Introduction
Thyroid cancer accounts for 1–2% of all cancer cases
worldwide, representing the most prevalent type of endocrine
malignancy (1,2). Most thyroid carcinomas are derived from
follicular cells and are classified into three broad categories
based on their differentiation levels: i) Differentiated thyroid
cancer (DTC), including papillary thyroid cancer (PTC) and
follicular thyroid cancer (FTC); ii) poorly DTC and
undifferentiated thyroid cancer; and iii) anaplastic thyroid cancer
(ATC) (1). ATC is a rare, but
aggressive, form of thyroid malignancy. Indeed, despite the fact
that it only accounts for <2% of all thyroid malignancies, it is
responsible for 20–50% of thyroid cancer-associated deaths (thyroid
cancer incidence and mortality in the United States, 1974–2013)
(1,2).
Due to the fast-growing and aggressive nature of the tumor, the
majority of patients with ATC are diagnosed with stage-IV disease,
at which point surgery is not possible (3). Moreover, due to its undifferentiated
nature, ATC is not sensitive to the conventional treatments used
for well-differentiated thyroid cancers, such as radioactive iodine
ablation, as thyroid-specific gene expression is lost through the
dedifferentiation process (4,5). Thus, the median survival rates in
several population-based studies have been reported to be between 3
and 6 months, without evidence of any improvement over several
decades (6,7). Treatments with widely used
chemotherapeutic agents, such as doxorubicin or cisplatin, as well
as more recent taxane-based therapies, are unable to improve the
poor overall survival rates, primarily due to inadequate control of
distant metastases (8,9). Novel strategies are currently under
investigation, including a major effort to identify innovative
approaches to ATC management.
Phytochemicals, a large class of chemical substances
naturally produced in plants, have been demonstrated to exhibit
several biological properties, including anticancer effects
(10,11). In this context, in the field of
thyroid cancer, resveratrol has been shown to sensitize tumor cells
to radioiodine therapy (12).
Furthermore, in the case of curcumin, the capacity to inhibit
invasion and migration via downregulation of the PI3K/Akt signaling
pathway has been reported in FTC cells (13) and inhibition of TGF-β1-induced
epithelial-to-mesenchymal transition (EMT) has been reported in PTC
cells (14). In consideration of
these findings, testing novel plant extracted compounds in thyroid
cancer therapy appears to be a promising strategy. Recently, among
the more novel extracted plant compounds with anticancer activity,
particular attention has been placed on 15,16-dihydrotanshinone I
(DHT), a tanshinone extracted from Salvia miltiorrhiza Bunge
(Danshen), one of the most frequently prescribed herbs in
Traditional Chinese Medicine (15).
DHT possesses anticancer activity in different types of cancer,
including colon (16), breast
(17) and gastric cancer (18), with an effective dose 50
(ED50) <10 µM (15–18). DHT
has been reported to be able to induce cell cycle arrest at the S
and G1 phases (17), as
well as apoptosis, acting primarily through the BCL2 family of
proteins (19). In addition, DHT has
also been tested in animal models, confirming its ability to
inhibit tumor growth in xenograft nude mouse models, without
adverse effects on other tissues (20,21).
Notably, DHT is effective in reducing cell proliferation and
aggressiveness, even in some drug-resistant tumor cell lines, such
as K562/ADR cells, and in p-glycoprotein-overexpressing HepG2
subclones (22). DHT has been
reported to enhance the effectiveness of irradiation, as the
combination of irradiation and DHT induces significantly greater
decrease in tumor growth in a nude xenograft mouse model of
cervical cancer (23).
Previously, Lal et al (24) showed that DHT treatment modifies the
activity of HuR, an RNA-binding protein involved in tumorigenesis
and cancer progression, altering its binding to mRNA targets in
vivo. In our previous studies, HuR was overexpressed in thyroid
cancer and that its downregulation resulted anticancer effects in
ATC cell lines (25–27).
Altogether, these findings indicate that DHT may be
a potential candidate for ATC treatment. Thus, in the present
study, the effects of DHT were examined in two human ATC cell
lines. As it has been established that DHT inhibits HuR-target
interactions (24), the aim of the
present study was to evaluate the effects of DHT on MAD2, a key
component of the MAD/BUB complex that regulates sister-chromatid
separation during metaphase to anaphase progression (28), and a known target of HuR with a
relevant role in cancer progression (27). The effect of the combination of DHT
with cisplatin were also investigated.
Materials and methods
Cell lines
SW1736 and 8505C cells, derived from ATC (29–31), were
cultured in RPMI-1640 medium (Euroclone S.p.A) supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 2 mM L-glutamine
(Euroclone S.p.A) and 50 mg/ml gentamicin (Gibco; Thermo Fisher
Scientific, Inc.). Cells were cultured in a humidified incubator
(5% CO2 and 95% air at 37°C). Both cell lines were
validated using short tandem repeat analysis and confirmed to be
mycoplasma-free. SW1736 and 8505C cells were treated with DMSO
(vehicle; Sigma-Aldrich; Merck KGaA), DHT (Selleck Chemicals) or
cisplatin (Cayman Chemical Company).
Cell viability
In order to test cell viability, the MTT assay was
used. SW1736 and 8505C cells (4×103 cells/well) were
seeded in 96-well plates. The following day, cells were treated
with DHT (0.5, 1, 2 or 3 µM) and cisplatin (1 µM) at different
concentrations. After 24, 48 or 72 h of incubation, 4 mg/ml MTT
(Sigma-Aldrich; Merck KGaA) was added to the cell medium and cells
were cultured for a further 4 h in the incubator in the dark. The
supernatant was removed, 100 µl/well DMSO (Sigma-Aldrich; Merck
KGaA) was added, and the absorbance at 570 nm was measured. All
experiments were performed as six technical repeats and cell
viability is expressed as the fold-change relative to the control
(DMSO-treated cells).
Cell cycle analysis
Cell cycle distribution was determined using flow
cytometry analysis of DNA content, as previously described
(32). Briefly, SW1736 and 8505C
cells were treated with 1.5 µM DHT or DMSO for 48 h. The cells were
collected and fixed in cold 70% ethanol, then stained with
propidium iodide solution containing RNase and Triton-X100 at 4°C
for 30 min. Flow cytometry analysis was performed on a FACSCalibur
instrument (BD Biosciences) using ModFit LT 5.0 analysis software
(Verity Software House, Inc.). A minimum of 2×104 cells
were analyzed for each sample. All experiments were performed in
triplicate.
Annexin V and propidium iodide
assay
To assess the effects of DHT on cell death, an
Annexin V and propidium iodide assay was performed using the
eBioscience Annexin V-FITC Apoptosis Detection kit (cat. no.
88-8005-74; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Briefly, DHT-treated SW1736 and 8505C
cells were washed with cold PBS and resuspended in 195 µl binding
buffer (BB; 10 mM; HEPES/NaOH, pH 7.4; 140 mM NaCl and 2.5 mM
CaCl2). A total of 5 µl fluorescein
isothiocyanate-conjugated Annexin V (Annexin V-FITC) was added and
samples were incubated for 10 min at room temperature. After
washing, cells were resuspended in 190 µl BB in which 10 µl
propidium iodide stock solution (final concentration 1 µg/ml) were
added. Flow cytometry analysis was performed on a FACSCalibur
(Becton-Dickinson and Company) and analyzed using the Summit
software (Beckman-Coulter). All experiments were performed in
triplicate.
Protein extraction and western
blotting
Total protein was extracted from SW1736 and 8505C
cells, treated with 1.5 µM DHT or DMSO, using a cell scraper and
lysis buffer (50 mM Tris HCl, pH 8; 120 mM NaCl; 5 mM EDTA; 1%
Triton; 1% NP40; 1 mM DTT), supplemented with
phenyl-methylsulphonyl fluoride and protease inhibitors. Lysates
were centrifuged at 13,000 × g for 10 min at 4°C, and supernatants
were quantified using a Bradford assay.
For western blot analysis, 30 µg protein was loaded
per lane on a 10% SDS gel, resolved using SDS-PAGE, then
transferred to nitrocellulose membranes (GE Healthcare). The
membranes were blocked at room temperature for 1 h using PBS-milk
(PBS; 0.1% Tween-20; 5% non-fat dry milk). The membranes were then
incubated overnight at 4°C with rabbit anti-actin antibody
(1:1,000; cat. no. A2066; Sigma-Aldrich; Merck KGaA), mouse
monoclonal anti-MAD2 (1:500; cat. no. sc-374131; Santa Cruz
Biotechnology, Inc.), mouse anti-E-cadherin (1:1,000; cat. no.
14472), mouse anti-N-cadherin (1:1,000; cat. no. 14215), rabbit
anti-vimentin (1:1,000; cat. no. 5741) (all Cell Signaling
Technology, Inc.) or Apoptosis Western Blot Cocktail
(pro/p17-caspase-3, cleaved PARP, muscle actin) (1:400; cat. no.
ab136812; Abcam). This cocktail is designed to study the induction
of apoptosis in response to various stimuli and includes monoclonal
antibodies specific for procaspase-3, caspase-3 and PARP. In
particular, the mouse PARP antibody of this cocktail detects only
the apoptosis-specific 89-kDa PARP fragment (cleaved PARP). The
following day, the membranes were incubated with
peroxidase-conjugated anti-rabbit or anti-mouse IgG secondary
antibody (both 1:4,000; cat. nos. A6154 and A9044, respectively;
both Sigma-Aldrich; Merck KGaA) for 2 h at room temperature. A
UVITEC Alliance LD (Uvitec Ltd.) western blot detection system with
SuperSignal Technology reagent (Thermo Fisher Scientific, Inc) and
the Alliance 1D Max software (Uvitec Ltd.) was used to visualize
the signals.
Colony formation assay
SW1736 and 8505C cells were treated with vehicle
(DMSO) or 1.5 µM DHT for 48 h, then seeded at 1.5×103
cells/plate. Colonies were left to form for 21 days, then washed
twice with PBS and fixed with pure methanol for 15 min at 4°C.
Cells were then washed twice with PBS-Triton X-0.1% (Sigma-Aldrich;
Merck KGaA) and incubated at room temperature with crystal violet
(Sigma-Aldrich; Merck KGaA) solution for 30 min. Crystal violet was
removed and stained colonies were washed, images were captured and
colonies were counted by eye.
Soft agar assay
The clonogenic ability of the SW1736 and 8505C cells
after treatment with 1.5 µM DHT was evaluated using a soft agar
assay. Briefly, after 48 h of treatment, cells were collected, and
1×104 cells were suspended in 4 ml complete medium
containing 0.25% agarose (Sigma-Aldrich), then seeded to the top of
a 1% agarose complete medium layer in 6-cm plates. The colonies
were counted by eye in four different fields, under a Leica
DMI-600B inverted microscope (Leica Microsystems Ltd.). Data are
representative of three independent experiments.
Transwell invasion assays
Transwell membranes coated with Matrigel™ were used
to measure the ability of cells to attach to the matrix, invade
into and through the matrix, and migrate towards a chemoattractant.
The invasion ability of ATC cells was evaluated after treatment for
72 h with 1.5 µM DHT. For each cell line, 2.5×104 live
cells per condition (DHT or vehicle) were separately re-suspended
in culture media containing 10% FBS and seeded in a 24-well plate
Transwell coated with Matrigel, as described by Justus et al
(33). RPMI-1640 medium supplemented
with 25% FBS was used as the chemoattractant in the lower chamber.
After 24 h, cells were fixed with 70% ethanol and stained with
crystal violet solution. After staining, the top of membrane was
gently scraped to remove the cells that had not migrated; the cells
that had migrated through the membrane toward the chemoattractant
were attached on the underside of the membrane. The number of cells
on the underside were counted in six different fields of view using
an inverted microscope (Leica DMI-600B). Data are representative of
the average of the sum of the cells counted in all these six fields
in three independent experiments.
High-throughput RNA sequencing and
analysis
RNA was extracted from SW1736 and 8505C cells
treated with vehicle (DMSO) or DHT using a RNeasy Mini kit (Qiagen
GmbH) according to the manufacturer's instructions. In total, ~1 µg
RNA (RNA integrity number >7) was used as the starting material
for preparation of the library using a Universal Plus mRNA-Seq kit
(cat. no. 0520-A01; Tecan Group, Ltd.) according to the
manufacturer's protocol. RNA samples were quantified, and the
quality was assessed using an Agilent 2100 Bioanalyzer RNA assay
(Agilent Technologies, Inc.) and the final libraries were checked
using both a Qubit 2.0 Fluorometer (Invitrogen; Thermo Fisher
Scientific, Inc.) and Agilent Bioanalyzer DNA assay. The library
final concentration was 68.7 nM. Libraries (1.4 nM) were then
prepared for sequencing using the single-end 75 bp mode on a
NextSeq 500 (Illumina, Inc.). The Bcl2Fastq version 2.20 in the
Illumina pipeline was used for processing the raw data (format
conversion and de-multiplexing); adapter sequences were masked with
Cutadapt version 1.11 (34) from raw
fastq data and the ERNE (35)
software was used to remove lower quality bases and adapters. Reads
were aligned to the reference hg38 genome/transcriptome using STAR
software (36). Finally, assembly and
quantitation of full-length transcripts representing multiple
spliced variants for each gene locus was performed using the
Stringtie tool (37). Differentially
expressed genes were defined as those with a log2 fold
change >1.5 or <-1.5. Raw and processed data are available on
the public online repository Gene Expression Omnibus (dataset no.
GSE168616).
Gene expression assays
A total of 500 ng total RNA from SW1736 and 8505,
extracted as described above, was reverse transcribed to cDNA using
random hexaprimers and SuperScript III (SSIII) reverse
transcriptase (Thermo Fisher Scientific, Inc.). Briefly, the first
strand cDNA synthesis was created in a total volume of 20 µl with
5X First-strand buffer, DTT 0.1 M, RNase OUT Recombinant RNase
Inhibitor (Thermo Fisher Scientific, Inc.), dNTPs 10 mM, random
primers (Thermo Fisher Scientific, Inc.) and SSIII reverse
transcriptase. The reverse transcription step involved incubation
at room temperature for 5 min, 50°C for 60 min, 70°C for 15 min and
45°C for 5 min. Quantitative PCR was performed using PowerUP Sybr
green master mix (Thermo Fisher Scientific, Inc.) on the
QuantStudio3 system (Applied Biosystems; Thermo Fisher Scientific,
Inc.), as previously described (38)
and following the Standard cycling mode (primer Tm
<60°C): 50°C for 2 min, 95°C for 2 min and 95°C for 15 sec, 60°C
for 15 sec and 72°C for 1 min for 40 cycles. The QuantStudio Design
and Analysis software (Applied Biosystems; Thermo Fisher
Scientific, Inc.), was used to calculate mRNA levels with the
2−∆∆Cq method (39) and
β-actin was used as reference. All experiments were performed in
triplicate. Oligonucleotide primers were purchased from
Sigma-Aldrich; Merck KGaA, and the sequences of the primers are
listed in Table I.
 | Table I.Reverse transcription-quantitative
PCR primer sequences. |
Table I.
Reverse transcription-quantitative
PCR primer sequences.
| Gene | Forward primer
sequence, 5′-3′ | Reverse primer
sequence, 5′-3′ |
|---|
| CDH1 |
AGCCTCAGGTCATAAACATCATTG |
CTCGCCCCGTGTGTTAGTTC |
| CDH2 |
CCATCACTCGGCTTAATGGT |
ACCCACAATCCTGTTCCACAT |
| VIM |
CAAATCGATGTGGATGTTTCCA |
AGGTTCTTGGCAGCCACACT |
| TWIST1 |
GCAGGACGTGTCCAGCTC |
CTGGCTCTTCCTCGCTGTT |
| ZEB1 |
TCAGTGTTCTTCACCGTCTCTTTC |
GTTTATTCTCTATCTTTTGCCGTATCTG |
| ZEB2 |
CAAGAGGCGCAAACAAGC |
GGTTGGCAATACCGTCATCC |
| ACTB |
TTGTTACAGGAAGTCCCTTGCC |
ATGCTATCACCTCCCCTGTGTG |
Statistical analysis
Data are presented as the mean ± standard deviation.
All results were analyzed using the unpaired Student's t-test or
one-way ANOVA in GraphPad Prism version 6 (GraphPad Software,
Inc.). After one-way ANOVA, the Dunnett's post hoc test was
performed. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of DHT on cell viability, cell
cycle progression and apoptosis
In the first set of experiments, the effects of DHT
on two human ATC cell lines (SW1736 and 8505C) were evaluated. To
assess the effects of DHT on cell viability using several doses of
DHT, an MTT assay was performed on ATC cells treated for 24, 48 or
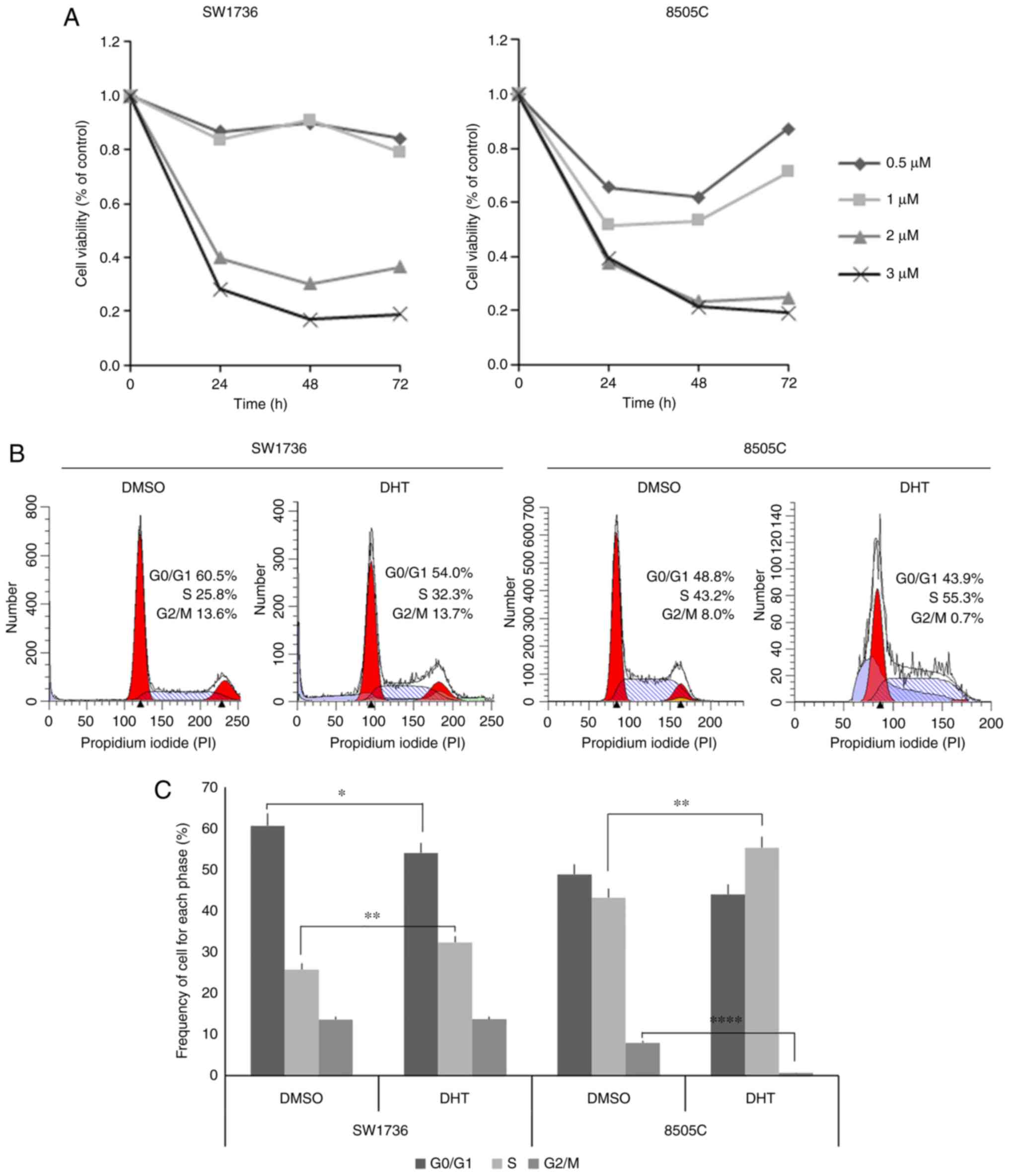
72 h. As shown in Fig. 1A, 2 and 3 µM DHT
treatment significantly reduced the viability of both ATC cell
lines at all time points. In SW1736 cells, doses <2 µM did not
significantly affect viability, whereas the 8505C cells were more
sensitive to DHT treatment, since they exhibited a ~50% reduction
in cell viability when treated with 0.5 and 1 µM DHT after 48 h.
However, the effects of DHT were transient, and cell viability
recovered at the 72-h time point. Based on these data, the median
ED50 of 1.5 µM was selected for treatment of both cell
lines in subsequent experiments.
The aforementioned experiments did not distinguish
whether the reduced viability was the effect of a decrease in cell
metabolism or cell number. To evaluate the effects of DHT on cell
cycle progression, flow cytometry analysis was performed after 72 h
of DHT treatment. As shown in Fig. 1B and
C, treated SW1736 and 8505C cells exhibited a slight reduction
in the proportion of cells in the G0/G1 phase
compared with vehicle-treated cells, significative only in SW1736
cells. By contrast, DHT treatment resulted in a significant
increase in the proportion of cells in the S phase from 25.8 to
32.3% and from 43.2 to 55.3% in SW1736 and 8505C cells,
respectively. Moreover, 8505C treated cells showed a significant
reduction in the proportion of cells in the G2/M. The
notable increase in cells with reduced DNA staining compared with
G0/G1 cells among treated cells was
hypothesized to reflect an increase in the degree of apoptosis, as
previously described (40).
To evaluate whether the decrease in cell viability
observed after the treatments was due to apoptotic cell death,
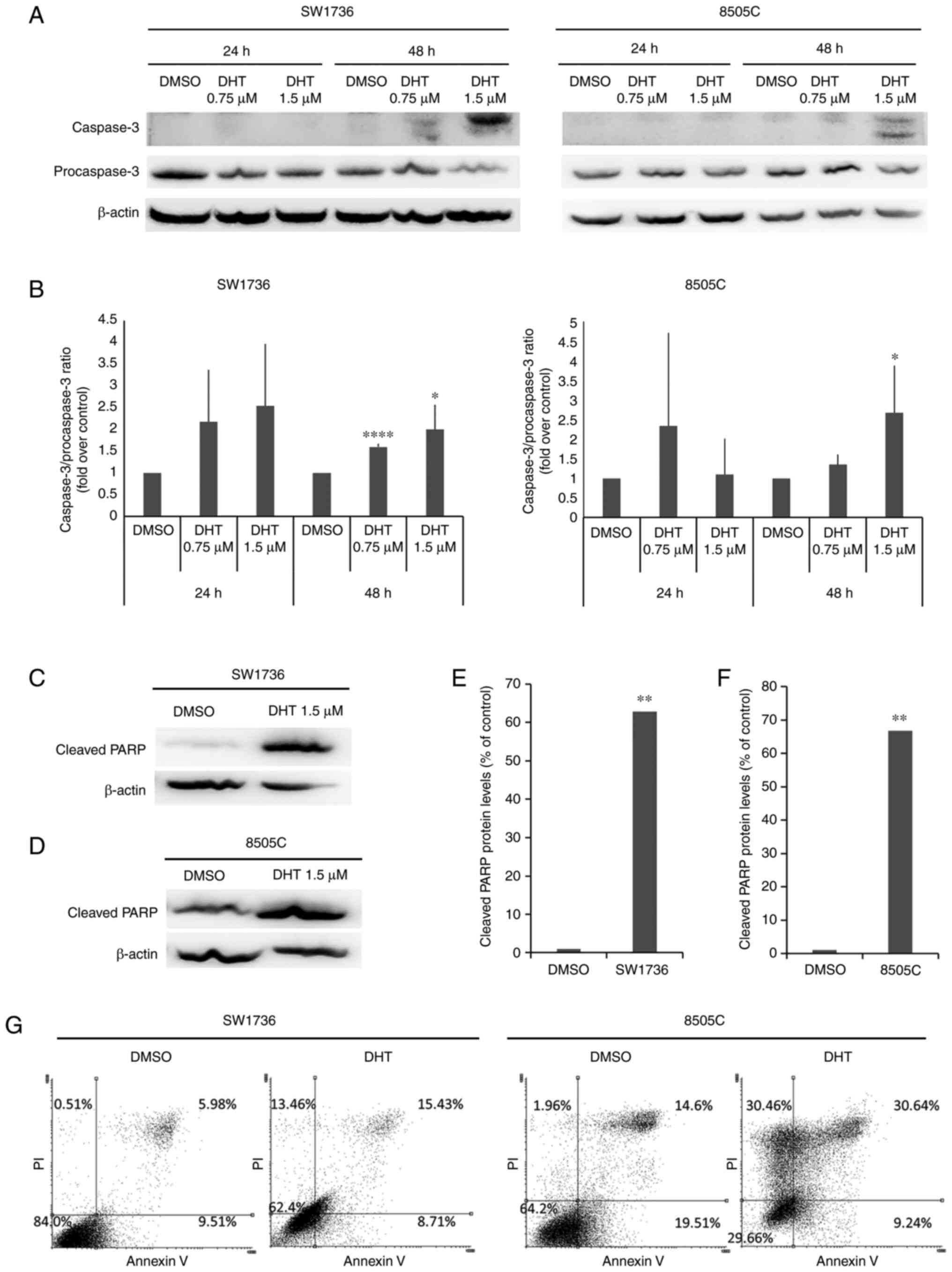
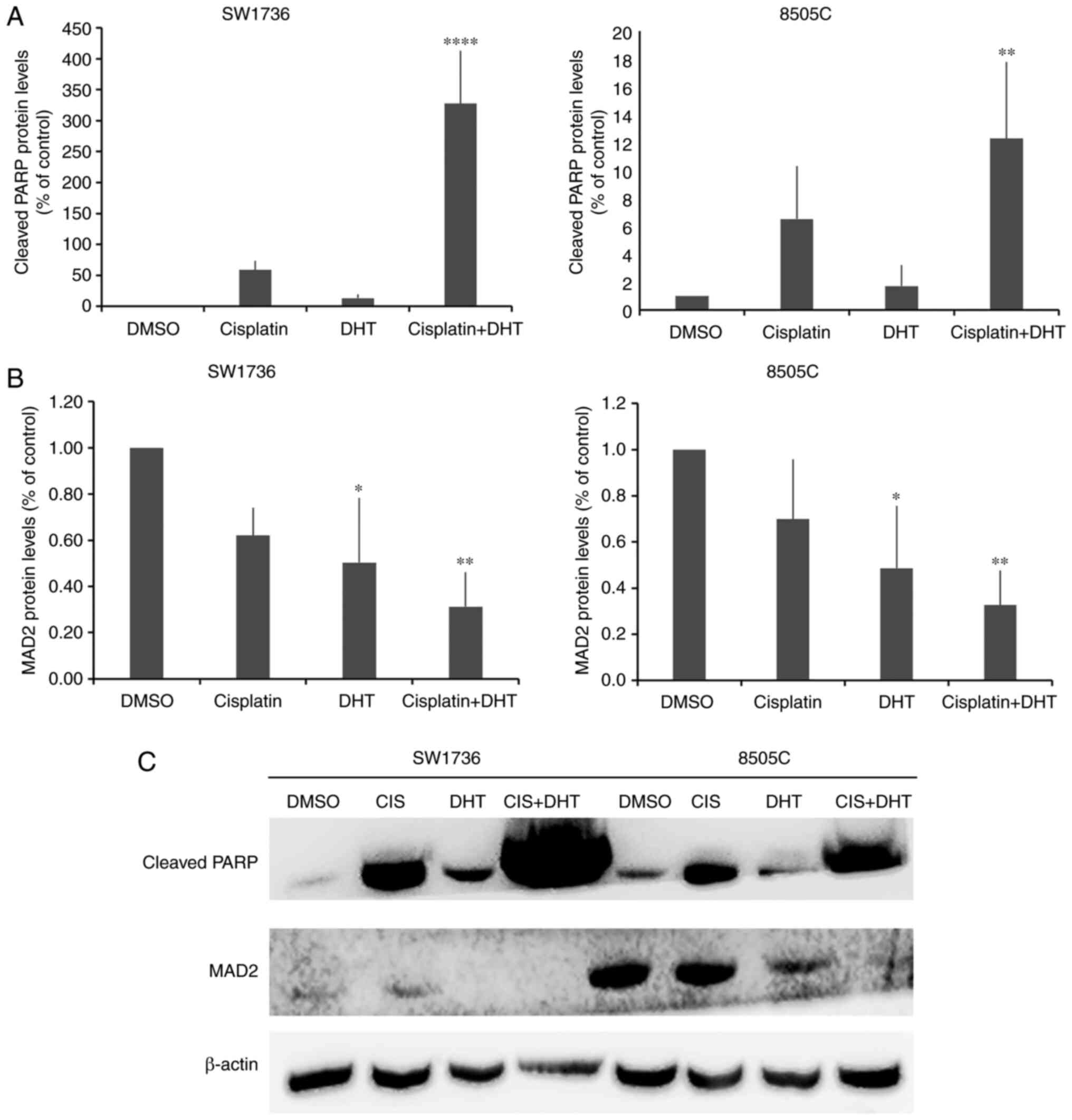
western blot analysis of caspase-3 activation (Fig. 2A and B) and cleaved-PARP protein
(Fig. 2C-F) was performed. Caspase-3
activation was evaluated after 24 and 48 h of treatment with 0.75
or 1.5 µM DHT. In both cell lines, 48-h treatment with 1.5 µM DHT
resulted in a significant increase in caspase-3 activation compared
with the DMSO control. These conditions also resulted in a ~60-fold
increase in the expression of cleaved-PARP levels compared with the
control in both cell lines (Fig. 2E and
F).
To better characterize the effects of DHT on cell
death, an Annexin V/propidium iodide assay was performed. As shown
in Fig. 2G, in both cell lines, 1.5
µM DHT treatment resulted in an increase in the proportion of
necrotic (top left area of the plot) and late apoptotic cells (top
right area of the plot).
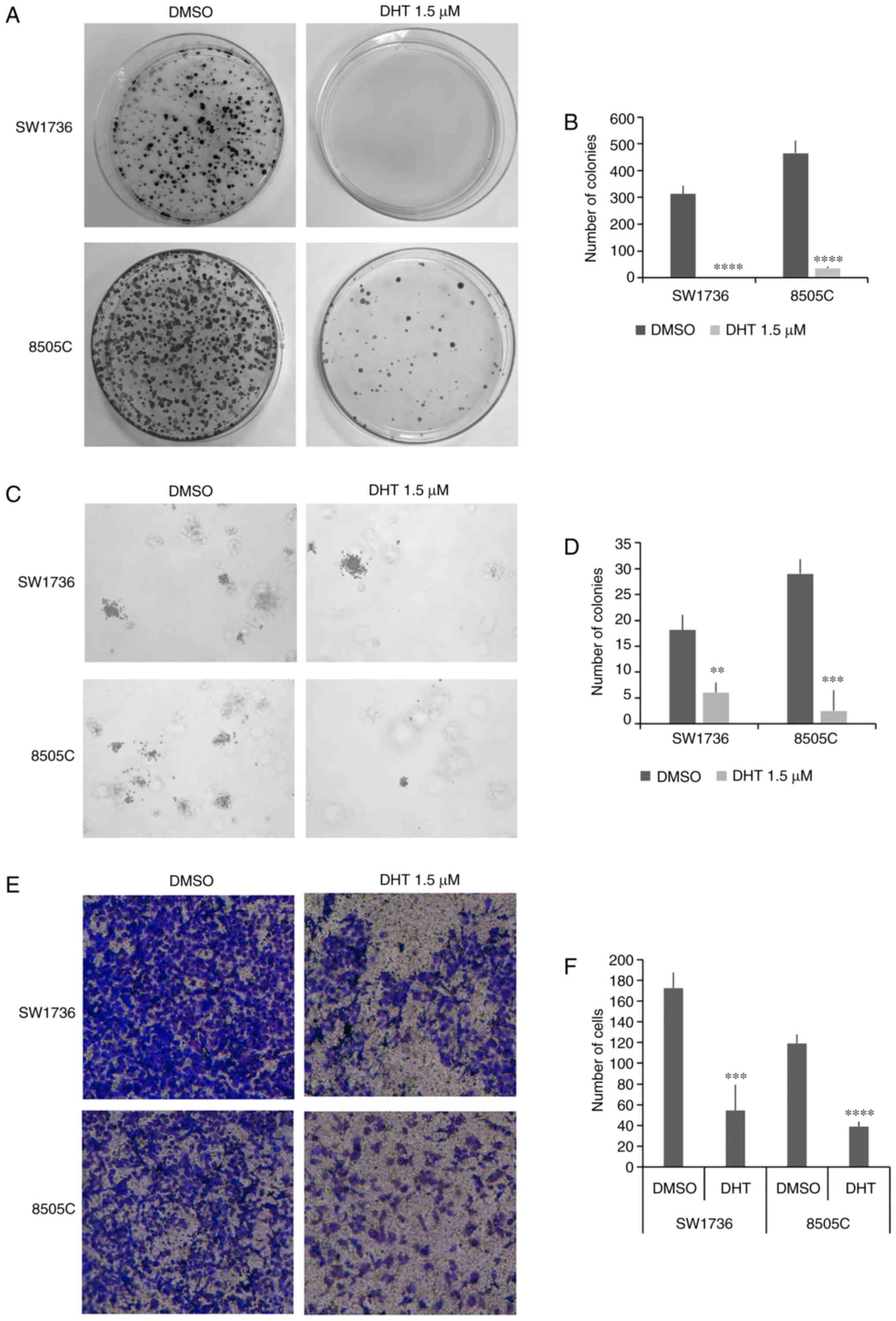
Effects of DHT on cell colony forming
ability and invasiveness
To assess the effects of DHT on markers of
aggressiveness in the two ATC cells, its influence on the ability
of cells to form colonies in an anchorage-dependent or independent
manner was assessed using a plate colony formation assay and a
soft-agar colony formation assay. As shown in Fig. 3A and B, there was a significant
reduction in the number of colonies in cells treated with 1.5 µM
DHT. In 8505C cells, DHT reduced the number of colonies by
~10-fold, and its effects were more potent on the SW1736 cells, in
which it completely abrogated colony formation ability. To better
evaluate the effects of DHT on tumor cell aggressiveness, the
anchorage-independent colony formation ability of SW1736 and 8505C
treated with DHT was investigated by performing a soft agar colony
formation assay. Treatment with 1.5 µM DHT for 48 h resulted in a
significant reduction in the number of colonies in both cell lines
relative to the respective vehicle-treated cells (Fig. 3C and D).
Since traversing the basement membrane by cancer
cells is an important step in the metastatic process, the effects
of DHT treatment on thyroid cancer cell invasiveness was assessed
using Transwell invasion assays. Treatment with 1.5 µM DHT resulted
in a significant decrease in cell invasion in both ATC cell lines
(Fig. 3E and F).
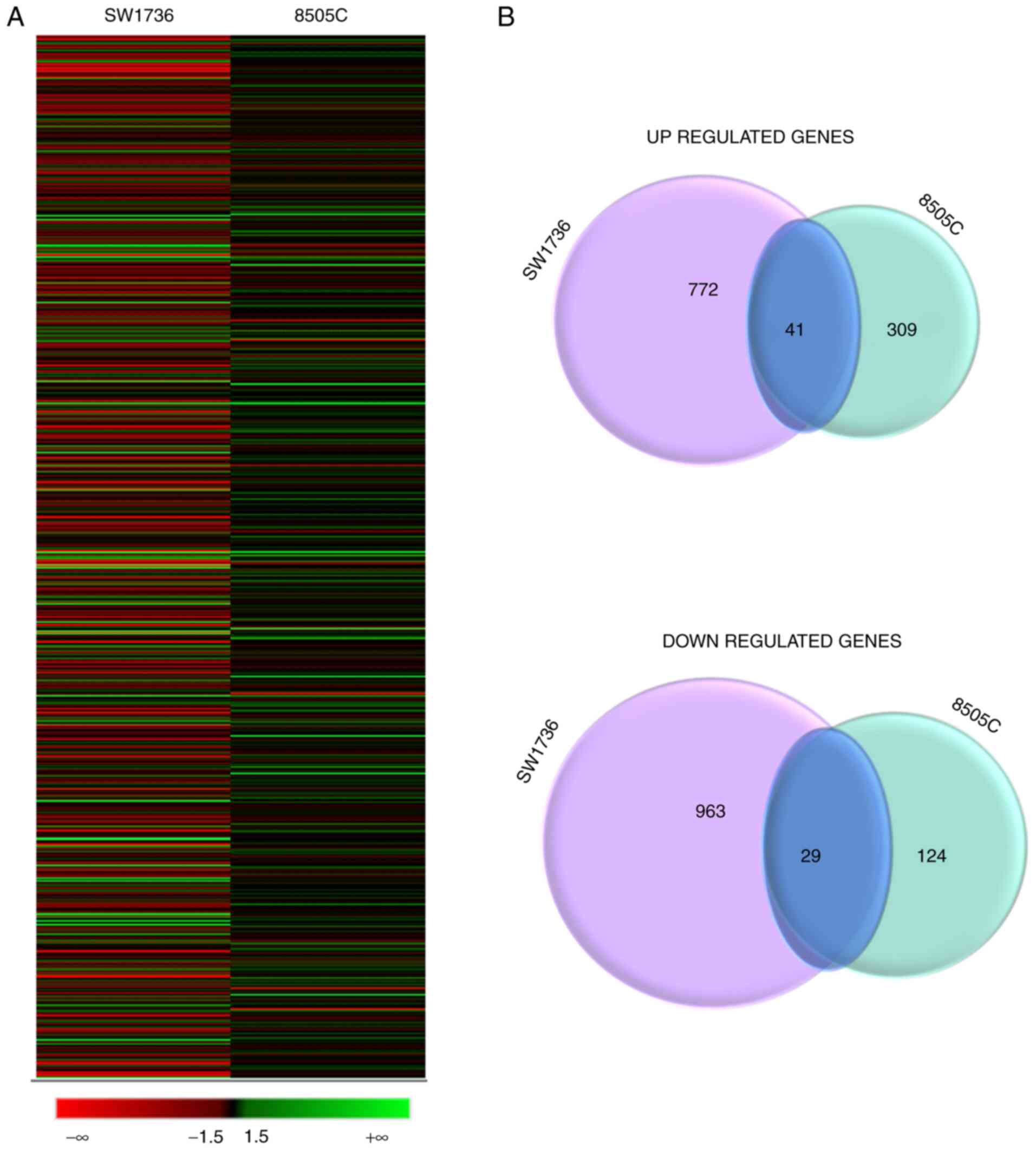
Effects of DHT on gene expression
To evaluate the effects of DHT on gene expression,
high-throughput RNA-seq analysis was performed on the SW1736 and
8505C cells treated with 1.5 µM DHT for 24 h. After filtering out
low quality reads, the comparison between cells treated with DMSO
or DHT showed that 1,805 and 503 genes were differentially
expressed (log2 fold change >1.5) in the DHT-treated
SW1736 and 8505C cells, respectively. In SW1736 cells, 813 genes
were upregulated and 992 were downregulated, whereas in 8505C, 350
genes were upregulated and 153 were downregulated in response to
DHT treatment. Among these, 41 genes were commonly upregulated and
29 commonly downregulated (Fig.
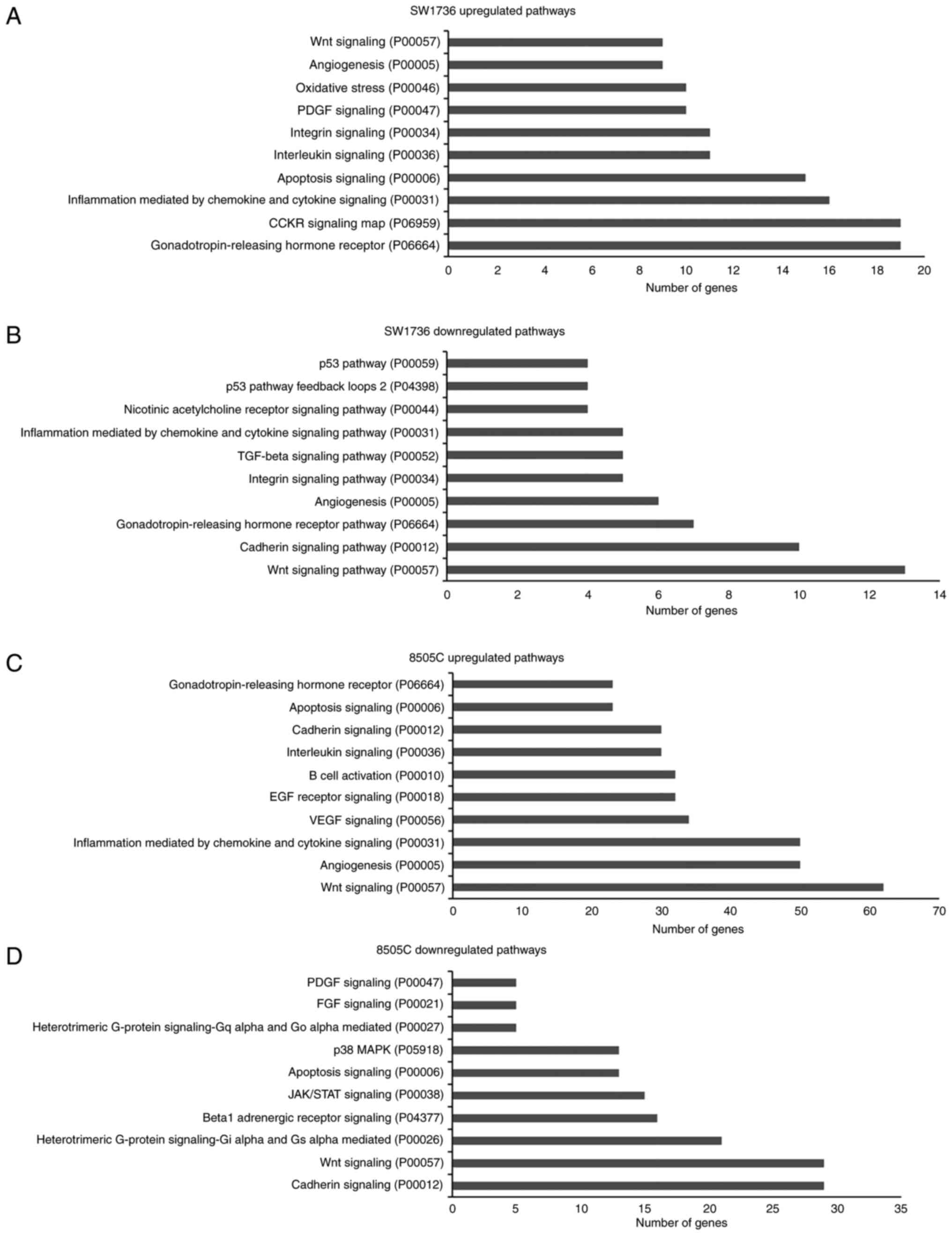
4).
Gene Ontology analysis was then performed using the
Panther Classification System (41).
Briefly, differential expressed gene lists for each cell line
(separately for up- and down-regulated genes) were loaded into
PANTHER (version 16.0) and mapped to its pathway database. When the
PANTHER pathway database was interrogated with the commonly
dysregulated genes, no pathway has been identified as significantly
deregulated. Regarding the altered genes in each cell line, the top
10 up- and downregulated pathways of each cell line are shown in
Fig. 5 (and Table SI,Table
SII,Table SIIITable SIV). Although the altered gene
expression involved different genes, the analysis identified some
commonly dysregulated pathways in both cell lines, which included
‘Wnt signaling’ (P00057), ‘apoptosis signaling’ (P00006) and
‘cadherin signaling pathway’ (P00012).
Effects of DHT on expression of EMT
markers
To characterize the effects of DHT on ATC cell
aggressiveness at the molecular level, the expression of five
universally recognized genes associated with EMT (42), a phenotypic change which endows cancer
cells with increased migratory and invasive capacity, was
analyzed.
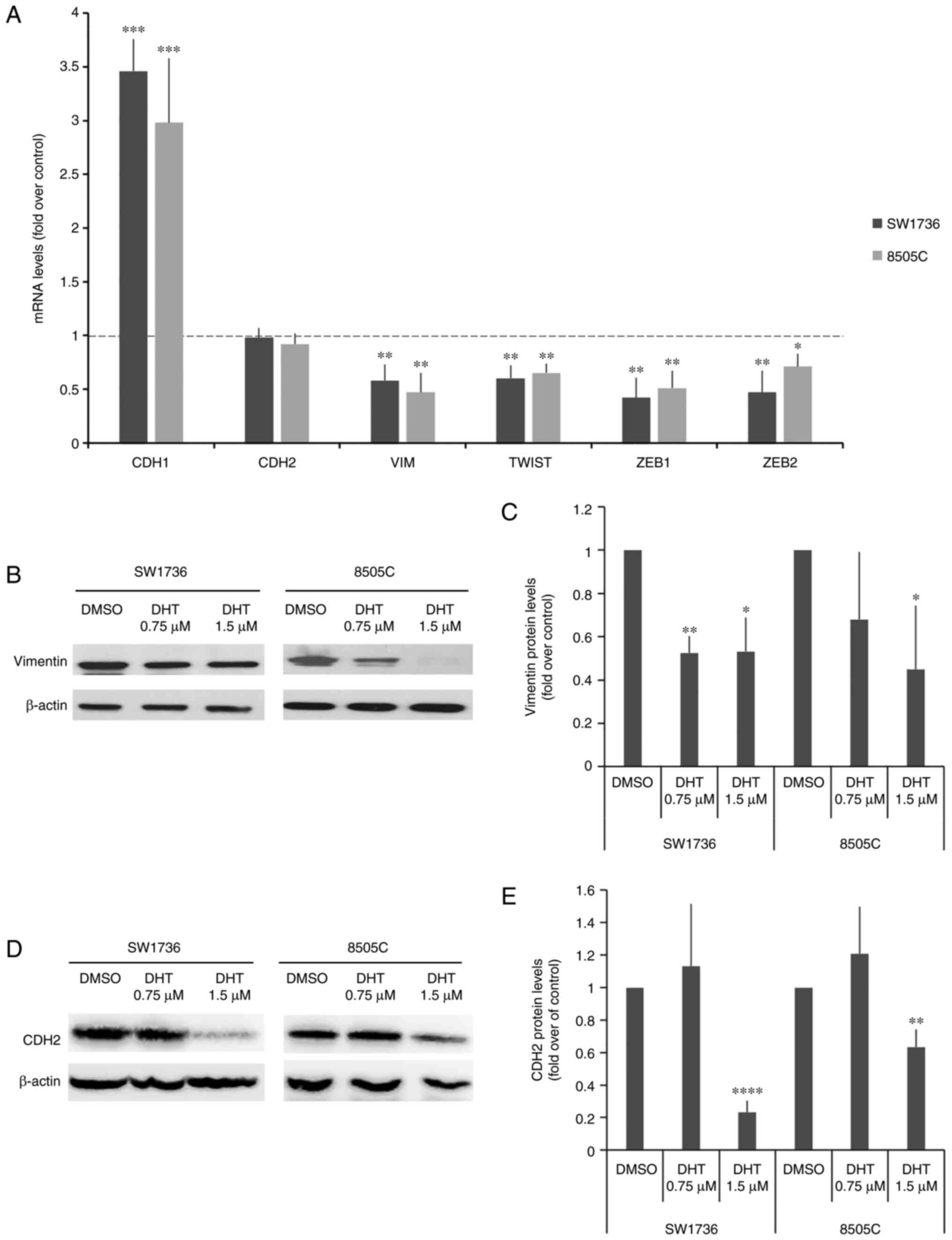
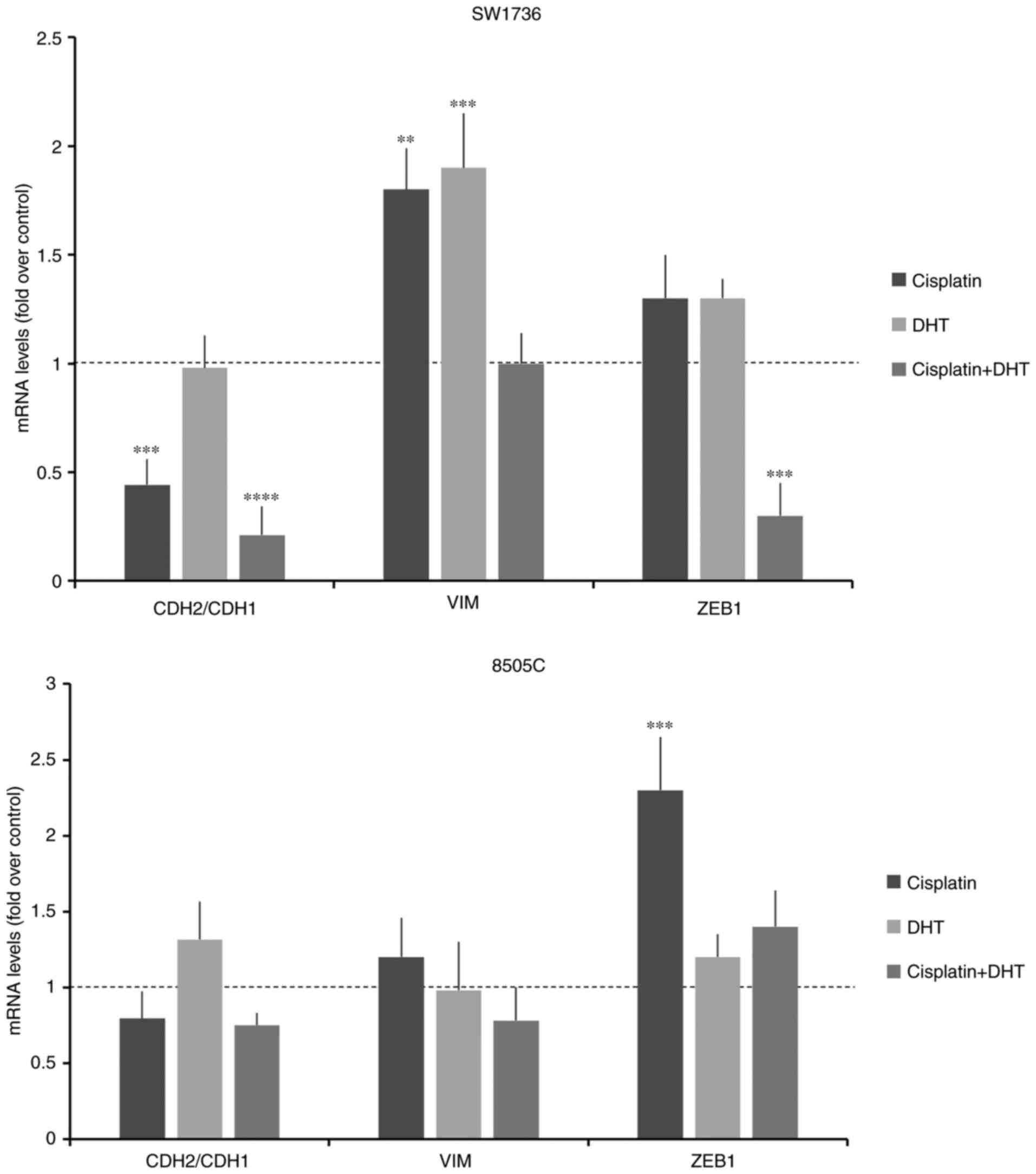
The changes in mRNA levels for the EMT-related genes
CDH1, CDH2, VIM, TWIST, ZEB1 and ZEB2 are shown in
Fig. 6A. Treatment with 1.5 µM DHT
resulted in a significant increase in the expression of
CDH1, a marker of the epithelial phenotype, in treated cells
compared with those treated with DMSO. Additionally, DHT treatment
significantly decreased the gene expression levels of all other
markers, which are associated with the mesenchymal phenotype, in
both cell lines, except for CDH2, confirming that DHT
modulated EMT.
Among the EMT-related genes assessed, CDH1,
CDH2 and VIM are particularly interesting, as they are
phenotypic markers of EMT. Thus, their protein expression levels
were also assessed. VIM and CDH2 protein expression levels were
significatively reduced following treatment with DHT (Fig. 6B and C). Despite the fact that CDH1
mRNA levels were increased following DHT treatment, the protein
expression levels remained undetectable in the treated cells (data
not shown).
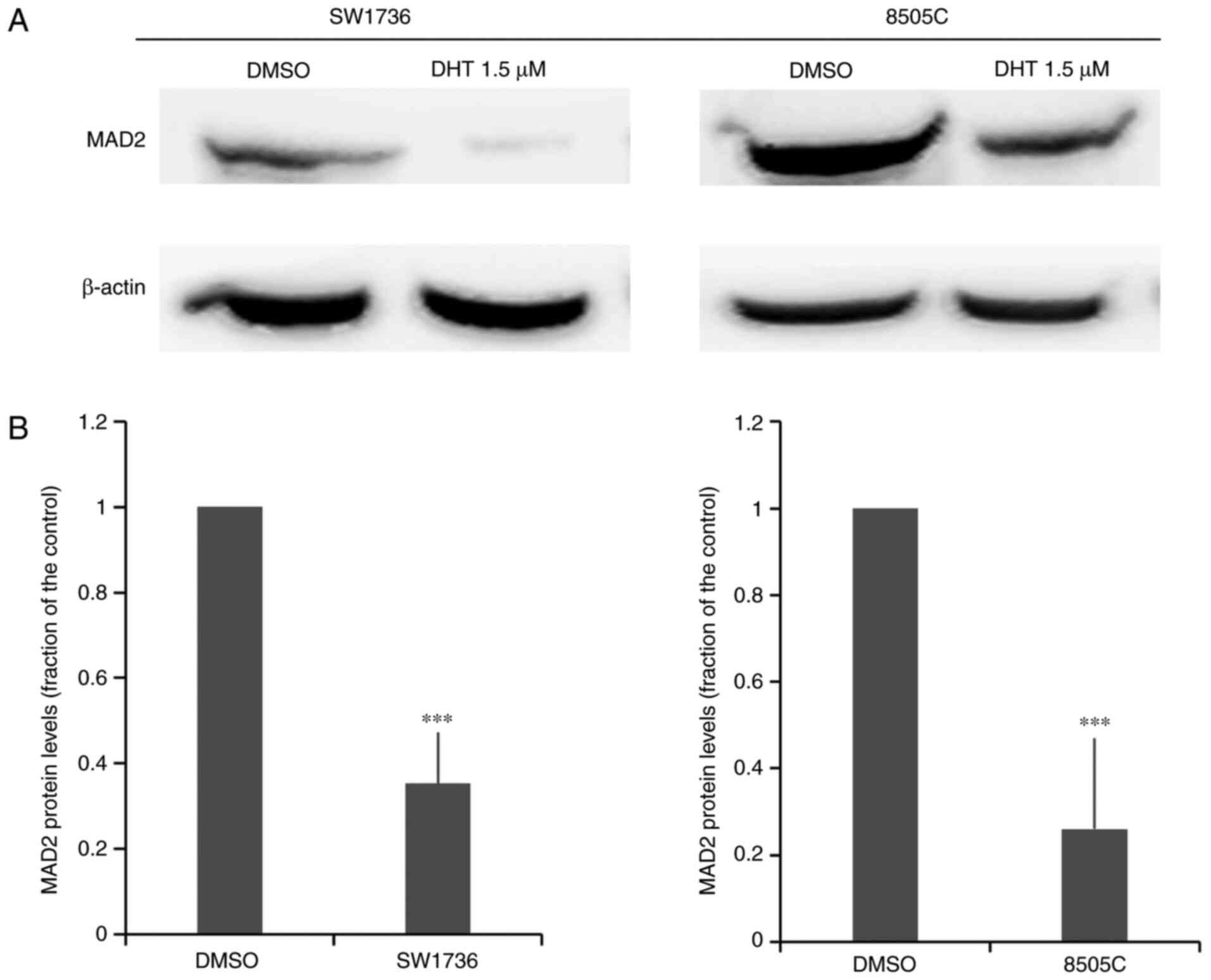
Effects of DHT on MAD2 expression
Since DHT is known to inhibit the activity of the
RNA-binding protein HuR (24), to
verify the involvement of HuR in the effects of DHT on viability,
apoptosis and tumor aggressiveness in ATC cells, MAD2, a target of
HuR, was then analyzed. MAD2, which is one of the major factors
involved in the spindle checkpoint, has been shown to be implicated
in cancer progression (43,44) and is overexpressed in thyroid
neoplasms (45). Thus, the effects of
DHT on MAD2 protein expression were examined using western blotting
(Fig. 7). After 48 h of treatment
with 1.5 µM DHT, there was a significant decrease in MAD2 protein
expression levels in both cell lines. This result demonstrated that
MAD2 may be involved in anticancer effect of DHT in ATC cell
lines.
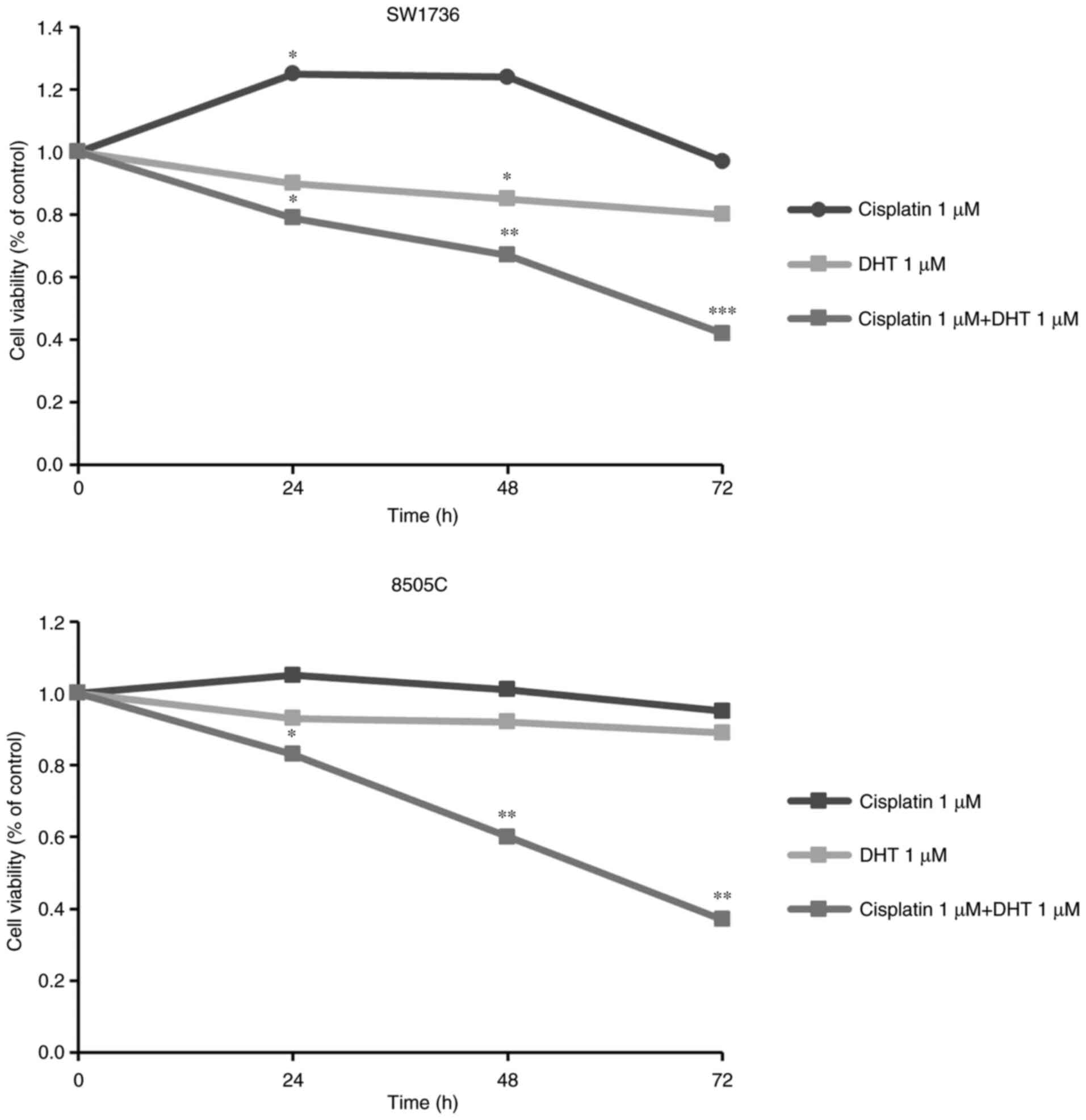
DHT administration increases the
sensitivity of ATC cells to cisplatin treatment
Based on the results of the DHT treatment, the
effects of its co-administration with cisplatin, one of the few
chemotherapeutic agents used for ATC treatment, were then examined.
The effect of their combined administration on the viability of
SW1736 and 8505C cells was assessed following 24, 48 or 72-h
treatment with cisplatin and DHT alone or combined. Since synergy
is considered as the interaction between two or more drugs, which
results in a total effect of the drugs, which is greater than the
sum of the individual effects of each compound, drug concentrations
of 1 µM for both compounds were used, as this concentration did not
exert any notable effects when the compounds were administered
alone.
As shown in Fig. 8,
DHT and cisplatin 1 µM alone did not affect cell viability after
72-h treatment, in either cell line. However, when co-administered
at the same individual concentration of 1 µM, there was a
significant decrease in cell viability of around 60–70% in both the
SW1736 and 8505C cells.
Thus, the effects of DHT-cisplatin combination on
apoptosis and other markers of tumor aggressiveness were assessed
next (Fig. 9). Western blot analysis
indicated that cleaved PARP protein levels were increased 6-fold
after cotreatment compared with 1 µM cisplatin alone in SW1736
cells. In 8505C cells, combined administration of DHT and cisplatin
resulted in a significant increase in cleaved-PARP protein
expression compared with either treatment alone. Similar to what
was observed when DHT was used at an ED50 dose, the
lower dose of 1 µM resulted in MAD2 downregulation in the ATC cell
lines. Moreover, cotreatment resulted in a greater decrease in MAD2
protein expression in the SW1736 and 8505C cells, compared with DHT
or cisplatin alone.
The effects of the combination of the two compounds
on the expression of EMT-related genes were then evaluated. During
EMT, CDH1 expression is significantly decreased, whereas CDH2
expression is increased. Thus, CDH2/CDH1 expression ratio is widely
considered a marker of EMT (46). As
shown in Fig. 10, in SW1736 cells,
DHT alone did not induce an increase in the CDH2/CDH1 ratio,
whereas cisplatin, alone and combined with DHT, resulted in a
significant reduction in the CHD2/CDH1 ratio. In SW1736, unlike
what observed using the ED50 dose of DHT (1.5 µM),
treatment with 1 µM DHT resulted in an increase in VIM mRNA
expression levels, compared with the control. When cells were
cotreated with DHT and cisplatin, the mRNA expression of VIM
returns to comparable levels with control, while ZEB1
decreased. In the 8505C cells, the observed effects on EMT-related
genes were not as prominent and the differences were not
significant.
Discussion
The post-diagnosis survival rate of ATC is <1
year due to its invasiveness and frequent metastasis, making it the
most lethal thyroid malignancy (47).
A study of Fan et al (48) has
focused on different trimodal treatment regimens, namely aggressive
radiotherapy in combination with surgery and systemic therapy, but
the prognosis of patients with ATC remains poor despite the use of
these aggressive multimodal therapies. For these reasons, the
search for novel approaches to the treatment of this aggressive
thyroid cancer is crucial, as it cannot be effectively managed by
the currently available therapies (49). Novel strategies can take advantage of
the molecular alterations that occur in these tumors (50). Promising results have been observed in
clinical trials of targeted therapy in ATC (50), underscoring the importance of a
selection of patients based on tumor molecular profiles. Additional
studies are focusing on the use of bioactive molecules that are
naturally present in plants, such as phytochemicals, some of which
are able to regulate certain cell physiological pathways, which may
promote malignant transformation or drug resistance (51,52).
Indeed, several studies have reported the anticancer proprieties of
several phytochemicals, including some acting on ATC cells
(12,13). Moreover, there are natural compounds
that may also improve cancer sensitivity to commonly used
chemotherapeutic agents (53,54). The lack of effective and standardized
therapeutic strategy for the management of ATC, and the promising
anticancer effects obtained in preclinical experimental models by
certain phytochemicals, highlight them as ideal candidates for
investigation as ATC treatments. Several studies have investigated
the use of phytochemicals for the treatment of ATC. For instance,
Schwertheim et al (55) and
Allegri et al (56)
independently demonstrated the antitumoral effects of curcumin, as
well as other phytochemicals, in different ATC cells.
In the present study, the effects of DHT were
examined. DHT is one of 40 tanshinones extracted from Salvia
miltiorrhiza, which not only has anticancer activity through
its cytotoxic properties, but also improves the sensitivity to
other anti-cancer agents drugs and exhibits synergism with other
chemotherapeutic regimens (15).
Interestingly, DHT has been shown to induce cell death via
autophagy in multidrug-resistant colon cancer cells (57) and to enhance irradiation-induced
cytotoxic effects, G2-phase arrest and apoptosis in HeLa
cells (23). Moreover, DHT was able
to sensitize tumors to irradiation in vivo (23). In the present study, it was
demonstrated for the first time that DHT exerts strong anticancer
activity against ATC cells. Indeed, a decrease in ATC cell
viability was observed, which was primarily due to the activation
of the apoptotic process, as indicated by a large increase in the
cleaved-PARP fraction. Moreover, cell cycle analysis demonstrated
that DHT induced S-phase arrest of ATC cells, which has already
been shown in other cancer models (17). The calculated DHT ED50 in
SW1736 and 8505C cells was almost 50-fold lower than those of
others phytochemicals (56),
highlighting the effectiveness of its antitumor function in these
cell lines.
The molecular mechanisms regulated by DHT remain to
fully elucidated. Tan et al (58) demonstrated that DHT inhibits glioma
cell proliferation through the activation of ferroptosis,
regulating the expression of ACSL4 and the GPX gene
family. Moreover, Wang et al (59) showed that DHT transcriptionally
repressed PIK3CA. These studies suggest that DHT primarily
exerts its functions by regulating gene expression. Thus, in the
present study, the effects of DHT on gene expression were evaluated
using high-throughput RNA-sequencing analysis on SW1736 and 8505C
cells. Despite the differences in the altered expression of genes,
the analysis identified certain commonly dysregulated pathways,
including the Wnt, apoptotic and cadherin signaling pathways.
These encouraging results obtained with regard to
cell viability and apoptosis are strengthened by those obtained
with regard to counteracting other features of aggressive tumor
behavior, as the invasiveness of a few aggressive cells that
survive can lead to dissemination and re-growth of metastasizing
tumor cells. In this context, the effects of DHT on several
features of tumor cell aggressiveness were analyzed by performing
colony formation, soft agar and invasion assays, whilst also
determining the expression of EMT-related genes. DHT significantly
reduced the colony formation ability of ATC cell lines, achieving
complete inhibition of colony formation of SW1736 cells. Moreover,
DHT treatment significantly reduced invasiveness of ATC cells,
indicating that this compound can affect mesenchymal motility. This
latter property was also confirmed by the effects obtained by
analyzing the effects on some key elements of the EMT process,
which is typical of aggressive neoplasia. Indeed, DHT treatment
resulted in an increase in CDH1 expression and a significant
decrease in VIM, TWIST, ZEB1 and ZEB2 expression
(components of the cytoskeleton, the extracellular matrix or
transcription factors), which are considered the five key
EMT-related genes (40). Our findings
showed that DHT regulated the mRNA levels of these genes. Thus, in
order to deepen our understanding of the molecular mechanism
underlying the effects of DHT, a second phase of our study was
performed based on a recent study by Lal et al (24), showing that DHT may alter the activity
of the RNA-binding protein HuR by inhibiting the HuR-mRNA binding
in xenograft tumors. In our previous study, the RNA-binding protein
HuR was overexpressed in thyroid cancer, and that its
downregulation resulted in anticancer effects in ATC cell lines
(25–27). Moreover, we also previously showed
that a HuR-specific inhibitor (CMLD-2) exerted antitumor effects on
ATC cells via downregulation of MAD2 (25–27). In
the present study, DHT may have exerted its antitumor effects, at
least partly, by decreasing the expression of the HuR target, MAD2,
a protein closely associated with ATC cell viability, survival and
aggressiveness (27). Bates et
al (60) and Pajuelo-Lozano et
al (61) demonstrated that MAD2
silencing results in reduced tumor aggressiveness and alteration of
several biological processes including EMT, in different cancer
types. Further experiments focused on the molecular mechanism
between MAD2 and EMT induction in thyroid cancer could better
highlight the role of MAD2 in thyroid cancer and EMT processes.
Furthermore, evaluating whether the effects of DHT are maintained
after knockdown of MAD2 would be an interesting experiment.
Nascimento et al (62) demonstrated that the combination of
MAD2 knockdown with cisplatin administration constituted an
efficient approach to the treatment of drug-resistant tumors. Since
the present findings indicated that DHT resulted in downregulation
of MAD2, the final part of this study was dedicated to the
investigation of a synergistic effect between DHT and cisplatin.
Seto et al (63) reported that
cisplatin was able to inhibit ATC progression and improved survival
outcomes of certain ATC patients. However, another study
demonstrated that cisplatin could activate autophagy-mediated
apoptosis resistance (64). Moreover,
chemotherapy resistance currently remains a significant hurdle in
ATC management (64). Recently, a
synergistic effect was demonstrated in thyroid cancer cells
cotreated with the phytochemical quercetin and a protein kinase
inhibitor (65). In the present
study, DHT exerted a synergistic effect with cisplatin, indicating
the potential benefits of the use of DHT as an adjuvant in a
multimodal therapeutic strategy, in order to improve the antitumor
effectiveness of other therapies. An important point regarding
these proposed compounds as anticancer drugs is the
absence/tolerance of toxicity on normal cells. In this regard, no
toxic effects have been reported when DHT is administered for the
treatment of xenograft tumors in vivo (20,21).
Moreover, the possibility of a toxic effect on normal thyroid cells
can be excluded in patients with ATC, since the use of anticancer
drugs is complementary to the surgical treatment to achieve total
thyroidectomy.
In conclusion, to the best of our knowledge, the
results of the present study were the first to demonstrate the
antitumor effects of DHT on ATC cells in vitro, alone and in
combination with cisplatin. Despite these encouraging results, this
study is limited by the lack of an in vivo model. Therefore,
further and more detailed studies on its effects on HuR regulation,
as well as in vivo investigation of this combination, are
needed.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. Raw and processed data are available on the public online
repository Gene Expression Omnibus (dataset no. GSE168616).
Authors' contributions
LA, FB, GD and DR conceived the study. LA designed
the methodology. LA, MN, RD, FC, FB and MC performed the
experiments and validated the data. FB, MN, FC and RD analyzed the
data. LA and FB wrote the original draft. GD, MC, DR reviewed and
edited the manuscript. GD and DR supervised the study. All authors
read and approved the final manuscript. LA, FB and GD confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Abe I and Lam AK: Anaplastic thyroid
carcinoma: Updates on WHO classification, clinicopathological
features and staging. Histol Histopathol. 36:239–248.
2021.PubMed/NCBI
|
|
2
|
Lim H, Devesa SS, Sosa JA, Check D and
Kitahara CM: Trends in thyroid cancer incidence and mortality in
the United States, 1974–2013. JAMA. 317:1338–1348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess K,
Sullivan DC, et al: Organization of the AJCC cancer staging manual.
AJCC Cancer Staging Manual. 31–37. 2017. View Article : Google Scholar
|
|
4
|
Nylén C, Mechera R, Maréchal-Ross I, Tsang
V, Chou A, Gill AJ, Clifton-Bligh RJ, Robinson BG, Sywak MS, Sidhu
SB and Glover AR: Molecular markers guiding thyroid cancer
management. Cancers (Basel). 12:21642020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Z, Zhang Y, Wang R, Zou K and Zou L:
Genetic alterations in anaplastic thyroid carcinoma and targeted
therapies. Exp Ther Med. 18:2369–2377. 2019.PubMed/NCBI
|
|
6
|
Goutsouliak V and Hay JH: Anaplastic
thyroid cancer in British Columbia 1985–1999: A population-based
study. Clin Oncol (R Coll Radiol). 17:75–78. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pezzi TA, Mohamed ASR, Sheu T, Blanchard
P, Sandulache VC, Lai SY, Cabanillas ME, Williams MD, Pezzi CM, Lu
C, et al: Radiation therapy dose is associated with improved
survival for unresected anaplastic thyroid carcinoma: Outcomes from
the National cancer data base. Cancer. 123:1653–1661. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prasongsook N, Kumar A, Chintakuntlawar
AV, Foote RL, Kasperbauer J, Molina J, Garces Y, Ma D, Wittich MAN,
Rubin J, et al: Survival in response to multimodal therapy in
anaplastic thyroid cancer. J Clin Endocrinol Metab. 102:4506–4514.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JH and Leeper RD: Treatment of locally
advanced thyroid carcinoma with combination doxorubicin and
radiation therapy. Cancer. 60:2372–2375. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hosseini A and Ghorbani A: Cancer therapy
with phytochemicals: Evidence from clinical studies. Avicenna J
Phytomedicine. 5:84–97. 2015.PubMed/NCBI
|
|
11
|
Shin HJ, Hwang KA and Choi KC: Antitumor
effect of various phytochemicals on diverse types of thyroid
cancers. Nutrients. 11:1252019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hosseinimehr SJ and Hosseini SA:
Resveratrol sensitizes selectively thyroid cancer cell to
131-iodine toxicity. J Toxicol. 2014:8395972014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu X, Qin J and Liu W: Curcumin inhibits
the invasion of thyroid cancer cells via down-regulation of
PI3K/Akt signaling pathway. Gene. 546:226–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Cheng X, Gao Y, Zhang C, Bao J,
Guan H, Yu H, Lu R, Xu Q and Sun Y: Curcumin inhibits metastasis in
human papillary thyroid carcinoma BCPAP cells via down-regulation
of the TGF-β/Smad2/3 signaling pathway. Exp Cell Res. 341:157–165.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Yu J, Zhong B, Lu J, Lu JJ, Li S
and Lu Y: Pharmacological activities of dihydrotanshinone I, a
natural product from Salvia miltiorrhiza Bunge. Pharmacol
Res. 145:1042542019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Yeung JH, Hu T, Lee WY, Lu L,
Zhang L, Shen J, Chan RL, Wu WK and Cho CH: Dihydrotanshinone
induces p53-independent but ROS-dependent apoptosis in colon cancer
cells. Life Sci. 93:344–351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai SL, Suk FM, Wang CI, Liu DZ, Hou WC,
Lin PJ, Hung LF and Liang YC: Anti-tumor potential of
15,16-dihydrotanshinone I against breast adenocarcinoma through
inducing G1 arrest and apoptosis. Biochem Pharmacol. 74:1575–1586.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng R, Chen J, Wang Y, Ge Y, Huang Z and
Zhang G: Dihydrotanshinone induces apoptosis of SGC7901 and MGC803
cells via activation of JNK and p38 signalling pathways. Pharm
Biol. 54:3019–3025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X, Li Q, He Y, Du H, Zhan Z, Zhao H,
Shi J, Ye Q and Hu J: 15,16-dihydrotanshinone I induces apoptosis
and inhibits the proliferation, migration of human osteosarcoma
cell line 143b in vitro. Anticancer Agents Med Chem. 17:1234–1242.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu JJ, Wu HH, Chen TH, Leung W and Liang
YC: 15,16-Dihydrotanshinone I from the functional food Salvia
miltiorrhiza exhibits anticancer activity in human HL-60
leukemia cells: In vitro and in vivo studies. Int J Mol Sci.
16:19387–19400. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang F, Ma J, Wang KS, Mi C, Lee JJ and
Jin X: Blockade of TNF-α-induced NF-κB signaling pathway and
anti-cancer therapeutic response of dihydrotanshinone I. Int
Immunopharmacol. 28:764–772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee DS and Lee SH: Biological activity of
dihydrotanshinone I: Effect on apoptosis. J Biosci Bioeng.
89:292–293. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ye Y, Xu W, Zhong W, Li Y and Wang C:
Combination treatment with dihydrotanshinone I and irradiation
enhances apoptotic effects in human cervical cancer by HPV E6
down-regulation and caspases activation. Mol Cell Biochem.
363:191–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lal P, Cerofolini L, D'Agostino VG, Zucal
C, Fuccio C, Bonomo I, Dassi E, Giuntini S, Di Maio D, Vishwakarma
V, et al: Regulation of HuR structure and function by
dihydrotanshinone-I. Nucleic Acids Res. 45:9514–9527. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baldan F, Mio C, Allegri L, Conzatti K,
Toffoletto B, Puppin C, Radovic S, Vascotto C, Russo D, Di Loreto C
and Damante G: Identification of tumorigenesis-related mRNAs
associated with RNA-binding protein HuR in thyroid cancer cells.
Oncotarget. 7:63388–63407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Allegri L, Mio C, Russo D, Filetti S and
Baldan F: Effects of HuR downregulation on anaplastic thyroid
cancer cells. Oncol Lett. 15:575–579. 2018.PubMed/NCBI
|
|
27
|
Allegri L, Baldan F, Roy S, Aubé J, Russo
D, Filetti S and Damante G: The HuR CMLD-2 inhibitor exhibits
antitumor effects via MAD2 downregulation in thyroid cancer cells.
Sci Rep. 9:73742019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Skoufias DA, Andreassen PR, Lacroix FB,
Wilson L and Margolis RL: Mammalian mad2 and bub1/bubR1 recognize
distinct spindle-attachment and kinetochore-tension checkpoints.
Proc Natl Acad Sci USA. 98:4492–4497. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heldin NE and Westermark B: The molecular
biology of the human anaplastic thyroid carcinoma cell.
Thyroidology. 3:127–131. 1991.PubMed/NCBI
|
|
30
|
Ito T, Seyama T, Hayashi Y, Hayashi T,
Dohi K, Mizuno T, Iwamoto K, Tsuyama N, Nakamura N and Akiyama M:
Establishment of 2 human thyroid-carcinoma cell-lines (8305c,
8505c) bearing P53 gene-mutations. Int J Oncol. 4:583–586.
1994.PubMed/NCBI
|
|
31
|
Landa I, Pozdeyev N, Korch C, Marlow LA,
Smallridge RC, Copland JA, Henderson YC, Lai SY, Clayman GL, Onoda
N, et al: Comprehensive genetic characterization of human thyroid
cancer cell lines: A validated panel for preclinical studies. Clin
Cancer Res. 25:3141–3151. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mio C, Lavarone E, Conzatti K, Baldan F,
Toffoletto B, Puppin C, Filetti S, Durante C, Russo D, Orlacchio A,
et al: MCM5 as a target of BET inhibitors in thyroid cancer cells.
Endocr Relat Cancer. 23:335–347. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Justus CR, Leffler N, Ruiz-Echevarria M
and Yang LV: In vitro cell migration and invasion assays. J Vis
Exp. 510462014.PubMed/NCBI
|
|
34
|
Martin M: Cutadapt removes adapter
sequences from high-throughput sequencing reads. EMBnet J.
17:10–12. 2011. View Article : Google Scholar
|
|
35
|
Del Fabbro C, Scalabrin S, Morgante M and
Giorgi FM: An extensive evaluation of read trimming effects on
Illumina NGS data analysis. PLoS One. 8:e850242013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dobin A, Davis CA, Schlesinger F, Drenkow
J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR: STAR:
Ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pertea M, Pertea GM, Antonescu CM, Chang
TC, Mendell JT and Salzberg SL: StringTie enables improved
reconstruction of a transcriptome from RNA-seq reads. Nat
Biotechnol. 33:290–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lombardo GE, Maggisano V, Celano M, Cosco
D, Mignogna C, Baldan F, Lepore SM, Allegri L, Moretti S, Durante
C, et al: Anti-hTERTsiRNA-Loaded nanoparticles block the growth of
anaplastic thyroid cancer xenograft. Mol Cancer Ther. 17:1187–1195.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Darzynkiewicz Z, Bruno S, Bino GD,
Gorczyca W, Hotz MA, Lassota P and Traganos F: Features of
apoptotic cells measured by flow cytometry. Cytometry. 13:795–808.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mi H, Ebert D, Muruganujan A, Mills C,
Albou LP, Mushayamaha T and Thomas PD: PANTHER version 16: A
revised family classification, tree-based classification tool,
enhancer regions and extensive API. Nucleic Acids Res.
49:D394–D403. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shakib H, Rajabi S, Dehghan MH, Mashayekhi
FJ, Safari-Alighiarloo N and Hedayati M: Epithelial-to-mesenchymal
transition in thyroid cancer: A comprehensive review. Endocrine.
66:435–455. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Genga KR, Filho FD, Ferreira FV, de Sousa
JC, Studart FS, Magalhães SM, Heredia FF and Pinheiro RF: Proteins
of the mitotic checkpoint and spindle are related to chromosomal
instability and unfavourable prognosis in patients with
myelodysplastic syndrome. J Clin Pathol. 68:381–387. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu L, Guo WC, Zhao SH, Tang J and Chen JL:
Mitotic arrest defective protein 2 expression abnormality and its
clinicopathologic significance in human osteosarcoma. APMIS.
118:222–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wada N, Yoshida A, Miyagi Y, Yamamoto T,
Nakayama H, Suganuma N, Matsuzu K, Masudo K, Hirakawa S, Rino Y, et
al: Overexpression of the mitotic spindle assembly checkpoint genes
hBUB1, hBUBR1 and hMAD2 in thyroid carcinomas with aggressive
nature. Anticancer Res. 28:139–144. 2008.PubMed/NCBI
|
|
46
|
Tanabe S, Aoyagi K, Yokozaki H and Sasaki
H: Gene expression signatures for identifying diffuse-type gastric
cancer associated with epithelial-mesenchymal transition. Int J
Oncol. 44:1955–1970. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu H, Sun Y, Ye H, Yang S, Lee SL and de
las Morenas A: Anaplastic thyroid cancer: Outcome and the
mutation/expression profiles of potential targets. Pathol Oncol
Res. 21:695–701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fan D, Ma J, Bell AC, Groen AH, Olsen KS,
Lok BH, Leeman JE, Anderson E, Riaz N, McBride S, et al: Outcomes
of multimodal therapy in a large series of patients with anaplastic
thyroid cancer. Cancer. 126:444–452. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bulotta S, Celano M, Costante G and Russo
D: Emerging strategies for managing differentiated thyroid cancers
refractory to radioiodine. Endocrine. 52:214–221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ljubas J, Ovesen T and Rusan M: A
Systematic review of phase II targeted therapy clinical trials in
anaplastic thyroid cancer. Cancers (Basel). 11:9432019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rigalli JP, Tocchetti GN, Arana MR,
Villanueva SS, Catania VA, Theile D, Ruiz ML and Weiss J: The
phytoestrogen genistein enhances multidrug resistance in breast
cancer cell lines by translational regulation of ABC transporters.
Cancer Lett. 376:165–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bespalov VG, Alexandrov VA, Semenov AL,
Vysochina GI, Kostikova VA and Baranenko DA: The inhibitory effect
of Filipendula ulmaria (L.) Maxim. on colorectal
carcinogenesis induced in rats by methylnitrosourea. J
Ethnopharmacol. 227:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Samec M, Liskova A, Kubatka P, Uramova S,
Zubor P, Samuel SM, Zulli A, Pec M, Bielik T, Biringer K, et al:
The role of dietary phytochemicals in the carcinogenesis via the
modulation of miRNA expression. J Cancer Res Clin Oncol.
145:1665–1679. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kapinova A, Kubatka P, Golubnitschaja O,
Kello M, Zubor P, Solar P and Pec M: Dietary phytochemicals in
breast cancer research: Anticancer effects and potential utility
for effective chemoprevention. Environ Health Prev Med. 23:362018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schwertheim S, Wein F, Lennartz K, Worm K,
Schmid KW and Sheu-Grabellus SY: Curcumin induces G2/M arrest,
apoptosis, NF-kappaB inhibition, and expression of differentiation
genes in thyroid carcinoma cells. J Cancer Res Clin Oncol.
143:1143–1154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Allegri L, Rosignolo F, Mio C, Filetti S,
Baldan F and Damante G: Effects of nutraceuticals on anaplastic
thyroid cancer cells. J Cancer Res Clin Oncol. 144:285–294. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hu T, Wang L, Zhang L, Lu L, Shen J, Chan
RL, Li M, Wu WK, To KK and Cho CH: Sensitivity of
apoptosis-resistant colon cancer cells to tanshinones is mediated
by autophagic cell death and p53-independent cytotoxicity.
Phytomedicine. 22:536–544. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tan S, Hou X and Mei L: Dihydrotanshinone
I inhibits human glioma cell proliferation via the activation of
ferroptosis. Oncol Lett. 20:1222020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang X, Xu X, Jiang G, Zhang C, Liu L,
Kang J, Wang J, Owusu L, Zhou L, Zhang L and Li W:
Dihydrotanshinone I inhibits ovarian cancer cell proliferation and
migration by transcriptional repression of PIK3CA gene. J Cell Mol
Med. 24:11177–11187. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bates M, Spillane CD, Gallagher MF, McCann
A, Martin C, Blackshields G, Keegan H, Gubbins L, Brooks R, Brooks
D, et al: The role of the MAD2-TLR4-MyD88 axis in paclitaxel
resistance in ovarian cancer. PLoS One. 15:e02437152020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pajuelo-Lozano N, Alcalá S, Sainz B Jr,
Perona R and Sanchez-Perez I: Targeting MAD2 modulates stemness and
tumorigenesis in human Gastric cancer cell lines. Theranostics.
10:9601–9618. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nascimento AV, Singh A, Bousbaa H,
Ferreira D, Sarmento B and Amiji MM: Overcoming cisplatin
resistance in non-small cell lung cancer with Mad2 silencing siRNA
delivered systemically using EGFR-targeted chitosan nanoparticles.
Acta Biomater. 47:71–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Seto A, Sugitani I, Toda K, Kawabata K,
Takahashi S and Saotome T: Chemotherapy for anaplastic thyroid
cancer using docetaxel and cisplatin: Report of eight cases. Surg
Today. 45:221–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ranganath R, Shah MA and Shah AR:
Anaplastic thyroid cancer. Curr Opin Endocrinol Diabetes Obes.
22:387–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Celano M, Maggisano V, Bulotta S, Allegri
L, Pecce V, Abballe L, Damante G and Russo D: Quercetin improves
the effects of sorafenib on growth and migration of thyroid cancer
cells. Endocrine. 67:496–498. 2020. View Article : Google Scholar : PubMed/NCBI
|