Introduction
Lung cancer has been identified as the leading cause
of cancer mortality in Taiwan over the past decade. In 2019, the
mortality rate of lung cancer was 19.3% and ranked first among
total cancer mortality according to the annual report of Ministry
of Health and Welfare (https://www.mohw.gov.tw/np-126-2.html). Despite
advances in surgery, radiation therapy, chemotherapy and targeted
therapy, treatment is largely unsuccessful; the 5-year survival for
patients with lung cancer is ~15-20% (1). Lung cancer can be classified into small
cell lung cancer (SCLC) and non-SCLC (NSCLC). NSCLC is subdivided
into adenocarcinoma, squamous cell carcinoma and large cell
carcinoma (2). NSCLC accounts for
~80% of all lung cancer cases (3).
More than 75% of patients with NSCLC develop metastasis during the
course of the disease, which is responsible for high mortality; for
example, in 2020, lung cancer was the leading cause of cancer
mortality, with an estimated 1.8 million deaths (18% of total
cancer deaths) globally (4–6). Innate or adaptive resistance to drug
treatments, including chemo- and targeted therapy, increases the
mortality rate of patients with lung cancer (7,8). Different
malignant characteristics of cancer cells may be interconnected.
For example, epithelial to mesenchymal transition (EMT), which is a
key step for motility, is acquired in cisplatin-resistant lung
cancer cells via the Akt/β-catenin/Snail-dependent pathway
(9). In addition to lung cancer,
similar connections between motility and drug resistance have also
been observed in other types of cancer, such as pancreatic and
breast cancer (10). These findings
highlight potential novel therapeutic strategies to treat lung
tumors by simultaneously targeting multiple malignant
characteristics.
The tumor suppressor p53 regulates biological
responses to DNA damage, hypoxia, nutrient deprivation and abnormal
expression of oncogenes (11–13) and serves a key role in tumor
suppression via the induction of arrest, senescence and apoptosis,
as well as by abrogating angiogenesis (14). The protein, which serves as a
transcriptional factor, is maintained at a low level and has a
short half-life under normal physiological conditions. Upon
activation, several routes are involved in induction of p53
signaling. p53 mRNA is induced via a transcriptional regulator,
such as CCAAT-enhancer-binding protein (15). p53 protein is stabilized by
phosphorylation and dissociation from MDM2 and p53 mRNA is
stabilized by binding of Wig-1 to its 3′-untranslated region (UTR)
(16). The activated p53 then
translocates to the nucleus and triggers transcription of target
genes, such as p21, p53 upregulated modulator of apoptosis (PUMA)
and Bax, to arrest the cell cycle or induce cellular apoptosis
(17). Abnormality in p53 is detected
in ~50% of all human tumors (18).
Furthermore, the function of p53 affects the sensitivity of tumor
cells to chemotherapeutic and/or radiotherapeutic agents (19). Furthermore, it has also been reported
that p53 inhibits the motility of cancer cells by inhibiting the
expression of MET or snail family transcriptional repressor 2
(Slug) (20,21). These results indicate that the
malfunction of p53 contributes to multiple malignancies in cancer
cells. Clinically, p53 has been considered as a therapeutic target
to restore its normal expression or abrogate its oncogenic
activity. In lung cancer, positive expression and mutation rates of
p53 in adenocarcinoma are half those in squamous cells (22). Therefore, an understanding of the
mechanisms underlying the pathogenic routes that cause p53
malfunction may facilitate the development of novel treatment
strategies for patients with lung cancer.
MicroRNAs (miRNAs or miRs) are a class of small RNA
that inhibit gene expression at the post-transcriptional level.
Mature miRNAs are composed of 17–25 nucleotides and primarily bind
to the complementary sequence of 3′-UTRs, followed by degradation
of target mRNA or abrogation of its translation (23). miRNAs regulate more than two-thirds of
all cellular processes, including proliferation, motility,
differentiation, metabolism, autophagy and apoptosis, and hence the
aberrant expression of miRNAs is hypothesized to be involved in the
development of various diseases, such as multiple sclerosis,
Parkinson's disease, Type II diabetes and cancer (24,25). The
design of specific inhibitors of target proteins, such as Ras and
Raf inhibitors, for the treatment of certain diseases is difficult
and time-consuming (26,27). However, due to the complementary
characteristics of nucleotides, the restoration of abnormally
expressed miRNAs with either agomir or antagomir represents a
promising option for future therapeutic strategies (28). To date, numerous studies have reported
that a large number of miRNAs are abnormally expressed in cancer
cells, which causes the dysregulation of various genes involved in
cancer pathogenesis, particularly metastasis or drug resistance
(29,30). For example, miR-10b is an oncomir that
is upregulated in breast, pancreatic, lung, esophageal, neck and
head, prostate and colorectal cancer, as well as melanoma,
hepatocellular carcinoma and glioma (31). The overexpression of miR-10b decreases
expression of HOXD10, zinc finger E-box binding homeobox 1,
Kruppel-like factor (KLF)4, epithelial (E-)cadherin and TIAM
Rac1-associated GEF 1 (Tiam1), which leads to malignant
transformation and development of invasive and metastatic
properties of inceptive benignant cancer cells (32–36).
miR-10b is also involved in drug resistance; for example, miR-10b
is involved in tamoxifen resistance via downregulation of histone
deacetylase (HDAC)4 in estrogen receptor-positive breast cancer
cells (37). In addition, high levels
of miR-10b confer 5-fluorouracil resistance to colorectal cancer
cells (38). Wu et al
(39) demonstrated that miR-10b
confers cisplatin resistance via targeting peroxisome
proliferator-activated receptor (PPAR)γ in esophageal cancer cells.
Furthermore, it has also been reported that higher miR-10b levels
are associated with resistance to neoadjuvant therapy and lower
survival rate in patients with pancreatic ductal adenocarcinoma
(40), suggesting an alternative
function of miR-10b. In lung cancer, high expression of miR-10b is
positively associated with malignancy and poor prognosis (41). It is therefore important to identify
lung cancer-associated miRNAs as biomarkers of treatment response
or pharmacological targets. Previous reports imply that miR-10b may
exhibit a wide range of functions, such as fibrosis, immune
regulation, neuron protection and muscle proliferation beyond
promoting motility (42–45). In addition, despite evidence of a
connection between miR-10b and malignancy of lung cancer, the genes
that coordinate or contribute to multiple oncogenic effects of
miR-10b in lung cancer cells have not yet been mechanistically
validated. The present review investigated p53 as a novel target of
miR-10b and its effect on drug tolerance and motility in lung
cancer cells.
Materials and methods
Cell lines and culture
Human lung cancer cell lines, including A549, H460,
CL1-0 and CL1-5, and non-tumor bronchial cell line Beas 2B were
maintained in RPMI-1640 medium (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 5% heat-inactivated fetal
bovine serum and incubated in a 5% CO2 incubator at
37°C.
Reverse transcription-quantitative
(RT-q)PCR of miRNA
In each cell line, the expression levels of
hsa-miR-10b (hsa-miR-10b-5p, unless otherwise specified, hereafter
referred to as miR-10b) were analyzed as previously described
(46). Briefly, miRNAs were extracted
using miRVANA® miRNA isolation kit (cat. no. AM1560;
Thermo Fisher Scientific, Inc.). A total of 5 µl total miRNA was
used for RT using a miRNA RT kit (cat. no. 4366596; Thermo Fisher
Scientific, Inc.), and 5X miR-10b or RNU6B probe. PCR was performed
with TaqMan PCR master mix kit (Thermo Fisher Scientific, Inc.),
using a 20X miR-10b or RNU6B probe. Both primers and probes of
miR-10b (cat. no. 002218) and RNU6B (cat. no. 001093) were provided
by Thermo Fisher Scientific, Inc. RNU6B served as an internal
control. The signals were read using an ABI StepOnePlus.
Thermocycling conditions were as follows: Initial denaturation at
95°C for 10 min; 95°C for 10 sec followed by 60°C for 1 min at 40
cycles. Subsequently, the comparative 2−ΔΔCq method
(46) was applied to quantify the
gene expression levels.
Ectopic expression of miR-10b-agomir
or antagomir and pmirGLO-p53 3′-UTR luciferase reporter vector and
assay
Human miR-10b-agomir
(5′-UACCCUGUAGAACCGAAUUUGUGUU-3′) was purchased from Thermo Fisher
Scientific, Inc. Both miR-10b antagomir and scramble (sc) RNA were
purchased from MDBio, Inc. sc served as miR-10b-agomir control. The
initial screening from multiple websites (TargetScan,
targetscan.org/vert_72/; miRbase, mirbase.org) did not reveal any
conserved binding region of p53 3′-UTR for miR-10b. However, two
partially matched regions (1,580-1,587 and 2,029-2,035 from
transcription start site) were targeted. The wild-type (WT) 1,035
bps of 3′- UTR of p53 (1,245–2280 from ttranscription start site;
forward, GCTAGCCATTCTCCACTTCTTGTTCCC and reverse,
GTCGACTAATCCCAGCACTCTGGGAGG), mutant 1,583 (CCA GGG A mutated to
CCA TTT A) or 2,033 (ACT GGG T mutated to ACT TTT T) or 1,583 +
2,033 dual mutant was cloned into the pmirGLOdual luciferase vector
(Promega Corporation). The transfection of 20 nM miR-10b-agomir, sc
and antagomir (both designed by MdBio, Inc.) was performed using
Oligofectamine and transfection of 1 µg luciferase reporter vector
was performed using Lipofectamine® 2000 reagent (both
Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C. After 16 h
transfection, the transfection medium was replaced with fresh
medium and incubated at 37°C for a further 24–48 h. Luciferase
activity of control and transfection cells was normalized with to
Renilla luciferase activity according to the protocol of
Dual-Luciferase Reporter assay system kit (Promega Corporation) and
a luminometer (Mini lumate LB 9506; Titertek-Berthold).
Cell proliferation assay
sc, miR-10b-agomir or antagomir-transfected A549 or
Beas 2B cells were seeded in 96-well plates (5×103
cells/well) overnight at 37°C followed by treatment with cisplatin
for 48 h at 37°C. In preliminary experiments, 2 µM cisplatin showed
a reduction of 20% viability of A549 [lethal dose
(LD)25] cells while 5 µM cisplatin showed reduction of
60% viability (>LD50). The LD25 of
cisplatin was used to test the viability of A549 when miR-10b was
reduced by antagomir in comparison with untransfected parental
cells. In additions, the higher dose of cisplatin (5 µM) was used
to verify the adaptive ability of A549 with increased miR-10b
expression levels. After 48 h, the medium was removed, washed with
1X PBS and incubated with serum-free medium containing 10 µl MTT
stock solution (5 mg/ml) at 37°C for 1 h, followed by washing with
1X PBS three times. The cells were then exposed to DMSO and the
absorbance at 570 nm was measured using a microplate photometer.
The absorbance values were normalized to those of sc as relative
fold values. Trypan blue exclusion assay was also performed to
determine viability. Briefly, the cells were trypsinized and
suspended in 1X PBS, then 20 µl cells were and mixed with equal
amount of 0.4% trypan blue solution (in PBS) and loaded on a
hemacytometer. The cells were then counted under a ZEISS
phase-contrast microscope at 40× or 100× magnification, and the
blue cells which stained by trypan blue were excluded.
In vitro migration assay
A total of 25,000 cells was added to the upper
compartment of a Boyden chamber (48)
with 0.5 and 10.0% serum-containing RPMI-1640 medium (Invitrogen;
Thermo Fisher Scientific, Inc.) in the bottom well at 37°C. The
upper and bottom chamber was separated by a silicon gasket and a
microporous membrane (pore size, 8 µm). After 10 h, cells on the
lower surface were fixed with 100% methanol and stained with 1X
crystal violet (Sigma-Aldrich; Merck KGaA) for 5 min at room
temperature, then washed with ddH2O. The cells were then
examined under a ZEISS phase-contrast microscope at 40× or 100×
magnification. Relative motility was expressed as a percentage of
cells on compound-treated wells compared with control wells
(n>3).
Protein extraction and western blot
analysis
Protein extraction and western blot procedures were
performed as previously described (49). Briefly, parental, sc- and mir-10b
agomir-transfected A549 or Beas 2B cells in the presence or absence
of cisplatin were collected and washed three times with ice-cold
PBS and lysed in RIPA buffer (25 mM Tris; 150 mM sodium chloride;
1% NP-40; 1% sodium deoxycholate; 0.1% SDS; pH 7.6). The cell
lysates were centrifuged at 14,000 × g for 20 min at 4°C, the
supernatant was collected and total protein concentration was
determined by the Bradford method. For western blot analysis, equal
amounts of proteins (50 µg/lane) were separated by 10–15%
SDS-polyacrylamide gel followed by electrophoretic transfer onto a
PVDF membrane (EMD Millipore). Following blocking with 5% milk in
1X TBS-Tween for 1 h at room temperature, the membrane was
incubated with primary antibodies (all 1:1,000 v/v) overnight at
4°C. The membrane was then reacted with horseradish
peroxidase-conjugated secondary antibody (1:5,000 v/v) for 1 h at
room temperature, and the blots were visualized using an ECL-Plus
detection kit (PerkinElmer, Inc.). The following antibodies were
used: Anti-ATM (G-12; cat. no. sc-377293; Santa Cruz Biotechnology,
Inc.), Anti-phosphorylated (phospho)-ATM (ser-1981) (cat. no.
sc-47739; Santa Cruz Biotechnology Co., Ltd.), Anti-p53 (DO-1; cat.
no. sc-126; Santa Cruz Biotechnology, Inc.), Phospho-p53 (Ser15;
cat. no. #9284; Cell Signaling Technology, Inc.), Anti-PTEN (A2B1;
cat. no. sc-7974; Santa Cruz Biotechnology, Inc.), PUMAα (B-6; cat.
no. sc-377015; Santa Cruz Biotechnology Co., Ltd.), Anti-Bax (B-9;
cat. no. sc-7480; Santa Cruz Biotechnology, Inc.), Monoclonal
Anti-β-Actin (cat. no. A2228; Sigma-Aldrich; MercK KGaA),
Phospho-Akt (Ser473) (D9E; cat no. 9271T; Cell Signaling
Technology, Inc.), Anti-Akt1 (B-1; cat. no. sc-5298; Santa Cruz
Biotechnology, Inc.), Goat anti-mouse IgG-HRP (cat. no. sc-2005;
Santa Cruz Biotechnology, Inc.) and goat anti-rabbit IgG-HRP (cat.
no. sc-2004; Santa Cruz Biotechnology, Inc.).
Meta-analysis
The OncoLnc database (oncolnc.org/) (50) was used to analyze the association
between expression levels of miR-10b and p53 in the clinic.
Expression levels of miR-10b or p53 in tumor tissue from 488
patients with lung adenocarcinoma (LUAD) were analyzed using SPSS
(v21; IBM Corp.) to calculate the Pearson's correlation (2-tailed)
between these two genes. Patients were subdivided into high and low
expression of miR-10b or p53 based on the median value. The
patients who exhibited the same expression trends of miR-10b and
p53 (high-high or low-low) were defined as cocurrent, while inverse
trends (low-high or high-low) between miR-10b and p53 were defined
as countercurrent. In addition, the survival probability was
estimated by the Kaplan-Meier plot method using SPSS (v21; IBM
Corp.) followed by Tarone-Ware test. P<0.05 was considered to
indicate a statistically significant difference.
Statistical analysis
Data are presented as the mean ± SD (n≥3) and were
analyzed using GraphPad Prism software version 8.0 (GraphPad
Software, Inc.) (51). One-way or
two-way ANOVA followed by Tukey's post hoc test was used to compare
differences between multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
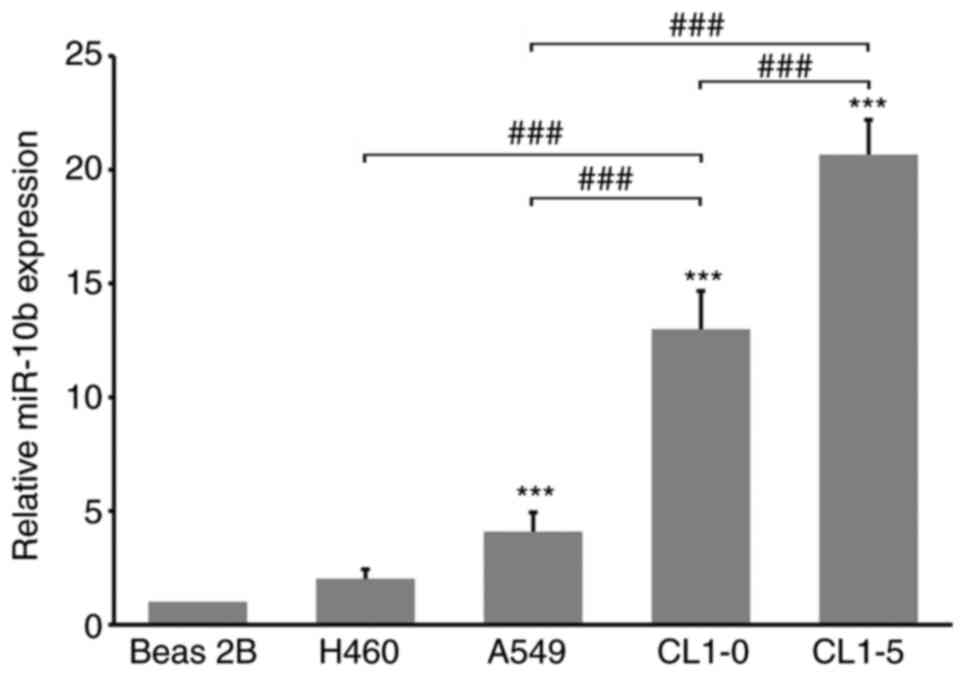
miR-10b exhibits higher expression in
highly invasive lung cancer cells
In order to characterize the role of miR-10b in lung
cancer cells the expression levels of miR-10b in different lung
cancer cells and non-tumor bronchial Beas 2B cells were analyzed.
miR-10b exhibited the highest levels in CL1-5 cells; these levels
were ~1.5-times greater than in CL1-0, 5-times greater than in A549
and 10-times greater than in Beas 2B cells. CL1-5 cells are known
to exhibit high migration/invasion ability and the present results
are consistent with those of previous reports concerning the
association between miR-10b expression levels and cellular motility
(Fig. 1) (33,52,53). CL
cells harbor an oncogenic-mutant p53 (21), these results imply a potential
association between p53 and miR-10b in lung cancer cells. In order
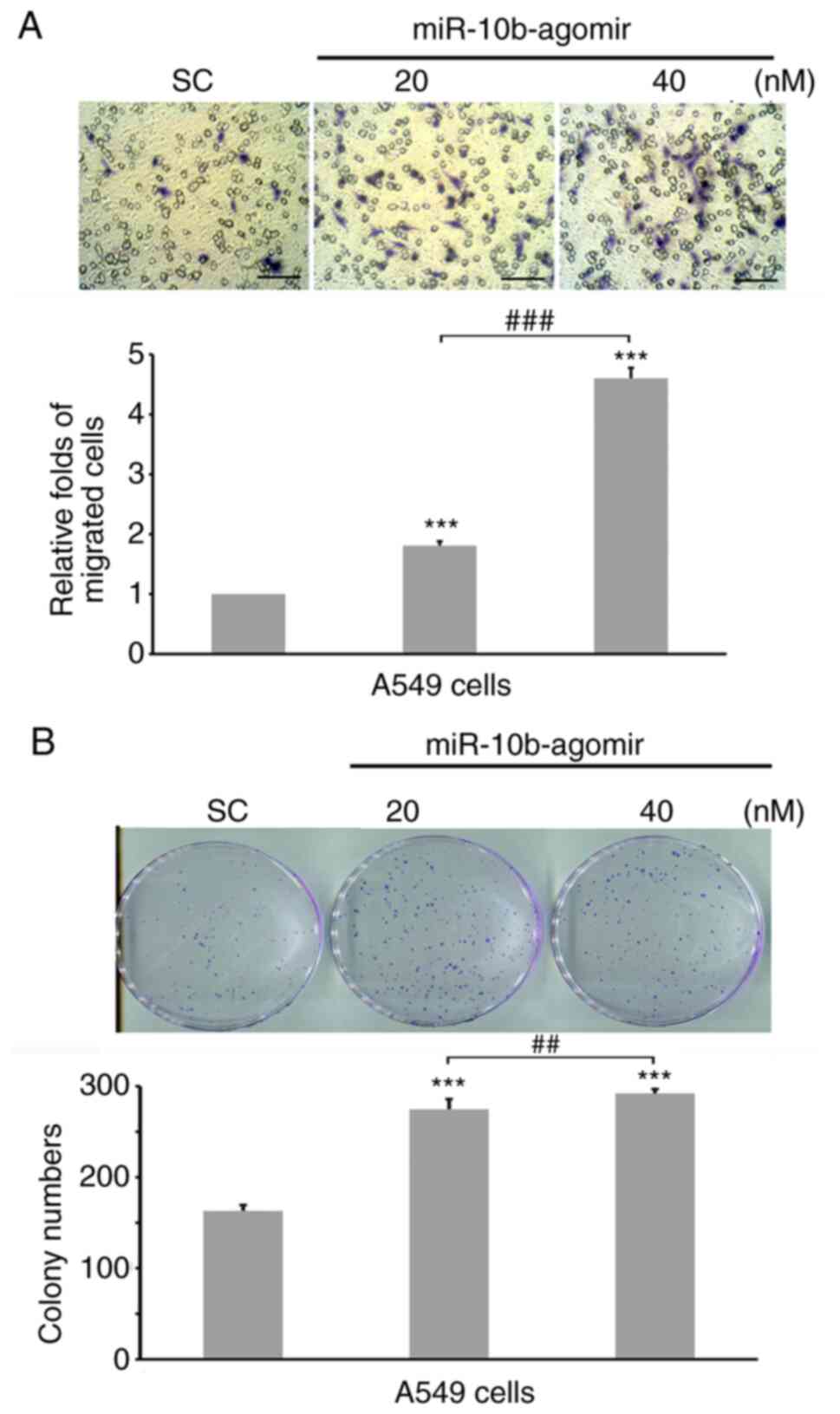
to confirm whether miR-10b is also involved in the motility of lung
cancer cells, 20 or 40 nM miR-10b-agomir were transfected into A549
cells, followed by invasion assay. Our preliminary experiments
demonstrated that 20 and 40 nM miR-10b-agomir inhibited p53
expression in a dose-dependent manner (data not shown), thus these
dosages were selected for subsequent experiments. Expression of
endogenous miR-10b in untransfected and sc transfections showed no
difference (Fig. S1), thus, in the
following experiments, sc was applied as control. Increasing
miR-10b levels significantly induced invasion of both types of cell
in a dose-dependent manner (Fig. 2A and
B). These results showed that miR-10b regulated the motility of
lung cancer cells, as has been demonstrated in other types of
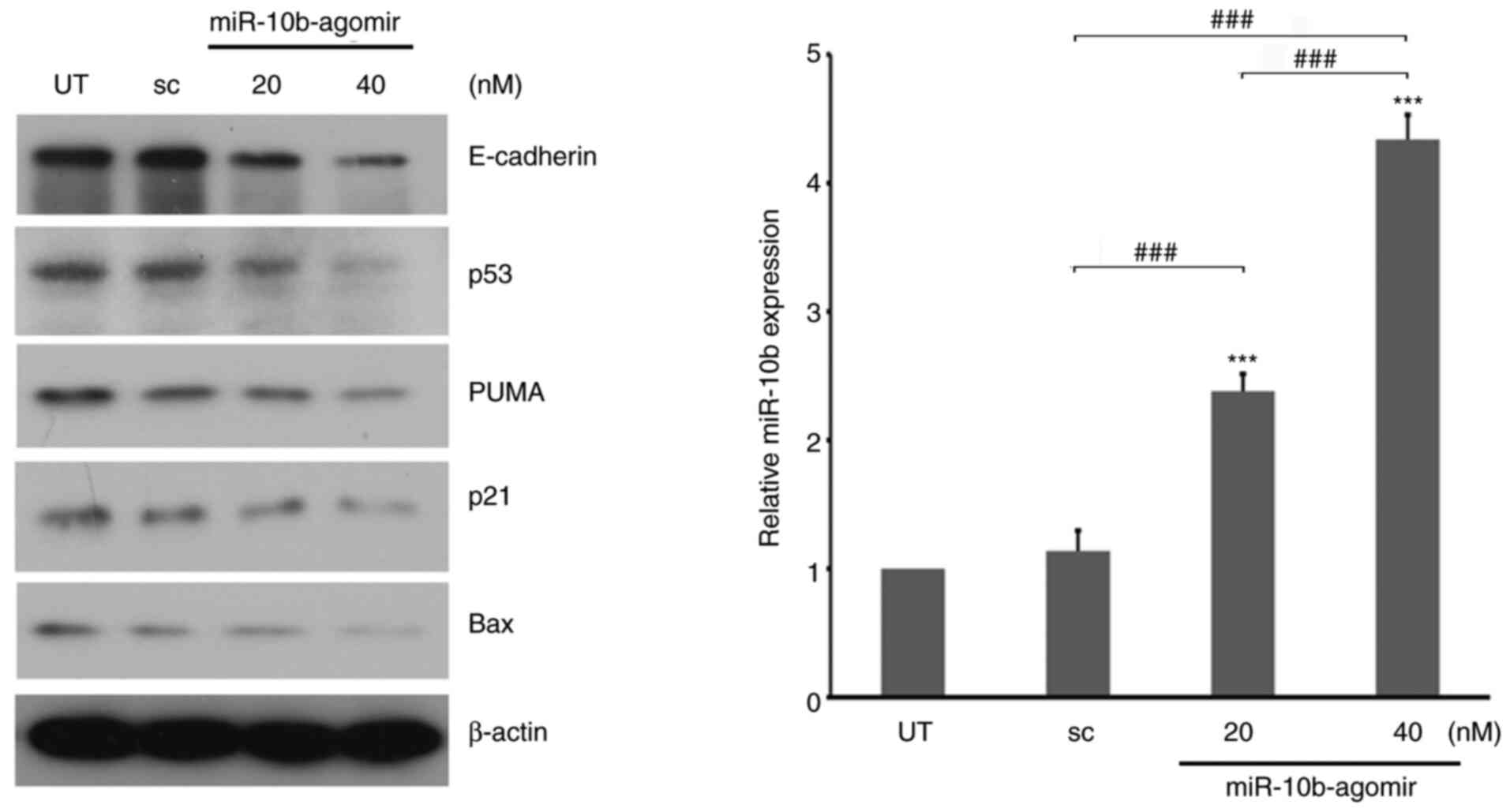
cancer (34,54). Protein expression levels were analyzed
following treatment with miR-10b. miR-10b-agomir decreased
expression levels of E-cadherin (Fig.
3), in accordance with past reports (35,55).
Levels of p53 and its downstream target genes, PUMA and Bax
(56), were also decreased in the
presence of miR-10b-agomir (Fig. 3).
These results suggested that, in addition to motility-associated
signaling, miR-10b is involved in the regulation of p53
pathways.
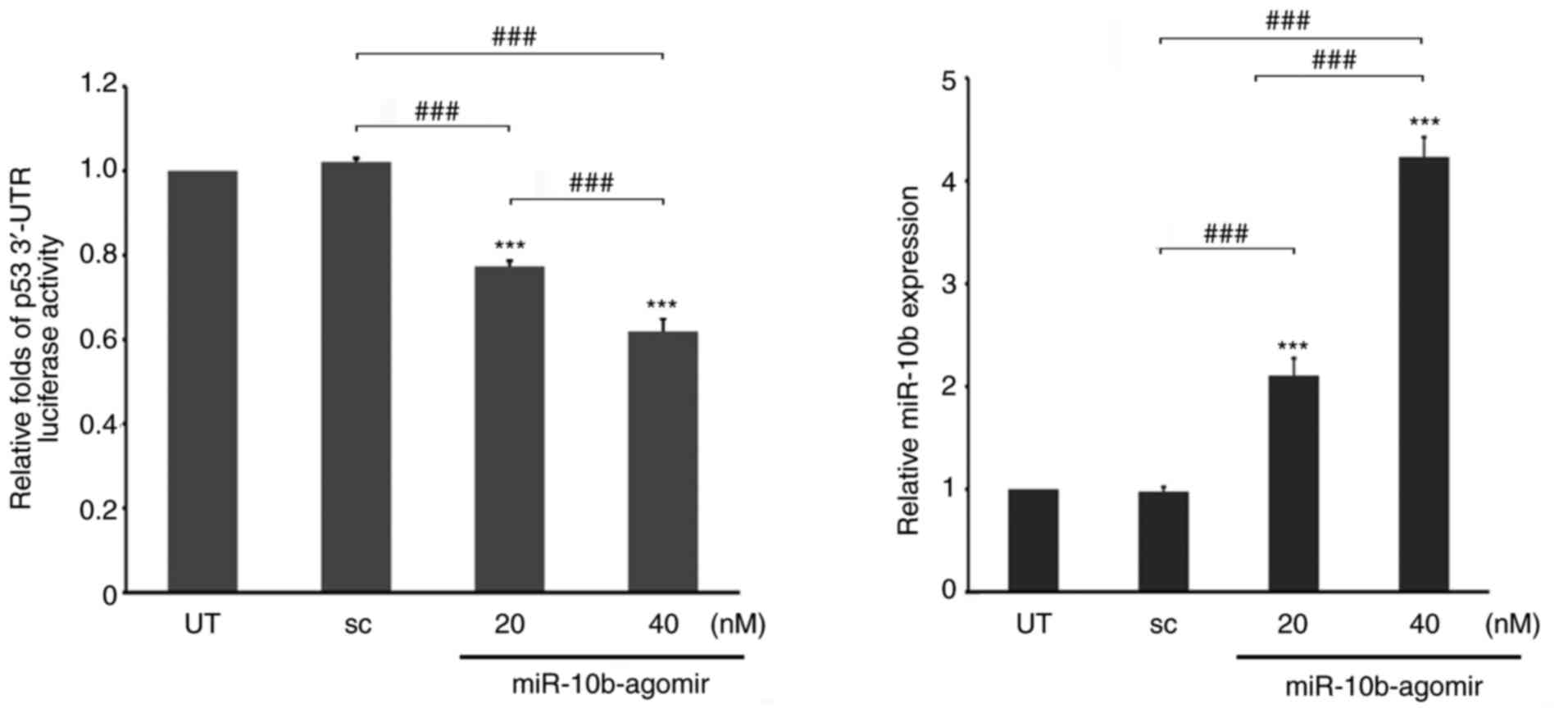
p53 is a direct target of miR-10b
In order to address whether miR-10b directly
regulates expression of p53, luciferase reporters carrying the
3′-UTR of p53 genes (1,000 bps) were treated with different doses
of miR-10b-agomir in A549 cells. These results revealed that the
ectopic expression of miR-10b significantly decreased the stability
of p53 3′-UTR in a dose-dependent manner (Fig. 4), indicating that miR-10b may directly
regulate p53 expression by binding to its 3′-UTR region. Therefore,
the binding sites of p53 3′-UTR for miR-10b were mapped. The
initial screening from multiple websites (TargetScan;
targetscan.org/vert_72/; miRbase, mirbase.org) did not reveal any
conserved binding region of p53 3′-UTR for miR-10b. However, two
partially matched regions (1,580-1,587 and 2,029-2,035 from
transcription start site) were identified. It was next determined
whether these regions were targeted by miR-10b. Two candidate
regions were identified that resembled the miR-10b-targeting
sequence of p53 3′-UTR and the corresponding mutations are depicted
in Fig. 5A. WT and single and double
mutants of p53 3′-UTR luciferase were either transfected alone or
co-transfected with miR-10b into A549 cells. These results
indicated that either 1,583 or 2,533 single mutant moderately
recovered the p53 3′-UTR stability, which was decreased by
miR-10b-agomir. However, simultaneous mutation of these two sites
significantly attenuated miR-10b-mediated instability of p53 3′-UTR
(Fig. 5B), which indicated that p53
was a target gene that was directly regulated by miR-10b.
Furthermore, these results also suggest the possibility that
miR-10b binds to its target gene via a consensus motif.
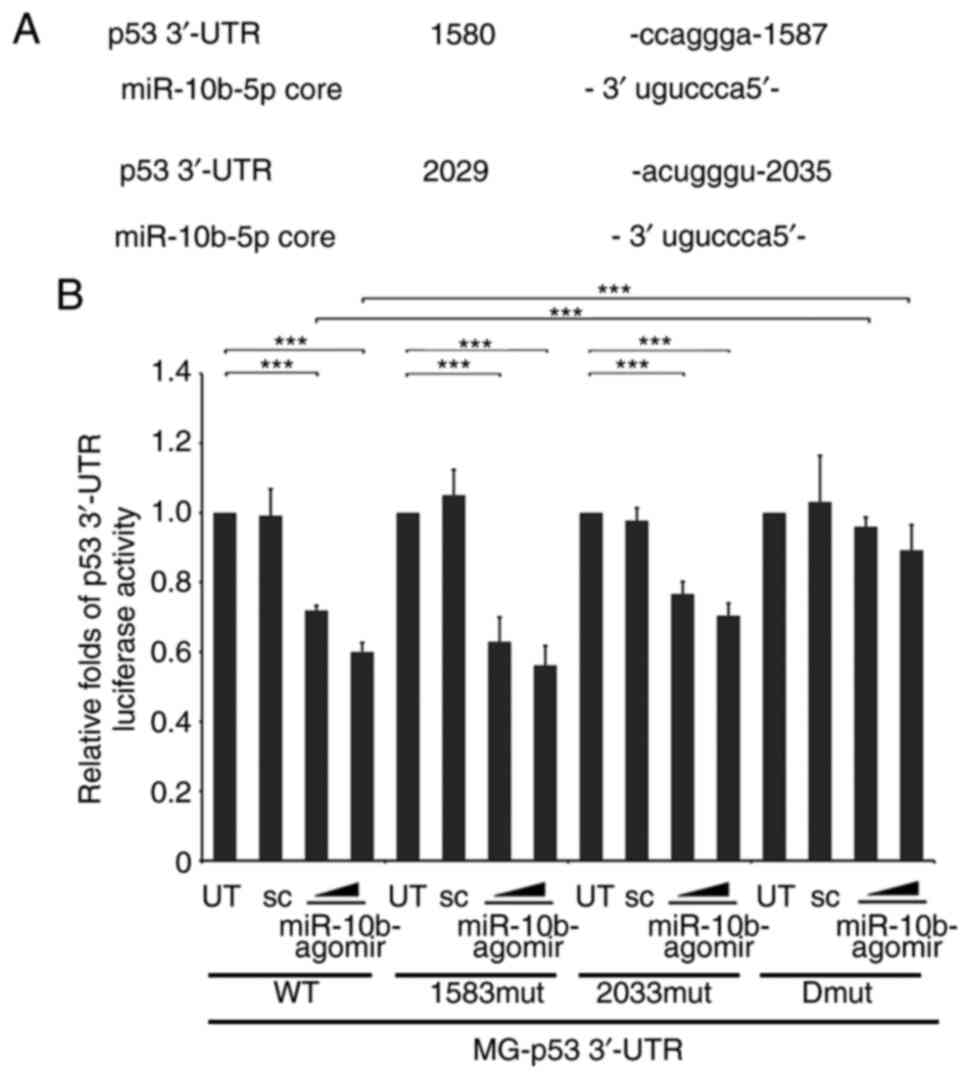 | Figure 5.Identification of target sites of
miR-10b in p53 3′-UTR. (A) Alignment between the miR-10b targeting
stem site and two consensus sites of p53 3′-UTR (1,580-1,587 and
2,029-2,035). Bold text indicates pairing miR-10b-core and
complementary target mRNA sequence. (B) WT, 1,583 or 2,023 mut or
1,583-2,023 Dmut pMGL-p53 3′-UTR luciferase reporter were
co-transfected with different doses of miR-10b-agomir into A549
cells, followed by analysis of luciferase activity. The luciferase
activity was then normalized to UT. ***P<0.001. miR, mircoRNA;
UTR, untranslated region; WT, wild-type; mut, mutant; sc, scramble;
Dmut, dual mutant. |
miR-10b is involved in upregulation of
p53 via 3′-UTR stability following treatment with cisplatin
In order to investigate whether miR-10b is involved
in the regulation of p53 expression under physiological conditions,
different doses of cisplatin, a chemotherapeutic drug for patients
with lung cancer and an inducer of p53 expression (57), was administered into A549 cells in the
presence or absence of transfection with WT or double-mutant p53
3′-UTR luciferase vector for 24 h. The results suggested that the
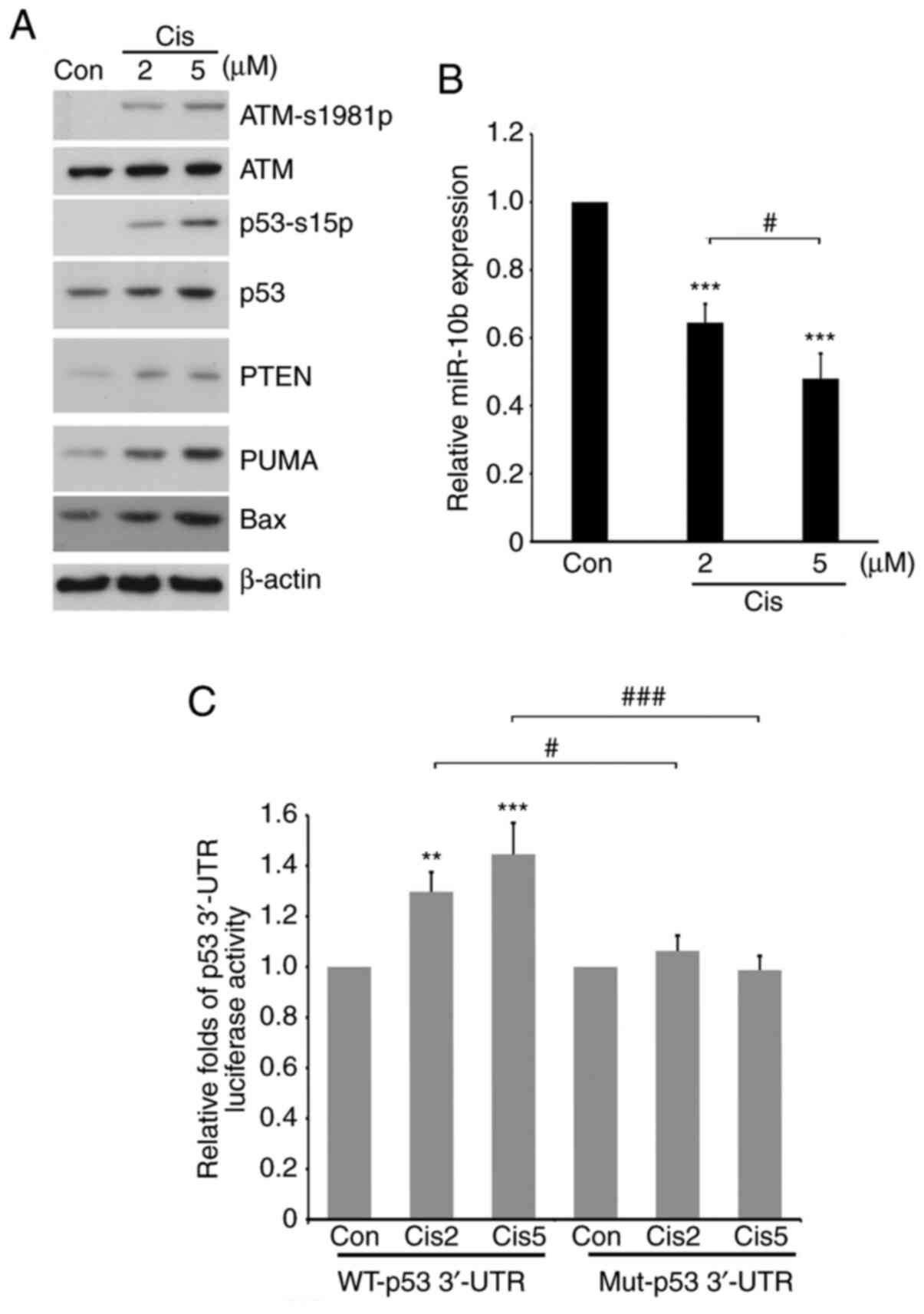
addition of cisplatin induced phosphorylation of ATM, an upstream
regulator of p53, and increased expression of p53 and its
downstream effectors, such as PUMA and Bax (Fig. 6A). Cisplatin decreased the expression
of miR-10b in a dose-dependent manner (Fig. 6B), suggesting an inverse association
between p53 and miR-10b in the presence of cisplatin. Furthermore,
the stability of WT, but not double-mutant p53 3′-UTR was increased
when treated with cisplatin (Fig.
6C), which suggested that miR-10b directly participated in the
upregulation of p53 following treatment with cisplatin. These
results indicate a novel mechanism underlying regulation of p53
expression levels following chemotherapy in NCSLC.
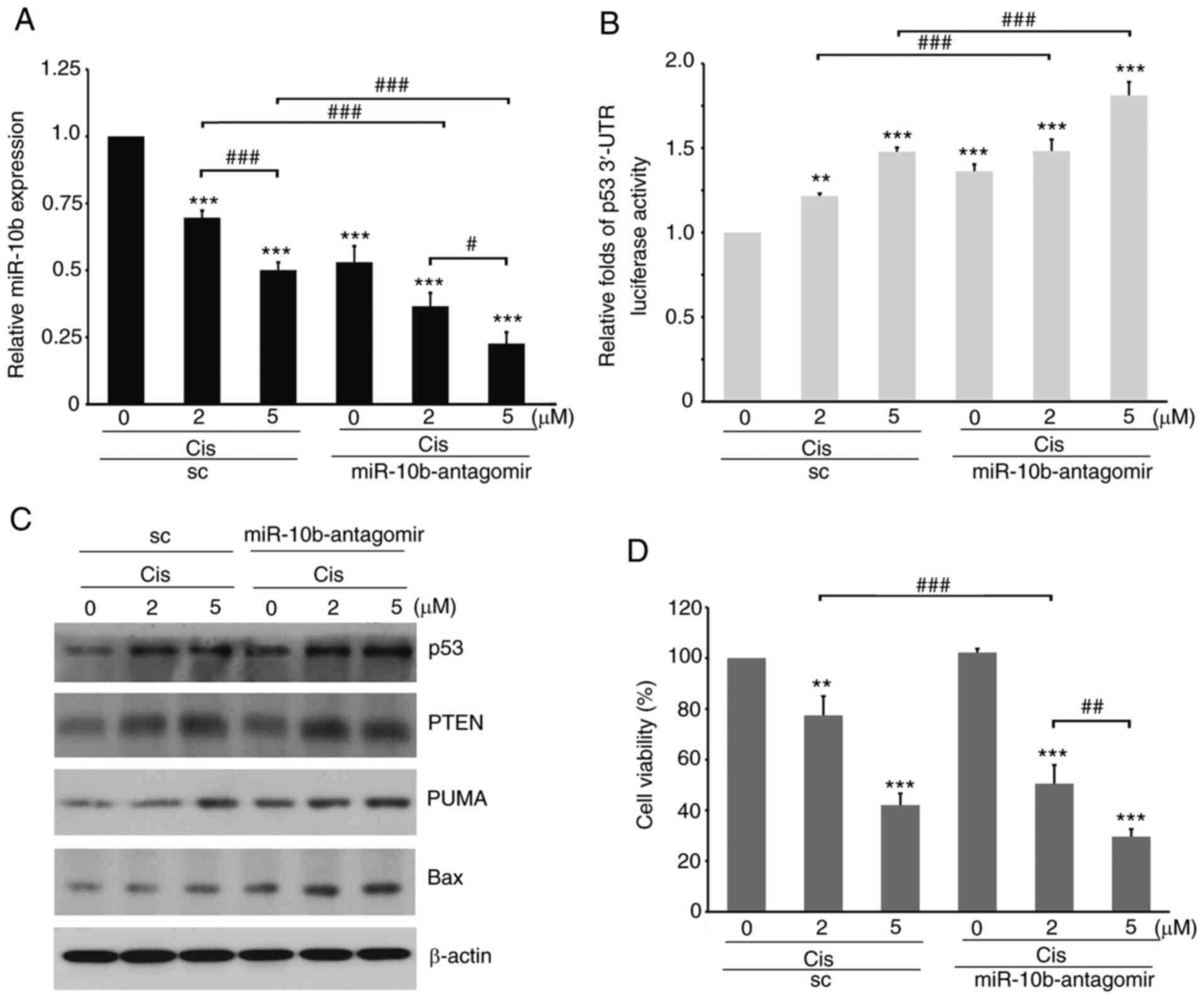
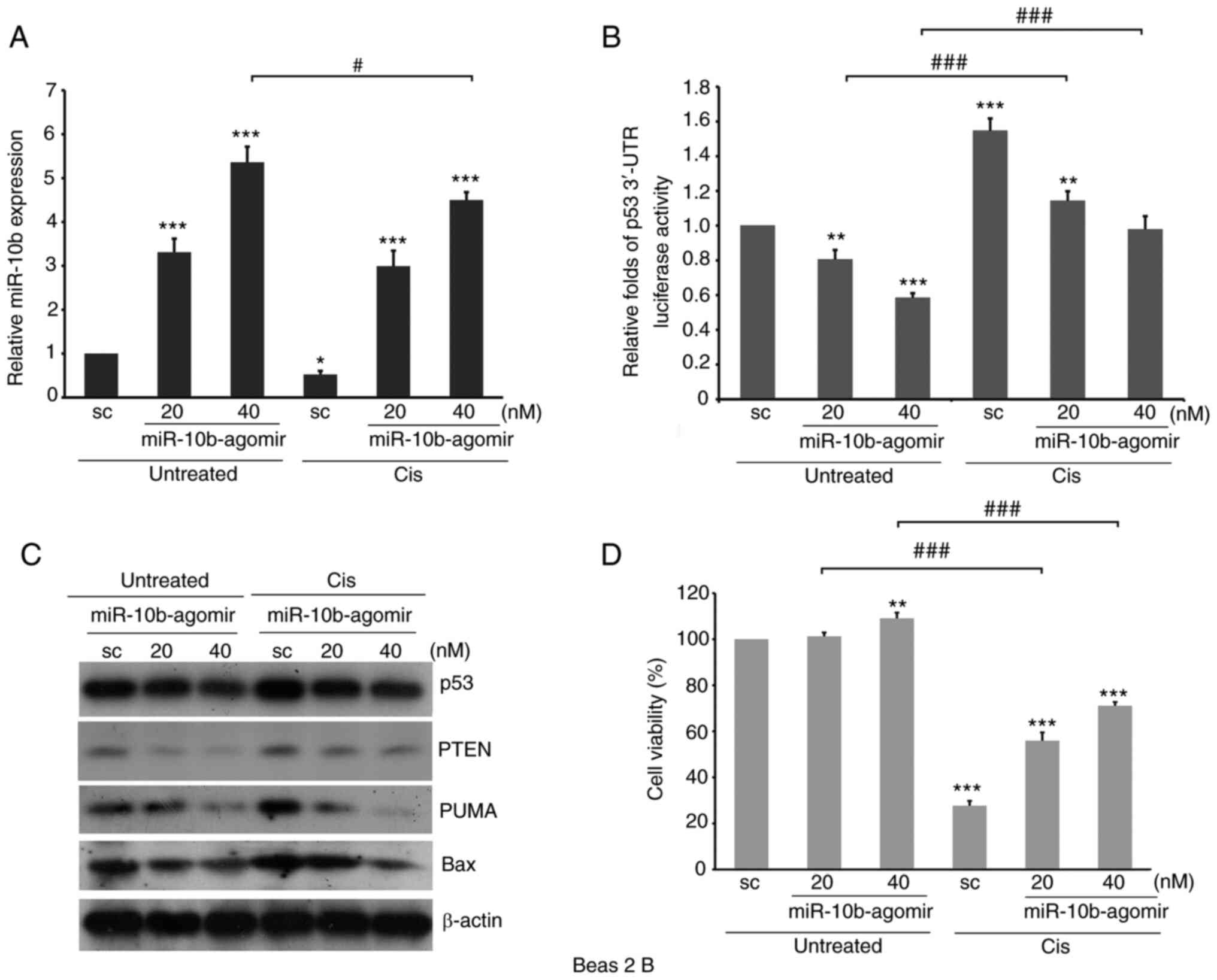
The expression level of p53 is involved in the
response to both chemotherapeutic drug treatments (58). Therefore, to characterize the role of
miR-10b in regulating the cell response to cisplatin, A549-p53
3′-UTR luciferase transfectants were transfected with
miR-10b-agomir, followed by treatment with cisplatin. Cisplatin
significantly decreased expression of endogenous miR-10b while
inducing the stability of p53 3′-UTR (Fig. 7A and B). By contrast, in cells with
ectopically expressed miR-10b-agomir, the stability of p53 3′-UTR
was low even in the presence of cisplatin. Furthermore, western
blotting revealed that p53 and its downstream effectors, such as
Bax, PUMA and PTEN, were decreased by miR-10b-agomir (Fig. 7C). On the other hand, treatment with
cisplatin increased levels of p53 signaling molecules; ectopic
expression of miR-10b-agomir attenuated such upregulation, which
activated Akt (Fig. 7C) and increased
the viability of lung cancer cells (Fig.
7D). By contrast, knockdown of miR-10b by antagomir increased
p53 3′-UTR stability and expression of p53 and its downstream
effectors (Fig. 8A and B);
furthermore, the decrease in miR-10b sensitized A549 cells to
cisplatin treatment even at a lower dose (Fig. 8C). Similar results were also observed
in non-tumorous bronchial Beas 2B cells (Fig. 9), suggesting a role of elevated
miR-10b in overcoming the therapeutic effects of cisplatin. These
results indicated that, in addition to serving as a canonical
pathway in the regulation of cell motility, miR-10b may also
participate in regulating the response to toxicity of
chemotherapeutic drugs, such as cisplatin, by directly affecting
the stability of p53 3′-UTR in tumorous and non-tumorous cells.
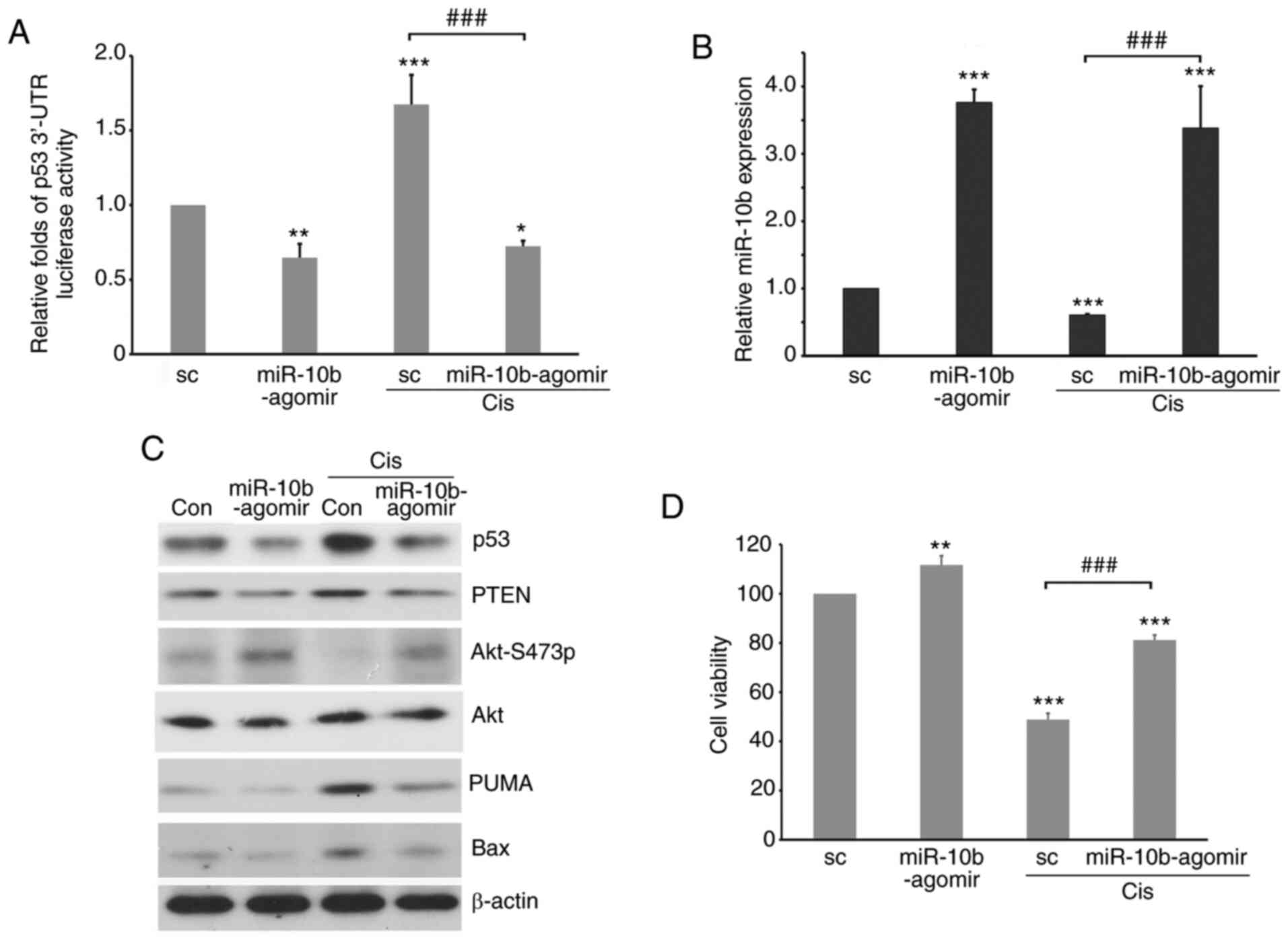 | Figure 7.Ectopic expression of miR-10b-agomir
decreases the stability of p53 3′-UTR and levels of p53 signaling
molecules induced by cis. MG-p53 3′-UTR luciferase
reporter-transfected A549 cells were transfected with
miR-10b-agomir, followed by treatment with cis for 48 h, and
subjected to (A) luciferase reporter assay to analyze p53 3′-UTR
stability, (B) reverse transcription-quantitative PCR to analyze
the expression levels of miR-10b, (C) western blotting to detect
expression levels of p53 and apoptosis-associated molecules or (D)
trypan blue exclusion assay to analyze cell viability. *P<0.05,
**P<0.01, ***P<0.001 vs. sc; ###P<0.001. miR,
microRNA; UTR, untranslated region; sc, scramble; PUMA, p53
upregulated modulator of apoptosis. |
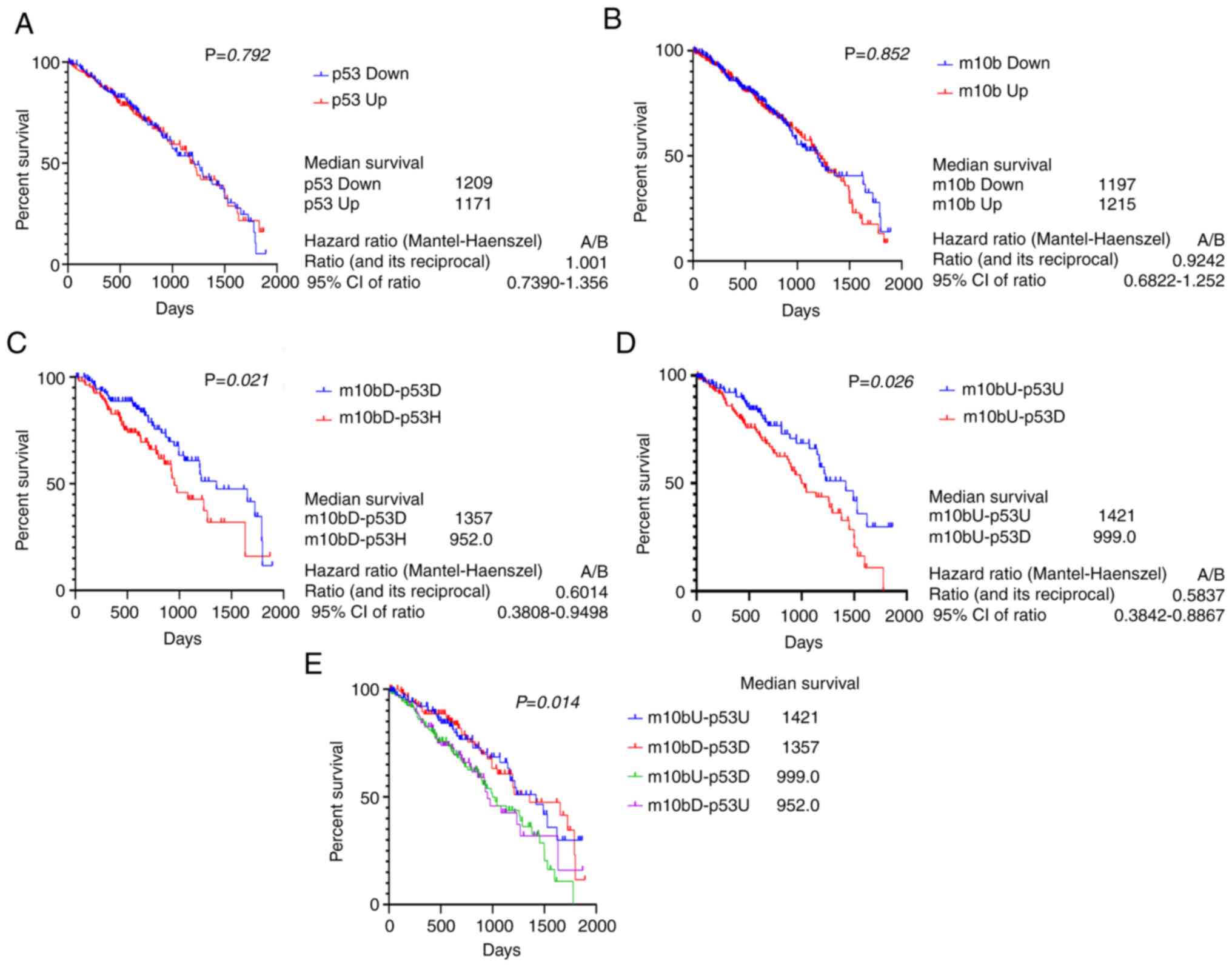
Countercurrent of miR-10b-p53 levels
are positively correlated with prognosis in patients with lung
cancer
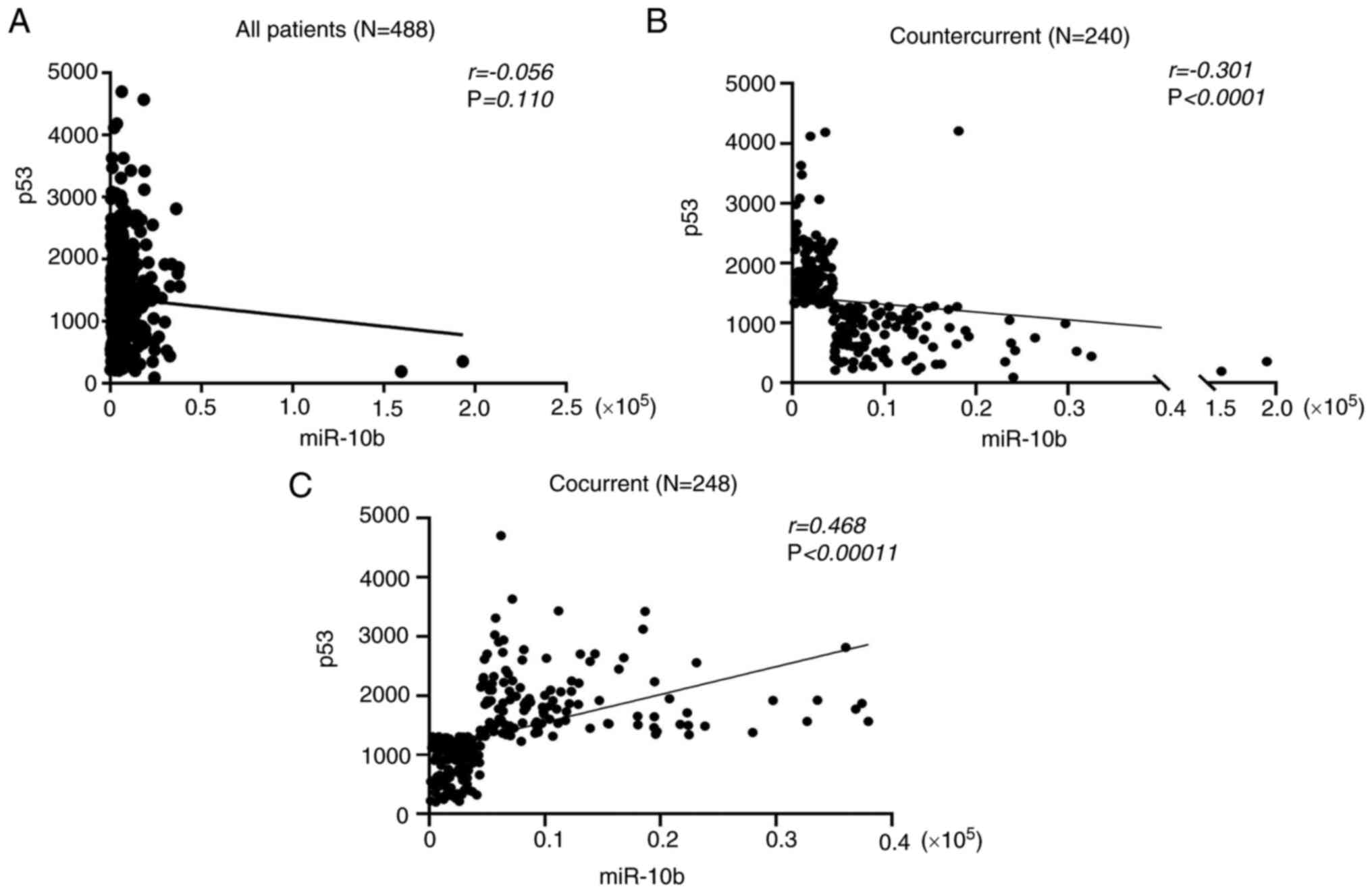
In order to understand the clinical relevance of the
association between miR-10b, p53 and the prognosis of patients with
lung cancer, Affymetrix microarray data of a lung adenocarcinoma
cancer cohort was analyzed using the Oncolnc database [data
originally derived from The Cancer Genome Atlas (TCGA) database].
In the preliminary analysis, the expression levels of miR-10b and
p53 showed no correlation in 488 patients with LUAD
(r=−0.056, Fig. 10A);
however, in a differentiated classification, the expression of
miR-10b and p53 could be categorized into two groups: Cocurrent
(same expression trend between miR-10b and p53) and countercurrent
(inverse expression trend between miR-10b and p53). The expression
association between miR-10b and p53 in the countercurrent group
indicated a moderate negative correlation (r=−0.301,
Fig. 10B); the cocurrent group of
miR-10b and p53 indicated a moderate positive correlation
(r=0.486, Fig. 10C). The
results showed that both the expression trends between miR-10b and
p53 were significant, suggesting that patients with LUAD exhibited
two distinct expression associations between miR-10b and p53.
Correlation between the miR-10b-p53 expression patterns and the
prognosis of patients with LUAD were analyzed. Kaplan-Meier plot
analysis indicated that neither miR-10b nor p53 expression-alone
was correlated with prognosis (Fig. 11A
and B). By contrast, patients with low miR-10b/high p53 status
displayed lower survival rate than those with low miR-10b/low p53
(median overall survival, 952 vs. 1,357 days, Fig. 11C) and high miR-10b/low p53 status
was associated with lower survival rate than high miR-10b/high p53
(median survival, 999 vs. 1,421 days, Fig. 11D). In addition, the survival rate of
patients with high miR-10b/low p53 was lower than for those with
high miR-10b-alone (median survival, 999 vs. 1,215 days; Fig. 11A and D). These results indicated
that the prognosis of patients with LUAD with countercurrent
expression of miR-10b and p53 was worse than those with cocurrent
expression of miR-10b and p53; low miR-10b/high p53 and high
miR-10b/low p53 were associated a similarly poor outcome (Fig. 11E).
Discussion
The miRNA miR-10b has been identified as a driver
for motility and a metastasis-associated oncomir in breast cancer
(33). The metastasis-promoting
characteristics have been confirmed in different types of cancer,
such as melanoma, hepatocellular carcinoma, glioma and acute
myeloid leukemia, as well as head and neck, endometrial, colorectal
and lung cancer (59). Several target
genes of miR-10b are associated with EMT or motility, such as
E-cadherin, HOXD10, HOXB3, KLF4 and itchy E3 ubiquitin protein
ligase (32,35,54,60,61).
Furthermore, miR-10b is involved in TGF-β-mediated EMT in breast
tumor and hyaluronan-induced migration/invasion in head and neck
tumor cells (62,63). In addition, past reports indicated
alternative roles of miR-10b in regulating cisplatin resistance via
targeting PPARγ or tamoxifen resistance via downregulation of HDAC4
(37,39). Chen et al (60) demonstrated that miR-10b simultaneously
induces proliferation and invasion via the inhibition of HOXB3 in
endometrial cancer cells. These results suggest a wide role of
miR-10b in promoting malignancy in various types of cancer cell. In
agreement with these results, the present study demonstrated that
fluctuations of endogenous miR-10b via agomir or antagomir
significantly affect the motility of A549, CL1-0 and CL1-5 cells
(data not shown). Furthermore, p53, a tumor suppressor with
multiple functions, was identified as a target gene of miR-10b. The
present results demonstrated the existence of two non-classical
binding sites of p53 3′-UTR (1,580-1,587 and 2,029-2,035) for
miR-10b and their regulation in p53 mRNA stability, which extends
knowledge of potential target genes and roles of miR-10b in
regulating physiological processes, such as drug response and
cellular growth (64,65). Lu et al (66) reported that mir-10 targets p53 in
colorectal cancer cells. The region 2,029-2,035 of p53 3′-UTR was
identified as a miR-10b regulatory element; this is consistent with
the present findings, which also identified another sequence
located in the region 1,580-1,587. Furthermore, Lu et al
revealed induction of stability of mutant p53 3′-UTR in the
presence of miR-10b. However, the present results showed that
ectopic expression of miR-10b decreased the stability of p53 3′-UTR
with a single mutation of either region 1,580-1,587 or 2,029-2,035
(data not shown). This difference may be attributed to differences
in the cell models used. In addition, Sun et al (67) reported that miR-10b regulates the p53
pathway via targeting p21 in glioblastoma, however, their results
also showed an induction of p53 following knockdown of miR-10b,
implying that miR-10b may target p53 to modulate the functions of
p53 pathway.
Several miRNAs have been shown to exert oncogenic
functions via targeting p53 expression. For example, miR-504
modulate cisplatin resistance by inhibiting p53 expression in
osteosarcoma cells (68), while
miR-675 induces invasion and metastasis by inhibiting p53
expression in colorectal cancer cells (69). Furthermore, miR-122 promotes lung
cancer cell development via inhibiting p53 expression (70). In addition, miR-125b induces cisplatin
resistance via the blockade of p53/Bax/apoptosis pathways in
nasopharyngeal cancer cells (71).
The present study demonstrated that ectopic expression of
miR-10b-agomir decreased cisplatin sensitivity of lung cancer cells
by directly inhibiting p53 levels. Since p53 is the primary
mediator of cellular transformation to tumor cells (72,73),
overexpression of miRNAs in different types of tumor may confer
malignancy by inhibiting p53 expression levels.
Despite the inverse association between miR-10b and
p53 in a cellular model in previous study (67), these two genes did not demonstrate a
negative correlation in a clinical investigation of patients with
LUAD from the TCGA database. By contrast, miR-10b and p53 showed
two distinct associations, termed cocurrent (positive correlation)
and countercurrent (negative correlation), which, to the best of
our knowledge, have not previously been observed between miR-10b
and its target genes or upstream regulators. On the other hand,
increasing evidence suggest an association between expression level
of miR-10b or p53 with the prognosis of various types of cancer;
for example, mir-10b is positively associated with the malignancies
of melanoma, glioblastoma and clear cell carcinoma, as well as
gastric, colorectal and lung cancer (35,54,67,74–76),
while p53 is positively associated with the malignancies of
colorectal (positive correlation), breast (negative correlation),
gastric (positive correlation) and lung cancer (negative
association) (49,77–79).
However, the present results showed that neither miR-10b nor
p53-alone exhibited prognostic value in patients with LUAD. Zhang
et al reported that levels of miR-10b are correlated with
lower survival rate in 73 patients with NSCLC (35). The present study analyzed 488
patients; larger sample sizes may reveal distinct patterns of
miR-10b expression levels and prognosis in patients with NSCLC. The
present results showed that patients with LUAD with cocurrence
between miR-10b and p53 showed better prognosis than those with
countercurrence. These results suggested that miR-10b and p53 may
be mutually exclusive regulators or that other regulators may
participate in modulating expression of both miR-10b and p53. p53
may serve as an upstream inhibitor of miR-10b following treatment
with Withaferin A (46) which
supports the hypothesis that p53 may downregulate miR-10b in the
presence of cisplatin. However, Bisio et al demonstrated
that p53 serves as an inducer of miR-10b expression in HCT cells
(80). This difference may be due to
the use of different cell lines and indicates the complexity of
functional correlations between miR-10b and p53. Moreover,
controversial studies also provide a potential explanation for the
distinct expression trends between miR-10b and p53 and the
subsequent survival ratio in patients with lung cancer. Further
investigation of the role of miR10b and p53 in regulating
tumorigenesis, malignancy and prognosis is required. Despite the
fact that miR-10b exhibited an inverse correlation with p53 between
non-tumor and tumorous cells, the highest level of miR-10b was
observed in CL cells, which harbor an oncogenic-mutant form of p53
that promotes cell invasion and chemoresistance by induing Slug and
Nrf2, respectively (21,81); whether miRNAs are involved in
oncogenic mutant p53-primed malignancy remains unclear. Changing
levels of miR-10b only marginally affected levels of p53 in CL
cells (data not shown), which suggested different regulatory
mechanisms between miR-10b and oncogenic mutant p53 may exist. In
addition, correlation analysis between p53 mutations and miR-10b
expression levels suggested no significant difference in miR-10b
expression levels between WT and mutated p53 (data not shown).
These results suggest that alternatively regulated mechanisms
between p53 and miR-10b exist. Moreover, the expression of miR-10b
was independent of the mutated status of p53 in patients with lung
cancer, which implies that miR-10b may serve as an upstream
regulator of p53 in patients with LUAD. Our previous report
indicated that Aurora-A and p53 exhibit an inverse association in
patients with NSCLC and that combining expression levels of p53 and
Aurora-A provides better prognostic potential than individual
consideration in patients with lung cancer (49). Similarly, the present study
demonstrated that combining miR-10b and p53 may provide a more
precise predictive value of patients with NSCLC with poor prognosis
who may benefit from specific targeted therapy. One limitation of
the present study is the lack of direct in vivo evidence to confirm
the association between miR-10b and p53 in drug-tolerance. The role
of miR-10b-p53 in mice bearing cisplatin-sensitive or resistant
lung tumors and the effects of miR-10b-antagomir in treating mice
with cisplatin-resistant lung tumor should be investigated in the
future.
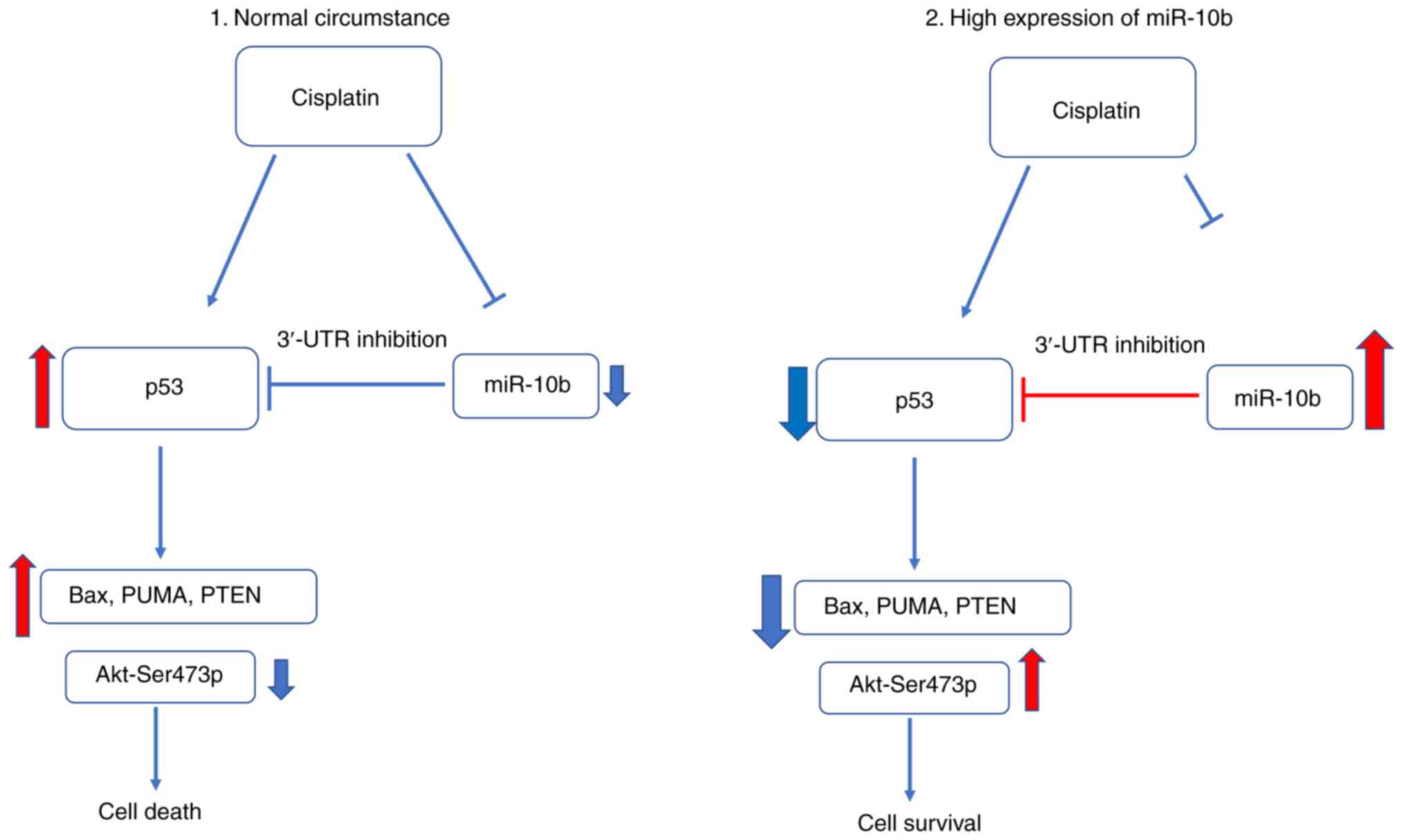
In summary, the present study identified p53 as a
target gene of miR-10b and revealed a pathway to increase p53
following treatment of cisplatin via downregulating levels of
miR-10b (Fig. 12). In addition, the
present study also revealed a novel function of miR-10b in
modulating drug tolerance in addition to promoting motility.
Furthermore, clinical investigation revealed that the combination
of miR-10b and p53 expression provided more precise prognosis of
patients with lung cancer. These findings improve understanding of
the role of miR-10b in regulating multiple malignancy in lung
cancer cells and offer more valuable prognostic prediction for
patients with lung cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Chung Shan
Medical University (grant no. CSMU-INT-106), Taichung Veterans
General Hospital (grant no. TCVGH-1073209D) and Show Chwan Memorial
Hospital (grant no. SRD109008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CCL performed qPCR, western blotting and data
analysis. TY, HJ and WT analyzed data. TY, SL and CCW participated
in experimental design and manuscript drafting. CCW, CCL and TYY
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
miR
|
microRNA
|
|
SCLC
|
small cell lung cancer
|
|
NSCLC
|
non-small cell lung cancer cell
|
|
LUAD
|
lung adenocarcinoma
|
|
UTR
|
untranslated region
|
|
PUMA
|
p53 upregulated modulator of
apoptosis
|
References
|
1
|
Bach PB, Mirkin JN, Oliver TK, Azzoli CG,
Berry DA, Brawley OW, Byers T, Colditz GA, Gould MK, Jett JR, et
al: Benefits and harms of CT screening for lung cancer: A
systematic review. JAMA. 307:2418–2429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brenner DR, McLaughlin JR and Hung RJ:
Previous lung diseases and lung cancer risk: A systematic review
and meta-analysis. PLoS One. 6:e174792011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hutchinson L: Practical assay to predict
survival. Nat Rev Clin Oncol. 9:1272012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ranpura V, Hapani S and Wu S:
Treatment-related mortality with bevacizumab in cancer patients: A
meta-analysis. JAMA. 305:487–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seton-Rogers S: Metastasis: Opposing
forces in invasion. Nat Rev Cancer. 11:624–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bray F, Ferlay J, Soerjomataram I, Siegel
R, Torre L and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Camidge DR, Pao W and Sequist LV: Acquired
resistance to TKIs in solid tumours: Learning from lung cancer. Nat
Rev Clin Oncol. 11:473–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Zhang G, Zhang H, Zhang F, Zhou B,
Ning F, Wang HS, Cai SH and Du J: Acquisition of
epithelial-mesenchymal transition phenotype and cancer stem
cell-like properties in cisplatin-resistant lung cancer cells
through AKT/β-catenin/Snail signaling pathway. Eur J Pharmacol.
723:156–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arumugam T, Ramachandran V, Fournier KF,
Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey
DJ and Choi W: Epithelial to mesenchymal transition contributes to
drug resistance in pancreatic cancer. Cancer Res. 69:5820–5828.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Toledo F and Wahl GM: Regulating the p53
pathway: In vitro hypotheses, in vivo veritas. Nat Rev Cancer.
6:909–913. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koumenis C, Alarcon R, Hammond E, Sutphin
P, Hoffman W, Murphy M, Derr J, Taya Y, Lowe SW, Kastan M and
Giaccia A: Regulation of p53 by hypoxia: Dissociation of
transcriptional repression and apoptosis from p53-dependent
transactivation. Mol Cell Biol. 21:1297–1310. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lakin N and Jackson S: Regulation of p53
in response to DNA damage. Oncogene. 18:7644–7655. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ravi R, Mookerjee B, Bhujwalla ZM, Sutter
CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL and Bedi A:
Regulation of tumor angiogenesis by p53-induced degradation of
hypoxia-inducible factor 1alpha. Genes Dev. 14:34–44.
2000.PubMed/NCBI
|
|
15
|
Wang X, Huang G, Mei S, Qian J, Ji J and
Zhang J: Over-expression of C/EBP-alpha induces apoptosis in
cultured rat hepatic stellate cells depending on p53 and peroxisome
proliferator-activated receptor-gamma. Biochem Biophys Res Commun.
380:286–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vilborg A, Glahder JA, Wilhelm MT, Bersani
C, Corcoran M, Mahmoudi S, Rosenstierne M, Grandér D, Farnebo M,
Norrild B and Wiman KG: The p53 target Wig-1 regulates p53 mRNA
stability through an AU-rich element. Proc Natl Acad Sci USA.
106:15756–15761. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vousden KH and Prives C: Blinded by the
light: The growing complexity of p53. Cell. 137:413–431. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soussi T and Beroud C: Assessing TP53
status in human tumours to evaluate clinical outcome. Nat Rev
Cancer. 1:233–240. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brown JM and Attardi LD: The role of
apoptosis in cancer development and treatment response. Nat Rev
Cancer. 5:231–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hwang CI, Matoso A, Corney DC,
Flesken-Nikitin A, Körner S, Wang W, Boccaccio C, Thorgeirsson SS,
Comoglio PM, Hermeking H and Nikitin AY: Wild-type p53 controls
cell motility and invasion by dual regulation of MET expression.
Proc Natl Acad Sci USA. 108:14240–14245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang SP, Wang WL, Chang YL, Wu CT, Chao
YC, Kao SH, Yuan A, Lin CW, Yang SC, Chan WK, et al: p53 controls
cancer cell invasion by inducing the MDM2-mediated degradation of
Slug. Nat Cell Biol. 11:694–704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gibbons DL, Byers LA and Kurie JM:
Smoking, p53 mutation, and lung cancer. Mol Cancer Res. 12:3–13.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cuperus JT, Fahlgren N and Carrington JC:
Evolution and functional diversification of MIRNA genes. The Plant
Cell. 23:431–442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y and Kowdley KV: MicroRNAs in common
human diseases. Genomics Proteomics Bioinformatics. 10:246–253.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lange A, Prenzler A, Frank M, Golpon H,
Welte T and von der Schulenburg JM: A systematic review of the
cost-effectiveness of targeted therapies for metastatic non-small
cell lung cancer (NSCLC). BMC Pulm Med. 14:1922014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Durrant DE and Morrison DK: Targeting the
Raf kinases in human cancer: The Raf dimer dilemma. Br J Cancer.
118:3–8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bouchie A: First microRNA mimic enters
clinic. Nat Biotechnol. 31:5772013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raza U, Zhang JD and Şahin Ö: MicroRNAs:
Master regulators of drug resistance, stemness, and metastasis. J
Mol Med. 92:321–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu Y, Yao J, Yu J, Wei Q and Cao X: The
association between abnormal microRNA-10b expression and cancer
risk: A meta-analysis. Sci Rep. 4:74982014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma Z, Chen Y, Min L, Li L, Huang H, Li J,
Yan Q, Song P, Dai L and Yao X: Augmented miR-10b expression
associated with depressed expression of its target gene KLF4
involved in gastric carcinoma. Int J Clin Exp Pathol. 8:5071–5079.
2015.PubMed/NCBI
|
|
33
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo Y, Lang X, Lu Z, Wang J, Li T, Liao Y,
Jia C, Zhao W and Fang H: MiR-10b directly targets ZEB1 and PIK3CA
to curb adenomyotic epithelial cell invasiveness via upregulation
of E-cadherin and inhibition of Akt phosphorylation. Cell Physiol
Biochem. 35:2169–2180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang J, Xu L, Yang Z, Lu H, Hu D, Li W,
Zhang Z, Liu B and Ma S: MicroRNA-10b indicates a poor prognosis of
non-small cell lung cancer and targets E-cadherin. Clin Transl
Oncol. 17:209–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moriarty CH, Pursell B and Mercurio AM:
miR-10b targets Tiam1 implications for Rac activation and carcinoma
migration. J Biol Chemistry. 285:20541–20546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ahmad A, Ginnebaugh KR, Yin S,
Bollig-Fischer A, Reddy KB and Sarkar FH: Functional role of
miR-10b in tamoxifen resistance of ER-positive breast cancer cells
through down-regulation of HDAC4. BMC Cancer. 15:5402015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nishida N, Yamashita S, Mimori K, Sudo T,
Tanaka F, Shibata K, Yamamoto H, Ishii H, Doki Y and Mori M:
MicroRNA-10b is a prognostic indicator in colorectal cancer and
confers resistance to the chemotherapeutic agent 5-fluorouracil in
colorectal cancer cells. Ann Surg Oncol. 19:3065–3071. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu K, Hu Y, Yan K, Qi Y, Zhang C, Zhu D,
Liu D and Zhao S: microRNA-10b confers cisplatin resistance by
activating AKT/mTOR/P70S6K signaling via targeting PPARγ in
esophageal cancer. J Cell Physiol. 235:1247–1258. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Setoyama T, Zhang X, Natsugoe S and Calin
GA: microRNA-10b: A new marker or the marker of pancreatic ductal
adenocarcinoma? Clin Cancer Res. 17:5527–5529. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang J, Sun C, Wang S, He Q and Li D:
MicroRNA miR-10b inhibition reduces cell proliferation and promotes
apoptosis in non-small cell lung cancer (NSCLC) cells. Mol Biosyst.
11:2051–2059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fang CY, Yu CC, Liao YW, Hsieh PL, Ohiro
Y, Chu PM, Huang YC, Yu CH and Tsai LL: miR-10b regulated by Twist
maintains myofibroblasts activities in oral submucous fibrosis. J
Formos Med Assoc. 119:1167–1173. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsukerman P, Stern-Ginossar N, Gur C,
Glasner A, Nachmani D, Bauman Y, Yamin R, Vitenshtein A, Stanietsky
N, Bar-Mag T, et al: MiR-10b downregulates the stress-induced cell
surface molecule MICB, a critical ligand for cancer cell
recognition by natural killer cells. Cancer Res. 72:5463–5472.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang L, Liu W, Zhang Y, Hu Z, Guo H, Lv J
and Du H: Dexmedetomidine had neuroprotective effects on
hippocampal neuronal cells via targeting lncRNA SHNG16 mediated
microRNA-10b-5p/BDNF axis. Mol Cell Biochem. 469:41–51. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu X, Li Z, Chen G and Wu WK: MicroRNA-10b
induces vascular muscle cell proliferation through Akt pathway by
targeting TIP30. Curr Vasc Pharmacol. 13:679–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lin CC, Yang TY, Lu HJ, Wan CK, Hsu SL and
Wu CC: Attenuating role of withaferin A in the proliferation and
migration of lung cancer cells via a p53-miR-27a/miR-10b pathway.
Oncol Lett. 21:2322021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Falasca M, Raimondi C and Maffucci T:
Boyden chamber. Cell Migration Springer. 87–95. 2011. View Article : Google Scholar
|
|
49
|
Yang TY, Teng CLJ, Lin TCC, Chen KC, Hsu
SL and Wu CC: Transcriptional repression of Aurora-A gene by
wild-type p53 through directly binding to its promoter with histone
deacetylase 1 and mSin3a. Int J Cancer. 142:92–108. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Anaya J: OncoLnc: Linking TCGA survival
data to mRNAs, miRNAs, and lncRNAs. PeerJ Computer Sci. 2:e672016.
View Article : Google Scholar
|
|
51
|
Mavrevski R, Traykov M, Trenchev I and
Trencheva M: Approaches to modeling of biological experimental data
with GraphPad Prism software. WSEAS Transactions Systems Control.
13:242–247. 2018.
|
|
52
|
Lund AH: miR-10 in development and cancer.
Cell Death Differ. 17:209–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liao CG, Kong LM, Zhou P, Yang XL, Huang
JG, Zhang HL and Lu N: miR-10b is overexpressed in hepatocellular
carcinoma and promotes cell proliferation, migration and invasion
through RhoC, uPAR and MMPs. J Transl Med. 12:2342014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang Y, Li Z, Zhao X, Zuo X and Peng Z:
miR-10b promotes invasion by targeting HOXD10 in colorectal cancer.
Oncol Lett. 12:488–494. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu Y, Zhao J, Zhang PY, Zhang Y, Sun SY,
Yu SY and Xi QS: MicroRNA-10b targets E-cadherin and modulates
breast cancer metastasis. Med Sci Monit. 18:BR299–BR308. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lee DH, Kim C, Zhang L and Lee YJ: Role of
p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer
cells. Biochem Pharmacol. 75:2020–2033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fan S, Smith ML, Rivet DJ II, Duba D, Zhan
Q, Kohn KW, Fornace AJ Jr and O'Connor PM: Disruption of p53
function sensitizes breast cancer MCF-7 cells to cisplatin and
pentoxifylline. Cancer Res. 55:1649–1654. 1995.PubMed/NCBI
|
|
58
|
Vazquez A, Bond EE, Levine AJ and Bond GL:
The genetics of the p53 pathway, apoptosis and cancer therapy. Nat
Rev Drug Discov. 7:979–987. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Huang Q, Song Q, Zhong W, Chen Y and Liang
L: MicroRNA-10b and the clinical outcomes of various cancers: A
systematic review and meta-analysis. Clin Chim Acta. 474:14–22.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen H, Fan Y, Xu W, Chen J, Xu C, Wei X,
Fang D and Feng Y: miR-10b inhibits apoptosis and promotes
proliferation and invasion of endometrial cancer cells via
targeting HOXB3. Cancer Biother Radiopharm. 31:225–231. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang S, Wu Y, Xu Y and Tang X: miR-10b
promoted melanoma progression through Wnt/β-catenin pathway by
repressing ITCH expression. Gene. 710:39–47. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Han X, Yan S, Weijie Z, Feng W, Liuxing W,
Mengquan L and Qingxia F: Critical role of miR-10b in transforming
growth factor-β1-induced epithelial-mesenchymal transition in
breast cancer. Cancer Gene Ther. 21:60–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bourguignon LY: Matrix hyaluronan-CD44
interaction activates microRNA and lncRNA signaling associated with
chemoresistance, invasion, and tumor progression. Front Oncol.
9:4922019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
El-Deiry WS: The role of p53 in
chemosensitivity and radiosensitivity. Oncogene. 22:7486–7495.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ho J, Ma W, Mao D and Benchimol S:
p53-Dependent transcriptional repression of c-myc is required for
G1 cell cycle arrest. Mol Cell Biol. 25:7423–7431. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lu C, Jiang W, Hui B, Rong D, Fu K, Dong
C, Tang W and Cao H: The circ_0021977/miR-10b-5p/P21 and P53
regulatory axis suppresses proliferation, migration, and invasion
in colorectal cancer. J Cell Physiol. 235:2273–2285. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sun B, Zhao X, Ming J, Liu X, Liu D and
Jiang C: Stepwise detection and evaluation reveal miR-10b and
miR-222 as a remarkable prognostic pair for glioblastoma. Oncogene.
38:6142–6157. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hu W, Chan CS, Wu R, Zhang C, Sun Y, Song
JS, Tang LH, Levine AJ and Feng Z: Negative regulation of tumor
suppressor p53 by microRNA miR-504. Mol Cell. 38:689–699. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu C, Chen Z, Fang J, Xu A, Zhang W and
Wang Z: H19-derived miR-675 contributes to bladder cancer cell
proliferation by regulating p53 activation. Tumor Biol. 37:263–270.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Manfè V, Biskup E, Rosbjerg A, Kamstrup M,
Skov AG, Lerche CM, Lauenborg BT, Odum N and Gniadecki R: miR-122
regulates p53/Akt signalling and the chemotherapy-induced apoptosis
in cutaneous T-cell lymphoma. PLoS One. 7:e295412012. View Article : Google Scholar
|
|
71
|
Le MT, Teh C, Shyh-Chang N, Xie H, Zhou B,
Korzh V, Lodish HF and Lim B: MicroRNA-125b is a novel negative
regulator of p53. Genes Dev. 23:862–876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Muller PA and Vousden KH: p53 mutations in
cancer. Nat Cell Biol. 15:2–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Stiewe T: The p53 family in
differentiation and tumorigenesis. Nat Rev Cancer. 7:165–167. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Bai M, Zhang H, Si L, Yu N, Zeng A and
Zhao R: Upregulation of serum miR-10b is associated with poor
prognosis in patients with melanoma. J Cancer. 8:2487–2491. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Khella HW, Daniel N, Youssef L, Scorilas
A, Nofech-Mozes R, Mirham L, Krylov SN, Liandeau E, Krizova A,
Finelli A, et al: miR-10b is a prognostic marker in clear cell
renal cell carcinoma. J Clin Pathol. 70:854–859. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang YY, Ye ZY, Zhao ZS, Li L, Wang YX,
Tao HQ, Wang HJ and He XJ: Clinicopathologic significance of
miR-10b expression in gastric carcinoma. Hum Pathol. 44:1278–1285.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang P, Liang J, Wang Z, Hou H, Shi L and
Zhou Z: The prognostic value of p53 positive in colorectal cancer:
A retrospective cohort study. Tumor Biol.
39:10104283177036512017.PubMed/NCBI
|
|
78
|
Santoro A, Vlachou T, Luzi L, Melloni G,
Mazzarella L, D'Elia E, Aobuli X, Pasi CE, Reavie L, Bonetti P, et
al: p53 loss in breast cancer leads to Myc activation, increased
cell plasticity, and expression of a mitotic signature with
prognostic value. Cell Rep. 26:624–638.e8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yildirim M, Kaya V, Demirpence O, Gunduz S
and Bozcuk H: Prognostic significance of p53 in gastric cancer: A
meta-analysis. Asian Pac J Cancer Prev. 16:327–332. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Bisio A, De Sanctis V, Del Vescovo V,
Denti MA, Jegga AG, Inga A and Ciribilli Y: Identification of new
p53 target microRNAs by bioinformatics and functional analysis. BMC
Cancer. 13:5522013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tung MC, Lin PL, Wang YC, He TY, Lee MC,
Yeh SD, Chen CY and Lee H: Mutant p53 confers chemoresistance in
non-small cell lung cancer by upregulating Nrf2. Oncotarget.
6:41692–41705. 2015. View Article : Google Scholar : PubMed/NCBI
|