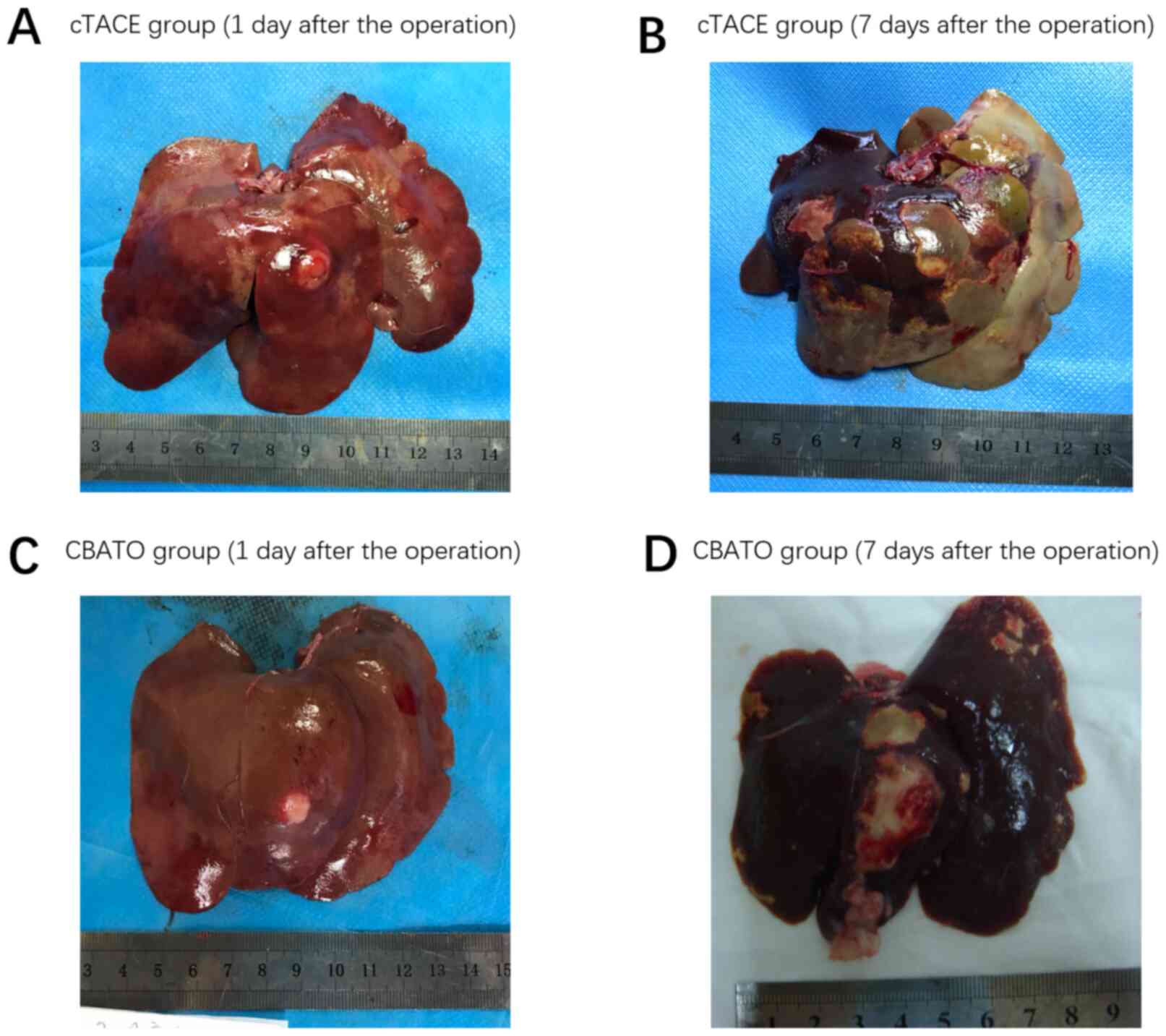
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jang JH, Lee JW, Hong JT and Jin YJ:
Transarterial chemoembolization for hepatocellular carcinoma: An
evidence-based review of its place in therapy. J Hepatocell
Carcinoma. 2:123–129. 2015.PubMed/NCBI
|
|
5
|
Liu YS, Ou MC, Tsai YS, Lin XZ, Wang CK,
Tsai HM and Chuang MT: Transarterial chemoembolization using
gelatin sponges or microspheres plus lipiodol-doxorubicin versus
doxorubicin-loaded beads for the treatment of hepatocellular
carcinoma. Korean J Radiol. 16:125–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie F, Zang J, Guo X, Xu F, Shen R, Yan L,
Yang J and He J: Comparison of transcatheter arterial
chemoembolization and microsphere embolization for treatment of
unresectable hepatocellular carcinoma: A meta-analysis. J Cancer
Res Clin Oncol. 138:455–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang S, Huang C, Li Z, Yang Y, Bao T,
Chen H, Zou Y and Song L: Comparison of pharmacokinetics and drug
release in tissues after transarterial chemoembolization with
doxorubicin using diverse lipiodol emulsions and CalliSpheres Beads
in rabbit livers. Drug Deliv. 24:1011–1017. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee KH, Liapi EA, Cornell C, Reb P, Buijs
M, Vossen JA, Ventura VP and Geschwind JF: Doxorubicin-loaded
QuadraSphere microspheres: Plasma pharmacokinetics and intratumoral
drug concentration in an animal model of liver cancer. Cardiovasc
Intervent Radiol. 33:576–582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ni JY, Xu LF, Wang WD, Sun HL and Chen YT:
Conventional transarterial chemoembolization vs microsphere
embolization in hepatocellular carcinoma: A meta-analysis. World J
Gastroenterol. 20:17206–17217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Zhou J, Zhu DD, Huang J, Sun JH,
Li TF, Shi CS, Sun ZC, Hou QM, Peng ZY, et al: CalliSpheres(R)
drug-eluting beads (DEB) transarterial chemoembolization (TACE) is
equally efficient and safe in liver cancer patients with different
times of previous conventional TACE treatments: a result from CTILC
study. Clin Transl Oncol. 21:167–177. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamaguchi T, Seki T, Komemushi A, Suwa K,
Tsuda R, Inokuchi R, Murata M, Yuki M, Harima Y and Okazaki K:
Acute necrotizing pancreatitis as a fatal complication following DC
Bead transcatheter arterial chemoembolization for hepatocellular
carcinoma: A case report and review of the literature. Mol Clin
Oncol. 9:403–407. 2018.PubMed/NCBI
|
|
12
|
Guan YS, He Q, Jin Y and Yao F:
Development of CalliSpheres® embolic microspheres.
Zhonghua Gan Zang Bing Za Zhi. 24:549–551. 2016.(In Chinese).
PubMed/NCBI
|
|
13
|
Chen G, Zhang D, Ying Y, Wang Z, Tao W,
Zhu H, Zhang J and Peng Z: Clinical investigation on transarterial
chemoembolization with indigenous drug-eluting beads in treatment
of unresectable hepatocellular carcinoma. Zhejiang Da Xue Xue Bao
Yi Xue Ban. 46:44–51. 2017.(In Chinese). PubMed/NCBI
|
|
14
|
Wu B, Zhou J, Ling G, Zhu D and Long Q:
CalliSpheres drug-eluting beads versus lipiodol transarterial
chemoembolization in the treatment of hepatocellular carcinoma: A
short-term efficacy and safety study. World J Surg Oncol.
16:692018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou GH, Han J, Sun JH, Zhang YL, Zhou TY,
Nie CH, Zhu TY, Chen SQ, Wang BQ, Yu ZN, et al: Efficacy and safety
profile of drug-eluting beads transarterial chemoembolization by
CalliSpheres® beads in Chinese hepatocellular carcinoma
patients. BMC Cancer. 18:6442018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cohen MH, Hirschfeld S, Flamm Honig S,
Ibrahim A, Johnson JR, OLeary JJ, White RM, Williams GA and Pazdur
R: Drug approval summaries: Arsenic trioxide, tamoxifen citrate,
anastrazole, paclitaxel, bexarotene. Oncologist. 6:4–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Min Z, Wang X, Hu M, Song D, Ren
Z, Cheng Y and Wang Y: Arsenic trioxide and sorafenib combination
therapy for human hepatocellular carcinoma functions via
up-regulation of TNF-related apoptosis-inducing ligand. Oncol Lett.
16:3341–3350. 2018.PubMed/NCBI
|
|
18
|
Zhang F, Zhang CM, Li S, Wang KK, Guo BB,
Fu Y, Liu LY, Zhang Y, Jiang HY and Wu CJ: Low dosage of arsenic
trioxide inhibits vasculogenic mimicry in hepatoblastoma without
cell apoptosis. Mol Med Rep. 17:1573–1582. 2018.PubMed/NCBI
|
|
19
|
Wang HY, Zhang B, Zhou JN, Wang DX, Xu YC,
Zeng Q, Jia YL, Xi JF, Nan X, He LJ, et al: Arsenic trioxide
inhibits liver cancer stem cells and metastasis by targeting
SRF/MCM7 complex. Cell Death Dis. 10:4532019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiu Y, Dai Y, Zhang C, Yang Y, Jin M, Shan
W, Shen J, Lu M, Tang Z, Ju L, et al: Arsenic trioxide reverses the
chemoresistance in hepatocellular carcinoma: A targeted
intervention of 14-3-3eta/NF-kappaB feedback loop. J Exp Clin
Cancer Res. 37:3212018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song P, Hai Y, Ma W, Zhao L, Wang X, Xie
Q, Li Y, Wu Z, Li Y and Li H: Arsenic trioxide combined with
transarterial chemoembolization for unresectable primary hepatic
carcinoma: A systematic review and meta-analysis. Medicine
(Baltimore). 97:e06132018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schiavone EL and Torrado OA: Determination
of arsenic in water by the silver diethyldithiocarbamate method.
Rev Sanid Milit Argent. 66:251–263. 1967.(In Spanish). PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cima G: AVMA Guidelines for the Euthanasia
of Animal. Javma - Journal of the American Veterinary Medical
Association. (2013 edition). 242:715–716. 2013.
|
|
25
|
Sharma BK, Srinivasan R, Chawla YK and
Chakraborti A: Vascular endothelial growth factor: Evidence for
autocrine signaling in hepatocellular carcinoma cell lines
affecting invasion. Indian J Cancer. 53:542–547. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou L, Wang DS, Li QJ, Sun W, Zhang Y and
Dou KF: Downregulation of the Notch signaling pathway inhibits
hepatocellular carcinoma cell invasion by inactivation of matrix
metalloproteinase-2 and −9 and vascular endothelial growth factor.
Oncol Rep. 28:874–882. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nouri YM, Kim JH, Yoon HK, Ko HK, Shin JH
and Gwon DI: Update on transarterial chemoembolization with
drug-eluting microspheres for hepatocellular carcinoma. Korean J
Radiol. 20:34–49. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang K, Zhou Q, Wang R, Cheng D and Ma Y:
Doxorubicin-eluting beads versus conventional transarterial
chemoembolization for the treatment of hepatocellular carcinoma. J
Gastroenterol Hepatol. 29:920–925. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang X, Jia S, Yang S and Yang Y, Yang T
and Yang Y: Arsenic trioxide induces G2/M arrest in hepatocellular
carcinoma cells by increasing the tumor suppressor PTEN expression.
J Cell Biochem. 113:3528–3535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang L, Wang L, Chen L, Cai GH, Ren QY,
Chen JZ, Shi HJ and Xie YH: As2O3 induces apoptosis in human
hepatocellular carcinoma HepG2 cells through a ROS-mediated
mitochondrial pathway and activation of caspases. Int J Clin Exp
Med. 8:2190–2196. 2015.PubMed/NCBI
|
|
31
|
Cui L, Gao B, Cao Z, Chen X, Zhang S and
Zhang W: Downregulation of B7-H4 in the MHCC97-H hepatocellular
carcinoma cell line by arsenic trioxide. Mol Med Rep. 13:2032–2038.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu H, Zhu GY, Xu RZ, Niu HZ, Lu Q, Li GZ,
Wang ZY, Zhang DS, Gu N and Teng GJ: Arterial embolization
hyperthermia using As2O3 nanoparticles in VX2 carcinoma-induced
liver tumors. PLoS One. 6:e179262011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tan B, Huang JF, Wei Q, Zhang H and Ni RZ:
Anti-hepatoma effect of arsenic trioxide on experimental liver
cancer induced by 2-acetamidofluorene in rats. World J
Gastroenterol. 11:5938–5943. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jordan O, Denys A, De Baere T, Boulens N
and Doelker E: Comparative study of chemoembolization loadable
beads: In vitro drug release and physical properties of DC bead and
hepasphere loaded with doxorubicin and irinotecan. J Vasc Interv
Radiol. 21:1084–1090. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Baere T, Plotkin S, Yu R, Sutter A, Wu
Y and Cruise GM: An in vitro evaluation of four types of
Drug-eluting microspheres loaded with doxorubicin. J Vasc Interv
Radiol. 27:1425–1431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hong K, Khwaja A, Liapi E, Torbenson MS,
Georgiades CS and Geschwind JF: New intra-arterial drug delivery
system for the treatment of liver cancer: preclinical assessment in
a rabbit model of liver cancer. Clin Cancer Res. 12:2563–2567.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gupta S, Wright KC, Ensor J, Van Pelt CS,
Dixon KA and Kundra V: Hepatic arterial embolization with
doxorubicin-loaded superabsorbent polymer microspheres in a rabbit
liver tumor model. Cardiovasc Intervent Radiol. 34:1021–1030. 2011.
View Article : Google Scholar : PubMed/NCBI
|