Introduction
Lung cancer is the most frequent cause of
cancer-associated mortality worldwide (1). Non-small cell lung cancer (NSCLC)
represents ~85% of all lung cancer types (2). Multiple genetic alterations have been
observed in patients with NSCLC, including mutations in the Kirsten
rat sarcoma (KRAS; 25–30%), epidermal growth factor receptor (EGFR;
10–35%), fibroblast growth factor (20%) and anaplastic lymphoma
kinase (5-7%) genes (3). The USA Food
and Drug Administration has approved numerous drugs such as
gefitinib and erlotinib against these mutated genes; however, the
5-year overall survival of patients with NSCLC remains low (~16%)
(3,4).
Therefore, there is an urgent need to identify novel oncogenes with
the aim of improving the survival of patients with NSCLC.
Heterogeneous nuclear ribonucleoprotein A2/B1
(hnRNPA2/B1) has two isoforms, A2 and B1, which are derived from
alternative splicing variants of the same gene (5). B1 has a 12-amino acid insertion at the
N-terminus of A2 (5). hnRNPA2/B1
serves major roles in RNA processing, splicing, transport and
stability through direct interaction with mRNAs (6). Previous studies have reported that
hnRNPA2/B1 has multiple functions in numerous cellular processes,
including proliferation (7),
metabolism (8), migration (9), survival and apoptosis (10). hnRNPA2/B1 also regulates the stability
of a number of downstream target genes such as CCR4-NOT
transcription complex subunit (CNOT)13, CNOT4 and CNOT6 through a
sequence-specific mRNA decay pathway (11). hnRNPA2B1 has also been reported to be
a novel nuclear DNA sensor that initiates antiviral innate immunity
(12). hnRNPA2/B1 is upregulated in
various types of human cancer, including lung (13), colon (14), breast (15), pancreatic (16), gastric (17,18), brain
(8,19)
and liver (20) cancer. hnRNPA2/B1
promotes pancreatic cancer progression by providing a link between
protein kinase B (AKT)/mammalian target of rapamycin and
KRAS-dependent mitogen-activated protein kinase
kinase/extracellular signal-regulated kinase (ERK) signaling
(21). hnRNPA2/B1 has been reported
to contribute to the tumorigenic potential of breast cancer cells
via signal transducer and activator of transcription 3 and ERK
signaling (22), although this has
been challenged by another study describing hnRNPA2/B1 as a
negative regulator of breast cancer metastasis (23). Since hnRNPB1 has been considered to be
an early detection marker for lung cancer (24), previous studies have demonstrated that
high expression levels of hnRNPA2/B1 lead to NSCLC through the
induction of cell proliferation and the epithelial-mesenchymal
transition (EMT) (25,26). However, the precise signaling
mechanism of the regulation of NSCLC growth and metastasis by
hnRNPA2/B1 remains elusive.
Our previous study demonstrated that hnRNPA2/B1
maintained the self-renewal and pluripotency of human embryonic
stem cells (hESCs) via the control of the G1/S phase
transition, and that hnRNPA2/B1 negatively regulated the EMT
process in hESCs (27). The present
study aimed to examine the effects of hnRNPA2/B1 on the survival,
number and migration of NSCLC cells, and to provide a mechanistic
insight into how hnRNPA2/B1 may regulate the survival, number and
migration of NSCLC cells.
Materials and methods
Cell culture
The human NSCLC cell lines NCI-H358, A549, NCI-H1703
and NCI-H460 were purchased from the Korean Cell Line Bank and
cultured according to the supplier's protocol. The 293T cell line
was obtained from the American Type Culture Collection and cultured
according to the supplier's protocol. Cell line authentication was
performed by short tandem repeat DNA profiling (http://cellbank.snu.ac.kr). NCI-H358, A549, NCI-H1703
and H460 cells were cultured in RPMI-1640 medium (Welgene, Inc.),
and 293T cells were cultured in DMEM (Welgene, Inc.). All cells
were supplemented with 10% fetal bovine serum (VWR International,
LLC) and an antibiotic-antimycotic (Welgene, Inc.). All cell lines
were cultured at 37°C in a humidified atmosphere containing 5%
CO2 and were monitored for mycoplasma contamination
monthly using a Mycoplasma PCR Detection Kit (Intron Biotechnology,
Inc.) according to the manufacturer's instructions. For anticancer
drug treatment, A549 cells were treated with dimethyl sulfoxide or
2 µM cisplatin (Sigma-Aldrich; Merck KGaA) in RPMI-1640 medium for
28 days. The medium was changed every 2 days. Well-isolated,
large-sized and propidium iodide (PI)-negative populations were
regarded as cisplatin-resistant cells in flow cytometric analysis.
All dead cells were washed out and remaining cells were subjected
to western blot analysis. Tumorsphere culture was performed using
A549 cells as previously described (28).
Flow cytometry
Flow cytometric analysis was performed as described
previously (29). To detect
apoptosis, cancer cells were analyzed with PI and Annexin V using
an Annexin V-FITC/PI Apoptosis kit (BD Biosciences) according to
the manufacturer's protocol. For cell cycle analysis, A549 cells
transfected with siCon or siA2B1 were analyzed with
bromodeoxyuridine (BrdU) as previously described (27). Briefly, A549 cells (1×106)
were treated with 30 mM BrdU (Thermo Fisher Scientific, Inc.) for 4
h at 48 h post-transfection. Detached cells were fixed in 70%
ethanol overnight at 4°C. The cells were subsequently suspended in
denaturation buffer (2 M HCl/0.5% Triton X-100) for 30 min and 0.1
M sodium borate for 2 min following washing. Then, the cells were
incubated with an Alexa 488-conjugated anti-BrdU antibody (1:200
dilution; cat. no. 364105; BioLegend, Inc.) for 30 min at room
temperature and incubated in an RNAase A (10 µg/ml) and PI (20
µg/ml) solution for 30 min at room temperature prior to flow
cytometric analysis.
Small interfering RNA (siRNA)
transfection
siRNA oligonucleotides targeting hnRNPA2/B1 (siA2B1)
were synthesized by Bioneer Corporation and used with AccuTarget™
Negative Control siRNA (Bioneer Corporation). The sequences of
siRNAs targeting human hnRNPA2/B1 were as follows: siA2B1 #1 sense,
5′-CGGUGGAAAUUUCGGACCTT-3′ and antisense,
5′-UGGUCCGAAUUUCCACCGTT-3′; #2 sense, 5′-CUGAAGUUGUUUAGGUUCUTT−3′
and antisense, 5′-AGAACCUAAACAACUUCAGTT−3′; and #3 sense,
5′-UGAGACAGUUUCUUAGCUUTT−3′ and antisense,
5′-AAGCTAAGAAACUGUCUCATT−3′. A549 cells (1.5×105) were
transfected with 100 nM control siRNA (siCon) or each siA2B1 using
Lipofectamine® RNAiMAX Transfection Reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The cells were incubated at 37°C for 48 h before
harvesting for analysis. For H460 cells, cells (1.5×105)
were transfected the second time at 36 h following the first
transfection and incubated for additional 36 h prior to subsequent
analysis.
Overexpression of hnRNPA2 in 293T
cells
The human hnRNPA2 gene was cloned into the
pCMV-Myc-DDK vector (OriGene Technologies, Inc.) with Myc and DDK
tags at the C-terminus. 293T (1×106) cells were
transiently transfected with pCMV-DDK or pCMV-hnRNPA2-Myc-DDK
plasmids (2 µg/well) in 6-well plates using polyethylenimine and
cultured at 37°C for 48 h following transfection. Transfected cells
were suspended in a lysis buffer [25 mM Tris-HCl (pH 7.5), 150 mM
NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 2 µg/ml
aprotinin, 100 µg/ml phenylmethylsulphonyl fluoride, 5 µg/ml
leupeptin, 1 mM NaF and 1 mM Na3VO4]. The
resulting cell lysates were centrifuged at 14,240 × g for 40 min at
4°C, collected and used for western blotting.
Clonogenic survival and migration
assays
siCon- or siA2B1-transfected A549 cells were
harvested at 2 days post-siRNA transfection. The number of viable
cells was determined by the trypan blue exclusion assay. The cells
(1×104) were plated in a 6-well plate with complete
medium. Following 7-day incubation, the cells were stained with
crystal violet (Sigma-Aldrich; Merck KGaA) at room temperature for
1 h, and the colonies were counted. Briefly, images of the plates
were captured by digital camera, and the number of colonies were
counted using CellCounter software (http://nghiaho.com/?page_id=1011). A cluster of ≥100
cells was counted as a colony. For the migration assays, A549 cells
were suspended in serum-free medium (2×104 cells/ml) and
placed in the upper chamber of Transwell inserts (Corning, Inc.),
whereas RPMI1640 containing 10% fetal bovine serum was placed in
the lower chamber. Following incubation at 37°C for 24 h, the cells
on the upper surface of the membrane were removed using cotton
swab. The cells on the lower surface of the membrane were fixed
with 4% paraformaldehyde at room temperature for 1 h. Crystal
violet staining was performed at room temperature for 1 h, and
cells were counted under a light microscope at ×100 magnification
in four sections per sample and three samples per group.
Sphere formation assay
siCon- or siA2B1-transfected A549 cells were
harvested at 24 h post-siRNA transfection. Cells (1×104)
were resuspended in TeSR™-E8™ medium (Stemcell Technologies, Inc.)
and seeded in low-attachment 6-well plates (Corning, Inc.). The
spheres were collected and counted under a light microscope at ×200
magnification after 5 days of culture.
Reverse transcription-quantitative PCR
(RT-qPCR)
The relative expression values of EMT-associated
markers were analyzed by RT-qPCR using the 2−ΔΔCq method
as previously described (30,31). Total RNAs were extracted from H358,
A549 and H1703 cells using the RNAiso Plus (Takara Bio, Inc.)
according to the manufacturer's protocol. cDNAs were generated from
total RNAs using 500 ng total RNAs and the PrimeScript RT Master
Mix (Takara Bio, Inc.) at 37°C for 15 min and 94°C for 5 sec. qPCR
was performed using Applied StepOne™ Real-Time PCR System with
SYBR®-Green (Thermo Fisher Scientific, Inc.). The
standard reaction contained 12.5 µl 2X PCR buffer
(PowerUP™-SYBR™-Green Master Mix; Thermo Fisher Scientific, Inc.),
2 µl cDNA template, and 10 pM of forward and reverse primers in a
total volume of 25 µl. The thermocycling conditions were 30 cycles
of 1 min at 95°C, 45 sec at 45–56°C and 1 min at 72°C. The target
gene-specific primers for human transcripts encoding E-cadherin,
hnRNPA2/B1, Snail, Twist, zinc finger E-box-binding homeobox 1
(ZEB1), human double minute 2 protein (HDM2) and GAPDH are
presented in Table S1. Each sample
was analyzed in triplicate.
Western blotting
A549 cells were lysed in lysis buffer at 4°C for 30
min. The protein concentration of each sample was determined by
bicinchoninic acid protein assay kit. Equal amounts of protein (60
µg) from each sample were separated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. The proteins were
transferred to nitrocellulose membranes, blocked with Tris-buffered
saline with 0.1% Tween-20 (TBST) with 5% skimmed milk for 1 h at
room temperature or TBST with 5% bovine serum albumin (MP
Biomedicals, Inc.) for phosphoproteins. The membranes were then
incubated with specific primary antibodies diluted to 1:1,000 in 5%
skimmed milk overnight at 4°C, followed by incubation with a
secondary antibody for 1 h at room temperature. β-actin (cat. no.
sc-84322, Santa Cruz Biotechnology, Inc.) or GAPDH (cat. no.
CSB-PA00025A0Rb; Cusabio Technology, LLC) antibodies were used as
the loading controls. HDM2 (cat. no. sc-965), cyclin-dependent
kinase (CDK)2 (cat. no. sc-6248), p21 (cat. no. sc-6246), glycogen
synthase kinase (GSK)-3β (cat. no. sc-7291), ERK1/2 (cat. no.
sc-514302), EGFR (cat. no. sc-1005), vimentin (cat. no. sc-5565),
Snail (cat. no. sc-28199), ZEB1 (cat. no. sc-25388), E-cadherin
(cat. no. sc-7870), phosphorylated (p)-CDK2/cell division cycle
(Cdc) 2 (T14/Y15; cat. no. sc-163), cyclin E (cat. no. sc-481),
Cdc25A (cat. no. sc-97), p27 (cat. no. sc-528), AKT1 (cat. no.
sc-1618), β-catenin (cat. no. sc-7199) and p-AKT1/2/3 (S473; cat.
no. sc-7985) primary antibodies were purchased from Santa Cruz
Biotechnology, Inc. FLAG antibody (cat. no. M185-3L) was purchased
from MBL International Co. Cyclin D1 (cat. no. 2978S), p-CDK2
(T160; cat. no. 2561S), p53 (cat. no. 2524S), p-p53 (S15; cat. no.
9284S), p-p53 (S20; cat. no. 9287S), p-HDM2 (S166; cat. no. 3521S),
p-GSK-3β (S9; cat. no. 9322S) and p-ERK1/2 (T202/Y204; cat. no.
9101S) primary antibodies were purchased from Cell Signaling
Technology, Inc. C-X-C chemokine receptor type 4 (CXCR4) primary
antibody (cat. no. CSB-PA006254GA01HU) was purchased from Cusabio
Technology, LLC. Major vault protein (MVP) antibody (cat. no.
OAAB16556) was purchased from Aviva Systems Biology Corp. Goat
anti-mouse IgG-HRP (1:5,000; cat. no. AP-124P) was purchased from
MilliporeSigma. Goat-anti-rabbit IgG-HRP (1:10,000; cat. no.
ab6721) was purchased from Abcam. Immunostained protein bands were
detected with an enhanced chemiluminescence kit (Advansta, Inc.).
Quantifications of Western Blots were performed using ImageJ
software (Version 1.8.0; National Institutes of Health).
Statistical analysis
Data are presented as the mean ± SD (n=3). The
unpaired Student's t-test was used to analyze the differences
between two populations. To compare the expression of five genes in
three cell lines, the non-parametric Kruskal-Wallis test was used,
followed by post hoc testing using the Mann-Whitney U test with the
Bonferroni correction. Analyses were performed with Stata/SE
version 13.1 (StataCorp, LLC.). P<0.05 was considered to
indicate a statistically significant difference.
Results
hnRNPA2/B1 drives the EMT, drug
resistance and cancer stemness in NSCLC cells
Previous studies have suggested that hnRNPA2/B1
regulates the stemness and EMT process of hESCs and NSCLC cells
(26,27). Among the three NSCLC cell lines
examined in the present study (NCI-H358, A549 and NCI-H1703),
EMT-associated markers were expressed at intermediate levels in
A549 cells (Fig. S1). The levels of
hnRNPA2/B1 were also detected at an intermediate level in A549
cells (Fig. S1), suggesting that the
role of hnRNPA2/B1 may be representative in A549 cells. hnRNPA2/B1
expression was also induced in the sphere cultures of A549 cells
(Fig. 1A), suggesting that it may be
induced during detachment stress. To determine whether hnRNPA2/B1
expression may be associated with NSCLC survival under stress
conditions, the expression levels of hnRNPA2/B1 were examined for
28 days in A549 cells under cisplatin treatment as previously
described (32). Cisplatin treatment
markedly induced cell death (Fig.
1B). When the proteins from the surviving cells were analyzed
by western blotting, the levels of mesenchymal markers Snail and
ZEB1 were upregulated, whereas the levels of the epithelial marker
E-cadherin were downregulated compared with those on day 0
(Fig. 1C). The levels of drug
resistance-associated markers MVP, CXCR4 and EGFR were also
upregulated 3 days after cisplatin treatment compared with those on
day 0 (Fig. 1C). Under cisplatin
treatment, hnRNPA2/B1 expression levels were also upregulated in
the surviving cells (Fig. 1C). These
results suggest that hnRNPA2/B1 expression may be induced in a
stressful environment.
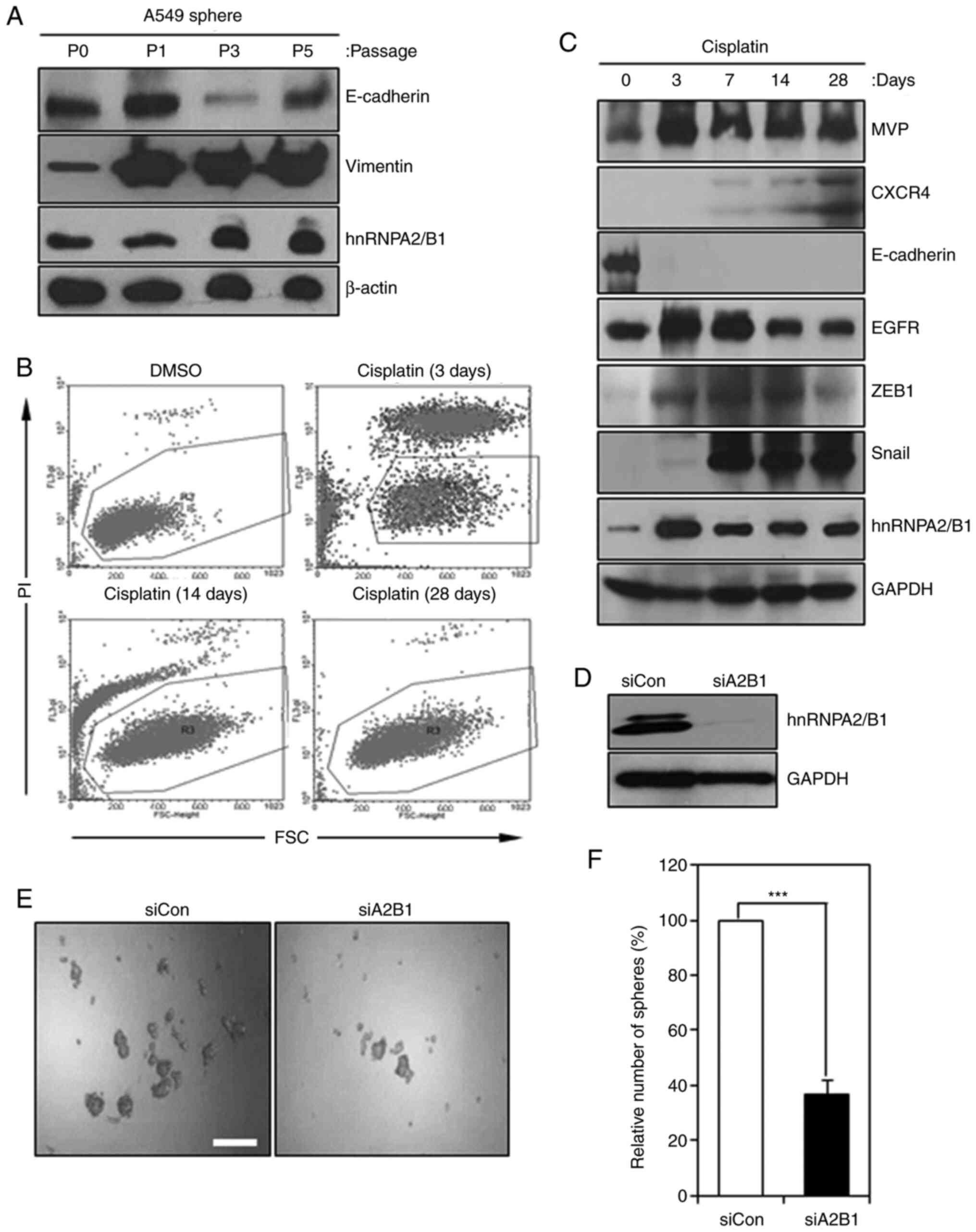 | Figure 1.hnRNPA2/B1 regulates the
epithelial-mesenchymal transition, drug resistance and cancer
stemness in non-small cell lung cancer cells. (A) hnRNPA2/B1
expression was induced in sphere cells of A549. Cancer cells were
cultured in sphere medium and subjected to western blot analysis to
detect the expression levels of vimentin, E-cadherin and
hnRNPA2/B1. β-actin was used as an internal control. (B and C)
hnRNPA2/B1 expression was induced in cisplatin-treated A549 cells.
A549 cells were treated with cisplatin for 28 days. (B) PI-negative
cells (gated) were harvested and subjected to (C) western blot
analysis to detect the expression levels of MVP, CXCR4, E-cadherin,
EGFR, ZEB1, Snail and hnRNPA2/B1. GAPDH was used as an internal
control. (D) Knockdown of hnRNPA2/B1 in A549 cells. Cells were
transfected with siCon or siA2B1. Knockdown efficiency was
determined by western blot analysis. (E) The sphere formation
assays of control siCon- or siA2B1-transfected A549 cells. siCon-
or siA2B1-transfected A549 cells were harvested after 24 h of siRNA
transfection and were subjected to the sphere forming assays for 5
days. Scale bar, 200 µm. (F) Statistical analysis of the data in E.
***P<0.005. siCon, control small interfering RNA; hnRNPA2/B1,
heterogeneous nuclear ribonucleoprotein A2/B1; siA2B1, small
interfering RNA targeting hnRNPA2B1; PI, propidium iodide; MVP,
major vault protein; CXC4, C-X-C chemokine receptor type 4; ZEB1,
zinc finger E-box-binding homeobox 1; FSC, forward scatter. |
Cancer stemness is associated with EMT and
anticancer drug resistance, and the sphere forming assay has been
previously used to enrich potential cancer stem cells in
vitro (28,33). To further analyze whether hnRNPA2/B1
may regulate the stemness of A549 cells, the effects of hnRNPA2/B1
knockdown by RNA interference on the sphere formation ability of
A549 cells were evaluated. The sphere forming capacity of
hnRNPA2/B1-knockdown A549 cells was decreased by ~63% compared with
that of the control A549 cells (Fig.
1D-F), suggesting that hnRNPA2/B1 may regulate the stemness of
A549 cells. Taken together, these results suggested that hnRNPA2/B1
may drive the EMT, drug resistance and cancer stemness in NSCLC
cells.
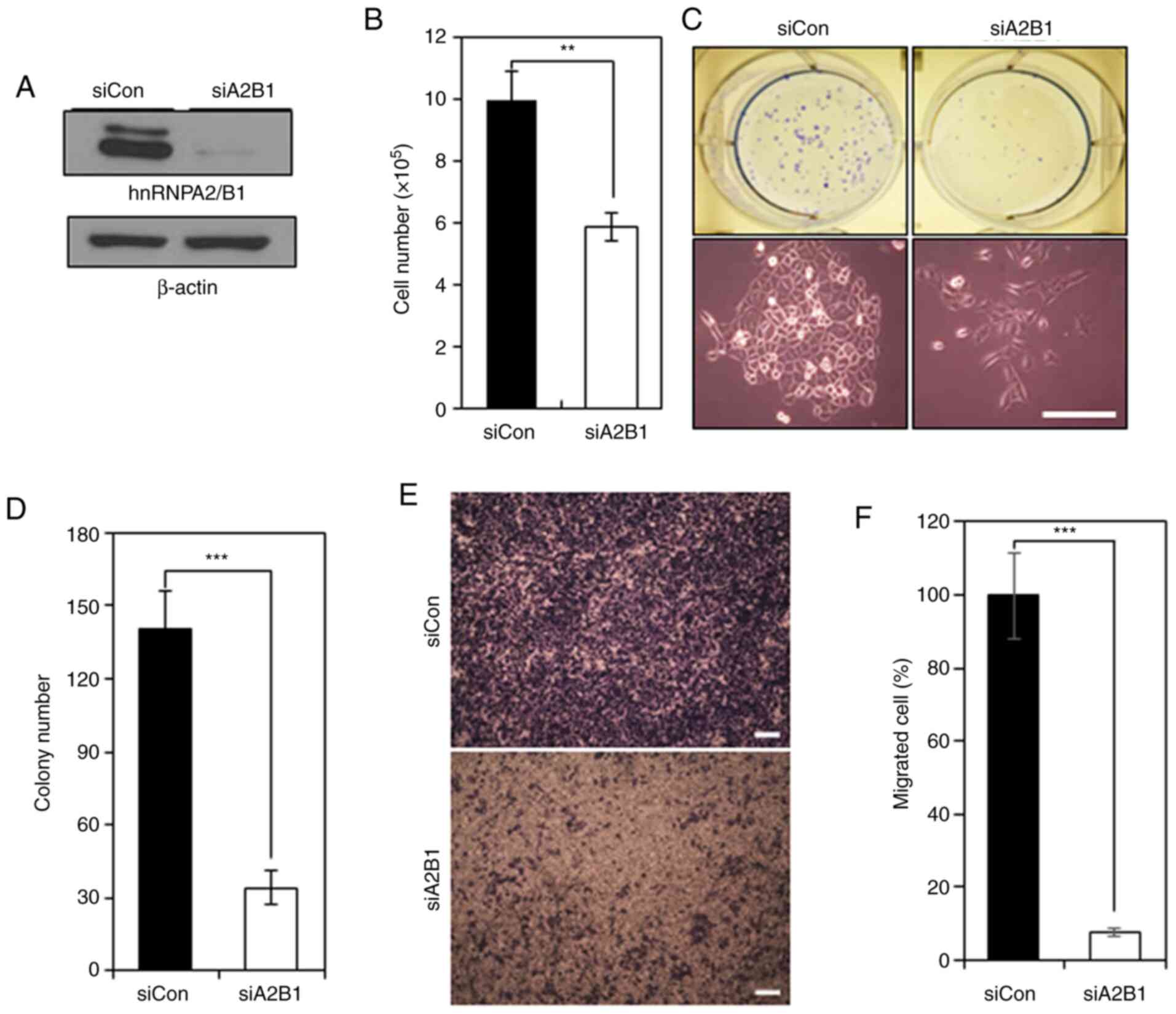
hnRNPA2/B1 is required for the
survival, proliferation and migration of NSCLC cells
To determine the role of hnRNPA2/B1 in NSCLC cells,
hnRNPA2/B1 was knocked down in A549 cells by RNA interference.
hnRNPA2/B1 expression was detected at low levels by western blot
analysis in A549 cells following hnRNPA2/B1 knockdown (Fig. 2A). hnRNPA2/B1 knockdown decreased the
number of A549 cells by ~41% compared with that in the control
group (Fig. 2B). hnRNPA2/B1 knockdown
decreased the number of A549 colonies by ~80% in the clonogenic
assay compared with that in the control group (Fig. 2C and D). However, the number of
Annexin V-positive cells was not altered in A549 cells following
hnRNPA2/B1 knockdown (Fig. S2),
suggesting that hnRNPA2/B1 may promote the clonogenic survival of
NSCLC cells irrespective of apoptosis. The migratory potential of
A549 cells was decreased by ~92% following hnRNPA2/B1 knockdown
compared with that in the control group (Fig. 2E and F). In addition, hnRNPA2/B1
knockdown decreased the expression levels of the mesenchymal marker
vimentin and increased the levels of the epithelial marker
E-cadherin compared with those in the control group (Fig. S3). Thus, hnRNPA2/B1 may positively
regulate the survival, proliferation and migration of NSCLC
cells.
hnRNPA2/B1 knockdown induces
G0/G1 phase arrest in NSCLC cells
Previous studies have reported that hnRNPA2/B1
promotes G1/S transition in carcinoma cells and hESCs
(7,27,34). The
results of the present study demonstrated that hnRNPA2/B1 knockdown
induced a substantial accumulation of A549 cells in the
G0/G1 phase compared with that of the control
cells; the proportion of hnRNPA2/B1-knockdown A549 cells in the
G0/G1 phase was increased by ~17%, whereas
the proportion of hnRNPA2/B1-knockdown A549 cells in the S phase
was decreased by ~26% (Fig. 3A and
B). Thus, hnRNPA2/B1 knockdown may inhibit A549 cell
proliferation by blocking the cell cycle progression from the
G1 to the S phase.
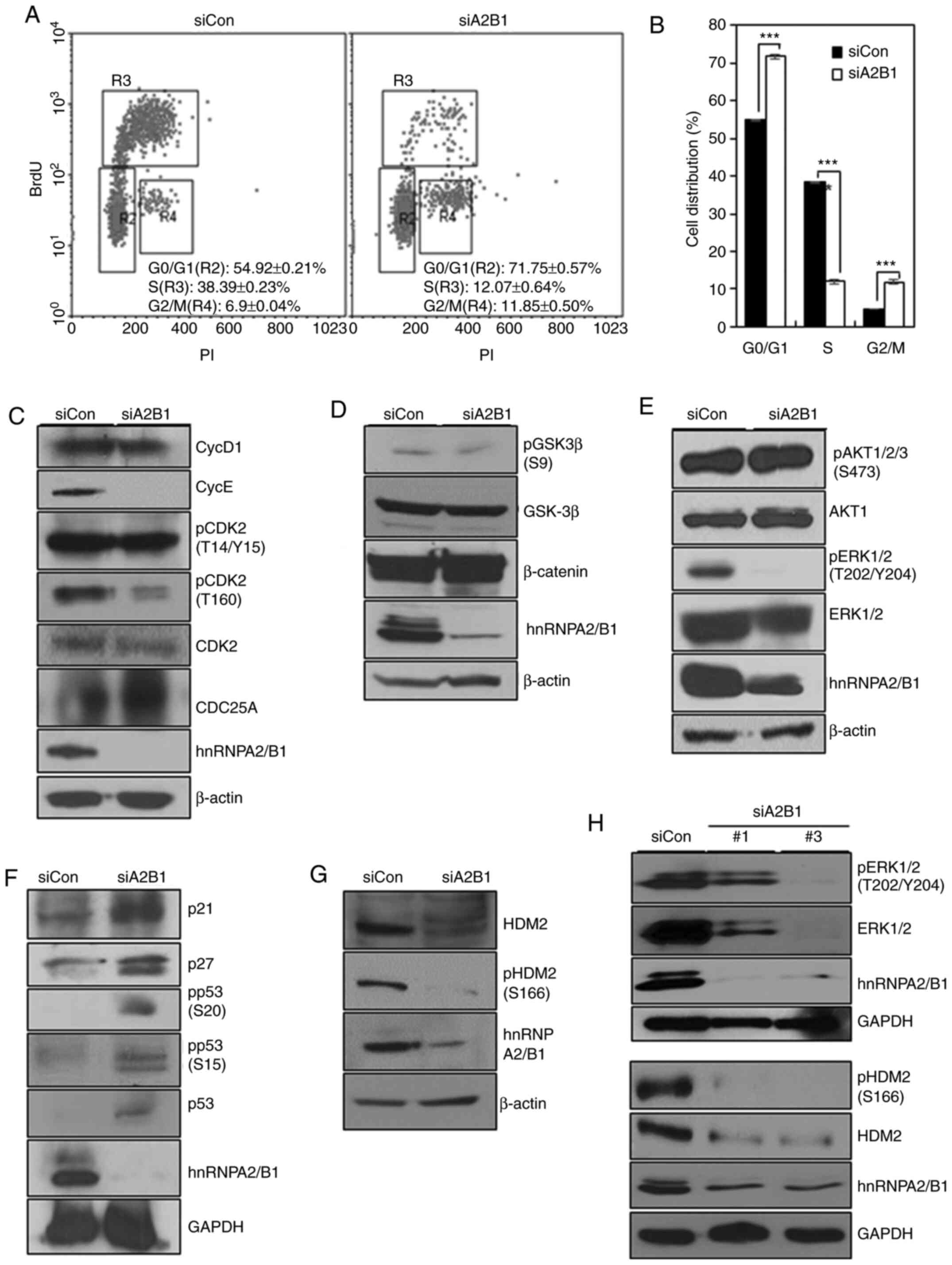 | Figure 3.hnRNPA2/B1 promotes the
G1/S phase transition through ERK signaling, suppresses
expression of p21 and p27, and prevents p53 activation via HDM2.
(A) Cell cycle analysis of BrdU incorporation of siCon- or
siA2B1-transfected A549 cells. (B) Statistical analysis of the data
presented in A. ***P<0.005. (C-H) Western blot analysis of the
protein expression levels of (C) cyclins, CDK2, p-CDK2 and Cdc25A,
(D) GSK-3β, p-GSK-3β and β-catenin, (E) Akt1, p-Akt1/2/3, ERK1/2
and p-ERK1/2, (F) p21, p27, p53 and p-p53, (G) HDM2 and p-HDM2, and
(H) ERK1/2, p-ERK1/2 (upper panels), HDM2 and p-HDM2 (lower panels)
in siCon- or siA2B1-transfected A549 cells. Two different siRNAs
(#1 and #3) against hnRNPA2/B1 were used in H. siCon, control small
interfering RNA; hnRNPA2/B1, heterogeneous nuclear
ribonucleoprotein A2/B1; siA2B1, small interfering RNA targeting
hnRNPA2B1; BrdU, bromodeoxyuridine; PI, propidium iodide; p-,
phosphorylated; Cdc25A, cell division cycle 25A; GSK-3β, glycogen
synthase kinase 3β. |
To examine the mechanism by which hnRNPA2/B1
promoted G1/S transition in NSCLC cells, cyclin
expression levels were analyzed in hnRNPA2/B1-knockdown A549 cells
by western blotting. Cyclin D1 expression levels were slightly
decreased, and cyclin E expression levels were markedly reduced in
A549 cells following hnRNPA2/B1 knockdown compared with those in
the control cells (Fig. 3C). Although
CDK2 dephosphorylation at T14/Y15 or Cdc25A degradation were not
altered, the levels of CDK2 phosphorylation at T160 were decreased
in A549 cells following hnRNPA2/B1 knockdown compared with those in
the control group (Fig. 3C). These
results suggested that cyclin E degradation and decreased
phosphorylation of CDK2 at T160 may be associated with the
G0/G1 phase arrest in NSCLC cells following
hnRNPA2/B1 knockdown.
hnRNPA2/B1 promotes G1/S
transition through ERK signaling
To determine how hnRNPA2/B1 regulated
G1/S transition in NSCLC cells, the present study
examined the GSK-3β/β-catenin, PI3K/AKT and MEK/ERK signaling
pathways. The levels of inhibitory phosphorylation of GSK-3β at S9
or β-catenin expression were not altered in hnRNPA2/B1-knockdown
A549 cells compared with those in the control group (Fig. 3D), suggesting that β-catenin signaling
was not involved in the G1/S transition of A549 cells
following hnRNPA2/B1 knockdown. Although hnRNPA2/B1 knockdown
decreases AKT signaling in pancreatic, cervical cancer cells and
hESCs (21,27,35), it
did not cause a significant change in AKT1/2/3 phosphorylation in
A549 cells (Fig. 3E). However,
hnRNPA2/B1 knockdown abolished ERK1/2 phosphorylation in A549 cells
compared with that in the control group (Fig. 3E). Taken together, these results
suggested that hnRNPA2/B1 may regulate G1/S transition
in NSCLC cells through the ERK signaling pathway.
hnRNPA2/B1 suppresses the expression
of p21 and p27, and prevents p53 activity via increased stability
and activation of HDM2
The transition from G1 into S phase is
also regulated by CDK inhibitors (36). In the present study, hnRNPA2/B1
knockdown increased the expression levels of p21 and p27 in A549
cells compared with those in the control group (Fig. 3F), indicating that hnRNPA2/B1 may
promote the G1/S transition in NSCLC cells through the
inhibition of p21 and p27 expression. Our previous studies reported
that hnRNPA2/B1 knockdown increased p53 expression and
phosphorylation at S15, suggesting that hnRNPA2/B1 also regulated
the G1/S phase transition by controlling p53 activity
(27). In the present study,
increased expression levels of p53 was detected in A549 cells
following hnRNPA2/B1 knockdown compared with those in the control
group, and the levels of phosphorylation of p53 at S15 and S20 were
also increased (Fig. 3F). N-terminal
phosphorylation of p53 at S15 and S20 reduces the affinity of p53
for its negative regulator HDM2, and it recruits the
transcriptional coactivators CBP/p300, leading to p53 stability and
activation; AKT and ERK1/2 physically associate with HDM2,
phosphorylate it at S166, and increase p53 degradation (37). hnRNPA2/B1 knockdown decreased the
stability of HDM2 in A549 cells and reduced the levels of HDM2
phosphorylation at S166 compared with those in the control group
(Fig. 3G). These results suggested
that the increased activation and stability of p53 protein may be
due to the decreased activation of HDM2 caused by reduced ERK
signaling activity (Fig. 3E-G).
Therefore, hnRNPA2/B1 knockdown may induce the
G0/G1 phase arrest through the activation of
p53, which is in turn induced by HDM2 inactivation.
To exclude the potential off-target effects of
siRNAs, hnRNPA2/B1 was also knocked down using two additional
siRNAs (Fig. S4), and the expression
and phosphorylation levels of ERK1/2 were decreased in A549 cells
with hnRNPA2/B1 knockdown using two siRNAs (Fig. 3H upper panels). The expression and
phosphorylation levels of HDM2 were also decreased in A549 cells
following hnRNPA2/B1 knockdown compared with those in the control
group (Fig. 3H, lower panels),
suggesting that the decreased activation of HDM2 was caused by
reduced activity of ERK signaling in hnRNPA2/B1-knockdown A549
cells.
To further examine the knockdown effects of
hnRNPA2/B1 on another NSCLC cell line, hnRNPA2/B1 was also depleted
in NCI-H460 cells. The expression and phosphorylation levels of ERK
and HDM2 tended to decrease in NCI-H460 cells following hnRNPA2/B1
knockdown compared with those in the control group, although the
knockdown efficiency of hnRNPA2/B1 was low in these cells (Fig. S5). Next, the mRNA expression level of
HDM2 in hnRNPA2/B1-knockdown A549 cells was analyzed by qPCR.
However, the mRNA expression levels of HDM2 were not significantly
changed in hnRNPA2/B1-knockdown cells compared with those in the
control A549 cells (Fig. S6),
suggesting that the effects of the hnRNPA2/B1-ERK-HDM2/p53
regulatory pathway were not mediated through the regulation of HDM2
mRNA transcription.
To further demonstrate whether hnRNPA2/B1 regulated
the G1/S phase transition through the suppression of p53
activity and the activation of HDM2, the effects of the
overexpression of hnRNPA2 on p53 and HDM2 were examined in 293T
cells. The overexpression of hnRNPA2 induced the dephosphorylation
of p53 at S15 and the phosphorylation of HDM2 at S166, which was
opposite to the effects of hnRNPA2/B1 knockdown (Fig. 4A). Taken together, these suggested
that hnRNPA2/B1 may promote the G1/S phase transition
through the suppression of p53 activity, which in turn is induced
by ERK-mediated HDM2 activation.
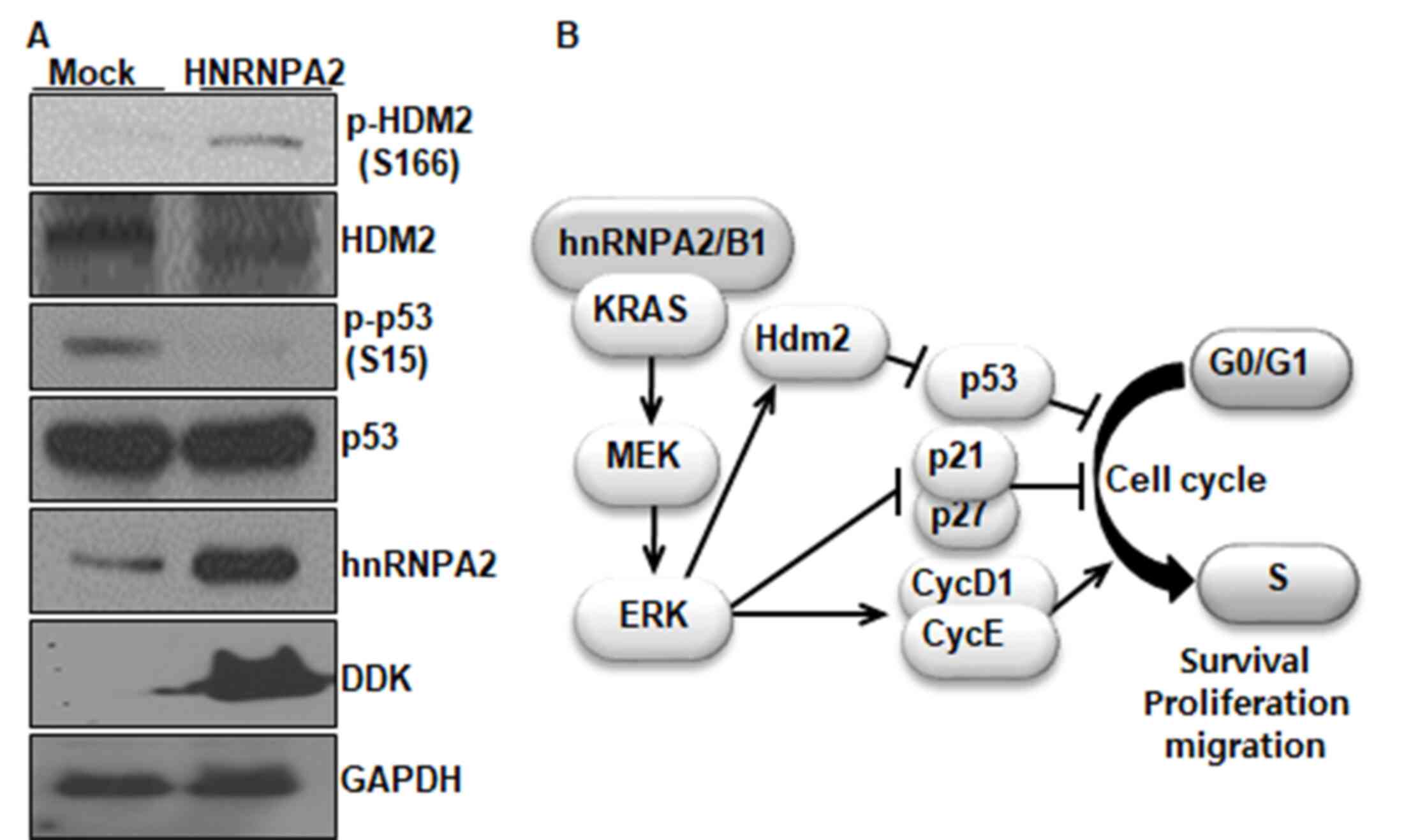 | Figure 4.Overexpression of hnRNPA2/B1
increases clonogenic survival and suppresses p53 activity via HDM2
activation in 293T cells. (A) Western blot analysis of the
expression levels of HDM2, p-HDM2, p53, p-p53, hnRNPA2 and DDK in
293T cells overexpressing DDK-tagged hnRNPA2. GAPDH was used as an
internal control. (B) The proposed model for the role of hnRNPA2/B1
in NSCLC cells. hnRNPA2/B1 is required for the survival,
proliferation and migration of NSCLC cells. hnRNPA2/B1 promotes the
G1/S phase transition via the ERK signaling pathway,
which induces cyclin D1 and E expression and inhibits that of p21
and p27. hnRNPA2/B1 also prevents p53 activity via ERK-mediated
HDM2 activation. hnRNPA2, heterogeneous nuclear ribonucleoprotein
A2; p-, phosphorylated; hnRNPA2/B1, heterogeneous nuclear
ribonucleoprotein A2/B1; NSCLC, non-small cell lung cancer; HDM2,
human double minute 2 protein; DDK, DBF4-dependent kinase. |
Discussion
hnRNPA2/B1 is associated with carcinogenesis through
its interactions with various proteins; however, its interaction
with p53 remains unclear. A549 cells exhibit wild-type p53, but
harbor a mutation in the KRAS gene (38). In our previous study, hnRNPA2/B1
knockdown was demonstrates to induce p53 expression and activation
in hESCs (27). In the present study,
hnRNPA2/B1 knockdown also induced p53 expression and activation in
A549 cells. Furthermore, hnRNPA2/B1 knockdown resulted in decreased
stability and phosphorylation of HDM2 in A549 cells compared with
that in the control group. These results were also supported by the
opposite effects observed following hnRNPA2 overexpression. Thus,
hnRNPA2/B1 may regulate the survival, proliferation and migration
of NSCLC cells via the p53/HDM2 pathway. Previous studies have
reported that hnRNPA2/B1 positively regulates AKT signaling in
pancreatic and cervical cancer cells (21,35).
However, the results of the present study demonstrated that
hnRNPA2/B1 expression was not involved in AKT signaling in A549
cells. Another study also revealed that AKT signaling is not
associated with hnRNPA2/B1 expression in A549 cells (39). Therefore, it was concluded that the
reduced phosphorylation of HDM2 at S166 was due to the decreased
phosphorylation of ERK1/2 induced by hnRNPA2/B1 knockdown,
suggesting that hnRNPA2/B1 may promote the survival, proliferation
and migration of NSCLC cells by preventing p53 activation, which is
induced by ERK-mediated HDM2 activation. This phenomenon was not
mediated through the regulation of HDM2 mRNA levels, suggesting
that hnRNPA2/B1 may regulate the activity of HDM2 at the
posttranscriptional level. A proposed model for the role of
hnRNPA2/B1 in NSCLC cells is presented in Fig. 4B. Further studies on different NSCLC
cell lines should be performed to verify the proposed model. The
present results also suggested that the hnRNPA2/B1/ERK/p53/HDM2
pathway may contain novel potential molecular targets for the
treatment of patients with NSCLC who exhibit wild-type p53 and
mutated KRAS.
Our previous study reported that hnRNPA2/B1 was
required for maintaining the epithelial phenotypes of hESCs
(27), suggesting that hnRNPA2/B1 may
inhibit the EMT in hESCs. A recent study has also reported that
hnRNPA2/B1 serves a role as a negative regulator in breast cancer
metastasis (23). By contrast, in
pancreatic cancer, hepatocellular carcinoma, prostate cancer and
NSCLC, hnRNPA2/B1 promotes the EMT and metastatic phenotypes
(26,40–42). In
the present study, hnRNPA2/B1 knockdown resulted in decreased
expression levels of vimentin, increased levels of E-cadherin and
reduced migration in NSCLC cells compared with those in the control
group, which was consistent with the results of the aforementioned
previous studies (26,40–42). Thus,
hnRNPA2/B1 exerts contradictory effects on cell proliferation, EMT,
invasion and migration. hnRNPA2/B1 serves as a key regulator of a
number of target genes and signaling pathways (6), such as the KRAS (21), PI3K/Akt (21) and STAT/ERK1/2 (22,40)
signaling pathways. Therefore, the different effects of hnRNPA2/B1
may be due to combined effects of the hnRNPA2/B1 downstream genes
and signaling pathways under different culture conditions and in
different cell lines. The detailed mechanism by which hnRNPA2/B1
serves different roles in cell proliferation, EMT, migration and
invasion remains elusive.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Hee Chul
Lee (College of Staten Island, New York, NY, USA) and Professor
Jeong-Hee Yang (Seoul National University Hospital, Seoul, Korea)
for their comments and proofreading the manuscript.
Funding
This study was supported by the National Research
Foundation of Korea (grant nos. 2018M2A2B3A02072345 and
2013R1A1A2012056).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MKK and CJR conceived and designed the study. MJC
contributed to the production of this manuscript following the peer
review. HML and HSC acquired and analyzed the data. CJR wrote the
manuscript. MKK revised the manuscript. YKP contributed to the
statistical analysis. MKK and CJR confirmed the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HDM2
|
human double minute 2 protein
|
|
hnRNPA2/B1
|
heterogeneous nuclear
ribonucleoprotein A2/B1
|
|
NSCLC
|
non-small cell lung carcinoma
|
|
hESCs
|
human embryonic stem cells
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Planchard D, Popat S, Kerr K, Novello S,
Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD,
et al: Metastatic non-small cell lung cancer: ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 29:iv192–iv237. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aran V and Omerovic J: Current approaches
in NSCLC targeting K-RAS and EGFR. Int J Mol Sci. 20:57012019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kozu T, Henrich B and Schafer KP:
Structure and expression of the gene (HNRPA2B1) encoding the human
hnRNP protein A2/B1. Genomics. 25:365–371. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goodarzi H, Najafabadi HS, Oikonomou P,
Greco TM, Fish L, Salavati R, Cristea IM and Tavazoie S: Systematic
discovery of structural elements governing stability of mammalian
messenger RNAs. Nature. 485:264–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He Y, Rothnagel JA, Epis MR, Leedman PJ
and Smith R: Downstream targets of heterogeneous nuclear
ribonucleoprotein A2 mediate cell proliferation. Mol Carcinog.
48:167–179. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
David CJ, Chen M, Assanah M, Canoll P and
Manley JL: HnRNP proteins controlled by c-Myc deregulate pyruvate
kinase mRNA splicing in cancer. Nature. 463:364–368. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moran-Jones K, Grindlay J, Jones M, Smith
R and Norman JC: hnRNP A2 regulates alternative mRNA splicing of
TP53INP2 to control invasive cell migration. Cancer Res.
69:9219–9227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Villarroya-Beltri C, Gutierrez-Vazquez C,
Sanchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N,
Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M and
Sánchez-Madrid F: Sumoylated hnRNPA2B1 controls the sorting of
miRNAs into exosomes through binding to specific motifs. Nat
Commun. 4:29802013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Geissler R, Simkin A, Floss D, Patel R,
Fogarty EA, Scheller J and Grimson A: A widespread
sequence-specific mRNA decay pathway mediated by hnRNPs A1 and
A2/B1. Genes Dev. 30:1070–1085. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Humphries F and Fitzgerald KA: hnRNPA2B1:
Fueling antiviral immunity from the nucleus. Mol Cell. 76:8–10.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu S, Sato M, Endo C, Sakurada A, Dong B,
Aikawa H, Chen Y, Okada Y, Matsumura Y, Sueoka E and Kondo T: hnRNP
B1 protein may be a possible prognostic factor in squamous cell
carcinoma of the lung. Lung Cancer. 41:179–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ushigome M, Ubagai T, Fukuda H, Tsuchiya
N, Sugimura T, Takatsuka J and Nakagama H: Up-regulation of hnRNP
A1 gene in sporadic human colorectal cancers. Int J Oncol.
26:635–640. 2005.PubMed/NCBI
|
|
15
|
Zhou J, Allred DC, Avis I, Martínez A, Vos
MD, Smith L, Treston AM and Mulshine JL: Differential expression of
the early lung cancer detection marker, heterogeneous nuclear
ribonucleoprotein-A2/B1 (hnRNP-A2/B1) in normal breast and
neoplastic breast cancer. Breast Cancer Res Treat. 66:217–224.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan-Sanders Y, Hammons GJ and Lyn-Cook BD:
Increased expression of heterogeneous nuclear ribonucleoprotein
A2/B1 (hnRNP) in pancreatic tissue from smokers and pancreatic
tumor cells. Cancer Lett. 183:215–220. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee CH, Lum JH, Cheung BP, Wong MS, Butt
YK, Tam MF, Chan WY, Chow C, Hui PK, Kwok FS, et al: Identification
of the heterogeneous nuclear ribonucleoprotein A2/B1 as the antigen
for the gastrointestinal cancer specific monoclonal antibody MG7.
Proteomics. 5:1160–1166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jing GJ, Xu DH, Shi SL, Li QF, Wang SY, Wu
FY and Kong HY: Aberrant expression and localization of hnRNP-A2/B1
is a common event in human gastric adenocarcinoma. J Gastroenterol
Hepatol. 26:108–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang Y, Shi SL, Li QF, Chen LY, Jing GJ,
Tan GW, Wang SY and Wu FY: The localization of hnRNP A2/B1 in
nuclear matrix and the aberrant expression during the RA-induced
differentiation of human neuroblastoma SK-N-SH cells. J Cell
Biochem. 112:1722–1729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mizuno H, Honda M, Shirasaki T, Yamashita
T, Yamashita T, Mizukoshi E and Kaneko S: Heterogeneous nuclear
ribonucleoprotein A2/B1 in association with hTERT is a potential
biomarker for hepatocellular carcinoma. Liver Int. 32:1146–1155.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barcelo C, Etchin J, Mansour MR, Sanda T,
Ginesta MM, Sanchez-Arévalo Lobo VJ, Real FX, Capellà G, Estanyol
JM, Jaumot M, et al: Ribonucleoprotein HNRNPA2B1 interacts with and
regulates oncogenic KRAS in pancreatic ductal adenocarcinoma cells.
Gastroenterology. 147:882–892.e8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Y, Sun Z, Deng J, Hu B, Yan W, Wei H
and Jiang J: Splicing factor hnRNPA2B1 contributes to tumorigenic
potential of breast cancer cells through STAT3 and ERK1/2 signaling
pathway. Tumour Biol. 39:10104283176943182017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Li H, Liu F, Gao LB, Han R, Chen C,
Ding X, Li S, Lu K, Yang L, et al: Heterogeneous nuclear
ribonucleoprotein A2/B1 is a negative regulator of human breast
cancer metastasis by maintaining the balance of multiple genes and
pathways. EBioMedicine. 51:1025832020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sueoka E, Goto Y, Sueoka N, Kai Y, Kozu T
and Fujiki H: Heterogeneous nuclear ribonucleoprotein B1 as a new
marker of early detection for human lung cancers. Cancer Res.
59:1404–1407. 1999.PubMed/NCBI
|
|
25
|
Katsimpoula S, Patrinou-Georgoula M,
Makrilia N, Dimakou K, Guialis A, Orfanidou D and Syrigos KN:
Overexpression of hnRNPA2/B1 in bronchoscopic specimens: A
potential early detection marker in lung cancer. Anticancer Res.
29:1373–1382. 2009.PubMed/NCBI
|
|
26
|
Tauler J, Zudaire E, Liu H, Shih J and
Mulshine JL: hnRNP A2/B1 modulates epithelial-mesenchymal
transition in lung cancer cell lines. Cancer Res. 70:7137–7147.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi HS, Lee HM, Jang YJ, Kim CH and Ryu
CJ: Heterogeneous nuclear ribonucleoprotein A2/B1 regulates the
self-renewal and pluripotency of human embryonic stem cells via the
control of the G1/S transition. Stem Cells. 31:2647–2658. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seo SR, Lee HM, Choi HS, Kim WT, Cho EW
and Ryu CJ: Enhanced expression of cell-surface B-cell
receptor-associated protein 31 contributes to poor survival of
non-small cell lung carcinoma cells. PLoS One. 12:e01880752017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi HS, Kim H, Won A, Kim JJ, Son CY, Kim
KS, Ko JH, Lee MY, Kim CH and Ryu CJ: Development of a decoy
immunization strategy to identify cell-surface molecules expressed
on undifferentiated human embryonic stem cells. Cell Tissue Res.
333:197–206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee HM, Seo SR, Kim J, Kim MK, Seo H, Kim
KS, Jang YJ and Ryu CJ: Expression dynamics of integrin α2, α3, and
αV upon osteogenic differentiation of human mesenchymal stem cells.
Stem Cell Res Ther. 11:2102020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee HM, Joh JW, Seo SR, Kim WT, Kim MK,
Choi HS, Kim SY, Jang YJ, Sinn DH, Choi GS, et al: Cell-surface
major vault protein promotes cancer progression through harboring
mesenchymal and intermediate circulating tumor cells in
hepatocellular carcinomas. Sci Rep. 7:132012017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shibue T and Weinberg RA: EMT, CSCs, and
drug resistance: The mechanistic link and clinical implications.
Nat Rev Clin Oncol. 14:611–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He Y, Brown MA, Rothnagel JA, Saunders NA
and Smith R: Roles of heterogeneous nuclear ribonucleoproteins A
and B in cell proliferation. J Cell Sci. 118:3173–3183. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi X, Ran L, Liu Y, Zhong SH, Zhou PP,
Liao MX and Fang W: Knockdown of hnRNP A2/B1 inhibits cell
proliferation, invasion and cell cycle triggering apoptosis in
cervical cancer via PI3K/AKT signaling pathway. Oncol Rep.
39:939–950. 2018.PubMed/NCBI
|
|
36
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Toledo F and Wahl GM: Regulating the p53
pathway: In vitro hypotheses, in vivo veritas. Nat Rev Cancer.
6:909–923. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gurtner K, Kryzmien Z, Koi L, Wang M,
Benes CH, Hering S, Willers H, Baumann M and Krause M:
Radioresistance of KRAS/TP53-mutated lung cancer can be overcome by
radiation dose escalation or EGFR tyrosine kinase inhibition in
vivo. Int J Cancer. 147:472–477. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen CY, Jan CI, Pi WC, Wang WL, Yang PC,
Wang TH, Karni R and Wang TC: Heterogeneous nuclear
ribonucleoproteins A1 and A2 modulate expression of Tid1 isoforms
and EGFR signaling in non-small cell lung cancer. Oncotarget.
7:16760–16772. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dai S, Zhang J, Huang S, Lou B, Fang B, Ye
T, Huang X, Chen B and Zhou M: HNRNPA2B1 regulates the
epithelial-mesenchymal transition in pancreatic cancer cells
through the ERK/snail signalling pathway. Cancer Cell Int.
17:122017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou ZJ, Dai Z, Zhou SL, Hu ZQ, Chen Q,
Zhao YM, Shi YH, Gao Q, Wu WZ, Qiu SJ, et al: HNRNPAB induces
epithelial-mesenchymal transition and promotes metastasis of
hepatocellular carcinoma by transcriptionally activating SNAIL.
Cancer Res. 74:2750–2762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stockley J, Villasevil ME, Nixon C, Ahmad
I, Leung HY and Rajan P: The RNA-binding protein hnRNPA2 regulates
β-catenin protein expression and is overexpressed in prostate
cancer. RNA Biol. 11:755–765. 2014. View Article : Google Scholar : PubMed/NCBI
|