Introduction
Glioma the most common type of primary brain tumor,
accounts for >70% of intracranial malignant tumors. According to
histopathological characteristics, World Health Organization
classifies glioma into four grades (1). Glioblastoma multiforme (GBM) belongs to
grade IV and is the most malignant glioma. The prognosis of GBM is
poor: In Switzerland and the USA, the 5-year survival rate of
patients with glioblastoma is only 2.9% (2). GBM is known for its resistance to
treatments such as temozolomide (TMZ), the most commonly used
chemotherapy drug to treat glioma (3,4). This
makes the development of novel drugs for treating glioma
essential.
Corilagin is the primary active constituent of the
matsumura leafflower herb (a Phyllanthus plant from the
Euphorbiaceae family), which has potential for various biological
activities, including antitumor, anti-inflammatory, and
hepatoprotective activity (5).
Corilagin inhibits malignant tumor cell proliferation and promotes
tumor cell apoptosis (6). Both in
vitro and in vivo tests have demonstrated that corilagin
exerts a significant inhibitory effect on ovarian and colon cancer,
hepatocellular (7) and esophageal
squamous cell carcinoma (8) and other
types of tumor cell. Corilagin inhibits breast cancer growth via
reactive oxygen species (ROS)-dependent apoptosis and autophagy
(9). Moreover, it induces apoptosis,
autophagy and ROS generation in gastric cancer cells in
vitro. (10). Our previous
research (11) demonstrated that
corilagin effectively inhibits the proliferation of U251 and
stem-like cells. It is reported that corilagin also affects
TMZ-resistant T98G glioma cells (12). Taken together, these findings suggest
the potential use of corilagin in glioma treatment. However, the
precise mechanism by which corilagin promotes U251 cell apoptosis
warrants further research.
A key feature of glioma, similar to that of other
advanced tumors, is increased protein synthesis and degradation
(13). This increase is due to the
role of proteasomes in the degradation of signaling molecules,
tumor suppressors, cyclin and apoptosis inhibitors; proteasomes are
thus associated with glioma occurrence and development. The 26S
proteasome is the primary proteolytic mechanism that regulates
protein degradation in eukaryotic cells. The 26S proteasome
comprises 20S catalytic and 19S regulatory particles. The 20S
proteasome is a cylindrical structure comprising α and β subunits
arranged in four stacked heteroheptamer rings (14). The two β-rings face three active sites
of the β1, β2, and β5 subunits, which face the core particle
(15). When stimulated by
inflammatory cytokines, such as IFN-γ, immune cells, express large
multifunctional peptidase (LMP)2 (β1i), multicatalytic
endopeptidase complex-like-1 (MECL-1; β2i) and LMP7 (β5i). These
three inducible subunits, β1i, β2i,and β5i, replace the
constitutive subunits β1, β2, and β5 to form immunoproteasomes
(16). Moreover, β1/β1i, β2/β2i, and
β5/β5i are responsible for caspase-, trypsin- and chymotrypsin-like
activity, respectively (17). Studies
(18–20) have shown that β1i also exhibits a
certain chymotrypsin-like activity. However, the inhibitory effect
of corilagin-induced U251 cell apoptosis mediated by proteasome has
yet to be investigated. The present study investigated the role of
proteasomes in corilagin-induced U251 cell apoptosis.
Materials and methods
Chemicals and reagents
Corilagin analytical standard (purity, >99%) was
purchased from Sigma-Aldrich (Merck KGaA) and TMZ from Selleck
Chemicals. Anti-β1, anti-β2, anti-β5, anti-β1i, anti-β2i and
anti-β5i antibodies were procured from Abcam; anti-β-actin and
horseradish peroxidase (HRP)-conjugated goat anti-mouse and
anti-rabbit IgG secondary antibodies were acquired from OriGene
Technologies, Inc.; MG-132, bortezomib, carfizomib and PR-957 were
procured from Selleck Chemicals. DMEM, streptomycin penicillin, and
fetal calf serum were obtained from HyClone (GE Healthcare Life
Sciences); trypsin was obtained from Gibco (Thermo Fisher
Scientific, Inc.). BCA protein assay kit was purchased from
Beyotime Institute of Biotechnology, an Annexin V/PI apoptosis
detection kit from BD Biosciences and proteasome activity detection
kit from Promega Corporation.
U251 cell line culture
U251 cells ware cultured at 37°C in 5%
CO2 in high glucose DMEM and 10% fetal bovine serum
supplemented with penicillin (100 U/ml) and streptomycin (100
µg/ml). In order to evaluate proteasome involvement in
corilagin-induced cell differentiation, cells were treated with
proteasome inhibitors MG-132 (0, 200 nM, 1, 5 µM) and bortezomib
(0, 5, 40, 100 nM) for 72 h and carfizomib (0, 100, 200 nM, 1 µM)
and PR-957 (0, 100 nM, 1, 10 µM) for 24 h at 37°C. In order to
prove whether corilagin increases TMZ-induced apoptosis of U251
cells, flow cytometry was performed using U251 cells treated with
increasing concentrations of corilagin (0, 25, 50, 100 and 200
µg/ml) and/or TMZ (0, 25, 50, 100 and 200 µg/ml) for 72 h at 37°C.
In order to demonstrate that proteasome inhibition mediated
corilagin-induced apoptosis, flow cytometry was performed using
U251 cells treated with corilagin + MG-132 (200 nM) and corilagin +
bortezomib (5 nM) for 72 h at 37°C.
Cell Counting Kit (CCK)-8 assay
A total of 100 µl cell suspension (5,000 cells/well)
was inoculated in a 96-well plate, which was placed in a 37°C cell
incubator for 24 h. Next, 10 µl corilagin at various concentrations
(0, 25, 50 and 100 µg/ml) was added to the wells. After 24, 48 and
72 h of incubation at 37°C, 10 µl CCK-8 reagent (Beyotime Institute
of Biotechnology) was added to each well, followed by incubation at
37°C for 2 h. The developed color was measured by determining the
absorbance at 450 nm wavelength (A). Finally, the cell survival
rate (%) was calculated as [(Aexperimental
well-Ablank well)/(Acontrol
well-Ablank well)] × 100%.
Clonogenic survival assay
U251 cells were treated with corilagin (0, 25, 50
and 100 µg/ml) for 48 h at 37°C. The cells in the logarithmic
growth phase were inoculated at a density of 500–1,000 cells/well
in a 6-well plate in 10% fetal bovine serum complete DMEM and
incubated for 7–14 days at 37°C. For each concentration, three
auxiliary wells were used. Every 3 days, cell growth was monitored
and the medium was changed. When a single clone grew to a visible
size, the medium was removed and cells were washed twice with
phosphate-buffered saline and fixed with methanol for 20 min at
room temperature. After the methanol was removed, the cells were
stained with 0.2% crystal violet for 30 min at room temperature and
images were captured with an inverted light microscope (Olympus
IX71) at ×100 magnification.
Apoptosis by flow cytometry
U251 cells were incubated with different
concentrations (0, 25, 50, 100 and 200 µg/ml) of corilagin for 24,
48, 72 and 96 h at 37°C. cells were collected and washed with
phosphate-buffered saline three times. For the detection of
apoptosis, cells were stained using a Annexin V/PI Apoptosis
Detection kit according to the manufacturer's instructions. Samples
were analyzed by flow cytometry (BD FACSAria; BD Biosciences). The
data were analyzed by FlowJo 7.6.1 software (FlowJo LLC).
Western blot analysis
Corilagin (0, 25, 50 and 100 µg/ml) was added to
U251 cells (30–40% density) at 37°C which were inoculated in 6-well
plates. After 48 h, proteins were extracted. Protein concentration
was determined using BCA protein assay kit. U251 cells were
collected and lysed using RIPA cell lysis buffer (Invitrogen;
Thermo Fisher Scientific, Inc.). Following two washes with PBS,
cells were lysed with lysis buffer [20 mM Tris-HCl (pH, 7.5), 150
mM NaCl, 1 mM EDTA, 1% NP-40] containing Complete Protease
Inhibitor Cocktail (Roche Applied Science). The cell lysates (40
µg) were separated by 12% SDS-PAGE and transferred onto a PVDF
membrane. Membranes were blocked with 5% skimmed milk for 1 h at
room temperature and incubated with primary antibodies at a
dilution of 1:1,000 overnight at 4°C. Antibodies against the
following proteins were used: β1, β2, β5, β1i, β2i, β5i and
β-actin. Luminata Forte Western HRP Substrate (EMD Millipore) was
used for the development of positive signals. The relative protein
expression of genes was quantified by ImageJ v.1.46r software
(National Institutes of Health) using β-actin as a control.
Assay for proteasome activity
The 26S proteasome activity was analyzed based on a
previously published method of Promega Corporation proteasome
activity assay kit (21). Cells were
harvested and 10,000 cells in high glucose complete DMEM were
incubated with detection reagents containing fluorogenic substrates
of caspase-, trypsin- and chymotrypsin-like activity at 37°C for 1
h. The caspase-like activity was determined with Z-LLEC (45 µM),
trypsin-like activity with Ac-RLR-AMC (40 µM) and chymotrypsin-like
activity with Suc-LLVY-AMC (18 µM) at 37°C for 1 h. The
fluorescence intensity was measured at the following wavelengths:
Excitation, 380 and emission, 460 nm.
Statistical analysis
Data were analyzed by one-way ANOVA followed by
Tukey's multiple comparisons test by GraphPad Prism 8.0.2 (GraphPad
Software, Inc.). Data are presented as the mean ± SEM (≥3).
P<0.05 was considered to indicate a statistically significant
difference.
Results
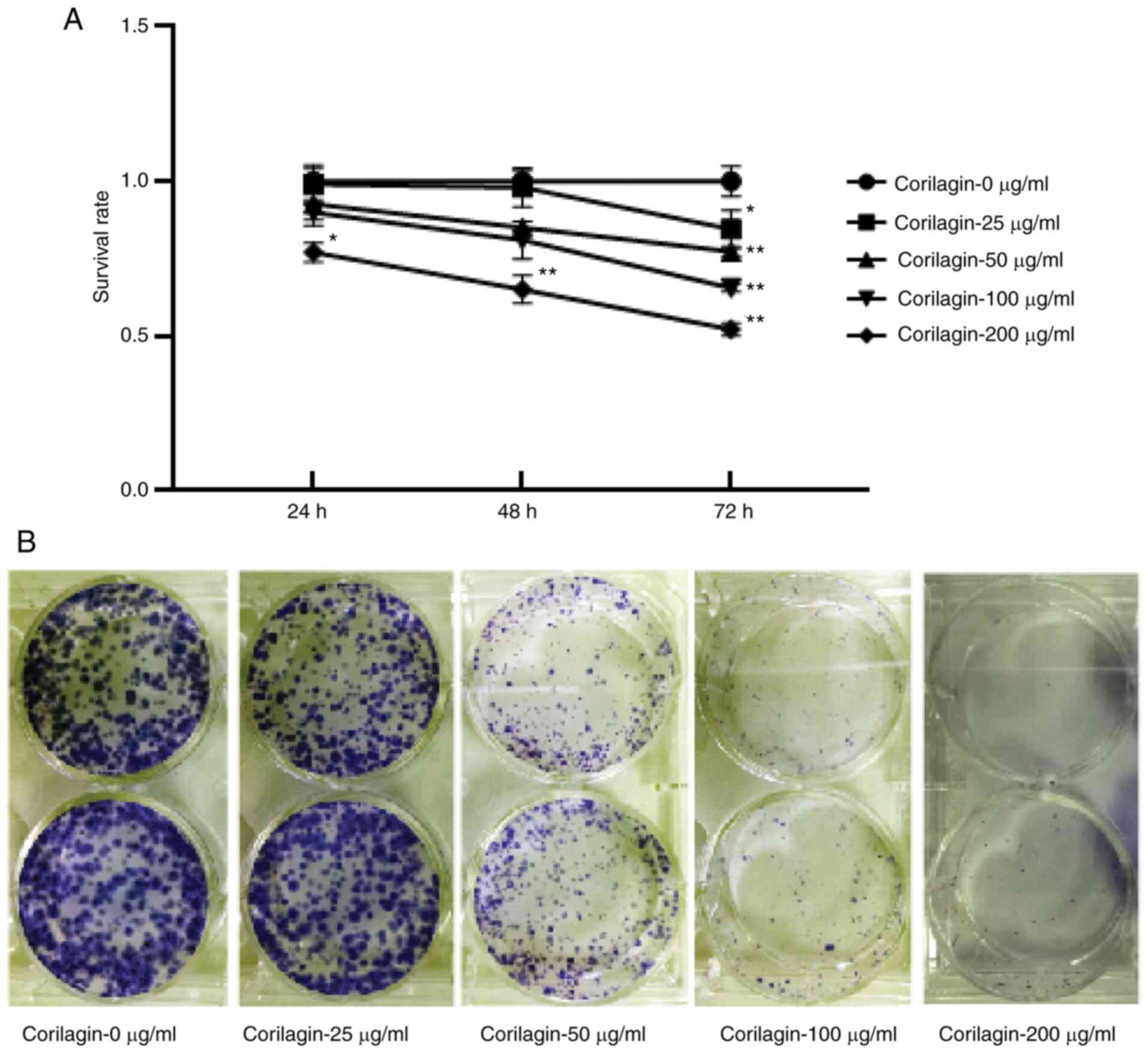
Corilagin inhibits U251
proliferation
In order to determine the role of corilagin in
regulating U251 growth, CCK-8 assay was performed to detect U251
cell survival following exposure to increasing corilagin
concentrations (0, 25, 50, 100 and 200 µg/ml) for 24, 48 and 72 h.
The results indicated that all concentrations of corilagin
significantly decreased survival over 72 h, and 200 µg/ml corilagin
at 24 and 48 h also significantly decreased the survival (Fig. 1A). Clone formation experiments on U251
cells treated with varying concentrations of corilagin for 48 h
also demonstrated that the number of cell clones decreased in a
dose-dependent manner (0, 25, 50, 100 and 200 µg/ml; Fig. 1B). Taken together, these data
suggested that corilagin inhibited U251 cell proliferation.
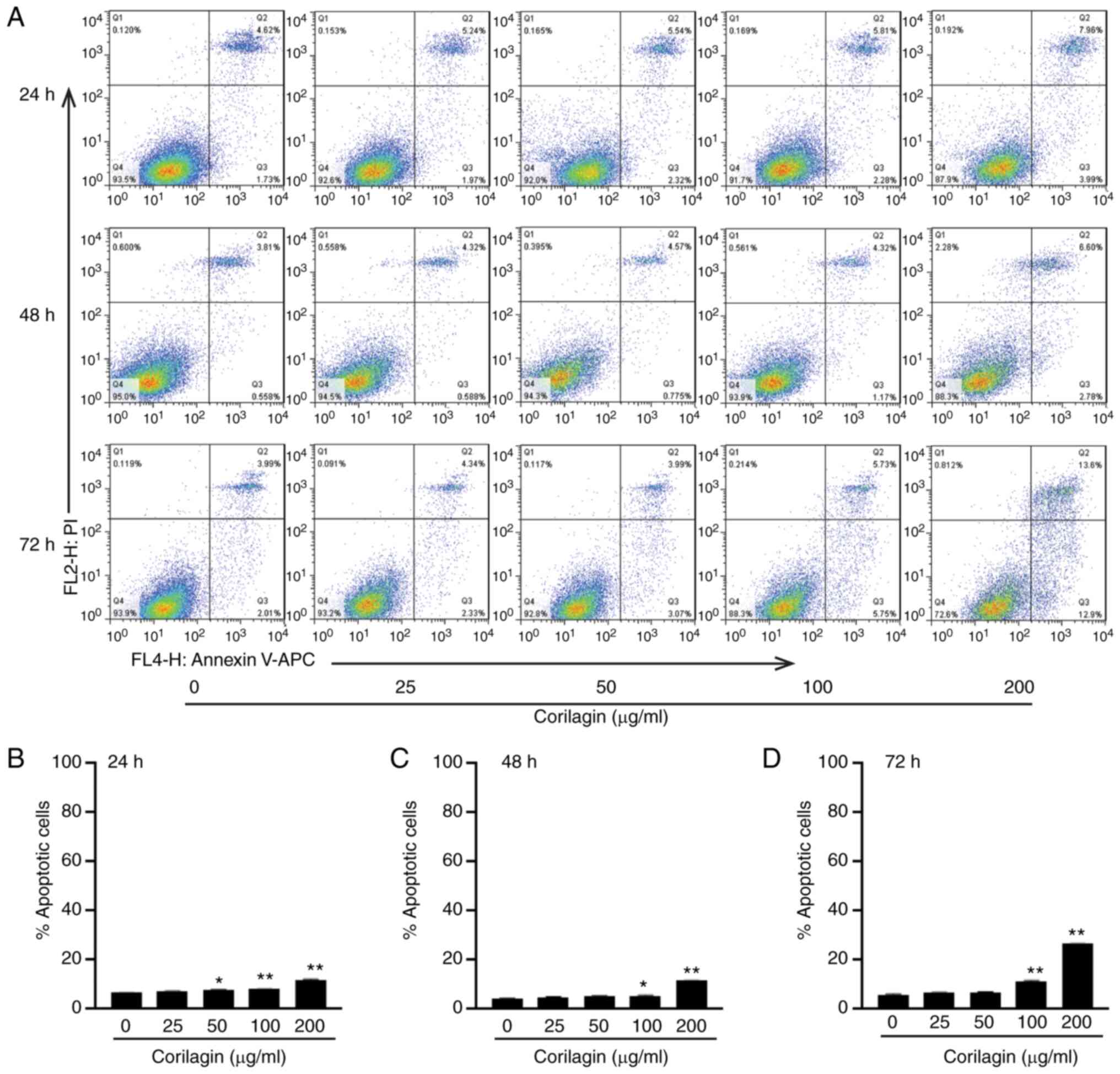
Corilagin promotes U251 apoptosis
In order to determine the role of corilagin in
regulating U251 apoptosis, apoptosis of U251 cells was assessed
using an Annexin V/PI staining kit. The results revealed that U251
cell apoptosis rates significantly increased following corilagin
treatment (0, 25, 50, 100 and 200 µg/ml) for 24, 48 and 72 h
(Fig. 2A). Following treatment with
200 µg/ml corilagin treatment for 24, 48, and 72 h, the ratio of
apoptotic cells significantly increased by 1.8-, 2.7- and 4.4-fold,
respectively (Fig. 2B-D).
Additionally, 50 µg/ml corilagin at 24 h and 100 µg/ml at all three
time points significantly increased the ratio of apoptotic cells.
Taken together, these results suggested that corilagin effectively
promoted U251 apoptosis.
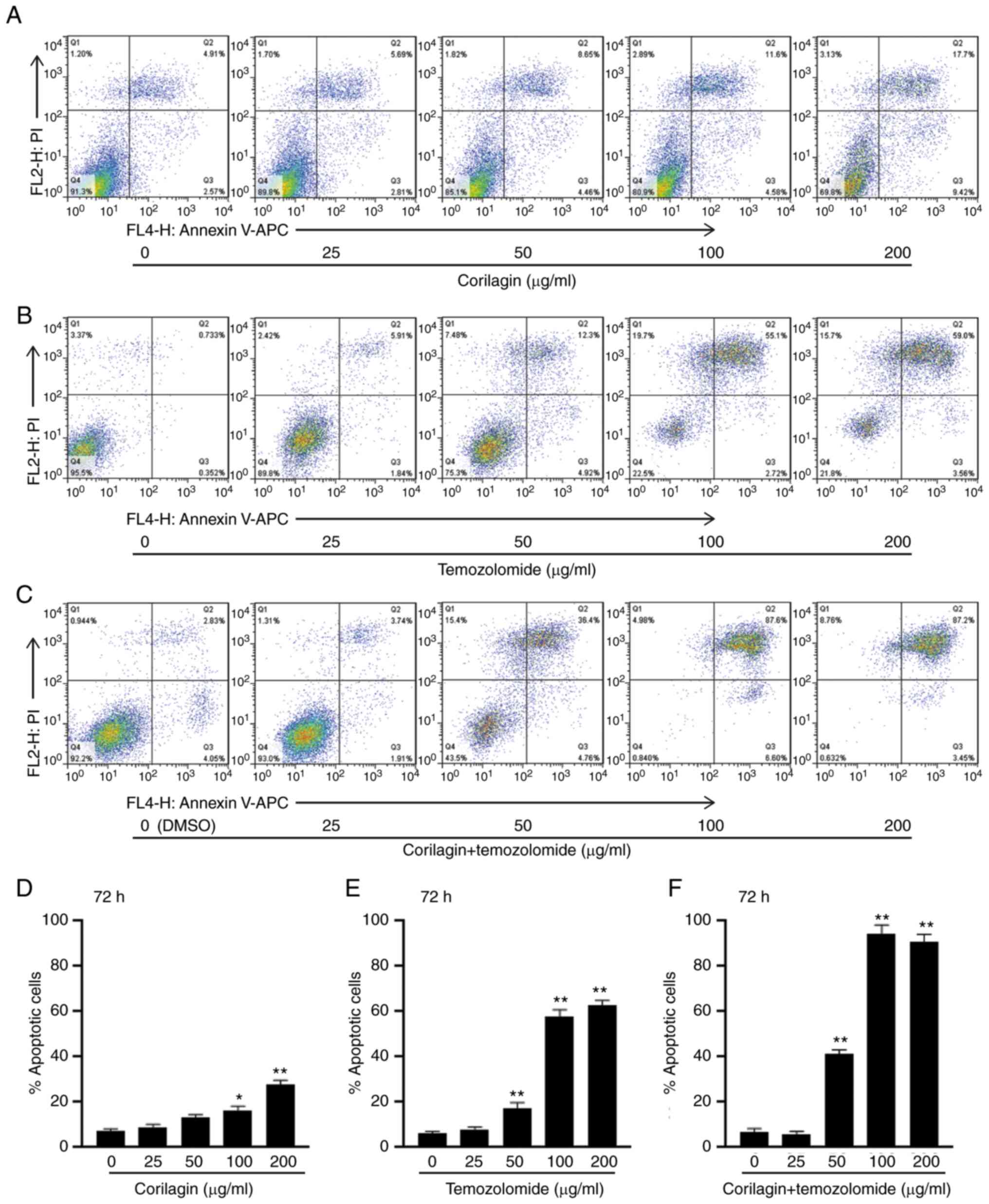
Corilagin combined with TMZ increases
apoptosis of U251 cells
In order to determine the role of corilagin + TMZ in
regulating U251 apoptosis, flow cytometry was performed. Images of
Annexin V/PI double staining of U251 cells treated with increasing
concentrations (0, 25, 50, 100 and 200 µg/ml) of corilagin, TMZ and
corilagin + TMZ for 72 h are shown in Fig. 3A-C. The percentage of apoptotic cells
(Annexin V+/PI+ and Annexin
V+/PI−) treated with corilagin, TMZ and
corilagin + TMZ was calculated (Fig.
3D-F). At 100 µg/ml corilagin + TMZ increased the apoptosis
rate from 58% in the TMZ-alone group to 94% Furthermore, 200 µg/ml
corilagin + TMZ increased the apoptosis rate from 62% in the
TMZ-alone group to 90% and 50 µg/ml corilagin + TMZ increased the
apoptosis rate from 17 to 40%. However, 50 µg/ml corilagin group
did not significantly increase the apoptosis rate compared with 0
µg/ml corilagin (Fig. 3E and F).
Taken together, these results suggested that corilagin + TMZ
increased apoptosis of U251 cells.
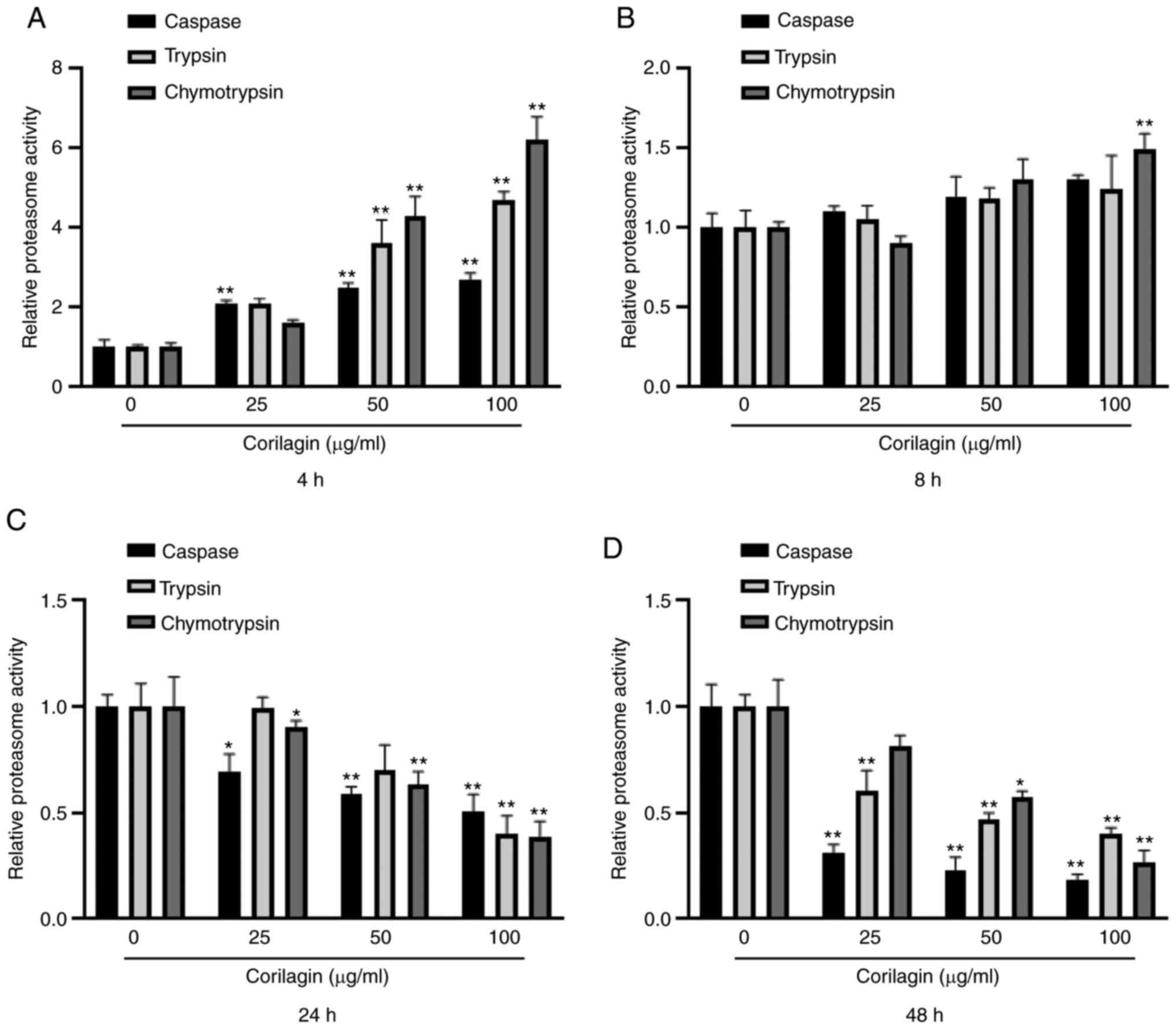
Corilagin decreases proteasome
activity
In order to determine the role of proteasome in
corilagin-induced U251 cell apoptosis, the effect of corilagin on
proteasome activity was investigated. U251 cells were treated with
corilagin at different concentrations (0, 25, 50 and 100 µg/ml) and
durations (4, 8, 24 and 48 h). The proteasome activity, including
caspase-, trypsin- and chymotrypsin-like activity, was measured
using fluorescent-labeled peptides. At 4 h, corilagin treatment
significantly increased caspase-, trypsin- and chymotrypsin-like
activity in a dose-dependent manner (Fig.
4A). At 8 h, chymotrypsin-like activity was significantly
increased by 100 µg/ml corilagin (Fig.
4B). At 24 and 48 h, caspase-, trypsin- and chymotrypsin-like
activities significantly decreased in a dose-dependent manner
(Fig. 4C and D). These results
suggested that corilagin-induced apoptosis was associated with
proteasome activity.
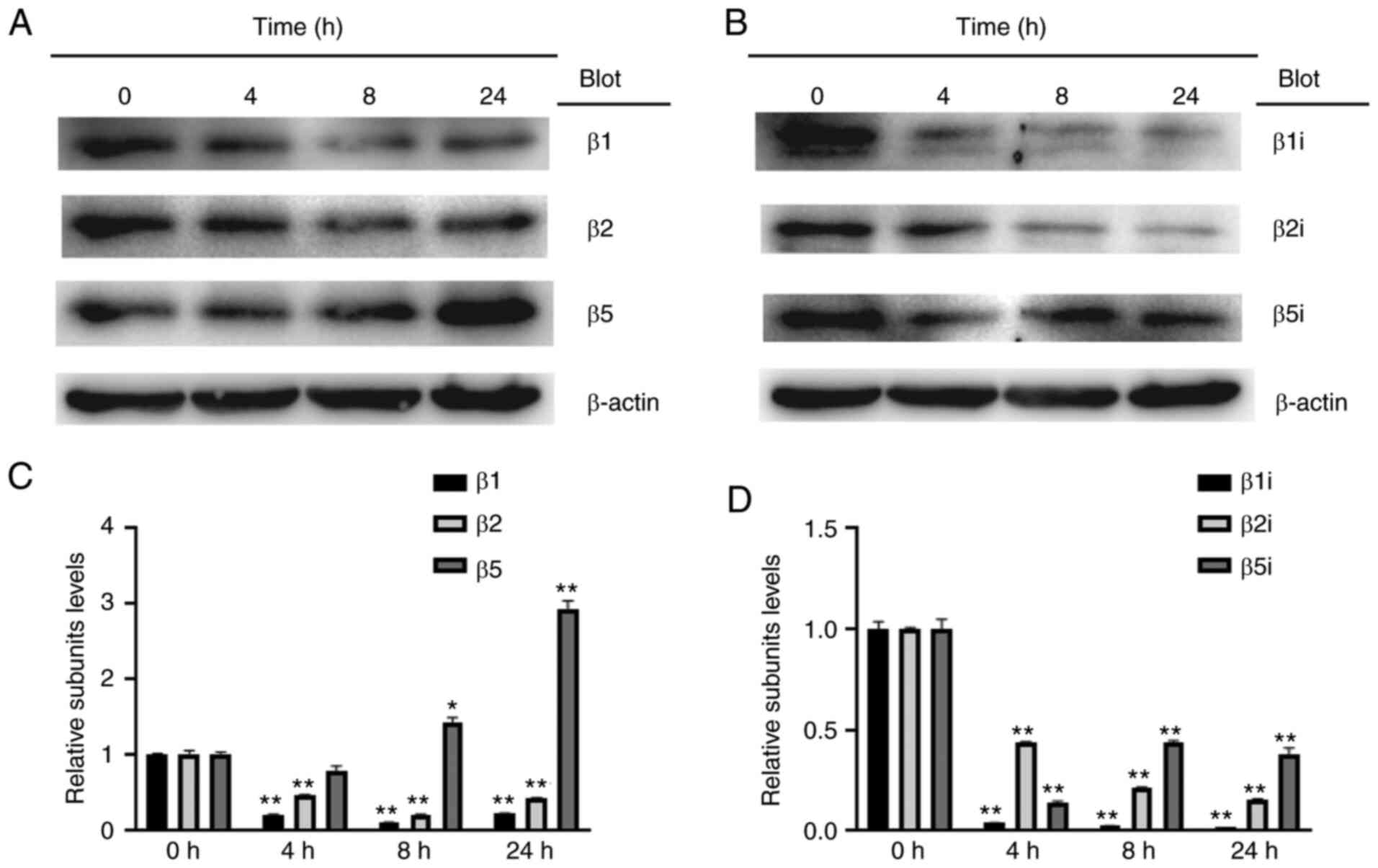
Corilagin decreases expression levels
of proteasome subunits
In order to determine the effect of corilagin on
proteasome catalytic subunit expression levels, U251 cells were
treated with corilagin (100 µg/ml) for different durations (4, 8
and 24 h). The protein expression levels of the constitutive (i.e.,
β1, β2, and β5; Fig. 5A) and
inducible proteasome catalytic subunits (i.e., β1i, β2i and β5i;
Fig. 5B) were detected by western
blotting. Corilagin treatment significantly decreased the protein
levels of constitutive subunits (β1 and β2) and immunosubunits
(β1i, β2i and β5i) but increased those of the constitutive subunit
β5 (Fig. 5C and D), indicating that
the corilagin-induced decrease in proteasome activity in U251 cells
was primarily mediated by decreased expression levels of proteasome
subunits β1/β1i, β2i and β5i.
Proteasome inhibitors promote U251
apoptosis
In order to determine the role of proteasome in
corilagin-mediated regulation of U251 apoptosis, the apoptotic rate
of U251 cells treated with proteasome inhibitors MG-132 (0, 200 nM,
1, 5 µM; Fig. 6B), bortezomib (0, 5,
40, 100 nM; Fig. 6E) for 72 h and
carfizomib (0, 100, 200 nM, 1 µM; Fig.
6H) and PR-957 (0, 100, 1, 10 µM; Fig. 6K) for 24 h was assessed using an
Annexin V/PI staining kit. Before detecting apoptosis, the effects
of proteasome inhibitors on the activity of three proteasome
enzymes was assessed. MG-132 and bortezomib significantly inhibited
caspase- and chymotrypsin-like activity; lower concentrations of
bortezomib were needed to achieve this inhibitory effect (Fig. 6A and D). Carfilzomib and PR-957
significantly inhibited chymotrypsin-like enzyme activity and
carfilzomib also significantly inhibited caspase-like enzyme
activity at a concentration of 1 µM (Fig.
6G and J). The apoptosis assay results showed that all
inhibitors increased the percentage of apoptotic cells in a
dose-dependent manner (Fig. 6C, F, I and
L).
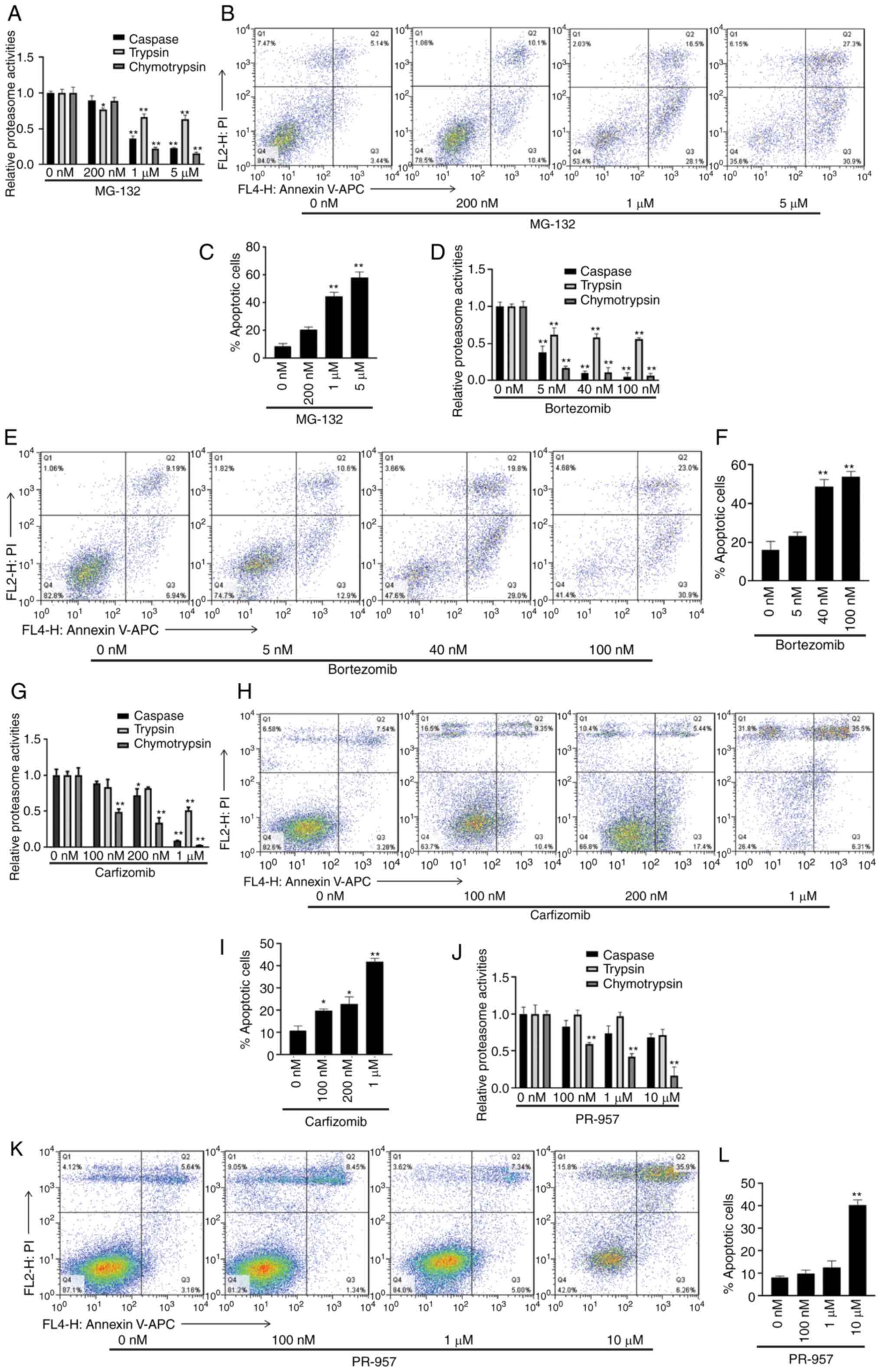 | Figure 6.MG-132, Bortezomib, carfizomib and
PR-957 induce U251 apoptosis. (A) Caspase-, trypsin- and
chymotrypsin-like proteasome activity of U251 cells treated with
MG-132 (100, 500 nM, 1 and 5 µM) for 72 h were measured. (B) Flow
cytometry analysis of U251 cells treated with increasing
concentrations of MG-132 for 72 h. (C) Ratio of apoptotic (Annexin
V+/PI+ and Annexin
V+/PI−) of MG-132-treated U251 cells was
calculated. (D) Proteasome activity, (E) flow cytometry and (F)
ratio of apoptotic U251 treated with bortezomib (0, 5, 40 and 100
nM) for 72 h. (G) Proteasome activity, (H) flow cytometry and (I)
ratio of apoptotic cells of U251 treated with carfizomib (0, 100,
200 nM, 1 µM) for 24 h. (J) Proteasome activity, (K) flow cytometry
and (L) ratio of apoptotic U251 cells treated with PR-957 (0, 100
nM, 1, 10 µM) for 24 h. Data are presented as the mean ± SEM
(n=5/group). *P<0.05, **P<0.01) vs. 0 nM. |
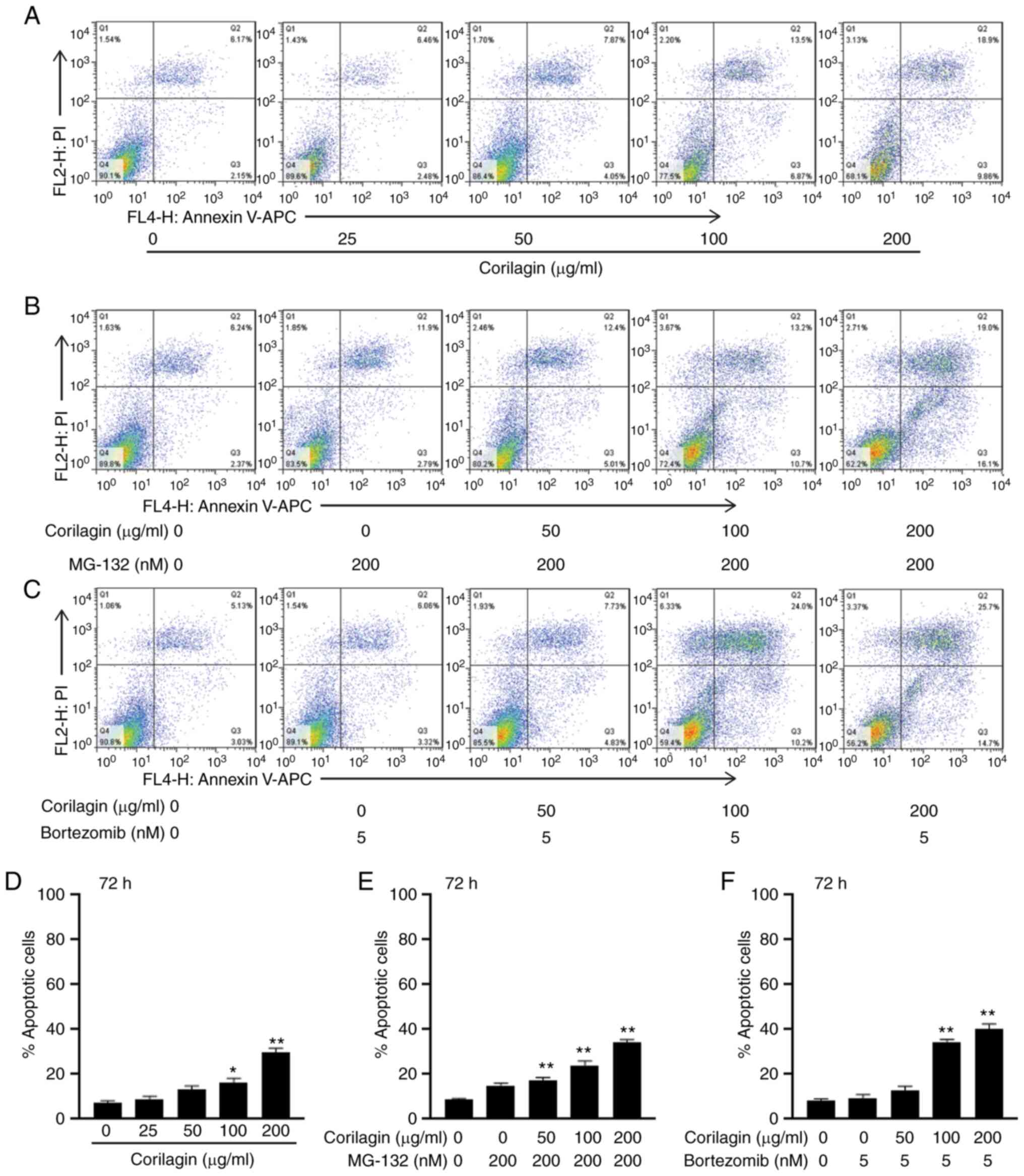
Proteasome inhibitors increase
corilagin-induced apoptosis of U251 cells
In order to demonstrate that proteasome inhibition
mediated corilagin-induced apoptosis, flow cytometry was performed
using U251 cells treated with corilagin + MG-132 (200 nM) and
corilagin + bortezomib (5 nM). Images of Annexin V/PI double
staining of U251 cells treated with increasing concentrations (0,
25, 50, 100 and 200 µg/ml) of corilagin, corilagin + MG-132 (200
nM), and corilagin + bortezomib (5 nM) for 72 h are shown in
Fig. 7A-C. The percentages of
apoptotic cells (Annexin V+/PI+ and Annexin
V+/PI−) of cells treated with corilagin,
corilagin + MG-132 and corilagin + bortezomib were calculated
(Fig. 7D-F). The results showed that
corilagin + MG-132 (200 nM) increased the percentage of apoptotic
cells compared with corilagin-alone (50, 100 and 200 ug/ml) from
13, 16 and 27 to 17, 23 and 34%, respectively (Fig. 7E). Corilagin + bortezomib (5 nM)
increased the percentage of apoptotic cells to 12, 34 and 40%
(Fig. 7F). These results indicated
that proteasome inhibitors increased corilagin-induced
apoptosis.
Discussion
The present study investigated the role of
proteasome activation in corilagin-induced U251 cell apoptosis.
Corilagin primarily stimulated U251 cell apoptosis and decreased
the activity and expression levels of proteasome subunits,
including immunosubunits. U251 cells were treated with proteasome
inhibitors, which were shown to promote apoptosis in these cells.
In order to demonstrate that proteasome inhibition mediates
corilagin-induced apoptosis, U251 cells were treated with corilagin
in combination with the proteasome inhibitors MG-132 and
bortezomib. The results of Annexin V/PI staining analysis for
corilagin, corilagin + MG-132 and corilagin + bortezomib indicated
that proteasome inhibitors increased corilagin-induced apoptosis.
The present study therefore identified a novel potential mechanism
for proteasomes in corilagin-induced U251 cell apoptosis.
The present study demonstrated that corilagin
effectively promoted the apoptosis of glioma U251 cells. Research
has indicated that corilagin inhibits the growth of U251 and
TMZ-resistant T98G glioma cells (12). Qiu et al (8) determined that corilagin downregulates
the E3 ubiquitin ligase RING finger protein 8 in the
ubiquitin-proteasome pathway, which disrupts the DNA damage repair
response and promotes cell death in esophageal squamous cell cancer
cells.
During corilagin-induced U251 cell apoptosis, the
proteasome catalytic subunit expression levels were all
downregulated in the present study, except for those of β5.
However, proteasome activity first increased and then decreased,
particularly at 4 h, at which time proteasome activity was notably
increased. This appears to contradict the decreased expression
levels. The proteasome is a biological complex composed of multiple
molecules, the assembly of which is complex and strictly regulated
by various mechanisms, such as the assembly of proteasome activator
200 (22) and Adc17, the absence of
which aggravates proteasome defects (23). The levels of proteasome are affected
by proteasome subunits and assembly chaperones (17). Proteasome activity is also regulated
by phosphorylation of 26S subunits (24) and by protein kinases that have been
reported to stimulate proteasomal activity (25). For example, increased cAMP levels and
protein kinase A activation cause phosphorylation of the 19S
subunit Rpn6 (26), which increases
peptide, ATP and Ubiquitin conjugate hydrolysis rates (27). The inconsistency between changes in
proteasome activity and catalytic subunit expression levels require
further analysis. It was hypothesized that in the early stage (4 h)
of corilagin intervention in cells, proteasome subunit expression
levels were affected and proteasome assembly was altered, but the
proteasome activity was stimulated in response to external stress.
After 8 h, proteasome function became increasingly damaged, causing
proteasome activity to continue to decline, resulting in decreased
physiological cell activity, finally leading to apoptosis.
In glioma cells, the expression levels of
immunosubunits are high (28), but
their mechanism of involvement in glioma occurrence and development
remains unclear. Studies have revealed that immunoproteasomes
regulate T helper cell differentiation (29,30).
Several factors, including IFN-γ and TNF-α, promote catalytic
subunit expression to produce immunoproteasomes and thereby affect
the enzymatic activity of their substrates (17) and alter cell function (31). Notably, in the present study, β5
expression was upregulated and β5i expression was downregulated
during corilagin intervention. It was hypothesized that corilagin
changed the proteasome structure. The assembly of proteasome
subunits is complex and strictly regulated; it is achieved by the
controlled expression of proteasome subunits by a common
transcription factor (such as Rpn4) in yeast (32). Cells adjust proteasome-mediated
degradation by regulating proteasome levels via coordinated
expression of proteasome subunits and assembly chaperones (17).
In order to demonstrate that corilagin promoted
apoptosis by interfering with proteasome activity, U251 cells were
treated with four proteasome inhibitors. Proteasome inhibitors
inhibited proteasome activity and promoted apoptosis of U251 cells.
Proteasome inhibitors are widely used to study the function of
proteasomes and are employed as anticancer drugs to treat various
types of cancer (18,33,34). The
following proteasome inhibitors were used in the present study:
MG-132, a reversible inhibitor of β1, β2, and β5; bortezomib, a
reversible inhibitor of β5, β5i and β1i; carfilzomib, an
irreversible inhibitor of β5 and β5i and PR-957, a specific
irreversible inhibitor of β5i (35,36). Yoo
et al (37) reported that
glioma stem cells are sensitive to proteasome inhibitors. MG-132
has also been used in glioma research (38,39). Both
bortezomib and carfilzomib have been approved for the treatment of
multiple myeloma (40) and their use
has also been reported in glioma research (40,41).
The International Journal of Radiation Oncology, Biology,
Physics published a phase-II clinical trial in 2018 (42) that evaluated the efficacy and safety
of bortezomib in combination with TMZ and local radiotherapy for
GBM. Bortezomib inhibits cell adhesion, angiogenesis and
cytokine-mediated intercellular communication, thereby affecting
the tumor microenvironment (33).
Bortezomib and TMZ in combination with local radiotherapy is
particularly beneficial for people with O-6-methylguanine-DNA
methyltransferase methylation and its side effects (such as
lymphopenia, neutropenia and thrombocytopenia) are generally mild
(42). PR-957, which specifically
inhibits β5i activity (35), is used
to study autoimmune (43) and
inflammatory disease (36). To the
best of our knowledge, however, it has not been used in the study
of glioma treatment. The present study demonstrated that PR-957
effectively promoted U251 cell apoptosis and its mechanism of
action warrants further investigation. Given the inhibitory effect
of proteasome inhibitors on glioma, future studies should
investigate whether corilagin can be combined with proteasome
inhibitors to improve its therapeutic effects on glioma.
Acknowledgements
Not applicable.
Funding
The present study was supported by Project of
Scientific Developmental Program of Shandong Provincial
Administration of Traditional Chinese Medicine (grant no.
2019-0479), Project of Health and Family Planning Commission of
Shandong Province (grant nos. 2019WS362 and 2019WS361), Teacher
Support Fund of Jining Medical University (grant no.
JYFC2018FKJ107), General Project of Jining Science and Technology
Bureau (grant no. 2016-56-60), Scientific Research Project of
Jining Medical University (grant no. JY2015KJ022) and Nursery
Research Program of Affiliated Hospital of Jining Medical
University (grant nos. MP-2015-003 and MP-2018-012).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XQ and FJ designed the experiments. JL and WM
performed western blot analysis, activity assays and cell
experiments. PC performed flow cytometry. XQ and DP analyzed data
and wrote the manuscript. XQ and FJ authenticated all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gusyatiner O and Hegi ME: Glioma
epigenetics: From subclassification to novel treatment options.
Semin Cancer Biol. 51:50–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohgaki H and Kleihues P: Epidemiology and
etiology of gliomas. Acta Neuropathol. 109:93–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Q, Zhou Y, Chen J, Huang N, Wang Z
and Cheng Y: Gene therapy for drug-resistant glioblastoma via
lipid-polymer hybrid nanoparticles combined with focused
ultrasound. Int J Nanomedicine. 16:185–199. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Deng Y, Zheng Z, Huang W, Chen L,
Tong Q and Ming Y: Corilagin, a promising medicinal herbal agent.
Biomed Pharmacother. 99:43–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu C, Huang H, Choi HY, Ma Y, Zhou T, Peng
Y, Pang K, Shu G and Yang X: Anti-esophageal cancer effect of
corilagin extracted from phmllanthi fructus via the mitochondrial
and endoplasmic reticulum stress pathways. J Ethnopharmacol.
269:1137002021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wan LF, Shen JJ, Wang YH, Zhao W, Fang NY,
Yuan X and Xue BY: Extracts of Qizhu decoction inhibit hepatitis
and hepatocellular carcinoma in vitro and in C57BL/6 mice by
suppressing NF-κB signaling. Sci Rep. 9:14152019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiu F, Liu L, Lin Y, Yang Z and Qiu F:
Corilagin inhibits esophageal squamous cell carcinoma by inducing
DNA damage and down-regulation of RNF8. Anticancer Agents Med Chem.
19:1021–1028. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tong Y, Zhang G, Li Y, Xu J, Yuan J, Zhang
B, Hu T and Song G: Corilagin inhibits breast cancer growth via
reactive oxygen species-dependent apoptosis and autophagy. J Cell
Mol Med. 22:3795–3807. 2018. View Article : Google Scholar
|
|
10
|
Iweala EEJ, Xu J, Zhang G, Tong Y, Yuan J,
Li Y and Song G: Corilagin induces apoptosis, autophagy and ROS
generation in gastric cancer cells in vitro. Int J Mol Med.
43:967–979. 2019.PubMed/NCBI
|
|
11
|
Yang WT, Li GH, Li ZY, Feng S, Liu XQ, Han
GK, Zhang H, Qin XY, Zhang R, Nie QM and Jin F: Effect of corilagin
on the proliferation and NF-κB in U251 glioblastoma cells and U251
glioblastoma stem-like Cells. Evid Based Complement Alternat Med.
2016:14183092016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Milani R, Brognara E, Fabbri E, Finotti A,
Borgatti M, Lampronti I, Marzaro G, Chilin A, Lee KK, Kok SH, et
al: Corilagin induces high levels of apoptosis in the
temozolomide-resistant T98G glioma cell line. Oncol Res.
26:1307–1315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee J, Kim J, Kim EM, Kim U, Kang AR, Park
JK and Um HD: p21WAF1/CIP1 promotes p53 protein
degradation by facilitating p53-Wip1 and p53-Mdm2 interaction.
Biochem Biophys Res Commun. 543:23–28. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakata E, Eisele MR and Baumeister W:
Molecular and cellular dynamics of the 26S proteasome. Biochim
Biophys Acta Proteins Proteom. 1869:1405832021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bard JAM, Goodall EA, Greene ER, Jonsson
E, Dong KC and Martin A: Structure and function of the 26S
proteasome. Annu Rev Biochem. 87:697–724. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murata S, Takahama Y, Kasahara M and
Tanaka K: The immunoproteasome and thymoproteasome: Functions,
evolution and human disease. Nat Immunol. 19:923–931. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rousseau A and Bertolotti A: Regulation of
proteasome assembly and activity in health and disease. Nat Rev Mol
Cell Biol. 19:697–712. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ettari R, Pallio G, Pizzino G, Irrera N,
Zappalà M, Maiorana S, Di Chio C, Altavilla D, Squadrito F and
Bitto A: Non-covalent immunoproteasome inhibitors induce cell cycle
arrest in multiple myeloma MM.1R cells. J Enzyme Inhib Med Chem.
34:1307–1313. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuzina ES, Kudriaeva AA, Maltseva DV and
Belogurov AA Jr: Peptidyl aldehyde specifically interacts with
immunosubunit β1i proteasome: In vitro and in vivo effects. Bull
Exp Biol Med. 161:69–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun C, Mo M, Wang Y, Yu W, Song C, Wang X,
Chen S and Liu Y: Activation of the immunoproteasome protects
SH-SY5Y cells from the toxicity of rotenone. Neurotoxicology.
73:112–119. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vilchez D, Boyer L, Morantte I, Lutz M,
Merkwirth C, Joyce D, Spencer B, Page L, Masliah E, Berggren WT, et
al: Increased proteasome activity in human embryonic stem cells is
regulated by PSMD11. Nature. 489:304–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Toste Rêgo A and da Fonseca PCA:
Characterization of fully recombinant human 20S and 20S-PA200
proteasome complexes. Mol Cell. 76:138–147.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanssum A, Zhong Z, Rousseau A, Krzyzosiak
A, Sigurdardottir A and Bertolotti A: An inducible chaperone adapts
proteasome assembly to stress. Mol Cell. 55:566–577. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Collins GA and Goldberg AL: The logic of
the 26S proteasome. Cell. 169:792–806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuo CL and Goldberg AL: Ubiquitinated
proteins promote the association of proteasomes with the
deubiquitinating enzyme Usp14 and the ubiquitin ligase Ube3c. Proc
Natl Acad Sci USA. 114:E3404–E3413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lokireddy S, Kukushkin NV and Goldberg AL:
cAMP-induced phosphorylation of 26S proteasomes on Rpn6/PSMD11
enhances their activity and the degradation of misfolded proteins.
Proc Natl Acad Sci USA. 112:E7176–E7185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Myeku N, Clelland CL, Emrani S, Kukushkin
NV, Yu WH, Goldberg AL and Duff KE: Tau-driven 26S proteasome
impairment and cognitive dysfunction can be prevented early in
disease by activating cAMP-PKA signaling. Nat Med. 22:46–53. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Min L, Zeng X, Li B, Tao B, Shi J, Zhang
W, Sun Q, Jing C and Wang X: Overexpression of immunoproteasome
low-molecular-mass polypeptide 7 and inhibiting role of
next-generation proteasome inhibitor ONX 0912 on cell growth in
glioma. Neuroreport. 30:1031–1038. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qin XY, Zhang YL, Chi YF, Yan B, Zeng XJ,
Li HH and Liu Y: Angiotensin II regulates Th1 T cell
differentiation through angiotensin II type 1 receptor-PKA-mediated
activation of proteasome. Cell Physiol Biochem. 45:1366–1376. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ettari R, Previti S, Bitto A, Grasso S and
Zappalà M: Immunoproteasome-selective inhibitors: A promising
strategy to treat hematologic malignancies, autoimmune and
inflammatory diseases. Curr Med Chem. 23:1217–1238. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Welk V, Coux O, Kleene V, Abeza C,
Trümbach D, Eickelberg O and Meiners S: Inhibition of proteasome
activity induces formation of alternative proteasome complexes. J
Biol Chem. 291:13147–13159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mannhaupt G, Schnall R, Karpov V, Vetter I
and Feldmann H: Rpn4p acts as a transcription factor by binding to
PACE, a nonamer box found upstream of 26S proteasomal and other
genes in yeast. FEBS Lett. 450:27–34. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zarfati M, Avivi I, Brenner B, Katz T and
Aharon A: Extracellular vesicles of multiple myeloma cells utilize
the proteasome inhibitor mechanism to moderate endothelial
angiogenesis. Angiogenesis. 22:185–196. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park JE, Miller Z, Jun Y, Lee W and Kim
KB: Next-generation proteasome inhibitors for cancer therapy.
Transl Res. 198:1–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huber EM, Basler M, Schwab R, Heinemeyer
W, Kirk CJ, Groettrup M and Groll M: Immuno- and constitutive
proteasome crystal structures reveal differences in substrate and
inhibitor specificity. Cell. 148:727–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Muchamuel T, Basler M, Aujay MA, Suzuki E,
Kalim KW, Lauer C, Sylvain C, Ring ER, Shields J, Jiang J, et al: A
selective inhibitor of the immunoproteasome subunit LMP7 blocks
cytokine production and attenuates progression of experimental
arthritis. Nat Med. 15:781–787. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoo YD, Lee DH, Cha-Molstad H, Kim H, Mun
SR, Ji C, Park SH, Sung KS, Choi SA, Hwang J, et al: Glioma-derived
cancer stem cells are hypersensitive to proteasomal inhibition.
EMBO Rep. 18:150–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Su L, Guo W, Lou L, Nie S, Zhang Q, Liu Y,
Chang Y, Zhang X, Li Y and Shen H: EGFR-ERK pathway regulates CSN6
to contribute to PD-L1 expression in glioblastoma. Mol Carcinog.
59:520–532. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang XF, Zhao ZJ, Liu JJ, Yang XH, Gao Y,
Zhao S, Shi S, Huang KQ and Zheng HC: SAHA and/or MG132 reverse the
aggressive phenotypes of glioma cells: An in vitro and vivo study.
Oncotarget. 8:3156–3169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tang JH, Yang L, Chen JX, Li QR, Zhu LR,
Xu QF, Huang GH, Zhang ZX, Xiang Y, Du L, et al: Bortezomib
inhibits growth and sensitizes glioma to temozolomide (TMZ) via
down-regulating the FOXM1-Survivin axis. Cancer Commun (Lond).
39:812019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang M, Lu L, Ying M, Ruan H, Wang X,
Wang H, Chai Z, Wang S, Zhan C, Pan J and Lu W: Enhanced
Glioblastoma targeting ability of carfilzomib enabled by a
DA7R-modified lipid nanodisk. Mol Pharm. 15:2437–2447. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kong XT, Nguyen NT, Choi YJ, Zhang G,
Nguyen HN, Filka E, Green S, Yong WH, Liau LM, Green RM, et al:
Phase 2 study of bortezomib combined with temozolomide and regional
radiation therapy for upfront treatment of patients with newly
diagnosed glioblastoma multiforme: Safety and efficacy assessment.
Int J Radiat Oncol Biol Phys. 100:1195–1203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu H, Wan C, Ding Y, Han R, He Y, Xiao J
and Hao J: PR-957, a selective inhibitor of immunoproteasome
subunit low-MW polypeptide 7, attenuates experimental autoimmune
neuritis by suppressing Th17-cell differentiation and
regulating cytokine production. FASEB J. 31:1756–1766. 2017.
View Article : Google Scholar : PubMed/NCBI
|