Introduction
Acid extrusion refers to an acid/base transport
process by which cells move intracellular acids out of, or
extracellular base equivalents into, the cytosol (1). In normal cells, acid extrusion rarely
changes extracellular pH (pHo) due to a large reservoir
of systemic buffers. However, in tumors, acid extrusion lowers
pHo in microenvironments as it counteracts excessive
CO2, H+ and lactate produced by high
metabolic activity in cancer cells (2,3).
Furthermore, blood perfusion is limited in tumors and
membrane-bound carbonic anhydrase (CA) IX contributes to
extracellular CO2 hydration (4,5). As a
consequence, the microenvironments surrounding cancer cells are
acidic while intracellular pH (pHi) is normal or
slightly higher than normal (6).
Remarkably, cancer cells thrive in acidic environments and undergo
adaptations to promote survival and proliferation, such that acidic
pHo stimulates cell growth, migration and invasion
(3). Abnormal pH gradient in cancer
cells has been a focus as a potential target for anticancer
therapies (7,8).
Na/HCO3 transporters (NBCs) are
acid-extruding proteins that move HCO3– into
cells and compensate intracellular H+ (9). There are five different NBCs (NBCe1,
NBCe2, NBCn1, NDCBE, and NCBE), each of which exhibits distinct
cell or tissue expression, biochemical and pharmacological
properties (10–12). These transporters are of particular
interest in cancer research because CO2-dependent acid
production from high metabolism and excessive glycolysis
corresponds to approximately half of the extracellular acids that
cancer cells generate (13). Studies
on cultured cancer cells in vitro or implanted in
vivo have identified NBC-mediated acid extrusion mechanisms in
a variety of cancer cells and their involvement in cell growth and
progression (2,14). Notably, NBCn1/SLC4A7 was identified as
a new marker for human breast cancer (15) and its contribution to cancer
progression has been recognized in MCF17 breast cancer cells
(16,17) and breast cancer cells from patients
(18). A study using knockout mice
(19) has further provided evidence
that NBCn1 stimulates ErbB2-induced breast cancer development and
tumor growth. Similarly, NBCe1/SLC4A4 has been revealed to regulate
proliferation, migration and invasion of LS174T colon cancer cells
and MDA-MB-231 breast cancer cells (20). McIntyre et al (21) screened a variety of cancer cell lines
and reported the importance of NBCe1 and controversial SLC4A9 (AE4)
for the growth of colon and breast cancer cell lines, as well as
glioma. In addition, inhibition of NDCBE/SLC4A8 or NCBE/SLC4A10 has
been revealed to decrease breast cancer cell growth (22).
As in other cancers, acidic microenvironments in
prostate cancer are also considered to be an important prognostic
factor (23). Acidic pHo
can be used as a robust imaging biomarker for aggressive prostate
cancer (24). Pharmacological
inhibition or knockdown of several acid extrusion transporters,
such as Na/H exchangers NHEs (25),
V-ATPases (26,27) and monocarboxylate transporters MCTs
(28), prevents prostate cancer
progression in vivo and in vitro. Furthermore,
increasing systemic buffers by NaHCO3 has been shown to
reduce a transition from intraductal carcinoma to invasive cancer
in a mouse model (29). Regarding
NBCs, NBCe1 is associated with prostate cancer (30,31). NBCe1
is one of the gene products upregulated in a mouse model of
prostate cancer induced by a deletion of the tumor suppressor gene
Atbf1 (30). Increased copy
numbers of the SLC4A4 gene are found in patients with prostate
cancer (31). In a preliminary study
by our group it was demonstrated that NBCe1 was expressed in LNCaP
and PC3 prostate cancer cells and regulated acid extrusion in these
cells (32). However, further studies
are required.
In this study, NBCe1 expression levels in multiple
human prostate cancer cells, NBCe1-mediated pH recovery from
intracellular acidification, and its effects on cell proliferation
and death were investigated. Prostate cancer was focused on because
no study has been reported on the role of this transporter in
prostate cancer, despite the fact that prostate cancer is the
second most frequent cancer and the fifth leading cause of
cancer-related deaths among men (33). The results revealed that, among NBCs,
NBCe1 played a key role in acid extrusion in prostate cancer cells
and affected cell proliferation and viability. NBCe1 localization
to the epithelial cells in prostatic glands and its extensive
expression in acinar and duct adenocarcinoma were also
demonstrated. The present results demonstrate the importance of
NBCe1 for pHi regulation and growth of prostate cancer
cells and leads to the possibility to develop NBCe1-mediated pH
regulation as a potential target for anticancer treatment.
Materials and methods
Cell culture
Authenticated LNCaP cells (LNCaP-FGC) were purchased
from American Type Culture Collection ATCC (cat. no. CRL-1740), and
PC3, 22RV1, C4-2, DU145 and RWPE1 were provided by Dr Carlos Moreno
and Dr Wei Zou at the Winship Cancer Institute of Emory University.
Cells were previously authenticated by each investigator's
laboratory. Cells were maintained in RPMI-1640 medium (ATCC)
supplemented with 10% fetal bovine serum and 1% pen/strep (Thermo
Fisher Scientific, Inc.) in a 5% CO2-equilibrated 37°C
incubator. For hypoxic exposure, cells were incubated in a
humidified atmosphere of 1% O2, 5% CO2 at
37°C. N-cyanosulphonamide S0859 (cat. no. 1019331-10-2; Millipore
Sigma; Merck KGaA) was added to media in experiments that required
pharmacological inhibition of NBCs. Viable cells were counted using
the trypan blue exclusion assay (34). Percent of viable cells was calculated
by the ratio of live cell number to total cell number (live cells +
trypan blue-stained cells).
Measurements of pHi
Cells at the density of 1–2×105 were
plated in a 60-mm dish containing a poly-lysine-coated coverslip
and incubated for 2 days. Cells on a coverslip were loaded with 6.5
µM of 2,7-bis(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl
ester (BCECF-AM; cat. no. B1170; Thermo Fisher Sientific, Inc.) for
20 min and mounted in a closed perfusion chamber affixed to the
stage of a Zeiss Axiovert inverted microscope. The dye was
alternately excited with 440 and 490 nm lights using a Lambda LS/30
Xenon Arc lamp (Sutter Instruments), and 535 nm emission lights
from both excitations were captured using a Nikon camera and
analyzed using Nikon NIS Elements AR 3.0 imaging software (both
from Nikon Corporation). The emission ratio 490/440 nm was
calculated and converted to a pH value according to the nigericin
method (35). The chamber was
perfused with HEPES-buffered solution (mM: 140 NaCl, 1 KCl, 1.2
MgCl2, 1 CaCl2, 8.8 sucrose, 10 HEPES, pH
7.4) and then with a solution containing 5% CO2, 28 mM
HCO3− (NaHCO3 replaced NaCl).
Solutions contained 100 µM of amiloride to block endogenous NHEs.
S0859 at 50 µM was added in experiments that required inhibition of
NBCs. For Na+-free
CO2/HCO3− solution, LiCl replaced
NaCl and choline bicarbonate replaced NaHCO3. The rate
of pHi recovery (dpH/dt; pHi change per sec ×
10−4) was calculated by drawing a slope in the first 4
min of recovery from a CO2-induced acidification.
Reverse transcription-quantitative
(RT-q)PCR
Total RNAs from the aforementioned cells were
isolated using RNeasy Mini kit (Qiagen, Inc.) and transcribed using
SuperScript III First-Strand Synthesis System (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. qPCR
was performed using Applied Biosystems SYBR Green PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with the
following primers purchased from OriGene Technologies, Inc.: SLC4A4
(cat. no. HP232301), SLC4A5 (cat. no. HP214119), SLC4A7 (cat. no.
HP207103), SLC4A8 (cat. no. HP227521), SLC4A10 (cat. no. HP214322),
β-actin ACTB (cat. no. HP204660), and 18S RNA (cat. no. HP220445).
The sequences are listed Table SI.
Reactions were performed using an ABI Prism 7900HT Sequence
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Amplification was achieved at 50°C for 2 min and 95°C for 10
min for an initial denaturation, and then 40 cycles at 95°C for 15
sec and 60°C for 1 min. The quantification cycle (Cq) was
determined using the software SDS 2.4 supplied with the instrument.
Cq values of NBCs relative to a geometric mean from reference genes
ACTB and 18S RNA were calculated, and fold changes relative to
NBCe1 were determined using the 2−ΔΔCq method (36).
Immunoblotting
Cells were homogenized in ice-cold buffer (10 mM
Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100), supplemented
with 1× protein inhibitor cocktail (Thermo Fisher Scientific, Inc.)
and 1 mM phenylmethylsulfonyl fluoride. Cells were centrifuged at
13,200 × g for 10 min at 4°C to remove cell debris and supernatants
were collected. Protein concentration was determined using Bradford
reagents (Millipore Sigma; Merck KGaA). A total of 15 µg of
proteins from samples were separated on a 4–15% SDS-polyacrylamide
gel and blotted to a nitrocellulose membrane. The blot was
incubated with mouse anti-human SLC4A4 monoclonal antibody (cat.
no. sc-515543; Santa Cruz Biotechnology, Inc.) for 2 h at room
temperature. The dilution was 1:500 with the blocking buffer (5%
nonfat dry milk and 0.05% Tween-20 in TBS). The blot was washed and
incubated with a goat horseradish peroxidase-conjugated antibody to
mouse IgG (1:1,000 dilution; cat. no. 12-349; Millipore Sigma;
Merck KGaA) for 2 h at room temperature. Immunoreactive bands were
visualized using ECL chemiluminescence (Thermo Fisher Scientific,
Inc.). The blot was striped and reprobed with rabbit anti-human
β-actin polyclonal antibody (product code ab8227; 1:1,000 dilution;
Abcam) for 1 h at room temperature. Densitometric analysis of
immunoreactive bands was performed using ImageJ as previously
described (37). NBCe1 pixel
intensity was normalized to β-actin intensity after background
subtraction.
Small interfering (si)RNA-mediated
NBCe1 knockdown
Cells at the density of 1–2×104 were
plated in 24-well plates and transfected with siRNA
oligonucleotides the following day. The siRNA 27-mer duplexes
targeting human SLC4A4 (cat. no. SR305704) and the scrambled
negative control duplex (cat. no. SR30004) were purchased from
OriGene Technologies, Inc. (Table
SII). Three SLC4A4 siRNA duplexes were pooled at equal
concentrations for transfection (total 10 and 20 nM). Transfection
was performed with Lipofectamine RNAiMax (Thermo Fisher) according
to the manufacturer's instructions. After transfection, cells were
incubated in hypoxia for 72–96 h in a 37°C incubator, and the
efficacy of knockdown was determined by immunoblotting.
Lactate dehydrogenase (LDH) release
assay
Cell death was measured using the LDH release assay
as previously described (38) with
slight modification. Briefly, cells in 24-well plates were
incubated with 1% Triton X-100 or water for 45 min and LDH released
from cells was quantitated using CyQuant LDH Cytotoxicity Assay Kit
(Thermo Fisher Scientific, Inc.). The amount of formazan produced
by LDH-mediated NADH oxidation was determined by absorbances at 490
and 680 nm (background). Background absorbance at 680 nm was
subtracted and absorbance in media only (no cells) was also
subtracted. Cell death was calculated as a percentage of
spontaneous LDH release to total LDH release.
Prostate cancer samples
The formalin-fixed, paraffin-embedded human prostate
carcinoma tissue microarrays containing 41 cases of prostate cancer
and 9 cases of normal prostate tissue were purchased from US Biolab
Corporation, Inc. (cat. no. PRO501). Information on pathology
grade, Gleason grade, Gleason score, TNM classification and
clinical stages are available on the company website. The purpose
of using human tissue samples in this study was to examine the
characteristics of cancerous tissue, not to develop treatments;
thus, a respective Intuitional Review Board was not required for
the use of the tissue microarrays in our study.
Sphere formation assay
A sphere formation assay was performed as previously
described by Zhang et al (39)
with slight modification. Falcon 8-well chamber slides (product no.
354118; Corning, Inc.) were precoated with 50 µl of LDEV-free
growth factor-reduced Geltrex (cat. no. A1413201; Thermo Fisher
Scientific, Inc.). Cells were plated at 3,000 cells/well in the
aforementioned culture medium supplemented with 1% Geltrex. One day
later, the cells were treated with 0 or 100 µM of S0859 and
incubated at 37°C for 6 days to form spheres. Images of spheres at
a magnification of ×10 were captured using a Keyence BZ-X700
fluorescence microscope (Keyence Corporation). To quantify sphere
growth, the number of spheres was counted and Feret diameters were
measured using the FIJI version of ImageJ.
Immunohistochemistry
The tissue microarrays were heated and subjected to
deparaffinization in xylene, rehydration in graded series of
ethanol, and rinsing with distilled water. The slides were then
heat treated with the target retrieval solution DIVA Decloaker
(Biocare Medical) using an electric pressure cooker for 20 min.
After washing, the slides were blocked with Background Sniper
(Biocare Medical) at room temperature for 10 min, washed and
incubated with anti-NBCe1/SLC4A4 antibody (product no. HPA035628;
Millipore Sigma; Merck KGaA) diluted at 1:200 at 4°C overnight. The
slides were washed and then incubated with MACH 2 Rabbit AP-Polymer
(cat. no. RALP525; Biocare Medical) for 30 min at room temperature.
The slides were stained with the chromogen solution Warp Red
(BioCare Medical) at room temperature for 7 min. Nuclei were
counterstained with hematoxylin at room temperature for 45 sec.
Digital images of stained slides were captured using a Biotek
Lionheart FX microscope. The images were then evaluated by a
histopathologist.
Statistical analysis
Data were reported as the mean ± standard error of
the mean (SEM). The significance of the difference between means
was determined using: i) Unpaired, two-tailed Student's
t-test for comparison of dpH/dt and immunoblotting in
normoxia vs. hypoxia, control vs. knockdown, and control vs. S0859
treatment; ii) paired, two-tailed Student's t-test for comparison
of dpH/dt before and after Na+ addition, as well as
S0859 sensitivity; iii) one-way ANOVA with Turkey post hoc test for
comparison of NBC qPCR; and iv) two-way ANOVA with Fisher's LSD or
Sidak post hoc test for comparison of viable cell number and cell
death over time after S0859 treatment. P<0.05 was considered to
indicate a statistically significant difference. Analysis was
performed using GraphPad Prism 7 (GraphPad Software, Inc.) and
Microsoft Office Excel add-in Analysis ToolPak (Microsoft
Corporation).
Results
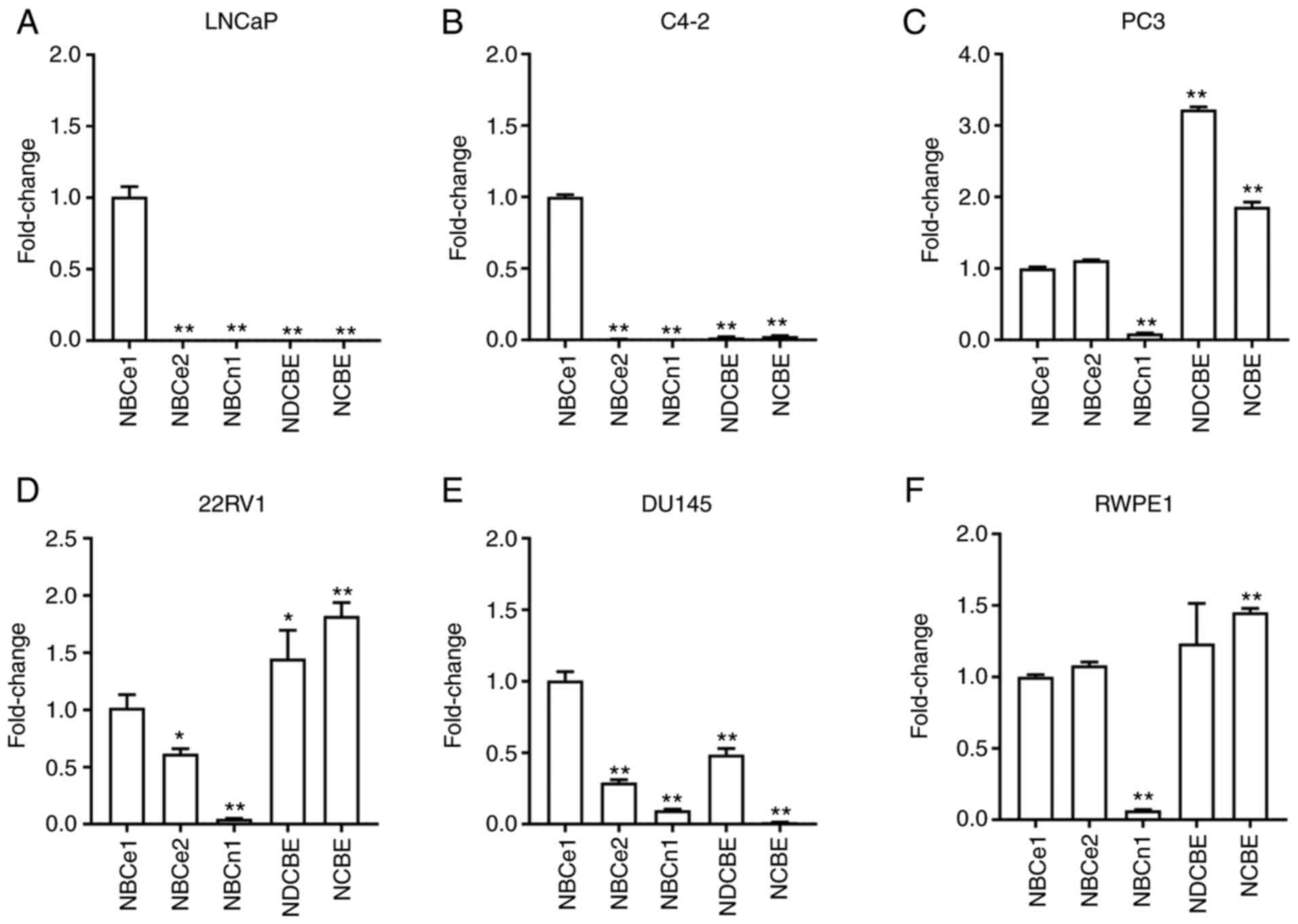
NBC expression levels are heterogenous
in different prostate cancer cells
qPCR with primers specific to each member of NBCs
was performed to determine their expression levels in human
prostate cancer cell lines LNCaP, C4-2, PC3, 22RV1, DU145, and the
prostate cell line RWPE-1. The expression ratios of each isoform
relative to NBCe1 after being normalized to a geometric mean of
reference genes ACTB and 18S RNA is presented in Fig. 1A-F. Notably, NBCe1 expression levels
were predominantly high in LNCaP cells and its subline C4-2 cells
(P<0.01 for both; n=4/group). NBCe1 expression was also observed
in other cells, but its level was not predominant and detected
together with NBCe2, NDCBE, NCBE and weakly with NBCn1. The
expression profiles in PC3, 22RV1, and DU145 were to a certain
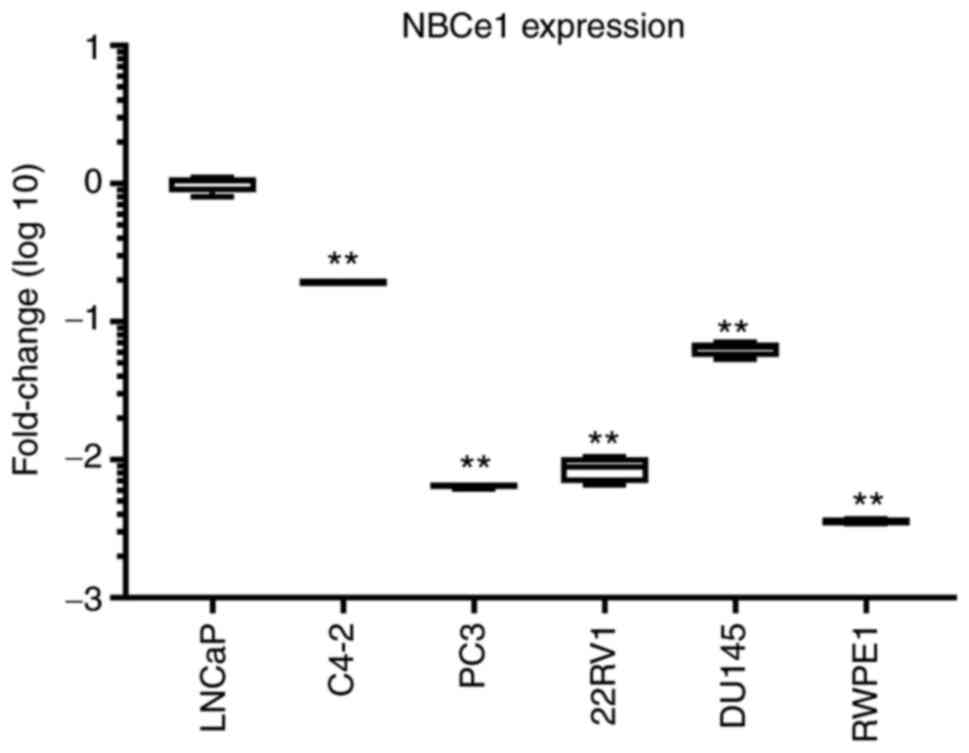
extent similar to that in RWPE1. The comparison of NBCe1 expression
levels among all six different cell lines is presented in Fig. 2. The level was significantly higher in
LNCaP cells.
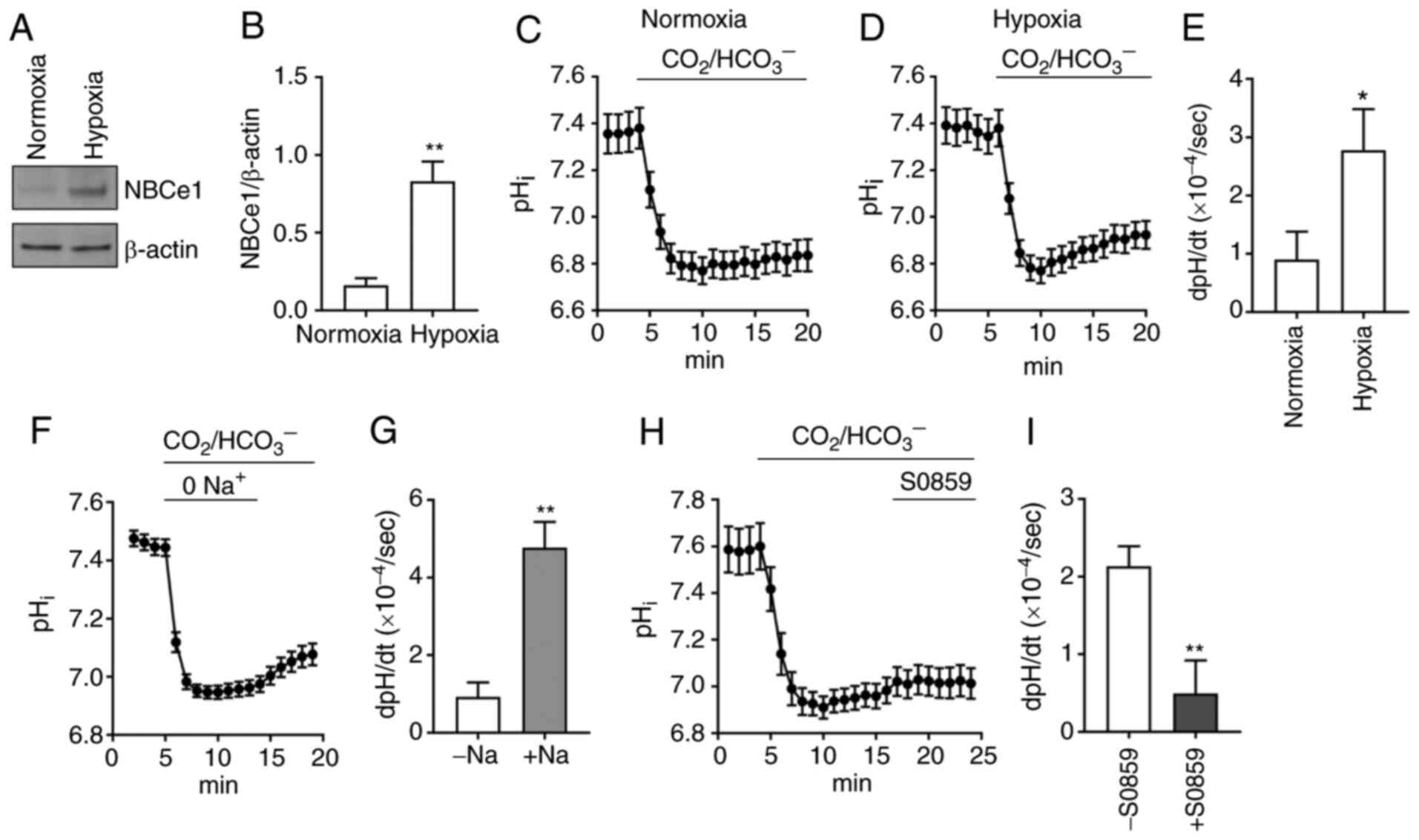
NBCe1 is responsible for acid
extrusion in LNCaP cells
Because NBCe1 is highly abundant in LNCaP cells,
this cell line was focused on for analysis of
Na/HCO3-dependent acid extrusion and its response to
hypoxia. Immunoblotting data from cells incubated in normoxia vs.
hypoxia (1% O2, 5% CO2) for 4 days are
presented in Fig. 3A. NBCe1 was
markedly upregulated in hypoxia. Densitometric quantitation of
immunoreactive NBCe1 normalized to β-actin resulted in a 5.1-fold
increase (P<0.01, Student's t-test; n=3; Fig. 3B). In parallel experiments,
pHi measurement with the fluorescence dye BCECF was
performed to assess whether acid extrusion is enhanced in hypoxia.
The average pHi traces (n=11 cells/group) when cells
were perfused with 5% CO2, 28 mM HCO3– (plus
100 µM of amiloride to block endogenous NHEs) are presented in
Fig. 3C and D. Comparison of
pHi recovery rates resulted in a 3-fold increase in
hypoxia (0.90±0.48 ×10−4 dpHi/sec in normoxia
vs. 2.78±0.71 ×10−4 dpHi/sec in hypoxia;
P<0.05, Student's t-test; Fig.
3E). The properties of the pHi recovery from a
CO2-induced acidification were further evaluated by
assessing its Na+ dependence and S0859 sensitivity. An
average pHi recovery in the absence and presence of
Na+ (n=11) is presented in Fig. 3F. The recovery was minimal in
Na+-free CO2/HCO3−
solution, indicating that the major acid extrusion in LNCaP cells
is dependent upon Na+. The recovery was increased when
Na+ was applied. The dpH/dt in this
Na+-containing solution was 5-fold higher than the value
in Na+-free solution (P<0.05, paired Student's
t-test; Fig. 3G), indicating that
Na/HCO3 transport largely governs acid extrusion in
hypoxia. In other experiments, the sensitivity to the
Na/HCO3 inhibitor S0859 (50 µM) was examined (Fig. 3H). This concentration was selected
based on a previous study (40) where
S0859 at >30 µM fully inhibited NBCs in cardiomyocytes.
Comparison of pHi recoveries in the absence vs. presence
of S0859 revealed a significant inhibition by the drug. The average
inhibition was 77% (P<0.01, Student's t-test; n=16 cells/group;
Fig. 3I). Collectively with the
predominant expression of NBCe1, these pHi data
demonstrated that NBCe1 plays a major role in acid extrusion in
LNCaP cells.
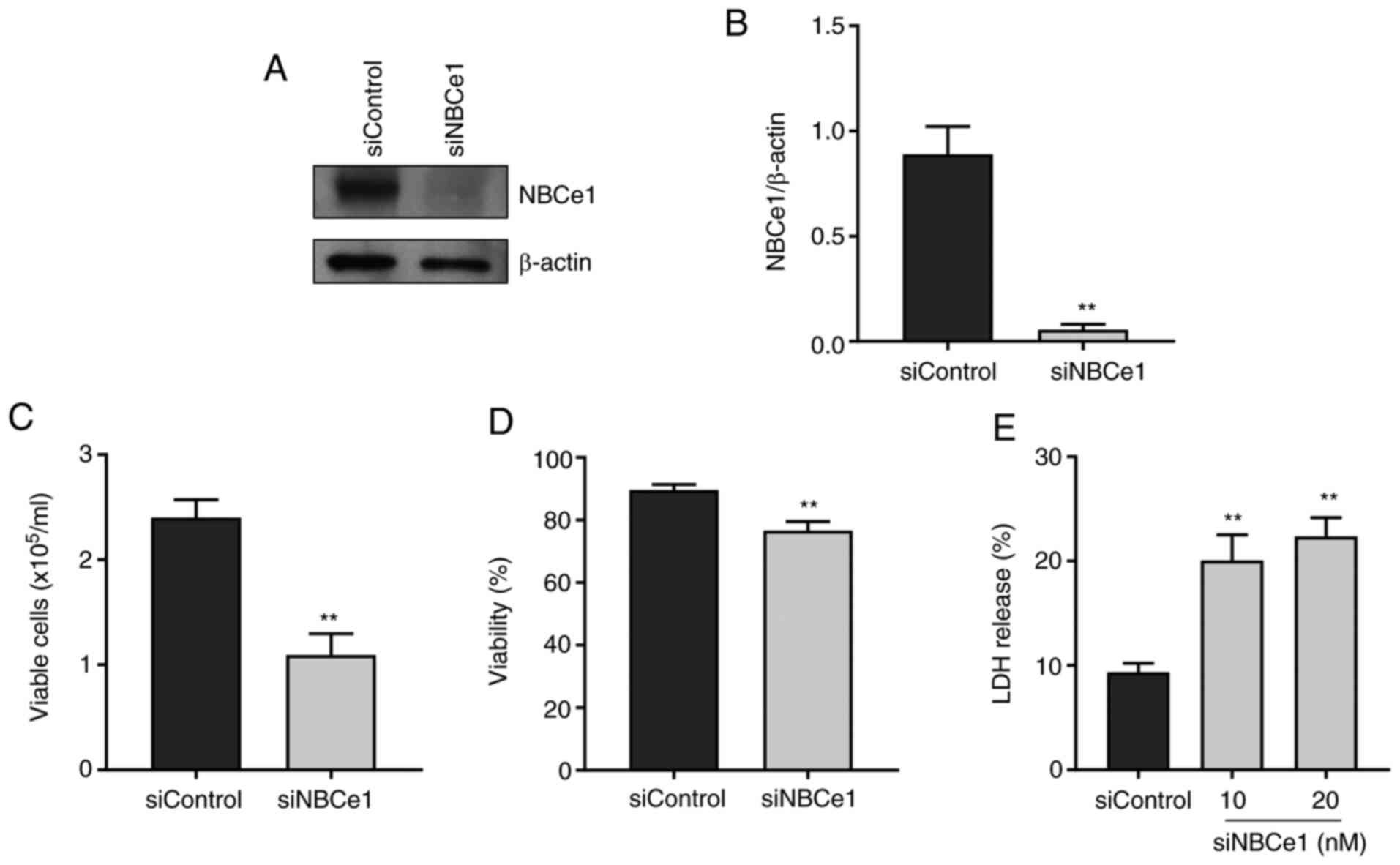
NBCe1 contributes to LNCaP cell
proliferation and viability
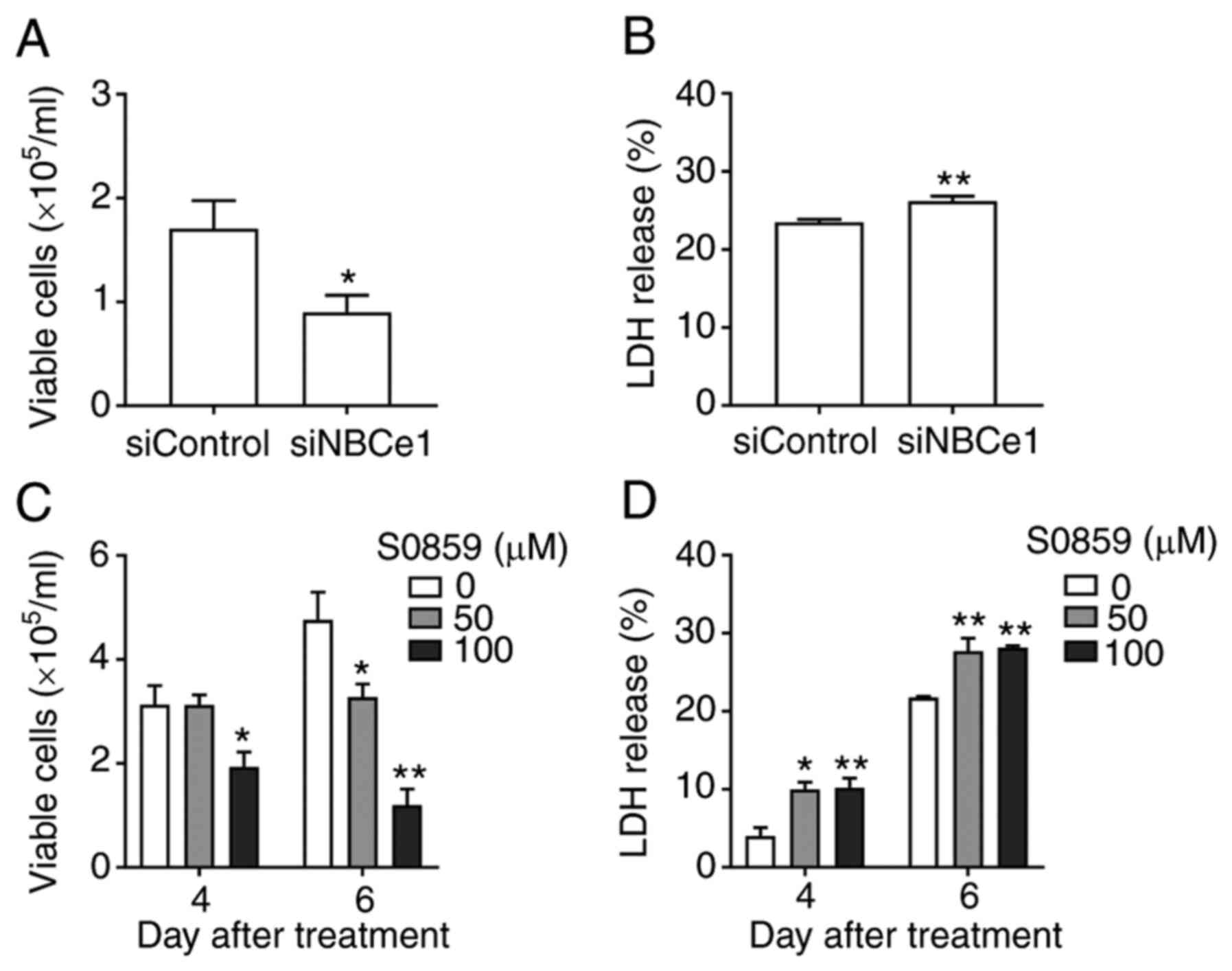
To examine whether NBCe1 affects growth and survival
of LNCaP cells, NBCe1 gene expression was disrupted using siRNA
oligonucleotides. The knockdown efficacy determined by
immunoblotting 96 h after transfection is presented in Fig. 4A. Compared to the control siRNA/random
27-mers, the siRNA/NBCe1 decreased NBCe1 protein levels by over 90%
(P<0.01, Student's t-test; n=3; Fig.
4B). In parallel experiments, the number of viable cells in the
trypan blue exclusion assay was counted. As shown in Fig. 4C, the knockdown decreased the number
of viable cells by 54% (from 2.4×105 cells/ml to
1.1×105 cells/ml; P<0.01; n=6/group) when determined
at 4 days after treatment. The cell viability (i.e., percentage of
viable cell to total cells) was also decreased, but the magnitude
of the change was relatively small (13%; P<0.01; Fig. 4D), implying that the decrease in cell
number is not tightly related to the decrease in viability.
Consistent with this implication, the knockdown caused 10–12% cell
death, determined by the LDH release assay (n=5 at 10 nM and n=6 at
20 nM of siRNA/NBCe1), markedly smaller than the percent change in
cell number (Fig. 4E). Doubling the
amounts of siRNA/NBCe1 oligonucleotides for transfection did not
further increase the cell death (P>0.05), indicating that the
knockdown has reached a maximum level of cell death.
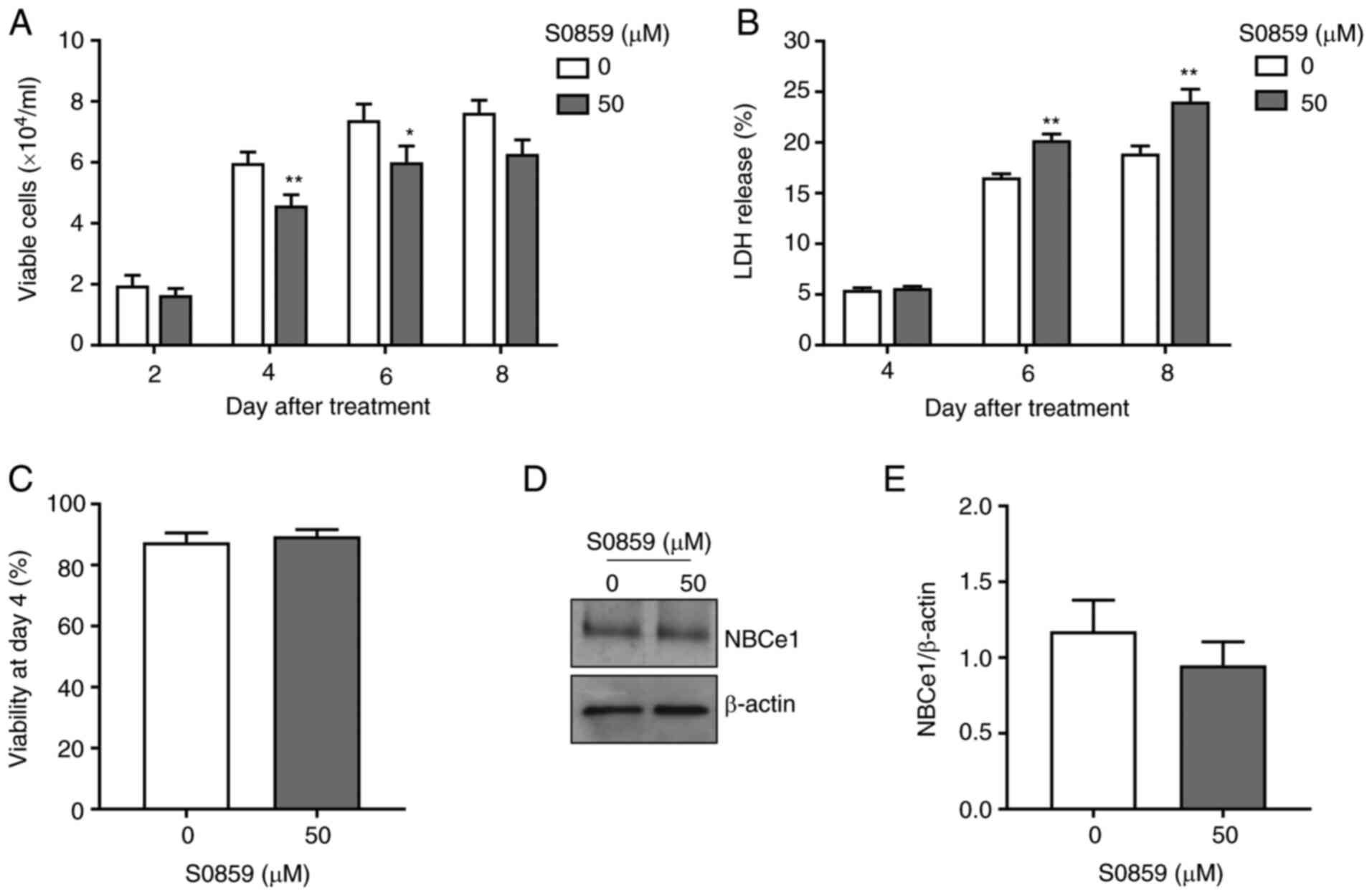
Next, LNCaP cells were treated with 50 µM of S0859
to assess whether pharmacological inhibition of NBCe1 produces
similar effects. As shown in Fig. 5A,
S0859 treatment decreased the number of viable cells (23% at 4 days
after treatment, P<0.01, n=15/group; and 19% at 6 days after
treatment, P<0.05, n=6/group). These decreases were smaller than
the decrease by the aforementioned NBCe1 knockdown. Furthermore,
cell death was not observed at 4 days after treatment but increased
at 6 days after treatment (Fig. 5B).
Consistent with this lack of cell death at 4 days after treatment,
the viability was unchanged during the same treatment days
(Fig. 5C) and NBCe1 protein levels
were also unaltered (Fig. 5D and E).
Thus, the pharmacological inhibition of NBCe1 decreases cell
proliferation, similar to the knockdown, but the two methods appear
to have different mechanisms affecting cell death.
NBCe1 contributes to PC3 cell
proliferation and viability
The qPCR results revealed the most exclusively
abundant expression of NBCe1 in LNCaP and C4-2 cells, but moderate
co-expression with other NBCs in cells such as PC3. This leads to
the possibility that NBCe1 contribution to cell proliferation and
viability may vary depending upon cell types. To address this
possibility, NBCe1 knockdown was performed in PC3 cells and cell
numbers were counted. As shown in Fig.
6A, the knockdown decreased the number of viable cells by 48%
(from 1.71×105 to 0.89×105 cells/ml at 4 days
after transfection; P<0.05, n=9/group). The knockdown also
caused a small increase in cell death (3%; Fig. 6B). Thus, NBCe1 affected the growth and
viability of PC3 cells, similar to those in LNCaP cells. Next,
cells were treated with S0859, which should inhibit all NBCs, and
viable cell number and cell death at 4 days after treatment were
assessed. Interestingly, S0859 had no effect at 50 µM but decreased
viable cell numbers at 100 µM (38% decrease; P<0.05, n=4/group;
Fig. 6C). The decrease was more
severe at 6 days after treatment (31% decrease at 50 µM and 75%
decrease at 100 µM; P<0.01 for both, n=3-8/group). As
anticipated, S0859 caused a small increase cell death (6% at both
concentrations; Fig. 6D). A higher
amount of S0859 was required to decrease the proliferation of PC3
cells, in comparison to LNCaP cells. Conclusively, NBCe1 knockdown
in PC3 cells decreased cell proliferation to the level similar to
that by the same knockdown in LNCaP cells, indicating that NBCe1
significantly affects PC3 cell growth.
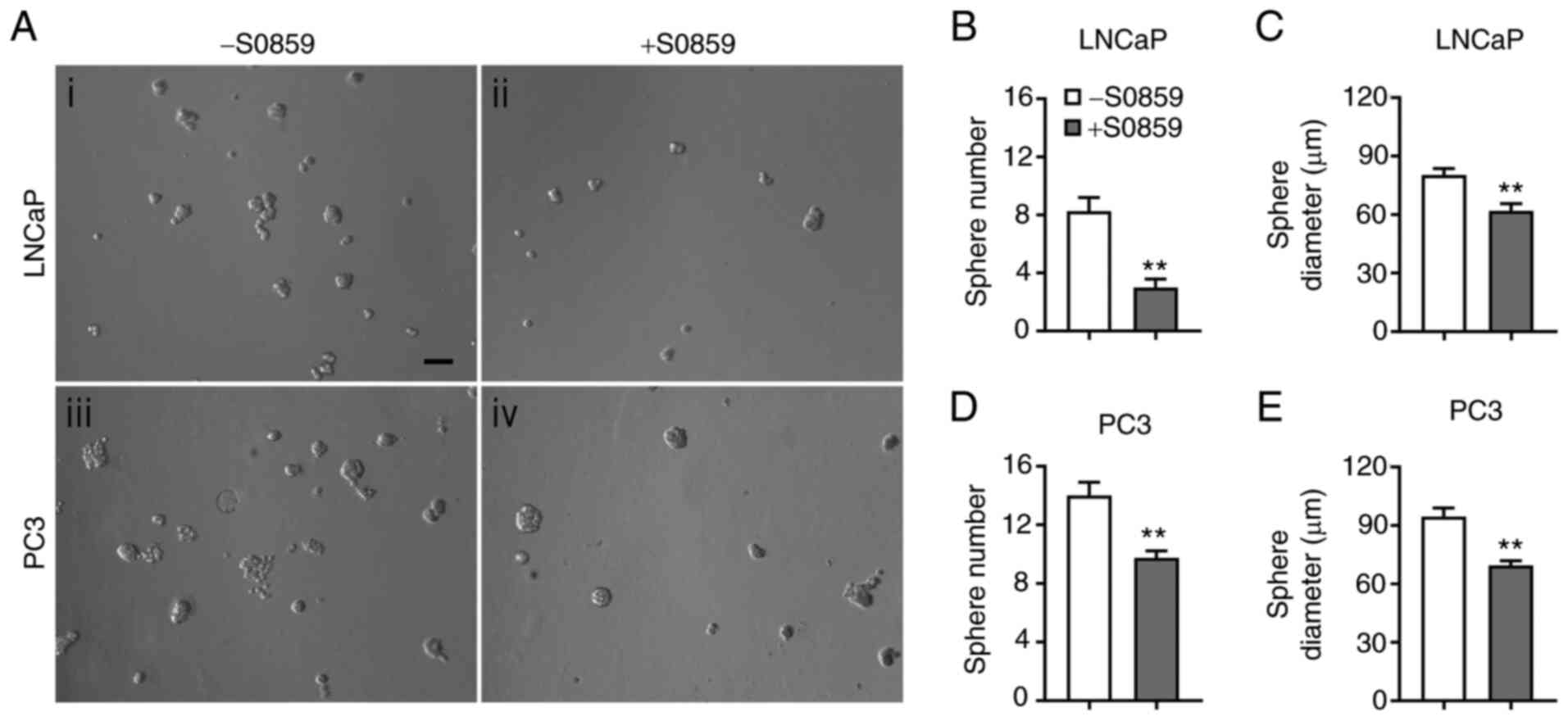
S0859 decreases LNCaP and PC3 cell
spheres in 3D cultures
The effects of NBC inhibition on LNCaP and PC3 cell
growth were further examined in 3D cultures. The images of cell
spheres formed 6 days after treatment with 100 µM of S0859 or none
are presented in Fig. 7A. Compared to
the control, S0859 decreased sphere formation in both cell lines.
The number of LNCaP cell spheres was decreased by 64% (P<0.01,
n=4/group; Fig. 7B) and the Feret
diameter was decreased by 23% (P<0.01, n=13–34 spheres/group;
Fig. 7C). Similarly, the number of
PC3 cell spheres was decreased by 31% (P<0.01, n=4/group;
Fig. 7D) and the Feret diameter was
decreased by 27% (P<0.01, n=39–56 spheres/group; Fig. 7E). Thus, similar as in 2D cultures,
pharmacological inhibition of NBCe1 reduces LNCaP and PC3 cell
growth in 3D cultures.
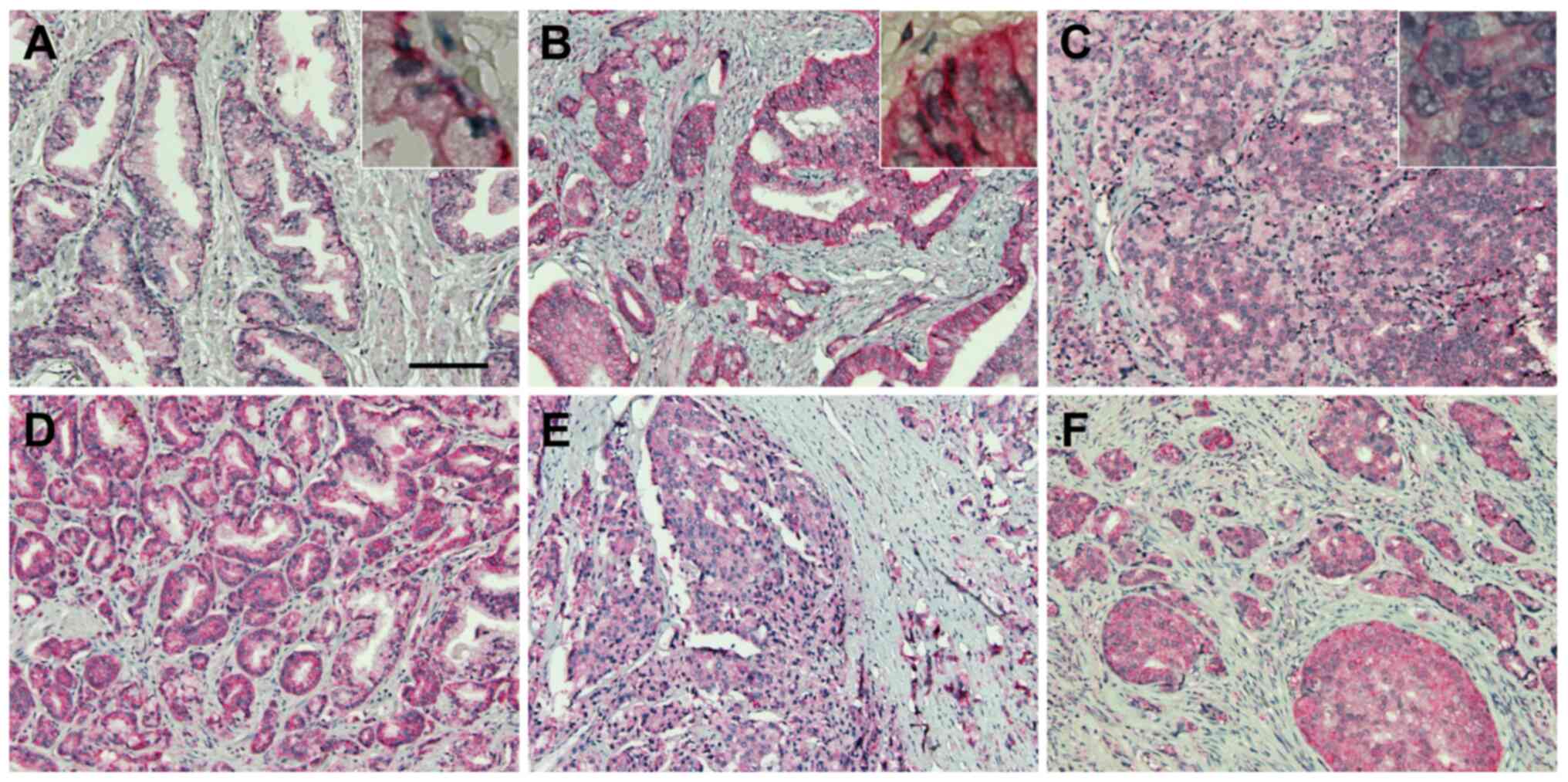
NBCe1 expression is robust in
prostatic adenocarcinoma
The robust expression of NBCe1 in LNCaP cells led us
to a localization study of this transporter in human prostate
tissue and prostatic cancer. For this experiment, NBCe1
immunohistochemistry was performed on human prostate cancer tissue
microarrays containing 9 cases of normal prostate tissue and 41
cases of prostate cancer (aforementioned in the Materials and
methods). NBCe1 was localized to the basolateral side of the
glandular epithelial cells in normal prostate (Fig. 8A), consistent with its basolateral
localization in a variety of secretory glands (11). In prostatic cancer, NBCe1 was highly
abundant in adenocarcinoma in Gleason grades 3–5 (Fig. 8B-F). The plasma membrane staining
progressively disappeared in higher Gleason grades, consistent with
the lost ability of cancer cells to form glands in more advanced
tumor stages.
Discussion
The significance and novelty of our study are as
follows: i) Despite reports on multiple acid extrusion mechanisms
and their involvement in cancer cell growth and progression, no
investigation has been made on prostate cancer. Our study, for the
first time, provides an expression profile of NBCs among different
prostate cancer cell lines. ii) NBCe1 knockdown and inhibition
decrease LNCaP and PC3 cell proliferation and viability. The
decrease in PC3 cell growth by the knockdown is notable given that
PC3 cells possess other NBCs in addition to NBCe1. This further
indicates that, among different NBCs, NBCe1 is the key transporter
affecting cell proliferation. iii) NBCe1 is extensively expressed
in human prostate adenocarcinoma. The result provides important
immunohistochemical evidence of NBCe1 expression/localization in
human prostate tissue and prostatic cancer.
In this study, high expression of NBCe1 was
identified in LNCaP and C4-2 cells, but weak to moderate expression
in PC3, 22RV1 and DU145 cells. The latter cells also express other
NBCs in addition to NBCe1. LNCaP and 22RV1 are androgen-responsive
and their growth is inhibited by androgen withdrawal, whereas C4-2,
PC3, DU145 are androgen-irresponsive and their growth is
independent of androgen (41). Thus,
the expression of NBCs including NBCe1 does not correlate with
androgen responsiveness in these cells. It is interesting to note
that neuron-specific NDCBE and NCBE are expressed in PC3, 22RV1 and
DU145 cells. Tai et al (42)
have reported that LNCaP cells are similar to adenocarcinoma
characterized by lack of basal cells and proliferation of malignant
tumor cells with luminal differentiation, whereas PC3 cells are
characteristic of neuroendocrine carcinoma. In our study, NBCe1
upregulation was observed in LNCaP cells under hypoxic conditions.
Literature search and database analysis have revealed a similar
upregulation in prostate cancer (30,31). NBCe1
was one of the gene products stimulated in a mouse model of
prostate cancer developed by a deletion of the tumor suppressor
gene Atbf1 (30). The increase
was 1.7–1.8 fold in mRNA expression; nonetheless, NBCe1 was the
only NBC that was increased in response to cancer development and
other NBCs were unaffected. The human genome array database in the
Oncomine Research (www.oncomine.org) revealed NBCe1 mRNA upregulation in
prostate carcinoma. The increase was 2.1-fold, but it was ranked in
top 1% among 8,603 measured genes. Furthermore, a whole-genome
sequencing of 27 prostate cancer patients revealed a focal
amplification of SLC4A4 gene (31).
The amplification occurred only 15% among patients, but the result
supports the idea that excessive NBCe1 activity may accelerate
extracellular acidification and promote microenvironments favorable
for cancer growth.
By what mechanism would NBCe1 be upregulated? NBCe1
upregulation is dependent on the hypoxia-inducing factor 1α (HIF1α)
in LS174T colon cancer cells (21).
HIF1α primarily promotes glucose consumption and glycolysis in
control of cell metabolism, whereas HIF2α promotes fatty acid
storage (43). HIF1α involvement in
NBCe1 upregulation implies that the upregulation is an upstream
event from the transporter's response to intracellular acid load.
Thus, while the upregulation offers an advantage when cancer cells
actively proliferate with a high rate of metabolic acid production,
intracellular acid load itself is unlikely the prime cause of this
upregulation. NBCe1 gene expression was stimulated by the
TGF-β/Smad4 signaling in mouse astrocytes (44). Given that TGF-β/Smad4 regulates
proto-oncogene Src (45), a
non-receptor tyrosine kinase associated with advanced malignancy in
human cancers, it is notable that NBCe1 is stimulated by Src
(46).
NBCe1 knockdown decreased the proliferation of both
LNCaP and PC3 cells. The effects were substantial as the cell
numbers were decreased by 48–54%. The knockdown also decreased cell
death; however, the magnitude of change was relatively small
(3-13%). There is not enough information on the cellular mechanisms
underlying NBCe1 involvement in cell proliferation and cytotoxicity
in other cells, and this makes it difficult to apprehend the
molecular events following NBCe1 knockdown. Nonetheless, it is
noteworthy that NBCe1 binds to IRBIT (IP3
receptor-binding protein released with IP3), which
regulates intracellular Ca2+ release from IP3
receptor (47,48). IRBIT is involved in cell death by
binding to Bcl2l10 and facilitating massive Ca2+
transfer to mitochondria (49). Thus,
it is possible that NBCe1 knockdown redistributes IRBIT in the
cytosol, such that its capacity to interact with Bcl2l10 is
enhanced. Similar to the knockdown, prolonged treatment of S0859
also decreased the growth of LNCaP and PC3 cells, consistent with
its effects in other cancer cell lines (20,21,50). S0859
was more potent in LNCaP than PC3 cells, because the treatment at
50 µM decreased viable LNCaP cell numbers but had no effect on PC3
cells when measured 4 days after treatment. A higher concentration
was required to alter PC3 cell numbers. S0859 also increased cell
death in both cell types, but the change was relatively small
compared to its effects on cell growth. Thus, S0859 primarily
inhibits cell proliferation, rather than cell death in prostate
cancer cells.
Na+ and
HCO3–-dependent acid extrusion was identified
in LNCaP cells, confirming the expression of active NBCe1 in these
cells. In our study, S0859 at 50 µM inhibited pHi
recovery from acidification by 77%. Heidtmann et al
(51) have reported that, in voltage
clamp recordings of Xenopus oocytes expressing NBCe1, S0859
at this concentration inhibited 90% of the electrogenic current
(IC50 of 9 µM). The authors also observed an 80%
inhibition of the current in mouse astrocytes, in which NBCe1 is
highly expressed (52). Thus, the
percent inhibition that was observed in LNCaP cells is comparable
to the inhibitions in NBCe1-expressing oocytes and native
astrocytes. The high level of inhibition further suggests that
NBCe1 plays a major role in acid extrusion in LNCaP cells while
other acid-extruding transporters are minimally involved. S0859 has
been reported to inhibit MCTs in the Xenopus oocyte
expression (51). In our experiment,
the pHi recovery in Na+-free
CO2/HCO3− solution was small,
indicating that acid extrusion in LNCaP cells is largely
Na+-dependent. This further suggests that MCTs play a
minor role in LNCaP cells. Hypoxia-inducible MCT4 was relatively
low in LNCaP cells, compared to PC3 as well as RWPE-1 and WPE1
prostate epithelial cell lines (53).
The results from our study lead to a discussion on a
possible role of NBCe1 in human prostate cancer. Prostatic
glandular epithelial cells are proliferated to premalignant
prostate intraepithelial neoplasia (PIN) that consequently develops
into intraductal carcinoma and invasive prostate cancer. Hypoxia
and acidosis are induced in PIN as cell proliferation occurs, and
HIF1α is activated (54). HIF1α
promotes NBCe1 upregulation along with other acid extrusion
proteins such as NHEs (25,55), V-ATPases (26) and MCTs (28). Membrane-bound CA IX is also
upregulated (56). The upregulation
of these proteins moves HCO3− from cell
surfaces to the inside of cancer cells and leaves H+ at
the outer side of the membrane, and acidic microenvironments are
exacerbated. Extracellular acidification is additionally
facilitated via a molecular interaction between NBCe1 and CA IX
(57). Consequently, acidic
microenvironments promote cancer cell survival and proliferation
(33). In addition, NBCe1 may
contribute to cancer cell migration and invasion because this
transporter is capable of facilitating cell migration in colon and
breast cancer cell lines (20). The
migration may occur in collaboration with NHE1, which is
concentrated at the leading edge of the lamellipodium and
contributes to cell migration (58).
In summary, the present study demonstrates the
importance of NBCe1 for acid extrusion in prostate cancer cells and
its contribution to cell growth. The decreased cell proliferation
and viability by NBCe1 knockdown and inhibition are in good
agreement with the current understanding that disrupting
intracellular acid-base homeostasis suppresses cancer cell growth
and progression (7,8). NBCe1 is proposed as a potential target
protein for a hypoxia-activated prodrug that is delivered to
hypoxic regions and kills cancer cells (59). In addition, given risks of prostate
cancer and systemic pH disturbance with age (60), our study may provide a basis for
future investigation of a pathological connection between the two
age-related health issues. The present study was performed in cell
culture models and additional assessments are required to confirm
the involvement of NBCe1 in cancer cell growth in vivo.
Thus, a future study will be to test whether abolishing or
inhibiting NBCe1 reduces prostate cancer growth in animal
models.
Supplementary Material
Supporting Data
Acknowledgements
We thank Dr Wei Zhou and Dr Carlos Moreno for
providing prostate cell lines and Dr Deepa Kodandera at the Emory
Yerkes Histology and Molecular Pathology Laboratory for
immunohistochemistry. We also thank Dr Baotong Zhang for technical
advice on 3D culture and Dr Thomas Chun for discussion about human
prostate cancer treatment and prevention. Reda Zafar is a medical
student at Tuoro College of Osteopathic Medicine, New York and
participated in the study as a summer intern.
Funding
This work was supported in part by Emory University
Winship Cancer Pilot Grant no. 00068255 (IC).
Authors' contributions
All the authors contributed to the conception and
design of the study. JML, SL, RZ and IC acquired the data. JML, SL,
RZ, ES analyzed and interpreted the data. ES drafted the
manuscript. ES and IC critically revised the manuscript for
important intellectual content. All authors read and approved the
final manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Ethics approval and consent to
participate
The formalin-fixed, paraffin-embedded human prostate
carcinoma tissue microarrays containing 41 cases of prostate cancer
and 9 cases of normal prostate tissue were purchased from US Biolab
Corporation, Inc. All tissues were collected under the highest
ethical standards with the donor being informed completely and with
their consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boron WF: Regulation of intracellular pH.
Adv Physiol Educ. 28:160–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parks SK, Cormerais Y and Pouyssegur J:
Hypoxia and cellular metabolism in tumour pathophysiology. J
Physiol. 595:2439–2450. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Corbet C and Feron O: Tumour acidosis:
From the passenger to the driver's seat. Nat Rev Cancer.
17:577–593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Svastová E, Hulíková A, Rafajová M,
Zat'ovicová M, Gibadulinová A, Casini A, Cecchi A, Scozzafava A,
Supuran CT, Pastorek J and Pastoreková S: Hypoxia activates the
capacity of tumor-associated carbonic anhydrase IX to acidify
extracellular pH. FEBS Lett. 577:439–445. 2004. View Article : Google Scholar
|
|
5
|
Pastorekova S and Gillies RJ: The role of
carbonic anhydrase IX in cancer development: Links to hypoxia,
acidosis, and beyond. Cancer Metastasis Rev. 38:65–77. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Webb BA, Chimenti M, Jacobson MP and
Barber DL: Dysregulated pH: A perfect storm for cancer progression.
Nat Rev Cancer. 11:671–677. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fais S, Venturi G and Gatenby B:
Microenvironmental acidosis in carcinogenesis and metastases: New
strategies in prevention and therapy. Cancer Metastasis Rev.
33:1095–1108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parks SK and Pouysségur J: Targeting pH
regulating proteins for cancer therapy-Progress and limitations.
Semin Cancer Biol. 43:66–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi I: SLC4A transporters. Curr Top
Membr. 70:77–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aalkjaer C, Boedtkjer E, Choi I and Lee S:
Cation-coupled bicarbonate transporters. Compr Physiol.
4:1605–1637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parker MD and Boron WF: The divergence,
actions, roles, and relatives of sodium-coupled bicarbonate
transporters. Physiol Rev. 93:803–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Yang J and Chen LM: Structure and
function of SLC4 family HCO-3 transporters. Front Physiol.
6:3552015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gatenby RA and Gillies RJ: A
microenvironmental model of carcinogenesis. Nat Rev Cancer.
8:56–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee D and Hong JH: The fundamental role of
bicarbonate transporters and associated carbonic anhydrase enzymes
in maintaining Ion and pH homeostasis in non-secretory organs. Int
J Mol Sci. 21:3392020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahmed S, Thomas G, Ghoussaini M, Healey
CS, Humphreys MK, Platte R, Morrison J, Maranian M, Pooley KA,
Luben R, et al: Newly discovered breast cancer susceptibility loci
on 3p24 and 17q23.2. Nat Genet. 41:585–590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boedtkjer E, Moreira JM, Mele M, Vahl P,
Wielenga VT, Christiansen PM, Jensen VE, Pedersen SF and Aalkjaer
C: Contribution of Na+,HCO3(−)-cotransport to cellular pH control
in human breast cancer: A role for the breast cancer susceptibility
locus NBCn1 (SLC4A7). Int J Cancer. 132:1288–1299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gorbatenko A, Olesen CW, Loebl N,
Sigurdsson HH, Bianchi C, Pedraz-Cuesta E, Christiansen J and
Pedersen SF: Oncogenic p95HER2 regulates Na+-HCO3- cotransporter
NBCn1 mRNA stability in breast cancer cells via 3′UTR dependent
processes. Biochem J. 473:4027–4044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee S, Axelsen TV, Andersen AP, Vahl P,
Pedersen SF and Boedtkjer E: Disrupting Na+,
HCO3−-cotransporter NBCn1 (Slc4a7) delays
murine breast cancer development. Oncogene. 35:2112–2122. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee S, Axelsen TV, Jessen N, Pedersen SF,
Vahl P and Boedtkjer E: Na+,
HCO3−-cotransporter NBCn1 (Slc4a7)
accelerates ErbB2-induced breast cancer development and tumor
growth in mice. Oncogene. 37:5569–5584. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parks SK and Pouyssegur J: The
Na(+)/HCO3(−) co-transporter SLC4A4 plays a role in growth and
migration of colon and breast cancer cells. J Cell Physiol.
230:1954–1963. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McIntyre A, Hulikova A, Ledaki I, Snell C,
Singleton D, Steers G, Seden P, Jones D, Bridges E, Wigfield S, et
al: Disrupting hypoxia-induced bicarbonate transport acidifies
tumor cells and suppresses tumor growth. Cancer Res. 76:3744–3755.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wong P, Kleemann HW and Tannock IF:
Cytostatic potential of novel agents that inhibit the regulation of
intracellular pH. Br J Cancer. 87:238–245. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fliegel L: Role of pH regulatory proteins
and dysregulation of ph in prostate cancer. Reviews of Physiology.
Biochemistry and Pharmacology Springer Berlin Heidelberg; Berlin,
Heidelberg: pp. 1–26. 2020
|
|
24
|
Korenchan DE, Bok R, Sriram R, Liu K,
Santos RD, Qin H, Lobach I, Korn N, Wilson DM, Kurhanewicz J and
Flavell RR: Hyperpolarized in vivo pH imaging reveals
grade-dependent acidification in prostate cancer. Oncotarget.
10:6096–6110. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dykes SS, Gao C, Songock WK, Bigelow RL,
Woude GV, Bodily JM and Cardelli JA: Zinc finger E-box binding
homeobox-1 (Zeb1) drives anterograde lysosome trafficking and tumor
cell invasion via upregulation of Na+/H+ Exchanger-1 (NHE1). Mol
Carcinog. 56:722–734. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Michel V, Licon-Munoz Y, Trujillo K,
Bisoffi M and Parra KJ: Inhibitors of vacuolar ATPase proton pumps
inhibit human prostate cancer cell invasion and prostate-specific
antigen expression and secretion. Int J Cancer. 132:E1–E10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu W, Wang L, Wang Y, Xu X, Zou P, Gong M,
Zheng J, You J, Wang H, Mei F and Pei F: A novel tumor metastasis
suppressor gene LASS2/TMSG1 interacts with vacuolar ATPase through
its homeodomain. J Cell Biochem. 114:570–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pertega-Gomes N and Baltazar F: Lactate
transporters in the context of prostate cancer metabolism: What do
we know? Int J Mol Sci. 15:18333–18348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ibrahim-Hashim A, Cornnell HH, Abrahams D,
Lloyd M, Bui M, Gillies RJ and Gatenby RA: Systemic buffers inhibit
carcinogenesis in TRAMP mice. J Urol. 188:624–631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun X, Fu X, Li J, Xing C, Frierson HF, Wu
H, Ding X, Ju T, Cummings RD and Dong JT: Deletion of atbf1/zfhx3
in mouse prostate causes neoplastic lesions, likely by attenuation
of membrane and secretory proteins and multiple signaling pathways.
Neoplasia. 16:377–389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang C, Niu L, Xiao Z, Zheng C, Shen Y,
Shi Y and Han X: Whole-genome sequencing of prostate cancer reveals
novel mutation-driven processes and molecular subgroups. Life Sci.
254:1172182020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee S, Li JM and Choi I: Sodium
bicarbonate cotransporter NBCe1 affects the growth and motility of
prostate cancer cell lines LNCaP and PC3. FASEB J.
32:IB4112018.
|
|
33
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. Feb 4–2021.(Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Strober W: Trypan blue exclusion test of
cell viability. Curr Protoc Immunol. 111:A3.B.1–A3.B.3. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thomas JA, Buchsbaum RN, Zimniak A and
Racker E: Intracellular pH measurements in Ehrlich ascites tumor
cells utilizing spectroscopic probes generated in situ.
Biochemistry. 18:2210–2218. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cooper DS, Yang HS, He P, Kim E,
Rajbhandari I, Yun CC and Choi I: Sodium/bicarbonate cotransporter
NBCn1/slc4a7 increases cytotoxicity in magnesium depletion in
primary cultures of hippocampal neurons. Eur J Neurosci.
29:437–446. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park HJ, Gonzalez-Islas CE, Kang Y, Li JM
and Choi I: Deletion of the Na/HCO3 transporter NBCn1
protects hippocampal neurons from NMDA-induced seizures and
neurotoxicity in mice. Sci Rep. 9:159812019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang B, Ci X, Tao R, Ni JJ, Xuan X, King
JL, Xia S, Li Y, Frierson HF, Lee DK, et al: Klf5 acetylation
regulates luminal differentiation of basal progenitors in prostate
development and regeneration. Nat Commun. 11:9972020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ch'en FF, Villafuerte FC, Swietach P,
Cobden PM and Vaughan-Jones RD: S0859, an N-cyanosulphonamide
inhibitor of sodium-bicarbonate cotransport in the heart. Br J
Pharmacol. 153:972–982. 2008. View Article : Google Scholar
|
|
41
|
Marchiani S, Tamburrino L, Nesi G,
Paglierani M, Gelmini S, Orlando C, Maggi M, Forti G and Baldi E:
Androgen-responsive and -unresponsive prostate cancer cell lines
respond differently to stimuli inducing neuroendocrine
differentiation. Int J Androl. 33:784–793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tai S, Sun Y, Squires JM, Zhang H, Oh WK,
Liang CZ and Huang J: PC3 is a cell line characteristic of
prostatic small cell carcinoma. Prostate. 71:1668–1679. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Majmundar AJ, Wong WJ and Simon MC:
Hypoxia-inducible factors and the response to hypoxic stress. Mol
Cell. 40:294–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Khakipoor S, Ophoven C, Schrödl-Häußel M,
Feuerstein M, Heimrich B, Deitmer JW and Roussa E: TGF-β signaling
directly regulates transcription and functional expression of the
electrogenic sodium bicarbonate cotransporter 1, NBCe1 (SLC4A4),
via Smad4 in mouse astrocytes. Glia. 65:1361–1375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kubiczkova L, Sedlarikova L, Hajek R and
Sevcikova S: TGF-β - an excellent servant but a bad master. J
Transl Med. 10:1832012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Namkoong E, Shin YH, Bae JS, Choi S, Kim
M, Kim N, Hwang SM and Park K: Role of sodium bicarbonate
cotransporters in intracellular pH regulation and their regulatory
mechanisms in human submandibular glands. PLoS One.
10:e01383682015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shirakabe K, Priori G, Yamada H, Ando H,
Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G and Mikoshiba K:
IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein,
specifically binds to and activates pancreas-type
Na+/HCO3− cotransporter 1 (pNBC1). Proc Natl Acad Sci
USA. 103:9542–9547. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee SK, Boron WF and Parker MD: Relief of
autoinhibition of the electrogenic Na-HCO(3) [corrected]
cotransporter NBCe1-B: Role of IRBIT vs. amino-terminal truncation.
Am J Physiol Cell Physiol. 302:C518–C526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bonneau B, Ando H, Kawaai K, Hirose M,
Takahashi-Iwanaga H and Mikoshiba K: IRBIT controls apoptosis by
interacting with the Bcl-2 homolog, Bcl2l10, and by promoting
ER-mitochondria contact. Elife. 5:e198962016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Andersen AP, Flinck M, Oernbo EK, Pedersen
NB, Viuff BM and Pedersen SF: Roles of acid-extruding ion
transporters in regulation of breast cancer cell growth in a
3-dimensional microenvironment. Mol Cancer. 15:452016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Heidtmann H, Ruminot I, Becker HM and
Deitmer JW: Inhibition of monocarboxylate transporter by
N-cyanosulphonamide S0859. Eur J Pharmacol. 762:344–349. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Majumdar D and Bevensee MO: Na-coupled
bicarbonate transporters of the solute carrier 4 family in the
nervous system: Function, localization, and relevance to neurologic
function. Neuroscience. 171:951–972. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sanità P, Capulli M, Teti A, Galatioto GP,
Vicentini C, Chiarugi P, Bologna M and Angelucci A: Tumor-stroma
metabolic relationship based on lactate shuttle can sustain
prostate cancer progression. BMC Cancer. 14:1542014. View Article : Google Scholar
|
|
54
|
Zhong H, Semenza GL, Simons JW and De
Marzo AM: Up-regulation of hypoxia-inducible factor 1alpha is an
early event in prostate carcinogenesis. Cancer Detect Prev.
28:88–93. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chatterjee S, Schmidt S, Pouli S, Honisch
S, Alkahtani S, Stournaras C and Lang F: Membrane androgen receptor
sensitive Na+/H+ exchanger activity in prostate cancer cells. FEBS
Lett. 588:1571–1579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ambrosio MR, Di Serio C, Danza G, Rocca
BJ, Ginori A, Prudovsky I, Marchionni N, Del Vecchio MT and
Tarantini F: Carbonic anhydrase IX is a marker of hypoxia and
correlates with higher Gleason scores and ISUP grading in prostate
cancer. Diagn Pathol. 11:452016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Svastova E, Witarski W, Csaderova L, Kosik
I, Skvarkova L, Hulikova A, Zatovicova M, Barathova M, Kopacek J,
Pastorek J and Pastorekova S: Carbonic anhydrase IX interacts with
bicarbonate transporters in lamellipodia and increases cell
migration via its catalytic domain. J Biol Chem. 287:3392–3402.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schwab A, Fabian A, Hanley PJ and Stock C:
Role of ion channels and transporters in cell migration. Physiol
Rev. 92:1865–1913. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
O'Connor LJ, Cazares-Körner C, Saha J,
Evans CN, Stratford MR, Hammond EM and Conway SJ: Design, synthesis
and evaluation of molecularly targeted hypoxia-activated prodrugs.
Nat Protoc. 11:781–794. 2016. View Article : Google Scholar
|
|
60
|
Frassetto L and Sebastian A: Age and
systemic acid-base equilibrium: Analysis of published data. J
Gerontol A Biol Sci Med Sci. 51:B91–B99. 1996. View Article : Google Scholar : PubMed/NCBI
|