Introduction
Melanoma, which remains the leading cause of skin
cancer-related mortality in patients, is characterized by high
levels of metastasis (1). In 2017,
the age-standardized prevalence rate of melanoma was 0.9 per
100,000 in China (2). As a type of
malignant tumor, cutaneous malignant melanoma (CMM) is derived from
melanocytes in the skin (3).
Worldwide, the morbidity of CMM is increasing and the age of onset
is decreasing (4), which may be
associated with genetic factors and increased exposure to UV,
amongst other risk factors (5). The
majority of patients with CMM can be cured by early detection and
treatment (6), including surgical
resection (7) and chemo- and
radiotherapy (8); however, the
prognosis of patients with CMM remains poor following late
detection.
Non-coding regulatory RNAs can be divided into two
groups: Small non-coding RNAs (≤200 nucleotides in length) and long
non-coding RNAs (lncRNAs; >200 nucleotides in length) (9). lncRNAs regulate gene expression via
numerous molecular mechanisms, including RNA degradation, microRNA
(miRNA/miR) sequestration and transcriptional and translational
activation or repression (10).
Accumulating evidence has indicated that numerous lncRNAs play
roles in the tumorigenesis of various cancer types, including CMM.
For example, lncRNA forkhead box D3 antisense RNA 1 was previously
discovered to increase CMM cell proliferation, invasion and
migration by regulating the miR-325/mitogen-activated protein
kinase kinase kinase 2 axis (11). In
addition, the lncRNA FOXF1 adjacent non-coding developmental
regulatory RNA was reported to inhibit the migration and invasion
of CMM cells (12). The lncRNA TINCR
ubiquitin domain containing (TINCR) was also found to function as a
tumor suppressor in various types of cancer. For instance, a
previous study revealed that Sp1 transcription factor-induced
upregulation of TINCR expression sponged miR-7-5p expression and
promoted the development of colorectal cancer (13). It has also been shown that the
activation of TINCR by the methylation of histone 3 on lysine 27
induced epithelial-mesenchymal transition in breast cancer by
targeting miR-125b (14).
Furthermore, TINCR was reported to serve a tumor suppressive role
over the proliferation, invasion and migration of prostate cancer
cells by regulating thyroid hormone receptor interactor 13
(15). However, to the best of our
knowledge, the precise function of TINCR in CMM remains unknown,
which will be investigated in the present study.
Materials and methods
Patient samples
In total, 60 patients with CMM (age range, 51–75
years; 46 men and 14 women) who underwent surgical resection at The
China-Japan Union Hospital of Ji Lin University (Jilin, China)
between December 2016 and January 2018 were enrolled in the current
study. The site of CMM was distributed on the back (38 cases), head
(11 cases), leg (7 cases), chest (2 cases), buttock (1 case) and
hand (1 case). CMM and adjacent normal tissues (2 cm from tumor
tissues) were extracted and immediately stored at −80°C. All
patients provided written informed consent prior to participation
in the study. The inclusion criteria were histopathological
confirmation of CMM. Exclusion criteria included uncontrolled
infections, autoimmune disease, the usage of systemic
corticosteroids and systemic therapy for CMM before the enrollment.
The current study protocol was approved by the Ethical Committee of
The China-Japan Union Hospital of Ji Lin University (approval no.
CJUHJLU20161102).
Cell lines and culture
Human CMM cell lines (M14, A375 and MV3) were
purchased from the American Type Culture Collection. Human
immortalized keratinocytes, HaCaT, were obtained from The Cell Bank
of Type Culture Collection of The Chinese Academy of Sciences.
HaCaT cells were authenticated by using STR profiling. Cells were
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and 1% penicillin/streptomycin (Sigma-Aldrich; Merck KGaA),
and maintained in an incubator with 5% CO2 at 37°C.
Bioinformatics analysis
The expression levels of TINCR in benign nevus,
atypical nevus, melanoma in situ, vertical growth phase
melanoma and metastatic growth phase melanoma tissues were analyzed
using data obtained from the GSE4587 dataset (16) in the Gene Expression Omnibus (GEO)
database (https://www.ncbi.nlm.nih.gov/gds/?term=). The
expression level of TINCR in 95 individuals, representing 27
different tissues, was retrieved from the Human Protein Atlas
RNA-sequencing (seq) normal tissues project published by Fagerberg
et al (17). Expression data
of TINCR in 558 normal and 461 skin cutaneous melanoma (SKCM)
tissues deposit in The Cancer Genome Atlas (TCGA) (TCGA-SKCM) were
downloaded from the Gene Expression Profiling Interactive Analysis
database (http://gepia.cancer.pku.cn) (18).
Cell transfection
pcDNA3.1 empty vector (control; 2 µg),
pcDNA3.1-TINCR vector (2 µg), miR-424-5p mimic (50 nM,
5′-CAGCAGCAAUUCAUGUUUUGAA-3′), miR-negative control (NC) mimic (50
nM, 5′-UCGCUUGGUGCAGGUCGGGAATT-3′), miR-424-5p inhibitor (50 nM,
5′-UCCAAAACAUGAAUUGCUGCUG-3′) or miR-NC inhibitor (50 nM,
5′-UUCUCCGAACGUGUCACGUTT-3′) (all from Guangzhou RiboBio Co., Ltd.)
were mixed with Lipofectamine® 3000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) in serum-free RPMI-1640 medium and
incubated for 15 min at room temperature. Following the incubation,
the mixture was added into each well of a 6-well plate
(1×106 cells/well) and incubated for 48 h at 37°C. A
total of 48 h after the transfection, the cells were harvested for
use in subsequent experiments.
For LATS1 knockdown, small interfering RNA (si)
control (siControl, 50 nM, 5′-UUCUCCGAACGUGUCACGUTT-3′) and siLATS1
(50 nM, 5′-GGUGAAGUCUGUCUAGCAA-3′) (both from Guangzhou RiboBio
Co., Ltd.) were mixed with Lipofectamine® RNAiMax
transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
in serum-free RPMI-1640 medium and incubated for 5 min at room
temperature. Following the incubation, the mixture was added into
each well of a 6-well plate (1×106 cells/well) and
incubated for 48 h at 37°C. A total of 48 h after the transfection,
the cells were harvested for use in subsequent experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from CMM tissues and cell
lines using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) and reverse transcribed into cDNA using a
PrimeScript RT reagent kit (Takara Bio, Inc.) according to the
manufacturer's instructions. qPCR was subsequently performed using
SYBR Premix Ex Taq (Takara Bio, Inc.) on a CFX96 Real-Time PCR
system (Bio-Rad Laboratories, Inc.). The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95°C for
1 min; followed by 45 cycles of denaturation at 95°C for 15 sec,
annealing at 60°C for 31 sec and elongation at 72°C for 30 sec, and
final extension at 72°C for 5 min. Expression levels were analyzed
using the 2−ΔΔCq method (19) and the relative expression levels of
TINCR, large tumor suppressor kinase 1 (LATS1), cellular
communication network factor 2 (CTGF), cellular communication
network factor 1 (CCN1), AXL receptor tyrosine kinase (AXL) and
miR-424-5p were normalized to GAPDH and U6, respectively. The
primer sequences are listed in Table
I.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Forward | Reverse |
|---|
| miR-424-5p |
5′-GCGGCGGCAGCAGCAATTCATG-3′ |
5′-ATCCAGTGCAGGGTCCGAGG-3′ |
| U6 |
5′-CTCGCTTCGGCAGCACATA-3′ |
5′-AACGATTCACGAATTTGCGT-3′ |
| TINCR |
5′-GGACAACCTTAGCGTGTTCA-3′ |
5′-TTGGATCAAAGAAGGGAAGG-3′ |
| LATS1 |
5′-AAACCAGGGAATGTGCAGCAA-3′ |
5′-CATGCCTCTGAGGAACTAAGGA-3′ |
| CTGF |
5′-AAAAGTGCATCCGTACTCCCA-3′ |
5′-CCGTCGGTACATACTCCACAG-3′ |
| CCN1 |
5′-GGTCAAAGTTACCGGGCAGT-3′ |
5′-GGAGGCATCGAATCCCAGC-3′ |
| AXL |
5′-ATCAGCTTCGGCTAGGCAG-3′ |
5′-TCCGCGTAGCACTAATGTTCT-3′ |
| GAPDH |
5′-GAAGGTGAAGGTCGGAGTC-3′ |
5′-GAAGATGGTGATGGGATTTC-3′ |
Western blotting
Total protein was extracted from A375 and MV3 cells
using RIPA lysis buffer (Beyotime Institute of Biotechnology).
Protein concentration was quantified using a BCA assay kit
(Beyotime Institute of Biotechnology) and 20 µg protein/lane was
separated via 10% SDS-PAGE. The separated proteins were
subsequently transferred onto PVDF membranes and blocked with 5%
non-fat milk for 2 h at room temperature. The membranes were then
incubated with the following primary antibodies at 4°C overnight:
Anti-LATS1 (1:1,000; cat. no. ab243656; Abcam), anti-phosphorylated
(p)-Yes 1 associated transcriptional regulator (YAP; 1:1,000; cat.
no. ab76252; Abcam), anti-YAP (1:1,000; cat. no. ab52771; Abcam)
and anti-β-actin (1:1,000; cat. no. ab8226; Abcam). Following the
primary antibody incubation, the membranes were incubated with
HRP-conjugated anti-mouse (1:10,000; cat. no. ab6289; Abcam) or
anti-rabbit (1:10,000; cat. no. ab6721; Abcam) secondary antibodies
for 2 h at room temperature. Protein bands were visualized using an
ECL reagent (Pierce; Thermo Fisher Scientific, Inc.) and
densitometric analysis was performed using ImageJ software (version
1.8.0; National Institutes of Health). β-actin was used as the
internal loading control.
Cell Counting Kit-8 (CCK-8) assay
The proliferation of A375 and MV3 cells was measured
following 1, 2, 3 and 4 days of transfection, respectively. After
the transfection, 10 µl CCK-8 reagent (DojindoDojindo Molecular
Technologies, Inc.) was added into each well and further incubated
for 4 h at 37°C, according to the manufacturer's instructions. The
optical density value was measured at a wavelength of 450 nm using
a microplate reader (Bio-Rad Laboratories, Inc.).
Flow cytometric analysis of
apoptosis
A375 and MV3 cells were seeded into 12-well plates
(1×105 cells/well) and cultured for 48 h at 37°C prior
to being harvested via digestion using 0.025% trypsin (Thermo
Fisher Scientific, Inc.). The cells were washed with PBS and
subsequently incubated with 5 µl Annexin V-FITC and 5 µl PI from an
Annexin V/Dead Cell Apoptosis kit (Thermo Fisher Scientific, Inc.)
for 15 min in the dark at 37°C. Apoptotic cells were analyzed
within 1 h using a FACSCalibur flow cytometer (BD Biosciences). The
results were analyzed using FlowJo software version 10.2 (FlowJo
LLC). The apoptotic rate was calculated as the percentage of early
+ late apoptotic cells. In total, 10,000 events were analyzed in
each group.
Transwell assay
The invasion of A375 and MV3 cells was analyzed
using Matrigel Transwell chambers (8-µm pore size; BD Biosciences).
Briefly, the upper Transwell chamber was precoated with 100 µl
Matrigel (BD Biosciences) at 37°C for 5 h. Then, 2×105
cells suspended in serum-free RPMI-1640 medium were plated into the
upper chamber. The lower chamber was filled with RPMI-1640 medium
supplemented with 20% FBS. Following 48 h of incubation, the cells
remaining in the upper chamber were removed, while the invasive
cells in the lower chamber were fixed with 4% formaldehyde for 15
min at room temperature and stained with 0.1% crystal violet for 20
min at room temperature. The invasive cells were visualized
(magnification, ×100) using a light microscope.
Dual luciferase reporter assay
The 3′untranslated region (UTR) of LATS1 was cloned
into pGL3 dual luciferase reporter vector (pGL3-LATS1; Promega
Corporation). The binding sites between TINCR and miR-424-5p were
predicted using miRDB (http://mirdb.org). The wild-type (WT) 3′UTR of TINCR
was cloned into a pGL3 vector (pGL3-TINCR-WT). In addition, two
site mutations were introduced into the pGL3-TINCR-WT sequence
using a Quick Site-Directed Mutation kit (Agilent Technologies,
Inc.) to generate the pGL3-TINCR-mutant (pGL3-TINCR-MUT) vector. To
study the association between miR-424-5p and TINCR, cells
(1×105 cells/well) were co-transfected with
pGL3-TINCR-WT (0.4 mg) or pGL3-TINCR-MUT (0.4 mg) and miR-424-5p
mimic (20 mM) or miR-NC mimic (20 mM) using Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 48 h. To
investigate the association between TINCR, miR-424-5p and LATS1,
cells were transfected with pGL3-LATS1 and miR-424-5p mimic or
miR-NC mimic and pcDNA3.1-TINCR. Following 48 h of transfection,
the relative firefly and Renilla luciferase activities were
determined using a Dual Luciferase Reporter assay system (Promega
Corporation). The firefly luciferase activity was normalized to
Renilla luciferase activity.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6.0 software (GraphPad Software, Inc.) and the data are
presented as the mean ± SD. Paired Student's t-tests were used to
determine the statistical differences between normal and tumor
tissues, while unpaired Student's t-tests were used to determine
statistical differences between two experimental groups of cells. A
one-way ANOVA followed by a Tukey's post hoc test was used to
determine the statistical differences between ≥3 groups. Pearson
correlation analysis was used to study the association between
expression levels of miR-424-5p and TINCR in CMM samples from
TCGA-SKCM using StarBase V2.0 (http://starbase.sysu.edu.cn/). This analysis was also
used to study the expression levels of miR-424-5p and TINCR in the
collected CMM samples. The categorical data in Table II were analyzed using a χ2
test. Every experiment was repeated three times. P<0.05 was
considered to indicate a statistically significant difference.
 | Table II.Association between
clinicopathological characteristics and TINCR expression in
patients with cutaneous malignant melanoma (n=60). |
Table II.
Association between
clinicopathological characteristics and TINCR expression in
patients with cutaneous malignant melanoma (n=60).
|
|
| TINCR |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factor | Patients
(n=60) | High (n=30) | Low (n=30) | P-value |
|---|
| Age, years |
|
|
| 0.601 |
|
≤60 | 35 | 16 | 19 |
|
|
>60 | 25 | 14 | 11 |
|
| Sex |
|
|
| 0.361 |
|
Male | 46 | 21 | 25 |
|
|
Female | 14 | 9 | 5 |
|
| TNM stage |
|
|
| 0.011a |
|
I–II | 13 | 11 | 2 |
|
|
III–IV | 47 | 19 | 28 |
|
| Distant
metastasis |
|
|
| 0.779 |
|
Yes | 18 | 8 | 10 |
|
| No | 42 | 22 | 20 |
|
Results
TINCR expression is downregulated in
CMM tissues
Analysis of RNA-seq data representing 27 different
tissues from 95 human individuals revealed that the lncRNA, TINCR,
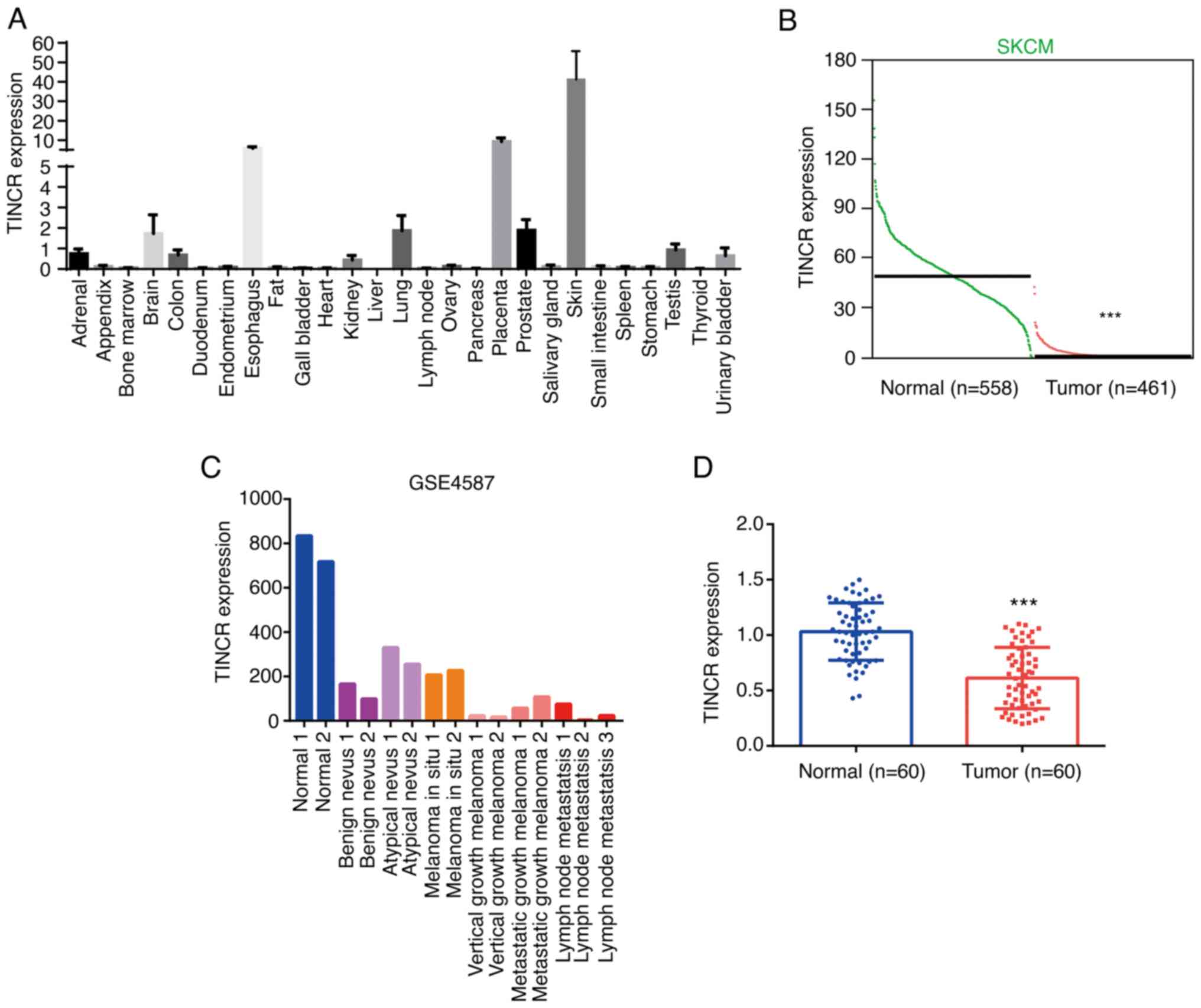
was found to be predominantly expressed in skin tissue (Fig. 1A). Analysis of the expression data of
TINCR in tumor and normal tissues from Gene Expression Profiling
Interactive Analysis database (http://gepia.cancer.pku.cn) identified that the
expression levels of TINCR were significantly downregulated in CMM
tissues (n=461) compared with the normal tissues (n=558) (Fig. 1B). Using the GSE4587 dataset, TINCR
expression was also found to be markedly downregulated in benign
nevus and melanoma in situ tissues, while the lowest TINCR
expression was observed in melanomas with lymph node metastasis
(Fig. 1C). Similar to these results,
in the tissues collected from patients with CMM, TINCR expression
was downregulated in tumor tissues compared with the adjacent
normal tissues (n=60; Fig. 1D).
Patients with CMM were divided into low (n=30) and high expression
(n=30) groups according to the median expression value of TINCR.
TINCR expression levels were not associated with age, sex or
distant metastasis, while low TINCR expression was associated with
an advanced TNM stage (Table II).
These results suggested that TINCR may play a tumor suppressive
role in the development of CMM.
TINCR overexpression reduces
proliferation and invasion, and induces apoptosis in CMM cell
lines
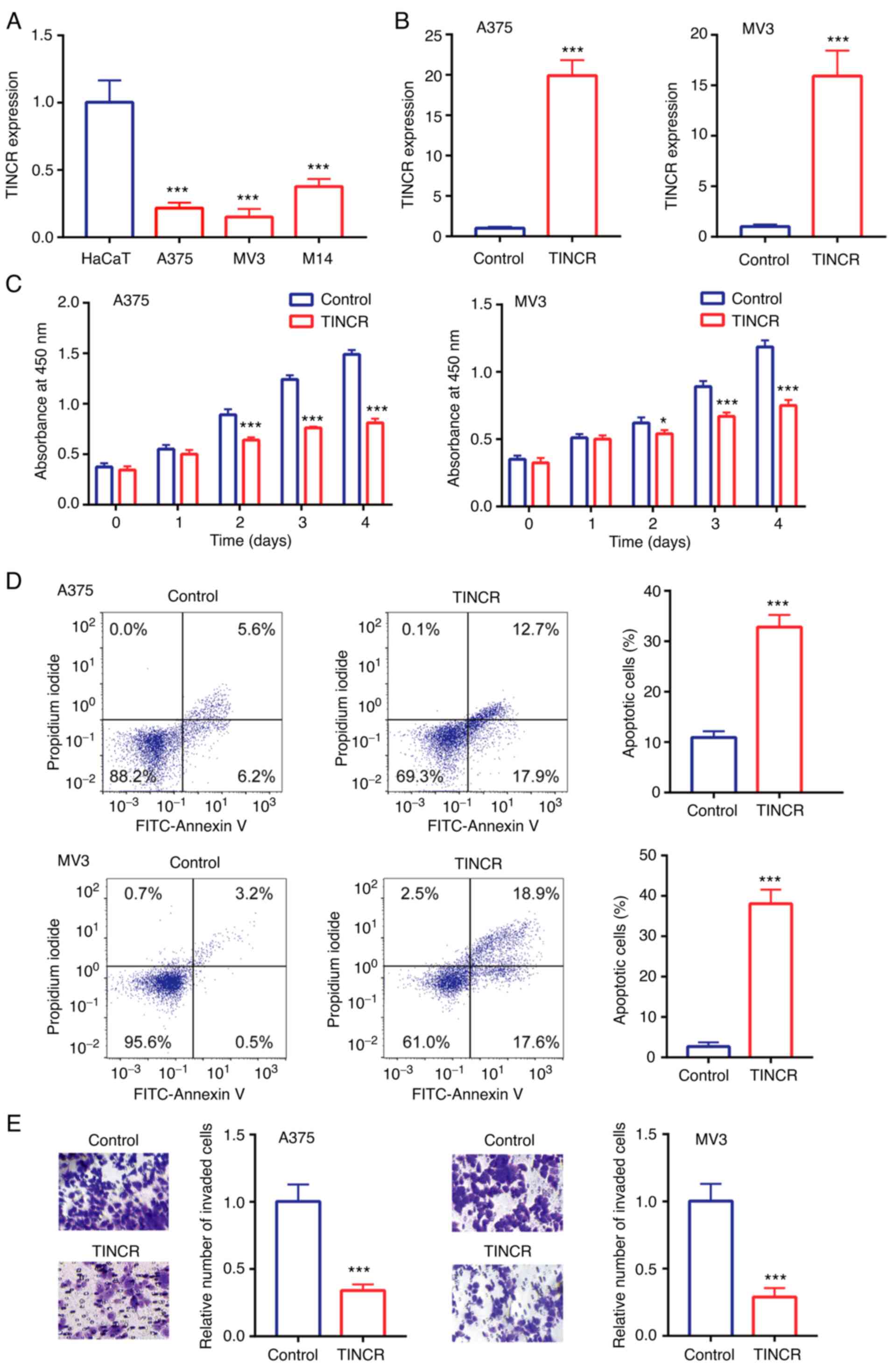
Compared with the human immortalized keratinocytes,
HaCaT, TINCR expression levels were significantly downregulated in
the human CMM cell lines, M14, A375 and MV3 (Fig. 2A). As A375 and MV3 cells showed
relatively lower TINCR expression levels compared with M14 cells,
these two cell lines were selected for use in the subsequent
experiments.
In A375 and MV3 cells, compared with the control
group, TINCR expression levels were upregulated following the
overexpression of TINCR (Fig. 2B). In
addition, the overexpression of TINCR significantly reduced cell
proliferation (Fig. 2C), induced cell
apoptosis (Fig. 2D) and reduced cell
invasion (Fig. 2E).
miR-424-5p interacts with TINCR in CMM
cell lines
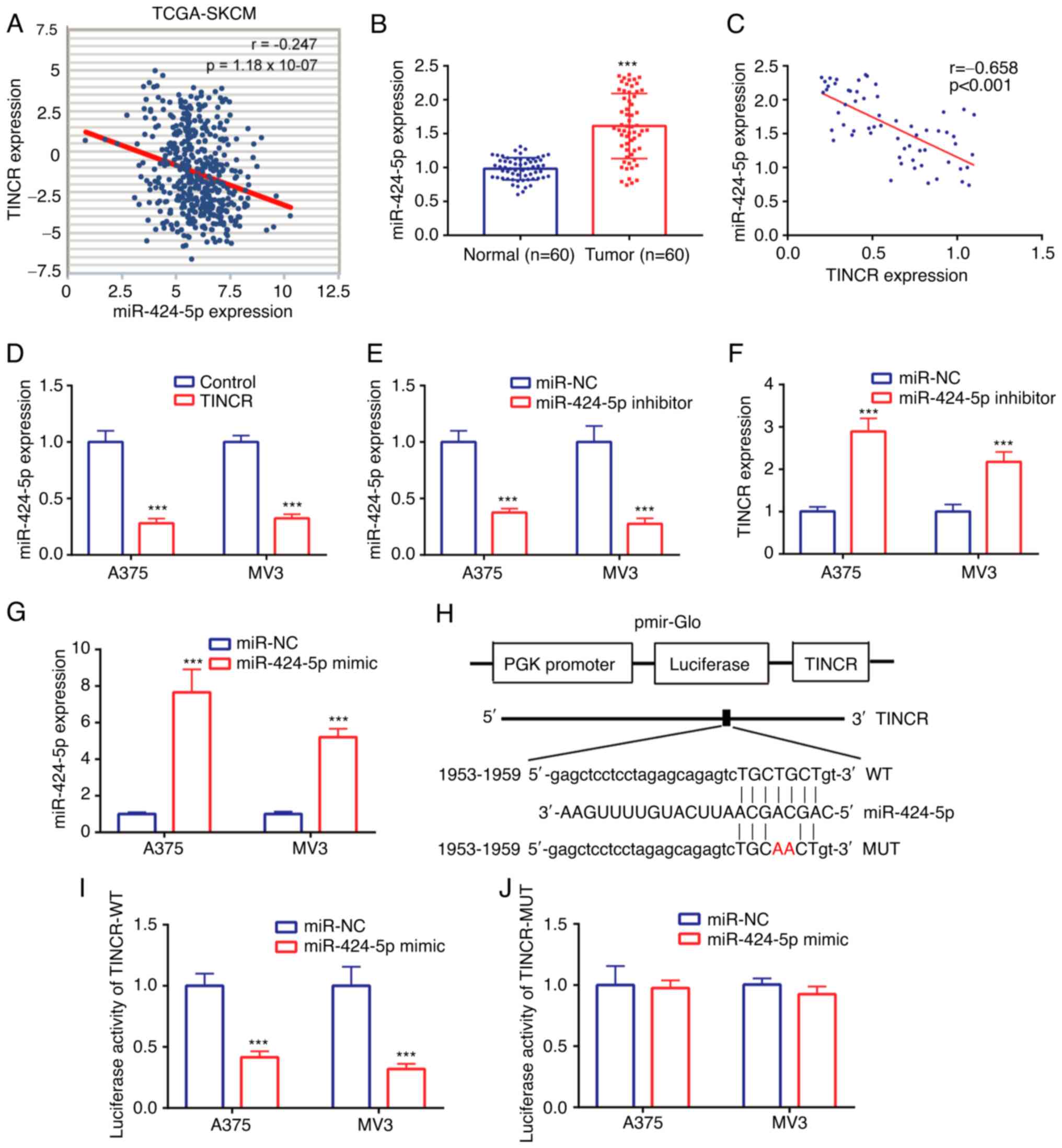
Data from TCGA-SKCM dataset identified a weak
negative correlation (r=−0.247) between TINCR and miR-424-5p
expression in CMM tissues (Fig. 3A).
In the tissues collected from patients with CMM, miR-424-5p
expression levels were found to be upregulated in CMM tissues
compared with the normal tissues (Fig.
3B). Similarly, a moderate negative correlation (r=−0.658) was
also noted between TINCR and miR-424-5p expression in the CMM
tissues (Fig. 3C).
The regulatory relationship between TINCR and
miR-424-5p was further investigated in CMM cell lines, A375 and
MV3. The expression levels of miR-424-5p were downregulated in the
TINCR overexpression group compared with the control group
(Fig. 3D). Next, the expression of
miR-424-5p was knocked down using a miR-424-5p inhibitor, and the
results revealed that the expression levels of miR-424-5p were
lower in the miR-424-5p inhibitor group compared with the miR-NC
inhibitor group (Fig. 3E).
Transfection with the miR-424-5p inhibitor upregulated TINCR
expression levels compared with the miR-NC inhibitor group
(Fig. 3F). These findings suggested
that TINCR and miR-424-5p may repress the expression of each other
in the CMM cell lines, A375 and MV3.
The relationship between TINCR and miR-424-5p was
further verified in CMM cell lines, A375 and MV3, using a dual
luciferase reporter assay. miR-424-5p was successfully
overexpressed using a miR-424-5p mimic, which was evidenced by the
higher miR-424-5p expression in the miR-424-5p mimic group compared
with the miR-NC mimic group (Fig.
3G). The binding sites between TINCR and miR-424-5p are
presented in Fig. 3H. In A375 and MV3
cells transfected with the pGL3-TINCR-WT vector, the relative
luciferase activity was discovered to be significantly decreased
following the co-transfection with the miR-424-5p mimic compared
with the miR-NC mimic (Fig. 3I).
However, in A375 and MV3 cells transfected with the pGL3-TINCR-MUT
vector, the relative luciferase activity was not significantly
different between the miR-424-5p mimic and miR-NC mimic groups
(Fig. 3J). These findings indicated
that miR-424-5p may interact with TINCR in the CMM cell lines, A375
and MV3.
TINCR upregulates LATS1 expression by
sponging miR-424-5p in CMM cell lines
In A375 and MV3 cells, TINCR overexpression
upregulated the mRNA expression levels of LATS1 (Fig. 4A), as well as the protein expression
levels of LATS1 and the p-YAP/YAP ratio, compared with the control
group (Fig. 4B and C). Next,
expression levels of YAP signaling-related molecules were detected.
In A375 and MV3 cells, TINCR overexpression downregulated the mRNA
expression levels of CTGF, CCN1 and AXL compared with the control
group (Fig. 4D and E). These results
suggested the potential positive regulatory relationships between
TINCR and LATS1, TINCR and the p-YAP/YAP ratio, as well as TINCR
and YAP signaling-related molecules.
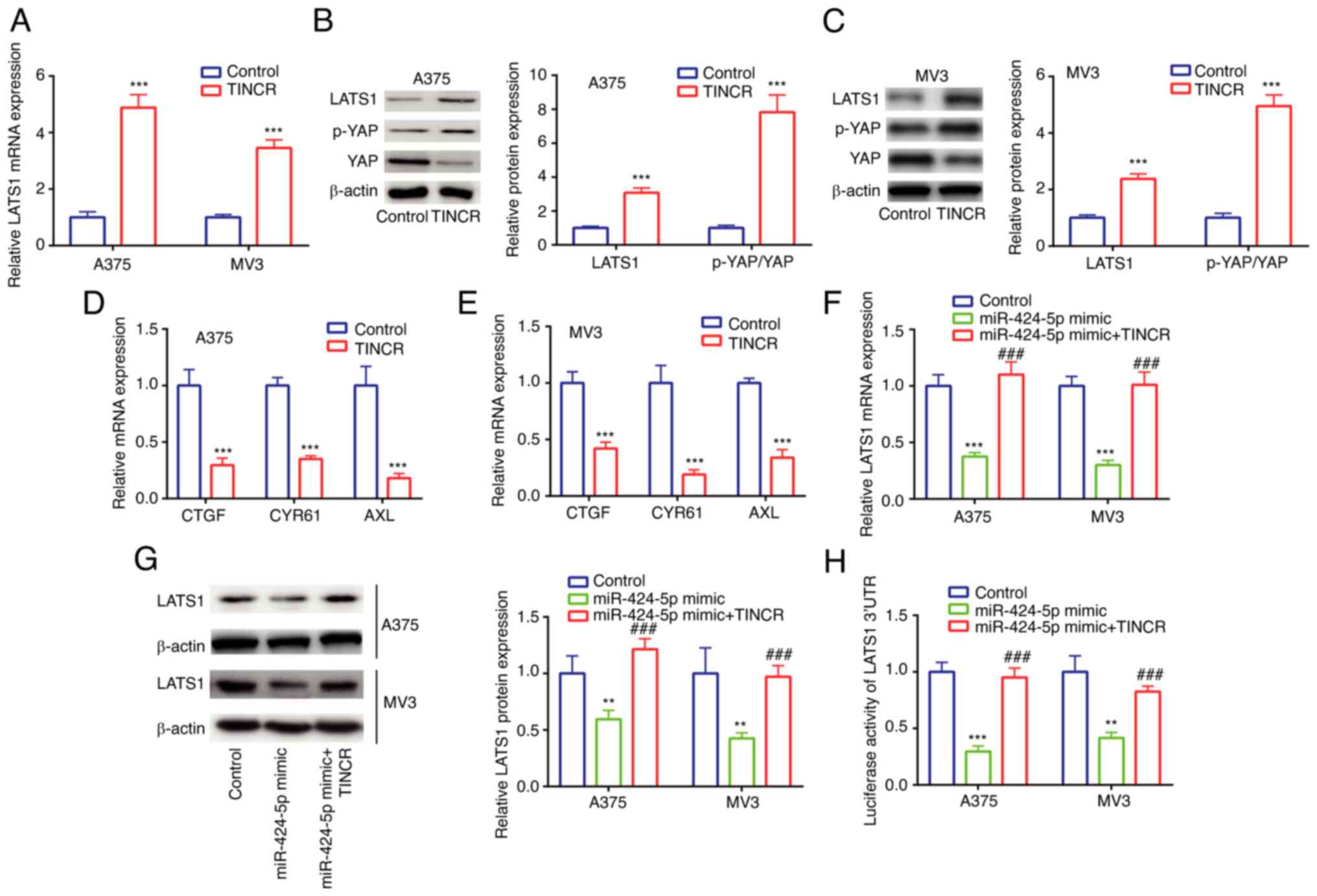 | Figure 4.TINCR promotes LATS1 expression by
sponging miR-424-5p in cutaneous malignant melanoma cell lines. (A)
LATS1 mRNA expression in control group and TINCR overexpression
group was determined via RT-qPCR. LATS1 protein expression and
p-YAP/YAP ratio in control group and TINCR overexpression group
were determined via western blotting in (B) A375 and (C) MV3 cells.
CTGF, CCN1 and AXL mRNA expression in control group and TINCR
overexpression group was determined via RT-qPCR in (D) A375 and (E)
MV3 cells. LATS1 (F) mRNA and (G) protein expression in control
group, miR-424-5p mimic group and miR-424-5p mimic + TINCR group
was determined via RT-qPCR and western blotting. (H) Luciferase
activity of LATS1 3′UTR in control group, miR-424-5p mimic group
and miR-424-5p mimic + TINCR group was determined using a dual
luciferase activity assay. **P<0.01, ***P<0.001 vs. control
(pcDNA3.1, miR-NC mimic + pcDNA3.1); ###P<0.001 vs.
miR-424-5p mimic. miR, microRNA; RT-qPCR, reverse
transcription-quantitative PCR; TINCR, TINCR ubiquitin domain
containing; LATS1, large tumor suppressor kinase 1; p-,
phosphorylated; YAP, Yes 1 associated transcriptional regulator;
UTR, untranslated region; CTGF, cellular communication network
factor 2; CCN1, cellular communication network factor 1; AXL, AXL
receptor tyrosine kinase. |
In A375 and MV3 cells, compared with the miR-NC
mimic group, transfection with the miR-424-5p mimic significantly
downregulated the mRNA and protein expression levels of LATS1,
which were then rescued by TINCR overexpression (Fig. 4F and G). Similar trends were also
observed in the relative luciferase activity of the LATS1 3′UTR
vector (Fig. 4H). These findings
suggested the existence of a regulatory relationship among TINCR,
miR-424-5p and LATS1.
TINCR reduces the proliferation and
invasion, and induces the apoptosis of CMM cell lines by regulating
LATS1 expression
In A375 and MV3 cells, compared with the siControl
group, siLATS1 downregulated the mRNA expression levels of LATS1
(Fig. 5A). Moreover, in A375 and MV3
cells, compared with the control group, TINCR overexpression
upregulated the mRNA expression levels of LATS1, which were
reversed following the co-transfection with siLATS1 (Fig. 5B). These findings further indicated
the positive relationship between TINCR and LATS1 in CMM cell
lines, A375 and MV3.
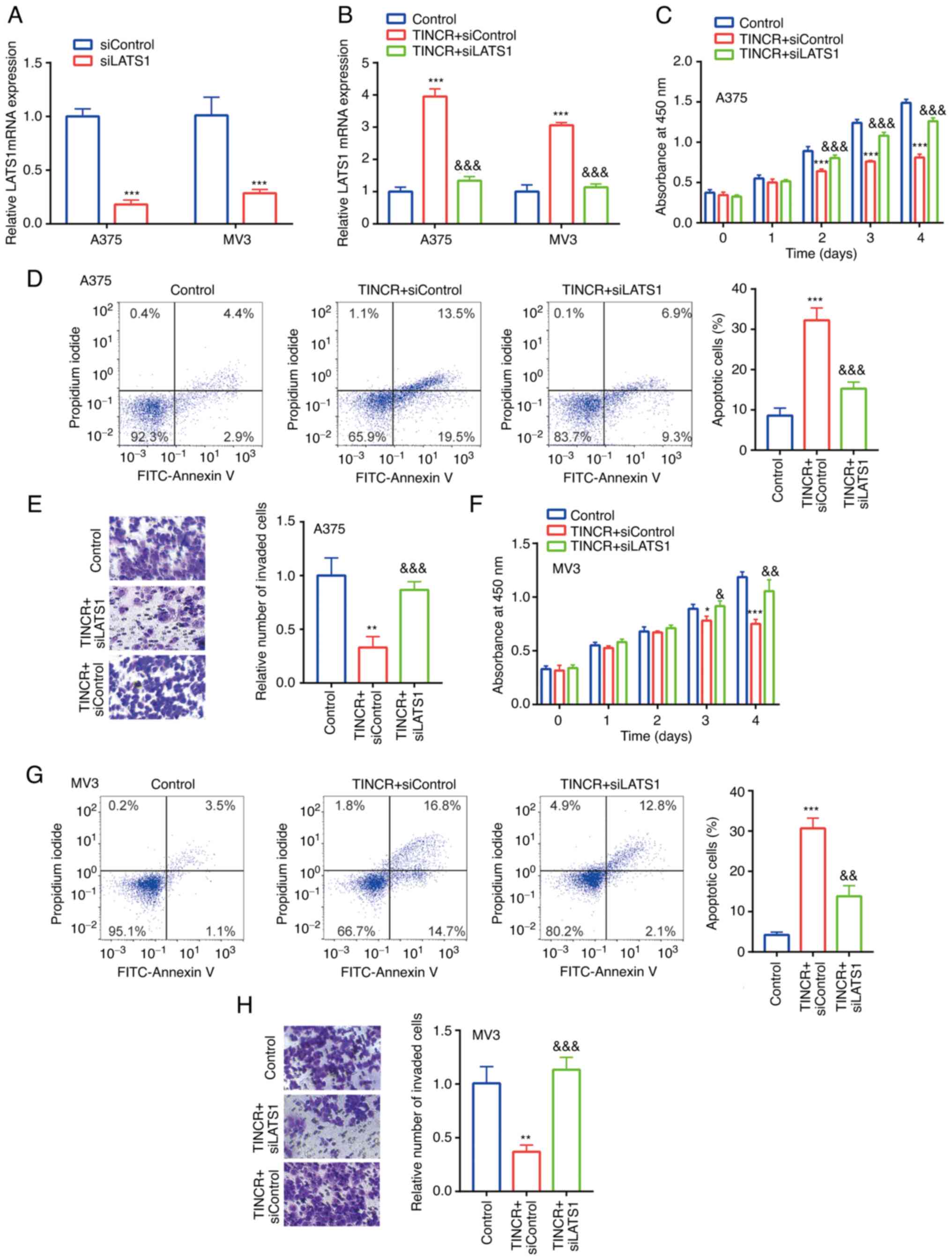 | Figure 5.TINCR reduces the proliferation and
induces the apoptosis of CMM cell lines by regulating LATS1. (A)
LATS1 mRNA expression in siControl group and siLATS1 group was
determined via RT-qPCR. ***P<0.001 vs. siControl. (B) LATS1 mRNA
expression in control group, TINCR + siControl group and TINCR +
siLATS1 group was determined via RT-qPCR. (C) In A375 cells,
proliferation in control group, TINCR + siControl group and TINCR+
siLATS1 group was examined using a Cell Counting Kit-8 assay. (D)
Cell apoptosis in control group, TINCR + siControl group and TINCR+
siLATS1 group was examined via flow cytometry. (E) Cell invasion in
control group, TINCR + siControl group and TINCR + siLATS1 group
was examined using Transwell assays (magnification, ×100). (F) In
MV3 cells, proliferation in control group, TINCR + siControl group
and TINCR + siLATS1 group was examined using a Cell Counting Kit-8
assay. (G) Cell apoptosis in control group, TINCR + siControl group
and TINCR + siLATS1 group was examined via flow cytometry. (H) Cell
invasion in control group, TINCR + siControl group and TINCR +
siLATS1 group was examined using a Transwell assay (magnification,
×100). *P<0.05, **P<0.01, ***P<0.001 vs. control (pcDNA3.1
+ siControl); &P<0.05,
&&P<0.01,
&&&P<0.001 vs. TINCR + siControl.
RT-qPCR, reverse transcription-quantitative PCR; TINCR, TINCR
ubiquitin domain containing; LATS1, large tumor suppressor kinase
1; si, small interfering RNA. |
The functions of TINCR and LATS1 in CMM cell lines
were subsequently investigated. In A375 cells, compared with the
control group, TINCR overexpression significantly reduced
proliferation (Fig. 5C), induced
apoptosis (Fig. 5D) and decreased
invasion (Fig. 5E), which were all
partially reversed following the co-transfection with siLATS1.
Similar effects were observed in the proliferation (Fig. 5F), apoptosis (Fig. 5G) and invasion (Fig. 5H) of MV3 cells following combined
TINCR overexpression and siLATS1 transfection.
Discussion
The metastasis of melanoma further promotes the
progression of the disease (20). The
results of the present study revealed that TINCR expression levels
were downregulated in CMM tissues from the GSE4587 dataset obtained
from the GEO database, as well as in collected CMM tissues. Low
expression of TINCR was observed in tumors of advanced stage in
comparison with those of early stage. TINCR overexpression was
found to decrease the proliferation and invasion, and induce the
apoptosis of CMM cell lines, indicating the potential tumor
suppressive role of TINCR in CMM.
It is well-known that lncRNAs regulate gene
expression by promoting RNA degradation, miRNA sequestration and
transcriptional and translational activation or repression
(10). Using next generation
sequencing, a previous study discovered that TINCR was negatively
correlated with miR-424-5p expression in CMM tissues, which was
found to be associated with the invasive and aggressive phenotype
of CMM (21). However, to the best of
our knowledge, studies reporting the precise function of miR-424-5p
or the correlation between TINCR and miR-424-5p in the development
of CMM have not been conducted.
miR-424-5p has been shown to function as an oncogene
in numerous cancer types. For example, miR-424-5p promoted lung
metastasis in thyroid cancer via inactivation of the Hippo
signaling pathway (22); miR-424-5p
increased cell proliferation in gastric cancer by targeting Smad3
via regulation of TGF-β signaling (23); and serum miR-424-5p expression levels
were found to be increased in patients with colorectal cancer
(24). Consistent with the reported
oncogenic role of miR-424-5p in the aforementioned cancer types,
miR-424-5p expression levels were also discovered to be upregulated
in CMM tissues in the current study. In addition, miR-424-5p was
identified to interact with TINCR in CMM cell lines, indicating the
oncogenic role of miR-424-5p in the progression of CMM. Based on
these results, the expression of target genes of miR-424-5p that
were regulated by TINCR were analyzed.
LATS1 was previously identified as a target of
miR-424-5p (25) and was reported to
serve a tumor suppressive role in numerous cancer types; for
example, LATS1 suppressed the development of breast cancer by
maintaining cell identity (26);
miR-103a-3p induced the malignant progression of thyroid cancer via
Hippo signaling by targeting LATS1 (27); and previous mutation analyses on LATS1
highlighted its tumor suppressive role in numerous human cancer
types, including stomach adenocarcinoma, uterine corpus endometrial
carcinoma and bladder urothelial carcinoma (28). The results of the present study
revealed that LATS1 expression was positively regulated by TINCR in
CMM cell lines. Subsequently, the functional relationship between
TINCR and LATS1 in CMM cell lines was investigated.
LATS1 is a core member of the Hippo/YAP signaling
pathway (29). The Hippo/YAP
signaling pathway is known to regulate cell proliferation and
invasion (30–32). AXL, CTGF and CCN1 are all downstream
molecules that are associated with the Hippo/YAP signaling pathway
(33). The findings of the current
study demonstrated that TINCR overexpression activated Hippo
signaling and repressed the activity of YAP, as well as the
expression levels of AXL, CTGF and CCN1 by regulating LATS1
expression in CMM cells. Rescue assays were performed and the
results revealed that LATS1 knockdown could reverse the effect of
TINCR overexpression on the proliferation, invasion and apoptosis
of CMM cells.
However, there remains a limitation to the present
study. For instance, there were differences between cell lines in
protein expression levels reported in western blot analyses, which
could be attributed possibly to morphology, gene expression or
other cell line characteristics. This will be addressed in future
work in more detail.
In conclusion, the present data suggested that TINCR
may attenuate the proliferation and invasion, and enhance the
apoptosis of CMM cells by regulating the miR-424-5p/LATS1 signaling
axis. These results suggested that TINCR may play a tumor
suppressive role in CMM.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH, YJ, XC and CS performed the experimentations and
data analysis. XH, YJ and JS designed the experiments. XH and JS
were responsible for confirming the authenticity of the raw data.
JS supervised the experimentations and prepared the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent prior
to participation in the study. The current study protocol was
approved by the Ethical Committee of The China-Japan Union Hospital
of Ji Lin University (approval no. CJUHJLU20161102).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miller AJ and Mihm MC Jr: Melanoma. N Engl
J Med. 355:51–65. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu Y, Wang Y, Wang L, Yin P, Lin Y and
Zhou M: Burden of melanoma in China, 1990–2017: Findings from the
2017 global burden of disease study. Int J Cancer. 147:692–701.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aubuchon MM, Bolt LJ, Janssen-Heijnen ML,
Verleisdonk-Bolhaar ST, van Marion A and van Berlo CL:
Epidemiology, management and survival outcomes of primary cutaneous
melanoma: A ten-year overview. Acta Chir Belg. 117:29–35. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karlsson P, Boeryd B, Sander S, Westermark
P and Rosdahl I: Increasing incidence of cutaneous malignant
melanoma in children and adolescents 12–19 years of age in Sweden
1973–92. Acta Derm Venereol. 78:289–292. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Veierød MB, Weiderpass E, Thörn M, Hansson
J, Lund E, Armstrong B and Adami HO: A prospective study of
pigmentation, sun exposure, and risk of cutaneous malignant
melanoma in women. J Natl Cancer Inst. 95:1530–1538. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lees VC and Briggs JC: Effect of initial
biopsy procedure on prognosis in Stage 1 invasive cutaneous
malignant melanoma: Review of 1086 patients. Br J Surg.
78:1108–1110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tseng WW, Fadaki N and Leong SP:
Metastatic tumor dormancy in cutaneous melanoma: Does surgery
induce escape? Cancers (Basel). 3:730–746. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JH, Hahn EW and Tokita N: Combination
hyperthermia and radiation therapy for cutaneous malignant
melanoma. Cancer. 41:2143–2148. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li PF, Chen SC, Xia T, Jiang XM, Shao YF,
Xiao BX and Guo JM: Non-coding RNAs and gastric cancer. World J
Gastroenterol. 20:5411–5419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moran VA, Perera RJ and Khalil AM:
Emerging functional and mechanistic paradigms of mammalian long
non-coding RNAs. Nucleic Acids Res. 40:6391–6400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Gao J, Yu Y, Zhao Z and Pan Y:
LncRNA FOXD3-AS1 promotes proliferation, invasion and migration of
cutaneous malignant melanoma via regulating miR-325/MAP3K2. Biomed
Pharmacother. 120:1094382019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen XE, Chen P, Chen S, Lu J, Ma T, Shi G
and Sheng L: Long non-coding RNA FENDRR inhibits migration and
invasion of cutaneous malignant melanoma cells. Biosci Rep.
40:BSR201911942020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu S, Wang D, Shao Y, Zhang T, Xie H,
Jiang X, Deng Q, Jiao Y, Yang J, Cai C and Sun L: SP1-induced
lncRNA TINCR overexpression contributes to colorectal cancer
progression by sponging miR-7-5p. Aging. 11:1389–1403. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong H, Hu J, Zou K, Ye M, Chen Y, Wu C,
Chen X and Han M: Activation of LncRNA TINCR by H3K27 acetylation
promotes Trastuzumab resistance and epithelial-mesenchymal
transition by targeting MicroRNA-125b in breast cancer. Mol Cancer.
18:32019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong L, Ding H, Li Y, Xue D and Liu Y:
LncRNA TINCR is associated with clinical progression and serves as
tumor suppressive role in prostate cancer. Cancer Manag Res.
10:2799–2807. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith AP, Hoek K and Becker D:
Whole-genome expression profiling of the melanoma progression
pathway reveals marked molecular differences between nevi/melanoma
in situ and advanced-stage melanomas. Cancer Biol Ther. 9:1018–29.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fagerberg L, Hallström BM, Oksvold P,
Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S,
Danielsson A, Edlund K, et al: Analysis of the human
tissue-specific expression by genome-wide integration of
transcriptomics and antibody-based proteomics. Mol Cell Proteomics.
13:397–406. 2014. View Article : Google Scholar
|
|
18
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PcR and
the 2(-delta deltac(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mo J, Zhao X, Dong X, Liu T, Zhao N, Zhang
D, Wang W, Zhang Y and Sun B: Effect of EphA2 knockdown on melanoma
metastasis depends on intrinsic ephrinA1 level. Cell Oncol (Dordr).
43:655–667. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Babapoor S, Wu R, Kozubek J, Auidi D,
Grant-Kels JM and Dadras SS: Identification of microRNAs associated
with invasive and aggressive phenotype in cutaneous melanoma by
next-generation sequencing. Lab Invest. 97:636–648. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu X, Fu Y, Zhang G, Zhang D, Liang N, Li
F, Li C, Sui C, Jiang J, Lu H, et al: miR-424-5p promotes anoikis
resistance and lung metastasis by inactivating Hippo signaling in
thyroid cancer. Mol Ther Oncolytics. 15:248–260. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wei S, Li Q, Li Z, Wang L, Zhang L and Xu
Z: miR-424-5p promotes proliferation of gastric cancer by targeting
Smad3 through TGF-β signaling pathway. Oncotarget. 7:75185–75196.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sahami-Fard MH, Kheirandish S and Sheikhha
MH: Expression levels of miR-143-3p and −424-5p in colorectal
cancer and their clinical significance. Cancer Biomark. 24:291–297.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Liu H, Hou L, Wang G, Zhang R,
Huang Y, Chen X and Zhu J: Circular RNA_LARP4 inhibits cell
proliferation and invasion of gastric cancer by sponging miR-424-5p
and regulating LATS1 expression. Mol Cancer. 16:1512017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Furth N, Pateras IS, Rotkopf R, Vlachou V,
Rivkin I, Schmitt I, Bakaev D, Gershoni A, Ainbinder E, Leshkowitz
D, et al: LATS1 and LATS2 suppress breast cancer progression by
maintaining cell identity and metabolic state. Life Sci Alliance.
1:e2018001712018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang ML, Sun WH, Wu HQ, Liu ZD and Wang
P: Knockdown of microRNA-103a-3p inhibits the malignancy of thyroid
cancer cells through Hippo signaling pathway by upregulating LATS1.
Neoplasma. 67:1266–1278. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu T, Bachman J and Lai ZC: Mutation
analysis of large tumor suppressor genes LATS1 and LATS2 supports a
tumor suppressor role in human cancer. Protein Cell. 6:6–11. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Wang G, Chu SJ, Zhu JS, Zhang R,
Lu WW, Xia LQ, Lu YM, Da W and Sun Q: Loss of large tumor
suppressor 1 promotes growth and metastasis of gastric cancer cells
through upregulation of the YAP signaling. Oncotarget.
7:16180–16193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ehmer U and Sage J: Control of
proliferation and cancer growth by the hippo signaling pathway. Mol
Cancer Res. 14:127–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Janse van Rensburg HJ and Yang X: The
roles of the hippo pathway in cancer metastasis. Cell Signal.
28:1761–1772. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu S, Zhang M, Huang L, Ma Z, Gong X, Liu
W, Zhang J, Chen L, Yu Z, Zhao W and Liu Y: ERK1 indicates good
prognosis and inhibits breast cancer progression by suppressing
YAP1 signaling. Aging. 11:12295–12314. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brodowska K, Al-Moujahed A, Marmalidou A,
Horste M, Cichy J, Miller J, Evangelos E and Vavvas D: The
clinically used photosensitizer Verteporfin (VP) inhibits YAP-TEAD
and human retinoblastoma cell growth in vitro without light
activation. Exp Eye Res. 124:67–73. 2014. View Article : Google Scholar : PubMed/NCBI
|