|
1
|
Szabo C: Gaseotransmitters: New frontiers
for translational science. Sci Transl Med. 2:59ps542010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kabil O and Banerjee R: Enzymology of H2S
biogenesis, decay and signaling. Antioxid Redox Signal. 20:770–782.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kimura H: Hydrogen sulfide: Its
production, release and functions. Amino Acids. 41:113–121. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mani S, Untereiner A, Wu L and Wang R:
Hydrogen sulfide and the pathogenesis of atherosclerosis. Antioxid
Redox Signal. 20:805–817. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu D, Wang J, Li H, Xue M, Ji A and Li Y:
Role of hydrogen sulfifide in ischemia-reperfusion injury. Oxid Med
Cell Longev. 2015:1869082015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szabó C: Hydrogen sulphide and its
therapeutic potential. Nat Rev Drug Discov. 6:917–935. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang R: Physiological implications of
hydrogen sulfide: A whiff exploration that blossomed. Physiol Rev.
92:791–896. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fagone P, Mazzon E, Bramanti P, Bendtzen K
and Nicoletti F: Gasotransmitters and the immune system: Mode of
action and novel therapeutic targets. Eur J Pharmacol. 834:92–102.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mintz J, Vedenko A, Rosete O, Shah K,
Goldstein G, Hare JM, Ramasamy R and Arora H: Current advances of
nitric oxide in cancer and anticancer therapeutics. Vaccines
(Basel). 9:942021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Motterlini R and Otterbein LE: The
therapeutic potential of carbon monoxide. Nat Rev Drug Discov.
9:728–743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lazarević M, Battaglia G, Jevtić B,
Đedović N, Bruno V, Cavalli E, Miljković Đ, Nicoletti F, Momčilović
M and Fagone P: Upregulation of tolerogenic pathways by the
hydrogen sulfide donor GYY4137 and impaired expression of
H2S-producing enzymes in multiple sclerosis.
Antioxidants (Basel). 9:6082020. View Article : Google Scholar
|
|
12
|
Lazarević M, Mazzon E, Momčilović M,
Basile MS, Colletti G, Petralia MC, Bramanti P, Nicoletti F and
Miljković Đ: The H2S donor GYY4137 stimulates reactive
oxygen species generation in BV2 cells while suppressing the
secretion of TNF and nitric oxide. Molecules. 23:29662018.
View Article : Google Scholar
|
|
13
|
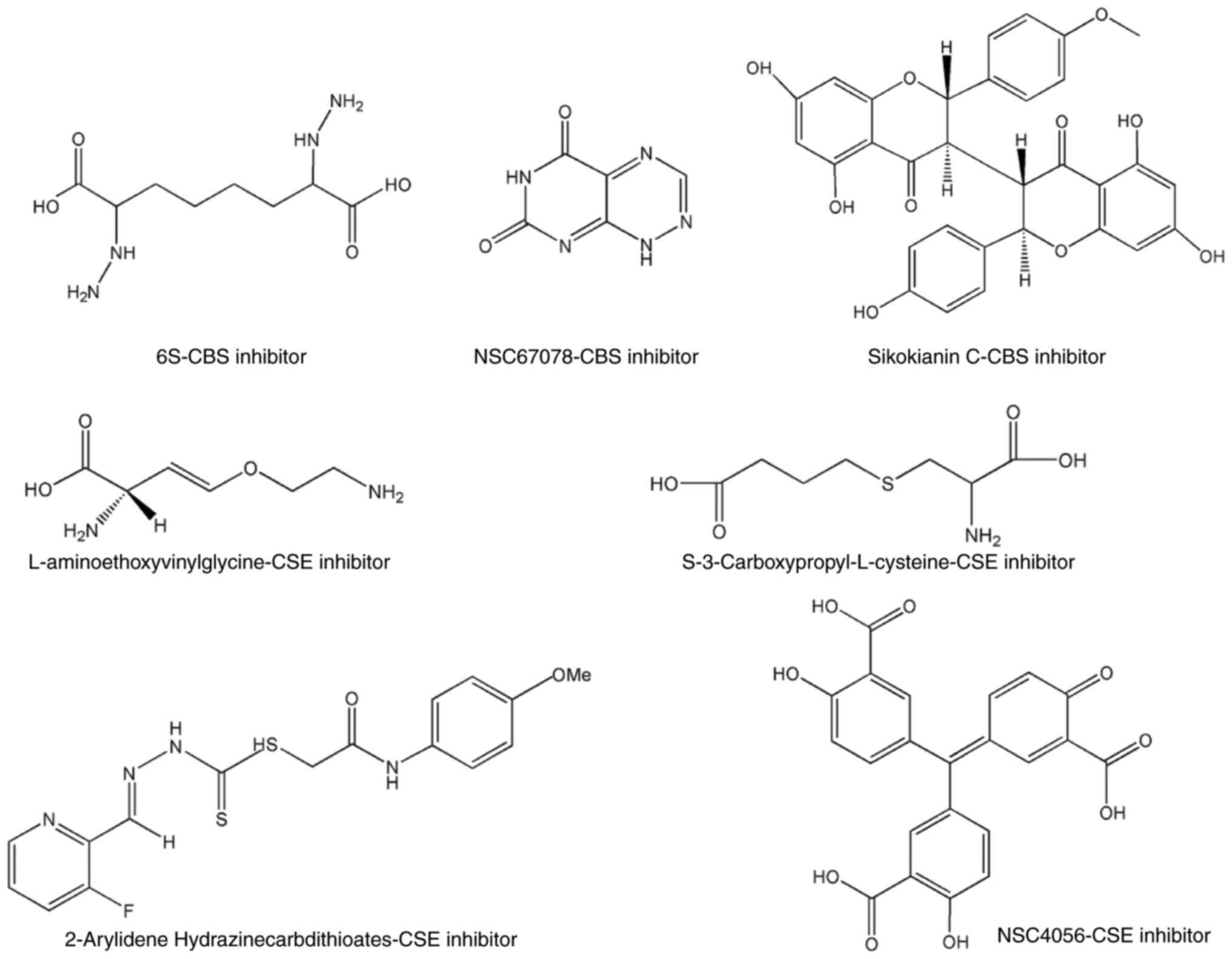
Gao L and Williams JL: Nitric
oxide-donating aspirin induces G2/M phase cell cycle arrest in
human cancer cells by regulating phase transition proteins. Int J
Oncol. 41:325–330. 2012.PubMed/NCBI
|
|
14
|
Furuhashi S, Sugita H, Takamori H, Horino
K, Nakahara O, Okabe H, Miyake K, Tanaka H, Beppu T and Baba H: NO
donor and MEK inhibitor synergistically inhibit proliferation and
invasion of cancer cells. Int J Oncol. 40:807–815. 2012.PubMed/NCBI
|
|
15
|
McMurtry V, Saavedra JE, Nieves-Alicea R,
Simeone AM, Keefer LK and Tari AM: JS-K, a nitric oxide-releasing
prodrug, induces breast cancer cell death while sparing normal
mammary epithelial cells. Int J Oncol. 38:963–971. 2011.PubMed/NCBI
|
|
16
|
Maksimovic-Ivanic D, Fagone P, McCubrey J,
Bendtzen K, Mijatovic S and Nicoletti F: HIV-protease inhibitors
for the treatment of cancer: Repositioning HIV protease inhibitors
while developing more potent NO-hybridized derivatives? Int J
Cancer. 140:1713–1726. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rothweiler F, Michaelis M, Brauer P, Otte
J, Weber K, Fehse B, Doerr HW, Wiese M, Kreuter J, Al-Abed Y, et
al: Anticancer effects of the nitric oxide-modified saquinavir
derivative saquinavir-NO against multidrug-resistant cancer cells.
Neoplasia. 12:1023–1030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nikolic I, Saksida T, Mangano K, Vujicic
M, Stojanovic I, Nicoletti F and Stosic-Grujicic S: Pharmacological
application of carbon monoxide ameliorates islet-directed
autoimmunity in mice via anti-inflammatory and anti-apoptotic
effects. Diabetologia. 57:980–990. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fagone P, Mangano K, Coco M, Perciavalle
V, Garotta G, Romao CC and Nicoletti F: Therapeutic potential of
carbon monoxide in multiple sclerosis. Clin Exp Immunol.
167:179–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fagone P, Mangano K, Quattrocchi C,
Motterlini R, Di Marco R, Magro G, Penacho N, Romao CC and
Nicoletti F: Prevention of clinical and histological signs of
proteolipid protein (PLP)-induced experimental allergic
encephalomyelitis (EAE) in mice by the water-soluble carbon
monoxide-releasing molecule (CORM)-A1. Clin Exp Immunol.
163:368–374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Módis K, Wolanska K and Vozdek R: Hydrogen
sulfide in cell signaling, signal transduction, cellular
bioenergetics and physiology in C. elegans. Gen Physiol
Biophys. 32:1–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wallace JL, Ferraz JG and Muscara MN:
Hydrogen sulfide: An endogenous mediator of resolution of
inflammation and injury. Antioxid Redox Signal. 17:58–67. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu D, Si W, Wang M, Lv S, Ji A and Li Y:
Hydrogen sulfide in cancer: Friend or foe? Nitric Oxide. 50:38–45.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ngowi EE, Afzal A, Sarfraz M, Khattak S,
Zaman SU, Khan NH, Li T, Jiang QY, Zhang X, Duan SF, et al: Role of
hydrogen sulfide donors in cancer development and progression. Int
J Biol Sci. 17:73–88. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH,
Li L, Moore PK and Deng LW: The slow-releasing hydrogen sulfide
donor, GYY4137, exhibits novel anti-cancer effects in vitro and in
vivo. PLoS One. 6:e210772011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu S, Gao Y, Huang X and Wang X: GYY4137,
a hydrogen sulfide (H2S) donor, shows potent
anti-hepatocellular carcinoma activity through blocking the STAT3
pathway. Int J Oncol. 44:1259–1267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sonke E, Verrydt M, Postenka CO, Pardhan
S, Willie CJ, Mazzola CR, Hammers MD, Pluth MD, Lobb I, Power NE,
et al: Inhibition of endogenous hydrogen sulfide production in
clear-cell renal cell carcinoma cell lines and xenografts restricts
their growth, survival and angiogenic potential. Nitric Oxide.
49:26–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oláh G, Módis K, Törö G, Hellmich MR,
Szczesny B and Szabo C: Role of endogenous and exogenous nitric
oxide, carbon monoxide and hydrogen sulfide in HCT116 colon cancer
cell proliferation. Biochem Pharmacol. 149:186–204. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hellmich MR and Szabo C: Hydrogen sulfide
and cancer. Handb Exp Pharmacol. 230:233–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dilek N, Papapetropoulos A, Toliver-Kinsky
T and Szabo C: Hydrogen sulfide: An endogenous regulator of the
immune system. Pharmacol Res. 161:1051192020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Szabo C: Hydrogen sulfide, an endogenous
stimulator of mitochondrial function in cancer cells cells. Cells.
10:2202021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
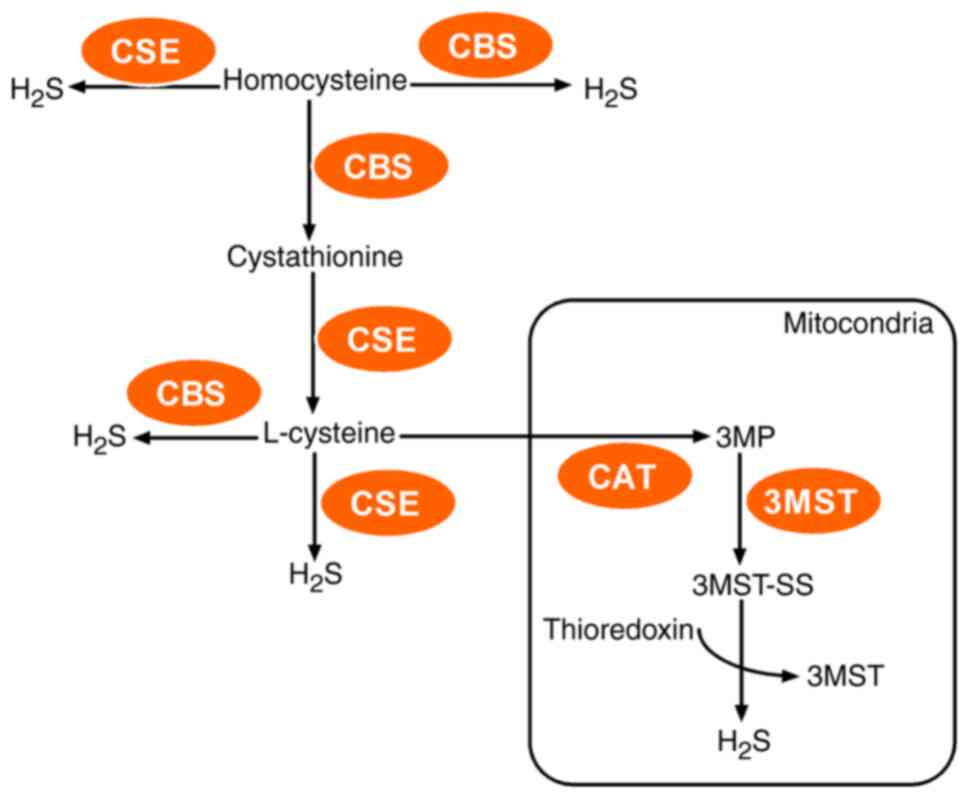
Jhee KH and Kruger WD: The role of
cystathionine beta-synthase in homocysteine metabolism. Antioxid
Redox Signal. 7:813–822. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu H, Blake S, Chan KT, Pearson RB and
Kang J: Cystathionine β-synthase in physiology and cancer. Biomed
Res Int. 2018:32051252018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ereño-Orbea J, Majtan T, Oyenarte I, Kraus
JP and Martínez-Cruz LA: Structural basis of regulation and
oligomerization of human cystathionine β-synthase, the central
enzyme of transsulfuration. Proc Natl Acad Sci USA.
110:E3790–E3799. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Taoka S and Banerjee R: Characterization
of NO binding to human cystathionine beta-synthase: Possible
implications of the effects of CO and NO binding to the human
enzyme. J Inorg Biochem. 87:245–251. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Niu WN, Yadav PK, Adamec J and Banerjee R:
S-glutathionylation enhances human cystathionine β-synthase
activity under oxidative stress conditions. Antioxid Redox Signal.
22:350–361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Renga B: Hydrogen sulfide generation in
mammals: The molecular biology of cystathionine-β-synthase (CBS)
and cystathionine-γ-lyase (CSE). Inflamm Allergy Drug Targets.
10:85–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Szabo C, Coletta C, Chao C, Módis K,
Szczesny B, Papapetropoulos A and Hellmich MR: Tumor-derived
hydrogen sulfide, produced by cystathionine-β-synthase, stimulates
bioenergetics, cell proliferation, and angiogenesis in colon
cancer. Proc Natl Acad Sci USA. 110:12474–12479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Phillips CM, Zatarain JR, Nicholls ME,
Porter C, Widen SG, Thanki K, Johnson P, Jawad MU, Moyer MP,
Randall JW, et al: Upregulation of cystathionine-β-synthase in
colonic epithelia reprograms metabolism and promotes
carcinogenesis. Cancer Res. 77:5741–5754. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bhattacharyya S, Saha S, Giri K, Lanza IR,
Nair KS, Jennings NB, Rodriguez-Aguayo C, Lopez-Berestein G, Basal
E, Weaver AL, et al: Cystathionine beta-synthase (CBS) contributes
to advanced ovarian cancer progression and drug resistance. PLoS
One. 8:e791672013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo H, Gai JW, Wang Y, Jin HF, Du JB and
Jin J: Characterization of hydrogen sulfide and its synthases,
cystathionine β-synthase and cystathionine γ-lyase, in human
prostatic tissue and cells. Urology. 79:483.e1–e5. 2012. View Article : Google Scholar
|
|
42
|
Chiku T, Padovani D, Zhu W, Singh S,
Vitvitsky V and Banerjee R: H2S biogenesis by human cystathionine
gamma-lyase leads to the novel sulfur metabolites lanthionine and
homolanthionine and is responsive to the grade of
hyperhomocysteinemia. J Biol Chem. 284:11601–11612. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Messerschmidt A, Worbs M, Steegborn C,
Wahl MC, Huber R, Laber B and Clausen T: Determinants of enzymatic
specificity in the Cys-Met-metabolism PLP-dependent enzymes family:
Crystal structure of cystathionine gamma-lyase from yeast and
intrafamiliar structure comparison. Biol Chem. 384:373–386. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mikami Y, Shibuya N, Ogasawara Y and
Kimura H: Hydrogen sulfide is produced by cystathionine γ-lyase at
the steady-state low intracellular Ca(2+) concentrations. Biochem
Biophys Res Commun. 431:131–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheung SH, Kwok WK, To KF and Lau JY:
Anti-atherogenic effect of hydrogen sulfide by over-expression of
cystathionine gamma-lyase (CSE) gene. PLoS One. 9:e1130382014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yuan S, Yurdagul A Jr, Peretik JM, Alfaidi
M, Al Yafeai Z, Pardue S, Kevil CG and Orr AW: Cystathionine
γ-lyase modulates flow-dependent vascular remodeling. Arterioscler
Thromb Vasc Biol. 38:2126–2136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ivanciuc T, Sbrana E, Casola A and
Garofalo RP: Cystathionine γ-lyase deficiency enhances airway
reactivity and viral-induced disease in mice exposed to side-stream
tobacco smoke. Pediatr Res. 86:39–46. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mani S, Li H, Untereiner A, Wu L, Yang G,
Austin RC, Dickhout JG, Lhoták Š, Meng QH and Wang R: Decreased
endogenous production of hydrogen sulfide accelerates
atherosclerosis. Circulation. 127:2523–2534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pan LL, Liu XH, Gong QH, Yang HB and Zhu
YZ: Role of cystathionine γ-lyase/hydrogen sulfide pathway in
cardiovascular disease: A novel therapeutic strategy? Antioxid
Redox Signal. 17:106–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fu X, Zhou K, Gao Q, Zheng S, Chen H, Li
P, Zhang Y, Suo K, Simoncini T and Wang T: 17β-estradiol attenuates
atherosclerosis development: The possible role of hydrogen sulfide.
Int J Cardiol. 167:1061–1063. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lambertini E, Penolazzi L, Angelozzi M,
Grassi F, Gambari L, Lisignoli G, De Bonis P, Cavallo M and Piva R:
The expression of cystathionine gamma-lyase is regulated by
estrogen receptor alpha in human osteoblasts. Oncotarget.
8:101686–101696. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu X, Yan Q, Liu X, Li P, Li X, Chen Y,
Simoncini T, Liu J, Zhu D and Fu X: 17β-Estradiol nongenomically
induces vascular endothelial H2S release by promoting
phosphorylation of cystathionine γ-lyase. J Biol Chem.
294:15577–15592. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
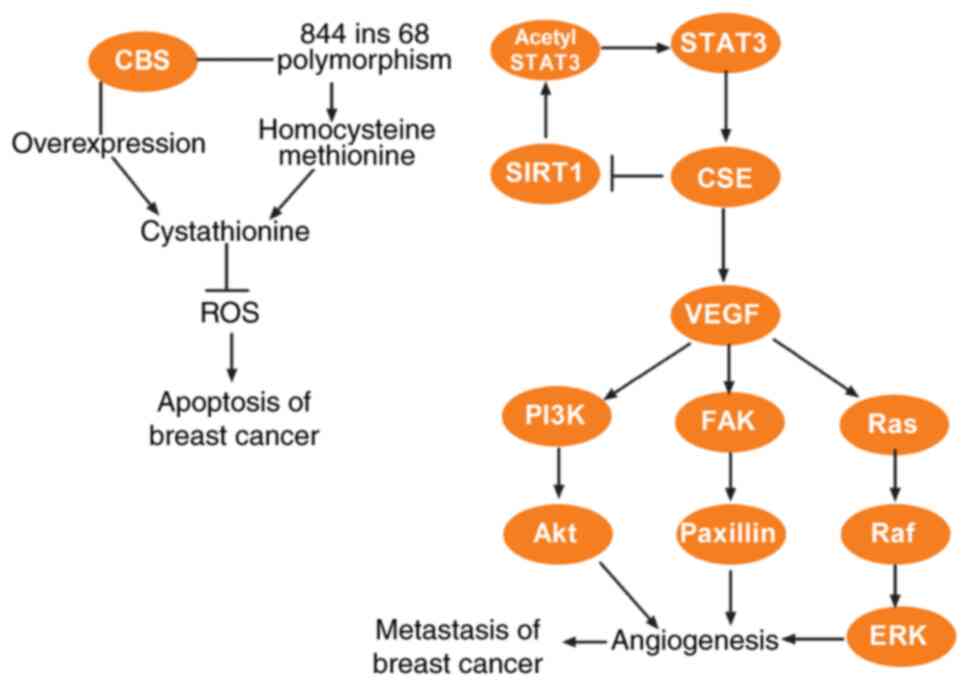
You J, Shi X, Liang H, Ye J, Wang L, Han
H, Fang H, Kang W and Wang T: Cystathionine-γ-lyase promotes
process of breast cancer in association with STAT3 signaling
pathway. Oncotarget. 8:65677–65686. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang L, Shi H, Liu Y, Zhang W, Duan X, Li
M, Shi X and Wang T: Cystathionine-γ-lyase promotes the metastasis
of breast cancer via the VEGF signaling pathway. Int J Oncol.
55:473–487. 2019.PubMed/NCBI
|
|
55
|
Hanaoka K, Sasakura K, Suwanai Y,
Toma-Fukai S, Shimamoto K, Takano Y, Shibuya N, Terai T, Komatsu T,
Ueno T, et al: Discovery and mechanistic characterization of
selective inhibitors of H2S-producing enzyme:
3-Mercaptopyruvate sulfurtransferase (3MST) targeting active-site
cysteine persulfide. Sci Rep. 7:402272017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nagahara N: Regulation of mercaptopyruvate
sulfurtransferase activity via intrasubunit and intersubunit
redox-sensing switches. Antioxid Redox Signal. 19:1792–1802. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Módis K, Asimakopoulou A, Coletta C,
Papapetropoulos A and Szabo C: Oxidative stress suppresses the
cellular bioenergetic effect of the 3-mercaptopyruvate
sulfurtransferase/hydrogen sulfide pathway. Biochem Biophys Res
Commun. 433:401–407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jeddi S, Gholami H, Gheibi S, Kashfi K and
Ghasemi A: Altered gene expression of hydrogen sulfide-producing
enzymes in the liver and muscles tissues of hyperthyroid rats. J
Cell Physiol. 234:17937–17945. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Huang CW and Moore PK: H2S synthesizing
enzymes: Biochemistry and molecular aspects. Handb Exp Pharmacol.
230:3–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li M, Xu C, Shi J, Ding J, Wan X, Chen D,
Gao J, Li C, Zhang J, Lin Y, et al: Fatty acids promote fatty liver
disease via the dysregulation of 3-mercaptopyruvate
sulfurtransferase/hydrogen sulfide pathway. Gut. 67:2169–2180.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Augsburger F and Szabo C: Potential role
of the 3-mercaptopyruvate sulfurtransferase (3-MST)-hydrogen
sulfide (H2S) pathway in cancer cells. Pharmacol Res.
154:1040832020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sen S, Kawahara B, Gupta D, Tsai R,
Khachatryan M, Roy-Chowdhuri S, Bose S, Yoon A, Faull K,
Farias-Eisner R and Chaudhuri G: Role of cystathionine β-synthase
in human breast cancer. Free Radic Biol Med. 86:228–238. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sen S, Kawahara B, Mahata SK, Tsai R, Yoon
A, Hwang L, Hu-Moore K, Villanueva C, Vajihuddin A, Parameshwar P,
et al: Cystathionine: A novel oncometabolite in human breast
cancer. Arch Biochem Biophys. 604:95–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kraus JP, Oliveriusová J, Sokolová J,
Kraus E, Vlcek C, de Franchis R, Maclean KN, Bao L, Bukovsk,
Patterson D, Paces V, et al: The human cystathionine beta-synthase
(CBS) gene: Complete sequence, alternative splicing, and
polymorphisms. Genomics. 52:312–324. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tsai MY, Bignell M, Yang F, Welge BG,
Graham KJ and Hanson NQ: Polygenic influence on plasma
homocysteine: Association of two prevalent mutations, the 844ins68
of cystathionine beta-synthase and A(2756)G of methionine synthase,
with lowered plasma homocysteine levels. Atherosclerosis.
149:131–137. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Weiner AS, Boyarskikh UA, Voronina EN,
Selezneva IA, Sinkina TV, Lazarev AF, Petrova VD and Filipenko ML:
Polymorphisms in the folate-metabolizing genes MTR, MTRR, and CBS
and breast cancer risk. Cancer Epidemiol. 36:e95–e100. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Stevens VL, McCullough ML, Pavluck AL,
Talbot JT, Feigelson HS, Thun MJ and Calle EE: Association of
polymorphisms in one-carbon metabolism genes and postmenopausal
breast cancer incidence. Cancer Epidemiol Biomarkers Prev.
16:1140–1147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gallegos-Arreola MP, Figuera-Villanueva
LE, Ramos-Silva A, Salas-González E, Puebla-Pérez AM, Peralta-Leal
V, García-Ortiz JE, Dávalos-Rodríguez IP and Zúñiga-González GM:
The association between the 844ins68 polymorphism in the CBS gene
and breast cancer. Arch Med Sci. 10:1214–1224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ryu CS, Kwak HC, Lee JY, Oh SJ, Phuong NT,
Kang KW and Kim SK: Elevation of cysteine consumption in
tamoxifen-resistant MCF-7 cells. Biochem Pharmacol. 85:197–206.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ryu CS, Kwak HC, Lee KS, Kang KW, Oh SJ,
Lee KH, Kim HM, Ma JY and Kim SK: Sulfur amino acid metabolism in
doxorubicin-resistant breast cancer cells. Toxicol Appl Pharmacol.
255:94–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Thornburg JM, Nelson KK, Clem BF, Lane AN,
Arumugam S, Simmons A, Eaton JW, Telang S and Chesney J: Targeting
aspartate aminotransferase in breast cancer. Breast Cancer Res.
10:R842008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chao C, Zatarain JR, Ding Y, Coletta C,
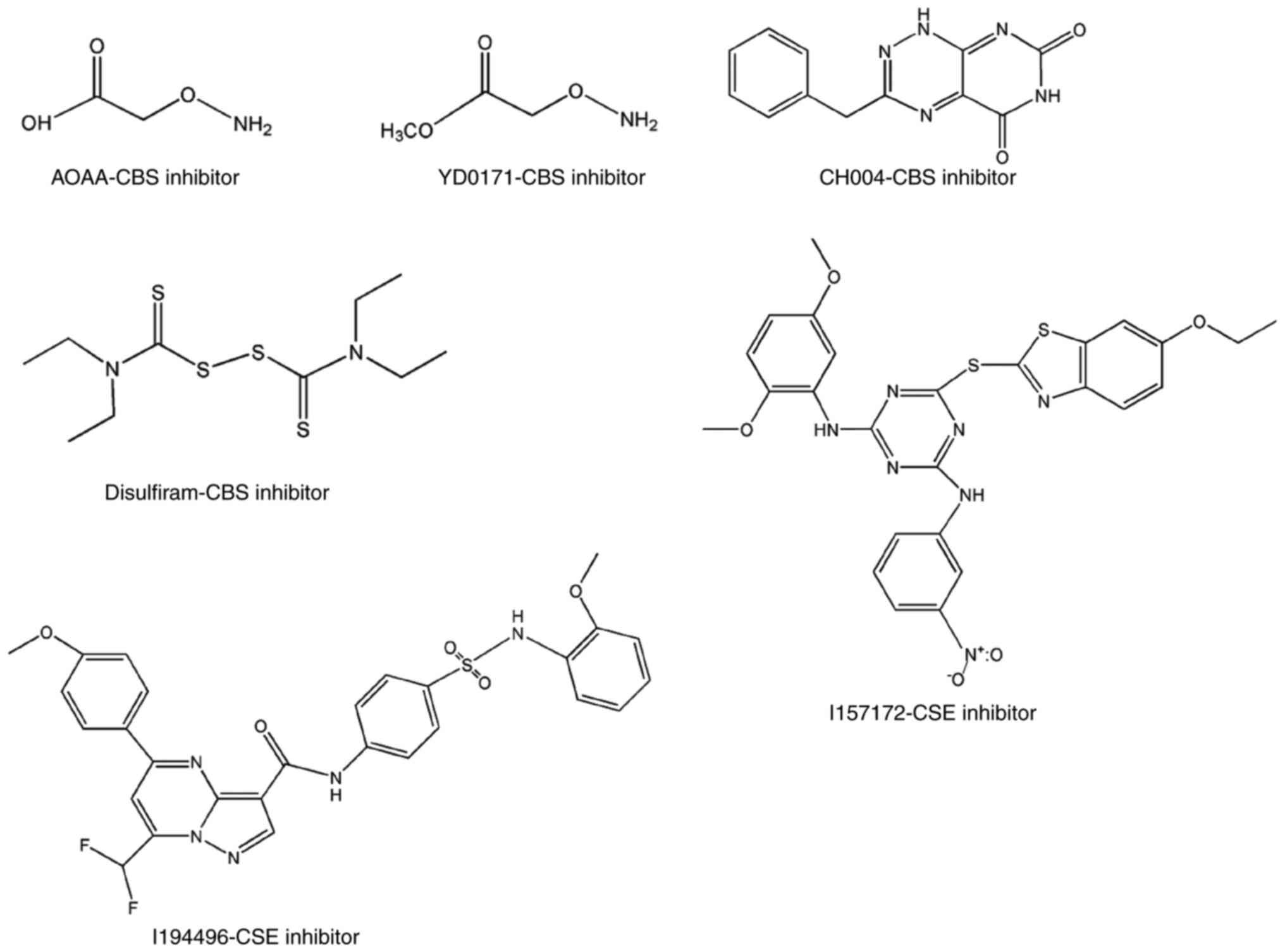
Mrazek AA, Druzhyna N, Johnson P, Chen H, Hellmich JL,
Asimakopoulou A, et al: Cystathionine-beta-synthase inhibition for
colon cancer: Enhancement of the efficacy of aminooxyacetic acid
via the prodrug approach. Mol Med. 22:361–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hellmich MR, Coletta C, Chao C and Szabo
C: The therapeutic potential of cystathionine β-synthetase/hydrogen
sulfide inhibition in cancer. Antioxid Redox Signal. 22:424–448.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
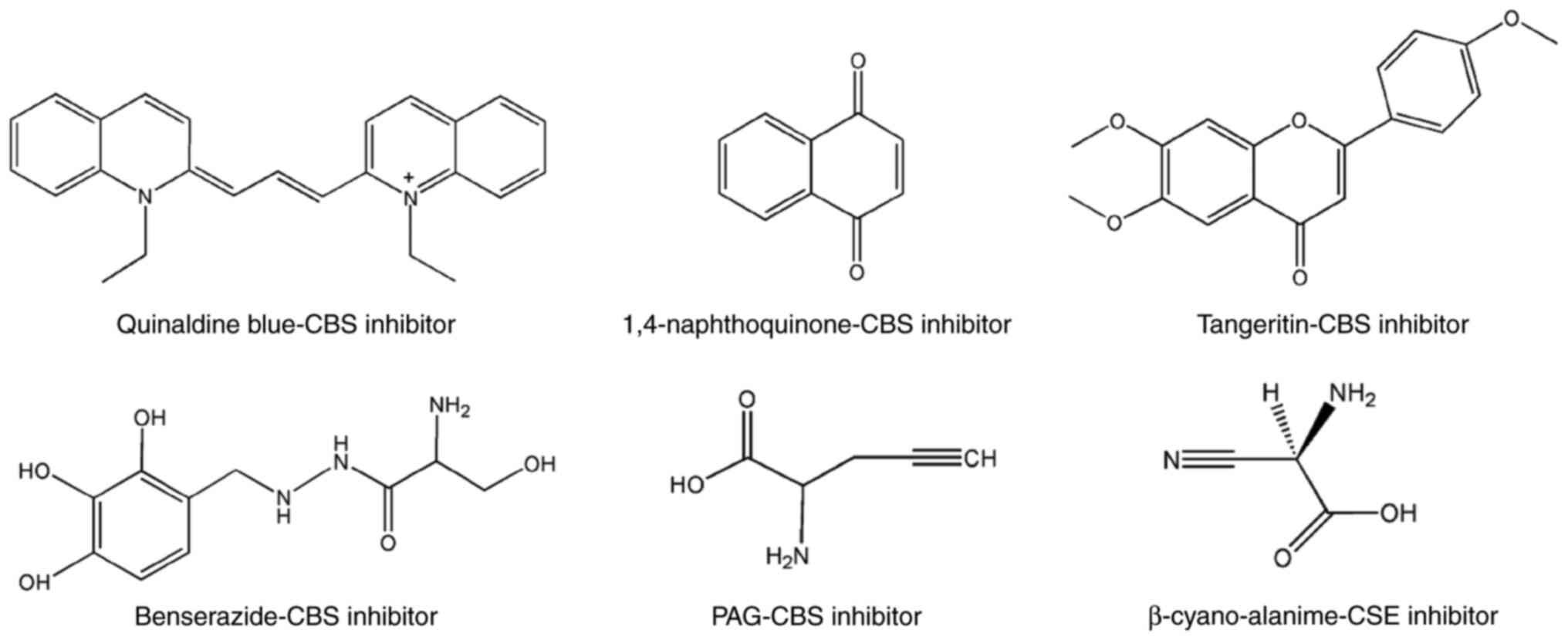
Asimakopoulou A, Panopoulos P, Chasapis
CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA
and Papapetropoulos A: Selectivity of commonly used pharmacological
inhibitors for cystathionine β synthase (CBS) and cystathionine γ
lyase (CSE). Br J Pharmacol. 169:922–932. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhou Y, Yu J, Lei X, Wu J, Niu Q, Zhang Y,
Liu H, Christen P, Gehring H and Wu F: High-throughput
tandem-microwell assay identifies inhibitors of the hydrogen
sulfide signaling pathway. Chem Commun. 49:11782–11784. 2013.
View Article : Google Scholar
|
|
76
|
Wang L, Cai H, Hu Y, Liu F, Huang S, Zhou
Y, Yu J, Xu J and Wu F: A pharmacological probe identifies
cystathionine β-synthase as a new negative regulator for
ferroptosis. Cell Death Dis. 9:10052018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zuhra K, Augsburger F, Majtan T and Szabo
C: Cystathionine-β-synthase: Molecular regulation and
pharmacological inhibition. Biomolecules. 10:6972020. View Article : Google Scholar
|
|
78
|
Marechal D, Brault V, Leon A, Martin D,
Lopes Pereira P, Loaëc N, Birling MC, Friocourt G, Blondel M and
Herault Y: Cbs overdosage is necessary and sufficient to induce
cognitive phenotypes in mouse models of down syndrome and interacts
genetically with Dyrk1a. Hum Mol Genet. 28:1561–1577. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chen D, Cui QC, Yang H and Dou QP:
Disulfiram, a clinically used anti-alcoholism drug and
copper-binding agent, induces apoptotic cell death in breast cancer
cultures and xenografts via inhibition of the proteasome activity.
Cancer Res. 66:10425–10433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Safi R, Nelson ER, Chitneni SK, Franz KJ,
George DJ, Zalutsky MR and McDonnell DP: Copper signaling axis as a
target for prostate cancer therapeutics. Cancer Res. 74:5819–5831.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zha J, Chen F, Dong H, Shi P, Yao Y, Zhang
Y, Li R, Wang S, Li P, Wang W and Xu B: Disulfiram targeting
lymphoid malignant cell lines via ROS-JNK activation as well as
Nrf2 and NF-κB pathway inhibition. J Transl Med. 12:1632014.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Papaioannou M, Mylonas I, Kast RE and
Brüning A: Disulfiram/copper causes redox-related proteotoxicity
and concomitant heat shock response in ovarian cancer cells that is
augmented by auranofin-mediated thioredoxin inhibition.
Oncoscience. 1:21–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Cong J, Wang Y, Zhang X, Zhang N, Liu L,
Soukup K, Michelakos T, Hong T, DeLeo A, Cai L, et al: A novel
chemoradiation targeting stem and nonstem pancreatic cancer cells
by repurposing disulfiram. Cancer Lett. 409:9–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Malcolm R, Olive MF and Lechner W: The
safety of disulfiram for the treatment of alcohol and cocaine
dependence in randomized clinical trials: Guidance for clinical
practice. Expert Opin Drug Saf. 7:459–472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Jiao Y, Hannafon BN and Ding WQ:
Disulfiram's anticancer activity: Evidence and mechanisms.
Anticancer Agents Med Chem. 16:1378–1384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
DeVito EE, Babuscio TA, Nich C, Ball SA
and Carroll KM: Gender differences in clinical outcomes for cocaine
dependence: Randomized clinical trials of behavioral therapy and
disulfiram. Drug Alcohol Depend. 145:156–167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Feng J, Weitner M, Shi W, Zhang S,
Sullivan D and Zhang Y: Identification of additional anti-persister
activity against Borrelia burgdorferi from an FDA drug library.
Antibiotics (Basel). 4:397–410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Thorson MK, Majtan T, Kraus JP and Barrios
AM: Identification of cystathionine β-synthase inhibitors using a
hydrogen sulfide selective probe. Angew Chem Int Ed Engl.
52:4641–4644. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Druzhyna N, Szczesny B, Olah G, Módis K,
Asimakopoulou A, Pavlidou A, Szoleczky P, Gerö D, Yanagi K, Törö G,
et al: Screening of a composite library of clinically used drugs
and well-characterized pharmacological compounds for cystathionine
β-synthase inhibition identifies benserazide as a drug potentially
suitable for repurposing for the experimental therapy of colon
cancer. Pharmacol. Res. 113:18–37. 2016.
|
|
90
|
Niu W, Wu P, Chen F, Wang J, Shang X and
Xu C: Discovery of selective cystathionine β-synthase inhibitors by
high-throughput screening with a fluorescent thiol probe.
Medchemcomm. 8:198–201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
McCune CD, Chan SJ, Beio ML, Shen W, Chung
WJ, Szczesniak LM, Chai C, Koh SQ, Wong PT and Berkowitz DB:
‘Zipped synthesis’ by cross-metathesis provides a cystathionine
β-synthase inhibitor that attenuates cellular H2S levels and
reduces neuronal infarction in a rat ischemic stroke model. ACS
Central Sci. 2:242–252. 2016. View Article : Google Scholar
|
|
92
|
Sekiguchi F, Sekimoto T, Ogura A and
Kawabata A: Endogenous hydrogen sulfide enhances cell proliferation
of human gastric cancer AGS cells. Biol Pharm Bull. 39:887–890.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Hu Y, Wang L, Han X, Zhou Y, Zhang T, Wang
L, Hong T, Zhang W, Guo XX, Sun J, et al: Discovery of a bioactive
inhibitor with a new scaffold for cystathionine γ-lyase. J Med
Chem. 62:1677–1683. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bhattacharjee A, Sinha A, Ratia K, Yin L,
Delgado-Rivera L, Petukhov PA, Thatcher GRJ and Wardrop DJ:
2-Arylidene hydrazinecarbodithioates as potent, selective
inhibitors of cystathionine γ-lyase (CSE). ACS Med Chem Lett.
8:1241–1245. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yadav PK, Vitvitsky V, Kim H, White A, Cho
US and Banerjee R: S-3-Carboxypropyl-l-cysteine specifically
inhibits cystathionine γ-lyase-dependent hydrogen sulfide
synthesis. J Biol Chem. 294:11011–11022. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang L, Shi H, Zhang X, Zhang X, Liu Y,
Kang W, Shi X and Wang T: I157172, a novel inhibitor of
cystathionine γ-lyase, inhibits growth and migration of breast
cancer cells via SIRT1-mediated deacetylation of STAT3. Oncol Rep.
41:427–436. 2019.PubMed/NCBI
|