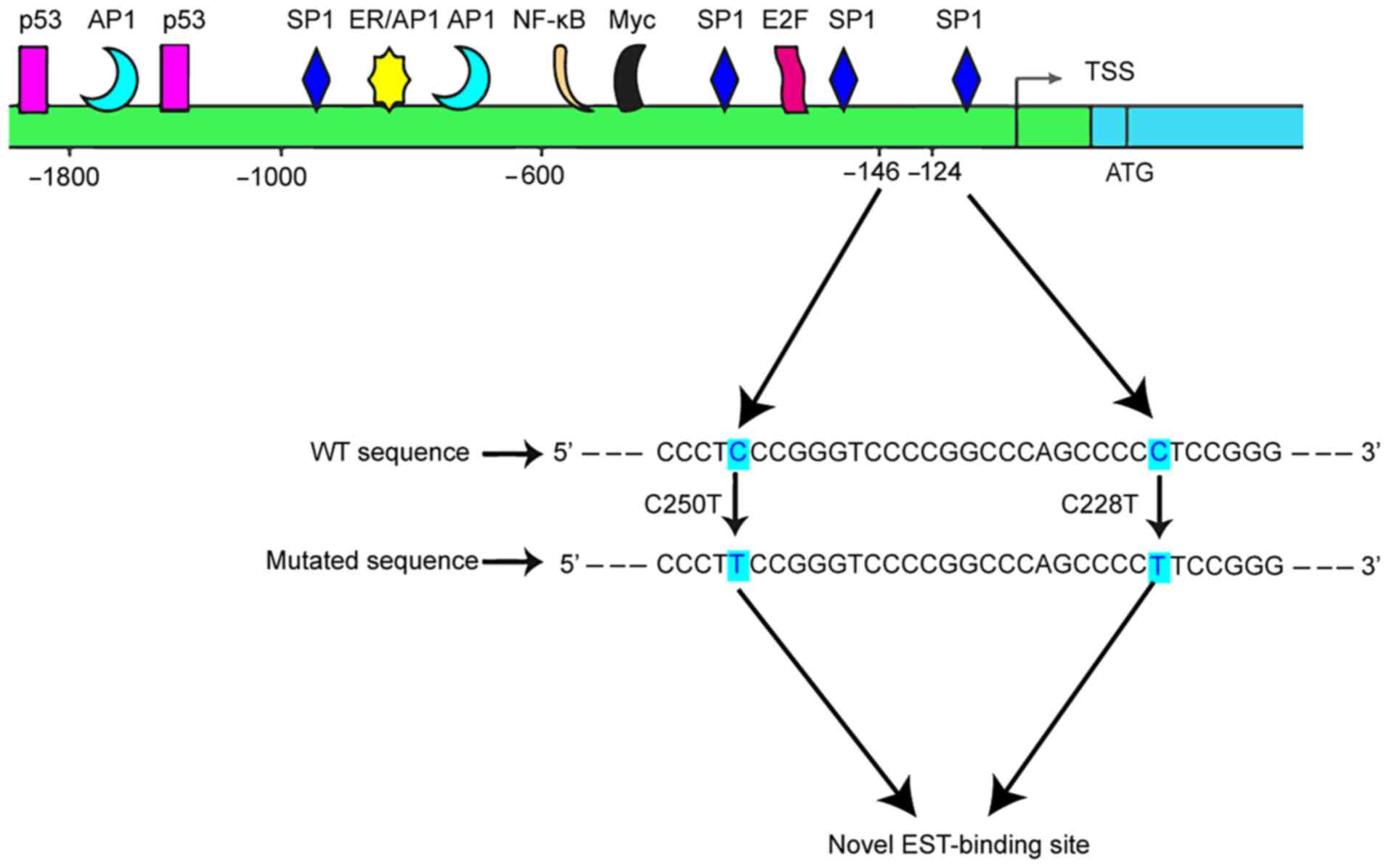
Lung cancer seriously endangers human health, and
has the highest morbidity and mortality rates among all malignant
tumors worldwide (1). While the
mechanisms underlying lung cancer pathogenesis have not been fully
elucidated (2), previous studies have
revealed that it may be associated with tobacco consumption, living
environment, genetic factors and the abnormal regulation of
oncogenes and tumor-suppressor genes (3–5).
According to its histological characteristics, lung
cancer is mainly divided into two major categories: Small cell lung
cancer (SCLC) and non-small cell lung cancer (NSCLC), which account
for ~15 and ~85% cases, respectively (6). In the early stage of NSCLC, surgical
treatment can achieve the best prognosis for patients (7). Unfortunately, the majority of patients
diagnosed with NSCLC are at an advanced stage and therefore require
comprehensive treatment. Over the past few decades, while the
development of novel treatments, innovative surgical procedures and
effective clinical management have improved the survival of
patients with NSCLC to some extent, the 5-year survival rate
remains poor at 10–15% (8–11). Therefore, improvements in therapeutic
strategies are urgently required. Moreover, the discovery of
molecular heterogeneity in NSCLC suggests the complexity of this
cancer (12).
Basic biology suggests that telomere maintenance is
an attractive candidate mechanism for potential cancer risk
(13). The telomere is a special
heterochromatic structure consisting of repetitive nucleotide
sequences (TTAGGG) and a telomere-associated protein complex at the
end of the linear chromosome, referred to as Shelterin. Moreover,
it can protect the ends of chromosomes from end-to-end fusion and
degradation, and then serves an important role in ensuring genomic
stability and integrity (14). The
length and stability of telomeres can determine the cell lifespan
and are closely associated with cellular aging and tumor formation
(15). In most normal cells, the
telomere becomes progressively shorter after each cell division,
and when it is shortened to a certain critical point, cells stop
dividing and cellular senescence is triggered. This is considered
as a strong tumor suppressor mechanism in humans (16–18).
However, most immortal tumor cells can overcome their fate of
senescence via telomere length maintenance, mainly by expressing or
re-activating telomerase, which adds nucleotides to the ends of
telomeres to extend the telomere length and cell proliferative
potential (19).
Telomerase is a ribonucleoprotein enzyme composed of
multi-subunits, and its core holoenzyme includes a catalytic
protein subunit referred to as human telomerase reverse
transcriptase (hTERT encoded by the TERT gene on chromosome
5p15.33) and an RNA component known as human telomerase RNA (hTERC
encoded by TERC gene on chromosomal region 3q26) (20,21). The
TERT gene is stringently repressed, which consequently leads
to telomerase silencing. Therefore, TERT regulation is the
rate-limiting factor for telomerase activity (22). Previous studies have demonstrated
that, compared with TERC, the correlation between hTERT
expression and telomerase has a consistency of 88.9% (23). Telomerase activity is hardly
detectable in most somatic cells, apart from those in the spinal
cord and peripheral blood or bone marrow (24). However, TERT expression and
telomerase activity can be detectable in up to 90% of tumor
tissues, which may cause immortal cells to sustain tumor growth
(19). Thus, targeting telomerase is
more universal compared with most other cancer targets, and it
represents a critical target specific to cancer cells.
Accumulating evidence has shown that genetic factors
serve an important role in regulating TERT expression, which
is associated with TERT point mutations, DNA amplification,
rearrangement and transcription, and predicted telomerase activity
(25–28).
The aim of the present review was to summarized the
clinical significance of telomerase activity in NSCLC and discuss
the mechanism underlying telomerase activity regulation by
TERT, as well as its effect on NSCLC. The mechanism of
action and the current research progress of targeted telomerase
drugs in NSCLC were also evaluated.
As aforementioned, increased telomerase activity has
been observed in various types of human malignancies, including
NSCLC tissues. Several studies have reported that hTERT mRNA
and telomerase activity were significantly higher in cancerous
tissue compared with those in the lung parenchyma free from
neoplastic infiltration (29–32), which confirms that telomerase activity
may serve an important role in the tumorigenesis of lung cancer. A
large number of early studies examined the possibility of using
telomerase activity as a tumor marker in NSCLC, and the expression
rates of telomerase in each study are presented in Table I. Moreover, the correlation between
telomerase activity and clinical characteristics of NSCLC has been
extensively investigated (Table I),
indicating that telomerase activity is significantly associated
with the prognosis of patients with NSCLC and may be used as a
diagnostic or prognostic indicator for these patients (31,33–39). In
general, it has been suggested that telomerase activity is
independent of age, sex, smoking history, and the histological
characteristics of the tumor, but it was found to be significantly
associated with tumor stage (34,36–38,40,41),
the grade of differentiation (32,38–40) and
lymph node metastasis (31,36,41).
However, in other studies, it was indicated that the stage of tumor
status was not associated with telomerase activity (33,42).
Currently, the majority of studies report the
prevalence and the prognostic characteristics of the TERT
promoter mutation in NSCLC and its potential as a clinical
biomarker. It has been demonstrated that TERT promoter
mutations have a low frequency in a very small proportion of
patients with NSCLC, whereas some studies did not identify
mutations in the TERT promoter (55,58–61) and
suggested that it may be a prognostic marker for NSCLC patients.
For example, Ma et al (55)
revealed that TERT promoter mutations occurred repeatedly in
2.57% of patients with NSCLC, and most mutations were observed in
elderly patients. Yuan et al (58) reported similar findings, suggesting
that the TERT promoter mutation was clinically age related
and that the incidence of the TERT promoter mutation was
very low, at ~5.8%. Moreover, Jung et al (26) studied the regional mutations and
clinical characteristics of the TERT promoter. The authors
found that the rate of the TERT promoter mutation was 2.2%
(4/188 NSCLC cases), which was closely associated with regional
lymph node infiltration (P<0.01), and further survival analysis
suggested a poor prognosis.
Single-nucleotide polymorphisms (SNPs) are DNA
sequence polymorphisms caused by variations in a single nucleotide
at the genomic level (62). The
rs2853669 SNP is located in the promoter region of the TERT
gene, and it is significantly associated with telomere and survival
in NSCLC cases with an EGFR mutation (63). Moreover, the rs2853669 T/C allele is
significantly associated with a shorter relative telomere length
(as opposed to C/C and T/T; P=0.039 and P=0.023, respectively, in
patients without EGFR mutations) and lower TERT mRNA
expression (compared with C/C and T/T; both P<0.001)
(63).
Epigenetic therapy has recently become a popular and
promising treatment for a variety of cancer types. Among these
therapies, histone deacetylase (HDAC) inhibition has been
attracting considerable attention (28,68,69).
Several studies have shown that HDAC inhibitors, such as chidamide
and vorinostat, may inhibit telomerase activity via epigenetic
alteration in NSCLC cells (70,71).
The gene amplification function is one of the most
common mechanisms of oncogene activation (73). TERT gene amplification has been
observed in some tumor cells including lung cancer, cervical
tumors, breast cancers, and neuroblastomas, suggesting that the
TERT site may be a common amplification target during
oncogenesis (74). TERT
amplification may be one factor causing the hTERT
upregulation and telomerase activation in cancer (73,74). For
example, Takuma et al (75)
reported that TERT gene amplification appeared to serve a
critical role in inducing hTERT mRNA in hepatocellular
carcinoma, but no significant correlation was identified between
hTERT gene amplification and its mRNA expression. Some
studies have suggested that low-level amplification may be the
reason for the lack of correlation with its expression (76,77).
The mechanism of gene structure rearrangement
involves a DNA double-strand break repair process, in which complex
conversational transfer of repeating units occurs within or between
alleles. Genomic rearrangements can result in inverted
orientations, tandem duplications, interchromosomal changes,
deletions, and amplification. Furthermore, genomic rearrangement
can affect the chromosomal region of the 5p15.33 proximal
TERT gene (80).
Rearrangements involving TERT caused by hundreds of tandem
duplications and templated insertions activate the TERT
promoter and induce a strong upregulation of TERT (81). The TERT coding sequence can be
juxtaposed with a strong enhancer element via the rearrangement of
TERT, resulting in extensive chromatin remodeling and DNA
methylation in affected regions (80). Moreover, Peifer et al (80) observed that the remodeling of the
genomic environment eliminated transcriptional silencing of
TERT and placed telomerase activation at the center of this
tumor transformation. TERT rearrangement in neuroblastoma
suggests a poor prognosis and is associated with other telomere
maintenance mechanisms, including alternative lengthening of
telomeres and MYCN amplification (82). However, further studies are required
to understand whether TERT rearrangement occurs in NSCLC and
its potential clinical implications.
Standard and targeted cancer therapies are almost
universally failing in patients with advanced cancer due to
plasticity/heterogeneity of the tumor and acquired or intrinsic
drug resistance (83). Previous
studies have reported that combination therapy with drug telomerase
inhibition may be an effective strategy for the treatment of
drug-sensitive and drug-resistant cancer types (83–86).
Telomerase is silenced in normal cells, but is reactivated in 90%
of tumor cells (19); thus, it is
considered an attractive target for cancer therapies. Moreover,
targeting telomerase is more pervasive, specific, and critical to
cancer cells compared with most other targets.
Telomerase complexes provide multiple potential
sites for inhibitor development (87). In NSCLC, different telomerase
inhibitors including 6-thio-2′-deoxyguanosine (6-thio-dg),
BIBR1532, imetelstat, and telomerase-derived peptide, destroy or
block different components and action pathways of telomerase,
thereby blocking telomerase activity and ultimately limiting the
growth and development of tumors (Table
II). In addition, several telomerase inhibitors have been used
in preclinical models and clinical trials (Table III).
6-thio-dg is a nucleoside analogue and telomerase
substrate recognized by telomerase, and it is incorporated into
newly synthesized telomeres. 6-thio-dg causes telomere dysfunction
and rapid cell death in tumor cells that are telomerase-positive
and in fibroblasts expressing hTERT, but not in telomerase-negative
cells (88). Moreover, 6-thio-dg may
be a novel telomerase-dependent anticancer therapy (83,86). In a
mouse xenograft study based on A549 lung cancer cells, 6-thio-dg
reduced tumor growth and this effect was more pronounced compared
with that induced by 6-thioguanine treatment (88). Mender et al (83) used human xenograft, syngeneic and
genetically engineered mouse lung cancer models to detect the
effects of 6-thio-dg on targeted therapy of chemotherapy-resistant
human lung cancer cells and mouse models. The results revealed that
erlotinib-, gemcitabine/cisplatin- and
paclitaxel/carboplatin-resistant cells were highly sensitive to
6-thio-dg, which indicated that 6-thio-dg could prolong disease
control with minimal toxicity in patients with drug-resistant lung
cancer (83).
BIBR1532 is a potent, selective, and
non-competitive small-molecule inhibitor of telomerase that induces
senescence in human cancer cells. Its mechanism is similar to that
of non-nuclear human immunodeficiency virus 1 (HIV1) reserve
transcriptase inhibitors (84,89).
BIBR1532 inhibits natural and recombinant human telomerase,
including the human telomerase RNA components and TERT, by
interfering with the enzyme's processing ability (89). A study of the effects of BIBR1532
combined with chemotherapy on drug-resistant leukemia and breast
cancer cells and their parental cells revealed that cells treated
with BIBR1532 exhibited gradually shortened telomeres, had a
reduced proliferative ability and were sensitive to chemotherapy
(84). It has been shown that a
KRAS mutation can increase telomerase activity, hTERT
expression and telomere length in lung adenocarcinoma cells (Calu-3
cell line) (90). However, BIBR1532
reduced telomere length and inhibited the proliferation, colony
formation and migration of Kras-mutant Calu-3 cells.
Specifically, BIBR1532 increased the sensitivity of
Kras-mutant Calu-3 cells to chemotherapy drugs (90).
BIBR1532 has the potential to be used as a
radiosensitizer in the clinical setting (91). Ding et al (91) studied the effect of BIBR1532 on the
NSCLC cellular response to radio-sensitization and observed
increased IR-induced telomere dysfunction, disruption of
chromosomal stability, inhibition of the
ataxia-telangiectasia-mutated/checkpoint kinase 1 (ATM/CHK1)
pathway, and reduced DNA damage repair. This study also
demonstrated that BIBR1532 at non-toxic dose level, interfered with
telomerase function and effectively enhanced the radio-sensitivity
of NSCLC cells.
Imetelstat is a competitive inhibitor of telomerase
activity, which leads to the shortening of telomeres in most cancer
cells (92–94), thereby reducing tumor growth.
Imetelstat considers the template region or the active site of the
telomerase as the target, and directly binds to the RNA component
of the telomerase at the active site of the enzyme (92,95,96).
Imetelstat has been shown to be effective in vivo in the
treatment of lung metastasis in xenograft animal models (80). Moreover, Frink et al (94) studied the efficacy of imetelstat in
NSCLC cell lines, and found that the short telomeres were more
sensitive compared with the long telomeres, and that continuous
application led to inhibition of continuous telomere shortening and
eventual growth inhibition. A clinical trial evaluated imetelstat
as a transformation maintenance therapy in patients with advanced
NSCLC. The results demonstrated that patients with short telomeres
administered imetelstat treatment tended to have longer median
progression-free survival (PFS) and overall survival (OS); however,
patients with long telomeres given imetelstat treatment had no
improvement in median PFS or OS. Furthermore, maintenance therapy
with imetelstat did not improve PFS in patients with advanced NSCLC
after first-line treatment (97).
Therefore, the clinical development of imetelstat in NSCLC requires
further investigation.
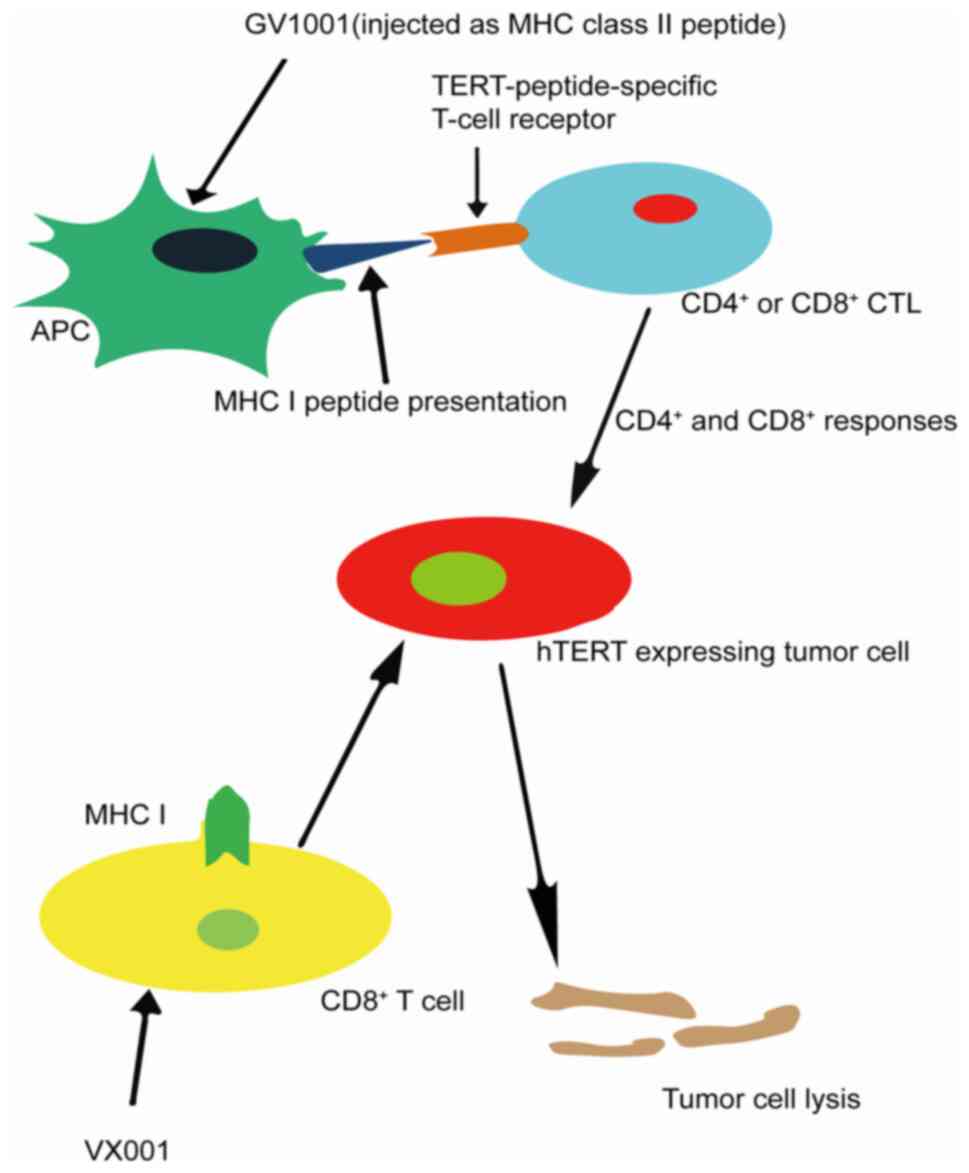
The first step in cancer immunotherapy is to
identify the target tumor-associated antigens. The
telomerase-derived peptide is one of the tumor-associated antigens
that bind to human leukocyte antigen class I and class II
molecules, effectively activating CD8+ and
CD4+ T-cell subsets, and telomerase is widely expressed
in tumors (Fig. 2) (98). Therefore, telomerase is an attractive
target antigen for cancer immunotherapy. The safety, immune
response and antitumor effects of several vaccines based on
telomerase-derived peptides have been evaluated in numerous types
of cancer (99). In total, three
hTERT vaccines, GV1001, Vx-001 and GRNVAC1, have been successfully
used to induce anti-telomerase immune responses in cancer patients
(100), and GV1001 and Vx-001 have
also been studied in NSCLC.
An early phase I/II study examined the safety,
tolerability and clinical response of a combination of telomerase
peptide GV1001 (hTERT: 611-626) and HR2822 (hTERT: 540–548) in
patients with NSCLC, and observed that these were both immunogenic
and safe for patients with NSCLC. In addition, the induction of a
GV1001-specific immune response may lead to an objective tumor
response (101). A phase III trial
in patients vaccinated following radiation and chemotherapy, and an
8-year update on a previous I/II trial in patients with NSCLC after
inoculation of telomerase peptide GV1001, revealed that the
patients' immune tolerance was good, and there was immunity in most
patients with NSCLC and the establishment of a lasting memory T
cells. The high immune response rate and low toxicity support the
concept of combining chemoradiotherapy with immunization (99). Other studies reported that T-regulated
cells and myeloid-derived suppressor cells are associated with the
impaired clinical efficacy of the vaccine response and the
environment of toggle-induced cytokines (102).
Vx-001 is a restricted telomerase-specific
antitumor vaccine, which is composed of a 9-mer Cryptic TERT (572)
peptide and its optimized variant TERT (572Y). Early studies have
shown that the Vx-001 vaccine is well-tolerated and can induce
T-cell-specific immune response, which is closely associated with
an improvement in clinical prognosis (103–105).
A randomized, double-blind, phase II clinical trial
examined the clinical activity of the Vx-001 cancer vaccine as
maintenance immunotherapy following chemotherapy in patients with
stage IV NSCLC. The primary endpoint of the study was OS. The
results did not reach the primary endpoint, and it was found that
Vx-001 significantly prolonged the survival of patients with NSCLC.
The median OS was 14.3 months in the vaccine Vx-001 patients vs.
11.3 months in patients in the placebo groups. The 6-month disease
control rates were 33.7 vs. 25.7% in the vaccine Vx-001 group vs.
the placebo groups, respectively. OS was significantly prolonged in
29.2% of the vaccinated patients compared with those who did not
respond. These authors suggested that Vx-001 induced a specific
CD8+ T-cell immune response (106).
A powerful antitumor strategy in humans involves
inhibiting telomerase and maintaining shorter telomeres over longer
evolutionary periods. Moreover, abnormal TERT gene
disinhibition/telomerase reactivation is key to the malignant
transformation of human cells (107).
Recent studies have reported that telomerase
expression and patterns are unique among histopathological types of
lung cancer and can predict the prognosis of patients (108). Telomerase can be used as a predictor
of disease recurrence and cancer-related death in patients with
early NSCLC after surgery (109). As
aforementioned, numerous studies have revealed that telomerase and
hTERT can be used as diagnostic markers of NSCLC. Although
most studies suggest that these factors can also be used as
prognostic indicators, certain studies have not confirmed their
effect on prognosis. Therefore, further studies are required to
determine whether telomerase can be used as a prognostic factor of
NSCLC. Early diagnosis is important for the prognosis of patients
with NSCLC, particularly in the diagnosis of advanced NSCLC, and
upregulation of TERT may be considered as an early molecular
event in lung cancer (108).
Therefore, the combination use of TERT with other lung
cancer-related factors can be considered in the early stage to
improve the diagnostic rate of patients with NSCLC.
Tumor formation is a multifactorial, staged process
that requires multiple gene transformations, including mutations in
several oncogenes and inactivation of ≥2 tumor-suppressor genes, as
well as alterations in apoptotic regulation and DNA repair genes
(112). TERT is both an
effector and a regulator in cancer, and it can interact with other
target genes to regulate the proliferation of tumor cells (113). Ret finger protein like 3 (RFPL3) is
a tumor-specific hTERT promoter-binding protein that can
promote the growth of lung cancer by activating hTERT
expression (114). Moreover,
EGF upregulates the expression levels of RFPL3 and hTERT
proteins in NSCLC cells via the MEK pathway, promotes cell
proliferation and inhibits apoptosis. RFPL3 overexpression
increases the expression level of hTERT and is associated
with MEK signaling proteins (115).
The transcription co-activator CREB-binding protein (CBP) is a
novel hTERT-binding protein that contributes to the
upregulation of hTERT expression and tumor growth.
Furthermore, upregulation of CBP may predict poor prognosis in
human lung cancer (116).
Interleukin (IL)-6 is an important cytokine in the development of
lung cancer. TERT regulates the expression of numerous
genes, including IL-6, and may serve a unique role in lung
adenocarcinoma (117). Early studies
have reported that the TP53 gene mutation and high
telomerase activity co-induce the occurrence and low
differentiation of NSCLC (117).
Moreover, the co-occurrence of TP53 gene mutation and
telomerase activity may be associated with the malignancy of NSCLC
(118,119). Although it has been shown that
TERT can interact with its target genes in NSCLC, the
mechanism of interaction is not fully understood and needs to be
further examined.
The close relationship between telomerase and
tumors makes telomerase inhibition a novel and promising
therapeutic approach to cancer treatment. However, there are still
numerous challenges to be addressed with regards to research on the
mechanism of telomerase inhibition. First, the specific mechanism
underlying the role of the TERT gene in tumor development is
incompletely understood. For example, whether TERT and other
genes jointly influence the occurrence and development of tumors
remains unknown and, if the specific association between the
co-mutation of TERT gene and other genes is identified, it
may prove helpful in the selection of suitable telomerase
inhibitors. Second, the mechanism of telomerase reactivation before
carcinogenesis and the molecular mechanism of inhibition of
telomerase function after carcinogenesis are yet to be elucidated,
and require further clarification. Moreover, although high
telomerase activity is present in tumor tissues, telomerase is also
highly active in embryonic cells, bone marrow stem cells, germ
cells and activated immune cells (118). Therefore, telomerase inhibition may
also prove harmful against normal cells. The biological
characteristics of telomerase prolong the time lag between drug
administration and clinical response, which may lead to drug
toxicity in patients. Therefore, if an inhibitor that only targets
telomerase-positive tumor cells is developed, it may help improve
drug toxicity in patients.
Not applicable.
The present study was supported by Hubei Province
Health and Family Planning Scientific Research Project (grant nos.
WJ2015MB212 and WJ2018H201) to JC, and the National Innovation And
Entrepreneurship Training Program for College Students (grant no.
202010489017) to XCP.
All information provided in this review is
documented by relevant references.
XCP and JC contributed to this review with the
design. LY, MW, YHZ, LDY and NL reviewed the references. LY, JC and
XCP wrote the manuscript. LY, WZ, ZQY and NL designed and produced
the tables and figures. XCP and JC acquired the funding. All
authors read and approved the manuscript for publication.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang X, Yin H, Zhang L, Zheng D, Yang Y,
Zhang J, Jiang H, Ling X, Xin Y, Liang H, et al: The construction
and analysis of the aberrant lncRNA-miRNA-mRNA network in non-small
cell lung cancer. J Thorac Dis. 11:1772–1778. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qiu M, Chen YB, Jin S, Fang XF, He XX,
Xiong ZF and Yang SL: Research on circadian clock genes in
non-small-cell lung carcinoma. Chronobiol Int. 36:739–750. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu XJ, Chen G, Yang J, Yu GC, Zhu PF,
Jiang ZK, Feng K, Lu Y, Bao B and Zhong FM: Smoking alters the
evolutionary trajectory of non-small cell lung cancer. Exp Ther
Med. 18:3315–3324. 2019.PubMed/NCBI
|
|
5
|
Nigro E, Imperlini E, Scudiero O, Monaco
ML, Polito R, Mazzarella G, Orrù S, Bianco A and Daniele A:
Differentially expressed and activated proteins associated with non
small cell lung cancer tissues. Respir Res. 16:742015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Braicu C, Zimta AA, Harangus A, Iurca I,
Irimie A, Coza O and Berindan-Neagoe I: The function of non-coding
RNAs in lung cancer tumorigenesis. Cancers (Basel). 11:6052019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Narsule CK, Sridhar P, Nair D, Gupta A,
Oommen RG, Ebright MI, Litle VR and Fernando HC: Percutaneous
thermal ablation for stage IA non-small cell lung cancer: Long-term
follow-up. J Thorac Dis. 9:4039–4045. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Shen WX, Zhou LN, Tang M, Tan Y,
Feng CX, Li P, Wang LQ and Chen MB: The value of next-generation
sequencing for treatment in non-small cell lung cancer patients:
The observational, real-world evidence in China. Biomed Res Int.
2020:93871672020.PubMed/NCBI
|
|
9
|
Yeh J, Marrone KA and Forde PM:
Neoadjuvant and consolidation immuno-oncology therapy in stage III
non-small cell lung cancer. J Thorac Dis. 10 (Suppl 3):S451–S459.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan WL, Jain A, Takano A, Newell EW, Iyer
NG, Lim WT, Tan EH, Zhai W, Hillmer AM, Tam WL and Tan DSW: Novel
therapeutic targets on the horizon for lung cancer. Lancet Oncol.
17:e347–e362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith RA, Andrews KS, Brooks D, Fedewa SA,
Manassaram-Baptiste D, Saslow D, Brawley OW and Wender RC: Cancer
screening in the United States, 2017: A review of current American
Cancer Society guidelines and current issues in cancer screening.
CA Cancer J Clin. 67:100–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dagogo-Jack I and Shaw AT: Crizotinib
resistance: Implications for therapeutic strategies. Ann Oncol. 27
(Suppl 3):iii42–iii50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baird DM: Variation at the TERT locus and
predisposition for cancer. Expert Rev Mol Med. 12:e162010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meena J, Rudolph KL and Gunes C: Telomere
dysfunction, chromosomal instability and cancer. Recent Results
Cancer Res. 200:61–79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng D, Zhao Y, Zhang F, Zhang J, Wang S
and Zhu J: Engineering a humanized telomerase reverse transcriptase
gene in mouse embryonic stem cells. Sci Rep. 9:96832019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inada E, Saitoh I, Kubota N, Iwase Y,
Kiyokawa Y, Shibasaki S, Noguchi H, Yamasaki Y and Sato M: piggyBac
transposon-based immortalization of human deciduous tooth dental
pulp cells with multipotency and non-tumorigenic potential. Int J
Mol Sci. 20:49042019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muneer A and Minhas FA: Telomere biology
in mood disorders: An updated, comprehensive review of the
literature. Clin Psychopharmacol Neurosci. 17:343–363. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Campisi J: Aging, cellular senescence, and
cancer. Annu Rev Physiol. 75:685–705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng J, Funk WD, Wang SS, Weinrich SL,
Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, et al:
The RNA component of human telomerase. Science. 269:1236–1241.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakamura TM, Morin GB, Chapman KB,
Weinrich SL, Andrews WH, Lingner J, Harley CB and Cech TR:
Telomerase catalytic subunit homologs from fission yeast and human.
Science. 277:955–959. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsang JYS, Hui YK, Lee MA, Lacambra M, Ni
YB, Cheung SY, Wu C, Kwong A and Tse GMK: Association of
clinicopathological features and prognosis of TERT alterations in
phyllodes tumor of breast. Sci Rep. 8:38812018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mortazavi-Haghighat R, Taghipour-Khiabani
K, David S, Kerrigan CL and Philip A: Rapid and dynamic regulation
of TGF-beta receptors on blood vessels and fibroblasts during
ischemia-reperfusion injury. Am J Physiol Cell Physiol.
282:C1161–C1169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Pedro N, Díez M, García I, García J,
Otero L, Fernandez L, Garcia B, González R, Rincón S, Pérez D, et
al: Analytical validation of telomere analysis
technology® for the High-throughput analysis of multiple
telomere-associated variables. Biol Proced Online. 22:22020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barthel FP, Wei W, Tang M,
Martinez-Ledesma E, Hu X, Amin SB, Akdemir KC, Seth S, Song X, Wang
Q, et al: Systematic analysis of telomere length and somatic
alterations in 31 cancer types. Nat Genet. 49:349–357. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jung SJ, Kim DS, Park WJ, Lee H, Choi IJ,
Park JY and Lee JH: Mutation of the TERT promoter leads to poor
prognosis of patients with non-small cell lung cancer. Oncol Lett.
14:1609–1614. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leão R, Apolónio JD, Lee D, Figueiredo A,
Tabori U and Castelo-Branco P: Mechanisms of human telomerase
reverse transcriptase (hTERT) regulation: Clinical impacts in
cancer. J Biomed Sci. 25:222018. View Article : Google Scholar
|
|
28
|
da Silva EM, Selenica P, Vahdatinia M,
Pareja F, Da Cruz Paula A, Ferrando L, Gazzo AM, Dopeso H, Ross DS,
Bakhteri A, et al: TERT promoter hotspot mutations and gene
amplification in metaplastic breast cancer. NPJ Breast Cancer.
7:432021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen W, Xiong X, Zhou H and Zhou Q:
Expression of telomerase activity, telomerase RNA component and
telomerase catalytic subunit gene in lung cancer. Chin Med J
(Engl). 115:290–292. 2002.PubMed/NCBI
|
|
30
|
Dobija-Kubica K, Zalewska-Ziob M,
Brulinski K, Rogozinski P, Wiczkowski A, Gawrychowska A and
Gawrychowski J: Telomerase activity in non-small cell lung cancer.
Kardiochir Torakochirurgia Pol. 13:15–20. 2016.PubMed/NCBI
|
|
31
|
Liu H, Zhang W, Cai C, Xu J and Xu Y:
Telomerase activity in human non-small cell lung cancer (NSCLC).
Zhonghua Bing Li Xue Za Zhi. 29:89–91. 2000.(In Chinese).
PubMed/NCBI
|
|
32
|
Yang HZ, Hu CP and Su XL: Detection of
telomerase activity level in human non-small-cell lung cancer.
Hunan Yi Ke Da Xue Xue Bao. 26:549–550. 2001.(In Chinese).
PubMed/NCBI
|
|
33
|
Chen Q, He J and Yi H: Study on telomerase
activity and its clinical value in human non small cell lung
cancer. Hunan Yi Ke Da Xue Xue Bao. 26:221–222. 2001.(In Chinese).
PubMed/NCBI
|
|
34
|
Wang J, Liu X, Jiang W and Liang L:
Telomerase activity and expression of the telomerase catalytic
subunit gene in non-small cell lung cancer: Correlation with
decreased apoptosis and clinical prognosis. Chin Med J (Engl).
113:985–990. 2000.PubMed/NCBI
|
|
35
|
Fernandez-Marcelo T, Gomez A, Pascua I, de
Juan C, Head J, Hernando F, Jarabo JR, Calatayud J, Torres-Garcia
AJ and Iniesta P: Telomere length and telomerase activity in
non-small cell lung cancer prognosis: Clinical usefulness of a
specific telomere status. J Exp Clin Cancer Res. 34:782015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hara H, Yamashita K, Shinada J, Yoshimura
H and Kameya T: Clinicopathologic significance of telomerase
activity and hTERT mRNA expression in non-small cell lung cancer.
Lung Cancer. 34:219–226. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hashim M, Sayed M, Samy N and Elshazly S:
Prognostic significance of telomerase activity and some tumor
markers in non-small cell lung cancer. Med Oncol. 28:322–330. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Taga S, Osaki T, Ohgami A, Imoto H and
Yasumoto K: Prognostic impact of telomerase activity in non-small
cell lung cancers. Ann Surg. 230:715–720. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu TC, Lin P, Hsu CP, Huang YJ, Chen CY,
Chung WC, Lee H and Ko JL: Loss of telomerase activity may be a
potential favorable prognostic marker in lung carcinomas. Lung
Cancer. 41:163–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hsu CP, Miaw J, Hsia JY, Shai SE and Chen
CY: Concordant expression of the telomerase-associated genes in
non-small cell lung cancer. Eur J Surg Oncol. 29:594–599. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen KY, Lee LN, Yu CJ, Lee YC, Kuo SH and
Yang PC: Elevation of telomerase activity positively correlates to
poor prognosis of patients with non-small cell lung cancer. Cancer
Lett. 240:148–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Metzger R, Vallbohmer D, Muller-Tidow C,
Higashi H, Bollschweiler E, Warnecke-Eberz U, Brabender J, Baldus
SE, Xi H, Berdel WE, et al: Increased human telomerase reverse
transcriptase (hTERT) mRNA expression but not telomerase activity
is related to survival in curatively resected non-small cell lung
cancer. Anticancer Res. 29:1157–1162. 2009.PubMed/NCBI
|
|
43
|
Li L, Xiong YY, Liu L, Chen TX, Yao XF and
Wang YW: Relationships among expressions of hTERT, MDR1, MRP mRNA,
and C-myc protein in non-small cell lung cancer. Ai Zheng.
24:53–57. 2005.(In Chinese). PubMed/NCBI
|
|
44
|
Ding M, Li X and Qiu T: Combination of
multiple gene markers to detect circulating tumor cells in the
peripheral blood of patients with non-small cell lung cancer using
real-time PCR. Genet Mol Res. 14:13033–13040. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zalewska-Ziob M, Dobija-Kubica K,
Biernacki K, Adamek B, Kasperczyk J, Brulinski K and Ostrowska Z:
Clinical and prognostic value of hTERT mRNA expression in patients
with non-small-cell lung cancer. Acta Biochim Pol. 64:641–646.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang J, Liu X and Fang J: Expression and
clinical significance of telomerase catalytic subunit gene in lung
cancer and its correlations with genes related to drug resistance
and apoptosis. Zhonghua Zhong Liu Za Zhi. 21:350–353. 1999.(In
Chinese). PubMed/NCBI
|
|
47
|
Cong YS, Wen J and Bacchetti S: The human
telomerase catalytic subunit hTERT: Organization of the gene and
characterization of the promoter. Hum Mol Genet. 8:137–142. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Heidenreich B and Kumar R: TERT promoter
mutations in telomere biology. Mutat Res. 771:15–31. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Stern JL, Theodorescu D, Vogelstein B,
Papadopoulos N and Cech TR: Mutation of the TERT promoter, switch
to active chromatin, and monoallelic TERT expression in multiple
cancers. Genes Dev. 29:2219–2224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Akincilar SC, Unal B and Tergaonkar V:
Reactivation of telomerase in cancer. Cell Mol Life Sci.
73:1659–1670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Horn S, Figl A, Rachakonda PS, Fischer C,
Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, et al:
TERT promoter mutations in familial and sporadic melanoma. Science.
339:959–961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Heidenreich B, Rachakonda PS, Hemminki K
and Kumar R: TERT promoter mutations in cancer development. Curr
Opin Genet Dev. 24:30–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vinagre J, Almeida A, Populo H, Batista R,
Lyra J, Pinto V, Coelho R, Celestino R, Prazeres H, Lima L, et al:
Frequency of TERT promoter mutations in human cancers. Nat Commun.
4:21852013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hsu CP, Hsu NY, Lee LW and Ko JL: Ets2
binding site single nucleotide polymorphism at the hTERT gene
promoter-effect on telomerase expression and telomere length
maintenance in non-small cell lung cancer. Eur J Cancer.
42:1466–1474. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ma X, Gong R, Wang R, Pan Y, Cai D, Pan B,
Li Y, Xiang J, Li H, Zhang J, et al: Recurrent TERT promoter
mutations in non-small cell lung cancers. Lung Cancer. 86:369–373.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Griewank KG, Murali R, Schilling B,
Schimming T, Moller I, Moll I, Schwamborn M, Sucker A, Zimmer L,
Schadendorf D and Hillen U: TERT promoter mutations are frequent in
cutaneous basal cell carcinoma and squamous cell carcinoma. PLoS
One. 8:e803542013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Killela PJ, Reitman ZJ, Jiao Y, Bettegowda
C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL,
Giovanella BC, et al: TERT promoter mutations occur frequently in
gliomas and a subset of tumors derived from cells with low rates of
self-renewal. Proc Natl Acad Sci USA. 110:6021–6026. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yuan P, Cao JL, Abuduwufuer A, Wang LM,
Yuan XS, Lv W and Hu J: Clinical characteristics and prognostic
significance of TERT promoter mutations in cancer: A cohort study
and a meta-analysis. PLoS One. 11:e01468032016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li C, Hao L, Li Y, Wang S, Chen H, Zhang
L, Ke B, Yin Y, Suo H, Sun B, et al: Prognostic value analysis of
mutational and clinicopathological factors in non-small cell lung
cancer. PLoS One. 9:e1072762014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Schwaederle M, Krishnamurthy N, Daniels
GA, Piccioni DE, Kesari S, Fanta PT, Schwab RB, Patel SP, Parker BA
and Kurzrock R: Telomerase reverse transcriptase promoter
alterations across cancer types as detected by next-generation
sequencing: A clinical and molecular analysis of 423 patients.
Cancer. 124:1288–1296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cheng KA, Kurtis B, Babayeva S, Zhuge J,
Tantchou I, Cai D, Lafaro RJ, Fallon JT and Zhong M: Heterogeneity
of TERT promoter mutations status in squamous cell carcinomas of
different anatomical sites. Ann Diagn Pathol. 19:146–148. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Feuk L, Carson AR and Scherer SW:
Structural variation in the human genome. Nat Rev Genet. 7:85–97.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yuan P, Huang S, Bao FC, Cao JL, Sheng HX,
Shi L, Lv W and Hu J: Discriminating association of a common
telomerase reverse transcriptase promoter polymorphism with
telomere parameters in non-small cell lung cancer with or without
epidermal growth factor receptor mutation. Eur J Cancer. 120:10–19.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lewis KA and Tollefsbol TO: Regulation of
the telomerase reverse transcriptase subunit through epigenetic
mechanisms. Front Genet. 7:832016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sarne V, Huter S, Braunmueller S, Rakob L,
Jacobi N, Kitzwogerer M, Wiesner C, Obrist P and Seeboeck R:
Promoter methylation of selected genes in non-small-cell lung
cancer patients and cell lines. Int J Mol Sci. 21:45952020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Guilleret I, Yan P, Grange F, Braunschweig
R, Bosman FT and Benhattar J: Hypermethylation of the human
telomerase catalytic subunit (hTERT) gene correlates with
telomerase activity. Int J Cancer. 101:335–341. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Devereux TR, Horikawa I, Anna CH, Annab
LA, Afshari CA and Barrett JC: DNA methylation analysis of the
promoter region of the human telomerase reverse transcriptase
(hTERT) gene. Cancer Res. 59:6087–6090. 1999.PubMed/NCBI
|
|
68
|
Falkenberg KJ and Johnstone RW: Histone
deacetylases and their inhibitors in cancer, neurological diseases
and immune disorders. Nat Rev Drug Discov. 13:673–691. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mamdani H and Jalal SI: Histone
Deacetylase inhibition in non-small cell lung cancer: Hype or Hope?
Front Cell Dev Biol. 8:5823702020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wu YF, Ou CC, Chien PJ, Chang HY, Ko JL
and Wang BY: Chidamide-induced ROS accumulation and
miR-129-3p-dependent cell cycle arrest in non-small lung cancer
cells. Phytomedicine. 56:94–102. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li CT, Hsiao YM, Wu TC, Lin YW, Yeh KT and
Ko JL: Vorinostat, SAHA, represses telomerase activity via
epigenetic regulation of telomerase reverse transcriptase in
non-small cell lung cancer cells. J Cell Biochem. 112:3044–3053.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Krupitsky EM, Rybakova KV, Skurat EP,
Semenova NV and Neznanov NG: A double blind placebo controlled
randomized clinical trial of the efficacy and safety of pregabalin
in induction of remission in patients with alcohol dependence. Zh
Nevrol Psikhiatr Im S S Korsakova. 120:33–43. 2020.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhu CQ, Cutz JC, Liu N, Lau D, Shepherd
FA, Squire JA and Tsao MS: Amplification of telomerase (hTERT) gene
is a poor prognostic marker in non-small-cell lung cancer. Br J
Cancer. 94:1452–1459. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang A, Zheng C, Lindvall C, Hou M,
Ekedahl J, Lewensohn R, Yan Z, Yang X, Henriksson M, Blennow E, et
al: Frequent amplification of the telomerase reverse transcriptase
gene in human tumors. Cancer Res. 60:6230–6235. 2000.PubMed/NCBI
|
|
75
|
Takuma Y, Nouso K, Kobayashi Y, Nakamura
S, Tanaka H, Matsumoto E, Fujikawa T, Suzuki M, Hanafusa T and
Shiratori Y: Telomerase reverse transcriptase gene amplification in
hepatocellular carcinoma. J Gastroenterol Hepatol. 19:1300–1304.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang A, Zheng C, Hou M, Lindvall C,
Wallin KL, Angstrom T, Yang X, Hellstrom AC, Blennow E, Bjorkholm
M, et al: Amplification of the telomerase reverse transcriptase
(hTERT) gene in cervical carcinomas. Genes Chromosomes Cancer.
34:269–275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tsuda H, Akiyama F, Terasaki H, Hasegawa
T, Kurosumi M, Shimadzu M, Yamamori S and Sakamoto G: Detection of
HER-2/neu (c-erb B-2) DNA amplification in primary breast
carcinoma. Interobserver reproducibility and correlation with
immunohistochemical HER-2 overexpression. Cancer. 92:2965–2974.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kang JU, Koo SH, Kwon KC, Park JW and Kim
JM: Gain at chromosomal region 5p15.33, containing TERT, is the
most frequent genetic event in early stages of non-small cell lung
cancer. Cancer Genet Cytogenet. 182:1–11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Alidousty C, Baar T, Martelotto LG, Heydt
C, Wagener S, Fassunke J, Duerbaum N, Scheel AH, Frank S, Holz B,
et al: Genetic instability and recurrent MYC amplification in
ALK-translocated NSCLC: A central role of TP53 mutations. J Pathol.
246:67–76. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Peifer M, Hertwig F, Roels F, Dreidax D,
Gartlgruber M, Menon R, Kramer A, Roncaioli JL, Sand F, Heuckmann
JM, et al: Telomerase activation by genomic rearrangements in
high-risk neuroblastoma. Nature. 526:700–704. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bayard Q, Meunier L, Peneau C, Renault V,
Shinde J, Nault JC, Mami I, Couchy G, Amaddeo G, Tubacher E, et al:
Cyclin A2/E1 activation defines a hepatocellular carcinoma subclass
with a rearrangement signature of replication stress. Nat Commun.
9:52352018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kawashima M, Kojima M, Ueda Y, Kurihara S
and Hiyama E: Telomere biology including TERT rearrangements in
neuroblastoma: A useful indicator for surgical treatments. J
Pediatr Surg. 51:2080–2085. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Mender I, LaRanger R, Luitel K, Peyton M,
Girard L, Lai TP, Batten K, Cornelius C, Dalvi MP, Ramirez M, et
al: Telomerase-mediated strategy for overcoming non-small cell lung
cancer targeted therapy and chemotherapy resistance. Neoplasia.
20:826–837. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ward RJ and Autexier C: Pharmacological
telomerase inhibition can sensitize drug-resistant and
drug-sensitive cells to chemotherapeutic treatment. Mol Pharmacol.
68:779–786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Meng E, Taylor B, Ray A, Shevde LA and
Rocconi RP: Targeted inhibition of telomerase activity combined
with chemotherapy demonstrates synergy in eliminating ovarian
cancer spheroid-forming cells. Gynecol Oncol. 124:598–605. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang G, Wu LW, Mender I, Barzily-Rokni M,
Hammond MR, Ope O, Cheng C, Vasilopoulos T, Randell S, Sadek N, et
al: Induction of telomere dysfunction prolongs disease control of
therapy-resistant melanoma. Clin Cancer Res. 24:4771–4784. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Dikmen ZG, Gellert GC, Jackson S, Gryaznov
S, Tressler R, Dogan P, Wright WE and Shay JW: In vivo inhibition
of lung cancer by GRN163L: A novel human telomerase inhibitor.
Cancer Res. 65:7866–7873. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Mender I, Gryaznov S, Dikmen ZG, Wright WE
and Shay JW: Induction of telomere dysfunction mediated by the
telomerase substrate precursor 6-thio-2′-deoxyguanosine. Cancer
Discov. 5:82–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Pascolo E, Wenz C, Lingner J, Hauel N,
Priepke H, Kauffmann I, Garin-Chesa P, Rettig WJ, Damm K and
Schnapp A: Mechanism of human telomerase inhibition by BIBR1532, a
synthetic, non-nucleosidic drug candidate. J Biol Chem.
277:15566–15572. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu W, Yin Y, Wang J, Shi B, Zhang L, Qian
D, Li C, Zhang H, Wang S, Zhu J, et al: Kras mutations increase
telomerase activity and targeting telomerase is a promising
therapeutic strategy for Kras-mutant NSCLC. Oncotarget. 8:179–190.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ding X, Cheng J, Pang Q, Wei X, Zhang X,
Wang P, Yuan Z and Qian D: BIBR1532, a selective telomerase
inhibitor, enhances radiosensitivity of non-small cell lung cancer
through increasing telomere dysfunction and ATM/CHK1 inhibition.
Int J Radiat Oncol Biol Phys. 105:861–874. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Webb D, Gagnon MM and Rose T: Metabolic
enzyme activities in black bream (Acanthopagrus butcheri)
from the Swan-Canning Estuary, Western Australia. Comp Biochem
Physiol C Toxicol Pharmacol. 141:356–365. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gellert GC, Dikmen ZG, Wright WE, Gryaznov
S and Shay JW: Effects of a novel telomerase inhibitor, GRN163L, in
human breast cancer. Breast Cancer Res Treat. 96:73–81. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Frink RE, Peyton M, Schiller JH, Gazdar
AF, Shay JW and Minna JD: Telomerase inhibitor imetelstat has
preclinical activity across the spectrum of non-small cell lung
cancer oncogenotypes in a telomere length dependent manner.
Oncotarget. 7:31639–31651. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Herbert BS, Gellert GC, Hochreiter A,
Pongracz K, Wright WE, Zielinska D, Chin AC, Harley CB, Shay JW and
Gryaznov SM: Lipid modification of GRN163, an N3′-->P5′
thio-phosphoramidate oligonucleotide, enhances the potency of
telomerase inhibition. Oncogene. 24:5262–5268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Mender I, Senturk S, Ozgunes N, Akcali KC,
Kletsas D, Gryaznov S, Can A, Shay JW and Dikmen ZG: Imetelstat (a
telomerase antagonist) exerts offtarget effects on the
cytoskeleton. Int J Oncol. 42:1709–1715. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chiappori AA, Kolevska T, Spigel DR, Hager
S, Rarick M, Gadgeel S, Blais N, Von Pawel J, Hart L, Reck M, et
al: A randomized phase II study of the telomerase inhibitor
imetelstat as maintenance therapy for advanced non-small-cell lung
cancer. Ann Oncol. 26:354–362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Negrini S, De Palma R and Filaci G:
Anti-cancer immunotherapies targeting telomerase. Cancers (Basel).
12:22602020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Brunsvig PF, Kyte JA, Kersten C, Sundstrom
S, Moller M, Nyakas M, Hansen GL, Gaudernack G and Aamdal S:
Telomerase peptide vaccination in NSCLC: A phase II trial in stage
III patients vaccinated after chemoradiotherapy and an 8-year
update on a phase I/II trial. Clin Cancer Res. 17:6847–6857. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Relitti N, Saraswati AP, Federico S, Khan
T, Brindisi M, Zisterer D, Brogi S, Gemma S, Butini S and Campiani
G: Telomerase-based cancer therapeutics: A review on their clinical
trials. Curr Top Med Chem. 20:433–457. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Brunsvig PF, Aamdal S, Gjertsen MK,
Kvalheim G, Markowski-Grimsrud CJ, Sve I, Dyrhaug M, Trachsel S,
Moller M, Eriksen JA, et al: Telomerase peptide vaccination: A
phase I/II study in patients with non-small cell lung cancer.
Cancer Immunol Immunother. 55:1553–1564. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Hansen GL, Gaudernack G, Brunsvig PF,
Cvancarova M and Kyte JA: Immunological factors influencing
clinical outcome in lung cancer patients after telomerase peptide
vaccination. Cancer Immunol Immunother. 64:1609–1621. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Vetsika EK, Konsolakis G, Aggouraki D,
Kotsakis A, Papadimitraki E, Christou S, Menez-Jamet J,
Kosmatopoulos K, Georgoulias V and Mavroudis D: Immunological
responses in cancer patients after vaccination with the therapeutic
telomerase-specific vaccine Vx-001. Cancer Immunol Immunother.
61:157–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kotsakis A, Papadimitraki E, Vetsika EK,
Aggouraki D, Dermitzaki EK, Hatzidaki D, Kentepozidis N, Mavroudis
D and Georgoulias V: A phase II trial evaluating the clinical and
immunologic response of HLA-A2(+) non-small cell lung cancer
patients vaccinated with an hTERT cryptic peptide. Lung Cancer.
86:59–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Bolonaki I, Kotsakis A, Papadimitraki E,
Aggouraki D, Konsolakis G, Vagia A, Christophylakis C, Nikoloudi I,
Magganas E, Galanis A, et al: Vaccination of patients with advanced
non-small-cell lung cancer with an optimized cryptic human
telomerase reverse transcriptase peptide. J Clin Oncol.
25:2727–2734. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gridelli C, Ciuleanu T, Domine M, Szczesna
A, Bover I, Cobo M, Kentepozidis N, Zarogoulidis K, Kalofonos C,
Kazarnowisz A, et al: Clinical activity of a htert (vx-001) cancer
vaccine as post-chemotherapy maintenance immunotherapy in patients
with stage IV non-small cell lung cancer: Final results of a
randomised phase 2 clinical trial. Br J Cancer. 122:1461–1466.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yuan X, Larsson C and Xu D: Mechanisms
underlying the activation of TERT transcription and telomerase
activity in human cancer: Old actors and new players. Oncogene.
38:6172–6183. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Lantuejoul S, Soria JC, Moro-Sibilot D,
Morat L, Veyrenc S, Lorimier P, Brichon PY, Sabatier L, Brambilla C
and Brambilla E: Differential expression of telomerase reverse
transcriptase (hTERT) in lung tumours. Br J Cancer. 90:1222–1229.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Marchetti A, Bertacca G, Buttitta F,
Chella A, Quattrocolo G, Angeletti CA and Bevilacqua G: Telomerase
activity as a prognostic indicator in stage I non-small cell lung
cancer. Clin Cancer Res. 5:2077–2081. 1999.PubMed/NCBI
|
|
110
|
Tang H, Wang H, Cheng X, Fan X, Yang F,
Zhang M, Chen Y, Tian Y, Liu C, Shao D, et al: HuR regulates
telomerase activity through TERC methylation. Nat Commun.
9:22132018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Baena-Del Valle JA, Zheng Q, Esopi DM,
Rubenstein M, Hubbard GK, Moncaliano MC, Hruszkewycz A, Vaghasia A,
Yegnasubramanian S, Wheelan SJ, et al: MYC drives overexpression of
telomerase RNA (hTR/TERC) in prostate cancer. J Pathol. 244:11–24.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Bellutti F, Tigan AS, Nebenfuehr S,
Dolezal M, Zojer M, Grausenburger R, Hartenberger S, Kollmann S,
Doma E, Prchal-Murphy M, et al: CDK6 antagonizes p53-induced
responses during tumorigenesis. Cancer Discov. 8:884–897. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wu XQ, Huang C, He X, Tian YY, Zhou DX, He
Y, Liu XH and Li J: Feedback regulation of telomerase reverse
transcriptase: New insight into the evolving field of telomerase in
cancer. Cell Signal. 25:2462–2468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chen W, Lu J, Qin Y, Wang J, Tian Y, Shi
D, Wang S, Xiao Y, Dai M, Liu L, et al: Ret finger protein-like 3
promotes tumor cell growth by activating telomerase reverse
transcriptase expression in human lung cancer cells. Oncotarget.
5:11909–11923. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Lin C, Qin Y, Zhang H, Gao MY and Wang YF:
EGF upregulates RFPL3 and hTERT via the MEK signaling pathway in
nonsmall cell lung cancer cells. Oncol Rep. 40:29–38.
2018.PubMed/NCBI
|
|
116
|
Guo W, Lu J, Dai M, Wu T, Yu Z, Wang J,
Chen W, Shi D, Yu W, Xiao Y, et al: Transcriptional coactivator CBP
upregulates hTERT expression and tumor growth and predicts poor
prognosis in human lung cancers. Oncotarget. 5:9349–9361. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wang F, Fu P, Pang Y, Liu C, Shao Z, Zhu
J, Li J, Wang T, Zhang X and Liu J: TERT rs2736100T/G polymorphism
upregulates interleukin 6 expression in non-small cell lung cancer
especially in adenocarcinoma. Tumour Biol. 35:4667–4672. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Maniwa Y, Yoshimura M, Obayashi C, Inaba
M, Kiyooka K, Kanki M and Okita Y: Association of p53 gene mutation
and telomerase activity in resectable non-small cell lung cancer.
Chest. 120:589–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Kiyooka K, Maniwa Y and Okada M: Analysis
of mutant p53 and telomerase activity in non-small cell lung
cancer. Ann Thorac Cardiovasc Surg. 5:293–299. 1999.PubMed/NCBI
|