Introduction
Cancer is a lethal disease, responsible for ~9.6
million deaths annually, thus rendering it the second leading cause
of mortality globally (1). The
most common cancer types include lung, breast, colorectal,
prostate, skin (melanoma) and stomach cancer. The treatment
approach to cancer is multifactorial, with chemotherapy,
radiotherapy and surgery being the main methods of treatment
(1). The immune system plays a
major role in all aspects of cancer, including its origin,
development, metastasis, therapy and prevention. Cancer cells and
the immune system are in a constant crosstalk, wherein cancer cells
undergo three phases: i) Elimination; ii) equilibrium; and iii)
escape. In the elimination phase, immune cells, particularly innate
immune cells, are in constant surveillance, eliminating cells that
are abnormal. The elimination process induces cancer cells to
undergo immune-editing or sculpting, causing an increase in the
number of cells that have decreased immunogenicity and become
resistant to the immune surveillance process; this part is the
equilibrium phase. The cells that become resistant can escape the
immune system and develop into advanced-stage cancer (2).
Vulnerable populations require a continuous
implementation approach for cancer prevention
It is possible to identify subsets of vulnerable
populations who are at high risk of either developing cancer or who
have cancer, but require intervention for preventing cancer
progression. These populations include:
i) Aged individuals with ‘inflammaging’. There is
sufficient evidence regarding how age-related pathologies,
including cancer, cardiovascular diseases and type 2 diabetes, have
a common inflammatory background, involving the process termed as
‘inflammaging’. In inflammaging, there is a constant systemic
proinflammatory state with increased levels of circulating ILs,
including IL-6 and IL-1, as well as TNF-α and other inflammatory
markers. A chronic antigen load caused by infections, cellular
senescence, a dysregulated DNA damage response, altered gut
microbiota, meta-inflammation and some microRNAs (miRNAs/miRs) that
are associated with aging also influence the causative factors for
cancer, simultaneously influencing and aiding inflammaging, thereby
leading to cancer formation and progression (3). Along with increasing age, studies
have revealed that there is an enhancement in the number of natural
killer (NK) cell subpopulations, as well as a redistribution. The
re-distribution is characterized by an increase of
CD56bright cell populations, which are more immature,
and of CD56dim mature cells, with intrinsic, reduced
cytotoxic activity at single-cell level. Moreover, NK cells from
elderly populations produce less IFN-γ upon IL-2 stimulation
(4). This immunocompromising
nature may also influence the elderly population in becoming prone
to cancer development.
ii) Individuals with genetic risk variants, which
are prone to cancer development either caused by the variants
themselves or due to negative influences on the immune system
(5). The association between genes
and cancer is well known. For instance, the most commonly mutated
gene in all cancer types is p53. Moreover, inherited mutations in
the BRCA1 and BRCA2 genes are associated with hereditary breast and
ovarian cancer syndromes (5). The
study by Imai et al (6)
examined immune system weakness and cancer development. Between
1986 and 1990, these authors assessed the natural cytotoxic
activity of peripheral blood mononuclear cells using an
isotope-release assay in 3,625 residents of a Japanese population
who were mostly aged >40 years. These authors also conducted an
11-year follow-up survey for the cohort members, aiming to examine
cancer incidence and mortality. The follow-up results indicated
that medium and high cytotoxic activity of peripheral-blood
lymphocytes was associated with reduced cancer risk, whereas low
activity was associated with increased cancer risk (6).
iii) Individuals with lifestyle and metabolic
disorders: For >70 years, the association diabetes and cancer
has been hypothesized (7).
Epidemiological data have demonstrated that patients with diabetes
are at an increased risk of developing various types of cancer,
with an increased mortality rate. Several pathways have been
proposed for the association between diabetes and cancer,
including: a) Hyperglycaemia leading to increased cancer risk via
augmented oxidative stress and DNA damage; b) hyperinsulinemia, due
to exogenous insulin or insulin analogues [this view has been
challenged by a previously published study (7)]; and c) chronic microinflammation with
cytokine dysregulation (7).
Hyperglycaemia in diabetes generally favours malignant cell
proliferation by providing energy to the cancer cells. Increased
levels of chronic inflammatory markers, including IL-1β, IL-6 and
TNF-α, have been observed in diabetic patients, which may indicate
the activation of the immune response in the progression and
development of cancer cells. The uncontrolled proinflammatory
response environment in diabetes, which is caused by chronic
accumulation of glycated biomolecules and advanced glycation end
products, leads to a chronic inflammatory state induced by the
activation of the transcription factor NF-кB and the generation of
reactive oxygen species (ROS) in cells. These factors promote a
tumour-favourable microenvironment and potentially trigger immune
system overactivation, ultimately leading to cancer growth
(8,9). With regards to metabolic syndromes,
chronic inflammation and cancer, a chronic and stable background
inflammation has been proposed, referred to as a ‘hypothalamic
microinflammation’ (9). This is
caused by the hypothalamus atypically undergoing proinflammatory
signalling activation, occurring alongside increases in age and the
development of metabolic syndrome. This hypothalamic
microinflammation has also been reported to programmatically
control whole-body aging. Since aging is also associated with a
chronic inflammatory state, negatively associated with longevity
but positively with neurodegenerative diseases, an association
between the hypothalamus and a microinflammatory state leading to
cancer has become increasingly evident (10).
iv) Individuals with immune system weaknesses due to
a) aged individuals with inflammaging; b) individuals with genetic
risk variants; and c) individuals with lifestyle and metabolic
disorders.
v) Patients with cancer undergoing chemotherapy,
radiotherapy or surgery, which lead to therapy-induced immune
dysfunction (11–13). Chemotherapy or a chemo- and
radiotherapy combination have been reported to significantly delay
the immune recovery to pre-treatment baseline levels. Similarly,
surgery leads to a window of opportunity that allows the residual
cancer cells, including those that have undergone distant
metastases, to gain a foothold in the absence of NK cell
surveillance (12).
Since conventional therapies have an associated risk
of therapy-induced immune dysfunction, it is important to identify
an approach that is effective in the long term as an adjunct to the
other interventions, which would help maintain the normal function
of the immune system, thereby enhancing its immune surveillance and
antitumour properties, and ultimately playing a potential role in
cancer prevention.
A vaccine therapy approach for cancer
treatment
According to the Centers for Disease Control and
Prevention in the USA, a vaccine is a product that stimulates the
immune system of an individual to produce immunity against a
specific disease (14). Vaccines
in cancer may be therapeutic or preventive. Preventive cancer
vaccines include proteins, peptides, DNA or RNA that can elicit or
boost pre-existing antitumour immunity, leading to cancer
elimination and the production of long-term memory to prevent
tumour recurrence (15). The
purpose of a therapeutic cancer vaccine is to control the cancer
burden. Such vaccines include autologous patient-derived immune
cell vaccines, tumour antigen-expressing recombinant virus
vaccines, peptide vaccines, DNA vaccines and heterologous
whole-cell vaccines derived from established human tumour cell
lines (16). The personalized
dendritic cell vaccine sipuleucel-T (Provenge) and recombinant
viral prostate cancer vaccine PSA-TRICOM (Prostvac-VF) are widely
known vaccines in the pre-approval/authorized approval/late
clinical trial stages (17).
Vaccines are often administered with adjuvants, which help to
improve poorly immunogenic vaccines (18). Different types of novel adjuvants
have been identified and applied with cancer vaccines, which
include inorganic nanoparticles, organic molecules and polymers
(19). Pathogens stimulate a
‘danger sensing’ signal via pathogen-associated molecular patterns
(PAMPs). Inorganic nanoparticle-based adjuvants function in a
similar manner to PAMPs, thereby stimulating antitumour immunity.
Organic molecule-based adjuvants include small molecule-based
factors, such as modified PAMPs, and are novel ligands for pattern
recognition receptors (PRRs). Agonists of the Toll-like receptor
family, which are type I transmembrane proteins that regulate the
innate and adaptive immune responses (19), and agonists of stimulator of IFN
genes (20) are examples of
organic adjuvants. Polymer-based adjuvants concurrently help in
drug delivery and act as PAMPs for immune system activation. At
present, alum (a mixture of diverse aluminium salts) has been the
only approved adjuvant in humans and remains one of the most common
adjuvants in human vaccines. In addition to aluminium salts,
oil-in-water emulsions containing squalene (e.g., MF59 and AS03),
in vitro-assembled influenza-virus-like particles (e.g.,
virosomes) and the liposome-based adjuvant system AS01, are other
licensed adjuvants in human vaccines (21).
However, it remains unknown whether there is a
nutrition-based supplementation that can act as a potential vaccine
adjuvant to facilitate cancer treatment, which can be both
preventive and therapeutic.
β-glucan vaccine adjuvant approach for
cancer treatment through immune enhancement (B-VACCIEN)
β-glucans, as a result of their high
biocompatibility and tolerability and satisfactory safety profile,
possess numerous beneficial properties, establishing them as
promising adjuvant candidates (21–23).
Dietary phytochemical carbohydrates have been considered as
effective cancer-preventing and therapeutic adjuvants, which can be
supplemented continuously for a longer time period (24). Among such phytochemicals, saponins
and β-glucans are widely distributed in the plant kingdom, and in
their purified extract form, represent two of the most potent
immunological adjuvants when injected as a mixture with antigen, or
immunomodulators when orally ingested (25).
β-glucans are naturally occurring polysaccharides
that are constituents of the cell walls of yeast, fungi (including
mushrooms), some bacteria, seaweed and cereals (oat and barley)
(26). β-glucans are functional
bioactive compounds possessing hypocholesterolaemic, hypoglycaemic,
immunomodulatory, antitumor, antioxidant and anti-inflammatory
activities. Moreover, their macromolecular structure and
functionality vary, depending on the source (27). Yeast-derived 1,3-1,6 β-glucans have
been reported to exert more prominent biological response modifier
(BRM) effects compared with β-glucans from other sources, including
oats or barley (28). An
immunomodulator includes any molecule or substance capable of
interacting with the immune system, resulting in the up- or
downregulation of specific components of the immune response
(29). Immunomodulators comprise
of an array of synthetic, natural and recombinant molecules.
Natural molecules, including those found in curcumin, thyme, bay
leaf, resveratrol, ginseng, echinacea, aloe vera, astragalus,
goldenseal, flavonoids and essential oils, have been studied for
their immunomodulation properties as nutritional supplements.
However, direct comparison studies of individual immunomodulators
are limited. Vetvicka et al (26) indicated that, amongst >20,000
published studies, compared with other immunomodulators, glucan was
the most prominent one.
Glucans are BRMs that exert significant effects on
various components of the immune system. Glucans are recognized by
PRRs present on the membranes of immune cells, such as macrophages,
monocytes, dendritic cells and NK cells, with their key receptors
being Dectin-1 and complement receptor 3 (CR3; CD11b/CD18).
Additional receptors include Toll-2, lactosylceramides and the
scavenger receptor family (26).
In terms of cancer immunity, β-glucans have been
demonstrated to possess various functions, including: i) the
increase of infection resistance (which is of particular importance
in virus-associated cancer types); ii) exerting antitumour effects
by activating the adaptive and innate arms of the immune system;
iii) stimulating immune cells, including leukocytes, T helper (Th)
and NK cells; and v) exerting anticoagulant effects (30). β-glucans activate early innate
reactions by acting as PAMPs. Glucan-activated B cells have been
revealed to secrete proinflammatory lymphokines, including IL-8,
through the involvement of several molecules including Dectin-1
receptors, MAPK and NF-κB and activator protein-1 transcription
factors. β-glucans have also been demonstrated to be potent
cellular immunity activators. The effects of β-glucan against
various types of infection have been demonstrated, such as for
example Leishmania (L.) major, Leishmania donovani,
Candida albicans, Toxoplasma gondii, Streptococcus suis, Plasmodium
berghei, Staphylococcus aureus, Escherichia coli, Mesocestoides
corti, Trypanosoma cruzi, Eimeria vermiformis and Bacillus
anthracis (26).
The antitumour effects of β-glucans against a wide
variety of tumour types (26). The
mechanism of the antitumor activity induced by β-glucans is
proposed to occur via the enhancement of the immune system against
tumour cells, as well as by inhibiting tumour invasion and
progression via a complex modulation of the apoptotic and
angiogenic mechanisms. The antitumour mechanisms enhanced by
β-glucans involve several pathways. For instance, β-glucans bind
with specific receptors, such as Dectin-1, expressed on myeloid
cells, converting them into antigen presenting cells. This binding
also activates CD4+ and CD8+ T-cells, which
are stimulated to produce the proinflammatory cytokine, TNF-α, the
antitumor cytokine, IFN-γ, granzyme B and perforins, all of them
cytotoxic against cancer cells (31). The tumoricidal effects are also
induced by switching suppressive M2 macrophages into inflammatory
M1 macrophages and, in turn, activating Th1-type T cells, thereby
enabling cancer cell destruction via the secretion of
proinflammatory cytokines through these T-cells. Interactions
between β-glucan and polymorphonuclear cells induce the release of
ROS in the microenvironment, ultimately leading to tumour cell
death. β-glucans also activate NK cell cytotoxicity via the
production and release of proinflammatory cytokines and via
complement activation (31).
β-glucans have been demonstrated to effectively modify the tumour
microenvironment, resulting in significant reduction of primary
tumour growth and distant metastases (31). β-glucans have a strong synergy with
antibodies (Abs) that naturally occur in cancer (26).
β-glucans as adjuvants
Japan is a forerunner on the use of β-glucans, and
β-glucans from the Shiitake mushroom (lentinan) and Coriolus
versicolor (polysaccharide-K) have been licensed drugs since
1983. As of 2019, 177 clinical trials valuating β-glucans have been
listed in the United States database, ClinicalTrials.gov, for
cancer, cholesterol-lowering effects and immune-modulation
(26). Clinical trials on
β-glucans in combination with the other cancer therapies, including
monoclonal Abs (mAbs), have been reported mentioned in the review
article by Vetvicka et al (26), revealing significant tumour
regression, a favourable Ab response, elevated immune cell number
and function, fewer side-effects, decreased cancer-related fatigue
and an improved nutritional state (26). Thus, β-glucans can serve as
potential adjuvants with other cancer treatments.
β-glucans are potential adjuvants that aid
immunomodulation, in combination as well as when administered alone
(32). Since glucan receptors,
including Dectin-1, CR3, lactosylceramide, natural cytotoxicity
receptor p30 and scavenger receptors, are expressed on different
types of immune cells, including macrophages, NK cells and
neutrophils, β-glucans have a distinct affinity with these
receptors, according to their different chemical structure. Thus,
they are capable of triggering different host responses, making
them potential immune adjuvants. β-glucan particles derived from
Saccharomyces cerevisiae cell walls have also been suggested
to be used as vaccine adjuvant carriers for protein antigen
delivery, and for the targeted delivery of compounds to macrophages
and dendritic cells (33).
β-glucans have been reported as trained immunity-based adjuvants
for rabies vaccines and have been demonstrated to elicit B-cell and
T-cell specific responses in a study on canines (34).
With regards to adjuvant immunotherapy for cancer,
since both dendritic cell priming and check-point inhibitor
blockades have been revealed to be required for immunotherapy
(23), β-glucans serve as an ideal
candidate, as they induce dendritic cell priming and potentiate Abs
against immune checkpoint molecules (26). β-glucans have been used as
adjuvants in association with chemotherapy in different types of
cancer, including oestrogen receptor-negative human breast,
gastric, colorectal and non-small-cell lung cancer, as well as
haematological diseases. The advantages of β-glucans as effective
anticancer therapy adjuvants include the following: i) They are
non-immunogenic due to the absence of the protein and peptide
components; ii) they are non-toxic, as even doses up to 10 mg/kg
have been reported to be well-tolerated in vivo, with no
adverse effects; and iii) they provide the opportunity for
beneficial structural modifications, due to the presence of
multiple aldehyde and hydroxyl groups (35).
β-glucans can serve as effective adjuvants with
latest therapies, including mAb-based and immune checkpoint
targeted therapies for cancer. Combination therapy using β-glucan
and mAbs targeting immune checkpoint molecules, including
programmed cell death protein 1 (PD-1) and programmed death-ligand
1 (PD-L1), has been investigated in preclinical models with
promising antitumour efficacy, as reviewed by Vetvicka et al
(26). Several clinical trials
have evaluated the interaction of glucans with mAbs in humans.
β-glucan has been examined in association with pembrolizumab in
head and neck cancers, metastatic melanoma and breast cancer with
positive outcomes in terms of safety, tolerability and increased
survival. β-glucans function via the iC3b-receptor CR3
(CD11b/CD18), thereby enhancing leukocyte-mediated killing of
tumour cells that are coated with iC3b via naturally occurring
antitumor Abs (36).
Trained innate immunity (TRIM) is the innate immune
system memory induced by modulation of mature myeloid cells or
their bone marrow progenitors. This process helps mediating the
sustained increased responsiveness to secondary challenges
(37,38). It is important to note that
β-glucans are effective inducers of TRIM, specifically via
epigenetic reprogramming of the innate immune cells at the level of
bone marrow (central TRIM), as well as peripheral TRIM (37,38).
Vetvicka and Vetvickova (39)
reported that highly purified and active glucans exert significant
pleiotropic effects against cancer.
Cancer cell resistance is an important hurdle in
anticancer therapies. β-glucans are potential candidates for
overcoming treatment resistance in cancer. This effect has been
reported in treatment-resistant Lewis lung carcinoma (LL/2) cells,
in which Candida cell wall-derived-β-glucan exerted a
significant cytotoxic effect on both the parent cell line and
cancer stem cells derived from the parent cell line (40).
Chronic microinflammation, cancer and
β-glucans
Accumulating evidence has indicated that chronic
inflammation may lead to cancer development. Underlying infection
or inflammation have been linked to 25% of all cancer cases
(41). Any unresolved inflammation
on account of the failure in the precise control of the immune
response can continue to disrupt the cellular microenvironment,
leading to alterations in cancer-related genes and
post-translational modifications in key cell signalling proteins
involved in the cell cycle, DNA repair and apoptosis (41). The identification of mononuclear
inflammatory cells in close association with areas of hyperplasia
and cellular atypia has been demonstrated even at very early stages
of tumour development, further supporting the concept that
inflammation is a major driving force that contributes to tumour
initiation and/or initial tumour progression. The upregulation of
non-specific proinflammatory cytokines (IFN-γ, TNF, IL-1α/β or
IL-6) by immune cells, such as macrophages, mast cells and
neutrophils, has been shown to promote tumour development (41). The inflammatory processes elicited
by cancer itself are likely to be involved in their progression.
Inflammation is also the common mechanism of action for numerous
cancer risk factors, including infection, obesity, tobacco smoking,
alcohol consumption, exposure to microparticles, dysbiosis and
chronic inflammatory diseases, including pancreatitis and colitis.
The administration of certain anti-inflammatory drugs, including
aspirin, has also been reported to significantly reduce cancer
risk. Thus, preventing or reversing inflammation has been suggested
as a promising approach to cancer control (42).
Chronic-microinflammation culminating in cancer
likewise requires focusing on metabolic disorders, including
diabetes and cancer development. Several pathways have been
proposed for the association between diabetes and cancer,
including: i) hyperglycaemia leading to increased cancer risk via
augmented oxidative stress and DNA damage; and ii)
hyperinsulinemia, involving chronic microinflammation with cytokine
dysregulation, which both require further attention. The
uncontrolled proinflammatory response environment in diabetes
caused by the chronic accumulation of glycated biomolecules and
advanced glycation end products creates a chronic inflammatory
state, via the activation of the transcription factor NF-кB and ROS
generation in cells. Thus, a tumour-favourable microenvironment is
promoted and immune system overactivation is potentially triggered,
thereby leading to cancer growth. Moreover, with regards to chronic
inflammation and cancer, a state of chronic and stable background
inflammation has been proposed, known as ‘hypothalamic
microinflammation’ (10), which
occurs when the hypothalamus atypically undergoes proinflammatory
signalling activation, and is associated with age increase and the
development of metabolic syndrome.
β-glucans, particularly those that are
yeast-derived, aid in combatting chronic microinflammation, thereby
contributing to a cancer-preventive response, alongside their
metabolic balancing activities (43,44),
further adding to their effects in cancer prevention. A previous
study on a yeast-derived β-glucan identified its antioxidant
activity via H2O2 scavenging, as well as its
in vivo anti-inflammatory potential in terms of
myeloperoxidase activity and malondialdehyde and nitric oxide level
reduction (45). In another study,
the regular intake of β-glucan was demonstrated to exert an
anti-inflammatory effect, which occurred by acting on IL-6, a
pleiotropic cytokine that plays a pivotal role in acute phase
responses in the balancing of the pro- and anti-inflammatory
pathways (46).
Use of a β-glucan vaccine adjuvant
approach for cancer treatment
In the majority of the clinical trials on β-glucans
as a cancer treatment adjuvant an oral route of administration has
been used and also across different age groups, as reviewed by
Vetvicka et al (26),
indicating that β-glucan can be applied universally as an effective
treatment adjuvant (26,39). Following oral administration,
β-glucans directly interact with gastrointestinal mucosa cells and
are transferred into the general circulation. Vetvicka et al
(26) proposed a process of
β-glucan internalization after which it rapidly enters into the
systemic circulation. The solubility of β-glucan is a critical
factor for oral administration and the speed of transfer across the
gut is dependent on the physicochemical characteristics of glucan
(26). In this regard, an AFO-202
BRM glucan (BRMG) derived from a black yeast (Aureobasidium
pullulans AFO-202 strain) demonstrates high purity and
functionality. AFO-202 glucan is a water-soluble β-glucan which has
been used for human consumption for several decades (47) and, as a result of these
characteristics, it can serve as a potential β-glucan vaccine
adjuvant approach to treating cancer.
AFO-202 β-glucan has been revealed to be beneficial
in maintaining blood glucose levels and lipid levels in the normal
range in human studies (43,44),
assisting in the prevention of the metabolic-micro and chronic
inflammation axis that may ultimately lead to cancer. AFO-202
β-glucan has been proven to stimulate the production of IL-8 or
soluble Fas (sFas), although not that of IL-1β, IL-6, IFN-γ, TNF-α
or sFas ligand (sFasL) (47). IL-8
exerts anti-inflammatory activity and helps in T-cell recruitment,
as well as ROS metabolism enhancement. Moreover, IL-8 serves as a
barrier against invading microorganisms, with airway epithelial
release of IL-8 contributing to the immune defence of the host by
promoting neutrophil chemotaxis (48). Tumours have been demonstrated to
express FasL and downregulate Fas to escape from host immune
surveillance. Elevated sFasL serum levels are associated with
cancer progression (49).
Cytokines, including IL-1, IL-4 and IL-6, secreted
by immune cells in the tumour microenvironment are observed in a
wide range of solid tumour types, with the expression of their
receptors by cancer cells aiding in immune evasion (50). IL-6 promotes tumour growth, with
its elevated serum levels and expression in tumours being negative
prognostic markers for cancer patient survival (51).
While IFN-γ has been long considered as a central
player in antitumor immunity, it also has pro-tumorigenic roles.
For instance, IFN-γ-mediated activation of the nonclassical major
histocompatibility complex class Ia genes has been shown to aid in
melanoma cell evasion from cytotoxic T-lymphocyte (CTL)-mediated
cytolysis, in turn leading to clinical failure of melanoma peptide
vaccines (52). IFN-γ is also
associated with the influx of monocytic and granulocytic
myeloid-derived suppressor cells to the tumour microenvironment,
leading to the suppression of the anticancer T-cell response.
Furthermore, IFN-γ-induced PD-L1/2 ligands on cancer cells causes
their binding to their immune inhibitory receptor PD-1, finally
suppressing T and NK cell immune effector activities, thereby
promoting cancer progression (52).
TNF-α, primarily secreted by tumour-associated
macrophages, initiates chronic inflammation. TNF-α has a dual role:
wherein it causes tumour cell apoptosis when administered in high
doses, however, long-term low dose administration has been revealed
to accelerate tumour metastasis in a lung cancer cell line
(40). TNF-α also induces the
expression of angiogenic factors, thereby promoting tumour
angiogenesis and accelerating tumour metastasis via the
upregulation of tumour-associated calcium signal transduction
protein-2 via the ERK1/2 signalling pathway (40,53).
AFO-202 β-glucan, could play a key role in
preventing the cytokine imbalance-induced inflammation caused by
chemotherapy or other cancer therapies through the balancing of
anticancer cytokines activation and pro-tumorigenic cytokines
suppression, thus offering benefits in anticancer prevention and
therapeutics (11–13). It has been reported that the
Dectin-1-based recognition of tumour cells orchestrates innate
immune cell antitumour responses (54). The key receptor via which AFO-202
β-glucan exerts its biological response and modifying effects is
Dectin-1 (54), thereby suggesting
its potential use as a β-glucan vaccine adjuvant. AFO-202 β-glucan
has been shown to aid against infections. For example, AFO-202
β-glucan may enhance immunity against Leishmania amazonensis
and malaria through the increase of NK cell activity and cellular
immunity, extending its application potential for the suppression
of infection, apart from its anticancer effects (55). At present, the metabolic balancing
effects of this AFO-202 β-glucan (43,44)
and its vaccine adjuvant effects as a potential effector in
enhancing the immune response to the avian influenza A H5N1 and
H5N2 vaccines have been reported (56). The AFO-202 β-glucan has been
demonstrated to enhance the immune system in animal (57) and human clinical studies (58), increasing the eosinophil and
monocyte counts and decreasing the neutrophil-to-lymphocyte ratio
(NLR), through the increase in the lymphocyte-to-CRP (LCR) and
leukocyte-to-CRP (LeCR) ratios. Another strain of the
Aureobasidium pullulans, N-163 derived β-glucan has been
demonstrated to attenuate lipotoxicity (decrease in non-esterified
fatty acids (NEFA) (59) with
anti-inflammatory effects of significant control of IL6, D-Dimer
and NLR apart from anti-fibrotic effects in animal (60) and human clinical studies (58).
In tumour animal models, the comparative antitumour
effect of Aureobasidium pullulans-derived β-glucan has been
shown to be significantly higher than those of other glucan types
(61). The administration of the
AFO-202 β-glucan, lead to an increase in the anti-tumour immune
response and its maintenance at normal levels, similar to the
levels of control groups without chemotherapy administration
(62). The percentage of tumour
size decrease has been reported to be higher when A.
pullulans β-glucan was administered (63) along with chemotherapy than with
chemotherapy (64) alone. In
another study, 11 healthy human volunteers consumed 15 g AFO-202
β-glucan orally three times per day for 1 month. NK cell cytotoxic
activity was assessed using peripheral blood provided by the
volunteers before and after the intake. NK cell activity was
evaluated using the 51Cr release test with an effector
to target (E/T) ratio of 50/1, using peripheral blood mononuclear
cells as functional cells and K562 cells as target cells. The rate
of increase of the cytotoxic activity was 90.9% (62).
Fungal β-glucans have been shown to increase NK cell
activity in cancer patients of different age groups (65) and this further attests to the
significance of its application in aged individuals and in those
who are immunocompromised due to cancer.
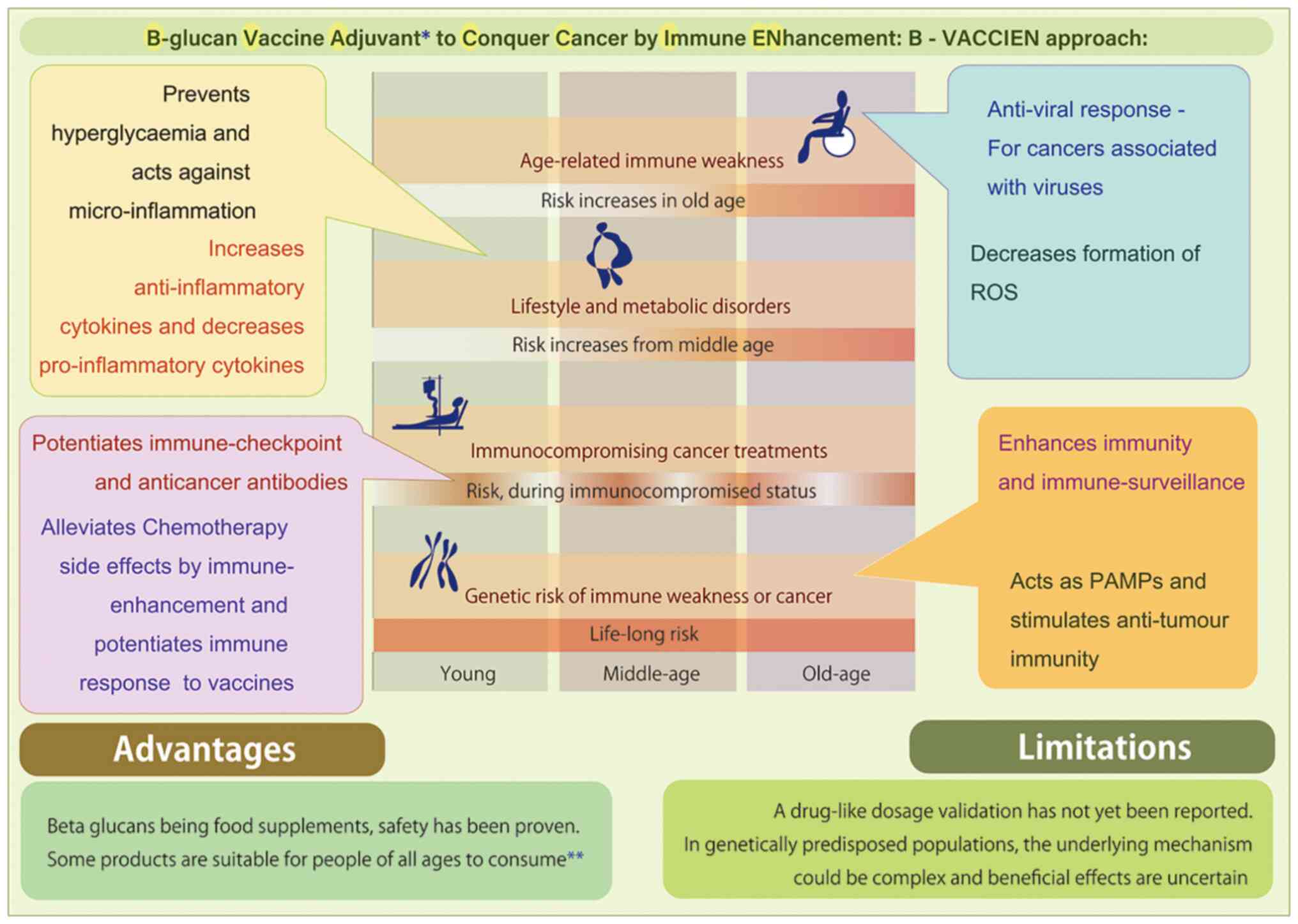
The evolution of the immune system includes a curve
upwards during cancer, with contributions from viruses and chronic
inflammation. With lifestyle and metabolic disorders having become
major healthcare-related issues in the latter half of the past
century, and with microinflammation serving as the underlying
mechanism leading to cancer in such individuals, senile immune
system weakness or inflammaging are unavoidable. These changes may
occur in any individual, even though they may not present with
chronic inflammation. All the aforementioned factors adversely
affect the immune system. Addressing this issue requires a holistic
approach that can potentially act against viral and other
infections, inflammation and metabolic disorders, in addition to
acting as a continuous supportive mechanism for the prevention of
the immune surveillance system weakening. Apart from these factors,
genetic components of the immune system or genetically prone cancer
types may further lead to immune system weakness. Genetics should
be also considered, as in these individuals vulnerable to cancer,
the time at which immune system weakness develops or the
deterioration of the cancer aggressiveness may occur remains
unknown. A continuous vaccine adjuvant approach could include the
use of food supplements, including β-glucan. Although it remains
unknown whether immunoenhancement will completely achieve treating
any cancer already formed, it is suggested as a potential strategy
to address the periodic or intermittent jeopardy to the immune
system. The duration of immune system weakness after surgery, as
well as the immune system weakness induced by chemo- or
radiotherapy, requires definite examination; immunosuppression is
considered a major reason for treatment failure in cancer (66). Treatment strategies to overcome
immune system weakness after cancer therapies require large-scale
translational and clinical research. It is hoped that this kind of
research will yield further insights into how chemotherapy, surgery
or radiotherapy-related cancer treatments can be supplemented by
B-VACCIEN (Fig. 1), to alleviate
side-effects. This goal can be achieved by effectively engaging the
immune system, in order to reduce cancer-adverse reaction-related
morbidity and mortality.
β-glucans are effective chemotherapy adjuvants, due
to their protective antioxidant effects against
chemotherapy-induced cytotoxicity (67). It is significant to underline that
a randomized phase I/II trial studying the side effects and optimal
dose of OPT-821 (a saponin-based immunoadjuvant OBI-821) with
vaccine therapy when given together with β-glucan, as well as the
examination of the effectiveness of this regimen in the treatment
of younger patients with neuroblastoma is currently ongoing
(68).
While several animal studies have demonstrated
promising results for the use of a β-glucan vaccine adjuvant
approach for the treatment of cancer, human studies fall short of
the expected outcome (69). This
may be attributed to the source of the β-glucan; careful selection
of the source and the process involved in the
extraction-purification is essential for an efficient antitumour
response. A comparative study on the various types of β-glucans in
different tumours and stages will be also essential for the
development of additional, more effective β-glucan vaccine adjuvant
therapeutics. Moreover, tumour microenvironment is extremely
complex and is a challenge for the successful combination of
β-glucan and various cancer therapies, including immune-modulating
Abs. The overexpression of some membrane complement regulatory
proteins can limit the β-glucan-primed immune cell infiltration
into tumours. Additional strategies of modifying the tumour
microenvironment are required to overcome these challenges
(35).
Conclusion
Several factors and pathogenic processes have been
identified, that can predispose an individual to a high risk of
developing cancer and/or enable the progression of cancer,
including: i) Chronic and microinflammation caused by infections,
aging or metabolic disorders, including diabetes; ii) genetic
causes; and iii) immune system weakness, either due to cancer or
cancer therapy. Therefore, the prevention of cancer in the general
population and in patients undergoing surgical or chemotherapeutic
treatments is practically feasible, only if a consistent and simple
approach can be followed, as for example a nutritional supplement
to combat the compromise of the immune system and chronic
microinflammation. The current review presented evidence of a BRMG,
with regards to its potential function as a β-glucan vaccine
adjuvant approach for the treatment of cancer through
immunoenhancement. This approach may aid in the treatment of cancer
in specific immunocompromised populations, as it induces a wide
variety of biological response modifications. For example, the BRMG
application may balance metabolic parameters, including blood
glucose and lipid levels, increase peripheral blood cell
cytotoxicity against cancer and alleviate chemotherapy-induced side
effects in animal models. Thus, the use of a β-glucan vaccine
adjuvant approach was suggested for the treatment of cancer via
immunoenhancement as a potential strategy for a long-term
prophylaxis in immunocompromised individuals or genetically prone
to cancer.
Acknowledgements
The authors would like to thank Mr. Yasunori Ikeue,
Mr. Mitsuru Nagataki and Mr. Takashi Onaka, (Sophy Inc.), for
providing the necessary technical clarifications, as well as
Loyola-ICAM College of Engineering and Technology (LICET) for
providing the necessary infrastructure for the present study.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
NI, VDD, KR and SJKA contributed to the conception
and design of the study. RS performed the literature search and
data analysis. SJKA and SP confirm the authenticity of all the raw
data. SJKA and SP drafted the manuscript. KSR, SV, HO, TK, GK, SS,
SRBK and MI performed the critical revision of the manuscript. All
authors contributed to manuscript revision, read, and approved the
submitted version.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
SJKA is a shareholder in GN Corporation, Japan which
in turn is a shareholder in the manufacturing company of the AFO
202 β-glucan.
References
|
1
|
World Health Organization (WHO), . Fact
sheet on cancer. https://www.who.int/news-room/factsheets/detail/cancer#:~:text=Cancer%20is%20the%20second%20leading,%2D%20and%20middle%2Dincome%20countriesAugust
23–2021
|
|
2
|
Kim R, Emi M and Tanabe K: Cancer
immunoediting from immune surveillance to immune escape.
Immunology. 121:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leonardi GC, Accardi G, Monastero R,
Nicoletti F and Libra M: Ageing: From inflammation to cancer. Immun
Ageing. 15:12018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Camous X, Pera A, Solana R and Larbi A: NK
cells in healthy aging and age-associated diseases. J Biomed
Biotechnol. 2012:1959562012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
National Cancer Institute: The genetics of
Cancer. https://www.cancer.gov/about-cancer/causes-prevention/geneticsAugust
23–2021PubMed/NCBI
|
|
6
|
Imai K, Matsuyama S, Miyake S, Suga K and
Nakachi K: Natural cytotoxic activity of peripheral-blood
lymphocytes and cancer incidence: An 11-year follow-up study of a
general population. Lancet. 356:1795–1799. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Y, Liu Y, Dong Y and Vadgama J:
Diabetes-associated dysregulated cytokines and cancer. Integr
Cancer Sci Ther. 3:370–378. 2016.PubMed/NCBI
|
|
8
|
Liefvendahl E and Arnqvist HJ: Mitogenic
effect of the insulin analogue glargine in malignant cells in
comparison with insulin and IGF-I. Horm Metab Res. 40:369–374.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esposito K, Nappo F, Marfella R, Giugliano
G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A and Giugliano D:
Inflammatory cytokine concentrations are acutely increased by
hyperglycemia in humans: Role of oxidative stress. Circulation.
106:2067–2072. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai D and Khor S: ‘Hypothalamic
Microinflammation’ paradigm in aging and metabolic diseases. Cell
Metab. 30:19–35. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang DH, Weaver MT, Park NJ, Smith B,
McArdle T and Carpenter J: Significant impairment in immune
recovery after cancer treatment. Nurs Res. 58:105–114. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Coffey JC, Wang JH, Smith MJ,
Bouchier-Hayes D, Cotter TG and Redmond HP: Excisional surgery for
cancer cure: Therapy at a cost. Lancet Oncol. 4:760–768. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Angka L, Khan ST, Kilgour MK, Xu R,
Kennedy MA and Auer RC: Dysfunctional natural killer cells in the
aftermath of cancer surgery. Int J Mol Sci. 18:17872017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Centers for Disease Control and
Prevention, . Immunization: The Basics. https://www.cdc.gov/vaccines/vac-gen/imz-basics.htmAugust
23–2021
|
|
15
|
Finn OJ: Vaccines for cancer prevention: A
practical and feasible approach to the cancer epidemic. Cancer
Immunol Res. 2:708–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Melief CJ, van Hall T, Arens R, Ossendorp
F and van der Burg SH: Therapeutic cancer vaccines. J Clin Invest.
125:3401–3412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomas S and Prendergast GC: Cancer
vaccines: A brief overview. Methods Mol Biol. 1403:755–761. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
British Society of Immunology, .
Adjuvants: Introduction. British Society of immunology. https://www.immunology.org/public-information/bitesized-immunology/vaccines-and-therapeutics/adjuvants-introductionAugust
23–2021
|
|
19
|
Hu HG and Li YM: Emerging adjuvants for
cancer immunotherapy. Front Chem. 8:6012020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Crunkhorn S: Strengthening the sting of
immunotherapy. Nat Rev Immunol. 20:5892020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pifferi C, Fuentes R and Fernández-Tejada
A: Natural and synthetic carbohydrate-based vaccine adjuvants and
their mechanisms of action. Nat Rev Chem. 25:1–20. 2021.PubMed/NCBI
|
|
22
|
De Maria L and Oestergaard LH:
Carbohydrate oxidases. Patent Ref. No: WO2011009747A1. https://patents.google.com/patent/WO2011009747A1/en
|
|
23
|
Lazarus MB, Nam Y, Jiang J, Sliz P and
Walker S: Structure of human O-GlcNAc transferase and its complex
with a peptide substrate. Nature. 27:564–567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ranjan A, Ramachandran S, Gupta N, Kaushik
I, Wright S, Srivastava S, Das H, Srivastava S, Prasad S and
Srivastava SK: Role of phytochemicals in cancer prevention. Int J
Mol Sci. 20:49812019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Raguindin PF, Itodo OA, Stoyanov J,
Dejanovic GM, Gamba M, Asllanaj E, Minder B, Bussler W, Metzger B,
Muka T, et al: A systematic review of phytochemicals in oat and
buckwheat. Food Chem. 5:1279822021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vetvicka V, Vannucci L, Sima P and Richter
J.: Beta glucan: Supplement or drug? from laboratory to clinical
trials. Molecules. 24:12512019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Du B, Meenu M, Liu H and Xu B: A concise
review on the molecular structure and function relationship of
β-glucan. Int J Mol Sci. 18:40322019. View Article : Google Scholar
|
|
28
|
Seya T, Takeda Y, Takashima K, Yoshida S,
Azuma M and Matsumoto M: Adjuvant immunotherapy for cancer: Both
dendritic cell-priming and check-point inhibitor blockade are
required for immunotherapy. Proc Jpn Acad Ser B Phys Biol Sci.
94:153–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kalafati L, Kourtzelis I,
Schulte-Schrepping J, Li X, Hatzioannou A, Grinenko T, Hagag E,
Sinha A, Has C, Dietz S, et al: Innate immune training of
granulopoiesis promotes anti-tumor activity. Cell. 183:771–785.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chaichian S, Moazzami B, Sadoughi F,
Kashani HH, Zaroudi M and Asemi Z: Functional activities of
beta-glucans in the prevention or treatment of cervical cancer. J
Ovarian Res. 13:242020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cognigni V, Ranallo N, Tronconi F, Morgese
F and Berardi R: Potential benefit of β-glucans as adjuvant therapy
in immuno-oncology: A review. Explor Target Antitumor Ther.
2:122–138. 2021.
|
|
32
|
Jin Y, Li P and Wang F: β-glucans as
potential immunoadjuvants: A review on the adjuvanticity,
structure-activity relationship and receptor recognition
properties. Vaccine. 36:5235–5244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mirza Z, Soto ER, Dikengil F, Levitz SM
and Ostroff GR: Beta-glucan particles as vaccine adjuvant carriers.
Methods Mol Biol. 1625:143–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Paris S, Chapat L, Martin-Cagnon N, Durand
PY, Piney L, Cariou C, Bergamo P, Bonnet JM, Poulet H, Freyburger L
and De Luca K: β-glucan as trained immunity-based adjuvants for
rabies vaccines in dogs. Front Immunol. 8:5644972020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang M, Kim JA and Huang AY: Optimizing
tumor microenvironment for cancer immunotherapy: β-glucan-based
nanoparticles. Front Immunol. 26:3412018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hong F, Hansen RD, Yan J, Allendorf DJ,
Baran JT, Ostroff GR and Ross GD: Beta-glucan functions as an
adjuvant for monoclonal antibody immunotherapy by recruiting
tumoricidal granulocytes as killer cells. Cancer Res. 15:9023–9031.
2003.PubMed/NCBI
|
|
37
|
Geller A and Yan J: Could the induction of
trained immunity by β-glucan serve as a defense against COVID-19?
Front Immunol. 11:17822020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ikewaki N, Iwasaki M, Kurosawa G, Rao KS,
Lakey-Beitia J, Preethy S and Abraham SJ: β-Glucans: Wide-spectrum
immune-balancing food-supplement-based enteric (β-WIFE) vaccine
adjuvant approach to COVID-19. Human Vaccin Immunother.
3:2808–2813. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vetvicka V and Vetvickova J: Glucans and
cancer: Comparison of commercially available β-glucans-Part IV.
Anticancer Res. 38:1327–1333. 2018.PubMed/NCBI
|
|
40
|
Sadeghi F, Peymaeei F, Falahati M, Safari
E, Farahyar S, Mohammadi SR and Roudbary M: The effect of
Candida cell wall beta-glucan on treatment-resistant LL/2
cancer cell line: In vitro evaluation. Mol Biol Rep. 47:3653–3661.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Eiró N and Vizoso FJ: Inflammation and
cancer. World J Gastrointest Surg. 4:62–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Todoric J, Antonucci L and Karin M:
Targeting inflammation in cancer prevention and therapy. Cancer
Prev Res (Phila). 9:895–905. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dedeepiya VD, Sivaraman G, Venkatesh AP,
Preethy S and Abraham SJ: Potential effects of nichi glucan as a
food supplement for diabetes mellitus and hyperlipidemia:
Preliminary findings from the study on three patients from India.
Case Rep Med. 2012:8953702012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ganesh JS, Rao YY, Ravikumar R,
Jayakrishnan GA, Iwasaki M, Preethy S and Abraham SJ: Beneficial
effects of black yeast derived 1-3, 1-6 beta glucan-nichi glucan in
a dyslipidemic individual of Indian origin-a case report. J Diet
Suppl. 11:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bacha U, Nasir M, Iqbal S and Anjum AA:
Nutraceutical, anti-inflammatory, and immune modulatory effects of
β-glucan isolated from yeast. Biomed Res Int. 2017:89726782017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Barera A, Buscemi S, Monastero R, Caruso
C, Caldarella R, Ciaccio M and Vasto S: β-glucans: Ex vivo
inflammatory and oxidative stress results after pasta intake. Immun
Ageing. 7:142016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ikewaki N, Fujii N, Onaka T, Ikewaki S and
Inoko H: Immunological actions of Sophy beta-glucan (beta-1,3-1,6
glucan), currently available commercially as a health food
supplement. Microbiol Immunol. 51:861–873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Qazi BS, Tang K and Qazi A: Recent
advances in underlying pathologies provide insight into
interleukin-8 expression-mediated inflammation and angiogenesis.
Int J Inflam. 2011:9084682011.PubMed/NCBI
|
|
49
|
Kozlowski M, Kowalczuk O, Sulewska A,
Dziegielewski P, Lapuc G, Laudanski W, Niklinska W, Chyczewski L,
Niklinski J and Laudanski J: Serum soluble fas ligand (sFasL) in
patients with primary squamous cell carcinoma of the esophagus.
Folia Histochem Cytobiol. 45:199–204. 2007.PubMed/NCBI
|
|
50
|
Setrerrahmane S and Xu H: Tumor-related
interleukins: Old validated targets for new anti-cancer drug
development. Mol Cancer. 16:1532017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chonov DC, Ignatova MMK, Ananiev JR and
Gulubova MV: IL-6 activities in the tumour microenvironment. Part
1. Open Access Maced J Med Sci. 7:2391–2398. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zaidi MR: The interferon-gamma paradox in
cancer. J Interferon Cytokine Res. 39:30–38. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhao P and Zhang Z: TNF-α promotes colon
cancer cell migration and invasion by upregulating TROP-2. Oncol
Lett. 15:3820–3827. 2018.PubMed/NCBI
|
|
54
|
Chiba S, Ikushima H, Ueki H, Yanai H,
Kimura Y, Hangai S, Nishio J, Negishi H, Tamura T, Saijo S, et al:
Recognition of tumor cells by dectin-1 orchestrates innate immune
cells for anti-tumor responses. Elife. 3:e041772014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yatawara L, Wickramasinghe S, Nagataki M,
Takamoto M, Nomura H, Ikeue Y, Watanabe Y and Agatsuma T:
Aureobasidium-derived soluble branched (1,3–1,6) beta-glucan
(Sophy beta-glucan) enhances natural killer activity in
Leishmania amazonensis-infected mice. Korean J Parasitol.
47:345–351. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Le T, Le T, Doan TH, Quyen D, Le KX, Pham
V, Nagataki M, Nomura H, Ikeue Y, Watanabe Y and Agatsuma T: The
adjuvant effect of sophy β-glucan to the antibody response in
poultry immunized by the avian influenza A H5N1 and H5N2 vaccines.
J Microbiol Biotechnol. 21:405–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ikewaki N, Raghavan K, Dedeepiya VD,
Suryaprakash V, Iwasaki M, Preethy S, Senthilkumar R and Abraham
SJK: Beneficial immune-regulatory effects of novel strains of
Aureobasidium pullulans AFO-202 and N-163 produced beta
glucans in Sprague Dawley rats. 02 August 2021, PREPRINT (Version
1) available at Research Square; doi: 10.21203/rs.3.rs-771315/v1.
View Article : Google Scholar
|
|
58
|
Raghavan K, Dedeepiya VD, Suryaprakash V,
Rao KS, Ikewaki N, Sonoda T, Levy GA, Iwasaki M, Senthilkumar R,
Preethy S and Abraham SJK: Beneficial effects of novel
Aureobasidium pullulans strains produced beta-1,3-1,6
glucans on interleukin-6 and D-Dimer levels in COVID-19 patients;
results of a randomized multiple-arm pilot clinical study. Biomed
Pharmacother. doi: 10.1016/j.biopha.2021.112243. 2021.(In print).
View Article : Google Scholar
|
|
59
|
Ikewaki N, Onaka T, Ikeue Y, Nagataki M,
Kurosawa G, Dedeepiya VD, Rajmohan M, Vaddi S, Senthilkumar R,
Preethy S and Abraham SJK: Beneficial effects of the AFO-202 and
N-163 strains of Aureobasidium pullulans produced 1,3-1,6
beta glucans on non-esterified fatty acid levels in obese diabetic
KKAy mice: A comparative study. bioRxiv. doi:
https://doi.org/10.1101/2021.07.22.453362.
|
|
60
|
Ikewaki N, Kurosawa G, Iwasaki M, Preethy
S, Dedeepiya VD, Vaddi S, Senthilkumar R, Levy GA and Abraham SJK:
Hepatoprotective effects of Aureobasidium pullulans derived
Beta 1,3-1,6 biological response modifier glucans in a STAM-animal
model of non-alcoholic steatohepatitis. bioRxiv. doi:
https://doi.org/10.1101/2021.07.08.451700.
|
|
61
|
Kimura Y, Sumiyoshi M, Suzuki T and
Sakanaka M: Antitumor and antimetastatic activity of a novel
water-soluble low molecular weight beta-1, 3-D-glucan (branch
beta-1,6) isolated from Aureobasidium pullulans 1A1 strain
black yeast. Anticancer Res. 26:4131–4141. 2006.PubMed/NCBI
|
|
62
|
Yano H, Takamoto M, Nagataki M,
Wickramasinghe S, Yatawara L, Mizobuchi S, Sasaguri S, Watanabe Y,
Azuma Y and Azuma K: Induction of NK activity using Sofy β-glucan.
Tosa Biological Society Annual Meeting 2006, Japan. https://b--glucan-org.translate.goog/society/%E5%9C%9F%E4%BD%90%E7%94%9F%E7%89%A9%E5%AD%A6%E4%BC%9A-2006%E5%B9%B4%E5%BA%A6%E4%BE%8B%E4%BC%9A-2/?_x_tr_sch=http&_x_tr_sl=ja&_x_tr_tl=en&_x_tr_hl=en&_x_tr_pto=nui,sc
|
|
63
|
Suzuki T, Kusano K, Kondo N, Nishikawa K,
Kuge T and Ohno N: Biological activity of high-purity
β-1,3-1,6-glucan derived from the black yeast Aureobasidium
pullulans: A literature review. Nutrients. 13:2422021.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ma L, Wang H, Wang C, Su J, Xie Q, Xu L,
Yu Y, Liu S, Li S, Xu Y and Li Z: Failure of elevating calcium
induces oxidative stress tolerance and imparts cisplatin resistance
in ovarian cancer cells. Aging Dis. 7:254–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Steimbach L, Borgmann AV, Gomar GG,
Hoffmann LV, Rutckeviski R, de Andrade DP and Smiderle FR: Fungal
beta-glucans as adjuvants for treating cancer patients-A systematic
review of clinical trials. Clin Nutr. 40:3104–3113. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Papaioannou NE, Beniata OV, Vitsos P,
Tsitsilonis O and Samara P: Harnessing the immune system to improve
cancer therapy. Ann Transl Med. 4:2612016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bayindir T, Iraz M, Kelles M, Kaya S, Tan
M, Filiz A, Toplu Y and Kalcioglu MT: The effect of beta glucan on
cisplatin ototoxicity. Indian J Otolaryngol Head Neck Surg.
66:131–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
National Cancer Institute, . OPT-821 with
Vaccine Therapy and Beta-Glucan in Treating Younger Patients with
High-Risk Neuroblastoma. https://www.cancer.gov/about-cancer/treatment/clinical-trials/search/v?id=NCI-2009-01362&r=1
|
|
69
|
Geller A, Shrestha R and Yan J:
Yeast-derived β-glucan in cancer: Novel uses of a traditional
therapeutic. Int J Mol Sci. 20:36182019. View Article : Google Scholar : PubMed/NCBI
|