Hypoxia-inducible factor (HIF)-2α is a member of the
hypoxia-inducible factor family. HIF-2α, also referred to as
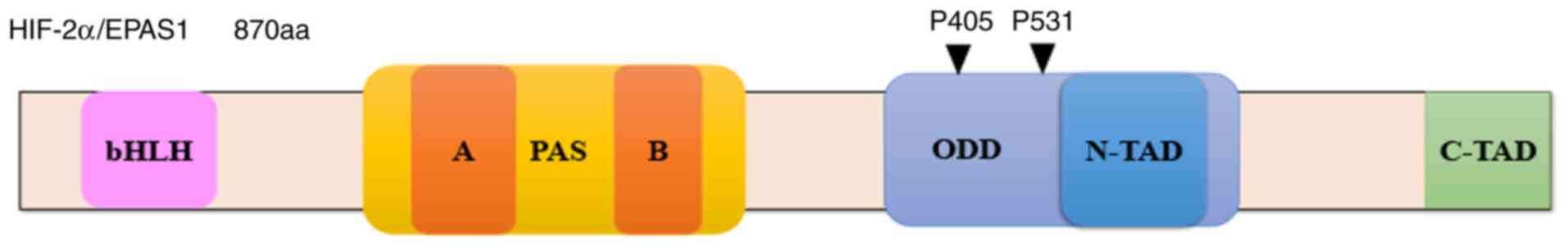
endothelial PAS domain protein-1 (EPAS1) (Fig. 1), was first identified in endothelial
cells as a transcription factor stimulated under hypoxic conditions
(1–3).
HIF-2α shares 48% amino acid sequence identity with HIF-1α and
enhances the expression of erythropoietin, vascular endothelial
growth factor (VEGF) and various glycolytic enzymes in vivo
(2). Despite this homology, HIF-α
expression differs under different oxygen concentrations. For
example, under normoxic and slightly hypoxic conditions, cells were
shown to express HIF-2α more obviously than HIF-1α (3). An increasing number of studies have
attempted to fully explore the role of HIF-α. HIF-2α expression has
been identified in a number of non-vascular endothelial sites, such
as the liver, kidney and sympathetic nervous system (4). Based on these findings, HIF-2α
expression was indicated to be strongly associated with systems
that were sensitive to hypoxia, including the vasculature and the
nervous system. Moreover, tumours exhibiting extensive angiogenesis
and high sensitivity to hypoxia have been demonstrated to be rich
in HIF-2α. Nuclear expression of HIF-2α has been reported in
various solid tumours of the bladder, brain, breast, colon, ovary,
pancreas, prostate and kidney (5).
HIF-2α was even found to be strongly expressed in subsets of
tumour-associated macrophages, despite its lack of expression in
the tumour (5). Although HIF-2α has
been shown to serve as an oncogene in most tumours, some studies
described opposite results, with inhibited tumour growth
accompanied by overexpression of HIF-2α (6). In addition, HIF-2α was confirmed to be
important in other types of diseases, such as preeclampsia
(7), hypoglycaemia (8), non-alcoholic fatty liver (9) and osteoarthritis (10).
The aim of the present review was to summarise our
current knowledge of the role of HIF-2α in lung cancer and provide
information on its initial discovery, structure, relationship with
different pathophysiological processes and, finally, its potential
as a targeted therapy strategy in lung cancer.
HIF-2α has been explored and confirmed to act as an
oncogene in various tumours from almost all human organ systems,
including renal carcinoma (15),
pancreatic cancer (16),
hepatocellular carcinoma (17),
colorectal adenocarcinoma (18), lung
cancer (19), neuroblastoma (20), osteosarcoma (21), breast cancer (22), bladder cancer (23) and oral squamous cell carcinoma
(24). However, some studies have
suggested that HIF-2α is not associated with tumours. Its
expression was strongly inhibited in small cell lung cancer cells
(25), and no significant effects in
functional tests in vitro were detected in hepatocellular
carcinoma cells with efficient downregulation of HIF-2α (26). In some clinical studies, HIF-2α was
identified as a tumour suppressor gene. For example, in large
neuroblastoma patient datasets, high expression levels of HIF-2α
were significantly associated with the expression of neuronal
differentiation genes and a more favourable prognosis (27), whereas xenograft studies revealed that
HIF-2α deficiency stimulated tumour growth and was correlated with
advanced tumour stage in colon cancer (28). Therefore, the findings of different
studies on the role of HIF-2α have been contradictory.
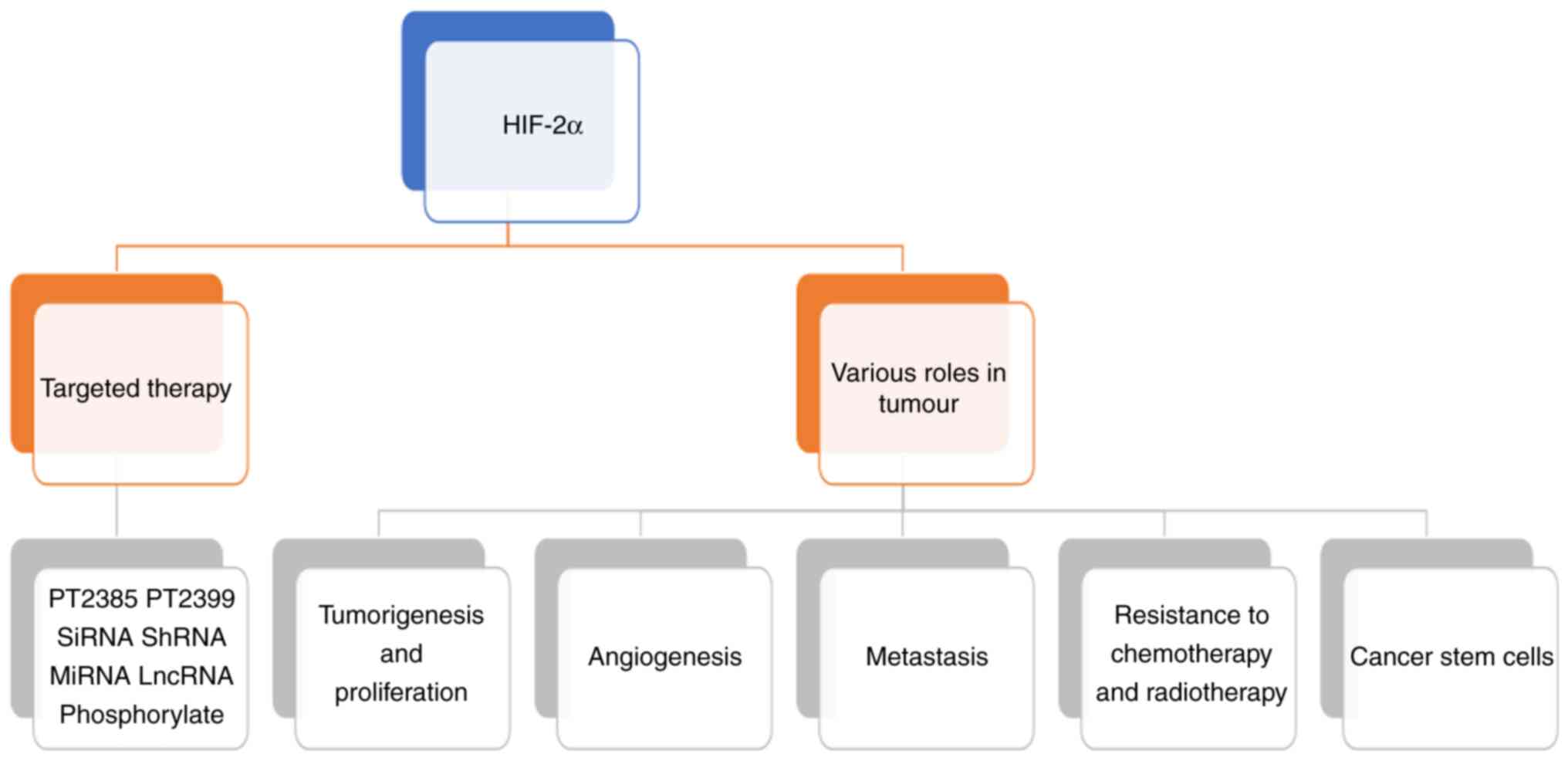
HIF-2α plays several roles in tumour-related
processes, including tumorigenesis and proliferation, angiogenesis,
metastasis, resistance to chemotherapy and radiotherapy, cancer
cell stemness and targeted therapy (Fig.
2).
First, high expression levels of HIF-2α have been
found to be closely associated with tumorigenesis and tumour cell
proliferation. It was demonstrated that the clonogenic survival
rate of head and neck squamous cancer cells decreased by ~30%
following HIF-2α silencing (29).
Furthermore, when PT2399 inhibited the expression of HIF-2α in
clear-cell renal cell carcinoma, 10 of the 18 examined cell lines
exhibited suppressed tumorigenic ability (11). Hypoxia-associated factor (HAF)
overexpression enhanced the expression of HIF-2α to increase the
proliferation and metastasis of clear-cell renal cell cancer cells,
which also confirmed its positive association with tumorigenesis
(30). The possible underlying
mechanisms are variable and may include lactate dehydrogenase A
(16), acetate-dependent acetyl-CoA
synthetase 2 (31), nuclear enriched
abundant transcript 1 (NEAT1) which is a nuclear long non-coding
(lnc)RNA (32) CREB-binding protein
(33) and sirtuin 1 (33).
Second, since it was first identified in blood
vessel walls and vascular smooth muscle cells (4). HIF-2α has been shown to participate in
cancer angiogenesis based on an increasing number of clinical
studies and experiments. Two examples are provided below.
Microvessel density (MVD) was found to be significantly higher in
tumours exhibiting high HIF-2α expression compared with that in
tumours with low HIF-2α expression among surgical specimens from
140 patients with non-small cell lung cancer (34). RAB11B-AS1, an lncRNA in breast cancer
cells, was induced by HIF-2α and could promote tumour angiogenesis
and distant metastasis without affecting primary tumour growth in
mice (35). These effects of HIF-2α
are most likely mediated via the HIF-2α/VEGF axis; for example, in
mesothelioma cells, VEGF secretion was enhanced via upregulation of
HIF-2α (36).
Third, metastasis is an important process in
tumours, and a number of studies have reported that HIF-2α promotes
cancer metastasis. In patients with clear-cell renal cell carcinoma
who underwent nephrectomy, increased levels of HIF-2α were strongly
associated with local invasion and distant metastasis (37). Similar findings were observed in
pancreatic cancer cells (16),
fibrosarcoma cells and animal models (31), non-small cell lung cancer cell lines
(38), breast carcinomas (39), hepatocellular carcinoma (40) and bladder cancer (23), among others.
Fourth, resistance to chemotherapy and radiotherapy
is responsible for tumour recurrence and treatment failure. In
targeted therapy and chemotherapy, HIF-2α is positively correlated
with treatment failure (41,42), but there remains controversy regarding
the role of HIF-2α in radiotherapy resistance. In breast cancer,
the protein and mRNA expression of HIF-2α was increased in a panel
of antioestrogen-resistant breast cells (41). EGFR is known to contribute to
antioestrogen resistance, and HIF-2α was shown to promote hypoxic
induction of EGFR in breast cancer cells (41). Knockdown of HIF-2α was found to be
positively associated with the increased treatment efficacy of
doxorubicin in hepatocellular carcinoma (17). There have been relatively few studies
on the association of radiotherapy and HIF-2α in cancer. Through
investigating HIF-2α isoform-deficient non-small cell lung cancer
cells, researchers demonstrated the strong radiosensitising effect
of HIF-1α, but not of HIF-2α (43).
In a continuous hyperfractionated accelerated radiotherapy
randomized trial, HIF-2α was associated with radiotherapy failure
in patients with head and neck cancer (44). However, further research on HIF-2α and
tumour radiotherapy is needed.
Finally, the cancer stem cell (CSC) theory has been
widely recognized, but there have been few studies on HIF-2α
expression in CSCs. Research has shown that HIF-2α maintains
self-renewal in leukaemia CSCs via Nanog and Sox2 (45). The overexpression of lncRNA HIF2PUT
suppressed the sphere-forming ability of osteosarcoma stem cells by
downregulating HIF-2α (21).
According to these findings, HIF-2α appears to promote the
proliferation of CSCs.
Given the important role of HIF-2α in tumours,
HIF-2α-targeted therapies have emerged. The most representative
HIF-2α-targeted therapies are PT2385 and PT2399 in renal cell
carcinoma (12). PT2385 prevents the
dimerization of HIF-2α and ARNT/HIF-1β, while PT2399 directly binds
to the HIF-2α PAS B domain (11,13).
PT2385 was utilized not only in renal cell carcinoma, but also in
liver cancer. The administration of PT2385 restored the
YTHDF2-programmed epigenetic machinery and inhibited the
progression and metastasis of liver cancer (46). siRNA (42,47) and
shRNA (48) are useful methods for
downregulating HIF-2α in cell or animal studies. lncRNAs (32) and microRNAs (miRNAs/miRs) (49), as upstream sequences, could similarly
regulate the level of HIF-2α. Finally, HIF-2α phosphorylation
modification is also a potential method for regulating the
expression of HIF-2α in basic research (50).
In recent years, an increasing number of studies
have shown that HIF-2α plays an important role in lung cancer
(51–53). Current studies have confirmed the
prognostic role of HIF-2α in patients with lung cancer; a high
level of HIF-2α was strongly associated with poor prognosis
(54). The Lung Cancer Consortium
identified a SNP (rs12614710) in HIF-2α/EPAS1 that was associated
with non-small cell lung cancer and achieved genome-wide
significance (52). Of note, the
findings of clinical studies on small cell lung cancer were
contrary to those of basic studies. The high expression level of
HIF-2α was found to be an independent poor prognostic index in
patients with small cell lung cancer (53), but the expression of HIF-2α was
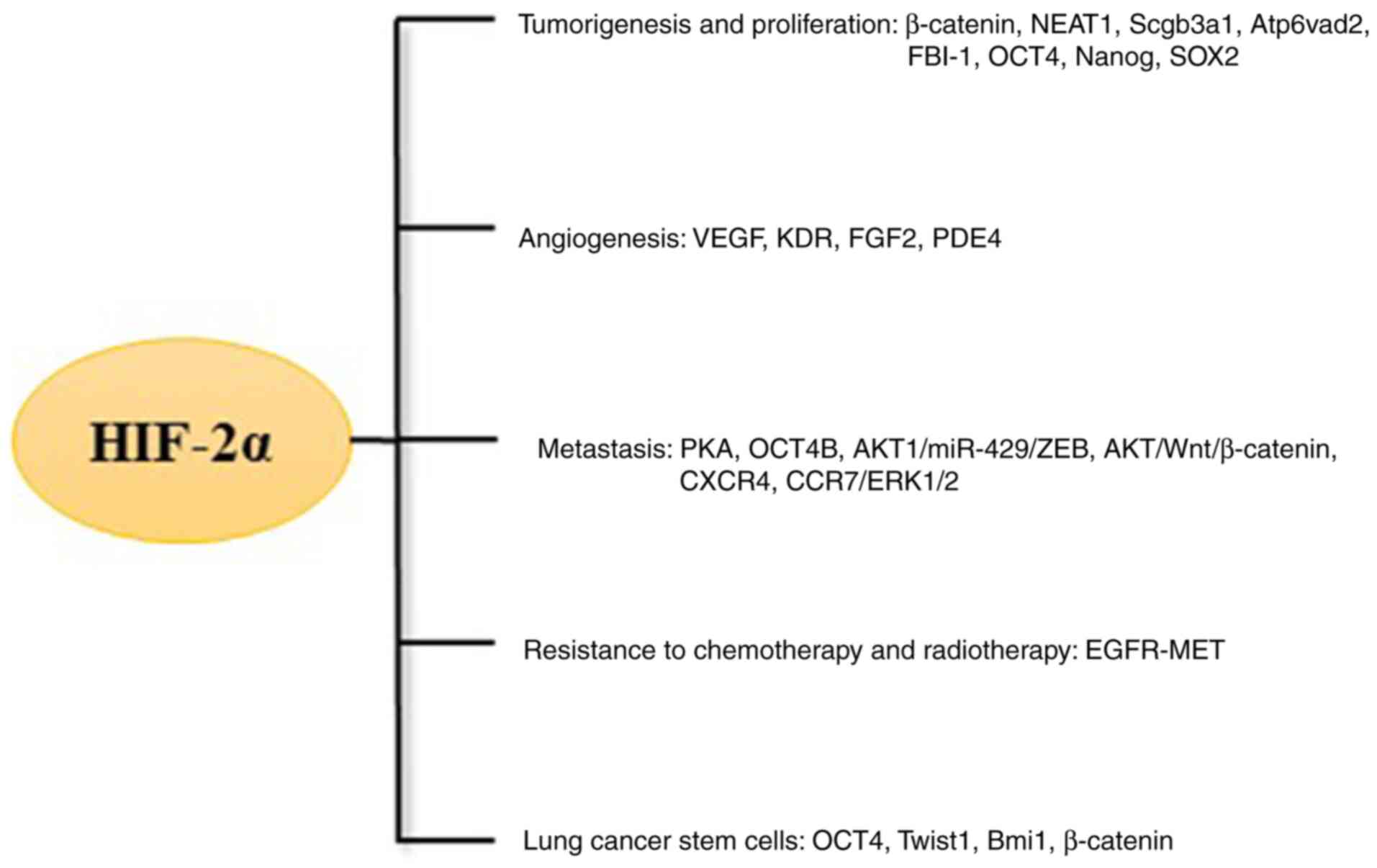
inhibited in small cell lung cancer cells (25). HIF-2α regulated genes controlling
important aspects of tumours, including tumorigenesis and
proliferation, angiogenesis, metastasis, resistance to chemotherapy
and radiotherapy and cancer cell stemness (Fig. 3).
The association between HIF-2α and tumorigenesis and
proliferation in lung cancer has been investigated by several
studies. The expression of HIF-2α was shown to promote the
occurrence and proliferation of lung cancer, and the mechanisms
were complex and variable. A clinical study on the association
between HIF-2α gene polymorphisms and tumour susceptibility in lung
adenocarcinoma was conducted in Japanese non-smoking females, and
suggested that the frequency of lung cancer in female Japanese
non-smokers with a minor G allele (A/G or G/G genotype) at
rs4953354 was markedly higher compared with that of healthy
controls (55). The genetic
polymorphism of HIF-2α affected the susceptibility of non-smokers
to lung adenocarcinoma, suggesting its importance in tumorigenesis
(55). The mutation probability of
EGFR in this type of population is significantly higher compared
with that of the general population, which suggests its potential
therapeutic significance (56).
According to a tissue microarray study of 140 patients with stage
I–III non-small cell lung cancer, immunohistological expression of
HIF-2α was positively associated with histology, tumour size and
stage, which further proved this hypothesis (57). It has been demonstrated that HIF-2α is
involved in the malignant transformation of normal cells. Studies
of arsenic-induced malignant transformation of bronchial epithelial
cells indicated that HIF-2α is involved in the inflammatory
response (58) and may block the
ATM/CHK-2 pathway activated by arsenic (59). In addition, the suppression of HIF-2α
prevented the effects of arsenic on benzopyrene-induced malignant
transformation, cell migration and chromosomal aberrations
(59). Downregulation or
overexpression of HIF-2α in cell experiments or animal models
provided more direct evidence. Knockdown of HIF-2α by shRNA
enhanced the colonization of A549 cells (60), and tumour proliferation was inhibited
in Lewis lung carcinoma mouse models through intravenous injection
of HIF-2α siRNA (61). The lncRNA
LINC01436 in non-small cell lung cancer cells, which acted as a
miR-30a-3p sponge, upregulated the expression of HIF-2α/EPAS1 to
promote cell proliferation in vitro (62). In addition, under hypoxic conditions,
HIF-2α was required in anaplastic lymphoma kinase (ALK) regulation
of the proliferation of ALK-rearranged non-small cell lung cancer
cells (63).
According to previous studies, the mechanisms
responsible for the HIF-2α involvement in the tumorigenesis and
proliferation of lung cancer cells were summarized. The most
important target protein was β-catenin (19,64). When
pcDNA3.1-HIF-2α was transfected into A549 lung cancer cells under
hypoxic conditions, the overexpression of HIF-2α enhanced the
expression of NEAT1. A preliminary study revealed that NEAT1 was
overexpressed in non-small cell lung cancer tissues and cell lines.
Finally, tumour progression was detected after upregulation of
NEAT1 via the miR-101-3p/SOX9/Wnt/β-catenin axis (19). Another study intuitively reflected the
association between HIF-2α and β-catenin. Overexpression of HIF-2α
was proven to increase the level of β-catenin to induce
morphological changes in lung cancer cells, while knockdown of
HIF-2α similarly inhibited β-catenin to reduce colony formation
under conditions of prolonged hypoxia (64). Of note, β-catenin was not the only
target gene of HIF-2α. When HIF-2α was abolished in established
KRAS (G12D)-driven murine non-small cell lung cancer mouse models,
the tumour burden was increased, which was linked to the depletion
of Scgb3a1 (65). The consumption of
Scgb3a1 appeared to be affected by HIF-2α, suggesting that it may
be involved in regulating proliferation. HIF-2α and the
macrophage-specific vacuolar ATPase subunit Atp6v0d2 were
negatively and positively associated with survival, respectively,
in a lung adenocarcinoma study; inhibiting the transcriptional
activity of HIF-2α decreased the tumour progression susceptibility
of Atp6v0d2−/− mice (51).
Therefore, Atp6v0d2 may play the same role as Scgb3a1. Factor
binding IST-1 (FBI-1) is an important oncogene in various tumours.
Overexpression of HIF-2α/EPAS1 increased FBI-1 expression to
enhance the survival and proliferation of human lung adenocarcinoma
cells, which suggested that FBI-1 may also be a target gene of
HIF-2α (66). Our team found that
HIF-2α could regulate octamer-binding transcription factor 4
(OCT4), Nanog and Sox2 in human lung adenocarcinoma A549 stem cell
spheres via the Wnt/β-catenin signalling pathway (unpublished
data). This finding requires further in-depth studies.
Consistent conclusions have been drawn regarding the
role of HIF-2α in tumorigenesis and cell proliferation in lung
cancer. The specific mechanism is complex, and HIF-2α may be
affected by a number of other factors. For example, patients with
lung cancer who can be surgically treated are generally evaluated
with positron emission tomography-computed tomography. An
interesting study suggested that HIF-2α expression was
significantly associated with cellular uptake of
fluorodeoxyglucose, and both factors were positively linked to
postoperative recurrence in patients with lung adenocarcinoma
(67). This result indicated that
HIF-2α may be associated not only with hypoxia-related diseases,
but also with certain detection methods or treatments.
Angiogenesis plays an important role in lung cancer,
while HIF-2α plays a key role in this process. HIF-2α may promote
the secretion of VEGF in tumours to enhance angiogenesis, but in a
hypoxic microenvironment, the association between MVD and the level
of HIF-2α needs more research. HIF-2α expression was positively
associated with MVD and poor outcome according to a tissue
microarray study of 140 patients with non-small cell lung cancer
(34). However, another study in lung
cancer animal models demonstrated that long-term hypoxia suppressed
MVD but increased the expression level of HIF-2α (68). In addition, HIF-2α could mobilize
circulating endothelial progenitor cells in lung tumorigenesis in
mice (69). VEGF is the best-known
target gene of HIF-2α in angiogenesis in lung cancer (70–72).
Researchers found that HIF-2α was an independent prognostic
indicator and linked to the intense vascularization activated by
VEGF/KDR in a study of 108 tissue samples evaluating the
immunohistochemical expression between non-small cell lung cancer
and normal lung tissues (72).
Long-term hypoxia plays a key role in the pathogenesis of chronic
obstructive pulmonary disease (COPD), and lung cancer is a
significant complication of COPD. It has been hypothesized that the
hypoxic microenvironment promotes angiogenesis in lung cancer, and
studies on this subject were undertaken. Tumour progression and
high HIF-2α-regulated VEGF-A and FGF2 angiogenesis (71) were detected in mice with lung cancer
exposed to hypoxic conditions, and these findings were partly
contrary to those of a previous study on HIF-2α and MVD (68). Targeted regulation of the HIF-2α-VEGF
axis provided more direct evidence. Downregulation of HIF-2α
through siRNA downregulated PDE4 and reduced the secretion of VEGF
to inhibit the growth of blood vessels in lung cancer animal models
(73). These data suggested that the
HIF-2α/VEGF axis is an important pathway in angiogenesis in lung
cancer. However, more research is necessary to explore regulatory
pathways other than VEGF that are involved in angiogenesis.
Clinicopathological and prognostic analyses indicate
that metastasis is an independent prognostic indicator of lung
cancer (53). When focusing on
tumorigenesis and cell proliferation in lung cancer, studies have
demonstrated that the expression of HIF-2α is closely associated
with and promotes lung cancer metastasis. A clinical trial
demonstrated that the expression of HIF-2α in lung cancer tissues
was positively associated with lymph node metastasis (57). Epithelial-to-mesenchymal transition
(EMT) was confirmed to participate in HIF-2α-associated lung cancer
metastasis, and other pathways were also involved (74–76).
EMT is an important characteristic in lung cancer
and is strongly associated with HIF-2α. A number of studies have
focused on the relationship between these factors. Downregulation
of HIF-1/2 inhibited hypoxia-mediated protein kinase A (PKA), and
downregulation of PKA prevented hypoxia-mediated EMT, cell
migration and invasion (76). In
addition, an interesting study focused on the association between
anaesthetics and lung cancer. Levobupivacaine induced the
proliferation and metastasis of A549 lung cancer cells in
vitro and in vivo through promoting EMT. According to
gene expression chips, quantitative (q)PCR and western blotting,
HIF-2α was upregulated in A549 cells after levobupivacaine
treatment (75). OCT4, as a stem cell
marker protein, was found to be associated with HIF-2α during
metastasis of lung cancer. High levels of OCT4B inhibited
epithelial barrier properties to reduce the migration and invasion
of lung cancer cells. Downregulation of OCT4B attenuated
HIF-2α-induced EMT to inhibit tumour metastasis in cell lines and
animal models (77). Of note,
mesothelial-to-mesenchymal transition (MMT) and
mesenchymal-to-epithelial transition (MET) also actively
participate in HIF-2α-related metastasis. Knockdown of EPAS1/HIF-2α
by shRNA significantly suppressed the peritoneal metastasis of lung
cancer in vivo, and the characteristics of MMT-inducing
factors were closely associated with HIF-2α (74). Findings have shown that there is a
special protein mediating metastasis through EMT and MET (78). Osteopontin (OPN) may be classified as
secretory OPN (sOPN) and intracellular/nuclear OPN (iOPN), both of
which promote metastasis through EMT and MET, respectively.
Intracellular OPN interacting with HIF-2α regulates the
AKT1/miR-429/ZEB pathway (78).
Further in vivo experiments demonstrated that increased
levels of OPN and MET in the nucleus were associated with tumour
metastasis (78).
β-catenin is not only involved in proliferation, but
also plays a role in the metastasis of lung cancer. Regulating
HIF-2α could regulate β-catenin in the same manner, thereby
affecting the migration of non-small cell lung cancer cells. The
AKT/Wnt/β-catenin pathway may be a possible underlying mechanism,
as evidence indicates that HIF-2α is necessary for Wnt activation
and AKT1 is activated under hypoxic conditions (64). It was previously demonstrated that
miRNAs may regulate HIF-2α. miRNA-184 inhibited metastasis, while
miRNA-574-5p enhanced metastasis; they both participated in
β-catenin signalling by suppressing EPAS1/HIF-2α according to
microarray and qPCR analyses of small cell lung cancer (79).
Chemokine receptors, such as CCR7 and CXCR4, are
induced and associated with metastasis in lung cancer cells under
hypoxia (38,80). To investigate HIF-dependent cell
invasion and migration, researchers knocked down HIF-1α or HIF-2α
in two lung cancer cell lines by lentiviral vector-mediated RNA
interference (RNAi). The results demonstrated that HIF-1α and
HIF-2α were strongly associated with upregulation of CXCR4 and
metastasis (38). CCR7 was positively
correlated with HIF-1α and HIF-2α, and both factors were associated
with lymph node metastasis as indicated in a study of 99 samples of
tissues from patients with non-small cell lung cancer stained by
immunohistochemistry (80). Further
studies revealed that downregulation of HIF-1α or HIF-2α led to a
decrease in CCR7 expression and inhibition of migration and
invasion, although HIF-1α played a more significant role. According
to this study, ERK1/2 was considered to be the downstream effector
of CCR7 (80). The connection between
HIF-2α and cytokines in lung cancer is a novel pathway implicated
in metastasis, and further in-depth research is required.
Ineffective treatments, including chemotherapy,
radiotherapy and targeted therapy, markedly restrict the efficacy
of lung cancer treatment, and it is crucial to explore the detailed
mechanisms involved. Investigators found that sensitivity to
cisplatin was enhanced after siRNA targeting HIF-2α was transfected
into A549 cells (81). Another study
focused on a wider variety of chemotherapy drugs. In the
vinorelbine, vinflunine and methotrexate groups, the proliferation
of lung cancer cells was effectively inhibited in both the 25 and
100 nmol/l siRNA HIF-1α and HIF-2α groups compared with the control
group (82). From these experiments,
it was inferred that HIF-2α expression indicated high resistance to
chemotherapy drugs. Pyruvate dehydrogenase kinase (PDK)4 was
detected early as a related gene involved in this process.
According to clinical samples and analyses, the level of PDK4 was
positively correlated with poor prognosis in lung adenocarcinoma,
whereas upregulating PDK4 through lentivirus infection
significantly increased the expression of EPSA1/HIF-2α and enhanced
resistance to cisplatin (54).
However, as regards sensitivity to radiotherapy, the role of HIF-2α
remains unclear. Isogenic H1299 non-small cell lung carcinoma cells
lacking HIF-1α, HIF-2α, or both, were constructed by CRISPR gene
editing. Double-HIF1/2 knockout cells exhibited the strongest
radiosensitivity, followed by HIF-1α knockout cells, while HIF-2α
had no radiosensitising effect (83).
A different result was obtained in patients with non-small cell
lung cancer, among whom patients with high HIF-2α expression levels
had a shorter survival time and a faster recovery of
carcinoembryonic antigen to the pre-radiation level (84). In recent years, targeted therapy
accounts for at least half of the effective treatments of non-small
lung cancer. Elucidating whether HIF-2α expression is related to
targeted therapy resistance may provide some useful indicators.
When non-small cell lung cancer cells expressing T790M EGFR were
treated with a tyrosine kinase inhibitor (TKI), the overexpression
of EPAS1/HIF-2α enhanced drug resistance, whereas the deregulation
of EPAS1/HIF-2α obviously increased drug efficacy. Knocking down
MET eliminated this EPAS1-dependent TKI resistance, which suggested
that EPAS1/HIF-2α may act as a bridge in this process (85). Regardless of whether HIF-2α is a
direct or indirect factor, it appears to be involved in treatment
failure, and its detailed mechanisms require further
clarification.
CSCs constitute a small part of the tumour mass and
are characterized by self-renewal, differentiation and the ability
to promote chemoresistance of tumours (86). CSCs have lower glucose and oxygen
consumption and lower intracellular reactive oxygen species and ATP
concentrations compared with other cells (87). Hypoxia is an important characteristic
of the tumour microenvironment, and studies have indicated that
HIF-2α is connected with lung CSCs (88). OCT4, as a homeobox transcription
factor, is an important stem cell marker in lung CSCs (89–93). Human
bronchial epithelial cells underwent EMT after long-term exposure
to arsenite, and then acquired a malignant stem cell-like phenotype
(94). Moreover, OCT4, Twist1 and
Bmi1 participate in arsenic-induced EMT, which is directly
regulated by HIF-2α (94). There have
been few studies on HIF-2α in lung CSCs, but the target protein
β-catenin, an important protein previously described in the tumour
signalling pathway, was found to be possibly linked to these cells.
Although there is no direct evidence of HIF-2α-mediated β-catenin
in lung CSCs, the Wnt/β-catenin pathway was activated in liver CSCs
(95) and lung CSCs (96). HIF-2α regulated the involvement of
β-catenin in tumorigenesis, proliferation and metastasis, which has
been described above. HIF-2α-mediated β-catenin regulation in lung
CSCs may provide a novel perspective for exploring the pathway
indicating that HIF-2α involved in maintaining the stemness of stem
cell.
Studies have indicated that some specific
characteristics of lung CSCs are related to HIF-2α. The expression
of CD133 and HIF-2α and cell invasion ability were detected
following CD133 siRNA transfection into lung cancer A549 stem
cells. Compared with that on CD133− CSCs, the expression
of HIF-2α was significantly enhanced on CD133+ CSCs. The
level of HIF-2α in CD133-si-A549 stem cells was decreased compared
with that in control-si-A549 stem cells, and cell invasion was
significantly reduced (88). Another
study on HIF-2α and radiation sensitivity revealed that a high
level of HIF-2α in lung CSCs was positively associated with its
radioresistance effect (84).
The hypoxic microenvironment of lung cancer provides
several perspectives for assessing HIFs. Several studies have
focused on lung CSCs and HIF expression. HIF-2α is different from
HIF-1α, but the upregulation of HIF-1 and HIF-2 in tumour sites
correlates with the expansion of the CSC population (86). However, more studies are required to
explore the function of HIF-2α in lung CSCs. The HIF-2α target
proteins summarized were only based on existing research, and there
may still be a number of mechanisms that remain unknown. Further
investigations of lung CSCs based on the signalling pathways or
target proteins previously summarized may be beneficial.
At present, the most mature HIF-2α-targeted therapy
is the application of PT2385 in renal clear-cell carcinoma, which
was tested in a phase III clinical trial and exhibited significant
efficacy (12). However, the
application of HIF-2α-targeted therapy in lung cancer is not yet
applicable in the clinical setting. Clinical studies, not only in
small cell lung cancer, but also in non-small cell lung cancer,
demonstrated that HIF-2α was negatively correlated with overall
survival (53,97). Moreover, according to a meta-analysis,
EPAS1/HIF-2α rs13419896 and rs4953344 gene polymorphisms were
associated with overall survival (98) and progression-free survival (99), respectively. These findings indicate
that HIF-2α-targeted therapy in lung cancer is meaningful and worth
exploring.
To date, few selectively targeted HIF-2α inhibitors
have been discussed in lung cancer. Although HIF-2α inhibitors for
lung cancer cells are currently being investigated in the
laboratory, clinically applicable HIF-2α-specific inhibitors have
yet to be developed. However, HIF-2α-targeted therapy may become a
promising treatment for lung cancer in the future.
Numerous studies have provided convincing evidence
that HIF-2α plays an important role in critical aspects of lung
cancer. HIF-2α is involved in tumorigenesis and proliferation,
angiogenesis, metastasis and resistance of lung cancer to drugs or
radiotherapy. Current studies on HIF-2α have indicated its role in
lung cancer cell conversion to CSCs, which was confirmed as the
main cause of treatment failure. Not only clinical evidence, but
also cell experiments and animal models, have demonstrated that a
high level of HIF-2α expression is correlated with the occurrence
and poor prognosis of lung cancer. In conclusion, the HIF-2α
signalling pathway may represent a useful biomarker for evaluating
lung cancer, as well as a promising tool for lung cancer
treatment.
However, a number of issues regarding HIF-2α remain
to be addressed. First, studies have mostly focused on non-small
cell lung cancer, whereas there are few studies on small cell lung
cancer. Moreover, in small cell lung cancer, clinical evidence and
laboratory studies may be contradictory, and it cannot be
definitively concluded that this is an important oncogene. The
reason for this phenomenon may be associated with the different
biological characteristics of non-small cell lung cancer and small
cell lung cancer. Second, although several HIF-2α-targeted genes
were described in the development of lung cancer, the exact
mechanisms remain elusive and the actual pathway may be unknown due
to the lack of comprehensive research. Particularly in studies on
lung CSCs, establishing the significance of HIF-2α requires more
clinical data and laboratory results, and its signalling pathways
and targeted genes should be thoroughly investigated. Third, the
development of clinical-level HIF-2α-targeted therapy is still
extremely challenging. HIF-2α does not exist alone, but is also
associated with HIF-1α and HIF-3α under normoxic and hypoxic
conditions. In particular, HIF-3α, as a regulatory factor, has been
less extensively explored than the other two subunits in lung
cancer. Finally, as previously discussed, HIF-2α plays a critical
role in other diseases, such as preeclampsia and non-alcoholic
fatty liver. Patients with lung cancer are prone to developing
various diseases, such as pulmonary embolism and diffuse
intracapillary coagulation, or this condition may be combined with
other diseases, particularly hypoxic diseases, such as obstructive
sleep apnoea/hypopnea syndrome. The role of HIF-2α may be
complicated and variable, but may be worth exploring in the context
of determining the cause for the high incidence of lung cancer. At
present, however, the road to developing a HIF-2α-targeted therapy
is long and further research on lung cancer is required.
Wen-Jun Wang would like to thank the continuous
encouragement, and support from Tao Huang.
This work was supported by the National Natural
Science Foundation of China (grant no. 81660493), and Natural
Science Foundation of Jiangxi Province (grant nos. 20171BAB205053,
20202ACBL206019).
Not applicable.
WJW and XQY developed and designed the idea and
wrote the manuscript. CO, BY, CC, XFX and WJW performed retrieving
articles and graphing. All authors read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Tian H, McKnight SL and Russell DW:
Endothelial PAS domain protein 1 (EPAS1), a transcription factor
selectively expressed in endothelial cells. Genes Dev. 11:72–82.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ema M, Taya S, Yokotani N, Sogawa K,
Matsuda Y and Fujii-Kuriyama Y: A novel bHLH-PAS factor with close
sequence similarity to hypoxia-inducible factor 1alpha regulates
the VEGF expression and is potentially involved in lung and
vascular development. Proc Natl Acad Sci USA. 94:4273–4278. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wiesener MS, Turley H, Allen WE, Willam C,
Eckardt KU, Talks KL, Wood SM, Gatter KC, Harris AL, Pugh CW, et
al: Induction of endothelial PAS domain protein-1 by hypoxia:
Characterization and comparison with hypoxia-inducible
factor-1alpha. Blood. 92:2260–2268. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Favier J, Kempf H, Corvol P and Gasc JM:
Cloning and expression pattern of EPAS1 in the chicken embryo.
Colocalization with tyrosine hydroxylase. FEBS Lett. 462:19–24.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Talks KL, Turley H, Gatter KC, Maxwell PH,
Pugh CW, Ratcliffe PJ and Harris AL: The expression and
distribution of the hypoxia-inducible factors HIF-1alpha and
HIF-2alpha in normal human tissues, cancers, and tumor-associated
macrophages. Am J Pathol. 157:411–421. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blancher C, Moore JW, Talks KL, Houlbrook
S and Harris AL: Relationship of hypoxia-inducible factor
(HIF)-1alpha and HIF-2alpha expression to vascular endothelial
growth factor induction and hypoxia survival in human breast cancer
cell lines. Cancer Res. 60:7106–7113. 2000.PubMed/NCBI
|
|
7
|
Rajakumar A, Whitelock KA, Weissfeld LA,
Daftary AR, Markovic N and Conrad KP: Selective overexpression of
the hypoxia-inducible transcription factor, HIF-2alpha, in
placentas from women with preeclampsia. Biol Reprod. 64:499–506.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brusselmans K, Bono F, Maxwell P, Dor Y,
Dewerchin M, Collen D, Herbert JM and Carmeliet P:
Hypoxia-inducible factor-2alpha (HIF-2alpha) is involved in the
apoptotic response to hypoglycemia but not to hypoxia. J Biol Chem.
276:39192–39196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Chen J, Huang J, Li Z, Gong Y, Zou
B, Liu X, Ding L, Li P, Zhu Z, et al: HIF-2α upregulation mediated
by hypoxia promotes NAFLD-HCC progression by activating lipid
synthesis via the PI3K-AKT-mTOR pathway. Aging (Albany NY).
11:10839–10860. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saito T, Fukai A, Mabuchi A, Ikeda T, Yano
F, Ohba S, Nishida N, Akune T, Yoshimura N, Nakagawa T, et al:
Transcriptional regulation of endochondral ossification by
HIF-2alpha during skeletal growth and osteoarthritis development.
Nat Med. 16:678–686. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen W, Hill H, Christie A, Kim MS,
Holloman E, Pavia-Jimenez A, Homayoun F, Ma Y, Patel N, Yell P, et
al: Targeting renal cell carcinoma with a HIF-2 antagonist. Nature.
539:112–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choueiri TK and Kaelin WG Jr: Targeting
the HIF2-VEGF axis in renal cell carcinoma. Nat Med. 26:1519–1530.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun DR, Wang ZJ, Zheng QC and Zhang HX:
Exploring the inhibition mechanism on HIF-2 by inhibitor PT2399 and
0X3 using molecular dynamics simulations. J Mol Recognit.
31:e27302018. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sasagawa T, Nagamatsu T, Morita K, Mimura
N, Iriyama T, Fujii T and Shibuya M: HIF-2α, but not HIF-1α,
mediates hypoxia-induced up-regulation of Flt-1 gene expression in
placental trophoblasts. Sci Rep. 8:173752018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Spirina LV, Yurmazov ZA, Gorbunov AK,
Usynin EA, Lushnikova NA and Kovaleva IV: Molecular protein and
expression profile in the primary tumors of clear cell renal
carcinoma and metastases. Cells. 9:16802020. View Article : Google Scholar
|
|
16
|
Cui XG, Han ZT, He SH, Wu XD, Chen TR,
Shao CH, Chen DL, Su N, Chen YM, Wang T, et al: HIF1/2α mediates
hypoxia-induced LDHA expression in human pancreatic cancer cells.
Oncotarget. 8:24840–24852. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He C, Sun XP, Qiao H, Jiang X, Wang D, Jin
X, Dong X, Wang J, Jiang H and Sun X: Downregulating
hypoxia-inducible factor-2α improves the efficacy of doxorubicin in
the treatment of hepatocellular carcinoma. Cancer Sci. 103:528–534.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cleven AH, Wouters BG, Schutte B, Spiertz
AJ, van Engeland M and de Bruïne AP: Poorer outcome in stromal
HIF-2 alpha- and CA9-positive colorectal adenocarcinomas is
associated with wild-type TP53 but not with BNIP3 promoter
hypermethylation or apoptosis. Br J Cancer. 99:727–733. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kong X, Zhao Y, Li X, Tao Z, Hou M and Ma
H: Overexpression of HIF-2α-dependent NEAT1 promotes the
progression of non-small cell lung cancer through
miR-101-3p/SOX9/Wnt/β-catenin signal pathway. Cell Physiol Biochem.
52:368–381. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Påhlman S and Mohlin S: Hypoxia and
hypoxia-inducible factors in neuroblastoma. Cell Tissue Res.
372:269–275. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao D, Wang S, Chu X and Han D: LncRNA
HIF2PUT inhibited osteosarcoma stem cells proliferation, migration
and invasion by regulating HIF2 expression. Artif Cells Nanomed
Biotechnol. 47:1342–1348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Chen Y, Bao L, Zhang B, Wang JE,
Kumar A, Xing C, Wang Y and Luo W: CHD4 promotes breast cancer
progression as a coactivator of hypoxia-inducible factors. Cancer
Res. 80:3880–3891. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Onita T, Ji PG, Xuan JW, Sakai H, Kanetake
H, Maxwell PH, Fong GH, Gabril MY, Moussa M and Chin JL:
Hypoxia-induced, perinecrotic expression of endothelial
Per-ARNT-Sim domain protein-1/hypoxia-inducible factor-2alpha
correlates with tumor progression, vascularization, and focal
macrophage infiltration in bladder cancer. Clin Cancer Res.
8:471–480. 2002.PubMed/NCBI
|
|
24
|
Lim E, Kuo CC, Tu HF and Yang CC: The
prognosis outcome of oral squamous cell carcinoma using HIF-2alpha.
J Chin Med Assoc. 80:651–656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Munksgaard Persson M, Johansson ME, Monsef
N, Planck M, Beckman S, Seckl MJ, Rönnstrand L, Påhlman S and
Pettersson HM: HIF-2α expression is suppressed in SCLC cells, which
survive in moderate and severe hypoxia when HIF-1α is repressed. Am
J Pathol. 180:494–504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shneor D, Folberg R, Pe'er J, Honigman A
and Frenkel S: Stable knockdown of CREB, HIF-1 and HIF-2 by
replication-competent retroviruses abrogates the responses to
hypoxia in hepatocellular carcinoma. Cancer Gene Ther. 24:64–74.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Westerlund I, Shi Y, Toskas K, Fell SM, Li
S, Surova O, Södersten E, Kogner P, Nyman U, Schlisio S and
Holmberg J: Combined epigenetic and differentiation-based treatment
inhibits neuroblastoma tumor growth and links HIF2α to tumor
suppression. Proc Natl Acad Sci USA. 114:E6137–E6146. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Imamura T, Kikuchi H, Herraiz MT, Park DY,
Mizukami Y, Mino-Kenduson M, Lynch MP, Rueda BR, Benita Y, Xavier
RJ and Chung DC: HIF-1alpha and HIF-2alpha have divergent roles in
colon cancer. Int J Cancer. 124:763–771. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coliat P, Ramolu L, Jégu J, Gaiddon C,
Jung AC and Pencreach E: Constitutive or induced HIF-2 addiction is
involved in resistance to Anti-EGFR treatment and radiation therapy
in HNSCC. Cancers (Basel). 11:16072019. View Article : Google Scholar
|
|
30
|
Koh MY, Nguyen V, Lemos R Jr, Darnay BG,
Kiriakova G, Abdelmelek M, Ho TH, Karam J, Monzon FA, Jonasch E and
Powis G: Hypoxia-induced SUMOylation of E3 ligase HAF determines
specific activation of HIF2 in clear-cell renal cell carcinoma.
Cancer Res. 75:316–329. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen R, Xu M, Nagati J and Garcia JA:
Coordinate regulation of stress signaling and epigenetic events by
Acss2 and HIF-2 in cancer cells. PLoS One. 12:e1902412017.
View Article : Google Scholar
|
|
32
|
Choudhry H, Albukhari A, Morotti M, Haider
S, Moralli D, Smythies J, Schödel J, Green CM, Camps C, Buffa F, et
al: Tumor hypoxia induces nuclear paraspeckle formation through
HIF-2α dependent transcriptional activation of NEAT1 leading to
cancer cell survival. Oncogene. 34:4482–4490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen R, Xu M, Nagati JS, Hogg RT, Das A,
Gerard RD and Garcia JA: The acetate/ACSS2 switch regulates HIF-2
stress signaling in the tumor cell microenvironment. PLoS One.
10:e1165152015.
|
|
34
|
Wu XH, Qian C and Yuan K: Correlations of
hypoxia-inducible factor-1α/hypoxia-inducible factor-2α expression
with angiogenesis factors expression and prognosis in non-small
cell lung cancer. Chin Med J (Engl). 124:11–18. 2011.PubMed/NCBI
|
|
35
|
Niu Y, Bao L, Chen Y, Wang C, Luo M, Zhang
B, Zhou M, Wang JE, Fang YV, Kumar A, et al: HIF2-Induced long
noncoding RNA RAB11B-AS1 Promotes hypoxia-mediated angiogenesis and
breast cancer metastasis. Cancer Res. 80:964–975. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sato A, Virgona N, Ando A, Ota M and Yano
T: A redox-silent analogue of tocotrienol inhibits cobalt(II)
chloride-induced VEGF expression via Yes signaling in mesothelioma
cells. Biol Pharm Bull. 37:865–870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kamai T, Tokura Y, Uematsu T, Sakamoto K,
Suzuki I, Takei K, Narimatsu T, Kambara T, Yuki H, Betsunoh H, et
al: Elevated serum levels of cardiovascular biomarkers are
associated with progression of renal cancer. Open Heart.
5:e0006662018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu YL, Yu JM, Song XR, Wang XW, Xing LG
and Gao BB: Regulation of the chemokine receptor CXCR4 and
metastasis by hypoxia-inducible factor in non small cell lung
cancer cell lines. Cancer Biol Ther. 5:1320–1326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Giatromanolaki A, Sivridis E, Fiska A and
Koukourakis MI: Hypoxia-inducible factor-2 alpha (HIF-2 alpha)
induces angiogenesis in breast carcinomas. Appl Immunohistochem Mol
Morphol. 14:78–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bangoura G, Yang LY, Huang GW and Wang W:
Expression of HIF-2alpha/EPAS1 in hepatocellular carcinoma. World J
Gastroenterol. 10:525–530. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alam MW, Persson CU, Reinbothe S, Kazi JU,
Rönnstrand L, Wigerup C, Ditzel HJ, Lykkesfeldt AE, Påhlman S and
Jögi A: HIF2α contributes to antiestrogen resistance via positive
bilateral crosstalk with EGFR in breast cancer cells. Oncotarget.
7:11238–11250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao D, Zhai B, He C, Tan G, Jiang X, Pan
S, Dong X, Wei Z, Ma L, Qiao H, et al: Upregulation of HIF-2α
induced by sorafenib contributes to the resistance by activating
the TGF-α/EGFR pathway in hepatocellular carcinoma cells. Cell
Signal. 26:1030–1039. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Moreno Roig E, Groot AJ, Yaromina A,
Hendrickx TC, Barbeau LMO, Giuranno L, Dams G, Ient J, Olivo
Pimentel V, van Gisbergen MW, et al: HIF-1α and HIF-2α differently
regulate the radiation sensitivity of NSCLC cells. Cells. 8:452019.
View Article : Google Scholar
|
|
44
|
Koukourakis MI, Bentzen SM, Giatromanolaki
A, Wilson GD, Daley FM, Saunders MI, Dische S, Sivridis E and
Harris AL: Endogenous markers of two separate hypoxia response
pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase
9) are associated with radiotherapy failure in head and neck cancer
patients recruited in the CHART randomized trial. J Clin Oncol.
24:727–735. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Das B, Pal B, Bhuyan R, Li H, Sarma A,
Gayan S, Talukdar J, Sandhya S, Bhuyan S, Gogoi G, et al: MYC
Regulates the HIF2α stemness pathway via Nanog and Sox2 to maintain
self-renewal in cancer stem cells. versus Non-stem cancer cells.
Cancer Res. 79:4015–4025. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hou J, Zhang H, Liu J, Zhao Z, Wang J, Lu
Z, Hu B, Zhou J, Zhao Z, Feng M, et al: YTHDF2 reduction fuels
inflammation and vascular abnormalization in hepatocellular
carcinoma. Mol Cancer. 18:1632019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao CX, Luo CL and Wu XH: Hypoxia
promotes 786-O cells invasiveness and resistance to sorafenib via
HIF-2α/COX-2. Med Oncol. 32:4192015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vukovic M, Guitart AV, Sepulveda C,
Villacreces A, O'Duibhir E, Panagopoulou TI, Ivens A,
Menendez-Gonzalez J, Iglesias JM, Allen L, et al: Hif-1α and Hif-2α
synergize to suppress AML development but are dispensable for
disease maintenance. J Exp Med. 212:2223–2234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jiang L, Shi S, Shi Q, Zhang H, Xia Y and
Zhong T: MicroRNA-519d-3p inhibits proliferation and promotes
apoptosis by targeting HIF-2α in cervical cancer under hypoxic
conditions. Oncol Res. 26:1055–1062. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pangou E, Befani C, Mylonis I, Samiotaki
M, Panayotou G, Simos G and Liakos P: HIF-2α phosphorylation by
CK1δ promotes erythropoietin secretion in liver cancer cells under
hypoxia. J Cell Sci. 129:4213–4226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu N, Luo J, Kuang D, Xu S, Duan Y, Xia
Y, Wei Z, Xie X, Yin B, Chen F, et al: Lactate inhibits ATP6V0d2
expression in tumor-associated macrophages to promote
HIF-2α-mediated tumor progression. J Clin Invest. 129:631–646.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang Z, Wei Y, Zhang R, Su L, Gogarten SM,
Liu G, Brennan P, Field JK, McKay JD, Lissowska J, et al:
Multi-Omics analysis reveals a HIF network and hub gene EPAS1
associated with lung adenocarcinoma. Ebiomedicine. 32:93–101. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Luan Y, Gao C, Miao Y, Li Y, Wang Z and
Qiu X: Clinicopathological and prognostic significance of
HIF-1alpha and HIF-2alpha expression in small cell lung cancer.
Pathol Res Pract. 209:184–189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yu S, Ren H, Li Y, Liang X, Ning Q, Chen
X, Chen M and Hu T: HOXA4-dependent transcriptional activation of
AXL promotes cisplatin-resistance in lung adenocarcinoma cells.
Anticancer Agents Med Chem. 18:2062–2067. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Iwamoto S, Tanimoto K, Nishio Y, Putra AC,
Fuchita H, Ohe M, Sutani A, Kuraki T, Hiyama K, Murakami I, et al:
Association of EPAS1 gene rs4953354 polymorphism with
susceptibility to lung adenocarcinoma in female Japanese
non-smokers. J Thorac Oncol. 9:1709–1713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sárosi V, Balikó Z, Smuk G, László T,
Szabó M, Ruzsics I and Mezősi E: The frequency of EGFR mutation in
lung adenocarcinoma and the efficacy of tyrosine kinase inhibitor
therapy in a hungarian cohort of patients. Pathol Oncol Res.
22:755–761. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gao ZJ, Wang Y, Yuan WD, Yuan JQ and Yuan
K: HIF-2α not HIF-1α overexpression confers poor prognosis in
non-small cell lung cancer. Tumour Biol. 39:10104283177096372017.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xu Y, Zhao Y, Xu W, Luo F, Wang B, Li Y,
Pang Y and Liu Q: Involvement of HIF-2α-mediated inflammation in
arsenite-induced transformation of human bronchial epithelial
cells. Toxicol Appl Pharmacol. 272:542–550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pang Y, Xu Y, Li H, Li Y, Zhao Y, Jiang R,
Shen L, Zhou J, Wang X and Liu Q: The inhibition of HIF-2α on the
ATM/Chk-2 pathway is involved in the promotion effect of arsenite
on benzo(a)pyrene-induced cell transformation. Toxicol Lett.
218:105–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhou Q, Chen T, Ibe JC, Raj JU and Zhou G:
Loss of either hypoxia inducible factor 1 or 2 promotes lung cancer
cell colonization. Cell Cycle. 10:2233–2234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kamlah F, Eul BG, Li S, Lang N, Marsh LM,
Seeger W, Grimminger F, Rose F and Hänze J: Intravenous injection
of siRNA directed against hypoxia-inducible factors prolongs
survival in a Lewis lung carcinoma cancer model. Cancer Gene Ther.
16:195–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yuan S, Xiang Y, Wang G, Zhou M, Meng G,
Liu Q, Hu Z, Li C, Xie W, Wu N, et al: Hypoxia-sensitive LINC01436
is regulated by E2F6 and acts as an oncogene by targeting
miR-30a-3p in non-small cell lung cancer. Mol Oncol. 13:840–856.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Martinengo C, Poggio T, Menotti M, Scalzo
MS, Mastini C, Ambrogio C, Pellegrino E, Riera L, Piva R, Ribatti
D, et al: ALK-dependent control of hypoxia-inducible factors
mediates tumor growth and metastasis. Cancer Res. 74:6094–6106.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hong CF, Chen WY and Wu CW: Upregulation
of Wnt signaling under hypoxia promotes lung cancer progression.
Oncol Rep. 38:1706–1714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mazumdar J, Hickey MM, Pant DK, Durham AC,
Sweet-Cordero A, Vachani A, Jacks T, Chodosh LA, Kissil JL, Simon
MC and Keith B: HIF-2alpha deletion promotes Kras-driven lung tumor
development. Proc Natl Acad Sci USA. 107:14182–14187. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang X, Cao P, Li Z, Wu D, Wang X and
Liang G: EPAS-1 mediates SP-1-dependent FBI-1 expression and
regulates tumor cell survival and proliferation. Int J Mol Sci.
15:15689–15699. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Higashi K, Yamagishi T, Ueda Y, Ishigaki
Y, Shimasaki M, Nakamura Y, Oguchi M, Takegami T, Sagawa M and
Tonami H: Correlation of HIF-1α/HIF-2α expression with FDG uptake
in lung adenocarcinoma. Ann Nucl Med. 30:708–715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yu L and Hales CA: Long-term exposure to
hypoxia inhibits tumor progression of lung cancer in rats and mice.
BMC Cancer. 11:3312011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kim WY, Perera S, Zhou B, Carretero J, Yeh
JJ, Heathcote SA, Jackson AL, Nikolinakos P, Ospina B, Naumov G, et
al: HIF2alpha cooperates with RAS to promote lung tumorigenesis in
mice. J Clin Invest. 119:2160–2170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shao JS, Sun J, Wang S, Chung K, Du JT,
Wang J, Qiu XS, Wang EH and Wu GP: HPV16 E6/E7 upregulates HIF-2α
and VEGF by inhibiting LKB1 in lung cancer cells. Tumour Biol.
39:10104283177171372017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Karoor V, Le M, Merrick D, Fagan KA,
Dempsey EC and Miller YE: Alveolar hypoxia promotes murine lung
tumor growth through a VEGFR-2/EGFR-dependent mechanism. Cancer
Prev Res (Phila). 5:1061–1071. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Giatromanolaki A, Koukourakis MI, Sivridis
E, Turley H, Talks K, Pezzella F, Gatter KC and Harris AL: Relation
of hypoxia inducible factor 1 alpha and 2 alpha in operable
non-small cell lung cancer to angiogenic/molecular profile of
tumours and survival. Br J Cancer. 85:881–890. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Pullamsetti SS, Banat GA, Schmall A,
Szibor M, Pomagruk D, Hänze J, Kolosionek E, Wilhelm J, Braun T,
Grimminger F, et al: Phosphodiesterase-4 promotes proliferation and
angiogenesis of lung cancer by crosstalk with HIF. Oncogene.
32:1121–1134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhen Q, Zhang Y, Gao L, Wang R, Chu W,
Zhao X, Li Z, Li H, Zhang B, Lv B and Liu J: EPAS1 promotes
peritoneal carcinomatosis of non-small-cell lung cancer by
enhancing mesothelial-mesenchymal transition. Strahlenther Onkol.
197:141–149. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chan SM, Lin BF, Wong CS, Chuang WT, Chou
YT and Wu ZF: Levobuipivacaine-Induced Dissemination of A549 lung
cancer cells. Sci Rep. 7:86462017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shaikh D, Zhou Q, Chen T, Ibe JC, Raj JU
and Zhou G: cAMP-dependent protein kinase is essential for
hypoxia-mediated epithelial-mesenchymal transition, migration, and
invasion in lung cancer cells. Cell Signal. 24:2396–2406. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lin SC, Chung CH, Chung CH, Kuo MH, Hsieh
CH, Chiu YF, Shieh YS, Chou YT and Wu CW: OCT4B mediates
hypoxia-induced cancer dissemination. Oncogene. 38:1093–1105. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jia R, Liang Y, Chen R, Liu G, Wang H,
Tang M, Zhou X, Wang H, Yang Y, Wei H, et al: Osteopontin
facilitates tumor metastasis by regulating epithelial-mesenchymal
plasticity. Cell Death Dis. 7:e25642016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhou R, Zhou X, Yin Z, Guo J, Hu T, Jiang
S, Liu L, Dong X, Zhang S and Wu G: Tumor invasion and metastasis
regulated by microRNA-184 and microRNA-574-5p in small-cell lung
cancer. Oncotarget. 6:44609–44622. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li Y, Qiu X, Zhang S, Zhang Q and Wang E:
Hypoxia induced CCR7 expression via HIF-1alpha and HIF-2alpha
correlates with migration and invasion in lung cancer cells. Cancer
Biol Ther. 8:322–330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gao ZJ, Yuan WD, Yuan JQ, Yuan K and Wang
Y: Downregulation of HIF-2α reverse the chemotherapy resistance of
lung adenocarcinoma A549 cells to Cisplatin. Med Sci Monit.
24:1104–1111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Stoleriu MG, Steger V, Mustafi M,
Michaelis M, Cinatl J, Schneider W, Nolte A, Kurz J, Wendel HP,
Schlensak C and Walker T: A new strategy in the treatment of
chemoresistant lung adenocarcinoma via specific siRNA transfection
of SRF, E2F1, Survivin, HIF and STAT3. Eur J Cardiothorac Surg.
46:877–886. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Moreno Roig E, Groot AJ, Yaromina A,
Hendrickx TC, Barbeau LMO, Giuranno L, Dams G, Ient J, Olivo
Pimentel V, van Gisbergen MW, et al: HIF-1α and HIF-2α differently
regulate the radiation sensitivity of NSCLC Cells. Cells. 8:452019.
View Article : Google Scholar
|
|
84
|
Sun JC, He F, Yi W, Wan MH, Li R, Wei X,
Wu R and Niu DL: High expression of HIF-2α and its
anti-radiotherapy effect in lung cancer stem cells. Genet Mol Res.
14:18110–18120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhen Q, Liu JF, Liu JB, Wang RF, Chu WW,
Zhang YX, Tan GL, Zhao XJ and Lv BL: Endothelial PAS
domain-containing protein 1 confers TKI-resistance by mediating
EGFR and MET pathways in non-small cell lung cancer cells. Cancer
Biol Ther. 16:549–557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hajizadeh F, Okoye I, Esmaily M, Ghasemi
Chaleshtari M, Masjedi A, Azizi G, Irandoust M, Ghalamfarsa G and
Jadidi-Niaragh F: Hypoxia inducible factors in the tumor
microenvironment as therapeutic targets of cancer stem cells. Life
Sci. 237:1169522019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ye XQ, Li Q, Wang GH, Sun FF, Huang GJ,
Bian XW, Yu SC and Qian GS: Mitochondrial and energy
metabolism-related properties as novel indicators of lung cancer
stem cells. Int J Cancer. 129:820–831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Gao Y, Feng J, Wu L, Zhan S and Sun J:
Expression and pathological mechanism of MMP-9 and HIF-2α in
CD133(+) lung cancer stem cells. Zhonghua Yi Xue Za Zhi.
95:2607–2611. 2015.(In Chinese). PubMed/NCBI
|
|
89
|
Wei-Hua W, Ning Z, Qian C and Dao-Wen J:
ZIC2 promotes cancer stem cell traits via up-regulating OCT4
expression in lung adenocarcinoma cells. J Cancer. 11:6070–6080.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang X, Hu F, Li C, Zheng X, Zhang B,
Wang H, Tao G, Xu J, Zhang Y and Han B: OCT4&SOX2-specific
cytotoxic T lymphocytes plus programmed cell death protein 1
inhibitor presented with synergistic effect on killing lung cancer
stem-like cells in vitro and treating drug-resistant lung cancer
mice in vivo. J Cell Physiol. 234:6758–6768. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang X, Zhang Y, Xu J, Wang H, Zheng X,
Lou Y and Han B: Antigen presentation of the Oct4 and Sox2 peptides
by CD154-activated B lymphocytes enhances the killing effect of
cytotoxic T lymphocytes on tumor stem-like cells derived from
cisplatin-resistant lung cancer cells. J Cancer. 9:367–374. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Phiboonchaiyanan PP and Chanvorachote P:
Suppression of a cancer stem-like phenotype mediated by
alpha-lipoic acid in human lung cancer cells through
down-regulation of β-catenin and Oct-4. Cell Oncol (Dordr).
40:497–510. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kobayashi I, Takahashi F, Nurwidya F, Nara
T, Hashimoto M, Murakami A, Yagishita S, Tajima K, Hidayat M,
Shimada N, et al: Oct4 plays a crucial role in the maintenance of
gefitinib-resistant lung cancer stem cells. Biochem Biophys Res
Commun. 473:125–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Xu Y, Li Y, Pang Y, Ling M, Shen L, Yang
X, Zhang J, Zhou J, Wang X and Liu Q: EMT and stem cell-like
properties associated with HIF-2α are involved in arsenite-induced
transformation of human bronchial epithelial cells. PLoS One.
7:e377652012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wang R, Sun Q, Wang P, Liu M, Xiong S, Luo
J, Huang H, Du Q, Geller DA and Cheng B: Notch and Wnt/β-catenin
signaling pathway play important roles in activating liver cancer
stem cells. Oncotarget. 7:5754–5768. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhu JY, Yang X, Chen Y, Jiang Y, Wang SJ,
Li Y, Wang XQ, Meng Y, Zhu MM, Ma X, et al: Curcumin suppresses
lung cancer stem cells via inhibiting Wnt/β-catenin and Sonic
Hedgehog Pathways. Phytother Res. 31:680–688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Li C, Lu HJ, Na FF, Deng L, Xue JX, Wang
JW, Wang YQ, Li QL and Lu Y: Prognostic role of hypoxic inducible
factor expression in non-small cell lung cancer: A meta-analysis.
Asian Pac J Cancer Prev. 14:3607–3612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Putra AC, Eguchi H, Lee KL, Yamane Y,
Gustine E, Isobe T, Nishiyama M, Hiyama K, Poellinger L and
Tanimoto K: The A Allele at rs13419896 of EPAS1 is associated with
enhanced expression and poor prognosis for non-small cell lung
cancer. PLoS One. 10:e01344962015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
de Haas S, Delmar P, Bansal AT, Moisse M,
Miles DW, Leighl N, Escudier B, Van Cutsem E, Carmeliet P, Scherer
SJ, et al: Genetic variability of VEGF pathway genes in six
randomized phase III trials assessing the addition of bevacizumab
to standard therapy. Angiogenesis. 17:909–920. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ma H, Liu B, Wang S and Liu J:
MicroRNA-383 is a tumor suppressor in human lung cancer by
targeting endothelial PAS domain-containing protein 1. Cell Biochem
Funct. 34:613–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sato M, Tanaka T, Maeno T, Sando Y, Suga
T, Maeno Y, Sato H, Nagai R and Kurabayashi M: Inducible expression
of endothelial PAS domain protein-1 by hypoxia in human lung
adenocarcinoma A549 cells. Role of Src family kinases-dependent
pathway. Am J Respir Cell Mol Biol. 26:127–134. 2002. View Article : Google Scholar : PubMed/NCBI
|