Introduction
Malignant glioma is the most common primary
intracranial tumor, accounting for approximately 80% of central
nervous system malignancies (1). At
present, the treatment of glioma is still mainly based on surgical
tumor removal supplemented by radiation therapy, chemotherapy, and
other comprehensive treatment methods (2). Glioma is characterized by rapid
proliferation, aggressive growth, and strong cellular heterogeneity
(3,4),
which is the main reason why glioma is difficult to remove
completely by surgery and why glioma is associated with high
recurrence rates and poor prognosis. There are numerous measures of
glioma prognosis, such as overall survival (OS) at one year,
progression-free survival, and median survival. Of these, median
survival is the most common and widely accepted metric for
establishing the prognosis of glioma multiforme. The median
survival of patients with glioblastoma multiforme (5–8), the most
malignant glioma, is only 1 to 2 years (9). Therefore, it is urgent to investigate
the regulatory mechanisms of glioma growth and explore new
therapeutic targets.
Patients with cancer suffer from mental and physical
stress, which causes an adverse stress response in the body and
seriously affects clinical treatment and prognosis. Epidemiologic
and clinical experimental studies have revealed that chronic stress
can promote the progression of tumor and is closely related to poor
prognosis (10–12). The hypothalamic-pituitary-adrenal
(HPA) axis and the sympathetic nervous system (SNS) are activated
by chronic stress, which is characterized by increased secretion of
glucocorticoid (GC) and catecholamines (13). Numerous studies have revealed that GC
signaling activation may contribute to progression of solid tumors,
primarily through i) inducing anti-apoptosis activity and
chemotherapy resistance and ii) disrupting antitumor immunity
(14–16). It has been reported that the
catecholamines, especially noradrenaline (NE), can alter downstream
signaling pathways by binding to membrane receptors and are
involved in the regulation of tumor growth. It has been revealed
that NE binds to β-adrenergic receptors (ADRBs) and activates the
cAMP/PKA signaling pathway in various cancers, such as ovarian,
prostate, and pancreatic cancer (17–20).
Propranolol, an ADRB inhibitor, has been demonstrated to inhibit
tumor growth and metastasis (21,22), which
provides a new strategy for the treatment of tumors. In addition,
catecholamines can also directly promote cancer cell proliferation
through α-adrenergic receptors (ADRAs) (23). However, findings reporting the
involvement of chronic stress in glioma are rare.
Numerous studies have revealed the changes of some
genes and signaling pathways involved in the occurrence and
development of glioma. The predominant signaling pathways involved
in glioma cell regulation include the RAS/RAF/ERK pathway, the
p53/MDM2/p21 pathway, and the phosphatidylinositol 3-kinase
(PI3K)/Akt/mTOR pathway (24–26). Studies have revealed that inhibition
of PI3K/Akt signaling can increase autophagy levels of tumor cells,
and thereby restrain cell proliferation and induce apoptosis
(27–29). Akt, also known as protein kinase B, is
one of the most important downstream target kinases in the PI3K
signal transduction pathway. It is in the central link of the
PI3K/Akt pathway, and thus it plays an important role in a series
of biological activities such as apoptosis, survival, and
proliferation (30). However, there
are few studies on the regulatory effects of the PI3K/Akt signaling
pathway on the chronic stress-induced proliferation of gliomas.
In the present study, the effect of chronic stress
on the malignant behavior of glioma was explored and its
accelerative role in glioma cell proliferation was elucidated. To
investigate whether chronic stress and stress hormone-induced
glioma cell proliferation are regulated by the PI3K/Akt signaling
pathway in vivo and in vitro, chronic stress animal
experiments and cytological experiments were used. The present
study was performed to investigate the targets and molecular
mechanisms of chronic stress in regulating glioma progression and
to provide new targets for the treatment of glioma.
Materials and methods
Animal model
A total of 10 male BALB/c nude mice (6–8 weeks old;
weight, ~18 g) were used in the present study. The feeding
conditions were as follows: Temperature, 18–22°C; relative
humidity, 50–60%; 10–14 h light/dark cycle. Feed was added 3–4
times a week and water was changed 2–3 times a week. All of the
animal experiments were approved (approval no. 2016-0002) by the
Institutional Animal Care and Use Committee of the Academy of
Military Medicine Sciences (Beijing, China). The experimental group
was divided into the chronic restraint stress group and the control
group, with 5 rats in each group. A restraint stress procedure was
adopted based on previous studies (17,31). The
animals in the stress group were subjected to restraint adaptation
for 1 week, which was gradually extended to 6 h per day. Next,
U87MG glioma cells were injected subcutaneously into the mice of
both groups at 2×106 cells per mouse. Then, the stress
group was subjected to restraint stress for 21 days, 6 h per day (9
am to 3 pm). Since the restrained group could not eat and drink
normally during restraint, the control mice were also deprived of
water and food during the restraining period. The animals in the
control group were also treated with water and food deprivation
during the restraint period. The health and behavior of animals
were monitored and observed every Wednesday and Saturday at 9 a.m.
The checks included the growth of the xenograft (measurement of
tumor size), the general condition of the mice (weight and mental
status), abdominal breathing (breathing rate), and paw and toe
characteristics (whether the paws and toes had fight damage).
Adequate food and water was ensured and mice were sacrificed after
5 weeks. The specific criteria for euthanasia applied in this
experiment were according to the Guidelines for the Review of
Humane Endpoints in Animal Experiments (GB/T1.1-2009; www.chinesestandard.net/PDF.aspx/GBT1.1-2009);
rapid cervical dislocation for euthanasia when the tumor
metastasizes or grows rapidly to the point of ulceration, causing
infection or necrosis, and observation of the experimental mice for
two to three min without voluntary respiration and no blink reflex,
was considered as having succumbed. The tumor samples were
collected and embedded in paraffin for subsequent analysis.
Cell culture and intervention
The glioma cell lines U87MG and LN229 were purchased
from the Chinese National Infrastructure of Cell Line Resource.
U87MG cells were cultured in MEM (Sigma-Aldrich; Merck KGaA) medium
containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin, 100 U/ml streptomycin, and
100 µg/ml nonessential amino acids. LN229 cells were cultured in
DMEM (Sigma-Aldrich; Merck KGaA) medium containing 10% FBS, 100
U/ml penicillin, and 100 U/ml streptomycin. All cells were cultured
in a humidified incubator containing 5% CO2 at 37°C.
U87MG cells were used as ‘glioblastoma of unknown origin’ and
identified by short tandem repeat analysis.
Stress hormones (GC and NE), an Akt-specific
inhibitor (perifosine), or receptor antagonists were added to the
culture medium for intervention. Based on successful
activation/inhibition in previous publications (32,33), the
concentrations of all drugs were selected as follows: GC (10
µmol/l), NE (10 µmol/l), perifosine (10 µmol/l; cat. no. S1037),
eplerenone [mineralocorticoid receptor (MR) antagonist, 5.2 µmol/l;
cat. no. S1707], mifepristone (GR receptor antagonist, 5.2 µmol/l;
cat. no. S2606), phentolamine (ADRA antagonist, 0.2 µmol/l; cat.
no. S2038), propranolol (ADRB antagonist, 24 µmol/l; cat. no.
S4076), atenolol (ADRB1 antagonist, 0.5 µmol/l; cat. no. S4817),
and higenamine hydrochloride (ADRB2 antagonist, 1.4 µmol/l; cat.
no. S3960). An equal volume of dimethyl sulfoxide (DMSO) was used
as the control. The inhibitor and antagonists were purchased from
Selleck Chemicals.
Immunohistochemistry
Hematoxylin-eosin (H&E) staining and
immunohistochemistry were performed as previously described
(28). Briefly, paraffin-embedded
sections were deparaffinized in xylene and rehydrated, followed by
antigen retrieval in sodium citrate. After blocking with 1% BSA
(BioFroxx), the sections were incubated with anti-Ki67 (1:200; cat.
no. A11005) and anti-p-Akt (1:100; cat. no. AP0004; both from
ABclonal Biotech Co., Ltd.) overnight at 4°C. A DAB kit (ZLI-9018;
ZSGB-BIO; OriGene Technologies, Inc.) was utilized to stain until
the desired stain intensity was developed. Sections were then
counterstained with hematoxylin, dehydrated, and mounted.
ELISA
Serums were collected from the stressed mice and the
control mice. The serum concentrations of GC and NE were determined
using a mouse GC ELISA kit (cat. no. D721183) and a mouse NE ELISA
kit (cat. no. D751020; both from Sangon Biotech, Co., Ltd.),
respectively, following the manufacturer's instructions. The ELISA
kits were purchased from Shanghai Guduo Biological Technology Co.,
Ltd.
Colony formation assay
Glioma cells were inoculated into a 6-well plate at
2,000 cells/well. GC, NE, Akt signaling inhibitor, or DMSO was
added and the culture solution was changed every 3 days. After
culturing for 7–10 days, the cells were fixed with 2 ml of 4%
paraformaldehyde for 15 min at 4°C and stained at room temperature
with 10% crystal violet solution for 30 min. The colonies with
>10 cells were counted under a low-power light microscope and
representative images were captured.
Cell Counting Kit-8 (CCK-8) assay
Cells in logarithmic growth phase were inoculated
into a 96-well plate at 500 cells/well in quintuplicate and
incubated. After the cells adhered to the wall, GC, NE, inhibitors,
and antagonists were added. After continuous culture for 0, 24, and
48 h, the drug-containing medium was discarded, and freshly
prepared solution containing 10 µl CCK-8 (Dojindo Molecular
Technologies, Inc.) was added into each well. After incubation at
37°C for 1 h, the OD value at 450 nm was measured by an enzyme
mapping instrument (Varioskan Flash; Thermo Fisher Scientific,
Inc.). The experiment was repeated three times.
Cell cycle experiment
The cells were digested to single cells and
centrifuged at 1,000 rpm for 5 min to collect cell precipitates.
Then 70% ethanol was added and cells were fixed overnight at 4°C.
After washing with PBS, the cells were resuspended in 500 µl PBS
containing 50 µg/ml PI, 100 µg/ml RNase A, and 0.2% Triton X-100
and incubated for 30 min at room temperature. Flow cytometry
(CytomicsFC500; Beckman Coulter) was used to detect 20,000-30,000
cells. The results were analyzed by cell cycle fitting software
ModFit LT 4.1 (Verity Software House).
Western blot analysis
The cells were lysed in RIPA buffer (Biosharp)
containing protease inhibitors and centrifuged at 12,000 × g for 15
min at 4°C. The pellet was discarded and loading buffer (Tiangen
Biotech Co., Ltd.) was added to the supernatant. Protein
determination was performed by bicinchoninic acid assay. Samples
were boiled for 5 min and proteins (30 µg) were separated by 10 or
12% SDS-PAGE, followed by transfer to a PVDF membrane using a dry
transfer system (Bio-Rad Laboratories, Inc.). The membrane was
blocked in 5% milk at room temperature for 1 h, washed 3 times in
1X TBST (0.5% Tween-20), and incubated at 4°C for 8 h with rabbit
anti-PI3K (1:1,000; product no. 4255; Cell Signaling Technology,
Inc.), rabbit anti-p-PI3K (1:1,000; product no. 13857; Cell
Signaling Technology, Inc.), rabbit anti-Akt (1:1,000; cat. no.
51077-1-AP; ProteinTech Group, Inc.), mouse anti-p-Akt (1:1,000;
cat. no. 66444-1-lg; ProteinTech Group, Inc.), or mouse anti-GAPDH
(1:5,000; cat. no. 60004-1-lg; ProteinTech Group, Inc.). After
washing three times in 1X TBST, membranes were incubated with
HRP-labeled goat anti-rabbit IgG (1:5,000; cat. no. ZB-2301;
ZSJQ-Bio) or goat anti-mouse IgG (1:5,000; cat. no. ZB-2305;
ZSJQ-Bio) for 2 h at room temperature. Protein bands were
visualized with an ECL western blotting substrate kit (Thermo
Fisher Scientific, Inc.). The protein molecular weights of PI3K
(110 kDa) and Akt (57 kDa) were so close to their corresponding
phosphorylated proteins, p-PI3K (100 kDa) and p-Akt (60 kDa), that
the same protein strip needed to be washed with stripping buffer
and then re-incubated with the antibody as well as exposed,
followed by image acquisition and analysis by ImageQuant LAS 4000
(GE Healthcare Bio-Sciences; Cytiva).
siRNA transfection and quantitative
(q)PCR
The siRNAs targeting ADRB1 and ADRB2 were
synthesized by TsingKe Biological Technology. Sequences of siRNAs
targeting ADRB1 were as follows: Sense, 5′-CCGAUAGCAGGUGAACUCGAA−3′
and antisense, 5′-GGCUAUCGUCCACUUGAGCUU−3′. Sequences of siRNAs
targeting ADRB2 were as follows: Sense, 5′-CAGAGUGGAUAUCACAUGGAA−3′
and antisense, 5′-GUCUCACCUAUAGUGUACCUU−3′. siRNA or siRNA negative
control (2.2 pmol) was diluted in 200 µl of serum-free medium and
mixed with a pipette. INTERFERin® (Polyplus
transfection) reagent (12 µl) was added, followed by vortex for 10
sec and spinning twice. After 10 min of incubation at room
temperature, the transfection mixture was added to the
serum-containing medium and the cells were incubated for 48 h
before performing the experiments, with the following groups: Ctrl
group, siRNA negative control and siRNA group. For siRNA against
Akt, the product Akt1/2 siRNA (h) (product no. sc-43609) was used,
purchased from Santa Cruz Biotechnology, Inc. siRNA or siRNA
negative control was diluted to 20 µmol/l with sterilized
ddH2O. The transfection mixture was added to
serum-containing medium, and the cells were incubated for 12 h
before performing the experiments with the following groups: Ctrl
group, siRNA negative control and Akt1/2 siRNA (h) group. The siRNA
negative control was purchased from TsingKe Biological Technology.
Sequences of siRNA negative control were as follows: SiRNA negative
control forward, 5′-UUCUCCGAACGUGUCACGUTT−3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT−3′. Successful siRNA knockdown was
verified by qPCR. Total RNA from cells or tissues was extracted
using TRIzol reagent (Takara Biotechnology Co., Ltd.) according to
the manufacturer's instructions. Reverse transcription (ABScriptII
One Step RT-qPCR; Abclonal Biotech Co., Ltd.) reactions were
performed with SYBR-Green. Real-time PCR was performed using a
LightCycler 96 Real-time PCR System (Roche Diagnostics) with TB
Green Premix Ex Taq kit (Takara Biotechnology Co., Ltd.). The
thermocycling conditions were as follows: Initial denaturation at
95°C for 10 min, denaturation at 95°C for 5 sec, annealing at 60°C
for 30 sec and extension at 72°C for 35 sec, for 40 cycles. β-Actin
was used as the internal control. All samples were normalized to
the internal controls, and fold changes were calculated via the
relative quantification method (2−ΔΔCq) (34). The primer sequences used were as
follows: ADRB1 forward, 5′-GACGCTCACCAACCTCTTCA-3′ and reverse,
ACTTGGGGTCGTTGTAGCAG; ADRB2 forward, 5′-TGATCGCAGTGGATCGCTAC-3′ and
reverse, 5′-CCACCTGGCTAAGGTTCTGG-3′; AKT1 forward,
5′-ACTGTCATCGAACGCACCTT-3′ and reverse, 5′-TCGGAGCCCCCGAGTTG-3′;
AKT2 forward, 5′-CCTCTGCAAAGAGGGCATCA-3′ and reverse,
5′-GAGGATGAGCTCGAAGAGGC-3′. β-actin forward,
5′-AATCGTGCGTGACATTAAGGAG-3′, and reverse,
5′-ACTGTGTTGGCGTACAGGTCTT-3′.
Statistical analysis
All of the data are listed as the mean ± standard
deviation (SD). The continuous variables were evaluated for
normality before comparison for statistical differences. Paired
Student's t-tests were used for comparisons between two groups.
One-way analysis of variance (ANOVA) was performed to compare the
values among multiple groups. Significant results of ANOVA were
subjected to Tukey's post hoc test. All of the differences were
two-sided. Statistical analysis was conducted with GraphPad Prism
5.0 software (GraphPad Software, Inc.) and P<0.05 was considered
to indicate a statistically significant difference.
Results
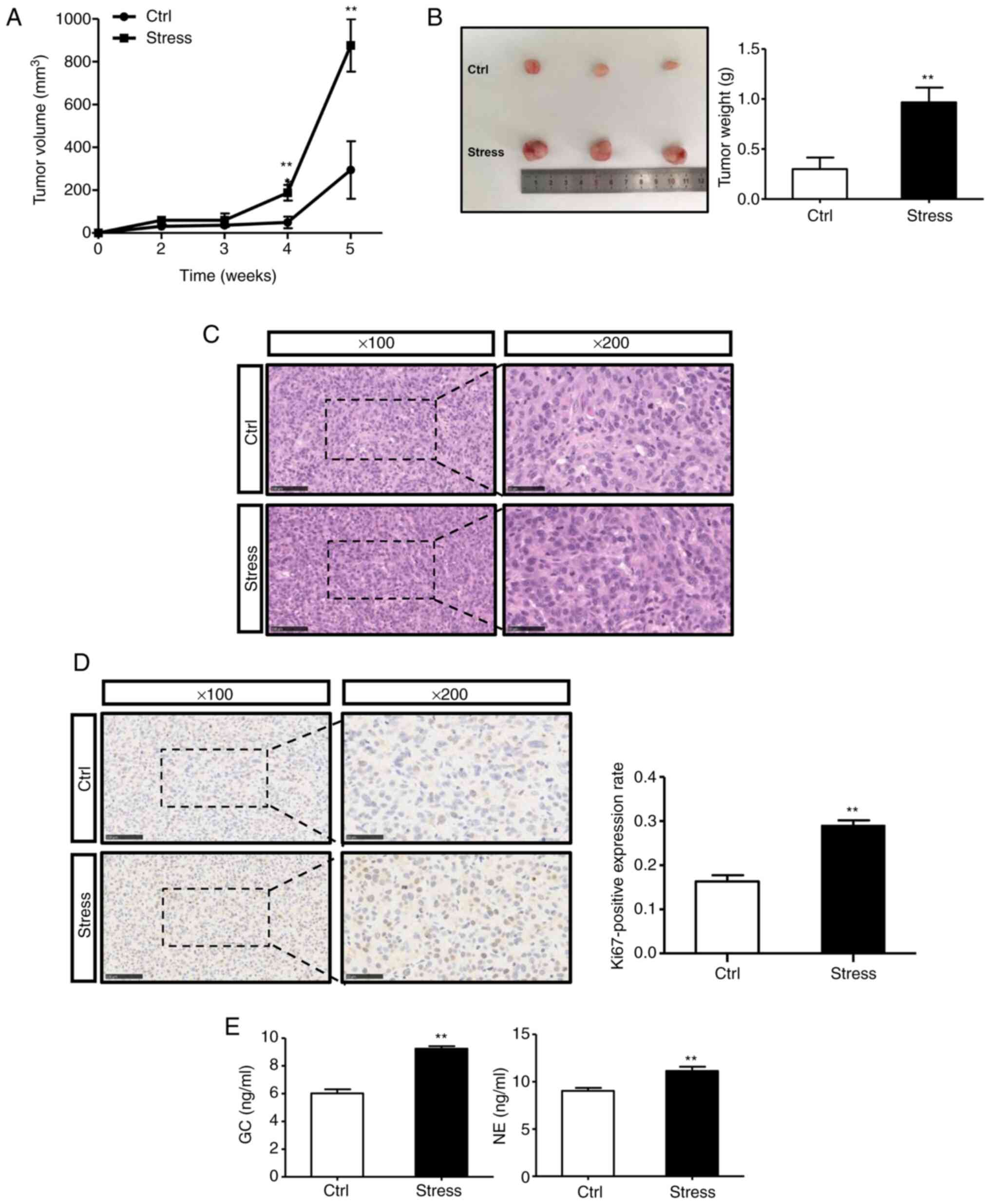
Chronic stress promotes glioma growth
in vivo, accompanied by an increase in serum GC and NE levels
To explore the effects of chronic stress on glioma
growth, U87MG cells were subcutaneously injected into nude mice and
a well-established chronic restraint stress model was adopted as
previously described (17,31). Xenograft growth was monitored. The
results revealed that the xenograft tumors in the stress group were
larger than those in the control group at 4 weeks post-implantation
(Fig. 1A). At 5 weeks
post-inoculation, the tumors were removed and weighed, and the
difference between the two groups was significant (Fig. 1B). Furthermore, although H&E
staining revealed no significant difference in the morphology of
tumor tissues between the two groups (Fig. 1C), immunohistochemical staining
revealed that the positive expression rate of Ki67 in the stress
group was higher than that in the control group (Fig. 1D). Thus, the results indicated that
chronic stress could enhance the tumor burden in the mouse model.
As GC and NE are two of the most important stress hormones, their
concentrations in the serum from control and stressed mice were
examined. The serum levels of both GC and NE were higher in the
chronic stress group (Fig. 1E).
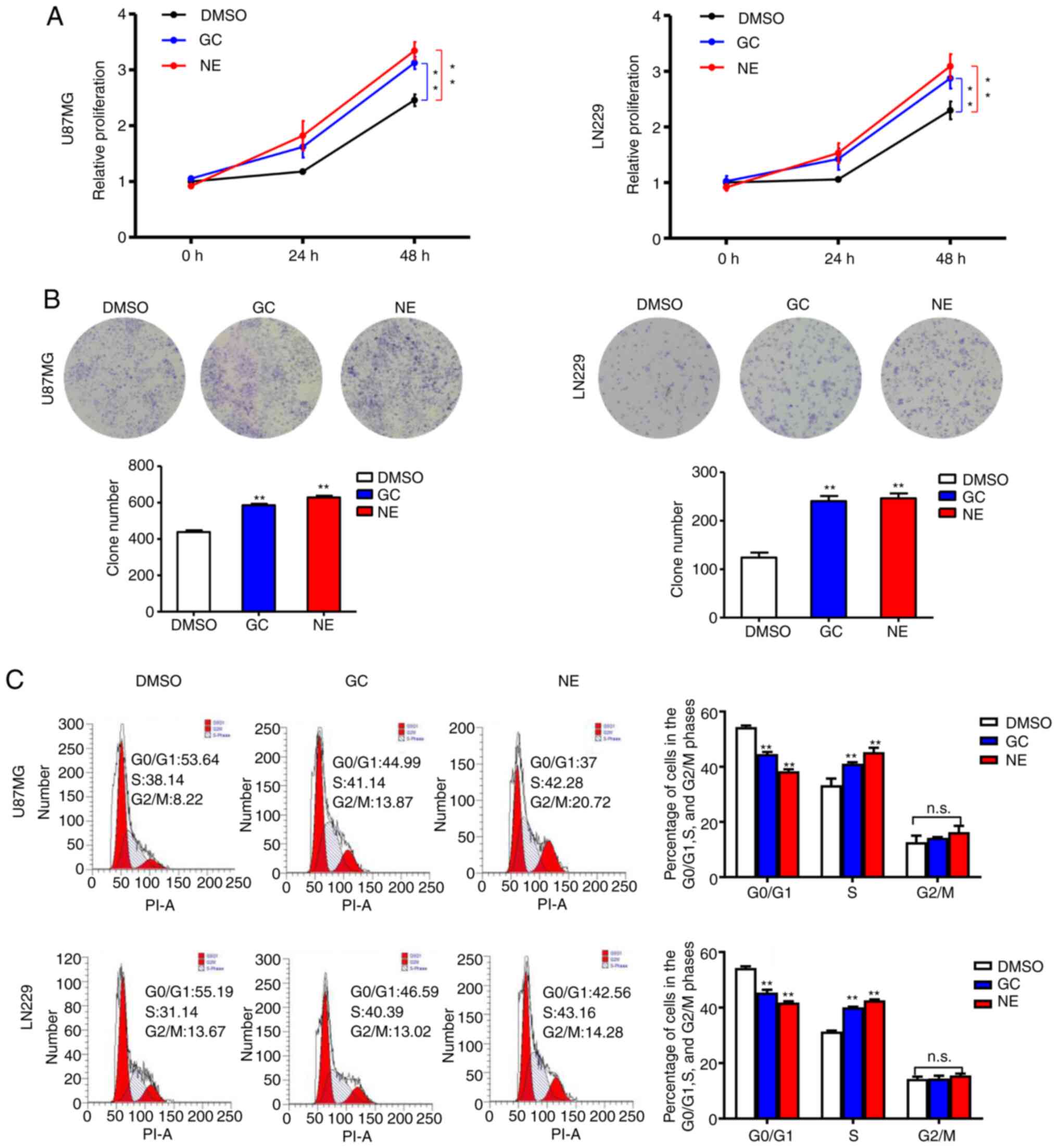
GC and NE enhance glioma cell
proliferation in vitro
To further characterize the role of chronic stress
in regulating the malignant behavior of glioma cells, the stress
hormones GC and NE were added to U87MG and LN229 cells in
vitro. The CCK-8 assay was used to examine cell growth.
Compared with the control group, the proliferation rate of the GC-
and NE-treated cells was increased (Fig.
2A). Consistently, the results of the colony formation assay
also revealed that the GC- and NE-treated cells formed more
colonies (Fig. 2B). Furthermore, the
cell cycle distribution was determined by flow cytometric analysis.
The data revealed a decrease in G0/G1-phase
cells and an enhanced S-phase transition after GC or NE
intervention (Fig. 2C). Collectively,
these results supported the theory that stress could accelerate
glioma cell proliferation by increasing the concentrations of GC
and NE.
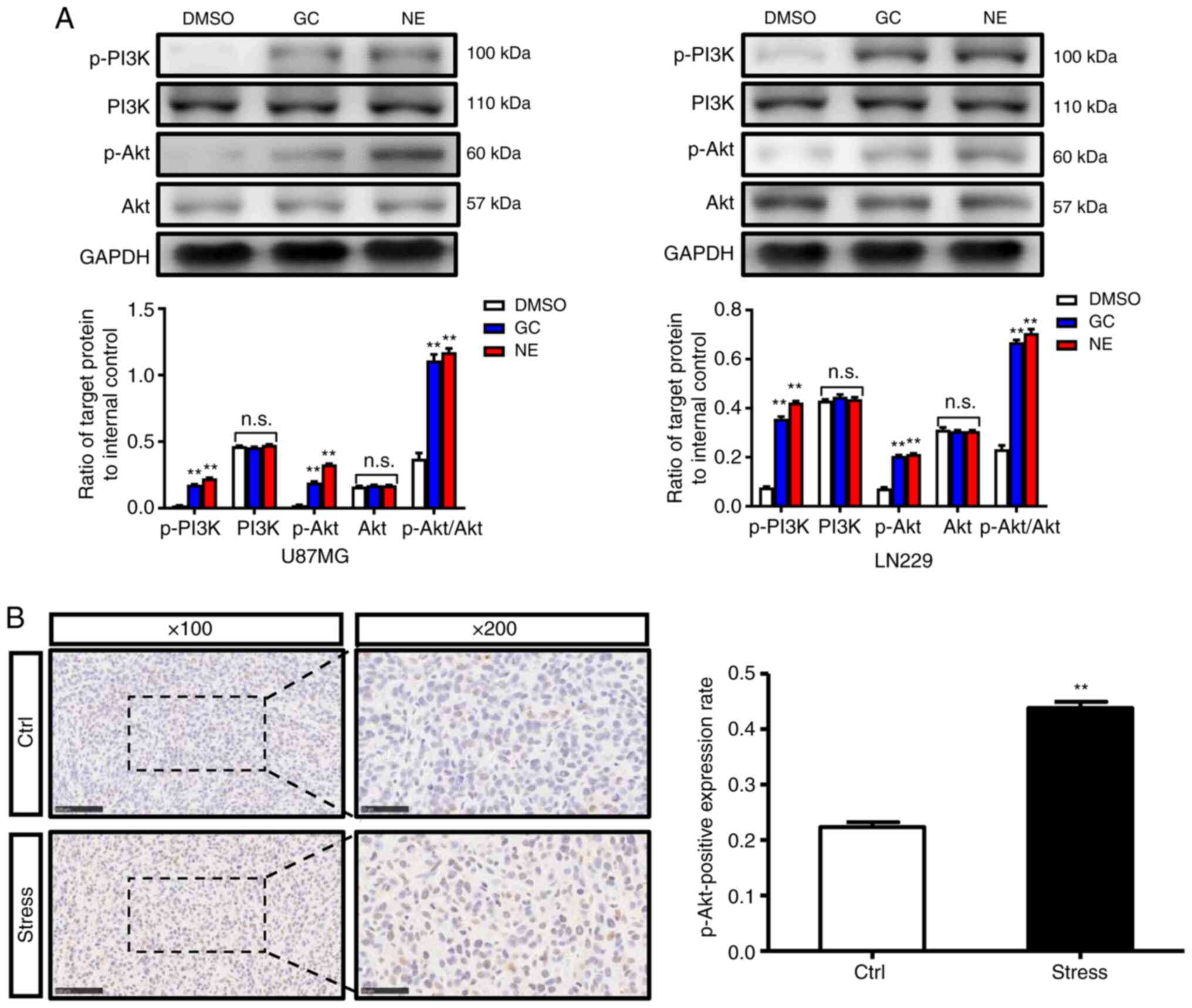
PI3K/Akt signaling is activated by
chronic stress
Considering PI3K/Akt signaling is hyperactivated in
various cancers and plays a critical role in cell proliferation and
survival (27,28), the influence of chronic stress on
PI3K/Akt signaling was evaluated. Western blot results revealed
that compared with the DMSO group, the expression levels of p-PI3K
and p-Akt in U87MG and LN229 cells were increased after GC or NE
intervention, indicating the PI3K/Akt pathway was activated
(Fig. 3A). Moreover, the
immunohistochemical results further confirmed that the positive
expression rate of p-Akt in xenografts in stressed mice was higher
than that in xenografts in control mice (Fig. 3B). The aforementioned results
indicated that the PI3K/Akt signaling pathway may have a regulatory
role in chronic stress-induced glioma proliferation.
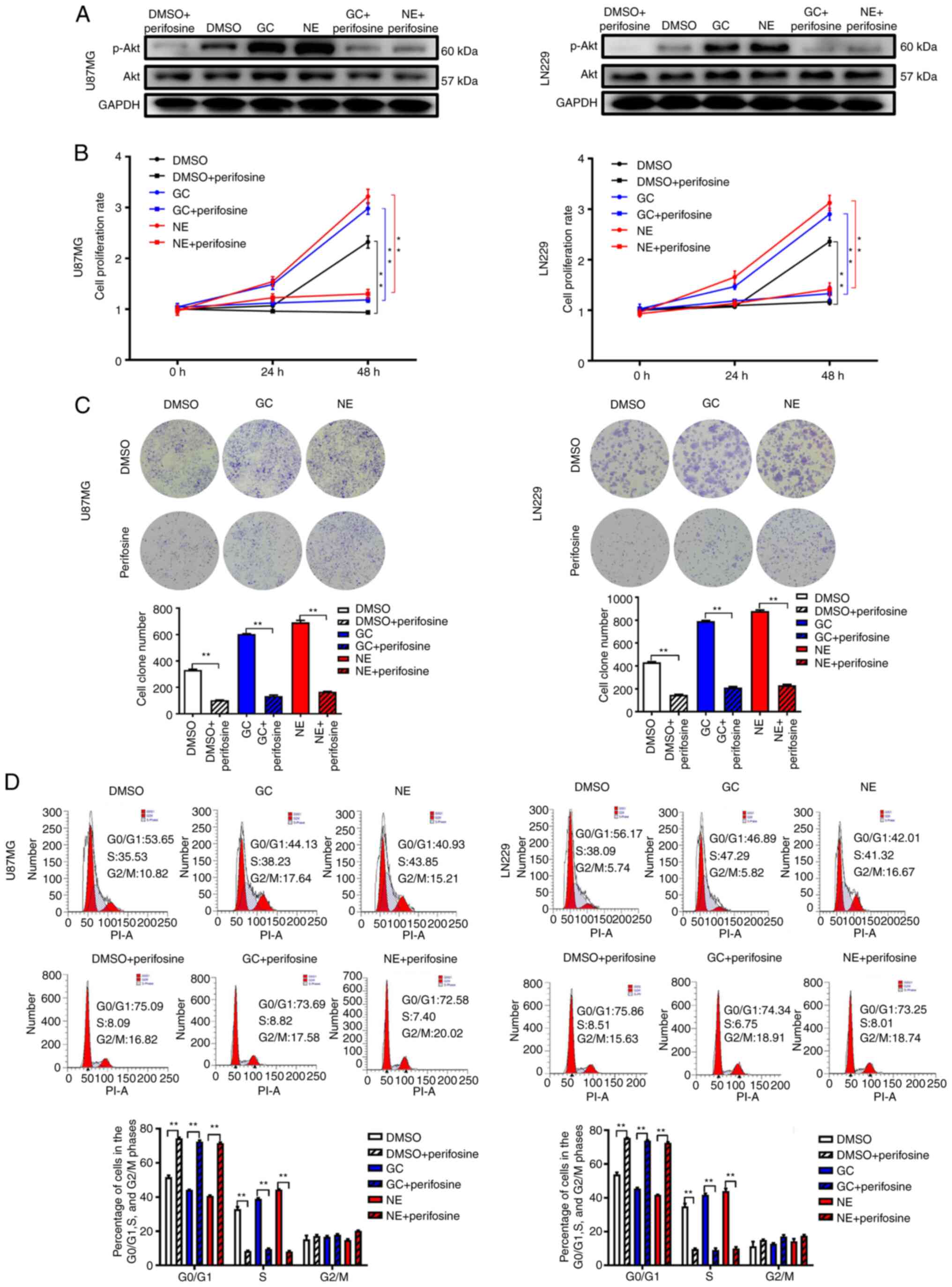
Chronic stress promotes glioma cell
proliferation via Akt signaling
To elucidate the role of Akt signaling in chronic
stress-induced glioma cell proliferation, the Akt-specific
inhibitor perifosine was used. Firstly, the influence of perifosine
on the p-Akt levels was determined by western blot analysis. The
results revealed that perifosine could effectively inhibit the
expression of p-Akt (Figs. 4A and
S1A). As anticipated, the reduction
in p-Akt levels significantly inhibited the GC- and NE-induced
growth and colony formation of U87MG and LN229 cells (Fig. 4B and C). In addition, the cell cycle
analysis revealed an increased proportion of
G0/G1-phase cells and a decreased proportion
of S-phase cells upon treatment with GC or NE combined with
perifosine compared with GC or NE treatment alone (Fig. 4D). Experiments using siRNA targeting
Akt revealed the same results (Fig.
S1B-D). The stress hormones GC and NE were able to promote the
proliferation of glioma cells, while inhibition of the Akt
signaling pathway significantly reduced the proliferation of glioma
cells. Although inhibition of the Akt signaling pathway failed to
completely eliminate the difference in proliferation between cells
in the GC and NE groups and control cells, the ratio of GC +
perifosine/DMSO + perifosine or NE + perifosine/DMSO + perifosine
was decreased compared with that of GC/DMSO or NE/DMSO in the
number of clones and S-phase cells (Fig.
S2). In conclusion, these data indicated that inhibition of
p-Akt expression, at least to some extent, inhibited stress
hormone-induced glioma cell proliferation.
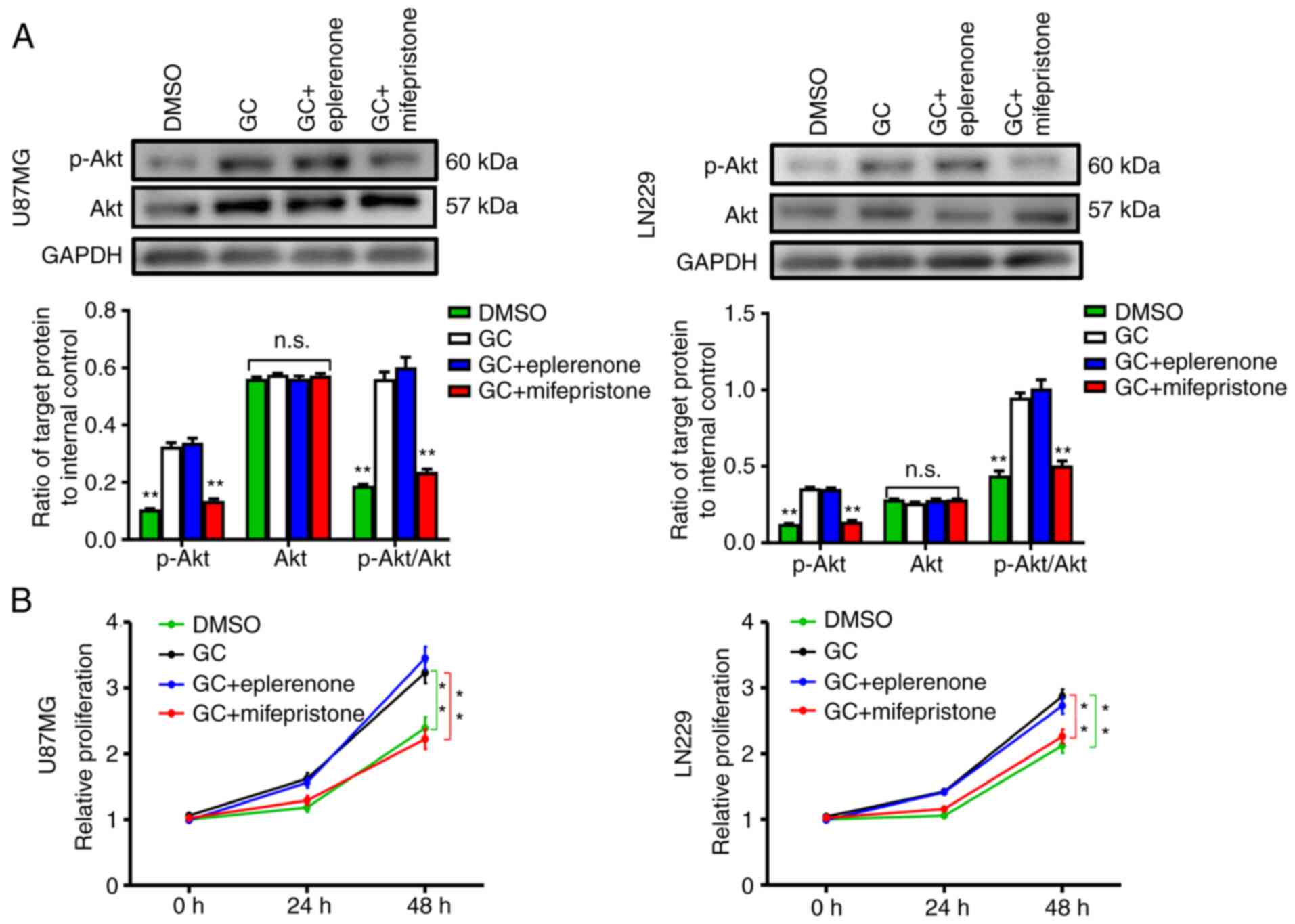
GR and ADRBs are required for GC- and
NE-mediated Akt activation and glioma cell proliferation
GC directly binds to MR or GR (35). Different antagonists were used to
determine the receptor type that participates in the GC-mediated
Akt activation and cell proliferation. Compared with the GC-treated
group, the GR antagonist mifepristone could reduce the expression
of p-Akt, while the MR antagonist eplerenone revealed no effect
(Fig. 5A). Consistently, CCK-8 assay
results revealed that mifepristone, but not eplerenone, reduced the
distinct growth capacity of U87MG and LN229 cells between the GC
group and the DMSO group (Fig.
5B).
Adrenergic receptors are classified as ADRAs and
ADRBs. The p-Akt expression levels and cell growth rate were
evaluated under different antagonist treatments. The results
revealed that propranolol, the antagonist targeting ADRBs, not only
abrogated the activation of p-Akt, but also abolished the
discrepant glioma cell growth triggered by NE treatment (Fig. 6A and B). Considering ADRB1 and ADRB2
are the main ADRBs in glioma tissues (36), specific antagonists were further
added. Lower p-Akt levels and reduced cell growth were observed in
cells in which ADRB1 and ADRB2 were inhibited (Fig. 6C and D), suggesting both receptors are
required for the biological function of NE in glioma cells.
Moreover, intervention with low concentrations of ADRB1 and ADRB2
antagonists alone and in combination revealed that the two drugs
exerted synergistic effects (Fig.
S3). Experiments using siRNA targeting ADRB1/2 confirmed these
results (Fig. S4).
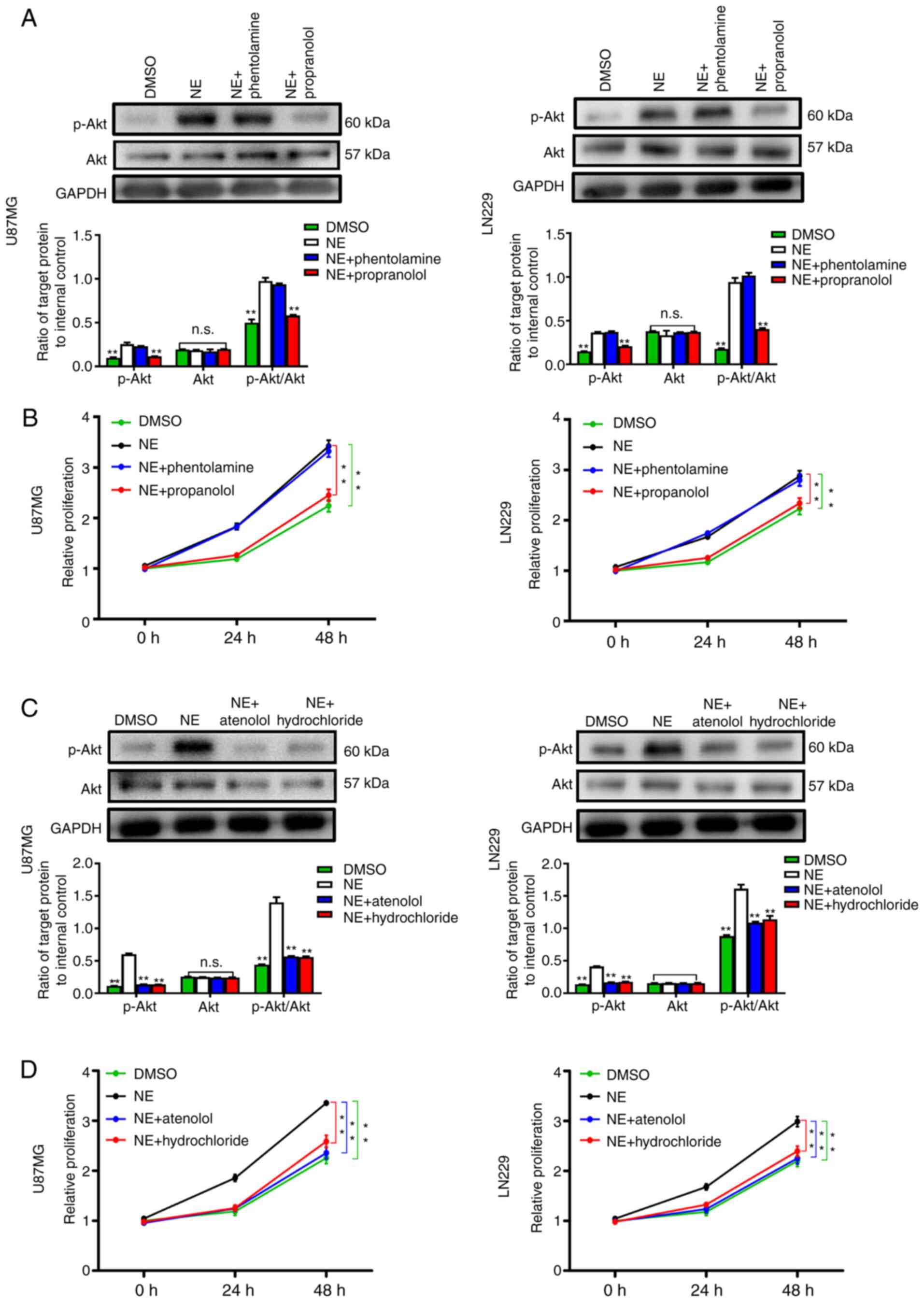 | Figure 6.NE promotes glioma cell progression
via ADRBs. (A) Western blot assay and statistical analysis of p-Akt
levels in U87MG and LN229 cells treated with NE with or without
ADRA antagonist (phentolamine, 0.2 µmol/l) or ADRB antagonist
(propranolol, 24 µmol/l). (B) CCK-8 results of U87MG and LN229
cells after NE treatment with or without adrenergic receptor
antagonists. (C) Western blot assay and statistical analysis of
p-Akt levels in U87MG and LN229 cells treated with NE with or
without ADRB1 antagonist (atenolol, 0.5 µmol/l) or ADRB2 antagonist
(hydrochloride, 1.4 µmol/l). (D) CCK-8 results of U87MG and LN229
cells after NE treatment with or without ADRB antagonists. Data are
presented as the mean ± SD (n=3). **P<0.01. NE, norepinephrine;
ADRB, β-adrenergic receptor; p-, phosphorylated; ADRA, α-adrenergic
receptor; CCK-8, Cell Counting Kit-8; n.s., no significance. |
Discussion
Patients with cancer suffer from persistent mental
and physical stress, which causes adverse stress reactions of the
body and affects clinical treatment and prognosis. A large number
of epidemiological investigations and clinical trials have revealed
that chronic stress is closely related to the occurrence and
development of a variety of tumors (37–39).
However, findings reporting the involvement of chronic stress in
glioma are rare. In the present study, it was revealed that chronic
stress promoted glioma cell proliferation by activating PI3K/Akt
signaling, indicating a stimulative role of chronic stress in
glioma progression.
Periodic immobilization is a well-established
chronic stress paradigm, in which stressed mice are restrained in a
confined space that prevents them from moving freely or turning
around but does not unduly compress them. This method has already
been demonstrated to induce high levels of HPA axis and SNS
activity characteristic of chronic stress (21,40,41). Thus,
this paradigm was adopted to evaluate the effect of chronic stress
on glioma growth in vivo. U87MG cells were injected
subcutaneously into the mice, which were subjected to restraint
stress for 21 days. It was revealed that the xenografts from the
stressed mice exhibited enhanced tumor growth (larger size and
higher weight). Consistently, the number of Ki67+ cells
was significantly increased in the chronic stress group compared
with that of the control group. These results indicated that
chronic stress promoted glioma growth in vivo.
Chronic stress can induce aberrant persistent
activation of the HPA axis and the SNS, leading to enhanced release
of GC and the simultaneous elevation of catecholamine levels
(42,43). In the present study, it was also
demonstrated that the serum levels of GC and NE in chronic stressed
mice were increased compared with control mice. Numerous studies
have reported that stress-induced hormones contribute to tumor
progression through a number of important biological processes,
such as exerting antiapoptotic effects, inducing chemotherapy
resistance, and disrupting antitumor immunity (14,44–46). The
concentrations of these stress-related hormones are 1 nmol/l in
normal human circulation, reaching up to 100 nmol/l under stress
conditions and 10 µmol/l in the tumor microenvironment (47). To further verify whether chronic
stress has the same stimulatory effect on glioma proliferation
in vitro, the glioma cell lines U87MG and LN229 were treated
with the stress hormones GC and NE at 10 µmol/l. The experimental
results revealed that after intervention with stress hormones, the
cell growth rate and clone number were significantly increased
compared with the control group, and more cells were in the S
phase. The aforementioned results were in agreement with the
results of the animal studies, revealing that chronic stress also
promoted glioma proliferation in vitro.
The activation of PI3K/Akt signaling can promote the
proliferation of cancer cells and inhibit their apoptosis. Studies
have revealed that inhibition of the activity of the PI3K/Akt
signaling pathway can promote autophagy in tumor cells, thereby
suppressing cell proliferation and inducing apoptosis (29,46,48).
Consistently, the PI3K/Akt signaling pathway can inhibit anticancer
drug-induced autophagy in U87MG cells (49). In the present study, it was also
revealed that inhibiting PI3K/Akt signaling decreased glioma cell
growth, colony formation, and S-phase transition. However, whether
the PI3K/Akt signaling pathway plays a regulatory role in the
chronic stress-induced proliferation of glioma cells remains
unclear. Herein, the changes in the levels of PI3K/Akt
signaling-related proteins upon chronic stress in vitro and
in vivo were detected by western blotting and
immunohistochemical assays. The results revealed that the signaling
pathway was activated. Furthermore, perifosine, an Akt inhibitor,
was used to block the pathway in vitro. Functional assays
revealed that in U87MG cells, the number of clones increased up to
1.81-fold after GC treatment compared with the control, whereas
after inhibition of Akt signaling, the number of GC-induced clones
increased only 1.31-fold. In the cell cycle analysis, the number of
S-phase cells increased to 1.25-fold after GC treatment compared
with the control, while there was no proliferation trend of
GC-induced S-phase cells compared with the control after inhibition
of Akt signaling. Notably, similar results were obtained in LN229
cells and after NE treatment. In conclusion, these data indicated
that inhibition of p-Akt expression, at least to some extent,
suppressed stress hormone-induced glioma cell proliferation.
It has been reported that stress hormones are
involved in the regulation of signaling pathways in tumor cells
through binding to their receptors, among which GC primarily binds
to GR (14), and NE has been revealed
to combine with ADRA or ADRB (23,50).
Herein, in order to investigate which receptor is required for the
biological effects of GC and NE in glioma, the hormone binding to
the receptors was reduced by using receptor antagonists of the
GC-related receptors GR and MR and the NE-related receptors ADRA
and ADRB. The results of protein immunoblotting experiments
demonstrated that GR and ADRB antagonists could effectively reduce
the expression of p-Akt. Consistently, CCK-8 results revealed that
the two antagonists decreased the proliferation of glioma cells.
Although the ADRBs can be divided into β1, β2, and β3 receptors,
studies have reported that glioma tissues mainly express β1- and
β2-adrenergic receptors (35,51,52).
Therefore, the β1- and β2-receptors separately were further
blocked. CCK-8 results revealed that this reduced the proliferation
of glioma cells, indicating that both receptors are required for
the regulation of glioma cell growth by NE. Collectively, our data
indicated that GC mainly bound to GR and NE bound to ADRB1 and
ADRB2 to exert their biological effects in glioma. In clinical
practice, patients with glioma are bound to suffer from negative
emotions and stress due to the disease. Therefore, glioma treatment
can be combined with surgery and targeted blockade of the PI3K/Akt
signaling pathway or blockade of GC/NE signaling and their
corresponding receptors GR/ADRBs to achieve a more desirable
therapeutic effect. Nevertheless, the molecular mechanisms by which
the PI3K/Akt signaling pathway is activated after binding of stress
hormones to receptors remain unknown.
In summary, our experiments are the first to explore
the ability of chronic stress and stress-induced hormones to
promote glioma proliferation in vivo and in vitro.
Mechanistically, GC and NE mainly bound to GR and ADRBs and further
activated the PI3K/Akt signaling pathway. The findings of the
present study may provide potential therapeutic targets and
facilitate the development of new strategies to protect patients
with glioma from the detrimental effects of stress on tumor
progression. However, there are still shortcomings in our
experiments, such as not considering the sample size adequately in
order to adhere to the 3R principle of animal welfare (Reduction,
Replacement, Refinement) (53), and
thus the sample size will be increased accordingly to render the
data more scientific and reliable when the same problems will be
encountered in future experiments.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported (grant nos.
81702454, 31771290 and 31571173) by the National Natural Science
Foundation of China.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZQZ performed the experiments. ZQZ and XW analyzed
and interpreted the data as well as critically revised the
manuscript for important intellectual content. ZQZ performed the
statistical analysis and drafted the manuscript. YSZ and LJQ
provided administrative and technical support and also supervised
the study. ZQZ, XW, BHX, YZ, FX, SDW, CX and YW contributed to the
conception and design of the study. All authors read and approved
the final manuscript and agree to be accountable for all aspects of
the research.
Ethics approval and consent to
participate
All of the animal experiments were approved
(approval no. 2016-0002) by the Institutional Animal Care and Use
Committee of the Academy of Military Medicine Sciences (Beijing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GC
|
glucocorticoid
|
|
NE
|
noradrenaline
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
GR
|
glucocorticoid receptor
|
|
MR
|
mineralocorticoid receptor
|
|
ADRA
|
α-adrenergic receptor
|
|
ADRB
|
β-adrenergic receptor
|
|
HPA
|
hypothalamic-pituitary-adrenal
|
|
SNS
|
sympathetic nervous system
|
|
FBS
|
fetal bovine serum
|
|
DMSO
|
dimethyl sulfoxide
|
|
SD
|
standard deviation
|
|
MR
|
mineralocorticoid receptor
|
References
|
1
|
Ostrom QT, Gittleman H, Fulop J, Liu M,
Blanda R, Kromer C, Wolinsky Y, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the united states in 2008–2012. Neuro Oncol. 17
(Suppl 4):iv1–iv62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea
D, et al: Bevacizumab plus radiotherapy-temozolomide for newly
diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cuddapah VA, Robel S, Watkins S and
Sontheimer H: A neurocentric perspective on glioma invasion. Nat
Rev Neurosci. 15:455–465. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen J, McKay RM and Parada LF: Malignant
glioma: Lessons from genomics, mouse models, and stem cells. Cell.
149:36–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gately L, McLachlan SA, Dowling A and
Philip J: Life beyond a diagnosis of glioblastoma: A systematic
review of the literature. J Cancer Surviv. 11:447–452. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lacroix M, Abi-Said D, Fourney DR,
Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch
SJ, Holland E, et al: A multivariate analysis of 416 patients with
glioblastoma multiforme: Prognosis, extent of resection, and
survival. J Neurosurg. 95:190–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carlsson SK, Brothers SP and Wahlestedt C:
Wahlestedt, Emerging treatment strategies for glioblastoma
multiforme. EMBO Mol Med. 6:1359–1370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Witthayanuwat S, Pesee M, Supaadirek C,
Supakalin N, Thamronganantasakul K and Krusun S: Survival analysis
of glioblastoma multiforme. Asian Pac J Cancer Prev. 19:2613–2617.
2018.PubMed/NCBI
|
|
9
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Graham J, Ramirez A, Love S, Richards M
and Burgess C: Stressful life experiences and risk of relapse of
breast cancer: Observational cohort study. BMJ. 324:14202002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chida Y, Hamer M, Wardle J and Steptoe A:
Do stress-related psychosocial factors contribute to cancer
incidence and survival? Nat Clin Pract Oncol. 5:466–475. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Magnon C, Hall SJ, Lin J, Xue X, Gerber L,
Freedland SJ and Frenette PS: Autonomic nerve development
contributes to prostate cancer progression. Asian J Androl.
15:713–714. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gray JD, Kogan JF, Marrocco J and McEwen
BS: Genomic and epigenomic mechanisms of glucocorticoids in the
brain. Nat Rev Endocrinol. 13:661–673. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang H, Xia L, Chen J, Zhang S, Martin V,
Li Q, Lin S, Chen J, Calmette J, Lu M, et al:
Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced
antitumor immunity. Nat Med. 25:1428–1441. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Volden PA and Conzen SD: The influence of
glucocorticoid signaling on tumor progression. Brain Behav Immun.
30 (Suppl):S26–S31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Skor MN, Wonder EL, Kocherginsky M, Goyal
A, Hall BA, Cai Y and Conzen SD: Glucocorticoid receptor antagonism
as a novel therapy for triple-negative breast cancer. Clin Cancer
Res. 19:6163–6172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thaker PH, Han LY, Kamat AA, Arevalo JM,
Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori
M, et al: Chronic stress promotes tumor growth and angiogenesis in
a mouse model of ovarian carcinoma. Nat Med. 12:939–944. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park SY, Kang JH, Jeong KJ, Lee J, Han JW,
Choi WS, Kim YK, Kang J, Park CG and Lee HY: Norepinephrine induces
VEGF expression and angiogenesis by a hypoxia-inducible factor-1α
protein-dependent mechanism. Int J Cancer. 128:2306–2316. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Radu M, Semenova G, Kosoff R and Chernoff
J: PAK signalling during the development and progression of cancer.
Nat Rev Cancer. 14:13–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park MH, Lee HS, Lee CS, You ST, Kim DJ,
Park BH, Kang MJ, Heo WD, Shin EY, Schwartz MA and Kim EG:
p21-Activated kinase 4 promotes prostate cancer progression through
CREB. Oncogene. 32:2475–2482. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Le CP, Nowell CJ, Kim-Fuchs C, Botteri E,
Hiller JG, Ismail H, Pimentel MA, Chai MG, Karnezis T, Rotmensz N,
et al: Chronic stress in mice remodels lymph vasculature to promote
tumour cell dissemination. Nat Commun. 7:106342016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Na Z, Qiao X, Hao X, Fan L, Xiao Y, Shao
Y, Sun M, Feng Z, Guo W, Li J, et al: The effects of beta-blocker
use on cancer prognosis: A meta-analysis based on 319,006 patients.
Onco Targets Ther. 11:4913–4944. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lamkin DM, Sung HY, Yang GS, David JM, Ma
JC, Cole SW and Sloan EK: α2-Adrenergic blockade mimics the
enhancing effect of chronic stress on breast cancer progression.
Psychoneuroendocrinology. 51:262–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao J, Liu X, Yang F, Liu T, Yan Q and
Yang X: By inhibiting Ras/Raf/ERK and MMP-9, knockdown of EpCAM
inhibits breast cancer cell growth and metastasis. Oncotarget.
6:27187–27198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Das S: MDM2 Inhibition in a subset of
sarcoma cell lines increases susceptibility to radiation therapy by
inducing senescence in the polyploid cells. Adv Radiat Oncol.
5:250–259. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
O'Donnell JS, Massi D, Teng MWL and
Mandala M: PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux.
Semin Cancer Biol. 48:91–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jia X, Wen Z, Sun Q, Zhao X, Yang H, Shi X
and Xin T: Apatinib suppresses the proliferation and apoptosis of
gastric cancer cells via the PI3K/Akt signaling pathway. J buon.
24:1985–1991. 2019.PubMed/NCBI
|
|
28
|
Chen H, Zhou L, Wu X, Li R, Wen J, Sha J
and Wen X: The PI3K/AKT pathway in the pathogenesis of prostate
cancer. Front Biosci (Landmark Ed). 21:1084–1091. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Costa RLB, Han HS and Gradishar WJ:
Targeting the PI3K/AKT/mTOR pathway in triple-negative breast
cancer: A review. Breast Cancer Res Treat. 169:397–406. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ediriweera MK, Tennekoon KH and Samarakoon
SR: Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer:
Biological and therapeutic significance. Semin Cancer Biol.
59:147–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cui B, Luo Y, Tian P, Peng F, Lu J, Yang
Y, Su Q, Liu B, Yu J, Luo X, et al: Stress-induced epinephrine
enhances lactate dehydrogenase A and promotes breast cancer
stem-like cells. J Clin Invest. 129:1030–1046. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen D, Tan Y, Li Z, Li W, Yu L, Chen W,
Liu Y, Liu L, Guo L, Huang W and Zhao Y: Organoid cultures derived
from patients with papillary thyroid cancer. J Clin Endocrinol
Metab. 106:1410–1426. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu R, Li X, Peng C, Gao R, Ma L, Hu J, Luo
T, Qing H, Wang Y, Ge Q, et al: miR-196b-5p-enriched extracellular
vesicles from tubular epithelial cells mediated aldosterone-induced
renal fibrosis in mice with diabetes. BMJ Open Diabetes Res Care.
8:e0011012020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhe D, Fang H and Yuxiu S: Expressions of
hippocampal mineralocorticoid receptor (MR) and glucocorticoid
receptor (GR) in the single-prolonged stress-rats. Acta Histochem
Cytochem. 28(41): 89–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang D, Ma Q, Shen S and Hu H: Inhibition
of pancreatic cancer cell proliferation by propranolol occurs
through apoptosis induction: The study of beta-adrenoceptor
antagonist's anticancer effect in pancreatic cancer cell. Pancreas.
38:94–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Surman M and Janik ME: Stress and its
molecular consequences in cancer progression. Postepy Hig Med Dosw
(Online). 71:485–499. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Umamaheswaran S, Dasari SK, Yang P,
Lutgendorf SK and Sood AK: Stress, inflammation, and eicosanoids:
An emerging perspective. Cancer Metastasis Rev. 37:203–211. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang X, Zhang Y, He Z, Yin K, Li B, Zhang
L and Xu Z: Chronic stress promotes gastric cancer progression and
metastasis: An essential role for ADRB2. Cell Death Dis.
10:7882019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Starr LR, Dienes K, Li YI and Shaw ZA:
Chronic stress exposure, diurnal cortisol slope, and implications
for mood and fatigue: Moderation by multilocus HPA-Axis genetic
variation. Psychoneuroendocrinology. 100:156–163. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Verbeek E, Colditz I, Blache D and Lee C:
Chronic stress influences attentional and judgement bias and the
activity of the HPA axis in sheep. PLoS One. 14:e02113632019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kvetnansky R, Sabban EL and Palkovits M:
Catecholaminergic systems in stress: Structural and molecular
genetic approaches. Physiol Rev. 89:535–606. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chetty S, Friedman AR, Taravosh-Lahn K,
Kirby ED, Mirescu C, Guo F, Krupik D, Nicholas A, Geraghty A,
Krishnamurthy A, et al: Stress and glucocorticoids promote
oligodendrogenesis in the adult hippocampus. Mol Psychiatry.
19:1275–1283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: Function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging (Albany NY). 8:603–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
D'Alterio C, Scala S, Sozzi G, Roz L and
Bertolini G: Paradoxical effects of chemotherapy on tumor relapse
and metastasis promotion. Semin Cancer Biol. 60:351–361. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Obradović MMS, Hamelin B, Manevski N,
Couto JP, Sethi A, Coissieux MM, Münst S, Okamoto R, Kohler H,
Schmidt A and Bentires-Alj M: Glucocorticoids promote breast cancer
metastasis. Nature. 567:540–544. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shin KJ, Lee YJ, Yang YR, Park S, Suh PG,
Follo MY, Cocco L and Ryu SH: Molecular mechanisms underlying
psychological stress and cancer. Curr Pharm Des. 22:2389–2402.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Butler DE, Marlein C, Walker HF, Frame FM,
Mann VM, Simms MS, Davies BR, Collins AT and Maitland NJ:
Inhibition of the PI3K/AKT/mTOR pathway activates autophagy and
compensatory Ras/Raf/MEK/ERK signalling in prostate cancer.
Oncotarget. 8:56698–56713. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang H, Zhu Y, Sun X, He X, Wang M, Wang
Z, Wang Q, Zhu R and Wang S: Curcumin-loaded layered double
hydroxide nanoparticles-induced autophagy for reducing glioma cell
migration and invasion. J Biomed Nanotechnol. 12:2051–2062. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cole SW and Sood AK: Molecular pathways:
Beta-adrenergic signaling in cancer. Clin Cancer Res. 18:1201–1206.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Homburger V, Lucas M, Rosenbaum E, Vassent
G and Bockaert J: Presence of both beta1-and beta2-adrenergic
receptors in a single cell type. Mol Pharmacol. 20:463–469.
1981.PubMed/NCBI
|
|
52
|
Schwalbe T, Huebner H and Gmeiner P:
Development of covalent antagonists for β1-and β2-adrenergic
receptors. Bioorg Med Chem. 27:2959–2971. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
O'Connor MD: The 3R principle: Advancing
clinical application of human pluripotent stem cells. Stem Cell Res
Ther. 4:212013. View Article : Google Scholar : PubMed/NCBI
|