Introduction
Breast cancer (BC) is the most common malignant
tumor among women throughout the world (1). The development of adjuvant therapy
has improved the prognosis of patients with BC. Indeed, the 5-year
overall survival (OS) rate of BC patients without metastasis
currently exceeds 80% (2).
However, 20–30% of patients with BC develop metastases after
primary tumor treatment (3).
Patients with recurrent BC are classified according to the
immunohistochemical detection of conventional target molecules such
as the estrogen receptor (ER), progesterone receptor (PgR), and
human epidermal growth factor receptor 2 (HER2). Although various
drugs have been developed and are available for the treatment of
patients with recurrent BC, they are still insufficient to cure and
only 5% of those patients achieve long-term disease control
(4). From this point of view,
development of new biomarkers or therapeutic target molecules for
the purpose of improving the prognosis of BC patients is
required.
Phosphofructokinase (PFK), which catalyzes the
formation of fructose 1,6-bisphosphate and adenosine diphosphate
from fructose 6-phosphate and adenosine triphosphate, is one of the
key regulating enzymes in the glycolytic pathway (5). PFK is a complex tetrameric enzyme
that has three isoforms: Liver (PFKL), muscle (PFKM), and platelet
(PFKP) (6). The activity of PFK is
regulated by quantitative and isozymic changes secondary to altered
gene expression during neoplastic transformation (7). Among the three isoforms, the
expression and regulatory mechanisms of PFKP have been
studied in several malignancies, including brain tumor, renal and
bladder cancer, in which the increased expression of PFKP
has been associated with the progression of cancer cells (8–10).
In BC cells, hypoxia inducible factor 1 subunit α, a
major transcriptional regulator of the cellular response to
hypoxia, and kruppel-like factor 4, a transcription factor that
regulates the expression of several genes involved in cell cycle
regulation and differentiation, activated the transcription of
PFKP and enhanced glycolytic metabolism (7,11).
Furthermore, in a triple-negative BC cell line, PFKP regulated
extracellular lactate production via lactate dehydrogenase A enzyme
(12). However, it has not been
evaluated whether PFKP promotes malignant features of BC across
subtypes. The present study aimed to investigate the functional
roles of PFKP in BC cells and the significance of PFKP
expression in patients with BC.
Materials and methods
Sample collection
A total of 13 BC cell lines (BT-20, BT-474, BT-549,
HCC1419, HCC1954, Hs578T, MCF7, MDA-MB-231, MDA-MB-361, MDA-MB-415,
MDA-MB-468, SK-BR-3, and ZR-75-1) and two non-cancerous breast
epithelial cell lines (MCF-10A and MCF-12A) were obtained. BT-549,
HCC1419, HCC1954 and Hs578T cell lines were purchased from the
Japanese Collection of Research Bioresources Cell Bank. BT-474,
MCF7, and MCF-12A were kindly provided by Professor David Sidransky
from Johns Hopkins University (Baltimore, USA). The other cell
lines were all purchased from the American Type Culture Collection.
All cell lines were stored at −80°C using a cell preservation
solution (CELLBANKER®; Mitsubishi Chemical Medicine
Corporation) and cultured in RPMI-1640 medium (Sigma-Aldrich; Merck
KGaA) supplemented with 10% fetal bovine serum (FBS; Corning, Inc.)
and incubated in an atmosphere of 5% carbon dioxide at 37°C
(13).
Primary BC and non-cancerous specimens were also
collected from 167 patients histologically diagnosed with BC after
undergoing surgery at Nagoya University Hospital (Nagoya, Japan)
from March 2002 to May 2007. Surveillance data for more than five
years after surgery for all 167 patients were available. All
specimens were immediately resected to a diameter of approximately
1.5 mm and stored at −80°C. Non-cancerous specimens were resected
≥3 cm from the edge of the tumor (14). All specimens were histologically
diagnosed as BC and classified using the Union for International
Cancer Control (UICC) staging system (8th edition). Postoperative
adjuvant therapy was determined on the basis of the condition of
the patient, pathological features, cancer subtype, and discretion
of physicians (14).
A total of 167 female patients were enrolled in the
present study; there were no male participants. The median age was
52 years (range, 26–78 years). The median follow-up duration was
100 months (range, 8–155 months), including fatalities. The tumor
(T) categories were Tis (ductal carcinoma in situ), 7; T1,
70; T2, 75; T3, 9; and T4, 6. A total of 82 patients (49%) had
lymph node metastases. The UICC stages were as follows: Stage 0, 7;
stage I, 47; stage II, 78; stage III, 34; and stage IV, 1. Among
the 167 patients, 127 (76%) were ER-positive and 40 (24%) were
ER-negative. There were 115 (69%) PgR-positive and 52 (31%)
PgR-negative patients. A total of 39 patients (23%) were
HER2-positive and 119 patients were (71%) HER2-negative. A total of
9 patients had unknown HER2 status. Of the 167 patients, 12
patients received neoadjuvant chemotherapy, such as anthracycline
and taxane. Tumor response to neoadjuvant chemotherapy was assessed
with the use of Response Evaluation Criteria in Solid Tumors,
version 1.1 (15); partial
response occurred in 8 patients and stable disease occurred in 4
patients. None of the patients had a pathological complete
response.
The present study was conducted in accordance with
the principles of the Declaration of Helsinki and was approved
(approval no. 2019-0028) by the Institutional Review Board and
Ethics Committee of Nagoya University Hospital. All patients
provided written informed consent for the use of their clinical
samples and data.
Reverse transcription-quantitative polymerase
chain reaction (RT-qPCR). PFKP mRNA expression levels were
evaluated by RT-qPCR. RNA was extracted from cell lines
(8×106 cells per cell line) using RNeasy Mini Kit
(Qiagen GmbH), as well as from BC and non-cancerous specimens from
167 patients. cDNA was synthesized as previously described
(13). Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) mRNA levels were quantified to normalize
expression levels. The primers specific for each gene were as
follows: PFKP forward, 5′-GGGCCAAGGTGTACTTCATC-3′ and
reverse, 5′-TGGAGACACTCTCCCAGTCG-3′ (which generated a 90-bp
product); GAPDH forward, 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse,
5′-GAAGATGGTGATGGGATTTC-3′ (which generated a 226-bp product)
(14). RT-qPCR was performed using
an ABI StepOnePlus real-time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) as previously described (13). Each cell line sample was examined
three times. The mRNA relative expression level of PFKP was
obtained by dividing the value of each sample by the corresponding
GAPDH value (13).
PCR array analysis
To determine the correlation between the expression
levels of PFKP and 84 cancer-related genes in BC cell lines,
PCR array analysis was conducted using RT2 Profiler PCR
Array Human Oncogenes and Tumor Suppressor Genes (cat. no. 330231;
GeneGlobe ID PAHS-502Z; Qiagen GmbH) according to the
manufacturer's protocol. The relative expression levels of these
genes in each sample were obtained by dividing them by the
corresponding GAPDH value.
PFKP knockdown using PFKP-specific
small interfering RNAs (siRNAs)
For PFKP knockdown, MCF7, SK-BR-3, and
MDA-MB-231 cell lines were transfected with three types of siRNAs
specific for PFKP, named ‘siPFKP’. Their sequences
were as follows: siPFKP−1, 5′-UAUUAAUGUCAAUAAUACGUG-3′;
siPFKP−2, 5′-GGAGCAAUUGAUACCCAAATT-3′; and siPFKP−3,
5′-GGAUCACUGCAAAACUCAATT-3′ (Hokkaido System Science Co., Ltd.).
AccuTarget™ Fluorescein-labeled Negative Control siRNA (cat. no.
SN-1023; Cosmo Bio Co., Ltd.) served as the control nontargeting
siRNA, named ‘siControl’. BC cells were seeded in antibiotic-free
RPMI-1640 medium supplemented with 10% FBS; a total of 24 h after
seeding, cells were transfected with the corresponding siRNAs (80
pmol for 6-well plates and 400 pmol for 10-cm dishes) in the
presence LipoTrust EX Oligo (Hokkaido System Science Co., Ltd.).
After transfection, cells were cultured in antibiotic-free
RPMI-1640 medium with 10% FBS in an atmosphere of 5% carbon dioxide
at 37°C for 72 h. Knockdown efficiency was determined using
RT-qPCR.
Western blot analysis
Western blotting was performed by the Simple Western
technique using the WES instrument (ProteinSimple), according to
the manufacturer's protocol. Cells were incubated in RIPA lysis
buffer, and the lysates were stored at −30°C. Protein concentration
was assessed using a BCA protein assay kit (Thermo Fisher
Scientific, Inc.). Using a 12–230 kDa Separation module (cat. no.
SM-W004; ProteinSimple), protein samples (6 µg/lane), biotin
ladder, primary antibody, secondary antibody, blocking reagent,
chemiluminescent substrate, and wash buffer were prepared and
dispensed into the assay plate. Antibody Diluent II (cat. no.
042-203; ProteinSimple) attached to the Detection module was used
as a blocking reagent. Then, the assay plate was loaded into the
instrument, and the protein was separated into individual
capillaries. Using the instrument, the protein was anchored to the
inner wall of the capillary. Protein separation and detection was
performed automatically on individual capillaries. The duration of
incubation of the primary and secondary antibodies was 30 min at
room temperature. Detection was performed by chemiluminescence with
luminol (cat. no. 043-311) and peroxide (cat. no. 043-379; both
from ProteinSimple) attached to the Detection module. Anti-PFKP
antibody (1:50; product no. 12746; Cell Signaling Technology, Inc.)
and anti-β-actin antibody (1:50; cat. no. ab6276; Abcam) were used
as primary antibodies. Streptavidin HRP (cat. no. 042-414) and
anti-mouse or anti-rabbit secondary antibodies (anti-mouse, cat.
no. 042-205; and anti-rabbit, cat. no. 042-206) (all from
ProteinSimple) were selected according to the corresponding primary
antibody (16,17).
Proliferation assay
Cell proliferation was evaluated using the Cell
Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies, Inc.). MCF7
(3×103 cells/well), SK-BR-3 (3×103
cells/well), and MDA-MB-231 (3×103 cells/well) cells,
which were transfected with siPFKP or siControl, were seeded
into 96-well plates with RPMI-1640 medium containing 10% FBS and 1%
antibiotic [Antibiotic-Antimycotic (100X); Thermo Fisher
Scientific, Inc.]. Each sample was applied to six wells, and the
optical density (450 nm) of each well was measured 2 h after adding
10 µl of CCK-8 solution up to 5 days after seeding (14).
Invasiveness assay
Invasiveness in Matrigel was determined using
BioCoat Matrigel Invasion Chambers (pore size 8-µm; Corning Inc.)
according to the manufacturer's protocol. Each chamber was
precoated with 500 µl of serum-free RPMI-1640 medium in an
atmosphere of 5% carbon dioxide at 37°C for 2 h. After
transfection, MCF7 (2.5×104 cells/well), SK-BR-3
(2.5×104 cells/well), and MDA-MB-231 (2.5×104
cells/well) cells were suspended in 750 µl of serum-free RPMI-1640
medium and seeded in the upper chambers. The lower chamber was
filled with 750 µl of RPMI-1640 medium containing 10% FBS and 1%
antibiotics. After 72 h of incubation in an atmosphere of 5% carbon
dioxide at 37°C, cells were fixed with 99% methanol for 5 sec at
room temperature, and stained with Solution I and II in Diff Quik
(cat. no. 16920; Sysmex) for 5 sec at room temperature. Cells on
the membrane were counted in ten randomly selected microscopic
fields with a magnification of ×100 using an upright microscope
(Olympus Corporation) (14).
Migration assay
Migration of MCF7, SK-BR-3, and MDA-MB-231 cells was
determined using a gap closure assay. After transfection, MCF7
(4.9×104 cells/well), SK-BR-3 (4.9×104
cells/well), and MDA-MB-231 (4.9×104 cells/well) cells
were seeded into each well of a 35-mm dish with a culture insert
with a cell-free gap width of 500-µm (Ibidi GmbH) using RPMI-1640
medium containing 10% FBS and 1% antibiotics. Because
siPFKP-transfected cells did not proliferate sufficiently
under serum starvation, cells were cultured in 10% FBS. After 24 h,
in the state of 100% confluence, the insert was removed and images
of the wound were captured after 0, 4, 8, 12, 18, 24, 36 and 48 h.
Wound widths at each time-point were assessed 20 times/well at
100-µm intervals using an inverted microscope at a magnification of
×40 magnification (Olympus Corporation) (14).
Kaplan-Meier survival analysis using
Kaplan-Meier Plotter
The website of the Kaplan-Meier Plotter (http://kmplot.com/analysis/index.php?p=background) was
used to analyze relapse-free survival (RFS) and OS for patients
with BC with respect to the expression of PFPK by
classifying its expression levels into the upper quartile or to
other quartiles (18).
Statistical analysis
Numeric variables between two groups were compared
using the Mann-Whitney test. Comparisons between multiple groups
were performed using ANOVA followed by Tukey's post hoc test and
the Kruskal-Wallis test for parametrical and non-parametrical
continuous variables, respectively. All values obtained from each
cell line were used to compare the PFKP expression levels
between the two groups. Spearman's rank correlation test was
performed to evaluate the correlation between PFKP and
cancer-related gene expression levels in the PCR array analysis.
Data are presented in each graph as the mean ± standard error of
the mean (SEM). The ratio of PFKP mRNA expression levels
between cancerous and non-cancerous specimens was presented as mean
± standard deviation (SD). The association between PFKP mRNA
expression and clinicopathological factors were analyzed using the
χ2 test. Disease-free survival (DFS) and OS were
calculated using the Kaplan-Meier method, and survival curves were
compared using the log-rank test. Although RT-qPCR was conducted
three times, proliferation, invasion, and migration assays were
performed once. All statistical analyses were performed using JMP
15 software (SAS Institute, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
PFKP mRNA expression levels in BC and
non-cancerous cell lines and its association with cancer-related
genes in BC cell lines
PFKP mRNA expression levels in 13 BC cell
lines and two non-cancerous cell lines are demonstrated in Fig. 1A. ER, PgR, and HER2 statuses of the
cell lines have been evaluated in previous studies (19,20).
PFKP mRNA expression levels in ER-negative cell lines were
significantly higher than those in ER-positive BC cells (P=0.003).
In addition, PFKP in triple-negative cell lines revealed
higher mRNA expression levels than that in the other cell lines
(P=0.038). Subsequent PCR array analysis revealed that PFKP
mRNA expression levels were positively correlated with those of
several well-known oncogenes, such as transforming growth factor-β1
(TGFB1) (correlation coefficient 0.758, P=0.003) and MYC
proto-oncogene (MYC) (correlation coefficient 0.648,
P=0.017) (Fig. 1B). The
correlation between PFKP mRNA expression levels and those of
84 cancer-related genes is revealed in Table SI.
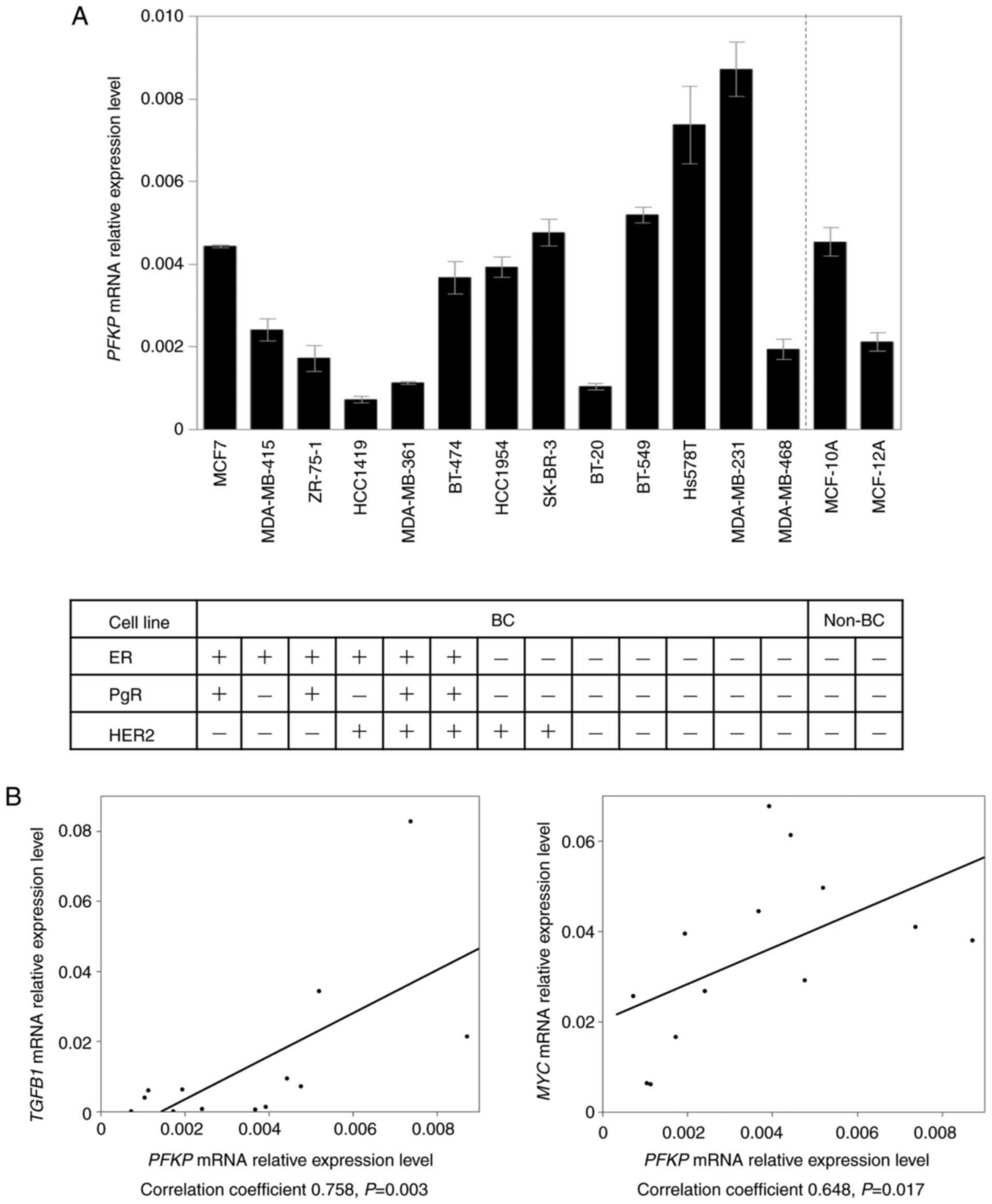 | Figure 1.PFKP mRNA expression in 13
breast cancer and two non-cancerous cell lines, and the correlation
between PFKP and cancer-related gene expression in PCR array
analysis. (A) PFKP mRNA expression levels in ER-negative
cell lines were significantly higher than those of ER-positive
cells. Error bars, mean ± SEM. (B) The mRNA expression level of
PFKP exhibited a significant positive correlation with the
levels of TGFB1 and MYC in various cell lines. PFKP,
platelet isoform of phosphofructokinase; BC, breast cancer; non-BC,
non-cancerous breast; ER, estrogen receptor; HER2, human epidermal
growth factor receptor 2; TGFB1, transforming growth factor-β1;
MYC, MYC proto-oncogene; PgR, progesterone receptor. |
Effects of PFKP knockdown in BC cell
lines
Considering the results of PFKP mRNA
expression levels, PFKP protein expression was evaluated in
representative BC cell lines to differentiate cell lines between
high and low PFKP levels. Among these cell lines, MCF7 represents
ER-positive/HER2-negative, SK-BR-3 represents
ER-negative/HER2-positive and MDA-MB-231 represents triple-negative
cells. HCC1419, which expressed the lowest mRNA expression level,
was used as a negative control (Fig.
2A). Knockdown cells tended to exhibit decreased levels in
PFKP mRNA expression (Fig.
2B), which was confirmed with the protein expression levels
(Fig. 2C).
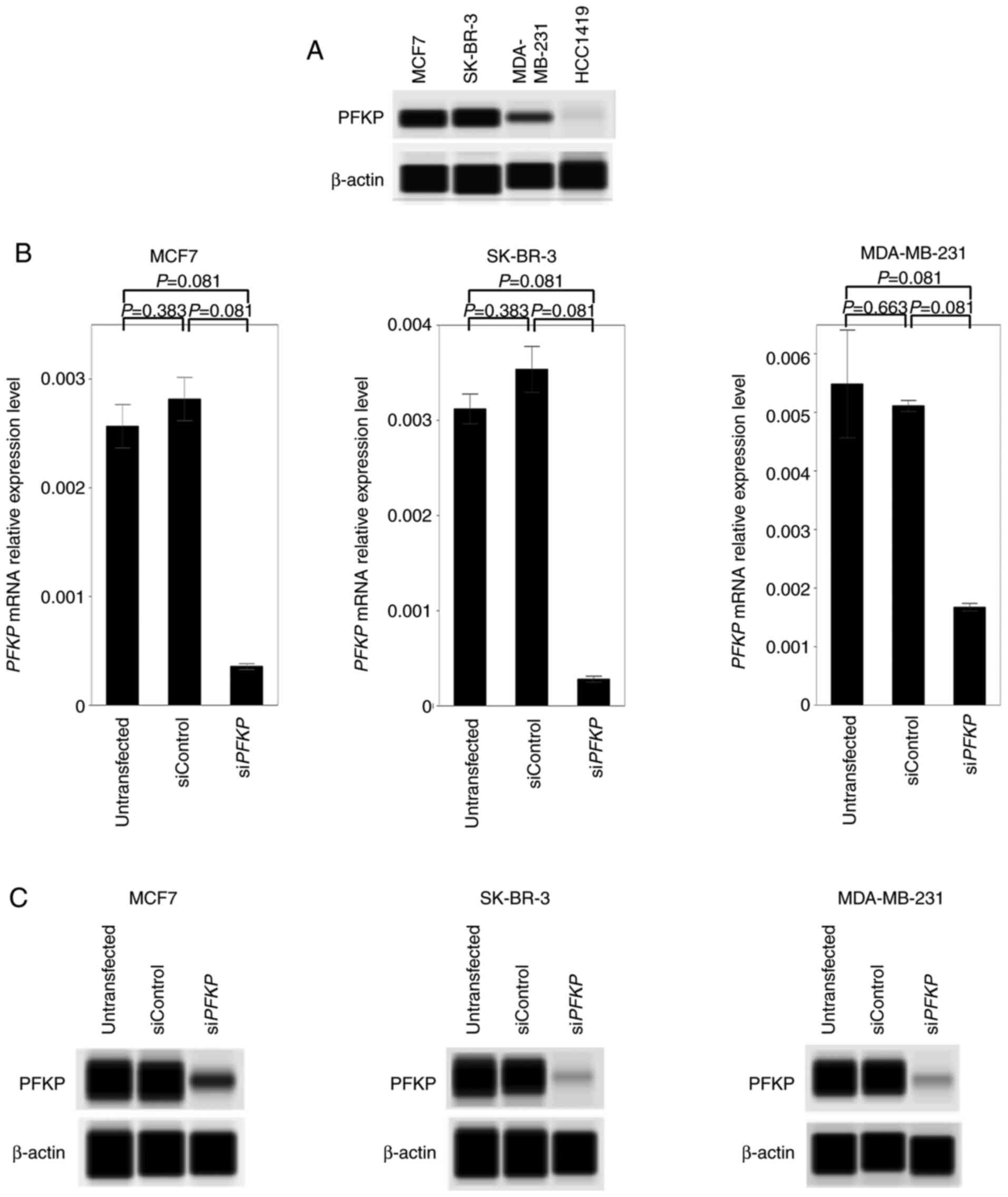 | Figure 2.PFKP expression, and knockdown of
PFKP mRNA and PFKP protein with siPFKP in BC cell
lines. (A) PFKP expression in representative BC cell lines. PFKP
expression was observed in MCF7 (ER-positive/HER2-negative),
SK-BR-3 (ER-negative/HER2-positive), and MDA-MB-231
(triple-negative), whereas PFKP was not detected in HCC1419 cells,
which exhibited the lowest expression level of PFKP. (B)
Validation of PFKP knockdown efficacies in mRNA expression
levels. Knockdown cells tended to exhibit lower PFKP
expression levels in MCF7, SK-BR-3, and MDA-MB-231 cell lines.
Error bars, mean ± SEM. Kruskal-Wallis test. (C) Western blotting
using the Simple Western technique confirmed the inhibition of PFKP
in MCF7, SK-BR-3 and MDA-MB-231 cell lines. PFKP, platelet isoform
of phosphofructokinase; si, small interfering; BC, breast cancer;
ER, estrogen receptor; HER2, human epidermal growth factor receptor
2. |
To determine the tumor-progressive roles of PFKP in
BC cells, cell proliferation, invasiveness and migration were
evaluated in the knockdown cells. Compared with the untransfected
and siControl-transfected cells, proliferation was significantly
inhibited in siPFKP-transfected MCF7 and SK-BR-3 cells
during the entire study period (P<0.001). Proliferation of
MDA-MB-231 cells transfected with siPFKP resulted in
significant inhibition on day 5 (P<0.01; Fig. 3A). In the invasiveness assay, fewer
siPFKP-than siControl-transfected or untransfected MCF7,
SK-BR-3, and MDA-MB-231 cells passed the Matrigel (P<0.001;
Fig. 3B). Moreover, the migration
ability of SK-BR-3 and MDA-MB-231 cells was inhibited after
siPFKP transfection (P<0.01; Fig. 3C). siPFKP-transfected MCF7
cells did not exhibit sufficient proliferation to perform the
migration assay, as revealed in Fig.
3A.
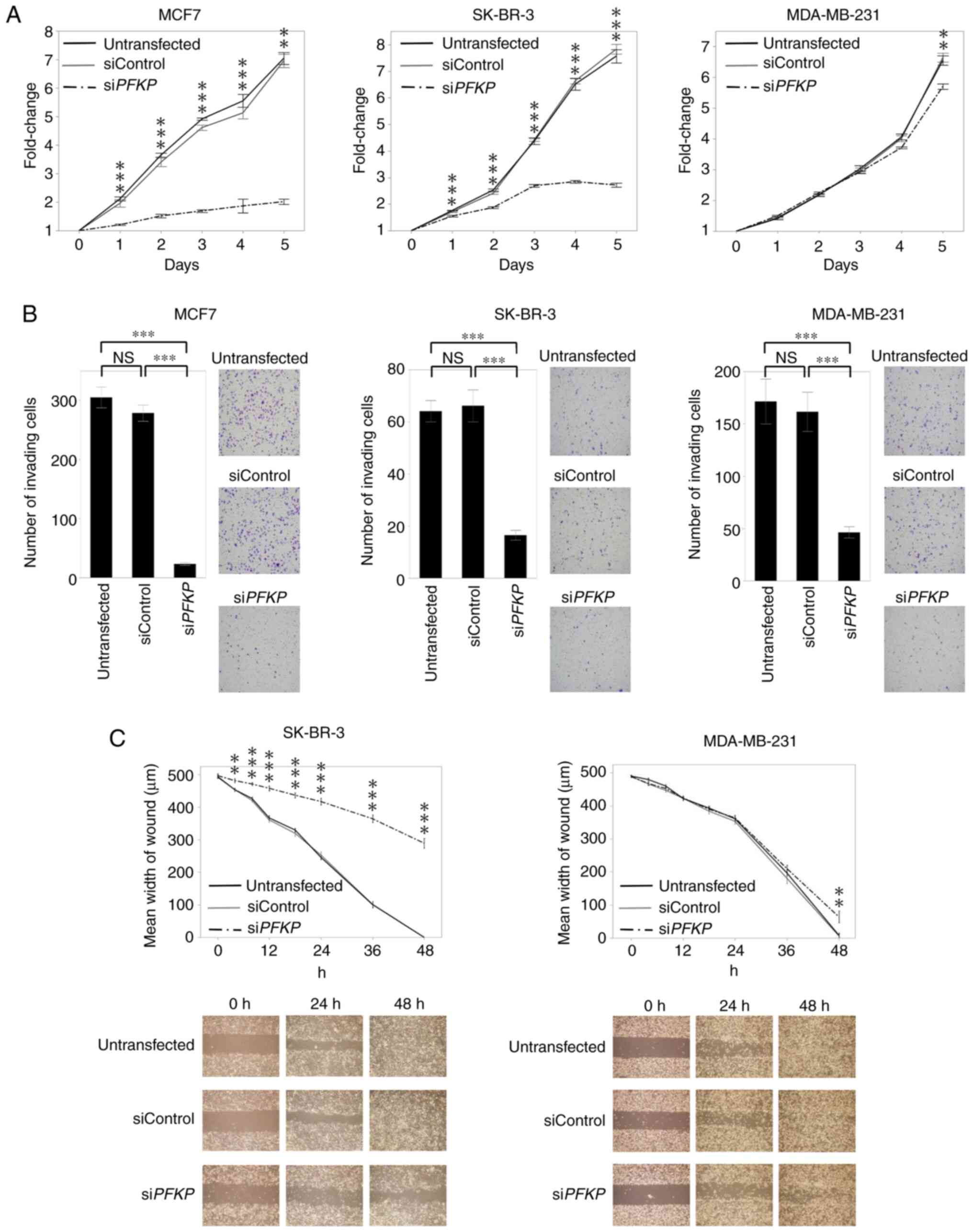 | Figure 3.Functional analysis in breast cancer
cell lines using knockdown cells. (A) Proliferation assay:
siPFKP cells revealed significantly decreased proliferation
in MCF7, SK-BR-3, and MDA-MB-231 cells, compared with untransfected
and siControl cells. (B) Invasiveness assay: Inhibiting PFKP in
MCF7, SK-BR-3, and MDA-MB-231 cells significantly decreased the
number of invading cells. (C) Migration assay: The migration
ability was inhibited in siPFKP cells in SK-BR-3 and
MDA-MB-231 cell lines. Error bars mean ± SEM. ANOVA followed by
Tukey's post hoc test (for A, B, and C). **P<0.01 and
***P<0.001. PFKP, platelet isoform of phosphofructokinase; N.S.,
not significant; si, small interfering. |
Association between PFKP mRNA
expression levels and clinicopathological factors
PFKP mRNA expression levels in both BC and
non-cancerous specimens were evaluated. The ratio of PFKP
mRNA expression levels between cancerous and non-cancerous
specimens was defined as the ‘C/N ratio’. Accordingly, the mean C/N
ratio (± SD) was 1.82±3.26, and the C/N ratio was >1 in 69
patients (41%). PFKP mRNA expression levels in patients with
T2/T3/T4 (n=90) were significantly higher than those revealed in
patients with Tis/T1 (n=77; P=0.049). Similarly, patients with
lymph node metastases (n=82) exhibited higher PFKP mRNA
expression levels than those without lymph node metastases (n=85;
P=0.048; Fig. 4A). Furthermore,
patients with stage II/III/IV (n=113) exhibited higher PFKP
expression levels than those with stage 0/I (n=54; P=0.011;
Fig. 4A). Regarding conventional
biomarkers, ER-negative specimens (n=40) revealed higher
PFKP mRNA expression levels than ER-positive specimens
(n=127; P=0.002). PgR-negative specimens (n=52) exhibited
significantly higher PFKP mRNA expression than PgR-positive
specimens (n=115; P<0.001; Fig.
4B). There was no significant difference between the
HER2-positive (n=39) and HER2-negative specimens in terms of their
PFKP mRNA expression (n=119; P=0.088; Fig. 4B).
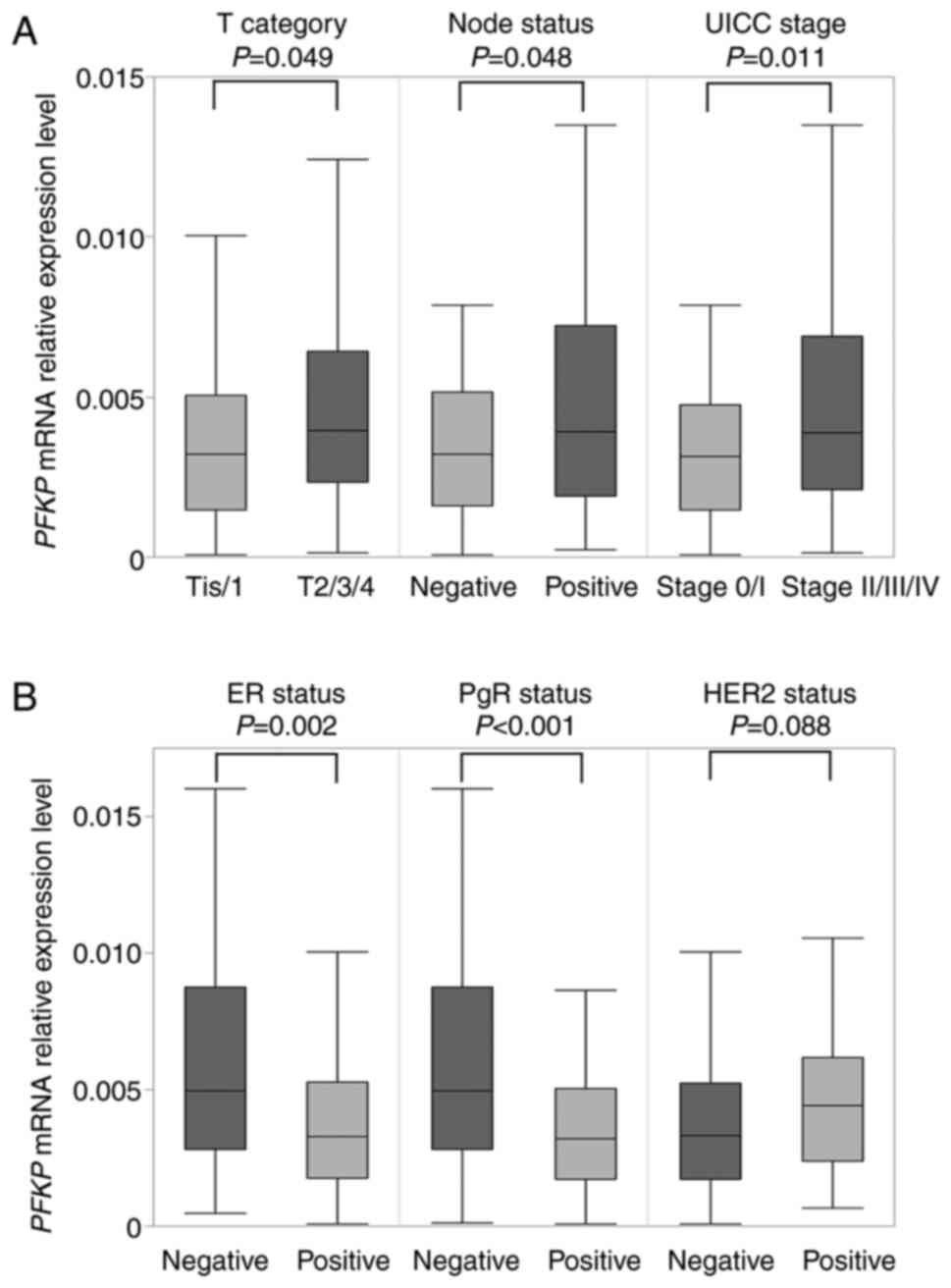 | Figure 4.Association between PFKP mRNA
expression and clinicopathological factors. (A) The mRNA expression
level of PFKP was significantly higher in the patients with
T2/T3/T4, lymph node metastases, or stage II/III/IV than those with
Tis/T1, without lymph node metastases, or stage 0/I, respectively.
(B) ER-negative and PgR-negative specimens exhibited higher
PFKP mRNA expression than ER-positive and PgR-positive
specimens, respectively, but no significant difference was observed
in HER2 status. PFKP, platelet isoform of phosphofructokinase; Tis,
tumor in situ; UICC, Union for International Cancer Control;
ER, estrogen receptor; PgR, progesterone receptor; HER2, human
epidermal growth factor receptor 2. |
The patients were grouped in the highest quartile of
PFKP mRNA expression into a ‘high-PFKP group’ (n=42)
and the remaining patients in other quartiles to a ‘medium-low
PFKP group’ (n=125). The association between
clinicopathological factors and PFKP expression is revealed
in Table I. As anticipated, the
high-PFKP group included more patients with T2/T3/T4
(P=0.023) and with more advanced UICC pathological stages
(P=0.001). In addition, the high-PFKP group had more
ER-negative and PgR-negative patients than the medium-low
PFKP group (P=0.004 and P<0.001, respectively).
 | Table I.Associations between PFPK mRNA
expression and the clinicopathological characteristics of 167
patients with breast cancer. |
Table I.
Associations between PFPK mRNA
expression and the clinicopathological characteristics of 167
patients with breast cancer.
|
| Expression of
PFKP |
|
|---|
|
|
|
|
|---|
|
Characteristics | High-PFKP
group (n=42) | Medium-low
PFKP group (n=125) | P-value |
|---|
| Age (years) | 52 (27–76) | 52 (26–78) | 0.813 |
| Histology |
|
| 0.794 |
|
DCIS | 1 (2.4%) | 6 (4.8%) |
|
|
IDC | 38 (90.5%) | 110 (88.0%) |
|
|
ILC | 3 (7.1%) | 3 (2.4%) |
|
|
Other | 0 (0%) | 6 (4.8%) |
|
| UICC T factor |
|
| 0.023a |
|
Tis/T1 | 1/12 (31.0%) | 6/58 (51.2%) |
|
|
T2/T3/T4 | 23/4/2 (69.0%) | 52/5/4 (48.8%) |
|
| Lymph node
status |
|
| 0.055 |
|
Positive | 26 (61.9%) | 56 (44.8%) |
|
|
Negative | 16 (38.1%) | 69 (55.2%) |
|
| UICC pathological
stage |
|
| 0.001a |
|
0/I | 1/4 (11.9%) | 6/43 (39.2%) |
|
|
II/III/IV | 27/10/0
(88.1%) | 51/24/1
(60.8%) |
|
| ER status |
|
| 0.004a |
|
Positive | 25 (59.5%) | 102 (81.6%) |
|
|
Negative | 17 (40.5%) | 23 (18.4%) |
|
| PgR status |
|
|
<0.001a |
|
Positive | 19 (45.2%) | 96 (76.8%) |
|
|
Negative | 23 (54.8%) | 29 (23.2%) |
|
| HER2 status |
|
| 0.118 |
|
Positive | 13 (31.0%) | 26 (20.8%) |
|
|
Negative | 25 (59.5%) | 94 (75.2%) |
|
|
Unknown | 4 (9.5%) | 5 (4.0%) |
|
| Adjuvant
therapy |
|
| 0.214 |
|
Endocrine therapy alone | 13 (30.9%) | 44 (35.2%) |
|
|
Chemotherapy alone | 12 (28.6%) | 18 (14.4%) |
|
|
Endocrine and
chemotherapy | 13 (31.0%) | 51 (40.8%) |
|
|
None | 4 (9.5%) | 12 (9.6%) |
|
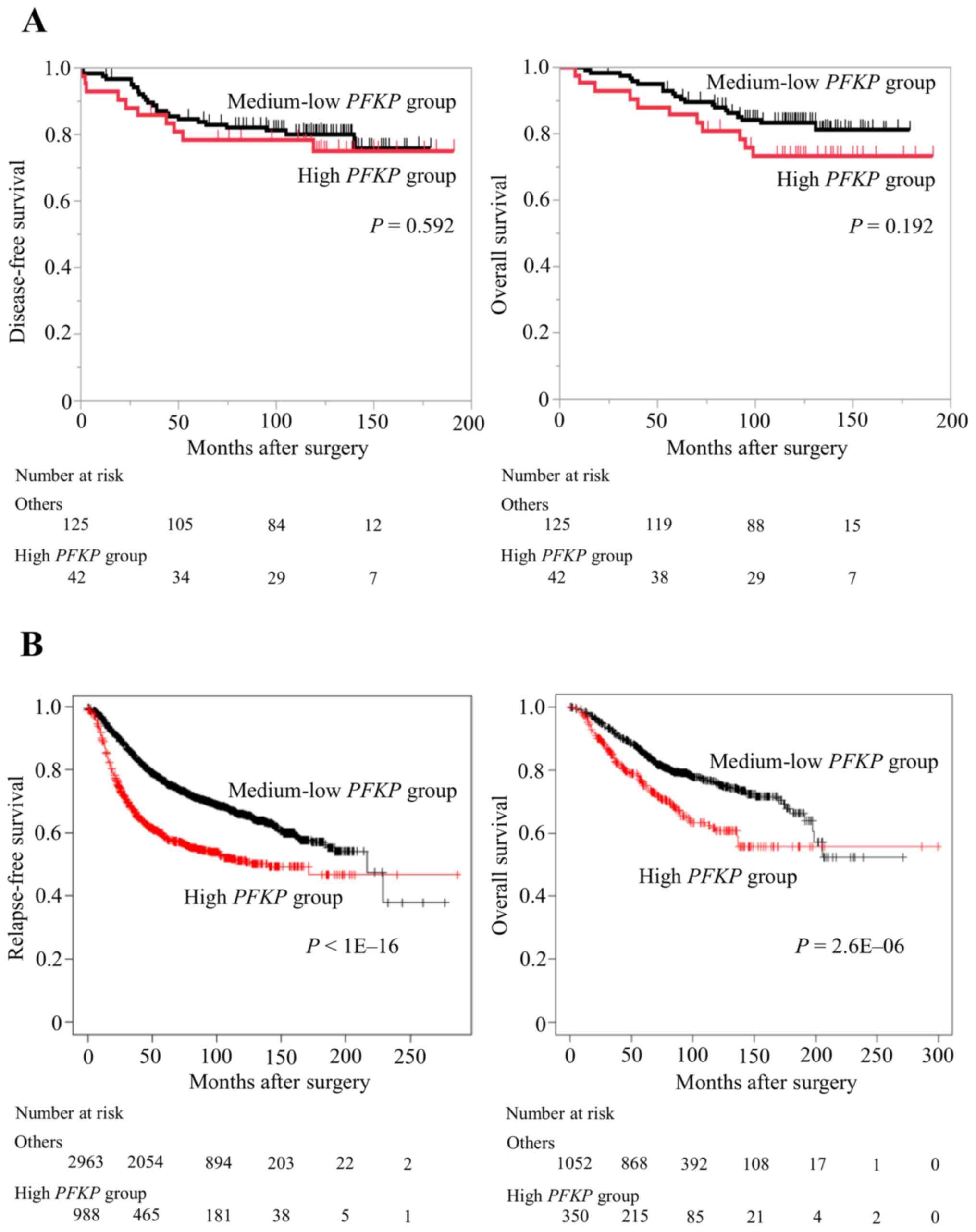
When prognosis was evaluated in our cohort, there
were no significant differences in terms of DFS or OS between these
two groups (Fig. 5A). Due to the
small sample size of our cohort, the effect of PFKP
expression on prognosis was subsequently investigated using the
Kaplan-Meier Plotter website. Similarly, when patients were
assigned either to the upper quartile (high-PFKP group) or
to other quartiles (medium-low PFKP group), the
high-PFKP group exhibited significantly worse RFS (n=3951;
P<1E-16) and OS (n=1402; P=2.6E-06; Fig. 5B).
Discussion
The present study demonstrated that PFKP expression
contributes to tumor progression by promoting cellular
proliferation, invasiveness and migration in various subtypes of BC
cell lines. Furthermore, analysis of clinical samples revealed that
PFKP mRNA expression levels were higher in patients with
advanced pathological stage, which supported our in vitro
results.
The activity of glycolytic enzymes, such as
hexokinase, PFK, and pyruvate kinase, is several folds higher in
cancer cells than that in normal cells (21,22).
PFKP upregulation has been revealed to increase glycolytic
flux and promote tumor cell proliferation and tumor growth
(10). In hepatocellular
carcinoma, PFKP was revealed to be regulated by
Tat-activating regulatory DNA-binding protein via microRNA 520
(23). In glioblastoma,
phosphorylation of PFKP S386 via AKT activation promoted aerobic
glycolysis and tumor growth (9).
In addition to the transcription factors that directly upregulate
PFKP, there is crosstalk between glycolysis and oncogenic
signaling (24). From these
insights, the expression and functional roles of PFKP in BC were
investigated.
Regarding PFKP mRNA expression levels in BC
cell lines and non-cancerous cell lines, ER-negative BC cell lines
had significantly higher PFKP mRNA expression levels than
ER-positive BC cell lines. In addition, triple-negative BC cell
lines expressed higher levels of PFKP mRNA than the other
cell lines. Similarly, analysis of our clinical samples
demonstrated that PFKP mRNA expression levels in ER-negative
patients were significantly higher than those in ER-positive
patients, and its expression levels in patients negative for PgR
were also significantly higher than those found in patients with
PgR-positive results. These results are consistent with a recent
report revealing that triple-negative BC is more dependent on
glycolysis by upregulating several key glycolytic enzymes and
transporters, including PFK and the glucose transporter (24). To analyze the interactions between
PFKP and several oncogenic signaling pathways, the
correlation between the expression levels of cancer-related genes
and those of PFKP in BC cell lines were investigated using a
PCR array. Accordingly, several already known oncogenes were
coordinately expressed with PFKP in BC cell lines, such as
BAX, JUN, MYC, PRKCA, and TGFB1. Among them, previous
studies demonstrated that TGFB1 and MYC may be
correlated with PFKP (25,26).
TGFB1 was revealed to induce
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 expression
through activation of p38 MAPK and PI3K/AKT signaling pathways that
complement and converge with Smad signaling activation (25), which promotes the synthesis of
fructose 2,6-bisphosphate, an activator of PFKP (27). Myc has been revealed to
suppress the level of thioredoxin-interacting protein, which is a
negative regulator of glucose uptake and glycolysis gene
expression, and activates aerobic glycolysis in BC (26). Although further mechanistic
investigation is required, these results would provide important
insights into understanding the involvement of PFKP in
signaling pathways associated with BC progression.
In the present study, PFKP inhibition suppressed
cellular proliferation, invasiveness and migration in various
subtypes of BC cell lines, such as MCF7, SK-BR-3 and MDA-MB-231.
Because PFKP protein expression in MDA-MB-231 cells was relatively
low compared with that in MCF7 and SK-BR-3 cells, the effects of
PFKP inhibition on cell proliferation and migration in MDA-MB-231
cells was lower than those in MCF7 and SK-BR-3 cells, indicating
that cellular proliferative and migrating capacities are
proportional to PFKP expression levels. In clinical samples,
PFKP expression levels were higher in patients with larger
tumor sizes, positive lymph node metastases, or more advanced
stages. Aerobic glycolysis provides a material basis for growth and
proliferation of tumor cells (28), and large amounts of lactic acid
cause invasion of tumor tissues to the normal adjacent tissues
(29). In non-small cell lung
cancer (NSCLC) cell lines, PFKP was revealed to promote
proliferation, migration, invasion, epithelial-mesenchymal
transition, and glycolysis (30).
Furthermore, PFKP mRNA expression was associated with lymph
node metastasis in NSCLC tissues (30). A similar phenomenon could be caused
by PFKP in BC as it promotes lymph node metastasis, leading
to a more malignant phenotype. A previous study on PFK isoenzyme
patterns in BC tissue revealed a positive correlation between
increased pathological stages and the expression of PFKP (31), indicating that PFKP is involved in
promoting the malignant phenotype of BC regardless of the BC
subtype. Regarding prognosis, although there was no significant
difference in DFS or OS between the high-PFKP group and
medium-low PFKP group in our cohort, the analysis using the
public database demonstrated that patients with high PFKP
expression exhibited poorer RFS and OS. This discrepancy could be
due to the small sample size of our cohort and the effect of
adjuvant therapy. Interestingly, the high-PFKP group in our
cohort included patients with more advanced T factors and
pathological stages, which indicated an association between
PFKP and tumor progression. In summary, our results
indicated that PFKP promotes malignant cellular features and
contributes to a more advanced pathological stage, which leads to
poor prognosis. Noticeably, there is no drug approved for BC that
targets glycolytic enzymes. These results indicated that PFKP could
be a new therapeutic target molecule in BC.
However, the present study had some limitations.
Firstly, the mechanism of PFKP expression involved in tumor
progression has not been fully elucidated. Secondly, as
aforementioned, due to the small number of patients in our study
and use of adjuvant medication therapy such as endocrine therapy,
chemotherapy and molecular targeted therapy, the results of the
prognostic analysis in our cohort data did not coincide with those
in public databases. Finally, further in vivo studies are
required to demonstrate the potential therapeutic targets of
PFKP.
In conclusion, the present study revealed the
tumor-progressive roles of PFKP in various subtypes of BC cells
expressing PFKP. These data support the possibility of PFKP as a
therapeutic target in BC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TIn and MS conceived and designed the study. TIn,
MS, TIc, and IS conducted the experiments. TIn and MS analyzed the
data and wrote the manuscript. MS, DT, YT, NT, and TK contributed
to the acquisition of patient data. TIc, MK, MH, IS, DT, YT, NT,
YK, and TK contributed to the interpretation of the comprehensive
data and revised the manuscript for important intellectual content.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board and Ethics Committee of Nagoya University Hospital
(approval no. 2019-0028). Informed consent was obtained from all
patients included in the study.
Patient consent for publication
Participants in the present study provided written
informed consent for publication required by the Institutional
Review Board and Ethics Committee of Nagoya University
Hospital.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Allemani C, Matsuda T, Di Carlo V,
Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ,
Estève J, et al: Global surveillance of trends in cancer survival
2000–14 (CONCORD-3): Analysis of individual records for 37,513,025
patients diagnosed with one of 18 cancers from 322 population-based
registries in 71 countries. Lancet. 391:1023–1075. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cancer Genome Atlas Network, .
Comprehensive molecular portraits of human breast tumours. Nature.
490:61–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwata H: Future treatment strategies for
metastatic breast cancer: Curable or incurable? Breast Cancer.
19:200–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mor I, Cheung EC and Vousden KH: Control
of glycolysis through regulation of PFK1: Old friends and recent
additions. Cold Spring Harb Symp Quant Biol. 76:211–216. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dunaway GA, Kasten TP, Sebo T and Trapp R:
Analysis of the phosphofructokinase subunits and isoenzymes in
human tissues. Biochem J. 251:677–683. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moon JS, Kim HE, Koh E, Park SH, Jin WJ,
Park BW, Park SW and Kim KS: Krüppel-like factor 4 (KLF4) activates
the transcription of the gene for the platelet isoform of
phosphofructokinase (PFKP) in breast cancer. J Biol Chem.
286:23808–23816. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun CM, Xiong DB, Yan Y, Geng J, Liu M and
Yao XD: Genetic alteration in phosphofructokinase family promotes
growth of muscle-invasive bladder cancer. Int J Biol Markers.
31:e286–e293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee JH, Liu R, Li J, Zhang C, Wang Y, Cai
Q, Qian X, Xia Y, Zheng Y, Piao Y, et al: Stabilization of
phosphofructokinase 1 platelet isoform by AKT promotes
tumorigenesis. Nat Commun. 8:9492017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Zhang P, Zhong J, Tan M, Ge J, Tao
L, Li Y, Zhu Y, Wu L, Qiu J and Tong X: The platelet isoform of
phosphofructokinase contributes to metabolic reprogramming and
maintains cell proliferation in clear cell renal cell carcinoma.
Oncotarget. 7:27142–27157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng M, Yang D, Hou Y, Liu S, Zhao M, Qin
Y, Chen R, Teng Y and Liu M: Intracellular citrate accumulation by
oxidized ATM-mediated metabolism reprogramming via PFKP and CS
enhances hypoxic breast cancer cell invasion and metastasis. Cell
Death Dis. 10:2282019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Umar SM, Kashyap A, Kahol S, Mathur SR,
Gogia A, Deo SVS and Prasad CP: Prognostic and therapeutic
relevance of phosphofructokinase platelet-type (PFKP) in breast
cancer. Exp Cell Res. 396:1122822020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe M, Shibata M, Inaishi T, Ichikawa
T, Soeda I, Miyajima N, Takano Y, Takeuchi D, Tsunoda N, Kanda M,
et al: MZB1 expression indicates poor prognosis in estrogen
receptor-positive breast cancer. Oncol Lett. 20:1982020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shibata M, Kanda M, Tanaka H, Umeda S,
Miwa T, Shimizu D, Hayashi M, Inaishi T, Miyajima N, Adachi Y, et
al: Overexpression of Derlin 3 is associated with malignant
phenotype of breast cancer cells. Oncol Rep. 38:1760–1766. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fujita M, Somasundaram V, Basudhar D,
Cheng RYS, Ridnour LA, Higuchi H, Imadome K, No JH, Bharadwaj G and
Wink DA: Role of nitric oxide in pancreatic cancer cells exhibiting
the invasive phenotype. Redox Biol. 22:1011582019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harris VM: Protein detection by Simple
Western™ analysis. Methods Mol Biol. 1312:465–468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Györffy B, Lanczky A, Eklund AC, Denkert
C, Budczies J, Li Q and Szallasi Z: An online survival analysis
tool to rapidly assess the effect of 22,277 genes on breast cancer
prognosis using microarray data of 1,809 patients. Breast Cancer
Res Treat. 123:725–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Riaz M, van Jaarsveld MT, Hollestelle A,
Prager-van der Smissen WJ, Heine AA, Boersma AW, Liu J, Helmijr J,
Ozturk B, Smid M, et al: MiRNA expression profiling of 51 human
breast cancer cell lines reveals subtype and driver
mutation-specific miRNAs. Breast Cancer Res. 15:R332013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Subik K, Lee JF, Baxter L, Strzepek T,
Costello D, Crowley P, Xing L, Hung MC, Bonfiglio T, Hicks DG and
Tang P: The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67
and AR by immunohistochemical analysis in breast cancer cell lines.
Breast Cancer (Auckl). 4:35–41. 2010.PubMed/NCBI
|
|
21
|
Young CD and Anderson SM: Sugar and
fat-that's where it's at: Metabolic changes in tumors. Breast
Cancer Res. 10:2022008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moreno-Sánchez R, Rodríguez-Enríquez S,
Marín-Hernández A and Saavedra E: Energy metabolism in tumor cells.
FEBS J. 274:1393–1418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park YY, Kim SB, Han HD, Sohn BH, Kim JH,
Liang J, Lu Y, Rodriguez-Aguayo C, Lopez-Berestein G, Mills GB, et
al: Tat-activating regulatory DNA-binding protein regulates
glycolysis in hepatocellular carcinoma by regulating the platelet
isoform of phosphofructokinase through microRNA 520. Hepatology.
58:182–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Z, Jiang Q and Dong C: Metabolic
reprogramming in triple-negative breast cancer. Cancer Biol Med.
17:44–59. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rodríguez-García A, Samsó P, Fontova P,
Simon-Molas H, Manzano A, Castaño E, Rosa JL, Martinez-Outshoorn U,
Ventura F, Navarro-Sabaté À and Bartrons R: TGF-β1 targets Smad,
p38 MAPK, and PI3K/Akt signaling pathways to induce PFKFB3 gene
expression and glycolysis in glioblastoma cells. FEBS J.
284:3437–3454. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen L, O'Shea JM, Kaadige MR, Cunha S,
Wilde BR, Cohen AL, Welm AL and Ayer DE: Metabolic reprogramming in
triple-negative breast cancer through Myc suppression of TXNIP.
Proc Natl Acad Sci USA. 112:5425–5430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clem BF, O'Neal J, Tapolsky G, Clem AL,
Imbert-Fernandez Y, Kerr DA II, Klarer AC, Redman R, Miller DM,
Trent JO, et al: Targeting 6-phosphofructo-2-kinase (PFKFB3) as a
therapeutic strategy against cancer. Mol Cancer Ther. 12:1461–1470.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brand K: Aerobic glycolysis by
proliferating cells: Protection against oxidative stress at the
expense of energy yield. J Bioenerg Biomembr. 29:355–364. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gillies RJ, Robey I and Gatenby RA: Causes
and consequences of increased glucose metabolism of cancers. J Nucl
Med. 49 (Suppl 2):24S–42S. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang F, Li L and Zhang Z: Platelet isoform
of phosphofructokinase promotes aerobic glycolysis and the
progression of non-small cell lung cancer. Mol Med Rep. 23:742021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang G, Xu Z, Wang C, Yao F, Li J, Chen C
and Sun S: Differential phosphofructokinase-1 isoenzyme patterns
associated with glycolytic efficiency in human breast cancer and
paracancer tissues. Oncol Lett. 6:1701–1706. 2013. View Article : Google Scholar : PubMed/NCBI
|