Introduction
Breast cancer (BC) is the most common type of
malignant cancer worldwide, with an estimated 2.3 million new
cases; mortality for female breast cancer is 12.4 per 100,000 in
developing countries and 5.2 per 100,000 in developed countries,
respectively (1). Despite
developments in the treatment of BC, resistance to conventional
cytotoxic drugs is increasing and recurrent BC remains the leading
cause of cancer-associated mortality in women worldwide. BC is
considered to comprise a heterogeneous group of diseases with
distinct clinical, pathological and molecular features (2). Due to the heterogeneity of the tumor and
advancements in molecular-targeted drugs in the clinic, the
identification of novel prognostic and predictive factors is key
for the development of personalized medicine for patients with BC
(3). The occurrence, development and
metastasis of cancer are associated with cell proliferation and
decreased levels of apoptosis (4).
Therefore, an improved understanding of the mechanisms underlying
the proliferation and apoptosis of BC cells is essential for the
identification of diagnostic markers and development of novel
effective therapies for patients with BC.
Erythropoietin-producing hepatocellular receptors
(Ephs) comprise the largest family of receptor tyrosine kinases
(RTKs), which interact with ligands known as ephrins (5). The binding of Ephs and ephrins produces
bidirectional signals that affect both Eph- and ligand-expressing
cells. Various downstream signaling pathways are linked to
Eph/ephrin binding, including the Ras/MAPK, PI3K/AKT/mTOR, FAK/SRC,
ABL and RHO/RAC/CDC42 signaling pathways (5,6).
Eph/ephrin signaling has been reported to be key for cell
positioning and migration during the development of the central
nervous system and in the maintenance of long-term potentiation,
angiogenesis and stem cell differentiation (7). In humans, 14 members of the Eph family
have been characterized to date, which are subdivided into two
classes, A (EphA1-8 and EphA10) and B (EphB1-4 and EphB6), on the
basis of sequence homology, structure and binding affinity
(8). A number of Ephs are required to
synergistically maintain sophisticated tissue organization of the
central nervous system (5).
Upregulated expression levels of several Eph members, such as
EphA2, EphA10 and EphB4, have been detected in various types of
tumor, where they are involved in the regulation of cancer
proliferation, migration, invasion and angiogenesis (9–11).
EphA8 functions as a receptor for
glycosylphosphatidylinositol-anchored ephrin-A1-5 (12,13). Upon
ligand binding and activation of EphA8 in NIH3T3 and HEK293 cells,
p110γ PI3K is recruited in a TK activity-independent manner, which
promotes cell adhesion and migration (14). During early brain development, EphA8
serves a role in modulating apoptosis in a caspase-dependent manner
in ephrin-A-expressing neuroepithelial cells (15). The expression of EphA8 in neuronal
cells induces a sustained increase in MAPK activity, thereby
promoting neurite outgrowth (16). In
addition, previous studies have indicated that EphA8 expression is
dysregulated in several types of cancer (10,17). Our
previous study (17) also
demonstrated that EphA8 serves as an oncogene and contributes to
poor prognosis in gastric cancer by regulating the expression of a
disintegrin and metalloproteinase domain 10.
The present study aimed to investigate the
expression levels and clinicopathological significance of EphA8
protein in patients with BC. Moreover, the present study determined
the functional role of EphA8 in BC cells and the potential of EphA8
expression levels to predict response to adjuvant chemotherapy for
BC treatment.
Materials and methods
Patient clinical information and
tissue samples
A total of 151 BC, 61 unpaired paracancerous and 30
benign formalin-fixed and paraffin-embedded breast tissue samples
were obtained. The median age of the patients was 54.3 years
(female, range 27–86 years). Clinical characteristics were obtained
from patient medical records. None of the patients had received
radiotherapy, neo-adjuvant chemotherapy or immunotherapy before
surgery. All samples were obtained from Clinical Biobank in
Affiliated Hospital of Nantong University (Jiangsu, China). All
patients provided written informed consent. The study was approved
by Ethics Committee of Affiliated Hospital of Nantong
University.
Tissue microarray (TMA) and
immunohistochemistry (IHC) staining
IHC was performed to investigate EphA8 protein
expression levels in a TMA consisting of 242 4-µm-thick tissue
sections. The sections were dewaxed using xylene, followed by
rehydration in a descending alcohol series (100, 95, 80 and 70%
ethanol). Antigen retrieval was performed by microwave treatment
with citrate buffer (pH, 6.0) for 15 min at 750 W. Endogenous
peroxidase activity was blocked using 3% hydrogen peroxide solution
for 5 min at room temperature. The slides were incubated with EphA8
antibody (1:150; cat. no. 13724-1; ProteinTech Group, Inc.)
overnight at 4°C, followed by secondary antibody (1:500; cat. no.
ab7089; Abcam) at 37°C for 30 min. The labeled antigens were
visualized using a DAB Substrate kit (Abcam). EphA8 staining cells
were observed under light microscope with a digital camera (Nikon;
magnification, ×200). EphA8 staining intensity was scored as
follows: 0 (−, no staining), 1 (+, mild staining), 2 (++, medium
staining) or 3 (+++, intense staining). The percentage of
positively stained cells was multiplied by the intensity score to
give the final IHC score, which ranged from 0 to 300.
Cell lines and culture
The human BC cell lines HS-578T, LCC, MDA-MB-453 and
MCF-7 and the mammary epithelial cell line MCF-10A were purchased
from the Chinese Tissue Culture Collection Bioscience (Shanghai,
China). MCF-7 cell line was maintained in RPMI-1640 medium (Thermo
Fisher Scientific, Inc.) and other cell lines in DMEM (Corning,
Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C with 5% CO2.
Transfection and reagents
The short hairpin RNA (shRNA) was cloned into
pGreenPuro plasmids (Shanghai GenePharma Co., Ltd.) to establish
the pGreenPuro/EphA8 shRNA vector, which was then transfected into
BC cells with Lipofectamine® 3000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). The shRNA target
sequences and negative control (NC) shRNA are listed in Table SI. EphA8 was amplified using the
primers 5′-CTGGTGGTGCTTCTGCTCCT-3′ and 5′-TGCAGAGGCAGGAAGACAGG-3′.
Cells were transfected with50 nM pGreenPuro/EphA8 vector for 6 h,
followed by treatment with paclitaxel (0.25 µg/ml; Beijing Solarbio
Science & Technology Co., Ltd.) for 48 h separately or in
combination.pcDNA3.1 expressing EphA8 was synthesized and obtained
from GenePharma. Puromycin was obtained from Beijing Solarbio
Science & Technology Co., Ltd. Cells were cultured at 37°C with
5% CO2 for 48 h prior to subsequent experiments.
Cell Counting Kit (CCK)-8 assay
Cell proliferation assays in vitro were
performed using CCK-8 (Dojindo Molecular Technologies, Inc.)
according to the manufacturer's instructions. Cells were incubated
in 96-well plates at a density of 3×103 cells per well
for 24 h. Then, after specified time points (24, 48 and 72 h), 10
µl CCK-8 reagent was added and incubated for 1 h at 37°C. The
optical density at 450 nm was measured and the results were
expressed the mean ± SEM.
Western blotting
Proteins were extracted from BC cells using RIPA
lysis buffer (Beijing Solarbio Life Sciences) and the protein
concentration was determined with a BCA protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.). Proteins (30 µg/lane) were
separated in 4–12% SDS-polyacrylamide gels and transferred to
polyvinylidene difluoride membranes, followed by blocking in 5%
non-fat milk (in Tris-buffered saline with 0.1% Tween-20) for 2 h
at room temperature. Samples were incubated at 4°C overnight with
the following primary antibodies: EphA8 (1:1,000; cat. no. 13724-1;
ProteinTech Group, Inc.), AKT (1:2,000; cat. no. ab179463; Abcam),
phosphorylated (p)-AKT (1:500; cat. no. ab38449; Abcam), p53
(1:1,000; cat. no. ab33889; Abcam), Bax (1:1,000; cat. no. ab32503;
Abcam), Bcl-2 (1:1,000; cat. no. ab32124; Abcam)Caspase-3 (1:1,000;
cat. no. ab32351; Abcam), cleaved Caspase-3 (1:200; cat. no.
ab2302; Abcam) and GAPDH (1:10,000; cat. no. ab181602; Abcam).
Membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit or goat anti-mouse secondary antibodies from
ProteinTech Group, Inc. at room temperature for 2 h. Blots were
detected by enhanced chemiluminescence (Beyotime Institute of
Biotechnology). Images were captured using a BioSpectrum Gel
Imaging System (Analytik Jena AG) and analyzed using ImageJ 1.51j8
software (National Institutes of Health).
Flow cytometric analysis of
apoptosis
Cells were harvested and fixed in 75% ethanol
overnight at 4°C. Cells were centrifuged at 800 × g for 5 min at
37°C, and resuspended in binding buffer containing Annexin-V APC
and PI (both Nanjing KeyGen Biotech Co., Ltd.), which were excited
at 633 and 488 nm and emitted fluorescence at 660 and 610 nm,
respectively. Following double staining with Annexin V and PI,
cells were analyzed by flow cytometry (FACScan; BD Biosicences).
Apoptosis analysis was performed using flow cytometric software
(CellQuest Pro5.1; BD Biosciences).
Wound healing assay
For the wound healing assay, cells were cultivated
in 6-well culture plates (6×105 cells/well) and grown to
80–90% confluence overnight. The confluent monolayer of cells was
scratched with a sterile 200-µl micropipette tip and washed with
PBS buffer to clear cell debris. The scratched cells were incubated
in serum-free medium for 24 h at 37°C in a humidified incubator
with 5% CO2. Closed area of the wound was determined
under an inverted light microscopy (magnification, ×40) at 24
h.
Cell invasion assay
Transwell invasion assays were performed in 24-well
(pore size, 8 µm) Transwell plates according to the manufacturer's
instructions (Corning, Inc.). The bottom of the Transwell chamber
was coated with BD Matrigel Basement Membrane Matrix (BD
Biosciences) for 30 min at 37°C. The upper chamber was filled with
1×105 cells in RPMI-1640. The lower chamber was filled
with RPMI-1640 containing 15% fetal bovine serum (both Gibco;
Thermo Fisher Scientific, Inc.). Following incubation for 36 h at
37°C and staining with 0.1% crystal violet for 10 min at room
temperature, the number of cells invading through the Matrigel was
counted in four randomly selected light microscopic fields of view
(magnification, ×200).
Xenograft experiments
All animal experiments were performed with
5-week-old BALB/c-Nu female mice (weight, 18–23 g) purchased from
the Shanghai Experimental Animal Center of the Chinese Academy of
Sciences. A total of 24 mice were kept in housing conditions of
40–70% humidity in a 12-h dark/light cycle with free access to food
and water at 21–25°C. Mice were divided into two groups (n=12) and
injected subcutaneously with control or shEphA8 stably transfected
MCF-7 cells. Tumor growth was monitored externally using a vernier
caliper every three days. Tumor volume was calculated using the
following formula: V (mm3)=πab2/6. When BC
xenografts reached 100 mm3 in volume, mice were treated
with paclitaxel (30 mg/kg) or PBS (100 µl) via intraperitoneal
injection. At 15 days after injection, mice were sacrificed by
cervical dislocation and the tumor weight was measured. The present
study was approved by the Ethics Committee of Affiliated Hospital
of Nantong University (approval no. 2016-070).
Statistical analysis
Data are expressed as the mean ± SD (n≥3). For
statistical analysis of the association between EphA8 expression
levels and overall survival (OS), the cutoff for low/high
expression was determined using X-tile software program (tissuearray.org/rimmlab). Survival curves were
generated using the Kaplan-Meier method and analyzed by log-rank
test. Multivariate analysis by Cox regression analysis
(proportional hazards model) was also performed for the prognostic
factors. The significance of differences between groups was tested
using χ2 test for the clinical data of patients. Paired
two-sided t-test was used for the comparison between two paired
groups for the cell experiments. One-way ANOVA and Dunnett's
multiple comparisons test were used for comparisons between more
than two groups. The statistical analysis was performed using SPSS
version 20.0 (IBM Corp.). The graphs were constructed using
GraphPad Prism 7 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
EphA8 expression levels are
upregulated in BC compared with paracancerous and benign breast
tissue
In order to investigate the significance of EphA8 in
the progression of BC, EphA8 protein expression levels were
analyzed in 151 tumor, 61 paracancerous and 30 benign breast tissue
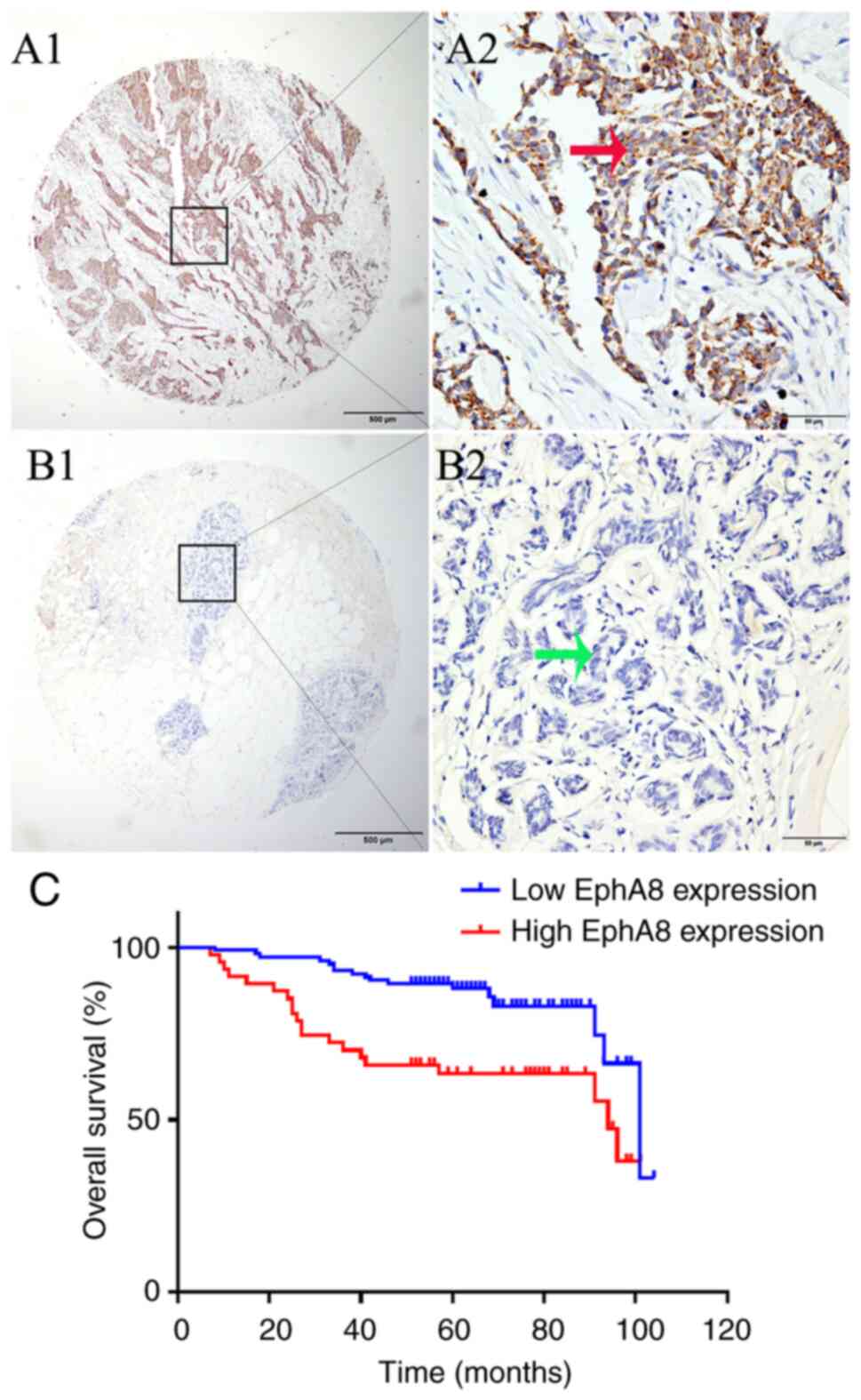
samples using IHC. Positive EphA8 staining was observed in the
cytoplasm and membrane of BC cells (Fig.
1A). The terminal duct lobular unit of the benign breast tissue
was negative for EphA8 staining (Fig.
1B). Tissues with EphA8 staining score ≥140 or <140 were
defined as high and low expression, respectively. High EphA8
expression was detected in 31.1% of BC but only 13.1% of
paracancerous and 16.7% of benign breast tissue samples. The
proportion of BC tissue with high EphA8 expression was
significantly increased compared with paracancerous and benign
breast tissue (χ2=8.772; P=0.0125; Table I).
 | Table I.Erythropoietin-producing
hepatocellular receptor A8 expression levels in BC, paracancerous
and benign tissue. |
Table I.
Erythropoietin-producing
hepatocellular receptor A8 expression levels in BC, paracancerous
and benign tissue.
| Tissue | n | Low expression
(%) | High expression
(%) | χ2 | P-value |
|---|
| Benign | 30 | 25 (83.3%) | 5 (16.7%) |
|
|
| Paracancerous | 61 | 53 (86.9) | 8 (13.1%) |
|
|
| BC | 151 | 104 (68.9%) | 47 (31.1%) |
|
|
| Total | 242 |
|
| 8.772 | 0.0125a |
The clinicopathological and histopathological
characteristics of patients with BC, which were classified
according to EphA8 protein expression levels, are summarized in
Table SII. High EphA8 expression
levels were significantly associated with tumor size and TNM stage
(both P<0.001). By contrast, no association was observed between
EphA8 expression levels and other clinicopathological parameters,
such as age at diagnosis, grade and nodal, estrogen and
progesterone receptor (ER and PR, respectively) and Ki-67 status.
These findings indicated that upregulated EphA8 expression levels
may be associated with tumor growth and poor TNM stage.
Upregulated EphA8 expression levels
are associated with poor survival in patients with BC
The association between EphA8 expression levels and
the prognosis of patients with BC was subsequently determined.
Kaplan-Meier survival curves demonstrated that patients with high
EphA8 expression had a significantly shorter OS time compared with
patients in the low EphA8 expression group (P<0.001; Fig. 1C). The survival curve with regard to
EphA8 expression showed good differentiation between high and low
expression with little overlap, indicating the reliability of EphA8
as a prognostic factor. These findings were also validated using
univariate Cox regression model (OS: HR, 2.733; 95% CI,
1.421–5.255; P=0.003). In multivariate Cox regression analysis,
subsequent to adjustment for grade, tumor size and PR status, high
EphA8 expression levels (P=0.022), age at diagnosis (P<0.001),
nodal (P=0.030) and ER status (P=0.014) were determined as
independent predictive factors of a poor outcome in patients with
BC (Table II). Together, these
results indicate that EphA8 expression is a negative prognostic
factor for survival in patients with BC.
 | Table II.Univariate and multivariate analysis
of prognostic factors for overall survival in breast cancer. |
Table II.
Univariate and multivariate analysis
of prognostic factors for overall survival in breast cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| EphA8 expression,
high vs. low | 2.733 | 1.421–5.255 | 0.003a | 2.385 | 1.131–5.031 | 0.022a |
| Age at diagnosis,
years, ≤ median vs. > median | 1.965 | 1.008–3.832 | 0.047a | 3.842 | 1.755–8.410 | 0.001a |
| NHG, I vs. II vs.
III | 1.526 | 0.954–2.440 |
|
|
|
|
| Tumor size, mm, ≤20
vs. >20 | 1.990 | 0.957–4.138 | 0.065 |
|
|
|
| Nodal status,
positive vs. negative | 5.880 | 2.835–12.194 |
<0.001a | 2.912 | 1.106–7.665 | 0.030a |
| TNM stage, 0/I/II
vs. III/IV | 5.121 | 2.647–9.908 |
<0.001a | 2.339 | 0.903–6.056 | 0.080 |
| ER status, negative
vs. positive | 0.376 | 0.193–0.734 | 0.004a | 0.395 | 0.188–0.831 | 0.014a |
| PR status, negative
vs. positive | 0.596 | 0.293–1.210 | 0.152 |
|
|
|
| Ki-67 status,
<14% vs. ≥14% | 2.949 | 1.482–5.866 | 0.002a | 1.370 | 0.652–2.877 | 0.406 |
EphA8 expression levels in BC cell
lines
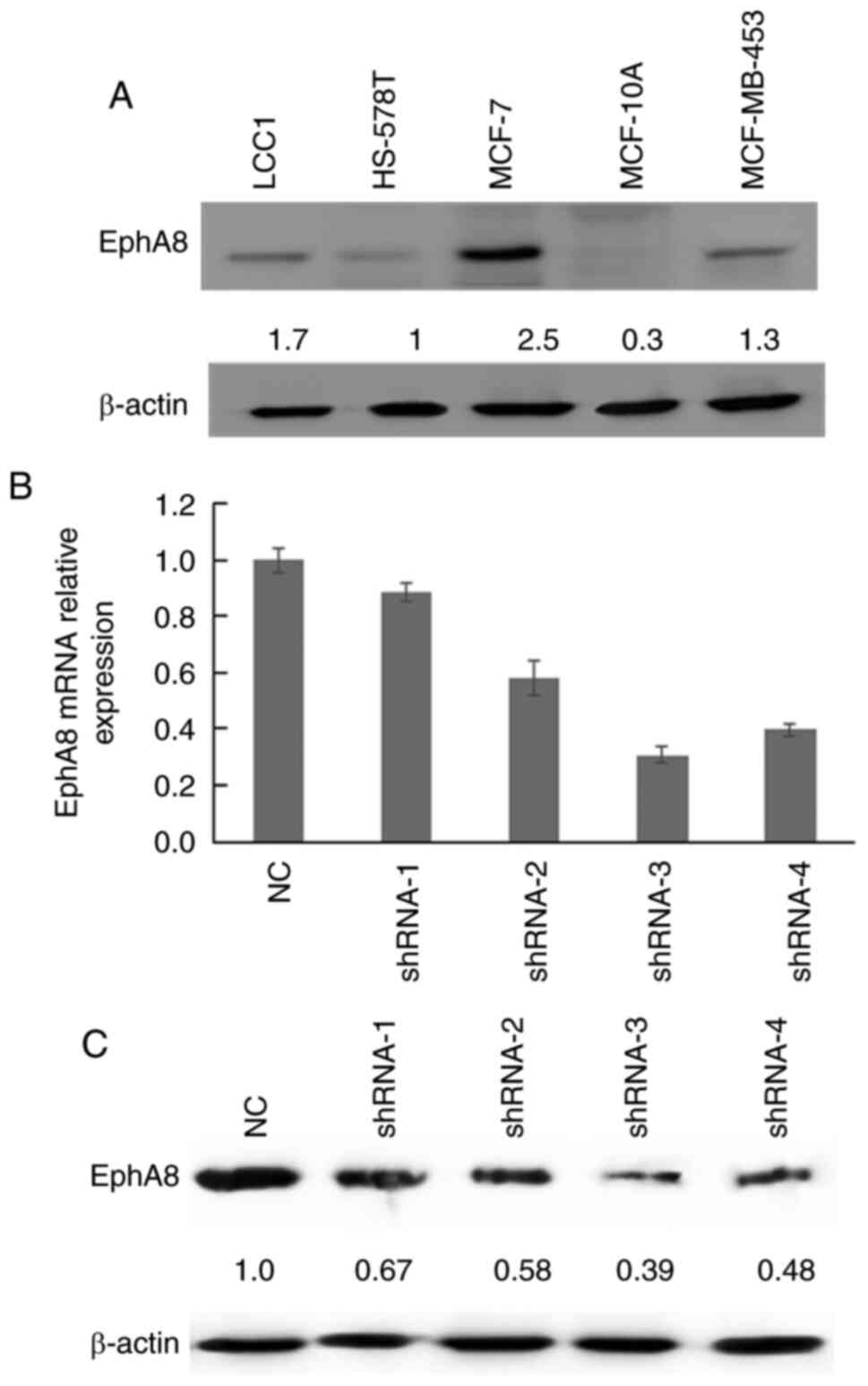
EphA8 protein expression levels were subsequently
analyzed in four BC cell lines. EphA8 expression levels were
significantly upregulated in BC cells compared with the normal
MCF-10A cell line. Protein expression levels of EphA8 were highest
in MCF-7 cells and lowest in HS-578T cells (Fig. 2A). Therefore, MCF-7 cells were
selected for transfection with four types of shEphA8 (shRNA 1–4)
and NC. The mRNA expression levels of EphA8 were downregulated in
shEphA8-transfected BC cells compared with the NC group, which
confirmed the successful transfection of shRNAs targeting EphA8
(Fig. 2B). Western blot analysis also
demonstrated that EphA8 protein expression levels were
downregulated in shEphA8-transfected cells compared with the NC
group (Fig. 2C). shRNA-3 demonstrated
the highest efficiency in silencing EphA8 expression and was used
in subsequent loss-of-function experiments.
EphA8 modulates proliferation,
apoptosis, invasion and migration of BC cells in vitro
The pGreenPuro vectors containing shRNA-3 were
transfected into the MCF-7 cell line, and puromycin was used to
establish the stable EphA8-knockdown MCF-7 cell line. CCK-8 assay
was performed to determine the effect of EphA8 on cell
proliferation. The proliferation of MCF-7 cells was significantly
decreased following knockdown of EphA8 with shEphA8 at each time
point (Fig. 3A). In order to
investigate whether EphA8 affected BC proliferation by regulating
apoptosis or the cell cycle, flow cytometry assay was performed.
The cells were treated and harvested 48 h later for analysis of
apoptosis and cell cycle distribution. The percentage of apoptotic
cells significantly increased (from 2.24 to 13.40%) in the EphA8
knockdown group (Fig. 3B). In order
to determine the effect of EphA8 on the migration and invasion of
BC cells, Matrigel invasion assay was performed. Following
knockdown of EphA8, the number of invasive MCF-7 cells was
significantly decreased compared with the control group (P<0.05,
Fig. 3C). In order to validate the
effect of shEphA8 on cell motility and migration, wound healing
assay was performed. Similarly, the knockdown of EphA8
significantly decreased the migratory ability of MCF-7 cells
compared with the control group (P<0.05, Fig. 3D). Conversely, overexpression of EphA8
was demonstrated to promote the proliferation, migration, and
invasion of HS-578T cells (Fig.
S1).
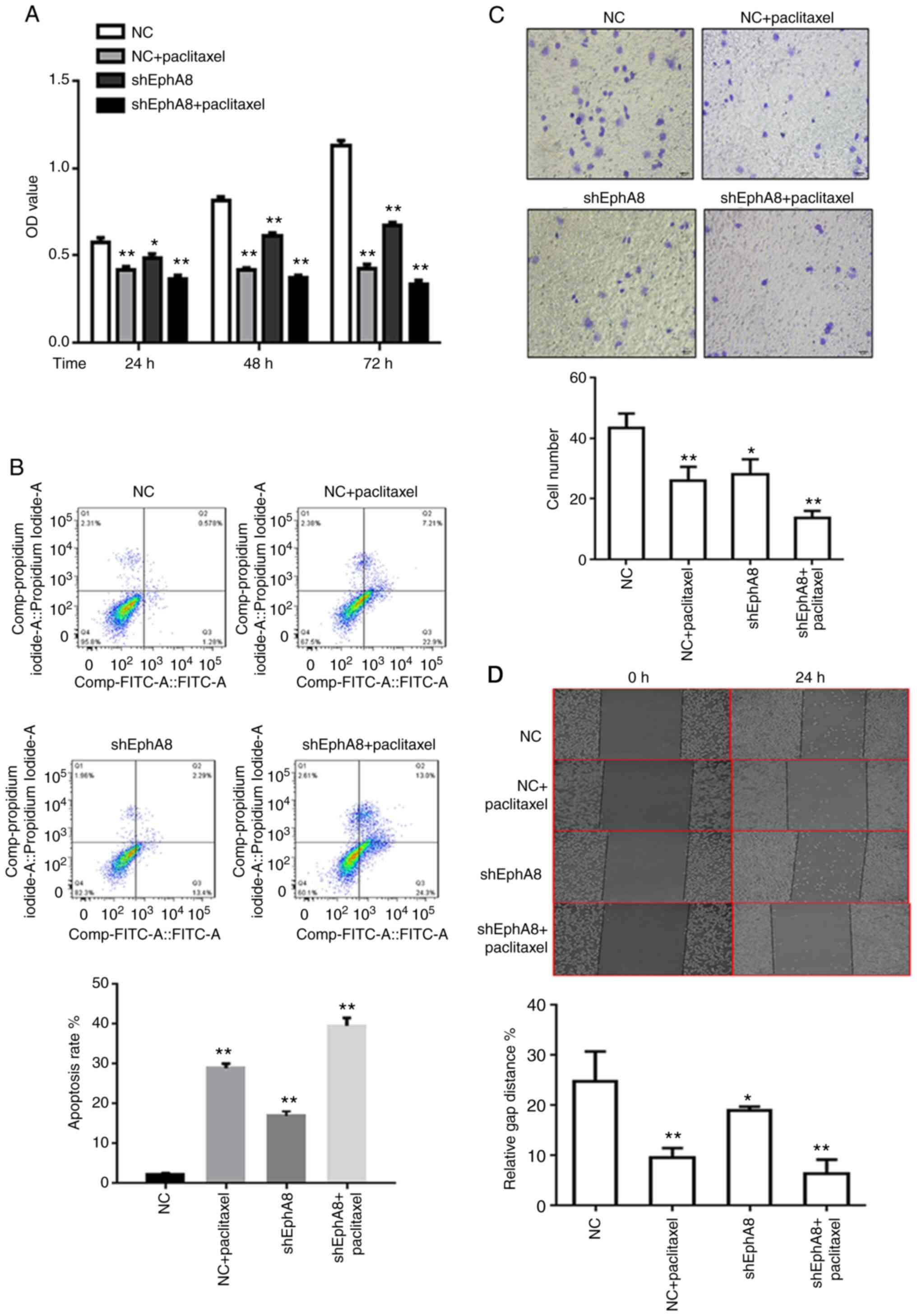 | Figure 3.EphA8 knockdown inhibits BC cell
proliferation and migration in vitro and enhances the
sensitivity of BC cells to paclitaxel. Effect of EphA8 knockdown
alone or in combination with paclitaxel treatment on BC cell (A)
proliferation, analyzed via Cell Counting Kit-8 assay; (B)
apoptosis, analyzed using Annexin V-FITC and PI staining followed
by flow cytometry; (C) invasion, analyzed via Transwell invasion
assay and (D) migration, analyzed via wound healing assay. Data are
presented as the mean ± SD (n=3). *P<0.05, **P<0.01. EphA8,
erythropoietin-producing hepatocellular receptor A8; BC, breast
cancer; sh, short hairpin; NC, negative control; OD, optical
density. |
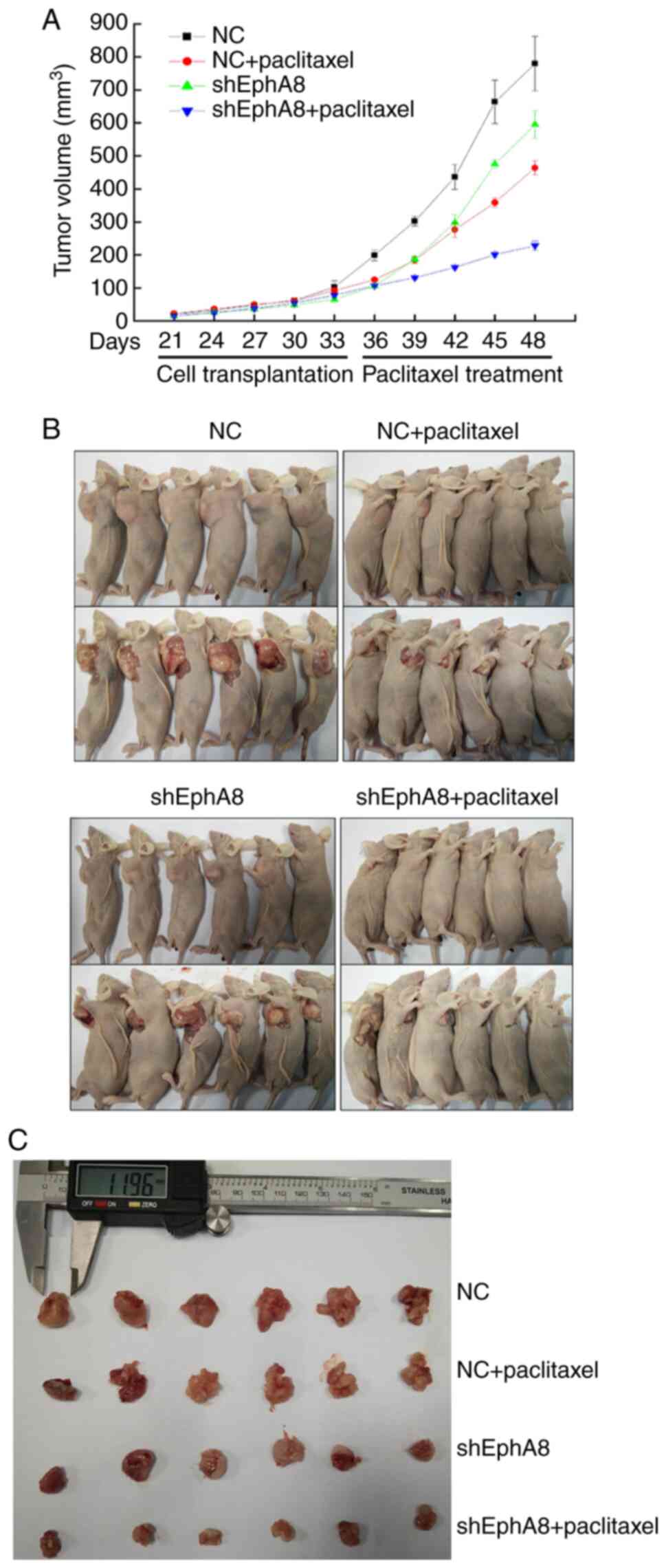
Knockdown of EphA8 inhibits
tumorigenicity of BC cells in vivo
In order to investigate the role of EphA8 in BC
in vivo, nude BALB/c mice were used to determine whether
EphA8 influenced the behavior of BC cells. Mice were injected
subcutaneously with NC- or shEphA8-transfected MCF-7 cells. Upon BC
xenografts reaching 100 mm3 in volume, mice were treated
with intraperitoneal injection of paclitaxel (30 mg/kg) or PBS (100
µl) once a week. Consistent with the in vitro findings, the
growth of BC cells in the shEphA8 group was notably slower compared
with NC (P<0.05, Fig. 4A).
Following 2 weeks of intraperitoneal injection, the tumors were
harvested (Fig. 4B and C); tumor
volume was decreased and tumor growth was notably inhibited
following paclitaxel administration (P<0.05, Fig. 4A). Taken together, these findings
suggested that EphA8 may serve an oncogenic role in the development
of BC.
Knockdown of EphA8 enhances
sensitivity of BC cells to paclitaxel chemotherapy
Chemotherapy is an important therapeutic regimen
used for the management of patients with advanced BC. Paclitaxel,
one of the most effective and widely used drugs, has shown good
efficacy against BC; however, long-term use of paclitaxel can
promote drug resistance (18).
Paclitaxel-based combination therapy increases the survival period
of patients with BC; therefore, current clinical treatments often
use this strategy (18). The combined
knockdown of EphA8 and treatment with paclitaxel significantly
decreased tumor cell proliferation, migration and invasion compared
with paclitaxel monotherapy in vitro (P<0.05; Fig. 3). Similarly, in vivo,
intraperitoneal injection of paclitaxel in mice in the shEphA8
group significantly impaired tumor growth compared with NC or PBS
injection groups (P<0.05; Fig. 4).
These in vitro and in vivo findings suggested that
knockdown of EphA8 may enhance the sensitivity of BC cells to
paclitaxel.
Knockdown of EphA8 regulates
expression levels of apoptosis-associated proteins both in vitro
and in vivo
The results of functional experiments revealed that
the transfection with shEphA8 significantly induced apoptosis in BC
cells. Therefore, changes in the expression levels of apoptotic
proteins following EphA8 knockdown were subsequently investigated.
Western blotting revealed that the expression levels of cleaved
Caspase-3 and Bax were upregulated, whereas Bcl-2 expression levels
were downregulated in the shEphA8 group both in vitro
(Fig. 5A) and in vivo
(Fig. 5B). The changes in expression
levels of apoptosis-associated proteins were more significant
following combined treatment with paclitaxel and transfection with
shEphA8 compared with either paclitaxel treatment or shEphA8
transfection-alone. As alterations in the PI3K/AKT signaling
pathway are among the most common genomic abnormalities in BC, and
the activation of the PI3K/AKT pathway suppresses the function of
p53 to inhibit mitochondrial pathway-induced apoptosis (19), the expression levels of p-AKT and p53
expression of in BC cells following the transfection with shEphA8
or paclitaxel treatment were investigated. The protein expression
levels of p-AKT were downregulated, whereas those of p53 were
upregulated in shEphA8-transfected or paclitaxel-treated cells
(Fig. 5). These results indicated
that knockdown of EphA8 enhanced activation of Caspase-3 and may be
involved in regulating the expression levels of p53 and Bax; this
may be a potential mechanism by which EphA8 regulates apoptosis in
BC. In addition, transfection of shEphA8 in combination with
paclitaxel treatment may exert a more significant effect compared
with paclitaxel treatment-alone; this effect may be due to enhanced
sensitivity of BC cells to chemotherapy.
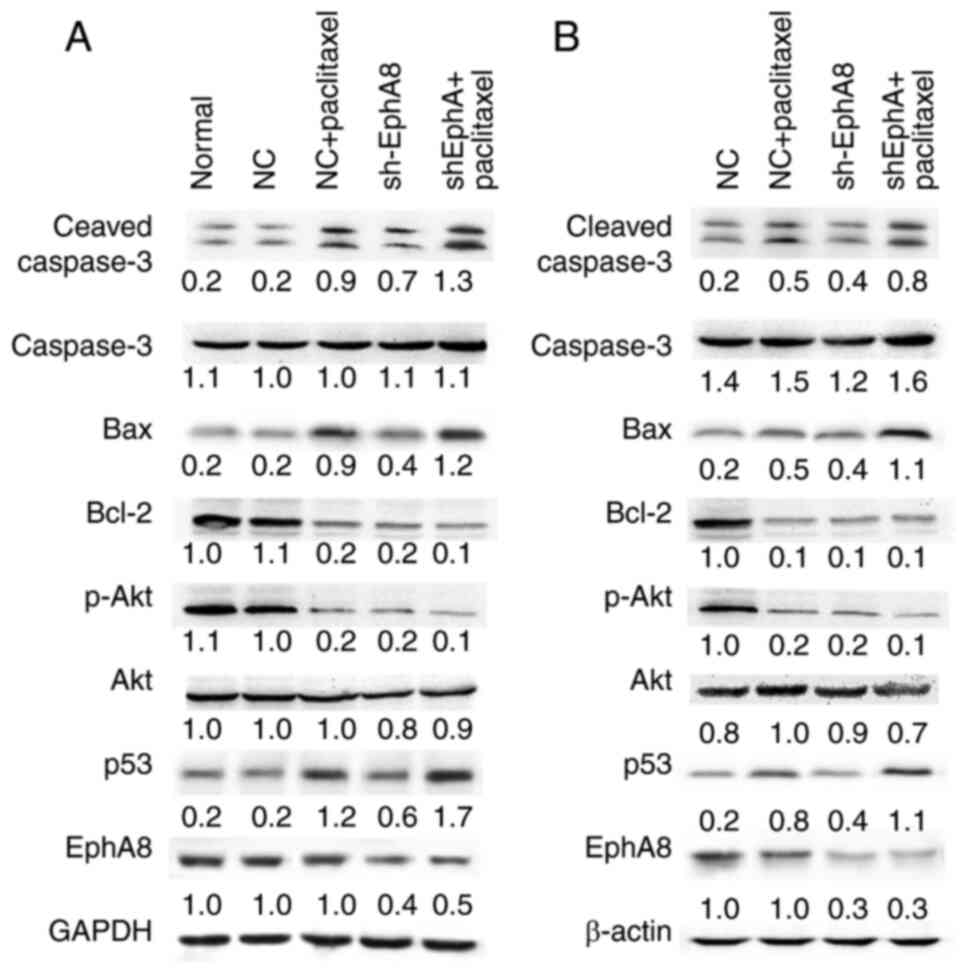 | Figure 5.Knockdown of EphA8 in combination
with paclitaxel treatment regulates expression levels of
apoptosis-associated proteins in breast cancer cells in
vitro and in vivo. (A) Western blot analysis of cleaved
Caspase-3, Bax, Bcl-2, p-AKT, p53 and EphA8 in shEphA8 stably
transfected MCF-7 cells compared with shNC cells in vitro.
GAPDH was used as the loading control. (B) Western blot analysis of
cleaved Caspase-3, Bax, Bcl-2, p-AKT, p53 and EphA8 in tumors of
nude mice from each group. β-actin was used as the loading control.
Densitometric values are listed below each blot. EphA8,
erythropoietin-producing hepatocellular receptor A8; p-,
phosphorylated; NC, negative control; sh, short hairpin. |
Discussion
RTKs are key regulators of signaling transduction
pathways and have been discovered to promote the malignant
progression of numerous types of solid tumor (20). EphA8 is a member of the Eph RTK
subfamily, which transduces signals via ligand-induced activation,
and has previously been shown to serve role in tumorigenesis
(21). Previous studies have
demonstrated that EphA8 promotes cell adhesion and migration via
the PI3K and MAPK kinase signaling pathways during normal embryonic
development (16,22). However, to the best of our knowledge,
the role of EphA8 in BC tumorigenesis and its potential molecular
mechanisms have not yet been investigated.
The present study demonstrated that expression
levels of EphA8 were upregulated in BC tissue and this was
significantly associated with tumor size and TNM stage.
Multivariate Cox regression analysis identified that EphA8 was an
independent predictive factor of poor outcome in patients with BC.
Subsequently, functional studies were performed to determine the
effect of knockdown of EphA8 on tumor biology; the results revealed
that knockdown of EphA8 inhibited proliferation and induced
apoptosis in BC cells. These findings suggested the potential of
EphA8 as a target for anticancer therapy in BC.
Ephs have previously been reported to be responsible
for cytoskeleton activity, cell adhesion, motility, invasion,
neo-angiogenesis, cell shape and epithelial-mesenchymal transition
(8,23). With such wide-ranging effects, the
altered function of Ephs has been implicated in tumorigenesis and
cancer progression. For example, the overexpression of Ephs is
associated with poor clinical outcome and cancer progression
(24,25). EphA2 and EphB4 are the two most
extensively studied members of the Eph RTK family in BC (20,26,27). The
upregulation of EphA2 expression levels is significantly associated
with poor prognosis and resistance to therapeutic agents in
HER2-positive patients with BC. The ephrin-A1/EphA2 signaling axis
regulates glutamine metabolism in HER2-overexpressing BC and EphA2
promotes BC tumorigenesis in the absence of ephrin-A1 (20). In addition, knockdown of EphA2 in
trastuzumab-resistant BC cells restores BC cell sensitivity to
trastuzumab treatment in vivo (26). Therefore, targeting EphA2 in BC may
serve as a novel strategy to inhibit tumorigenesis and reverse
trastuzumab resistance. Recent studies have suggested that EphB4
may be associated with tumor angiogenesis, growth and metastasis
(27,28). Downregulation of EphB4 expression
levels using small interfering RNAs or antisense oligonucleotides
has been demonstrated to inhibit malignant cell behavior of BC
(27). However, other studies have
reported conflicting results on the role of EphB4 in tumor
progression. For example, following stimulation of EphB4 with
ephrin-B2, EphB4 acts as a tumor suppressor in a xenograft model of
BC by activating a tumor suppressive pathway involving Abl family
TKs and the Crk adaptor protein (28). These conflicting findings may be due
to the bidirectional signaling of EphB4/ephrin and crosstalk with
other signaling pathways. Although the role of Eph in BC has been
reported in previous studies (24,27), the
exact role and signaling pathway of EphA8 remains unclear.
Due to the association between EphA8 expression
levels and poor survival in patients with BC in the present study,
the function of EphA8 in BC was investigated both in vivo
and in vitro. The findings of the present study revealed
that the knockdown of EphA8 significantly induced apoptosis of BC
cells. Activation of PI3K/AKT signaling regulates its downstream
proteins, including Bad, Bcl-2, Caspase-3 and other effector
proteins (29), and suppresses the
function of p53 to inhibit apoptosis via the mitochondrial pathway
(30,31). In addition, a previous study
discovered that EphA8 promotes integrin-mediated cell adhesion and
migration during normal embryonic development via the PI3K
signaling pathway (12). Thus, the
present study investigated the effects of EphA8 knockdown on the
activity of the PI3K/AKT signaling pathway. The protein expression
levels of p-AKT and Bcl-2 were downregulated in EphA8-knockdown BC
cells, whereas the expression levels of caspase-3, p53 and Bax were
upregulated. Based on this finding, it was hypothesized that EphA8
may inhibit apoptosis of BC cells, at least partly, by activating
the PI3K/AKT signaling pathway. Other members of the Eph family
activate the PI3K/AKT signaling pathway (32) and Eph expression patterns vary in
different types of tumor cell. For example, in BC stem cells, EphA8
is the most upregulated transcript relative to normal breast
epithelial cells (33).
One of the most significant challenges in the
treatment of BC is that cancer cells become insensitive or
resistant to radio- or chemotherapy-induced inhibition of cell
growth and induction of apoptosis. Taxanes are the most widely used
chemotherapeutic agents for the treatment of BC (18). Paclitaxel binds to β-tubulin to
mechanistically stabilize tubulin polymerization, which results in
G2/M arrest and subsequent apoptosis via the
mitochondrial pathway (18,34). Following long-term paclitaxel exposure
in BC therapy, abnormal activation of PI3K/AKT, NF-κB and MAPK
signaling induces paclitaxel resistance (35). Paclitaxel-based combination therapy
increases the survival period of patients with BC; therefore,
current clinical treatments often use this strategy (18). Thus, the present study analyzed the
effects of the combination of paclitaxel treatment and transfection
with shEphA8 on BC cells. Knockdown of EphA8 combined with
paclitaxel treatment increased the chemosensitivity of BC cells to
paclitaxel and significantly decreased tumor cell proliferation,
migration and invasion compared with paclitaxel monotherapy. These
results suggested that silencing of EphA8 may inhibit PI3K/AKT
signaling, which may increase the chemosensitivity of BC cells to
paclitaxel. These data provided an improved understanding of BC
progression and the effect of combined chemotherapy for BC
treatment. However, further studies are required to determine the
association between EphA8 and the PI3K/AKT signaling pathway and to
verify the potential application of these findings for BC
chemotherapy.
In conclusion, the findings of the present study
suggested that the expression levels of EphA8 may be upregulated in
BC and are associated with tumor size and TNM stage. Upregulated
expression levels of EphA8 were also shown to be a biomarker for a
poor prognosis in patients with BC. Moreover, knockdown of EphA8
expression inhibited tumorigenesis and induced apoptosis of BC
cells in vitro and in vivo. In addition, knockdown of
EphA8 increased the sensitivity of BC cells to chemotherapeutic
reagents by inhibiting PI3K/AKT signaling. These findings may have
important implications for understanding BC progression and
improving BC treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by research grants
from Maternal and Child Health Project of Jiangsu (grant no.
FRC201760), the Elite Program of Affiliated Hospital of Nantong
University, the 226 Talent Training Program of Nantong City and the
National Natural Science Foundation of China (grant no.
81702086).
Availability of data and materials
All data generated or analyzed in this study are
available from the corresponding author on reasonable request.
Authors' contributions
QN conceptualized the study. GHW and KN acquired and
analyzed the clinical data. CG and JC performed the in vitro
experiments. JH and XDW analyzed the data and prepared the figures.
GHW and XDW wrote, reviewed and revised the manuscript. GHW and QCN
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Affiliated Hospital of Nantong University (approval
no. 2016-070). All experiments complied with the Helsinki
declaration and institutional guidelines. All samples were obtained
from patients who had provided written informed consent for the use
of their tissue for the purposes of research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Eph
|
Erythropoietin-producing
hepatocellular receptor
|
|
BC
|
breast cancer
|
|
RTKs
|
receptor tyrosine kinases
|
|
shRNA
|
short hairpin RNA
|
|
CCK-8
|
Cell Counting Kit-8
|
|
OS
|
overall survival
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. Feb 4–2021.(Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Januškevičienė I and Petrikaite V:
Heterogeneity of breast cancer: The importance of interaction
between different tumor cell populations. Life Sci. 239:1170092019.
View Article : Google Scholar
|
|
3
|
Koren S and Bentires-Alj M: Breast tumor
heterogeneity: Source of fitness, hurdle for therapy. Mol Cell.
60:537–546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bratton MR, Duong BN, Elliott S, Weldon
CB, Beckman BS, McLachlan JA and Burow ME: Regulation of
ERalpha-mediated transcription of Bcl-2 by PI3K-AKT crosstalk:
Implications for breast cancer cell survival. Int J Oncol.
37:541–550. 2010.PubMed/NCBI
|
|
5
|
Lisabeth EM, Falivelli G and Pasquale EB:
Eph receptor signaling and ephrins. Cold Spring Harb Perspect Biol.
5:a0091592013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boyd AW, Bartlett PF and Lackmann M:
Therapeutic targeting of EPH receptors and their ligands. Nat Rev
Drug Discov. 13:39–62. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nikolov DB, Xu K and Himanen JP:
Eph/ephrin recognition and the role of Eph/ephrin clusters in
signaling initiation. Biochim Biophys Acta. 1834:2160–2165. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shiuan E and Chen J: Eph receptor tyrosine
kinases in tumor immunity. Cancer Res. 76:6452–6457. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pasquale EB: Eph receptors and ephrins in
cancer: Bidirectional signalling and beyond. Nat Rev Cancer.
10:165–180. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hafner C, Schmitz G, Meyer S, Bataille F,
Hau P, Langmann T, Dietmaier W, Landthaler M and Vogt T:
Differential gene expression of Eph receptors and ephrins in benign
human tissues and cancers. Clin Chem. 50:490–499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagano K, Maeda Y, Kanasaki S, Watanabe T,
Yamashita T, Inoue M, Higashisaka K, Yoshioka Y, Abe Y, Mukai Y, et
al: Ephrin receptor A10 is a promising drug target potentially
useful for breast cancers including triple negative breast cancers.
J Control Release. 189:72–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park S, Frisen J and Barbacid M: Aberrant
axonal projections in mice lacking EphA8 (Eek) tyrosine protein
kinase receptors. EMBO J. 16:3106–3114. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kandouz M: The Eph/Ephrin family in cancer
metastasis: Communication at the service of invasion. Cancer
Metastasis Rev. 31:353–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu C and Park S: The EphA8 receptor
regulates integrin activity through p110gamma
phosphatidylinositol-3 kinase in a tyrosine kinase
activity-independent manner. Mol Cell Biol. 21:4579–4597. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim Y, Park E, Noh H and Park S:
Expression of EphA8-Fc in transgenic mouse embryos induces
apoptosis of neural epithelial cells during brain development. Dev
Neurobiol. 73:702–712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gu C, Shim S, Shin J, Kim J, Park J, Han K
and Park S: The EphA8 receptor induces sustained MAP kinase
activation to promote neurite outgrowth in neuronal cells.
Oncogene. 24:4243–4256. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Xu Y, Jin Q, Wang W, Zhang S, Wang
X, Zhang Y, Xu X and Huang J: EphA8 is a prognostic marker for
epithelial ovarian cancer. Oncotarget. 7:20801–20809. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murray S, Briasoulis E, Linardou H,
Bafaloukos D and Papadimitriou C: Taxane resistance in breast
cancer: Mechanisms, predictive biomarkers and circumvention
strategies. Cancer Treat Rev. 38:890–903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coradini D, Biganzoli E, Ardoino I,
Ambrogi F, Boracchi P, Demicheli R, Daidone MG and Moliterni A: p53
status identifies triple-negative breast cancer patients who do not
respond to adjuvant chemotherapy. Breast. 24:294–297. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Youngblood VM, Kim LC, Edwards DN, Hwang
Y, Santapuram PR, Stirdivant SM, Lu P, Ye F, Brantley-Sieders DM
and Chen J: The Ephrin-A1/EPHA2 signaling axis regulates glutamine
metabolism in HER2-positive breast cancer. Cancer Res.
76:1825–1836. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lodola A, Giorgio C, Incerti M, Zanotti I
and Tognolini M: Targeting Eph/ephrin system in cancer therapy. Eur
J Med Chem. 142:152–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gu C and Park S: The p110 gamma PI-3
kinase is required for EphA8-stimulated cell migration. FEBS Lett.
540:65–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen J, Song W and Amato K: Eph receptor
tyrosine kinases in cancer stem cells. Cytokine Growth Factor Rev.
26:1–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vaught D, Brantley-Sieders DM and Chen J:
Eph receptors in breast cancer: Roles in tumor promotion and tumor
suppression. Breast Cancer Res. 10:2172008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mathot L, Kundu S, Ljungstrom V, Svedlund
J, Moens L, Adlerteg T, Falk-Sörqvist E, Rendo V, Bellomo C,
Mayrhofer M, et al: Somatic ephrin receptor mutations are
associated with metastasis in primary colorectal cancer. Cancer
Res. 77:1730–1740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhuang G, Brantley-Sieders DM, Vaught D,
Yu J, Xie L, Wells S, Jackson D, Muraoka-Cook R, Arteaga C and Chen
J: Elevation of receptor tyrosine kinase EphA2 mediates resistance
to trastuzumab therapy. Cancer Res. 70:299–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar SR, Singh J, Xia G, Krasnoperov V,
Hassanieh L, Ley EJ, Scehnet J, Kumar NG, Hawes D, Press MF, et al:
Receptor tyrosine kinase EphB4 is a survival factor in breast
cancer. Am J Pathol. 169:279–293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noren NK, Foos G, Hauser CA and Pasquale
EB: The EphB4 receptor suppresses breast cancer cell tumorigenicity
through an Abl-Crk pathway. Nat Cell Biol. 8:815–825. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choudhary GS, Al-Harbi S, Mazumder S, Hill
BT, Smith MR, Bodo J, His ED and Almasan A: MCL-1 and
BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be
overcome by preventing PI3K/AKT/mTOR activation in lymphoid
malignancies. Cell Death Dis. 6:e15932015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bernardi MP, Ngan SY, Michael M, Lynch AC,
Heriot AG, Ramsay RG and Phillips WA: Molecular biology of anal
squamous cell carcinoma: Implications for future research and
clinical intervention. Lancet Oncol. 16:e611–e621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dey N, De P and Leyland-Jones B:
PI3K-AKT-mTOR inhibitors in breast cancers: From tumor cell
signaling to clinical trials. Pharmacol Ther. 175:91–106. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li JY, Xiao T, Yi HM, Yi H, Feng J, Zhu
JF, Huang W, Lu SS, Zhou YH, Li XH and Xiao ZQ: S897
phosphorylation of EphA2 is indispensable for EphA2-dependent
nasopharyngeal carcinoma cell invasion, metastasis and stem
properties. Cancer Lett. 444:162–174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lucero M, Thind J, Sandoval J, Senaati S,
Jimenez B and Kandpal RP: Stem-like cells from invasive breast
carcinoma cell line MDA-MB-231 express a distinct set of Eph
receptors and ephrin ligands. Cancer Genomics Proteomics.
17:729–738. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Camblin AJ, Pace EA, Adams S, Curley MD,
Rimkunas V, Nie L, Tan G, Bloom T, Iadevaia S, Baum J, et al: Dual
inhibition of IGF-1R and ErbB3 enhances the activity of gemcitabine
and nab-paclitaxel in preclinical models of pancreatic cancer. Clin
Cancer Res. 24:2873–2885. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin YF, Tseng IJ, Kuo CJ, Lin HY, Chiu IJ
and Chiu HW: High-level expression of ARID1A predicts a favourable
outcome in triple-negative breast cancer patients receiving
paclitaxel-based chemotherapy. J Cell Mol Med. 22:2458–2468. 2018.
View Article : Google Scholar : PubMed/NCBI
|