Tumors are hypothesized to originate as a result of
and progress due to sequential genetic and epigenetic mutations of
cells. Tumors originate gradually from the tumor stem cells (TSCs)
that accrue several mutations (1–6). In recent
years, it has been indicated that the origin of tumors or TSCs
involves cell-cell fusion (1–6). It was originally hypothesized that tumor
cells possessed characteristics indicative of aneuploidy and
chromosomal disorders. Therefore, it was reasonable to hypothesize
that these features of tumors may be associated with cell-cell
fusion. It would be interesting to determine if the fusion of a
cancerous cell with a normal healthy cell, such as a migrating bone
marrow-derived cell (BMDCs), may give rise to unique features in
the resultant cell, such as increased tumor initiation, tumor
metastasis and/or drug resistance capacity.
Cell-cell fusion, also termed cell hybridization,
refers to the process of the fusion of two or more cells into a
single hybrid cell, with the formation of a single nucleus
possessing genetic information from two or more lineages (7). At the beginning of the process, the
membranes begin to fuse, followed by fusion of the cytoplasm and
the nuclei, ultimately resulting in the formation of a single cell
(8). In multicellular organisms, cell
fusion is a basic developmental and physiological process. The
fusion of a sperm and egg cell is one of the most classical
examples of cell fusion. In 2002, Mohler et al (9) first identified that the eff-1 gene was
essential for developmental cell fusion. In 2004, Shemer et
al successfully demonstrated that the expression of EFF-1
protein leads to cell fusion, and that it could cause independent
cell fusion in the absence of other proteins (10).
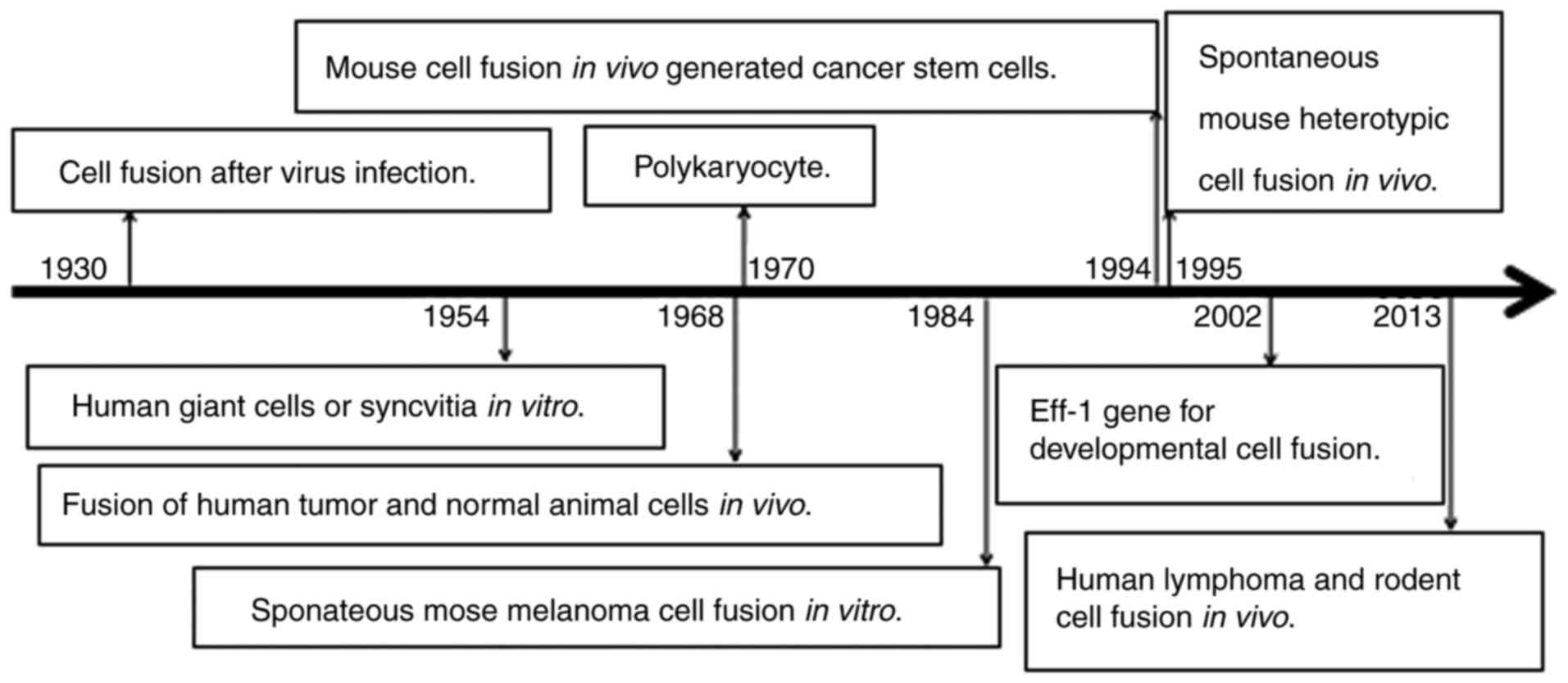
In recent years, increasing evidence of cell-cell
fusion and their underlying mechanisms have been reported (Table II; Fig.
1). In 2016, the interaction between the sperm protein Izumo
sperm-egg fusion 1 and egg the protein IZUMO1 receptor, JUNO was
revealed to mediate mouse fertilization (24). In 2017, cell-cell fusion was
demonstrated to be mediated by cell division cycle 42 pseudogene
1-Fus2p and spectraplakin-EFF-1 interactions in yeast and C.
elegans, respectively (25,26). In
2017, the myomaker, a myoblast fusion actor gene, was reported to
be involved in cell-cell fusion, leading to Carey-Fineman-Ziter
syndrome (27), and in 2017, lipid
raft-associated stomatin was reported to form a molecular assembly
that promoted membrane fusion (28).
A tumor is formed by the continuous proliferation of
transformed cells, and may progress to become more carcinogenic
through continuous evolution. Abnormal proliferation of
non-physiological fusion cells in multicellular organisms may be
one of the causes of tumor formation and progression. There is a
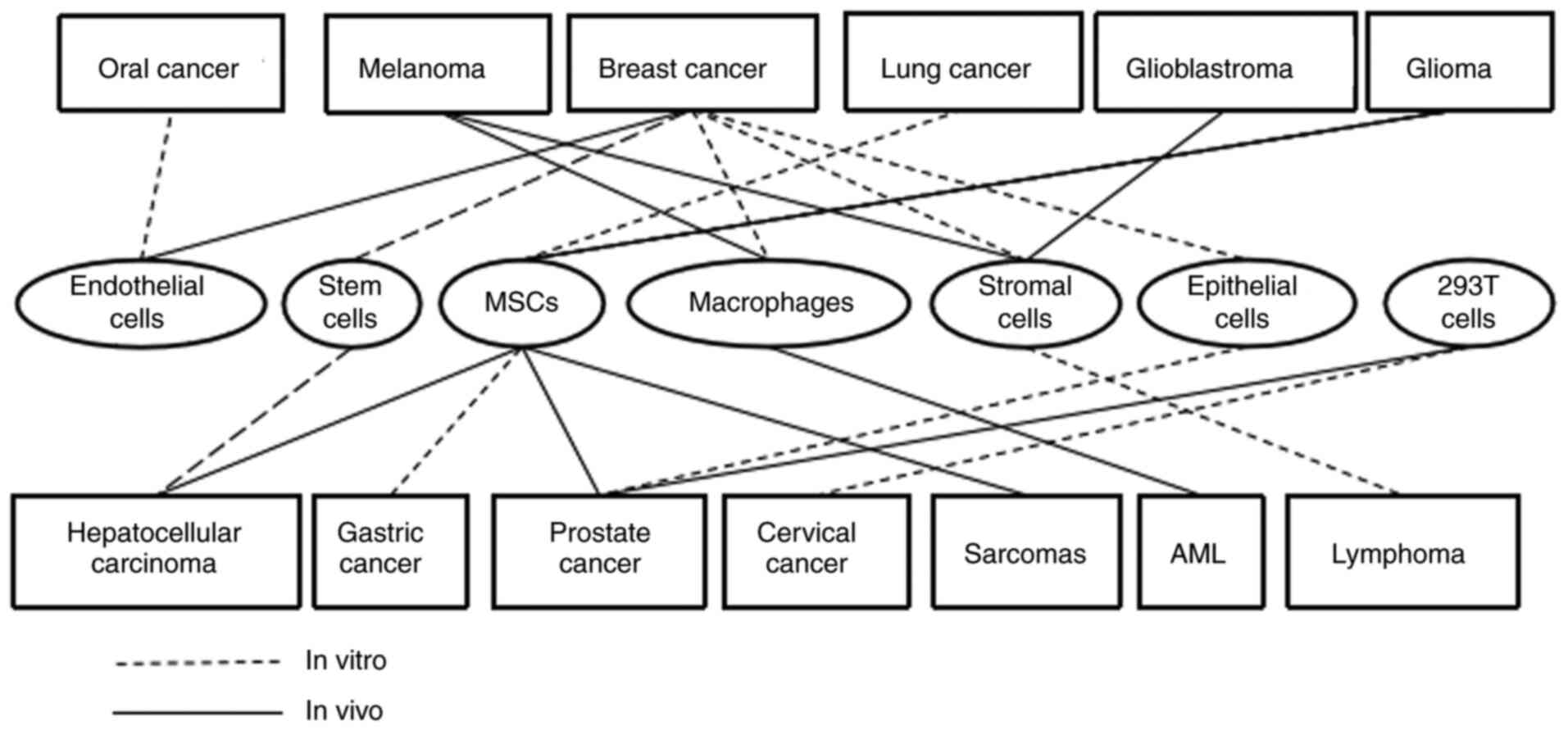
considerable body of knowledge supporting the occurrence of
spontaneous cell-cell fusion in vitro in cell cultures
between cancerous and other cell types as demonstrated in Table III and Fig. 2. These studies have investigated the
fusion of cancer cells with endothelial cells, BMDCs and epithelial
cells.
Endothelial cells line the inner side of blood and
lymphatic vessels, and cancer cells must cross this barrier to gain
access to the circulation, and cross again to exit and metastasize.
Fusions between cancerous and endothelial cells were revealed to
occur in vitro in co-cultures of human breast cancer cells
and endothelial cells (29). These
observations demonstrated a novel type of cancer-endothelial cell
interaction, which may be of fundamental importance in the process
of metastasis (29). Song et
al (30) demonstrated that the
oral cancer cell line SCC9 could spontaneously fuse with
co-cultured endothelial cells, and the resultant hybrid cells
exhibited continuous division and proliferation following
re-plating and thawing. Such hybrids express markers of both of the
parental cells, and undergo nuclear fusion, resulting in the
acquisition of novel properties, enhanced drug resistance and
improved survival potential. The hybrid cells comprised a
significant portion of the tumor composition as demonstrated by
immunostaining and FISH analysis, even though the hybrid cells and
SCC9 cells were inoculated with a ratio of 1:10,000 cells (30). These experimental findings provided
further evidence supporting the hypothesis that cell fusion may be
involved in cancer progression (31,32).
Human BMDCs, including embryonic stem cells (ESCs),
hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs),
macrophages and dendritic cells (DCs) have been reported to fuse
in vitro with various types of cancer cells spontaneously
(Table III; Fig. 2). Spontaneous in vitro
formation of heterotypic hybrids was revealed to occur between
human bone marrow-derived multipotent stromal cells and two
different breast cancer cell lines, MDA-MB-231 and MA11 cells
(33). The resultant fused cells
formed of hepatocellular carcinoma cells and hESCs, expressed both
cancer and stemness markers and exhibited increased drug resistance
and enhanced tumorigenesis (34).
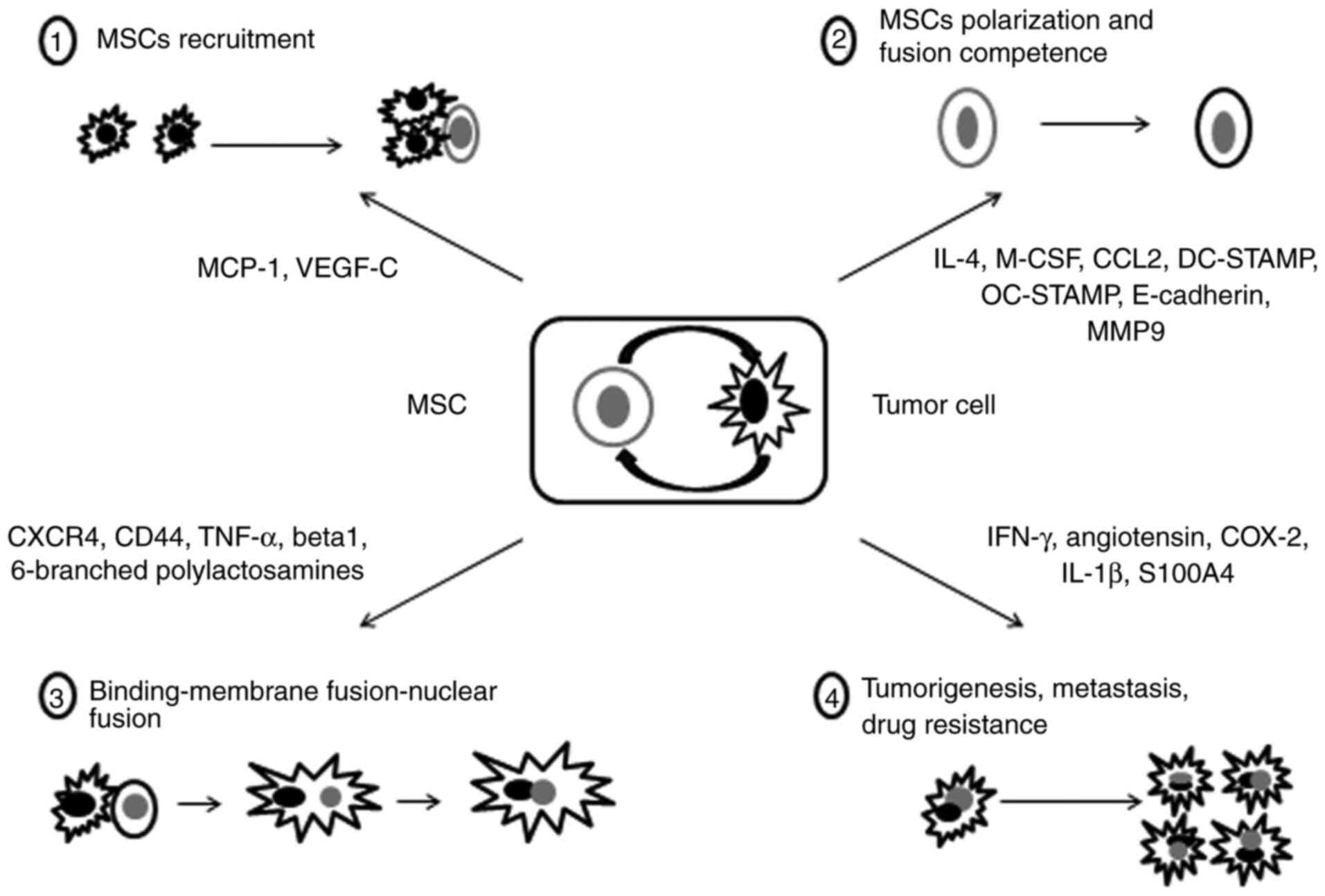
MSCs and breast cancer cell fusion resulted in hybrids with
enhanced migratory capacity, which promoted breast cancer
metastasis (35,36). In 2019, it was demonstrated that actin
cytoskeletal components served an important role in the cell fusion
between breast cancer cells and MSCs (37). Cell fusion between lung cancer cells
and MSCs provided a non-mutation-dependent mechanism that
contributed to the aberrant gene expression patterns, and gave rise
to highly malignant subpopulations with epithelial-mesenchymal
transition (EMT) and TSC-like properties (38). Cell fusion between hMSCs and gastric
cancer cells may contribute to the generation of tumorigenic
hybrids, with EMT and TSC-like properties (39). The spontaneous in vitro fusion
of mouse hMSCs and human SU3 glioma stem/progenitor cells is one of
the driving factors for glioma neovascularization (40). Cell fusion between MSCs and lung
cancer cells enhanced the metastatic capacity and characteristics
of cancer stem cells by undergoing EMT (41). The hybrid cells that were formed of
human liver cancer cells and mouse MSCs exhibited increased
expression of E-cadherin, vimentin, twist, snail, and matrix
metalloproteinase 2 and 9, were aneuploid, possessed enhanced
invasive and migratory capacities and generated an increased number
of metastatic liver and lung lesions (42). In 2020, prostate cancer cells were
revealed to exhibit characteristics associated with neuroendocrine
function and heterogeneity following fusion with bystander neural
stem cells in the tumor microenvironment (43). Macrophages serve an important role
during the development of cancer, such as in breast cancer and
melanoma (44). Macrophage-breast
cancer cell hybrids become more proliferative and invasive as they
undergo EMT and following increased activity of the Wnt/β-catenin
signaling pathway (45), and may also
acquire TSC properties (46). Fusion
between cancer cells and macrophages generates metastatic hybrids
with genetic and phenotypic characteristics of both maternal cells.
Fusion hybrids of macrophages and melanoma cells exhibited
upregulated expression of N-acetylglucosaminyltransferase V, β1-6
branching and were metastatic (47).
Melanoma-peritumoral stromal cell fusion may assist in explaining
the high rate of recurrence of melanomas in patients following
removal of the primary tumors (48,49).
Macrophage-cancer cell fusion was reported to generate a
subpopulation of radiotherapy-resistant cells with enhanced
DNA-repair capacity (50).
However, it is not always the case that fusion cells
will exhibit increased tumorigenicity or TSC-like properties. He
et al (51) reported in 2017
that hESCs and ovarian cancer cells can fuse in vitro
spontaneously, and the fused cells interestingly exhibited
epigenetic changes that led to inhibition of growth, which may
provide a novel direction for the treatment of ovarian cancer.
Although cell fusion between BMDCs and somatic cells may be the
origin of TSCs, the hybrid cells that form as a result of the
fusion of human HSCs and esophageal carcinoma cells did not
generate esophageal TSCs (52,53).
DC-cancer cell fusion vaccines are an attractive modality for the
treatment of several types of cancers, such as prostate, liver,
gastric, colorectal, lung and breast cancer (54–63). The
cytotoxic T chemokine interferon-induced protein-10 was
demonstrated to enhance the antitumor effects of DC/tumor cell
fusion vaccines by alleviating the immunosuppressive tumor
environment (64).
In addition, cancer cells can fuse with normal
epithelial cells. The hybrid cells derived from the spontaneous
fusion between the breast epithelial cell line M13SV1-EGFP-Neo and
two breast cancer cell lines, HS578T-Hyg and MDA-MB-435-Hyg, both
exhibited increased migratory capacity and increased drug
resistance towards chemotherapeutic drugs, such as doxorubicin and
paclitaxel. This finding further supported the hypothesis that cell
fusion may give rise to drug resistant and metastatic cells
(65). Human breast cancer cells and
breast epithelial cell fusion was observed and verified using a
Cre-loxP-based double fluorescence reporter system (35,66). The
fusion between human breast epithelial cells and breast cancer
cells gave rise to hybrid cells that possessed certain TSC or tumor
initiating cell-like properties, indicating that cell fusion may be
a mechanism underlying how tumor cells come to acquire a TSC
phenotype (67). Additionally, the
fusion of senescent human prostate epithelial cells and cancer
cells was reported to promote tumor development in prostate cancer
(68).
Tumor cells may fuse with several different types of
cells, including stromal cells, epithelial cells and endothelial
cells in vivo. Cell-cell fusion in vivo provides more
convincing evidence of the involvement of this process in cancer
development and progression than cell-cell fusion in vitro.
However, providing direct evidence of cell-cell fusion at the DNA
level is considerably more difficult, particularly for human
cell-human cell fusions in vivo. There are >30 reports of
cell-cell fusions in vivo between tumor cells and normal
cells, in most of which, macrophages or other BMDCs are a component
cell of the fusion (Table IV;
Fig. 2) (50). These reports primarily revealed cell
fusion between mouse-mouse cells or human-mouse cells, with only a
few reports demonstrating fusion between human-human cells.
However, due to the lack of specific DNA markers of
both fusion partner cells, the direct evidence of human-human cell
fusion in vivo remains lacking. Human-human cell fusion
in vivo was reported between human cancer cells and human
BMDCs (76,77). Studies have demonstrated the presence
of donor cell genes in recipient malignant cells after bone marrow
transplantation (BMT), supporting the possibility of
donor-recipient cell fusion in vivo (77). In a previous study, donor DNA was
detected in the recipient tumors by continued genetic analysis of
renal cell carcinoma specimens from allogeneic BMT patients who
developed secondary malignancies (78). Donor DNA was analyzed by laser capture
microdissection of the tumor cells followed by PCR. In another
study, patients receiving radiotherapy and immunosuppression prior
to transplantation increased the likelihood of recurrence of the
tumors and the donor BMDCs were found in the tumors of the patients
(76). Other researchers discovered
that early papillary renal cell carcinoma originated from male to
female HSC transplantation, and showed trisomy 17 characteristics,
which is common in early stage renal cell carcinoma and other types
of tumors; ~1% of trisomy 17 of the tumor cells also contained a Y
chromosome. It is worth noting that Y chromosome-containing and
chromosome 17 paired tumor cells clustered in the tumor during
mitotic anaphase. In addition, tumor cells containing the Y
chromosome appeared in ~10% of the tumor cells, indicating clonal
growth of these cells. As aforementioned, HSCs were associated with
tumor cells. However, it is possible that the tumor cells
originated from the donor HSCs alone, that no fusion had occurred,
and the Y chromosome was lost during tumor growth and proliferation
(79). In another similar study, the
tumor cells containing the Y chromosome were revealed in two
patients with intestinal cancer and one patient with lung cancer
who had previously received a male HSC transplant. The presence of
XXY or XXXY chromosome phenotypes detected by XY fluorescence in
situ hybridization analysis supported the notion that the
tumors originated from a cancer cell-BMDC fusion (77). The first and second pieces of
convincing evidence of human cell-human cell fusions in vivo
came from the detection of a short tandem repeat of parental cell
alleles (80,81). Both donor and recipient DNA were
detected in single cells of melanoma lymph-node and brain
metastases from sex mismatched BMT female cancer patients.
Not applicable.
The present study was supported by the National
Natural Science Foundation of China (81872412 to XHW, 81602303 to
XY, 31700736 to WXW), the National innovation and entrepreneurship
training program for College Students (202010489017 to PXC), the
Hubei Province Health and Family Planning Scientific Research
Project (WJ2016-Y-10 to PXC), the Jingzhou Science and Technology
Development Planning Project (JZKJ15063 to WXW), the Hubei Province
Scientific and Technological Research Project (Q20171306 to XWW),
the Hubei Province Natural Science Foundation of China (2017CFB786
to PXC, 2016CFB180 to WXW), and the Guangzhou Key Medical
Discipline Construction Project (CSZ).
Not applicable.
HWX designed and supervised the study. YFL, YYW, YYM
and XQL reviewed the references. XCP and MZ wrote the manuscript.
WQC, YZ, XWW and ZWM contributed to tables and figures. YYM, YX,
LSZ, LMY and SZC revised the manuscript. HWX and XCP acquired
funding. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Fonseca NA, Cruz AF, Moura V, Simões S and
Moreira JN: The cancer stem cell phenotype as a determinant factor
of the heterotypic nature of breast tumors. Crit Rev Oncol Hematol.
113:111–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xin HW, Hari DM, Mullinax JE, Ambe CW,
Koizumi T, Ray S, Anderson AJ, Wiegand GW, Garfield SH,
Thorgeirsson SS and Avital I: Tumor-initiating label-retaining
cancer cells in human gastrointestinal cancers undergo asymmetric
cell division. Stem Cells. 30:591–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xin HW, Ambe CM, Ray S, Kim BK, Koizumi T,
Wiegand GW, Hari D, Mullinax JE, Jaiswal KR, Garfield SH, et al:
Wnt and the cancer niche: Paracrine interactions with
gastrointestinal cancer cells undergoing asymmetric cell division.
J Cancer. 4:447–457. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xin HW, Ambe CM, Miller TC, Chen JQ,
Wiegand GW, Anderson AJ, Ray S, Mullinax JE, Hari DM, Koizumi T, et
al: Liver label retaining cancer cells are relatively resistant to
the reported anti-cancer stem cell drug metformin. J Cancer.
7:1142–11451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xin HW, Ambe CM, Hari DM, Wiegand GW,
Miller TC, Chen JQ, Anderson AJ, Ray S, Mullinax JE, Koizumi T, et
al: Label-retaining liver cancer cells are relatively resistant to
sorafenib. Gut. 62:1777–1786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hari D, Xin HW, Jaiswal K, Wiegand G, Kim
BK, Ambe C, Burka D, Koizumi T, Ray S, Garfield S, et al: Isolation
of live label-retaining cells and cells undergoing asymmetric cell
division via nonrandom chromosomal cosegregation from human
cancers. Stem Cells Dev. 20:1649–1658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kiberstis PA: Micromanaging muscle cell
fusion. Science. 356:280–281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Liu Q, Luo K, Chen X, Xiao J,
Zhang C, Tao M, Zhao R and Liu S: Cell fusion as the formation
mechanism of unreduced gametes in the gynogenetic diploid hybrid
fish. Sci Rep. 6:316582016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mohler WA, Shemer G, del Campo JJ, Valansi
C, Opoku-Serebuoh E, Scranton V, Assaf N, White JG and Podbilewicz
B: The type I membrane protein EFF-1 is essential for developmental
cell fusion. Dev Cell. 2:355–362. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shemer G, Suissa M, Kolotuev I, Nguyen KC,
Hall DH and Podbilewicz B: EFF-1 is sufficient to initiate and
execute tissue-specific cell fusion in C. elegans. Curr Biol.
14:1587–1591. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Forkner CE: The origin and fate of two
types of multi-nucleated giant cells in the circulating blood. J
Exp Med. 52:279–297. 1930. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Enders JF and Peebles TC: Propagation in
tissue cultures of cytopathogenic agents from patients with
measles. Proc Soc Exp Biol Med. 86:277–286. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barski G: ‘Hybrid’ cell clones isolated
from mixed cell cultures. C R Hebd Seances Acad Sci. 253:1186–1188.
1961.(In French). PubMed/NCBI
|
|
14
|
Furusawa E and Cutting W: Loss of
neurotropic pathogenicity and hemagglutinating property of Columbia
SK virus by in vitro cultivation in sarcoma 180 ascites cells. Proc
Soc Exp Biol Med. 109:417–421. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cascardo MR and Karzon DT: Measles virus
giant cell induction factor (fusion factor). Virology. 26:311–325.
1965. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harris H and Watkins JF: Hybrid cells
derived from mouse and man: Artificial heterokaryons of mammalian
cells from different species. Nature. 205:640–646. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldenberg DM: On the progression of
malignancy: A hypothesis. Klin Wochenschr. 46:898–899. 1968.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Poste G: Virus-induced polykaryocytosis
and the mechanism of cell fusion. Adv Virus Res. 16:303–356. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldenberg DM, Pavia RA and Tsao MC: In
vivo hybridisation of human tumour and normal hamster cells.
Nature. 250:649–651. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klein PA, Xiang JH and Kimura AK: Melanoma
cells growing in aggregates on a non-adhesive poly(HEMA) substrate
exhibit polykaryocytosis but do not develop an increased metastatic
capability. Clin Exp Metastasis. 2:287–295. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gibson AJ, Karasinski J, Relvas J, Moss J,
Sherratt TG, Strong PN and Watt DJ: Dermal fibroblasts convert to a
myogenic lineage in mdx mouse muscle. J Cell Sci. 108:207–214.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goldenberg DM, Gold DV, Loo M, Liu D,
Chang CH and Jaffe ES: Horizontal transmission of malignancy:
In-vivo fusion of human lymphomas with hamster stroma produces
tumors retaining human genes and lymphoid pathology. PLoS One.
8:e553242013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kato K, Satouh Y, Nishimasu H, Kurabayashi
A, Morita J, Fujihara Y, Oji A, Ishitani R, Ikawa M and Nureki O:
Structural and functional insights into IZUMO1 recognition by JUNO
in mammalian fertilization. Nat Commun. 7:121982016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Smith JA, Hall AE and Rose MD: Membrane
curvature directs the localization of Cdc42p to novel foci required
for cell-cell fusion. J Cell Biol. 216:3971–3980. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Y, Zhang Y, Li WJ, Jiang Y, Zhu Z, Hu
H, Li W, Wu JW, Wang ZX, Dong MQ, et al: Spectraplakin induces
positive feedback between fusogens and the actin cytoskeleton to
promote cell-cell fusion. Dev Cell. 41:107–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Di Gioia SA, Connors S, Matsunami N,
Cannavino J, Rose MF, Gilette NM, Artoni P, de Macena Sobreira NL,
Chan WM, Webb BD, et al: A defect in myoblast fusion underlies
Carey-Fineman-Ziter syndrome. Nat Commun. 8:160772017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee JH, Hsieh CF, Liu HW, Chen CY, Wu SC,
Chen TW, Hsu CS, Liao YH, Yang CY, Shyu JF, et al: Lipid
raft-associated stomatin enhances cell fusion. FASEB J. 31:47–59.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mortensen K, Lichtenberg J, Thomsen PD and
Larsson LI: Spontaneous fusion between cancer cells and endothelial
cells. Cell Mol Life Sci. 61:2125–2131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song K, Song Y, Zhao XP, Shen H, Wang M,
Yan TL, Liu K and Shang ZJ: Oral cancer/endothelial cell fusion
experiences nuclear fusion and acquisition of enhanced survival
potential. Exp Cell Res. 328:156–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Raj AT, Kheur S, Patil VR and Gupta AA:
Assessing the role of cell fusion in cancer metastasis. Oral Oncol.
90:124–125. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song K, Zhu F, Zhang Hz and Shang Zj:
Tumor necrosis factor-α enhanced fusions between oral squamous cell
carcinoma cells andendothelial cells via VCAM-1/VLA-4 pathway. Exp
Cell Res. 318:1707–1715. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rappa G, Mercapide J and Lorico A:
Spontaneous formation of tumorigenic hybrids between breast cancer
and multipotent stromal cells is a source of tumor heterogeneity.
Am J Pathol. 180:2504–2515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang R, Chen S, Li C, Ng KTP, Kong Cw,
Cheng J, Cheng SH, Li RA, Lo CM, Man K and Sun D: Fusion with stem
cell makes the hepatocellular carcinoma cells similar to liver
tumor--initiating cells. BMC Cancer. 16:562016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Noubissi FK, Harkness T, Alexander CM and
Ogle BM: Apoptosis-induced cancer cell fusion: A mechanism of
breast cancer metastasis. FASEB J. 29:4036–4045. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Melzer C, von der Ohe J and Hass R:
Enhanced metastatic capacity of breast cancer cells after
interaction and hybrid formation with mesenchymal stroma/stem cells
(MSC). Cell Commun Signal. 16:22018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Melzer C, von der Ohe J and Hass R:
Involvement of actin cytoskeletal components in breast cancer cell
fusion with human mesenchymal stroma/stem-like cells. Int J Mol
Sci. 20:8762019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu MH, Gao X, Luo D, Zhou XD, Xiong W and
Liu GX: EMT and acquisition of stem cell-like properties are
involved in spontaneous formation of tumorigenic hybrids between
lung cancer and bone marrow-derived mesenchymal stem cells. PLoS
One. 9:e878932014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xue J, Zhu Y, Sun Z, Ji R, Zhang X, Xu W,
Yuan X, Zhang B, Yan Y, Yin L, et al: Tumorigenic hybrids between
mesenchymal stem cells and gastric cancer cells enhanced cancer
proliferation, migration and stemness. BMC Cancer. 15:7932015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun C, Zhao D, Dai X, Chen J, Rong X, Wang
H, Wang A, Li M, Dong J, Huang Q and Lan Q: Fusion of cancer stem
cells and mesenchymal stem cells contributes to glioma
neovascularization. Oncol Rep. 34:2022–2030. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang LN, Kong CF, Zhao D, Cong XL, Wang
SS, Ma L and Huang YH: Fusion with mesenchymal stem cells
differentially affects tumorigenic and metastatic abilities of lung
cancer cells. J Cell Physiol. 234:3570–3582. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li H, Feng Z, Tsang TC, Tang T, Jia X, He
X, Pennington ME, Badowski MS, Liu AKM, Chen D, et al: Fusion of
HepG2 cells with mesenchymal stem cells increases cancer associated
and malignant properties: An in vivo metastasis model. Oncol
Rep. 32:539–547. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yin L, Hu P, Shi X, Qian W, Zhau HE,
Pandol SJ, Lewis MS, Chung LWK and Wang R: Cancer cell's
neuroendocrine feature can be acquired through cell-cell fusion
during cancer-neural stem cell interaction. Sci Rep. 10:12162020.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang H, Yang L, Wang D, Zhang Q and Zhang
L: Pro-tumor activities of macrohpages in the progression of
melanoma. Hum Vaccin Immunother. 13:1556–1562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang LN, Huang YH and Zhao L: Fusion of
macrophages promotes breast cancer cell proliferation, migration
and invasion through activating epithelial-mesenchymal transition
and Wnt/β-catenin signaling pathway. Arch Biochem Biophys.
676:1081372019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ding J, Jin W, Chen C, Shao Z and Wu J:
Tumor associated macrophage × cancer cell hybrids may acquire
cancer stem cell properties in breast cancer. PLoS One.
7:e419422012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chakraborty AK, Pawelek J, Ikeda Y,
Miyoshi E, Kolesnikova N, Funasaka Y, Ichihashi M and Taniguchi N:
Fusion hybrids with macrophage and melanoma cells up-regulate
N-acetylglucosaminyltransferase V, beta1-6 branching, and
metastasis. Cell Growth Differ. 12:623–630. 2001.PubMed/NCBI
|
|
48
|
Kemény LV, Kurgyis Z, Buknicz T, Groma G,
Jakab A, Zänker K, Dittmar T, Kemény L and Németh IB: Melanoma
cells can adopt the phenotype of stromal fibroblasts and
macrophages by spontaneous cell fusion in vitro. Int J Mol Sci.
17:8262016. View Article : Google Scholar
|
|
49
|
Kurgyis Z, Kemény LV, Buknicz T, Groma G,
Oláh J, Jakab A, Polyánka H, Zänker K, Dittmar T, Kemény L and
Németh IB: Melanoma-Derived BRAF (V600E) mutation in peritumoral
stromal cells: Implications for in vivo cell fusion. Int J Mol Sci.
17:9802016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lindström A, Midtbö K, Arnesson LG, Garvin
S and Shabo I: Fusion between M2-macrophages and cancer cells
results in a subpopulation of radioresistant cells with enhanced
DNA-repair capacity. Oncotarget. 8:51370–51386. 2017. View Article : Google Scholar
|
|
51
|
He K, Qu H, Xu LN, Gao J, Cheng FY, Xiang
P and Zhou CQ: Epigenetics changes caused by the fusion of human
embryonic stem cell and ovarian cancer cells. Biosci Rep.
36:e003782016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang Y, Fan H, Zhou B, Ju Z, Yu L, Guo L,
Han J and Lu S: Fusion of human umbilical cord mesenchymal stem
cells with esophageal carcinoma cells inhibits the tumorigenicity
of esophageal carcinoma cells. Int J Oncol. 40:370–377.
2012.PubMed/NCBI
|
|
53
|
Fan H and Lu S: Fusion of human bone
hemopoietic stem cell with esophageal carcinoma cells didn't
generate esophageal cancer stem cell. Neoplasma. 61:540–545. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim TB, Park HK, Chang JH, Choi IH, Kim
KH, Yoon SJ, Lee MS, Jung H and Kim CS: The establishment of
dendritic cell-tumor fusion vaccines for hormone refractory
prostate cancer cell. Korean J Urol. 51:139–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yoo C, Do HA, Jeong IG, Park H, Hwang JJ,
Hong JH, Cho JS, Choo MS, Ahn H and Kim CS: Efficacy of dendritic
cells matured early with OK-432 (Picibanil), prostaglandin E2, and
interferon-alpha as a vaccine for a hormone refractory prostate
cancer cell line. J Korean Med Sci. 25:1284–1290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kawada M, Ikeda H, Takahashi T, AIshizu A,
Ishikura H, Katoh H and Yoshiki T: Vaccination of fusion cells of
rat dendritic and carcinoma cells prevents tumor growth in vivo.
Int J Cancer. 105:520–526. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Matsumoto S, Saito H, Tsujitani S and
Ikeguchi M: Allogeneic gastric cancer cell-dendritic cell hybrids
induce tumor antigen (carcinoembryonic antigen) specific CD8(+) T
cells. Cancer Immunol Immunother. 55:131–139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Koido S, Hara E, Homma S, Torii A, Toyama
Y, Kawahara H, Watanabe M, Yanaga K, Fujise K, Tajiri H, et al:
Dendritic cells fused with allogeneic colorectal cancer cell line
present multiple colorectal cancer-specific antigens and induce
antitumor immunity against autologous tumor cells. Clin Cancer Res.
11:7891–7900. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang K, Gao PF, Yu PW, Rao Y and Zhou LX:
Study on biological characters of SGC7901 gastric cancer
cell-dendritic cell fusion vaccines. World J Gastroenterol.
12:3438–3441. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Imura K, Ueda Y, Hayashi T, Itoh T,
Shimizu K, Tamai H, Yano Y, Naito K, Kohara J, Nakane K, et al:
Induction of cytotoxic T lymphocytes against human cancer cell
lines using dendritic cell-tumor cell hybrids generated by a newly
developed electrofusion technique. Int J Oncol. 29:531–539.
2006.PubMed/NCBI
|
|
61
|
Zhang Y, Ma B, Zhou Y, Zhang M, Qiu X, Sui
Y, Zhang X, Ma B and Fan Q: Dendritic cells fused with allogeneic
breast cancer cell line induce tumor antigen-specific CTL responses
against autologous breast cancer cells. Breast Cancer Res Treat.
105:277–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Koido S, Tanaka Y, Tajiri H and Gong J:
Generation and functional assessment of antigen-specific T cells
stimulated by fusions of dendritic cells and allogeneic breast
cancer cells. Vaccine. 25:2610–2619. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Serhal K, Baillou C, Ghinea N, Fontanges
P, Dupuy FP, Lemoine FM and Lacave R: Characteristics of hybrid
cells obtained by dendritic cell/tumour cell fusion in a T-47D
breast cancer cell line model indicate their potential as
anti-tumour vaccines. Int J Oncol. 31:1357–1365. 2007.PubMed/NCBI
|
|
64
|
Hu Z, Chen J, Zhou S, Yang N, Duan S,
Zhang Z, Su J, He J, Zhang Z, Lu X and Zhao Y: Mouse IP-10 gene
delivered by folate-modified chitosan nanoparticles and
dendritic/tumor cells fusion vaccine effectively inhibit the growth
of hepatocellular carcinoma in mice. Theranostics. 7:1942–1952.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dittmar T, Schwitalla S, Seidel J,
Haverkampf S, Reith G, Meyer-Staeckling S, Brandt BH, Niggemann B
and Zänker KS: Characterization of hybrid cells derived from
spontaneous fusion events between breast epithelial cells
exhibiting stem-like characteristics and breast cancer cells. Clin
Exp Metastasis. 28:75–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ozel C, Seidel J, Meyer-Staeckling S,
Brandt BH, Niggemann B, Zänker KS and Dittmar T: Hybrid cells
derived from breast epithelial cell/breast cancer cell fusion
events show a differential RAF-AKT crosstalk. Cell Commun Signal.
10:102012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gauck D, Keil S, Niggemann B, Zänker KS
and Dittmar T: Hybrid clone cells derived from human breast
epithelial cells and human breast cancer cells exhibit properties
of cancer stem/initiating cells. BMC Cancer. 17:5152017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Bhatia B, Multani AS, Patrawala L, Chen X,
Calhoun-Davis T, Zhou J, Schroeder L, Schneider-Broussard R, Shen
J, Pathak S, et al: Evidence that senescent human prostate
epithelial cells enhance tumorigenicity: Cell fusion as a potential
mechanism and inhibition by p16INK4a and hTERT. Int J Cancer.
122:1483–1495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kerbel RS, Lagarde AE, Dennis JW and
Donaghue TP: Spontaneous fusion in vivo between normal host and
tumor cells: Possible contribution to tumor progression and
metastasis studied with a lectin-resistant mutant tumor. Mol Cell
Biol. 3:523–538. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chakraborty AK, Sodi S, Rachkovsky M,
Kolesnikova N, Platt JT, Bolognia JL and Pawelek JM: A spontaneous
murine melanoma lung metastasis comprised of host × tumor hybrids.
Cancer Res. 60:2512–2519. 2000.PubMed/NCBI
|
|
71
|
Luo F, Liu T, Wang J, Li J, Ma P, Ding H,
Feng G, Lin D, Xu Y and Yang K: Bone marrow mesenchymal stem cells
participate in prostate carcinogenesis and promote growth of
prostate cancer by cell fusion in vivo. Oncotarget. 7:30924–30934.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sun C, Dai X, Zhao D, Wang H, Rong X,
Huang Q and Lan Q: Mesenchymal stem cells promote glioma
neovascularization in vivo by fusing with cancer stem cells. BMC
Cancer. 19:12402019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jacobsen BM, Harrell JC, Jedlicka P,
Borges VF, Varella-Garcia M and Horwitz KB: Spontaneous fusion
with, and transformation of mouse stroma by, malignant human breast
cancer epithelium. Cancer Res. 66:8274–8279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Martin-Padura I, Marighetti P, Gregato G,
Agliano A, Malazzi O, Mancuso P, Pruneri G, Viale A and Bertolini
F: Spontaneous cell fusion of acute leukemia cells and macrophages
observed in cells with leukemic potential. Neoplasia. 14:1057–1066.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chitwood CA, Dietzsch C, Jacobs G, McArdle
T, Freeman BT, Banga A, Noubissi FK and Ogle BM: Breast tumor cell
hybrids form spontaneously in vivo and contribute to breast tumor
metastases. APL Bioeng. 2:0319072018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pawelek JM and Chakraborty AK: The cancer
cell-leukocyte fusion theory of metastasis. Adv Cancer Res.
101:397–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Harkness T, Weaver BA, Alexander CM and
Ogle BM: Cell fusion in tumor development: Accelerated genetic
evolution. Crit Rev Oncog. 18:19–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chakraborty A, Lazova R, Davies S,
Bäckvall H, Ponten F, Brash D and Pawelek J: Donor DNA in a renal
cell carcinoma metastasis from a bone marrow transplant recipient.
Bone Marrow Transplant. 34:183–186. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yilmaz Y, Lazova R, Qumsiyeh M, Cooper D,
Pawelek J and Donor Y: Chromosome in renal carcinoma cells of a
female BMT recipient: Visualization of putative BMT-tumor hybrids
by FISH. Bone Marrow Transplant. 35:1021–1024. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lazova R, Laberge GS, Duvall E, Spoelstra
N, Klump V, Sznol M, Cooper D, Spritz RA, Chang JT and Pawelek JM:
A melanoma brain metastasis with a donor-patient hybrid genome
following bone marrow transplantation: First evidence for fusion in
human cancer. PLoS One. 8:e667312013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
LaBerge GS, Duvall E, Grasmick Z, Haedicke
K and Pawelek J: A melanoma lymphnode metastasis with a
donor-patient hybrid genome following bone marrow transplantation:
A second case of leucocyte-tumor cell hybridization in cancer
metastasis. PLoS One. 12:e01685812017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Andersen TL, Boissy P, Sondergaard TE,
Kupisiewicz K, Plesner T, Rasmussen T, Haaber J, Kølvraa S and
Delaissé JM: Osteoclast nuclei of myeloma patients show chromosome
translocations specific for the myeloma cell clone: A new type of
cancer-host partnership? J Pathol. 211:10–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Clawson GA, Matters GL, Xin P,
Imamura-Kawasawa Y, Du Z, Thiboutot DM, Helm KF, Neves RI and
Abraham T: Macrophage-tumor cell fusions from peripheral blood of
melanoma patients. PLoS One. 10:e01343202015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Melzer C, von der Ohe J and Hass R: In
vivo cell fusion between mesenchymal stroma/stem-like cells and
breast cancer cells. Cancers (Basel). 110:1852019. View Article : Google Scholar
|
|
85
|
Hong S, Zhang P, Zhang H, Jia L, Qu X,
Yang Q, Rong F and Kong B: Enforced effect of tk-MCP-1 fusion gene
in ovarian cancer. J Exp Clin Cancer Res. 31:742012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang D, Li B, Shi J, Zhao L, Zhang X,
Wang C, Hou S, Qian W, Kou G, Wang H and Guo Y: Suppression of
tumor growth and metastasis by simultaneously blocking vascular
endothelial growth factor (VEGF)-A and VEGF-C with a
receptor-immunoglobulin fusion protein. Cancer Res. 70:2495–2503.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Tammela T, Zarkada G, Nurmi H, Jakobsson
L, Heinolainen K, Tvorogov D, Zheng W, FrancoC A, Murtomäki A,
Aranda E, et al: VEGFR-3 controls tip to stalk conversion at vessel
fusion sites by reinforcing Notch signalling. Nat Cell Biol.
13:1202–1213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Liang AL, Qian HL, Zhang TT, Zhou N, Wang
HJ, Men XT, Qi W, Zhang PP, Fu M, Liang X, et al: Bifunctional
fused polypeptide inhibits the growth and metastasis of breast
cancer. Drug Des Devel Ther. 9:5671–5686. 2015.PubMed/NCBI
|
|
89
|
Beha N, Harder M, Ring S, Kontermann RE
and Müller D: IL15-based trifunctional antibody-fusion proteins
with costimulatory TNF-superfamily ligands in the single-chain
format for cancer immunotherapy. Mol Cancer Ther. 18:1278–1288.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Weiler J and Dittmar T: Minocycline
impairs TNF-α-induced cell fusion of M13SV1-Cre cells with
MDA-MB-435-pFDR1 cells by suppressing NF-κB transcriptional
activity and its induction of target-gene expression of
fusion-relevant factors. Cell Commun Signal. 17:712019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Goldenberg DM, Zagzag D, Heselmeyer-Haddad
KM, Garcia LYB, Ried T, Loo M, Chang CH and Gold DV: Horizontal
transmission and retention of malignancy, as well as functional
human genes, after spontaneous fusion of human glioblastoma and
hamster host cells in vivo. Int J Cancer. 131:49–58. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lee SH, Lee YP, Kim SY, Jeong MS, Lee MJ,
Kang HW, Jeong HJ, Kim DW, Sohn EJ, Jang SH, et al: Inhibition of
LPS-induced cyclooxygenase 2 and nitric oxide production by
transduced PEP-1-PTEN fusion protein in raw 264.7 macrophage cells.
Exp Mol Med. 40:629–638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wolf S, Haase-Kohn C, Lenk J, Hoppmann S,
Bergmann R, Steinbach J and Pietzsch J: Expression, purification
and fluorine-18 radiolabeling of recombinant S100A4: A potential
probe for molecular imaging of receptor for advanced glycation
endproducts in vivo? Amino Acids. 41:809–820. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu XQ, Xin HY, Lyu YN, Ma ZW, Peng XC,
Xiang Y, Wang YY, Wu ZJ, Cheng JT, Ji JF, et al: Oncolytic herpes
simplex virus tumor targeting and neutralization escape by
engineering viral envelope glycoproteins. Drug Deliv. 25:1950–1962.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Foo CH, Rootes CL, Cowley K, Marsh GA,
Gould CM, Deffrasnes C, Cowled CJ, Klein R, Riddell SJ and
Middleton D: Dual microRNA screens reveal that the
immune-responsive miR-181 promotes henipavirus entry and cell-cell
fusion. PLoS Pathog. 12:e10059742016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hu C, He Y, Liu D, Zhao L, Fang S, Tan B,
Dong S, Wang Y, He T and Bi Y: Hypoxia preconditioning promotes the
proliferation and migration of urine-derived stem cells in
chronically injured liver of mice by upregulating CXCR4. Stem Cells
Dev. 15:526–536. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Luo Y, Zhu D, Lam DH, Huang J, Tang Y, Luo
X and Wang S: A double-switch cell fusion-inducible transgene
expression system for neural stem cell-based antiglioma gene
therapy. Stem Cells Int. 2015:6490802015. View Article : Google Scholar : PubMed/NCBI
|