin knockdown with lenvatinib resulted in greater apoptosis of
BHT101 cells compared with control cells treated with lenvatinib
alone. Under various lenvatinib doses, the combination of Livin
knockdown and lenvatinib showed a continuous increase in apoptosis
than in control cells treated with lenvatinib alone, showing a
significant intergroup difference at 20 µM. *P<0.05 vs. C. (B)
In a cell viability assay, after applying 40 or 80 µM lenvatinib,
the number of viable Livin-knockdown BHT101 cells, calculated by
absorbance, was significantly lower than that of negative control
cells. *P<0.05. siRNA, small interfering RNA; C, negative
control siRNA-transfected cells; L, Livin-specific
siRNA-transfected cells. |
Elevated Livin expression is associated with a high
Ki-67 labeling index and low apoptotic index in human ATC tissues.
Table I presents the
clinicopathological variables of the patients with ATC. The mean
size of ATC was 5.4±2.9 cm (range, 0.5–13.0 cm), and 12 patients
(52.2%) presented with PTC with ATC. The majority of patients
(22/23; 95.7%) had extrathyroidal extension, and 60.9% (14/23) had
a distant metastasis at the time of diagnosis. After surgery, 16
patients were treated with adjuvant radiotherapy and 2 patients
were treated with adjuvant chemotherapy. Formalin-fixed
paraffin-embedded biopsy tissues acquired from 23 patients with ATC
were used for immunohistochemical staining to examine Livin protein
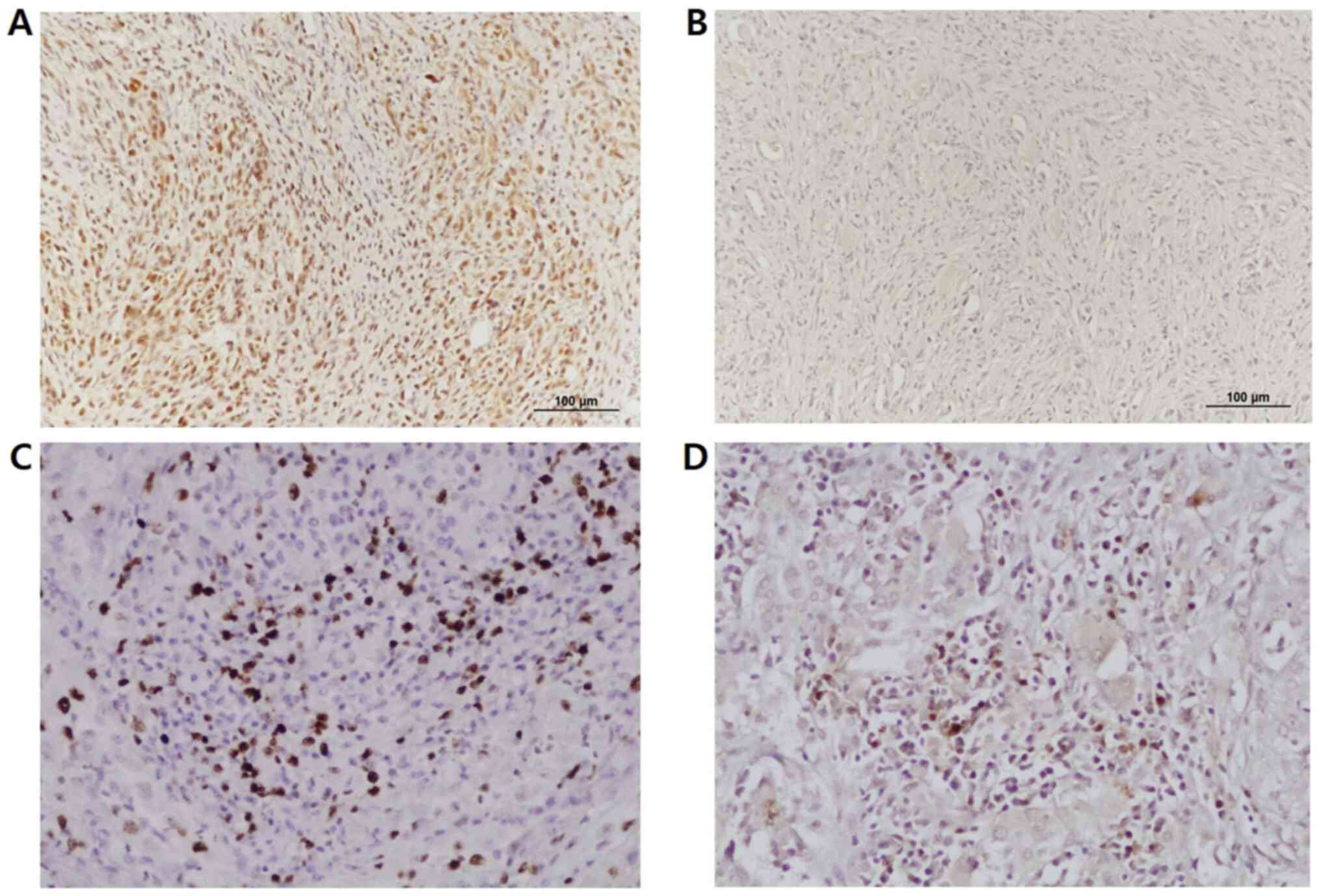
expression. Livin protein staining demonstrated a heterogenous
pattern, with predominantly nuclear and/or cytoplasmic dark brown
staining in tumor cells (high expression; Fig. 5A) rather than weak or no staining (low
expression; Fig. 5B). According to
our grading criteria, 30.4% of patients (7/23) exhibited high Livin
expression, while 69.6% of patients (16/23) exhibited low Livin
expression. Tumors with high Livin expression had significantly
higher Ki-67 labeling and lower apoptotic indexes compared with
those with low Livin expression (Table
II; P<0.02 and P<0.03, respectively; Fig. 5C and D). These findings indicated that
tumors with high Livin expression exhibited higher tumorigenic
activity, which potentiated tumor progression in ATC.
 | Table I.Clinicopathological variables of
patients with anaplastic thyroid carcinoma (n=23). |
Table I.
Clinicopathological variables of
patients with anaplastic thyroid carcinoma (n=23).
| Variable | Value |
|---|
| Mean age ± standard
deviation (range), years | 73.0±10.7
(37.0–85.0) |
| Sex, n (%) |
|
|
Male | 6
(26.1) |
|
Female | 17 (73.9) |
| Mean size ±
standard deviation (range), cm | 5.4±2.9
(0.5–13.0) |
| Extrathyroidal
extension, n (%) |
|
| No | 1 (4.3) |
|
Yes | 22 (95.7) |
| PTC component, n
(%) |
|
| No | 11 (47.8) |
|
Yes | 12 (52.2) |
| Distant metastasis,
n (%) |
|
| No | 9 (39.1) |
|
Yes | 14 (60.9) |
| Radiotherapy, n
(%) |
|
| No | 7 (30.4) |
|
Yes | 16 (69.6) |
| Chemotherapy, n
(%) |
|
| No | 21 (91.3) |
|
Yes | 2 (8.7) |
 | Table II.Association between Livin expression
and proliferation/apoptosis in patients with anaplastic thyroid
cancer. |
Table II.
Association between Livin expression
and proliferation/apoptosis in patients with anaplastic thyroid
cancer.
|
|
| Livin
expression |
|
|---|
|
|
|
|
|
|---|
| Parameter | Total (n=23) | Low (n=16) | High (n=7) | P-value |
|---|
| Ki-67 labeling
indexa | 39.3±10.6 | 35.9±9.6 | 47.0±8.8 | 0.02 |
| Apoptotic
indexa | 27.4±21.8 |
32.7±23.5 |
15.5±10.7 | 0.03 |
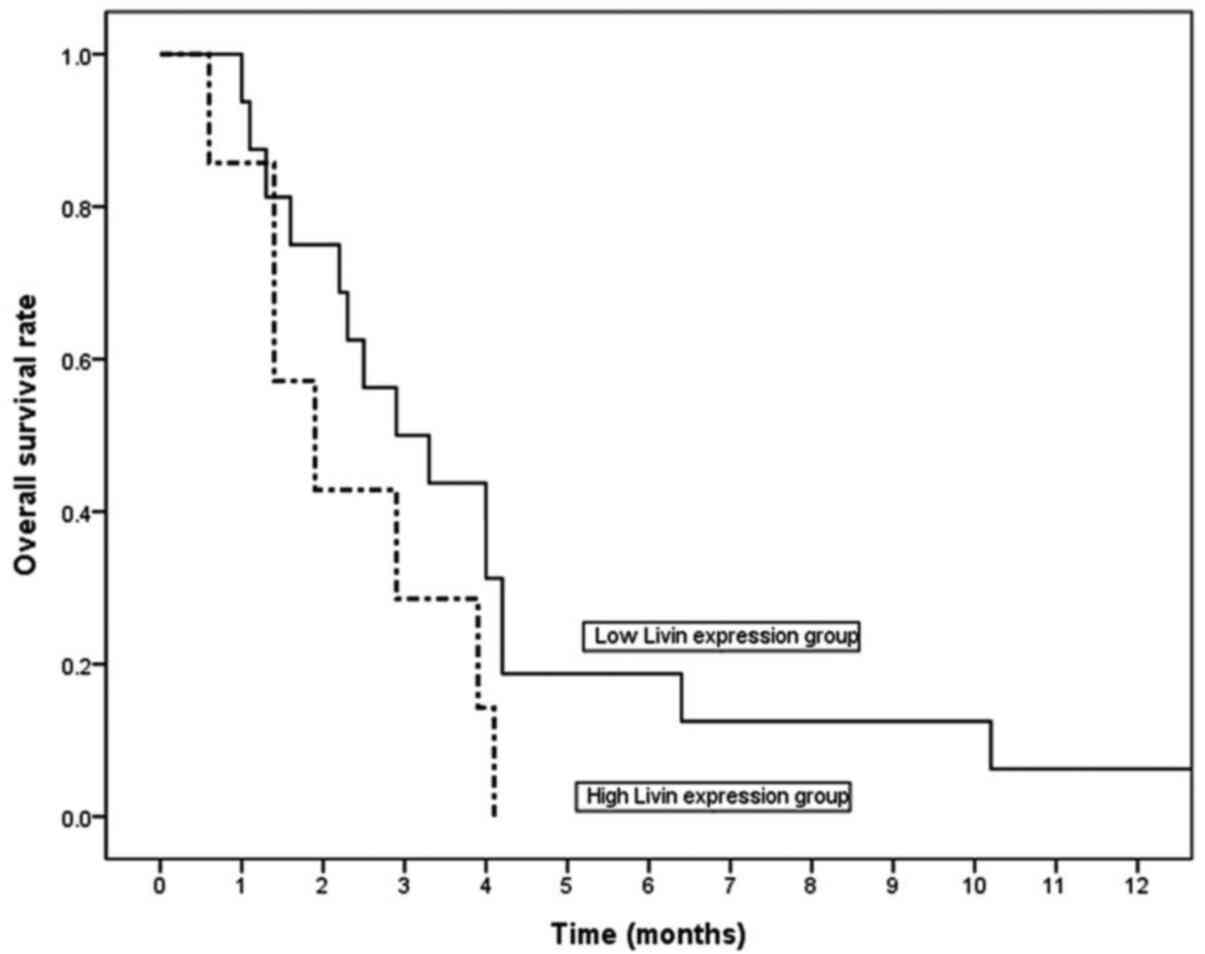
For the 23 patients with ATC who were enrolled in
the present study, the median survival duration was only 2.9
months. Livin expression was not associated with radiotherapy or
chemotherapy, and radiotherapy or chemotherapy were not associated
with an improved survival (P>0.05; data not shown). Patients
with a low Livin expression exhibited a longer median overall
survival time compared with patients with high expression (2.9
months in the low-expression group vs. 1.9 months in the
high-expression group). However, there was no significant
difference in overall survival when analyzed with Kaplan-Meier
curves and a log-rank test (P=0.12; Fig.
6).
Discussion
ATC is among the most serious malignant tumors for
which no effective treatment currently exists, and multimodal
treatment is required (6). Owing to
its rarity, most studies on ATC therapy are retrospective (3–5).
Therefore, it is difficult to identify the most efficacious
treatment regimen for ATC. Several studies have demonstrated that
longer survival can be expected in stage IVA and IVB patients, who
are eligible for total tumor resection with adjuvant
radiotherapy/concurrent chemoradiotherapy (30,31).
However, the majority of ATCs exhibit advanced disease progression
with regional or systemic metastasis at diagnosis, for which it may
be impossible to perform curative surgery. In patients with
non-resected ATC, radiation therapy or chemotherapy could be
considered. Pezzi et al (32)
reported improved survival outcome in non-resected ATC patients
with radiation therapy with a cumulative dose of >45 Gy.
Although conventional chemotherapy involving paclitaxel, docetaxel
or doxorubicin is employed, the benefit on survival is only
marginal, and the duration of the response is short (33,34).
Recently, various tyrosine kinase inhibitors have been approved for
cancer treatment. Lenvatinib is a multikinase inhibitor that
targets the vascular endothelial growth factor receptor 1–3,
fibroblast growth factor receptor 1–4, platelet-derived growth
factor receptor-α, and RET and KIT proto-oncogenes (35). Lenvatinib was approved for patients
with radioiodine-refractory differentiated thyroid cancer, based on
the results of the SELECT trial, which demonstrated marked
improvement in progression-free survival and response rate
(36). Although the efficacy of
lenvatinib in ATC is limited, several studies have shown that it
can be employed as a possible treatment for ATC. In preclinical
human thyroid cancer xenograft models, lenvatinib exhibited
significant antitumor activity in five ATC xenografts (35). Several case reports demonstrated its
possible efficacy as a therapeutic agent for ATC (37,38). A
phase II trial of lenvatinib in 17 ATC patients demonstrated a
median progression-free survival of 7.4 months and a median overall
survival of 10.6 months with manageable toxicities (39). The objective response rate was 24% and
the disease control rate was 94% in the 17 cases. Therefore, the
efficacy of lenvatinib in ATC cells was evaluated in the present
study.
Apoptosis involves the sensor phase, wherein cell
death signals are sensed by monitoring the extra- and intracellular
environment, and the effector phase, wherein cell death is caused
via mitochondrial apoptosis signaling pathways, which involve the
inhibition of downstream caspases (caspase-3, −7 and −9), leading
to their inactivation and degradation (7). Resistance to apoptosis can be acquired
by cancer cells through a variety of strategies. The IAP group
includes structurally related proteins with antiapoptotic potential
associated with tumorigenesis and tumor resistance to chemotherapy
and radiotherapy (40). IAP family
members include NAIP, c-IAP1, c-IAP2, XIAP, survivin, Apollon,
ILP-2 and Livin (40). Livin, a newly
discovered IAP (41), is not
expressed in most normal adult tissues but is expressed in several
cancer cell lines (17,18). Similar to other IAP family members,
Livin shows an antiapoptotic activity depending on the BIR domain
(41). Livin is essential for tumor
progression and poor prognosis in several tumors as it regulates
the tumor cell sensitivity to chemotherapy and radiotherapy
(9,13,14,42–46).
Therefore, inactivating Livin may help induce apoptosis and can be
considered a treatment modality for certain cancers.
To the best of our knowledge, no previous studies
have elucidated the role of Livin in ATC. In the present study,
decreased tumor cell invasion and increased apoptosis in
Livin-knockdown BHT101 cells were demonstrated. Apoptosis induced
by chemotherapy (lenvatinib) and radiotherapy was enhanced in
Livin-knockdown BHT101 cells. Furthermore, Livin-knockdown cells
demonstrated significantly increased expression of cleaved
caspase-3, cleaved caspase-7, and cleaved PARP following
radiotherapy. Lenvatinib is a potent angiogenesis inhibitor that
targets multiple receptor tyrosine kinases, including VEGF
receptors (35). Targeting tumor
angiogenesis can induce nutrient starvation and hypoxia, leading to
apoptosis in tumor cells (47). The
loss of the effect of suppressing apoptosis during Livin knockdown,
appears to strengthen the antitumor effect of lenvatinib and
radiotherapy. The obtained results implied that Livin plays a
pivotal role in ATC progression and resistance to chemotherapy and
radiotherapy.
Several studies have shown that Livin is associated
with the aggressive metastasis and prognosis of variable cancers
(17,19–22);
however, to the best of our knowledge, no studies have been
conducted on ATC patients. It is difficult to analyze ATC patients
because of the low incidence and short median survival rate. A
total of 10,960 patients underwent thyroid surgery between 2005 and
2015 at Chonnam National University Hwasun Hospital (Jeonnam,
Korea), of whom only 23 were ATC patients. For those 23 patients,
the median survival was only 2.9 months. There was no significant
difference in clinical aggressiveness owing to the small number of
patients and short survival time; the only differences noted were
histological aggressiveness, such as Ki-67 labeling index and
apoptotic index, between the high- and low-Livin expression groups.
Further studies with a higher number of patients are needed to
confirm the differences in clinical characteristics according to
Livin expression.
Several studies have identified Livin as a possible
diagnostic marker and therapeutic tool for malignant tumors
(48–61). El Ali et al (48) reported significant differences in the
levels of serum anti-Livin antibodies between patients with
gastrointestinal cancer and healthy subjects (48). Several studies have also suggested
that anti-Livin antibody may be a useful diagnostic marker for a
variety of tumors, including those of the gastrointestinal tract,
lung and breast (49–51); this also indicates that Livin may be a
tumor-associated antigen. Therefore, Livin can serve as a novel
immunotherapy target. Zhang et al (52) reported that Livin peptide could be
used as a novel substitute to trigger cell immunity by loading
dendritic cells in combination with chemotherapeutic agents in
cases of non-small cell lung cancer. In addition to its potential
as a direct immunotherapy agent, prognosis of ATC can be improved
by increasing chemosensitivity of ATC cells. The inhibition of
apoptosis is one mechanism of chemoresistance because several
chemotherapy drugs induce apoptosis (8). As a member of the IAP family, Livin has
been shown to cause chemoresistance in various cancers (53–57).
Furthermore, several studies have shown that Livin knockdown
increases chemosensitivity to multiple anticancer drugs, suggesting
its potential as an adjuvant therapeutic agent (58–61).
In conclusion, the present study demonstrated that
Livin is positively expressed in ATC and contributes to tumor
progression and chemoradioresistance in ATC. However, the current
study had some limitations, such as that it was produced using one
ATC cell line and that the number of enrolled patients was small.
Therefore, further studies including animal studies are needed to
support the present results. Although, in summary, the present
study suggests the possibility of Livin as a therapeutic target in
ATC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Chonnam
National University Hwasun Hospital Institute for Biomedical
Science (Hwasun, South Korea; grant no. HCRI 20044) and the
National Research Foundation of Korea grant funded by the Korea
government (Daejeon, South Korea; grant no. 2020R1F1A1076054).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
HKK and TMY analyzed the data and drafted the
manuscript. SAK performed the experimental study. KHL and TMY
analyzed the pathological data. TMY and YEJ participated in the
design of the study. EKJ, JKL, HCK and SCL contributed to the
interpretation of the data. KHL, JKL and SAK revised the manuscript
and data including western blotting at the request of reviewers.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Institutional Review Board of Chonnam National
University Hwasun Hospital (Hwasun, South Korea; approval no.
CNUHH-2020-042). Patients provided written informed consent for the
use of resected tissue specimens.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davies L and Welch HG: Current thyroid
cancer trends in the United States. JAMA Otolaryngol Head Neck
Surg. 140:317–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sherman SI: Thyroid carcinoma. Lancet.
361:501–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smallridge RC and Copland JA: Anaplastic
thyroid carcinoma: Pathogenesis and emerging therapies. Clin Oncol
(R Coll Radiol). 22:486–497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim TY, Kim KW, Jung TS, Kim JM, Kim SW,
Chung KW, Kim EY, Gong G, Oh YL, Cho SY, et al: Prognostic factors
for Korean patients with anaplastic thyroid carcinoma. Head Neck.
29:765–772. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pierie JP, Muzikansky A, Gaz RD, Faquin WC
and Ott MJ: The effect of surgery and radiotherapy on outcome of
anaplastic thyroid carcinoma. Ann Surg Oncol. 9:57–64. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smallridge RC, Ain KB, Asa SL, Bible KC,
Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal
MS, et al American Thyroid Association Anaplastic Thyroid Cancer
Guidelines Taskforce, : American Thyroid Association guidelines for
management of patients with anaplastic thyroid cancer. Thyroid.
22:1104–1139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Igney FH and Krammer PH: Death and
anti-death: Tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu B, Han M, Wen JK and Wang L:
Livin/ML-IAP as a new target for cancer treatment. Cancer Lett.
250:168–176. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sanna MG, da Silva Correia J, Ducrey O,
Lee J, Nomoto K, Schrantz N, Deveraux QL and Ulevitch RJ: IAP
suppression of apoptosis involves distinct mechanisms: The
TAK1/JNK1 signaling cascade and caspase inhibition. Mol Cell Biol.
22:1754–1766. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Allen SM, Florell SR, Hanks AN, Alexander
A, Diedrich MJ, Altieri DC and Grossman D: Survivin expression in
mouse skin prevents papilloma regression and promotes
chemical-induced tumor progression. Cancer Res. 63:567–572.
2003.PubMed/NCBI
|
|
12
|
Wang L, Zhang Q, Liu B, Han M and Shan B:
Challenge and promise: Roles for Livin in progression and therapy
of cancer. Mol Cancer Ther. 7:3661–3669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang H and Schimmer AD: Livin/melanoma
inhibitor of apoptosis protein as a potential therapeutic target
for the treatment of malignancy. Mol Cancer Ther. 6:24–30. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan B: Research progress on Livin protein:
An inhibitor of apoptosis. Mol Cell Biochem. 357:39–45. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun JG, Liao RX, Zhang SX, Duan YZ, Zhuo
WL, Wang XX, Wang ZX, Li DZ and Chen ZT: Role of inhibitor of
apoptosis protein Livin in radiation resistance in nonsmall cell
lung cancer. Cancer Biother Radiopharm. 26:585–592. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoon TM, Kim SA, Lee DH, Lee JK, Park YL,
Lee KH, Chung IJ, Joo YE and Lim SC: Livin enhances chemoresistance
in head and neck squamous cell carcinoma. Oncol Rep. 37:3667–3673.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanabe H, Yagihashi A, Tsuji N, Shijubo Y,
Abe S and Watanabe N: Expression of survivin mRNA and livin mRNA in
non-small-cell lung cancer. Lung Cancer. 46:299–304. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Augello C, Caruso L, Maggioni M, Donadon
M, Montorsi M, Santambrogio R, Torzilli G, Vaira V, Pellegrini C,
Roncalli M, et al: Inhibitors of apoptosis proteins (IAPs)
expression and their prognostic significance in hepatocellular
carcinoma. BMC Cancer. 9:1252009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gazzaniga P, Gradilone A, Giuliani L,
Gandini O, Silvestri I, Nofroni I, Saccani G, Frati L and Aglianò
AM: Expression and prognostic significance of LIVIN, SURVIVIN and
other apoptosis-related genes in the progression of superficial
bladder cancer. Ann Oncol. 14:85–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li CJ, Cong Y, Liu XZ, Zhou X, Shi X, Wu
SJ, Zhou GX and Lu M: Research progress on the livin gene and
osteosarcomas. Asian Pac J Cancer Prev. 15:8577–8579. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiang Y, Yao H, Wang S, Hong M, He J, Cao
S, Min H, Song E and Guo X: Prognostic value of Survivin and Livin
in nasopharyngeal carcinoma. Laryngoscope. 116:126–130. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun K, Liao Q, Chen Z, Chen T and Zhang J:
Expression of Livin and PlGF in human osteosarcoma is associated
with tumor progression and clinical outcome. Oncol Lett.
16:4953–4960. 2018.PubMed/NCBI
|
|
23
|
Wang R, Lin F, Wang X, Gao P, Dong K, Zou
AM, Cheng SY, Wei SH and Zhang HZ: Silencing Livin gene expression
to inhibit proliferation and enhance chemosensitivity in tumor
cells. Cancer Gene Ther. 15:402–412. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu S, Li X, Li Q, Liu H, Shi Y, Zhuo H,
Li C and Zhu H: Silencing Livin improved the sensitivity of colon
cancer cells to 5-fluorouracil by regulating crosstalk between
apoptosis and autophagy. Oncol Lett. 15:7707–7715. 2018.PubMed/NCBI
|
|
25
|
Miura K, Fujibuchi W, Ishida K, Naitoh T,
Ogawa H, Ando T, Yazaki N, Watanabe K, Haneda S, Shibata C, et al:
Inhibitor of apoptosis protein family as diagnostic markers and
therapeutic targets of colorectal cancer. Surg Today. 41:175–182.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jing C, Gao Z, Wang R, Yang Z, Shi B and
Hou P: Lenvatinib enhances the antitumor effects of paclitaxel in
anaplastic thyroid cancer. Am J Cancer Res. 7:903–912.
2017.PubMed/NCBI
|
|
28
|
Ogino H, Hanibuchi M, Kakiuchi S, Trung
VT, Goto H, Ikuta K, Yamada T, Uehara H, Tsuruoka A, Uenaka T, et
al: E7080 suppresses hematogenous multiple organ metastases of lung
cancer cells with nonmutated epidermal growth factor receptor. Mol
Cancer Ther. 10:1218–1228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoon TM, Kim SA, Park YL, Lee KH, Sung MW,
Lee JK, Lim SC, Chung IJ and Joo YE: Expression of the receptor
tyrosine kinase recepteur d'origine nantais and its association
with tumor progression in hypopharyngeal cancer. Head Neck.
35:1106–1113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goffredo P, Thomas SM, Adam MA, Sosa JA
and Roman SA: Impact of timeliness of resection and thyroidectomy
margin status on survival for patients with anaplastic thyroid
cancer: An analysis of 335 cases. Ann Surg Oncol. 22:4166–4174.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baek SK, Lee MC, Hah JH, Ahn SH, Son YI,
Rho YS, Chung PS, Lee YS, Koo BS, Jung KY, et al: Role of surgery
in the management of anaplastic thyroid carcinoma: Korean
nationwide multicenter study of 329 patients with anaplastic
thyroid carcinoma, 2000 to 2012. Head Neck. 39:133–139. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pezzi TA, Mohamed AS, Sheu T, Blanchard P,
Sandulache VC, Lai SY, Cabanillas ME, Williams MD, Pezzi CM, Lu C,
et al: Radiation therapy dose is associated with improved survival
for unresected anaplastic thyroid carcinoma: Outcomes from the
National Cancer Data Base. Cancer. 123:1653–1661. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haymart MR, Banerjee M, Yin H, Worden F
and Griggs JJ: Marginal treatment benefit in anaplastic thyroid
cancer. Cancer. 119:3133–3139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tiedje V, Stuschke M, Weber F, Dralle H,
Moss L and Führer D: Anaplastic thyroid carcinoma: Review of
treatment protocols. Endocr Relat Cancer. 25:R153–R161. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res. 2014:6387472014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schlumberger M, Tahara M, Wirth LJ,
Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff
AO, et al: Lenvatinib versus placebo in radioiodine-refractory
thyroid cancer. N Engl J Med. 372:621–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oishi K, Takabatake D and Shibuya Y:
Efficacy of lenvatinib in a patient with anaplastic thyroid cancer.
Endocrinol Diabetes Metab Case Rep. 2017:16–0136. 2017.PubMed/NCBI
|
|
38
|
Koyama S, Miyake N, Fujiwara K, Morisaki
T, Fukuhara T, Kitano H and Takeuchi H: Lenvatinib for anaplastic
thyroid cancer and lenvatinib-induced thyroid dysfunction. Eur
Thyroid J. 7:139–144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tahara M, Kiyota N, Yamazaki T, Chayahara
N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Y, et
al: Lenvatinib for Anaplastic Thyroid Cancer. Front Oncol.
7:252017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deveraux QL and Reed JC: IAP family
proteins - suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kasof GM and Gomes BC: Livin, a novel
inhibitor of apoptosis protein family member. J Biol Chem.
276:3238–3246. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chung CY, Park YL, Kim N, Park HC, Park
HB, Myung DS, Kim JS, Cho SB, Lee WS and Joo YE: Expression and
prognostic significance of Livin in gastric cancer. Oncol Rep.
30:2520–2528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li F, Yin X, Luo X, Li HY, Su X, Wang XY,
Chen L, Zheng K and Ren GS: Livin promotes progression of breast
cancer through induction of epithelial-mesenchymal transition and
activation of AKT signaling. Cell Signal. 25:1413–1422. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin X, Li HR, Lin XF, Yu ME, Tu XW, Hua
ZD, Lin M, Xu NL, Han LL and Chen YS: Silencing of Livin inhibits
tumorigenesis and metastasis via VEGF and MMPs pathway in lung
cancer. Int J Oncol. 47:657–667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xi RC, Sheng YR, Chen WH, Sheng L, Gang
JJ, Tong Z, Shan Z, Ying GH, Dong LC and Chen YS: Expression of
survivin and livin predicts early recurrence in non-muscle invasive
bladder cancer. J Surg Oncol. 107:550–554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen YS, Li HR, Lin M, Chen G, Xie BS, Xu
NL and Lin LF: Livin abrogates apoptosis of SPC-A1 cell by
regulating JNKI signaling pathway. Mol Biol Rep. 37:2241–2247.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hoshi T, Watanabe Miyano S, Watanabe H,
Sonobe RM, Seki Y, Ohta E, Nomoto K, Matsui J and Funahashi Y:
Lenvatinib induces death of human hepatocellular carcinoma cells
harboring an activated FGF signaling pathway through inhibition of
FGFR-MAPK cascades. Biochem Biophys Res Commun. 513:1–7. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
El Ali Z, Grzymisławski M, Majewski P,
Baumann-Antczak A and Kosowicz J: Anti-livin antibodies: Novel
markers of malignant gastrointestinal cancers. Pol Arch Med Wewn.
120:26–29. 2010.PubMed/NCBI
|
|
49
|
Yagihashi A, Asanuma K, Tsuji N, Torigoe
T, Sato N, Hirata K and Watanabe N: Detection of anti-livin
antibody in gastrointestinal cancer patients. Clin Chem.
49:1206–1208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yagihashi A, Asanuma K, Kobayashi D, Tsuji
N, Shijubo Y, Abe S, Hirohashi Y, Torigoe T, Sato N and Watanabe N:
Detection of autoantibodies to livin and survivin in Sera from lung
cancer patients. Lung Cancer. 48:217–221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yagihashi A, Ohmura T, Asanuma K,
Kobayashi D, Tsuji N, Torigoe T, Sato N, Hirata K and Watanabe N:
Detection of autoantibodies to survivin and livin in sera from
patients with breast cancer. Clin Chim Acta. 362:125–130. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang L, Xu Z, Chen X, Duan Y, Chen Z and
Sun J: Clinical benefits of Livin peptide-loaded DCs/CIKs combined
with chemotherapy in advanced non-small cell lung cancer. Am J
Cancer Res. 9:406–414. 2019.PubMed/NCBI
|
|
53
|
Yin L, Liu S, Li C, Ding S, Bi D, Niu Z,
Han L, Li W, Gao D, Liu Z, et al: CYLD downregulates Livin and
synergistically improves gemcitabine chemosensitivity and decreases
migratory/invasive potential in bladder cancer: The effect is
autophagy-associated. Tumour Biol. 37:12731–12742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang Z, Liu S, Ding K, Ding S, Li C, Lu J,
Gao D, Zhang T and Bi D: Silencing Livin induces apoptotic and
autophagic cell death, increasing chemotherapeutic sensitivity to
cisplatin of renal carcinoma cells. Tumour Biol. 37:15133–15143.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu Y, Guo Q, Zhang H, Li GH, Feng S, Yu
XZ, Kong LS, Zhao L and Jin F: Effect of siRNA-Livin on drug
resistance to chemotherapy in glioma U251 cells and
CD133+ stem cells. Exp Ther Med. 10:1317–1323. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhuang L, Shen LD, Li K, Yang RX, Zhang
QY, Chen Y, Gao CL, Dong C, Bi Q, Tao JN, et al: Inhibition of
livin expression suppresses cell proliferation and enhances
chemosensitivity to cisplatin in human lung adenocarcinoma cells.
Mol Med Rep. 12:547–552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zou AM, Wang HF, Zhu WF, Wang FX and Shen
JJ: Effect of RNAi-mediated silencing of Livin gene on biological
properties of colon cancer cell line LoVo. Genet Mol Res.
13:3832–3841. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang TS, Ding QQ, Guo RH, Shen H, Sun J,
Lu KH, You SH, Ge HM, Shu YQ and Liu P: Expression of Livin in
gastric cancer and induction of apoptosis in SGC-7901 cells by
shRNA-mediated silencing of Livin gene. Biomed Pharmacother.
64:333–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang X, Xu J, Ju S, Ni H, Zhu J and Wang
H: Livin gene plays a role in drug resistance of colon cancer
cells. Clin Biochem. 43:655–660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Oh BY, Kim KH, Chung SS and Lee RA:
Silencing the livin gene enhances the cytotoxic effects of
anticancer drugs on colon cancer cells. Ann Surg Treat Res.
91:273–277. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yuan D, Liu L, Xu H and Gu D: The effects
on cell growth and chemosensitivity by livin RNAi in non-small cell
lung cancer. Mol Cell Biochem. 320:133–140. 2009. View Article : Google Scholar : PubMed/NCBI
|