Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
most common cancer worldwide that causes cancer-related deaths. It
is an aggressive disease that is associated with high morbidity and
mortality, globally affecting 600,000 new patients every year
(1–3).
Although there has been some progress in HNSCC therapy in recent
years, the therapeutic effects of current treatment methods in
patients with HNSCC remain unsatisfactory.
Long non-coding RNAs (lncRNAs) are a class of
non-protein-coding transcripts that are more than 200 nucleotides
in length and possess the ability to regulate gene expression
(4). Accumulating evidence has shown
that lncRNAs participate in multiple biological processes,
including cell growth, differentiation, and metastasis (5–7). Notably,
several studies have reported that lncRNAs are usually deregulated
in cancers; this deregulation results in aberrant gene expression,
leading to the progression of cancers, including HNSCC. For
example, the lncRNA LNCAROD is upregulated in HNSCC; its knockdown
represses cell proliferation and mobility in vitro and
tumorigenesis in vivo (8). On
the other hand, LINC00460 overexpression promotes HNSCC cell
proliferation and invasion (9). The
role of lncRNAs in cancer development has been the focus of several
studies. However, the aberrant expression patterns and functional
roles of lncRNAs in HNSCC development are still not well
documented.
The lncRNA MIR4435-2 host gene (MIR4435-2HG) is
located on human chromosome 2q13, and it is a proven oncogenic
lncRNA that is implicated in various tumors (10). For example, MIR4435-2HG knockdown was
shown to suppress the proliferation and invasion of ovarian cancer
cells (11). MIR4435-2HG expression
is upregulated in glioblastoma tissues and is associated with
shorter overall survival in patients with glioblastoma (12). In addition, it serves as an oncogene
in colorectal cancer, oral squamous cell carcinoma, non-small cell
lung cancer, and gastric cancer (10,13–15).
However, the roles and mechanisms of action of MIR4435-2HG in HNSCC
remain unknown.
In the present study, we found that MIR4435-2HG
inhibition suppressed cell proliferation, cell migration, and
epithelial-mesenchymal transition (EMT) in vitro and
inhibited tumor growth in vivo. Moreover, we demonstrated
that MIR4435-2HG serves as an oncogene in HNSCC by regulating the
miR-382-5p/RNA-binding motif protein 3 (RBM3) axis. These results
suggest that MIR4435-2HG functions as a potential target for cancer
therapy.
Materials and methods
Tissues and cells
A total of 519 HNSCC tissues and 44 normal tissues
from the Gene Expression Profiling Interactive Analysis
(GEPIA)-HNSC database (http://gepia.cancer-pku.cn/) (16) were used to determine the RNA levels of
MIR4435-2HG. Eighteen HNSCC specimens and paired adjacent normal
tissues were acquired from patients. Our patients ranged in age
from 38 to 75, including 16 males and 2 females, and the cases were
collected between June 2014 and June 2016 at Jinshan Hospital,
Fudan University (Shanghai, China). This study was approved by the
Institutional Review Board of Jinshan Hospital of Fudan University,
and informed consent was obtained from all patients. The human
HNSCC cell lines CAL27 and SCC25 were obtained from American Type
Culture Collection (ATCC) and were cultured in Dulbecco's modified
Eagle's medium containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.).
Bioinformatics analyses
The expression level of MIR4435-2HG in HNSCC tissues
was determined using GEPIA. Correlation between the expression
level of MIR4435-2HG and patients with more advanced stage or
higher grade HNSCC and the prognosis based on the expression level
of MIR4435-2HG were determined using GEPIA. The potential binding
sites of MIR4435-2HG and miR-383-5p were predicted using miRDB
(17), and those of miR-383-5p and
RBM3 were predicted using TargetScan (18).
RNA extraction and quantitative
real-time polymerase chain reaction (qPCR)
Total RNA was extracted from tissues or cells using
the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse-transcribed to complementary DNA (cDNA) using the RevertAid
First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Inc.).
qPCR analysis was performed using Fluorescein qPCR Master Mix
(Thermo Fisher Scientific, Inc.). The relative expression levels of
the detected genes were determined using the 2−ΔΔCq
method (19). The expression levels
of MIR4435-2HG and RBM3 were normalized to those of glyceraldehyde
3-phosphate dehydrogenase, (GAPDH) and the expression level of
miR-383-5p was normalized to that of U6.
Cell transfection
Short-hairpin RNA (shRNA) sequences targeting
MIR4435-2HG were synthesized by Sangong Biotech (Shanghai). 293T
cells were co-transfected with a validated vector and lentiviral
packaging vectors pMD and psPAX2 using Lipofectamine 2000 (Thermo
Fisher Scientific, Inc.). Virus particles were harvested and
purified after 48 h. The complete sequence of MIR4435-2HG was
subcloned into a pcDNA3.1(+) vector to construct the
pcDNA3.1-MIR4435-2HG vector (GenePharma). miR-383-5p mimics and
their corresponding negative control (control mimics) were obtained
from RiboBio. Cell transfection was performed using Lipofectamine
2000 Transfection Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. A series of
experimental operations were performed at 48 h after
transfection.
Western blot assay
Tissues and cells were homogenized or lysed using
RIPA buffer supplemented with a protease inhibitor cocktail (Roche,
Applied Science). Protein concentrations was quantified by BCA
protein quantification kit. Protein extracts were loaded onto 12%
acrylamide gels and transferred onto polyvinylidene fluoride
membranes (Millipore) for 30 min. The membrane was blocked with a
5% skim milk solution for 1 h at room temperature and incubated
with the primary antibodies overnight at 4°C. Antibodies against
RBM3 (cat. no. 14363-1-AP, 1:1,000, 17 kDa), E-cadherin (E-cad,
cat. no. 20874-1-AP, 1:10,000, 97 kDa), vimentin (Vim, cat. no.
60330-1-Ig, 1:5,000, 54 kDa), and GAPDH (cat. no. 60004-1-Ig,
1:20,000, 36 kDa) were acquired from Proteintech. Finally, the
membrane was incubated with secondary antibodies (#7076 and #7074,
Cell Signaling Technology) for 1 h at room temperature and washed
three times. Protein bands were visualized using an
ECL-chemiluminescence kit. Detection of intensity of the specific
band was conducted by ImageJ Software (National Institutes of
Health, Bethesda, MD, USA).
Cell proliferation assays
Cell viability was measured using the Cell Counting
Kit-8 (CCK-8; Beyotime Institute of Biotechnology) assay. The
viability of the transfected cells was determined for 5 days after
plating the cells onto 96-well plates. After incubation with the
CCK-8 solution, absorbance was measured at 450 nm. For the colony
formation assay, transfected HNSCC cells were plated on to 6-well
plates at a density of 300 cells per well and incubated for 12
days. The colonies were fixed, stained, and then counted under a
microscope (CX21; Olympus, Tokyo, Japan) at ×100 magnification.
Transwell migration and invasion
assays
Cells were loaded into the upper chamber coated with
Matrigel mix for the invasion assay or without the Matrigel mix for
the migration assay. After incubation for 24 h, the cells in the
upper membrane of the chambers were removed using cotton wool, and
the cells that had migrated or invaded through the membrane were
fixed with methanol and stained with 0.5% hematoxylin. The cells
were counted under a microscope (CX21; Olympus, Tokyo, Japan) in
three randomly selected microscopic fields at ×100
magnification.
Luciferase reporter assay
The cDNA fragments of MIR4435-2HG or 3′-untranslated
region (UTR) of RBM3 containing the putative miR-383-5p binding
site were inserted into pmirGLO dual luciferase vectors. CAL27 and
SCC25 cells were co-transfected with wild-type (WT) or mutated (MT)
MIR4435-2HG or RBM3 3′-UTR reporter plasmids and miR-383-5p mimics
or control mimics. After incubation for 48 h, the luciferase assay
was performed using the Dual Luciferase Reporter Assay System
(Promega Corp.).
RNA immunoprecipitation (RIP)
assay
The RIP assay was performed using the Magna RIP
RNA-Binding Protein Immunoprecipitation Kit (Millipore). CAL27 and
SCC25 cells were lysed with RIPA lysis buffer and then incubated
with magnetic beads conjugated with antibodies against IgG (Abcam
ab172730, 1:50) and AGO2 (Abcam, ab186733, 1:50). The
co-precipitated RNAs were analyzed using qPCR.
HNSCC xenograft model
Four-week-old BALB/C nude mice (18–20 g, n=10) were
obtained from Shanghai Laboratory Animal Center (Shanghai, China)
and were conducted in accordance with the appropriate ethical
standards and national guidelines, and which were used to establish
the HNSCC xenograft model. CAL27 cells stably infected with
shMIR4435-2HG or shNC lentivirus were subcutaneously injected into
the left dorsal flanks of mice. The sizes of the xenografts were
checked every week for 35 days, and the volume (V) was determined
(V=0.5 × length × width2). After 35 days, the mice were
sacrificed by cervical dislocation. All experimental procedures and
protocols were approved by the Ethics Committee of Jinshan Hospital
of Fudan University.
Immunohistochemistry
Tumor tissues from the shNC and shMIR4435-2HG groups
were fixed in 4% paraformaldehyde, dehydrated, embedded in
paraffin, cut into 4-µm-thick sections, and analyzed via
immunohistochemical analysis using antibodies against Ki67 (Santa
Cruz Biotechnology).
Statistical analysis
Data are expressed as mean ± standard deviation.
Statistical analysis was performed using GraphPad Prism 5.0
software (GraphPad Software, Inc.). Student's t-test was used to
determine significant differences between the groups. P<0.05 was
considered to indicate statistical significance.
Results
MIR4435-2HG expression is upregulated
in HNSCC
To determine the expression level of MIR4435-2HG in
HNSCC, we analyzed its expression in HNSCC and normal tissues using
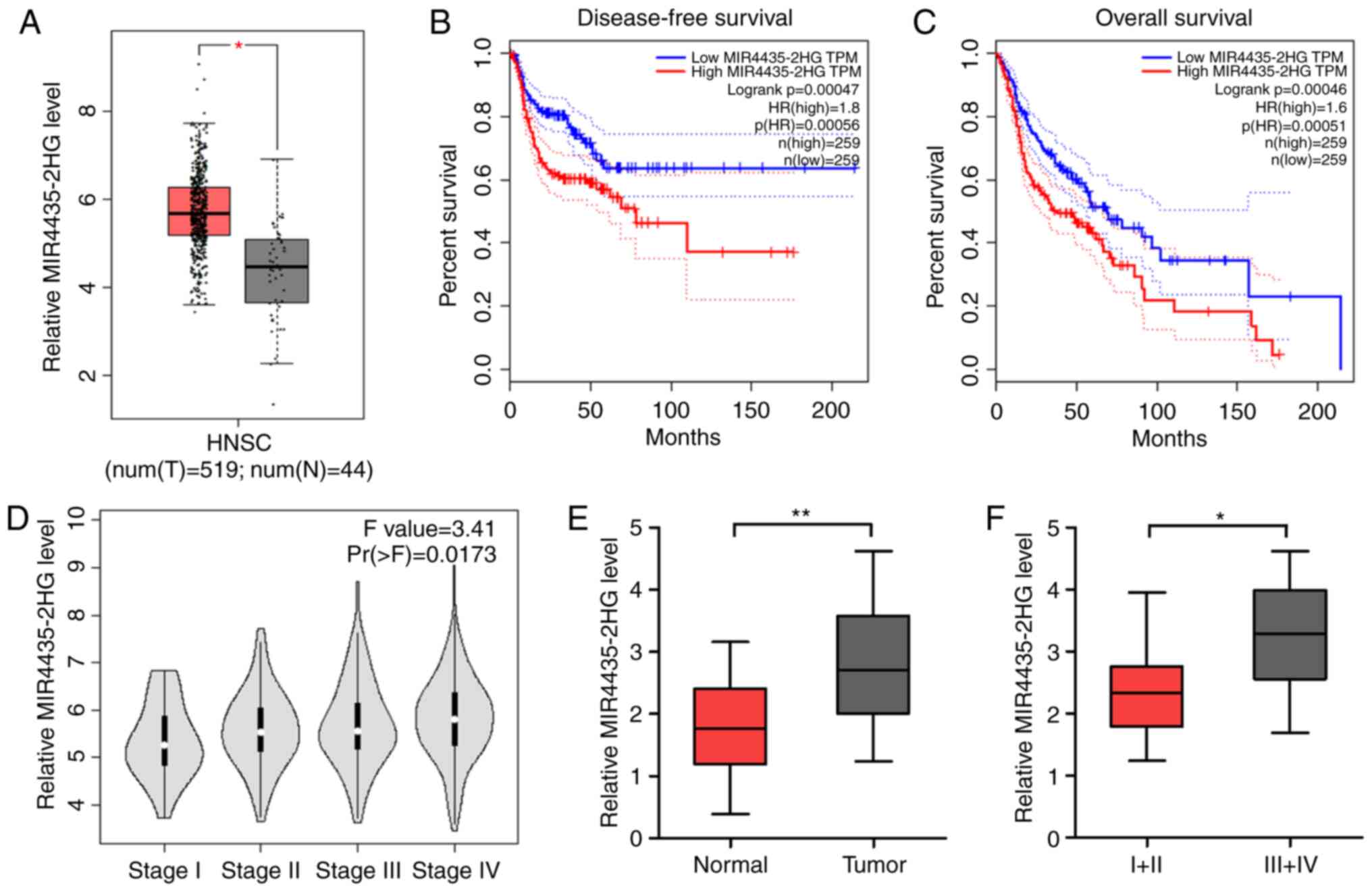
a GEPIA dataset. GEPIA analysis revealed that the expression level
of MIR4435-2HG was substantially increased in HNSCC tissues
compared with that in normal tissues (Fig. 1A). In addition, the higher expression
level of MIR4435-2HG was associated with poorer disease-free
survival and overall survival in all HNSCC cases (Fig. 1B and C). Furthermore, analysis of
public data revealed that upregulated MIR4435-2HG expression was
clearly discernible among the clinical stages, with considerably
higher expression levels in patients with more advanced stage or
higher grade HNSCC (Fig. 1D). To
confirm these results, we determined the expression level of
MIR4435-2HG in 18 HNSCC tissues and normal tissues using qPCR. The
results showed that MIR4435-2HG expression was significantly
upregulated in the tumor tissues compared with that in the normal
tissues (Fig. 1E) and increased in
the later stages of the cancer (Fig.
1F). We also analyzed the relationship between MIR4435-2HG
level and age, sex, tumor size and lymph node metastasis stage, but
there was no statistical significance (data not shown). These
results suggest that MIR4435-2HG plays an important role in the
tumorigenesis and progression of HNSCC.
MIR4435-2HG knockdown suppresses HNSCC
cell proliferation, migration, and invasion in vitro
Next, we conducted loss-of-function assays in HNSCC
cells to examine the function of MIR4435-2HG in HNSCC cells. We
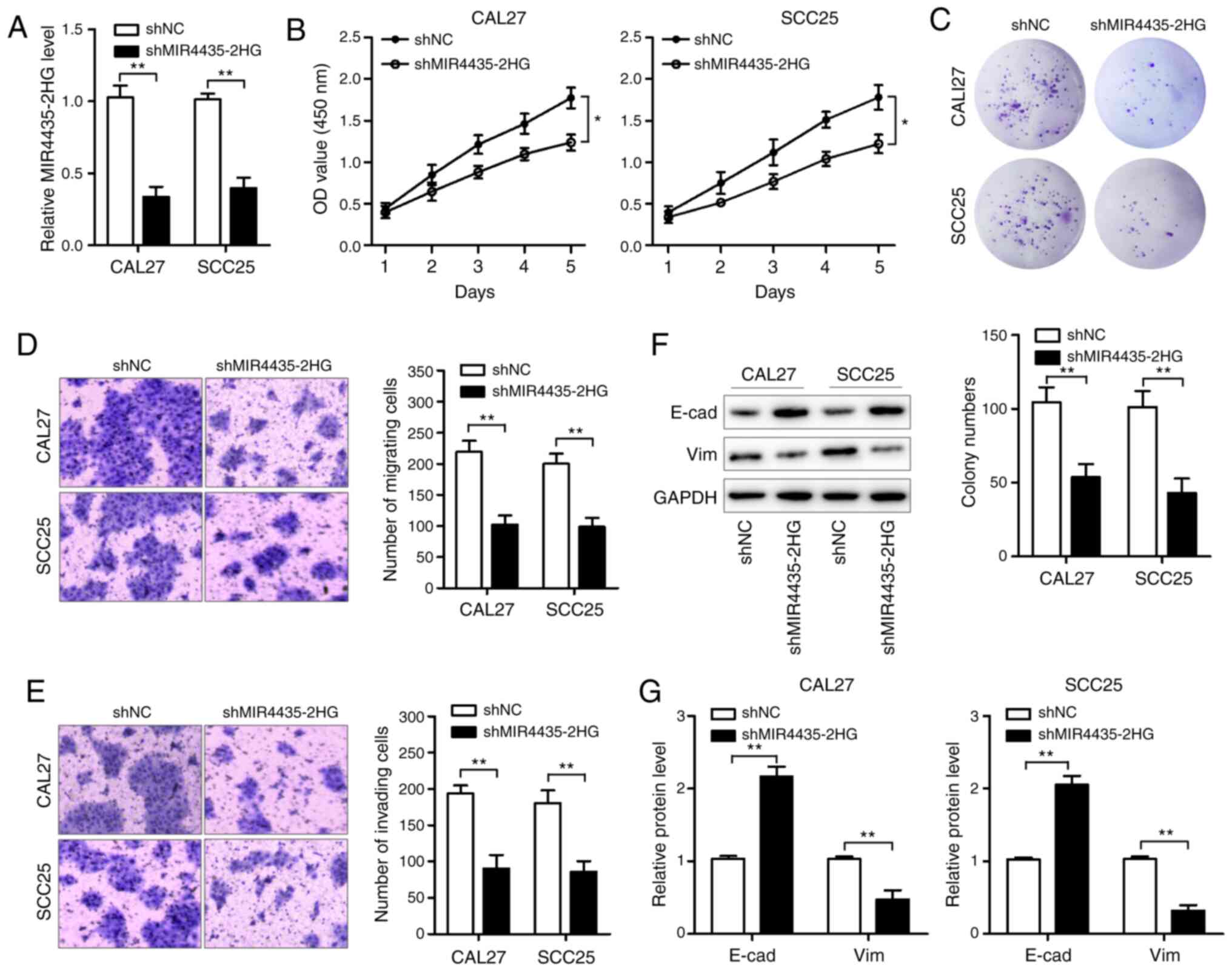
infected CAL27 and SCC25 cells (20–22) with
lentiviral particles carrying MIR4435-2HG shRNA and successfully
verified the significant decrease in the expression level of
MIR4435-2HG in the established cell lines using qPCR (Fig. 2A). Then, we determined the effects of
MIR4435-2HG knockdown on cell viability using the CCK-8 and colony
formation assays. As shown in Fig. 2B and
C, MIR4435-2HG knockdown obviously suppressed cell
proliferation and resulted in a marked decrease in the colony
formation ratio of CAL27 and SCC25 cells. In addition, the
Transwell assay results revealed that MIR4435-2HG knockdown
significantly weakened the migratory and invasive abilities of
CAL27 and SCC25 cells (Fig. 2D and
E). We found that E-cadherin (E-cad) expression was enhanced,
whereas vimentin (Vim) expression was decreased in the CAL27 and
SCC25 cells after MIR4435-2HG knockdown (Fig. 2F and G), suggesting that MIR4435-2HG
knockdown inhibits EMT in HNSCC cells. Collectively, these results
revealed that MIR4435-2HG negatively regulates HNSCC cell
proliferation, migration, and invasion in vitro.
MIR4435-2HG knockdown attenuates tumor
growth in vivo
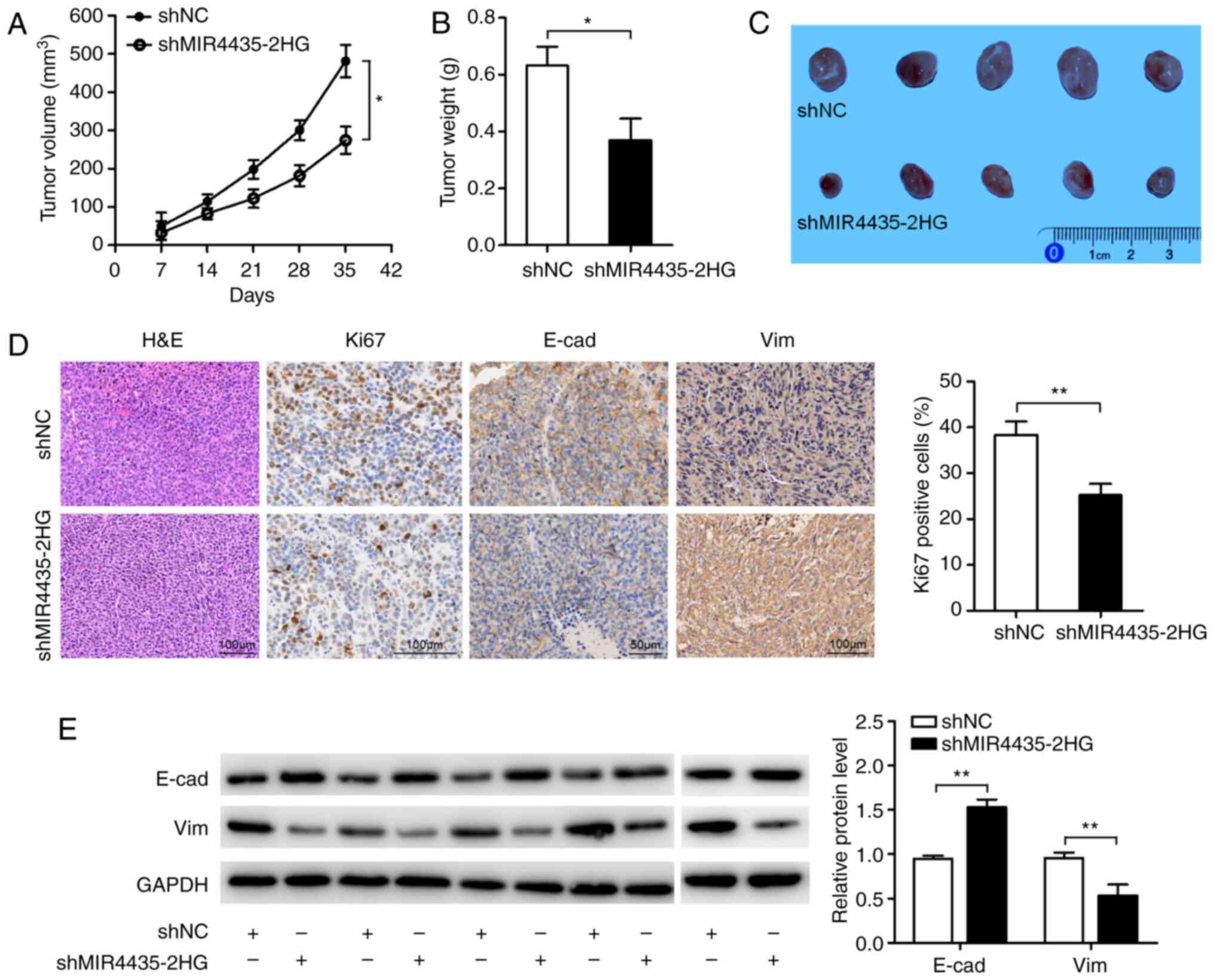
To further investigate the tumorigenic effects of
MIR4435-2HG on HNSCC cells in vivo, we established a tumor
xenograft mouse model by subcutaneously inoculating CAL27 cells
into BALB/C nude mice according to the in vitro results and
based on reports as previously described (23–25). As
shown in Fig. 3A-C, both the volumes
and weights of the tumors in the shMIR4435-2HG group were
significantly decreased compared with those in the shNC group.
Immunohistochemical staining for the cell proliferation marker Ki67
revealed lower percentages of Ki67-positive tumor cells and showed
the membrane localization of E-cad as well as lower expression of
Vim in the shMIR4435-2HG group compared with that in the shNC group
(Fig. 3D). Western blot analysis
revealed that E-cad expression was enhanced, whereas Vim expression
levels were reduced in the shMIR4435-2HG group (Fig. 3E). These results indicate that
MIR4435-2HG enhances the tumorigenicity of HNSCC cells in
vivo.
MIR4435-2HG acts as a competing
endogenous RNA (ceRNA) and competitively binds miR-383-5p
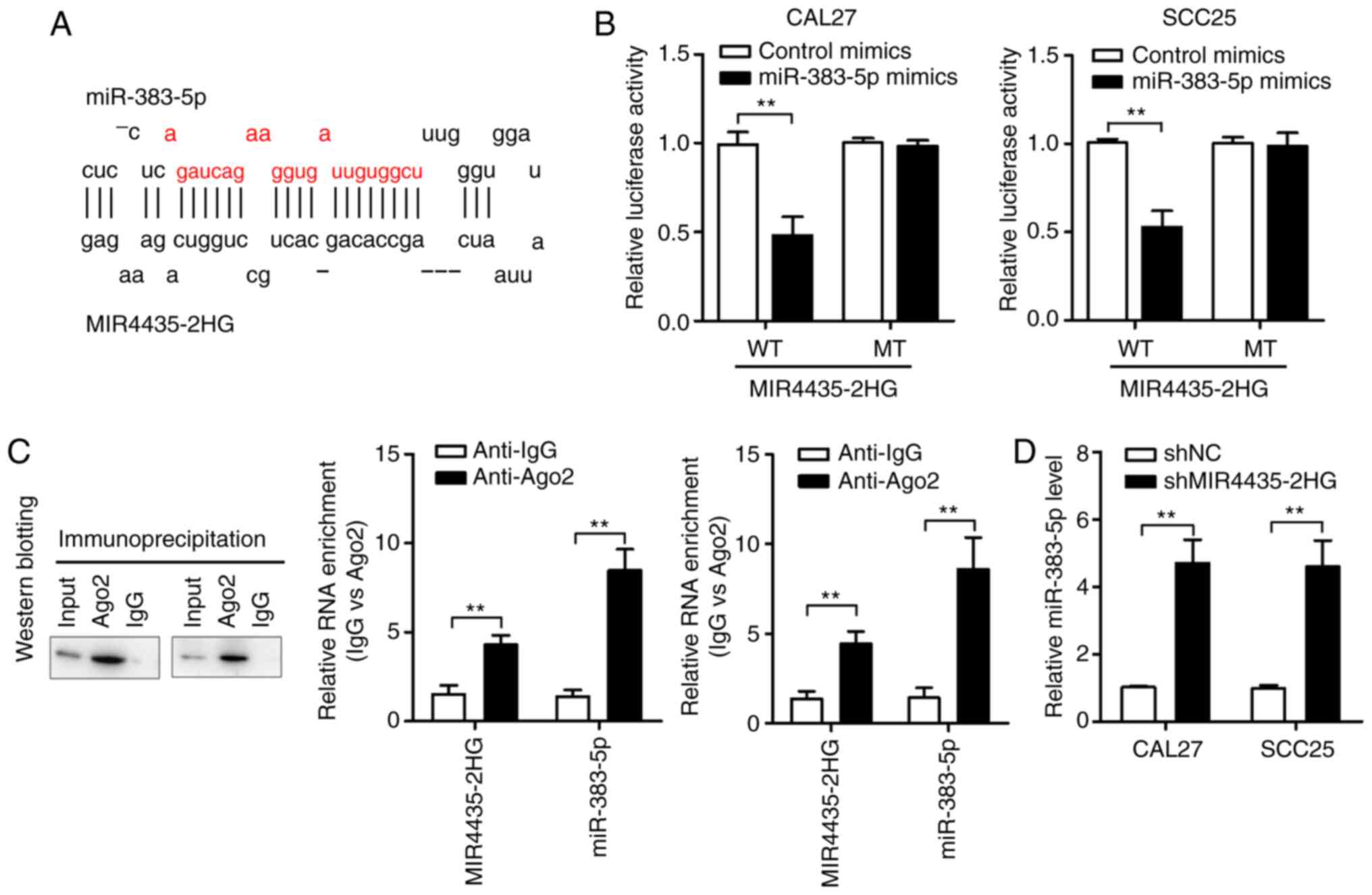
Using the online software miRDB, we found that
MIR4435-2HG forms complementary base pairs with miR-383-5p
(Fig. 4A). We first performed the
luciferase reporter assay to confirm the direct binding between
MIR4435-2HG and miR-383-5p. The results showed that HNSCC cells
transfected with miR-383-5p mimics had significantly decreased
luciferase activity compared with those transfected with
MIR4435-2HG-WT; on the other hand, the luciferase activities of
HNSCC cells transfected with miR-383-5p mimics and MIR4435-2HG-MT
remained unaffected (Fig. 4B).
Furthermore, we examined miR-383-5p and MIR4435-2HG on magnetic
beads conjugated to the anti-Ago2 antibody. RIP assay results
showed that MIR4435-2HG was preferentially enriched in
Ago2-containing miRNPs compared with than in control IgG
immunoprecipitates. Similarly, miR-383-5p was detected at a higher
level than control anti-IgG (Fig.
4C). Therefore, MIR4435-2HG is present in Ago2-containing
miRNPs, possibly by associating with miR-383-5p. This result is
consistent with our bioinformatics analysis and luciferase assay
results. In addition, MIR4435-2HG knockdown significantly increased
miR-383-5p expression (Fig. 4D).
These results suggest that MIR4435-2HG acts as a sponge for
miR-383-5p in HNSCC cells.
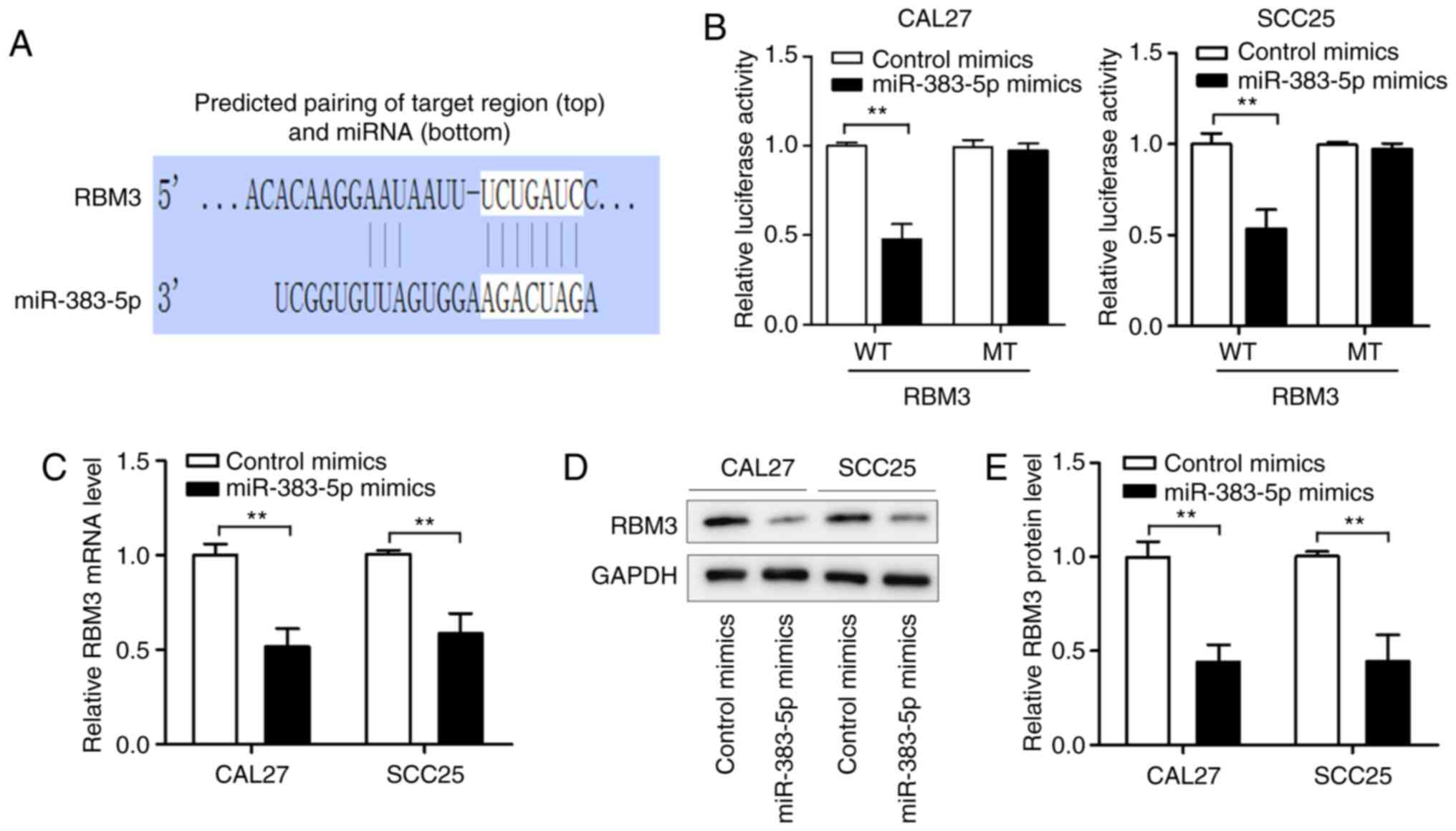
RBM3 is a target of miR-383-5p
We identified RBM3 as a potential target of
miR-383-5p using TargetScan (Fig.
5A). After co-transfection with luciferase reporter vectors and
miR-383-5p mimics, the luciferase activity of RBM3-WT was
significantly reduced, whereas the activity of RBM3-MT was
unaffected by the miR-383-5p mimic (Fig.
5B). Subsequent experiments revealed that miR-383-5p
overexpression significantly reduced RBM3 expression at the mRNA
and protein levels (Fig. 5C-E) in the
CAL27 and SCC25 cell lines. These data suggest that miR-383-5p
inhibits RBM3 expression in HNSCC cells by directly targeting the
3′-UTR of RBM3.
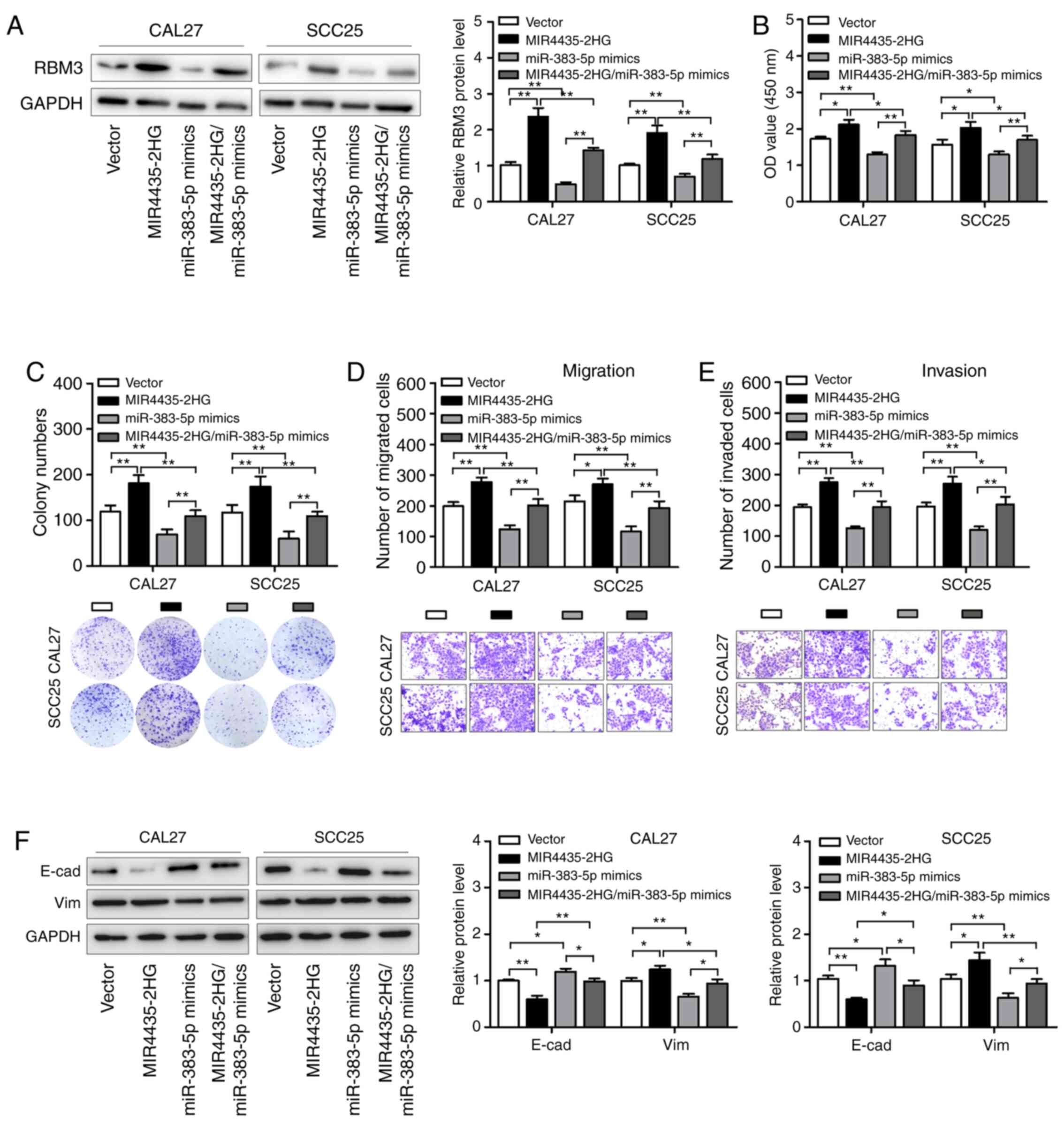
miR-383-5p reverses the
tumor-promoting roles of MIR4435-2HG in HNSCC cells by regulating
RBM3
Recent studies have shown that lncRNAs function as
ceRNAs by competitively binding with miRNAs during tumorigenesis.
We speculated that MIR4435-2HG affects the biological behavior of
HNSCC cells by regulating the miR-383-5p/RBM3 axis. Western
blotting showed that MIR4435-2HG overexpression promoted RBM3
expression in HNSCC cells; however, co-transfection with miR-383-5p
mimics reduced this effect (Fig. 6A).
This result indicates that MIR4435-2HG regulates the protein
expression level of RBM3 by influencing miR-383-5p expression in
HNSCC cells. In addition, we observed that the transfection of
HNSCC cells with miR-383-5p mimics abrogated the promotive effects
of MIR4435-2HG on cell colony formation, migration, and invasion
(Fig. 6B-E). Moreover, we observed
that MIR4435-2HG decreased E-cad expression and increased Vim
expression; these changes were abolished in HNSCC cells
co-transfected with MIR4435-2HG and miR-383-5p (Fig. 6F). These results suggest that
miR-383-5p reverses the function of MIR4435-2HG in HNSCC
development by regulating RBM3 expression.
Discussion
Recently, studies have shown that the lncRNA
MIR4435-2 host gene (MIR4435-2HG) is abnormally expressed and
functions as an oncogene in ovarian cancer (11), colorectal cancer (13), gastric cancer (10), and hepatocellular carcinoma (26); however, its function in head and neck
squamous cell carcinoma (HNSCC) remains unknown. In the present
study, we showed that the expression level of MIR4435-2HG was
upregulated in HNSCC tissues and cell lines. MIR4435-2HG knockdown
suppressed HNSCC cell proliferation, migration and invasion.
Epithelial-mesenchymal transition (EMT) is a process
in which cancer cells lose their polarity and cell-cell adhesion,
develop into mesenchymal fibroblast-like cells, and then confer
metastatic properties to cancer cells by increasing their ability
to migrate and invade. In cancer cells, EMT is a complex
reprogramming process characterized by inhibition of the above
cortical markers and upregulation of mesenchymal markers (27). Thus, we also determined the role of
MIR4435-2HG on HNSCC cell EMT and found that MIR4435-2HG knockdown
inhibited HNSCC cell EMT by enhancing E-cadherin and reducing
vimentin expression levels. Based on the results of previous
research (13), MIR4435-2HG may serve
as an miRNA sponge and exhibit its functions in several tumor
types. We further revealed that the MIR4435-2HG/miR-383-5p/RBM3
axis is a potential competing endogenous RNA (ceRNA) regulatory
network in HNSCC.
Increasing evidence shows that lncRNAs can serve as
ceRNAs in various cancers. For example, the lncRNA LINC00460
promotes tumor growth and metastasis in patients with
hepatocellular carcinoma by upregulating the expression of
miR-342-3p-dependent AGR2 (28). The
lncRNA OGFRP1 acts as a ceRNA to facilitate prostate cancer
progression by regulating the expression level of SARM1 via
miR-124-3p (29). The lncRNA
HNF1A-AS1 serves as a ceRNA in gastric cancer by promoting the
activation of the PI3K/AKT signaling pathway by sponging miR-30b-3p
(30). MIR4435-2HG has also been
observed to play a role in several cancers through this regulatory
mechanism. For example, MIR4435-2HG knockdown suppressed ovarian
cancer cell proliferation, invasion, and migration through the
miR-128-3p/CDK14 axis (11),
MIR4435-2HG promoted the proliferation and invasion of glioblastoma
cells by regulating the miR-1224-5p/TGFBR2 axis (12), and MIR4435-2HG acted as a ceRNA to
upregulate YAP1 expression by sponging miR-206 and promoted
colorectal cancer growth and metastasis (13). To investigate the mechanism of
MIR4435-2HG regulation in HNSCC, bioinformatics analysis indicated
that miR-383-5p, a tumor suppressor in several types of cancers,
including triple-negative breast cancer, ovarian cancer, and
gastric cancer (31–33), has potential MIR4435-2HG binding
sites. In the present study, we demonstrated that MIR4435-2HG
serves as a ceRNA and competitively binds miR-383-5p using
luciferase reporter and RIP assays. In addition, we demonstrated
that MIR4435-2HG knockdown induces miR-383-5p expression. These
results confirm that miR-383-5p is a direct target of
MIR4435-2HG.
RNA-binding motif protein 3 (RBM3) is an RNA- and
DNA-binding protein whose gene encodes two alternatively spliced
RNA transcripts and maps to Xp11.23 (34). RBM3 plays a key role in tumor
progression. In a previous study, RBM3 was shown to promote the
proliferation of hepatocellular carcinoma cells in an SCD-circRNA
2-dependent manner (35). RBM3
expression was found to be upregulated in human breast cancer
tissues compared with that in adjacent normal tissues (36). RBM3 also exhibited this function in
other cancers such as glioblastoma, non-small cell lung cancer, and
urinary bladder cancer (37–39). In addition, it has been reported to
act as a target of miR-383-5p in triple-negative breast cancer
(31). In the present study,
bioinformatics analysis and luciferase reporter assay results
showed that RBM3 could function as a direct target of miR-383-5p in
HNSCC. Moreover, we demonstrated that miR-383-5p mimics decreased
RBM3 expression in HNSCC cells, which was attenuated by
MIR4435-2HG. Furthermore, miR-383-5p mimics abrogated the promotive
effects of MIR4435-2HG on cell proliferation, migration, invasion,
and EMT. These results suggest that RBM3 is involved in the effect
of MIR4435-2HG in HNSCC, and the detailed function of RBM3 in HNSCC
will be explored in subsequent research.
We demonstrated that MIR4435-2HG affected HNSCC
progression partly through regulating the miR-383-5p/RBM3 axis.
Through bioinformatics analysis, it can be predicted that
MIR4435-2HG can bind to multiple miRNAs, and one miRNA can also
bind to multiple target mRNAs. In this study, we only selected a
potential miRNA and mRNA that play a role in HNSCC, confirming that
MIR4435-2HG can partially affect the HNSCC invasion process by
regulating the miR-383-5p/RBM3 axis. In our next study, we will
continue to study the role of MIR4435-2HG in HNSCC, further
identify potential miRNAs and mRNAs by MIR4435-2HG, and their roles
in HNSCC. In subsequent research, we also will focus on the impact
of MIR4435-2HG on metastasis and the expression pattern of EMT
marker genes should be examined in the presence of TGF-β, as TGF-β1
could induce EMT of HNSCC cells. In conclusion, we found that
MIR4435-2HG expression is upregulated in HNSCC cells and that its
knockdown suppresses cell proliferation, invasion, and EMT in
vitro and inhibits tumor growth and EMT in vivo.
Mechanistically, the MIR4435-2HG/miR-383-5p/RBM3 axis may play key
roles in HNSCC progression, thereby functioning as a novel
therapeutic target for cancer treatment.
Acknowledgements
Not applicable.
Funding
This research was supported by the Jinshan Health
Planning Committee Fund (grant no. JSKJ-KTMS-2018-06) and Youth
Project Initiation Fund from Jinshan Hospital, Fudan University
(grant no. JYQN-JC-202103).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SW and TQ contributed to the study conception and
design. SW and XC performed the experiments, analyzed the data and
wrote the manuscript. TQ revised this manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of Jinshan Hospital of Fudan University, and informed consent
was obtained from all patients. Moreover, all experimental
procedures and protocols for the animal study were approved by the
Ethics Committee of Jinshan Hospital of Fudan University (JIEC
2021-S20).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rothenberg SM and Ellisen LW: The
molecular pathogenesis of head and neck squamous cell carcinoma. J
Clin Invest. 122:1951–1957. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tuttle TR, Mierzwa ML, Wells SI, Fox SR
and Ben-Jonathan N: The cyclic GMP/protein kinase G pathway as a
therapeutic target in head and neck squamous cell carcinoma. Cancer
Lett. 370:279–285. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lan T, Ma W, Hong Z, Wu L, Chen X and Yuan
Y: Long non-coding RNA small nucleolar RNA host gene 12 (SNHG12)
promotes tumorigenesis and metastasis by targeting miR-199a/b-5p in
hepatocellular carcinoma. J Exp Clin Cancer Res. 36:112017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu S, Zhang J, Yin L, Wang X, Zheng Y,
Zhang Y, Gu J, Yang L, Yang J, Zheng P, et al: The lncRNA RUNX1-IT1
regulates C-FOS transcription by interacting with RUNX1 in the
process of pancreatic cancer proliferation, migration and invasion.
Cell Death Dis. 11:4122020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhong J, Tu X, Kong Y, Guo L, Li B, Zhong
W, Cheng Y, Jiang Y and Jiang Q: LncRNA H19 promotes odontoblastic
differentiation of human dental pulp stem cells by regulating
miR-140-5p and BMP-2/FGF9. Stem Cell Res Ther. 11:2022020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Q, Wu J, Huang H, Jiang Y, Huang Y,
Fang H, Zheng G, Zhou X, Wu Y, Lei C and Hu D: lncRNA LIFR-AS1
suppresses invasion and metastasis of non-small cell lung cancer
via the miR-942-5p/ZNF471 axis. Cancer Cell Int. 20:1802020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ban Y, Tan P, Cai J, Li J, Hu M, Zhou Y,
Mei Y, Tan Y, Li X, Zeng Z, et al: LNCAROD is stabilized by m6A
methylation and promotes cancer progression via forming a ternary
complex with HSPA1A and YBX1 in head and neck squamous cell
carcinoma. Mol Oncol. 14:1282–1296. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie X, Xiong G, Wang Q, Ge Y and Cui X:
Long non-coding RNA LINC00460 promotes head and neck squamous cell
carcinoma cell progression by sponging miR-612 to up-regulate AKT2.
Am J Transl Res. 11:6326–6340. 2019.PubMed/NCBI
|
|
10
|
Wang H, Wu M, Lu Y, He K, Cai X, Yu X, Lu
J and Teng L: LncRNA MIR4435-2HG targets desmoplakin and promotes
growth and metastasis of gastric cancer by activating Wnt/β-catenin
signaling. Aging (Albany NY). 11:6657–6673. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu L, Wang A, Gao M, Duan X and Li Z:
LncRNA MIR4435-2HG triggers ovarian cancer progression by
regulating miR-128-3p/CKD14 axis. Cancer Cell Int. 20:1452020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu H, Zhang B, Yang Y, Li Z, Zhao P, Wu W,
Zhang H and Mao J: LncRNA MIR4435-2HG potentiates the proliferation
and invasion of glioblastoma cells via modulating
miR-1224-5p/TGFBR2 axis. J Cell Mol Med. 24:6362–6372. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong X, Yang Z, Yang H, Li D and Qiu X:
Long non-coding RNA MIR4435-2HG promotes colorectal cancer
proliferation and metastasis through miR-206/YAP1 Axis. Front
Oncol. 10:1602020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang S, Li C, Liu J, Geng F, Shi X, Li Q,
Lu Z and Pan Y: Fusobacterium nucleatum promotes
epithelial-mesenchymal transiton through regulation of the lncRNA
MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway. FEBS J.
287:4032–4047. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang M, He X, Huang X, Wang J, He Y and
Wei L: LncRNA MIR4435-2HG-mediated upregulation of TGF-β1 promotes
migration and proliferation of nonsmall cell lung cancer cells.
Environ Toxicol. 35:582–590. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar
|
|
17
|
Chen Y and Wang X: miRDB: An online
database for prediction of functional microRNA targets. Nucleic
Acids Res. 48:D127–D131. 2020. View Article : Google Scholar
|
|
18
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Foki E, Stanisz I, Kadletz L, Kotowski U,
Seemann R, Schmid R and Heiduschka G: HS-173, a selective PI3K
inhibitor, induces cell death in head and neck squamous cell
carcinoma cell lines. Wien Klin Wochenschr. 133:26–31. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khalil A and Jameson MJ: Downregulation of
IGF1R expression inhibits growth and enhances cisplatin sensitivity
of head and neck squamous cell carcinoma cells in vitro. Horm
Cancer. 10:11–23. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li SJ, Yang XN and Qian HY: Antitumor
effects of WNT2B silencing in GLUT1 overexpressing cisplatin
resistant head and neck squamous cell carcinoma. Am J Cancer Res.
5:300–308. 2014.PubMed/NCBI
|
|
23
|
Zhuang Z, Yu P, Xie N, Wu Y, Liu H, Zhang
M, Tao Y, Wang W, Yin H, Zou B, et al: MicroRNA-204-5p is a tumor
suppressor and potential therapeutic target in head and neck
squamous cell carcinoma. Theranostics. 10:1433–1453. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang Y, Cao W, Wu K, Qin X, Wang X, Li Y,
Yu B, Zhang Z, Wang X, Yan M, et al: LncRNA LINC00460 promotes EMT
in head and neck squamous cell carcinoma by facilitating
peroxiredoxin-1 into the nucleus. J Exp Clin Cancer Res.
38:3652019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu Y, Wang Y, Diao P, Zhang W, Li J, Ge H,
Song Y, Li Z, Wang D, Liu L, et al: Therapeutic targeting of BRD4
in head neck squamous cell carcinoma. Theranostics. 9:1777–1793.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kong Q, Liang C, Jin Y, Pan Y, Tong D,
Kong Q and Zhou J: The lncRNA MIR4435-2HG is upregulated in
hepatocellular carcinoma and promotes cancer cell proliferation by
upregulating miRNA-487a. Cell Mol Biol Lett. 24:262019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galle E, Thienpont B, Cappuyns S, Venken
T, Busschaert P, Van Haele M, Van Cutsem E, Roskams T, van Pelt J,
Verslype C, et al: DNA methylation-driven EMT is a common mechanism
of resistance to various therapeutic agents in cancer. Clin
Epigenetics. 12:272020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hong H, Sui C, Qian T, Xu X, Zhu X, Fei Q,
Yang J and Xu M: Long noncoding RNA LINC00460 conduces to tumor
growth and metastasis of hepatocellular carcinoma through
miR-342-3p-dependent AGR2 up-regulation. Aging (Albany NY).
12:10544–10555. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan K, Hou L, Liu T, Jiao W, Ma Q, Fang Z,
Zhang S, Song D, Liu J, Gao X and Fan Y: lncRNA OGFRP1 functions as
a ceRNA to promote the progression of prostate cancer by regulating
SARM1 level via miR-124-3p. Aging (Albany NY). 12:8880–8892. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu HT, Ma RR, Lv BB, Zhang H, Shi DB, Guo
XY, Zhang GH and Gao P: LncRNA-HNF1A-AS1 functions as a competing
endogenous RNA to activate PI3K/AKT signalling pathway by sponging
miR-30b-3p in gastric cancer. Br J Cancer. 122:1825–1836. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tian Y, Xia S, Ma M and Zuo Y: LINC00096
promotes the proliferation and invasion by sponging miR-383-5p and
regulating RBM3 expression in triple-negative breast cancer. Onco
Targets Ther. 12:10569–10578. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang J, Xie C, Liu Y, Shi Q and Chen Y:
Up-regulation of miR-383-5p suppresses proliferation and enhances
chemosensitivity in ovarian cancer cells by targeting TRIM27.
Biomed Pharmacother. 109:595–601. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei C and Gao JJ: Downregulated miR-383-5p
contributes to the proliferation and migration of gastric cancer
cells and is associated with poor prognosis. PeerJ. 7:e78822019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao G, Shi X, Long Y, Yao Z, Shen J and
Shen L: The prognostic and clinicopathological significance of RBM3
in the survival of patients with tumor: A Prisma-compliant
meta-analysis. Medicine. 99:e200022020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dong W, Dai ZH, Liu FC, Guo XG, Ge CM,
Ding J, Liu H and Yang F: The RNA-binding protein RBM3 promotes
cell proliferation in hepatocellular carcinoma by regulating
circular RNA SCD-circRNA 2 production. EBioMedicine. 45:155–167.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen P, Yue X, Xiong H, Lu X and Ji Z:
RBM3 upregulates ARPC2 by binding the 3′UTR and contributes to
breast cancer progression. Int J Oncol. 54:1387–1397.
2019.PubMed/NCBI
|
|
37
|
Fuentes-Fayos AC, Vazquez-Borrego MC,
Jimenez-Vacas JM, Bejarano L, Pedraza-Arévalo S, L-López F,
Blanco-Acevedo C, Sánchez-Sánchez R, Reyes O, Ventura S, et al:
Splicing machinery dysregulation drives glioblastoma
development/aggressiveness: Oncogenic role of SRSF3. Brain.
143:3273–3293. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Melling N, Bachmann K, Hofmann B, El
Gammal AT, Reeh M, Mann O, Moebius C, Blessmann M, Izbicki JR and
Grupp K: Prevalence and clinical significance of RBM3
immunostaining in non-small cell lung cancers. J Cancer Res Clin
Oncol. 145:873–879. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boman K, Segersten U, Ahlgren G, Eberhard
J, Uhlén M, Jirström K and Malmström PU: Decreased expression of
RNA-binding motif protein 3 correlates with tumour progression and
poor prognosis in urothelial bladder cancer. BMC Urol. 13:172013.
View Article : Google Scholar : PubMed/NCBI
|