Introduction
Esophageal cancer (EC) is the eighth most common
cancer and the sixth most common cause of cancer-related deaths
worldwide (1,2). Radical surgery is the preferred
treatment for EC, however 80% of patients with EC are unable to
undergo radical surgery by the time of diagnosis (3). Therefore, radiotherapy or chemotherapy
maintenance is the main treatment for patients with advanced EC
(4). Despite the rapid and ongoing
development of chemotherapeutic drug development and multimodal
therapies that have facilitated treatment for patients with EC, the
prognoses and outcomes typically remain unsatisfactory. High
incidences of chemotherapeutic drug resistance have become one of
the important causes of failure in the treatment of EC (5). Therefore, it is urgent and necessary to
develop innovative treatment strategies for EC.
5-Fluorouracil (5-FU) is one of the most common
chemotherapeutic drugs used in the treatment of EC (6). 5-FU inhibits thymidylate synthase and
interferes with DNA synthesis through its metabolite FdUMP. In
addition, its metabolite FdUTP can affect cell metabolism in
numerous manners, such as transcription, translation and
post-translation modification through the incorrect addition into
the RNA (7). 5-FU is characterized by
a narrow therapeutic index due to its toxicity to normal cells, and
cancer cells are easily resistant to 5-FU. These two factors are
the main obstacles to the clinical application of 5-FU (8). Hence, there is an urgent need to
discover other drug reagents to be used in combination with 5-FU to
improve the sensitivity of cancer cells to 5-FU and reduce its
toxicity to normal cells.
Melatonin (MLT) is an amine hormone produced in the
conarium of mammals and humans (9).
In recent years, studies have revealed that MLT has a number of
physiological functions, such as promoting sleep, regulating jet
lag, anti-aging effects, regulating immunity and antitumor effects
(10–13). Further biological function studies
have revealed that MLT can regulate the proliferation, cycle and
apoptosis of cancer cells (14–16). MLT
increases the efficacy of chemotherapeutic drugs by increasing its
sensitivity of chemotherapy drugs, reducing the probability of drug
resistance, and reducing the toxicity to normal cells (17–20).
However, the mechanisms and dynamics of MLT and its effects on the
chemotherapeutic effect of 5-FU in EC have rarely been examined and
reported.
Methyl-transferase EZH2 is a component of polycomb
repressive complex 2. Accumulating studies have indicated that EZH2
is upregulated in several types of human cancers (21). Our previous research also revealed
that EZH2 was upregulated in samples afflicted by EC and EZH2 was
confirmed as an oncogene in esophageal cancer via biological
experiments (22). Recent studies
have revealed that EZH2 promoted chemotherapeutic drug resistance
in numerous cancers (23,24). In addition, previous studies also
revealed that melatonin inhibited the tumorigenicity of
glioblastoma stem-like cells via the EZH2 signaling axis (25,26).
Hence, it was hypothesized that MLT and 5-FU combination inhibits
cell proliferation and promotes apoptosis by regulating the
expression of EZH2.
The present study sought to examine whether or not
MLT affects the chemotherapeutic effects of 5-FU, namely via its
ability to increase the sensitivity of EC cells to 5-FU and its
subsequent downstream effects. It was hypothesized that MLT may
play a role in sensitizing or synergizing EC cellular response to
5-FU treatment. EC cells were treated with MLT and 5-FU alone or in
combination and the effects of MLT and 5-FU combination on cell
proliferation, migration, invasion and apoptosis in EC-9706 and
EC-109 cells were analyzed. The present study also observed the
effects of MLT and 5-FU co-treatment on EZH2 expression to
determine the potential molecular mechanisms.
Materials and methods
Cell culture and transfection
Human EC cell lines (EC-9706 and EC-109) and an
immortalized normal esophagus epithelial cell line (HET-1A) were
obtained from The Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Cell were cultured in RPMI-1640 medium
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 100 U/ml of penicillin and streptomycin (Gibco; Thermo Fisher
Scientific, Inc.). Cells were incubated in a humidified atmosphere
at 37°C and 5% CO2. Cells were transfected with the
following small interfering RNAs (siRNAs; transfection
concentration 2 µg/ml): EZH2-specific si-RNA (si-EZH2-1,
5′-AAGACTCTGAATGCAGTTGCT-3′; si-EZH2-2,
5′-GGAUGGUACUUUCAUUGAATT-3′), siRNA negative control (si-NC,
5′-UUCUCCGAACGUGUCACGUTT-3′) (all from Shanghai GenePharma Co.,
Ltd.). si-EZH2-1 (hereafter referred to as si-EZH2) was used for
further experiments. The pcDNA3.1 empty vector (pcDNA-NC) and
pcDNA3.1-EZH2 overexpression vector (pcDNA-EZH2; transfection
concentration 2 µg/ml) were designed and produced by Shanghai
GeneChem Co., Ltd.
Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to transfect cells with si-RNA
and pcDNA-EZH2 according to the manufacturer's protocols. Cells
were incubated at 37°C and 5% CO2, for 48 h for RT-qPCR
and 72 h for western blotting. All transfection experiments were
repeated three times. The transfection efficiency was confirmed by
reverse transcription-quantitative PCR (RT-qPCR) analysis.
EZH2 expression in EC from the cancer
genome atlas (TCGA) database
EZH2 expression in EC was extracted from the public
database starBase 3.0 online tool (http://starbase.sysu.edu.cn/) (27).
RNA extraction and reverse
transcription RT-qPCR
TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from cells.
Subsequently, samples were treated with DNase I and total RNA was
quantified using a NanoDrop 2000. Samples were reverse transcribed
into cDNA. A total of 20 µl qPCR reaction mixture was run on an ABI
7500 Fast RT-qPCR system for qPCR. GAPDH was used as the internal
control. The following primer pairs were used for the qPCR: EZH2
forward, 5′-AGGACGGCTCCTCTAACCAT-3′ and reverse,
5′-CTTGGTGTTGCACTGTGCTT-3′; and GAPDH forward,
5′-GGGAGCCAAAAGGGTCAT-3′ and reverse, 5′-GAGTCCTTCCACGATACCAA-3′.
Relative expression levels were calculated using the
2−ΔΔCq method (28).
Detailed steps were previously described in a published study
(29).
Cell proliferation assay
Cell proliferation assays were performed using Cell
Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology)
reagent. Briefly, 5×103 cells were seeded into 96-well
plates with 100 µl of medium. Cells were incubated overnight at
37°C. Upon reaching 70–80% confluence, fresh medium containing 0,
0.125, 0.25, 0.5, 1 and 2 mM melatonin (Sigma-Aldrich; Merck KGaA)
or 0, 10, 20, 40, 60 and 80 mM 5-FU (Sigma-Aldrich; Merck KGaA)
were added. The cells were further incubated for 2 h with 10 µl of
CCK-8 solution per well and for another 24, 48, 72 and 96 h. The
absorbance (optical density) was measured at a wavelength of 450 nm
using a Tecan microplate reader (Tecan Group, Ltd.).
Cell migration and invasion assay
Cell migration and invasion were assessed using
Transwell assays. EC-9706 cells were treated with 1 mM melatonin
and 40 mM 5-FU and EC-109 cells was treated with 1 mM melatonin and
60 mM 5-FU, then harvested and resuspended in serum-free medium
(5×105 cells/ml). For the Transwell migration assays,
5×104 cells were seeded in the upper chamber. For the
invasion assays, Transwell inserts were pre-coated with Matrigel
solution and polymerized in the upper chamber. The upper chamber
was filled with FBS-free medium and the lower chamber was filled
with 600 µl medium with 10% FBS. Cells were incubated for 24 at
37°C. Cells that had migrated or invaded to the lower surface of
the membrane were fixed for 20 min with 4% (v/v) paraformaldehyde,
stained with 0.1% crystal violet (Sigma-Aldrich; Merck KGaA) for 20
min, and counted from five independent visual fields by an inverted
microscope (magnification, ×100; Olympus Corp.).
Cell apoptosis assay
Cell apoptosis assays were performed using an
Annexin V/PI double-staining assay (Sigma-Aldrich; Merck KGaA). In
brief, after EC-9706 cells were treated with 1 mM melatonin and 40
mM 5-FU and EC-109 cells were treated with 1 mM melatonin and 60 mM
5-FU for 24 h, the cells (2×105 cells/well) were
harvested and washed with PBS. Then, the cells were resuspended
with 400 µl 1X binding buffer, followed by the addition of 5 µl
Annexin V-FITC and incubation at room temperature for 15 min and
the addition and incubation of 10 µl PI on an ice bath for 5 min.
Cells were then analyzed using a flow cytometer (Beckman Coulter,
Inc.). Data were analyzed using CytExpert software (version 2.3;
Beckman Coulter, Inc.).
Western blotting
Proteins were extracted from EC cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology) containing
protease K inhibitor. The protein concentration was determined with
a BCA protein assay kit (Beijing Solarbio Life Sciences). Proteins
(30 µg/lane) were separated via 12% SDS-PAGE and transferred onto
PVDF membranes. Subsequently, the membranes were blocked with 5%
skim milk for 2 h at room temperature and washed three times with
PBS. Samples were incubated at 4°C overnight with the following
primary antibodies: Anti-Bcl-2 (1:500; product code ab32124),
anti-Mcl-1 (1:500; product code ab32087), and anti-caspase-3
(1:500; product code ab32351; all from Abcam). Membranes were then
incubated 2 h at room temperature with horseradish
peroxidase-conjugated immunoglobulin G secondary antibody (1:2,000;
product code ab205718, Abcam). GAPDH (1:1,000; product code
ab181602; Abcam) was used as an internal control gene. Protein
bands were visualized using an ECL kit (Beyotime Institute of
Biotechnology). A Chemidoc EQ system (Bio-Rad Laboratories, Inc.)
was used to quantify western blot results. The densitometry of
protein signals was analyzed with Quantity One software (version
4.62; Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analyses were conducted using SPSS 19.0
software (IBM Corp.) and illustrated using GraphPad Prism 5.0
(GraphPad Software, Inc.). Comparisons between two groups were
performed using unpaired Student's t-test, Comparisons of
differences among ≥3 groups were performed using one-way ANOVA
followed by Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
MLT and 5-FU combination enhances the
inhibition of cell proliferation
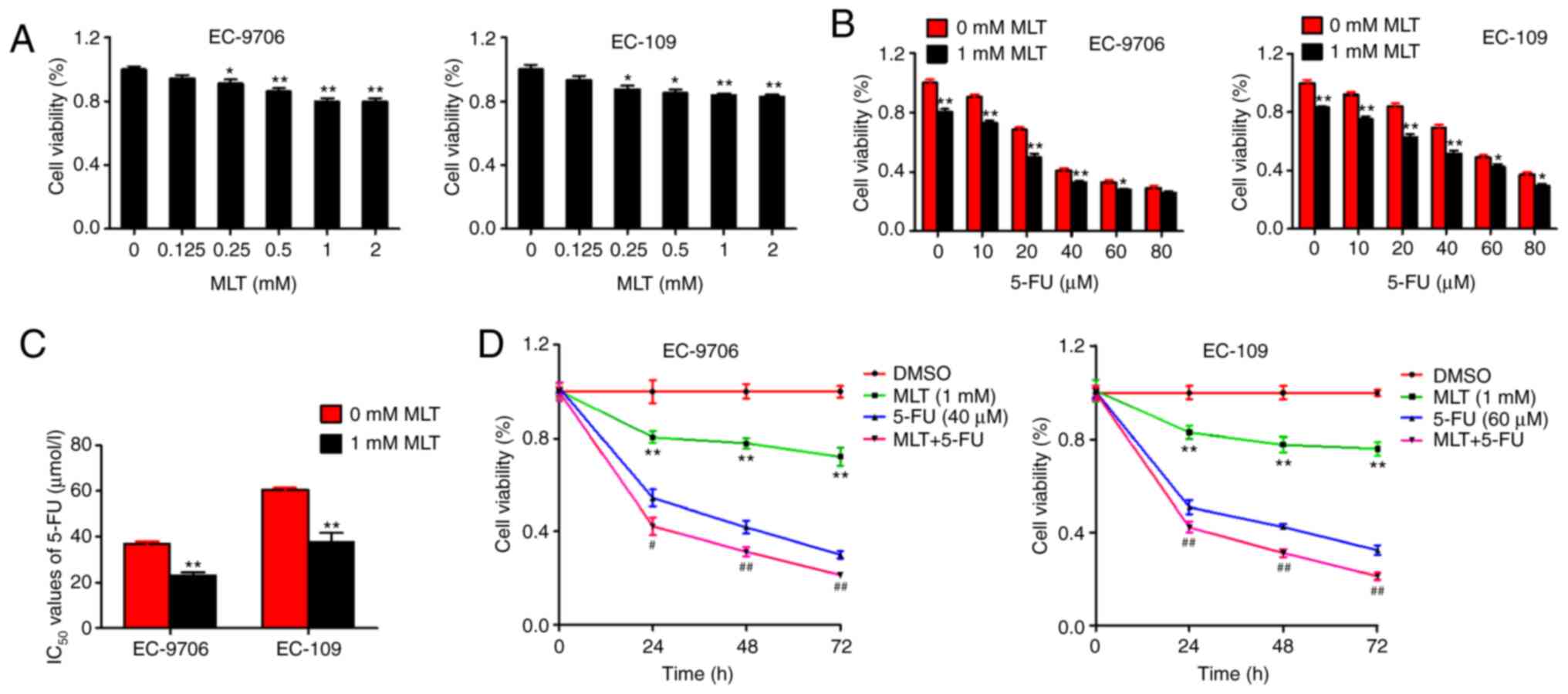
The present study first observed the effects of MLT
on cell proliferation in EC-9706 and EC-109 cells. MLT (0–2 mM)
produced significant inhibition of cell activity in a
dose-dependent manner in EC-9706 and EC-109 cells (Fig. 1A). Subsequently, to determine whether
MLT enhances 5-FU-mediated inhibition of cell proliferation in
EC-9706 and EC-109 cells, cells were treated with various
concentrations of 5-FU alone or in combination with MLT. 5-FU alone
inhibited cell activity in a dose-dependent manner. However,
compared with 5-FU alone, the MLT and 5-FU combination
significantly enhanced 5-FU-mediated inhibition of cell activity in
EC-9706 and EC-109 cells (Fig.
1B).
Next, the present study analyzed the effects of MLT
and 5-FU combination treatment on the IC50 values of
5-FU in EC-9706 and EC-109 cells. Compared with 5-FU alone, the
IC50 values of MLT and 5-FU combination were
significantly decreased (38.14 and 37.76 in EC-9706 and EC-109
cells, respectively) (Fig. 1C). In
addition, the inhibition of MLT and 5-FU on cell activity occurred
in a time-dependent manner either alone or in combination (Fig. 1D). These results suggested that MLT
may enhance the chemotherapeutic effects of 5-FU in EC.
Effects of MLT and 5-FU combination on
cell migration, invasion and apoptosis
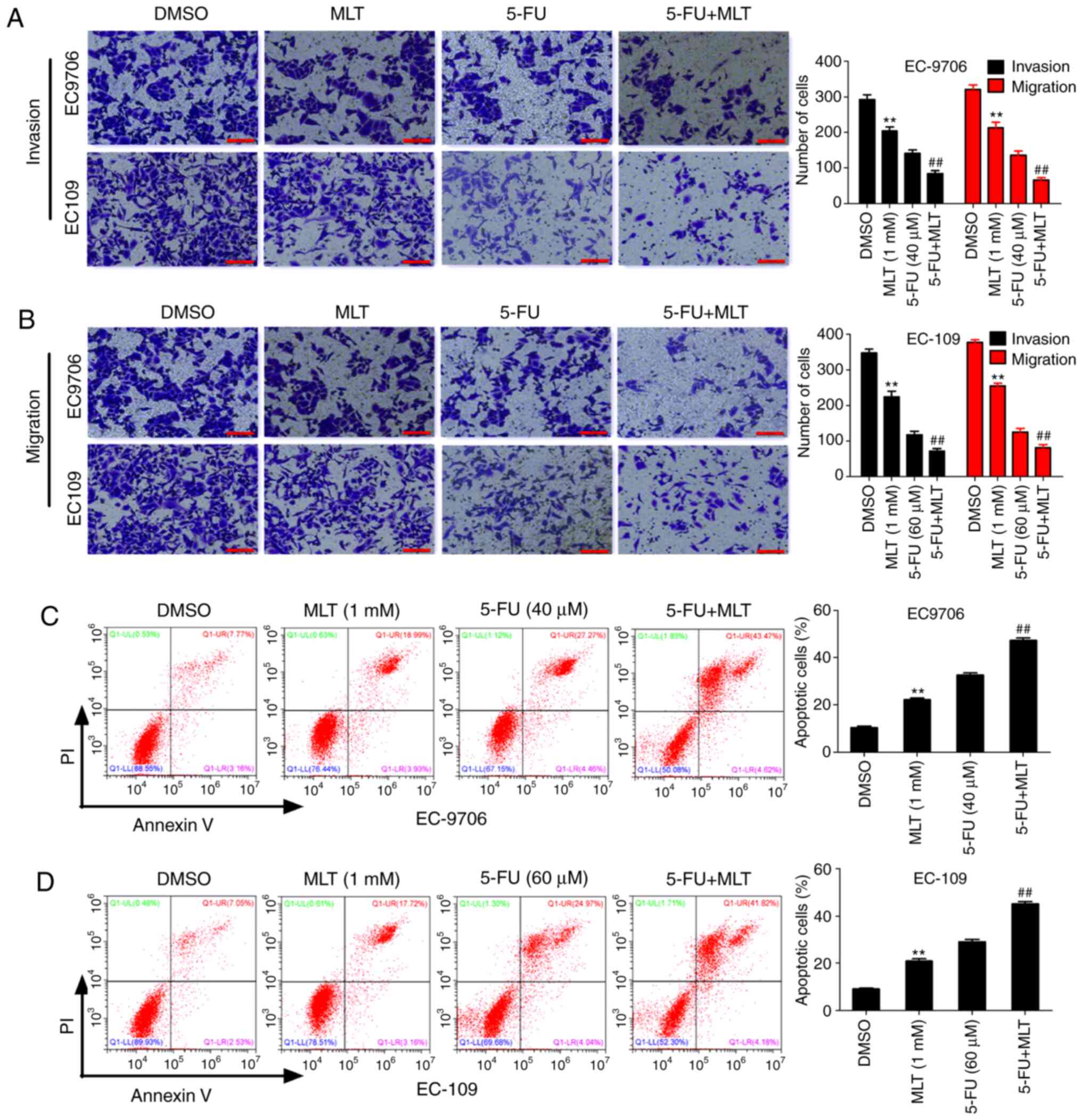
To further investigate the effects of MLT and 5-FU
on the biological function in EC, the present study first evaluated
the effects of MLT and 5-FU on cell invasion and migration using
Transwell assays. The results revealed that MLT treatment
significantly inhibited invasion and migration of EC-9706 and
EC-109 cells compared with the DMSO group. Similarly, compared with
5-FU alone, the MLT and 5-FU combination significantly inhibited
invasion and migration of EC-9706 and EC-109 cells (Fig. 2A and B). These results indicated that
MLT enhanced 5-FU-mediated inhibition of cell invasion and
migration. Subsequently, the effects of MLT and 5-FU on cell
apoptosis were assessed by flow cytometric analysis. The results
revealed that MLT treatment significantly promoted cell apoptosis
of EC-9706 and EC-109 cells compared with the DMSO group.
Similarly, compared with 5-FU alone, the MLT and 5-FU combination
significantly promoted apoptosis of EC-9706 and EC-109 cells
(Fig. 2C and D). These results
suggested that MLT and 5-FU synergistically promoted apoptosis in
EC.
Role of EZH2 in EC
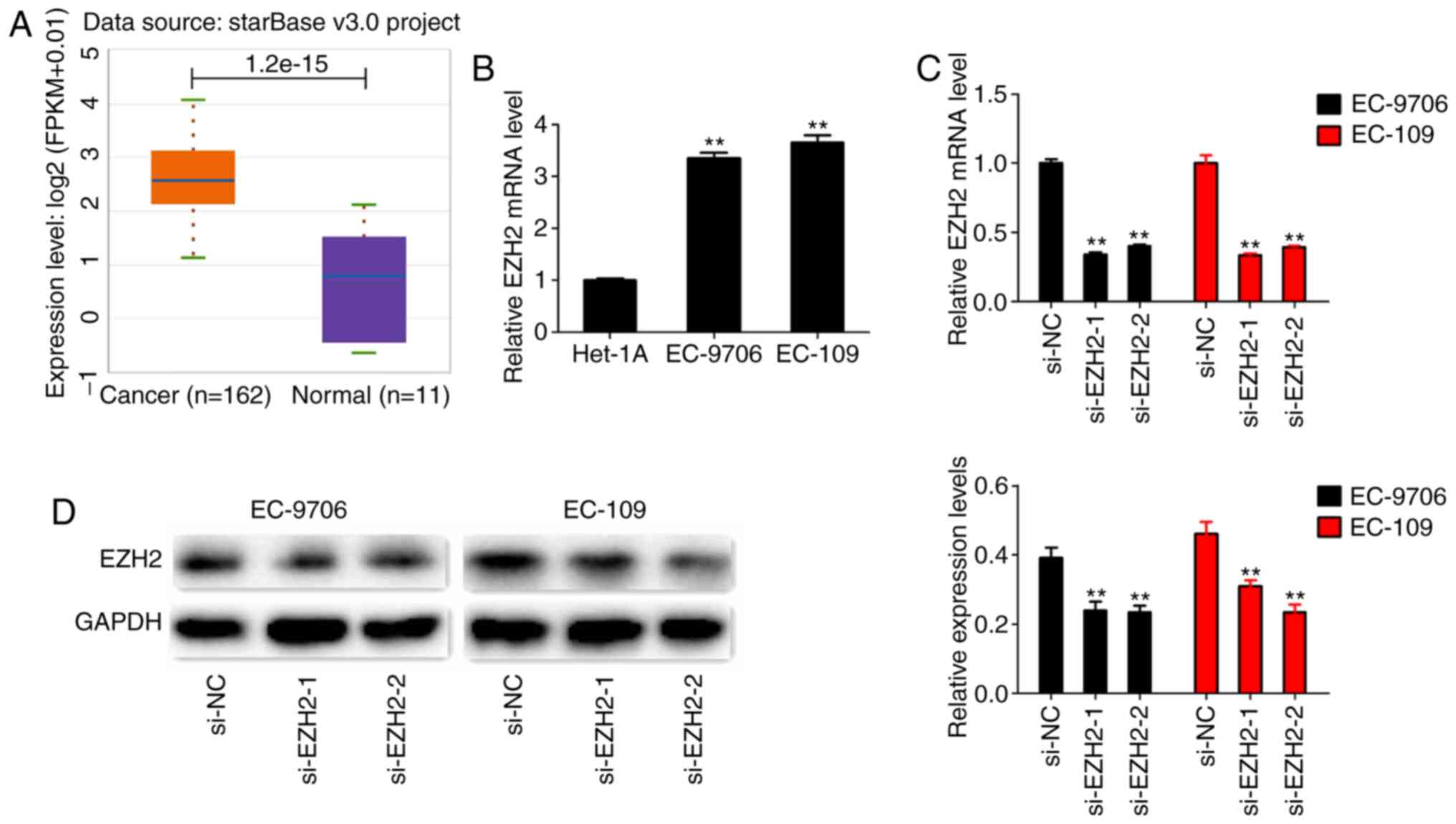
To investigate the expression levels of EZH2 in EC,
the present study first extracted the expressional data of EZH2 in
EC from TCGA database. The results revealed that the levels of EZH2
expression assessed in tumor tissues was higher compared with
normal tissues (Fig. 3A).
Subsequently, the levels of EZH2 expression were assessed in EC
cell lines (EC-9706 and EC-109), and in a normal cell line
(HET-1A). Both EC cell lines exhibited significantly higher levels
of EZH2 expression compared with HET-1A cells (Fig. 3B). To further investigate the roles of
EZH2 in EC, two EZH2 siRNAs were designed to knockdown EZH2.
RT-qPCR and western blotting results revealed that EZH2 expression
was significantly decreased in EC-9706 and EC-109 cells transfected
with si-EZH2 (Fig. 3C and D). Next,
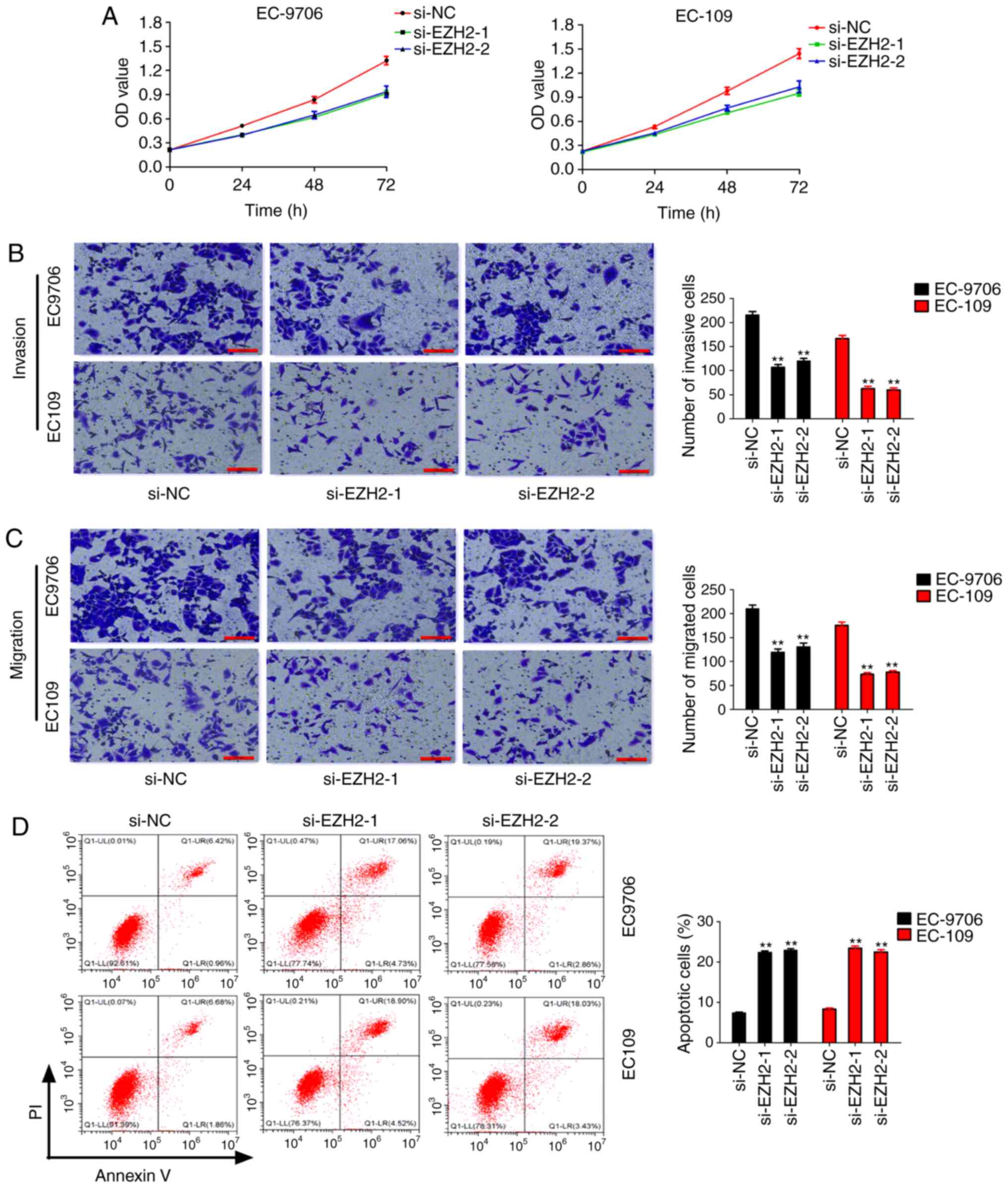
the present study further investigated the effects of silencing
EZH2 expression on the biological function in EC. CCK-8 assays
revealed that EZH2 knockdown significantly inhibited cell
proliferation of EC-9706 and EC-109 cells (Fig. 4A). Transwell assays revealed that EZH2
knockdown significantly inhibited cell invasion and migration of
EC-9706 and EC-109 cells (Fig. 4B and
C). However, silencing of EZH2 expression significantly
promoted apoptosis in EC-9706 and EC-109 cells (Fig. 4D). These results suggested that EZH2
may act as an oncogene in EC.
MLT and 5-FU combination suppresses
EZH2 expression
To further confirm the promotion of apoptosis in EC
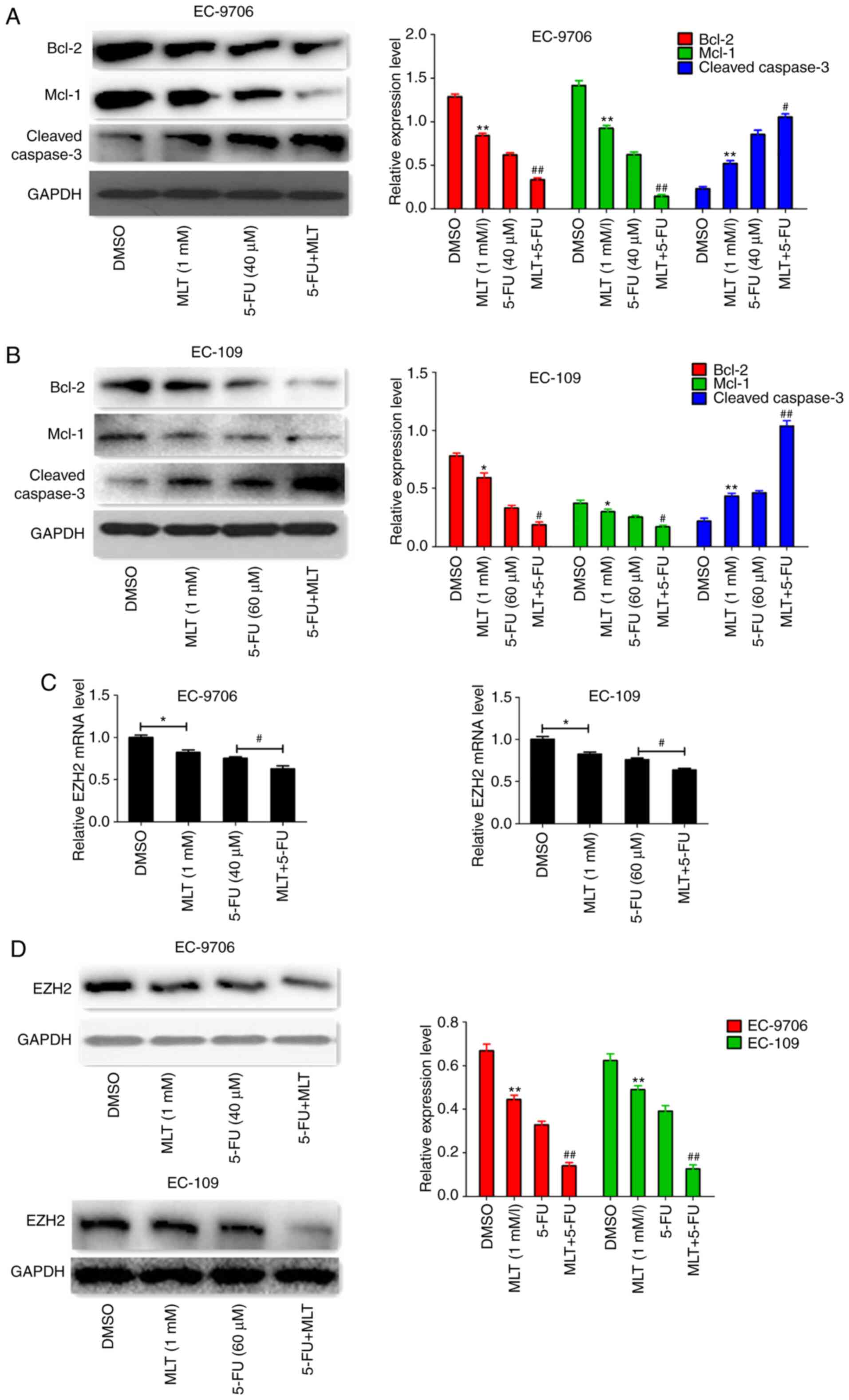
by MLT and 5-FU, protein expression levels were determined using
western blotting. The results indicated that MLT and 5-FU
co-treatment significantly inhibited the protein expression of
Bcl-2 and Mcl-1, but enhanced cleaved caspase-3 expression in
EC-9706 and EC-109 cells (Fig. 5A and
B). Subsequently, the effects of MLT and 5-FU co-treatment on
EZH2 expression were assessed. The results revealed that MLT and
5-FU co-treatment significantly inhibited EZH2 expression at the
mRNA and protein level in EC-9706 and EC-109 cells (Fig. 5C and D).
MLT and 5-FU combination inhibits cell
proliferation and promotes apoptosis by regulating EZH2
expression
The aforementioned results suggested that MLT and
5-FU combination significantly inhibited EZH2 expression. Thus, the
present study hypothesized that MLT and 5-FU combination
contributed to the chemotherapeutic effects of 5-FU by regulating
EZH2 expression. To test this hypothesis, si-EZH2 or pcDNA-EZH2
were co-treated alongside MLT and 5-FU in EC-9706 and EC-109 cells.
The results indicated that co-treatment of MLT, 5-FU and si-EZH2
synergistically inhibited EZH2 expression at the mRNA and protein
level and further inhibited cell proliferation, invasion and
migration, but promoted apoptosis of EC-9706 and EC-109 cells
(Fig. 6A-F). In addition,
co-treatment of MLT, 5-FU and si-EZH2 significantly inhibited the
protein expression of Bcl-2 and Mcl-1, but enhanced the expression
of cleaved caspase-3 (Fig. 6G).
However, EZH2 overexpression significantly enhanced EZH2 expression
at the mRNA and protein level, and reversed the effects of MLT and
5-FU on cell proliferation, invasion, migration, apoptosis and
related protein expression in EC-9706 and EC-109 cells (Fig. 7A-H). These results suggested that MLT
and 5-FU combination inhibited cell proliferation and promoted
apoptosis by regulating EZH2 expression.
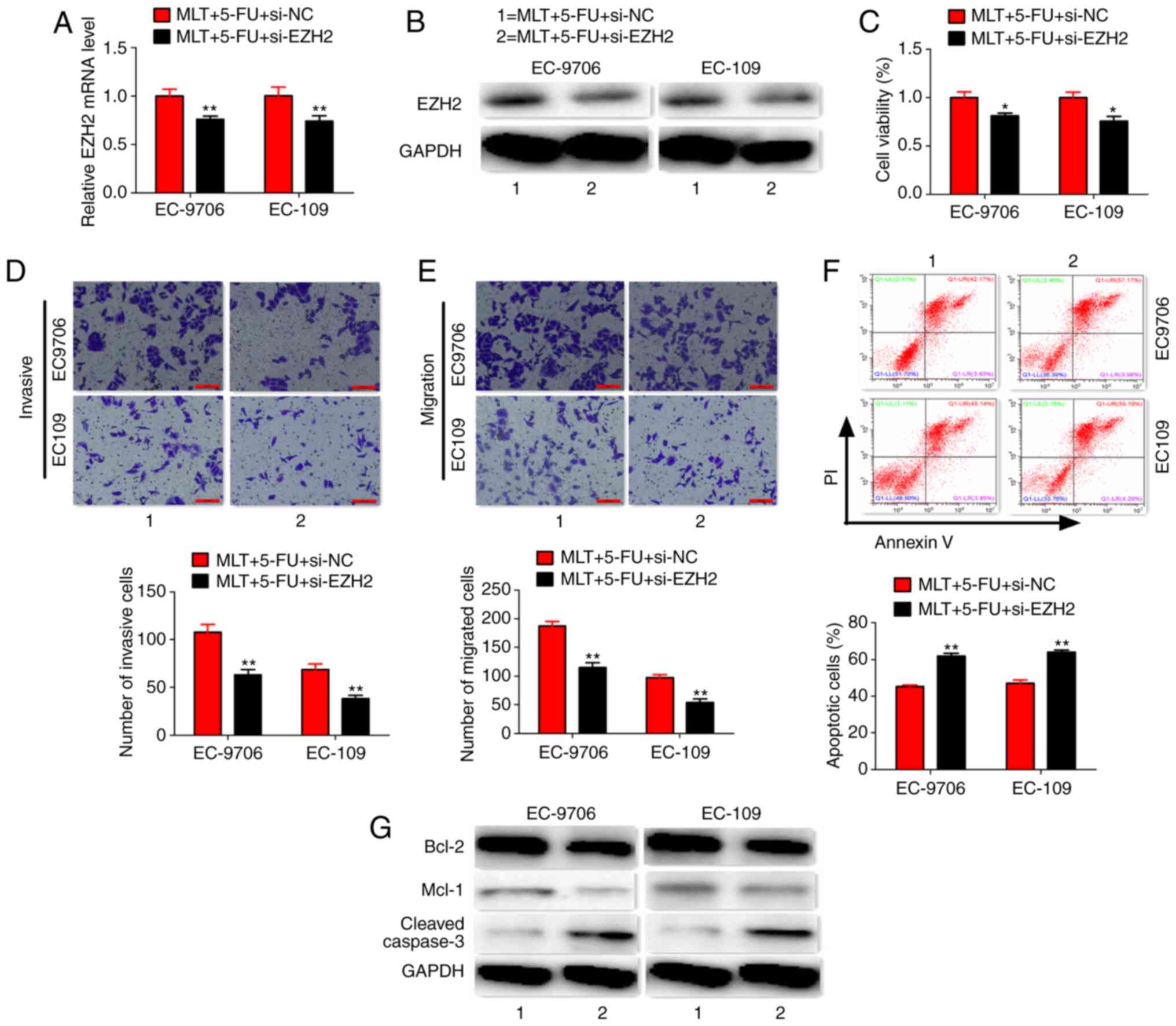 | Figure 6.Co-treatment of MLT, 5-FU and
si-EZH2. (A and B) Effects of MLT, 5-FU and si-EZH2 co-treatment on
EZH2 expression. (C-F) Effects of MLT, 5-FU and si-EZH2
co-treatment on cell viability, invasion, migration and apoptosis
(scale bars, 100 µm). (G) Effects of MLT, 5-FU and si-EZH2
co-treatment on protein expression. *P<0.05 and **P<0.01 vs.
MLT + 5-FU + si-NC group. EZH2, histone-lysine N-methyltransferase
EZH2; MLT, melatonin; 5-FU, 5-fluorouracil; si, small interfering
RNA; NC, negative control. |
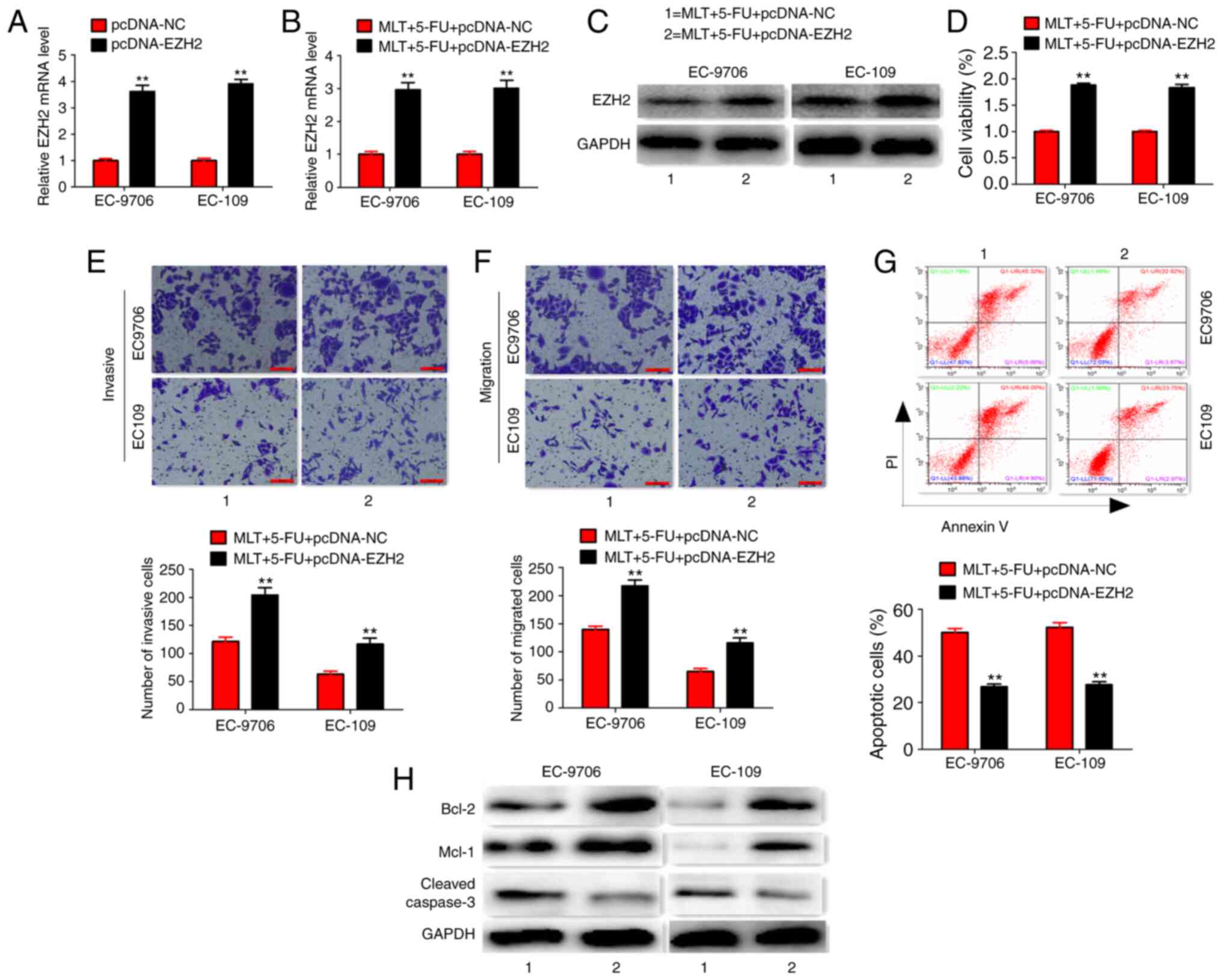 | Figure 7.Co-treatment of MLT, 5-FU and
pcDNA-EZH2. (A) Effects of pcDNA-EZH2 treatment on EZH2 expression
(B and C) Effects of MLT, 5-FU and pcDNA-EZH2 co-treatment on EZH2
expression. (D-G) Effects of MLT, 5-FU and pcDNA-EZH2 co-treatment
on cell viability, invasion, migration and apoptosis (scale bars,
100 µm). (H) Effects of MLT, 5-FU and pcDNA-EZH2 co-treatment on
protein expression. **P<0.01 vs. MLT + 5-FU + pcDNA-NC group.
EZH2, histone-lysine N-methyltransferase EZH2; MLT, melatonin;
5-FU, 5-fluorouracil; NC, negative control. |
Discussion
China has been identified to have a relatively high
incidence rate of EC, with ~50% of new cases occurring each year.
Although the incidence of EC has declined in recent years, the
mortality rate remains fourth among malignant tumors, and the
overall survival and 5-year survival rate of patients with EC
remain low (30). Early diagnosis of
EC is difficult due to its non-obvious clinical symptoms. The
majority of patients with EC already present with locally advanced
cancer or distant metastasis (3).
Therefore, chemotherapy for the purpose of controlling the spread
plays an important role in EC treatment. Although chemotherapeutic
drugs for EC have made significant progress, 5-FU is still an
important first-line chemotherapeutic drug (6). Therefore, it is urgent to improve the
chemotherapeutic efficacy of 5-FU.
Recently, it was revealed that MLT exerts antitumor
roles in studies of different types of human cancers, including
pancreatic, liver, breast and colorectal cancer (18,31,32).
Studies have revealed that MLT can reduce the cell activity in
bladder and colorectal cancer in a dose-dependent manner within a
range of concentrations (33,34). The present study determined that MLT
could also reduce the cell activity of EC-9706 and EC-109 in a
dose-dependent manner within a certain concentration range. Recent
studies have revealed that the combination of MLT and
chemotherapeutic agents increased the efficacy of these agents.
Xiang et al (17) revealed
that the abnormal secretion of MLT inhibited aplasia Ras homology
member I expression and mediated STAT3-induced paclitaxel
resistance in breast cancer. Leja-Szpak et al (20) demonstrated that MLT and its metabolite
enhanced gemcitabine chemosensitivity in pancreatic carcinoma
cells. Hence, the present study assessed whether MLT combined with
5-FU can improve the chemotherapy sensitivity of 5-FU in EC.
To confirm the present hypothesis, cells were
co-treated with 5-FU and MLT. MLT and 5-FU combination
significantly enhanced 5-FU-mediated inhibition of cell activity
and significantly decreased the IC50 of 5-FU in EC-9706
and EC-109 cells. Although no study has demonstrated that MLT
increases 5-FU sensitivity in EC cells, a study has revealed that
MLT enhanced 5-FU sensitivity and inhibited tumor cell growth in
colorectal cancer cells (34).
Apoptosis or programmed cell death is a basic physiological process
that plays a key role in cell development and tissue homeostasis
(35). Flow cytometric assays
revealed that the effects of MLT and 5-FU on the sensitivity of EC
cells was related to the induction of apoptosis. Bcl-2 and Mcl-1
are important apoptosis-regulating genes, while the expression of
cleaved caspase-3 directly indicates the level of apoptosis. The
effects of MLT and 5-FU combination on the protein levels of Bcl-2,
Mcl-1 and cleaved caspase-3 further confirmed its effect in
promoting apoptosis.
The present results and TCGA database analysis
revealed that EZH2 was upregulated in EC samples. Subsequently,
EZH2 was confirmed as an oncogene in EC via biological experiments.
Wang et al (23) revealed that
EZH2 contributed to 5-FU resistance in gastric cancer by
epigenetically suppressing F-box protein 32 expression. Rastgoo
et al (24) determined that
the EZH2/miR-138 axis contributed to drug resistance in multiple
myeloma by downregulating RBPMS. In addition, previous studies also
revealed that melatonin inhibited the tumorigenicity of
glioblastoma stem-like cells via the EZH2 signaling axis (25,26).
Hence, the present study hypothesized that the MLT and 5-FU
combination inhibited cell proliferation and promoted apoptosis by
regulating EZH2 expression. First, the present study confirmed that
MLT and 5-FU significantly inhibited EZH2 expression via RT-qPCR
and western blotting assays. Next, the present study confirmed that
MLT and 5-FU combination improved the sensitivity of 5-FU to EC
cells by downregulating EZH2 expression through co-treatment
experiments. As an important oncogene, EZH2 may affect the
malignancy of tumors through multiple pathways. In a previous study
it was revealed that melatonin inhibited glioblastoma stem-like
cells via the EZH2-NOTCH1 signaling axis (25). Another study revealed that melatonin
inhibited glioblastoma stem-like cells via AKT-EZH2-STAT3 signaling
axis (26). One of our ongoing
studies also revealed that EZH2 effected the development of ESCC by
activating the JKA2/STAT3 signaling pathway. Hence, it was
hypothesized that the effects of melatonin and 5-FU combination on
the malignancy of esophageal cancer may have been achieved by
regulating the EZH2/JKA2/STAT3 signaling pathway. However, this
hypothesis requires further experiments for confirmation.
In conclusion, the present experimental approach
demonstrated that MLT enhanced 5-FU-mediated inhibition of cell
proliferation via the promotion of apoptosis by regulating EZH2
expression in EC cells. This combination treatment may potentially
be a more effective treatment option in EC chemotherapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical
Science Research Project of Henan Province (grant no.
LHGJ20191039); Science and Technology Project of Henan Province
(grant no. 212102310121).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MengtZ, MenglZ and RL performed the majority of the
experiments in the study. YZ and RZ contributed to the analysis of
the experimental data. MengtZ and YZ contributed to the study
design, manuscript writing and provided experimental funding
support. All authors read and approved the manuscript and agree to
be accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tu CC and Hsu PK: The frontline of
esophageal cancer treatment: Questions to be asked and answered.
Ann Transl Med. 6:832018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kimura M, Ishiguro H, Tanaka T and
Takeyama H: Advanced esophageal cancer with tracheobronchial
fistula successfully treated by esophageal bypass surgery. Int J
Surg Case Rep. 9:115–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li B, Hong P, Zheng CC, Dai W, Chen WY,
Dai W, Chen WY, Yang QS, Han L, Tsao SW, et al: Identification of
miR-29c and its target FBXO31 as a key regulatory mechanism in
esophageal cancer chemoresistance: Functional validation and
clinical significance. Theranostics. 9:1599–1613. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siersema PD: Esophageal cancer awareness
issue 2019. Endoscopy. 51:291–292. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang N, Yin Y, Xu SJ and Chen WS:
5-Fluorouracil: Mechanisms of resistance and reversal strategies.
Molecules. 13:1551–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vodenkova S, Buchler T, Cervena K,
Veskrnova V, Vodicka P and Vymetalkova V: 5-Fluorouracil and other
fluoropyrimidines in colorectal cancer: Past, present and future.
Pharmacol Ther. 206:1074472020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Acuna-Castroviejo D, Escames G, Venegas C,
Diaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX
and Reiter RJ: Extrapineal melatonin: Sources, regulation, and
potential functions. Cell Mol Life Sci. 71:2997–3025. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Claustrat B and Leston J: Melatonin:
Physiological effects in humans. Neurochirurgie. 61:77–84. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Talib WH: Melatonin and cancer hallmarks.
Molecules. 23:5182018. View Article : Google Scholar
|
|
12
|
Cardinali DP: Melatonin: Clinical
perspectives in neurodegeneration. Front Endocrinol (Lausanne).
10:4802019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang C, Yang XZ, Xu MJ, Huang GY, Zhang
Q, Cheng YX, He L and Ren HY: Melatonin promotes cheliped
regeneration, digestive enzyme function, and immunity following
autotomy in the Chinese mitten crab, eriocheir sinensis. Front
Physiol. 9:2692018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou N, Wei ZX and Qi ZX: Inhibition of
autophagy triggers melatonin-induced apoptosis in glioblastoma
cells. BMC Neurosci. 20:632019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu K, Wang J, Liu H, Zhao J and Lu W:
Melatonin promotes the proliferation of chicken sertoli cells by
activating the ERK/Inhibin alpha subunit signaling pathway.
Molecules. 25:12302020. View Article : Google Scholar
|
|
16
|
Song J, Ma SJ, Luo JH, Zhang H, Wang RX,
Liu H, Li L, Zhang ZG and Zhou RX: Melatonin induces the apoptosis
and inhibits the proliferation of human gastric cancer cells via
blockade of the AKT/MDM2 pathway. Oncol Rep. 39:1975–1983.
2018.PubMed/NCBI
|
|
17
|
Xiang S, Dauchy RT, Hoffman AE, Pointer D,
Frasch T, Blask DE and Hill SM: Epigenetic inhibition of the tumor
suppressor ARHI by light at night-induced circadian melatonin
disruption mediates STAT3-driven paclitaxel resistance in breast
cancer. J Pineal Res. 67:e125862019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Q, Sun Z, Du L, Xu C, Wang Y, Yang B,
He N, Wang J, Ji K, Liu Y and Liu Q: Melatonin sensitizes human
colorectal cancer cells to γ-ray ionizing radiation in vitro and in
vivo. Int J Mol Sci. 19:39742018. View Article : Google Scholar
|
|
19
|
Hao J, Fan W, Li Y, Tang R, Tian C, Yang
Q, Zhu T, Diao C, Hu S, Chen M, et al: Melatonin synergizes
BRAF-targeting agent vemurafenib in melanoma treatment by
inhibiting iNOS/hTERT signaling and cancer-stem cell traits. J Exp
Clin Cancer Res. 38:482019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leja-Szpak A, Nawrot-Porąbka K, Góralska
M, Jastrzębska M, Link-Lenczowski P, Bonior J, Pierzchalski P and
Jaworek J: Melatonin and its metabolite
N1-acetyl-N2-formyl-5-methoxykynuramine (afmk) enhance
chemosensitivity to gemcitabine in pancreatic carcinoma cells
(PANC-1). Pharmacol Rep. 70:1079–1088. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bae WK and Hennighausen L: Canonical and
non-canonical roles of the histone methyltransferase EZH2 in
mammary development and cancer. Mol Cell Endocrinol. 382:593–597.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Yang X, Li R, Zhang R, Hu D, Zhang
Y and Gao L: LncRNA SNHG6 inhibits apoptosis by regulating EZH2
expression via the sponging of MiR-101-3p in esophageal
squamous-cell carcinoma. Onco Targets Ther. 13:11411–11420. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C, Li X, Zhang J, Ge Z, Chen H and Hu
J: EZH2 contributes to 5-FU resistance in gastric cancer by
epigenetically suppressing FBXO32 expression. Onco Targets Ther.
11:7853–7864. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rastgoo N, Pourabdollah M, Abdi J, Reece D
and Chang H: Dysregulation of EZH2/miR-138 axis contributes to drug
resistance in multiple myeloma by downregulating RBPMS. Leukemia.
32:2471–2482. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng X, Pang B, Gu G, Gao T, Zhang R,
Pang Q and Liu Q: Melatonin inhibits glioblastoma stem-like cells
through suppression of EZH2-NOTCH1 signaling axis. Int J Biol Sci.
13:245–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen X, Hao A, Li X, Du Z, Li H, Wang H,
Yang H and Fang Z: Melatonin inhibits tumorigenicity of
glioblastoma stem-like cells via the AKT-EZH2-STAT3 signaling axis.
J Pineal Res. 61:208–217. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Z, Jiang C and Yuan Y: TCGA based
integrated genomic analyses of ceRNA network and novel subtypes
revealing potential biomarkers for the prognosis and target therapy
of tongue squamous cell carcinoma. PLoS One. 14:e02168342019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Y, Li R, Ding X, Zhang K and Qin W:
Upregulation of long non-coding RNA SNHG6 promote esophageal
squamous cell carcinoma cell malignancy and its diagnostic value.
Am J Transl Res. 11:1084–1091. 2019.PubMed/NCBI
|
|
30
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reiter RJ, Rosales-Corral SA, Tan DX,
Acuna-Castroviejo D, Qin L, Yang SF and Xu K: Melatonin, a full
service anti-cancer agent: Inhibition of initiation, progression
and metastasis. Int J Mol Sci. 18:8432017. View Article : Google Scholar
|
|
32
|
Hill SM, Belancio VP, Dauchy RT, Xiang S,
Brimer S, Mao L, Hauch A, Lundberg PW, Summers W, Yuan L, et al:
Melatonin: An inhibitor of breast cancer. Endocr Relat Cancer.
22:R183–R204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen YT, Yang CC, Shao PL, Huang CR and
Yip HK: Melatonin-mediated downregulation of ZNF746 suppresses
bladder tumorigenesis mainly through inhibiting the AKT-MMP-9
signaling pathway. J Pineal Res. 66:e125362019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang
Z, Li Z, Feng X, Hao J, Zhang K, et al: Melatonin synergizes the
chemotherapeutic effect of 5-fluorouracil in colon cancer by
suppressing PI3K/AKT and NF-κB/iNOS signaling pathways. J Pineal
Res. 62:e123802017. View Article : Google Scholar
|
|
35
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019. View Article : Google Scholar : PubMed/NCBI
|