Introduction
Liver cancer is the most common type of primary
liver cancer. It usually occurs in people with chronic liver
diseases, such as cirrhosis caused by hepatitis B or hepatitis C
infection (1). In 2012, over 70,000
cases were diagnosed worldwide, and the mortality rate due to liver
cancer and its incidence are increasing annually. Among these
cases, over 80% occurred in East Asia and sub-Saharan Africa
(1). Although chemotherapy is an
important therapeutic method for liver cancer in combination with
surgery and radiotherapy, its side effects are obvious, such as
drug resistance and toxicity (2).
Traditional Chinese medicine (TCM) is a system of medical care that
has been developed in China over thousands of years (3–7). It has
been used for treating cancers in clinical application due to
advantages such as low toxicity, high efficacy, moderate costs, and
high acceptability (8–10). In recent years, TCM has been widely
accepted as a type of complementary and alternative medicine (CAM)
in numerous countries around the world, such as the United States,
Canada, and the European Union (11).
Kaempferol (KF), or 3,4′,5,7-tetrahydroxyflavone, is
a natural flavonoid found in numerous herbal medicines and fruits
(12). A few studies reported that
the application of KF may reduce the risk of various diseases, such
as cancers, diabetes, cardiovascular disorders, bacterial
infection, and viral infection (13,14). In
vitro studies along with some animal testing have demonstrated
the wide range of potential antitumor properties of KF (15,16).
Antitumor effects have been identified for certain malignant cancer
cells (including breast cancer, ovarian cancer, leukemia, as well
as bladder, gastric, colorectal, pancreatic and lung cancer),
revealing an ability to interrupt cell growth, limit angiogenesis,
induce apoptosis, and reduce the ability to metastasize (17,18).
Doxorubicin (DOX) is a chemotherapy medication used
to treat numerous cancers. It works through interaction with DNA by
the intercalation and inhibition of macromolecular biosynthesis,
leading to cellular apoptosis. However, the therapeutic effects of
DOX are hampered by its significant side effects and acquired drug
resistance (19). In addition to hair
loss, bone marrow suppression, vomiting, rash, and other slight
side effects, DOX can induce serious allergic reactions, heart
damage, radiation recall, treatment-related leukemia, and hand-foot
syndrome (20–22). Numerous studies have revealed that the
combination of chemotherapy with various types of TCMs can reduce
the side effects of DOX and enhance its drug sensitivity. For
example, Lin et al revealed that Astragaloside IV could
significantly reduce DOX-induced cardiotoxicity (23). Li et al revealed that oridonin
could assist DOX against aggressive breast cancer by promoting
apoptosis and suppressing angiogenesis (24). Tanshinone IIA, a compound found in the
plant Danshen, exhibited the capacity to overcome DOX resistance in
gastric cancer cells (25). As a
result, a question arises regarding whether the combination of
doxorubicin with KF could lead to a better therapeutic outcome than
monotherapies.
Materials and methods
Reagents
KF was purchased from Sigma-Aldrich; Merck KGaA
(cat. no. K0133) and was initially dissolved in dimethyl sulfoxide
(DMSO) into a 50 mg/ml solution according to the manufacturer's
instructions, and then diluted in Dulbecco's modified of Eagle's
medium (DMEM) (Gibco/BRL; Thermo Fisher Scientific, Inc.) to the
desired working concentrations. DOX was purchased from
Sigma-Aldrich; Merck KGaA (cat. no. D1515) and directly dissolved
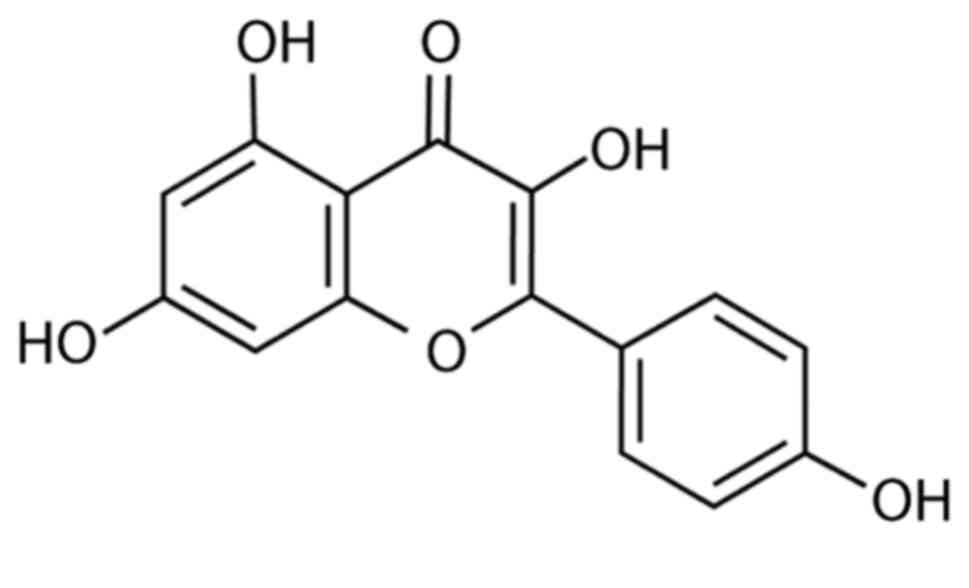
in DMEM into the desired concentration. The structure of KF is
presented in Fig. 1.
Cell lines and culture
Liver cancer cell lines Huh-7, Huh-1, HepG2,
HepG2.2.15, SK-Hep-1, PLC/PRF/5, HLE, HLF, and Hep3B were purchased
from the Chinese Academy of Sciences affiliated cell bank. The
HepG2 and SK-Hep-1 cell lines were authenticated via STR profiling
method. The normal hepatocyte cell line was purchased from Lonza
Group, Ltd. Hepatocyte cells were cultured in the growth medium
supplied by the manufacturer and liver cancer cells were maintained
in DMEM medium (cat. no. 11965084) supplemented with 10% fetal
bovine serum (FBS; cat. no. 12483020; both from Gibco/BRL; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin, and 100 mg/ml
streptomycin at 37°C in a humidified incubator with 5%
CO2.
Cell viability
Cells were seeded in cell culture medium at a
concentration of 3×104 cells/100 µl/well in microtiter
plates (tissue culture grade, 96 wells, flat bottom) and incubated
for 24 h at 37°C and 5% CO2. After the incubation
period, 10 µl of MTT-labeling reagent was then added to each well
to a final concentration of 0.5 mg/ml. The cells were then
incubated for an additional 4 h at 37°C in a humidified atmosphere.
Solubilization solution (Sigma-Aldrich; Merck KGaA) was then added
at 100 µl per well and allowed to stand overnight in the incubator
in a humidified atmosphere. After overnight incubation, the cells
were checked to ensure the complete solubilization of purple
formazan crystals, and absorbance was then measured at a wavelength
570 nm using a microplate (ELISA) reader.
Colony formation assay
Cells were separated into a 6-well plate at a
density of 500 cells/well and cultured for 10 days until most of
the single colonies contained >50 cells per colony. The growth
medium was refreshed every three days. After 10 days of growth, the
colonies were then stained with 0.25% crystal violet for at least
15 min at room temperature, washed three times with
phosphate-buffered solution (PBS), and dried at room temperature.
The number of colonies was then counted under a light microscope
(magnification, ×100).
Fluorescence-activated cell sorting
analysis (FACS)
To validate the cell cycle progression and apoptotic
ratio, flow cytometry was performed. Liver cancer cells were seeded
at a density of 2×105 cells per dish. After culturing
for 48 h (37°C, 5% CO2), cells were harvested and fixed
in 75% ethanol overnight at 4°C. The cells were then stained with
50 µg/ml propidium iodine (PI) solution and incubated at 4°C for 1
h in the incubator. PI-stained cells were then analyzed by flow
cytometry (FACSCalibur, BD Biosciences). Cell cycle analysis was
then performed using the accompanying flow cytometric software
(CellQuest Pro, BD Biosciences).
Hoechst 33258 staining
Hoechst 33258 staining kit (Dojindo Molecular
Technologies, Inc.) was used to detect changes in cellular
apoptosis. Cells were cultured in 12-well plates and incubated at
37°C for 24 h. Then reagents were then added and cells were
incubated at 37°C for another 24 h. The cells were then fixed in 4%
methanol and permeabilized for 20 min at 4°C and stained with
Hoechst 33258 reagent at room temperature for 15 min. After washing
three times with PBS, the morphological changes of liver cancer
cells and nuclear chromatin agglutination were validated with a
fluorescence microscope (magnification, ×400). The images were then
acquired and documented.
Mitochondrial apoptosis detection
A fluorometric mitochondrial apoptosis detection kit
was purchased from BioVision, Inc. (cat. no. K250). Cells
(2×105 cells/ml) were seeded in 12-well plates and
incubated at 37°C for 24 h. After incubation with reagents at 37°C
for another 24 h, the MitoCapture reagent diluted at 1:1,000 in
pre-warmed incubation buffer was then added. Cells were incubated
with the MitoCapture working solution in the incubator at 37°C for
20 min and washed with pre-warmed incubation buffer. After washing,
cells were visualized and documented with fluorescence microscopy
(magnification, ×40).
Wound healing assay
Liver cancer cells were seeded in 6-well plates and
cultured until confluent, generally after 24 h. A 100-µl pipette
tip was used to produce a straight scratch to simulate a wound.
Then, the scratch was washed with pre-warmed PBS to remove cell
debris. After washing, cells were cultured in DMEM which contained
1% FBS in order to inhibit cell proliferation. Images were captured
under a light microscope (magnification, ×40) at 24, 48, and 72 h
after wounding.
Transwell assay
A 6.5-mm Transwell with an 8-µm pore polyester
membrane insert was used to perform this experiment. Before being
applied to the Transwell insert, the Matrigel was pre-cooled on
ice, and then 100 µl was used for one chamber. Then, the Transwell
inserts were placed in the incubator for 30 min at 37°C to complete
precoating. Next, 5×104 cells were seeded in the upper
chamber and cultured in DMEM without serum. DMEM with 10% FBS was
added to the lower chamber. Cells were administered with
chemotherapeutic agents at 37°C for 48 h and then fixed in 4%
methanol for 15 min and stained with 0.25% crystal violet for 15
min at room temperature. Subsequently, invasive cells in the lower
chamber were visualized and documented with light microscopy
(magnification, ×100).
Western blotting
After being treated with drugs for 24 h, the cells
were lysed in an ice bath for 30 min in RIPA Lysis Buffer (Solarbio
Life Sciences). The lysates were centrifuged at 10,000 × g for 10
min at 4°C and the supernatant was harvested. Protein
concentrations of the supernatants were estimated using a DC
Protein assay kit (Bio-Rad Laboratories, Inc.). Then, 30 µg protein
sample per lane was loaded into the 10% SDS-PAGE which was
performed to separate the various proteins by their molecular
weight. The separated proteins were then transferred onto Bio-Rad
polyvinylidene difluoride membranes in a semi-dry transmembrane
device. The transferred membranes were firstly soaked in the
blocking solution (with 3% BSA) for 1 h at room temperature and
then incubated with corresponding primary antibodies at 4°C
overnight. These antibodies which were purchased from Cell
Signaling Technology, Inc. included: Bax (1:1,000; product no.
5023), Bcl-2 (1:1,000; product no. 3498), cytochrome c
(1:1,000; product no. 4280), Bid (1:1,000; product no. 2002),
pro-caspase-3 (1:1,000; product no. 9662), cleaved caspase-3
(1:1,000; product no. 9664), cleaved caspase-8 (1:1,000; product
no. 8592), cleaved caspase-9 (1:1,000; product no. 9509), MMP-2
(1:1,000; product no. 40994), MMP-9 (1:1,000; product no. 13667),
PI3K (1:1,000; product no. 4249), Akt (1:2,000; product no. 4685),
mTOR (1:1,000; product no. 2983), S6K (1:1,000; product no. 9202),
and β-actin (1:1,000; product no. 4970). After they were washed
with TBST solution three times, the membranes were incubated with
HRP-labeled secondary antibody (1:3,000; product no. 7074; Cell
Signaling Technology, Inc.) at 37°C for 1 h and then washed with
TBST three more times. Finally, protein bands were then detected by
an ECL detection kit (cat. no. RPN2209; Cytiva). The protein
expression level was analyzed by ImageJ 1.53 (National Institutes
of Health).
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). Differences between the two groups were compared using
unpaired Student's t-test, while differences between multiple
groups were compared by Tukey's post hoc tests following ANOVA,
using SPSS 19.0 (IBM Corp.). Results with a P-value <0.05 were
considered to indicate a statistically significant difference.
Results
Inhibitive effect on proliferation by
KF and DOX in liver cancer cells and toxicity assessment in normal
cells
The inhibitive effect of KF on the proliferation of
liver cancer cells was assessed by MTT assay. The viability of
Huh-7, Huh-1, HepG2, HepG2.2.15, SK-Hep-1, PLC/PRF/5, HLE, HLF, and
Hep3B cells treated with increasing concentrations of KF was
detected at 24, 48, and 72 h post-treatment. As revealed (Fig. 2A-I), KF had an inhibitive effect on
the nine cell lines in time- and dose-dependent manners. After
treatment with 40 µM KF for 72 h, the growth inhibition rate of
HepG2 cells was 47.15% (Fig. 2C)
while in SK-Hep-1 cells it was 52.05% (Fig. 2E). Due to it being close to the
IC50 of both cell lines, 40 µM KF was used for future
experiments. After treatment with KF for 72 h, the growth
inhibitory effect on hepatocytes (Fig.
2J) was not noticeable compared with the control group.
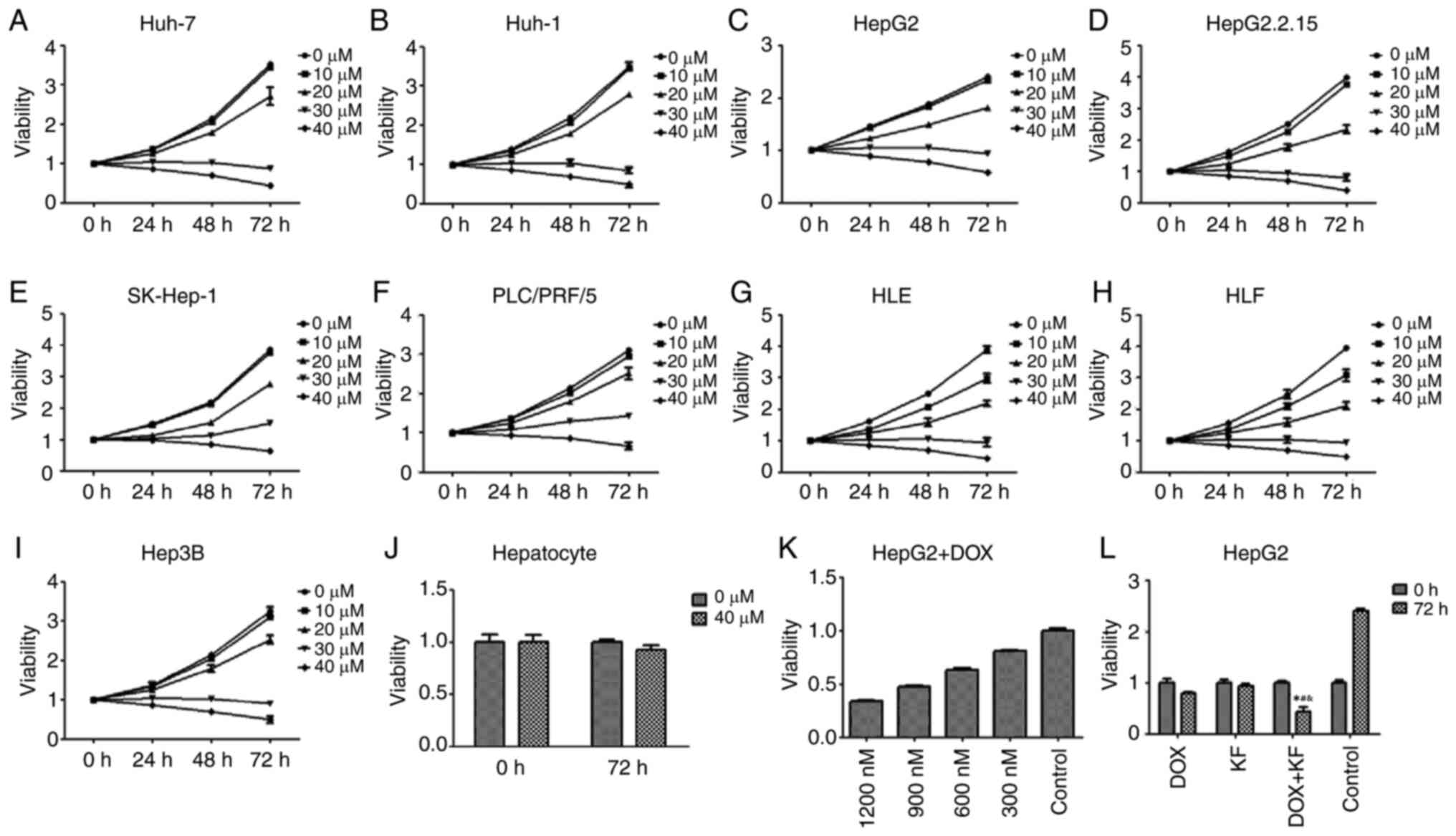 | Figure 2.Growth inhibitory effect of KF and
doxorubicin. (A-I) Cells were treated with various concentrations
of KF for 24, 48, and 72 h, and cell viability was determined by
MTT assay. (J) Normal hepatocytes were treated with 0 and 40 µM of
KF and the cell viability was determined by MTT assay. (K) Cells
were treated with various concentrations of DOX for 24 h, and cell
viability was determined by MTT assay. (L) Four treatment groups
(DOX, KF, DOX + KF, and Control) were assessed in cells for 72 h,
and cell viability was determined by MTT assay. The data represent
the mean ± SD. (n=3). *P<0.05 compared with the DOX group;
#P<0.05 compared with the KF group;
&P<0.05 compared with the Control group. KF,
Kaempferol; DOX, doxorubicin. |
HepG2, which is one of the most commonly used liver
cancer cell lines, was used to determine the effect of KF and the
combined therapy on cell proliferation and apoptosis in the next
experiment. As a positive control, the inhibitory effect of DOX at
24 h was detected in HepG2 cells (Fig.
2K). The effect was also revealed to be in a dose-dependent
manner. At a concentration of 900 nM, doxorubicin had a similar
inhibitory effect as KF at 40 µM. Four treatment groups (the DOX
group, KF group, DOX+KF combination group and control group) were
then detected for changes in cellular viability. The result
revealed that the combination group had a more substantial
inhibitive effect in HepG2 cells (Fig.
2L). The examination of the four treatment groups in HepG2
cells revealed that a combination treatment could significantly
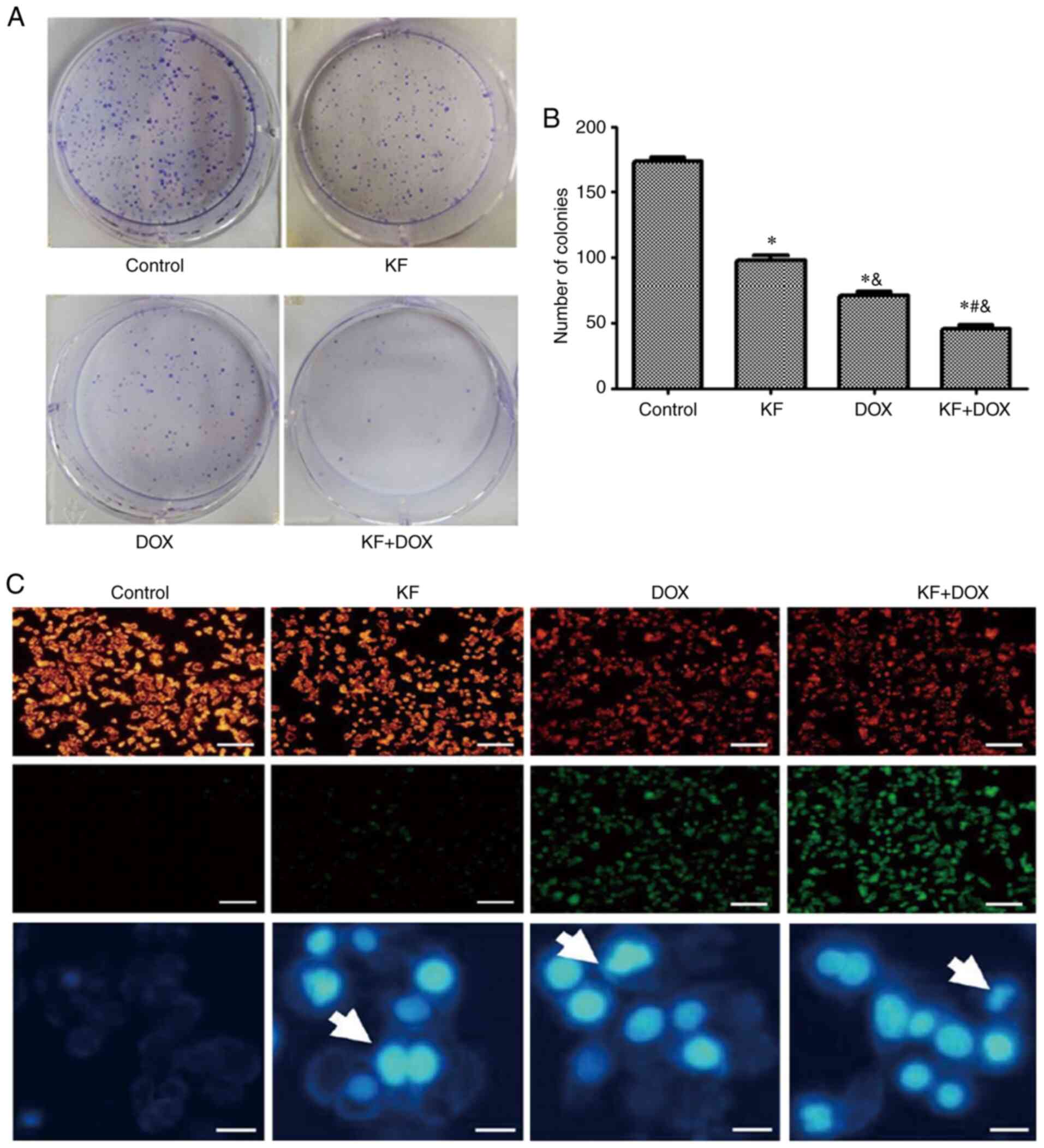
inhibit the colony formation of the liver cancer cells (Fig. 3A and B).
KF and DOX induce apoptosis in HepG2
cells
To ascertain whether KF and DOX in combination
induce apoptosis in HepG2 cells, mitochondrial potential staining
was performed. Marked changes in mitochondrial potential were
observed in the combined treatment group as compared with slight
changes in the KF group (Fig. 3C).
Furthermore, DAPI staining also revealed an increased number of
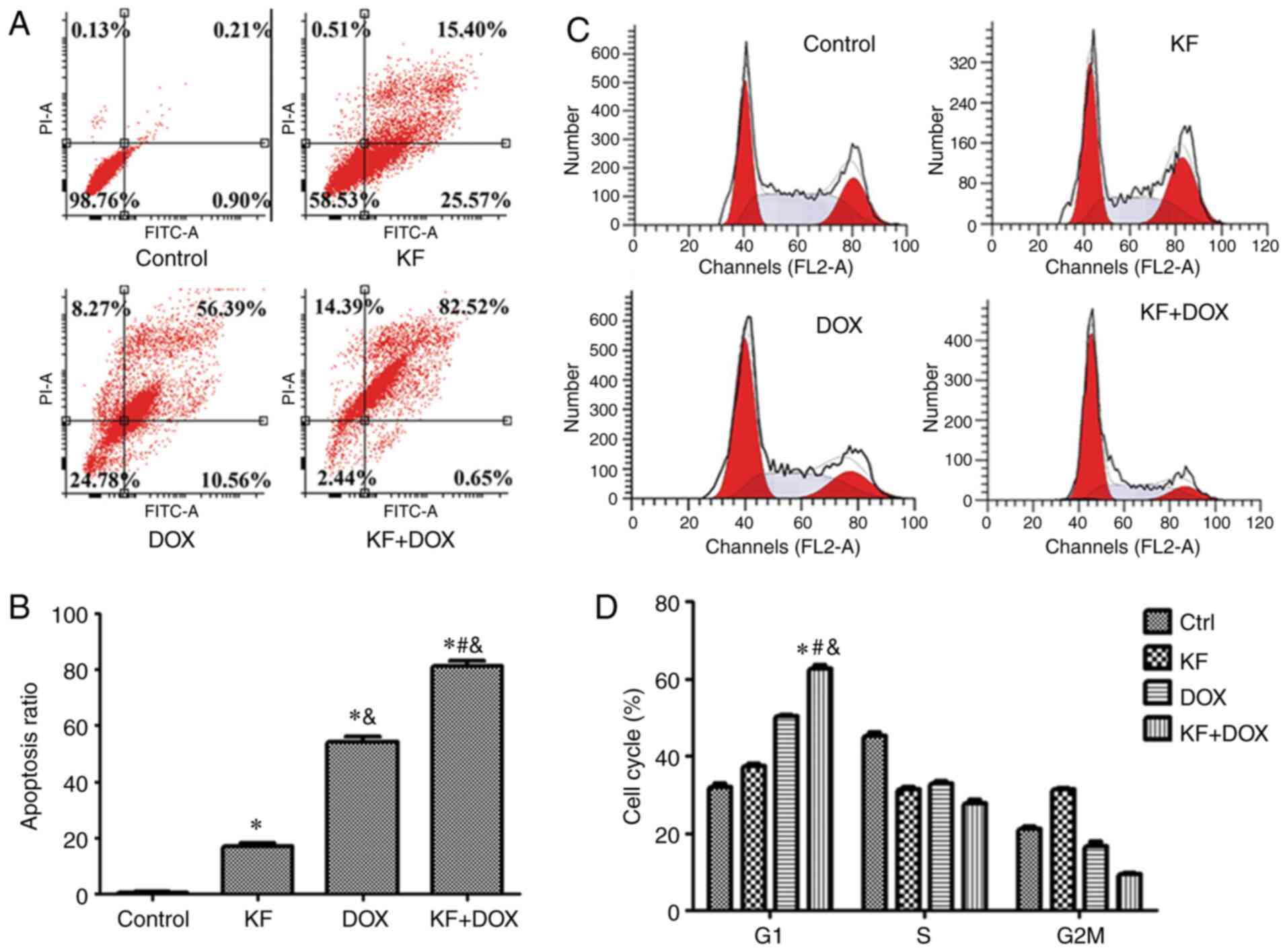
highlighted nuclei, indicative of apoptosis (Fig. 3C). The cellular apoptotic ratio was
then measured by Annexin V-FITC/PI double-staining and analyzed by
flow cytometry. The percentage of HepG2 cells was significantly
increased in the combination group, and in the two monotherapy
groups the apoptotic ratio was also observed to be increased
compared with the control group (Fig. 4A
and B).
KF and DOX induce cell cycle arrest in
HepG2 cells
To investigate whether cell cycle arrest contributed
to cell proliferation and colony formation inhibition, the cell
cycle of HepG2 cells was analyzed using flow cytometry.
Administration of KF and DOX arrested liver cancer cells in the G1
phase and accordingly decreased the cell ratio in the G2/M and S
phases (Fig. 4C and D).
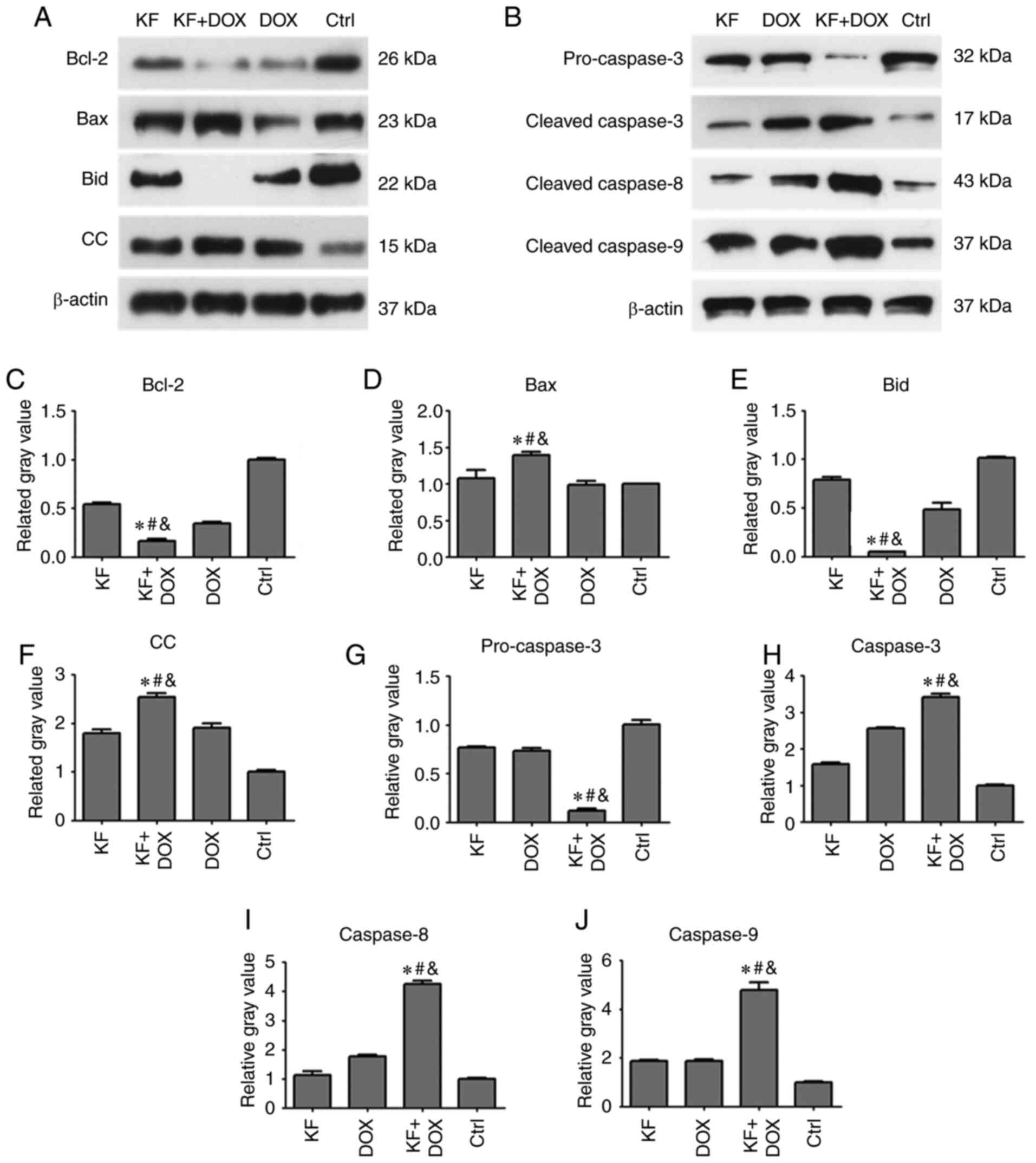
Effect of KF on the production of
apoptotic proteins in HepG2 cells
To determine changes in the level of apoptotic
proteins impacted by drug treatments in HepG2 cells, the expression
levels of apoptotic proteins, such as caspase-3, caspase-8,
caspase-9, Bcl-2, Bax, Bid, and cytochrome c were detected
by western blotting assay. The cells were separated into four
groups: The KF, DOX, combination of DOX and KF group, and control
group (Fig. 5A and B). Following a
72-h treatment, protein expression levels of Bcl-2, Bid, and
pro-caspase-3 were clearly reduced in the combined treatment group
compared with KF and DOX single-treatment groups (Fig. 5C, E and G). The protein expression
levels of Bax, cytochrome c (CC), caspase-3, caspase-8, and
caspase-9 were upregulated in the combined treatment group compared
to KF and DOX single-treatment groups (Fig. 5D, F and H-J).
KF inhibits migration of SK-Hep-1
cells
To study the functions of KF on cell migration and
invasion, the liver adenocarcinoma cell line, SK-Hep-1, which had a
higher level of malignancy was used. The migration was assessed by
monolayer scratch wound healing assay. The results revealed
impaired wound closure of cells treated with KF and/or DOX. The
combination group exhibited the largest opening area from the
scratch, while KF treatment alone had a weaker effect than the
combined treatment (Fig. 6A and B).
This finding indicates that KF alone or in combination with DOX can
inhibit the motility of liver cancer cells.
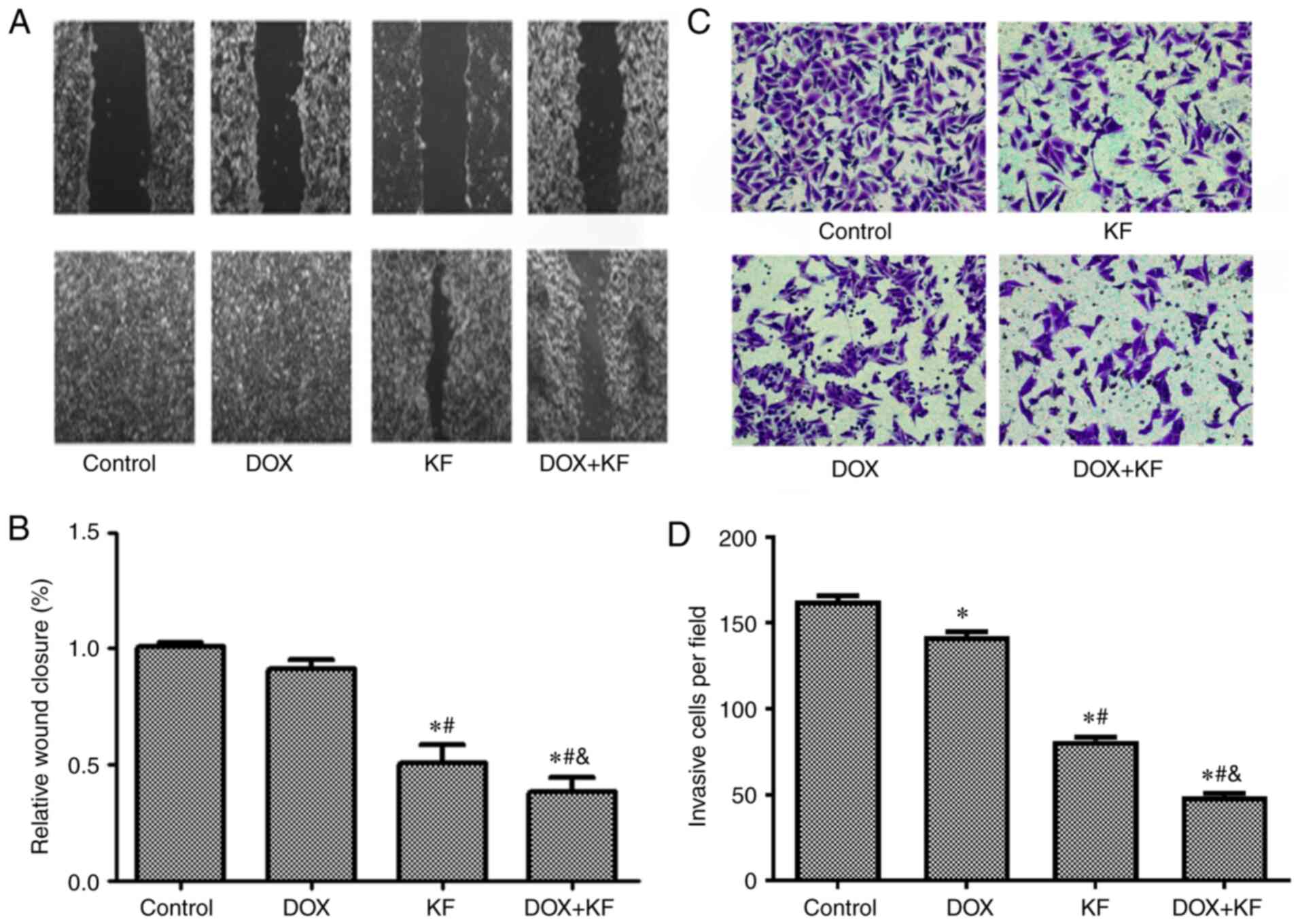 | Figure 6.Inhibitive effect of DOX and KF on
the migration and invasion of liver cancer cells. (A and B) In the
wound healing experiment, the SK-Hep-1 cells were administered with
chemotherapeutic agents for 72 h, and then the changes in the
scratched wound width were observed and recorded under a microscope
(scale bar, 1,000 µm). Changes in the wound widths are presented in
the histograms of B. (C and D) For the Transwell invasion
experiment, the SK-Hep-1 cells were administered with
chemotherapeutic agents for 48 h, and then the cells were removed
and fixed in methanol, stained with cell labeling dye, and recorded
under a microscope (Scale bar, 400 µm). The number of invasive
cells were presented in the histograms of D. The data represent the
mean ± SD (n=3). *P<0.05 compared to the Control group;
#P<0.05 compared to the DOX group;
&P<0.05 compared to the KF group. DOX,
doxorubicin; KF, Kaempferol. |
KF inhibits invasion of SK-Hep-1
cells
To further evaluate the function of KF on the
invasion capability, Transwell chamber assay was performed using
SK-Hep-1 cells. The results from the Transwell invasion experiments
indicated that KF alone and combined treatment could restrict the
invasive ability of liver cancer cells (Fig. 6C and D) and significantly decrease the
number of invasive cells.
Effect of KF on the expression of
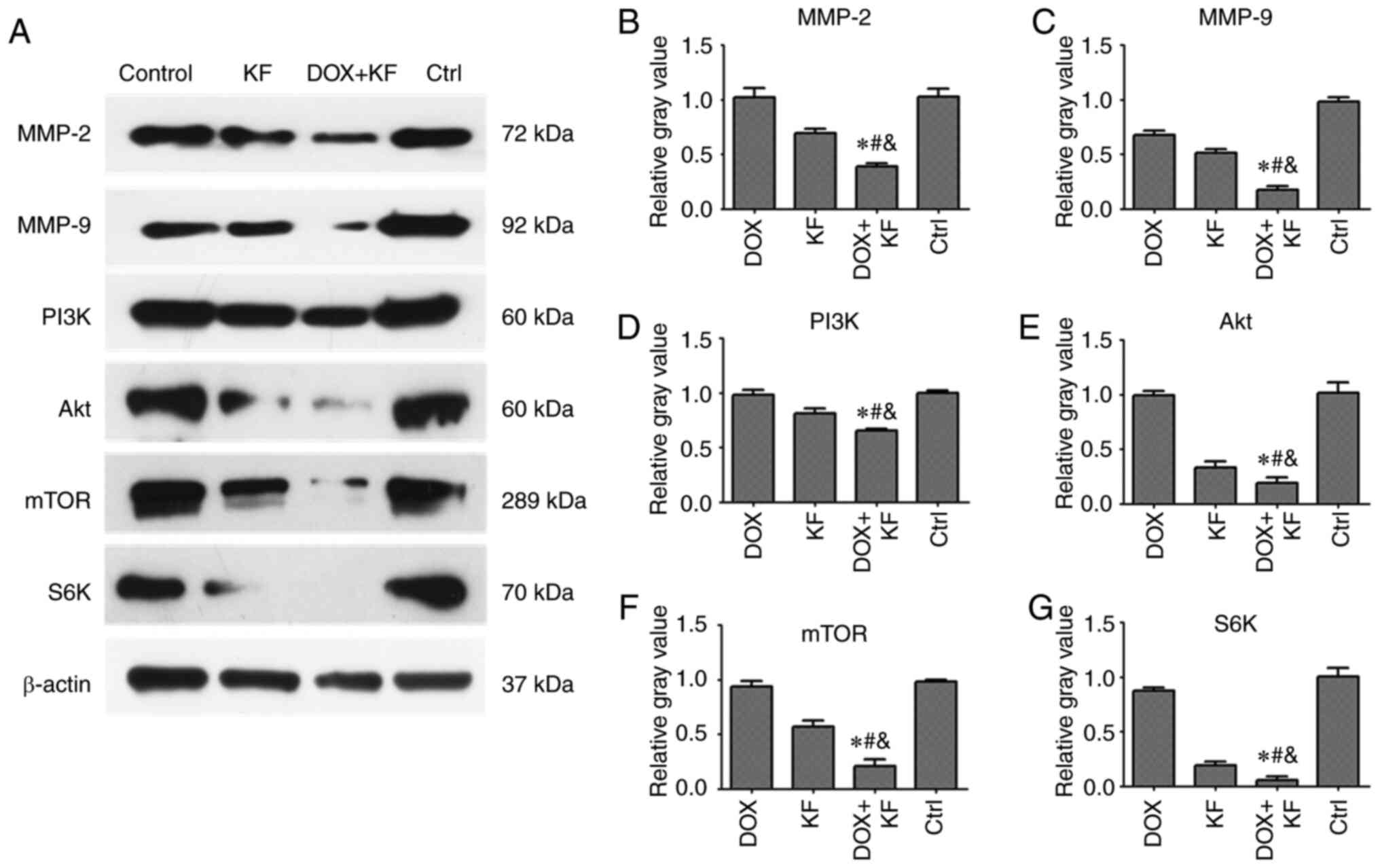
migration and invasion-related proteins in SK-Hep-1 cells
To validate the expression of migration and
invasion-related proteins affected by KF and the combination group
in liver cancer cells, the expression levels of MMP-2, MMP-9, PI3K,
Akt, mTOR, and S6K were measured by western blotting assay. There
were four groups: The DOX group, KF group, combination of DOX and
KF group, and control group. Following treatment for 72 h,
downregulated levels of MMP-2, MMP-9, PI3K, Akt, mTOR, and S6K were
observed in the combined treatment group compared with the group
treated with either KF or DOX alone in SK-Hep-1 cells (Fig. 7A-G).
Discussion
Liver cancer is one of the most lethal
gastrointestinal malignancies worldwide. It is typically
characterized by late-stage presentation, which limits treatment
options and results in poor prognoses (26). At present, surgery and chemotherapy
are the main preferred therapeutic strategies for advanced liver
cancer. However, with metastasis, and given the drug-resistant
nature of terminal liver cancer, patients with advanced liver
cancer are generally not suitable for surgery. In addition, there
have been few effective chemotherapeutic agents available for liver
cancer treatment (27). To date,
sorafenib was the only approved targeted drug for advanced liver
cancer, and numerous attempts using other monoclonal antibodies or
small-molecule tyrosine kinase inhibitors failed to demonstrate
their efficacy (28). Thus,
combination chemotherapeutic strategies were investigated to
contribute to the development of a more effective treatment for
liver cancer. Some studies have reported that traditional Chinese
medicine has the ability to inhibit liver cancer growth by inducing
apoptosis and suppressing invasion through the blocking of crucial
cellular signaling pathways (29,30). KF
was reported to be able to inhibit numerous cancers both in
vitro and in vivo (31).
Hepatocellular carcinoma, as a major cancer worldwide, has also
been reported to be inhibited by KF by several research groups.
Seydi et al reported that KF exhibited selective
cytotoxicity toward hepatocellular carcinoma cells in a rat model
(32). Han et al revealed that
KF could induce autophagic cell death in hepatocellular carcinoma
cells by activating adenosine 5′-monophosphate (AMP)-activated
protein kinase (AMPK) signaling (33). Another study indicated that KF induced
apoptosis in HepG2 cells via activation of the endoplasmic
reticulum stress pathway (34).
However, these studies were only performed using monotherapy in
liver cancer cells, and no data showing the effects of combination
therapy of KF with conventional chemotherapeutic agents were
available. Thus, in the present study it was examined whether KF or
a combination of KF and DOX could achieve more robust antitumor
effects.
In our initial studies, liver cancer cells treated
with KF alone exhibited inhibitive effects in time- and
dose-dependent manners. KF was then used in combination with DOX.
The results indicated that a combination of KF and DOX exhibited a
significantly stronger growth inhibition than either KF or DOX
alone (P<0.05).
Previous studies have revealed that flavonoids can
induce cell death by activating apoptosis and mitochondrial
signaling pathways, and inhibit cell migration and invasion by
suppressing mTOR signaling pathways (35,36). The
results of the present study revealed that KF could induce
apoptosis in liver cancer cells by inducing changes in the protein
expression levels of critical factors involved in the mitochondrial
apoptotic signaling pathways. Furthermore, combination treatment
exhibited a more significant effect on apoptotic activity. However,
through wound healing and Transwell invasion assays, it was
observed that KF exhibited obvious inhibitive activities on liver
cancer cells. Combination treatment also revealed higher inhibitive
activity on migration and invasion-related proteins (including
MMP-2, MMP-9, PI3K, Akt, mTOR, and S6K) than in the case of KF or
doxorubicin treatment alone.
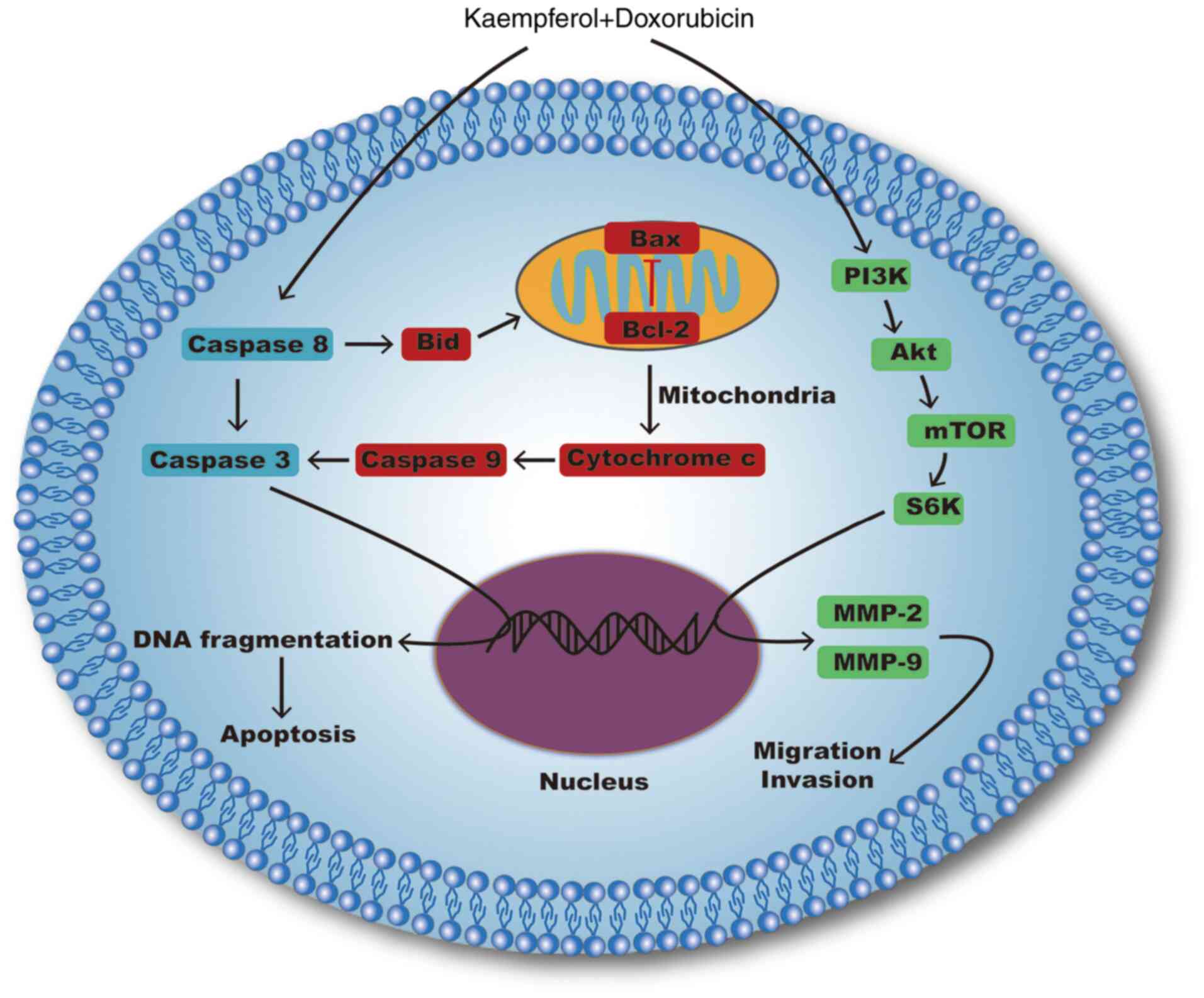
Notably, our research indicated that KF could
inhibit the proliferation of liver cancer cells by activating
mitochondrial signaling pathways and suppressing migration and
invasion by inhibiting the PI3K/mTOR/MMP signaling pathway
(Fig. 8). By contrast, KF treatment
on normal cells revealed markedly low toxicity, which suggests that
KF may be a safer complementary medicine for clinical application.
Furthermore, combined treatment with DOX and KF induced a higher
inhibitive effect than either of the monotherapies. In a future
study, the safety and efficacy of this combined therapy in
vivo will be confirmed, in order to determine the theoretical
basis on its clinical application. Additionally, the effects of KF
combined with other chemotherapeutics will be explored as well. It
is theorized that KF as a hypotoxic compound could affect carcinoma
cells by interrupting cell survival signaling pathways and could
compensate for the shortcomings of the present monotherapies.
Moreover, the merit of the multi-targeting effect of KF could lead
to a less drug-resistant response. Therefore, KF, as an adjuvant
medicine, combined with other chemotherapeutics, even targeted
drugs could potentially be a more effective approach to advance the
therapeutic outcomes and quality of life of patients with liver
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Fund Program
for the Scientific Activities of Selected Returned Overseas
Professionals in Shanxi Province.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
GY, JS and MZ conceived and designed the
experiments. GY and JX performed the experiments. GY and BA
analyzed the data. BA, GY, JX and JS contributed to the statistical
analysis. GY and JS wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2012. View Article : Google Scholar
|
|
2
|
Anwanwan D, Singh SK, Singh S, Saikam V
and Singh R: Challenges in liver cancer and possible treatment
approaches. Biochim Biophys Acta Rev Cancer. 1873:1883142020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiao B and Gao JJ: Intensive research on
the prospective use of complementary and alternative medicine to
treat systemic lupus erythematosus. Drug Discov Ther. 7:167–171.
2013.PubMed/NCBI
|
|
4
|
Huang H, Peng X and Zhong C: Idiopathic
pulmonary fibrosis: The current status of its epidemiology,
diagnosis, and treatment in China. Intractable Rare Dis Res.
2:88–93. 2013.PubMed/NCBI
|
|
5
|
Gao J, Inagaki Y, Li X, Kokudo N and Tang
W: Research progress on natural products from traditional Chinese
medicine in treatment of Alzheimer's disease. Drug Discov Ther.
7:46–57. 2013.PubMed/NCBI
|
|
6
|
Melendez-Martinez AJ, Nascimento AF, Wang
Y, Liu C, Mao Y and Wang XD: Effect of tomato extract
supplementation against high-fat diet-induced hepatic lesions.
Hepatobiliary Surg Nutr. 2:198–208. 2013.PubMed/NCBI
|
|
7
|
Tao Z, Gao J, Zhang G, Xue M, Yang W, Tong
C and Yuan Y: Shufeng Jiedu Capsule protect against acute lung
injury by suppressing the MAPK/NF-κB pathway. BioSci Trends.
8:45–51. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi FH, Wang ZX, Cai PP, Zhao L, Gao JJ,
Okudo N, Li AY, Han JQ and Tang W: Traditional Chinese medicine and
related active compounds: A review of their role on hepatitis B
virus infection. Drug Discov Ther. 7:212–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia J, Inagaki Y, Gao J, Qi F, Song P, Han
G, Sawakami T, Gao B, Luo C, Kokudo N, et al: Combination of
Cinobufacini and Doxorubicin increases apoptosis of hepatocellular
carcinoma cells through the Fas- and mitochondria-mediated
pathways. Am J Chin Med. 45:1537–1556. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xia J, Rong L, Sawakami T, Inagaki Y, Song
P, Hasegawa K, Sakamoto Y and Tang W: Shufeng Jiedu Capsule and its
active ingredients induce apoptosis, inhibit migration and
invasion, and enhances doxorubicin therapeutic efficacy in
hepatocellular carcinoma. Biomed Pharmacother. 99:921–930. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wong R, Sagar CM and Sagar SM: Integration
of Chinese medicine into supportive cancer care: A modern role for
an ancient tradition. Cancer Treat Rev. 27:235–246. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holland TM, Agarwal P, Wang Y, Leurgans
SE, Bennett DA, Booth SL and Morris MC: Dietary flavonols and risk
of Alzheimer dementia. Neurology. 29:e1749–e1756. 2020. View Article : Google Scholar
|
|
13
|
Veeresham C, Rama Rao A and Asres K:
Aldose reductase inhibitors of plant origin. Phytotherapy Research.
28:317–333. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khalil MI and Sulaiman SA: The potential
role of honey and its polyphenols in preventing heart diseases: A
review. Afr J Tradit Complement Altern Med. 7:315–321. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen AY and Chen YC: A review of the
dietary flavonoid, kaempferol on human health and cancer
chemoprevention. Food Chem. 138:2099–2107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niestroy J, Barbara A, Herbst K, Rode S,
van Liempt M and Roos PH: Single and concerted effects of
benzo[a]pyrene and flavonoids on the AhR and Nrf2-pathway in the
human colon carcinoma cell line Caco-2. Toxicol In Vitro.
25:671–683. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calderón-Montaño JM, Burgos-Morón E,
Pérez-Guerrero C and López-Lázaro M: A review on the dietary
flavonoid kaempferol. Mini Rev Med Chem. 11:298–344. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SH and Choi KC: Anti-cancer effect and
underlying mechanism(s) of kaempferol, a phytoestrogen, on the
regulation of apoptosis in diverse cancer cell models. Toxicol Res.
29:229–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Speth PA, van Hoesel QG and Haanen C:
Clinical pharmacokinetics of doxorubicin. Clin Pharmacokinet.
15:15–31. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chatterjee K, Zhang J, Honbo N and
Karliner JS: Doxorubicin cardiomyopathy. Cardiology. 115:155–162.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaczmarek A, Brinkman BM, Heyndrickx L,
Vandenabeele P and Krysko DV: Severity of doxorubicin-induced small
intestinal mucositis is regulated by the TLR-2 and TLR-9 pathways.
J Pathol. 226:598–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bun S, Yunokawa M, Tamaki Y, Shimomura A,
Shimoi T, Kodaira M, Shimizu C, Yonemori K, Fujiwara Y, Makino Y,
et al: Symptom management: The utility of regional cooling for
hand-foot syndrome induced by pegylated liposomal doxorubicin in
ovarian cancer. Support Care Cancer. 26:2161–2166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin J, Fang L, Li H, Li Z, Lyu L, Wang H
and Xiao J: Astragaloside IV alleviates doxorubicin induced
cardiomyopathy by inhibiting NADPH oxidase derived oxidative
stress. Eur J Pharmacol. 859:1724902019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J, Wu Y, Wang D, Zou L, Fu C, Zhang J
and Leung GP: Oridonin synergistically enhances the anti-tumor
efficacy of doxorubicin against aggressive breast cancer via
pro-apoptotic and anti-angiogenic effects. Pharmacol Res.
146:1043132019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu Z, Chen L, Xiao Z, Zhu Y, Jiang H, Jin
Y, Gu C, Wu Y, Wang L, Zhang W, et al: Potentiation of the
anticancer effect of doxorubicinin drug-resistant gastric cancer
cells by tanshinone IIA. Phytomedicine. 51:58–67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Caldwell S and Park SH: The epidemiology
of hepatocellular cancer: From the perspectives of public health
problem to tumor biology. Journal of Gastroenterology. 44 (Suppl
19):S96–S101. 2009. View Article : Google Scholar
|
|
27
|
El-Serag HB, Marrero JA, Rudolph L and
Reddy KR: Diagnosis and treatment of hepatocellular carcinoma.
Gastroenterology. 134:1752–1763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gnoni A, Santini D, Scartozzi M, Russo A,
Licchetta A, Palmieri V, Lupo L, Faloppi L, Palasciano G, Memeo V,
et al: Hepatocellular carcinoma treatment over sorafenib:
Epigenetics, microRNAs and microenvironment. Is there a light at
the end of the tunnel? Expert Opin Ther Targets. 19:1623–1635.
2015. View Article : Google Scholar
|
|
29
|
Han G, Xia J, Gao J, Inagaki Y, Tang W and
Kokudo N: Anti-tumor effects and cellular mechanisms of
resveratrol. Drug Discov Ther. 9:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anand David AV, Arulmoli R and Parasuraman
S: Overviews of biological importance of quercetin: A bioactive
flavonoid. Pharmacogn Rev. 10:84–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ren J, Lu Y, Qian Y, Chen B, Wu T and Ji
G: Recent progress regarding kaempferol for the treatment of
various diseases. Exp Ther Med. 18:2759–2776. 2019.PubMed/NCBI
|
|
32
|
Seydi E, Salimi A, Rasekh HR, Mohsenifar Z
and Pourahmad J: Selective cytotoxicity of luteolin and kaempferol
on cancerous hepatocytes obtained from Rat model of hepatocellular
carcinoma: Involvement of ROS-mediated mitochondrial targeting.
Nutr Cancer. 70:594–604. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han B, Yu YQ, Yang QL, Shen CY and Wang
XJ: Kaempferol induces autophagic cell death of hepatocellular
carcinoma cells via activating AMPK signaling. Oncotarget.
8:86227–86239. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo H, Ren F, Zhang L, Zhang X, Yang R,
Xie B, Li Z, Hu Z, Duan Z and Zhang J: Kaempferol induces apoptosis
in HepG2 cells via activation of the endoplasmic reticulum stress
pathway. Mol Med Rep. 13:2791–2800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang WW, Chiu YJ, Fan MJ, Lu HF, Yeh HF,
Li KH, Chen PY, Chung JG and Yang JS: Kaempferol induced apoptosis
via endoplasmic reticulum stress and mitochondria-dependent pathway
in human osteosarcoma U-2 OS cells. Mol Nutr Food Res.
54:1585–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu G, Liu X, Li H, Yan Y, Hong X and Lin
Z: Kaempferol inhibits proliferation, migration, and invasion of
liver cancer HepG2 cells by down-regulation of microRNA-21. Int J
Immunopathol Pharmacol. 32:20587384188143412018. View Article : Google Scholar : PubMed/NCBI
|