Introduction
Acute myeloid leukemia (AML) is common in adults and
children (1), but is typically
considered a disease of the elderly population (2). AML is characterized by the rapid growth
of the myeloid lineage of blood cells and the malignant
transformation of hematopoietic stem/progenitor cells (3,4). Malignant
precursor cells accumulate in the blood and bone marrow, resulting
in acute symptoms, including anemia, infections, bleeding and
bruising, bone pain, bone marrow failure and death (5). Elderly patients with AML have a markedly
less favorable prognosis due to increased resistance to standard
cytotoxic agents (6–8). In addition, patients with AML who
respond to chemotherapy often relapse later in life (7,9,10). Yu et al (11) reported that relapse typically occurred
within the first 3 years from the end of chemotherapy in young
patients. Therefore, preventing chemoresistance in a selective
manner and identifying a novel therapeutic strategy are important
for improving the cure rate of AML.
The PI3K/AKT signaling pathway serves an important
role in maintaining cell proliferation and survival, and
dysregulation of the signaling pathway is involved in various
malignancies, including AML (12–16).
Directly stimulating the mitochondrial apoptosis signaling pathway
is a novel therapeutic strategy to target cancer cells (17). The BCL2 protein family regulates the
mitochondrial apoptosis signaling pathway, and aberrant
upregulation of BCL2 is related to carcinogenesis and drug
resistance (18). BCL2 overexpression
has also been reported in AML (19).
Moreover, BCL2 overexpression can increase leukemia fitness, render
intrinsic chemoresistance, and contribute to the survival of
minimal residual quiescent leukemia stem cells that are responsible
for AML relapse (20,21). Activation of the PI3K/AKT/mTOR
signaling pathway and BCL2 upregulation are related to
stroma-mediated AML survival (22–26).
LY294002, an inhibitor of PI3K, is widely used to
study the role of the PI3K/AKT signaling pathway in transformed
cells (27,28). In some cancer cell lines, LY294002 can
induce apoptosis and increase sensitivity to chemotherapeutic drugs
(29–31). It is reported that LY294002 enhances
chemosensitivity of K562 cells to Adriamycin (32). ABT199 is a second-generation, specific
antagonist of BCL2 (3). At nanomolar
concentrations, ABT199 induces apoptosis in various chemosensitive
and chemoresistant AML stem and progenitor cells, and inhibits
leukemic progression (3). In
addition, a combination of ABT199 hypomethylating agents showed an
encouraging response in patients with newly diagnosed AML (33). At present, the most promising drugs
for targeted treatment of AML are inhibitors that regulate
metabolism or signaling pathways (34). However, it is difficult for
single-target inhibitors to produce significant and sustained
effects. The scientific and reasonable combination of multi-pathway
or multi-target drugs is a research hotspot. Therapeutic strategies
targeting the key molecules in the PI3K/AKT and cell apoptosis
signaling pathways, such as LY294002 and ABT199, may improve
therapeutic efficacy in patients with AML.
The aim of the present study was to investigate
whether LY294002 and ABT199 exerted a synergistic effect on AML
cell apoptosis and the cell cycle. The result of the present study
may provide insight for the combined application of LY294002 and
ABT199 in the treatment of AML, thus providing a novel therapeutic
strategy for the disease.
Materials and methods
Cell lines and cell culture
Human erythroleukemia (K562) and promyelocytic
leukemia (HL60) cell lines were purchased from Wuhan Punosai Life
Technology Co., Ltd. The K562 cell line was isolated and
established from human leukemia cells, which can grow in
vitro over a long period of time. Based on the characteristics
of a short proliferation cycle, as well as stable growth and
metabolism, the K562 cell line is a commonly used model cell line
in biomedical research (35). The
HL60 cell line is typically used to study how certain blood cells
form, providing continuous human cells for the study of molecular
events in granulocyte differentiation and the physiological effects
of this process, drug action and viral components (36). The human myeloid leukemia cell line
(KG1a) was purchased from Shanghai Xinyu Biological Technology Co.,
Ltd. The KG1a cell line is morphologically similar to AML,
displaying significant polymorphisms (37). K562, HL60 and KG1a cells were cultured
in medium (IMDM medium for K562 cells; RPMI-1640 medium for HL60
and KG1a cells) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 1% 100 IU/ml penicillin and 100 mg/l
streptomycin (Beijing Solarbio Science and Technology Co., Ltd.) at
37°C in a humidified atmosphere with 5% CO2.
Reagents
PI3K inhibitor (LY294002) and BCL2 inhibitor
(ABT199) were purchased from Biyuntian Technology, Inc. To make a
10 mM stock solution, 25 mg LY294002 was dissolved in 8.13 ml DMSO.
Similarly, to make a 10 mM stock solution, 25 mg ABT199 was
dissolved in 2.9 ml DMSO. Cell medium was used for the preparation
of a concentration gradient of LY294002 and ABT199. The
concentration gradient of LY294002 was as follows: 0.5, 0.57, 0.97,
1.5, 2.5 and 5 µM. The concentration gradient of ABT199 was as
follows: 3, 8, 15, 20, 30 and 50 nM. The related effects of
LY294002 and ABT199 have been previously investigated (38,39).
Stable compounds are considered optimal for drug research. It has
been hypothesized that LY294002 and ABT199 do not undergo
degradation or other alterations in activity in the medium, and
their chemical properties are relatively stable.
Cell counting kit-8 (CCK-8) assay
K562, HL60 and KG1a cell suspensions were prepared
and seeded (100 µl/well; three replicate wells) into 96-well
plates. Cells were cultured for 24 h to allow adherence.
Subsequently, the cells were treated with LY294002 and ABT199 for
24, 36, 48 or 72 h. Then, 10 µl CCK-8 solution (Biyuntian
Technology, Inc.) was added to each well and cultured for 1 h.
Absorbance was measured at a wavelength of 450 nm using a
microplate reader (Bio-Rad Laboratories, Inc.).
Dose-effect relationship of single and
combination treatment of drugs on cells
The cell activity value was calculated according to
the optical density value obtained via the CCK-8 assay. Based on
the cell activity value, the IC50 value was determined
using an online calculator (www.aatbio.com/tools/ic50-calculator). The smaller the
IC50 value, the more suitable the treatment was for
selection. A synergistic effect was observed when the inhibitory
rate of the combination treatment was greater than the sum of the
inhibitory rates of the two single drugs, which had reference
significance. In the present study, Jin's Formula was used to
evaluate the synergistic effect (40). The formula is as follows: Q=Eab/(Ea +
Eb-Ea × Eb), where Ea is the inhibition rate of LY294002 treatment,
Eb is the inhibition rate of ABT19 treatment and Eab is the
inhibition rate of LY294002 and ABT199 treatment. Q<0.85
indicates that the combined effect of the two agents is
antagonistic, 0.85≤Q<1.15 indicates that the combined effect of
the two agents is additive and Q≥1.15 indicates that the combined
effect of the two agents is synergistic. Based on Jin's Formula,
the Q-value in the three cell lines was ≥1.15, which indicated a
synergistic effect of LY294002 and ABT199.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from K562, HL60 and KG1a
cells using TRIzol® according to the manufacturer's
protocol. RNA concentration and purity were determined using a
nucleic acid concentration analyzer. Total RNA was
reverse-transcribed into cDNA using the SuperScript III reverse
transcription kit (Invitrogen; Thermo Fisher Scientific, Inc.).
Subsequently, qPCR was performed using an ABI 7300 Real-time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with
SYBR® Green PCR Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The sequences of reverse and forward
primers for all of the genes analyzed were as follows: Skp2
(forward: ATGCCCCAATCTTGTCCATCT, reverse: CACCGACTGAGTGATAGGTGT);
P27 (forward: AGGAGGAGATAGAAGCGCAGA, reverse: GTGCGGACTTGGTACAGGT);
Bcl-2 (forward: AGATGGGAACACTGGTGGAG, reverse:
CTTCCCCAAAAGAAATGCAA); Bax (forward: AGGGTTTCATCCAGGATCGAGCA,
reverse: CAGCTTCTTGGTGGACGCATC); Caspase-3 (forward:
ACATCTCCCGGCGGCGGGCCGCGGA, reverse: CTTCTACAACCGCCTCACAATAGCA);
Caspase-9 (forward: AGTTGGCTACTCGCCATGGACGAAG, reverse:
TTTGCTGCTTGCCTGTTAGTTCGCA); β-actin (forward:
GACAGGATGCAGAAGGAGATTACT, reverse: TGATCCACATCTGCTGGAAGGT). The
following thermocycling conditions were used for qPCR: 5 min at
95°C; followed by 40 cycles of 10 sec at 95°C, 20 sec at 58°C, 20
sec at 72°C and 15 sec at 95°C; 60 sec at 60°C; and final extension
for 15 sec at 95°C. All reactions were performed in triplicate.
mRNA expression levels were quantified using the 2−ΔΔCq
method (41) and normalized to the
internal reference gene β-actin.
Western blot analysis
Total protein was extracted from K562, HL60 and KG1a
cells and mixed with pyrolysis liquid. Maximum power ultrasonic was
used for cell crushing in the ice bath (3×10 sec). Protein
concentrations were determined using the BCA protein quantitative
method. Equivalent amounts of protein (25 µg) were separated via
12% SDS-PAGE and electro-transferred onto a PVDF membrane in 1X
protein transfer membrane solution in ice water for 1.5 h.
Following blocking in PBS supplemented with 5% skimmed dry milk at
room temperature for 1 h, the membranes were incubated at 4°C
overnight with primary antibodies of Skp2 (monoclonal antibody,
1:500, bs-1096R, BIOSS), P27 (polyclonal antibody, 1:200, DF6090,
Affinity Biosciences), Bcl2 (polyclonal antibody, 1:500, bs-0032R,
BIOSS), Bax (polyclonal antibody, 1:500, bs-0127R, BIOSS), cleaved
casepase-3 (polyclonal antibody, 1:500, bs-0081R, BIOSS), cleaved
casepase-9 (polyclonal antibody, 1:500, bs-0049R, BIOSS),
Procasepase-3 (monoclonal antibody, 1:500, sc-7272, Santa Cruz
Biotechnology) and Procasepase-9 (monoclonal antibody, 1:500,
sc-70506, Santa Cruz Biotechnology). Subsequently, the membranes
were incubated with HRP-conjugated secondary antibodies (1:5,000,
ZB-2305, ZSGB-BIO) at room temperature for 1 h. Protein bands were
visualized using enhanced ECL chemiluminescence reagents followed
by exposure to X-ray film. The protocol was repeated three times by
using ImageJ software.
Statistical analysis
Statistical analyses were performed using SPSS
statistical software (SPSS, Inc.). Data are presented as the mean ±
SD. ANOVA and Dunnett's post hoc test was used for comparison
analysis between the two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Determination of optimal concentration
and duration of LY294002 and ABT199 combination treatment
To identify the optimal concentration and duration
of LY294002 and ABT199 combination treatment, the IC50
values of different concentrations of LY294002, ABT199 or LY294002
and ABT199 combination treatment at 24, 36, 48 and 72 h in K562,
HL60 and KG1a cells were calculated. A synergistic effect was
observed when the inhibitory rate of the combination treatment was
greater than the sum of the inhibitory rates of the two single
drugs.
In K562 cells (Table
I), the IC50 value of LY294002 (1.433 µM) was the
lowest following treatment for 48 h. At 48 h, the IC50
value of ABT199 was 22.498 nM. Therefore, the combination of
LY294002 <1.433 µM and ABT199 <22.498 nM at 48 h was used as
the screening criteria. The combination of 0.97 µM LY294002 and
18.222 nM ABT199 at 48 h was considered to be the optimal
concentration and duration of drug combination action in K562 cells
(Table II).
 | Table I.IC50 value of different
contention of single LY294002 and ABT199 at different time points
in K562, HL60 and KG1a cells. |
Table I.
IC50 value of different
contention of single LY294002 and ABT199 at different time points
in K562, HL60 and KG1a cells.
|
| K562 cells | HL60 cells | KG1a cells |
|---|
|
|
|
|
|
|---|
| IC50
value | LY294002 (µM) | ABT199 (nM) | LY294002 (µM) | ABT199 (nM) | LY294002 (µM) | ABT199 (nM) |
|---|
| 24 h | 1.637 | 23.666 | 3.373 | 1100.611 | 2.794 | 36.294 |
| 36 h | 1.612 | 24.445 | 3.472 | 957.016 | 2.942 | 313.530 |
| 48 h | 1.433 | 22.498 | 3.893 | 262.94 | 2.853 | 389.674 |
| 72 h | 1.547 | 22.128 | 4.577 | 464.444 | 6.758 | 378.516 |
 | Table II.IC50 value of different
contention of LY294002 and ABT199 combination at different time
points in K562 cells. |
Table II.
IC50 value of different
contention of LY294002 and ABT199 combination at different time
points in K562 cells.
| IC50
value | ABT199 (nM) |
|---|
|
|
|
|---|
| LY294002 (µM) | 24 h | 36 h | 48 h | 72 h |
|---|
| 0.5 | 23.285 | 25.654 | 25.230 | 69.294 |
| 0.57 | 23.162 | 26.747 | 40.195 | 127.106 |
| 0.97 | 25.122 | 25.184 | 18.222 | 32.125 |
| 1.5 | 25.339 | 25.339 | 23.048 | 21.069 |
| 2.5 | 25.347 | 25.704 | 28.156 | 18.440 |
| 5 | 24.202 | 25.731 | 77.801 | 23.194 |
In HL60 cells (Table
I), the IC50 value of ABT199 (262.94 nM) was the
lowest after treatment for 48 h. At 48 h, the IC50 value
of LY294002 was 3.893 µM. Therefore, the combination of LY294002
<3.893 µM and ABT199 <262.94 nM at 48 h was used as the
screening criteria. The combination of 0.57 µM LY294002 and 22.476
nM ABT199 at 48 h was considered the optimal concentration and
duration of drug combination action in HL60 cells (Table III).
 | Table III.IC50 value of different
contention of LY294002 and ABT199 combination at different time
points in HL60 cells. |
Table III.
IC50 value of different
contention of LY294002 and ABT199 combination at different time
points in HL60 cells.
| IC50
value | ABT199 (nM) |
|---|
|
|
|
|---|
| LY294002 (µM) | 24 h | 36 h | 48 h | 72 h |
|---|
| 0.5 | 42.234 | 183.449 | 24.275 | 37.273 |
| 0.57 | 21.314 | 183.569 | 22.476 | 160.804 |
| 0.97 | 56.324 | 196.308 | 26.998 | 81.574 |
| 1.5 | 18.712 | 35.984 | 28.015 | 60.768 |
| 2.5 | 19.210 | 43.297 | 28.457 | 39.534 |
| 5 | 23.052 | 28.282 | 63.810 | 19.902 |
In KG1a cells (Table
I), the IC50 value of LY294002 (2.794 µM) was the
lowest after treatment for 24 h. At 24 h, the IC50 value
of ABT199 (36.294 nM) was also the lowest. Therefore, the
combination of LY294002 <2.794 µM and ABT199 <36.294 nM at 24
h was used as the screening criteria. The combination of 0.97 µM
LY294002 and 23.141 nM ABT199 at 24 h was considered to be the
optimal concentration and duration of drug combination action in
KG1a cells (Table IV).
 | Table IV.IC50 value of different
concentrations of LY294002 and ABT199 combination at different time
points in KG1a cells. |
Table IV.
IC50 value of different
concentrations of LY294002 and ABT199 combination at different time
points in KG1a cells.
| IC50
value | ABT199 (nM) |
|---|
|
|
|
|---|
| LY294002 (µM) | 24 h | 36 h | 48 h | 72 h |
|---|
| 0.5 | 39.457 | 228.364 | 106.779 | 130.807 |
| 0.57 | 24.414 | 167.608 | 208.388 | 24.812 |
| 0.97 | 23.141 | 52.986 | 198.294 | 16.557 |
| 1.5 | 32.621 | 26.569 | 104.024 | 41.357 |
| 2.5 | 23.782 | 21.719 | 75.506 | 15.062 |
| 5 | 25.547 | 17.115 | 39.875 | 16.435 |
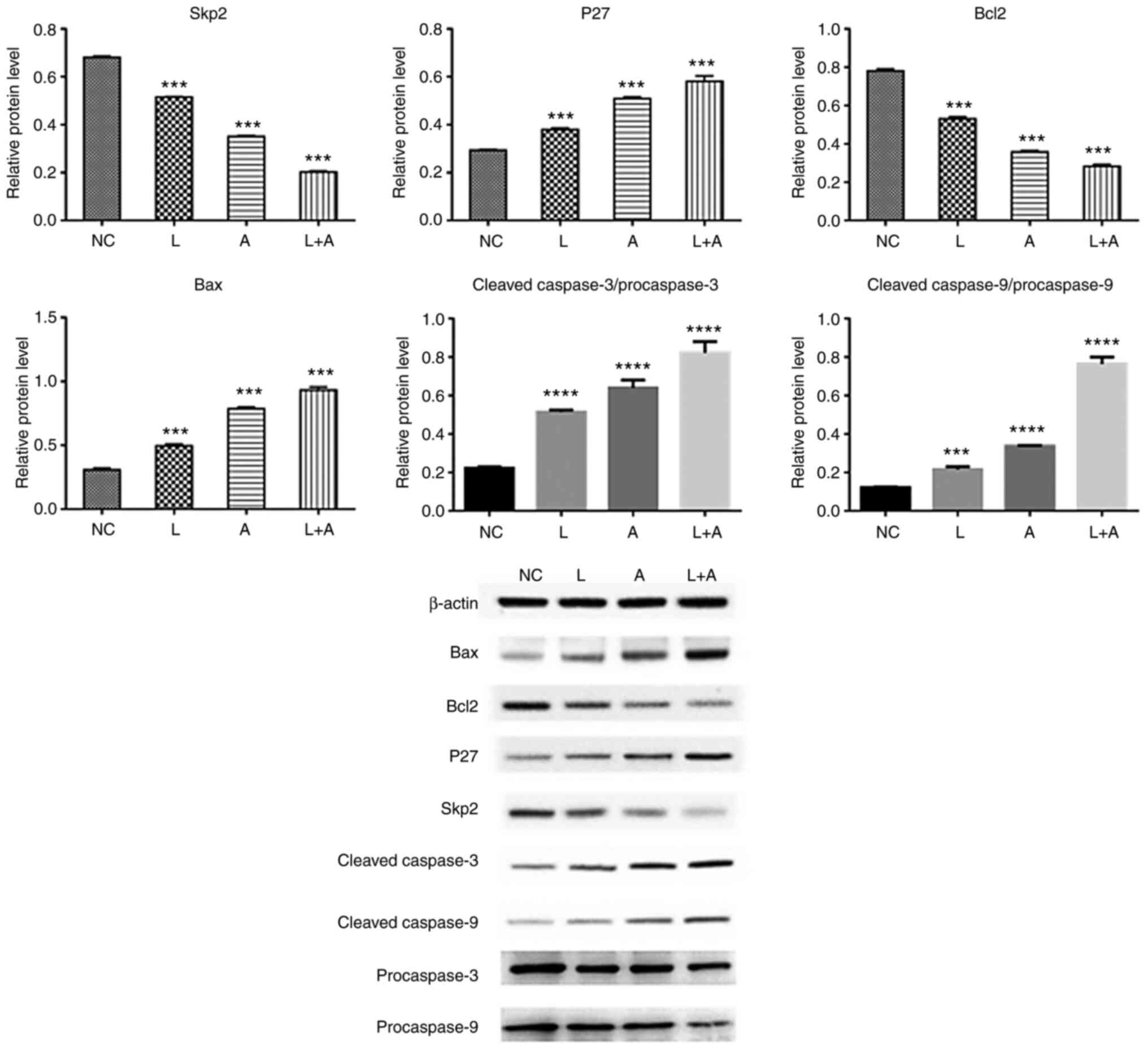
RT-qPCR
To further investigate the effects of LY294002 and
ABT199 combination treatment on K562, HL60 and KG1a cells at the
molecular level, six cell cycle-related molecular markers [S-phase
kinase associated protein 2 (Skp2), p27, Bcl2, Bax, cleaved
caspase-3 and caspase-9] were evaluated. The primer sequences are
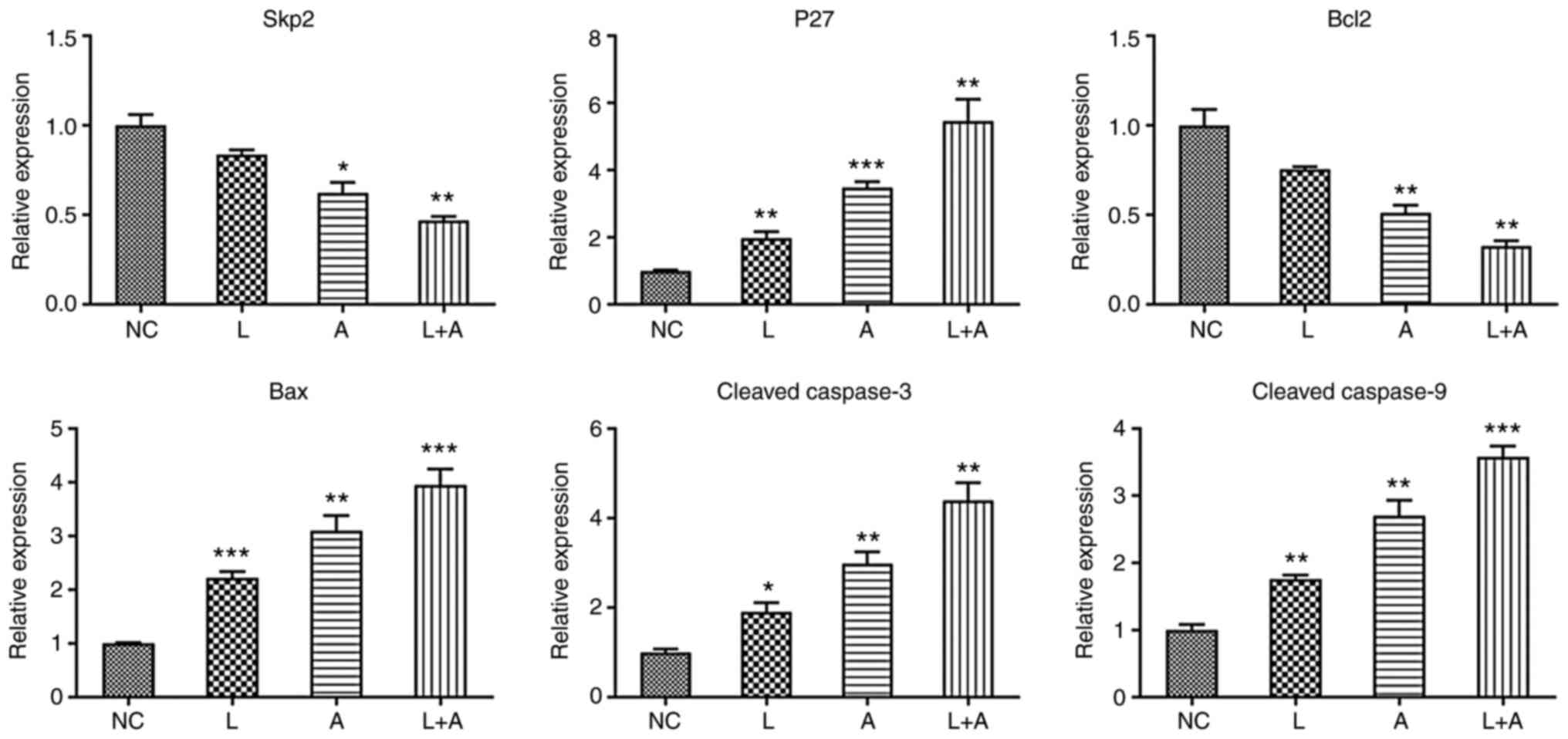
shown in Table V. In K562 cells
(Fig. 1), Skp2 and Bcl2 expression
levels were significantly downregulated after LY294002 and ABT199
combination treatment. p27, Bax, cleaved caspase-3 and caspase-9
expression levels were markedly upregulated by single and
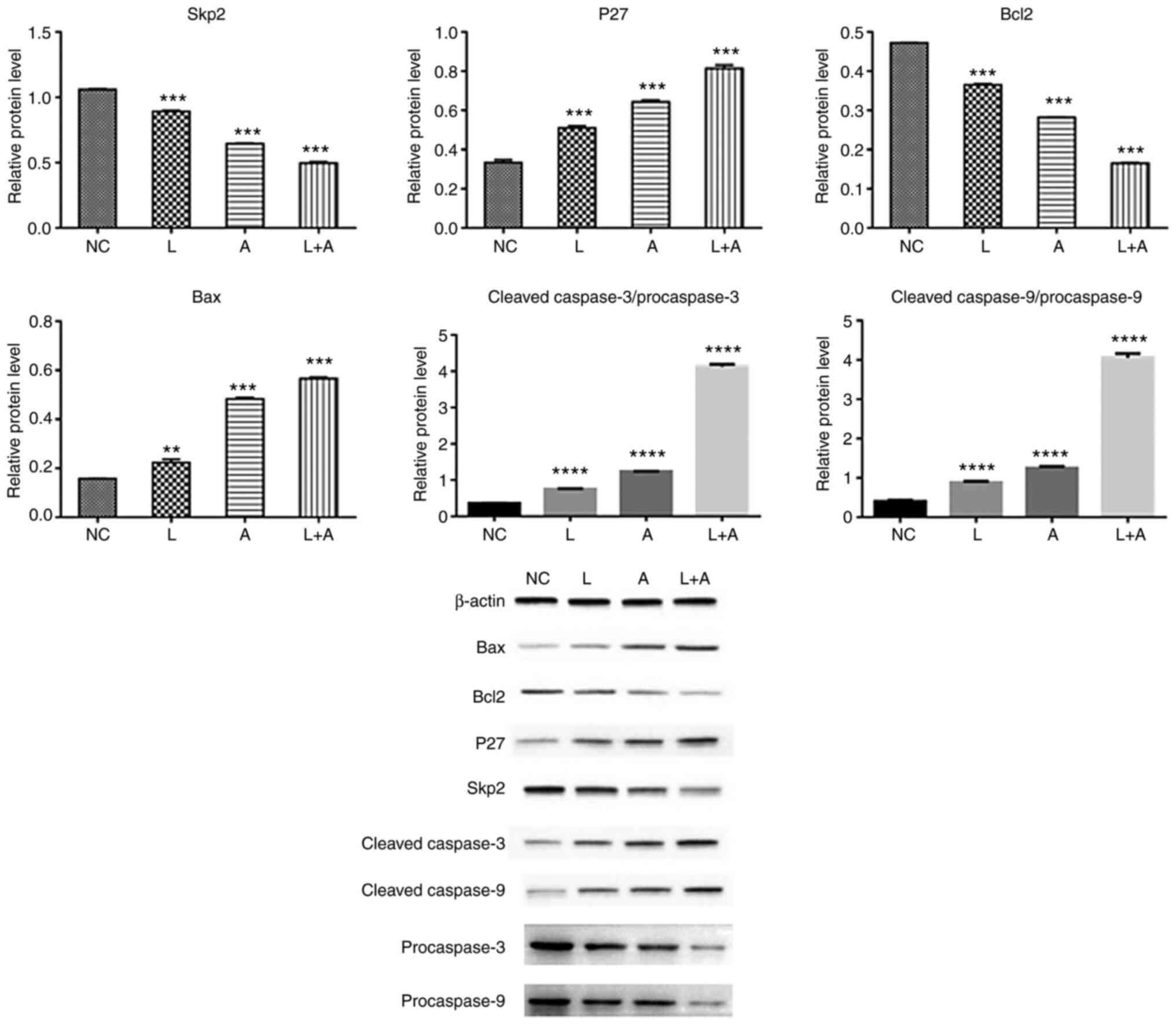
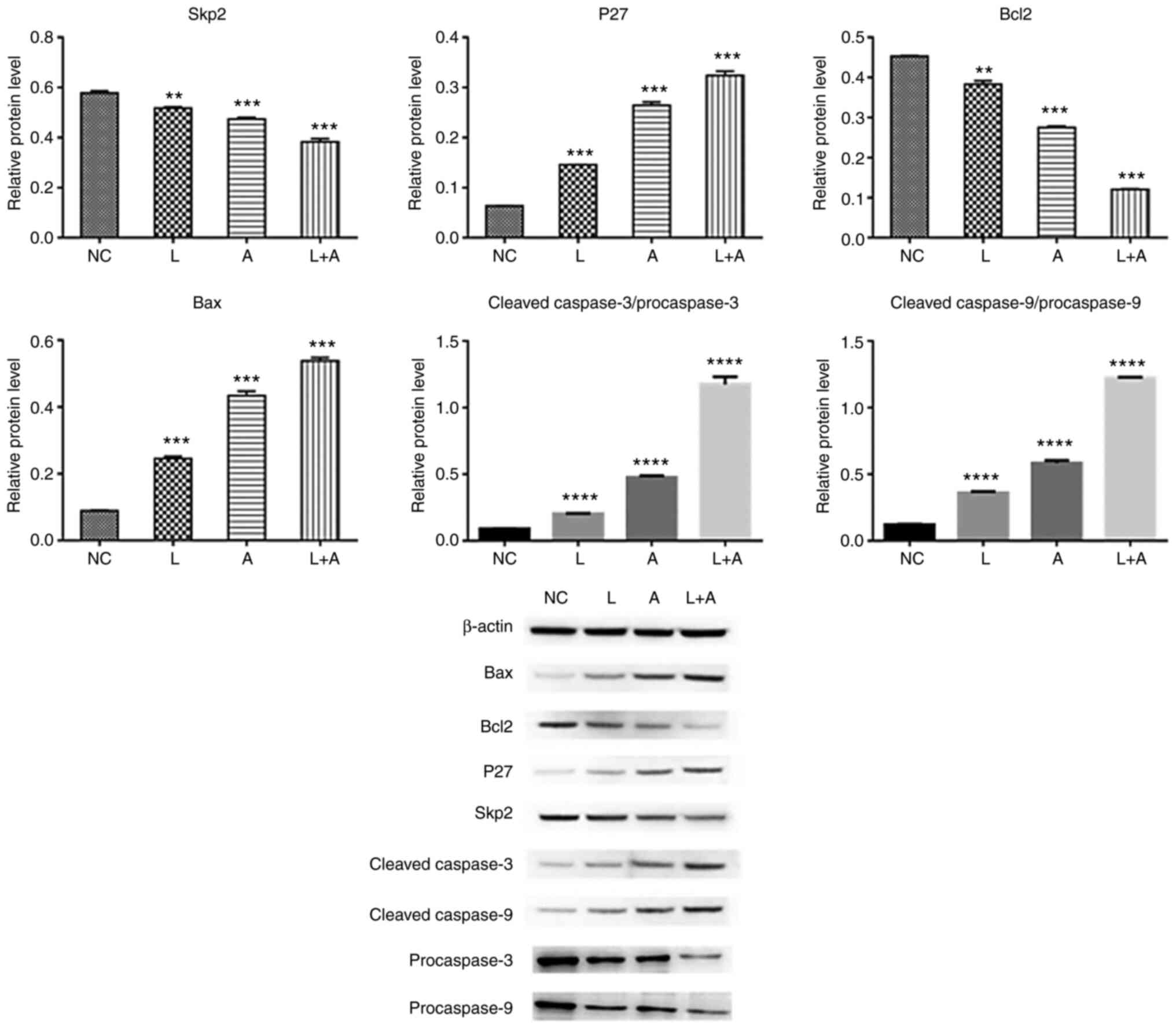
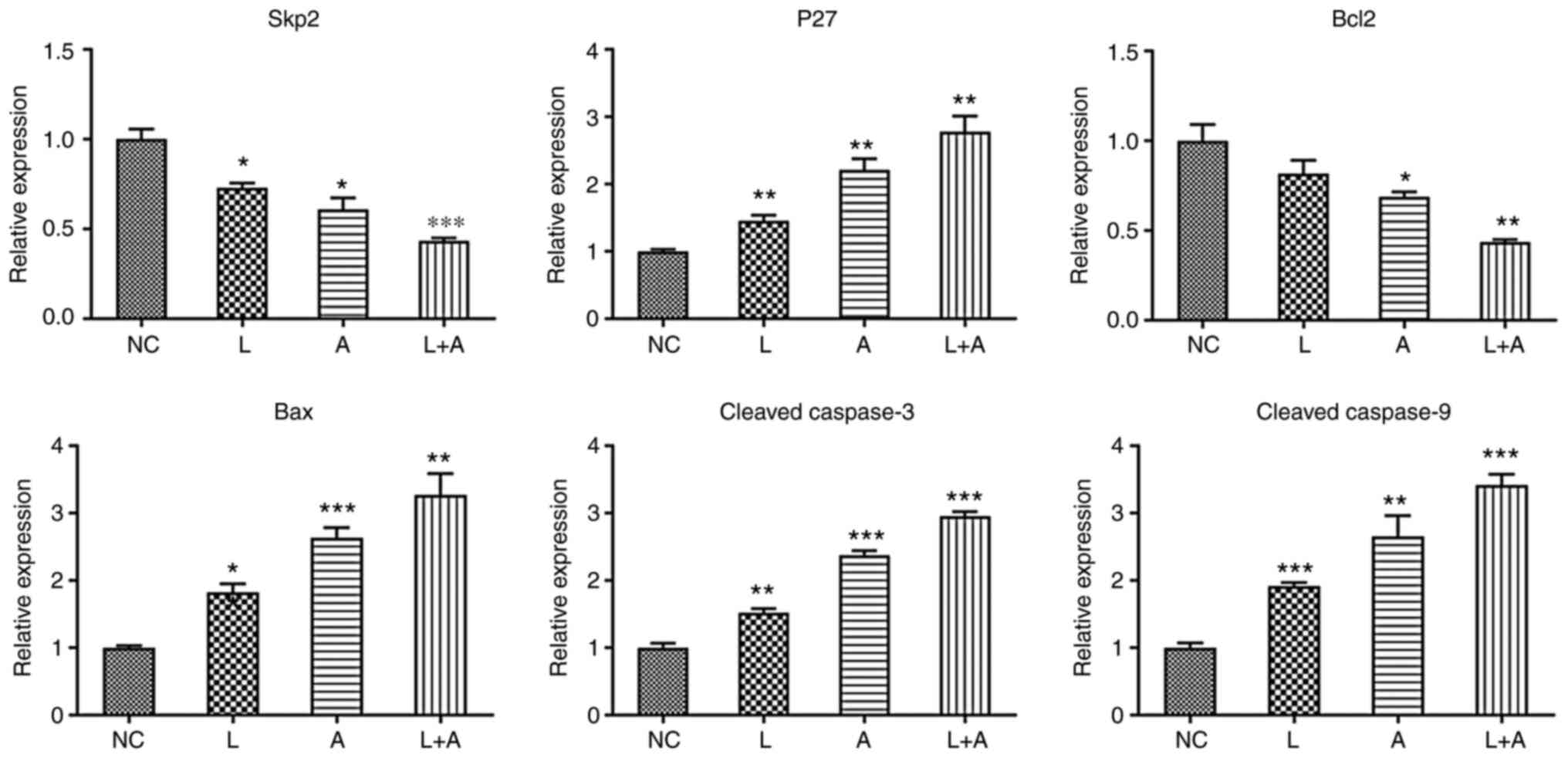
combination treatment with LY294002 and ABT199. In both HL60
(Fig. 2) and KG1a (Fig. 3) cells, Skp2 and Bcl2 expression
levels were significantly downregulated in the single ABT199
treatment group and the combined treatment group. p27, Bax, cleaved
caspase-3 and caspase-9 expression levels were significantly
upregulated in the single LY294002, single ABT199 and combined
treatment groups.
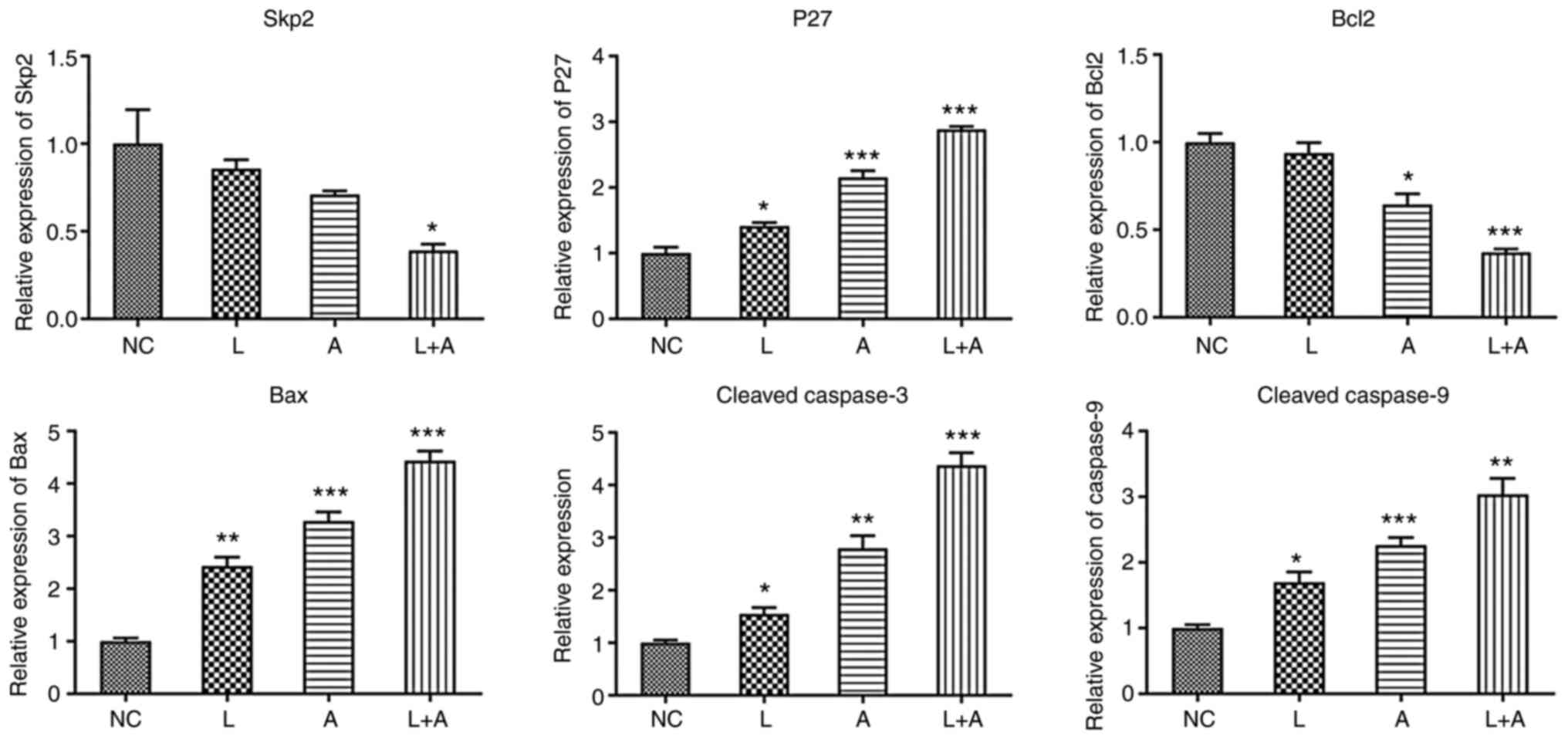 | Figure 1.Skp2, p27, Bcl2, Bax, cleaved
caspase-3 and cleaved caspase-9 mRNA expression levels in different
drug treatment groups in K562 cells. *P<0.05, **P<0.01 and
***P<0.001. Skp2, S-phase kinase associated protein 2; NC,
normal controls; L, LY294002; A, ABT199; L+A, LY294002+ ABT199. |
 | Figure 2.Skp2, p27, Bcl2, Bax, cleaved
caspase-3 and cleaved caspase-9 mRNA expression levels in different
drug treatment groups in HL60 cells. *P<0.05, **P<0.01 and
***P<0.001. Skp2, S-phase kinase associated protein 2; NC,
normal controls; L, LY294002; A, ABT199; L+A, LY294002+ ABT199. |
 | Figure 3.Skp2, p27, Bcl2, Bax, cleaved
caspase-3 and cleaved caspase-9 mRNA expression levels in different
drug treatment groups in KG1a cells. *P<0.05, **P<0.01 and
***P<0.001. Skp2, S-phase kinase associated protein 2; NC,
normal controls; L, LY294002; A, ABT199; L+A, LY294002+ ABT199. |
 | Table V.Primer sequences used in RT-qPCR. |
Table V.
Primer sequences used in RT-qPCR.
| Target name | Primer | Sequences |
|---|
| β-actin | F |
GACAGGATGCAGAAGGAGATTACT |
|
| R |
TGATCCACATCTGCTGGAAGGT |
| Skp2 | F |
ATGCCCCAATCTTGTCCATCT |
|
| R |
CACCGACTGAGTGATAGGTGT |
| P27 | F |
AGGAGGAGATAGAAGCGCAGA |
|
| R |
GTGCGGACTTGGTACAGGT |
| Bcl-2 | F |
AGATGGGAACACTGGTGGAG |
|
| R |
CTTCCCCAAAAGAAATGCAA |
| Bax | F |
AGGGTTTCATCCAGGATCGAGCA |
|
| R |
CAGCTTCTTGGTGGACGCATC |
| Caspase-3 | F |
ACATCTCCCGGCGGCGGGCCGCGGA |
|
| R |
CTTCTACAACCGCCTCACAATAGCA |
| Caspase-9 | F |
AGTTGGCTACTCGCCATGGACGAAG |
|
| R |
TTTGCTGCTTGCCTGTTAGTTCGCA |
Western blotting. To assess the protein expression
levels of Skp2, P27, Bcl2, Bax, procaspase-3, procaspase-9, cleaved
caspase-3 and caspase-9 following LY294002 and ABT199 combination
treatment, western blotting was performed in K562 (Fig. 4), HL60 (Fig.
5) and KG1a (Fig. 6) cells. The
results demonstrated that Skp2 and Bcl2 protein expression levels
were significantly decreased in the single LY294002, single ABT199
and combined treatment groups in all three cell lines. The protein
expression levels of p27 and Bax, the ratio of cleaved
procaspase-3/procaspase-3 and cleaved procaspase-9/procaspase-9
were remarkably increased in the single LY294002, single ABT199 and
combined treatment groups, and significantly higher compared with
single drug treatment.
Discussion
LY294002 blocked the proliferation of primary AML
blasts by inhibiting AKT-induced survival signaling pathways and
induced cell death (42–44). In addition, LY294002 induced AML cell
apoptosis (42). Treatment with
LY294002 led to a dose-dependent decrease in the phosphorylation of
AKT, mTOR, eukaryotic translation initiation factor 4E binding
protein 1, ribosomal protein S6 kinase B1 and ribosomal protein S6,
which was associated with reduced cell viability due to increased
apoptosis (45). Zhou et al
(32) and Manda-Handzlik et al
(36) reported that LY294002 in
combination with conventional chemotherapeutic drugs increased the
sensitivity of AML cells to apoptosis. ABT199 can impair
mitochondrial respiration and energy production in human leukemia
stem cells (20). Clinical trials
have demonstrated that ABT199 is a promising drug for the treatment
of hematopoietic malignancy and chronic lymphocytic leukemia
(46–48). Roche et al (27) reported that ABT199 showed promising
single-agent activity in samples derived from patients with AML.
Several clinical trials of hypomethylator-based combinations
(ABT199 + decitabine/azacytidine) have doubled the response rate,
improving the survival of patients with AML (33). In addition, ABT199 and ONC212
combination treatment was highly synergistic in the AML xenograft
model (49). At the molecular level,
LY294002 and ABT199 combination treatment significantly decreased
Skp2 and Bcl2 expression levels, but markedly increased p27, Bax,
cleaved caspase-3 and caspase-9 expression levels in K562, HL-60
and KG1a cells. Skp2 is involved in leukemia cell proliferation and
is associated with chronic myeloid leukemia (50,51).
Kojima et al (43) and Park
et al (44) reported that Skp2
expression was increased in leukemia and AML. The p27 gene is
located within a high incidence translocation region of leukemic
chromosomes (52). p27 expression
levels can serve as a prognostic reference to predict the outcomes
of patients with pediatric acute lymphoblastic leukemia,
particularly for disease recurrence (52). High p27 expression has a favorable
prognostic impact in patients with AML (53). Bax is frequently associated with
therapy resistance and is an attractive target for the development
of anti-AML agents (3). It is
reported that the apoptotic network of inactivation of BAX mediated
resistance to BCL2 inhibition in AML (47). In human acute promyelocytic leukemia,
cleaved caspase-3 induces apoptosis and decreases cell
proliferation (54). During normal
hematopoiesis, caspase-9 is not required for cell apoptosis
(55). In AML, a mutation in
caspase-9 has been identified (56).
Furthermore, it has been demonstrated that caspase-9 serves a
non-redundant role in the pathogenesis of T-therapy-related AML
(57).
The present study indicated that LY294002 and ABT199
served a synergistic role in inhibiting the cell cycle, which
suggested LY294002 and ABT199 combination treatment may serve as a
novel therapeutic strategy for AML. However, the present study had
a number of limitations. Future studies should use an animal model
of AML to further investigate the effect of LY294002 and ABT199
combination treatment on cell apoptosis and the cell cycle in AML.
Moreover, the functional study of BCR-ABL, sphingosine kinase
(SphK)1 and SphK2 in all cell lines should be conducted in future
studies.
Acknowledgements
This study was funded by Anhui University Natural
Science key project (KJ2019A0307).
Funding
This study was funded by Anhui University Natural
Science key project (KJ2019A0307).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YHG and WJW conceived and designed the study. YHG
was responsible for sample collection and WJW provided
administrative support. YBG and LLZ collected and collated data and
conducted analysis and interpretation thereof. JL and YLY
contributed to data analysis and interpretation. YBG and YLY wrote
and revised the manuscript. All authors approved of the final
manuscript. In addition, the authenticity of all the raw data was
assessed by YBG and YLY to ensure its legitimacy.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Conflicts of interest
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AML
|
acute myeloid leukemia
|
|
BCL2
|
B-cell leukemia/lymphoma 2
|
|
Bax
|
BCL2-associated X
|
|
caspase-3
|
caspase 3
|
|
caspase-9
|
caspase 9
|
|
DMSO
|
dimethyl sulfoxide
|
|
EDTA
|
ethylenediamine tetraacetic acid
|
|
EIF4EBP1
|
eukaryotic translation initiation
factor 4E binding protein 1
|
|
P27
|
p27 protein
|
|
PBS
|
phosphate buffer
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
PI
|
propyl iodide solutions
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
mTOR
|
rapamycin kinase
|
|
RPS6
|
ribosomal protein S6
|
|
RPS6KB1
|
ribosomal protein S6 kinase B1
|
|
Skp2
|
S-phase kinase associated protein
2
|
References
|
1
|
Sakamoto KM, Grant S, Saleiro D, Crispino
JD, Hijiya N, Giles F, Platanias L and Eklund EA: Targeting novel
signaling pathways for resistant acute myeloid leukemia. Mol Genet
Metab. 114:397–402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Horner MJ, Ries LAG, Krapcho M, Neyman N,
Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A,
Miller BA, et al: SEER Cancer Statistics Review, 1975–2006.
National Cancer Institute; Bethesda, MD: 2009
|
|
3
|
Pan R, Hogdal LJ, Benito JM, Bucci D, Han
L, Borthakur G, Cortes J, DeAngelo DJ, Debose L, Mu H, et al:
Selective BCL-2 inhibition by ABT-199 causes on-target cell death
in acute myeloid leukemia. Cancer Discov. 4:362–375. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stuani L, Sabatier M and Sarry JE:
Exploiting metabolic vulnerabilities for personalized therapy in
acute myeloid leukemia. BMC Biol. 17:572019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Staudt D, Murray HC and McLachlan T:
Targeting oncogenic signaling in mutant FLT3 acute myeloid
leukemia: The path to least resistance. Int J Mol Sci. 19:31982018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kantarjian HM: Therapy for elderly
patients with acute myeloid leukemia: A problem in search of
solutions. Cancer. 109:1007–1010. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kantarjian H, O'Brien S, Cortes J, Giles
F, Faderl S, Jabbour E, Garcia-Manero G, Wierda W, Pierce S, Shan J
and Estey E: Results of intensive chemotherapy in 998 patients age
65 years or older with acute myeloid leukemia or high-risk
myelodysplastic syndrome: Predictive prognostic models for outcome.
Cancer. 106:1090–1098. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nazha A and Ravandi F: Acute myeloid
leukemia in the elderly: Do we know who should be treated and how?
Leuk Lymphoma. 55:979–987. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Döhner H, Estey EH, Amadori S, Appelbaum
FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson
RA, et al: Diagnosis and management of acute myeloid leukemia in
adults: Recommendations from an international expert panel, on
behalf of the European LeukemiaNet. Blood. 115:453–474. 2010.
View Article : Google Scholar
|
|
10
|
Lerch E, Espeli V, Zucca E, Leoncini L,
Scali G, Mora O, Bordoni A, Cavalli F and Ghielmini M: Prognosis of
acute myeloid leukemia in the general population: Data from
southern Switzerland. Tumori. 95:303–310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu S, Xiong Y, Xu J, Liang X, Fu Y, Liu D,
Yu X and Wu D: Identification of dysfunctional gut microbiota
through rectal swab in patients with different severity of acute
pancreatitis. Dig Dis Sci. 65:3223–3237. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park S, Chapuis N, Tamburini J, Bardet V,
Cornillet-Lefebvre P, Willems L, Green A, Mayeux P, Lacombe C and
Bouscary D: Role of the PI3K/AKT and mTOR signaling pathways in
acute myeloid leukemia. Haematologica. 95:819–828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vanhaesebroeck B, Stephens L and Hawkins
P: PI3K signalling: The path to discovery and understanding. Nat
Rev Mol Cell Biol. 13:195–203. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fransecky L, Mochmann LH and Baldus CD:
Outlook on PI3K/AKT/mTOR inhibition in acute leukemia. Mol Cell
Ther. 3:22015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lindblad O, Cordero E, Puissant A,
Macaulay L, Ramos A, Kabir NN, Sun J, Vallon-Christersson J,
Haraldsson K, Hemann MT, et al: Aberrant activation of the
PI3K/mTOR pathway promotes resistance to sorafenib in AML.
Oncogene. 35:5119–5131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kulsoom B, Shamsi TS, Afsar NA, Memon Z,
Ahmed N and Hasnain SN: Bax, Bcl-2, and Bax/Bcl-2 as prognostic
markers in acute myeloid leukemia: Are we ready for Bcl-2-directed
therapy? Cancer Manag Res. 10:403–416. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bhola PD and Letai A: Mitochondria-judges
and executioners of cell death sentences. Mol Cell. 61:695–704.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
DeStefano CB and Hourigan CS:
Personalizing initial therapy in acute myeloid leukemia:
Incorporating novel agents into clinical practice. Ther Adv
Hematol. 9:109–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lagadinou ED, Sach A, Callahan K, Rossi
RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O'Dwyer
KM, et al: BCL-2 inhibition targets oxidative phosphorylation and
selectively eradicates quiescent human leukemia stem cells. Cell
Stem Cell. 12:329–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Campos L, Rouault JP, Sabido O, Oriol P,
Roubi N, Vasselon C, Archimbaud E, Magaud JP and Guyotat D: High
expression of bcl-2 protein in acute myeloid leukemia cells is
associated with poor response to chemotherapy. Blood. 81:3091–3096.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Konopleva M, Konoplev S, Hu W, Zaritskey
AY, Afanasiev BV and Andreeff M: Stromal cells prevent apoptosis of
AML cells by up-regulation of anti-apoptotic proteins. Leukemia.
16:1713–1724. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsunaga T, Takemoto N, Sato T, Takimoto
R, Tanaka I, Fujimi A, Akiyama T, Kuroda H, Kawano Y, Kobune M, et
al: Interaction between leukemic-cell VLA-4 and stromal fibronectin
is a decisive factor for minimal residual disease of acute
myelogenous leukemia. Nat Med. 9:1158–1165. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hazlehurst LA, Argilagos RF and Dalton WS:
Beta1 integrin mediated adhesion increases Bim protein degradation
and contributes to drug resistance in leukaemia cells. Br J
Haematol. 136:269–275. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tabe Y, Jin L, Tsutsumi-Ishii Y, Xu Y,
McQueen T, Priebe W, Mills GB, Ohsaka A, Nagaoka I, Andreeff M and
Konopleva M: Activation of integrin-linked kinase is a critical
prosurvival pathway induced in leukemic cells by bone
marrow-derived stromal cells. Cancer Res. 67:684–694. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kojima K, McQueen T, Chen Y, Jacamo R,
Konopleva M, Shinojima N, Shpall E, Huang X and Andreeff M: p53
activation of mesenchymal stromal cells partially abrogates
microenvironment-mediated resistance to FLT3 inhibition in AML
through HIF-1α-mediated down-regulation of CXCL12. Blood.
118:4431–4439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roche S, Koegl M and Courtneidge SA: The
phosphatidylinositol 3-kinase alpha is required for DNA synthesis
induced by some, but not all, growth factors. Proc Natl Acad Sci
USA. 91:9185–9189. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shivakrupa R, Bernstein A, Watring N and
Linnekin D: Phosphatidylinositol 3′-kinase is required for growth
of mast cells expressing the kit catalytic domain mutant. Cancer
Res. 63:4412–4419. 2003.PubMed/NCBI
|
|
29
|
West KA, Castillo SS and Dennis PA:
Activation of the PI3K/Akt pathway and chemotherapeutic resistance.
Drug Resist Updat. 5:234–248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martelli AM, Tabellini G, Bortul R,
Tazzari PL, Cappellini A, Billi AM and Cocco L: Involvement of the
phosphoinositide 3-kinase/Akt signaling pathway in the resistance
to therapeutic treatments of human leukemias. Histol Histopathol.
20:239–252. 2005.PubMed/NCBI
|
|
31
|
Luo J, Manning BD and Cantley LC:
Targeting the PI3K-Akt pathway in human cancer: Rationale and
promise. Cancer Cell. 4:257–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou F, Mei H, Wu Q and Jin R: Expression
of histone H2AX phosphorylation and its potential to modulate
adriamycin resistance in K562/A02 cell line. J Huazhong Univ Sci
Technolog Med Sci. 31:154–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Daver N, Cortes J, Kantarjian H and
Ravandi F: Acute myeloid leukemia: Advancing clinical trials and
promising therapeutics. Expert Rev Hematol. 9:433–445. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pollyea DA, Stevens BM, Jones CL, Winters
A, Pei S, Minhajuddin M, D'Alessandro A, Culp-Hill R, Riemondy KA,
Gillen AE, et al: Venetoclax with azacitidine disrupts energy
metabolism and targets leukemia stem cells in patients with acute
myeloid leukemia. Net Med. 24:1859–1866. 2018. View Article : Google Scholar
|
|
35
|
Li H, Li J, Cheng J, Chen X, Zhou L and Li
Z: AML-derived mesenchymal stem cells upregulate CTGF expression
through the BMP pathway and induce K562-ADM fusiform transformation
and chemoresistance. Oncol Rep. 42:1035–1046. 2019.PubMed/NCBI
|
|
36
|
Manda-Handzlik A, Bystrzycka W, Wachowska
M, Sieczkowska S, Stelmaszczyk-Emmel A, Demkow U and Ciepiela O:
The influence of agents differentiating HL-60 cells toward
granulocyte-like cells on their ability to release neutrophil
extracellular traps. Immunol Cell Biol. 96:413–425. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
She M, Niu X, Chen X, Li J, Zhou M, He Y,
Le Y and Guo K: Resistance of leukemic stem-like cells in AML cell
line KG1a to natural killer cell-mediated cytotoxicity. Cancer
Lett. 318:173–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Kuramitsu Y, Baron B, Kitagawa T,
Tokuda K, Akada J, Maehara SI, Maehara Y and Nakamura K: PI3K
inhibitor LY294002, as opposed to wortmannin, enhances AKT
phosphorylation in gemcitabine-resistant pancreatic cancer cells.
Int J Oncol. 50:606–612. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chiou JT, Lee YC, Huang CH, Shi YJ, Wang
LJ and Chang LS: Autophagic HuR mRNA degradation induces survivin
and MCL1 downregulation in YM155-treated human leukemia cells.
Toxicol Appl Pharmacol. 387:1148572020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu H, Huang M, Ren D, He J, Zhao F, Yi C
and Huang Y: The synergistic effects of low dose fluorouracil and
TRAIL on TRAIL-resistant human gastric adenocarcinoma AGS cells.
Biomed Res Int. 2013:2938742013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu Q, Simpson SE, Scialla TJ, Bagg A and
Carroll M: Survival of acute myeloid leukemia cells requires PI3
kinase activation. Blood. 102:972–980. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kojima K, Shimanuki M, Shikami M, Samudio
IJ, Ruvolo V, Corn P, Hanaoka N, Konopleva M, Andreeff M and
Nakakuma H: The dual PI3 kinase/mTOR inhibitor PI-103 prevents p53
induction by Mdm2 inhibition but enhances p53-mediated
mitochondrial apoptosis in p53 wild-type AML. Leukemia.
22:1728–1736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Park S, Chapuis N, Bardet V, Tamburini J,
Gallay N, Willems L, Knight ZA, Shokat KM, Azar N, Viguié F, et al:
PI-103, a dual inhibitor of Class IA phosphatidylinositide 3-kinase
and mTOR, has antileukemic activity in AML. Leukemia. 22:1698–1706.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen W, Drakos E, Grammatikakis I,
Schlette EJ, Li J, Leventaki V, Staikou-Drakopoulou E, Patsouris E,
Panayiotidis P, Medeiros LJ and Rassidakis GZ: mTOR signaling is
activated by FLT3 kinase and promotes survival of FLT3-mutated
acute myeloid leukemia cells. Mol Cancer. 9:2922010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Souers AJ, Leverson JD, Boghaert ER,
Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH,
Fairbrother WJ, et al: ABT-199, a potent and selective BCL-2
inhibitor, achieves antitumor activity while sparing platelets. Nat
Med. 19:202–208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Phase I study of ABT-199 (GDC-0199) in
patients with relapsed/refractory non-Hodgkin lymphoma, . Responses
observed in diffuse large B-cell (DLBCL) and follicular lymphoma
(FL) at higher cohort doses. Clin Adv Hematol Oncol. 12((8 Suppl
16)): S18–S19. 2014.
|
|
48
|
Roberts AW, Davids MS, Pagel JM, Kahl BS,
Puvvada SD, Gerecitano JF, Kipps TJ, Anderson MA, Brown JR,
Gressick L, et al: Targeting BCL2 with venetoclax in relapsed
chronic lymphocytic leukemia. N Engl J Med. 374:311–322. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nii T, Prabhu VV, Ruvolo V, Madhukar N,
Zhao R, Mu H, Heese L, Nishida Y, Kojima K, Garnett MJ, et al:
Imipridone ONC212 activates orphan G protein-coupled receptor
GPR132 and integrated stress response in acute myeloid leukemia.
Leukemia. 33:2805–2816. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen JY, Wang MC and Hung WC:
Bcr-Abl-induced tyrosine phosphorylation of Emi1 to stabilize Skp2
protein via inhibition of ubiquitination in chronic myeloid
leukemia cells. J Cell Physiol. 226:407–413. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sabri S, Keyhani M and Akbari MT: Whole
exome sequencing of chronic myeloid leukemia patients. Iran J
Public Health. 45:346–352. 2016.PubMed/NCBI
|
|
52
|
Yue ZX, Gao RQ, Gao C, Liu SG, Zhao XX,
Xing TY, Niu J, Li ZG, Zheng HY and Ding W: The prognostic
potential of coilin in association with p27 expression in pediatric
acute lymphoblastic leukemia for disease relapse. Cancer Cell Int.
18:1062018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Haferlach C, Bacher U, Kohlmann A,
Schindela S, Alpermann T, Kern W, Schnittger S and Haferlach T:
CDKN1B, encoding the cyclin-dependent kinase inhibitor 1B (p27), is
located in the minimally deleted region of 12p abnormalities in
myeloid malignancies and its low expression is a favorable
prognostic marker in acute myeloid leukemia. Haematologica.
96:829–836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fasihi-Ramandi M, Moridnia A, Najafi A and
Sharifi M: Inducing apoptosis and decreasing cell proliferation in
human acute promyelocytic leukemia through regulation expression of
CASP3 by Let-7a-5p blockage. Indian J Hematol Blood Transfus.
34:70–77. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Marsden VS, O'Connor L, O'Reilly LA, Silke
J, Metcalf D, Ekert PG, Huang DCS, Cecconi F, Kuida K, Tomaselli
KJ, et al: Apoptosis initiated by Bcl-2-regulated caspase
activation independently of the cytochrome c/Apaf-1/caspase-9
apoptosome. Nature. 419:634–637. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Endo A, Tomizawa D, Aoki Y, Morio T,
Mizutani S and Takagi M: EWSR1/ELF5 induces acute myeloid leukemia
by inhibiting p53/p21 pathway. Cancer Sci. 107:1745–1754. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cahan P and Graubert TA: Integrated
genomics of susceptibility to alkylator-induced leukemia in mice.
BMC Genomics. 11:6382010. View Article : Google Scholar : PubMed/NCBI
|