Introduction
The rhomboid gene family encodes a heterogeneous
group of polytopic proteins, with and without protease activity,
which is conserved throughout evolution (1). A total of 14 human rhomboid-like
proteins have been described, of which five are classified as
active proteases [rhomboid-like 1 (RHBDL1), RHBDL2, RHBDL3, RHBDL4
and presenilin-associated rhomboid-like] and nine as
pseudoproteases [inactive rhomboid proteins (iRhoms) 1–2, Derlins
1–3, UBA domain-containing 2 (UBAC2), rhomboid domain containing
(RHBDD)2, RHBDD3 and transmembrane protein 115] (2). Rhomboid pseudoproteases lack catalytic
activity and are primarily located in the endoplasmic reticulum
(ER) and Golgi apparatus (3). The
functional role of these pseudoproteases includes the ability to
recognize and recruit target proteins to regulate their subcellular
fate, turnover and degradation, affecting various signaling
pathways and pathophysiological processes (2). A number of rhomboid pseudoproteases are
associated with neoplastic disease (including iRhom1, iRhom2 and
Derlin1) via activation of diverse cancer signaling pathways, such
as WNT, HIF-1, VEGF and EGFR signaling (4–7).
Our previous studies determined that RHBDD2
expression is increased in advanced stages of breast and colorectal
cancer (8,9). Our subsequent study demonstrated that
RHBDD2 abrogation in breast cancer cell lines is associated
with cell proliferation and modulation of the unfolded protein
response pathway (10). Further
analysis showed that increased RHBDD2 expression is
associated with the proliferative stages of mammary gland
development (11), however, the
mechanistic role of such upregulation remains to be determined.
Recently, high-throughput proteomic approaches identified RHBDD2 as
a novel putative interactor of WW domain-containing oxidoreductase
(WWOX) (12). However, proteins that
interact with RHBDD2 and their associated functions have not been
defined yet in normal and breast cancer cells. WWOX has been
described as a tumor suppressor that is frequently altered in
breast cancer; its function is mediated by its interactions with
cancer-associated proteins in luminal-like breast cancer cells
(13). This ability to interact with
multiple proteins is due to WW domains within its protein structure
(14–16). For example, WWOX serves as an
inhibitor of TGFβ signaling by binding to SMAD3 via its WW domains
and modulating nuclear translocation of this transcription factor,
thus decreasing promoter occupation and transcriptional activation
(17). In addition, several studies
using conditional ablation animal models have shown that WWOX
serves an essential role in cell proliferation and differentiation
during murine mammary gland development (17–20).
Given that RHBDD2 is a cancer-associated protein
overexpressed in breast cancer cells that may be involved in
protein trafficking, WWOX is a tumor suppressor involved in mammary
cell proliferation and differentiation and RHBDD2 has been
described as a putative interactor of WWOX, it was hypothesized
that RHBDD2-WWOX protein interaction may serve as a negative
regulator of WWOX tumor suppressor activity by their sequestering
in the Golgi compartment. The present study aimed to corroborate
the RHBDD2-WWOX interaction and determine whether this affects
proliferation and differentiation processes in normal mammary and
breast cancer cells via modulation of the TGFβ signaling
pathway.
Materials and methods
Cell lines, culture and
differentiation
HC11 cells (proliferating cells) were grown at 37°C
to subconfluence in RPMI-1640 (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FCS (Natocor) and 5 µg/ml insulin
(Sigma-Aldrich; Merck KGaA). Then, confluent HC11 cells (competent
cells) were maintained in RPMI-1640 with 2% FCS and 5 µg/ml insulin
for 3 days, after which 5 µg/ml ovine prolactin (PRL;
Sigma-Aldrich; Merck KGaA) was added for 3 days (differentiated
cells). MCF7 and T47D breast cancer cells (proliferating
PRL− cells; American Type Culture Collection, Manassas,
VA, USA) were grown to subconfluence in DMEM (Sigma-Aldrich; Merck
KGaA) with 10% FCS at 37°C in a humidified atmosphere with 5%
CO2. T47D confluent cells were grown at 37°C and treated
with PRL (3 µg/ml) in DMEM with 2% FCS for 3 days.
Subcellular fractions
HC11 and T47D cells were grown at 37°C in 10-cm
plastic dishes until subconfluence, harvested by trypsinization and
then washed 3 times with cold PBS at 300 × g for 5 min at 4°C. The
cells were subsequently incubated at 4°C with 2 ml 10 mM HEPES (pH,
7.9; Sigma-Aldrich; Merck KGaA), 150 mM NaCl (Merck KGaA), 50 mM
Tris-HCl (Merck KGaA), 1 mM PMSF and 1% NP-40 buffer supplemented
with protease inhibitor cocktail (both Sigma-Aldrich; Merck KGaA).
Cells were transferred to a dounce homogenizer (Sigma-Aldrich;
Merck KgaA) and 10 strokes were applied while cell lysis was
verified under a phase-contrast microscope at ×10 magnification.
Homogenized cells were centrifuged at 300 × g for 5 min at 4°C to
obtain supernatant (cytoplasmic and membrane fraction) and pellet
(nuclear fraction). Supernatant was ultracentrifuged at 100,000 × g
for 45 min at 4°C using an ultracentrifuge (Beckman Coulter, Inc.;
cat. no. LE-80K) to obtain the corresponding cytoplasmic and
membrane fractions. Protein concentration was measured by Bradford
assay (Bio-Rad Laboratories, Inc.) and samples were stored at
−70°C.
Small interfering (si)RNA assay
MCF7 and T47D cell lines were cultured at 37°C on
12-well plates to 60% confluence in Opti-MEM I Reduced Serum Medium
(Gibco; Thermo Fisher Scientific, Inc.) and transiently transfected
with 40 pmol/µl siRNA mixed with Lipofectamine®
according to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc.). siRNA (length, 21 nucleotides) against
RHBDD2 mRNA (RHBDD2-siRNA, 5′-CUGUGUUGGGUACUUUGAUdTdT-3′)
was used as previously described (8).
In addition, AccuTarget™ biotin-labeled negative control siRNA
(5′-CCUACGCCACCAAUUUCGUdTdT-3′; Bioneer Corporation), which
exhibits no homology to any human genome sequence, was used as a
control. Cells were incubated at 37°C for 72 h.
RNA isolation and reverse
transcription-quantitative (RT-q)PCR
Total RNA was isolated from HC11, MCF7 and T47D cell
lines using TRIzol™ solution (Thermo Fisher Scientific, Inc.)
according to manufacturer's instructions. RNA was reverse
transcribed to cDNA using the SuperScript™ Reverse Transcriptase
kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. RT-qPCR was performed using PerfeCTa
SYBR-Green SuperMix (Quantabio); primer sets are listed in Table SI. PCR conditions were as follows:
Initial denaturation at 95°C for 3 min and 40 cycles of
denaturation (95°C 40 sec), annealing (55°C, 30 sec) and extension
(72°C, 30 sec). Data were captured and analyzed using the Agilent
AriaMx Real-Time PCR System 1.5 software (Agilent Technologies,
Inc.). Gene expression levels were calculated as 2−ΔΔCq
values using the housekeeping gene RNA18S as a reference (21).
Antibodies (Abs)
The primary Abs were as follows: Rabbit anti-RHBDD2
(cat. no. TA306891; OriGene Technologies, Inc.), rabbit anti-WWOX
(Aldaz Lab) (20), mouse anti-WWOX
(cat. no. sc-374449; Santa Cruz Biotechnology, Inc.), mouse
anti-human β-casein (cat. no. cat. no. sc-53189; Santa Cruz
Biotechnology, Inc.), mouse anti-mouse β-casein (cat. no.
sc-166520; Santa Cruz Biotechnology, Inc.) and mouse
anti-follistatin (FST; cat. no. sc-365003; Santa Cruz
Biotechnology, Inc.). Secondary Ab were as follows: Goat
anti-rabbit IgG (Sigma-Aldrich; Merck KGaA), donkey anti-rabbit IgG
Cy3-conjugated (cat. no. 711165152; Jackson ImmunoResearch
Laboratories, Inc.), goat anti-mouse IgG biotinylated (cat. no.
BA9200; Vector Laboratories, Inc.; Maravai LifeSciences) and
anti-mouse IgG BP-CFL 488 (Santa Cruz Biotechnology, Inc.).
Co-immunoprecipitation (Co-IP)
The cells were lysed with lysis buffer (50 nM
Tris-HCl, pH, 7.4; 150 mM NaCl; 2 mM PMSF; and 1% NP-40). RHBDD2
and WWOX protein were immunoprecipitated from cell lysates. In
order to obtain RHBDD2 and WWOX immune complexes, 300 µl homogenate
was incubated overnight with 5 µl Ab at 4°C (1:60). The immune
complexes were isolated with protein A Sepharose CL-4B, which had
been washed with cold lysis buffer followed by centrifugation at
10,000 × g for 30 sec at 4°C. A total of 300 µl lysates
precipitated with corresponding Ab (containing the immune
complexes) were incubated with 50 µl protein A Sepharose-CL-4B
(Sigma-Aldrich; Merck KGaA; cat no GE17-0963-02) for 1 h on a
rocking platform at 4°C and centrifuged at 10,000 × g for 30 sec at
4°C. The pellets were washed 5 times with 500 µl lysis buffer. In
order to release the immune complexes, pellets were boiled for 10
min in Laemmli's buffer followed by centrifugation at 10,000 × g
for 5 min at 4°C. The supernatants containing the isolated and
purified immune complexes were analyzed by SDS-PAGE followed by
western blot analysis.
SDS-PAGE and western blot
analysis
The aforementioned cell lysates and immune complexes
were diluted in 25% SDS, 10% glycerol and 2-mercaptoethanol (2:1),
heated at 90°C for 5 min and separated in discontinuous 4–12, 5%
acrylamide mini-gels. The protein concentration was measured by the
Bradford method and 50 µg protein was loaded per lane. Following
electrophoresis, gels were blotted onto nitrocellulose transfer
membranes (Whatman plc; Cytiva) in wet conditions. Membranes were
blocked with 3% powdered milk in 0.05% PBS/Tween-20 at 4°C
overnight and washed in 0.05% PBS/Tween-20. Membranes were
incubated primary Ab (anti-RHBDD2, 1:1,000; anti-WWOX, 1:2,000) at
4°C overnight. Following washing, membranes were incubated with the
appropriate secondary Ab and protein bands were visualized by
chemiluminescence on radiographic plates using the EasySee Western
Blot kit (cat. no. DW101-01, TransGen Biotech Co., Ltd.). Loading
controls such as ACTB and GAPDH were not included in the IP assays
because they were not detected in the specifics
immunoprecipitated.
Immunofluorescence and confocal
microscopy
In order to evaluate the subcellular localization of
RHBDD2, WWOX and FST protein, fluorescence immunohistochemistry
analysis of proliferating HC11 and T47D cells was performed. Cells
were grown at 37°C on 100 mm2 cover glass to 70%
confluence (or 100% in differentiated cells) and fixed for 1 h.
with 4% formaldehyde at room temperature or cold acetone (100%).
The cell membrane was permeabilized with 0.01% Triton for 10 min at
room temperature. Then, cells were incubated overnight with primary
Ab (anti-RHBDD2, 1:150; anti-WWOX, 1:150; anti-FST, 1:50) at 4°C.
Cells were incubated for 2 h with the appropriate secondary Ab at
room temperature, and nuclei were stained with propidium iodide
(1:100) or DAPI for 1 h at room temperature. Finally, cells were
visualized under an immunofluorescence microscope at 10 and 40×
magnifications, and images were captured by Micrometrics SE Premium
4.5 software (Unitron Ltd.). Then, RHBDD2 and WWOX co-localization
was viewed under a confocal immunofluorescence microscope at ×10
and ×40 magnifications (Confocal FluoView™ 1000) and images were
acquired at red and green signal channels using FluoView FV1000
software (Olympus Latin America, Inc.). Co-localization analysis
was performed with the JaCoP application on ImageJ software 1.8.0
(National Institutes of Health). Mander's overlap coefficient (MOC)
was used to quantify the degree of co-localization between
fluorophores. Pearson's correlation coefficient was calculated
between the mean intensity values of the overlapping green (WWOX
localization) and red signals (RHBDD2 localization).
In silico analysis of RHBDD2 and WWOX
expression in normal and breast cancer cells
In order to evaluate the relevance of the combined
RHBDD2 and WWOX mRNA expression between normal tissue
and primary breast carcinoma, The Cancer Genome Atlas (TCGA)-BRCA
dataset was analyzed. Briefly, RHBDD2 and WWOX
expression profiles from 1,211 breast samples (114 normal and 1,097
tumor samples) and their intrinsic subtypes were retrieved from the
University of California Santa Cruz Xena browser (xena.ucsc.edu/). Primary invasive breast carcinoma was
classified as low or high WWOX mRNA expression according to the
median expression value (7.85) of the normalized profile. In
silico prediction of RHBDD2 protein structure was performed
with PROTTER 1.0 software (wlab.ethz.ch/protter/start/).
Statistical analysis
Data are presented as the mean ± SEM (measured in
triplicate). Kolmogorov-Smirnov and Shapiro-Wilk tests were used to
evaluate the distribution of the obtained data. RT-qPCR data
analysis was performed using Mann-Whitney U test in R software
3.6.2 (r-project.org/). RHBDD2 and WWOX expression
levels from in silico analysis were using Mann-Whitney U
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
RHBDD2 and WWOX mRNAs are
differentially modulated in differentiated and proliferating human
and mouse mammary cells
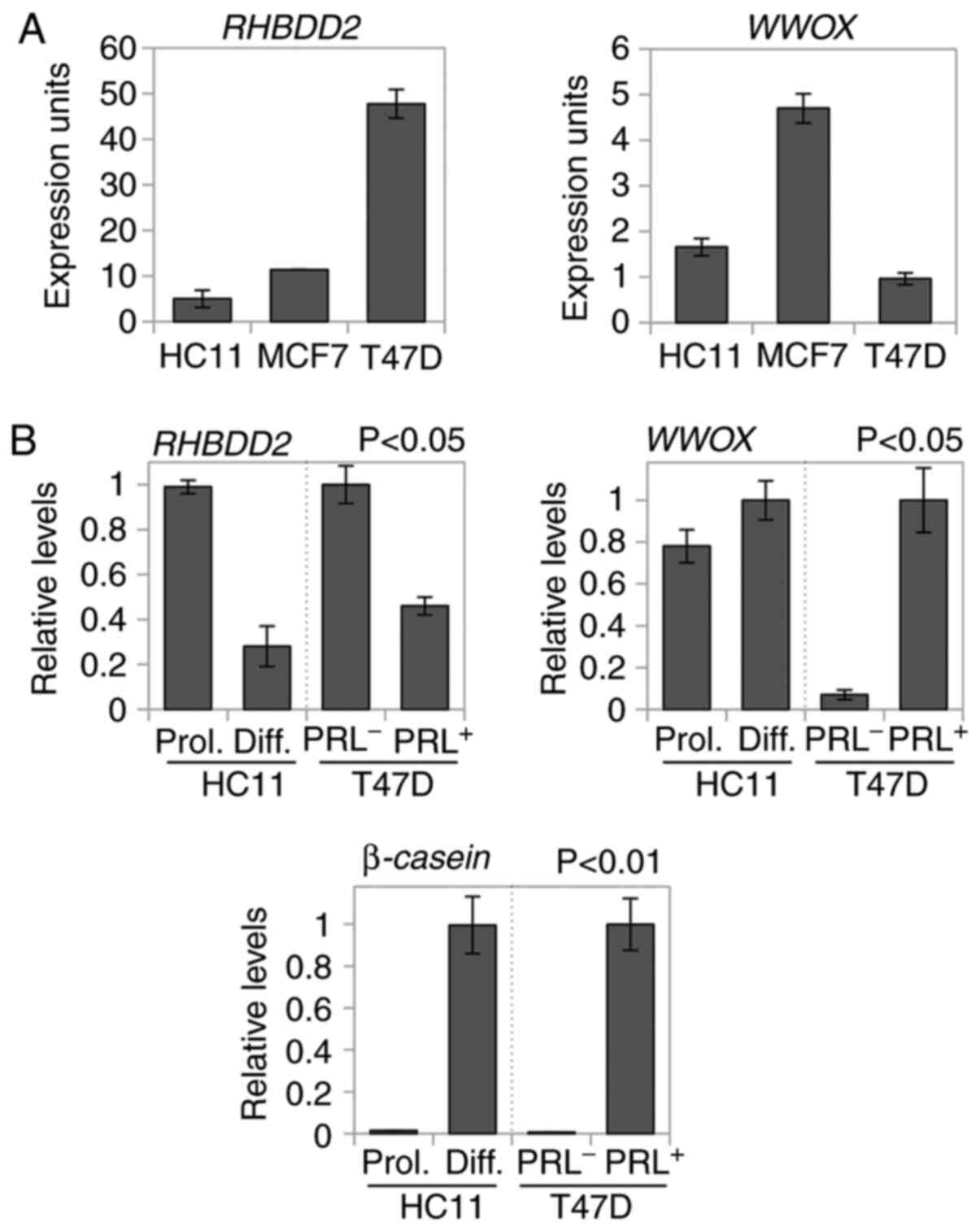
Expression of both RHBDD2 and WWOX transcripts in
normal and breast cancer cell lines was evaluated by RT-qPCR. The
highest levels of RHBDD2 expression were observed in T47D
breast cancer cell line, while WWOX mRNA was highly
expressed in MCF7 breast cancer cells (Fig. 1A). HC11 and T47D proliferative cells
showed significantly increased RHBDD2 expression levels
compared with differentiated or PRL-treated counterparts
(P<0.05; Fig. 1B). WWOX was
highly expressed in differentiated HC11 and PRL-treated T47D cells
compared with the proliferating and PRL− cells,
respectively (P<0.05; Fig. 1B).
PRL-induced differentiation was confirmed by elevated β-casein
expression in differentiated cells compared with proliferating
cells (Fig. 1B).
RHBDD2-WWOX interaction increases in
proliferative normal and breast cancer cells
In order to investigate whether RHBDD2 and WWOX
proteins physically interact, co-IP was investigated in normal
mammary and breast cancer cell models. Both proteins were
co-immunoprecipitated in HC11 and MCF7 whole cell lysates using
anti-WWOX and anti-RHBDD2 Ab (Figs.
2A and S1). In order to evaluate
whether the interaction between the aforementioned proteins was
modulated by differentiation, co-IP analysis of differentiated and
proliferative HC11 cells was performed. RHBDD2-WWOX IP was more
abundant in proliferating HC11 mammary cells compared with
differentiating cells (Fig. 2B),
which may have been due to RHBDD2 upregulation in the
proliferating cells (Fig. 1B).
Subcellular fractions from proliferative, differentiated or
PRL-treated HC11 and T47D cells were co-immunoprecipitated with
anti-WWOX Ab and immunoblotted with anti-RHBDD2 Ab. Strong
co-expression of both proteins was detected in the membrane
fractions of proliferating cells in both cell lines compared with
the cytoplasmic fractions (Fig. 2C).
HC11 differentiated and T47D PRL-treated cells showed lower
co-expression in both subcellular fractions than their respective
proliferative and PRL− counterparts (Fig. 2C).
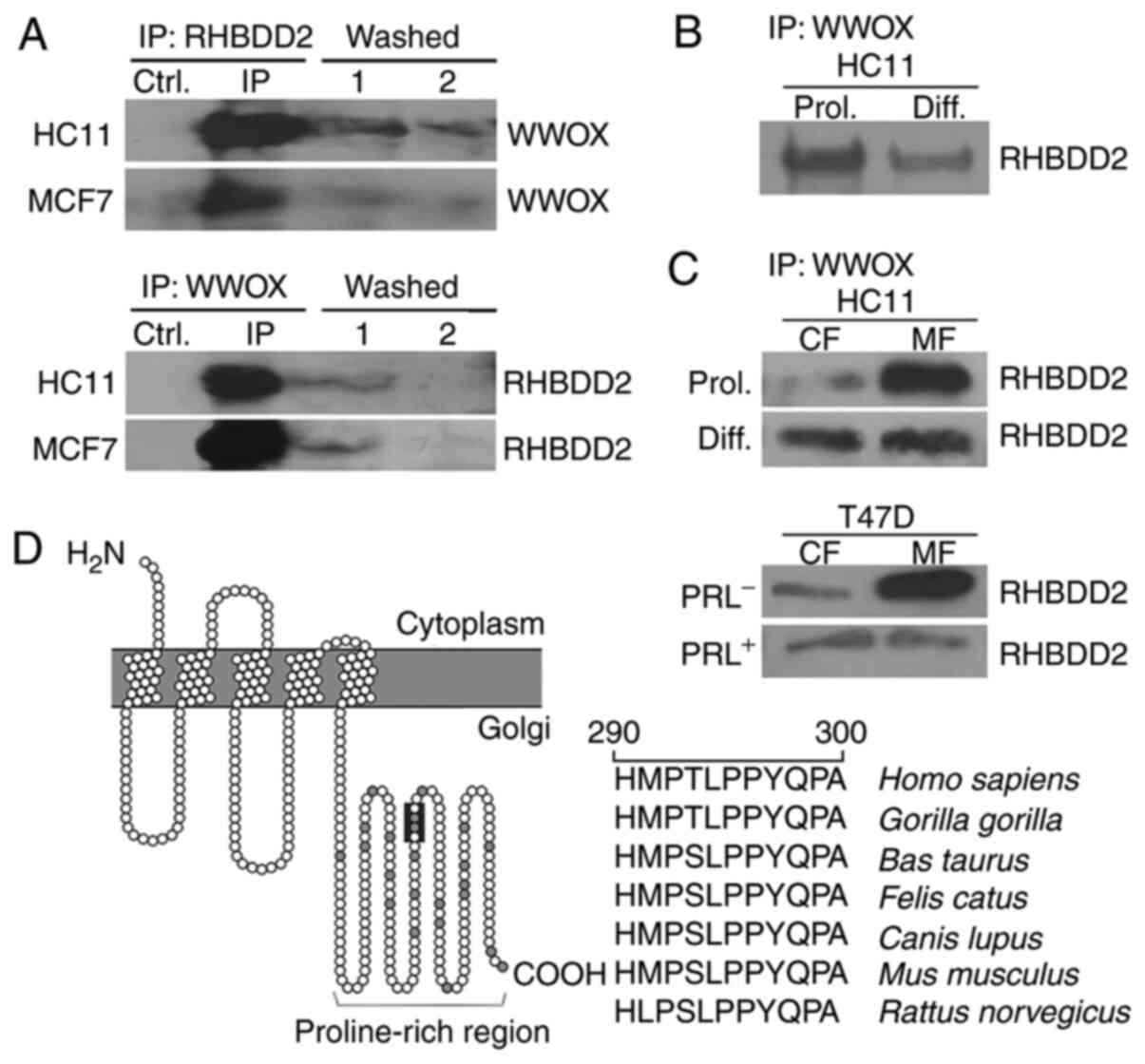 | Figure 2.RHBDD2 and WWOX co-IP analysis in
normal mammary and breast cancer cell lines. (A) Co-IP of
endogenous WWOX and RHBDD2 from HC11 and MCF7 cells. Whole-cell
lysates were immunoprecipitated with anti-RHBDD2 Ab and
immunoblotted with anti-WWOX Ab or immunoprecipitated with
anti-WWOX Ab and immunoblotted with anti-RHBDD2 Ab. In both IP
assays, non-primary Ab Ctrl and IP washes with unbound protein
(washed 1 and 2) were included as non-specific Ctrls. (B) Co-IP of
endogenous WWOX and RHBDD2 from HC11 Prol and Diff cells.
Whole-cell fraction lysates were immunoprecipitated with anti-WWOX
and immunoblotted with anti-RHBDD2 Ab. (C) Co-IP of endogenous WWOX
and RHBDD2 from CF and MF derived from Prol., PRL−,
Diff. or PRL+ HC11 and T47D cells. (D) RHBDD2 protein
structure prediction using PROTTER software. A conserved LPPY amino
acid sequence was identified in the carboxyl-terminal region (dark
grey) of RHBDD2 protein. RHBDD2, rhomboid domain containing 2;
WWOX, WW domain-containing oxidoreductase; IP, immunoprecipitation;
PRL, prolactin; Prol., proliferative; Diff., differentiated; Ab,
antibody; Ctrl, control; CF, cytoplasmic fraction; MF, membrane
fraction. |
The ability of WWOX protein to ubiquitously interact
with multiple proteins is attributed to the presence of two WW
domains. WW domains are small protein modules that bind to
proline-rich ligand consensus motifs, such as PPXY and LPXY
(14–16). In order to predict RHBDD2 protein
structure in silico, PROTTER software was used. In
silico analysis of the RHBDD2 primary sequence suggested that
it is an integral and polytopic protein containing 5 transmembrane
domains and a proline-rich region (PRR) in the C-terminus (Fig. 2D). RHBDD2 harbored a conserved LPPY
motif located in the PRR that directly interacted with the WW
domains of WWOX. This LPPY motif is phylogenetically conserved
across different species (Fig. 2D).
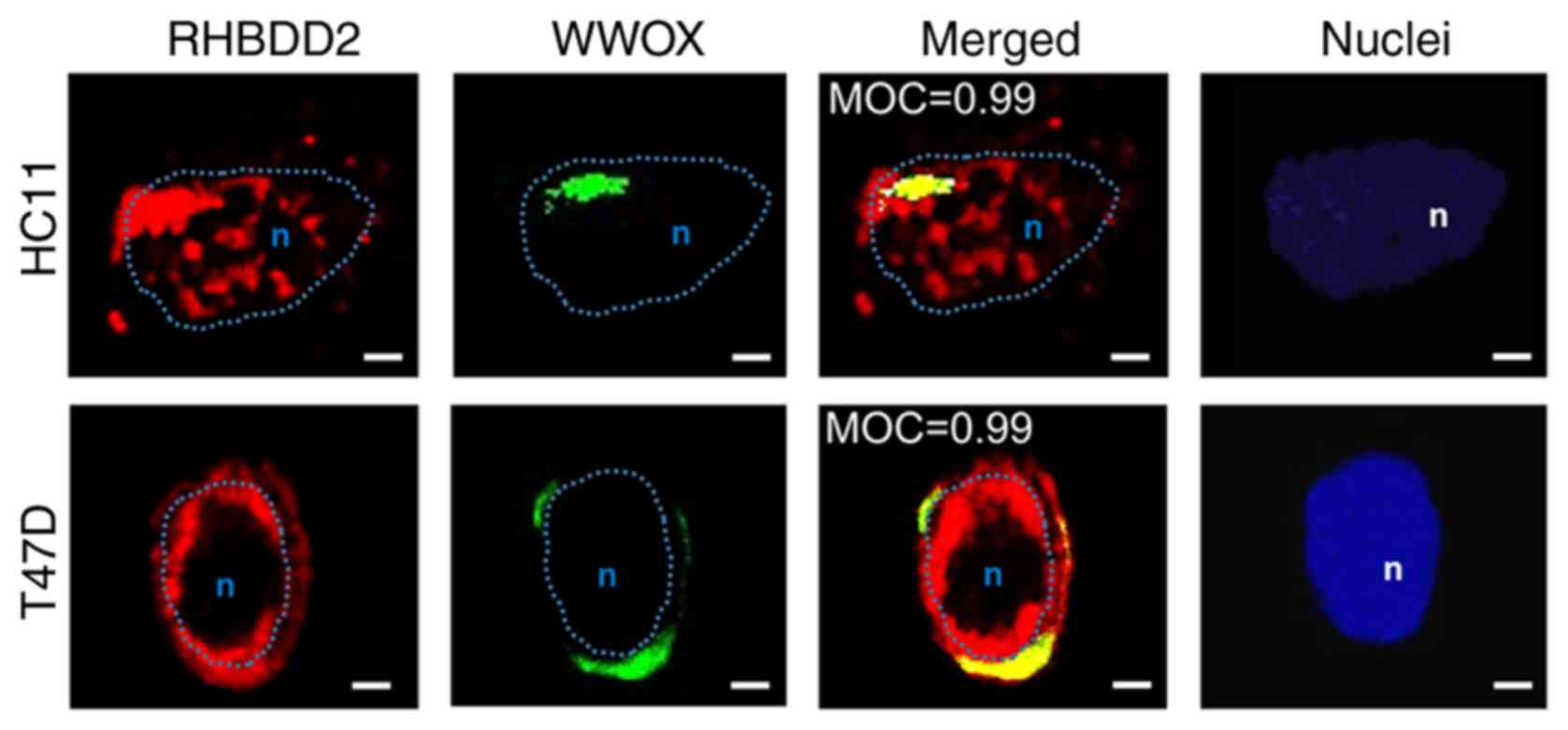
Next, localization of the endogenous RHBDD2-WWOX complex was
analyzed in proliferating HC11 and T47D proliferating; observed
juxtanuclear co-localization of both proteins was observed
(Fig. 3). Mander's test showed
significant WWOX-RHBDD2 co-localization coefficients in both
proliferating cell lines (MOC=0.99).
RHBDD2 modulates the TGFβ signaling
pathway by interacting with WWOX
In order to determine whether RHBDD2-WWOX protein
interaction was associated with modulation of the TGFβ/SMAD3
pathway during differentiation and/or proliferation of mammary
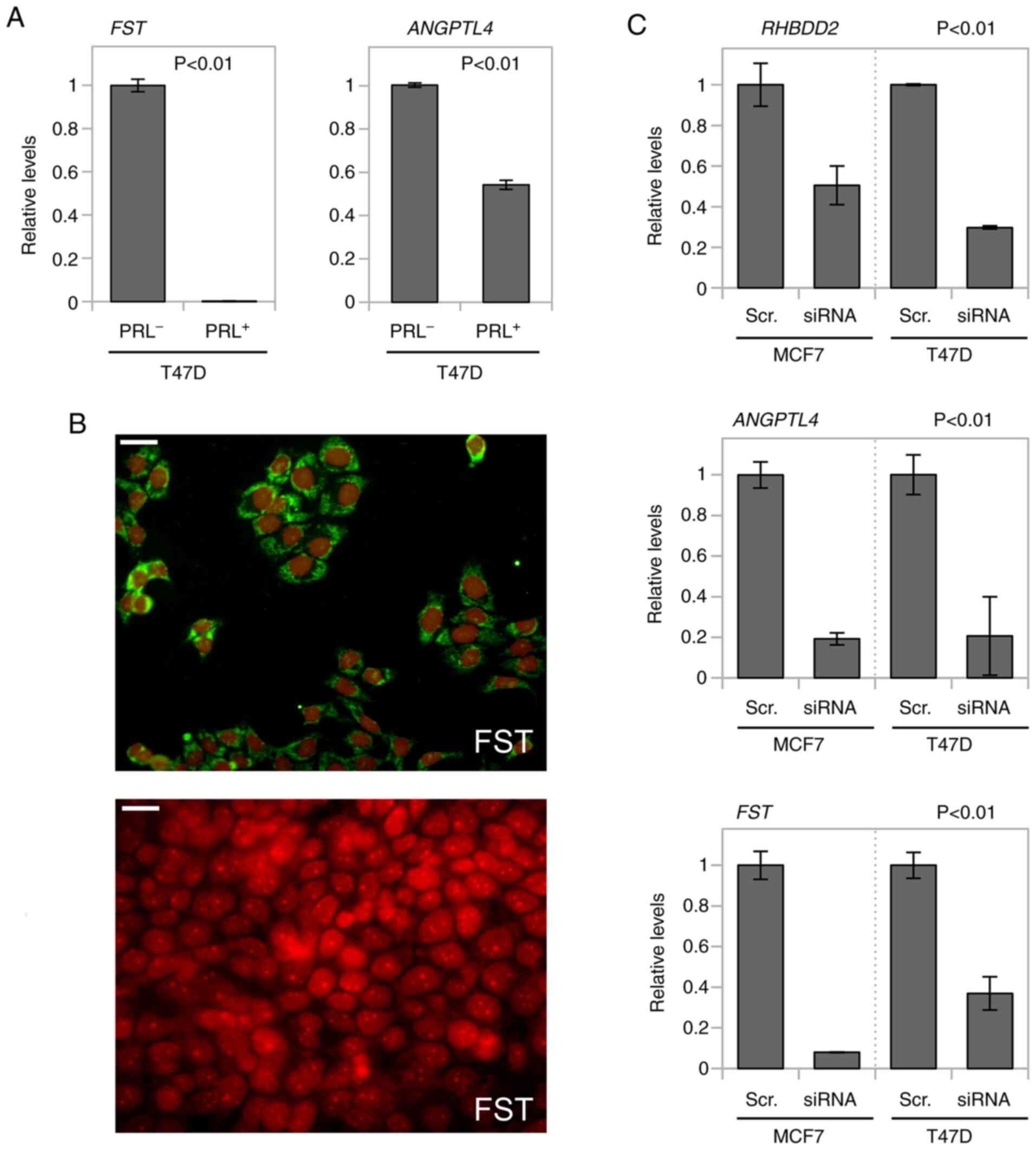
cells, expression levels of two SMAD3 target genes [FST and
angiopoietin-like 4 (ANGPTL4)] were evaluated in T47D cells.
Increased FST and ANGPTL4 mRNA expression levels were
detected in T47D proliferating PRL− cells compared with
PRL-treated counterparts (P<0.01; Fig.
4A). FST upregulation in T47D proliferating cells was
corroborated by immunofluorescence (Fig.
4B).
Furthermore, the effects of RHBDD2 silencing
on modulation of these TGFβ/SMAD3 target genes were evaluated in
breast cancer cells with elevated endogenous RHBDD2
expression. A siRNA-mediated approach was used to transiently
induce RHBDD2 gene expression abrogation in MCF7 and T47D
cells (Fig. 4C). FST and
ANGPTL4 mRNA was significantly downregulated in
RHBDD2-silenced cells compared with scrambled control siRNA
(P<0.01; Fig. 4C).
RHBDD2 overexpression in luminal A
breast cancer with high WWOX expression
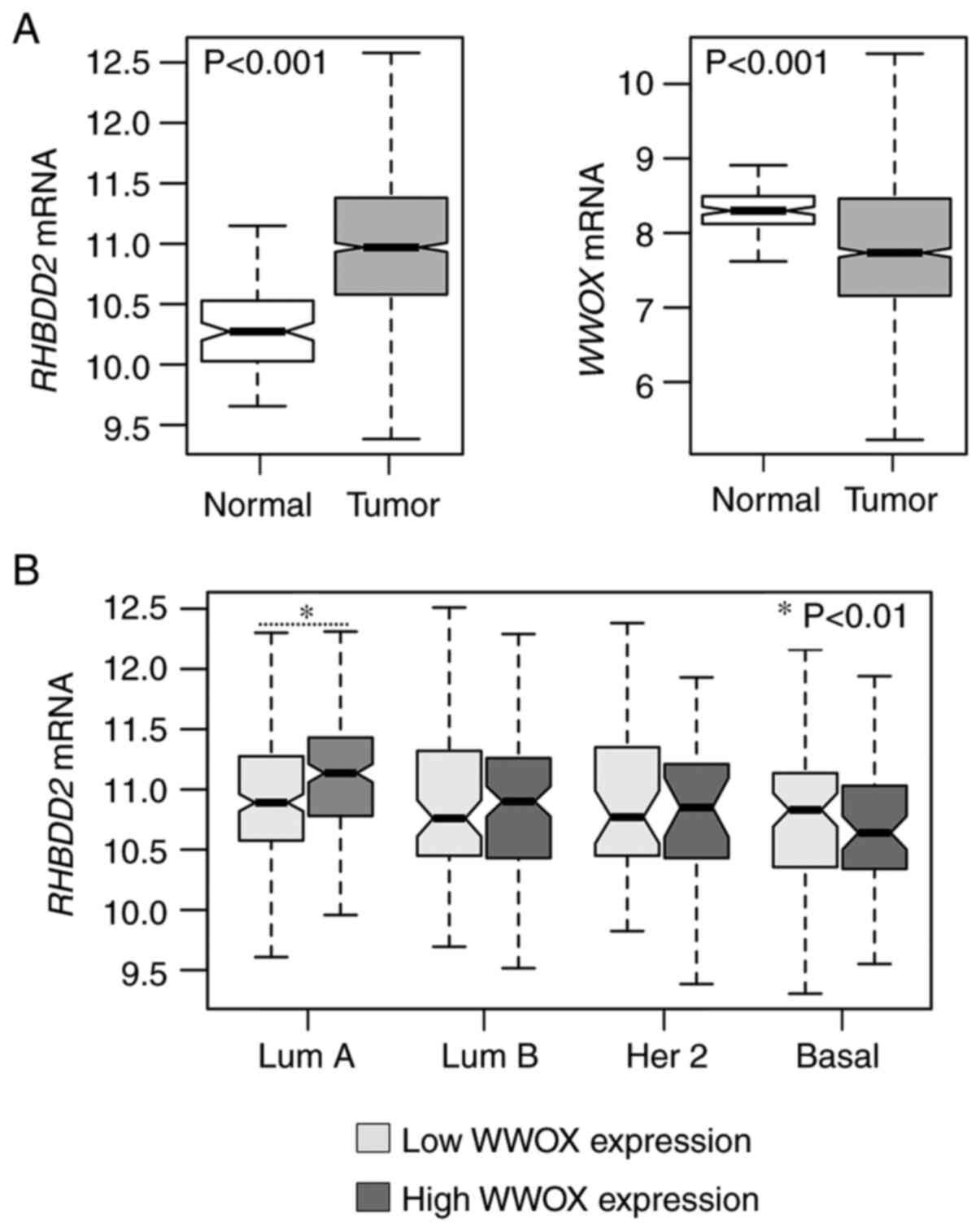
RHBDD2 and WWOX mRNA expression was
evaluated in normal and breast cancer samples obtained from the
TCGA-BRCA project (n=1,211). Primary invasive breast carcinoma
showed consistent upregulation of RHBDD2 and downregulation
of WWOX (both P<0.001) compared with normal samples
(Fig. 5A). In order to assess whether
RHBDD2 overexpression was associated with WWOX
expression levels, WWOX mRNA profiles were classified as low- or
high-expression according to each intrinsic subtype. RHBDD2
expression was significantly upregulated in luminal A breast
carcinoma, which was the intrinsic subgroup with the highest
WWOX expression levels (P<0.01; Fig. 5B).
Discussion
Our previous studies determined that the
RHBDD2 gene is overexpressed in advanced stages of breast
and colorectal cancer, suggesting a role in tumor progression and
chemoresistance to neoadjuvant therapy (8,9,22). Under normal physiological conditions,
RHBDD2 expression is detected not only in embryonic and developing
rat tissue but also in proliferating adult rat tissue (11). Regarding the mammary gland, increased
Rhbdd2 expression is associated with the pregnancy stage (11). RHBDD2 encodes one of nine known
rhomboid pseudoproteases whose functional roles are defined by
binding to target proteins (2).
Recently, RHBDD2 was detected among multiple WWOX protein
interactors under physiological conditions using a proteomic scale
approach (12). The present study
evaluated the expression levels and interaction between RHBDD2 and
WWOX in proliferating and differentiated normal mammary and breast
cancer cells to define the mechanistic role of RHBDD2.
RHBDD2 and WWOX mRNA expression levels
were detected in both luminal-like breast cancer cell lines (MCF7
and T47D). MCF7 is the breast cancer cell line with the highest
WWOX mRNA expression levels and is characterized by its high
dependence on estradiol for growth (23). Here, two PRL-responsive mammary
epithelial cell lines (HC11 and T47D) were used to analyze
RHBDD2 and WWOX expression levels and protein
interaction in differentiated and proliferating cells. HC11 mouse
mammary epithelial cells were used as a normal differentiation
model due to their ability to produce β-casein under lactogenic
hormone stimulation and to produce extracellular matrix during
differentiation (24). HC11 cells
only produce β-casein when lactogenic hormones are added to
confluent cells, not to proliferating HC11 cells (24). T47D luminal-like breast cancer cells
can be induced either to proliferate or differentiate; following
under PRL stimulation, these cells underwent differentiation, which
is characterized by morphological changes, production of lipid
vesicles and expression of β-casein (25–27).
Proliferating normal and breast cancer proliferating showed
significantly increased RHBDD2 mRNA levels compared with
their differentiated counterparts. WWOX mRNA was primarily
expressed in differentiated cells. These results were consistent
with previous studies in rat mammary gland development and human
breast cancer cells, indicating that RHBDD2 is highly
expressed in proliferating cells (10,11,28). Co-IP
suggested that RHBDD2-WWOX protein interaction primarily occured in
the membrane fraction of proliferating cells and decreased in
differentiated cells. Immunodetection of both proteins in the
membrane-enriched fraction was consistent with previous studies
that demonstrated that WWOX and RHBDD2 are localized to perinuclear
regions overlapping the Golgi compartment (20,28). The
co-localization results corroborated co-IP data suggesting RHBDD2
and WWOX interacting proteins reside, at least transiently, in the
same cellular compartment of proliferating cells. Moreover,
physical interaction between both proteins was supported by
identification of a conserved LPPY motif at the C-terminus region
of RHBDD2 that directly interact with the WW domains of WWOX.
Human WWOX primarily localizes to juxtanuclear
regions significantly overlapping Golgi compartment (20); here, RHBDD2 displayed a vesicular
distribution consistent with endosomal compartmentalization. Our
previous studies demonstrated that RHBDD2 protein is primarily
located in the Golgi apparatus of HC11, MCF7 and T47D cells
(11,28) is and associated with vesicle SNAP
receptor transport vesicles, suggesting a putative role in protein
trafficking (28). Among the known
WWOX interactors, several proteins are associated with protein
trafficking from ER exit sites to Golgi and from late endosomes to
lysosomes, such as SEC23-interacting protein, secretory carrier
membrane protein 3 and vesicular, overexpressed in cancer,
pro-survival protein 1 (VOPP1) (12). Furthermore, Bonin et al
(29) reported that VOPP1 physically
interacts with WWOX and that upon binding, WWOX translocates to the
VOPP1-containing lysosomal compartment, serving as a negative
regulator of WWOX tumor suppressor activity. WWOX expression loss
is common in various types of cancer and is implicated in normal
mammary gland proliferation and differentiation processes. Our
previous study reported that WWOX modulates the TGFβ signaling
pathway in normal breast cells by binding and sequestering SMAD3 in
the cytoplasmic compartment (17). In
order to investigate the role of RHBDD2-WWOX protein interaction in
the modulation of the TGFβ/SMAD3 pathway, expression of SMAD3
target genes was evaluated in proliferating and differentiated T47D
cells and following transient RHBDD2 abrogation. Increased
gene expression levels of FST and ANGPTL4 were
detected in proliferating T47D cells compared with their
differentiated counterparts. In addition, FST and
ANGPTL4 mRNA was significantly downregulated in
RHBDD2 transiently silenced cells. High FST and
ANGPTL4 expression suggested that the TGFβ/SMAD3 signaling
pathway was active in proliferating T47D cells and RHBDD2
depletion in luminal-like breast cancer cells may affect cell
proliferation by modulating TGFB/SMAD3 signaling via WWOX
interactions.
It was hypothesized that RHBDD2 expression
may contribute to unfavorable clinical breast cancer outcome due to
its inhibitory effect on WWOX tumor suppressor activity.
Luminal breast cancer is an intrinsic subtype characterized by
tumors that are predominantly regulated by estrogen receptors and
respond to endocrine therapy (30).
In addition, WWOX expression positively correlates with expression
levels of hormone receptors and its expression is significantly
decreased or lost in ER− breast cancer (31). The present results showed a consistent
upregulation of RHBDD2 and downregulation of WWOX in
primary invasive breast carcinoma compared with normal samples.
RHBDD2 expression was significantly upregulated in luminal A
primary invasive breast carcinoma, which is the intrinsic subtype
with the highest WWOX expression levels. These data suggest
that RHBDD2 overexpression may influence breast cancer
progression in luminal A tumors with WWOX expression favoring their
interaction. The most common mechanism leading to loss of WWOX
expression is genomic loss via gross chromosomal deletions and
rearrangements (13). Mechanisms
involving epigenetic silencing by promoter hypermethylation and
degradation have also been described (13). The present findings identified
post-translational sequestration of WWOX by RHBDD2 as an
alternative mechanism underlying inhibition of the
tumor-suppressive properties of WWOX.
In summary, the present results indicated that
RHBDD2 interacted with WWOX protein; this interaction may serve a
role in the TGFβ/SMAD3 signaling pathway, thus impacting the
proliferation and differentiation of normal and neoplastic mammary
epithelial cells. RHBDD2 overexpression in advanced breast cancer
may promote the TGFβ/SMAD3 signaling pathway by sequestering WWOX
in the Golgi apparatus or other membrane vesicles. RHBDD2-WWOX
interaction promoted activation of SMAD3 target genes involved in
mammary cell proliferation and breast cancer progression, promoting
the development of luminal A breast carcinoma.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Edith Kordon
(Institute for Physiology, Molecular Biology and Neurosciences,
Buenos Aires, Argentina) for kindly providing the HC11 cell
line.
Funding
The present study was supported by the National
Agency of Scientific and Technological Promotion (grant nos.
PICT-2017-0418 and PICT-2018-01403,) and CONICET (grant no.
PIP0159) and the Leukemia and Lymphoma Society Specialized Center
of Research (grant no. 7016-18).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MCA and VAF conceptualized the study and designed
the experiments. Experiments were performed by VAF, RC and SP. MCA
and EL performed statistical and bioinformatic analysis. MCA, VFA
and CMA wrote the manuscript. MCA and VAF confirm the authenticity
of all the raw data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Freeman M: Rhomboid proteases and their
biological functions. Annu Rev Genet. 42:191–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adrain C and Cavadas M: The complex life
of rhomboid pseudoproteases. FEBS J. 287:4261–4283. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bergbold N and Lemberg MK: Emerging role
of rhomboid family proteins in mammalian biology and disease.
Biochim Biophys Acta. 1828:2840–2848. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan Z, Zou H, Tian F, Grandis JR, Mixson
AJ, Lu PY and Li LY: Human rhomboid family-1 gene silencing causes
apoptosis or autophagy to epithelial cancer cells and inhibits
xenograft tumor growth. Mol Cancer Ther. 7:1355–1364. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zou H, Thomas SM, Yan Z, Grandis JR, Vogt
A and Li L: Human rhomboid family-1 gene RHBDF1 participates in
GPCR-mediated transactivation of EGFR growth signals in head and
neck squamous cancer cells. FASEB J. 23:425–432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Z, Liu F, Zhang ZS, Shu F, Zheng Y,
Fu L and Li LY: Human rhomboid family-1 suppresses
oxygen-independent degradation of hypoxia-inducible factor-1α in
breast cancer. Cancer Res. 74:2719–2730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan H, Wei R, Xiao Y, Song Y, Wang J, Yu
H, Fang T, Xu W and Mao S: RHBDF1 regulates APC-mediated
stimulation of the epithelial-to-mesenchymal transition and
proliferation of colorectal cancer cells in part via the
Wnt/β-catenin signalling pathway. Exp Cell Res. 368:24–36. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abba MC, Lacunza E, Nunez MI, Colussi A,
Isla-Larrain M, Segal-Eiras A, Croce MV and Aldaz CM: Rhomboid
domain containing 2 (RHBDD2): A novel cancer-related gene
over-expressed in breast cancer. Biochim Biophys Acta.
1792:988–997. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lacunza E, Canzoneri R, Rabassa ME,
Zwenger A, Segal-Eiras A, Croce MV and Abba MC: RHBDD2: A
5-fluorouracil responsive gene overexpressed in the advanced stages
of colorectal cancer. Tumour Biol. 33:2393–2399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lacunza E, Rabassa ME, Canzoneri R,
Pellon-Maison M, Croce MV, Aldaz CM and Abba MC: Identification of
signaling pathways modulated by RHBDD2 in breast cancer cells: A
link to the unfolded protein response. Cell Stress Chaperones.
19:379–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferretti VA, Canzoneri R, Barbeito CG,
Croce MV, Abba MC and Lacunza E: Spatiotemporal expression of
rhomboid domain containing 2 (Rhbdd2) during rat development. Acta
Histochem. 117:635–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hussain T, Lee J, Abba MC, Chen J and
Aldaz CM: Delineating WWOX protein interactome by tandem affinity
purification-mass spectrometry: Identification of top interactors
and key metabolic pathways involved. Front Oncol. 8:5912018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aldaz CM, Ferguson BW and Abba MC: WWOX at
the crossroads of cancer, metabolic syndrome related traits and CNS
pathologies. Biochim Biophys Acta. 1846:188–200. 2014.PubMed/NCBI
|
|
14
|
Sudol M: Structure and function of the WW
domain. Prog Biophys Mol Biol. 65:113–132. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chan DC, Bedford MT and Leder P: Formin
binding proteins bear WWP/WW domains that bind proline-rich
peptides and functionally resemble SH3 domains. EMBO J.
15:1045–1054. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ludes-Meyers JH, Kil H, Bednarek AK, Drake
J, Bedford MT and Aldaz CM: WWOX binds the specific proline-rich
ligand PPXY: Identification of candidate interacting proteins.
Oncogene. 23:5049–5055. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferguson BW, Gao X, Zelazowski MJ, Lee J,
Jeter CR, Abba MC and Aldaz CM: The cancer gene WWOX behaves as an
inhibitor of SMAD3 transcriptional activity via direct binding. BMC
Cancer. 13:5932013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferguson BW, Gao X, Kil H, Lee J,
Benavides F, Abba MC and Aldaz CM: Conditional Wwox deletion in
mouse mammary gland by means of two cre recombinase approaches.
PLoS One. 7:e366182012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abdeen SK, Salah Z, Khawaled S and Aqeilan
RI: Characterization of WWOX inactivation in murine mammary gland
development. J Cell Physiol. 228:1391–1396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bednarek AK, Keck-Waggoner CL, Daniel RL,
Laflin KJ, Bergsagel PL, Kiguchi K, Brenner AJ and Aldaz CM: WWOX,
the FRA16D gene, behaves as a suppressor of tumor growth. Cancer
Res. 61:8068–8073. 2001.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Palma S, Raffa CI, Garcia-Fabiani MB,
Ferretti VA, Zwenger A, Perez Verdera PV, Llontop A, Rojas Bilbao
E, Cuartero V, Abba MC and Lacunza E: RHBDD2 overexpression
promotes a chemoresistant and invasive phenotype to rectal cancer
tumors via modulating UPR and focal adhesion genes. Biochim Biophys
Acta. 1866:1658102020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bednarek AK, Laflin KJ, Daniel RL, Liao Q,
Hawkins KA and Aldaz CM: WWOX, a novel WW domain-containing protein
mapping to human chromosome 16q23.3–24.1, a region frequently
affected in breast cancer. Cancer Res. 60:2140–2145.
2000.PubMed/NCBI
|
|
24
|
Chammas R, Taverna D, Cella N, Santos C
and Hynes NE: Laminin and tenascin assembly and expression regulate
HC11 mouse mammary cell differentiation. J Cell Sci. 107:1031–1040.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shiu RP and Paterson JA: Alteration of
cell shape, adhesion, and lipid accumulation in human breast cancer
cells (T-47D) by human prolactin and growth hormone. Cancer Res.
44:1178–1186. 1984.PubMed/NCBI
|
|
26
|
Chambon M, Rochefort H, Vial HJ and
Chalbos D: Progestins and androgens stimulate lipid accumulation in
T47D breast cancer cells via their own receptors. J Steroid
Biochem. 33:915–922. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ronen D, Altstock RT, Firon M, Mittelman
L, Sobe T, Resau JH, Vande Woude GF and Tsarfaty I: Met-HGF/SF
mediates growth arrest and differentiation in T47D breast cancer
cells. Cell Growth Differ. 10:131–140. 1999.PubMed/NCBI
|
|
28
|
Canzoneri R, Rabassa ME, Gurruchaga A,
Ferretti V, Palma S, Isla-Larrain M, Croce MV, Lacunza E and Abba
MC: Alternative splicing variant of RHBDD2 is associated with cell
stress response and breast cancer progression. Oncol Rep.
40:909–915. 2018.PubMed/NCBI
|
|
29
|
Bonin F, Taouis K, Azorin P, Petitalot A,
Tariq Z, Nola S, Bouteille N, Tury S, Vacher S, Bièche I, et al:
VOPP1 promotes breast tumorigenesis by interacting with the tumor
suppressor WWOX. BMC Biol. 16:1092018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fontes-Sousa M, Amorim M, Salta S, Palma
De Sousa S, Henrique R and Jerónimo C: Predicting resistance to
endocrine therapy in breast cancer: It's time for epigenetic
biomarkers (Review). Oncol Rep. 41:1431–1438. 2019.PubMed/NCBI
|
|
31
|
Nunez MI, Ludes-Meyers J, Abba MC, Kil H,
Abbey NW, Page RE, Sahin A, Klein-Szanto AJ and Aldaz CM: Frequent
loss of WWOX expression in breast cancer: Correlation with estrogen
receptor status. Breast Cancer Res Treat. 89:99–105. 2005.
View Article : Google Scholar : PubMed/NCBI
|